A DODTA–TPB-Based Potentiometric Sensor for Anionic Surfactants: A Computational Design and Environmental Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Sensor Preparation
2.3. Measuring Setup
2.4. Computational Details
3. Results and Discussion
3.1. Computational Analysis
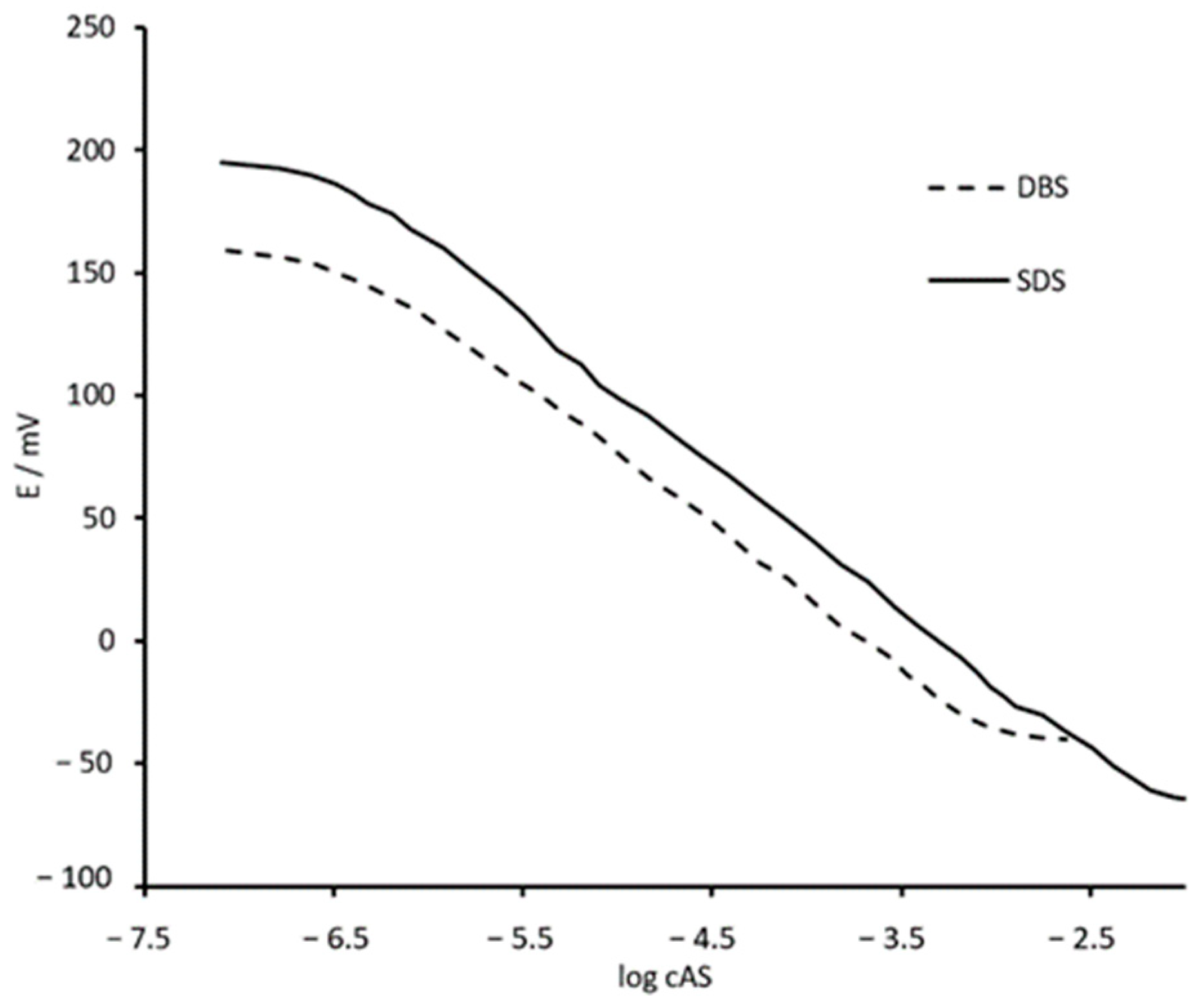
3.2. Potentiometric Sensor Characterization
3.3. Interference Study
3.4. Potentiometric Titrations of Model and Environmental Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Presedence Research Surfactants Market Size, Share and Trends 2025 to 2034. Available online: https://www.precedenceresearch.com/surfactants-market#:~:text=Report%20Code%20%3A%201728-,Surfactants%20Market%20Size%20and%20Forecast%202025%20to%202034,5.36%25%20from%202025%20to%202034 (accessed on 10 May 2025).
