Next-Generation Chemical Sensors: The Convergence of Nanomaterials, Advanced Characterization, and Real-World Applications
Abstract
1. Introduction
2. Materials for Advanced Chemical Sensors
2.1. Carbon-Based Nanomaterials
2.2. Metal and Metal Oxide Nanostructures
2.3. Silicon Nanowires and Semiconductor Materials
2.4. MOFs and Hybrid Composites
2.5. Emerging Materials
3. Fabrication Technologies and Sensor Architectures
3.1. Miniaturization Techniques
3.2. Additive Manufacturing and Printing Technologies
3.3. Wearable and Stretchable Sensor Designs
4. Advanced Characterization and Performance Evaluation
4.1. Spectroscopic and Microscopic Tools
4.2. Electrochemical and Impedance-Based Techniques
4.3. In-Field and Real-Time Validation
5. Application Domains in the United States
5.1. Environmental Monitoring
5.2. Healthcare and Biomedical Diagnostics
5.3. Food Safety and Quality Assurance
5.4. Industrial Process Control
5.5. Smart Cities and IoT-Integrated Sensing Networks
6. Challenges, Limitations, and Future Perspectives
- (a)
- Self-calibrating and self-powered sensors leveraging energy harvesting technologies (e.g., triboelectric, piezoelectric, or biofuel cells) to enable autonomous long-term operation [258].
- (b)
- Multimodal sensing platforms integrating chemical, physical, and biological transduction mechanisms for comprehensive environmental and physiological monitoring [272].
- (c)
- Flexible, stretchable, and biodegradable sensors to expand wearable and implantable applications while minimizing environmental impact [273].
- (d)
- Digital twin frameworks, combining sensor data with real-time simulation models for predictive maintenance, disease progression modeling, and smart city management [274].
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | Two-Dimensional |
| AFM | Atomic Force Microscopy |
| AI | Artificial Intelligence |
| AM | Additive Manufacturing |
| CNT | Carbon Nanotube |
| CO | Carbon Monoxide |
| CO2 | Carbon Dioxide |
| CVD | Chemical Vapor Deposition |
| DIW | Direct Ink Writing |
| DPV | Differential Pulse Voltammetry |
| EIS | Electrochemical Impedance Spectroscopy |
| EPA | Environmental Protection Agency |
| FDM | Fused Deposition Modeling |
| FET | Field-Effect Transistor |
| FDA | Food and Drug Administration |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| GDPR | General Data Protection Regulation |
| GO | Graphene Oxide |
| HIPAA | Health Insurance Portability and Accountability Act |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| IoT | Internet of Things |
| KPFM | Kelvin Probe Force Microscopy |
| LD | Linear Dichroism |
| LOD | Limit of Detection |
| LPR | Linear Polarization Resistance |
| MEMS | Microelectromechanical Systems |
| ML | Machine Learning |
| MOF | Metal–Organic Framework |
| MOS | Metal Oxide Semiconductor |
| MWCNT | Multi-Walled Carbon Nanotube |
| MXene | Transition Metal Carbide or Nitride (general formula Mn+1XnTx) |
| NEMS | Nanoelectromechanical Systems |
| NIH | National Institutes of Health |
| NIL | Nanoimprint Lithography |
| NO | Nitric Oxide |
| NO2 | Nitrogen Dioxide |
| NOx | Nitrogen Oxides |
| NSF | National Science Foundation |
| OPC | Optical Particle Counter |
| PANI | Polyaniline |
| PDMS | Polydimethylsiloxane |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PET | Polyethylene Terephthalate |
| ppb | Parts Per Billion |
| ppm | Parts Per Million |
| ppt | Parts Per Trillion |
| PPy | Polypyrrole |
| QCM | Quartz Crystal Microbalance |
| rGO | Reduced Graphene Oxide |
| SAM | Self-Assembled Monolayer |
| SEM | Scanning Electron Microscopy |
| SiNW | Silicon Nanowire |
| SLA | Stereolithography |
| SPR | Surface Plasmon Resonance |
| STEM-EELS | Scanning Transmission Electron Microscopy–Electron Energy Loss Spectroscopy |
| SWCNT | Single-Walled Carbon Nanotube |
| TMD | Transition Metal Dichalcogenide |
| UV | Ultraviolet |
| VOC | Volatile Organic Compound |
| XPS | X-ray Photoelectron Spectroscopy |
References
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Janata, J.; Josowicz, M. Conducting Polymers in Electronic Chemical Sensors. Nat. Mater. 2003, 2, 19–24. [Google Scholar] [CrossRef]
- Yavari, F.; Koratkar, N. Graphene-Based Chemical Sensors. J. Phys. Chem. Lett. 2012, 3, 1746–1753. [Google Scholar] [CrossRef]
- Tang, R.; Shi, Y.; Hou, Z.; Wei, L. Carbon Nanotube-Based Chemiresistive Sensors. Sensors 2017, 17, 882. [Google Scholar] [CrossRef] [PubMed]
- Gosai, A.; Khondakar, K.; Ma, X.; Ali, M.A. Application of Functionalized Graphene Oxide Based Biosensors for Health Monitoring: Simple Graphene Derivatives to 3D Printed Platforms. Biosensors 2021, 11, 384. [Google Scholar] [CrossRef]
- Shahriari, S.; Sastry, M.; Panjikar, S.; Singh Raman, R. Graphene and Graphene Oxide as a Support for Biomolecules in the Development of Biosensors. NSA 2021, 14, 197–220. [Google Scholar] [CrossRef]
- Zhu, C.; Gerald, R.E.; Huang, J. Metal-Organic Framework Materials Coupled to Optical Fibers for Chemical Sensing: A Review. IEEE Sens. J. 2021, 21, 19647–19661. [Google Scholar] [CrossRef]
- Gittins, J.W.; Ge, K.; Balhatchet, C.J.; Taberna, P.-L.; Simon, P.; Forse, A.C. Understanding Electrolyte Ion Size Effects on the Performance of Conducting Metal–Organic Framework Supercapacitors. J. Am. Chem. Soc. 2024, 146, 12473–12484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lu, L.; Chang, Y.; Liu, M. Gas Sensing Based on Metal-Organic Frameworks: Concepts, Functions, and Developments. J. Hazard. Mater. 2022, 429, 128321. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.K.; Kumar, A.; Mahapatra, C. Smart Nano-Hybrid Metal-Organic Frameworks: Revolutionizing Advancements, Applications, and Challenges in Biomedical Therapeutics and Diagnostics. Hybrid. Adv. 2025, 9, 100406. [Google Scholar] [CrossRef]
- Carter, E.A.; Hungerford, J.T.; Joshi, J.N.; DeWitt, S.J.A.; Jiang, X.; Marszalek, B.; Lively, R.P.; Walton, K.S. Chemical Stability of MIL-101(Cr) upon Adsorption of SO2 and NO2 under Dry and Humid Conditions. Ind. Eng. Chem. Res. 2023, 62, 8864–8872. [Google Scholar] [CrossRef]
- Kong, X.-J.; Li, J.-R. An Overview of Metal–Organic Frameworks for Green Chemical Engineering. Engineering 2021, 7, 1115–1139. [Google Scholar] [CrossRef]
- Gaba, S.; Sahu, M.; Chauhan, N.; Jain, U. Unlocking the Potential of Low-Dimensional MoS2 as a Smart Nanoplatform for Environmental Technologies, Therapeutic Strategies, and Biomedical Sensing. Talanta Open 2025, 12, 100498. [Google Scholar] [CrossRef]
- Wang, W.; Wu, C.; Li, Z.; Liu, K. Interface Engineering of 2D Materials toward High-Temperature Electronic Devices. Adv. Mater. 2025, 37, 2418439. [Google Scholar] [CrossRef]
- Environments and Contaminants—Criteria Air Pollutants. Available online: https://Www.Epa.Gov/Americaschildrenenvironment/Environments-and-Contaminants-Criteria-Air-Pollutants#:~:text=The%20six%20most%20common%20air,the%20Criteria%20Air%20Pollutants%20Indicators (accessed on 1 July 2025).
- United States Government Accountability Office. Air Quality Sensors: Policy Options to Help Address Implementation Challenges; GAO-24-106393; U.S. GAO: Washington, DC, USA, 2024.
- Sheffield, Z.; Paul, P.; Krishnakumar, S.; Pan, D. Current Strategies and Future Directions of Wearable Biosensors for Measuring Stress Biochemical Markers for Neuropsychiatric Applications. Adv. Sci. 2025, 12, 2411339. [Google Scholar] [CrossRef]
- Xu, J.; Fang, Y.; Chen, J. Wearable Biosensors for Non-Invasive Sweat Diagnostics. Biosensors 2021, 11, 245. [Google Scholar] [CrossRef]
- Zeng, F.; Pang, C.; Tang, H. Sensors on Internet of Things Systems for the Sustainable Development of Smart Cities: A Systematic Literature Review. Sensors 2024, 24, 2074. [Google Scholar] [CrossRef] [PubMed]
- Zong, B.; Wu, S.; Yang, Y.; Li, Q.; Tao, T.; Mao, S. Smart Gas Sensors: Recent Developments and Future Prospective. Nano-Micro Lett. 2025, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Chemical Sensors Market Report by Product Type (Electrochemical, Optical, Pellistor/Catalytic Bead, Semiconductor, and Others), Analyte (Solid, Liquid, Gas), Application (Industrial, Environmental Monitoring, Medical, Defense and Homeland Security, and Others), and Region 2025–2033. SR112025A1579. Available online: https://www.giiresearch.com/report/imarc1792430-chemical-sensors-market-report-by-product-type.html#:~:text=List%20of%20Tables,research%20and%20development%20(R&D) (accessed on 3 August 2025).
- Deb, T. Environmental Health and Safety Software Market to Reach USD 99.8 Billion by 2034. Available online: https://media.market.us/environmental-health-and-safety-software-market-news/ (accessed on 30 July 2025).
- Vo, D.-K.; Trinh, K.T.L. Advances in Wearable Biosensors for Healthcare: Current Trends, Applications, and Future Perspectives. Biosensors 2024, 14, 560. [Google Scholar] [CrossRef] [PubMed]
- Cernat, A.; Groza, A.; Tertis, M.; Feier, B.; Hosu-Stancioiu, O.; Cristea, C. Where Artificial Intelligence Stands in the Development of Electrochemical Sensors for Healthcare Applications-A Review. Trends Anal. Chem. 2024, 181, 117999. [Google Scholar] [CrossRef]
- Wang, C.; He, T.; Zhou, H.; Zhang, Z.; Lee, C. Artificial Intelligence Enhanced Sensors—Enabling Technologies to next-Generation Healthcare and Biomedical Platform. Bioelectron. Med. 2023, 9, 17. [Google Scholar] [CrossRef]
- Noopur, J. New Study Revolutionizes Healthcare with Green Wearable Sensors. Available online: https://www.azosensors.com/news.aspx?newsID=15984 (accessed on 3 August 2025).
