Anti-Aging Potential of Illyrian Iris Rhizome Extract: Preliminary Chemical and Biological Profiling and Chemosensor Analysis via GC/MS and UHPLC-DAD-MS/MS Combined with HPTLC Bioautography
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
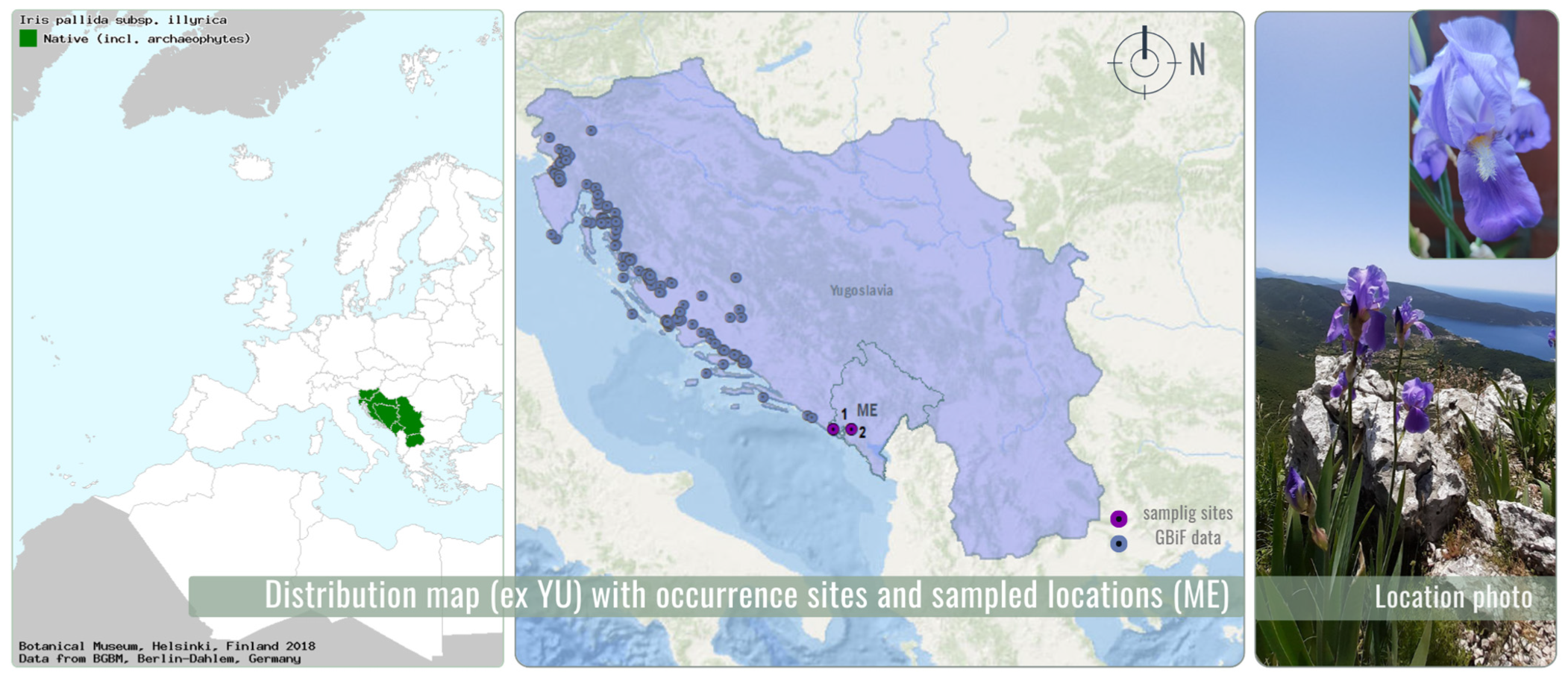
2.2. Plant Material
2.2.1. Plant Material Collection
2.2.2. Conventional and Green Extraction of Plant Material
2.2.3. Solid-Phase Extraction (SPE)
2.3. Hydrodistillation of Illyrian Iris Rhizome
2.4. HTPLC Analysis
2.4.1. Phenolic Compound Detection
2.4.2. Terpenoid Compound Detection
2.4.3. Flavonoid Detection
2.5. Bioautography Assays
2.5.1. HTPLC-DPPH Assay
2.5.2. HTPLC-Tyrosinase Assay
2.6. Spectrophotometric Assays
2.6.1. DPPH-Radical-Scavenging Assay (RSA)
2.6.2. Tyrosinase Inhibition Assay
2.7. LC-MS Analysis
2.8. GC/MS and GC/FID Analysis
2.9. HaCaT Cell Viability Assay
2.9.1. Cell Culture Maintenance
2.9.2. Cell Viability Evaluation (MTT Assay)
3. Results and Discussion
3.1. HPTLC Analysis
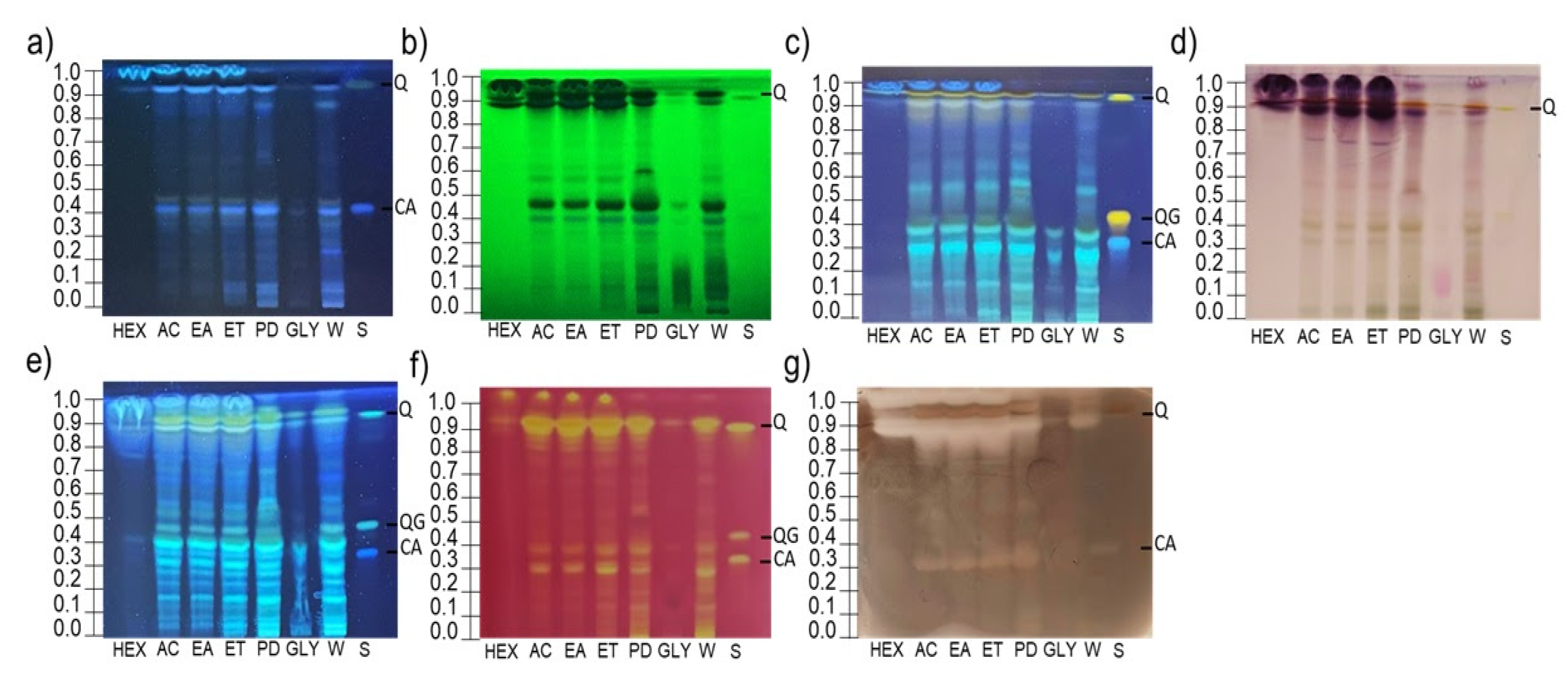
3.2. Bioautography Assays
3.2.1. HPTLC-DPPH
3.2.2. HPTLC-Tyrosinase
3.3. Spectrophotometric Assays
