Abstract
Nanomaterials’ special features enable their extensive application in chemical and biochemical nanosensors for food assays; food packaging; environmental, medicinal, and pharmaceutical applications; and photoelectronics. The analytical strategies based on novel nanomaterials have proved their pivotal role and increasing interest in the assay of key food components. The choice of transducer is pivotal for promoting the performance of electrochemical sensors. Electrochemical nano-transducers provide a large active surface area, enabling improved sensitivity, specificity, fast assay, precision, accuracy, and reproducibility, over the analytical range of interest, when compared to traditional sensors. Synthetic routes encompass physical techniques in general based on top–down approaches, chemical methods mainly relying on bottom–up approaches, or green technologies. Hybrid techniques such as electrochemical pathways or photochemical reduction are also applied. Electrochemical nanocomposite sensors relying on conducting polymers are amenable to performance improvement, achieved by integrating redox mediators, conductive hydrogels, and molecular imprinting polymers. Carbon-based or metal-based nanoparticles are used in combination with ionic liquids, enhancing conductivity and electron transfer. The composites may be prepared using a plethora of combinations of carbon-based, metal-based, or organic-based nanomaterials, promoting a high electrocatalytic response, and can accommodate biorecognition elements for increased specificity. Nanomaterials can function as pivotal components in electrochemical (bio)sensors applied to food assays, aiming at the analysis of bioactives, nutrients, food additives, and contaminants. Given the broad range of transducer types, detection modes, and targeted analytes, it is important to discuss the analytical performance and applicability of such nanosensors.
1. Introductory Aspects: Characterization, Classification, and Synthesis of Nanomaterials
Nanomaterials show special mechanical, optical, and electrocatalytic features, enabling their extensive application in numerous fields: selective and sensitive chemical and biochemical assays; food packaging; environmental, agricultural, medicinal, and pharmaceutical applications; and photoelectronics.
The first classification relies on their chemical nature. Organic nanomaterials are synthesized using phospholipids, polymers, and hybrids as precursors [1]. Organic compounds can be converted into nanoscale structures, and organic nanoparticles or polymers are represented by liposomes, micelles, dendrimers, and ferritin [2,3]. Nanocapsule micelles and nanoliposomes possess hollow inner parts and are non-toxic, biodegradable, and sensitive to heat, electromagnetic radiation, and light [4,5].
Lipid-Based Nanomaterials
Relying on their peculiarities, lipid-based vesicles such as liposomes, microvesicles, exosomes, and liposome–nanomaterial hybrids offer biocompatible, multifunctional biosensing membrane surfaces in rapid biomarker detection, promoting the sensitivity and lowering the detection limit of the assay. The term “vesicles” is used mainly in the case of liposomes [6].
Liposomes are constituted by submicron vesicles enclosing an aqueous mass, surrounded by a membrane made of cholesterol or a phospholipid bilayer [7].
Thin-film hydration involves lipid dissolution in an organic solvent, with subsequent solvent evaporation. Eventually, the lipid film is dispersed in an aqueous medium. In the reverse-phase evaporation method, the lipid material is dissolved in the organic solvent, then mixed with an aqueous solution, followed by ultrasound treatment and solvent evaporation under reduced pressure. Finally, the lipid is converted to a gel and is then hydrated, forming vesicles [8].
Solid lipid nanoparticles possess a spherical shape and a 50–1000 nm diameter. They are formed of a solid lipid core enclosing a bioactive compound and a surfactant coating to stabilize the whole structure; the mentioned advantages of solid lipid nanoparticles are biocompatibility, a lack of toxicity, the possibility of preparation on a large scale without requiring organic solvents, and promoting the stability of the active ingredient [9]. Solid lipid nanoparticles can be synthesized using high-pressure homogenization, which relies on fragmentation. As a result of high cavitation and shear forces, the liquid lipid particles are fragmented to a submicrometer size, achieving the nanodispersion of the lipid material even at concentrations of up to 40% by weight [10].
The microemulsion technique uses a solid lipid dissolved in a partially hydrosoluble organic solvent. Solid lipid nanoparticles precipitate after solvent removal, upon the water dilution of the microemulsion [11]. Other methods considered simpler have also been reported, such as high-speed stirrer or ultrasound dispersion, the membrane squeezing technique, and solvent-based emulsification/evaporation [9,12].
A dendrimer represents a covalently assembled macromolecule, individualized as a nanoparticle. Dendrimers present three major components, a core, interior layers (also named generations, constituted of repetitive units, radically attached to the inner core), and an external component (also named terminal functionality) attached to the outer interior layers (generations). Two main techniques have been reported for dendrimer preparation, divergent and convergent synthesis. In the divergent method, the reaction begins at the core and involves two steps: the activation of the surface functional groups and the addition of branching monomer units. In the convergent technique, the reaction begins from the exterior and encompasses the attachment of external functional groups to the inner layer, followed by the attachment of the latter to the core [13].
Ferritin can be synthesized as a core–shell-structured material, which, in comparison to individual nanoparticles, presents a series of advantages: the shell can promote the material’s biocompatibility and stability, whereas the core possesses favorable electrocatalytic, optical, and electromagnetic features [14].
Another important category is represented by inorganic nanoparticles, composed of metals and their alloys, various oxides (metal and semiconductor oxides), composites, and carbon-based nanomaterials [1], although other authors typically include metal-based or metal-oxide-based nanomaterials, semiconductors, and ceramic nanomaterials in this category [5].
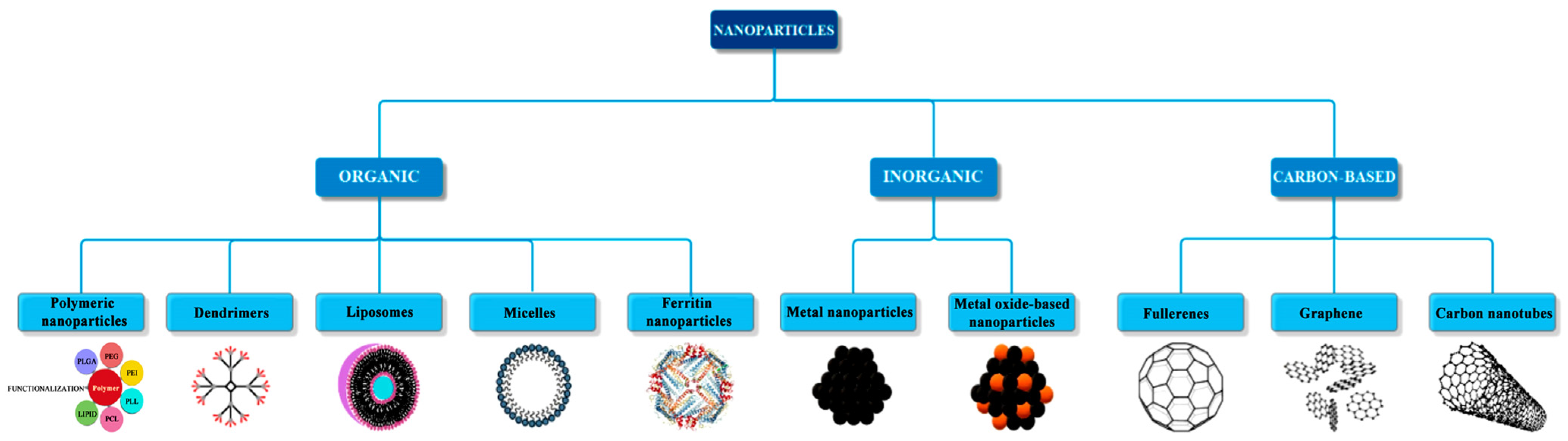
Below, the general classification of nanoparticles based on their nature is presented (Figure 1) [15].
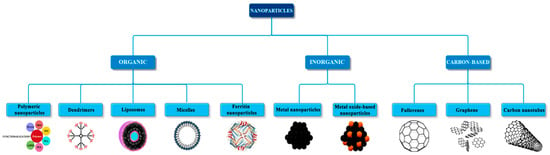
Figure 1.
Classification of nanomaterials based on their nature, from [15], MDPI, 2018.
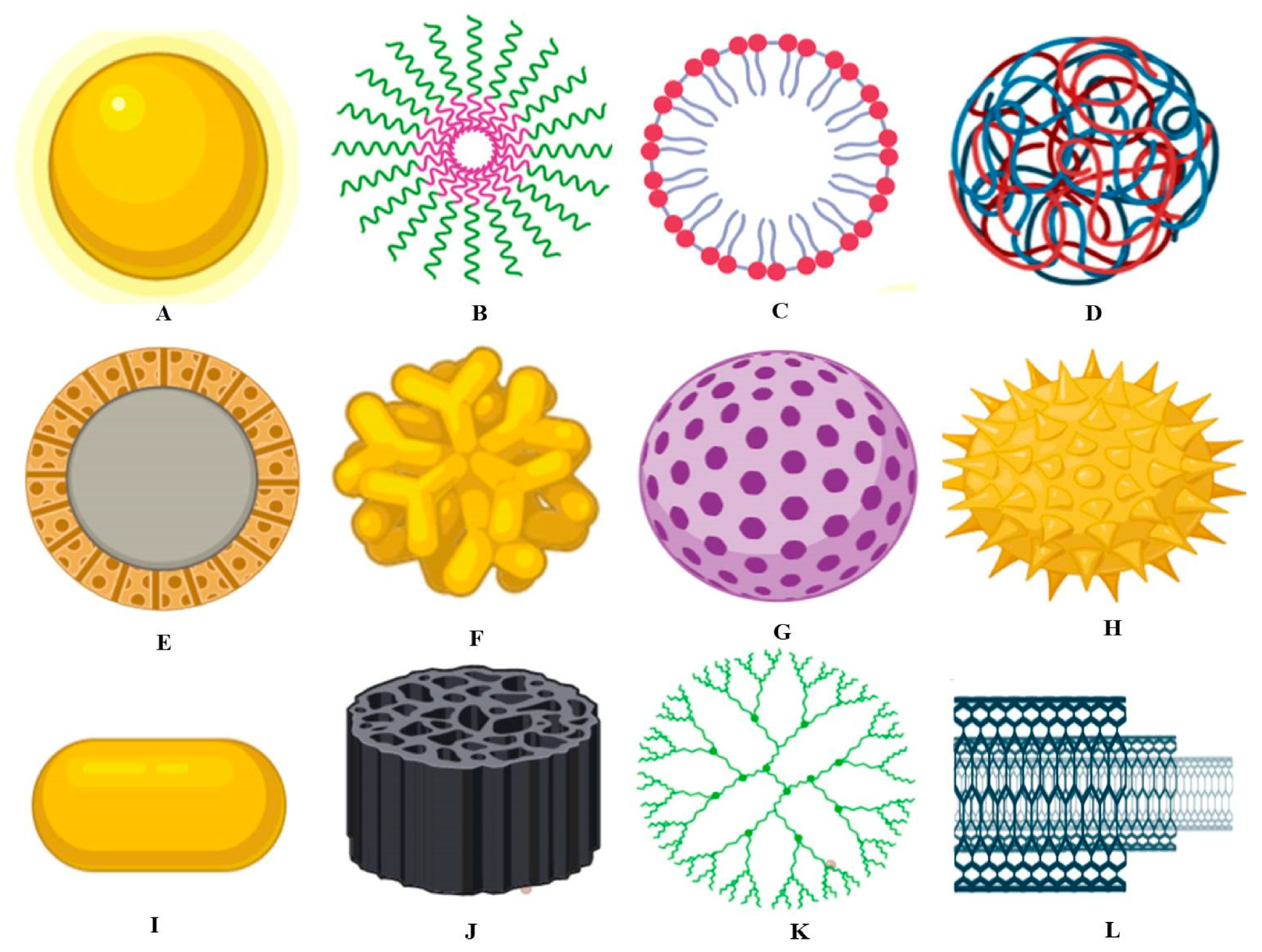
Figure 2 details the different types of nanomaterials applied in the food industry [16].
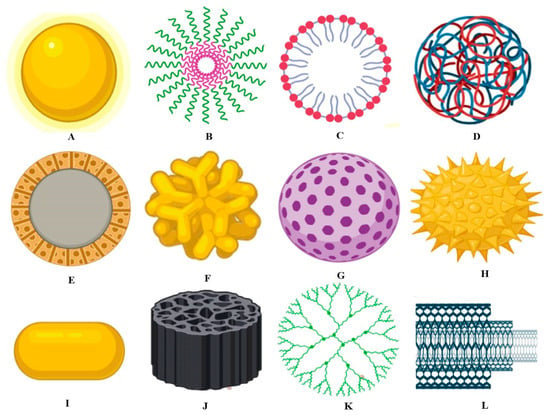
Figure 2.
(A) Metallic nanoparticle, (B) polymeric micelle, (C) nanoliposome, (D) polymeric nanoparticle, (E) solid-core mesoporous nanoparticle, (F) branched gold nanoparticle, (G) mesoporous nanoparticle, (H) surface-functionalized nanoparticle, (I) nanorod, (J) porous silica nanoparticle, (K) dendrimer, and (L) carbon nanotube, from [16], MDPI 2020.
Metal-Based Nanoparticles
The features that individualize metal-based nanoparticles are their broad size ranges (10 to 100 nm), high surface-to-volume ratio, significant charge densities, reactivity, and cylindrical and round forms, but it should be mentioned that they are also susceptible to environmental factors (moisture, sunlight, heat, and air). Most metal nanoparticles are based on aluminum, silver, gold, iron, lead, cobalt, zinc, cadmium, or copper [17].
Electrochemical pathways and chemical or photochemical reduction are usually applied in the synthesis and stabilization of metal-based nanoparticles [18].
Recently, gold nanoparticles were synthesized by a technique based on cationic reverse micelles, obtaining spheres, triangles, rings, rods, and truncated decahedrons. In the reverse micelle method, nano-sized water droplets are dispersed in an organic medium, using a surfactant. The precursors (tetrachloroauric acid and hydrazine) are dissolved into the reverse micelles’ water core, at a fixed water amount. The polar reactants interact in the water pool, and auric ions are reduced, followed by the nucleation process and nanoparticle development [19].
In metal oxide nanoparticles, stability is conferred by strong electrostatic interactions between the positive metal ions and the negative oxygen ions. Metal-oxide-based nanomaterials are prepared via the modification of the properties of their metal correspondents. When exposed to the action of oxygen at room temperature, nanoparticles of iron are rapidly converted to Fe2O3 nanoparticles, endowed with more significant reactivity, when compared to the corresponding metal nanoparticles. Other types of metal oxide nanoparticles often synthesized, including aluminum oxide (Al2O3), titanium oxide (TiO2), and zinc oxide (ZnO), are reported to possess improved features, when compared to their metal homologs [4].
Synthetic routes that can be applied include co-precipitation, the sol–gel technique, microemulsion, hydrothermal and solvothermal methods, and ultrasound treatment. Metal salts or organometallic complexes can be employed as precursors. Surfactants and polymers enable the control of particle development and aggregation and the induction of the self-assembled system. The synthesis can be performed in an aqueous medium or in an organic solvent system [20].
The sol–gel method is based on dispersing solid nanoparticles in a liquid medium, leading to the formation of a gel possessing nano-sized pores. The sol–gel features are impacted by factors such as temperature, pH, the presence of catalysts, time, and solvent type [21].
For metal oxide nanoparticle synthesis, a metal alkoxide sol is subjected to hydrolysis, and subsequently, condensation to a rigid gel takes place. The thermal treatment of gels leads to metal oxide nanoparticles. Hydrothermal and solvothermal techniques are efficient methods enabling the control of crystallinity, form, or dimension. The chemical process is carried out in a closed environment, at an elevated temperature and pressure. The hydrothermal method employs water, whereas the solvothermal technique makes use of organic solvents. The method can be coupled with microwave treatment to enhance the reaction speed [22].
Hence, conventional synthetic pathways encompass hydrothermal and solvothermal techniques, the sol–gel method, pyrolysis of the metal homolog, chemical precipitation, wet chemical synthesis, thermal decomposition, and microwave synthesis. The green synthesis of metal oxide nanoparticles, also denoted as biosynthesis, has become popular, as it overcomes the shortcomings of toxic chemicals. It can employ plant leaf extracts, algae, biopolymers, or active biosurfactants, imparting elevated specificity, biodegradability, and biocompatibility [23].
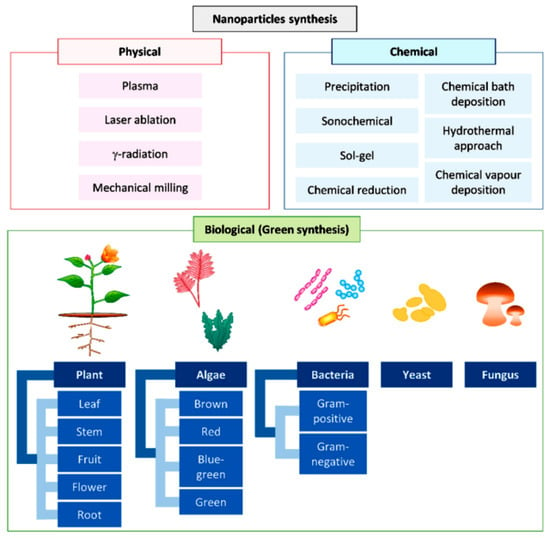
Figure 3.
Different approaches used for the synthesis of nanoparticles, from [24,25], MDPI 2023, 2021.
Hydrothermal, solvothermal, electrochemical, photochemical, microwave, microemulsion, pyrolysis, redox, co-precipitation, sol–gel, and chemical vapor deposition approaches, in which nanomaterials are produced through the combination of single atoms or molecules, are classified as bottom–up approaches, and the other techniques, ball milling, arc discharge, laser ablation, sonication, and nanolithography, in which nanomaterials are prepared by reducing molecule sizes to the nanoscale, are categorized as top–down methods [5,24]. Physical synthesis, in general based on top–down approaches, requires high energy input and elevated costs; nevertheless, it imparts high purity and exerts control on shape, size, and crystallinity. Chemical techniques relying mainly on bottom–up approaches are compatible with production on a large scale; nevertheless, they may involve high energy input, as well as toxic instrumentation, reagents, and by-products. Green synthesis is environmental friendly, characterized by the simplicity of the procedure, but necessitates control to ensure an aseptic cultivation environment [24].
Ceramic nanoparticles are made of inorganic materials, oxides like alumina, titania, zirconia, silica, or carbonates, carbides, and phosphates, prepared by heating at elevated temperatures and subsequent quick cooling. Crystalline, amorphous, dense, porous, or hollow forms can be obtained, characterized as robust materials, with chemical and high-temperature resistance [26].
Silica nanoparticles can be prepared by applying various processes like the sol–gel technique, flame synthesis, and reverse microemulsion (in which particles constituted by nano-sized polar droplets are dispersed by an appropriate surfactant, in a continuous phase represented by a non-polar organic solvent). The sol–gel technique is classed as a widely employed technology for silica nanoparticle synthesis, enabling the control of morphology, size, and particle distribution. The process relies on forming a colloidal suspension and promoting gelation in the continuous liquid phase. The precursors can be inorganic salts (chlorides, sulfides, nitrates) or organic compounds such as alkoxides [27].
Mechanical milling is used for the synthesis of oxide- and carbide-reinforced aluminum alloys, aluminum/nickel/magnesium/copper-based nanoalloys, or other nanocomposites [28].
Semiconductor nanomaterials have gained increasing interest, given their chemical, physical, electronic, optical, and mechanical properties. Semiconductor core–shell nanomaterials have found extensive application in agricultural and food processing, sustainable energy, medical, and environmental applications [29].
Nanomaterials have been classified based on their magnetic features into the following:
- Concentrated magnetic semiconductor nanomaterials are binary compounds such as EuTe (antiferromagnetic) presenting a spontaneous magnetic order.
- Non-magnetic semiconductor nanomaterials are not composed of magnetic ions but function relying on the charge of electrons.
- Diluted magnetic semiconductor nanomaterials present a few magnetic impurities added to the host matrix. Several diamagnetic positive ions in the host matrix undergo aleatoric replacement by magnetic doping cations. These semiconductor materials are endowed with magnetic properties that combine the features of ordinary and magnetic semiconductors [2,5].
Quantum dots are semiconductor nanocrystals (composed of a binary compound such as CdSe or InP) with diameters below 10 nm [1,30,31,32].
