Recent Advances in Resistive Gas Sensors: Fundamentals, Material and Device Design, and Intelligent Applications
Abstract
1. Introduction
2. Fundamentals of Resistive Gas Sensors
2.1. Working Principle
2.2. Classification
2.2.1. Classification by Sensitive Material Type
2.2.2. Classification by Device Structure Form
2.3. Performance Evaluation of Sensors
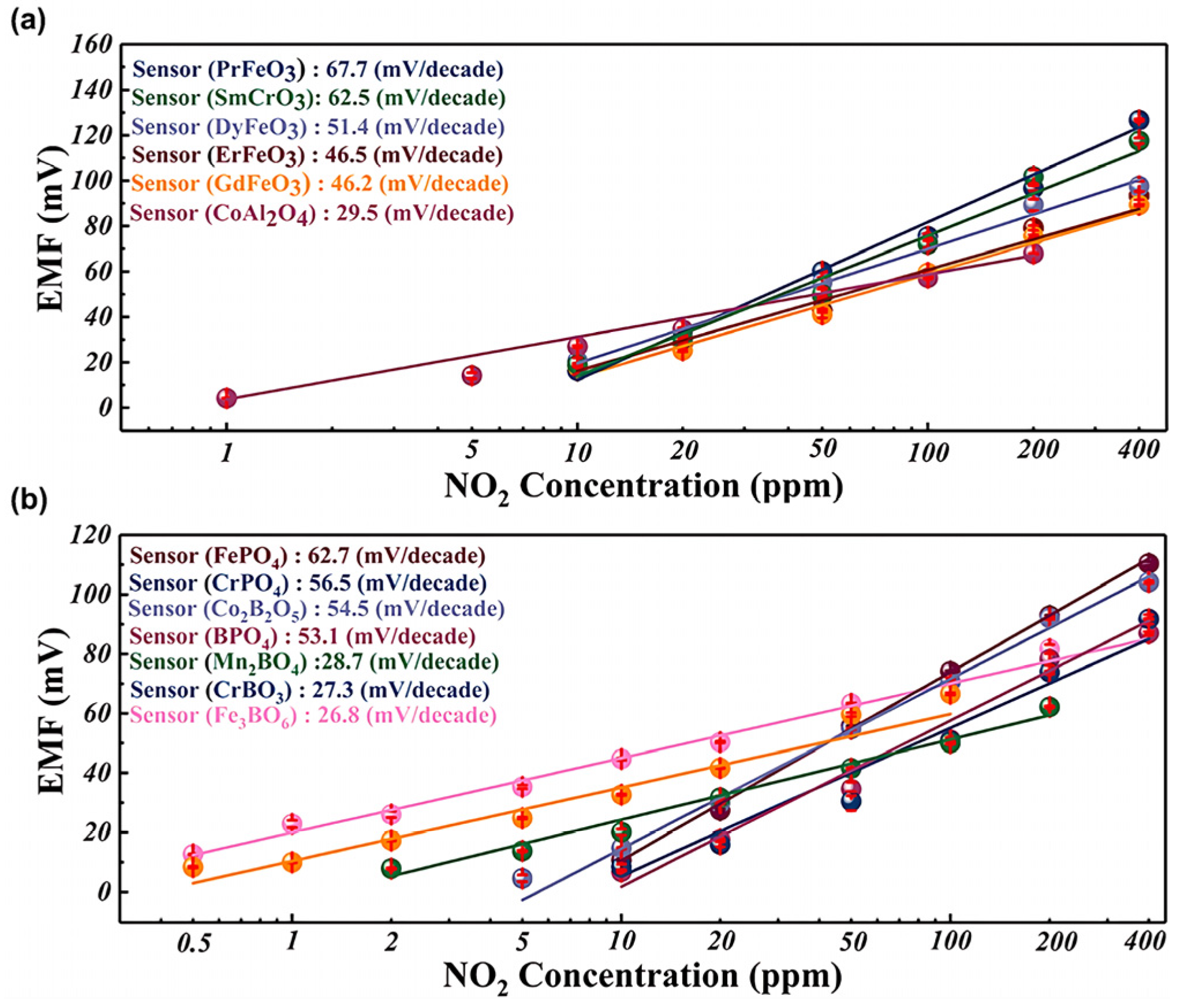
2.3.1. Sensitivity
2.3.2. Selectivity
2.3.3. Response and Recovery Time
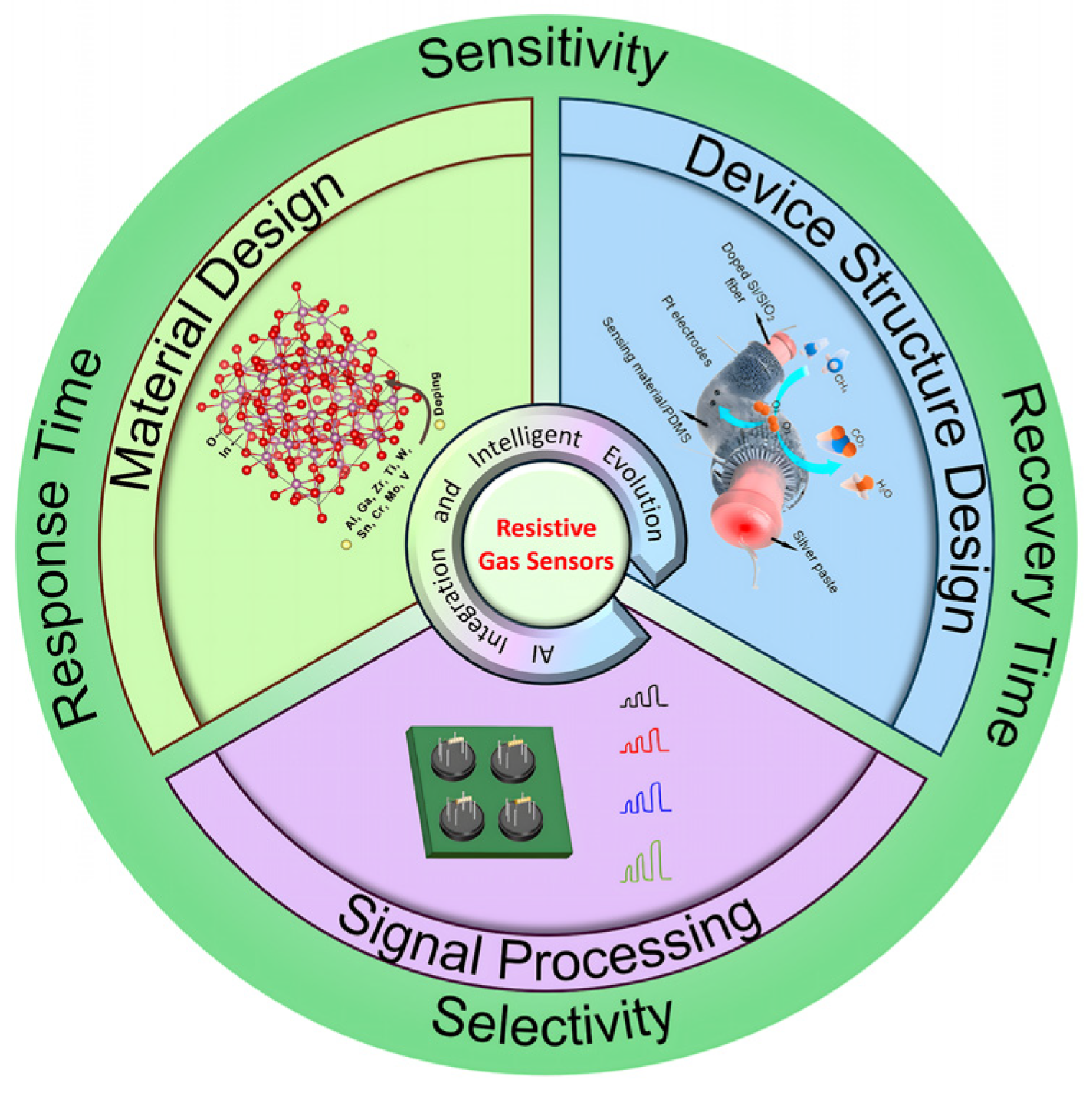
3. Key Technological Advancements
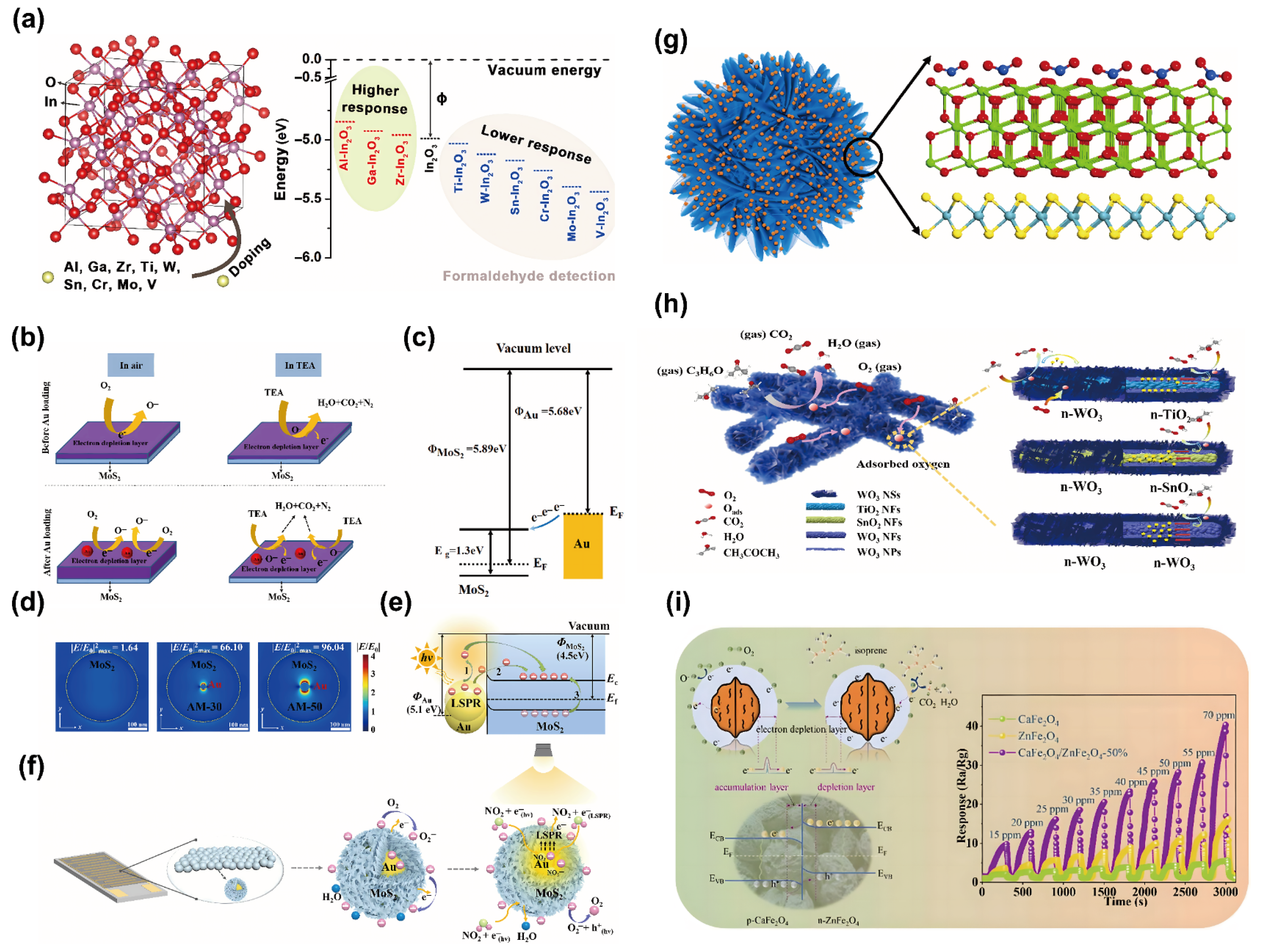
3.1. Material Design
- (i)
