Sensors and Biosensors as Viable Alternatives in the Determination of Contaminants in Corn: A Review (2021–2025)
Abstract
1. Introduction
2. Sensors and Biosensors in the Food Industry
Challenges and Perspectives
3. Sensors and Biosensors for the Determination of Contaminants on Corn
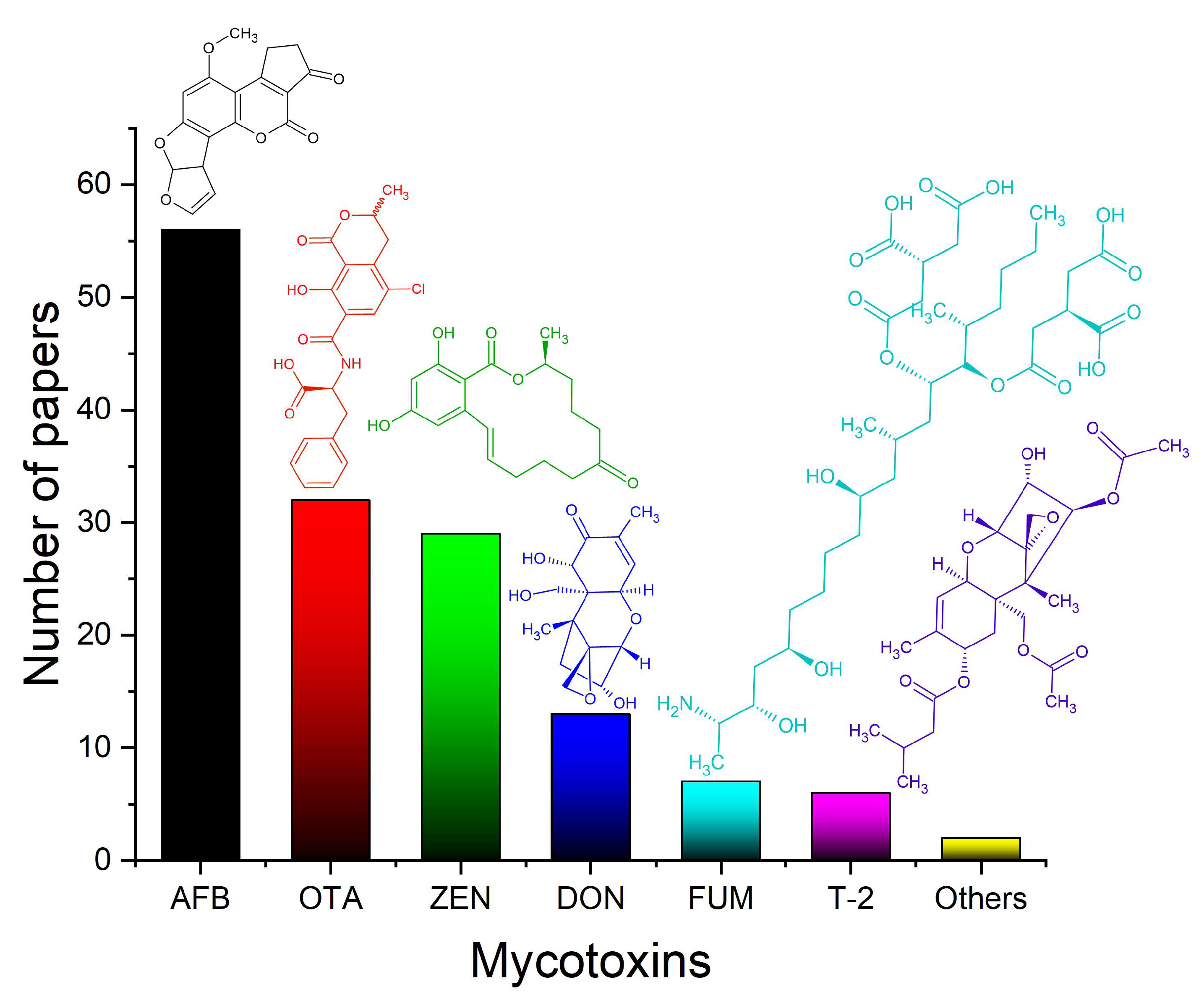
3.1. Mycotoxins
3.1.1. Interdisciplinary Research and Simultaneous Determinations
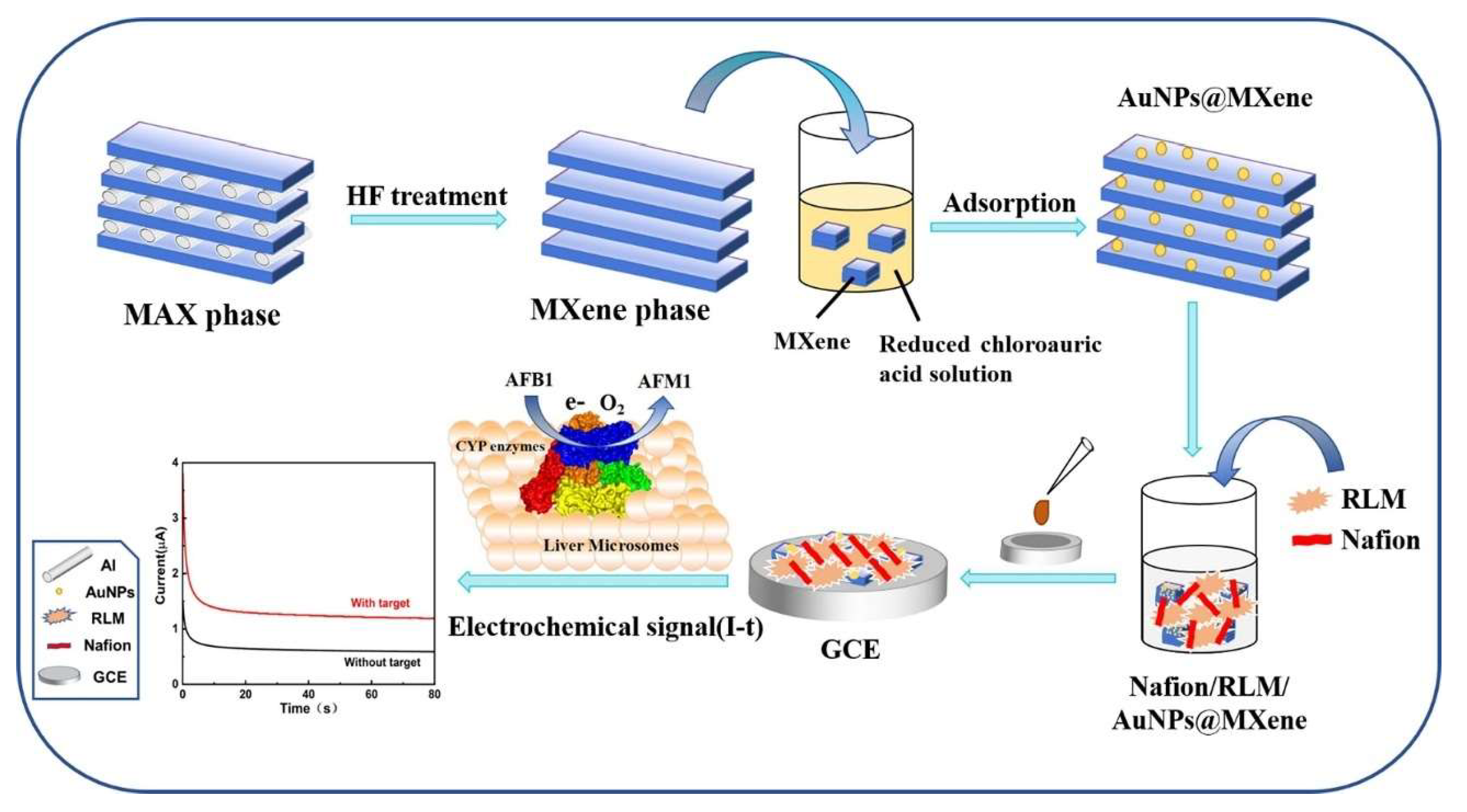
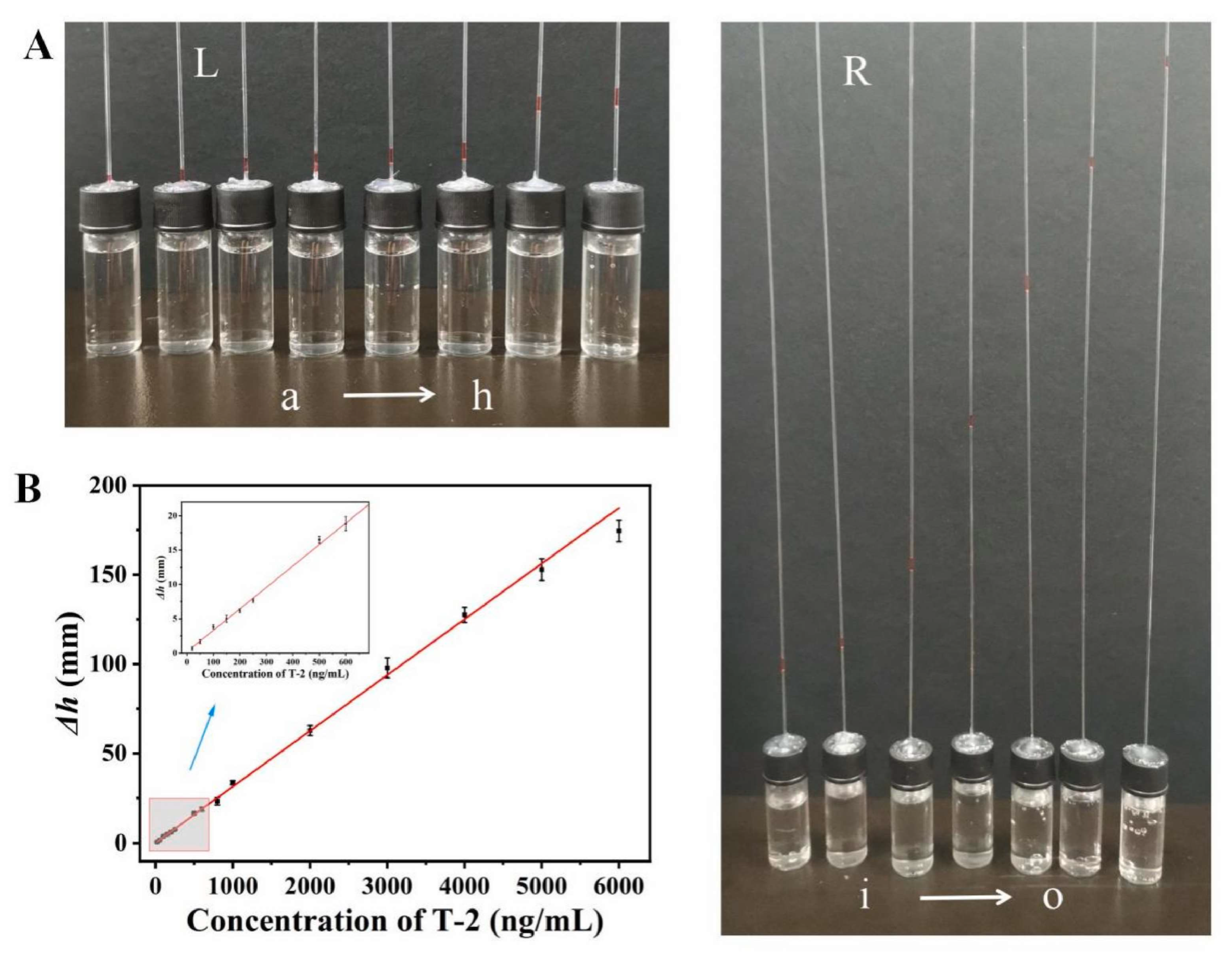
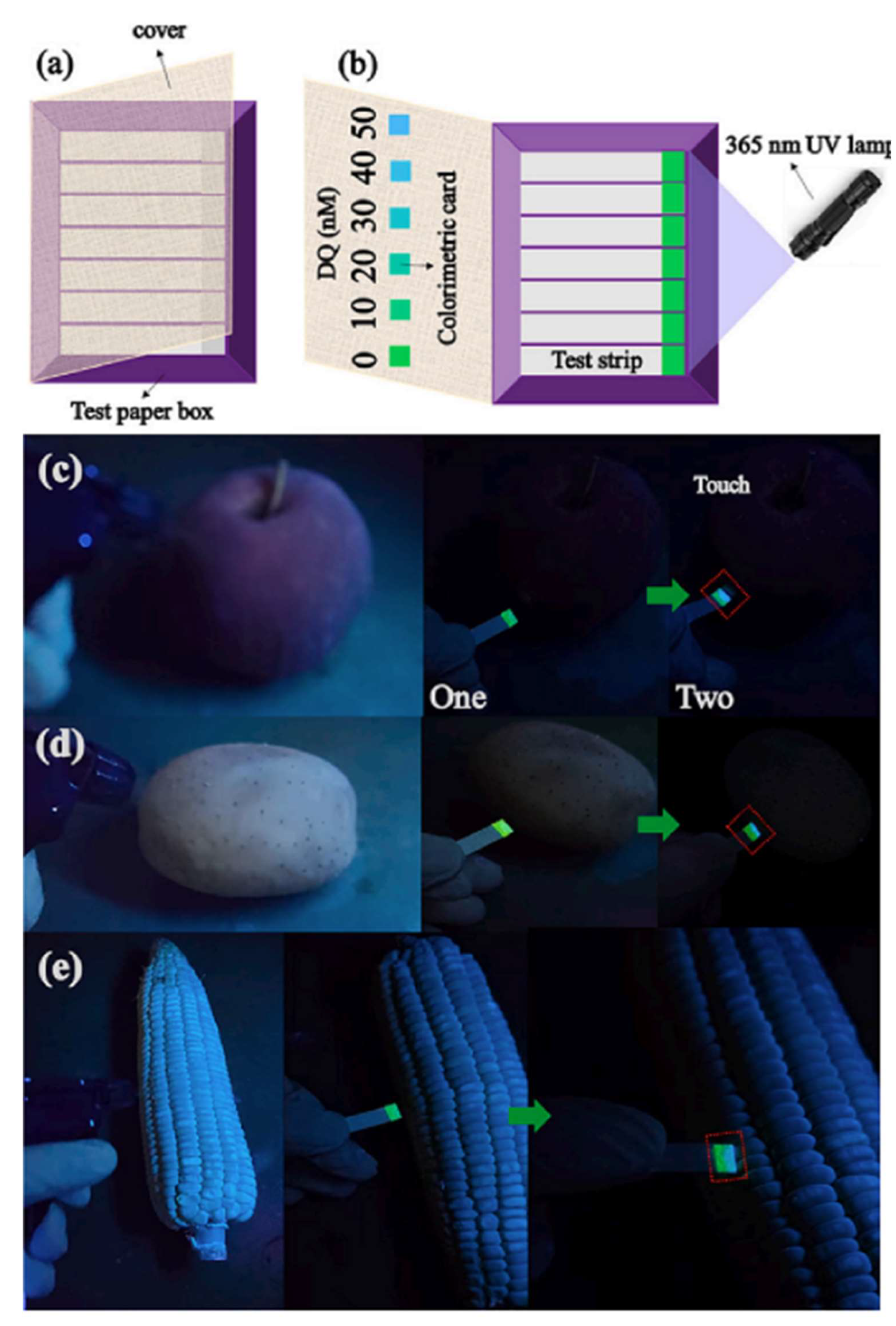
3.1.2. Non-Traditional and Innovative Systems
3.2. Pesticides
3.3. Other Contaminants
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Lara, S.; Serna-Saldivar, S.O. Corn History and Culture. In Corn; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–18. ISBN 9780128119716. [Google Scholar]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global Maize Production, Consumption and Trade: Trends and R&D Implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Sheng, S.; Li, T.; Liu, R. Corn Phytochemicals and Their Health Benefits. Food Sci. Hum. Wellness 2018, 7, 185–195. [Google Scholar] [CrossRef]
- Díaz-Gómez, J.L.; Castorena-Torres, F.; Preciado-Ortiz, R.E.; García-Lara, S. Anti-Cancer Activity of Maize Bioactive Peptides. Front. Chem. 2017, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, L.; Tang, J.; Deng, J.; Hao, E.; Bai, G.; Tang, P.L.; Yang, J.; Li, H.; Yao, L.; et al. Research on the Mechanism and Material Basis of Corn (Zea mays L.) Waste Regulating Dyslipidemia. Pharmaceuticals 2024, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Subedi, K.D.; Ma, B.L. Corn Crop Production: Growth, Fertilization and Yield. In Corn Crop Production: Growth, Fertilization and Yield; Danforth, A.T., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 1–85. ISBN 978-1-60741-955-6. [Google Scholar]
- Kazerooni, E.G.; Sharif, A.; Nawaz, H.; Rehman, R.; Nisar, S. Maize (Corn)-A Useful Source of Human Nutrition and Health: A Critical Review. Int. J. Chem. Biochem. Sci. 2019, 15, 35–41. [Google Scholar]
- Corn|USDA Foreign Agricultural Service. Available online: https://fas.usda.gov/data/production/commodity/0440000 (accessed on 29 November 2024).