They are defined as nanoclusters composed of several hundred to thousand atoms, forming binary compounds like SiC, CdTe, CdSe, GaAs, or InAs or ternary compounds such as InGaN, InGaP, or InGaAs. They exhibit a reverse relationship between size and band gap: as the size increases, the band gap and the emission wavelength diminish [33].
Colloidal silicon quantum dots are viewed as viable alternatives to metal-based quantum dots, mainly in optical applications, and were prepared by the reductive thermal treatment of hydrogen silsesquioxane [34].
Another technique usually applied to quantum dot synthesis is colloidal synthesis, using metal chalcogenides or semiconductor nanoparticles. The dissolution of precursors in a solvent is followed by the addition of appropriate capping ligands, nucleation, and the development of quantum dots. Other methods for synthesizing quantum dots are epitaxial growth (the growth of a crystalline film appropriately oriented on a substrate), molecular beam epitaxy, and electrochemical synthesis [35].
Carbon-based quantum dots are another type of nanomaterial that are extensively used due to their excellent electrical, optical, magnetic, thermal, and mechanical properties, remarkable stability, and lack of toxicity, becoming pivotal components of smart nanomaterials [1,36].
Carbon-based nanomaterials include carbon nanotubes, fullerenes, graphene, and carbon nanofibers. These nanomaterials are versatile, endowed with high conductivity, mechanical robustness, high electron mobility, and anisotropic thermal conductivity [1,37,38].
A fullerene is an allotropic form of carbon present in a hollow sphere, ellipsoid, or tube shape. Fullerenes have a structure similar to that of graphite, additionally encompassing some pentagonal or even heptagonal rings, giving rise to porous structures. Spherical fullerenes are also named buckyballs, whereas cylindrical ones are defined as carbon nanotubes or buckytubes. Buckyball clusters, or buckyballs involving less than 300 carbon atoms, are currently denoted as endohedral fullerenes, including the most significant fullerene category, C60 [39].
In recent years, graphene, constituted of sp2-hybridized carbon atoms arranged in a honeycomb lattice, forming a two-dimensional single planar layer, has gained increasing interest due to its high specific surface, mechanical resistance, physicochemical features, and excellent electronic and thermal conductivity [40]. Different techniques can be applied in graphene synthesis, such as mechanical or liquid exfoliation, chemical vapor deposition, and the chemical and electrochemical Hummers’ method [41]. Unfortunately, mechanical exfoliation, applied to obtain graphene nanosheets, presents several shortcomings, requiring a long time and being characterized by low efficiency [42]. Also, some difficulties cannot be avoided, like the occurrence of defects and contaminating by-products [43].
Chemical vapor deposition is applied to prepare graphene nanosheets at a large scale, while the laser-induced graphene method is currently applied at a laboratory scale [42]. It employs synthetic or natural polymers that are converted into graphene using CO2 or UV lasers. The technique relies on the carbonization and gasification of the polymer. Under the action of a high-energy laser, the polymer is graphitized, and the subsequent gas generation yields multilayered graphene. Laser-induced graphene has high porosity and is endowed with elevated electrical conductivity and excellent electrochemical activity [44]. The growth of graphene can be also epitaxially performed by the thermal decomposition of silicon carbide, represented by crystals forming hexagonal basal planes, enabling the development of a graphene thin film from the carbon atoms that remain after silicon desorption from the surface [45].
In the Hummers’ method, graphite or graphene oxidation is performed using potassium permanganate, sodium nitrate, and sulfuric acid. Hydrazine serves to reduce graphene oxide to graphene [43].
Graphite oxide can be produced by adding oxygenated groups, hydroxyl, carbonyl, carboxyl, or epoxy, to the graphite surface by electrochemical oxidation. Graphite oxide is less conductive than graphite; its electrical conductivity depends on the oxygen level. Graphite oxide preserves, nevertheless, the layered structure of graphite, but its interplanar distance is larger and has lower regularity [46].
Carbon nanotubes are endowed with enhanced electric conductivity without the associated significant heating due to their dielectric anisotropy [47]. The rolling of graphene sheets leads to tubular shapes. The external diameters of multi-walled carbon nanotubes range from 0.4 to 2 nm [48], while those of single-walled carbon nanotubes range from 2 to 100 nm [49].
Catalyzed chemical vapor deposition, as a technique enabling the deposition of a solid material from a vapor, using a chemical process in the proximity of a heated substrate surface, is used for carbon nanotube synthesis [50].
The synthesis of single- or multiple-walled carbon nanotubes implies the decomposition of a carbon-based precursor (carbon monoxide, hydrocarbons, or alcohol), catalyzed by transition-metal-based nanostructures relying on cobalt, nickel, or iron. Discharge using electric arc (or plasma arching) and laser ablation (relying on graphite irradiation and subsequent conversion into vapor carbon atoms) implies functionalization, doping, filling, or chemical addition [51].
Carbon nanotubes have been used in many applications, with their incorporation in nanosensors relying on their outstanding structural, physical, and chemical features. These properties are responsible for the nanotubes’ type, wall nature, diameter, and length. Nevertheless, as their main shortcomings, their insolubility and tendency to agglomerate have been mentioned [1].
Nanodiamonds are characterized by increased surface areas, tunable surface structures, and a lack of toxicity, enabling their use in biosensors [1]. Carbon nanodiamonds are nanostructured tetrahedral materials 5–10 nm in size. sp3-hybridized carbon atoms attached by covalent bonds constitute a crystalline diamond core. Plasma-assisted chemical vapor deposition, a detonation process, high-energy ball milling (which uses material collision with balls, under shaking to reduce particle size), and laser ablation can be applied in their synthesis [52].
Carbon nanofibers and nanospheres and mesoporous carbon possess excellent properties like one-direction accelerated electron transfer, pivotal for obtaining nanosensors with a low detection time [53,54].
In electrospinning, a high-voltage-driven synthesis method conducted by an electrohydrodynamic mechanism, small fibers are generated from a polymer solution, and it is considered a suitable method for producing carbon nanofibers with different porosities and diameters; nevertheless, controlling the process may be difficult [1,55].
Nanowires, nanosheets, nanorods, and nanospheres of different geometries are prepared by hydrothermal and solvothermal methods [56,57,58,59]. Carbon nanorings are synthesized by performing ultrasonication and acid treatment as post-treatment processes that can induce alterations in the properties of the rings. Hence, in a novel study, chemical vapor deposition was applied, employing acetylene as the carbon precursor and NiO/Al2O3 as the catalyst [60].
Carbon-based quantum dots have gained increasing interest in electrochemical sensors given their excellent conductivity, diminished cytotoxicity, and favorable opto-electronic features. Carbon quantum dot-based electrochemical sensors were integrated into cheap, disposable sensitive electrodes [61]. The solvothermal method implies subjecting the material to high pressure and temperature, promoting the nucleation and development of carbon dots. The hydrothermal method relies on the carbonization of the organic material, using a batch reactor resistant to high temperatures, while counteracting vapor emissions to elevate the reaction pressure [62]. Hydrothermal carbon dots exhibit high photoluminescence and quantum yield. Their synthesis involves the use of non-toxic renewable precursors and solvents, leading to chemical stability and a lack of toxicity. Nano-carbon dot synthesis implies four steps: polymerization, aromatization, nucleation, and growth. Hydrothermal carbonization is regarded as a non-expensive and green technique making use of a closed autoclave under controlled, elevated temperature [63,64].
The term composite nanomaterials refers to multiphasic nanoparticles and nanostructured materials, presenting one phase in the nanoscale range. They can combine different nanoparticle types, or they can combine nanoparticles with larger-sized materials or with bulk-type materials (in the case of hybrid nanofibers). More complex structures can be incorporated, such as metal–organic frameworks. The composites may be prepared using a plethora of combinations of carbon-based, metal-based, or organic-based nanomaterials, integrating metals, ceramics, or polymers [65].
Polymeric nanoparticles have received attention in health-related applications but are also largely applied to detect contaminants in both the gaseous and liquid state of aggregation. Nanocomposites prepared from graphene, carbon nanotubes or metal nanoparticles, or mixed nanocomposites possess enhanced electrochemical activity. The features of the derived nanosensors, sensitivity, selectivity, and biocompatibility, are highly impacted by both the nature of the matrix and the filler material [21].
Nanocomposite-modified screen-printed carbon electrodes can be synthesized from graphene, graphite oxide, and polymers, such as polyethyleneimide, gelatin, and chitosan [66].
Electrochemical nanocomposite sensors relying on conducting polymers have proved their outstanding ability to detect various biomolecules and contaminants like heavy metals, mycotoxins, and pesticides. Performance improvement can be achieved by integrating redox mediators, conductive hydrogels, and molecular imprinting polymers.
Carbon paste can be modified by incorporating dopants such as metal nanoparticles, ionic liquids, or specific compounds, promoting the electrochemical response. Employing golden nanoparticles or carbonaceous materials (carbon nanotubes, graphene) as dopants promotes conductivity and the analytical signal. The synthesis of composite nanomaterials implies amalgamating carbon paste with carbon nanotubes, graphene, or metallic oxides, promoting electronic transmission, stability, and sensitivity. Carbon paste can be incorporated into a matrix of nanomaterials such as graphene oxide or carbon nanotubes. Polyaniline, polypyrrole, poly (3,4-ethylenedioxythiophene), polyazulene, and polyactylene are frequently used carbon pastes that can present many morphologies, such as flakes, sheets, rods, or particle-like structures, enabling their extensive use in diverse commercial processes. A shortcoming mentioned is their low solubility [67].
Molecular organic frameworks frequently incorporated in nanosensors are represented by crystalline porous polymers formed by coordinate bonds. The sol–gel technique is most often used to synthesize molecular organic frameworks [68]. The approaches encompass the engineering of the pore surfaces at the molecular level by sol–gel precursors; exerting control of the position and development of molecular organic framework crystals on the inorganic support; employing molecular organic framework crystals as templates for sol–gel processes in the pores but also on the outer surfaces; and using sol–gel-derived inorganic templates for designing molecular organic framework structures [69].
The solvothermal process under controlled temperature and time uses metal ions and organic linkers. Other approaches to prepare metal organic frameworks encompass mechanochemical, electrochemical, sonochemical, microwave-assisted, and layer-by-layer growth [70]. Metal organic frameworks are applied for their outstanding adsorption abilities due to their distinguished physicochemical features and enhanced inner porosity. The grafting of functional groups such as -COOH and -NH2 can be performed in situ or post preparation or purification [71,72]. These properties render metal organic frameworks suitable candidates for co-immobilizing biological ligands, exploiting strong interactions established between the functional groups of the metal organic frameworks and the biological ligands, such as stacking, hydrogen bonds, and ionic forces, which have the potential to be helpful in the development of biosensors [73]. The resulting biosensors have proved their potential in assessing food safety [68].
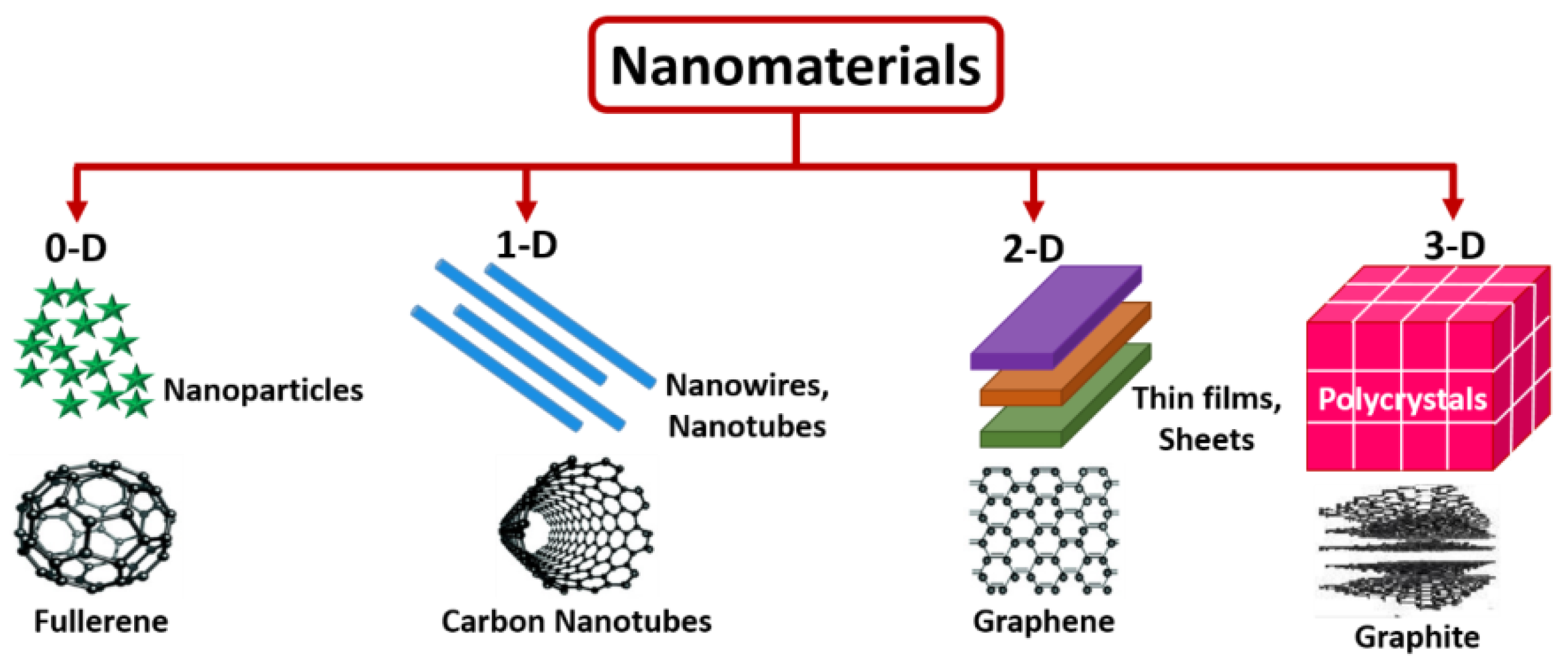
Based of their dimension, nanomaterials are classified as zero-dimensional (nanoparticles and quantum dots), one-dimensional (nanotubes, nanorods, nanofibers, nanopillars, or nanowires), two-dimensional (graphene nanosheets, nanoplates, or nanopores), or three-dimensional (nanocomposites and complex hierarchical structures, nanoprisms, or nanoflowers) [74,75].
One-dimensional nanomaterials possess a spherical or quasi-spherical shape, with a diameter smaller than 100 nm. Carbon nanodots, carbon quantum dots [76], graphene quantum dots [77], fullerenes [78], polymer dots [79], inorganic quantum dots [80], and noble metal nanoparticles [81] are typically classed as 0D nanomaterials frequently incorporated in sensors. The reduction in size highly improves their features, when compared to their bulk counterparts. One-dimensional nanomaterials are characterized by a high length-to-diameter ratio, leading to adaptable electrical, chemical, mechanical, and magnetic properties. Single-walled carbon nanotubes are porous nanostructures consisting of sp2-hybridized atoms [82].
The significant progresses in the application 2D graphene sheets has proved the pivotal role of 2D nanomaterials, which are constituted by planar atomic layers, bonded by weak interactions like van der Waals forces [83]. Such materials are endowed with excellent mechanical strength but also malleability due to the presence of strong covalent bonds between the atoms of the same plane and low atomic thickness [82,84].
Three-dimensional nanomaterials can be developed following the organization of zero-dimensional, one-dimensional, or two-dimensional nanostructures. The morphologies are complex and rely on the growth conditions. The resulting structures can be in the form of cubes, spheres, rods, cross-linked nanorods, foams, or hierarchical dendrites [82,85].
Figure 4 depicts 0-D, 1-D, 2-D, and 3-D nanostructured materials [86].
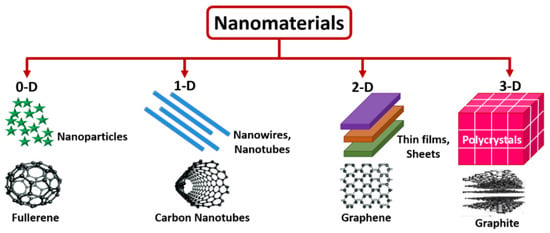
Figure 4.
Schematic representation of 0-D, 1-D, 2-D, and 3-D nanostructured materials, from [86], MDPI 2022.
The functionalization and surface modification of nanomaterials become crucial for improving analytical performance. For carbon-based nanomaterials, the introduction of functional groups such as carboxyl, hydroxyl, or amino promotes the redox process underlying the analytical signal. Metal nanoparticles often require stabilization with ligands, polymers, or surfactants, hampering aggregation and ensuring an even distribution on the electrode surface. Carbon-based and metal-based nanomaterials and composites can be integrated into advanced electrochemical detection platforms, enhancing analytical performance in food safety and promoting reproducibility, stability, and biocompatibility. Sensors incorporating composites of carbonaceous materials and metal-based nanoparticles are used to determine bioactives, nutrients, food additives, and contaminants in foods and beverages. Polymer–nanoparticle hybrids integrate the adaptability and molecular recognition features of polymers with the high conductivity and catalytic activity of nanoparticles. The resulting sensors can detect complex bioactive molecules present in food samples. Polymer coatings hamper non-specific adsorption, improving selectivity [87].
2. Electrochemical Methods Based on Nanotechnology
2.1. General Aspects Concerning Transducers and Detection Modes
Electrochemical sensors, considered the most widely applied sensors, rely on the electron flow generated or consumed during a (bio)chemical reaction to yield a measurable electrochemical signal. Electrochemical nanosensors rely on the interaction between the nanomaterial, the biological element, and the target analyte, which consumes or generates charged species, ions, or electrons, with the analytical signal being quantified as the current intensity, voltage, or impedance [88]. Biorecognition elements such as enzymes, nucleic acids, and microorganisms can be associated with electrochemical transducers, imparting specificity to the analytical response [89,90].
Electrochemical transducers are highly sensitive, compatible with novel miniaturization technologies related to nanoscale fabrication, and prone to modification by biorecognition elements; have low power requirements, mechanical robustness, and cost efficacy; rely on fast assays and on simplicity of the analytical procedure. Nanoparticles impart large reaction surfaces, enhancing analytical performance [91].
Metal and metal oxide nanoparticles, graphene and its derivates, and carbon nanotubes become a part of nano-transducers to increase the electrode surface and sensitivity. The nanostructured electrochemical transducers have distinctive characteristics such as a high surface-to-volume ratio and outstanding electrocatalytic activity. Nanomaterials promote the electrochemical response by driving faster electron transfer but also represent immobilization matrices: they enable the immobilization of high enzyme amounts, preserving biocatalytic activity and improving sensitivity. Electrochemical nanobiosensors possess synergistic features, intertwining specific biorecognition and the aforementioned electronic characteristics [91].