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh Electron Mobility in Suspended Graphene. Solid. State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, Y.-W.; Stormer, H.L.; Kim, P. Experimental Observation of the Quantum Hall Effect and Berry’s Phase in Graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef]
- Nag, A.; Simorangkir, R.B.V.B.; Gawade, D.R.; Nuthalapati, S.; Buckley, J.L.; O’Flynn, B.; Altinsoy, M.E.; Mukhopadhyay, S.C. Graphene-Based Wearable Temperature Sensors: A Review. Mater. Des. 2022, 221, 110971. [Google Scholar] [CrossRef]
- Behrent, A.; Borggraefe, V.; Baeumner, A.J. Laser-Induced Graphene Trending in Biosensors: Understanding Electrode Shelf-Life of This Highly Porous Material. Anal. Bioanal. Chem. 2024, 416, 2097–2106. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Cheng, H.-M. The Reduction of Graphene Oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Ragab, H.M.; Diab, N.S.; Aleid, G.M.; Aziz, R.A.; Obeidat, S.T.; Yusof, N.; Farea, M.A. Selective H2S Sensor with CdS@PPy/rGO Nanocomposite for Sustainable Air Quality Monitoring. Diam. Relat. Mater. 2025, 154, 112155. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, D.; Liang, R.-P.; Qiu, J.-D. Graphene-Based Optical Nanosensors for Detection of Heavy Metal Ions. Trends Anal. Chem. 2018, 102, 280–289. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Kim, S.; Min, D.-H. Biosensors Based on Graphene Oxide and Its Biomedical Application. Adv. Drug Deliv. Rev. 2016, 105, 275–287. [Google Scholar] [CrossRef]
- Collins, P.G.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme Oxygen Sensitivity of Electronic Properties of Carbon Nanotubes. Science 2000, 287, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, T.; Hallander, P.; Liu, F.; Poot, T.; Åkermo, M. Sensing Abilities of Embedded Vertically Aligned Carbon Nanotube Forests in Structural Composites: From Nanoscale Properties to Mesoscale Functionalities. Compos. Part B Eng. 2023, 255, 110587. [Google Scholar] [CrossRef]
- Oliveira, I.E.; Silva, R.M.; Silva, C.G.; Silva, R.F. Modelling VA-CNT Surface Morphology for Pollutant Adsorption from Aqueous Media. Nanoscale Adv. 2025, 7, 1714–1726. [Google Scholar] [CrossRef]
- Saleh, T.A.; Fadillah, G. Recent Trends in the Design of Chemical Sensors Based on Graphene–Metal Oxide Nanocomposites for the Analysis of Toxic Species and Biomolecules. Trends Anal. Chem. 2019, 120, 115660. [Google Scholar] [CrossRef]
- Speranza, G. Carbon Nanomaterials: Synthesis, Functionalization and Sensing Applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [PubMed]
- Sainz-Urruela, C.; Vera-López, S.; Paz San Andrés, M.; Díez-Pascual, A.M. Surface Functionalization of Graphene Oxide with Tannic Acid: Covalent vs Non-Covalent Approaches. J. Mol. Liq. 2022, 357, 119104. [Google Scholar] [CrossRef]
- Guo, Z.; Chakraborty, S.; Monikh, F.A.; Varsou, D.; Chetwynd, A.J.; Afantitis, A.; Lynch, I.; Zhang, P. Surface Functionalization of Graphene-Based Materials: Biological Behavior, Toxicology, and Safe-By-Design Aspects. Adv. Biol. 2021, 5, 2100637. [Google Scholar] [CrossRef]
- Khan, A.; Alamry, K.A. Surface Modified Carbon Nanotubes: An Introduction. In ACS Symposium Series; Aslam, J., Hussain, C.M., Aslam, R., Eds.; American Chemical Society: Washington, DC, USA, 2022; Volume 1424, pp. 1–25. ISBN 978-0-8412-9749-4. [Google Scholar]
- Dong, R.; Yang, M.; Zuo, Y.; Liang, L.; Xing, H.; Duan, X.; Chen, S. Conducting Polymers-Based Gas Sensors: Principles, Materials, and Applications. Sensors 2025, 25, 2724. [Google Scholar] [CrossRef]
- Khan, M.; Refati, M.F.A.D.; Arup, M.M.R.; Islam, M.A.; Mobarak, M.H. Conductive Polymer-Based Electronics in Additive Manufacturing: Materials, Processing, and Applications. Adv. Polym. Technol. 2025, 2025, 4234491. [Google Scholar] [CrossRef]
- Pargoletti, E.; Cappelletti, G. Breakthroughs in the Design of Novel Carbon-Based Metal Oxides Nanocomposites for VOCs Gas Sensing. Nanomaterials 2020, 10, 1485. [Google Scholar] [CrossRef]
- Channabasavana Hundi Puttaningaiah, K.P. Innovative Carbonaceous Materials and Metal/Metal Oxide Nanoparticles for Electrochemical Biosensor Applications. Nanomaterials 2024, 14, 1890. [Google Scholar] [CrossRef]
- Onyinye Okechukwu, V.; Olayiwola Idris, A.; Umukoro, E.H.; Azizi, S.; Maaza, M. Exploring the Contribution of Intelligent Nanomaterials in Gas Sensing. ChemistrySelect 2024, 9, e202304703. [Google Scholar] [CrossRef]
- Saranya, M.; Ramachandran, R.; Wang, F. Graphene-Zinc Oxide (G-ZnO) Nanocomposite for Electrochemical Supercapacitor Applications. J. Sci. Adv. Mater. Devices 2016, 1, 454–460. [Google Scholar] [CrossRef]
- Tayebi, M.; Kolaei, M.; Tayyebi, A.; Masoumi, Z.; Belbasi, Z.; Lee, B.-K. Reduced Graphene Oxide (RGO) on TiO2 for an Improved Photoelectrochemical (PEC) and Photocatalytic Activity. Sol. Energy 2019, 190, 185–194. [Google Scholar] [CrossRef]
- Zhang, T.; Li, L.; Sun, X.; Shi, Y.; Cheng, W.; Pan, L. Recent Advances in Nanomaterials for Wearable Devices: Classification, Synthesis, and Applications. Nanotechnology 2025, 36, 232003. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Joo, S.W.; Jung, J.H.; Mandal, T.K. Unlocking the Future: Carbon Nanotubes as Pioneers in Sensing Technologies. Chemosensors 2025, 13, 225. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. ZnO Nanostructured Materials and Their Potential Applications: Progress, Challenges and Perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, X. Nanomaterial ZnO Synthesis and Its Photocatalytic Applications: A Review. Nanomaterials 2025, 15, 682. [Google Scholar] [CrossRef]
- Janotti, A.; Van De Walle, C.G. Fundamentals of Zinc Oxide as a Semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc Oxide Nanostructures: Growth, Properties and Applications. J. Phys. Condens. Matter 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Jamal, M.R.; Sameer, O.N.; Ahmed, K.I. A Review on Tin Dioxide Gas Sensor: The Role of the Metal Oxide Doping, Nanoparticles, and Operating Temperatures. World J. Adv. Res. Rev. 2022, 14, 51–62. [Google Scholar] [CrossRef]
- Bârsan, N.; Hübner, M.; Weimar, U. Conduction Mechanisms in SnO2 Based Polycrystalline Thick Film Gas Sensors Exposed to CO and H2 in Different Oxygen Backgrounds. Sens. Actuators B Chem. 2011, 157, 510–517. [Google Scholar] [CrossRef]
- Li, G.; Hou, J.; Hilal, M.; Kim, H.; Chen, Z.; Cui, Y.; Kim, J.-H.; Cai, Z. Development of High-Performance Ethanol Gas Sensors Based on La2O3 Nanoparticles-Embedded Porous SnO2 Nanofibers. Sensors 2024, 24, 6839. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-F.; Liu, S.-B.; Meng, F.-L.; Liu, J.-Y.; Jin, Z.; Kong, L.-T.; Liu, J.-H. Metal Oxide Nanostructures and Their Gas Sensing Properties: A Review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, S.; Li, N.; Li, J.; Cheng, M.; Wei, T.; Liu, Q.; Li, W.; Dong, Y.; Zhang, Y.; et al. Designing One-Dimensional Hierarchical Cu@Cu2O/CuO Core-Shell Heterostructure for Highly Sensitive Detection of NO2 at Room Temperature. Sens. Actuators B Chem. 2023, 378, 133118. [Google Scholar] [CrossRef]
- Franco, M.A.; Conti, P.P.; Andre, R.S.; Correa, D.S. A Review on Chemiresistive ZnO Gas Sensors. Sens. Actuators Rep. 2022, 4, 100100. [Google Scholar] [CrossRef]
- Sowmya, B.; John, A.; Panda, P.K. A Review on Metal-Oxide Based p-n and n-n Heterostructured Nano-Materials for Gas Sensing Applications. Sens. Int. 2021, 2, 100085. [Google Scholar] [CrossRef]
- Scarpelli, F.; Mastropietro, T.F.; Poerio, T.; Godbert, N. Mesoporous TiO2 Thin Films: State of the Art. In Titanium Dioxide—Material for a Sustainable Environment; Yang, D., Ed.; InTech: London, UK, 2018; ISBN 978-1-78923-326-1. [Google Scholar]
- Eddy, D.R.; Permana, M.D.; Sakti, L.K.; Sheha, G.A.N.; Solihudin; Hidayat, S.; Takei, T.; Kumada, N.; Rahayu, I. Heterophase Polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for Efficient Photocatalyst: Fabrication and Activity. Nanomaterials 2023, 13, 704. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas Sensors Based on TiO2 Nanostructured Materials for the Detection of Hazardous Gases: A Review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, K.; Korpal, S.; Dalal, J.; Kumar, A.; Supreet; Kumar, S.; Duhan, S. A Comprehensive Study on Photocatalysis: Materials and Applications. CrystEngComm 2024, 26, 4886–4915. [Google Scholar] [CrossRef]