- Smil, V. Detonator of the Population Explosion. Nature 1999, 400, 415. [Google Scholar] [CrossRef]
- Evenson, R.E.; Gollin, D. Assessing the Impact of the Green Revolution, 1960 to 2000. Science 2003, 300, 758–762. [Google Scholar] [CrossRef]
- Li, Y.; Hallerman, E.M.; Peng, Y. How Can China Prepare for the Domestic Cultivation of Bt Maize? Trends Food Sci. Technol. 2018, 73, 87–88. [Google Scholar] [CrossRef]
- Zikankuba, V.L.; Mwanyika, G.; Ntwenya, J.E.; James, A. Pesticide Regulations and Their Malpractice Implications on Food and Environment Safety. Cogent Food Agric. 2019, 5, 1601544. [Google Scholar] [CrossRef]
- Malhotra, K.; Aman, Z. World Agronomy: A Study of Pesticides Usage and Its Harmful Effects. Int. Res. J. Adv. Eng. Manag. 2024, 2, 1992–2001. [Google Scholar] [CrossRef]
- Ali, S.; Hameed, A.; Muhae-Ud-Din, G.; Ikhlaq, M.; Ashfaq, M.; Atiq, M.; Ali, F.; Zia, Z.U.; Naqvi, S.A.H.; Wang, Y. Citrus Canker: A Persistent Threat to the Worldwide Citrus Industry—An Analysis. Agronomy 2023, 13, 1112. [Google Scholar] [CrossRef]
- Goellner, K.; Loehrer, M.; Langenbach, C.; Conrath, U.; Koch, E.; Schaffrath, U. Phakopsora Pachyrhizi, the Causal Agent of Asian Soybean Rust. Mol. Plant Pathol. 2010, 11, 169–177. [Google Scholar] [CrossRef]
- Aladhadh, M. A Review of Modern Methods for the Detection of Foodborne Pathogens. Microorganisms 2023, 11, 1111. [Google Scholar] [CrossRef]
- Zhou, B.; Ye, Q.; Chen, M.; Li, F.; Xiang, X.; Shang, Y.; Wang, C.; Zhang, J.; Xue, L.; Wang, J.; et al. Novel Species-Specific Targets for Real-Time PCR Detection of Four Common Pathogenic Staphylococcus Spp. Food Control 2022, 131, 108478. [Google Scholar] [CrossRef]
- Li, Q.; Qin, D.; Zhu, J.; Yang, X.; Lu, Z.; Ye, S.; Zhang, Y.; Yang, H.; Wang, Z.; Shen, J.; et al. Development and Validation of an ELISA Kit for the Detection of Staphylococcus Aureus Enterotoxin A, B, C1, C2, C3, D, E from Food Samples. Food Control 2024, 166, 110630. [Google Scholar] [CrossRef]
- Quintanilla-Villanueva, G.E.; Sánchez-Álvarez, A.; Núñez-Salas, R.E.; Rodríguez-Delgado, M.M.; Luna-Moreno, D.; Villarreal-Chiu, J.F. Recent Advances in Monitoring Microbial Toxins in Food Samples by HPLC-Based Techniques: A Review. Analytica 2024, 5, 512–537. [Google Scholar] [CrossRef]
- Rusin, M.; Domagalska, J.; Rogala, D.; Razzaghi, M.; Szymala, I. Concentration of Cadmium and Lead in Vegetables and Fruits. Sci. Rep. 2021, 11, 11913. [Google Scholar] [CrossRef]
- Nolvachai, Y.; Amaral, M.S.S.; Marriott, P.J. Foods and Contaminants Analysis Using Multidimensional Gas Chromatography: An Update of Recent Studies, Technology, and Applications. Anal. Chem. 2023, 95, 238–263. [Google Scholar] [CrossRef]
- Andreu, V.; Picó, Y. Determination of Pesticides and Their Degradation Products in Soil: Critical Review and Comparison of Methods. TrAC Trends Anal. Chem. 2004, 23, 772–789. [Google Scholar] [CrossRef]
- Narenderan, S.T.; Meyyanathan, S.N.; Babu, B. Review of Pesticide Residue Analysis in Fruits and Vegetables. Pre-Treatment, Extraction and Detection Techniques. Food Res. Int. 2020, 133, 109141. [Google Scholar] [CrossRef]
- Clark, L.C.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. New York Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Bollella, P.; Katz, E. Biosensors—Recent Advances and Future Challenges. Sensors 2020, 20, 6645. [Google Scholar] [CrossRef]
- Venkatachalam, D.; Biswal, A.; Sellamuthu, P.S.; Sadiku, S.E. Introduction to Food Quality Monitoring Using Various Sensor Technologies. In Sensor Technologies for Food Quality and Safety; Royal Society of Chemistry: London, UK, 2025; pp. 1–21. ISBN 978-1-83767-478-7. [Google Scholar]
- Bankole, O.E.; Verma, D.K.; Chávez González, M.L.; Ceferino, J.G.; Sandoval-Cortés, J.; Aguilar, C.N. Recent Trends and Technical Advancements in Biosensors and Their Emerging Applications in Food and Bioscience. Food Biosci. 2022, 47, 101695. [Google Scholar] [CrossRef]
- Adachi, G.; Imanaka, N. Chemical Sensors. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 1995; Volume 21, pp. 179–262. ISBN 978-0-444-82178-2. [Google Scholar]
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical Sensors: Definitions and Classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Nagel, B.; Dellweg, H.; Gierasch, L.M. Glossary for Chemists of Terms Used in Biotechnology (IUPAC Recommendations 1992). Pure Appl. Chem. 1992, 64, 143–168. [Google Scholar] [CrossRef]
- Oliveira, A.E.F.; Pereira, A.C. Biosensor and Food Industry—Review. Rev. Virtual Química 2016, 8, 1311–1333. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Rab, S.; Pratap Singh, R.; Suman, R. Sensors for Daily Life: A Review. Sens. Int. 2021, 2, 100121. [Google Scholar] [CrossRef]
- Yousefi, H.; Su, H.-M.; Imani, S.M.; Alkhaldi, K.; Filipe, C.D.M.; Didar, T.F. Intelligent Food Packaging: A Review of Smart Sensing Technologies for Monitoring Food Quality. ACS Sens. 2019, 4, 808–821. [Google Scholar] [CrossRef]
- Nath, S. Advancements in Food Quality Monitoring: Integrating Biosensors for Precision Detection. Sustain. Food Technol. 2024, 2, 976–992. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Moon, Y.K.; Kim, T.H.; Park, S.W.; Kim, K.B.; Kang, Y.C.; Lee, J.H. A New Strategy for Detecting Plant Hormone Ethylene Using Oxide Semiconductor Chemiresistors: Exceptional Gas Selectivity and Response Tailored by Nanoscale Cr2O3 Catalytic Overlayer. Adv. Sci. 2020, 7, 1903093. [Google Scholar] [CrossRef]
- Poorahong, S.; Oin, W.; Buapoon, S.; Nijpanich, S.; Harding, D.J.; Siaj, M. Construction of an Electrochemical PH Sensor Using One-Pot Synthesis of a Molybdenum Diselenide/Nitrogen Doped Graphene Oxide Screen-Printed Electrode. RSC Adv. 2024, 14, 14616–14623. [Google Scholar] [CrossRef]
- Krzyczmonik, P.; Socha, E.; Skrzypek, S. Electrochemical Detection of Glucose in Beverage Samples Using Poly(3,4-Ethylenedioxythiophene)-Modified Electrodes with Immobilized Glucose Oxidase. Electrocatalysis 2018, 9, 380–387. [Google Scholar] [CrossRef]
- Samphao, A.; Kunpatee, K.; Prayoonpokarach, S.; Wittayakun, J.; Švorc, Ľ.; Stankovic, D.M.; Zagar, K.; Ceh, M.; Kalcher, K. An Ethanol Biosensor Based on Simple Immobilization of Alcohol Dehydrogenase on Fe3O4@Au Nanoparticles. Electroanalysis 2015, 27, 2829–2837. [Google Scholar] [CrossRef]
- Ali, M.E.; Hashim, U.; Mustafa, S.; Che Man, Y.B.; Adam, T.; Humayun, Q. Nanobiosensor for the Detection and Quantification of Pork Adulteration in Meatball Formulation. J. Exp. Nanosci. 2014, 9, 152–160. [Google Scholar] [CrossRef]
- Peveler, W.J.; Yazdani, M.; Rotello, V.M. Selectivity and Specificity: Pros and Cons in Sensing. ACS Sens. 2016, 1, 1282–1285. [Google Scholar] [CrossRef] [PubMed]
- Nikmanesh, Y.; Farhadi, M.; Taherian, M.; Asban, P.; Kiani, F.; Mohammadi, M.J. The Health Endpoint Due to Exposure Organophosphorus Toxicant. Clin. Epidemiol. Glob. Health 2024, 25, 101508. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Nian, B.; Yu, C.; Maimaiti, D.; Chai, M.; Yang, X.; Zang, X.; Xu, D. Mechanisms of Neurotoxicity of Organophosphate Pesticides and Their Relation to Neurological Disorders. Neuropsychiatr. Dis. Treat. 2024, 20, 2237–2254. [Google Scholar] [CrossRef]
- Kumaran, A.; Vashishth, R.; Singh, S.; U, S.; James, A.; Chellam, P.V. Biosensors for Detection of Organophosphate Pesticides: Current Technologies and Future Directives. Microchem. J. 2022, 178, 107420. [Google Scholar] [CrossRef]
- Dhull, V.; Gahlaut, A.; Dilbaghi, N.; Hooda, V. Acetylcholinesterase Biosensors for Electrochemical Detection of Organophosphorus Compounds: A Review. Biochem. Res. Int. 2013, 2013, 731501. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic Evolution of Ligands by Exponential Enrichment: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In Vitro Selection of RNA Molecules That Bind Specific Ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef]
- Anıl, İ.U.; Sezgintürk, M.K. MIP-Based Sensing Strategies for the Diagnosis of Prostate and Lung Cancers. Talanta Open 2025, 11, 100432. [Google Scholar] [CrossRef]
- Garg, M.; Pamme, N. Strategies to Remove Templates from Molecularly Imprinted Polymer (MIP) for Biosensors. TrAC Trends Anal. Chem. 2024, 170, 117437. [Google Scholar] [CrossRef]
- Ashley, J.; Shahbazi, M.-A.; Kant, K.; Chidambara, V.A.; Wolff, A.; Bang, D.D.; Sun, Y. Molecularly Imprinted Polymers for Sample Preparation and Biosensing in Food Analysis: Progress and Perspectives. Biosens. Bioelectron. 2017, 91, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Xu, Y.; Sayada, J.; Zareef, M.; Shoaib, M.; Chen, X.; Li, H.; Chen, Q. Progress of Machine Learning-Based Biosensors for the Monitoring of Food Safety: A Review. Biosens. Bioelectron. 2025, 267, 116782. [Google Scholar] [CrossRef]
- Wasilewski, T.; Kamysz, W.; Gębicki, J. AI-Assisted Detection of Biomarkers by Sensors and Biosensors for Early Diagnosis and Monitoring. Biosensors 2024, 14, 356. [Google Scholar] [CrossRef] [PubMed]
- European Commission Pesticide Database. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/mrls/searchpr (accessed on 4 August 2025).
- Zhang, K. FDA Foods Program Compendium of Analytical Laboratory Methods: Chemical Analytical Manual (CAM). Available online: https://www.fda.gov/media/114240/download (accessed on 30 June 2025).
- Laying down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs. Available online: https://eur-lex.europa.eu/eli/reg/2006/401/2014-07-01 (accessed on 30 June 2025).
- Chen, W.; Yang, Y.; Fu, K.; Zhang, D.; Wang, Z. Progress in ICP-MS Analysis of Minerals and Heavy Metals in Traditional Medicine. Front. Pharmacol. 2022, 13, 891273. [Google Scholar] [CrossRef]
- Jackson, B.P.; Punshon, T. Recent Advances in the Measurement of Arsenic, Cadmium, and Mercury in Rice and Other Foods. Curr. Environ. Health Rep. 2015, 2, 15–24. [Google Scholar] [CrossRef]
- Abdelmonem, B.H.; Kamal, L.T.; Elbaz, R.M.; Khalifa, M.R.; Abdelnaser, A. From Contamination to Detection: The Growing Threat of Heavy Metals. Heliyon 2025, 11, e41713. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bezerra, M.A.; Santos, A.S.; dos Santos, W.N.L.; Novaes, C.G.; de Oliveira, O.M.C.; Oliveira, M.L.; Garcia, R.L. Atomic Absorption Spectrometry—A Multi Element Technique. TrAC Trends Anal. Chem. 2018, 100, 1–6. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 333/2007; Laying Down the Methods of Sampling and Analysis for the Official Control of the Levels of Lead, Cadmium, Mercury, Inorganic Tin, 3-MCPD and Benzo(a)pyrene in Foodstuffs. European Union: Brussels, Belgium, 2007.