3.3.1. RSA Assay
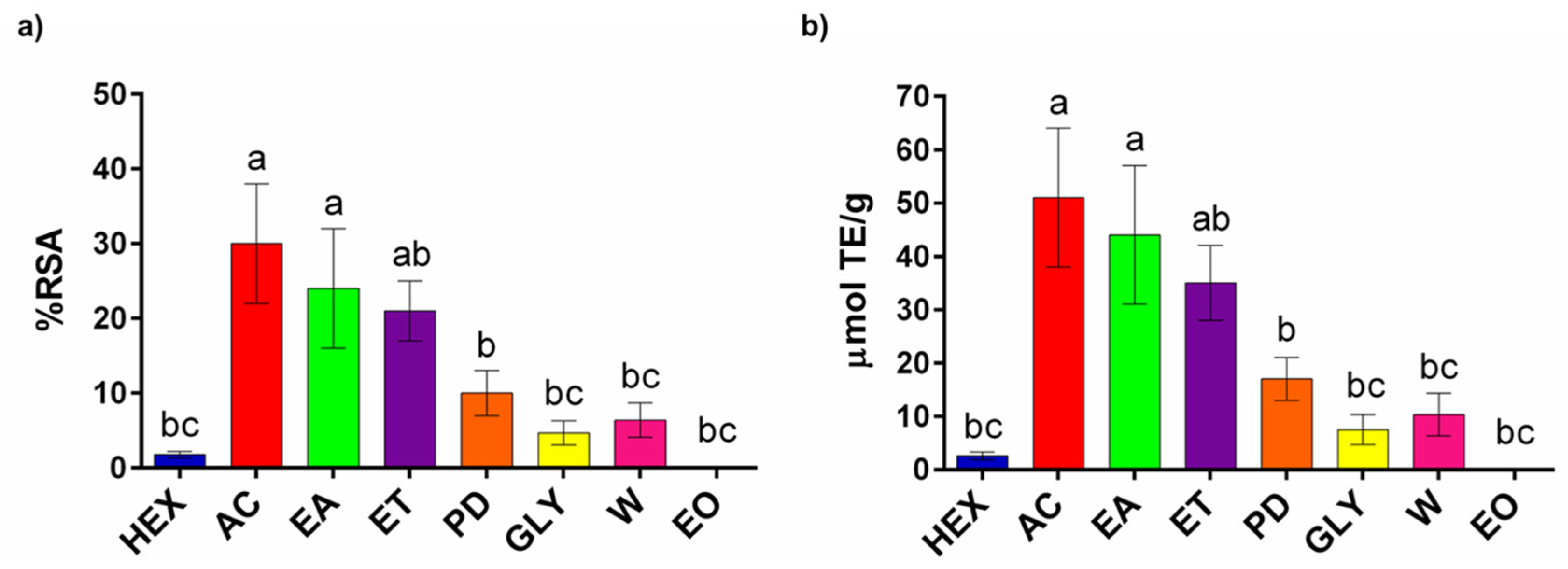
3.3.2. Tyrosinase Inhibition Assay

3.4. LC-MS Analysis
3.4.1. Phenolic Compounds
3.4.2. Xanthones
3.4.3. Benzophenone Derivatives
3.4.4. Triterpenoids
3.4.5. Other Compounds
3.5. GC/MS and GC/FID Analysis
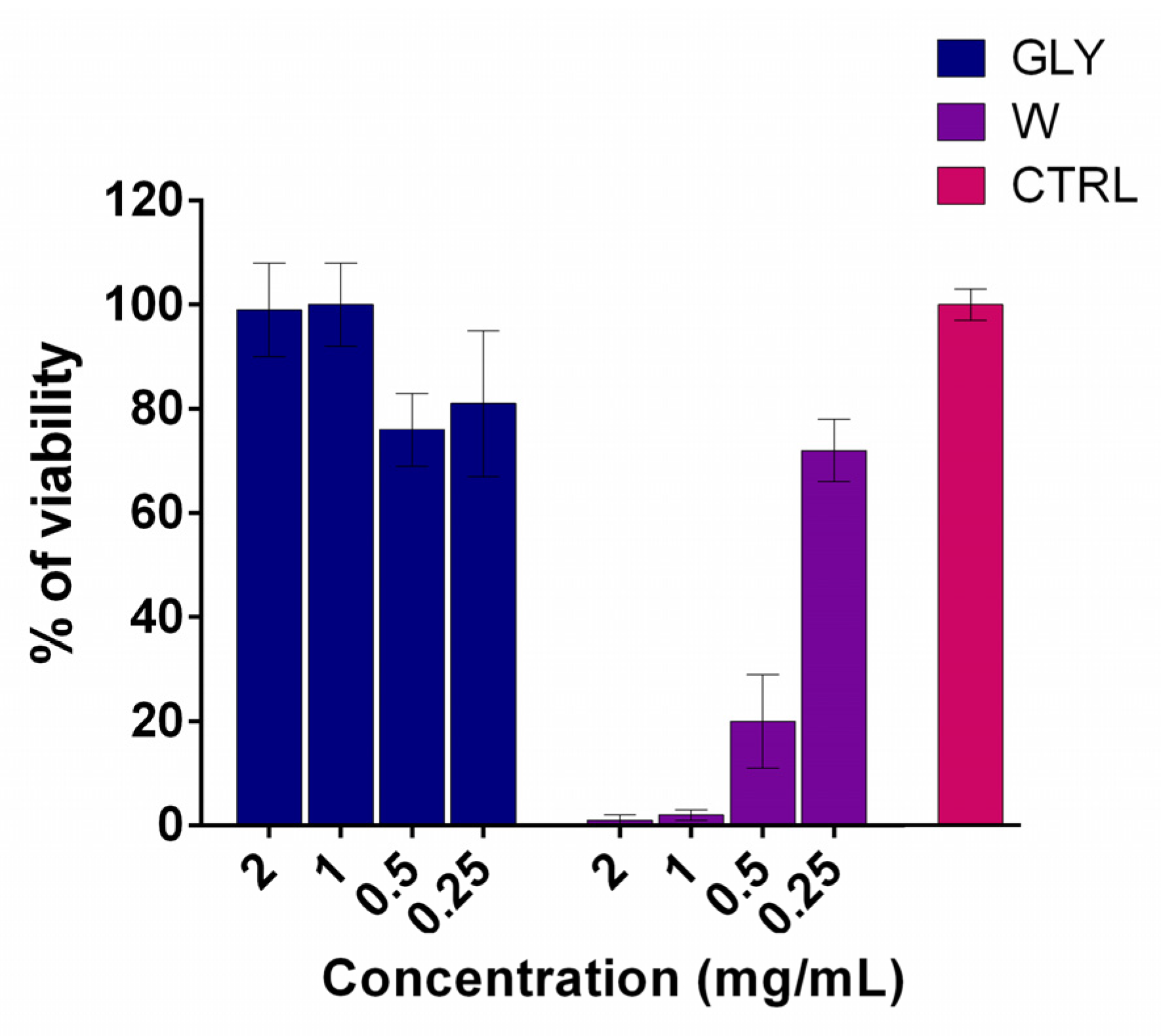
3.6. HaCaT Cells Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Acetone extract |
| CA | Chlorogenic acid |
| CTRL | Control cells |
| DAD | Diode array detector |
| DMSO | Dimethyl sulfoxide |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EA | Ethyl acetate extract |
| ET | Ethanol extract |
| EO | Essential oil |
| ESI | Electrospray ionization |
| FBS | Fetal bovine serum |
| FID | Flame ionization detector |
| GC | Gass chromatography |
| GLY | Glycerol extract |
| HaCaT | Immortalized human keratinocytes |
| HEX | n-Hexane |
| HPTLC | High-performance thin-layer chromatography |
| KA | Kojic acid |
| L-DOPA | 3,4-Dihydroxy-L-phenylalanine |
| LC | Liquid chromatography |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NEAAs | Non-essential amino acids |