- Au NPs increased the thickness of the electron depletion layer on the MoS2/C surface (Figure 4b), thereby enhancing the adsorption capacity for oxygen molecules and providing more active sites for the TEA sensing reaction. This mechanism is crucial for achieving a high response magnitude.
- (ii)
3.2. Device Structure Design
3.2.1. Thickness Control of Sensitive Films
3.2.2. Electrode Spacing Design
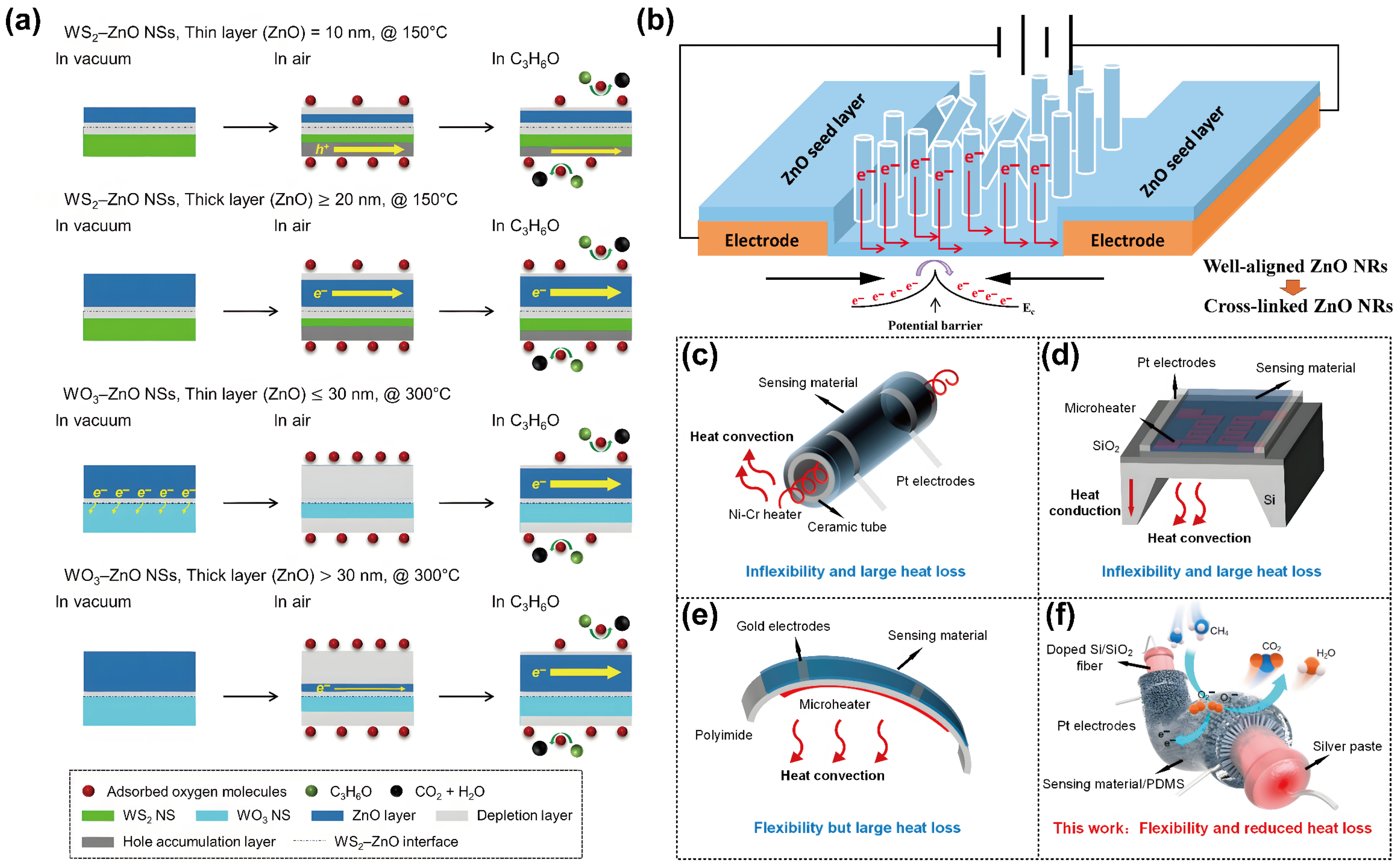
3.2.3. Sensor Array Integration
3.2.4. Integrated Microheater Design
3.3. Signal Processing
3.3.1. Multivariate Response Pattern Decoding
3.3.2. Response Drift Compensation
3.3.3. Baseline Normalization and Dynamic Calibration
4. AI Integration and Intelligent Evolution