- Fahimi-Kashani, N.; Orouji, A.; Ghamsari, M.; Sahoo, S.K.; Hormozi-Nezhad, M.R. Plasmonic Noble Metal (Ag and Au) Nanoparticles: From Basics to Colorimetric Sensing Applications. In Gold and Silver Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–58. ISBN 978-0-323-99454-5. [Google Scholar]
- Dykman, L.; Khlebtsov, N. Gold Nanoparticles in Biomedical Applications: Recent Advances and Perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef]
- Guo, L.; Jackman, J.A.; Yang, H.-H.; Chen, P.; Cho, N.-J.; Kim, D.-H. Strategies for Enhancing the Sensitivity of Plasmonic Nanosensors. Nano Today 2015, 10, 213–239. [Google Scholar] [CrossRef]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, G.; Nagashima, K.; Takahashi, T.; Hosomi, T.; Yanagida, T. Metal–Oxide Nanowire Molecular Sensors and Their Promises. Chemosensors 2021, 9, 41. [Google Scholar] [CrossRef]
- Raj, V.J.; Ghosh, R.; Girigoswami, A.; Girigoswami, K. Application of Zinc Oxide Nanoflowers in Environmental and Biomedical Science. BBA Adv. 2022, 2, 100051. [Google Scholar] [CrossRef]
- El-Toni, A.M.; Habila, M.A.; Labis, J.P.; ALOthman, Z.A.; Alhoshan, M.; Elzatahry, A.A.; Zhang, F. Design, Synthesis and Applications of Core–Shell, Hollow Core, and Nanorattle Multifunctional Nanostructures. Nanoscale 2016, 8, 2510–2531. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.S.; Lonkar, S.; Singh, K.K.; Swaminathan, S.; Abdala, A. Recent Advances in Graphene Based Gas Sensors. Sens. Actuators B Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Liang, Y.; Li, H.; Zhao, X.; Xue, L.; Tang, L.; Xue, F.; Yu, T.; Yang, Y. Crystal Facets-Controlled NiO/SnO2 p-n Heterostructures with Engineered Surface and Interface towards Triethylamine Sensing. J. Alloys Compd. 2023, 947, 169503. [Google Scholar] [CrossRef]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire Nanosensors for Highly Sensitive and Selective Detection of Biological and Chemical Species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef]
- Zafar, S.; D’Emic, C.; Jagtiani, A.; Kratschmer, E.; Miao, X.; Zhu, Y.; Mo, R.; Sosa, N.; Hamann, H.; Shahidi, G.; et al. Silicon Nanowire Field Effect Transistor Sensors with Minimal Sensor-to-Sensor Variations and Enhanced Sensing Characteristics. ACS Nano 2018, 12, 6577–6587. [Google Scholar] [CrossRef]
- Stern, E.; Klemic, J.F.; Routenberg, D.A.; Wyrembak, P.N.; Turner-Evans, D.B.; Hamilton, A.D.; LaVan, D.A.; Fahmy, T.M.; Reed, M.A. Label-Free Immunodetection with CMOS-Compatible Semiconducting Nanowires. Nature 2007, 445, 519–522. [Google Scholar] [CrossRef]
- Janićijević, Ž.; Nguyen-Le, T.-A.; Baraban, L. Extended-Gate Field-Effect Transistor Chemo- and Biosensors: State of the Art and Perspectives. Next Nanotechnol. 2023, 3–4, 100025. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Yang, P. Semiconductor Nanowires for Energy Conversion. Chem. Rev. 2010, 110, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Arlett, J.L.; Myers, E.B.; Roukes, M.L. Comparative Advantages of Mechanical Biosensors. Nat. Nanotech 2011, 6, 203–215. [Google Scholar] [CrossRef]
- Chaste, J.; Eichler, A.; Moser, J.; Ceballos, G.; Rurali, R.; Bachtold, A. A Nanomechanical Mass Sensor with Yoctogram Resolution. Nat. Nanotech 2012, 7, 301–304. [Google Scholar] [CrossRef]
- Gao, N.; Zhou, W.; Jiang, X.; Hong, G.; Fu, T.-M.; Lieber, C.M. General Strategy for Biodetection in High Ionic Strength Solutions Using Transistor-Based Nanoelectronic Sensors. Nano Lett. 2015, 15, 2143–2148. [Google Scholar] [CrossRef]
- Yan, R.; Park, J.-H.; Choi, Y.; Heo, C.-J.; Yang, S.-M.; Lee, L.P.; Yang, P. Nanowire-Based Single-Cell Endoscopy. Nat. Nanotech 2012, 7, 191–196. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, T.; Wang, H.; Wang, Z.; Hou, L.; Jiang, J.; Xu, T. Applications of Nanomaterial Technology in Biosensing. J. Sci. Adv. Mater. Devices 2024, 9, 100694. [Google Scholar] [CrossRef]
- Imash, A.; Smagulova, G.; Kaidar, B.; Keneshbekova, A.; Kazhdanbekov, R.; Velasco, L.F.; Mansurov, Z. Chemoresistive Gas Sensors Based on Electrospun 1D Nanostructures: Synergizing Morphology and Performance Optimization. Sensors 2024, 24, 6797. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Sun, L.; Campbell, M.G.; Dincă, M. Electrically Conductive Porous Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2016, 55, 3566–3579. [Google Scholar] [CrossRef]
- Sheberla, D.; Bachman, J.C.; Elias, J.S.; Sun, C.-J.; Shao-Horn, Y.; Dincă, M. Conductive MOF Electrodes for Stable Supercapacitors with High Areal Capacitance. Nat. Mater. 2017, 16, 220–224. [Google Scholar] [CrossRef]
- Campbell, M.G.; Sheberla, D.; Liu, S.F.; Swager, T.M.; Dincă, M. Cu3 (Hexaiminotriphenylene)2: An Electrically Conductive 2D Metal–Organic Framework for Chemiresistive Sensing. Angew. Chem. Int. Ed. 2015, 54, 4349–4352. [Google Scholar] [CrossRef]
- Zuliani, A.; Khiar, N.; Carrillo-Carrión, C. Recent Progress of Metal–Organic Frameworks as Sensors in (Bio)Analytical Fields: Towards Real-World Applications. Anal. Bioanal. Chem. 2023, 415, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Hupp, J.T. Metal−Organic Frameworks as Sensors: A ZIF-8 Based Fabry−Pérot Device as a Selective Sensor for Chemical Vapors and Gases. J. Am. Chem. Soc. 2010, 132, 7832–7833. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Banga, I.K.; Muthukumar, S.; Prasad, S. Engineering the ZIF-8 Pore for Electrochemical Sensor Applications─A Mini Review. ACS Omega 2022, 7, 26993–27003. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, Y.; Lyu, L.; Hou, Q.; Caro, J.; Wang, H. Flexible Polypropylene-Supported ZIF-8 Membranes for Highly Efficient Propene/Propane Separation. J. Am. Chem. Soc. 2020, 142, 20915–20919. [Google Scholar] [CrossRef] [PubMed]
- Salman, F.; Kazıcı, H.Ç.; Gülcan, M. Comparative of MIL101(Cr) and Nano -MIL101(Cr) Electrode as an Electrochemical Hydrogen Peroxide Sensor. Electroanalysis 2022, 34, 1598–1609. [Google Scholar] [CrossRef]
- Wang, W.; Chen, D.; Li, F.; Xiao, X.; Xu, Q. Metal-Organic-Framework-Based Materials as Platforms for Energy Applications. Chem. 2024, 10, 86–133. [Google Scholar] [CrossRef]
- Crivello, C.; Sevim, S.; Graniel, O.; Franco, C.; Pané, S.; Puigmartí-Luis, J.; Muñoz-Rojas, D. Advanced Technologies for the Fabrication of MOF Thin Films. Mater. Horiz. 2021, 8, 168–178. [Google Scholar] [CrossRef]
- Jajko, G.; Sevillano, J.J.G.; Calero, S.; Makowski, W.; Kozyra, P. The Boost of Toluene Capture in UiO-66 Triggered by Structural Defects or Air Humidity. J. Phys. Chem. Lett. 2023, 14, 5618–5623. [Google Scholar] [CrossRef]
- Shahzadi, S.; Akhtar, M.; Arshad, M.; Ijaz, M.H.; Janjua, M.R.S.A. A Review on Synthesis of MOF-Derived Carbon Composites: Innovations in Electrochemical, Environmental and Electrocatalytic Technologies. RSC Adv. 2024, 14, 27575–27607. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, D.; Wang, G. Covalent Organic Frameworks for Chemical and Biological Sensing. Molecules 2022, 27, 2586. [Google Scholar] [CrossRef]
- Jiang, D.; Tan, V.G.W.; Gong, Y.; Shao, H.; Mu, X.; Luo, Z.; He, S. Semiconducting Covalent Organic Frameworks. Chem. Rev. 2025, 125, 6203–6308. [Google Scholar] [CrossRef] [PubMed]
- Kuila, T.; Bose, S.; Khanra, P.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Recent Advances in Graphene-Based Biosensors. Biosens. Bioelectron. 2011, 26, 4637–4648. [Google Scholar] [CrossRef]
- Late, D.J.; Huang, Y.-K.; Liu, B.; Acharya, J.; Shirodkar, S.N.; Luo, J.; Yan, A.; Charles, D.; Waghmare, U.V.; Dravid, V.P.; et al. Sensing Behavior of Atomically Thin-Layered MoS2 Transistors. ACS Nano 2013, 7, 4879–4891. [Google Scholar] [CrossRef]
- Sarkar, D.; Liu, W.; Xie, X.; Anselmo, A.C.; Mitragotri, S.; Banerjee, K. MoS2 Field-Effect Transistor for Next-Generation Label-Free Biosensors. ACS Nano 2014, 8, 3992–4003. [Google Scholar] [CrossRef]
- Ran, J.; Gao, G.; Li, F.-T.; Ma, T.-Y.; Du, A.; Qiao, S.-Z. Ti3C2 MXene Co-Catalyst on Metal Sulfide Photo-Absorbers for Enhanced Visible-Light Photocatalytic Hydrogen Production. Nat. Commun. 2017, 8, 13907. [Google Scholar] [CrossRef]
- Yu, S.; Li, P.; Ding, H.; Liang, C.; Wang, X. 2D MXenes-Based Gas Sensors: Progress, Applications, and Challenges. Small Methods 2025, 2402179. [Google Scholar] [CrossRef]
- Kumar, P.; Kataria, S.; Subaharan, K.; Chandel, M.; Sahu, B.K.; Sharma, P.; Shanmugam, V. Sensing Nature’s Alarm: SnO2/MXene Gas Sensor Unveils Methyl Jasmonate Signatures of Plant Insect Stress. Nanoscale 2024, 16, 10675–10681. [Google Scholar] [CrossRef]