| NPR | Natural product reagent |
| PBS | Phosphate-buffered saline |
| PD | 1,3-Propanediol extract |
| PTFE | Polytetrafluoroethylene |
| Q | Quercetin |
| QG | Quercetin-3-O-glucoside |
| RF | Retention factor |
| RS | Radical scavenging |
| SPE | Solid-phase extraction |
| TE | Trolox equivalent |
| UHPLC | Ultra-high-performance liquid chromatography |
| UV | Ultraviolet |
| WFO | World Flora online |
References
- WFO (World Flora Online). Iris pallida subsp. illyrica (Tomm. ex Vis.) K. Richt. 2025. Available online: http://www.worldfloraonline.org/taxon/wfo-0000790617 (accessed on 5 June 2025).
- Pliny the Elder. Natural History; Jones, W.H.S., Translator; Original work published ca. 77 CE; William Heinemann Cambridge: Cambridge, MA, USA, 1938; Volume 6, Books XX–XXIII. [Google Scholar]
- Dioscorides, P.D.m.m.l.q.K.C.G. (Ed.) Medicorum Graecorum Quae Exstant; Officina Libraria Car. Cnoblochii: Lipsiae, Germany, 1829–1830; Volume 30, O.w.p.c.C. [Google Scholar]
- WFO (World Flora Online). Iris × Florentina L. 2025. Available online: https://www.worldfloraonline.org/taxon/wfo-0000783335 (accessed on 5 June 2025).