- Munkvold, G.P.; Arias, S.; Taschl, I.; Gruber-Dorninger, C. Mycotoxins in Corn: Occurrence, Impacts, and Management. In Corn; Elsevier: Amsterdam, The Netherlands, 2019; pp. 235–287. ISBN 9780128119716. [Google Scholar]
- Shultz, S. Corn. J. Agric. Food Inf. 2008, 9, 101–114. [Google Scholar] [CrossRef]
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global Maize Production, Utilization, and Consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef]
- Qu, M.; He, Y.; Xu, W.; Liu, D.; An, C.; Liu, S.; Liu, G.; Cheng, F. Array-Optimized Artificial Olfactory Sensor Enabling Cost-Effective and Non-Destructive Detection of Mycotoxin-Contaminated Maize. Food Chem. 2024, 456, 139940. [Google Scholar] [CrossRef] [PubMed]
- Ranbir; Singh, G.; Kaur, N.; Singh, N. Machine Learning Driven Metal Oxide-Based Portable Sensor Array for on-Site Detection and Discrimination of Mycotoxins in Corn Sample. Food Chem. 2025, 464, 141869. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Cerón, M.L.; Feng, K.; Wang, D.; Camarada, M.B.; Liao, X. Anchoring Black Phosphorus Quantum Dots over Carboxylated Multiwalled Carbon Nanotubes: A Stable 0D/1D Nanohybrid with High Sensing Performance to Ochratoxin A. Appl. Surf. Sci. 2022, 583, 152429. [Google Scholar] [CrossRef]
- Zeng, Y.; Camarada, M.B.; Lu, X.; Tang, K.; Li, W.; Qiu, D.; Wen, Y.; Wu, G.; Luo, Q.; Bai, L. Detection and Electrocatalytic Mechanism of Zearalenone Using Nanohybrid Sensor Based on Copper-Based Metal-Organic Framework/Magnetic Fe3O4-Graphene Oxide Modified Electrode. Food Chem. 2022, 370, 131024. [Google Scholar] [CrossRef]
- Mao, L.; Xue, X.; Xu, X.; Wen, W.; Chen, M.; Zhang, X.; Wang, S. Heterostructured CuO-g-C3N4 Nanocomposites as a Highly Efficient Photocathode for Photoelectrochemical Aflatoxin B1 Sensing. Sens. Actuators B Chem. 2021, 329, 129146. [Google Scholar] [CrossRef]
- Veenuttranon, K.; Lu, X.; Chen, J. Ultrasensitive Electrochemical Sensing for Simultaneous Rapid Detection of Zearalenone and Ochratoxin A in Feedstuffs and Foodstuffs. Chem. Eng. J. 2024, 497, 154807. [Google Scholar] [CrossRef]
- Khansili, N.; Krishna, P.M. Cerium Oxide Bentonite Nanocomposite-Based Colorimetric Paper Sensor for Aflatoxins Detection in Cereal Nuts, Oilseed and Legumes. J. Food Compos. Anal. 2023, 122, 105476. [Google Scholar] [CrossRef]
- Huang, H.; Ouyang, W.; Feng, K.; Camarada, M.B.; Liao, T.; Tang, X.; Liu, R.; Hou, D.; Liao, X. Rational Design of Molecularly Imprinted Electrochemical Sensor Based on Nb2C-MWCNTs Heterostructures for Highly Sensitive and Selective Detection of Ochratoxin A. Food Chem. 2024, 456, 140007. [Google Scholar] [CrossRef]
- Feng, X.; Yuan, R.; Liu, L.; Ding, L.; Long, L.; Wang, K. Construction of Dual-Signal Output Sensing Platform for Different Scene of Rapid and Sensitive Ochratoxin A Detection in Corn. Talanta 2025, 282, 126991. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, C.; Tong, X.; Duan, N.; Wang, Z.; Wu, S. A Portable Paper-Based Aptasensor for Simultaneous Visual Detection of Two Mycotoxins in Corn Flour Using Dual-Color Upconversion Nanoparticles and Cu-TCPP Nanosheets. Food Chem. 2023, 404, 134750. [Google Scholar] [CrossRef] [PubMed]
- Guo, K. Design and Fabrication of a Molecularly Imprinted Electrochemical Sensor with High Sensitivity for Zearalenone Assessment in Maize. Int. J. Electrochem. Sci. 2024, 19, 100612. [Google Scholar] [CrossRef]
- Khansili, N.; Krishna, P.M. Curcumin Functionalized TiO2 Modified Bentonite Clay Nanostructure for Colorimetric Aflatoxin B1 Detection in Peanut and Corn. Sens. Bio-Sens. Res. 2022, 35, 100480. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Li, Z.; Wang, T.; Sun, Q.; Le, T.; Jirimutu. A Novel Fluorescent Sensing Platform Based on Nitrogen-Doped Carbon Quantum Dots for Rapid and Sensitive Detection of Aflatoxin B1 in Corn Flour. LWT 2023, 185, 115130. [Google Scholar] [CrossRef]
- Chi, H.; Liu, G. A Fluorometric Sandwich Biosensor Based on Molecular Imprinted Polymer and Aptamer Modified CdTe/ZnS for Detection of Aflatoxin B1 in Edible Oil. LWT 2023, 180, 114726. [Google Scholar] [CrossRef]
- Wang, J.; Xia, M.; Wei, J.; Jiao, T.; Chen, Q.; Chen, Q.; Chen, X. Dual-Signal Amplified Cathodic Electrochemiluminescence Aptsensor Based on a Europium-Porphyrin Coordination Polymer for the Ultrasensitive Detection of Zearalenone in Maize. Sens. Actuators B Chem. 2023, 382, 133532. [Google Scholar] [CrossRef]
- Yan, H.; He, B.; Ren, W.; Suo, Z.; Xu, Y.; Xie, L.; Li, L.; Yang, J.; Liu, R. A Label-Free Electrochemical Immunosensing Platform Based on PEI-RGO/Pt@Au NRs for Rapid and Sensitive Detection of Zearalenone. Bioelectrochemistry 2022, 143, 107955. [Google Scholar] [CrossRef]
- Liu, B.; Peng, J.; Wu, Q.; Zhao, Y.; Shang, H.; Wang, S. A Novel Screening on the Specific Peptide by Molecular Simulation and Development of the Electrochemical Immunosensor for Aflatoxin B1 in Grains. Food Chem. 2022, 372, 131322. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Sumana, G.; Marquette, C.A. Gold Nanobipyramids Integrated Ultrasensitive Optical and Electrochemical Biosensor for Aflatoxin B1 Detection. Talanta 2021, 222, 121578. [Google Scholar] [CrossRef]
- Zhong, T.; Li, S.; Li, X.; JiYe, Y.; Mo, Y.; Chen, L.; Zhang, Z.; Wu, H.; Li, M.; Luo, Q. A Label-Free Electrochemical Aptasensor Based on AuNPs-Loaded Zeolitic Imidazolate Framework-8 for Sensitive Determination of Aflatoxin B1. Food Chem. 2022, 384, 132495. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Hu, X.; Shi, Y.; Liang, N.; Huang, X.; Wang, X.; Shen, T.; Zou, X.; Shi, J. Simple Design Concept for Dual-Channel Detection of Ochratoxin A Based on Bifunctional Metal–Organic Framework. ACS Appl. Mater. Interfaces 2022, 14, 5615–5623. [Google Scholar] [CrossRef]
- Duan, F.; Rong, F.; Guo, C.; Chen, K.; Wang, M.; Zhang, Z.; Pettinari, R.; Zhou, L.; Du, M. Electrochemical Aptasensing Strategy Based on a Multivariate Polymertitanium-Metal-Organic Framework for Zearalenone Analysis. Food Chem. 2022, 385, 132654. [Google Scholar] [CrossRef]
- Wei, M.; Yue, S.; Liu, Y. An Amplified Electrochemical Aptasensor for Ochratoxin A Based on DNAzyme-Mediated DNA Walker. J. Electroanal. Chem. 2021, 891, 115269. [Google Scholar] [CrossRef]
- Wang, K.; He, B.; Xie, L.; Li, L.; Yang, J.; Liu, R.; Wei, M.; Jin, H.; Ren, W. Exonuclease III-Assisted Triple-Amplified Electrochemical Aptasensor Based on PtPd NPs/PEI-RGO for Deoxynivalenol Detection. Sens. Actuators B Chem. 2021, 349, 130767. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, W.; Zhang, Y.; Lu, X.; Yang, Q.; Zhang, W. An Accurate and Ultrasensitive Ratiometric Electrochemical Aptasensor for Determination of Ochratoxin A Based on Catalytic Hairpin Assembly. Food Chem. 2023, 423, 136301. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, D.; Li, Y.; Chen, T.; You, T. Label-Free Ratiometric Homogeneous Electrochemical Aptasensor Based on Hybridization Chain Reaction for Facile and Rapid Detection of Aflatoxin B1 in Cereal Crops. Food Chem. 2022, 373, 131443. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, Y.; Li, Z.; Lu, X.; Qi, C.; Yang, Q.; Zhang, W. DNAzyme-Driven Tripedal DNA Walker for Ratiometric Electrochemical Aptasensor Ultrasensitive Detection of Aflatoxin B1. Food Control 2024, 164, 110573. [Google Scholar] [CrossRef]
- Liang, X.; Zhao, F.; Xiao, C.; Yue, S.; Huang, Y.; Wei, M. A Ratiometric Electrochemical Aptasensor for Ochratoxin A Detection. J. Chin. Chem. Soc. 2021, 68, 1271–1278. [Google Scholar] [CrossRef]
- Yang, H.; Du, L.; Geng, L.; Liu, X.; Xu, Z.; Liu, R.; Liu, W.; Zuo, H.; Chen, Z.; Wang, X.; et al. A Novel Yeast-Based Biosensor for the Quick Determination of Deoxynivalenol. Anal. Chim. Acta 2024, 1315, 342760. [Google Scholar] [CrossRef]
- Lerdsri, J.; Thunkhamrak, C.; Jakmunee, J. Development of a Colorimetric Aptasensor for Aflatoxin B1 Detection Based on Silver Nanoparticle Aggregation Induced by Positively Charged Perylene Diimide. Food Control 2021, 130, 108323. [Google Scholar] [CrossRef]
- Jiang, W.; Yang, Q.; Duo, H.; Wu, W.; Hou, X. Ionic Liquid-Enhanced Silica Aerogels for the Specific Extraction and Detection of Aflatoxin B1 Coupled with a Smartphone-Based Colorimetric Biosensor. Food Chem. 2024, 447, 138917. [Google Scholar] [CrossRef] [PubMed]
- Pal, T.; Aditya, S.; Mathai, T.; Mukherji, S. Polyaniline Coated Plastic Optic Fiber Biosensor for Detection of Aflatoxin B1 in Nut, Cereals, Beverages, and Body Fluids. Sens. Actuators B Chem. 2023, 389, 133897. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, J.; Li, X.; Hu, Q.; Qian, J.; Hao, N.; Wang, K. Region Separation Type Bio-Photoelectrode Based All-Solid-State Self-Powered Aptasensor for Ochratoxin A and Aflatoxin B1 Detection. Sens. Actuators B Chem. 2022, 364, 131897. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, J.; Liu, S.; Li, B. A Label-Free Visual Aptasensor for Zearalenone Detection Based on Target-Responsive Aptamer-Cross-Linked Hydrogel and Color Change of Gold Nanoparticles. Food Chem. 2022, 389, 133078. [Google Scholar] [CrossRef]
- Singh, A.K.; Dhiman, T.K.; Lakshmi, G.B.V.S.; Solanki, P.R. Dimanganese Trioxide (Mn2O3) Based Label-Free Electrochemical Biosensor for Detection of Aflatoxin-B1. Bioelectrochemistry 2021, 137, 107684. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Sameiyan, E.; Zahraee, H.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. A Simple and Robust Aptasensor Assembled on Surfactant-Mediated Liquid Crystal Interface for Ultrasensitive Detection of Mycotoxin. Anal. Chim. Acta 2023, 1270, 341478. [Google Scholar] [CrossRef]
- Singh, H.; Deep, A.; Puri, S.; Khatri, M.; Bhardwaj, N. UiO-66-NH2 MOF-Based Fluorescent Aptasensor for Detection of Zearalenone in Cereals. Food Control 2024, 163, 110497. [Google Scholar] [CrossRef]
- Zhang, X.; Zhi, H.; Zhu, M.; Wang, F.; Meng, H.; Feng, L. Electrochemical/Visual Dual-Readout Aptasensor for Ochratoxin A Detection Integrated into a Miniaturized Paper-Based Analytical Device. Biosens. Bioelectron. 2021, 180, 113146. [Google Scholar] [CrossRef]
- He, Y.; Wang, H.; Yu, Z.; Tang, X.; Zhou, M.; Guo, Y.; Xiong, B. A Disposable Immunosensor Array Using Cellulose Paper Assembled Chemiresistive Biosensor for Simultaneous Monitoring of Mycotoxins AFB1 and FB1. Talanta 2024, 276, 126145. [Google Scholar] [CrossRef]
- Demirbakan, B.; Köseer, N.T.; Uzman, E.; Özay, Ö.; Özay, H.; Sezgintürk, M.K. A Single-Use Electrochemical Biosensor System for Ultrasensitive Detection of Aflatoxin B1 in Rice, Corn, Milk, Peanut, Chili Pepper Samples. J. Food Compos. Anal. 2024, 136, 106701. [Google Scholar] [CrossRef]
- Demirbakan, B.; Köseer, N.T.; Özay, Ö.; Özay, H.; Sezgintürk, M.K. An Unusual Impedimetric Biosensor Design Based on 3-MPDS for Highly Sensitive Detection of AFB1 in Food Samples. Food Biosci. 2024, 62, 105022. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, X.; Huang, X.; Xu, F.; Gu, C.; Yu, S.; Zhang, X.; Qian, J. Simultaneous Detection of Multiple Mycotoxins Using MXene-Based Electrochemical Aptasensor Array and a Self-Developed Multi-Channel Portable Device. Talanta 2024, 278, 126450. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Cai, S.; Deng, Y.; Luo, F.; Huang, J.; Lin, Z. Portable T-2 Toxin Biosensor Based on Target-Responsive DNA Hydrogel Using Water Column Height as Readout. Talanta 2024, 276, 126203. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, S.; Chen, X.; Sun, D.; Zuo, X.; Liu, C.; Zhang, Q.; Li, S.; Ye, H.; Kong, D. Magnetically Controlled Fluorescence Biosensor for Simultaneous Detection of Aflatoxin B1 and Its Toxin-Producing Gene AflD. Food Biosci. 2025, 64, 105972. [Google Scholar] [CrossRef]
- Qin, Y.; Li, S.; Wang, Y.; Peng, Y.; Han, D.; Zhou, H.; Bai, J.; Ren, S.; Li, S.; Chen, R.; et al. A Highly Sensitive Fluorometric Biosensor for Fumonisin B1 Detection Based on Upconversion Nanoparticles-Graphene Oxide and Catalytic Hairpin Assembly. Anal. Chim. Acta 2022, 1207, 339811. [Google Scholar] [CrossRef]