- Lim, K.R.G.; Handoko, A.D.; Nemani, S.K.; Wyatt, B.; Jiang, H.-Y.; Tang, J.; Anasori, B.; Seh, Z.W. Rational Design of Two-Dimensional Transition Metal Carbide/Nitride (MXene) Hybrids and Nanocomposites for Catalytic Energy Storage and Conversion. ACS Nano 2020, 14, 10834–10864. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.N.; Vij, V.; Kemp, K.C.; Kim, K.S. Engineered Carbon-Nanomaterial-Based Electrochemical Sensors for Biomolecules. ACS Nano 2016, 10, 46–80. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Ang, B.C.; Haseeb, A.S.M.A.; Baharuddin, A.A.; Wong, Y.H. Review—Conducting Polymers as Chemiresistive Gas Sensing Materials: A Review. J. Electrochem. Soc. 2020, 167, 037503. [Google Scholar] [CrossRef]
- Ding, B.; Wang, M.; Yu, J.; Sun, G. Gas Sensors Based on Electrospun Nanofibers. Sensors 2009, 9, 1609–1624. [Google Scholar] [CrossRef] [PubMed]
- Thali, B.G.; Agrahari, D.S.; Kamble, R.M. Current Advancement in Electrochemical Potential of Polypyrrole-Based Composite Materials for Supercapacitor Application: A Mini Review. ChemistrySelect 2025, 10, e202502298. [Google Scholar] [CrossRef]
- Jain, R.; Jadon, N.; Pawaiya, A. Polypyrrole Based next Generation Electrochemical Sensors and Biosensors: A Review. Trends Anal. Chem. 2017, 97, 363–373. [Google Scholar] [CrossRef]
- Sim, D.; Huang, T.; Kim, S.S. Peptide-Functionalized Carbon Nanotube Chemiresistors: The Effect of Nanotube Density on Gas Sensing. Sensors 2023, 23, 8469. [Google Scholar] [CrossRef]
- Tao, H.; Kaplan, D.L.; Omenetto, F.G. Silk Materials—A Road to Sustainable High Technology. Adv. Mater. 2012, 24, 2824–2837. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Hu, J.; Dong, M. Recent Advances in Two-Dimensional Nanomaterials for Sustainable Wearable Electronic Devices. J. Nanobiotechnol 2024, 22, 63. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft Lithography for Micro- and Nanoscale Patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef]
- Wilbur, J.L.; Kumar, A.; Biebuyck, H.A.; Kim, E.; Whitesides, G.M. Microcontact Printing of Self-Assembled Monolayers: Applications in Microfabrication. Nanotechnology 1996, 7, 452–457. [Google Scholar] [CrossRef]
- Chou, S.Y.; Krauss, P.R.; Renstrom, P.J. Nanoimprint Lithography. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 1996, 14, 4129–4133. [Google Scholar] [CrossRef]
- Guo, L.J. Nanoimprint Lithography: Methods and Material Requirements. Adv. Mater. 2007, 19, 495–513. [Google Scholar] [CrossRef]
- Grzelczak, M.; Vermant, J.; Furst, E.M.; Liz-Marzán, L.M. Directed Self-Assembly of Nanoparticles. ACS Nano 2010, 4, 3591–3605. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef] [PubMed]
- Kajbafvala, A.; Bahmanpour, H.; Maneshian, M.H.; Li, M. Self-Assembly Techniques for Nanofabrication. J. Nanomater. 2013, 2013, 158517. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-Based Microfluidic Point-of-Care Diagnostic Devices. Lab. Chip 2013, 13, 2210. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The Present and Future Role of Microfluidics in Biomedical Research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully Integrated Wearable Sensor Arrays for Multiplexed in Situ Perspiration Analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef]
- Maji, M.; Mandal, M.K.; Ghosh Chaudhuri, R. Covalent Organic Frameworks: A State-of-the-Art from Design, Synthesis to Gas Sensing Application with the Prospect of Ammonia Gas Detection. Adv. Sustain. Syst. 2025, 9, e00215. [Google Scholar] [CrossRef]
- Derby, B. Inkjet Printing of Functional and Structural Materials: Fluid Property Requirements, Feature Stability, and Resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Rosati, G.; Ravarotto, M.; Scaramuzza, M.; De Toni, A.; Paccagnella, A. Silver Nanoparticles Inkjet-Printed Flexible Biosensor for Rapid Label-Free Antibiotic Detection in Milk. Sens. Actuators B Chem. 2019, 280, 280–289. [Google Scholar] [CrossRef]
- Yan, C.; Wang, J.; Kang, W.; Cui, M.; Wang, X.; Foo, C.Y.; Chee, K.J.; Lee, P.S. Highly Stretchable Piezoresistive Graphene–Nanocellulose Nanopaper for Strain Sensors. Adv. Mater. 2014, 26, 2022–2027. [Google Scholar] [CrossRef]
- Secor, E.B.; Prabhumirashi, P.L.; Puntambekar, K.; Geier, M.L.; Hersam, M.C. Inkjet Printing of High Conductivity, Flexible Graphene Patterns. J. Phys. Chem. Lett. 2013, 4, 1347–1351. [Google Scholar] [CrossRef]
- Truby, R.L.; Lewis, J.A. Printing Soft Matter in Three Dimensions. Nature 2016, 540, 371–378. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D Printing of Conducting Polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef]
- Yang, H.; Leow, W.R.; Chen, X. 3D Printing of Flexible Electronic Devices. Small Methods 2018, 2, 1700259. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-Invasive Wearable Electrochemical Sensors: A Review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Torrente-Rodríguez, R.M.; Tu, J.; Yang, Y.; Min, J.; Wang, M.; Song, Y.; Yu, Y.; Xu, C.; Ye, C.; IsHak, W.W.; et al. Investigation of Cortisol Dynamics in Human Sweat Using a Graphene-Based Wireless mHealth System. Matter 2020, 2, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Someya, T.; Bao, Z.; Malliaras, G.G. The Rise of Plastic Bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; De Ávila, B.E.-F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Stoppa, M.; Chiolerio, A. Wearable Electronics and Smart Textiles: A Critical Review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef]
- Wu, G.; Du, H.; Cha, Y.L.; Lee, D.; Kim, W.; Feyzbar-Khalkhali-Nejad, F.; Oh, T.-S.; Zhang, X.; Kim, D.-J. A Wearable Mask Sensor Based on Polyaniline/CNT Nanocomposites for Monitoring Ammonia Gas and Human Breathing. Sens. Actuators B Chem. 2023, 375, 132858. [Google Scholar] [CrossRef]
- Ray, T.R.; Choi, J.; Bandodkar, A.J.; Krishnan, S.; Gutruf, P.; Tian, L.; Ghaffari, R.; Rogers, J.A. Bio-Integrated Wearable Systems: A Comprehensive Review. Chem. Rev. 2019, 119, 5461–5533. [Google Scholar] [CrossRef]
- Miller, P.R.; Narayan, R.J.; Polsky, R. Microneedle-Based Sensors for Medical Diagnosis. J. Mater. Chem. B 2016, 4, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel Bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef]
- Heo, J.S.; Eom, J.; Kim, Y.; Park, S.K. Recent Progress of Textile-Based Wearable Electronics: A Comprehensive Review of Materials, Devices, and Applications. Small 2018, 14, 1703034. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Ma, K.; Luo, Y.; Tang, S.; Liu, T.; Liu, J.; Leung, M.; Yang, J.; Hui, R.; Xiong, Y.; et al. Textile Electronics for Wearable Applications. Int. J. Extrem. Manuf. 2023, 5, 042007. [Google Scholar] [CrossRef]
- Baer, D.R.; Engelhard, M.H.; Johnson, G.E.; Laskin, J.; Lai, J.; Mueller, K.; Munusamy, P.; Thevuthasan, S.; Wang, H.; Washton, N.; et al. Surface Characterization of Nanomaterials and Nanoparticles: Important Needs and Challenging Opportunities. J. Vac. Sci. Technol. A 2013, 31, 050820. [Google Scholar] [CrossRef]
- Desimoni, E.; Brunetti, B. X-Ray Photoelectron Spectroscopic Characterization of Chemically Modified Electrodes Used as Chemical Sensors and Biosensors: A Review. Chemosensors 2015, 3, 70–117. [Google Scholar] [CrossRef]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc Oxide Nanostructures for NO2 Gas–Sensor Applications: A Review. Nano-Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Tang, L.; Lu, J.; Li, J. Application of Graphene-Modified Electrode for Selective Detection of Dopamine. Electrochem. Commun. 2009, 11, 889–892. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on Carbon Nanotubes and Graphene Raman Spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef]
- Homola, J. Surface Plasmon Resonance Sensors for Detection of Chemical and Biological Species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; Analytical Techniques in the Sciences, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 978-0-470-85427-3. [Google Scholar]
- Foo, M.L.; Matsuda, R.; Kitagawa, S. Functional Hybrid Porous Coordination Polymers. Chem. Mater. 2014, 26, 310–322. [Google Scholar] [CrossRef]
- Egerton, R.F. Physical Principles of Electron Microscopy; Springer International Publishing: Cham, Swtzerland, 2016; ISBN 978-3-319-39876-1. [Google Scholar]
- He, S.; Song, B.; Li, D.; Zhu, C.; Qi, W.; Wen, Y.; Wang, L.; Song, S.; Fang, H.; Fan, C. A Graphene Nanoprobe for Rapid, Sensitive, and Multicolor Fluorescent DNA Analysis. Adv. Funct. Mater. 2010, 20, 453–459. [Google Scholar] [CrossRef]