- Oztas, F.; Turkmen, A.; Oztas, H.; Turkmen, M. The Medical Properties of Iris and its Usage in Pharmaceutical, Perfumery and Cosmetic Industries. In Medical Research and Its Applications; BookPI: London, UK, 2024; pp. 114–124. [Google Scholar]
- Bicchi, C.; Joulain, D. A comprehensive review on essential oils and extracts from Iris rhizomes. Phytochem. Rev. 2024, 24, 1629–1665. [Google Scholar] [CrossRef]
- Crișan, I.; Cantor, M. New perspectives on medicinal properties and uses of Iris sp. Hop Med. Plants 2016, 24, 24–36. [Google Scholar]
- Sadgrove, N.J.; Padilla-González, G.F.; Phumthum, M. Fundamental Chemistry of Essential Oils and Volatile Organic Compounds, Methods of Analysis and Authentication. Plants 2022, 11, 789. [Google Scholar] [CrossRef]
- Mykhailenko, O. Composition of Volatile Oil of Iris pallida Lam. from Ukraine. Turk. J. Pharm. Sci. 2018, 15, 85–90. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Korinek, M.; Ivanauskas, L.; Bezruk, I.; Myhal, A.; Petrikaitė, V.; El-Shazly, M.; Lin, G.-H.; Lin, C.-Y.; Yen, C.-H.; et al. Qualitative and Quantitative Analysis of Ukrainian Iris Species: A Fresh Look on Their Antioxidant Content and Biological Activities. Molecules 2020, 25, 4588. [Google Scholar] [CrossRef] [PubMed]
- Khatib, S.; Faraloni, C.; Bouissane, L. Exploring the Use of Iris Species: Antioxidant Properties, Phytochemistry, Medicinal and Industrial Applications. Antioxidants 2022, 11, 526. [Google Scholar] [CrossRef]
- Wollenweber, E.; Stevens, J.F.; Klimo, K.; Knauft, J.; Frank, N.; Gerhäuser, C. Cancer chemopreventive in vitro activities of isoflavones isolated from Iris germanica. Planta Medica 2003, 69, 15–20. [Google Scholar] [CrossRef]
- Saric Medic, B.; Jerković-Mujkić, A.; Cubara, B.; Durmic, A.; Kurtović, J.; Bajrovic, K.; Omeragic, E.; Dedic, M.; Bogunić, F.; Pojskic, L. Crude Extracts of Three Iris Species as Sources of MRSA Antimicrobial Compounds. Eur. J. Biol. 2024, 83, 182–188. [Google Scholar] [CrossRef]
- Roger, B.; Jeannot, V.; Fernandez, X.; Cerantola, S.; Chahboun, J. Characterisation and Quantification of Flavonoids in Iris germanica L. and Iris pallida Lam. Resinoids from Morocco. Phytochem. Anal. 2012, 23, 450–455. [Google Scholar] [CrossRef]
- Al-Snafi, A. The medical importance of Iris pallida—A review. Int. J. Biol. Pharm. Sci. Arch. 2021, 1, 190–196. [Google Scholar] [CrossRef]
- Hoang, L.; Beneš, F.; Fenclová, M.; Kronusová, O.; Švarcová, V.; Řehořová, K.; Baldassarre Švecová, E.; Vosátka, M.; Hajšlová, J.; Kaštánek, P.; et al. Phytochemical Composition and In Vitro Biological Activity of Iris spp. (Iridaceae): A New Source of Bioactive Constituents for the Inhibition of Oral Bacterial Biofilms. Antibiotics 2020, 9, 403. [Google Scholar] [CrossRef]
- Euro+Med PlantBase. Iris pallida subsp. illyrica. Available online: https://en.wikipedia.org/wiki/Iris_pallida_subsp._illyrica (accessed on 5 June 2025).
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps (World Soil Resources Reports No. 106); FAO: Rome, Italy, 2015; Available online: http://www.fao.org/3/i3794en/I3794en.pdf (accessed on 5 June 2025).
- Holmgren, P.K.; Holmgren, N.H. Additions to Index Herbariorum (Herbaria), Edition 8 Fourteenth Series. Taxon 2003, 52, 385–389. [Google Scholar] [CrossRef]
- Iris pallida subsp. illyrica (Tomm. ex Vis.) K.Richt. in GBIF Secretariat. GBIF Backbone Taxonomy. Checklist Dataset. 2023. Available online: https://www.gbif.org/species/5299102 (accessed on 5 June 2025).
- Đurđa, I.; Filip, A.; Milan, S.; Tatjana, S.; Ristivojevic, M.; Petar, R. Innovative analytical methodology for skin anti-aging compounds discovery from plant extracts: Integration of High-Performance Thin-Layer Chromatography-in vitro spectrophotometry bioassays with multivariate modeling and molecular docking. J. Chromatogr. A 2025, 1742, 465640. [Google Scholar] [CrossRef]
- Todorović, P.; Krstić Ristivojević, M.; Jović, M.; Ivković, Đ.; Nestorović Živković, J.; Gašić, U.; Dimkić, I.; Stojiljković, I.; Ristivojević, P. Antimicrobial Effect of Boswellia serrata Resin’s Methanolic Extracts Against Skin Infection Pathogens. Processes 2025, 13, 850. [Google Scholar] [CrossRef]
- Milica, J.; Petar, R.; Ilija, C.; Dušanka, M.-O. Assessing radical scavenging capacity of Sempervivum tectorum L. leaf extracts: An integrated high-performance thin-layer chromatography/in silico/chemometrics approach. J. Chromatogr. A 2023, 1703, 464082. [Google Scholar] [CrossRef]
- Taibon, J.; Ankli, A.; Schwaiger, S.; Magnenat, C.; Boka, V.I.; Simões-Pires, C.; Aligiannis, N.; Cuendet, M.; Skaltsounis, A.L.; Reich, E.; et al. Prevention of False-Positive Results: Development of an HPTLC Autographic Assay for the Detection of Natural Tyrosinase Inhibitors. Planta Med. 2015, 81, 1198–1204. [Google Scholar] [CrossRef]
- Sachett, A.; Gallas-Lopes, M.; Conterato, G.; Herrmann, A.; Piato, A. Antioxidant Activity by DPPH Assay: In Vitro Protocol. 2021. Available online: https://www.protocols.io/view/antioxidant-activity-by-dpph-assay-in-vitro-protoc-q26g783n9lwz/v1 (accessed on 21 April 2025).