4.1. Machine Learning-Assisted Design of Novel Material Compositions
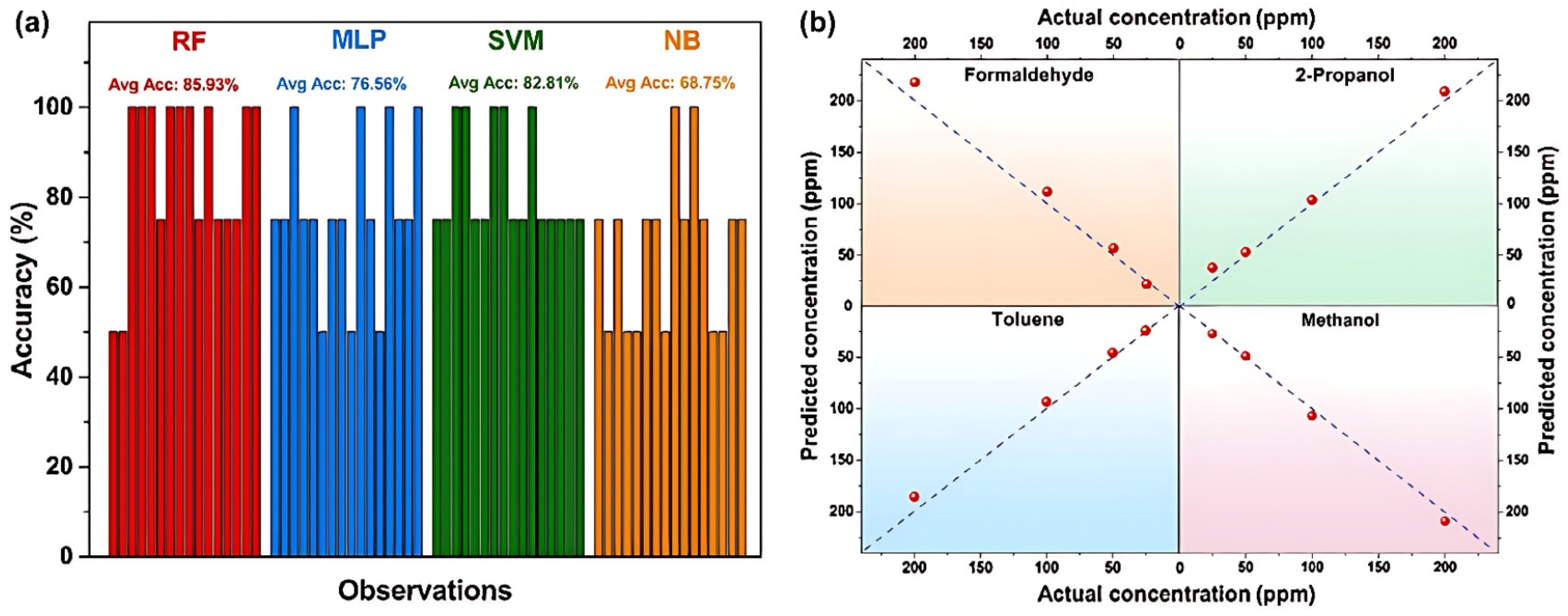
4.2. Intelligent Recognition and Signal Processing
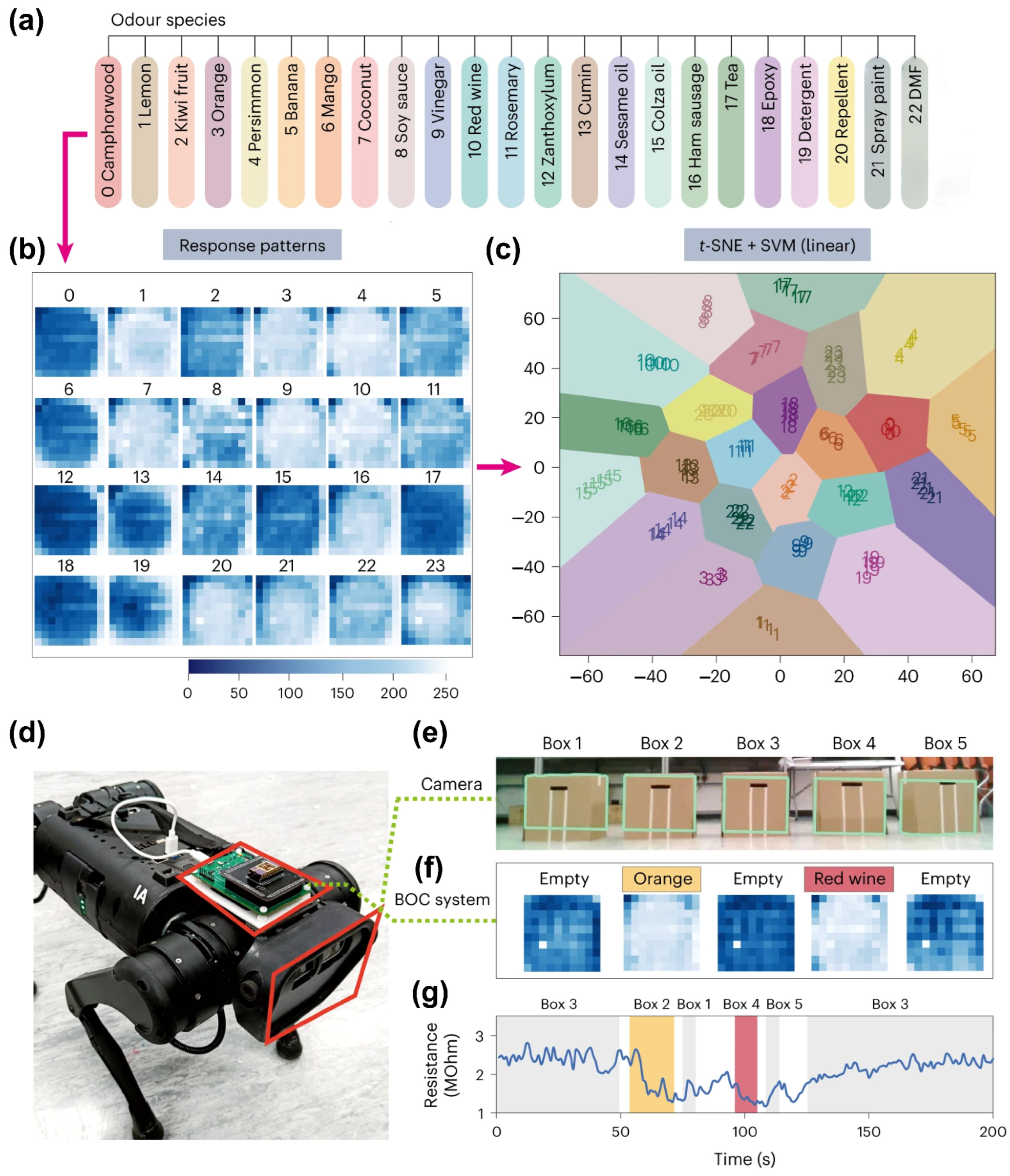
4.3. Intelligent Sensor System
5. Application of Resistive Gas Sensors
5.1. Environmental and Air Pollution Monitoring
5.2. Industrial Safety and VOCs Detection
5.3. Healthcare and Breath Analysis
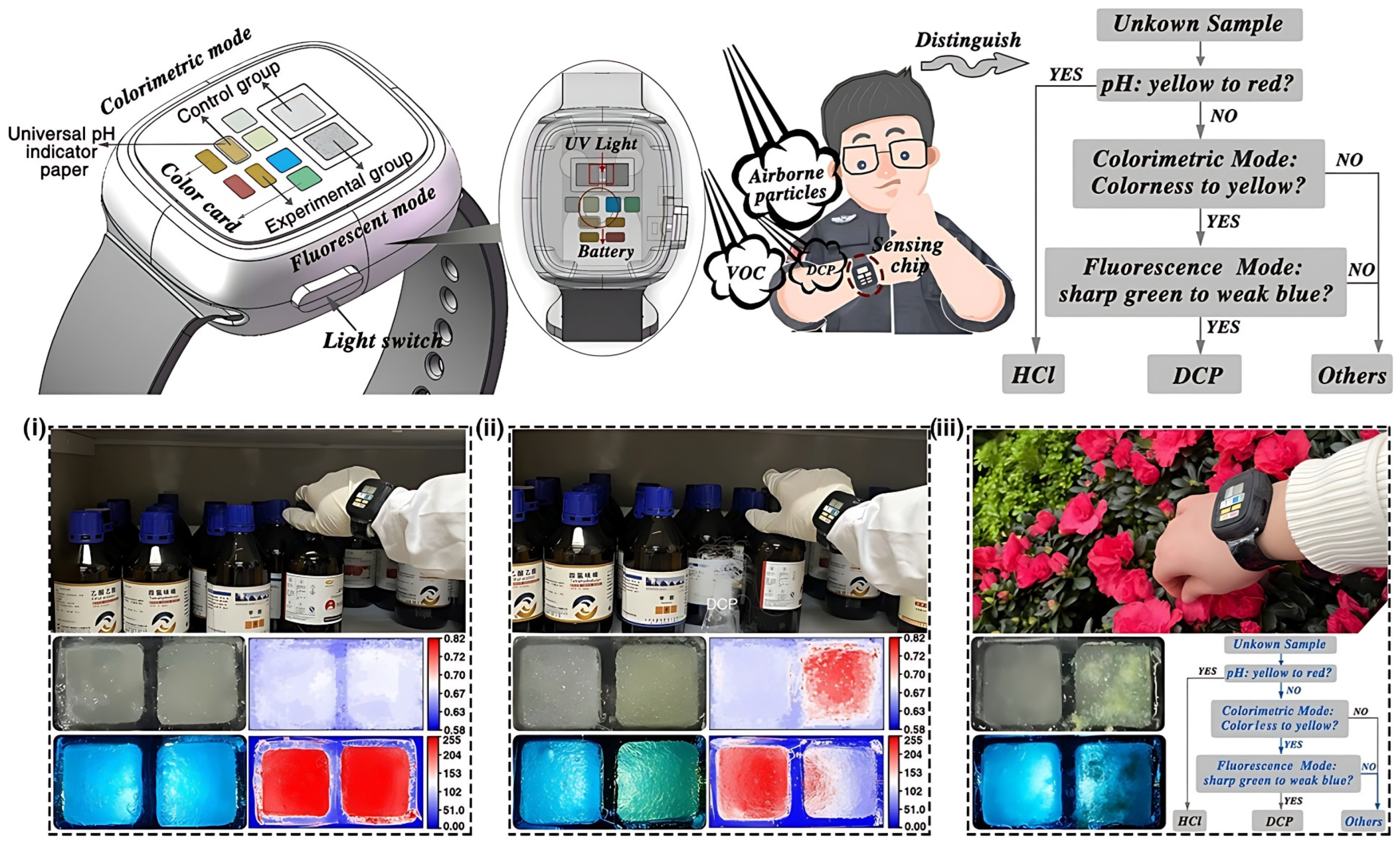
5.4. Smart Cities and Wearable Systems
6. Conclusions and Perspectives
- (1)
- High Power Consumption: Elevated operating temperatures (200–400 °C) for metal oxide sensors increase energy demands and limit integration in portable systems.