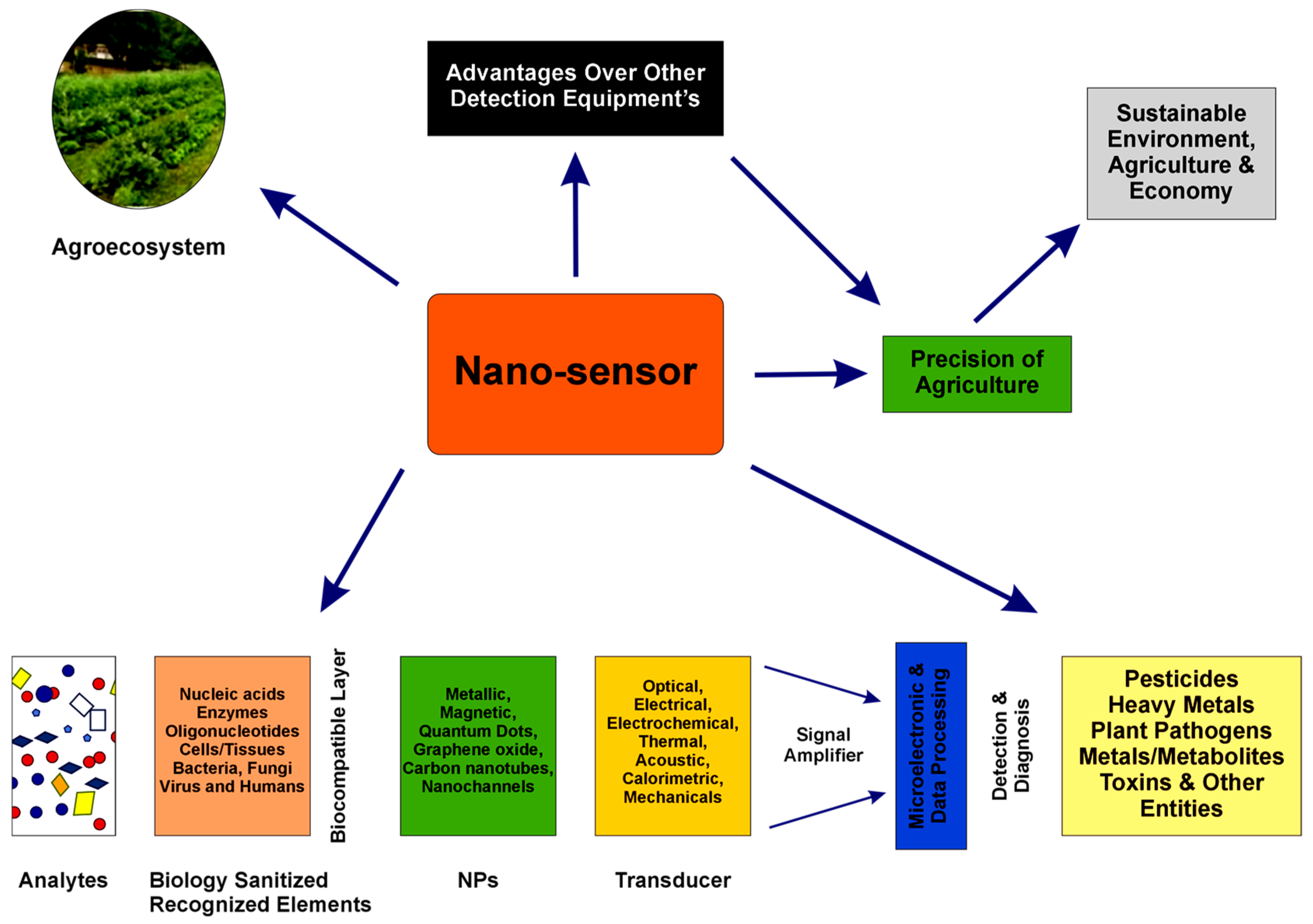
In Figure 5, the main principles of development, integrating various transducers and biorecognition elements, as well as the most important analyte classes targeted, are presented [92].
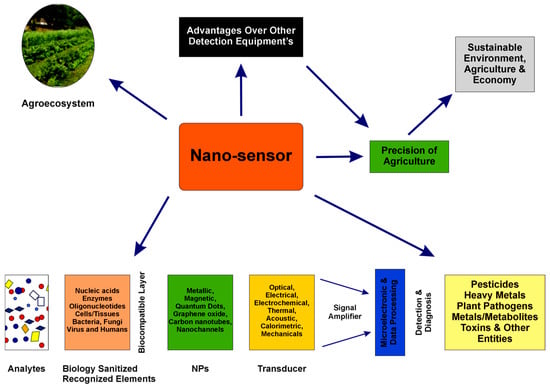
Figure 5.
Nanotechnology-based biosensors’ characteristics and applications in agriculture, from [92], MDPI 2023.
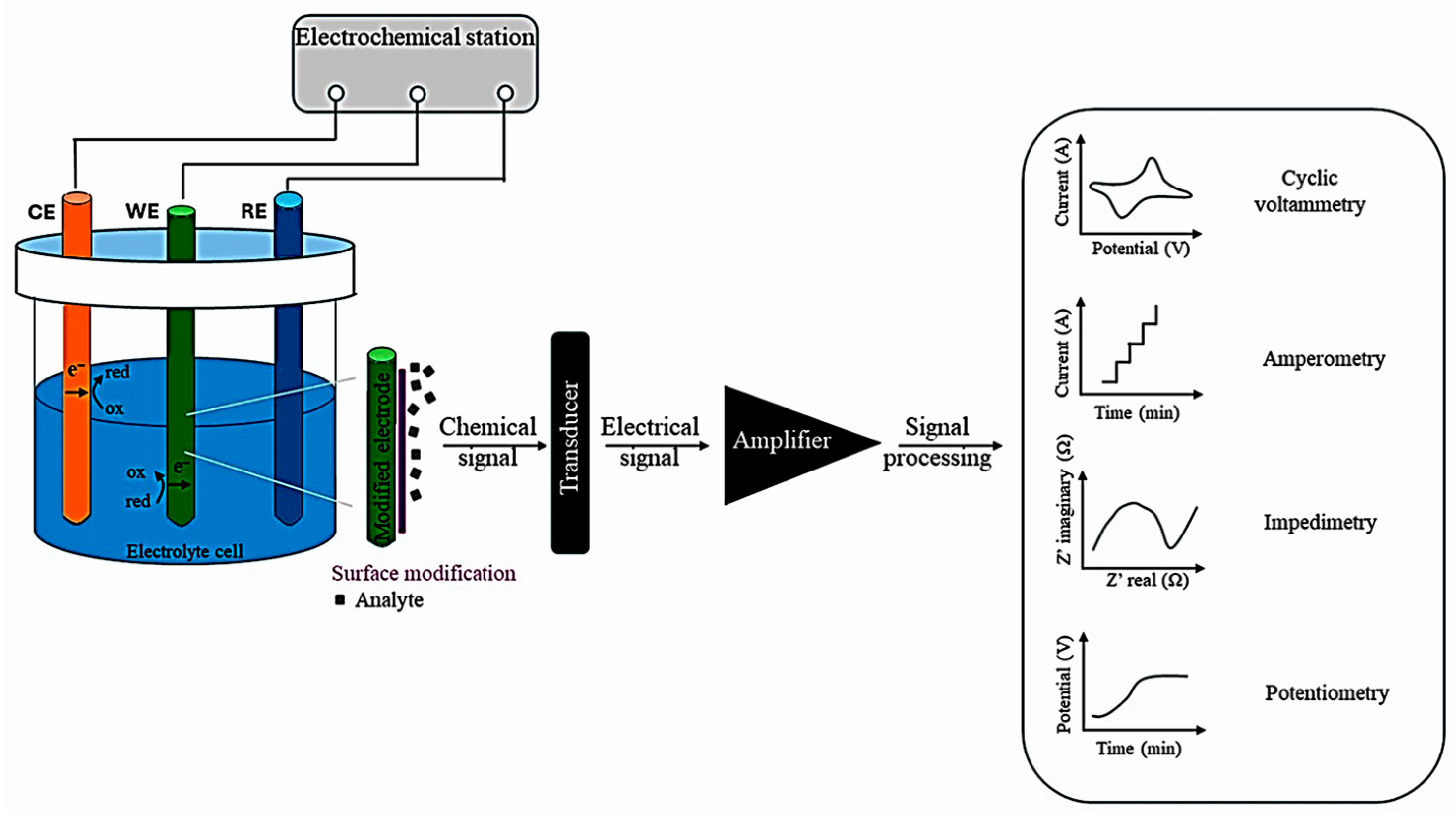
Figure 6 depicts the basis of an electrochemical assay. A three-electrode electrochemical cell is presented; the electrical signal generated by the receptor–analyte interaction is converted into a measurable value by a physicochemical transducer. The specific manner in which the analyte bonds to the sensing element induces a change in its current intensity, potential, conductivity, or charge. The principles of the detection modes are depicted in the graphs [93].
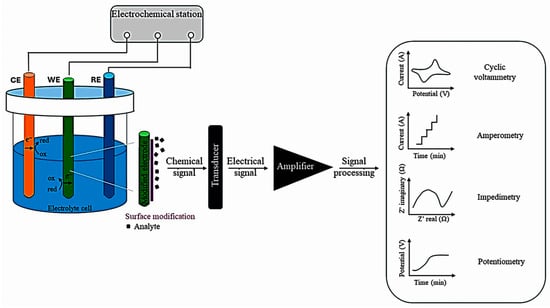
Figure 6.
The main components of electrochemical sensing mechanisms, from [93], MDPI, 2025.
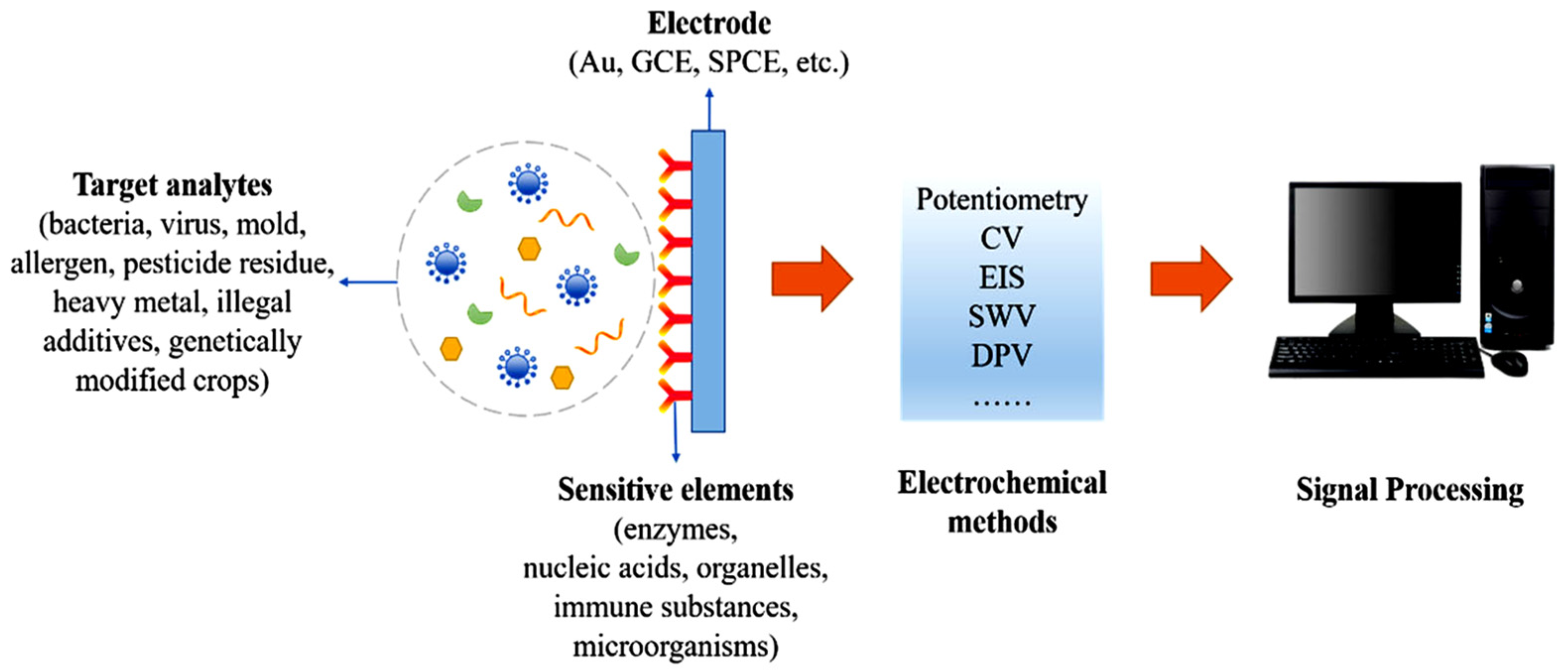
Figure 7 presents the components of electrochemical biosensors. To detect and quantify contaminants present in foodstuffs, transducers made of metal, carbonaceous materials, and composites can accommodate various biorecognition elements like biocatalysts, nucleic acids, antibodies, or microorganisms. Combining the specificity of the biorecognition and the sensitivity of the electrochemical transducer leads to a selective and fast real-time assay [94].
Figure 7.
Essential component parts of an electrochemical biosensor, detailed in the case of the assay of food contaminants, from [94], MDPI 2022.
Carbon nanotubes with their particular tubular structure and tunable sidewalls are also biocompatible, prone to undergoing enzyme modification. It has been reported that carbon nanotube-based biosensors possess improved sensitivity and electron transfer speed and lower detection limits, when compared to conventional carbon-based electrodes. Carbon nanotubes can undergo functionalization by hydroxyl, carbonyl, amino, or carboxyl groups. Grafting polar groups will enhance the carbon nanotubes’ solubility, biocatalytic activity, and biocompatibility, with the immobilization of the biorecognition element on the electrochemical transducer’s surface being facilitated by covalent linkage. Carbon nanotubes can be mixed with metal-based nanoparticles, with surface modification being performed more effectively than when relying only on one nanomaterial type. The immobilization procedure has to confer reproducibility and stability, enabling long-term usage and stability during storage. Enzyme immobilization is viewed as a key aspect impacting measurement accuracy, sensor-to-sensor reproducibility, and operational lifetime. The immobilization matrix may represent a support only or may also be involved in mediating signal transduction [91].
Enzyme immobilization techniques encompass physical adsorption (relying on van der Waals forces or hydrogen bonding), physical entrapment in polymer matrices, or covalent attachment. Cross-linking employs bifunctional agents, most often glutaraldehyde, forming a “bridge” between the protein macromolecule and the electrode surface. In self-assembled monolayers, long-chain alkylthiols, disulphides, or amines are used. The sol–gel technique can provide an environment comparable to that of the enzyme in solution. The immobilization method applied should consider the enzyme’s nature, the type of transducer, and the physicochemical characteristics of the analyte, as well as the operation conditions of the biosensor [91].
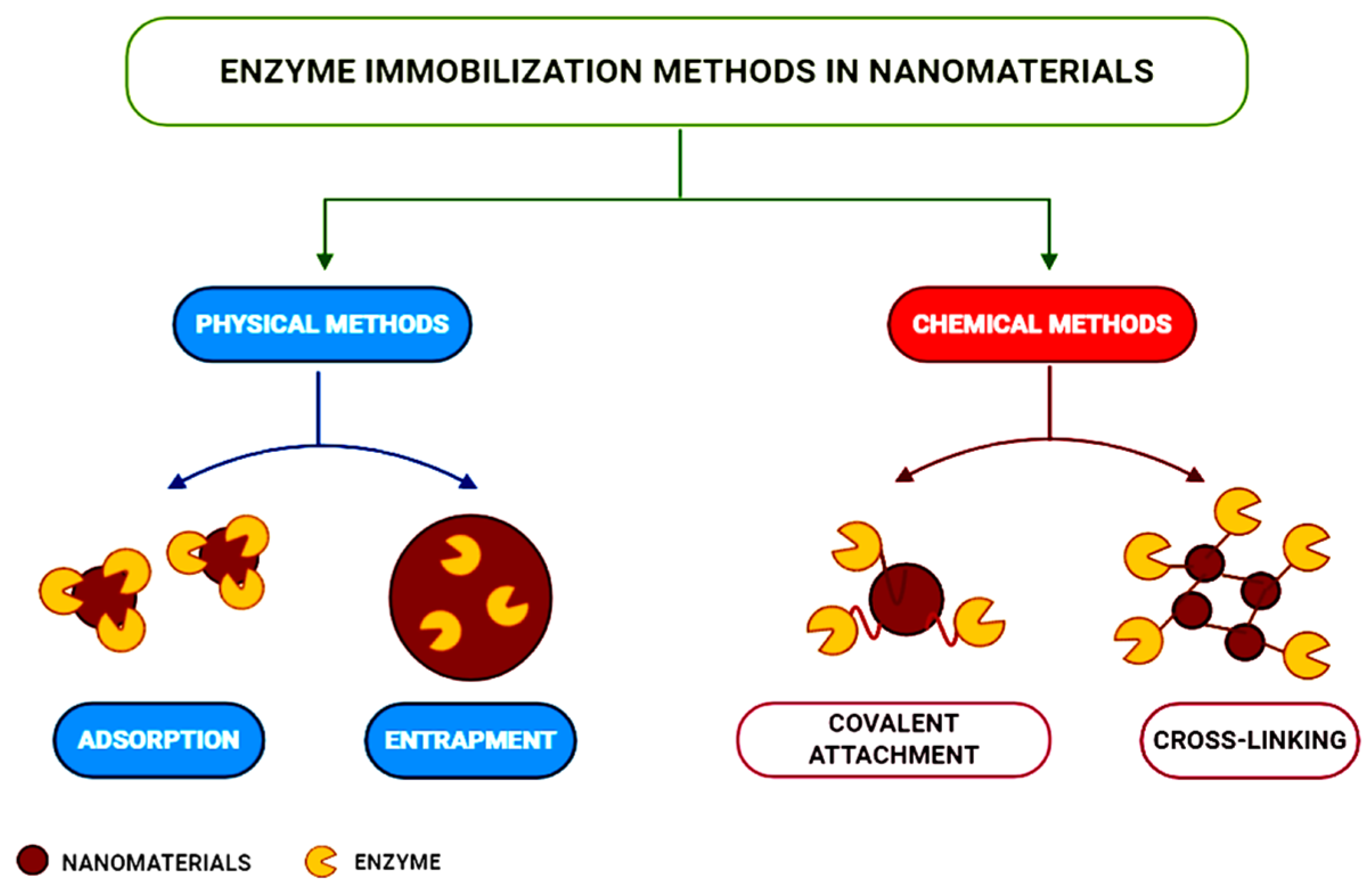
The main physical and chemical enzyme immobilization techniques applied in developing nanomaterials are presented in Figure 8 [95].
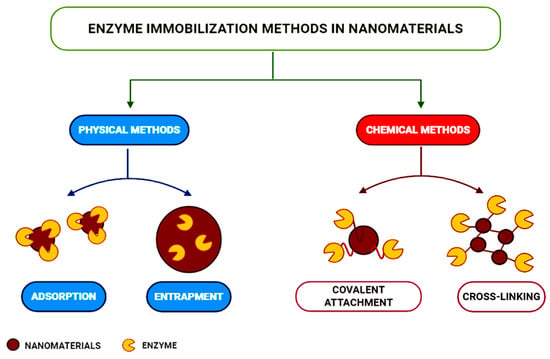
Figure 8.
Enzyme immobilization techniques applied to nanomaterials, from [95], MDPI 2021.
The sensing devices can be electronically gated, rendering them sensitive to the attachment of a single biorecognition element molecule, which can be a protein (enzyme) or a nucleic acid [96].
In other biosensing approaches, a lipid bilayer is attached to an ultrathin polymeric support, yielding a lipid–polymer complex. In this manner, various “channels” can be introduced into the membrane, enabling selective solute motion, for assay purposes. Also, enzymes can be encapsulated inside microscopic or nano-sized artificial cells [97].
Organic or inorganic polymers capable of biorecognition can be obtained by molecular imprinting. Molecular recognition is achieved via template-shaped cavities present in polymer matrices with a memory of the template compounds. There are numerous possibilities of obtaining sorbents selective for organic compounds belonging to different classes. Molecularly imprinted polymers can be successfully integrated into electrochemical sensors due to their stability and ease of synthesis [98].
In electrochemical devices, novel component parts with advantageous physicochemical features are sought for incorporation as solvents, electrolytes, modifiers, or even suitable electrode materials. Ionic liquids are characterized, besides their ionic nature, by high conductivity, thermal stability, and low volatility. Their versatility and adaptable physicochemical characteristics are due to the possibility of replacing the cation or anion of the salt. Graphene, expanded graphite, graphene oxide–multi-walled carbon nanotubes, and metal-based nanoparticles are used in combination with ionic liquids to increase conductivity and enhance electron transfer. Their practical application cannot be significantly extended given their relatively high cost [98].
Voltammetry is an electrochemical technique relying on the measurement of current intensity, under controlled potential variation. It has mainly been applied to measure redox potentials and electrochemical reaction rates, but it can also be broadly applied for analytical purposes [33,99].
Cyclic voltammetry (CV) relies on linearly sweeping the potential over time. Differently from linear sweep voltammetry (LSV), after the established maximum potential is attained, the working electrode’s potential is varied in the opposite direction, eventually reaching the initial value. Differential pulse voltammetry (DPV) is considered a derivative of linear sweep voltammetry or staircase voltammetry, using regular voltage pulses superimposed on a linear or stairstep variation in the potential. The electrochemical reaction is analyzed more precisely and the sensitivity can be increased due to the lowering of the capacitive current [100].
The small step sizes result in narrower voltammetric peaks and a better discrimination of electroactive analytes with close oxidation potentials. One limitation of this method as a stepping technique is the experiment duration, which is higher than in ramping techniques such as amperometry or cyclic voltammetry [101].
Square wave voltammetry (SWV) applies combined square wave and staircase potential to a stationary electrode. Like in the case of differential pulse voltammetry, increased sensitivity can be achieved due to the minimized contribution of the nonfaradaic current. The recording of a differential intensity current plot and the time between potential reversal and current sampling increases sensitivity more than in other electroanalytical techniques but also increases the duration [102].