- Williams, D.B.; Carter, C.B. Transmission Electron Microscopy; Springer: Boston, MA, USA, 1996; ISBN 978-0-306-45324-3. [Google Scholar]
- Chen, C.; Kang, Y.; Huo, Z.; Zhu, Z.; Huang, W.; Xin, H.L.; Snyder, J.D.; Li, D.; Herron, J.A.; Mavrikakis, M.; et al. Highly Crystalline Multimetallic Nanoframes with Three-Dimensional Electrocatalytic Surfaces. Science 2014, 343, 1339–1343. [Google Scholar] [CrossRef]
- García, R. Dynamic Atomic Force Microscopy Methods. Surf. Sci. Rep. 2002, 47, 197–301. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA; Chichester, UK, 2022; ISBN 978-1-119-33406-4. [Google Scholar]
- Radi, A.-E. Electrochemical Aptamer-Based Biosensors: Recent Advances and Perspectives. Int. J. Electrochem. 2011, 2011, 1–17. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Hussain, A.; Abbas, N.; Ali, A. Inkjet Printing: A Viable Technology for Biosensor Fabrication. Chemosensors 2022, 10, 103. [Google Scholar] [CrossRef]
- Yang, Y.; Song, Y.; Bo, X.; Min, J.; Pak, O.S.; Zhu, L.; Wang, M.; Tu, J.; Kogan, A.; Zhang, H.; et al. A Laser-Engraved Wearable Sensor for Sensitive Detection of Uric Acid and Tyrosine in Sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar] [CrossRef]
- Yoo, E.-H.; Lee, S.-Y. Glucose Biosensors: An Overview of Use in Clinical Practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef]
- Huang, C.-W.; Lin, C.; Nguyen, M.K.; Hussain, A.; Bui, X.-T.; Ngo, H.H. A Review of Biosensor for Environmental Monitoring: Principle, Application, and Corresponding Achievement of Sustainable Development Goals. Bioengineered 2023, 14, 58–80. [Google Scholar] [CrossRef]
- Marx, K.A. Quartz Crystal Microbalance: A Useful Tool for Studying Thin Polymer Films and Complex Biomolecular Systems at the Solution−Surface Interface. Biomacromolecules 2003, 4, 1099–1120. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.S.K.; Saad, M.A.H.S.; Al-Marri, M.J.; Kumar, A. Quartz Crystal Microbalance (QCM) Study of Electrochemical CO2 Reduction on Sn Electrocatalysts. Int. J. Hydrogen Energy 2025, 136, 1142–1151. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Acharyya, D.; Dutta, K. Resistive and Capacitive Measurement of Nano-Structured Gas Sensors. In Environmental Nanotechnology; Dasgupta, N., Ranjan, S., Lichtfouse, E., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2019; Volume 21, pp. 25–62. ISBN 978-3-319-98707-1. [Google Scholar]
- Mathew, S.; Chintagumpala, K. Design and Characterization of PDMS-CB-ZnO-Ternary Composite Based Flexible Capacitance Pressure Sensors for Wearable Applications. Flex. Print. Electron. 2025, 10, 025004. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Liu, Z.; Fu, J.; Shan, T.; Yang, X.; Lei, Q.; Yang, Y.; Li, D. Flexible Capacitive Pressure Sensors for Wearable Electronics. J. Mater. Chem. C 2022, 10, 1594–1605. [Google Scholar] [CrossRef]
- Turner, A.P.F. Biosensors—Sense and Sensitivity. Science 2000, 290, 1315–1317. [Google Scholar] [CrossRef]
- Odobašić, A.; Šestan, I.; Begić, S. Biosensors for Determination of Heavy Metals in Waters. In Biosensors for Environmental Monitoring; Rinken, T., Kivirand, K., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-78923-823-5. [Google Scholar]
- Bandodkar, A.J.; Jeang, W.J.; Ghaffari, R.; Rogers, J.A. Wearable Sensors for Biochemical Sweat Analysis. Annu. Rev. Anal. Chem. 2019, 12, 1–22. [Google Scholar] [CrossRef]
- Delgado, A.; Briciu-Burghina, C.; Regan, F. Antifouling Strategies for Sensors Used in Water Monitoring: Review and Future Perspectives. Sensors 2021, 21, 389. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Pan, Z.; Li, D. Development and Characterization of a Highly Sensitive Fluorometric Transducer for Ultra Low Aqueous Ammonia Nitrogen Measurements in Aquaculture. Comput. Electron. Agric. 2018, 150, 364–373. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.-T.; Li, D.-W.; Long, Y.-T. Recent Developments and Applications of Screen-Printed Electrodes in Environmental Assays—A Review. Anal. Chim. Acta 2012, 734, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Dincer, C.; Bruch, R.; Kling, A.; Dittrich, P.S.; Urban, G.A. Multiplexed Point-of-Care Testing—xPOCT. Trends Biotechnol. 2017, 35, 728–742. [Google Scholar] [CrossRef]
- Zhu, J.; Cho, M.; Li, Y.; He, T.; Ahn, J.; Park, J.; Ren, T.-L.; Lee, C.; Park, I. Machine Learning-Enabled Textile-Based Graphene Gas Sensing with Energy Harvesting-Assisted IoT Application. Nano Energy 2021, 86, 106035. [Google Scholar] [CrossRef]
- Villa, T.; Gonzalez, F.; Miljievic, B.; Ristovski, Z.; Morawska, L. An Overview of Small Unmanned Aerial Vehicles for Air Quality Measurements: Present Applications and Future Prospectives. Sensors 2016, 16, 1072. [Google Scholar] [CrossRef]
- Snyder, E.G.; Watkins, T.H.; Solomon, P.A.; Thoma, E.D.; Williams, R.W.; Hagler, G.S.W.; Shelow, D.; Hindin, D.A.; Kilaru, V.J.; Preuss, P.W. The Changing Paradigm of Air Pollution Monitoring. Environ. Sci. Technol. 2013, 47, 11369–11377. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.C.; Lee, J.D.; Edwards, P.M.; Shaw, M.D.; Evans, M.J.; Moller, S.J.; Smith, K.R.; Buckley, J.W.; Ellis, M.; Gillot, S.R.; et al. Evaluating the Performance of Low Cost Chemical Sensors for Air Pollution Research. Faraday Discuss. 2016, 189, 85–103. [Google Scholar] [CrossRef]
- Castell, N.; Dauge, F.R.; Schneider, P.; Vogt, M.; Lerner, U.; Fishbain, B.; Broday, D.; Bartonova, A. Can Commercial Low-Cost Sensor Platforms Contribute to Air Quality Monitoring and Exposure Estimates? Environ. Int. 2017, 99, 293–302. [Google Scholar] [CrossRef]
- Sousan, S.; Koehler, K.; Hallett, L.; Peters, T.M. Evaluation of the Alphasense Optical Particle Counter (OPC-N2) and the Grimm Portable Aerosol Spectrometer (PAS-1.108). Aerosol Sci. Technol. 2016, 50, 1352–1365. [Google Scholar] [CrossRef]
- Kumar, P.; Skouloudis, A.N.; Bell, M.; Viana, M.; Carotta, M.C.; Biskos, G.; Morawska, L. Real-Time Sensors for Indoor Air Monitoring and Challenges Ahead in Deploying Them to Urban Buildings. Sci. Total Environ. 2016, 560–561, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.M.; Lippard, S.J. Tools and Tactics for the Optical Detection of Mercuric Ion. Chem. Rev. 2008, 108, 3443–3480. [Google Scholar] [CrossRef]
- Honeychurch, K.C.; Hart, J.P.; Cowell, D.C. Voltammetric Behavior and Trace Determination of Lead at a Mercury-Free Screen-Printed Carbon Electrode. Electroanalysis 2000, 12, 171–177. [Google Scholar] [CrossRef]
- English, P.B.; Richardson, M.J.; Garzón-Galvis, C. From Crowdsourcing to Extreme Citizen Science: Participatory Research for Environmental Health. Annu. Rev. Public Health 2018, 39, 335–350. [Google Scholar] [CrossRef]
- Clements, A.L.; Griswold, W.G.; Rs, A.; Johnston, J.E.; Herting, M.M.; Thorson, J.; Collier-Oxandale, A.; Hannigan, M. Low-Cost Air Quality Monitoring Tools: From Research to Practice (A Workshop Summary). Sensors 2017, 17, 2478. [Google Scholar] [CrossRef] [PubMed]
- Hajat, A.; Hsia, C.; O’Neill, M.S. Socioeconomic Disparities and Air Pollution Exposure: A Global Review. Curr. Environ. Health Rep. 2015, 2, 440–450. [Google Scholar] [CrossRef]
- Reid, C.E.; Brauer, M.; Johnston, F.H.; Jerrett, M.; Balmes, J.R.; Elliott, C.T. Critical Review of Health Impacts of Wildfire Smoke Exposure. Environ. Health Perspect. 2016, 124, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Wildfire Smoke Air Monitoring Response Technology (WSMART). Available online: https://Www.Epa.Gov/Air-Sensor-Toolbox/Wildfire-Smoke-Air-Monitoring-Response-Technology-Wsmart#:~:text=Compact%20monitoring%20system%20that%20utilizes,Battery%20powered (accessed on 1 August 2025).
- Seesaard, T.; Kamjornkittikoon, K.; Wongchoosuk, C. A Comprehensive Review on Advancements in Sensors for Air Pollution Applications. Sci. Total Environ. 2024, 951, 175696. [Google Scholar] [CrossRef]
- Mohapatra, A.; Trinh, T. Early Wildfire Detection Technologies in Practice—A Review. Sustainability 2022, 14, 12270. [Google Scholar] [CrossRef]
- Jaffe, D.A.; O’Neill, S.M.; Larkin, N.K.; Holder, A.L.; Peterson, D.L.; Halofsky, J.E.; Rappold, A.G. Wildfire and Prescribed Burning Impacts on Air Quality in the United States. J. Air Waste Manag. Assoc. 2020, 70, 583–615. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency Evaluation of Emerging Air Sensor Performance. Available online: https://www.epa.gov/air-sensor-toolbox/evaluation-emerging-air-sensor-performance (accessed on 9 July 2025).