- (2)
- Humidity Cross-Sensitivity: Water vapor adsorption alters baseline resistance by >20% at 70%RH, obscuring target gas signals in humid environments.
- (3)
- Drift-Induced Calibration Burden: Long-term material degradation causes signal drift, necessitating frequent recalibration that disrupts continuous monitoring.
- (4)
- Fabrication Inconsistency: Nanomaterial synthesis variations yield >30% performance deviation in sensor arrays, hindering mass production.
- (1)
- Hybrid Material Systems: We emphasize room-temperature operable heterojunctions to eliminate microheaters while maintaining ppb-level sensitivity.
- (2)
- Embedded AI Compensation: We recommend integrating machine learning algorithms into sensor nodes for real-time humidity/drift correction.
- (3)
- Structural Optimization and Standardization: We advocate MEMS-based batch fabrication with optimized geometries to enhance response speed and batch consistency.
- (4)
- Multimodal Sensing Fusion: We highlight the coupling of resistive units with complementary transducers to decouple overlapping gas responses. In practical applications, resistive-type gas sensors have demonstrated wide-ranging utility across multiple domains, including environmental monitoring of hazardous gases such as VOCs and NOx, healthcare diagnostics, intelligent residential systems, and precision agriculture. To enhance selectivity and interference resistance, sensor arrays coupled with machine learning algorithms have been developed to achieve precise identification of mixed gases. The integration of edge computing and embedded artificial intelligence has further advanced real-time monitoring capabilities, facilitating their incorporation into IoT ecosystems. Looking ahead, the convergence of self-smart resistive gas sensors with low-power chip technology is expected to expand their utility in wearable devices and industrial safety applications.
- (1)
- Adaptive Sensing Systems: We suggest exploring lightweight algorithms for on-device learning to autonomously calibrate drift and humidity interference, potentially reducing maintenance costs.
- (2)
- Hybrid Sensing Platforms: We propose combining resistive units with low-cost optical/electrochemical modules to improve cross-validation capability for complex gas mixtures.
- (3)
- Energy Harvesting Integration: We recommend investigating micro-energy harvesters (e.g., solar/motion) to alleviate power constraints in wearable applications.
- (4)
- Accessible Manufacturing: We encourage developing inkjet printing or roll-to-roll processes for low-cost sensor array fabrication.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, Y.; Wei, Y.; Zhu, C.; Cao, J.; Han, D.-M. Ratiometric Fluorescent Signals-Driven Smartphone-Based Portable Sensors for Onsite Visual Detection of Food Contaminants. Coord. Chem. Rev. 2022, 458, 214442. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, L.; Ni, W.; Cheng, W.; Yang, Z.; Xu, S.; Wang, T.; Zhang, B.; Xuan, F. NiO/ZnO Nanocomposites for Multimodal Intelligent MEMS Gas Sensors. ACS Sens. 2025, 10, 2531–2541. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Zeng, M.; Wang, T.; Wu, Y.; Ni, W.; Chen, L.; Yang, J.; Hu, N.; Zhang, B.; Xuan, F.; et al. Gas Sensor Drift Compensation Using Semi-Supervised Ensemble Classifiers with Multi-Level Features and Center Loss. ACS Sens. 2025, 10, 2906–2918. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Mei, H.; Yang, R.; Meng, K.; Shi, L.; Zhao, J.; Zhang, B.; Xuan, F.; Wang, T.; Zhang, T. Olfactory Diagnosis Model for Lung Health Evaluation Based on Pyramid Pooling and SHAP-Based Dual Encoders. ACS Sens. 2024, 9, 4934–4946. [Google Scholar] [CrossRef]
- Mei, H.; Peng, J.; Wang, T.; Zhou, T.; Zhao, H.; Zhang, T.; Yang, Z. Overcoming the Limits of Cross-Sensitivity: Pattern Recognition Methods for Chemiresistive Gas Sensor Array. Nano-Micro Lett. 2024, 16, 269. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Wang, T.; Wu, Y.; Liu, X.; Li, Z.; Yang, R.; Zhang, K.; Yang, J.; Zeng, M.; Hu, N.; et al. Multi-Task Deep Learning Model for Quantitative Volatile Organic Compounds Analysis by Feature Fusion of Electronic Nose Sensing. Sens. Actuators B Chem. 2024, 417, 136206. [Google Scholar] [CrossRef]
- Wang, B.; Yu, Q.; Zhang, S.; Wang, T.; Sun, P.; Chuai, X.; Lu, G. Gas Sensing with Yolk-Shell LaFeO3 Microspheres Prepared by Facile Hydrothermal Synthesis. Sens. Actuators B Chem. 2018, 258, 1215–1222. [Google Scholar] [CrossRef]
- Zainab, Y.; Mohd, N.H.; Ahsanul, K.; Zaiki, A. Gas sensors: A review. Sens. Transducers 2014, 168, 61–75. [Google Scholar]
- Tonezzer, M.; Izidoro, S.C.; Moraes, J.P.A.; Dang, L.T.T. Improved Gas Selectivity Based on Carbon Modified SnO2 Nanowires. Front. Mater. 2019, 6, 277. [Google Scholar] [CrossRef]
- Luo, Y.; Abidian, M.R.; Ahn, J.-H.; Akinwande, D.; Andrews, A.M.; Antonietti, M.; Bao, Z.; Berggren, M.; Berkey, C.A.; Bettinger, C.J.; et al. Technology Roadmap for Flexible Sensors. ACS Nano 2023, 17, 5211–5295. [Google Scholar] [CrossRef]
- Kim, D.-H.; Cha, J.-H.; Lim, J.Y.; Bae, J.; Lee, W.; Yoon, K.R.; Kim, C.; Jang, J.-S.; Hwang, W.; Kim, I.-D. Colorimetric Dye-Loaded Nanofiber Yarn: Eye-Readable and Weavable Gas Sensing Platform. ACS Nano 2020, 14, 16907–16918. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, H.; Ding, Q.; Tao, K.; Shi, W.; Liu, C.; Chen, J.; Wu, J. A Self-Powered, Rechargeable, and Wearable Hydrogel Patch for Wireless Gas Detection with Extraordinary Performance. Adv. Funct. Mater. 2023, 33, 2300046. [Google Scholar] [CrossRef]
- Anju; Saini, L.K.; Pandey, M. Quantum Chemical Analysis of Porphyrin-Based Sensors: Adsorption and Sensing Capabilities of Pure, Protonated, and Metallic Porphyrins Insights into Volatile Organic Compounds (VOCs). Mater. Today Commun. 2024, 41, 110989. [Google Scholar] [CrossRef]
- Elia, E.A.; Stylianou, M.; Agapiou, A. Investigation on the Source of VOCs Emission from Indoor Construction Materials Using Electronic Sensors and TD-GC-MS. Environ. Pollut. 2024, 348, 123765. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Li, Z.; Zheng, H.; Jiang, X.; Wu, H.; Zhou, H.; Liu, H. Disposable, Strain-Insensitive, and Room-Temperature-Operated Flexible Humidity and VOC Sensor with Enhanced Sensitivity and Selectivity through Interface Control. Sens. Actuators B Chem. 2024, 399, 134831. [Google Scholar] [CrossRef]