- ISO 2271:1989; Surface Active Agents, Detergents, Determination of Anionic-Active Matter by Manual or Mechanical Direct Two-phase Titration Procedure. ISO: Geneva, Switzerland, 1989.
- ISO 7875-1:1996; Water Quality-Determination of Surfactant-Part 1: Determination of Anionic Surfactants by Measurement of the Methylene Blue Index (MBAS). ISO: Geneva, Switzerland, 1996. Available online: https://www.iso.org/standard/24784.html (accessed on 7 August 2018).
- Wyrwas, B.; Zgoła-Grześkowiak, A. Continuous flow methylene blue active substances method for the determination of anionic surfactants in river water and biodegradation test samples. J. Surfactants Deterg. 2014, 17, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Shin, Y.G.; Kirkham, M.B.; Jeong, S.S.; Lee, J.G.; Kim, H.S.; Yang, J.E. A Simplified Method for Anionic Surfactant Analysis in Water Using a New Solvent. Toxics 2022, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Shin, Y.G.; Kim, H.S.; Kirkham, M.B.; Yang, J.E. Screening of a Novel Solvent for Optimum Extraction of Anionic Surfactants in Water. Toxics 2022, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ródenas-Torralba, E.; Reis, B.F.; Morales-Rubio, Á.; De La Guardia, M. An environmentally friendly multicommutated alternative to the reference method for anionic surfactant determination in water. Talanta 2005, 66, 591–599. [Google Scholar] [CrossRef]
- Lengyel, J.; Krtil, J. Radiometric determination of anionic surfactants by two-phase titration method with the use of131I-Rose Bengal as indicator. J. Radioanal. Nucl. Chem. Lett. 1986, 103, 51–61. [Google Scholar] [CrossRef]
- Miller, C.; Bageri, B.S.; Zeng, T.; Patil, S.; Mohanty, K.K. Modified Two-Phase Titration Methods to Quantify Surfactant Concentrations in Chemical-Enhanced Oil Recovery Applications. J. Surfactants Deterg. 2020, 23, 1159–1167. [Google Scholar] [CrossRef]
- Zdrachek, E.; Bakker, E. Potentiometric Sensing. Anal. Chem. 2021, 93, 72–102. [Google Scholar] [CrossRef]
- Kovács, B.; Csóka, B.; Nagy, G.; Ivaska, A. All-solid-state surfactant sensing electrode using conductive polymer as internal electric contact. Anal. Chim. Acta 2001, 437, 67–76. [Google Scholar] [CrossRef]
- Khedr, A.M.; Abu Shawish, H.M.; Gaber, M.; Abed Almonem, K.I. Potentiometric Determination of Alkyl Dimethyl Hydroxyethyl Ammonium Surfactant by a New Chemically Modified Carbon Past Electrode. J. Surfactants Deterg. 2014, 17, 183–190. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E.; Bühlmann, P. Selectivity of Potentiometric Ion Sensors. Anal. Chem. 2000, 72, 1127–1133. [Google Scholar] [CrossRef]
- Mihali, C.; Vaum, N. Use of Plasticizers for Electrochemical Sensors. In Recent Advances in Plasticizers; InTech: London, UK, 2012. [Google Scholar]
- Mikhelson, K.N.; Peshkova, M.A. Advances and trends in ionophore-based chemical sensors. Russ. Chem. Rev. 2015, 84, 555–578. [Google Scholar] [CrossRef]