- Li, M.; Li, D.Y.; Li, Z.Y.; Hu, R.; Yang, Y.H.; Yang, T. A Visual Peroxidase Mimicking Aptasensor Based on Pt Nanoparticles-Loaded on Iron Metal Organic Gel for Fumonisin B1 Analysis in Corn Meal. Biosens. Bioelectron. 2022, 209, 114241. [Google Scholar] [CrossRef]
- Zhan, C.; Lu, P.; Dong, Y.; Chen, R.; Yu, D.; Chen, Y. Magnetic Relaxation Switching Immunosensor Based on Polystyrene Microcolumn and Tyramine Signal Amplification for Ultrasensitive and User-Friendly Detection of Aflatoxin B1 in Corn. Food Chem. 2024, 460, 140362. [Google Scholar] [CrossRef]
- Gong, Q.; Meng, S.; Liu, D.; You, T. Direct Z-Scheme NiTiO3/Polyaniline Heterojunction Based Photoelectrochemical Aptasensor for the Efficient Detection of Ochratoxin A in Corn and Soil. Sens. Actuators B Chem. 2024, 401, 134976. [Google Scholar] [CrossRef]
- Li, X.; Meng, F.; Li, Z.; Li, R.; Zhang, Y.; Zhang, M. Dual-Signal Aptasensor Based on Zr-MOF for Ultrasensitive Detection of AFB1 in Corn. Sens. Actuators B Chem. 2023, 394, 134372. [Google Scholar] [CrossRef]
- Subak, H.; Selvolini, G.; Macchiagodena, M.; Ozkan-Ariksoysal, D.; Pagliai, M.; Procacci, P.; Marrazza, G. Mycotoxins Aptasensing: From Molecular Docking to Electrochemical Detection of Deoxynivalenol. Bioelectrochemistry 2021, 138, 107691. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, X.; Song, Y.; Zhang, J.; Han, Q. CRISPR-Cas12a-Based Aptasensor for Sensitive and Selective FB1 Detection. J. Food Compos. Anal. 2023, 123, 105615. [Google Scholar] [CrossRef]
- Tang, J.; Liu, J.; Wang, F.; Yao, Y.; Hu, R. Colorimetric and Photothermal Dual-Mode Aptasensor with Redox Cycling Amplification for the Detection of Ochratoxin A in Corn Samples. Food Chem. 2024, 439, 137968. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, J.; Li, D.; Wang, D. Enzyme-Free Autocatalysis-Driven DNA Cascade Circuits for Amplified Electrochemical Sensing of Ochratoxin A in Food. J. Food Compos. Anal. 2025, 140, 107279. [Google Scholar] [CrossRef]
- Kang, K.; Zhang, H.; Jia, L.; Tan, X.; Wang, B.; Gao, X.; Fu, Y.; Niu, L.; Ji, X. Enhanced Sensitivity for Aflatoxin B1 Detection in Food through a H-Zn1Cd5S-Based Photoelectrochemical Aptasensor. Sens. Actuators B Chem. 2024, 413, 135894. [Google Scholar] [CrossRef]
- Jahangiri–Dehaghani, F.; Zare, H.R.; Shekari, Z. Simultaneous Measurement of Ochratoxin A and Aflatoxin B1 Using a Duplexed-Electrochemical Aptasensor Based on Carbon Nanodots Decorated with Gold Nanoparticles and Two Redox Probes Hemin@HKUST-1 and Ferrocene@HKUST-1. Talanta 2024, 266, 124947. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.; Wu, T.; Zhang, X.; Ren, X.; Feng, R.; Du, Y.; Yong Lee, J.; Liu, X.; Wei, Q. Zirconium Based Metal–Organic Frameworks with Aggregation-Induced Electrochemiluminescence for Sensitive Analysis of Aflatoxin B1 by Signal Dual-Amplification Strategy. Chem. Eng. J. 2024, 500, 157308. [Google Scholar] [CrossRef]
- He, C.; Wang, L.; Shen, D.; Zhang, J.; Zheng, L.; Yao, H.; Feng, G.; Fang, J. A G-Quadruplex Dual-Signal Strategy for on-Site Detection of OTA in Moldy Foods. Microchem. J. 2024, 201, 110746. [Google Scholar] [CrossRef]
- Zhu, W.; Ji, G.; Chen, R.; Xiang, Y.; Ji, S.; Zhang, S.; Gao, Z.; Liu, H.; Wang, Y.; Han, T. A Fluorescence Aptasensor Based on Hybridization Chain Reaction for Simultaneous Detection of T-2 Toxins and Zearalenone1. Talanta 2023, 255, 124249. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, H.; Yang, Y.; Yang, P.; Yan, X.; Mu, Y.; Jin, Q.; Yang, P.; Gao, W. A Comb-Shaped Microfluidic Aptasensor for Rapid and Sensitive on-Site Simultaneous Detection of Aflatoxin B1 and Deoxynivalenol. Food Chem. 2025, 473, 143072. [Google Scholar] [CrossRef]
- Wu, M.; Ma, Y.; Huang, Y.; Zhang, X.; Dong, J.; Sun, D. An Ultrasensitive Electrochemical Aptasensor Based on Zeolitic Imidazolate Framework-67 Loading Gold Nanoparticles and Horseradish Peroxidase for Detection of Aflatoxin B1. Food Chem. 2024, 456, 140039. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.; Liu, J.; Yan, K.; Zhang, J. Near-Infrared-Driven Dual-Photoelectrode Photoelectrochemical Sensing for Fumonisin B1: Integrating a Photon up-Conversion Bio-Photocathode with an Enhanced Light-Capturing Photoanode. Talanta 2025, 282, 127047. [Google Scholar] [CrossRef]
- Chen, M.-M.; Liu, Y.; Zhao, S.; Jiang, J.; Zhang, Q.; Li, P.; Tang, X. Carbon Nanospheres Bridging in Perovskite Quantum Dots/BiOBr: An Efficient Heterojunction for High-Performance Photoelectrochemical Sensing of Deoxynivalenol. Carbon N. Y. 2024, 221, 118919. [Google Scholar] [CrossRef]
- Wang, X.; Jia, X.; Wang, Y.; Li, S.; Ren, S.; Wang, Y.; Han, D.; Qin, K.; Chang, X.; Zhou, H.; et al. A Facile Dual-Mode Immunosensor Based on Speckle Ag-Doped Nanohybrids for Ultrasensitive Detection of Ochratoxin A. Food Chem. 2024, 439, 138102. [Google Scholar] [CrossRef]
- Fan, Y.; Amin, K.; Jing, W.; Lyu, B.; Wang, S.; Fu, H.; Yu, H.; Yang, H.; Li, J. A Novel Recjf Exo Signal Amplification Strategy Based on Bioinformatics-Assisted Truncated Aptamer for Efficient Fluorescence Detection of AFB1. Int. J. Biol. Macromol. 2024, 254, 128061. [Google Scholar] [CrossRef] [PubMed]
- Damphathik, C.; Songsiriritthigul, C.; Lerdsri, J.; Jakmunee, J.; Wongnongwa, Y.; Jungsuttiwong, S.; Ortner, A.; Kalcher, K.; Samphao, A. A Novel Immunosensor Based on Cobalt Oxide Nanocomposite Modified Single Walled Carbon Nanohorns for the Selective Detection of Aflatoxin B1. Talanta 2023, 258, 124472. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.S.; Kummari, S.; Catanante, G.; Gobi, K.V.; Marty, J.L.; Goud, K.Y. A Label-Free Impedimetric Immunosensor for Zearalenone Based on CS-CNT-Pd Nanocomposite Modified Screen-Printed Disposable Electrodes. Sens. Actuators B Chem. 2023, 377, 133077. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, Y.; Tan, H.; Yang, Y.; Wang, X.; Liu, X. Ratiometric Electrochemical and Impedimetric Dual-Mode Aptasensor Based on Thionine-Functionalized Ti3C2Tx MXene/Pt and Au Nanoparticle Composites for Reliable Detection of Aflatoxin B1. Sens. Actuators B Chem. 2025, 423, 136758. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, Y.; Wang, S.; Dong, Y. Simultaneous Determination of Zearalenone and Ochratoxin A Based on Microscale Thermophoresis Assay with a Bifunctional Aptamer. Anal. Chim. Acta 2021, 1155, 338345. [Google Scholar] [CrossRef]
- Li, Z.; Xu, H.; Zhang, Z.; Miao, X. DNA Tetrahedral Scaffold-Corbelled 3D DNAzyme Walker for Electrochemiluminescent Aflatoxin B1 Detection. Food Chem. 2023, 407, 135049. [Google Scholar] [CrossRef]
- Sun, Y.; Qi, S.; Dong, X.; Qin, M.; Zhang, Y.; Wang, Z. Colorimetric Aptasensor Targeting Zearalenone Developed Based on the Hyaluronic Acid-DNA Hydrogel and Bimetallic MOFzyme. Biosens. Bioelectron. 2022, 212, 114366. [Google Scholar] [CrossRef]
- Lin, X.; Li, C.; Meng, X.; Yu, W.; Duan, N.; Wang, Z.; Wu, S. CRISPR-Cas12a-Mediated Luminescence Resonance Energy Transfer Aptasensing Platform for Deoxynivalenol Using Gold Nanoparticle-Decorated Ti3C2Tx MXene as the Enhanced Quencher. J. Hazard. Mater. 2022, 433, 128750. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Guo, H.; Ye, H.; Zhang, Y.; Wang, Z. Aptamer-Locker Probe Coupling with Truncated Aptamer for High-Efficiency Fluorescence Polarization Detection of Zearalenone. Sens. Actuators B Chem. 2023, 380, 133356. [Google Scholar] [CrossRef]
- Xiong, J.; He, S.; Zhang, S.; Qin, L.; Yang, L.; Wang, Z.; Zhang, L.; Shan, W.; Jiang, H. A Label-Free Aptasensor for Dual-Mode Detection of Aflatoxin B1 Based on Inner Filter Effect Using Silver Nanoparticles and Arginine-Modified Gold Nanoclusters. Food Control 2023, 144, 109397. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, M.; Peng, D.; Zheng, H.; Qin, H.; Xiao, J.; Wu, Y.; Yang, N. A Self-Supported Electrochemical Immunosensor Based on Cu2O/CuO@AuNPs Heterostructures for Sensitive and Selective Detection of Ochratoxin A in Food. Talanta 2025, 287, 127657. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, D.; Li, Y.; Ma, S.; Wang, M.; You, T. Hairpin DNA Assisted Dual-Ratiometric Electrochemical Aptasensor with High Reliability and Anti-Interference Ability for Simultaneous Detection of Aflatoxin B1 and Ochratoxin A. Biosens. Bioelectron. 2021, 174, 112654. [Google Scholar] [CrossRef]
- He, Y.; Zhang, D.; Wu, Q.; Du, G.; Liu, R.; Zhou, X.; Zhang, Y. Highly Sensitive Fluorescent Aptasensor Based on Magnetic Metal-Organic Framework for Aflatoxin B1 Detection. Talanta 2025, 287, 127620. [Google Scholar] [CrossRef]
- Liu, J.; Suo, Z.; Liu, Y.; He, B.; Wei, M. An Electrochemical Apta-Assay Based on Hybridization Chain Reaction and Aflatoxin B1-Driven Ag-DNAzyme as Amplification Strategy. Bioelectrochemistry 2023, 149, 108322. [Google Scholar] [CrossRef]
- Wei, Q.; Huang, C.; Lu, P.; Zhang, X.; Chen, Y. Combining Magnetic MOFs as a Highly Adsorbent with Homogeneous Chemiluminescent Immunosensor for Rapid and Ultrasensitive Determination of Ochratoxin A. J. Hazard. Mater. 2023, 441, 129960. [Google Scholar] [CrossRef]
- Suo, Z.; Niu, X.; Liu, R.; Xin, L.; Liu, Y.; Wei, M. A Methylene Blue and Ag+ Ratiometric Electrochemical Aptasensor Based on Au@Pt/Fe-N-C Signal Amplification Strategy for Zearalenone Detection. Sens. Actuators B Chem. 2022, 362, 131825. [Google Scholar] [CrossRef]
- Li, W.; Xu, L.; Zhang, X.; Ding, Z.; Xu, X.; Cai, X.; Wang, Y.; Li, C.; Sun, D. Fabrication of a High-Performance Photoelectrochemical Aptamer Sensor Based on Er-MOF Nanoballs Functionalized with Ionic Liquid and Gold Nanoparticles for Aflatoxin B1 Detection. Sens. Actuators B Chem. 2023, 378, 133153. [Google Scholar] [CrossRef]
- Wen, J.; Fan, Y.-Y.; Li, J.; Yang, X.-W.; Zhang, X.-X.; Zhang, Z.-Q. A G-Triplex and G-Quadruplex Concatemer-Enhanced Fluorescence Probe Coupled with Hybridization Chain Reaction for Ultrasensitive Aptasensing of Ochratoxin A. Anal. Chim. Acta 2023, 1272, 341503. [Google Scholar] [CrossRef]
- Xia, M.; Wang, J.; Li, S.; Lin, A.; Yao, Q.; Guo, Z.; Chen, X.; Chen, Q.; Chen, X. A Sensitive Electrochemiluminescence Resonance Energy Transfer System between Ru-MOFs and Bi2S3 for Deoxynivalenol Detection. Sens. Actuators B Chem. 2023, 393, 134192. [Google Scholar] [CrossRef]
- Qiao, M.; Wan, Z.; Wang, X.; Suo, Z.; Liu, Y.; Wei, M. A Novel Fluorescent Aptasensor Based on H-Shaped DNA Nanostructure and Hollow Carbon-Doped Nitrogen Nanospheres for Sensitive Detection of AFB1. Food Control 2024, 162, 110430. [Google Scholar] [CrossRef]
- Ge, Y.; Liu, P.; Chen, Q.; Qu, M.; Xu, L.; Liang, H.; Zhang, X.; Huang, Z.; Wen, Y.; Wang, L. Machine Learning-Guided the Fabrication of Nanozyme Based on Highly-Stable Violet Phosphorene Decorated with Phosphorus-Doped Hierarchically Porous Carbon Microsphere for Portable Intelligent Sensing of Mycophenolic Acid in Silage. Biosens. Bioelectron. 2023, 237, 115454. [Google Scholar] [CrossRef]
- Ren, W.; Pang, J.; Ma, R.; Liang, X.; Wei, M.; Suo, Z.; He, B.; Liu, Y. A Signal On-off Fluorescence Sensor Based on the Self-Assembly DNA Tetrahedron for Simultaneous Detection of Ochratoxin A and Aflatoxin B1. Anal. Chim. Acta 2022, 1198, 339566. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, K.; Zhang, K.; Li, P.; Wang, L.; Wu, C.; Xu, K. Ultrasensitive Aflatoxin B1 Detection Based on Vertical Organic Electrochemical Transistor. Food Chem. 2025, 464, 141648. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; He, B.; Liu, Y.; Wang, L.; Liang, Y.; Wang, J.; Jin, H.; Wei, M.; Ren, W.; Suo, Z.; et al. A Dual-Signal Mode Electrochemical Aptasensor Based on Tetrahedral DNA Nanostructures for Sensitive Detection of Citrinin in Food Using PtPdCo Mesoporous Nanozymes. Food Chem. 2024, 460, 140739. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-J.; Wang, G.-Q.; Zheng, J.-Y.; Yang, H.-Y.; Wang, A.-J.; Mei, L.-P.; Feng, J.-J.; Cheang, T.Y. Z-Scheme Cu2MoS4/CdS/In2S3 Nanocages Heterojunctions-Based PEC Aptasensor for Ultrasensitive Assay of Fumonisin B1 via Signal Amplification with Hollow PtPd–CoSnO3 Nanozyme. Biosens. Bioelectron. 2023, 230, 115293. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Suo, Z.; He, B.; Liu, Y.; Wei, M.; Jin, H. An Innovative Electrochemical Aptasensor Based on the Dual Signal Amplification Strategy of Gold Nanowires and Bifunctional DNA Nanoflowers. Sens. Actuators B Chem. 2023, 377, 132995. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, J.; Huo, B.; Huang, H.; Bai, J.; Peng, Y.; Li, S.; Han, D.; Ren, S.; et al. A Fluorescence Aptasensor for the Sensitive Detection of T-2 Toxin Based on FRET by Adjusting the Surface Electric Potentials of UCNPs and MIL-101. Anal. Chim. Acta 2021, 1160, 338450. [Google Scholar] [CrossRef]