- Yan, H.; Zhou, Y.-G. Electrical Sensing of Volatile Organic Compounds in Exhaled Breath for Disease Diagnosis. Curr. Opin. Electrochem. 2022, 33, 100922. [Google Scholar] [CrossRef]
- Pathak, A.K.; Viphavakit, C. A Review on All-Optical Fiber-Based VOC Sensors: Heading towards the Development of Promising Technology. Sens. Actuators A Phys. 2022, 338, 113455. [Google Scholar] [CrossRef]
- Acharyya, S.; Bhowmick, P.K.; Guha, P.K. Selective Identification and Quantification of VOCs Using Metal Nanoparticles Decorated SnO2 Hollow-Spheres Based Sensor Array and Machine Learning. J. Alloys Compd. 2023, 968, 171891. [Google Scholar] [CrossRef]
- Shen, Y.; Tissot, A.; Serre, C. Recent progress on MOF-based optical sensors for VOC sensing. Chem. Sci. 2022, 13, 13978–14007. [Google Scholar] [CrossRef]
- Guo, M.; Zhao, T.; Cui, Z. Adsorption Behavior and Sensing Performance of VOCs on Monolayer XC (X = Ge, Si). Surf. Interfaces 2024, 51, 104607. [Google Scholar] [CrossRef]
- Ghazi, M.; Janfaza, S.; Tahmooressi, H.; Tasnim, N.; Hoorfar, M. Selective Detection of VOCs Using Microfluidic Gas Sensor with Embedded Cylindrical Microfeatures Coated with Graphene Oxide. J. Hazard. Mater. 2022, 424, 127566. [Google Scholar] [CrossRef] [PubMed]
- Bogris, A.; Herdt, A.; Syvridis, D.; Elsaber, W. Mid-Infrared Gas Sensor Based on Mutually Injection Locked Quantum Cascade Lasers. IEEE J. Select. Top. Quantum Electron. 2017, 23, 8–14. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yao, W.; Deng, Q. Performance Evaluation of a Portable Infrared Gas Analyser Based on Fourier Infrared Spectral Analysis Technique. Metrol. Sci. Technol. 2025, 69, 53–59, 41. [Google Scholar]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal Oxides for Solid-State Gas Sensors: What Determines Our Choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- El-Muraikhi, M.D.; Ayesh, A.I.; Mirzaei, A. Review of Nanostructured Bi2O3, Bi2WO6, and BiVO4 as Resistive Gas Sensors. Surf. Interfaces 2025, 60, 106003. [Google Scholar] [CrossRef]
- Ghafarinia, V.; Amiri Raeiz, M.; Barami, S. A General Gas-Dependent Resistivity Model for Metal Oxide Semiconductor Gas Sensors Considering the Kinetics of Charge Transfer on the Surface. Sens. Actuators B Chem. 2023, 395, 134488. [Google Scholar] [CrossRef]
- Gupta, S.; Zou, H. Implementing an Analytical Model to Elucidate the Impacts of Nanostructure Size and Topology of Morphologically Diverse Zinc Oxide on Gas Sensing. Chemosensors 2025, 13, 38. [Google Scholar] [CrossRef]
- Hierlemann, A.; Gutierrez-Osuna, R. Higher-Order Chemical Sensing. Chem. Rev. 2008, 108, 563–613. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef]
- Du, X.; Wang, Y.; Mu, Y.; Gui, L.; Wang, P.; Tang, Y. A New Highly Selective H2 Sensor Based on TiO2 /PtO−Pt Dual-Layer Films. Chem. Mater. 2002, 14, 3953–3957. [Google Scholar] [CrossRef]
- Duan, P.; Wang, H.; Peng, Q.; Chen, S.; Zhou, H.; Duan, Q.; Jin, K.; Sun, J. Ultra-Effective Room Temperature Gas Discrimination Based on Monolithic Pd@MOF-Derived Porous Nanocomposites: An Exclusive Scheme with Photoexcitation. J. Mater. Chem. A 2024, 12, 3896–3909. [Google Scholar] [CrossRef]
- Zhou, Q.; Sussman, A.; Chang, J.; Dong, J.; Zettl, A.; Mickelson, W. Fast Response Integrated MEMS Microheaters for Ultra Low Power Gas Detection. Sens. Actuators A Phys. 2015, 223, 67–75. [Google Scholar] [CrossRef]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent Advances in Energy-Saving Chemiresistive Gas Sensors: A Review. Nano Energy 2021, 79, 105369. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Wang, J.; Li, X. A Front-Side Microfabricated Thermoresistive Gas Flow Sensor for High-Performance, Low-Cost and High-Yield Volume Production. Micromachines 2020, 11, 205. [Google Scholar] [CrossRef]
- Zhang, R.; Pang, W.; Feng, Z.; Chen, X.; Chen, Y.; Zhang, Q.; Zhang, H.; Sun, C.; Yang, J.J.; Zhang, D. Enabling Selectivity and Fast Recovery of ZnO Nanowire Gas Sensors through Resistive Switching. Sens. Actuators B Chem. 2017, 238, 357–363. [Google Scholar] [CrossRef]
- Mousavi, S.; Zeinali, S. VOC s detection using resistive gas nanosensor based on MIL-101(Cr) as a metal organic framework. Sens. Actuators A Phys. 2022, 346, 113810. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Y.; Shi, L.; Li, G.-D.; Sun, L.; Zou, X. Revealing the Relationship between Energy Level and Gas Sensing Performance in Heteroatom-Doped Semiconducting Nanostructures. ACS Appl. Mater. Interfaces 2018, 10, 29795–29804. [Google Scholar] [CrossRef]
- Niu, F.; Zhou, F.; Wang, Z.; Wei, L.; Hu, J.; Dong, L.; Ma, Y.; Wang, M.; Jia, S.; Chen, X.; et al. Synthesizing Metal Oxide Semiconductors on Doped Si/SiO2 Flexible Fiber Substrates for Wearable Gas Sensing. Research 2023, 6, 0100. [Google Scholar] [CrossRef]
- Ren, W.; Zhao, C.; Niu, G.; Zhuang, Y.; Wang, F. Gas Sensor Array with Pattern Recognition Algorithms for Highly Sensitive and Selective Discrimination of Trimethylamine. Adv. Intell. Syst. 2022, 4, 2200169. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Jiang, C.; Liu, A.; Xia, B. Quantitative Detection of Formaldehyde and Ammonia Gas via Metal Oxide-Modified Graphene-Based Sensor Array Combining with Neural Network Model. Sens. Actuators B Chem. 2017, 240, 55–65. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Theory of Power Laws for Semiconductor Gas Sensors. Sens. Actuators B Chem. 2008, 128, 566–573. [Google Scholar] [CrossRef]
- Sivaperuman, K.; Thomas, A.; Thangavel, R.; Thirumalaisamy, L.; Palanivel, S.; Pitchaimuthu, S.; Ahsan, N.; Okada, Y. Binary and Ternary Metal Oxide Semiconductor Thin Films for Effective Gas Sensing Applications: A Comprehensive Review and Future Prospects. Prog. Mater. Sci. 2024, 142, 101222. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Xie, L.; Li, X.; Lin, D.; Zhu, Z. Low-Temperature and Highly Sensitivity H2S Gas Sensor Based on ZnO/CuO Composite Derived from Bimetal Metal-Organic Frameworks. Ceram. Int. 2020, 46, 15858–15866. [Google Scholar] [CrossRef]