- Ivkovic, D.; Cvijetic, I.; Radoicic, A.; Stojkovic-Filipovic, J.; Trifkovic, J.; Krstic Ristivojevic, M.; Ristivojevic, P. NADES-Based Extracts of Selected Medicinal Herbs as Promising Formulations for Cosmetic Usage. Processes 2024, 12, 992. [Google Scholar] [CrossRef]
- Roux, D.G.; Maihs, A.E. Selective spray reagents for the identification and estimation of flavonoid compounds associated with condensed tannins. J. Chromatogr. A 1960, 4, 65–74. [Google Scholar] [CrossRef]
- Özlem, E.; Shipra, N.; Mohammed Ali, S.; Ismael, O.; John, J.W.; Helen, S.; Young Hae, C. Identification of anti-inflammatory and antimicrobial compounds from leaves and rhizome of Iris pseudacorus collected in Ireland bogland using chemical profiling techniques. Fitoterapia 2025, 185, 106671. [Google Scholar] [CrossRef]
- Angelika, M.; Mirosława, K.-B.; Piotr, M.; Anna, K.; Adam, K.; Aleksandra, K. Iris pseudacorus as an easily accessible source of antibacterial and cytotoxic compounds. J. Pharm. Biomed. Anal. 2021, 195, 113863. [Google Scholar] [CrossRef]
- Duka, I.; Males, Z.; Bojić, M.; Hrusevar, D.; Mitic, B. Chemical Fingerprinting, Total Phenolics and Antioxidant Activity of Some Iris Taxa. Croat. Chem. Acta 2020, 93, 49–56. [Google Scholar] [CrossRef]
- Won, Y.; Kim, H.-H.; Jeong, S.-H.; Bhosale, P.B.; Abusaliya, A.; Heo, J.-D.; Seong, J.-K.; Ahn, M.-J.; Kim, H.-J.; Kim, G.-S. The Effects of Iridin and Irigenin on Cancer: Comparison with Well-Known Isoflavones in Breast, Prostate, and Gastric Cancers. Int. J. Mol. Sci. 2025, 26, 2390. [Google Scholar] [CrossRef] [PubMed]
- Sıcak, Y.; Büyüksakallı, H.; Malkoçoğlu, S.; Özler, M.; Öztürk, M. Antioxidant, Anticholinesterase Inhibitory and Tyrosinase Inhibitory Activities of Iris xanthospuria Extracts Growing in Köyceğiz Region. J. Ongoing Chem. Res. 2017, 3, 22–31. [Google Scholar]
- Mahmoud, S.; Ayoub, I.; Watanabe, M.; Devkota, H.; Singab, A.N. Metabolic profiling, antioxidant, and enzyme inhibition potential of Iris pseudacorus L. from Egypt and Japan: A comparative study. Sci. Rep. 2023, 13, 5233. [Google Scholar] [CrossRef]
- Miastkowska, M.; Sikora, E. Anti-Aging Properties of Plant Stem Cell Extracts. Cosmetics 2018, 5, 55. [Google Scholar] [CrossRef]
- Gyeong Han, J.; Tae Hoon, K. Tyrosinase inhibitors isolated from Iris bungei collected in Mongolia. Phytochem. Lett. 2024, 60, 167–173. [Google Scholar] [CrossRef]
- Andrei, M.; Gokhan, Z.; Adriano, M.; Ahmet, U.; Erdogan, G.; Gianina, C.; Abdurrahman, A. Biological effects and chemical characterization of Iris schachtii Markgr. extracts: A new source of bioactive constituents. Food Chem. Toxicol. 2018, 112, 448–457. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Sieniawska, E.; Widelski, J.; Urjin, O.; Głowniak, P.; Skalicka-Woźniak, K. Major secondary metabolites of Iris spp. Phytochem. Rev. 2015, 14, 51–80. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Han, G.; Bai, G.; Wu, Y.; Zhou, Y.; Yao, W.; Li, L. Comparative Transcriptome Analysis to Identify Candidate Genes Related to Chlorogenic Acid and Flavonoids Biosynthesis in Iridaceae. Forests 2022, 13, 1632. [Google Scholar] [CrossRef]
- Fabian, A.; Božena, M.; Stefanie, M.; Željan, M.; Olaf, K.; Dario, H.; Franz, B. Metabolic profiling of rhizomes of native populations of the strictly endemic Croatian species Iris adriatica. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2019, 153, 317–324. [Google Scholar] [CrossRef]
- Jerome, M.; Erica, L.; Hugues, B.; Carlo, B.; Patrizia, R. A metabolomic approach to quality determination and authentication of raw plant material in the fragrance field. Iris rhizomes: A case study. J. Chromatogr. A 2014, 1368, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.-J.; Li, R.; Wang, X.; Ye, W.-C. New Isoflavonoid Glycosides from the Rhizomes of Iris leptophylla Lingelsh. J. Integr. Plant Biol. 2007, 49, 213–217. [Google Scholar] [CrossRef]
- Jia, Y.-W.; Zeng, Z.-Q.; Shi, H.-L.; Liang, J.; Liu, Y.-M.; Tang, Y.-X.; Liao, X. Characterization of in vitro metabolites of irisflorentin by rat liver microsomes using high-performance liquid chromatography coupled with tandem mass spectrometry. Biomed. Chromatogr. 2016, 30, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Kostić, A.Ž.; Gašić, U.M.; Pešić, M.B.; Stanojević, S.P.; Barać, M.B.; Mačukanović-Jocić, M.P.; Avramov, S.N.; Tešić, Ž.L. Phytochemical Analysis and Total Antioxidant Capacity of Rhizome, Above-Ground Vegetative Parts and Flower of Three Iris Species. Chem. Biodivers. 2019, 16, e1800565. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A. Pharmacology and Phytochemistry of Isoflavonoids from Iris Species. J. Pharmacol. Clin. Res. 2017, 3, 10–17. [Google Scholar] [CrossRef]
- Extraction and Formulation of Plant Substances. Dissertation Zur Erlangung des Grades Doktor der Naturwissenschaften (Dr. rer. Nat.) der Fakultät für Chemie und Pharmazie Universität Regensburg, Pierre DEGOT, Bains-Les-Bains, Januar 2022. Available online: https://epub.uni-regensburg.de/52268/1/Promotion_DP.pdf (accessed on 5 June 2025).