- Dou, X.; Wu, G.; Ding, Z.; Xie, J. Construction of a Nanoscale Metal-Organic Framework Aptasensor for Fluorescence Ratiometric Sensing of AFB1 in Real Samples. Food Chem. 2023, 416, 135805. [Google Scholar] [CrossRef] [PubMed]
- Vijitvarasan, P.; Cheunkar, S.; Oaew, S. A Point-of-Use Lateral Flow Aptasensor for Naked-Eye Detection of Aflatoxin B1. Food Control 2022, 134, 108767. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, C.; Zhao, F.; Suo, Z.; Liu, Y.; Wei, M.; Jin, B. Ratiometric Fluorescent Aptasensor Based on DNA-Gated Fe3O4@Uio-66-NH2 and Exo I-Assisted Signal Amplification. Anal. Chim. Acta 2025, 1340, 343665. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; He, B.; Wang, Y.; Zhao, R.; Zhang, Y.; Bai, C.; Wei, M.; Jin, H.; Ren, W.; Suo, Z.; et al. ZIF-8 Labelled a New Electrochemical Aptasensor Based on PEI-PrGO/AuNWs for DON Detection. Talanta 2024, 267, 125257. [Google Scholar] [CrossRef]
- Zhao, L.; Suo, Z.; He, B.; Huang, Y.; Liu, Y.; Wei, M.; Jin, H. A Fluorescent Aptasensor Based on Nitrogen-Doped Carbon Supported Palladium and Exonuclease III-Assisted Signal Amplification for Sensitive Detection of AFB1. Anal. Chim. Acta 2022, 1226, 340272. [Google Scholar] [CrossRef]
- Mu, Z.; Ma, L.; Wang, J.; Zhou, J.; Yuan, Y.; Bai, L. A Target-Induced Amperometic Aptasensor for Sensitive Zearalenone Detection by CS@AB-MWCNTs Nanocomposite as Enhancers. Food Chem. 2021, 340, 128128. [Google Scholar] [CrossRef]
- Wu, H.; Wang, H.; Wu, J.; Han, G.; Liu, Y.; Zou, P. A Novel Fluorescent Aptasensor Based on Exonuclease-Assisted Triple Recycling Amplification for Sensitive and Label-Free Detection of Aflatoxin B1. J. Hazard. Mater. 2021, 415, 125584. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Nan, M.; Li, Y.; Yun, J.; Wang, Y.; Bi, Y. Novel Colorimetric Aptasensor Based on Unmodified Gold Nanoparticle and SsDNA for Rapid and Sensitive Detection of T-2 Toxin. Food Chem. 2021, 348, 129128. [Google Scholar] [CrossRef]
- Yan, H.; He, B.; Zhao, R.; Ren, W.; Suo, Z.; Xu, Y.; Xie, D.; Zhao, W.; Wei, M.; Jin, H. Electrochemical Aptasensor Based on CRISPR/Cas12a-Mediated and DNAzyme-Assisted Cascade Dual-Enzyme Transformation Strategy for Zearalenone Detection. Chem. Eng. J. 2024, 493, 152431. [Google Scholar] [CrossRef]
- Fang, H.; Zhan, S.; Feng, L.; Chen, X.; Guo, Q.; Guo, Y.; He, Q.; Xiong, Y. Chemical Modification of M13 Bacteriophage as Nanozyme Container for Dramatically Enhanced Sensitivity of Colorimetric Immunosensor. Sens. Actuators B Chem. 2021, 346, 130368. [Google Scholar] [CrossRef]
- Bi, X.; Li, L.; Luo, L.; Liu, X.; Li, J.; You, T. A Ratiometric Fluorescence Aptasensor Based on Photoinduced Electron Transfer from CdTe QDs to WS2 NTs for the Sensitive Detection of Zearalenone in Cereal Crops. Food Chem. 2022, 385, 132657. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Li, L.; Liu, X.; Luo, L.; Cheng, Z.; Sun, J.; Cai, Z.; Liu, J.; You, T. Inner Filter Effect-Modulated Ratiometric Fluorescence Aptasensor Based on Competition Strategy for Zearalenone Detection in Cereal Crops: Using Mitoxantrone as Quencher of CdTe QDs@SiO2. Food Chem. 2021, 349, 129171. [Google Scholar] [CrossRef]
- Ming, P.; Lai, H.; Liu, Y.; Wang, J.; You, F.; Sun, D.; Zhai, H. Aptasensor Development for T-2 Toxin Detection Utilizing a Dual Signal Amplification Strategy: Synergistic Effects of Bimetallic Oxide (Ce-In)Ox and COFTAPB-DMTP. Sens. Actuators B Chem. 2023, 396, 134602. [Google Scholar] [CrossRef]
- Yan, H.; He, B.; Zhao, R.; Ren, W.; Suo, Z.; Xu, Y.; Zhang, Y.; Bai, C.; Yan, H.; Liu, R. Electrochemical Aptasensor Based on Ce3NbO7/CeO2@Au Hollow Nanospheres by Using Nb.BbvCI-Triggered and Bipedal DNA Walker Amplification Strategy for Zearalenone Detection. J. Hazard. Mater. 2022, 438, 129491. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, B.; Cui, X.; Chao, X.; Song, F.; Chen, H.; He, B. An Electrochemical Aptamer-Sensing Strategy Based on a Ti3C2Tx MXene Synergistic Ti-MOF Amplification Signal for Highly Sensitive Detection of Zearalenone. Food Chem. 2024, 461, 140828. [Google Scholar] [CrossRef]
- Wang, K.; Yan, X.; Wu, J.; Qi, J.; Ning, M.; Li, M.; Sun, R.; Wang, Z.; Yuan, Y.; Yue, T. A Fluorescent Aptasensor for Deoxynivalenol Detection Based on Nb.BbvCI-Assisted Targeted-Responsive Three-Way Junctions Integrated DNA Walking Machine. Food Chem. 2025, 467, 142365. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Yang, H.; Li, R.; Cai, R.; Tan, W. An “off-on” Electrochemical Luminescence Biosensor with Aggregation-Induced Emission for Ultrasensitive Detection of Aflatoxin B1. Sens. Actuators B Chem. 2023, 380, 133407. [Google Scholar] [CrossRef]
- Li, Y.-L.; Chen, Y.; Xie, F.; Li, Q.; Yang, T.; Yang, Y.; Hu, R. Smartphone-Based Dual-Mode Aptasensor with Bifunctional Metal-Organic Frameworks as Signal Probes for Ochratoxin A Detection. Food Chem. 2025, 464, 141540. [Google Scholar] [CrossRef]
- Jafari, S.; Burr, L.; Migliorelli, D.; Galve, R.; Marco, M.-P.; Campbell, K.; Elliott, C.; Suman, M.; Sturla, S.J.; Generelli, S. Smartphone-Based Magneto-Immunosensor on Carbon Black Modified Screen-Printed Electrodes for Point-of-Need Detection of Aflatoxin B1 in Cereals. Anal. Chim. Acta 2022, 1221, 340118. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, L.; Xin, S.; Yang, Q.; Wu, W.; Hou, X. Poly (Ionic Liquid) Cross-Linked Hydrogel Encapsulated with AuPt Nanozymes for the Smartphone-Based Colorimetric Detection of Zearalenone. Food Chem. X 2024, 22, 101471. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, M.; Li, Z.; Liao, W.; Chen, B.; Yang, T.; Hu, R.; Yang, Y.; Meng, S. Highly Sensitive and Convenient Aptasensor Based on Au NPs@Ce-TpBpy COF for Quantitative Determination of Zearalenone. RSC Adv. 2022, 12, 17312–17320. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, Y.; Xu, H.; Lu, X.; Ma, X.; Zhang, W. Exonuclease III Assisted Electrochemical Aptasensor Simultaneous Detection of Aflatoxin B1 and Ochratoxin a in Grains. LWT 2024, 201, 116211. [Google Scholar] [CrossRef]
- Alsulami, T.; Nath, N.; Flemming, R.; Wang, H.; Zhou, W.; Yu, J. Development of a Novel Homogeneous Immunoassay Using the Engineered Luminescent Enzyme NanoLuc for the Quantification of the Mycotoxin Fumonisin B1. Biosens. Bioelectron. 2021, 177, 112939. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Sun, D.; Pu, H. CRISPR/Cas12a and G-Quadruplex DNAzyme-Driven Multimodal Biosensor for Visual Detection of Aflatoxin B1. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 302, 123121. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, V.; Boisjoli, S.; DeRosa, M.C. Adsorption–Desorption Nano-Aptasensors: Fluorescent Screening Assays for Ochratoxin A. RSC Adv. 2022, 12, 13727–13739. [Google Scholar] [CrossRef]
- Naidoo, L.; Uwaya, G.E.; Meier, F.; Bisetty, K. A Novel MB-Tagged Aptasensor for Aflatoxin B1 Detection in Food Using Fe3O4 Nanoparticles Substantiated with in Silico Modelling. Biosens. Bioelectron. X 2023, 15, 100416. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, S.; Wei, Z.; Lou, X. Evanescent Wave Optical-Fiber Aptasensor for Rapid Detection of Zearalenone in Corn with Unprecedented Sensitivity. Biosensors 2022, 12, 438. [Google Scholar] [CrossRef]
- Feng, B.-B.; Suo, Z.-G.; Wei, M.; Liu, Y.; Jin, H.-L. A Novel Electrochemical Aptasensor Based on Rolling Circle Amplification-Driven Ag+-DNAzyme Amplification for Ochratoxin A Detection. Chin. J. Anal. Chem. 2023, 51, 100217. [Google Scholar] [CrossRef]
- Wang, B.; Ren, X.; Gao, Z.; Ma, H.; Wang, H.; Wu, D.; Wei, Q. Double Quenching Electrochemiluminescence Aptsensor Based on Free Radical Elimination and Resonance Energy Transfer for the Sensitive Detection of Zearalenonea. Sens. Actuators B Chem. 2024, 418, 136329. [Google Scholar] [CrossRef]
- Cui, J.; Wu, B.; Li, Z.; Bai, Y.; Kan, L.; Wang, M.; He, L.; Du, M. Hierarchical CoCoPBA@PCN-221 Nanostructure for the Highly Sensitive Detection of Deoxynivalenol in Foodstuffs. Food Chem. 2023, 403, 134370. [Google Scholar] [CrossRef]
- Yao, H.; Du, S.; Yang, L.; Ding, Y.; Shen, H.; Qiu, Y.; Dai, G.; Mo, F. A Magnetic Graphene Oxide and UiO-66 Based Homogeneous Dual Recognition Electrochemical Aptasensor for Accurate and Sensitive Detection of Aflatoxin B1. Talanta 2024, 273, 125915. [Google Scholar] [CrossRef]
- Sun, X.; Sun, J.; Ye, Y.; Ji, J.; Sheng, L.; Yang, D.; Sun, X. Metabolic Pathway-Based Self-Assembled Au@MXene Liver Microsome Electrochemical Biosensor for Rapid Screening of Aflatoxin B1. Bioelectrochemistry 2023, 151, 108378. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Li, J.; Fan, L.; Wen, J.; Zhang, J.; Zhang, Z. A Label-Free Aptasensor Based on a Dual-Emission Fluorescent Strategy for Aflatoxin B1 Detection. Sens. Actuators B Chem. 2021, 346, 130561. [Google Scholar] [CrossRef]
- Jing, P.; Wen, T.; Li, J.; Cai, W.; Yang, B.; Kong, Y. Highly Reliable Chiral Discrimination of Tryptophan Enantiomers through Two Different Modes: Electrochemistry and Temperature. Anal. Chem. 2023, 95, 8569–8577. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Lang, W.; Sun, C.; Huang, Y.; Wu, P.; Cai, C.; Xing, B. Near Infrared-II Photothermal and Colorimetric Synergistic Sensing for Intelligent Onsite Dietary Myrosinase Profiling. Anal. Chem. 2023, 95, 3856–3863. [Google Scholar] [CrossRef] [PubMed]
- Dacey, G.C.; Ross, I.M. The Field Effect Transistor. Bell Syst. Technol. J. 1955, 34, 1149–1189. [Google Scholar] [CrossRef]
- Verdian, A.; Rouhbakhsh, Z.; Fooladi, E. An Ultrasensitive Platform for PCB77 Detection: New Strategy for Liquid Crystal-Based Aptasensor Fabrication. J. Hazard. Mater. 2021, 402, 123531. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Z. Simple and Rapid Detection of Ibuprofen─A Typical Pharmaceuticals and Personal Care Products─by a Liquid Crystal Aptasensor. Langmuir 2022, 38, 282–288. [Google Scholar] [CrossRef]
- Koczula, K.M.; Gallotta, A. Lateral Flow Assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef]
- Rahbar, M.; Zou, S.; Baharfar, M.; Liu, G. A Customized Microfluidic Paper-Based Platform for Colorimetric Immunosensing: Demonstrated via HCG Assay for Pregnancy Test. Biosensors 2021, 11, 474. [Google Scholar] [CrossRef]
- FAO. Pesticides Use and Trade 1990-2021. Faostat Anal. Br. 70 2023, 70, 1–12. [Google Scholar]
- Zhang, L.; Rana, I.; Shaffer, R.M.; Taioli, E.; Sheppard, L. Exposure to Glyphosate-Based Herbicides and Risk for Non-Hodgkin Lymphoma: A Meta-Analysis and Supporting Evidence. Mutat. Res. Mutat. Res. 2019, 781, 186–206. [Google Scholar] [CrossRef]
- Chung, Y.-L.; Hou, Y.-C.; Wang, I.-K.; Lu, K.-C.; Yen, T.-H. Organophosphate Pesticides and New-Onset Diabetes Mellitus: From Molecular Mechanisms to a Possible Therapeutic Perspective. World J. Diabetes 2021, 12, 1818–1831. [Google Scholar] [CrossRef]
- Fucic, A.; Duca, R.C.; Galea, K.S.; Maric, T.; Garcia, K.; Bloom, M.S.; Andersen, H.R.; Vena, J.E. Reproductive Health Risks Associated with Occupational and Environmental Exposure to Pesticides. Int. J. Environ. Res. Public Health 2021, 18, 6576. [Google Scholar] [CrossRef]
- Eddleston, M. Poisoning by Pesticides. Medicine 2024, 52, 390–393. [Google Scholar] [CrossRef]
- EPA. Announces Proposed Registration of New Pesticide Florylpicoxamid. Available online: https://www.epa.gov/pesticides/epa-announces-proposed-registration-new-pesticide-florylpicoxamid (accessed on 30 June 2025).