Stripping voltammetry, applied mainly in the case of heavy metal ions, implies two main steps: pre-concentration at the working electrode by reduction and the removal of the accumulated metal from the electrode’s surface, by oxidation via a Faradaic reaction, giving rise to heavy metal ions in the solution. This final step yields an analytical signal (current intensity) proportional to the concentration of the heavy metal present in the solution [103].
Amperometric sensors rely on the measurement of the current intensity, following an oxidative or reductive process involving an electroactive analyte, at a fixed potential value [86,104]. The resulting current is proportional to the analyte’s concentration.
Potentiometric sensors rely on the measurement of a working or indicator electrode’s potential with respect to a reference, and they can be applied to the assay of biological, food, or agricultural samples. The analytical signal promoted by ion accumulation at ion-selective electrodes or field-effect transistors is logarithmically correlated to the concentration of the analyte. The latter can be an ionic species (H+, NH4+, Na+, K+, Ca2+, heavy metal ions) or another analyte (molecular chemical species) involved in a chemical process that releases or consumes an ionic species (antibiotics, pesticides) [33].
Conductometric sensors rely on the electrical conductivity variation that is associated with a chemical/biochemical reaction. This change often occurs after a biochemical event. For electrochemical detection, electrochemical impedance spectroscopy (EIS) is also applied. It is useful for studying the electrode–electrolyte interface but also for quantitation purposes. It implies the application of a small sinusoidal potential across the electrochemical cell. The transduction system involves an electrochemical cell to which a low-amplitude signal is applied, and its dependence on frequency is recorded. The resulting current can differ over time (phase shift) with respect to the applied voltage, and the voltage-to-intensity ratio is the impedance, illustrating the opposition of the electrochemical components of the cell to the flow of charged chemical species—electrons or ions [105].
The next subsections will discuss electrochemical methods (voltammetry, amperometry, potentiometry, conductometry, impedimetry) in detail, focusing on the electroactivity and analytical performance imparted by the properties of the nano-transducer, as well as those of the analyte and matrix components. The applicability to a wide range of food samples is presented for each analyte family.
2.2. Linear Sweeping Techniques—Linear Sweep Voltammetry and Cyclic Voltammetry Using Nanosensors
Antioxidants and preserving agents
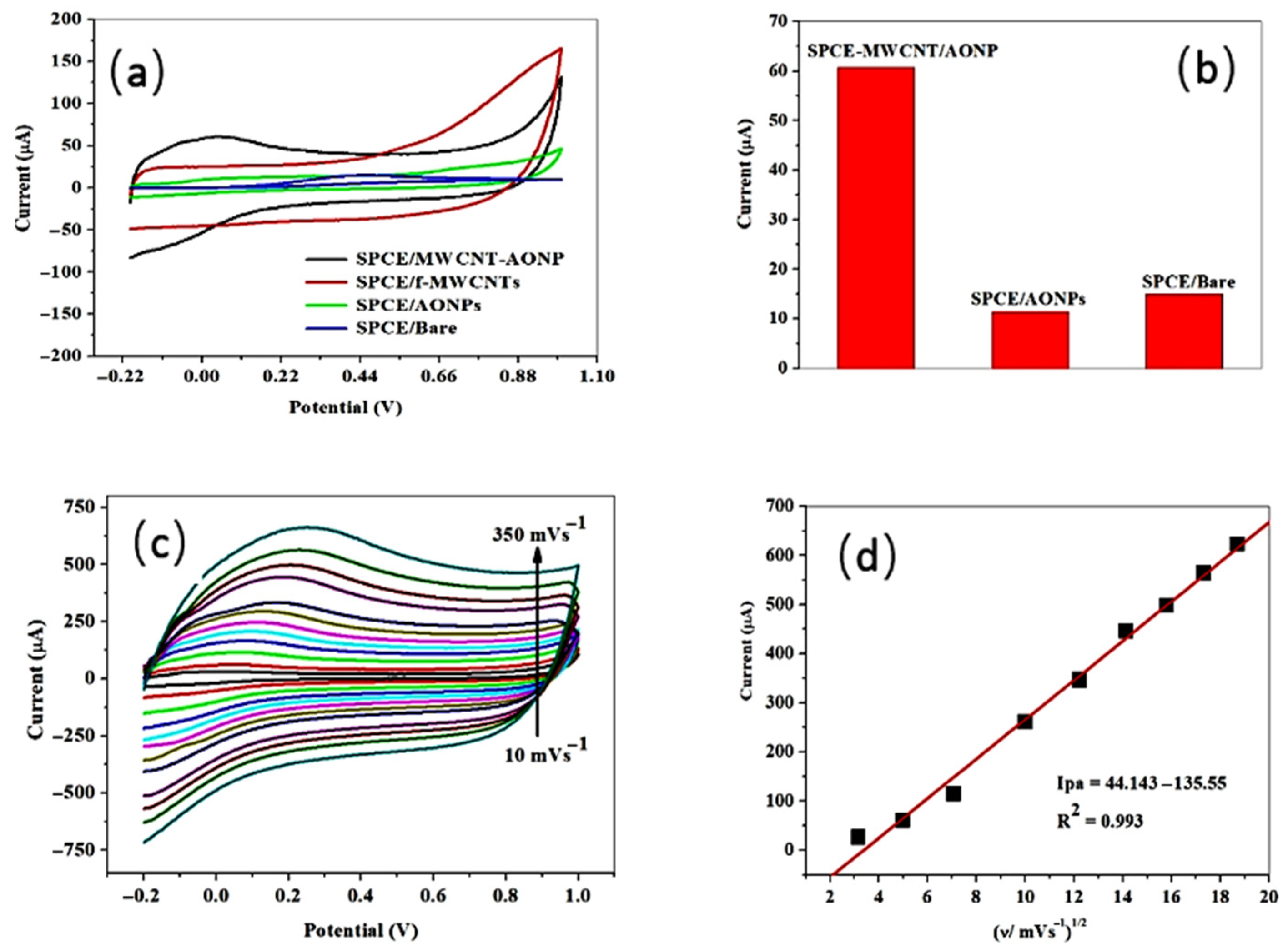
In the cyclic voltammetric assay of ascorbic acid at unmodified and modified electrodes, the analytical signal as the peak current intensity varied as follows: screen-printed carbon electrode/antimony oxide nanoparticle/multi-walled carbon nanotubes (with an intensity of the anodic peak of 60.71 µA and a peak potential of 0.032 V), screen-printed carbon electrode/antimony oxide nanoparticle (with an intensity of the anodic peak of 11.36 µA and a peak potential of 0.034 V), and a bare screen-printed carbon electrode (with an intensity of the anodic peak of 14.96 µA and a peak potential of 0.45 V). The screen-printed carbon electrode/functionalized multi-walled carbon nanotubes did not give a signal for ascorbic acid. The highest current response observed at the nanocomposite electrode (Figure 9) was assigned to the synergism exerted between the two types of nanomaterials used for screen-printed electrode modification. The nanocomposite electrode that gave the highest electrocatalytic activity was applied to the analysis of oranges, with very good recoveries of between 99.12 and 107.76% and a precision illustrated by a relative standard deviation value of 3.52% [106].
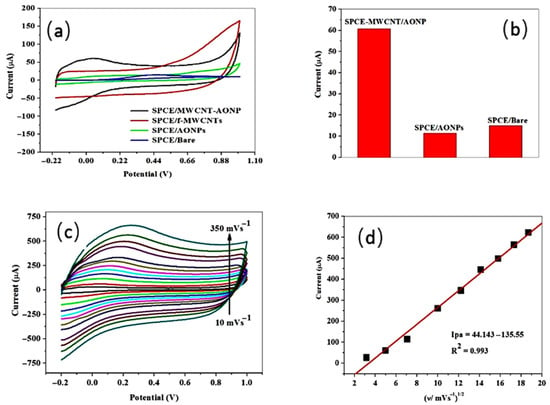
Figure 9.
Comparative cyclic voltammetric determination at modified and bare electrodes: (a) ascorbic acid detection, (b) comparative voltammetric responses of bare and modified electrodes, (c) scan rate influence investigation between 10 and 350 mV s−1, and (d) the linear dependence between the intensity of the anodic peak and the square root of the scan rate, showing the diffusion-controlled process, from [106], MDPI 2022.
Apoferritin, apoferritin–biomimetic platinum nanoparticles, and apoferritin–biomimetic platinum nanoparticles/Ti3C2 were used for glassy carbon modification to assess the analytical performance in the cyclic voltammetric assay of nitrite. The apoferritin–biomimetic platinum nanoparticles/Ti3C2 hybrid nanomaterial yielded the highest analytical signal, as a peak current, in a potential range of 0.7–0.9 V. MXene as a titanium carbide 2D nanomaterial (nanosheets) imparted a high specific area, an efficient electron transfer rate, and outstanding conductivity and therefore promoted the redox process consisting of nitrite depletion to generate the current response. Moreover, it was noticed that Ti3C2 nanosheets can contribute a large number of active sites to load apoferritin–biomimetic platinum nanoparticles. The linear calibration of the cyclic voltammetric response versus NaNO2 concentration ranged from 0 to 20 mM in spiked milk samples [14].
Food dyes
With linear sweep voltammetry at a Cu/Fe/NiO nanocomposite transducer, in the potential range of 110 mV–290 mV, a linear increase in the peak current was obtained for Tartrazine concentrations of between 10 and 500 µM. Cu/Fe/NiO used for glassy carbon modification gave a high oxidation peak, improving the conductivity and surface area, with respect to the bare electrode. The voltammogram had an oxidation peak current directly correlated with the Tartrazine level, which was due to the oxidation of the hydroxyl group present in the structure of the dye to a carbonyl group. pH changes can impact the local ionic force and distribution of charge in the proximity of the working electrode. The sensors function at a pH value for which the redox reaction of interest has the best sensitivity and specificity. Also, pH influences the presence and concentration of interferents in the analyzed matrix. So, a sodium phosphate buffer solution was used as the electrolyte, and the influence of the pH value was investigated in the range 3.0–12.0 at a scan rate of 0.01 mV s−1 and 90 μM Tartrazine. The maximum peak current was obtained at pH 6.0. In the LSV analysis of soft drink samples, the oxidative current had a direct relationship with concentration, at Tartrazine levels tested up to 400 µM [107].
Pesticides
Carbosulfan was detected in food products, relying on a green, simple, inexpensive, selective, and sensitive electrochemical method employing silver nanoparticle-modified laser-reduced graphene oxide, grown on a green recyclable polyethylene terephthalate substrate. The adaptable sensor was developed using green laser printing. Laser-reduced graphene oxide imparted outstanding conductivity, and the silver nanoparticles provided specific analyte adsorption sites. The electrochemical sensor was characterized by a highly electroactive surface and sensitivity. The deposition of silver nanoparticles increased the electroactive surface area and conductivity. The investigation of the electrochemical features and surface characterization were performed via electrochemical impedance spectroscopy, cyclic voltammetry, scanning electron microscopy, X-ray photoelectron spectroscopy, energy-dispersive X-ray spectroscopy, and Raman spectroscopy.
The maximum cathodic cyclic voltammetric peak of carbosulfan was obtained when 0.200 mL of a graphene oxide 2 mg ml−1 suspension was deposited on a polyethylene terephthalate substrate, chosen for its outstanding chemical and thermal features, over rigid glass or silicon substrates. For higher graphene oxide concentrations, the conductivity of the sensor decreased, as the excess laser-reduced graphene oxide prevented electron transport between the carbosulfan molecules and the sensor’s surface. The optimized concentration of silver nitrate, yielding the maximum cathodic peak current, was 50 µg ml−1. The silver nitrate solution volume deposited on the laser-reduced graphene oxide surface was 50 µL, and the silver reduction time was five minutes. For a concentration of 2.0 × 10−8 mol L−1 carbosulfan, the optimum adsorption time was 20 min.
Carbosulfan underwent irreversible reduction at the laser-reduced graphene oxide/silver nanoparticle sensor, with the absence of an oxidation peak during the reverse scan. The underlying electrochemical mechanism was investigated, and it was stipulated that silver nanoparticles are oxidized to Ag+, followed by the subsequent oxidation of carbosulfan by silver ions, releasing a phenolic compound but also a silver salt originating from the N-S moiety of carbosulfan. The salt was eventually reduced to metallic silver, which generates the cathodic signal. It was asserted that carbosulfan participates in the electrode process indirectly, via the redox interplay with silver nanoparticles. The cyclic voltammograms present a major cathodic peak at −0.75 V, assigned to the reduction of (R)2N-S-Ag to (R)2N-S- after the reaction of carbosulfan with the silver ions electrochemically generated from the silver nanoparticles, on the surface of laser-reduced graphene oxide/silver nanoparticles. In the voltammetric assay of carbamate pesticides which do not contain sulfur (carbofuran or carbaryl), no reduction peaks were noticed under similar conditions. This points towards the pivotal involvement of sulfur in carbosulfan detection, via cathodic peaks ascribed to the analyte adsorption on the silver nanoparticles present in the nanosensor’s structure. Silver nanoparticles present on laser-reduced graphene oxide behaved as efficient electron mediators, enabling selective carbosulfan reduction. Despite the sensitive character of the differential pulse voltammetric technique, the authors opined that this cannot lead to appreciable results in this case, given the strong carbosulfan adsorption. Hence, cyclic voltammetry was applied to the carbosulfan assay.
Under optimized conditions for carbosulfan detection (position of the cathodic voltammetric peak at −750 mV, with 200 μL of a 2 mg ml−1 suspension of graphene oxide deposited on the polyethylene terephthalate substrate), the laser-reduced graphene oxide/silver nanoparticle sensor exhibited a well-contoured cyclic voltammetric peak, with a linear dynamic range of 0.01–10 mg kg−1 and a detection limit of 0.005 mg kg−1 calculated according to the three-sigma criteria. The analytical applicability of the developed cyclic voltammetric carbosulfan sensor was proved in the assay of apples, oranges, and basmati rice, with a relative standard deviation smaller than 5% and 90% to 105% recovery. The recovery experiment was performed at pH = 13.0 in 0.1 mol L−1 KOH. It was inferred that the novel sensor gives no important interferent effects. Alongside its selectivity and sensitivity, the sensor was reproducible and had long-term stability [108].
Metal ions
A voltammetric portable biosensor applied to the quantitation of copper ions in wine relied on the use of glycine as a biorecognition element and agarose as an immobilization agent. Carbon-based screen-printed electrodes were used as cyclic voltammetric transducers. The linear range of the analytical response corresponded to 0.2–1.0 mg L−1. The sensitivity obtained was 11.05 × 10−5, and the detection limit was 0.041 ppm. The sensors had a very short response time of 30 s, with the potential to apply the technique to the analysis of wine [99].
2.3. Differential Pulse Voltammetry Based on Nanosensors
Food dyes
At a bare glassy carbon electrode, very small oxidation waves were noticed for Sunset Yellow, Tartrazine, Ponceau 4R, and Allura Red, pointing towards low electroactivities. Porous carbon was employed as a modifier for a glassy carbon coating, and it was synthesized from CaCO3 nanoparticles as a hard template and starch as a carbon precursor. The quantitation of these analytes was performed at pH 7.0 in 0.1 M phosphate buffer. The differential pulse voltammograms were recorded in a potential range of between 0.10 and 1.10 V. The pulse amplitude was 40 mV, the pulse width was 40 ms, the accumulation potential was 0.10 V, the accumulation time before performing each determination was 4 min, and the scan rate was 40 mV s−1. Moreover, the analytical signal increase imparted by porous carbon depended on the starch/nano-CaCO3 weight ratio. The porous carbon sample prepared in a starch-to-nano-CaCO3 weight ratio of 1:1 was the most active in food dye differential pulse voltammetric oxidation and optimized the signals by 89.4-fold, 79.3-fold, 47.3-fold, and 50.7-fold for Sunset Yellow, Tartrazine, Ponceau 4R, and Allura Red, respectively. The detection limits were 1.4, 3.5, 2.1, and 1.7 µg L−1 for Sunset Yellow, Tartrazine, Ponceau 4R, and Allura Red. In the analysis of real samples, except for the analytical signals assigned to the studied food colorants, no other oxidation signals were noticed, and the RSD values were smaller than 5%. Hence, the analytical sensing platform was applied to drink samples, with precision and selectivity [109].
A copper/iron/nickel oxide nanocomposite-modified working electrode was developed for differential pulse voltammetric Tartrazine detection in soft drinks. First, cyclic voltammetry was used to investigate the electrochemical behavior of the dye at the Cu/Fe/NiO nanocomposite-modified working electrode. The oxidation peak current increased linearly with the dye concentration. A broad linear range of 0–500 µM was obtained in sodium phosphate, with respect to a silver/silver chloride reference, at a 0.01 mV s−1 scan rate. Tartrazine yielded irreversible oxidation peaks, each concentration resulting in distinctive peak currents. The electro-oxidation at the nanocomposite electrode yielded a sharp peak with a maximum current of 7124.96 µA at a 500 µM concentration. The electrochemical performance was ascribed to the enhanced conductivity, fast electron transfer, antifouling properties, and elevated electrochemical activity of the nanocomposite-based working electrode. Differential pulse voltammetry at the copper/iron/nickel oxide nanocomposite electrode was applied to obtain the calibration plot of Tartrazine and for real sample quantitation purposes. In 0.1 M sodium phosphate buffer solution, pH = 6.0, with the increase in the dye concentration, the anodic peak had a marked increase between 0 and 500 µM. The pulse voltammetric assay ascertained the accuracy and steady analytical performance of the electrochemical sensor. An excellent correlation coefficient of 0.9945 was obtained, with the technique enabling the assay of soft drinks [107].
Alkaloids
At a miniaturized boron-doped diamond electrode, theobromine had a diffusion-controlled, well-defined, irreversible oxidation peak at a significantly high potential value, +1.2 V versus the silver/silver chloride reference, in 0.1 M H2SO4. The optimization of the analytical parameters was performed for differential pulse voltammetry, with a modulation time of 25 ms and a modulation amplitude of 100 mV. An increase in the modulation amplitude can cause a displacement of the peak potential towards more negative values, with a moderate broadening of the half-peak width. An increase in the modulation time resulted in a diminution in the background current, so it was considered that the aforementioned values ensured both sensitivity and selectivity. Under optimized experimental conditions, the linear range of the analytical response corresponded to 0.99–54.5 μM, with a sensitivity of 0.07 μA/μM, in differential pulse and square wave voltammetry. The complex voltammetric investigation resulted in low detection limits of 0.42 and 0.51 μM, with other analytical parameters revealing precision, such as RSD values of 2.5 and 1.7%, accounting for intra-day repeatability, in DPV and SWV, respectively. The miniaturized boron-doped diamond electrode showed its specificity and practical viability in the voltammetric assay of theobromine in chocolate samples, with reported mass percentages of between 0.75 and 2.24% and between 0.69 and 2.15% using differential and square wave voltammetry, respectively, the results being consistent with those obtained by potentiometric reference titration [110].
A fullerene/MWCNT/Nafion-modified glassy carbon electrode was developed for the differential pulse voltammetric assay of caffeine, an alkaloid of interest in the food and pharmaceutical industries, being present in coffee, tea, and soft drinks [111,112].
Cyclic voltammetry and electrochemical impedance spectroscopy using a ferricyanide/ferrocyanide redox couple were first applied to study the electrochemical features of the developed electrode, like ionic exchange capacity and conductivity. For the studied analyte, an irreversible oxidation peak versus the silver/silver chloride reference was noticed, at pH = 1.0 in 0.1 M HClO4 electrolyte, which yielded the best signal with respect to form and intensity. Compared to the glassy carbon electrode and the Nafion/glassy carbon electrode, the oxidation peak increased at the fullerene/multi-walled carbon nanotube/Nafion/glassy carbon electrode, and the oxidation potential had a negative shift from 1.522 to 1.393 V, proving an enhanced electrocatalytic effect due to the high specific area. The electron transfer was confirmed as a diffusion-controlled process, with a linear dependence of the peak current intensity on the square root of the potential sweep rate. For trace caffeine analysis by differential pulse voltammetry, the optimized parameters had the following values: an initial potential of +1.0 V, a final potential of +1.7 V, a pulse amplitude of 50 mV, a step potential of 5 mV, and an equilibrium time of 5 s. The differential voltammetric oxidation peak current had a linear range of increase with concentration for 10–1000 µM caffeine. The reported detection limit was 7.289 × 10−8 M, at a signal-to-noise ratio of 3 [111].