- Kulapina, E.G.; Ovchinskii, V.A. New modified electrodes for the separate determination of anionic surfactants. J. Anal. Chem. 2000, 55, 169–174. [Google Scholar] [CrossRef]
- Sakač, N.; Madunić-Čačić, D.; Marković, D.; Ventura, B.D.; Velotta, R.; Ptiček Siročić, A.; Matasović, B.; Sermek, N.; Đurin, B.; Šarkanj, B.; et al. The 1,3-Dioctadecyl-1H-imidazol-3-ium Based Potentiometric Surfactant Sensor for Detecting Cationic Surfactants in Commercial Products. Sensors 2022, 22, 9141. [Google Scholar] [CrossRef]
- Vladimirova, N.; Puchkova, E.; Dar’in, D.; Turanov, A.; Babain, V.; Kirsanov, D. Predicting the Potentiometric Sensitivity of Membrane Sensors Based on Modified Diphenylphosphoryl Acetamide Ionophores with QSPR Modeling. Membranes 2022, 12, 953. [Google Scholar] [CrossRef] [PubMed]
- Turyshev, E.S.; Kopytin, A.V.; Zhizhin, K.Y.; Kubasov, A.S.; Shpigun, L.K.; Kuznetsov, N.T. Potentiometric quantitation of general local anesthetics with a new highly sensitive membrane sensor. Talanta 2022, 241, 123239. [Google Scholar] [CrossRef]
- Fizer, O.; Fizer, M.; Sidey, V.; Studenyak, Y. Predicting the end point potential break values: A case of potentiometric titration of lipophilic anions with cetylpyridinium chloride. Microchem. J. 2021, 160, 105758. [Google Scholar] [CrossRef]
- Bakker, E.; Bühlmann, P.; Pretsch, E. Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 1. General Characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [CrossRef]
- Kumar, V.; Suri, R.; Mittal, S. Review on new ionophore species for membrane ion selective electrodes. J. Iran. Chem. Soc. 2023, 20, 509–540. [Google Scholar] [CrossRef]
- Cuartero, M.; Más-Montoya, M.; Soledad García, M.; Curiel, D.; Ortuño, J.A. New carbazolo[1,2-a]carbazole derivative as ionophore for anion-selective electrodes: Remarkable recognition towards dicarboxylate anions. Talanta 2014, 123, 200–206. [Google Scholar] [CrossRef]
- Krivačić, S.; Speck, A.; Kassal, P.; Bakker, E. Towards mass-production of ion-selective electrodes by spotting: Optimization of membrane composition and real-time tracking of membrane drying. Sens. Actuators B Chem. 2025, 423, 136759. [Google Scholar] [CrossRef]
- Sakač, N.; Madunić-Čačić, D.; Marković, D.; Hok, L.; Vianello, R.; Vrček, V.; Šarkanj, B.; Đurin, B.; Della Ventura, B.; Velotta, R.; et al. Potentiometric Surfactant Sensor for Anionic Surfactants Based on 1,3-dioctadecyl-1H-imidazol-3-ium tetraphenylborate. Chemosensors 2022, 10, 523. [Google Scholar] [CrossRef]
- Abd El-Rahman, M.K.; Zaazaa, H.E.; Eldin, N.B.; Moustafa, A.A. Just-Dip-It (Potentiometric Ion-Selective Electrode): An Innovative Way of Greening Analytical Chemistry. ACS Sustain. Chem. Eng. 2016, 4, 3122–3132. [Google Scholar] [CrossRef]
- Glumac, N.; Fizer, M.; Sakač, N.; Marković, D.; Vrban, L.; Vianello, R.; Šarkanj, B.; Sakač, M.K.; Jozanović, M. Study of a 1,3-dioctadecyl-1H-1,2,3-triazol-3-ium cation for potentiometric surfactants sensing applications. J. Mol. Liq. 2025, 432, 127831. [Google Scholar] [CrossRef]