- Yousefsani, B.S.; Boozari, M.; Shirani, K.; Jamshidi, A.; Dadmehr, M. A review on phytochemical and therapeutic potential of Iris germanica. J. Pharm. Pharmacol. 2021, 73, 611–625. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Al-Musayeib, N.M. New Constituents from the Rhizomes of Egyptian Iris germanica L. Molecules 2012, 17, 2587–2598. Molecules 2012, 17, 2587–2598. [Google Scholar] [CrossRef]
- Sabrin, I.; Abdulrahman, A.-A.; Amgad, K.; Gamal, M. Antioxidant α-amylase inhibitors flavonoids from Iris germanica rhizomes. Rev. Bras. Farmacogn. 2017, 27, 170–174. [Google Scholar] [CrossRef]
- Iwashina, T.; Mizuno, T. Flavonoids and Xanthones from the Genus Iris: Phytochemistry, Relationships with Flower Colors and Taxonomy, and Activities and Function. Nat. Prod. Commun. 2020, 15, 1934578X20937151. [Google Scholar] [CrossRef]
- Badiali, C.; Petruccelli, V.; Brasili, E.; Pasqua, G. Xanthones: Biosynthesis and Trafficking in Plants, Fungi and Lichens. Plants 2023, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, A.-K.; Hideki, T.; Munekazu, I. A xanthone C-glycoside from Iris nigricans. Phytochemistry 1995, 38, 729–731. [Google Scholar] [CrossRef]
- Remali, J.; Sahidin, I.; Aizat, W.M. Xanthone Biosynthetic Pathway in Plants: A Review. Front. Plant Sci. 2022, 13, 809497. [Google Scholar] [CrossRef] [PubMed]
- Koul, N.; Sharma, V.; Dixit, D.; Ghosh, S.; Sen, E. Bicyclic triterpenoid Iripallidal induces apoptosis and inhibits Akt/mTOR pathway in glioma cells. BMC Cancer 2010, 10, 328. [Google Scholar] [CrossRef]
- Fuchs, D.; Hamberg, M.; Skold, C.; Wheelock, A.; Wheelock, C. An LC-MS/MS workflow to characterize 16 regio- and stereoisomeric trihydroxyoctadecenoic acids (TriHOMEs). J. Lipid Res. 2018, 59, 2025–2033. [Google Scholar] [CrossRef]
- Song, C.; Zhang, Y.; Manzoor, M.A.; Li, G. Identification of alkaloids and related intermediates of Dendrobium officinale by solid-phase extraction coupled with high-performance liquid chromatography tandem mass spectrometry. Front. Plant Sci. 2022, 13, 952051. [Google Scholar] [CrossRef]
- Almaarri, K.; Zedan, T.; Albatal, N. Chemical Analysis of Essential Oils of Some Syrian Wild Iris Species. Am. J. Biochem. Mol. Biol. 2013, 3, 38–49. [Google Scholar] [CrossRef]
- Friščić, M.; Maleš, Ž.; Maleš, I.; Duka, I.; Radonić, A.; Mitić, B.; Hruševar, D.; Jurić, S.; Jerković, I. Gas Chromatography–Mass Spectrometry Analysis of Volatile Organic Compounds from Three Endemic Iris Taxa: Headspace Solid-Phase Microextraction vs. Hydrodistillation. Molecules 2024, 29, 4107. [Google Scholar] [CrossRef]
- Jevtović, S.Č.; Stojković, J.P.; Mitić, Z.S.; Niketić, M.S.; Stojanović, G.S. The Chemical Composition of Essential Oil and Headspace Volatiles of Balkan Endemic Taxon Achillea ×vandasii Velen. Nat. Prod. Commun. 2024, 19, 1934578X241264624. [Google Scholar] [CrossRef]
- Shuna, Z.; Oon-Doo, B.; Young Jin, C.; Sang-Moo, K. Pretreatments for the Efficient Extraction of Bioactive Compounds from Plant-Based Biomaterials. Crit. Rev. Food Sci. Nutr. 2014, 54, 1283–1297. [Google Scholar] [CrossRef]
- Hala, I.A.-J. Variation in essential oil composition of Iris nigricans Dinsm. (Iridaceae) endemic to Jordan at different flowering stages. Arab. J. Chem. 2016, 9, S1190–S1196. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Pino, J.A.; Mesa, J.; Muñoz, Y.; Martí, M.P.; Marbot, R. Volatile components from mango (Mangifera indica L.) cultivars. J. Agric. Food Chem. 2005, 53, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.X.; Li, X.N.; Liang, Y.Z.; Fang, H.Z.; Huang, L.F.; Guo, F.Q. Comparative analysis of chemical components of essential oils from different samples of Rhododendron with the help of chemometrics methods. Chemom. Intell. Lab. Syst. 2006, 82, 218–228. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, X.; Jia, H.; Le, W.; Xiang, P. Effect of freeze-thaw treatment on the yield and quality of tiger nut oil. Food Chem. X 2024, 23, 101733. [Google Scholar] [CrossRef] [PubMed]
- Krick, W.; Marner, F.J.; Jaenicke, L. Isolation and structure determination of the precursors of alpha-irone and gamma-irone and homologous compounds from Iris pallida and Iris florentina. Z. Naturforsch. 2014, 38, 179–184. [Google Scholar] [CrossRef]
- Olha, M.; Kovalyov, V.; Orlova, T. Chemical composition of the essential oil of several Iris species. Thai J. Pharm. Sci. 2020, 44, 179–185. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and Biological Functions of Terpenoids in Plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar] [CrossRef]
- Natalia, D.; Florence, N.; Dinesh, A.N.; Irina, O. Plant Volatiles: Recent Advances and Future Perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Paul, I.; Sarkar, M.; Bhadoria, P. Floral secondary metabolites in context of biotic and abiotic stress factors. Chemoecology 2022, 32, 49–68. [Google Scholar] [CrossRef]