The specificity of the sensor in the presence of interferents commonly incorporated into drinks and pharmaceuticals was tested, with the tolerance limit being reported as the concentration ratio of the potential interferent imparting a ±5.0% relative error to the analytical signal value. The oxidation peak of caffeine is mildly impacted by citric acid, as this compound did not exert a significant influence on the caffeine peak current at more than 100-fold excess. Nevertheless, it was found that dopamine and sulfite ions exerted the highest interfering effect, hindering the selectivity of the sensor [111].
Pesticides
A portable graphene oxide–gold nanoparticle-modified screen-printed carbon electrode enabled the highly sensitive and selective assay of carbofuran. The optimization of the method’s analytical parameters, such as the graphene oxide and gold nanoparticle loading and working solution pH, was performed. The optimum amount of graphene oxide ranged between 1.6 and 18.4 mg, and the golden nanoparticles’ concentration ranged between 164 and 836 ppm. The working pH value was studied in the range 2.0–12.0, as the pH value influences the dissociation equilibrium of citric acid to citrate, with a disruption in the negative charge on citrate leading to the nanoparticles’ aggregation, lowering the golden nanoparticles’ stability. The exploration of the graph of the response surface proved that the highest peak current resulted from the central composite design experiment using 18.4 mg graphene oxide and 836 ppm gold nanoparticles. The working pH did not exert a significant impact on the peak current, given the reduced value of the pH coefficient and the plots of graphene oxide–pH and golden nanoparticles–pH. The results point out that pH acts as an independent variable in the developed technique. Moreover, 7.4 was considered as a pH value compatible with enzyme assays or immunoassays. In differential pulse voltammetry performed on golden nanoparticles/graphene oxide screen-printed carbon electrodes, after condition optimization (0.1 M phosphate buffer pH 7.4, accumulation time of 60 s, and accumulation potential of +0.0 V), carbofuran was detected over a broad linear range of 1–250 µM, with detection and quantitation limits of 0.22 and 0.72 µM, respectively, with application to real samples of cucumber and rice. The technique was characterized by the simplicity of the analytical procedure and was cost-effective and sensitive [113].
A hybrid ferrocene–thiophene modified by carbon nanotube sensor was synthesized by applying Click chemistry to perform the surface modification of the carbon nanotube with thiophene–ferrocene groups. An azide-functionalized carbon nanotube was employed as the core material for surface modification. Different concentrations of the carbon nanotube-modified ferrocene-thiophene hybrid nanosensor (0.5, 1.0, 1.5, and 2.0 mg/mL) were obtained in aqueous medium by ultrasonication at room temperature for 30 min.
The modification was performed by dropping 10 μL of the hybrid ferrocene–thiophene-modified carbon nanotube sensor dispersion on the surface of a glassy carbon electrode, followed by oven drying. The differential pulse voltammetric method was applied to study the electrochemical features of the unmodified glassy carbon electrode, hybrid ferrocene–thiophene-modified carbon nanotube/glassy carbon electrode, and electropolymerized ferrocene–thiophene-modified carbon nanotube on glassy carbon electrode in the presence of 5 μM parathion and chlorantraniliprole, at a pH of 7.0, in phosphate buffer solution. After the addition of 5.0 μM parathion, the electropolymerized ferrocene–thiophene-modified carbon nanotube on glassy carbon electrode nanosensor exhibited one reduction peak at −0.18 V, whereas the bare glassy carbon electrode and the carbon nanotube-modified glassy carbon electrode did not exhibit any significant electrochemical response in the presence of 5.0 μM parathion. The measured peak intensity value of the electropolymerized ferrocene–thiophene-modified carbon nanotube on the glassy carbon electrode sensor was 50.5 μA, corresponding to a cathodic signal present at −0.18 V. The high selectivity of the electropolymerized ferrocene–thiophene-modified carbon nanotube on glassy carbon electrode sensor proved the enzyme-like activity of the hybrid nanosensor, with the most significant responses when compared to the other studied electrodes. Therefore, it can be concluded that the electropolymerized ferrocene–thiophene-modified carbon nanotube on glassy carbon electrode sensor showed improved electrocatalytic activity towards parathion, as compared to the bare glassy carbon electrode, carbon nanotube-modified glassy carbon electrode, and hybrid ferrocene–thiophene-modified carbon nanotube/glassy carbon electrode. Chlorantraniliprole did not show any voltammetric responses at the bare glassy carbon electrode, whereas the carbon nanotube/glassy carbon electrode and the hybrid ferrocene–thiophene-modified carbon nanotube/glassy carbon electrode yielded peak intensities of 4.6 μA and 17.58 μA, respectively. On the other hand, the electropolymerized ferrocene–thiophene-modified carbon nanotube/glassy carbon electrode showed the most significant voltammetric signal for chlorantraniliprole, with a peak intensity of 24.7 μA and positive potential shift of 16 mV. The peak intensity value for the electropolymerized ferrocene–thiophene-modified carbon nanotube/glassy carbon electrode was around 1.40 and 5.37 times more sensitive than that for the hybrid ferrocene–thiophene-modified carbon nanotube/glassy carbon electrode and the carbon nanotube/glassy carbon electrode, respectively, proving the most enhanced electrocatalytic activity. The applications on real samples encompassed the analysis of parathion and chlorantraniliprole in soil, tomato, and apple samples, with good agreement between the spiked and recovered amounts (97.21–104.64%) [114].
Mycotoxins
A sensitive and selective patulin sensor was developed relying on a graphene screen-printed electrode modified with manganese–zinc sulfide quantum dots coated with a patulin molecularly imprinted polymer. The manganese–zinc sulfide quantum dots coated with a patulin molecularly imprinted polymer core–shell highly promote the redox process due to the synergistic interplay between the outstanding electrocatalytic activity and the high electrical conductivity of the manganese–zinc sulfide quantum dots coated with patulin molecularly imprinted polymer that improves the electron transfer between patulin and the electrode surface. Ferrocyanide functioned as a redox mediator for patulin oxidation, promoting sensitivity. Patulin gave well-shaped differential pulse voltammetric peaks at approximately +150 mV. The patulin-imprinted Origami 3D electrochemical paper-based analytical device developed exhibited a broad range of analytical responses, 0.001–25 μM, and a 0.2 nM detection limit. The analytical viability of the Origami 3D-ePAD, as a ready-to-use analytical device in food safety, was confirmed in the assay of fruits and certified reference materials with very good accuracy (interday error of 1.11%) and precision (relative standard deviation smaller than 4.1%) [115].
Feed additives
Microwave-synthesized carbon dots were used as reductants for gold nanoparticle synthesis, in view of the modification of a glassy carbon electrode. Cyclic voltammetry was applied to investigate ractopamine electrochemical behavior, before the analyte’s quantitation by differential pulse voltammetry. Both the glassy carbon electrode and carbon dots/glassy carbon electrode showed no noticeable response, pointing towards the absence of the carbon dots’ influence on the analyte’s electro-reduction. With the presence of carbon dot-reduced gold nanoparticles used for glassy carbon modification, a notable electrochemical response was recorded between 0.2 and 1.0 V, alongside a cathodic (reduction) peak potential located at about 0.5 V. In the presence of 0.2 mg/L ractopamine, the well-defined reduction current confirms the electrocatalytic reduction of the analyte at the carbon dots/golden nanoparticles/glassy carbon electrode. Differential pulse voltammetric determinations showed a linear range of analytical response of 0.01–32.5 mg/L, with an excellent linear correlation coefficient of 0.9999 and a detection limit as low as 1.2 μg/L. The reported sensor is characterized by stability, sensitivity, and good reproducibility.
Given the low ractopamine level in pork meat, which could induce assay errors, recovery tests were applied by adding 0.10 mg/L into the analyzed sample. The average recovery of ractopamine ranged between 96.7 and 102%, proving good accuracy in the pork meat assay [116].
2.4. Square Wave Voltammetry Based on Nanosensors
Preserving agents
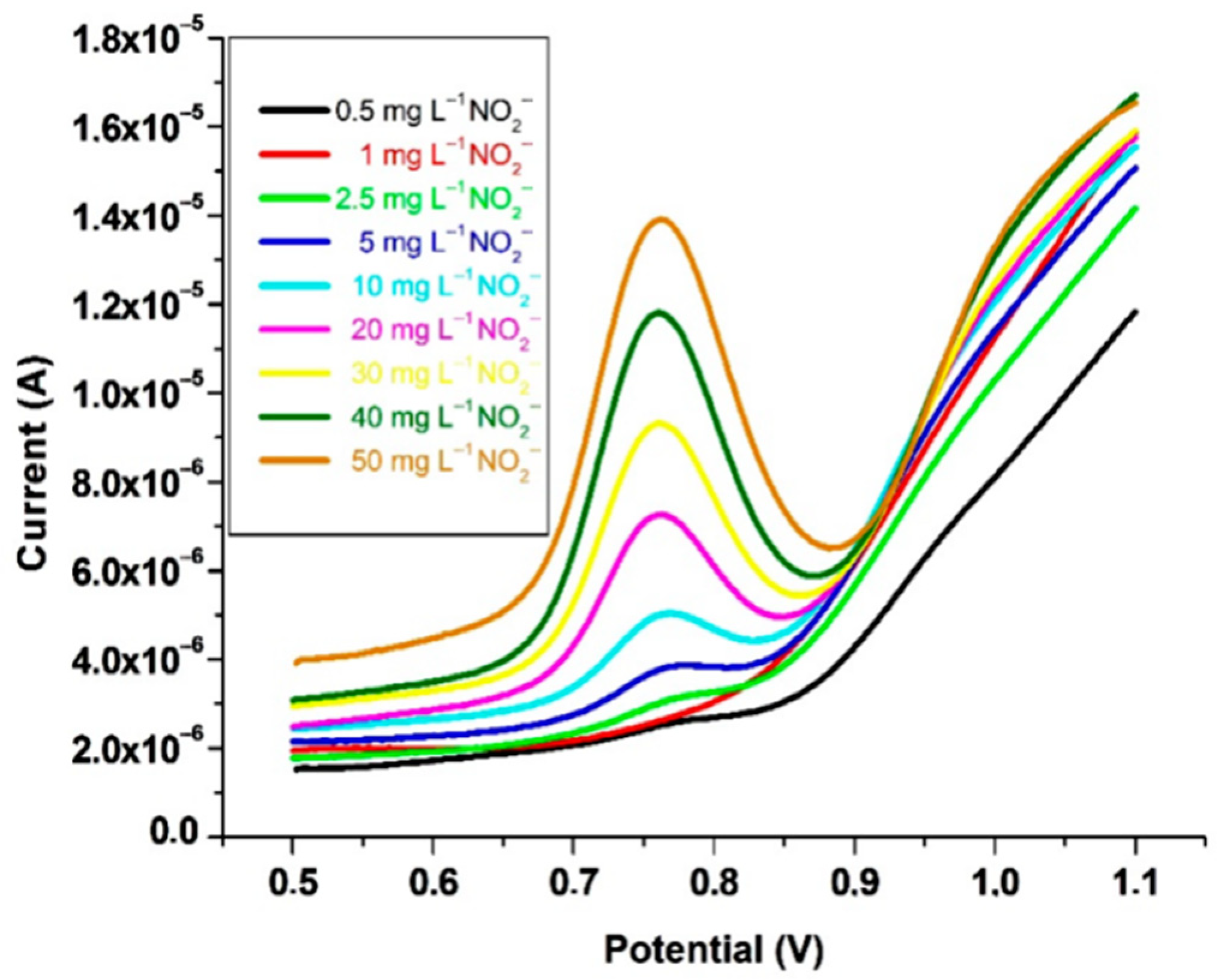
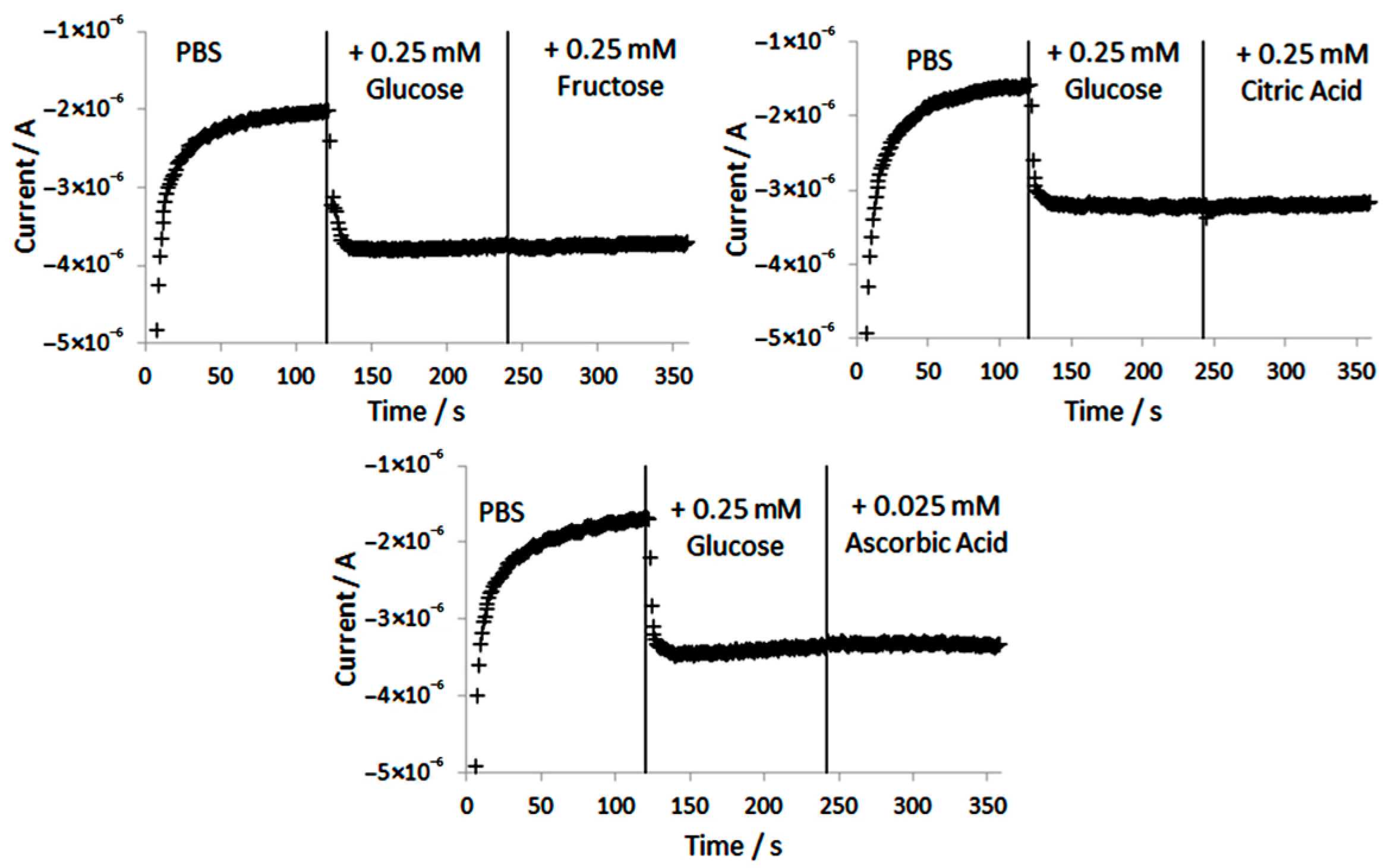
A gold working electrode was functionalized with p-aminothiophenol and modified with gold nanoparticles for the square wave voltammetric assay of nitrite in pH 4 buffer (Figure 10). The developed voltammetric technique showed its analytical viability in the assay of sausages, with results consistent with those provided by colorimetry. The 100 mg·L−1 ascorbic acid gave a square voltammetric signal at 0.97 V, which was close to that of nitrite, 0.76 V. Ascorbic acid oxidase inhibited the ascorbic acid interference, and the accuracy was illustrated by 100.5% nitrite recovery [117].
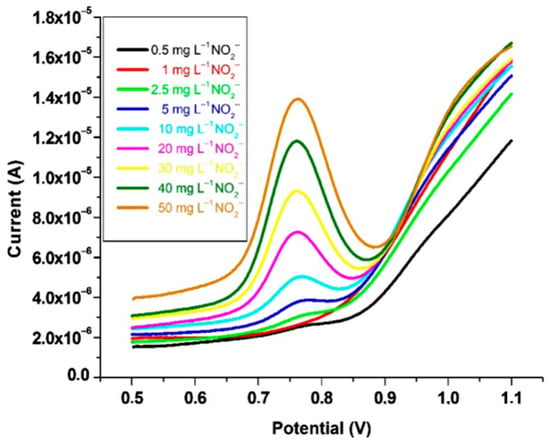
Figure 10.
Square wave voltammograms at gold/polyaminothiophenol–nanogold-modified electrode for 0.5–50 mg·L−1 nitrite, from [117], MDPI 2016.
Food dyes
Studies performed on food dyes revealed that combining carbon-based nanomaterials with metal and metal oxide nanoparticles can often promote the electrocatalytic effect more than metals or metal oxides only. Graphene and mesoporous titanium dioxide were used for carbon paste modification, aiming at dye quantification in traces, by square wave voltammetry. The limits of detection for Tartrazine and Sunset Yellow simultaneous determination were 8 nmoles/L and 6 nmoles/L, respectively, in the assay of sweets and ice cream [118], while in the case of Ponceau 4R and Allura Red, the limits of detection were lower, 1 nmol/L and 0.3 nmol/L for the simultaneous assay [119]. Amaranth, Sunset Yellow, Tartrazine, Ponceau 4R, and Allura Red were determined by DPV and/or SWV, using glassy carbon-modified electrodes with single-walled carbon nanotubes plus titanium nanoparticles [120], graphene plus Fe3O4 nanoparticles [121], graphene plus nickel nanoparticles [122], graphene plus iron/nickel oxide nanoparticles [123], and reduced graphene oxide plus gold nanoparticles [124]. Nevertheless, it is opined that these sensitivities require complex electrode development, which can involve lower robustness [98].
2.5. Anodic Stripping Voltammetry Based on Nanosensors
Heavy metal ions
In another study, a glassy carbon electrode modified with silver nanoparticles, bismuth nanoparticles, multi-walled carbon nanotubes, and Nafion was applied for sensitive Cd(II) and Pb(II) detection, with limits of detection as low as 25.12 ppb and 20.55 ppb, respectively. Cyclic voltammetry was first used to investigate the electrochemical features of the electrode, revealing the nanoparticles’ adherence to the electrode’s surface, an observation consistent with the high anodic peak obtained. The agglomeration of silver nanoparticles significantly affects the stability of Ag+, which can be oxidized to Ag2+, a redox process observed also during the stability study of the electrode. This tendency of the silver monovalent cation to undergo oxidation could be confirmed during the reverse scan on the voltammogram, with the occurrence of two cathodic peaks. The electrode was stable for 50 scans. The deposition, accumulation time, and potential scan rate underwent optimization, with confirmed values of 80 s, 60 s, and 100 mV s−1, respectively. After ashing and digestion in aqua regia (HNO3/HCl = 1/3), the rice samples were analyzed by anodic stripping voltammetry, with contents ranging between 85.52 ppb and 259.44 ppb (in husk) for cadmium and between 124.64 (grain) and 756.23 (stalk) for lead [125].
The simultaneous determination of Sn(II) and Pb(II) was performed by a bismuth nanoparticle-modified screen-printed graphene electrode, on a paper-based analytical device fabricated by a simple analytical procedure, coupled with a portable potentiostat. The optimization of the experimental conditions revealed a 0.25% w/w percentage of bismuth nanoparticles in the graphene ink, a deposition potential of −1.1 V, and a deposition time of 80 s. The best resolution and peak height for both metals were obtained using 0.1 M oxalic acid and 0.2 mM cetyl trimethylammonium bromide in the supporting electrolyte solution. Under optimized conditions, the linear range of the analytical response was 10–250 ng mL−1 for both ionic species, and the limits of detection (as three times the standard deviation divided by the slope) were 0.26 ng mL−1 and 0.44 ng mL−1 for Sn(II) and Pb(II), respectively. This sensor enabled the simultaneous determination of Sn(II) and Pb(II) in canned food samples, mushrooms, and bamboo shoots. After grinding, samples were digested with a 2% v/v nitric acid solution. Subsequently, the pH was brought to 7.0, employing a sodium hydroxide solution, diluted with oxalic acid and cetyltrimethylammonium bromide. The cationic surfactant enabled an improvement in peak separation and promoted the square wave anodic stripping voltammetric sensitive detection of both ions. The results provided by the electrochemical method were in accordance with those obtained by a standardized technique. Hence, it was inferred that the developed electrochemical analytical tool can be amended for the on-site detection of Sn(II) and Pb(II) in real samples [126].