- Amann, A.; Smith, D. (Eds.) Volatile Biomarkers: Non-Invasive Diagnosis in Physiology and Medicine, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-0-444-62620-2. [Google Scholar]
- Kharitonov, S.A.; Barnes, P.J. Exhaled Markers of Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2001, 163, 1693–1722. [Google Scholar] [CrossRef]
- Minh, T.D.C.; Blake, D.R.; Galassetti, P.R. The Clinical Potential of Exhaled Breath Analysis for Diabetes Mellitus. Diabetes Res. Clin. Pract. 2012, 97, 195–205. [Google Scholar] [CrossRef]
- Righettoni, M.; Tricoli, A.; Pratsinis, S.E. Si:WO3 Sensors for Highly Selective Detection of Acetone for Easy Diagnosis of Diabetes by Breath Analysis. Anal. Chem. 2010, 82, 3581–3587. [Google Scholar] [CrossRef] [PubMed]
- Sonner, Z.; Wilder, E.; Heikenfeld, J.; Kasting, G.; Beyette, F.; Swaile, D.; Sherman, F.; Joyce, J.; Hagen, J.; Kelley-Loughnane, N.; et al. The Microfluidics of the Eccrine Sweat Gland, Including Biomarker Partitioning, Transport, and Biosensing Implications. Biomicrofluidics 2015, 9, 031301. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Valdés-Ramírez, G.; Bandodkar, A.J.; Windmiller, J.R.; Wang, J. Epidermal Biofuel Cells: Energy Harvesting from Human Perspiration. Angew. Chem. Int. Ed. 2013, 52, 7233–7236. [Google Scholar] [CrossRef]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Yin Yin Nyein, H.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous Sweat Extraction and Analysis Applied to Cystic Fibrosis and Glucose Monitoring Using a Fully Integrated Wearable Platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef]
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D.-H. Wearable/Disposable Sweat-Based Glucose Monitoring Device with Multistage Transdermal Drug Delivery Module. Sci. Adv. 2017, 3, e1601314. [Google Scholar] [CrossRef]
- Wyllie, A.L.; Fournier, J.; Casanovas-Massana, A.; Campbell, M.; Tokuyama, M.; Vijayakumar, P.; Warren, J.L.; Geng, B.; Muenker, M.C.; Moore, A.J.; et al. Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Yakoh, A.; Pimpitak, U.; Rengpipat, S.; Hirankarn, N.; Chailapakul, O.; Chaiyo, S. Paper-Based Electrochemical Biosensor for Diagnosing COVID-19: Detection of SARS-CoV-2 Antibodies and Antigen. Biosens. Bioelectron. 2021, 176, 112912. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Kuroki, Y.; Nitta, H.; Chouhan, P.; Toma, K.; Sawada, S.; Takeuchi, S.; Sekita, T.; Akiyoshi, K.; Minakuchi, S.; et al. Mouthguard Biosensor with Telemetry System for Monitoring of Saliva Glucose: A Novel Cavitas Sensor. Biosens. Bioelectron. 2016, 84, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable Sensors: Modalities, Challenges, and Prospects. Lab. Chip 2018, 18, 217–248. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Gao, W.; Shahpar, Z.; Emaminejad, S.; Challa, S.; Chen, K.; Fahad, H.M.; Tai, L.-C.; Ota, H.; Davis, R.W.; et al. A Wearable Electrochemical Platform for Noninvasive Simultaneous Monitoring of Ca2+ and pH. ACS Nano 2016, 10, 7216–7224. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable Non-Invasive Epidermal Glucose Sensors: A Review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef]
- Gao, W.; Ota, H.; Kiriya, D.; Takei, K.; Javey, A. Flexible Electronics toward Wearable Sensing. Acc. Chem. Res. 2019, 52, 523–533. [Google Scholar] [CrossRef]
- Ben Halima, H.; Lakard, B.; Jaffrezic-Renault, N. Microneedle-Based Sensors for Wearable Diagnostics. Chemosensors 2025, 13, 68. [Google Scholar] [CrossRef]
- Erdem, A.; Eksin, E.; Senturk, H.; Yildiz, E.; Maral, M. Recent Developments in Wearable Biosensors for Healthcare and Biomedical Applications. Trends Anal. Chem. 2024, 171, 117510. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Emerging Point-of-Care Technologies for Food Safety Analysis. Sensors 2019, 19, 817. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Hua, L.; Guo, Z.; Jia, F.; Xu, X.; Wang, S.; Lu, Z. Nano-Engineered Fiber-Based Sensing Frontiers: Revolutionizing on-Site Pesticide Detection for Global Food-Environment Nexus Challenges. Coord. Chem. Rev. 2025, 538, 216710. [Google Scholar] [CrossRef]
- Hara, T.O.; Singh, B. Electrochemical Biosensors for Detection of Pesticides and Heavy Metal Toxicants in Water: Recent Trends and Progress. ACS EST Water 2021, 1, 462–478. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Scognamiglio, V.; Moscone, D. Nanomaterials in Electrochemical Biosensors for Pesticide Detection: Advances and Challenges in Food Analysis. Microchim. Acta 2016, 183, 2063–2083. [Google Scholar] [CrossRef]
- Li, Y.; Schluesener, H.J.; Xu, S. Gold Nanoparticle-Based Biosensors. Gold Bull. 2010, 43, 29–41. [Google Scholar] [CrossRef]
- Mauriz, E.; Calle, A.; Montoya, A.; Lechuga, L. Determination of environmental organic pollutants with a portable optical immunosensor. Talanta 2006, 69, 359–364. [Google Scholar] [CrossRef]
- Kuswandi, B.; Wicaksono, Y.; Jayus; Abdullah, A.; Heng, L.Y.; Ahmad, M. Smart Packaging: Sensors for Monitoring of Food Quality and Safety. Sens. Instrumen. Food Qual. 2011, 5, 137–146. [Google Scholar] [CrossRef]
- Valdez, M.; Gupta, S.K.; Lozano, K.; Mao, Y. ForceSpun Polydiacetylene Nanofibers as Colorimetric Sensor for Food Spoilage Detection. Sens. Actuators B Chem. 2019, 297, 126734. [Google Scholar] [CrossRef]
- Bao, F.; Liang, Z.; Deng, J.; Lin, Q.; Li, W.; Peng, Q.; Fang, Y. Toward Intelligent Food Packaging of Biosensor and Film Substrate for Monitoring Foodborne Microorganisms: A Review of Recent Advancements. Crit. Rev. Food Sci. Nutr. 2024, 64, 3920–3931. [Google Scholar] [CrossRef]
- Verma, N.; Bhardwaj, A. Biosensor Technology for Pesticides—A Review. Appl. Biochem. Biotechnol. 2015, 175, 3093–3119. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, J.; Tatam, R.P. Optical Gas Sensing: A Review. Meas. Sci. Technol. 2013, 24, 012004. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal Oxides for Solid-State Gas Sensors: What Determines Our Choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Bao, X.; Chen, L. Recent Progress in Distributed Fiber Optic Sensors. Sensors 2012, 12, 8601–8639. [Google Scholar] [CrossRef]
- Akyildiz, I.F.; Su, W.; Sankarasubramaniam, Y.; Cayirci, E. Wireless Sensor Networks: A Survey. Comput. Netw. 2002, 38, 393–422. [Google Scholar] [CrossRef]
- Kelly, R.G.; John, R.; David, W.; Rudolph, G. Corrosion Technology. In Electrochemical Techniques in Corrosion Science and Engineering; Marcel Dekker: New York, NY, USA, 2003; ISBN 978-0-8247-9917-5. [Google Scholar]
- Bertolini, L.; Elsener, B.; Pedeferri, P.; Redaelli, E.; Polder, R.B. Corrosion of Steel in Concrete: Prevention, Diagnosis, Repair; Bertolini, L., Ed.; Wiley-VCH: Weinheim, Germany, 2004; ISBN 978-3-527-30800-2. [Google Scholar]
- Shevtsov, D.; Cao, N.L.; Nguyen, V.C.; Nong, Q.Q.; Le, H.Q.; Nguyen, D.A.; Zartsyn, I.; Kozaderov, O. Progress in Sensors for Monitoring Reinforcement Corrosion in Reinforced Concrete Structures—A Review. Sensors 2022, 22, 3421. [Google Scholar] [CrossRef]
- Fan, L.; Bao, Y. Review of Fiber Optic Sensors for Corrosion Monitoring in Reinforced Concrete. Cem. Concr. Compos. 2021, 120, 104029. [Google Scholar] [CrossRef]
- Claßen, J.; Aupert, F.; Reardon, K.F.; Solle, D.; Scheper, T. Spectroscopic Sensors for In-Line Bioprocess Monitoring in Research and Pharmaceutical Industrial Application. Anal. Bioanal. Chem. 2017, 409, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Jamrógiewicz, M. Application of the Near-Infrared Spectroscopy in the Pharmaceutical Technology. J. Pharm. Biomed. Anal. 2012, 66, 1–10. [Google Scholar] [CrossRef]
- Villadsen, J.; Nielsen, J.H.; Lidén, G.; Nielsen, J.H. Bioreaction Engineering Principles, 3rd ed.; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-9687-9. [Google Scholar]
- Lee, I.; Lee, K. The Internet of Things (IoT): Applications, Investments, and Challenges for Enterprises. Bus. Horiz. 2015, 58, 431–440. [Google Scholar] [CrossRef]
- Brida, P.; Krejcar, O.; Selamat, A.; Kertesz, A. (Eds.) Smart Sensor Technologies for IoT; MDPI: Basel, Switzerland, 2021; ISBN 978-3-0365-2463-4. [Google Scholar]
- Howard, A.; Matarić, M.J.; Sukhatme, G.S. Mobile Sensor Network Deployment Using Potential Fields: A Distributed, Scalable Solution to the Area Coverage Problem. In Distributed Autonomous Robotic Systems 5; Asama, H., Arai, T., Fukuda, T., Hasegawa, T., Eds.; Springer: Tokyo, Japan, 2002; pp. 299–308. ISBN 978-4-431-65943-3. [Google Scholar]
- Shao, Y.; Wei, L.; Wu, X.; Jiang, C.; Yao, Y.; Peng, B.; Chen, H.; Huangfu, J.; Ying, Y.; Zhang, C.J.; et al. Room-Temperature High-Precision Printing of Flexible Wireless Electronics Based on MXene Inks. Nat. Commun. 2022, 13, 3223. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Morawska, L.; Martani, C.; Biskos, G.; Neophytou, M.; Di Sabatino, S.; Bell, M.; Norford, L.; Britter, R. The Rise of Low-Cost Sensing for Managing Air Pollution in Cities. Environ. Int. 2015, 75, 199–205. [Google Scholar] [CrossRef]
- Catlett, C.E.; Beckman, P.H.; Sankaran, R.; Galvin, K.K. Array of Things: A Scientific Research Instrument in the Public Way: Platform Design and Early Lessons Learned. In Proceedings of the 2nd International Workshop on Science of Smart City Operations and Platforms Engineering, Pittsburgh, PA, USA, 18–21 April 2017; ACM: Pittsburgh, PA, USA, 2017; pp. 26–33. [Google Scholar]
- Cepa, J.J.; Pavón, R.M.; Caramés, P.; Alberti, M.G. A Review of Gas Measurement Practices and Sensors for Tunnels. Sensors 2023, 23, 1090. [Google Scholar] [CrossRef]
- Kim, G.-S.; Son, Y.-S.; Lee, J.-H.; Kim, I.-W.; Kim, J.-C.; Oh, J.-T.; Kim, H. Air Pollution Monitoring and Control System for Subway Stations Using Environmental Sensors. J. Sens. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Mead, M.I.; Popoola, O.A.M.; Stewart, G.B.; Landshoff, P.; Calleja, M.; Hayes, M.; Baldovi, J.J.; McLeod, M.W.; Hodgson, T.F.; Dicks, J.; et al. The Use of Electrochemical Sensors for Monitoring Urban Air Quality in Low-Cost, High-Density Networks. Atmos. Environ. 2013, 70, 186–203. [Google Scholar] [CrossRef]
- Raza, U.; Kulkarni, P.; Sooriyabandara, M. Low Power Wide Area Networks: An Overview. IEEE Commun. Surv. Tutor. 2017, 19, 855–873. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Yang, B.D.; Su, Y.; Tran, P.L.; Joe, P.; Anderson, E.; Xia, J.; Doraiswamy, V.; Dehdashti, B.; Feng, X.; et al. Conformal Piezoelectric Energy Harvesting and Storage from Motions of the Heart, Lung, and Diaphragm. Proc. Natl. Acad. Sci. USA 2014, 111, 1927–1932. [Google Scholar] [CrossRef]
- Lau, B.P.L.; Marakkalage, S.H.; Zhou, Y.; Hassan, N.U.; Yuen, C.; Zhang, M.; Tan, U.-X. A Survey of Data Fusion in Smart City Applications. Inf. Fusion 2019, 52, 357–374. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal′ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A Roadmap for Graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Ervin, M.H.; Le, L.T.; Lee, W.Y. Inkjet-Printed Flexible Graphene-Based Supercapacitor. Electrochim. Acta 2014, 147, 610–616. [Google Scholar] [CrossRef]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical Biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [CrossRef]
- Haupt, K.; Mosbach, K. Molecularly Imprinted Polymers and Their Use in Biomimetic Sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.J.; Han, M.; Desroches, P.E.; Manasa, C.S.; Dennaoui, J.; Quigley, A.F.; Kapsa, R.M.I.; Moulton, S.E.; Guijt, R.M.; Greene, G.W.; et al. Antifouling Strategies for Electrochemical Biosensing: Mechanisms and Performance toward Point of Care Based Diagnostic Applications. ACS Sens. 2021, 6, 1482–1507. [Google Scholar] [CrossRef]
- Benn, T.M.; Westerhoff, P. Nanoparticle Silver Released into Water from Commercially Available Sock Fabrics. Environ. Sci. Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef]
- Tao, H.; Brenckle, M.A.; Yang, M.; Zhang, J.; Liu, M.; Siebert, S.M.; Averitt, R.D.; Mannoor, M.S.; McAlpine, M.C.; Rogers, J.A.; et al. Silk-Based Conformal, Adhesive, Edible Food Sensors. Adv. Mater. 2012, 24, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. Available online: https://Www.Fda.Gov/Medical-Devices (accessed on 6 August 2025).