- Yuan, W.; Shi, G. Graphene-based gas sensors. J. Mater. Chem. A 2013, 1, 10078. [Google Scholar] [CrossRef]
- Lu, G.; Ocola, L.E.; Chen, J. Reduced graphene oxide for room-temperature gas sensors. Nanotechnology 2009, 20, 445502. [Google Scholar] [CrossRef]
- Liu, B.; Liu, X.; Yuan, Z.; Jiang, Y.; Su, Y.; Ma, J.; Tai, H. A flexible NO2 gas sensor based on polypyrrole/nitrogen-doped multiwall carbon nanotube operating at room temperature. Sens. Actuators B Chem. 2019, 295, 86–92. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, Z.; Zeng, D.; Xie, C. Metal-Oxide-Semiconductor Based Gas Sensors: Screening, Preparation, and Integration. Phys. Chem. Chem. Phys. 2017, 19, 6313–6329. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, Y.; Xu, J.; Debliquy, M. Room temperature conductive type metal oxide semiconductor gas sensors for NO2 detection. Sens. Actuators A Phys. 2019, 289, 118–133. [Google Scholar] [CrossRef]
- Lee, E.; VahidMohammadi, A.; Prorok, B.C.; Yoon, Y.S.; Beidaghi, M.; Kim, D.-J. Room Temperature Gas Sensing of Two-Dimensional Titanium Carbide (MXene). ACS Appl. Mater. Interfaces 2017, 9, 37184–37190. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Yavari, F.; Koratkar, N. Graphene-Based Chemical Sensors. J. Phys. Chem. Lett. 2012, 3, 1746–1753. [Google Scholar] [CrossRef]
- Wang, T.; Huang, D.; Yang, Z.; Xu, S.; He, G.; Li, X.; Hu, N.; Yin, G.; He, D.; Zhang, L. A Review on Graphene-Based Gas/Vapor Sensors with Unique Properties and Potential Applications. Nano-Micro Lett. 2016, 8, 95–119. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Choi, Y.N.; Bae, J.M.; Oh, T.S. Carbon Dioxide Sensitivity of La-Doped Thick Film Tin Oxide Gas Sensor. Ceram. Int. 2012, 38, S657–S660. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kulkarni, S.B.; Mathe, V.L. A Multifunctional ZnO Thin Film Based Devices for Photoelectrocatalytic Degradation of Terephthalic Acid and CO2 Gas Sensing Applications. Sens. Actuators B Chem. 2018, 274, 1–9. [Google Scholar] [CrossRef]
- Abdelmounaïm, C.; Amara, Z.; Maha, A.; Mustapha, D. Effects of Molarity on Structural, Optical, Morphological and CO2 Gas Sensing Properties of Nanostructured Copper Oxide Films Deposited by Spray Pyrolysis. Mater. Sci. Semicond. Process. 2016, 43, 214–221. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, K.; Zou, J.; Wang, X.; Sun, L.; Wang, T.; Zhang, Q. Functionalized Horizontally Aligned CNT Array and Random CNT Network for CO2 Sensing. Carbon 2017, 117, 263–270. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A Survey on Gas Sensing Technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, M.; Duan, L.; Zheng, L.; Nguyen, V.; Hung, C.M.; Nguyen, D. Gas classification system based on hybrid waveform modulation technology on FPGA. Sens. Actuators B Chem. 2025, 435, 137637. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor Metal Oxide Gas Sensors: A Review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, Y.; Zhang, H.; Chen, Z.; Zhao, H.; Wu, G.; Zheng, W.; Xue, C.; Yin, Z.; Gao, L. A Fully Integrated Electronic Fabric-Enabled Multimodal Flexible Sensors for Real-Time Wireless Pressure-Humidity-Temperature Monitoring. Int. J. Extrem. Manuf. 2024, 6, 065502. [Google Scholar] [CrossRef]
- Zong, B.; Wu, S.; Yang, Y.; Li, Q.; Tao, T.; Mao, S. Smart Gas Sensors: Recent Developments and Future Prospective. Nano-Micro Lett. 2025, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Weber, I.C.; Güntner, A.T. Catalytic filters for metal oxide gas sensors. Sens. Actuators B Chem. 2022, 356, 131346. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Applications and Advances in Electronic-Nose Technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, K.; Liu, K.; Xu, J.; Zheng, Z. Metal Oxide Resistive Sensors for Carbon Dioxide Detection. Coord. Chem. Rev. 2022, 472, 214758. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, M.; Sun, B.; Song, P. Efficient Detection of Triethylamine by In2O3@Co3O4 Core-Shell Nanofibers Synthesized by Coaxial Electrospinning. Sens. Actuators B Chem. 2025, 439, 137831. [Google Scholar] [CrossRef]
- Chojer, H.; Branco, P.T.B.S.; Martins, F.G.; Alvim-Ferraz, M.C.M.; Sousa, S.I.V. Development of Low-Cost Indoor Air Quality Monitoring Devices: Recent Advancements. Sci. Total Environ. 2020, 727, 138385. [Google Scholar] [CrossRef]
- Kong, Y.; Li, Y.; Cui, X.; Su, L.; Ma, D.; Lai, T.; Yao, L.; Xiao, X.; Wang, Y. SnO2 Nanostructured Materials Used as Gas Sensors for the Detection of Hazardous and Flammable Gases: A Review. Nano Mater. Sci. 2022, 4, 339–350. [Google Scholar] [CrossRef]
- Potyrailo, R.A.; Go, S.; Sexton, D.; Li, X.; Alkadi, N.; Kolmakov, A.; Amm, B.; St-Pierre, R.; Scherer, B.; Nayeri, M.; et al. Extraordinary Performance of Semiconducting Metal Oxide Gas Sensors Using Dielectric Excitation. Nat. Electron. 2020, 3, 280–289. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly Sensitive and Selective Gas Sensors Using P-Type Oxide Semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Wang, J.; Ni, G.; Liao, W.; Liu, K.; Chen, J.; Liu, F.; Zhang, Z.; Jia, M.; Li, J.; Fu, J.; et al. Subsurface Engineering Induced Fermi Level De-Pinning in Metal Oxide Semiconductors for Photoelectrochemical Water Splitting. Angew. Chem. Int. Ed. 2023, 62, e202217026. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, J. Synergistic Enhancement Effect on Surface Au Nanoparticles Decoration on Co-Doped SnO2 Nanobelts: High-Response and Selection for VOC Gas Sensing. Ceram. Int. 2025, 51, 2861–2870. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Wei, Z.; Hu, G.; Cui, Z.; Zhao, Z.; Zhang, Y.; Li, F.; Gong, F.; Wei, S. Engineering of Au Nanoparticles over Hollow MoS2/C Nanoreactor for Enhanced TEA Sensing at Low Temperature. Vacuum 2024, 224, 113114. [Google Scholar] [CrossRef]