Various polymeric nanocomposite sensors were developed to simultaneously determine Cu2+, Cd2+, and Zn2+. Screen-printed carbon electrodes underwent modification with nanocomposites, which were obtained with a mixture of graphene, graphite oxide, and polymers, such as polyethyleneimide, chitosan, or gelatin. The simultaneous detection of metal ions was performed by square wave anodic stripping voltammetry at the modified electrodes. These polymeric compounds possess -NH2 groups, enabling retention of divalent cations by the nanocomposite. Cyclic voltammetric characterization of the polyethyleneimide/graphite oxide/graphene electrode was performed using a 1 mM ferro-/ferricyanide redox couple in 0.1 M potassium chloride, between 50 mV s−1 and 300 mV s−1. For all modified electrodes, the intensities of the anodic and cathodic current peak increased linearly as the scan speed increased. Also, an Ipa/Ipc ratio close to 1 was noticed for all modified electrodes, pointing towards a diffusion-controlled reversible process.
Polyethyleneimide/graphite oxide/graphene electrode/screen-printed carbon electrodes were characterized by the most important increase in the surface area and an enhanced transfer rate, when compared to the bare electrode. The electrocatalytic effect was improved when compared to the chitosan- and gelatin-modified electrode. Nevertheless, the presence of chitosan imparted a 6 times greater surface area than in the case of gelatin, while the results concerning the transfer coefficient were close. It was found that a hydrogen bond imparts better electron conductivity than a covalent bond. Moreover, the conductance diminishes as the chain length of the hydrogen-bonded molecules augments, pointing towards the role of both molecular weights and hydrogen bridges in affecting the transport properties [127]. These effects were assigned to an improved activity of the graphite oxide/graphene borders and involvement in electron transfer and to the mutual influences of graphite oxide/graphene–polymer [128].
The electrode was introduced into the solution containing the metal ion, aiming at the analytes’ pre-concentration at the electrode’s surface at an applied potential of −1.2 V, for 200 s. After a quiet time of 10 s, square wave anodic stripping voltammetry was performed from −1.2 to 0.6 V at an increment of 4 mV, an amplitude of 60 mV, and a frequency of 25 Hz for the quantitative determination of Zn (II), Cd(II), and Cu(II).
This synergism of polyethyleneimide and graphite oxide/graphene is responsible for the outstanding conductive properties, the functional group enabling interaction with the studied metallic species, an improved surface, and excellent stability. The analytical performance of polyethyleneimide/graphite oxide/graphene/screen-printed carbon electrodes in the determination of the metals under study was assessed by square wave adsorptive stripping voltammetry. The highest analytical signals were obtained at pH 4.5 in 0.25 M acetate buffer solution. Also, the analytical signal increased in the potential range of −1.0 to −1.3 V (with respect to Ag/AgCl), then diminished due to hydrogen release. The best sensitivity, linked to a reasonably short analysis time, was associated with a −1.2 V deposition potential and 200 s deposition time.
Under condition optimization, an increase in the metal ions’ concentration led to a peak current elevation, with a linear range of analytical response of 0.1–50 μg L−1 for Zn(II), Cd(II), and Cu(II). The detection limits calculated relying on the 3σ value of the blank were 0.23 μg L−1, 0.53 μg L−1, and 1.52 μg L−1 for Zn(II), Cd(II), and Cu(II), respectively. The accuracy of the method was proved by the recovery values obtained in the assay of spiked water samples (which included mineral bottled water), ranging between 97% and 104%, and the precision was given by the relative standard deviation smaller than 4.29%. The interferents’ influence investigation was performed in acetate buffer 0.1 M pH 4.5, at a 50.0 μg L−1 concentration of the analyzed ionic species. The tolerance limit was defined as the interferent level, inducing less than 5% error. It was reported that 1000-fold higher weight amounts of Cl−, F−, PO43−, SO42−, Ca2+, Mg2+, K+, and Mn2+ or 500-fold weight ratios of Fe3+, As3+, and Hg2+ did not exert interference on the analysis of Zn(II), Cd(II), and Cu(II). These reported results prove that the sensor is characterized by sensitivity, specificity, and repeatability [66].
2.6. Amperometry Based on Nanosensors
Vitamins
A novel amperometric sensor for ascorbic acid determination relied on the modification of a glassy carbon electrode with polyaspartic acid, carboxylated multi-walled carbon nanotubes, and 1-butyl-3-methylimidazolium hexafluorophosphate as ionic liquid. To attain high analytical performance, optimization of the operating conditions was carried out. An optimum pH of 8.0 was validated, and the linear range corresponded to 5.3–2766.3 μM ascorbic acid, with a sensitivity of 19.64 μA mM−1. The carboxylated multi-walled carbon nanotube/ionic liquid/polyaspartic acid composite imparted outstanding electron transfer ability, a high surface area, and stability, with a low value of the operating potential of 200 mV, contributing to the anti-interference effect. The reported detection limit was 3.0 μM, enabling ascorbic acid determination in tablets, injection solution, and orange juice with accuracy proved by recovery values of between 98.9% and 101.6% [129].
The amperometric determination of thiamine was performed via an electrocatalytic screen-printed sensor, aiming at application in the assay of food supplements. The investigation of the electrocatalytic features revealed the analyte’s ability to be electroactive in its anionic form, the optimal pH value for the assay being 12.0. The screen-printed carbon electrode modified by cobalt phthalocyanine enabled an analytical signal current at a low working potential of 0 V vs. Ag/AgCl to be obtained, compared with +0.34 V for the unmodified screen-printed carbon electrodes, revealing the electrocatalytic ability imparted by the redox mediator. The low detection limit achieved, 6.3 ng mL−1, was partly assigned to the aforementioned low working potential, which gives small background currents. Moreover, constant potential amperometry enabled the use of a low-pass filter, markedly decreasing stirrer noise and allowing low current values to be set on the potentiostat. Controllable steady-state currents were noticed for all the concentrations studied. The method was selective for thiamine in the presence of other vitamins belonging to the B group [130].
Preserving agents
A Ti3C2 nanosheets functionalized with apoferritin–biomimetic platinum nanoparticle-based composite material was synthesized by employing apoferritin as a template and protein-activated biomineralization. The resulting nanohybrid had outstanding electrochemical potential in nitrite sensing. Through self-assembly, the preparation of the core–shell-structured apoferritin–biomimetic platinum nanoparticle was carried out, with subsequent loading onto Ti3C2 nanosheets by bioconjugation. The experimental results proved that the electrochemical biosensor based on the apoferritin–biomimetic platinum nanoparticle/Ti3C2 nanohybrid material was characterized by high sensitivity and selectivity and good stability. The designed apoferritin–biomimetic platinum nanoparticle/Ti3C2-based electrochemical biosensor was characterized by a broad detection range of 0.001–9 mM and a detection limit of 0.425 μM. The anti-interference ability was confirmed by the intensity–time plots obtained for sodium chloride, potassium chloride, calcium chloride, magnesium chloride, cupric chloride, ferric chloride, sodium carbonate, potassium nitrate, silver nitrate, ammonium ion, hydrochloric acid, acetic acid, sodium sulfate, sodium bromide, and sodium monohydrogen phosphate. By performing the synthesis of protein–nanomaterial hybrids, the biocompatibility of the hybrid sensors was promoted, anticipating the applicative viability of 2D bioengineered nanomaterials for complex media. Based on the Chinese national regulations for the nitrite levels in food (≤3 mg/kg for fresh meat, fresh fish, or grains and ≤2 mg/kg for salt), the minimum detection limit of nitrite by the electrochemical biosensor was calculated as 20 μg/kg, considerably lower than the stipulated food standards [14].
The electropolymerization process was applied for the synthesis of a polyneutral red/reduced graphene oxide paste electrode. The reduced graphene oxide nanosheets’ incorporation into the conducting neutral red polymer imparted synergistic effects with an improvement in the nitrite electrochemical response, great selectivity, a sensitivity of 0.01511 µA/µM, and a stable, wide linear range from 0 to 14,000 µM, as revealed by cyclic voltammetry and amperometry. The detection limit was as low as 17 nM. The achieved RSD values (3.56–4.78%) and recovery (90.00–96.66%) validated the applicability on cooked meat samples. The electrocatalytic analytical signal for the polyneutral red and reduced graphene oxide paste electrode showed a significant electrocatalytic response towards nitrite and a low electrocatalytic response in the presence of interferents. The fact that the electropolymerized dye on the reduced graphene oxide paste electrode had electroactive sites interacting with the analyte molecule gave specificity to the nanocomposite [131].
A nanosensor employing poly [2-(methacryloyloxy) ethyl] trimethylammonium chloride decorated with golden nanoparticles immobilized on a glassy carbon electrode was developed for sulfite analysis. The effective coordination capacity of the positively charged quaternary ammonium-based composite, exerted on the negatively charged sulfite anion, was confirmed by a zeta potential assay [132]. Before analyte quantitation using current–time plots, the surface conductivity of the modified glassy carbon electrode was investigated via electrochemical impedance spectroscopy and cyclic voltammetry, proving better performance with respect to the conductivity and charge transfer ability of the modified nanosensor. The peak intensity increased as the pH value increased, reaching an optimum at pH = 7.0, with the bisulfate anion present at acidic values being considered as less oxidizable than SO32− ions, which are more numerous at neutral pH [133]. When the pH value exceeded 7.0, a current decrease was recorded. It was ascribed to a lower number of protons available in the reaction environment. Also, it was found that oxygen-containing chemical species (OH- ions), tightly bonded, impeded the sulfite anions’ adsorption onto the modified electrode. The oxidation of SO32− on the gold nanoparticles–poly [2-(methacryloyloxy) ethyl] trimethylammonium chloride–glassy carbon electrode proceeded via first-order kinetics and followed a stepwise pathway facilitated by the charge interactions. An amperometric quantitative assay of sulfite was carried out, reaching a detection limit of 0.41 ± 0.003 μM (at a signal-to-noise ratio of 3), within a linear range corresponding to 6.66 μM–1020 μM. The modified nanoelectrode revealed excellent stability, repeatability, and specificity in the presence of common interferent species. The relative standard deviation values were 4.20%, 3.45%, and 3.35%, for 1.0 mM, 0.75 mM, and 0.5 mM sulfite levels, respectively. The accuracy was illustrated by the excellent recovery values obtained with tap water analysis of between 97.5% and 99.2% [132].
Mycotoxins
Nanobiosensors relying on gold nanoparticle-functionalized poly (3,4-ethylenedioxythiophene) were designed using layer-by-layer deposition on an indium tin oxide electrode, aiming at the immunoassay of aflatoxin B1. The monoclonal anti-aflatoxin antibodies were immobilized on the surface of poly (3,4-ethylenedioxythiophene)/gold nanoparticles/indium tin oxide using 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide/N-hydroxysuccinimide coupling. Before amperometric quantitation, scanning electron microscopy and contact angle measurements were applied to assess the surface morphology and features of the golden nanoparticles. SEM micrographs showed that poly (3,4-ethylenedioxythiophene) film acts as an efficient substrate that lowers the gold nanoparticles’ tendency to agglomerate. Cyclic voltammetry, electrochemical impedance spectroscopy, and Fourier Transform Infrared Spectroscopy confirmed efficient antibody immobilization. After the golden nanoparticles’ incorporation, a diminution in the electron transfer resistance value, alongside an increase in the peak current, illustrated the excellent electronic features of poly (3,4-ethylenedioxythiophene)-embedded golden nanoparticles. Relying on the anodic and cathodic peak potentials and applying Laviron’s method, the electron transfer coefficient and heterogeneous rate constant proved that the electron transfer between the reaction medium and the electrode is fast at the gold nanoparticles/poly (3,4-ethylenedioxythiophene)/indium tin oxide electrode, which was assigned to the presence of golden nanoparticles that proved to be biocompatible, prone to easy modification, and endowed with catalytic activity. The developed immunosensor showed a highly sensitive amperometric response of 3.72 μA ng mL−1 towards the AFB1 concentration with a linear range of analytical response of 1–25 ng mL−1 and limits of detection and quantification of 0.0045 ng mL−1 and 0.0156 ng mL−1, respectively. The accuracy of the bovine serum albumin/anti-AFB1/gold nanoparticles/poly (3,4-ethylenedioxythiophene)/indium tin oxide electrode was proved by recovery values of 96.13% and 94.5% in the assay of maize samples spiked with 30 ng mL−1 and 50 ng mL−1 AFB1, respectively [134].
Pesticides
Moreover, the amperometric analysis of carbofuran was performed by an immunosensor based on gold nanoparticles, a Prussian blue–multi-walled carbon nanotubes–chitosan nanocomposite film, and protein A layer-by-layer assembly technology. Both the golden nanoparticles and the Prussian blue–multi-walled carbon nanotubes–chitosan nanocomposite incorporated films promoted electroactivity and stability. The porous three-dimensional Prussian blue–multi-walled carbon nanotubes–chitosan nanocomposite film presented many amino and carboxyl groups for protein A cross-linking and also provided a high specific surface area for biorecognition element immobilization. Additionally, the ordered state of protein A in the self-assembled layer promoted its binding capacity to the antibody, also increasing carbofuran binding. The protein A-modified electrode resulted in a broader dynamic range and improved sensitivity, when compared with that lacking protein modification. This was assigned to protein A’s ability to supply functional molecules that can efficiently adsorb the Fc component of the antibody on solid surfaces. The detection limit was 0.021 ng/mL, and the method enabled quantitative determinations in cabbage and lettuce samples, with very good accuracy given by recovery values of 97.1–103.4% [135].
A novel molecularly imprinted polymer-based amperometric sensor integrated into a simple flow-injection system was proposed for the selective analysis of carbofuran in vegetables. For the development of the carbofuran sensor, a carbon paste electrode was modified with carbon nanotubes and gold-coated magnetite covered with a molecularly imprinted polymer. The modification of the transducer by the molecularly imprinted polymer was performed by applying electropolymerization using a 4-ter-butylcalix [8] arene-carbofuran supramolecular complex as the template. O-phenylenediamine was used as a functional monomer. The inclusion of the molecularly imprinted polymer for biorecognition imparted improved electrocatalytic properties to the electrode, increasing the selectivity and surface area, by modulation of the analytical signal via elution and readsorption. The sensor functioned using the amperometric detection mode, under optimized conditions that involved 0.1 M phosphate buffer pH 8.0 as the carrier, a flow rate of 0.5 mL min−1, and an applied potential of +0.50 V. The effect of the flow rate on the analytical signal was studied, in the range 0.1–1.5 mL min−1, showing a sample throughput increase but also a sensitivity decrease with the increase in the flow rate. Nevertheless, a high increase in the flow rate is associated with lowering both the reaction times and signal responses. An optimized flow rate of 0.5 mL min−1 was applied to obtain both appropriate sensitivity and sample throughput. When integrated into the FIA system, the developed imprinted sensor resulted in a linear dynamic range of 0.1–100 µM carbofuran, a sensitivity of 0.001 µA/µM, with a detection limit of 3.8 nM, and a quantification limit of 12.7 nM, calculated as three times the standard deviation of the blank and ten times the standard deviation of the blank, respectively. The sensor was selective towards carbofuran and had suitable precision, given by the relative standard deviation of 4.8%. Before sample analysis, liquid extraction was performed employing a blender and a centrifuge for separation and phosphate buffer as the extraction medium. The method was successfully applied to the carbofuran assay in samples of celery, cabbage, chili, onion, and peppermint. The relative differences between the results obtained with the sensor and those provided by HPLC were in the range 0.2–5.2%.
The influence of some organic interferents, phenol, hydroquinone, and caffeine, as well as inorganic chemical species, such as ammonium sulfate, magnesium sulfate, sodium carbonate, sodium nitrate, potassium chloride, sodium chloride, sodium acetate, and calcium chloride, on the analytical signal corresponding to 10 µM carbofuran was studied. Most of these interferents are commonly found in vegetables or similar samples. The molecularly imprinted polymer provides selectivity due to the presence of recognition or binding sites that are specific to the target analyte. The binding sites are generated during the polymerization step, and the cavities are evidenced after template removal. In this investigation, carbofuran was determined by amperometric detection of its hydrolysate obtained in alkaline medium, carbofuran phenol, so the chemical species chosen for the interference study should possess a structure related to that of carbofuran phenol. The tolerance limit was denoted as the maximum level of a foreign chemical species, resulting in a variance higher than ± 5%. The results showed that a 100-fold excess of magnesium sulfate, sodium carbonate, sodium nitrate, potassium chloride, sodium chloride, sodium acetate, or calcium chloride did not exert a notable influence on the analyte’s response. A 50-fold excess of ammonium sulfate, phenol, or caffeine or a 10-fold excess of hydroquinone did not exert interference on amperomeric carbofuran determination. The authors concluded that the sensor based on the molecularly imprinted polymer is specific, characterized by the simplicity of the procedure, with the sample’s preparation requiring mere extraction, without clean up or a pre-concentration step, with the minimization of organic waste [136].
2.7. Potentiometry Based on Nanosensors
The potentiometric detection mode relies on ion-selective electrodes or field-effect transducers and is characterized by the simplicity of the procedure, minimum sample pre-treatment, a broad dynamic and linear range, portability, multifunctionality, and cost-effectiveness, achieving very low detection limits, with determinations performed in turbid, viscous, or colored solutions [137].
Several heterodiazo dyes can function as ligands, forming stable complexes with some cations, enabling their detection in pharmaceutical samples, foodstuffs, or environmental samples by spectrophotometry [138,139]. Moreover, a ligand reported for its significant sensitivity and selectivity coefficient is 2-(5-Bromo-2-Pyridylazo)-5-[N-n-Propyl-N-(3-sulfopropyl)amino]aniline [140], and it was asserted that it can be incorporated into chitosan electrochemical membrane sensors.
Food nanopolymers
A selective nano-chitosan liquid membrane sensor was developed, relying on the association between chitosan and 2-(5-Bromo-2-Pyridylazo)-5-[N-n-Propyl-N-(3-sulfopropyl)amino]aniline. The characteristic slope of the analytical response was close to the theoretical Nernstian value (54 mV/decade); the wide linear range of the analytical response ranged between 1.0 × 10−7 and 1.0 × 10−2 M, with a limit of detection of 1.47 × 10−8 M, a short response time (10 s), and an extended lifespan (five months). Other key influencing factors investigated were the selectivity towards several inorganic cations and the impact of pH on the electrode potential value.
At pH values outside the 4.0–6.0 range, the dramatic diminution in the potential value was ascribed to the incompleteness of the complexation reaction or to the hydrolysis of chitosan cations [141,142].
At a pH lower than 4.0, the potential increased, which was ascribed to the retention of oxonium ions and nano-chitosan cations at the electrode’s surface. The electrode was successfully employed to quantify nano-chitosan in food supplements and fruit juices containing this nanopolymer. Both the calibration curve method and the standard addition method were applied. The calibration curve method led to better accuracy, revealed by recovery values of between 95.45 and 99.14%, whereas the standard addition method gave lower recoveries: 89.66–96.55%. The method was considered repeatable, the error being lower than 1%, with a Nernstian characteristic slope, a quick response of 10 s, and a reported lifetime of about 5 months [137].
Preserving agents
An extended-gate-type organic field-effect transistor was developed for lactate analysis consisting of 50 nm gold and was synthesized on a 125 μm thick polyethylene naphthalate film substrate by thermal evaporation. The extended-gate electrode of the organic field-effect transistor was modified with lactate oxidase layers and a horseradish peroxidase osmium redox polymer, placed on a flexible plastic film substrate. The change in the drain current over time was followed for progressive changes in the lactate concentration at pH 7.4, in a 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer solution. Before lactate addition, the organic field-effect transistor was operated for 1 h, at a drain–source voltage equal to the gate voltage, namely 2.5 V. The potentiometric sensor was sensitive and selective and responded to the enzyme reaction, with a linear range of analytical response between 0 and 1000 nM. The device exhibited both high selectivity and sensitivity. The control exerted on sensitivity was possible by shielding the enzyme active sites. The limits of detection and quantification were 66 nM and 220 nM, respectively, enabling practical applicability [143].