- Mijajlović, A.; Stanković, V.; Vlahović, F.; Đurđić, S.; Manojlović, D.; Stanković, D. The Cathodically Pretreated Boron-Doped Diamond Electrode as an Environmentally Friendly Electrochemical Tool for the Detection and Monitoring of Mesotrione in Food Samples. Food Chem. 2024, 447, 138993. [Google Scholar] [CrossRef]
- Balram, D.; Lian, K.-Y.; Sebastian, N.; Alharthi, S.S.; Al-Saidi, H.M. Synergy of β-Cyclodextrin Functionalized Carbon Black/CuFe2O4 Nanocomposite for Nanomolar Quantification of Neonicotinoid in Agricultural Crops. Measurement 2025, 242, 116088. [Google Scholar] [CrossRef]
- Ren, X.; Zeng, H.; Zhang, Q.; Cai, H.; Yang, W. Electrochemical Sensor Based on Molecularly Imprinted Polymer and Graphene Oxide Nanocomposite for Monitoring Glyphosate Content in Corn. Int. J. Electrochem. Sci. 2022, 17, 221292. [Google Scholar] [CrossRef]
- Dasriya, V.; Joshi, R.; Ranveer, S.; Dhundale, V.; Kumar, N.; Raghu, H.V. Rapid Detection of Pesticide in Milk, Cereal and Cereal Based Food and Fruit Juices Using Paper Strip-Based Sensor. Sci. Rep. 2021, 11, 18855. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhangsun, H.; Zhao, Y.; Zhuang, Y.; Xu, Z.; Bu, T.; Li, R.; Wang, L. Macro-Meso-Microporous Carbon Composite Derived from Hydrophilic Metal-Organic Framework as High-Performance Electrochemical Sensor for Neonicotinoid Determination. J. Hazard. Mater. 2021, 411, 125122. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Ji, B.-T.; Chen, J.-H.; Gao, L.-L.; Sun, Y.; Deng, Z.-P.; Zhao, B.; Li, J.-G. Ratiometric Emission of Tb(III)-Functionalized Cd-Based Layered MOFs for Portable Visual Detection of Trace Amounts of Diquat in Apples, Potatoes and Corn. Food Chem. 2024, 449, 139259. [Google Scholar] [CrossRef]
- Jeyaraman, A.; Karuppusamy, N.; Chen, T.-W.; Chen, S.-M.; Velmurugan, S.; Al-onazi, W.A.; Algarni, T.S.; Elshikh, M.S. Hard Template Assisted Synthesis of Iron-Cobalt Phosphide Core-Shell for the Enhanced Electrochemical Detection of Fenitrothion. Chem. Eng. J. 2024, 491, 151642. [Google Scholar] [CrossRef]
- Gupta, H.; Kaur, K.; Mohiuddin, I.; Singh, R.; Kaur, V. Cobalt/Aluminum Layered Double Hydroxide Intercalated with Rice Straw Based-Biochar for Recognizing Organophosphates in Cereal Crops. J. Lumin. 2025, 277, 120950. [Google Scholar] [CrossRef]
- Yi, L.; Wu, S.; Ren, G.; Zhou, Q.; Li, P.; Wang, Y.; Tian, X.; He, D.; Pan, Q. Glyphosate Detection Based on Eu Coordination Polymer through Competitive Coordination. Food Chem. 2025, 463, 141554. [Google Scholar] [CrossRef] [PubMed]
- Karuppaiah, B.; Jeyaraman, A.; Chen, S.-M.; Chavan, P.R.; Karthik, R.; Hasan, M.; Shim, J.-J. Effect of Bismuth Doping on Zircon-Type Gadolinium Vanadate: Effective Electrocatalyst for Determination of Hazardous Herbicide Mesotrione. Chemosphere 2023, 313, 137543. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.F.; Li, Y.X.; Dong, L.M.; Zheng, L.L.; Li, J.Z.; Shen, Y.; Xia, F. Photothermal-Induced Partial Leidenfrost Superhydrophobic Surface as Ultrasensitive Surface-Enhanced Raman Scattering Platform for the Detection of Neonicotinoid Insecticides. Sens. Actuators B Chem. 2021, 348, 130728. [Google Scholar] [CrossRef]
- He, P.; Zheng, S.; Li, Y.; Guo, H.; Yang, F. Pyridine-Substituted Cyanostilbene Macrocycle: A “Turn-on” Fluorescence Sensor for Pesticide Bromoxynil Octanoate. Microchem. J. 2025, 212, 113221. [Google Scholar] [CrossRef]
- Peng, X.; Yuan, Y.; Lu, A.; Wang, C.; Zu, C.; Zhang, H.; Bai, Z. A Highly Sensitive EC-SERS Sensor of PANI/RGO/Ag/Cu Film for Doubly Detecting Glyphosate Residues in Fresh Fruit. Food Chem. 2025, 487, 144787. [Google Scholar] [CrossRef]
- Kumar, M.; Dhiman, A.; Singh, G.; Kaur, N.; Singh, N. Pyrene Functionalized Organic Cation Receptor-Based “Turn-on” Fluorescence Approach for Monitoring of Chlorpyrifos in Food, Soil, and Water Samples. Anal. Chim. Acta 2025, 1336, 343488. [Google Scholar] [CrossRef]
- Tecuapa-Flores, E.D.; Thangarasu, P.; Narayanan, J. Electrochemical, Adsorption, and Bio-Imaging Studies: MWCNTs/Ag/Au NPs as a Potential Electrochemical Sensor for Glyphosate. Electrochim. Acta 2025, 529, 146352. [Google Scholar] [CrossRef]
- Li, X.-H.; Li, M.-Z.; Yang, X.-Y.; Wang, T.-Y.; Luo, Y.-H.; Kandegama, W.; Li, J.-Y.; Hao, G.-F.; Liu, C.-R. Ultra-Sensitive, Versatile and Portable Detection of Hydrazine in Eco-Environmental Systems Using a Smartphone-Integrated Ratiometric Fluorescent Sensor. J. Hazard. Mater. 2025, 492, 138172. [Google Scholar] [CrossRef]
- Zhou, B.; Li, X.; Zheng, X.; Liang, M.; Yang, Z.; Liu, A.; Chen, L. A Self-Reporting Electrochemical Sensor for Carbendazim in Food Based on Magnetic Molecularly Imprinted MOFs. Food Chem. 2025, 487, 144789. [Google Scholar] [CrossRef]
- Bai, L.; Li, Z.; Liu, Q.; Zhang, Z.; Tian, H.; Li, Z.; Han, J.; Hu, Y. Enoxacin-Embedded EuMOF-Based Ratio Fluorescent Sensing Platform Integrated with Paper-Based Sensor and Skin-Attachable Hydrogel for Glyphosate Detection in Foods. J. Hazard. Mater. 2025, 489, 137658. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wei, J.; Wu, C.; Lv, G.; Wu, L. ZrO2/CeO2/Polyacrylic Acid Nanocomposites with Alkaline Phosphatase-like Activity for Sensing. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 263, 120165. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zheng, X.; Wang, X.; Zhang, Z.; Qin, S.; Wang, X.; Jing, X. Deep Eutectic Solvent-Based Adhesive Tape Extraction Combined with Enzyme Inhibition Assay for the Determination and Distinction of Dithiocarbamate Pesticides in Food Samples. Talanta 2023, 260, 124601. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Yu, L.; Yang, C.; Zhu, J.; Wang, J.; Zheng, J.; Wang, F.; He, G.; Jiang, F.; et al. Confinement-Enhanced Microalgal Individuals Biosensing for Digital Atrazine Assay. Biosens. Bioelectron. 2023, 241, 115647. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Xie, L.; Zhu, L.; He, B.; Cao, X. RecJf Exonuclease-Catalyzed Signal Amplified Aptasensor for Sensitive Detection of Atrazine Using Ni6MnO8@C/Au Nanorods and COF@MOF Nanohybrids. Sens. Actuators B Chem. 2024, 398, 134769. [Google Scholar] [CrossRef]
- Tao, H.; Liu, F.; Ji, C.; Wu, Y.; Wang, X.; Shi, Q. A Novel Electrochemical Sensing Platform Based on the Esterase Extracted from Kidney Bean for High-Sensitivity Determination of Organophosphorus Pesticides. RSC Adv. 2022, 12, 5265–5274. [Google Scholar] [CrossRef]
- Dorozhko, E.V.; Gashevskay, A.S.; Korotkova, E.I.; Barek, J.; Vyskocil, V.; Eremin, S.A.; Galunin, E.V.; Saqib, M. A Copper Nanoparticle-Based Electrochemical Immunosensor for Carbaryl Detection. Talanta 2021, 228, 122174. [Google Scholar] [CrossRef]
- Liu, J.; Li, N.; Ye, L.; Zhou, L.; Chen, G.; Tang, J.; Zhang, H.; Yang, H. Triple Modal Aptasensor Arrays Driven by CHA-Mediated DNAzyme for Signal-Amplified Atrazine Pesticide Accumulation Monitoring in Agricultural Crops. J. Hazard. Mater. 2024, 476, 135172. [Google Scholar] [CrossRef] [PubMed]
- Tsounidi, D.; Soulis, D.; Manoli, F.; Klinakis, A.; Tsekenis, G. AChE-Based Electrochemical Biosensor for Pesticide Detection in Vegetable Oils: Matrix Effects and Synergistic Inhibition of the Immobilized Enzyme. Anal. Bioanal. Chem. 2023, 415, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Johnson, Z.T.; Jared, N.; Peterson, J.K.; Li, J.; Smith, E.A.; Walper, S.A.; Hooe, S.L.; Breger, J.C.; Medintz, I.L.; Gomes, C.; et al. Enzymatic Laser-Induced Graphene Biosensor for Electrochemical Sensing of the Herbicide Glyphosate. Glob. Chall. 2022, 6, 2200057. [Google Scholar] [CrossRef] [PubMed]
- Ballen, S.C.; Silva, D.M.; Machado, E.P.; Soares, A.C.; Correa, D.R.; dos Santos, H.C.; Jacques, R.A.; Steffens, J.; Steffens, C. Enhanced Detection of Atrazine and Simazine in Agricultural and Environmental Waters Using Graphene Oxide/Tyrosinase Nanobiosensors. Microchem. J. 2025, 214, 114000. [Google Scholar] [CrossRef]
- Agricultural Consumption of Pesticides Worldwide from 1990 to 2022. Available online: https://www.statista.com/statistics/1263077/global-pesticide-agricultural-use/ (accessed on 8 August 2025).