- Hu, J.; Liu, X.; Zhang, J.; Gu, X.; Zhang, Y. Plasmon-Activated NO2 Sensor Based on Au@MoS2 Core-Shell Nanoparticles with Heightened Sensitivity and Full Recoverability. Sens. Actuators B Chem. 2023, 382, 133505. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, J.; Yang, X.; Lei, X.; Sun, H.; Huang, Y.; Lu, H.; Ai, T.; Ma, F.; Chu, P.K. Edge-Enriched CeO2/MoS2 Heterostructure with Coupled Interface for Enabling Selective Room-Temperature NO2 Detection. Sens. Actuators B Chem. 2024, 419, 136443. [Google Scholar] [CrossRef]
- Liao, Q.; Sun, Q.; Cao, C.; Hu, J.; Wang, Y.; Li, S.; Xu, J.; Li, G.; Zhu, Y.; Wang, D. One-Dimensional Hierarchical Core-Shell Metal Oxide semiconductor@WO3 Nanocomposites for Ppb-Level Acetone Sensing. Sens. Actuators B Chem. 2024, 415, 136008. [Google Scholar] [CrossRef]
- Guo, W.; Huang, L.; Liu, X.; Wang, J.; Zhang, J. Enhanced Isoprene Gas Sensing Performance Based on P-CaFe2O4/n-ZnFe2O4 Heterojunction Composites. Sens. Actuators B Chem. 2022, 354, 131243. [Google Scholar] [CrossRef]
- Kurtishaj Hamzaj, A.; Donà, E.; M Santhosh, N.; Shvalya, V.; Košiček, M.; Cvelbar, U. Plasma-Modification of Graphene Oxide for Advanced Ammonia Sensing. Appl. Surf. Sci. 2024, 660, 160006. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Z.; Xu, L.; Wang, H.; Peng, S.; Zheng, L.; Yang, Z.; Wu, L.; Miao, J.-T. Preparation of Silver-Plated Carbon Nanotubes/Carbon Fiber Hybrid Fibers by Combining Freeze-Drying Deposition with a Sizing Process to Enhance the Mechanical Properties of Carbon Fiber Composites. Compos. Part A Appl. Sci. Manuf. 2021, 146, 106421. [Google Scholar] [CrossRef]
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S.; Joshi, D. Nanostructured Metal Oxide Semiconductor-Based Gas Sensors: A Comprehensive Review. Sens. Actuators A Phys. 2022, 341, 113578. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Mirzaei, A.; Kim, J.-H. Effect of ZnO Thickness on Gas Sensing Behavior of WS2-ZnO p-n Heterojunction Nanosheets towards Reducing Gases. J. Alloys Compd. 2024, 984, 173967. [Google Scholar] [CrossRef]
- Bai, J.; Zhao, C.; Gong, H.; Wang, Q.; Huang, B.; Sun, G.; Wang, Y.; Zhou, J.; Xie, E.; Wang, F. Debye-Length Controlled Gas Sensing Performances in NiO@ZnO p-n Junctional Core–Shell Nanotubes. J. Phys. D Appl. Phys. 2019, 52, 285103. [Google Scholar] [CrossRef]
- Kim, D.; Shin, W.; Hong, S.; Jeong, Y.; Jung, G.; Park, J.; Lee, J.-H. Effects of Electrode Structure on H2S Sensing and Low-Frequency Noise Characteristics in In2O3-Based Resistor-Type Gas Sensors. IEEE Sens. J. 2022, 22, 6311–6320. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Chen, H.-I.; Hsu, C.-S.; Huang, C.-C.; Wu, J.-S.; Chou, P.-C.; Liu, W.-C. Characteristics of ZnO Nanorods-Based Ammonia Gas Sensors with a Cross-Linked Configuration. Sens. Actuators B Chem. 2015, 221, 491–498. [Google Scholar] [CrossRef]
- Naganaboina, V.R.; Bonam, S.; Anandkumar, M.; Deshpande, A.S.; Singh, S.G. Improved Chemiresistor Gas Sensing Response by Optimizing the Applied Electric Field and Interdigitated Electrode Geometry. Mater. Chem. Phys. 2023, 305, 127975. [Google Scholar] [CrossRef]
- Fàbrega, C.; Casals, O.; Hernández-Ramírez, F.; Prades, J.D. A Review on Efficient Self-Heating in Nanowire Sensors: Prospects for Very-Low Power Devices. Sens. Actuators B Chem. 2018, 256, 797–811. [Google Scholar] [CrossRef]
- Pandit, N.A.; Alshehri, S.M.; Ahmad, T. CeO2/ZrO2 p-n heterojunction nanostructures for efficient NO2 gas sensing. J. Alloys Compd. 2024, 1004, 175782. [Google Scholar] [CrossRef]
- Ojha, B.; Aleksandrova, M.; Schwotzer, M.; Franzreb, M.; Kohler, H. Thermo-Cyclically Operated Metal Oxide Gas Sensor Arrays for Analysis of Dissolved Volatile Organic Compounds in Fermentation Processes: Part I—Morphology Aspects of the Sensing Behavior. Sens. Bio-Sens. Res. 2023, 40, 100558. [Google Scholar] [CrossRef]
- Haugen, J.-E.; Tomic, O.; Kvaal, K. A Calibration Method for Handling the Temporal Drift of Solid State Gas-Sensors. Anal. Chim. Acta 2000, 407, 23–39. [Google Scholar] [CrossRef]
- Hyeon, J.-S.; Kim, H.-J. Baseline Calibration Scheme Embedded in Single-Slope ADC for Gas Sensor Applications. Electronics 2024, 13, 1252. [Google Scholar] [CrossRef]
- Lee, Y.S.; Chen, J. A Robust Semi-Supervised Learning Scheme for Development of within-Batch Quality Prediction Soft-Sensors. Eng. Appl. Artif. Intell. 2024, 133, 107920. [Google Scholar] [CrossRef]
- Jirayupat, C.; Nagashima, K.; Hosomi, T.; Takahashi, T.; Samransuksamer, B.; Hanai, Y.; Nakao, A.; Nakatani, M.; Liu, J.; Zhang, G.; et al. Breath Odor-Based Individual Authentication by an Artificial Olfactory Sensor System and Machine Learning. Chem. Commun. 2022, 58, 6377–6380. [Google Scholar] [CrossRef]
- Zong, B.; Xu, Q.; Mao, S. Single-Atom Pt-Functionalized Ti3C2Tx Field-Effect Transistor for Volatile Organic Compound Gas Detection. ACS Sens. 2022, 7, 1874–1882. [Google Scholar] [CrossRef]
- Kim, I.; Kim, W.-S.; Kim, K.; Ansari, M.A.; Mehmood, M.Q.; Badloe, T.; Kim, Y.; Gwak, J.; Lee, H.; Kim, Y.-K.; et al. Holographic metasurface gas sensors for instantaneous visual alarms. Sci. Adv. 2021, 7, eabe9943. [Google Scholar] [CrossRef]
- Yuan, Z.; Luo, X.; Meng, F. Machine Learning-Assisted Research and Development of Chemiresistive Gas Sensors. Adv. Eng. Mater. 2024, 26, 2400782. [Google Scholar] [CrossRef]
- Zhang, Q.; Zeng, R.; Lu, Y.; Zhang, J.; Zhou, W.; Yu, J. Machine Learning-Based Prediction of the Adsorption Energy of CO on Boron-Doped Graphene. New J. Chem. 2022, 46, 10451–10457. [Google Scholar] [CrossRef]
- Wang, T.; Tan, X.; Wei, Y.; Jin, H. Accurate Bandgap Predictions of Solids Assisted by Machine Learning. Mater. Today Commun. 2021, 29, 102932. [Google Scholar] [CrossRef]
- Liu, B.; Vu-Bac, N.; Zhuang, X.; Fu, X.; Rabczuk, T. Stochastic Integrated Machine Learning Based Multiscale Approach for the Prediction of the Thermal Conductivity in Carbon Nanotube Reinforced Polymeric Composites. Compos. Sci. Technol. 2022, 224, 109425. [Google Scholar] [CrossRef]
- Geng, X.; Li, S.; Mei, Z.; Li, D.; Zhang, L.; Luo, L. Ultrafast Metal Oxide Reduction at Pd/PdO2 Interface Enables One-Second Hydrogen Gas Detection under Ambient Conditions. Nano Res. 2023, 16, 1149–1157. [Google Scholar] [CrossRef]