- Roberta, A.; Guido, F. Iris lutescens on serpentine soil: Volatile emission profiles in different organs of its two colour morphs. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2021, 155, 406–414. [Google Scholar] [CrossRef]
- Kemal, H.C.B.; Betül, D.; Ilkay Erdogan, O.; Murat, K.; Nazim, S.; Bilge, S. Composition of Volatiles from Three Iris Species of Turkey. J. Essent. Oil Res. 2011, 23, 66–71. [Google Scholar] [CrossRef]
- Pinto, S.; Leitao, G.; Oliveira, D.; Bizzo, H.; Ramos, D.; Coelho, T.; Silva, P.; Lourenço, M.; Leitão, S. Chemical Composition and Antimycobacterial Activity of the Essential Oil from Anemia tomentosa var. anthriscifolia. Nat. Prod. Commun. 2009, 4, 1675–1678. [Google Scholar] [CrossRef]
- Viljoen, A.M.; Kamatou, G.P.P.; Coovadia, Z.H.; Özek, T.; Başer, K.H.C. Rare sesquiterpenes from South African Pteronia species. S. Afr. J. Bot. 2010, 76, 146–152. [Google Scholar] [CrossRef]
- Bos, R.; Stojanova, A.S.; Woerdenbag, H.J.; Koulman, A.; Quax, W.J. Volatile components of the aerial parts of Artemisia pontica L. grown in Bulgaria. Flavour Fragr. J. 2005, 20, 145–148. [Google Scholar] [CrossRef]
- Weyerstahl, P.; Marschall-Weyerstahl, H.; Schröder, M.; Brendel, J.; Kaul, V.K. Functionalised silphiperfolenes from Artemisia laciniata. Phytochemistry 1991, 30, 3349–3352. [Google Scholar] [CrossRef]
- Choo, J.H.; Lee, H.G.; Lee, S.Y.; Kang, N.G. Iris Pallida Extract Alleviates Cortisol-Induced Decrease in Type 1 Collagen and Hyaluronic Acid Syntheses in Human Skin Cells. Curr. Issues Mol. Biol. 2023, 45, 353–363. [Google Scholar] [CrossRef]
| Peak no. | tR, Min (MS Signal) | λmax, nm | Molecular Ion in Negative ESI-MS Mode, [M-H]–, m/z | MS/MS Fragment Ions, m/z | Assignment (Reference) | Extracts | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EA | AC | ET | HEX | GLY | PD | W | ||||||
| 1 | 0.78 | - | 195 | 177, 128 (100%), 99 | Gluconic acid (a PubChem CID:10690) | - | - | - | - | - | - | + |
| 2 | 3.80 | - | 407 b 409 | 389, 359, 317, 287 (100%), 245, 193 b 391 (100%), 373, 355, 325, 313, 289, 195 | Iriflophenone hexoside ([38], a PubChem CID:184358) | + | + | + | - | - | - | + |
| 3 | 4.60 | 299 | 407 b 409 | 389, 359, 317, 287 (100%), 245, 193 b 391 (100%), 373, 355, 325, 313, 289, 195 | Iriflophenone hexoside ([38], a PubChem CID:184358) | + | + | + | - | - | - | + |
| 4 | 5.55 | - | 583 | 565, 493 (100%), 463, 421, 403, 331, 301, 259 | Neomangiferin ([33]) | + | + | + | - | + | + | + |
| 5 | 6.30 | - | 421 | 403, 331, 301, 259 (100%), 165 | Mangiferin ([33]) | + | + | + | - | + | - | + |
| 6 | 6.60 | 242, 259, 319, 366 | 421 | 403, 331, 301 (100%), 259 | Isomangiferin ([33]) | + | + | + | - | + | + | + |
| 7 | 6.75 | 277, 304 | 245 | 161, 151 (100%), 126, 107 | Iriflophenone (a PubChem CID:11311158) | + | + | + | + | + | + | - |
| 8 | 7.00 | - | b 167 | b 149, 125 (100%) | Dihydro-coumaric acid (a PubChem CID:10394) | + | + | + | + | - | + | + |
| 9 | 7.45 | 298 | 421 | 403, 331, 301 (100%), 259 | Nigricanside ([38]) | + | + | + | - | + | + | + |
| 10 | 7.45 | - | 461 | 415 (100%), 311 | n.i. | - | - | - | - | + | + | + |
| 11 | 8.10 | 248, 282, 322 | 435 | 345, 315 (100%), 272 | Irisxanthone ([39]) | + | + | + | - | + | + | + |
| 12 | 8.60 | - | c 729 b 685 | c 683 (100%) b 523 (100%), 361 | Irigenin-O-dihexoside ([40]) | + | + | + | - | + | + | + |
| 13 | 9.20 | - | c 537 b 493 | c 491 (100%), 423, 329 b 331 (100%) | Iristectoridin B ([37]) | + | + | + | - | + | + | + |
| 14 | 9.35 | - | 259 | 165 (100%) | O-methyl-iriflophenone ([38]) | + | + | + | + | + | + | + |
| 15 | 9.43 | 267, 340 | c 567 b 523 | c 521 (100%), 359 b 361 (100%) | Iridin ([39]) | + | + | + | + | + | + | + |
| 16 | 10.21 | 263, 323 | c 519 b 475 | c 473, 311 (100%) b 313 (100%), 298 | Irisolone hexoside ([40]) | + | + | + | + | + | + | + |
| 17 | 10.45 | - | b 505 | b 343 (100%), 328 | Hydroxy-dimethoxy-methylenedioxy-isoflavone-hexoside, irisleptophyllidin ([39]) | + | + | + | - | + | + | + |
| 18 | 10.80 | - | b 685 | b 523 (100%), 361 | Irigenin-O-dihexoside ([40]) | + | + | + | - | - | + | + |
| 19 | 10.84 | 272, 340 | c 505 b 461 | c459 (100%), 297, 207 b299 (100%) | Irilone-hexoside ([41]) | + | + | + | + | + | + | + |
| 20 | 11.10 | - | b 329 | b 314 (100%), 297, 269, 180 | Irisflogenin ([39]) | + | + | + | - | + | + | + |
| 21 | 11.25 | - | 583 | 537, 421 (100%) | Hexoside derivative of xanthone—mangiferin or nigricanside derivative | + | + | + | - | + | + | + |
| 22 | 11.62 | 267, 331 | 359 | 344 (100%)/345, 329 | Irigenin, isomer 1 ([38]) | + | + | + | - | - | - | + |