- EPA U.S. Environmental Protection Agency Air Sensor Toolbox. Available online: https://Www.Epa.Gov/Air-Sensor-Toolbox (accessed on 9 July 2025).
- Price, W.N.; Cohen, I.G. Privacy in the Age of Medical Big Data. Nat. Med. 2019, 25, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Broza, Y.Y.; Haick, H. Nanomaterial-Based Sensors for Detection of Disease by Volatile Organic Compounds. Nanomedicine 2013, 8, 785–806. [Google Scholar] [CrossRef]
- Qazi, S.; Raza, K. Smart Biosensors for an Efficient Point of Care (PoC) Health Management. In Smart Biosensors in Medical Care; Elsevier: Amsterdam, The Netherlands, 2020; pp. 65–85. ISBN 978-0-12-820781-9. [Google Scholar]
- Yang, Y.; Gao, W. Wearable and Flexible Electronics for Continuous Molecular Monitoring. Chem. Soc. Rev. 2019, 48, 1465–1491. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Noghanian, S.; Khan, Z.U.; Alzahrani, S.; Alharbi, S.; Alhartomi, M.; Alsulami, R. Wearable and Flexible Sensor Devices: Recent Advances in Designs, Fabrication Methods, and Applications. Sensors 2025, 25, 1377. [Google Scholar] [CrossRef]
- Abdullahi, I.; Longo, S.; Samie, M. Towards a Distributed Digital Twin Framework for Predictive Maintenance in Industrial Internet of Things (IIoT). Sensors 2024, 24, 2663. [Google Scholar] [CrossRef]
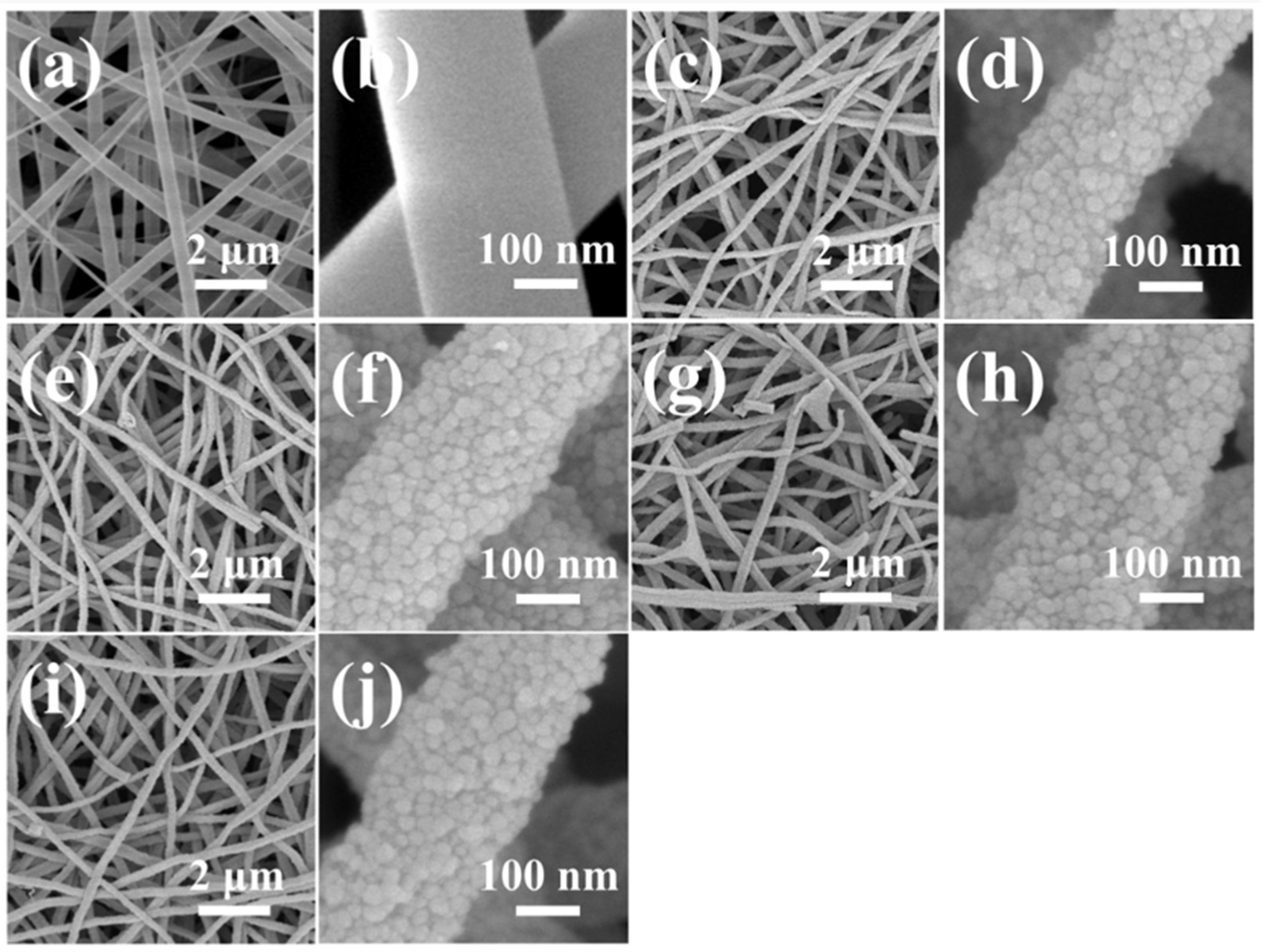
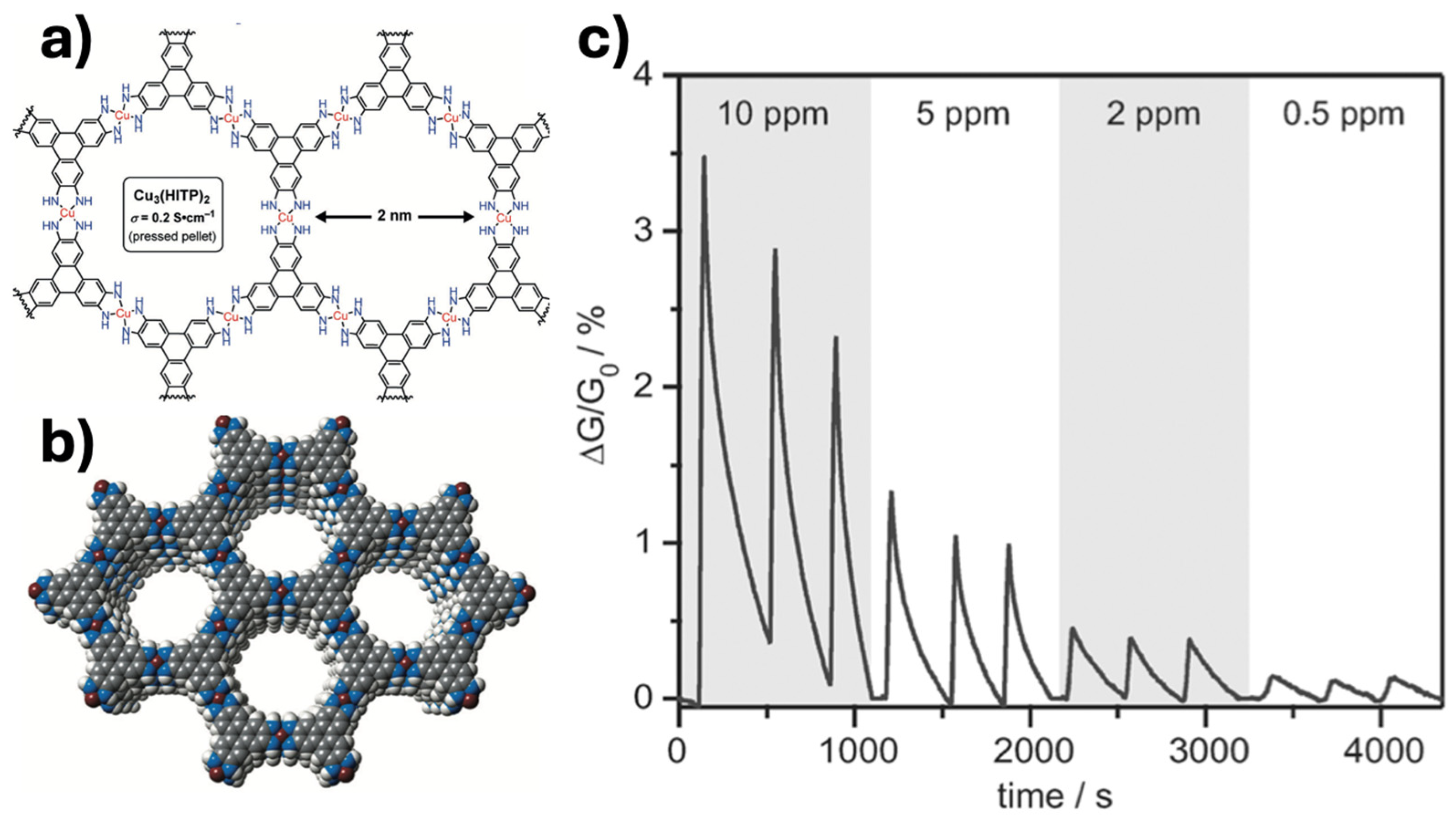
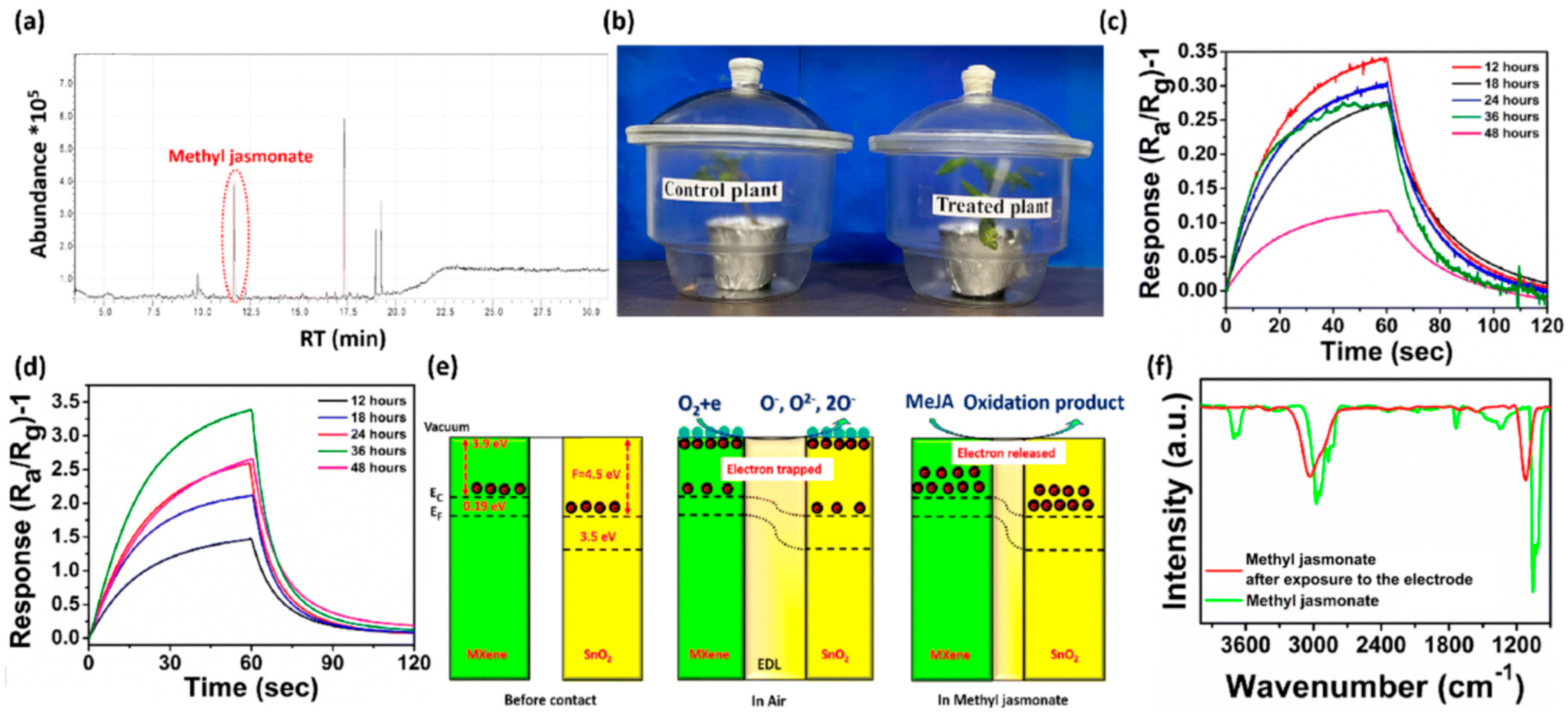
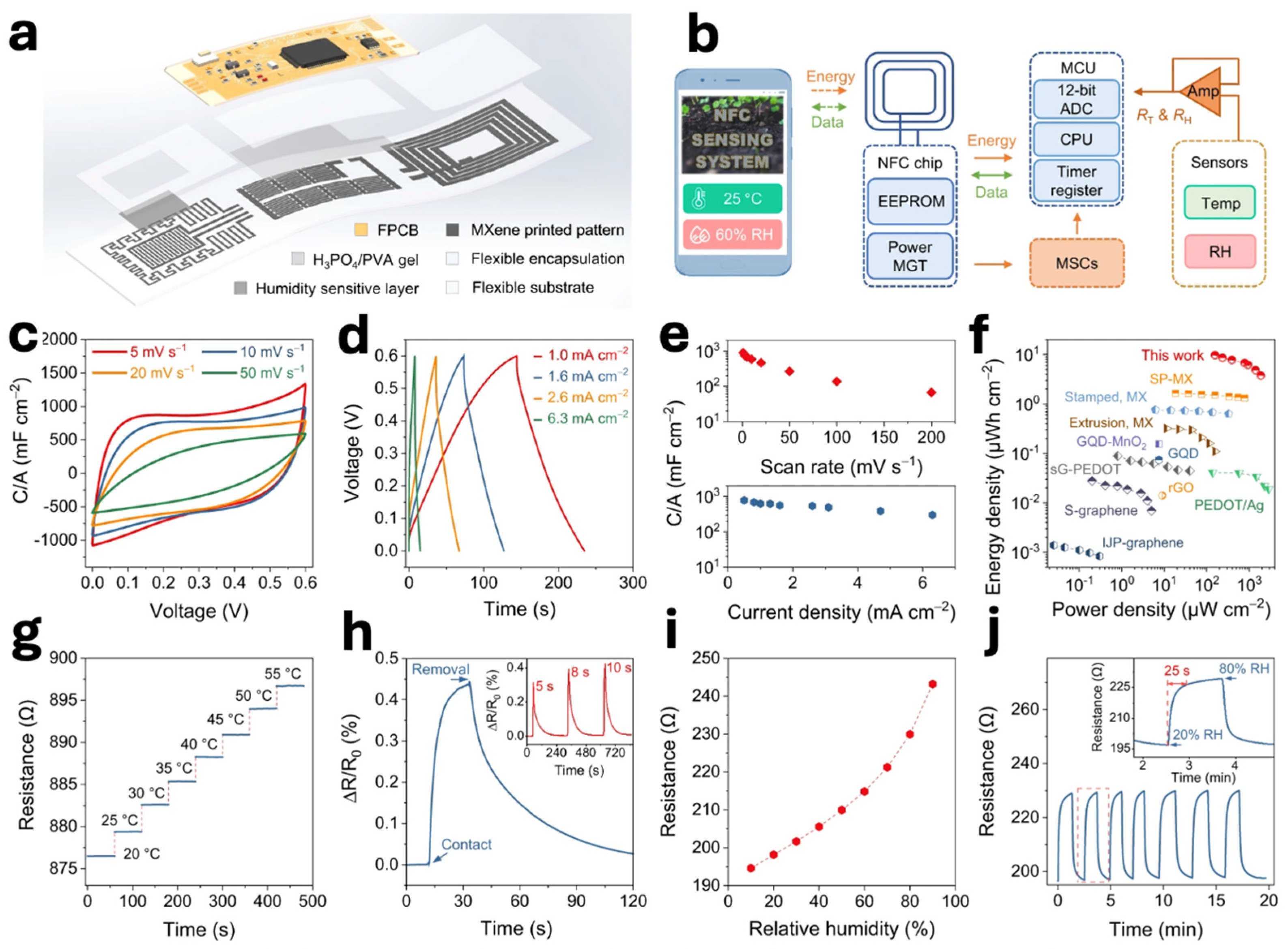
| Technique | Resolution (Typical) | Processing Complexity | Accuracy/Reproducibility | Advantages | Limitations |
|---|---|---|---|---|---|
| Soft lithography [127] | ~100 nm–few µm | Low (elastomeric stamps, PDMS) | Moderate | Low-cost, flexible substrates, biointegration | Limited long-term stability, deformation |