- Wang, B.; Li, W.; Lu, Q.; Zhang, Y.; Yu, H.; Huang, L.; Wang, T.; Liang, X.; Liu, F.; Liu, F.; et al. Machine Learning-Assisted Development of Sensitive Electrode Materials for Mixed Potential-Type NO2 Gas Sensors. ACS Appl. Mater. Interfaces 2021, 13, 50121–50131. [Google Scholar] [CrossRef]
- Krivetskiy, V.V.; Andreev, M.D.; Efitorov, A.O.; Gaskov, A.M. Statistical Shape Analysis Pre-Processing of Temperature Modulated Metal Oxide Gas Sensor Response for Machine Learning Improved Selectivity of Gases Detection in Real Atmospheric Conditions. Sens. Actuators B Chem. 2021, 329, 129187. [Google Scholar] [CrossRef]
- Iwata, K.; Abe, H.; Ma, T.; Tadaki, D.; Hirano-Iwata, A.; Kimura, Y.; Suda, S.; Niwano, M. Application of Neural Network Based Regression Model to Gas Concentration Analysis of TiO2 Nanotube-Type Gas Sensors. Sens. Actuators B Chem. 2022, 361, 131732. [Google Scholar] [CrossRef]
- Ogbeide, O.; Bae, G.; Yu, W.; Morrin, E.; Song, Y.; Song, W.; Li, Y.; Su, B.; An, K.; Hasan, T. Inkjet-Printed rGO/Binary Metal Oxide Sensor for Predictive Gas Sensing in a Mixed Environment. Adv. Funct. Mater. 2022, 32, 2113348. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Z.; Chan, C.L.J.; Wan, Z.; Ye, W.; Tang, W.; Ma, Z.; Ren, B.; Zhang, D.; Song, Z.; et al. Biomimetic Olfactory Chips Based on Large-Scale Monolithically Integrated Nanotube Sensor Arrays. Nat. Electron. 2024, 7, 157–167. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, Y.; Zhang, Y.; Ding, X.; Zhang, H.; Cao, T.; Qu, Z.; Ren, J.; Li, L.; Guo, Z.; et al. Modular Assembly of MXene Frameworks for Noninvasive Disease Diagnosis via Urinary Volatiles. ACS Nano 2022, 16, 17376–17388. [Google Scholar] [CrossRef]
- Van Den Broek, J.; Klein Cerrejon, D.; Pratsinis, S.E.; Güntner, A.T. Selective Formaldehyde Detection at Ppb in Indoor Air with a Portable Sensor. J. Hazard. Mater. 2020, 399, 123052. [Google Scholar] [CrossRef]
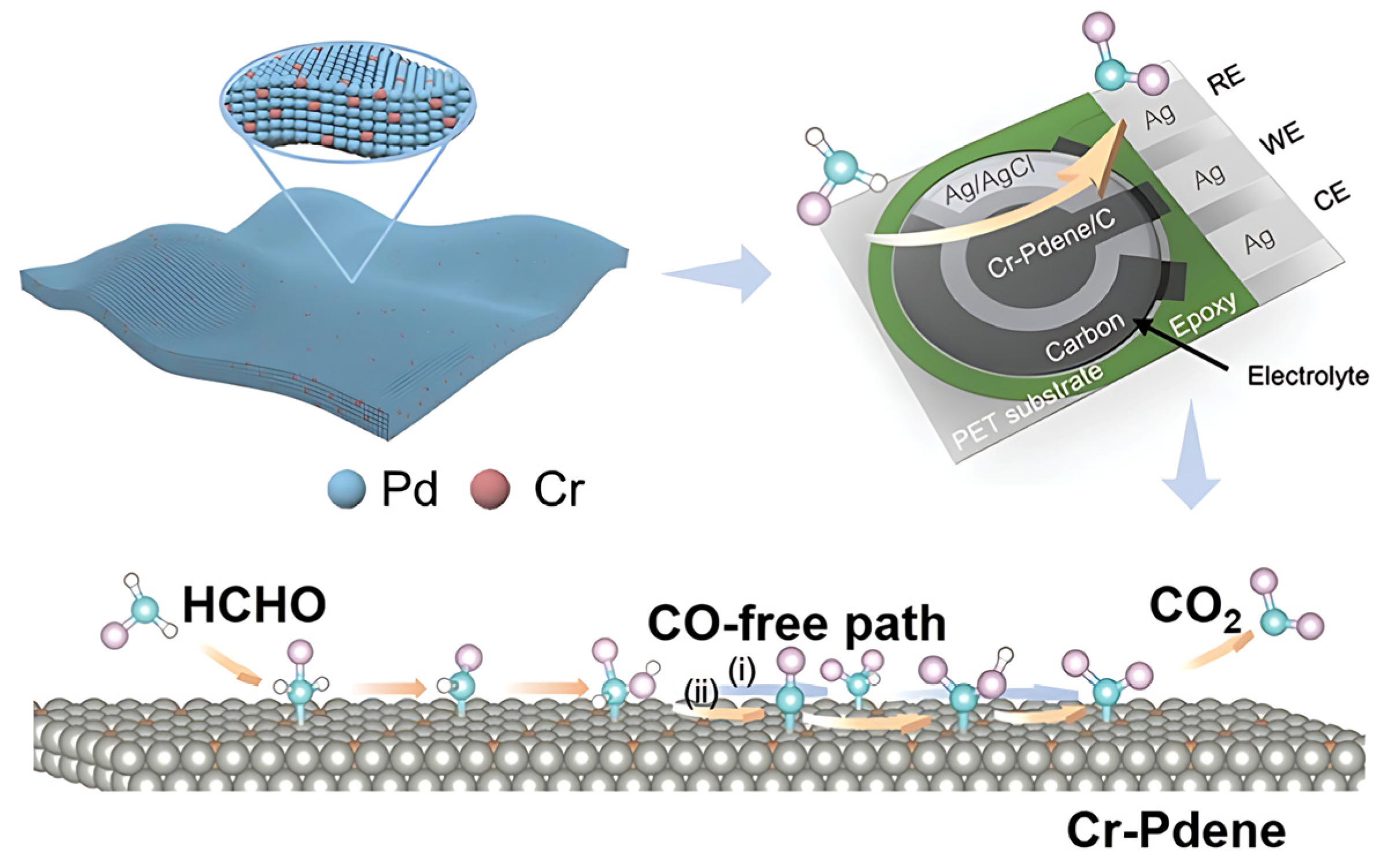
- Zhang, J.; Lv, F.; Li, Z.; Jiang, G.; Tan, M.; Yuan, M.; Zhang, Q.; Cao, Y.; Zheng, H.; Zhang, L.; et al. Cr-Doped Pd Metallene Endows a Practical Formaldehyde Sensor New Limit and High Selectivity. Adv. Mater. 2021, 34, 2105276. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Moon, Y.K.; Wang, J.; Lee, J.-H. Exclusive Detection of Volatile Aromatic Hydrocarbons Using Bilayer Oxide Chemiresistors with Catalytic Overlayers. Nat. Commun. 2023, 14, 233. [Google Scholar] [CrossRef]
- Qin, Z.; Ouyang, C.; Zhang, J.; Wan, L.; Wang, S.; Xie, C.; Zeng, D. 2D WS2 Nanosheets with TiO2 Quantum Dots Decoration for High-Performance Ammonia Gas Sensing at Room Temperature. Sens. Actuators B Chem. 2017, 253, 1034–1042. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, D.; Sun, Q.; Zheng, S.; Sun, J.; Wang, Y. Hierarchical SnS2/SnO2 Nanoheterojunctions with Increased Active-Sites and Charge Transfer for Ultrasensitive NO2 Detection. Nanoscale 2018, 10, 7210–7217. [Google Scholar] [CrossRef] [PubMed]
- Acharyya, S.; Jana, B.; Nag, S.; Saha, G.; Guha, P.K. Single Resistive Sensor for Selective Detection of Multiple VOCs Employing SnO2 Hollowspheres and Machine Learning Algorithm: A Proof of Concept. Sens. Actuators B Chem. 2020, 321, 128484. [Google Scholar] [CrossRef]
- Acharyya, S.; Nag, S.; Guha, P.K. Ultra-Selective Tin Oxide-Based Chemiresistive Gas Sensor Employing Signal Transform and Machine Learning Techniques. Anal. Chim. Acta 2022, 1217, 339996. [Google Scholar] [CrossRef]
- Zhou, Q.; Geng, Z.; Yang, L.; Shen, B.; Kan, Z.; Qi, Y.; Hu, S.; Dong, B.; Bai, X.; Xu, L.; et al. A Wearable Healthcare Platform Integrated with Biomimetical Ions Conducted Metal–Organic Framework Composites for Gas and Strain Sensing in Non-Overlapping Mode. Adv. Sci. 2023, 10, 2207663. [Google Scholar] [CrossRef]
- Meng, Z.; Stolz, R.M.; Mendecki, L.; Mirica, K.A. Electrically-Transduced Chemical Sensors Based on Two-Dimensional Nanomaterials. Chem. Rev. 2019, 119, 478–598. [Google Scholar] [CrossRef]
- Song, Z.; Ye, W.; Chen, Z.; Chen, Z.; Li, M.; Tang, W.; Wang, C.; Wan, Z.; Poddar, S.; Wen, X.; et al. Wireless Self-Powered High-Performance Integrated Nanostructured-Gas-Sensor Network for Future Smart Homes. ACS Nano 2021, 15, 7659–7667. [Google Scholar] [CrossRef]
- Xiao, F.; Lei, D.; Liu, C.; Li, Y.; Ren, W.; Li, J.; Li, D.; Zu, B.; Dou, X. Coherent Modulation of the Aggregation Behavior and Intramolecular Charge Transfer in Small Molecule Probes for Sensitive and Long-term Nerve Agent Monitoring. Angew. Chem. Int. Ed. 2024, 63, e202400453. [Google Scholar] [CrossRef]



| Type | Materials | Analyte Gas/ Concentration (ppm) | T (°C) | Response | τres/τrec (s) | Refs. |
|---|---|---|---|---|---|---|
| n-type | SnO2 | CO2/1000 | 400 | 1.14 | - | [56] |
| ZnO | CO2/400 | 350 | 2.86 | 75/108 | [57] | |
| p-type | NiO | NO2/10 | 300 | 15.7 | - | [51] |
| CuO | CO2/100 | RT | 1.04 | 10/6 | [58] | |
| organic | PANI-based | NH3/1 | RT | - | 12/- | [53] |
| carbon-based | graphene | CO2/100 | RT | 1.26 | 8/10 | [59] |
| CNT | CO2/500 | RT | 1.09 | 33/46 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Xu, S.; Shi, X.; Zhu, J.; Xiong, H.; Wen, H. Recent Advances in Resistive Gas Sensors: Fundamentals, Material and Device Design, and Intelligent Applications. Chemosensors 2025, 13, 224. https://doi.org/10.3390/chemosensors13070224
Wang P, Xu S, Shi X, Zhu J, Xiong H, Wen H. Recent Advances in Resistive Gas Sensors: Fundamentals, Material and Device Design, and Intelligent Applications. Chemosensors. 2025; 13(7):224. https://doi.org/10.3390/chemosensors13070224
Chicago/Turabian StyleWang, Peiqingfeng, Shusheng Xu, Xuerong Shi, Jiaqing Zhu, Haichao Xiong, and Huimin Wen. 2025. "Recent Advances in Resistive Gas Sensors: Fundamentals, Material and Device Design, and Intelligent Applications" Chemosensors 13, no. 7: 224. https://doi.org/10.3390/chemosensors13070224
APA StyleWang, P., Xu, S., Shi, X., Zhu, J., Xiong, H., & Wen, H. (2025). Recent Advances in Resistive Gas Sensors: Fundamentals, Material and Device Design, and Intelligent Applications. Chemosensors, 13(7), 224. https://doi.org/10.3390/chemosensors13070224