- Benbrook, C.M. Trends in Glyphosate Herbicide Use in the United States and Globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef]
- Tarannum, N.; Gautam, A.; Chauhan, T.; Kumar, D. Nanomaterial Based Sensors for Detection of Food Contaminants: A Prospect. Sens. Technol. 2024, 2, 2373196. [Google Scholar] [CrossRef]
- Welch, C.M.; Compton, R.G. The Use of Nanoparticles in Electroanalysis: A Review. Anal. Bioanal. Chem. 2006, 384, 601–619. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Aladesanmi, O.T.; Oroboade, J.G.; Osisiogu, C.P.; Osewole, A.O. Bioaccumulation Factor of Selected Heavy Metals in Zea Mays. J. Heal. Pollut. 2019, 9, 191207. [Google Scholar] [CrossRef]
- Driehuis, F.; Wilkinson, J.M.; Jiang, Y.; Ogunade, I.; Adesogan, A.T. Silage Review: Animal and Human Health Risks from Silage. J. Dairy Sci. 2018, 101, 4093–4110. [Google Scholar] [CrossRef]
- Dhaked, R.; Singh, M.; Singh, P.G.P. Botulinum Toxin: Bioweapon & Magic Drug. Indian J. Med. Res. 2010, 132, 489–503. [Google Scholar] [PubMed]
- Sharma, V.; Jain, D.; Rai, A.R.; Kumari, P.; Nagar, V.; Kaur, A.; Singh, A.; Verma, R.K.; Pandey, H.; Sankhla, M.S. Toxicological Assessment and Concentration Analysis of Bisphenol A in Food Grade Plastics: A Systematic Review. Mater. Today Proc. 2023, 95, 18–25. [Google Scholar] [CrossRef]
- Vilarinho, F.; Sendón, R.; van der Kellen, A.; Vaz, M.F.; Silva, A.S. Bisphenol A in Food as a Result of Its Migration from Food Packaging. Trends Food Sci. Technol. 2019, 91, 33–65. [Google Scholar] [CrossRef]
- Jiang, H.; Lin, H.; Lin, J.; Yao-Say Solomon Adade, S.; Chen, Q.; Xue, Z.; Chan, C. Non-Destructive Detection of Multi-Component Heavy Metals in Corn Oil Using Nano-Modified Colorimetric Sensor Combined with near-Infrared Spectroscopy. Food Control 2022, 133, 108640. [Google Scholar] [CrossRef]
- Zhang, K.; Kwadzokpui, B.A.; Adade, S.Y.-S.S.; Lin, H.; Chen, Q. Quantitative and Qualitative Detection of Target Heavy Metals Using Anti-Interference Colorimetric Sensor Array Combined with near-Infrared Spectroscopy. Food Chem. 2024, 459, 140305. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Ma, Y.; Luo, H.; Hou, J.; Hou, C.; Huo, D. Ultra-Sensitive Electrochemical Sensors through Self-Assembled MOF Composites for the Simultaneous Detection of Multiple Heavy Metal Ions in Food Samples. Anal. Chim. Acta 2024, 1289, 342155. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Li, N.; Liu, X.; Ma, Y.; Yang, S.; Luo, H.; Hou, C.; Huo, D. An Ultrasensitive Electrochemical Sensor Based on Antimonene Simultaneously Detect Multiple Heavy Metal Ions in Food Samples. Food Chem. 2023, 421, 136131. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, P.; Liang, Y.; Ma, Y.; Liu, Y.; Zhao, J.; Hou, J.; Hou, C.; Huo, D. A Sensitive Electrochemical Sensor Based on 3D Porous Melamine-Doped RGO/MXene Composite Aerogel for the Detection of Heavy Metal Ions in the Environment. Talanta 2023, 256, 124294. [Google Scholar] [CrossRef]
- Al Kaassamani, R.; Sawan, S.; Jaffrezic-Renault, N.; Maalouf, R. A Novel Electrochemical Sensor Based on Iron Oxide Nanoparticles Coated with Molecularly Imprinted Polymers for Bisphenol A Detection. Microchem. J. 2025, 208, 112632. [Google Scholar] [CrossRef]
- Lin, H.; Jiang, H.; He, P.; Haruna, S.A.; Chen, Q.; Xue, Z.; Chan, C.; Ali, S. Non-Destructive Detection of Heavy Metals in Vegetable Oil Based on Nano-Chemoselective Response Dye Combined with near-Infrared Spectroscopy. Sens. Actuators B Chem. 2021, 335, 129716. [Google Scholar] [CrossRef]
- Shi, Y.; Dong, F.; Rodas-Gonzalez, A.; Wang, G.; Yang, L.; Chen, S.; Zheng, H.B.; Wang, S. Simultaneous Detection of Heavy Metal Ions in Food Samples Using a Hypersensitive Electrochemical Sensor Based on APTES-Incubated MXene-NH2@CeFe-MOF-NH2. Food Chem. 2025, 475, 143362. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Zhao, P.; Liang, Y.; Ma, Y.; Liu, H.; Hou, J.; Hou, C.; Huo, D. Sulfhydryl-Functionalized 3D MXene-AuNPs Enabled Electrochemical Sensors for the Selective Determination of Pb2+, Cu2+ and Hg2+ in Grain. Food Chem. 2024, 446, 138770. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, P.; Hu, Z.; Liang, Y.; Han, H.; Yang, M.; Luo, X.; Hou, C.; Huo, D. Amino-Functionalized Multilayer Ti3C2Tx Enabled Electrochemical Sensor for Simultaneous Determination of Cd2+ and Pb2+ in Food Samples. Food Chem. 2023, 402, 134269. [Google Scholar] [CrossRef]
- Yadav, N.; Narang, J.; Singh Rana, J.; Kumar Chhillar, A.; Mohan, H. Development of an Ultrasensitive Electrochemical Aptasensor Based on Aptamer/RGO/SPGE for the Detection of Botulinum Neurotoxin A (BoTN/A) in Food Samples. Microchem. J. 2024, 207, 111916. [Google Scholar] [CrossRef]


| Type | Transducer | Toxin * | Sample | Linear Range (pg mL−1) ** | LOD (pg mL−1) ** | Ref. |
|---|---|---|---|---|---|---|
| Sensor | Optical | AFB1 DON ZEN | Maize | - | - | [63] |
| Sensor | Optical | FB1 OTA ZEN OA PAT | Corn | 2.0 × 104–9.0 × 104 | - | [64] |
| Sensor | Electrochemical | OTA | Corn grain | 1.61 × 105–4.04 × 106 | 1.21 × 104 | [65] |
| Sensor | Electrochemical | ZEN | Maize powder | 1.59 × 105–2.86 × 106 | 2.31 × 104 | [66] |
| Sensor | Photoelectrochemical | AFB1 | Maize | 10.0–1.0 × 106 | 6.8 | [67] |
| Sensor | Electrochemical | ZEN OTA | Corn flour | 4 × 104–1.02 × 107 1.6 × 105–4.1 × 107 | 1.4 × 104 4.5 × 104 | [68] |
| Sensor | Optical | ZEN | Corn | 1.0–30.0 μg Kg−1 | 0.5 μg Kg−1 | [69] |
| Sensor | Electrochemical | OTA | Corn | 1.61 × 104–4.04 × 106 | 1.45 × 103 | [70] |
| Sensor | Photoelectrochemical | OTA | Corn flour | 2.0 × 104–2.5 × 108 and 2.0 × 10−3–3.0 × 105 (PEC) | 8,33 × 103 and 8.0 × 10−4 (PEC) | [71] |
| Sensor | Optical | ZEN OTA | Corn flour | 50.0–1.0 × 105 100–1.0 × 104 | 4.40 × 102 98.0 | [72] |
| Sensor | Electrochemical | ZEN | Spiked maize extract | 0.1–1.0 × 105 | 3.4 × 10−2 | [73] |
| Sensor | Optical | AFB1 | Corn | 1.0 × 103–2.0 × 104 | 3.8 × 102 | [74] |
| Sensor | Optical | AFB1 | Corn flour | 5.0 × 102–1.0 × 105 | 1.3 × 102 | [75] |
| Sensor | Optical | AFB1 | Corn oil | 10.0–2.0 × 104 | 4.0 | [76] |
| Biosensor | Electrochemiluminescence | ZEN | Maize | 1.0 × 10−3–2 × 102 µg kg−1 | 9.75 × 10−5 µg kg−1 | [77] |
| Biosensor | Electrochemical | ZEN | Corn | 1.0–1.0 × 106 | 0.02 | [78] |
| Biosensor | Electrochemical | AFB1 | Corn | 10.0–2.0 × 104 | 0.94 | [79] |
| Biosensor | Electrochemical Optical | AFB1 | Maize | 31.23–7.81 × 103 (impedimetric) 31.23–1.56 × 105 (SPR) | 31.23 (impedimetric) 124.91 (SPR) | [80] |
| Biosensor | Electrochemical | AFB1 | Corn oil | 10.0–1.0 × 105 | 1.82 | [81] |
| Biosensor | Electrochemical Optical | OTA | Corn | 0.10–140.0 0.10–160.0 | 0.024 0.051 | [82] |
| Biosensor | Electrochemical | ZEN | Corn | 0.01–1 × 104 | 7 × 10−3 (EIS) 3.5 × 10−3 (DPV) | [83] |
| Biosensor | Electrochemical | OTA | Corn | 1.0–5.0 × 103 | 0.1 | [84] |
| Biosensor | Electrochemical | DON | Maize flour | 10.0–1.0 × 105 | 6.9 | [85] |
| Biosensor | Electrochemical | OTA | Maize | 0.1–5 × 104 | 0.081 | [86] |
| Biosensor | Electrochemical | AFB1 | Corn | 100.0–1 × 105 | 38.8 | [87] |
| Biosensor | Electrochemical | AFB1 | Corn flour | 0.1–1 × 104 | 0.061 | [88] |
| Biosensor | Electrochemical | OTA | Corn | 5–5 × 104 | 2.35 | [89] |
| Biosensor | Chemoluminescence | DON | Corn and cornmeal | 1.0–1.32 × 105 | 0.166 | [90] |
| Biosensor | Optical | AFB1 | Corn | 0.2 × 103–6 × 103 | 90.0 | [91] |
| Biosensor | Optical | AFB1 | Corn | 1.0 × 103–1.0 × 105 | 2.5 × 103 | [92] |
| Biosensor | Optical | AFB1 | Corn | 50–5.0 × 105 | 85 | [93] |
| Biosensor | Optical | OTA AFB1 | Corn flour | 1.0–5.0 × 105 1.0–1.0 × 106 | 0.33 | [94] |
| Biosensor | Optical | ZEN | Corn | 2.5 × 103–1.0 × 105 | 980.0 | [95] |
| Biosensor | Electrochemical | AFB1 | Spiked corn | 1.0–1.0 × 107 | 0.54 | [96] |
| Biosensor | Optical | OTA | Corn | 4.04 × 10−5–0.40 | 8.48 × 10−6 | [97] |
| Biosensor | Optical | ZEN | Corn flour | 10.0–1.0 × 105 | 5.0 | [98] |
| Biosensor | Electrochemical Optical | OTA | Corn | 0.01–2.0 × 105 | 0.025 | [99] |
| Biosensor | Electrochemical | AFB1 FB1 | Ground corn | 2.0–20.0 | 0.46 0.34 | [100] |
| Biosensor | Electrochemical | AFB1 | Corn | 1 × 10−4–0.5 | 1.9 × 10−4 | [101] |
| Biosensor | Electrochemical | AFB1 | Corn | 1 × 10−4–0.2 | 1.0 × 10−5 | [102] |
| Biosensor | Electrochemical | AFB1 ZEN OTA | Corn | 1.0 × 103–1.0 × 105 | 41.2 27.6 30.0 | [103] |
| Biosensor | Mechanical (pressure) | T-2 | Corn | 0.02–6.0 × 106 | 5.6 × 10−3 | [104] |
| Biosensor | Optical | AFB1 | Corn flour | 1.0 × 103–5.0 × 105 | 38.0 | [105] |
| Biosensor | Optical | FB1 | Corn | 32.0–5.0 × 105 | 12.1 | [106] |
| Biosensor | Optical | FB1 | Corn flour | 10.0–2.0 × 106 | 2.7 | [107] |
| Biosensor | Magnetic Relaxation Switching | AFB1 | Corn | 10.0–1.0 × 104 | 6.0 | [108] |
| Biosensor | Photoelectrochemical | OTA | Corn | 1.0–2.0 × 103 | 0.33 | [109] |
| Biosensor | Electrochemical Optical | AFB1 | Corn | 1.0–3.0 × 104 | 0.6 0.8 | [110] |
| Biosensor | Electrochemical | DON | Maize flour | 5.0 × 103–3.0 × 104 | 3.2 × 103 | [111] |
| Biosensor | Optical | FB1 | Corn flour | 1.80 × 104–3.61 × 105 | 1.21 × 104 | [112] |
| Biosensor | Thermometric Optical | OTA | Corn | 5.0–5 × 104 | 50.0 | [113] |
| Biosensor | Electrochemical | OTA | Corn | 1.0–1.0 × 105 | 0.135 | [114] |
| Biosensor | Photoelectrochemical | AFB1 | Moldy corn | 0.01–100.0 | 1.32 × 10−3 | [115] |
| Biosensor | Electrochemical | OTA AFB1 | Corn flour | 10.0–1 × 105 | 4.3 5.2 | [116] |
| Biosensor | Electrochemiluminescence | AFB1 | Corn | 1.0–1.0 × 105 | 0.79 | [117] |
| Biosensor | Thermometric Optical | DON | Moldy corn | 0–1.78 × 106 | 2.87 × 103 (colorimetric) 4.65 × 103 (temperature) | [118] |
| Biosensor | Optical | T-2 ZEN | Corn flour | 1.0–1.0 × 104 10.0–1 × 105 | 0.1 1.2 | [119] |
| Biosensor | Electrochemical | DON AFB1 | Corn extract | 3.0 × 102–3.0 × 106 | 0.172 7.74 | [120] |
| Biosensor | Electrochemical | AFB1 | Corn | 1.0–1.0 × 105 | 3.9 | [121] |
| Biosensor | Photoelectrochemical | FB1 | Corn paste | 1.0–1.0 × 105 | 0.13 | [122] |
| Biosensor | Photoelectrochemical | DON | Corn | ~50.0–1.0 × 106 | 34.3 | [123] |
| Biosensor | Optical | OTA | Corn | 30.0–3 × 103 (UV) 10.0–1.0 × 104 (fluorescence) | 23.5 (UV) 992.1 (fluorescence) | [124] |
| Biosensor | Optical | AFB1 | Corn oil | 1.0 × 103–4.0 × 105 | 5.7 × 10−2 | [125] |
| Biosensor | Electrochemical | AFB1 | Corn | 10.0–1.0 × 103 | 1.9 | [126] |
| Biosensor | Electrochemical | ZEN | Corn | 2.5 × 10−2–1.6 × 104 | 2.5 × 10−2 | [127] |
| Biosensor | Electrochemical | AFB1 | Corn | 10.0–3.0 × 104 (ACV) 3.0–3.0 × 104 (EIS) | 6.2 (ACV) and 0.8 (EIS) | [128] |
| Biosensor | Optical | ZEN OTA | Corn oil | 4.88 nM–5.0 µM (simultaneously) | 0.12 nM (simultaneously) | [129] |