Sugars
The potentiometric assay of glucose was performed via a platinized paper as support, developed using a 100 nm platinum layer sputtered on conventional filter paper to obtain the redox-sensitive substrate. The biocompatible polymeric membrane comprised polyvinyl alcohol and chitosan, containing the specific oxidase for biorecognition. The sensor relied on the detection of the hydrogen peroxide formed in the enzyme reaction with sensitivity, selectivity, and simplicity of procedure. The sensitivity was −119.6 ± 6.4 mV per concentration decade, the linear range corresponded to 0.03–1.0 mM, and the limit of detection was 0.02 mM, enabling the fast and accurate analysis of commercial fruit juices, with agreement with standard techniques [144].
Metal ions
A highly selective Pb2+ potentiometric PVC membrane sensor was developed, relying on glassy carbon electrode modification with 1,2-Bis(N’-benzoylthioureido) benzene as an ionophore and nanostructured reduced graphene oxide. The sensor exhibited a highly selective analytical response for the lead cation, with a linearity corresponding to 6.31 × 10−8–3.98 × 10−2 M and a Nernstian slope of 30.37 ± 0.62 mV per decade. The sensor functioned in a pH range of 4.0–8.0, with excellent sensitivity given by a detection limit of 2.51 × 10−8 M and a response time as short as 15 s. The match potential method applied in the presence of interfering species proved that the potentiometric sensor was selective. The structure of 1,2-Bis(N’-benzoylthioureido) benzene and its ability to function as a ligand for some common cations were studied via Density Functional Theory calculations. Pb2+ exhibited outstanding affinity for the 1,2-Bis(N’-benzoylthioureido) benzene ionophore. It could be applied to the quantification of the lead cation in raw milk with good agreement with Inductively Coupled Plasma Mass Spectrometry results and to the potentiometric titration of Pb2+ [145].
A derivatized multi-walled carbon nanotube-based carbon paste electrode was developed for the determination of lead ions with a broad linear response ranging from 5.9 × 10−10 to 1.0 × 10−2 M, a detection limit of 3.2 × 10−10 M, and a slope of 29.5 ± 0.3 mV per decade. The electrode operated over a broad pH range (2.5–6.5) with a fast response time, was selective, and could be used for more than 3 months, allowing for lead ion quantitation in samples such as black tea and mineral water [146].
The selective determination of cupric ions was performed via a novel potentiometric sensor relying on a polymeric membrane enclosing 4.0% ionophore, 33.0% poly(vinyl chloride), 63.0% bis(2–ethylhexyl)sebacate, and 1.0% potassium tetrakis(p–chlorophenyl)borate. A Nernstian response was obtained over a broad concentration range of 1.0 × 10−6–1.0 × 10−1 mol L−1 with a slope of 29.6 (±1.2) mV per concentration decade and a detection limit as low as 8.75 × 10−7 mol L−1. The sensor was cost-effective, as the ionophore was synthesized in the laboratory, and had a fast and stable potentiometric response, with good selectivity and repeatability. The working pH range was assessed by employing nitric acid (for the pH range 2.0–7.0) and sodium hydroxide (for the pH range pH 8.0–12.0) solutions. Cupric nitrate at concentrations of 1.0 × 10−2 or 1.0 × 10−3 mol L−1 was added to the aforementioned acid or base solutions, before performing the potentiometric measurements. The pH value did not affect the potentiometric response, in the range 5.0–10.0, with variations being noticed at a pH lower than 5.0 or higher than 9.0. The increased potential at a pH lower than 5.0 was assigned to the sensor’s response to protons. Moreover, a diminution in the recorded potential at a pH higher than 9.0 was ascribed to Cu(OH)2 generation.
The newly proposed sensor can be used as an indicator electrode, for the quantitation of cupric ions via potentiometric titration against EDTA, with application for bottled water samples. It was concluded that the developed potentiometric sensor can be applied with accuracy in resource-limited areas, stressing the significance of the trace elements’ assay, as related to their toxicity [147].
2.8. Conductometry Based on Nanosensors
Sugars
Conductometric sensors measure the changes in the electrolytic conductivity that result from the consumption of reactants or from the occurrence of products. The recorded steady-state response of the conductometric biosensor is due to the rate-limited kinetics of the enzyme reaction and the diffusive flow of the products away from the transducer, in the boundary layer. In the case of glucose determination catalyzed by glucose oxidase, the underlying chemical process gives a pH decrease, and a limit pH value is attained at the transducer’s surface. The developed biosensors rely on conductivity changes resulting from the enzyme-catalyzed oxidation. Gold and magnetic nanoparticles, as well as glucose oxidase adsorbed on poly(allylamine hydrochloride)-modified nanoparticles, were deposited on a planar interdigitated electrode, aiming at a glucose conductometric assay. The 3.03% nanoparticles suspension was homogenized in 100 μL ultrapure water, followed by coating of nanoparticles with a positively charged layer of poly(allylamine hydrochloride). The recovery of the resulting nanobeads was performed by low-intensity magnetic field application. The supernatant rich in poly(allylamine hydrochloride) was discarded, followed by nanoparticle rinsing with ultrapure water under mechanical stirring for 10 min and magnetic field separation [148]. The poly(allylamine hydrochloride)-coated gold nanoparticles and poly(allylamine hydrochloride)-coated magnetic nanoparticles were dispersed in phosphate buffer solution 20 mM, pH = 7.3, containing 6% bovine serum albumin, 4% glucose oxidase, and 10% glycerol, amounts reported after optimization [149].
After mixing for 1 h, these solutions were subject to centrifugation in the case of gold nanoparticles or decantation under magnetic field, for magnetic nanoparticles, followed by redispersion in 20 mM phosphate buffer and deposition of the obtained solution (0.2 μL) on the sensing area of the working electrode. For reference sensor preparation, poly(allylamine hydrochloride)-coated gold and magnetic nanoparticles were dispersed in 20 mM phosphate buffer (pH 7.3) containing 10% of BSA and 10% of glycerol. After separation and redispersion in 20 mM phosphate buffer, 0.2 μL of this solution was deposited onto the sensitive area of the reference sensor. The sensors were exposed to saturated glutaraldehyde vapor for 30 min and then dried at room temperature for 15–30 min and stored at 4 °C when not in use. The enzyme immobilization on the surface of nanoparticles increases the surface density of immobilized glucose oxidase, given the improved surface-to-volume ratio. To study the impact of glucose nanoparticles on the analytical response of the glucose enzyme sensor, the conductometric determinations were carried out using glucose oxidase-poly(allylamine hydrochloride)-coated nanoparticles and the enzyme directly cross-linked on the transducer.
The analytical response increased two times in the presence of nanoparticles: 75 μS, compared to 30 μS, for the sensor having only the enzyme directly cross-linked. The reported dynamic range corresponded to 0.1–1 mM with glucose oxidase-poly(allylamine hydrochloride)-coated magnetic nanoparticles. The limit of detection was 3 μM, calculated as three times the signal-to-background ratio, obtained by linear extrapolation of the calibration graph below 10−4 M, when glucose oxidase-functionalized magnetic nanoparticles were deposited on top of the interdigitated electrodes. The other detection limits were 50 μM when glucose oxidase was directly deposited on top of the interdigitated electrodes and 9 μM when glucose oxidase-functionalized gold nanoparticles were deposited on top of the interdigitated electrodes. Glucose oxidase-functionalized magnetic nanoparticles deposited on top of the interdigitated electrode also yielded best sensitivity in glucose detection, 70 μM/mM, compared to 45 μM/mM when using gold nanoparticles and 30 μM/mM with the enzyme directly cross-linked on the transducer. The intra-sensor reproducibility of the technique was illustrated by a relative standard deviation of 3%. It was reported that the conductometric biosensor prevented Faradaic processes, being prone to application in complex matrices, hampering the influence of redox species where the presence of interferents is expected [148].
2.9. Impedimetry Based on Nanosensors
In a faradaic impedance spectrum, the form of the Nyquist plot over a broad frequency domain encompasses a semicircle (in the region where the electrochemical process is governed by charge transfer) and a straight line (where the electrochemical process is mass transfer-controlled) [150]. The technique is applied to study the resistance to charge transfer, leading to information about the conductive properties of the electrode material and the influence of the electrode modifiers (mediator, biorecognition element, synergistic effect between biocatalyst and nanoparticles), as well as for quantitation purposes.
Preserving agents
Figure 11 depicts the impedance spectra of gold/polyaminothiophenol and gold/polyaminothiophenol–nanogold-modified electrodes. The radius of the semicircle for the gold/polyaminothiophenol transducer was lower when compared to that characterizing the gold/polyaminothiophenol–nanogold-modified electrode, showing the smaller charge transfer resistance and improved electroactivity of the former. This was assigned to the greater thickness of the gold/polyaminothiophenol–nanogold-modified electrode, correlated to an obstruction of the electron transfer by the monolayer coating the electrode. Nevertheless, the authors opined that while the impedance spectra showed moderately increased values of the charge transfer resistance upon gold nanoparticle modification, the gold/polyaminothiophenol–nanogold-modified electrode proved some analytical advantages such as sensitivity, stability, reproducibility, and repeatable application without coating requirement, prior to each application.
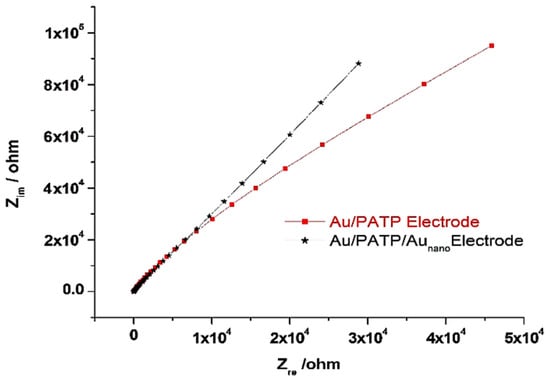
Figure 11.
Impedance measurements performed at the gold/polyaminothiophenol and gold/polyaminothiophenol–nanogold-modified electrodes by applying the potentiostat electrochemical impedance spectroscopy method in monomer-free solution, from [117], MDPI 2016.
These observations can be corroborated with the results obtained by cyclic voltammetry. The anodic and cathodic peak currents for both the gold/polyaminothiophenol and gold/polyaminothiophenol–nanogold-modified electrodes showed that both electrodes are endowed with good electroactivity and that the gold nano-coating did not induce activity lowering. For the gold/polyaminothiophenol electrode, the anodic and cathodic peaks occurred at 517.9 mV and 214.1 mV, respectively, whereas for the gold/polyaminothiophenol–nanogold-modified electrode, there was a mild peak potential shift that corresponded to 502.6 mV and 225.6 mV, respectively. These potential shifts and current increases were assigned to the improvement in the effective surface area of the modified electrode [117].
MoS2 nanosheets obtained by liquid exfoliation and lactate oxidase were used for glassy carbon modification, aiming at lactate biosensing. The glassy carbon/molybdenum disulfide/lactate oxidase biosensor was applied for redox lactate determination, mediated by hydroxymethylferrocene, and electrochemical impedance spectroscopy measurements enabled the study of the charge transfer at the electrode interface [151,152].
MoS2 nanosheets and lactate oxidase proved their synergistic activity in lactate biosensing. Using N-methyl pyrrolidone as the exfoliation solvent and MoS2 particles with a size of 90 nm, at a 7.5 mg mL−1 concentration, yielded the highest biosensor response. Electrochemical impedance spectroscopy studies proved that when the electrode was modified only with molybdenum disulfide, the electron transfer resistance value highly increased, whereas the presence of both molybdenum disulfide and lactate oxidase in the biosensor decreased the elevated electron transfer resistance, promoting the electrocatalytic response towards lactate. The Nyquist plot for the bare glassy carbon electrode showed an electron transfer resistance of 565 Ω. When using only lactate oxidase or molybdenum disulfide as a modifier, this value drastically increased, attaining 78,560 Ω and 154,000 Ω, respectively. In both cases, the electron transfer between the redox probe, ferro/ferricyanide, and the glassy carbon electrode surface was impeded, mainly for the glassy carbon/molybdenum disulfide system. Nevertheless, when the glassy carbon electrode was modified simultaneously with lactate oxidase and molybdenum disulfide, an intermediate value for the charge transfer resistance was recorded (112,000 Ω), proving the role of lactate oxidase in lowering the elevated electron transfer resistance imparted by MoS2 [151].
Pesticides
In another study, electrochemical impedance spectroscopy assays were performed at a bare glassy carbon electrode and at modified nanoelectrodes. The charge transfer resistance value corresponding to the bare electrode was 380 Ω, while that corresponding to the chitosan/glassy carbon electrode was slightly lower, 263 Ω, due to the electrostatic attraction between the positively charged chitosan and the negatively charged ferro-/ferricyanide system. For the reduced graphene oxide–chitosan/glassy carbon electrode, the recorded value was much lower, 74 Ω, which was due to the highly improved conductivity imparted by the reduced graphene oxide film. After the immobilization of the golden nanoparticles on the reduced graphene oxide–chitosan/glassy carbon electrode surface, the electron transfer resistance was further diminished, confirming the outstanding conductivity of golden nanoparticles. On the other hand, reduced graphene oxide accelerates the electron transfer between golden nanoparticles and the glassy carbon electrode.
After 3-carboxyphenylboronic modification on the surface of the golden nanoparticles/reduced graphene oxide–chitosan/glassy carbon electrode, the charge transfer resistance increased to 136 Ω. This was ascribed to the repulsion of carboxyphenylboronic acid by the negatively charged ferro-/ferricyanide system. After acetylcholinesterase immobilization on the surface of the carboxyphenylboronic acid/golden nanoparticles/graphene oxide–chitosan/glassy carbon electrode, the charge transfer resistance markedly increased to 945 Ω, as a result of the interface thickness increase, which showed that acetylcholinesterase underwent successful immobilization onto the carboxyphenylboronic acid/golden nanoparticles/graphene oxide–chitosan/glassy carbon electrode surface. After studying the features of modified electrodes, enzyme inhibition enabled the sensitive assay of organophosphorus and carbamate pesticides [153].
Food dyes
Highly sensitive electrochemical techniques for food dyes can rely on tuning porous carbon attributes. The charge transfer resistance notably diminished at the surface of porous carbon-modified glassy carbon electrodes, pointing towards an improvement in the electron transfer capacities of food colorants due to the presence of porous carbon as a modifier. The oxidation waves obtained at the surface of the porous carbon-modified glassy carbon electrode markedly increased, pointing towards porous carbon activity in food dye oxidation, as proved by electrochemical impedance spectroscopy and chronocoulometry. The authors concluded that the electro-oxidation signals of food dyes markedly increased, with the PC-2 modifier (based on 1:1 starch/nano-CaCO3 weight ratio) resulting in the most sensitive modified electrode, in the detection food dyes largely incorporated into drink samples [109].
Triglycerides
A conductive fluorine-doped tin oxide glass, onto which a sequence of films was deposited, was used for electrochemical detection of triglycerides. The transducer was developed using the sol–gel method and the dip coating technique. First, thin films containing silica and zirconia were deposited, followed by a silsesquioxane ionic film to stabilize and control the gold nanoparticle size, and eventually, Candida rugosa-sourced lipase was immobilized by entrapment in a silica film, to preserve its stability, forming the fluorine-doped tin oxide–silica–zirconia–gold nanoparticle–lipase electrode. Scanning electron microscopy with energy-dispersive X-ray analysis, X-ray photoelectron spectroscopy, UV–Vis spectroscopy, and optical profilometry were applied for film characterization. Cyclic voltammetry using the p-nitrophenyl palmitate as substrate confirmed lipase immobilization on the sensing platform. The analytical tool functioned viably in tributyrin biosensing, by electrochemical impedance spectroscopy, proving a detection limit as low as 1.86 μmol L−1 and an excellent sensitivity of 5.37 μΩ μmol−1 L. The low value of the apparent Michaelis constant of 22.69 μmol L−1 and the maximum apparent value of the rate, 85.57 μmol L−1 min−1, point towards a high affinity of the enzyme for tributyrin and its fast reaction with the substrate.
The film that yielded the best electrochemical response was the silica–zirconia–gold containing 10% silica and synthesized with five dips of silsesquioxane gold nanoparticle dispersion. On this transducer, a silica lipase film was deposited, preserving the activity and operational stability, as observed during p-nitrophenyl palmitate hydrolysis, monitored by cyclic voltammetry. UV–Vis spectroscopy ascertained the presence of ionic silsesquioxane-stabilized gold nanoparticles, with a 6.5 nm diameter. The fluorine-doped tin oxide–silica–zirconia–gold nanoparticle–lipase films were hydrophilic and homogeneous, without noticeable defects. The precision of the determination, as reproducibility, was assessed by electronic impedance spectroscopy, using three distinct electrodes in a solution containing 74.4 μmol L−1 tributyrin. The reported relative standard deviation was 5%, proving reproducibility. At the lower concentrations studied, 59.6 μmol L−1 and 49.7 μmol L−1 tributyrin, the values of the relative standard deviations were 4.9% and 3.6%, respectively.
For the analysis of real samples, the authors used 10 mL of a 0.01 mol L−1 of tributyrin ethanolic solution containing 0.5% surfactant (triton X) and 50 μL sunflower oil. Consequently, a volume of 50 μL of the sample was analyzed by electronic impedance spectrometry. The tributyrin level recovered was determined by applying the standard addition method. For spiked sunflower oil samples, the level of tributyrin recovered was 104%, pointing towards a suitable accuracy [154].
Mycotoxins
A gold electrode was subjected to modification with a cysteine self-assembled layer and carboxyl-functionalized carbon nanotubes covalently attached to cysteine to achieve anti-AFB1 tethering. In the presence of AFB1-containing samples, the analyte’s binding to anti-AFB1 results in a conductive ability change. The presence of carbon nanotubes in the transducer promotes electrical properties, and the charge transfer resistance can be correlated to the AFB1 concentration. The biosensor’s selectivity was tested by using samples containing ochratoxin A. The detection limit was as low as 0.79 pg·g−1, and the linear range of analytical response corresponded to 0.1–20 pg·g−1. The technique enabled the AFB1 assay in aflatoxin-contaminated corn flour at levels so low that they could not be assessed by ELISA. Also, the authors reported that the developed technique can be used for point-of-care testing in AFB1 determination [155].
The analytical platforms based on novel nanomaterials have proved their pivotal role and significance in the assay of key food components. Comparative assessments are focused on the detection mode or on the transducer type.
Voltammetric sensors are considered prominent in the assay of bioactive compounds [87] or in the analysis of food additives like azo dyes [98], given their increased sensitivity, selectivity, simplicity of procedure, and short response time.
Linear scan voltammetric detection techniques like cyclic voltammetry and linear sweep voltammetry are used for studying electrode features, as well as analytes’ electroactivity, but also for quantitation purposes. Differential pulse and square wave voltammetry minimize capacitive current, increasing sensitivity, and allow for the better discrimination of electroactive analytes with close oxidation potentials [101]. The sensing platforms provide precision and selectivity [109]. The recorded oxidation signals are increased due to both electron transfer and accumulation efficiency [98]. One limitation of stepping techniques is the experiment duration, which is higher than in ramping techniques such as amperometry or cyclic voltammetry [101]. In stripping voltammetry, the analyte’s pre-concentration, including from very diluted solutions, allows lower detection ranges than those in direct voltammetry. Before performing the assay, the analyte is accumulated by applying a potential sweep (linear or pulsed), involving passing a Faradaic current, resulting from the oxidation or reduction of the analyte. By extending the accumulation time, the detection limit can be lowered, allowing the determination of contaminants such as heavy metals [156]. Potentiometry has an extended linear range [146], and the selectivity is imparted by the presence of ionophore membranes [157]. It does not require current or potential modulation; nevertheless, alterations in ion activity or temperature can lead to deviations from the Nernst dependence [158].
Conductometric biosensors do not require the integration of a reference electrode and function at low-amplitude alternating voltage, hampering faradaic processes; they are prone to miniaturization and integration with low-cost thin-film technology. Challenges can be the requirement for specificity improvement, the control of the signal-to-noise ratio, and electrode polarization [159].
The type of transducer chosen is essential for designing a high-performance electrochemical sensor. Metal nanoparticles, metal oxide nanoparticles, and carbon-based nanomaterials offer advantages when compared to traditional materials given their uniqueness with respect to electrochemical and mechanical features [152,160].
Electrochemical nano-transducers provide a large active surface area, enabling improved sensitivity, specificity, fast assays, accuracy, and reproducibility, over the analytical range of interest [161].