- Cherinka, B.; Andrews, B.H.; Sánchez-Gallego, J.; Brownstein, J.; Argudo-Fernández, M.; Blanton, M.; Bundy, K.; Jones, A.; Masters, K.; Law, D.R.; et al. Marvin: A Tool Kit for Streamlined Access and Visualization of the SDSS-IV MaNGA Data Set. Astron. J. 2019, 158, 74. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. G16_C01 2016, Gaussian 16, Revision C.01; Gaussian Inc.: Wallin, UK, 2016. [Google Scholar]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780123751829. [Google Scholar]
- Marcus, Y.; Hefter, G. Ion Pairing. Chem. Rev. 2006, 106, 4585–4621. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.A.; Castellano, R.K.; Diederich, F. Interactions with Aromatic Rings in Chemical and Biological Recognition. Angew. Chem. Int. Ed. 2003, 42, 1210–1250. [Google Scholar] [CrossRef] [PubMed]
- Bühlmann, P.; Chen, L.D. Ion-Selective Electrodes With Ionophore-Doped Sensing Membranes. In Supramolecular Chemistry; John Wiley and Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Guilbault, G.G.; Durst, R.A.; Frant, M.S.; Freiser, H.; Hansen, E.H.; Light, T.S.; Pungor, E.; Rechnitz, G.; Rice, N.M.; Rohm, T.J.; et al. Recommendations for nomenclature of ion-selective electrodes. Pure Appl. Chem. 1976, 48, 127–132. [Google Scholar] [CrossRef]
- Madunić-Čačić, D.; Sak-Bosnar, M.; Samardžić, M.; Grabarić, Z. Determination of anionic surfactants in industrial effluents using a new highly sensitive surfactant-selective sensor. Sens. Lett. 2009, 7, 50–56. [Google Scholar] [CrossRef]
- Sakač, N.; Madunić-Čačić, D.; Marković, D.; Hok, L.; Vianello, R.; Šarkanj, B.; Đurin, B.; Hajdek, K.; Smoljan, B.; Milardović, S.; et al. Potentiometric Surfactant Sensor Based on 1,3-Dihexadecyl-1H-benzo[d]imidazol-3-ium for Anionic Surfactants in Detergents and Household Care Products. Molecules 2021, 26, 3627. [Google Scholar] [CrossRef] [PubMed]
- Samardžić, M.; Galović, O.; Petrušić, S.; Sak-Bosnar, M. The Analysis of Anionic Surfactants in Effluents Using a DDA-TPB Potentiometric Sensor. Int. J. Electrochem. Sci. 2014, 9, 6166–6181. [Google Scholar] [CrossRef]
- Buck, R.P.; Lindner, E. Recomendations for nomenclature of ion-selective electrodes (IUPAC recommendations 1994). Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]


| Cation Component | 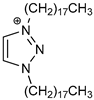 | 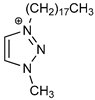 | 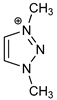 | 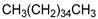 |
| ΔGBIND | −23.4 | −14.9 | −5.0 | −12.0 |
| AS * | Sensor Characteristic | Ion-Pair | ||||
|---|---|---|---|---|---|---|
| New DODTA-TPB | DMIC-TPB [37] | DODI-TPB [25] | DHBI-TPB [38] | DDA-TPB [39] | ||
| DBS | LOD (M) | 5.9 × 10−7 | 6.0 × 10−7 | 7.1 × 10−7 | 6.1 × 10−7 | 2.0 × 10−7 |
| Linear response range (M) | 8.1 × 10−7–6.1 × 10−4 | 8 × 10−7–6 × 10−4 | 6.3 × 10−7–3.2 × 10−4 | 8.9 × 10−7–4.1 × 10−3 | 2.5 × 10−7–1.2 × 10−3 | |
| Slope (mV/decade of activity) | −57.5 | −57.8 | −59.3 | −58.4 | −55.3 | |
| SDS | LOD (M) | 3.1 × 10−7 | 3.2 × 10−7 | 6.8 × 10−7 | 3.2 × 10−7 | 2.5 × 10−7 |