| 23 | 11.80 | 267, 340 | 359 | 344 (100%)/345, 329 | Irigenin, isomer 2 ([38]) | + | + | + | + | + | + | + |
| 24 | 11.90 | 267, 321 | b 313 | b 298 (100%)/299, 283 | Irisolone ([40]) | + | + | + | + | + | + | + |
| 25 | 12.60 | 272, 340 | 373 | 358 (100%)/359, 343 | Irisjaponin B ([39]) | + | + | + | + | - | + | + |
| 26 | 12.64 | - | b 387 | b 372, 357 (100%), 326 | Irisflorentin ([42]) | + | + | + | + | + | + | + |
| 27 | 12.85 | - | b 487 | b 469 (100%), 451 | Iriflorentale or iripallidal ([39]) | + | - | - | - | - | - | - |
| 28 | 13.00 | - | 327 | 309, 291, 239, 229 (100%), 221, 211, 193, 177, 171 | Methoxy-irilone ([43]) | + | + | + | - | + | + | + |
| 29 | 13.40 | - | 329 | 311, 293, 229, 211 (100%)/209, 171, 165, 127/125 | Trihydroxyoctadecenoic acid ([33]) | + | + | + | - | + | + | + |
| 30 | 13.63 | - | b 513 | b 495, 429, 359 (100%), 345, 313, 301 | Belamcandin n.i. derivative ([39]) | + | + | + | + | + | + | + |
| 31 | 13.80 | - | 329 | 311, 293, 229, 211/209, 171 (100%), 165, 127/125 | Trihydroxyoctadecenoic acid ([33]) | + | + | + | - | + | + | + |
| 32 | 13.84 | - | b 513 | b 495 (100%), 361 | Irigenin n.i. derivative | + | + | + | - | - | + | + |
| 33 | 14.00 | - | b 513 | b 495, 371, 359 (100%), 313, 303 | Belamcandin n.i. derivative ([39]) | + | + | + | + | - | + | + |
| 34 | 14.00 | - | b 274 | b 256 (100%), 230, 106, 102, 88 | Amino-hexadecane diol ([44]) | + | + | + | - | + | - | + |
| 35 | 14.65 | - | 565 | 519 (100%) | n.i. | + | + | + | + | + | + | + |
| 36 | 14.65 | - | b 485 | b 467 (100%), 449, 347, 323 | n.i. | + | + | + | - | - | + | - |
| 37 | 14.90 | - | b 679 | b 661, 541 (100%) | n.i. | - | - | + | + | + | - | - |
| 38 | 15.00 | - | b 497 | b 479, 359 (100%), 342, 331, 301 | Belamcandin n.i. derivative ([39]) | + | + | + | + | + | + | + |
| 39 | 15.35 | - | 531 | 485 (100%), 467 | Iriflorentale or iripallidal n.i. derivative ([39]) | + | + | + | + | - | + | - |
| 40 | 15.75 | - | 573 | 527, 499, 485 (100%), 467, 449, 431 | Iriflorentale or iripallidal n.i. derivative ([39]) | + | + | + | - | - | + | - |
| 41 | 16.70 | - | c 531 d 487 b 469 d 451 | c 485 (100%) - b 451 (100%), 433, 191 - | Iriflorentale n.i. derivative ([39]) | + | + | + | + | - | + | + |
| 42 | 17.20 | - | c 531 d 487 b 469 d 451 | c 485 (100%) - b 451 (100%), 433, 191 - | Iripallidal n.i. derivative ([39]) | + | + | + | + | + | + | + |
| 43 | 17.40 | - | b 668 | b 649 (100%), 631, 439, 421, 403 | n.i. | - | - | + | + | + | + | - |
| 44 | 18.20 | - | b 456 | b 437 (100%), 319 | n.i. | + | - | + | - | - | + | + |
| No. | tret, Min | Compound | RIexp | RIlit | Method of Identification | Content, % |
|---|---|---|---|---|---|---|
| 1. | 29.32 | Butylated hydroxy toluene | 1516 | 1518 [64] | RI, MS | 12.8 |
| 2. | 30.30 | α-Irone | 1541 | 1535 [61] | RI, MS | 9.2 |
| 3. | 30.61 | Silphiperfol-5-en-3-one B | 1549 | 1550 [62] | RI, MS | 2.2 |
| 4. | 38.96 | n-Tetradecanoic acid syn. Myristic acid | 1779 | 1780 [63] | RI, MS | 75.8 |
| Total identified (%) | 100.0 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojiljković, I.; Ivković, Đ.; Stanojević, J.; Zvezdanović, J.; Beloica, J.; Krstić Ristivojević, M.; Stanković, D.; Jakanovski, M.; Ristivojević, P. Anti-Aging Potential of Illyrian Iris Rhizome Extract: Preliminary Chemical and Biological Profiling and Chemosensor Analysis via GC/MS and UHPLC-DAD-MS/MS Combined with HPTLC Bioautography. Chemosensors 2025, 13, 319. https://doi.org/10.3390/chemosensors13090319
Stojiljković I, Ivković Đ, Stanojević J, Zvezdanović J, Beloica J, Krstić Ristivojević M, Stanković D, Jakanovski M, Ristivojević P. Anti-Aging Potential of Illyrian Iris Rhizome Extract: Preliminary Chemical and Biological Profiling and Chemosensor Analysis via GC/MS and UHPLC-DAD-MS/MS Combined with HPTLC Bioautography. Chemosensors. 2025; 13(9):319. https://doi.org/10.3390/chemosensors13090319
Chicago/Turabian StyleStojiljković, Ivana, Đurđa Ivković, Jelena Stanojević, Jelena Zvezdanović, Jelena Beloica, Maja Krstić Ristivojević, Dalibor Stanković, Mihajlo Jakanovski, and Petar Ristivojević. 2025. "Anti-Aging Potential of Illyrian Iris Rhizome Extract: Preliminary Chemical and Biological Profiling and Chemosensor Analysis via GC/MS and UHPLC-DAD-MS/MS Combined with HPTLC Bioautography" Chemosensors 13, no. 9: 319. https://doi.org/10.3390/chemosensors13090319
APA StyleStojiljković, I., Ivković, Đ., Stanojević, J., Zvezdanović, J., Beloica, J., Krstić Ristivojević, M., Stanković, D., Jakanovski, M., & Ristivojević, P. (2025). Anti-Aging Potential of Illyrian Iris Rhizome Extract: Preliminary Chemical and Biological Profiling and Chemosensor Analysis via GC/MS and UHPLC-DAD-MS/MS Combined with HPTLC Bioautography. Chemosensors, 13(9), 319. https://doi.org/10.3390/chemosensors13090319