| Biosensor | Electrochemiluminescence | AFB1 | Corn | 1.0 × 10−3–1.0 × 104 | 5.8 × 10−4 | [130] |
| Biosensor | Optical | ZEN | Corn | 1.0–2.0 × 105 | 0.8 | [131] |
| Biosensor | Optical | DON | Corn flour | 1.0 × 10−3–5.0 × 105 | 6.4 × 10−2 | [132] |
| Biosensor | Optical | ZEN | Corn | 10.0–1.0 × 105 | 4.0 | [133] |
| Biosensor | Optical | AFB1 | Corn | 2.0 × 104–4.0 × 105 | 1.22 × 104 | [134] |
| Biosensor | Electrochemical | OTA | Corn | 50.0–2.0 × 105 | 0.2 | [135] |
| Biosensor | Electrochemical | AFB1 OTA | Corn | 10.0–3.0 × 103 30.0–1.0 × 104 | 4.3 1.33 | [136] |
| Biosensor | Optical | AFB1 | Corn | 1.0–2.0 × 105 | 12.0 | [137] |
| Biosensor | Electrochemical | AFB1 | Corn flour | 1.0–5.0 × 104 | 0.416 | [138] |
| Biosensor | Chemiluminescence | OTA | Corn | 1.0 × 102–1.0 × 105 | 2.8 × 102 | [139] |
| Biosensor | Electrochemical | ZEN | Corn flour | 0.01–1.0 × 104 | 5.0 × 10−3 (MB/Ag+) 2.86 × 10−3 (MB) 1.07 × 10−5 (Ag+) | [140] |
| Biosensor | Photoelectrochemical | AFB1 | Corn | 5.0–1.0 × 104 | 19.6 × 10−3 | [141] |
| Biosensor | Optical | OTA | Powder corn | 20.0–2.0 × 103 | 8.0 | [142] |
| Biosensor | Electrochemiluminescence | DON | Corn | 0.01–500 µg kg−1 | 9 × 10−3 µg kg−1 | [143] |
| Biosensor | Optical | AFB1 | Corn flour | 50.0–5.0 × 105 | 23.0 | [144] |
| Biosensor | Electrochemical | MYA | Corn silage | 7.98 × 105–2.28 × 107 | 6.0 × 103 | [145] |
| Biosensor | Optical | OTA AFB1 | Corn | 10.0–1.0 × 105 50.0–1.0 × 105 | 5.0 10.0 | [146] |
| Biosensor | Electrochemical | AFB1 | Corn | 1.0 × 10−5–0.1 | 1.0 × 10−5 | [147] |
| Biosensor | Electrochemical | CIT | Corn meal | 10.0–1 × 107 | 7.67 (DPV) 1.57 (SWV) | [148] |
| Biosensor | Photoelectrochemical | FB1 | Corn | 0.1–10.0 | 0.0723 | [149] |
| Biosensor | Electrochemical | OTA | Cornmeal | 0.1–1.0 × 104 | 1.12 × 10−3 | [150] |
| Biosensor | Optical | T-2 | Corn flour | 1.0 × 102–1.0 × 104 | 87.0 | [151] |
| Biosensor | Optical | AFB1 | Corn | 0–3.33 × 103 | 80.0 | [152] |
| Biosensor | Optical | AFB1 | Corn | 0–5.0 × 104 | 4.56 × 103 | [153] |
| Biosensor | Optical | OTA | Corn flour | 5.0 × 102–1.0 × 106 | 3.08 × 102 | [154] |
| Biosensor | Electrochemical | DON | Corn flour | 10.0–1.0 × 104 | 2.0 | [155] |
| Biosensor | Optical | AFB1 | Spiked corn | 50.0–2.5 × 104 | 9.0 | [156] |
| Biosensor | Electrochemical | ZEN | Corn oil and corn flour | 1.0 × 10−2–1.0 × 103 | 3.64 × 10−3 | [157] |
| Biosensor | Optical | AFB1 | Corn | 1.0–1.0 × 106 | 0.19 | [158] |
| Biosensor | Optical | T-2 | Corn | 1.0 × 102–5.0 × 106 | 57.84 | [159] |
| Biosensor | Electrochemical | ZEN | Corn | 0.01–1.0 × 104 | 6.27 × 10−3 | [160] |
| Biosensor | Optical | DON | Corn | 1.0 × 102–3.0 × 105 | 13.67 | [161] |
| Biosensor | Optical | ZEN | Corn flour | 0.1–100.0 | 0.1 | [162] |
| Biosensor | Optical | ZEN | Corn flour | 0.32–320.0 | 0.32 | [163] |
| Biosensor | Electrochemical | T-2 | Corn | 5.0 × 10−4–5.0 × 103 | 7.6 × 10−5 | [164] |
| Biosensor | Electrochemical | ZEN | Spiked corn | 0.1–1.0 × 106 | 4.57 × 10−3 | [165] |
| Biosensor | Electrochemical | ZEN | Cornmeal | 1.0 × 10−2–1.0 × 104 | 1.64 × 10−3 | [166] |
| Biosensor | Optical | DON | Cornmeal | 10.0–1.0 × 105 | 9.0 | [167] |
| Biosensor | Electrochemiluminescence | AFB1 | Corn | 1.0–1.0 × 106 | 0.46 | [168] |
| Biosensor | Electrochemical Optical | OTA | Corn | 1.0 × 10−3–2.5 × 105 | 2.2 × 10−4 | [169] |
| Biosensor | Electrochemical | AFB1 | Corn extract | 57.0–1157.0 | 24.0 | [170] |
| Biosensor | Optical | ZEN | Corn | 1.0 × 103–2.5 × 105 | 7.0 × 102 | [171] |
| Biosensor | Electrochemical | ZEN | Corn flour | 1.0–1.0 × 104 | 0.389 | [172] |
| Biosensor | Electrochemical | OTA AFB1 | Corn flour | 1.0–5.0 × 105 | 0.564 0.229 | [173] |
| Biosensor | Optical | FB1 | Spiked corn | 5.33 × 102–6.81 × 103 | 79.0 | [174] |
| Biosensor | Optical | AFB1 | Maize | 10.0–100.0 5.0–1.0 × 104 5.0–2.0 × 104 | 0.85 (colorimetric) 0.79 (SERS) 1.65 (fluorescence) | [175] |
| Biosensor | Optical | OTA | Corn | 0–6.46 × 104 | 1.61 × 104 | [176] |
| Biosensor | Electrochemical | AFB1 | Corn flour | 5.0 × 10−4–5.0 × 10−3 | 0.43 × 10−3 | [177] |
| Biosensor | Optical | ZEN | Corn flour | 3.18 × 10−4–31.8 | 7.34 × 10−4 | [178] |
| Biosensor | Electrochemical | OTA | Corn flour | 0.5–5.0 × 104 | 38.0 × 10−3 | [179] |
| Biosensor | Electrochemiluminescence | ZEN | Corn flour | 0.5–1.0 × 105 | 0.37 | [180] |
| Biosensor | Electrochemical | DON | Corn | 1.0 × 10−3–1.0 × 103 | 0.14 × 10−3 | [181] |
| Biosensor | Electrochemical | AFB1 | Corn | 5.0–5.0 × 105 | 1.0 | [182] |
| Biosensor | Electrochemical | AFB1 | Corn | 3.12 × 103–1.46 × 107 | 8.74 × 10−2 | [183] |
| Biosensor | Optical | AFB1 | Corn flour | 0–3.3 × 102 and 3.33 × 101–3.75 × 105 | 10.0 | [184] |
| Type | Transducer | Toxin | Sample | Linear Range (µM) * | LOD (µM) * | Ref. |
|---|---|---|---|---|---|---|
| Sensor | Electrochemical | Mesotrione | Corn food products | 0.5–70.0 | 0.46 | [198] |
| Sensor | Electrochemical | Neonicotinoid | Corn | 0.16–2.86 2.86–220.86 | 2.0 × 10−3 | [199] |
| Sensor | Electrochemical | Glyphosate | Corn | 0–1.8 × 103 | 11.0 | [200] |
| Sensor | Optical | Lindane | Corn flakes and maize flour | 1.0 ppb–100.0 ppm | 10.0 ppb, each | [201] |
| Pretilachlor | ||||||
| Propiconazole | ||||||
| Sensor | Electrochemical | Imidacloprid | Corn | 0.5–60.0 | 0.026 | [202] |
| Thiamethoxam | 1.0–60.0 | 0.062 | ||||
| Dinotefuran | 0.5–60.0 | 0.010 | ||||
| Sensor | Optical | Diquat | Corn | 0–5 × 10−2 | 6.0 × 10−5 | [203] |
| Sensor | Electrochemical | Fenitrothion | Corn | 1.0 × 10−3–8.5 × 102 | 0.038 | [204] |
| Sensor | Optical | Dibutyl Phosphate (DBP) | Corn | 1.9–25.3 | 1.39 | [205] |
| Diphenyl Phosphate (DPP) | 1.17 | |||||
| Diethyl Chlorophosphate (DCP) | 1.29 | |||||
| Sensor | Optical | Glyphosate | Corn | 1.18–5.91 | 0.473 | [206] |
| Sensor | Electrochemical | Mesotrione | Corn | 0.1–261.0 | 4.5 × 10−2 | [207] |
| Sensor | Optical | Imidacloprid | Corn | 1.0 × 10−8–1.0 × 10−3 | 1.0 × 10−9 | [208] |
| Sensor | Optical | Bromoxynil octanoate | Corn | 0.1–0.8 (equivalent concentration) | 2.1 × 10−2 | [209] |
| Sensor | Optical/Electrochemical | Glyphosate | Corn | 1.0 × 10−2–1.0 (SERS) 1.0 × 10−1–1.0 (DPV) | 1.9 × 10−3 (SERS) 1.73 × 10−2 (DPV) | [210] |
| Sensor | Optical | Chlorpyrifos | Corn | 0–120.0 | 1.89 × 10−2 | [211] |
| Sensor | Electrochemical | Glyphosate | Corn | 1.0−40.0 (EIS) 5.0 × 10−4–7.4 × 10−4 (CV) 2.0 × 10−1−2.34 (SWV) | 6.4 × 10−1 (EIS) 4.0 × 10−4 (CV) 1.5 × 10−1 (SWV) | [212] |
| Sensor | Optical | Hydrazine | Corn | 0–100.0 | 4.5 × 10−3 | [213] |
| Sensor | Electrochemical | Carbendazim | Corn flour | 2.5 × 10−4–1.0 | 8.0 × 10−5 | [214] |
| Sensor | Optical | Glyphosate | Corn | 29.57–591.47 | 2.07 | [215] |
| Sensor | Optical | Methyl parathion | Corn | 7.60 × 10−5–7.60 × 10−2 | 2.1. 10−5 | [216] |
| Biosensor | Optical | Dithiocarbamates | Corn | 0.6–6.0 × 102 µg kg−1 | 0.2 µg kg−1 | [217] |
| Biosensor | Optical | Atrazine | Corn juice | 1.85 × 10−4–0.464 | 7.7 × 10−3 | [218] |
| Biosensor | Electrochemical | Atrazine | Corn | 1.85 × 10−7–1.85 × 10−2 | 2.27 × 10−8 | [219] |
| Biosensor | Electrochemical | Trichlorfon | Corn | 1.94 × 10−5–3.88 × 10−5 5.82 × 10−4–2.72 × 10−3 | 1.16 × 10−5 | [220] |
| Biosensor | Electrochemical | Carbaryl | Corn flour | 0.8–32.3 µg kg−1 | 0.08 µg kg−1 | [221] |
| Biosensor | Optical | Atrazine | Maize | 2.31 × 10−4–2.31 × 10−1 (colorimetric) 4.63 × 10−5–2.31 × 10−1 (fluorescence) | 3.43 × 10−5 (colorimetric) 1.16 × 10−3 (fluorescence) | [222] |
| Biosensor | Electrochemical | Carbofuran | Maize oil | 1.8.10−2–1.8 | 9.0 × 10−4 | [223] |
| Biosensor | Electrochemical | Glyphosate | Spiked corn residues | 10.0–260.0 | 3.03 | [224] |
| Biosensor | Cantilever | Atrazine | Corn crops | 4.96 × 10−6–4.63 × 10−1 4.96 × 10−6–4.96 × 10−1 | 6.49 × 10−6 3.87 × 10−5 | [225] |
| Simazine |
| Type | Transducer | Contaminant | Sample | Linear Range (pM) | LOD (pM) | Ref. |
|---|---|---|---|---|---|---|
| Sensor | Optical | Pb2+ | Corn oil | 4.83 × 104–4.83 × 105 | 2.41 × 104 | [236] |
| Hg2+ | 4.98 × 104–4.98 × 105 | 3.49 × 104 | ||||
| Sensor | Optical | Pb2+ | Corn oil | 9.65 × 104–2.41 × 103 | 1.45 × 103 | [237] |
| Hg2+ | 9.97 × 104–2.49 × 106 | 3.0 × 103 | ||||
| Sensor | Electrochemical | Cd2+ | Corn | 0.02–60 | 0.02 | [238] |
| Pb2+ | 0.032–60 | 0.032 | ||||
| Cu2+ | 0.018–60 | 0.018 | ||||
| Hg2+ | 0.041–60 | 0.041 | ||||
| Sensor | Electrochemical | Pb2+ | Corn | 0.1–2.0 × 102 | 0.042 | [239] |
| Cu2+ | 0.01 | |||||
| Hg2+ | 0.031 | |||||
| Sensor | Electrochemical | Zn2+ | Corn | 4.59 × 104–1.38 × 107 | 7.34 × 103 | [240] |
| Cd2+ | 2.67 × 104–8.0 × 106 | 4.0 × 103 | ||||
| Pb2+ | 1.45 × 104–4.34 × 106 | 1.40 × 103 | ||||
| Sensor | Electrochemical | Bisphenol A | Canned corn and corn | 5.0 × 106–7.3 × 107 | 3.8 × 105 | [241] |
| Sensor | Optical | Pb2+ | Corn oil | ~5 × 103 1.0–5.0 × 108 | ≤5.0 × 103 | [242] |
| Hg2+ | ||||||
| Sensor | Electrochemical | Pb2+ | Corn | 50.0–2.0 × 104 | 9.50 × 102 | [243] |
| Cd2+ | 50.0–1.5 × 104 | 6.90 × 102 | ||||
| Hg2+ | 10.0–1.5 × 104 | 3.30 × 102 | ||||
| Sensor | Electrochemical | Pb2+ | Maize | 4.83 × 103–6.27 × 106 | 3.38 × 102 | [244] |
| Cu2+ | 1.57 × 104–2.05 × 107 | 2.04 × 103 | ||||
| Hg2+ | 5.0 × 103–6.48 × 106 | 1.05 × 103 | ||||
| Sensor | Electrochemical | Cd2+ | Corn | 8.9 × 104–8.9 × 105 | 3.65 × 103 | [245] |
| Pb2+ | 2.41 × 104–4.83 × 105 | 1.45 × 103 | ||||
| Simultaneous | Cd2+ (8.9 × 104–8.9 × 105) and Pb2+ (4.83 × 104–4.83 × 105) | Cd2+ (4.27 × 103) and Pb2+ (1.45 × 103) | ||||
| Biosensor | Electrochemical | Butolinum neurotoxin A | Packed corn | 1.0–1.0 × 102 | 1.0 | [246] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teodoro, L.M.P.; Lacerda, L.R.G.; Santos, P.C.e.; Ferreira, L.F.; Franco, D.L. Sensors and Biosensors as Viable Alternatives in the Determination of Contaminants in Corn: A Review (2021–2025). Chemosensors 2025, 13, 299. https://doi.org/10.3390/chemosensors13080299
Teodoro LMP, Lacerda LRG, Santos PCe, Ferreira LF, Franco DL. Sensors and Biosensors as Viable Alternatives in the Determination of Contaminants in Corn: A Review (2021–2025). Chemosensors. 2025; 13(8):299. https://doi.org/10.3390/chemosensors13080299
Chicago/Turabian StyleTeodoro, Lívia M. P., Letícia R. G. Lacerda, Penelopy Costa e Santos, Lucas F. Ferreira, and Diego L. Franco. 2025. "Sensors and Biosensors as Viable Alternatives in the Determination of Contaminants in Corn: A Review (2021–2025)" Chemosensors 13, no. 8: 299. https://doi.org/10.3390/chemosensors13080299
APA StyleTeodoro, L. M. P., Lacerda, L. R. G., Santos, P. C. e., Ferreira, L. F., & Franco, D. L. (2025). Sensors and Biosensors as Viable Alternatives in the Determination of Contaminants in Corn: A Review (2021–2025). Chemosensors, 13(8), 299. https://doi.org/10.3390/chemosensors13080299