| Linear response range (M) | 4.1 × 10−7–5.1 × 10−3 | 4.0 × 10−7–5 × 10−3 | 5.9 × 10−7–4.1 × 10−3 | 4.6 × 10−7–5.1 × 10−3 | 3.2 × 10−7–4.6 × 10−3 | |
| Slope (mV/decade of activity) | −59.2 | −59.3 | −58.3 | −60.1 | −58.5 | |
| Interfering Anions | |
|---|---|
| Acetate | −3.72 |
| Benzoat | −3.84 |
| Bromide | −3.21 |
| Borate | −2.98 |
| Chloride | −3.83 |
| Carbonate | −4.14 |
| Dihydrogenphosphate | −4.01 |
| EDTA | −3.84 |
| Fluoride | −3.68 |
| Hydrogen carbonate | −4.02 |
| Hydrogen sulfate | −3.86 |
| Nitrate | −3.93 |
| Sulfate | −4.62 |
| Technical-Grade Anionic Surfactant | w (Surfactant) */% | n (Added)/µmol | n (Found) **/µmol | Recovery/% |
|---|---|---|---|---|
| SDS | 91.28 ± 0.31 | 50 | 50.21 ± 0.04 | 100.4 |
| DBS | 47.61 ± 0.25 | 50 | 50.13 ± 0.09 | 100.2 |
| LES | 28.74 ± 0.12 | 50 | 50.11 ± 0.05 | 100.2 |
| Commercial Detergents | w (ANIONIC SURFACTANT)/% | ||
|---|---|---|---|
| DODTA-TPB | Two-Phase Titration * | ||
| Powdered | sample 1 | 5.83 ± 0.21 | 5.91 |
| sample 2 | 6.74 ± 0.13 | 7.01 | |
| Handwashing | sample 3 | 13.85 ± 0.56 | 13.69 |
| sample 4 | 16.41 ± 0.06 | 16.08 | |
| Liquid/gel | sample 5 | 2.57 ± 0.06 | 2.66 |
| sample 6 | 2.72 ± 0.19 | 2.76 | |
| Sampling Place | Vial Test | Added SDS | Recovery% |
|---|---|---|---|
| River Drava | No A.S. * | 50 µmol | 96.5% |
| River Mura | No A.S. | 50 µmol | 95.8% |
| Lake Motičnjak | No A.S. | 50 µmol | 94.2% |
| Hydro accumulation Drava | No A.S. | 50 µmol | 94.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glumac, N.; Vrban, L.; Vianello, R.; Jozanović, M.; Fizer, M.; Sakač, M.K.; Velotta, R.; Iannotti, V.; Della Ventura, B.; Cvetnić, M.; et al. A DODTA–TPB-Based Potentiometric Sensor for Anionic Surfactants: A Computational Design and Environmental Application. Chemosensors 2025, 13, 321. https://doi.org/10.3390/chemosensors13090321
Glumac N, Vrban L, Vianello R, Jozanović M, Fizer M, Sakač MK, Velotta R, Iannotti V, Della Ventura B, Cvetnić M, et al. A DODTA–TPB-Based Potentiometric Sensor for Anionic Surfactants: A Computational Design and Environmental Application. Chemosensors. 2025; 13(9):321. https://doi.org/10.3390/chemosensors13090321
Chicago/Turabian StyleGlumac, Nada, Lucija Vrban, Robert Vianello, Marija Jozanović, Maksym Fizer, Marija Kraševac Sakač, Raffaele Velotta, Vincenzo Iannotti, Bartolomeo Della Ventura, Matija Cvetnić, and et al. 2025. "A DODTA–TPB-Based Potentiometric Sensor for Anionic Surfactants: A Computational Design and Environmental Application" Chemosensors 13, no. 9: 321. https://doi.org/10.3390/chemosensors13090321
APA StyleGlumac, N., Vrban, L., Vianello, R., Jozanović, M., Fizer, M., Sakač, M. K., Velotta, R., Iannotti, V., Della Ventura, B., Cvetnić, M., Marković, D., & Sakač, N. (2025). A DODTA–TPB-Based Potentiometric Sensor for Anionic Surfactants: A Computational Design and Environmental Application. Chemosensors, 13(9), 321. https://doi.org/10.3390/chemosensors13090321