| Nanoimprint lithography (NIL) [128,129] | <10 nm | Medium–high (thermal/UV curing) | High | Sub-10 nm resolution, high throughput | Mold fabrication cost, resist compatibility |
| Self-assembly [130,131,132] | Molecular–50 nm | Low (bottom-up, spontaneous) | Variable | Simple, scalable, tunable chemical functionalization | Less precise control, batch variability |
| Microfluidics integration [137] | ~10–100 µm | Medium (chip fabrication) | High | Precise liquid handling, multiplexed sensing | Fabrication requires cleanroom/PDMS handling |
| On-chip integration (electronics) | ~µm–mm scale | High (semiconductor processes) | High | Wireless, compact, low-power | Expensive, CMOS compatibility needed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machín, A.; Márquez, F. Next-Generation Chemical Sensors: The Convergence of Nanomaterials, Advanced Characterization, and Real-World Applications. Chemosensors 2025, 13, 345. https://doi.org/10.3390/chemosensors13090345
Machín A, Márquez F. Next-Generation Chemical Sensors: The Convergence of Nanomaterials, Advanced Characterization, and Real-World Applications. Chemosensors. 2025; 13(9):345. https://doi.org/10.3390/chemosensors13090345
Chicago/Turabian StyleMachín, Abniel, and Francisco Márquez. 2025. "Next-Generation Chemical Sensors: The Convergence of Nanomaterials, Advanced Characterization, and Real-World Applications" Chemosensors 13, no. 9: 345. https://doi.org/10.3390/chemosensors13090345
APA StyleMachín, A., & Márquez, F. (2025). Next-Generation Chemical Sensors: The Convergence of Nanomaterials, Advanced Characterization, and Real-World Applications. Chemosensors, 13(9), 345. https://doi.org/10.3390/chemosensors13090345