Bare electrodes are viewed as simpler, more robust, and more stable; nevertheless, higher sensitivity and improved selectivity are provided by modified electrodes and smart sensors [98]. Associating carbon-based nanomaterials with metal and metal oxide nanoparticles can enhance the electrocatalytic activity more than metals or metal oxides only [119]. Nanocomposites prepared from graphene, carbon nanotubes, or metal nanoparticles or mixed nanocomposites can be employed, possessing enhanced electrochemical activity [21]. Nanocomposite-modified screen-printed carbon electrodes can be prepared from graphene, graphite oxide, and polymers, such as polyethyleneimide, gelatin, and chitosan [66].
3. Analytical Performance Obtained for Some Electrochemical Nanosensors Applied to Food Compounds
In this section, the analytical performance obtained in the assays of some food products is presented and discussed. The comprehensive Table 1, Table 2, Table 3 and Table 4 point towards the most important analytical parameters, linear range, relative standard deviation illustrating precision, and limits of detection and determination that characterize the electroanalytical techniques applied to nutrients, bioactives, additives, and contaminants (pesticides, toxins, and heavy metals) present in a variety of analyzed samples. For each analyte category, the analytical methods alongside their performance are systematized—voltammetry, amperometry, potentiometry, and impedimetry. The targeted chemical species are analyzed in different matrices such as fruits and vegetables and derived products (juices), cereal and cereal-based products, milk, wine, beer, herbs, spices, mushrooms, and food supplements.

Table 1.
Analytical performance of some nanosensors applied to the assay of nutrients and bioactives.

Table 2.
Analytical performance of some nanosensors applied to the assay of mycotoxins.

Table 3.
Analytical performance of some nanosensors applied to the assay of pesticides.

Table 4.
Analytical performance of some nanosensors applied to the assay of metal ions.
A broad range of electroanalytical techniques based on various transducers allow for the determination of a plethora of analytes with very good analytical parameters. We notice the ability to adapt the electrode surface with specific nanomaterials or biorecognition elements. Hybrid materials incorporating polymer/metal/carbon nanostructures, or the synergism between the nanomaterial and the biorecognition element, allow for the achievement of good performance, such as a wide linear range, specificity, and sensitivity, with limits of detection down to the ppt level.
Attempts have been made to characterize and compare nanomaterial-based transducers. Metal nanoparticles are characterized by elevated activity, are rich in surface groups, and have tunable surfaces. The disadvantages encountered may be aggregation and elevated costs. Magnetic nanoparticles have tunable surfaces and are prone to functionalization and easy recovery. Nevertheless, the enzyme activity can be affected by magnetic fields. Molecular organic frameworks are environmentally friendly and have high porosity, specific surface area, and tunable surfaces. Reduced stability and elevated costs are the shortcomings involved. Mesoporous silica nanoparticles have high porosity, specific surface area, and tunable surfaces and are environmentally friendly and stable. Nevertheless, the difficulty in processing and the costs may be the disadvantages involved. Carbon-based nanomaterials are endowed with electrical and heat conductivity, specific surface area, and mechanical robustness. Difficulties encountered in functional group modification or processing and high costs are some of the reported shortcomings. Organic–inorganic hybrid nanomaterials are biocompatible, tunable, and prone to easy functionalization but can have low stability and be difficult to process. DNA nanomaterials are biocompatible and highly specific but can have low stability and high costs involved. Polymer nanoparticles are rich in surface groups but can have low stability [221].
Ceramic matrix nanocomposites, metal matrix nanocomposites, and polymer matrix nanocomposites present a series of advantageous properties, when compared to monolithic or microcomposite materials: an increased surface-to-volume ratio compatible with a small filler size and short distance between fillers, ductility without impairing mechanical strength, and enhanced optical features. The disadvantages associated with nanocomposites can be toughness and the influence on the performance that can be linked to nanoparticle inclusion in the bulk composite matrix. Also, a thorough understanding of the formulation/properties/structure interplay is required to achieve particle exfoliation and dispersion [222].
In the case of quantum dots, extensive lifetime may constitute an issue when rapid degradation is necessary [223].
Nanostructures can constitute viable sensing platforms to immobilize various biorecognition elements. Nevertheless, rigorous control of the immobilization conditions may be required for optimal analytical response. Aptamer immobilization at the surface of the platinum nanoparticles/iron-based metal–organic frameworks/glassy carbon electrode led to a faradaic signal inhibition and an increase in the resistance to change transfer, assigned to the obstruction of the electrode surface by aptamer strands and the presence of a negative charge on the phosphate backbone that exerted the rejection of the ferri-/ferrocyanide redox probe. The recorded Δratio increased with the increase in the aptamer amount up to 5.0 µM, which was assigned to the prevention of non-specific adsorption of aptamer molecules and to a favorable aptamer orientation. The Δratio increased with increasing immobilization time up to 12 h and then slowly diminished due to the thorough coverage of the active sites present on the modified electrode surface after 12 h. Moreover, the measured Δratio value of the aptasensor corresponding to aflatoxin M1 in the presence of aflatoxin B1 was important, pointing towards an aflatoxin B1 interference with aflatoxin M1 determination. No ochratoxin interference was noticed, so it was concluded that the impedimetric aptasensor cannot distinguish between aflatoxin types but can perform detection of aflatoxins among other mycotoxins. Also, the developed aptasensor could only be used once for aflatoxin M1 quantification, being considered disposable. In the case of aptasensors, it is considered that their strong binding ability, enhanced selectivity, thermal stability, ease of production and adaptation, low expenses, immunogenicity, and capacity to recognize a broad range of target analytes enable their use as alternatives to antibodies [191].
An impedimetric aptasensor has been developed relying on a gold electrode modified with electropolymerized neutral red and gold nanoparticles suspended in a dendrimeric polymer. The ochratoxin A-specific thiolated aptamer was covalently bonded to golden nanoparticles via Au-S linkage. The aptamer–analyte interaction resulted in an aptamer conformational change involving a transition from linear to guanine quadruplex, with subsequent strengthening of the surface layer and a charge transfer resistance increase. The elevated activity of gold nanoparticles in the signal transduction, as well as the improvement in the aptasensor features and the presence of both poly(neutral red) and thiolated aptamer, allowed for easy aptamer immobilization and ensured a highly sensitive response. The applicability of the aptasensor was confirmed in the assay of spiked dark and light beer samples. Below (Figure 12), the Nyquist plots and sensor calibration are presented [194].
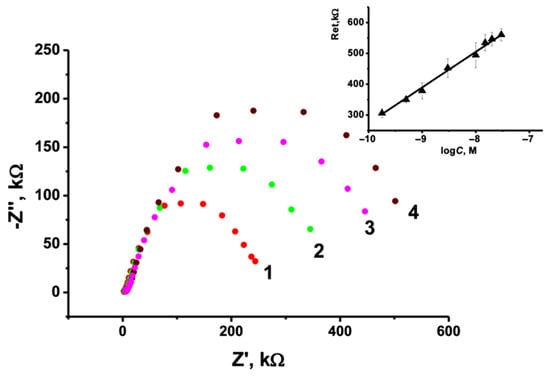
Figure 12.
Nyquist diagrams of impedance spectra obtained prior to (1) and after addition of 1.0 nM (2), 10 nM (3), and 100 nM (4) ochratoxin and the dependence of the charge transfer resistance on the logarithm of the analyte concentration (inset). Measurements were carried out in the presence of 0.01 M potassium ferricyanide and 0.01 M potassium ferrocyanide at 0.235 V vs. Ag/AgCl. Frequency range 0.04 Hz–100 kHz; ac voltage amplitude: 5 mV; from [194], MDPI, 2013.
In the case of the gold nanopopcorns/Nafion–multi-walled carbon nanotube impedimetric aptasensor, the dual amplification of Nafion–multi-walled carbon nanotubes and gold nanopopcorns led to the extremely selective and highly sensitive assay of ochratoxin. The inclusion of Nafion solution allowed for the Nafion–multi-walled carbon nanotubes’ even distribution in the film, stabilizing the sensor and obtaining in situ golden nanopopcorns with a uniform particle size. The improvement in both the conductivity and specific surface enabled loading of significant aptamer amounts, able to recognize and bind high amounts of ochratoxin. The developed gold nanopopcorns/Nafion–multi-walled carbon nanotube impedimetric aptasensor was label-free and had an outstanding sensitivity, proved by an LOD of 1 pg/mL. It was inferred that the developed sensor is an easy to use, inexpensive, accurate, and sensitive solution to quantify more contaminants present in other foodstuffs [195].
A novel nitrogen–copper molecular organic framework endowed with a high specific surface area and electrical conductivity functioned as an electrical signal probe but also as an efficient substrate for aptamer stabilization via amino and copper interactions [181].
Compared with titanium molecular frameworks prepared by using a single ligand, multivariate titanium metal–organic frameworks exhibit a porous structure assembled with multilayered nanosheets, leading to stronger aptamer bioaffinity and enhanced electroactivity [187].
A lead cation-dependent DNAzyme for specific recognition and a porphyrin-functionalized metal–organic framework, as a peroxidase mimic integrated into a highly stable electrochemical sensor, allowed for the single-step assay of this heavy metal in leafy vegetables, down to the parts-per-trillion level, by weight. The specific biorecognition resulted in an efficient anti-jamming capacity against interfering cations [212].
Nanomaterials can lose some exposed active sites due to stacking, and the incorporation of multiple layers of composites was considered a solution to obtain long-term stability. An acetylcholinesterase–chitosan/MXene/gold nanoparticles/MnO2/Mn3O4/glassy carbon electrode methamidophos sensor included a molecular organic framework-derived MnO2/Mn3O4 composite with a layered structure composed of stacked nanosheets. The latter provided an outstanding specific surface area, with excellent electrochemical features and high stability. It was asserted that combining MnO2/Mn3O4 and MXene/gold nanoparticles can lead to synergistic analytical response enhancement in the acetylcholinesterase-based biosensor [196].
In the next figure (Figure 13), the selectivity assay is presented, as exemplified for glucose determination by an amperometric biosensor based on the immobilization of glucose oxidase using a chitosan hydrogel onto highly ordered titanium dioxide nanotube arrays. The presence of potential interferents usually found in analyzed food samples like soft drinks and sauces did not exert a significant impact on the glucose analytical response. The changes in the recorded current intensity were minimal with ascorbic acid addition (reported as a minor interferent). The sensor relied on the cathodic reduction of hydrogen peroxide to water and not on hydrogen peroxide oxidation to oxygen, which causes interferences. Moreover, the presence of chitosan as a barrier promoted high sensitivity. Its significant biocompatibility and affinity for proteins favor enzyme protection [175].
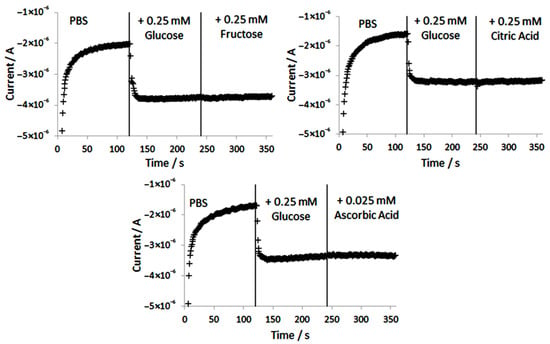
Figure 13.
Amperometric responses of the biosensor recorded in 0.1 M phosphate buffer solution pH 7.0 at a potential of −0.4 V vs. Ag/AgCl in the presence of 0.25 mM glucose and: 0.25 mM fructose (upper left), 0.25 mM citric acid (upper right), 0.025 mM ascorbic acid (below, center), respectively, from [175], MDPI, 2017.
Gold nanoparticles immobilized on the surface of fluorine-doped tin oxide can constitute a bridge for signal amplification via antigen–antibody interaction. The fluorine-doped tin oxide and golden nanoparticles are considered smart transducing elements, providing a synergetic effect and outstanding sensitivity in the femtomolar range, while the antibody imparted specificity to the sensor. Antibodies are considered as less impacted by the variations occurring in the experimental environment, when compared to other biorecognition elements such as enzymes [198].
A gold nanoparticle, poly(ionic liquid), and flavin mononucleotide-decorated carbon nanotube–MoS2 nanosheet composite was prepared for an ochratoxin assay, and the molecularly imprinted polymer for biorecognition was electro-synthesized via cyclic voltammetry. During electropolymerization, a magnetic field could promote the molecularly imprinted polymer orientation and the generation of uniform imprinting sites, allowing for the efficient recognition and rebinding of ochratoxin [190].
The figure below (Figure 14) is representative of the detailed voltammetric responses and analytical parameters which are obtained by using a hybrid sensor based on gold nanoparticles immobilized in a molecularly imprinted D-gluconate polymer. The method enabled the sensitive assay of gluconic acid, with application in Italian red wine, with good recoveries of up to 102.2% [167].
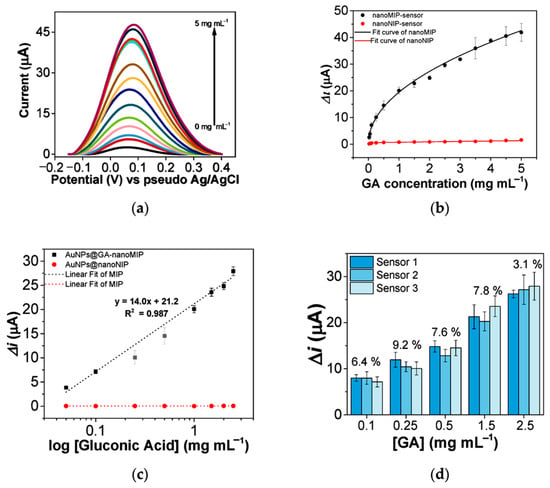
Figure 14.
(a) DPV responses at increasing analyte concentrations (0.025–5 mg/mL), in phosphate buffer solution, 5 mM, pH = 7.40. (b) Calibration curves obtained for the hybrid sensor (nanoMIP) and for the control sensor (non-imprinted, synthesized in an identical manner but in the absence of a template, denoted as nanoNIP). (c) Working linear range of sensors on a logarithmic scale (0.05–2.5 mg/mL concentration range). (d) Inter-sensor reproducibility for three hybrid sensors based on gold nanoparticles immobilized in the molecularly imprinted polymer (AuNPs@GA-nanoMIP sensors), determined at different concentrations, from [167], MDPI, 2025.
A glassy carbon electrode was modified with poly(amidoamine) dendrimers and functionalized with magnetic graphene oxide for the simultaneous determination of lead and cadmium ions. Such dendrimers are rich in amino groups and binding sites and have uniform morphology and versatility, promoting heavy metal ion extraction from the analyzed water samples. Graphene oxide nanosheets have good electronic properties; magnetite nanoparticles are endowed with adsorption capability, and, associated with the dendrimer selectivity, lead to sensitivity and specificity with minimized matrix effects [224].
4. Critical Conclusions and Future Outlook
Nanomaterials are applied in the food industry [225]. In addition to their use in food packaging [226] and in the screening of food products for pathogens [227], nanomaterials have been incorporated in sensors used for the assay of food nutrients, bioactives, additives, contaminants, and toxins [228].
Compared to traditional sensors, nanosensors provide enhanced sensitivity and specificity, accuracy, and precision [229]. They require lower power use, detect low analyte concentrations, and are characterized by a diminished distance between the target and sensor contact [230]. Nevertheless, nanosensor fabrication and handling are more complex than in a case of a larger one, and more expensive instrumentation/equipment may be required for nanoscale precision [231].
In nanobiosensors, the essential characteristics are the configuration, the immobilization efficiency and bioactivity maintenance, suitable for the biorecognition element, and the elevated conductivity of nano-tranducers [232].
Electrochemical (bio)sensors are regarded as a multibillion-dollar industry, employing portable electrodes, characterized by reproducibility, selectivity, cost-effectiveness, and ease of application on a large scale [99].
First-generation amperometric biosensors, with the enzyme directly immobilized on the transducer, were described as sensitive and rapidly responsive. Nevertheless, they operate at high potential values, may require the involvement of coenzymes, and suffer from the transducer’s fouling in the case of prolonged use. In second-generation enzyme sensors, mediators interact with the biocatalyst at the active site and then transfer electrons at the electrode’s surface. For instance, if the biocatalyzed reaction is an oxidation one, after the biorecognition event, the mediator is reduced and transported to the electrode surface and reconverted to its oxidized form [233].
The redox processes involving mediators (ferrocyanide, ferrocene derivatives, quinones, or conducting organic salts) take place at a lower potential than in the case of traditional co-substrates or products of enzyme reactions. In voltammetric and amperometric assays, mediators enable a decrease in the working potential versus unmodified electrodes, hampering the interferent effects [151,234].
Although the analytical response of the second-generation biosensors is oxygen-independent, leaching or interference of the redox mediator may constitute issues, caused by mediator selectivity. Third-generation biosensors function relying on the direct electron transfer between the enzyme and the electrode surface, without mediator or co-substrate involvement in the catalyzed conversion of the substrate. The electrocatalytic role of the redox enzyme promotes the electron transfer between the electrode surface and the analyte molecule [233].
Nanomaterials are suitable carriers to immobilize and co-immobilize enzymes, are characterized by adjustable dimensions and morphology, have an increased surface area, and are rich in active sites. Nanomaterials promote the efficacy of the biocatalyst and its stability and activity. In particular, nanozymes are characterized by increased catalytic activity and selectivity, enhancing the speed of biocatalytic reactions at the microscopic scale. Nevertheless, lowered enzyme efficiency can occur, given the spatial constraints, in the case of compartmentalized or positional co-immobilization [221]. Titania/electro-reduced graphene oxide nanohybrids highly increased the electrocatalytic activity and analytical signal of dyes, proved in the differential pulse voltammetric assay of Allura Red in milk drinks [235], as well as Ponceau 4R and Tartrazine in orange juice, with a sensitivity at the nanomolar level [236].
When compared to conventional carriers, nanocarriers are characterized by better chemical and thermal stability, withstanding degradation and enzyme inactivation. Nanomaterials play a protective role, extend the enzyme lifetime, and enhance the stability and recyclability of co-immobilized enzymes. Nanocarriers are biocompatible and can undergo surface modification/functionalization by introducing particular functional groups to improve both their interaction with enzymes and biocatalyst immobilization. Nanomaterials have a high specific surface area providing a larger number of sites for enzyme immobilization, in comparison to traditional carriers. This improves both the loading and density of the immobilized biocatalyst. Given the outstanding mass transfer properties, enzymes immobilized on nanocarriers can more effectively interact with the substrate, significantly promoting the catalytic efficacy [221].
Nevertheless, the toxicity and biocompatibility of nanomaterials should be considered when designing nanosensors. Another aspect that can impede the extended use of nanomaterials can be the cost. It is thought that adequate preparation techniques, associated with stability and biocompatibility testing, should be correlated with the integration of nanomaterials into enzyme sensors [221].
When applying nanostructures for electrode modification, the biocatalyst–nanoparticle interplay and the interface or coating layer thickness should be considered as aspects impacting electroactivity and the magnitude of the analytical response. Acetylcholinesterase immobilization on the surface of a carboxyphenylboronic acid/golden nanoparticles/graphene oxide–chitosan/glassy carbon electrode led to an increase in the charge transfer resistance, as a result of the interface thickness increase, but also proved the efficient biocatalyst immobilization on the carboxyphenylboronic acid/golden nanoparticles/graphene oxide–chitosan/glassy carbon electrode surface. Nevertheless, when compared to the bare glassy carbon electrode and to the other modified electrodes developed in the investigation, the highest oxidation current was achieved by the acetylcholinesterase/3-carboxyphenylboronic/gold nanoparticles/reduced graphene oxide sheets/glassy carbon electrode, with a peak potential of + 685 mV. It was concluded that the synergism of the reduced graphene oxide and golden nanoparticles enabled efficient electronic transport, resulting in high stability and repeatability, with a detection limit as low as 0.05 ppb for carbofuran [153]. In another study, MoS2 nanosheets and lactate oxidase, by their use in glassy carbon electrode modification, proved their synergistic and electrocatalytic activity in lactate biosensing, promoting electron transfer and analytical response. Lactate oxidase catalyzes the conversion of lactate to pyruvate, with the electrons which are part of the process being readily transferred to the soluble, oxidized form of the mediator, restoring the enzyme activity. Hydroxymethylferrocene re-oxidation at the electrode surface resulted in an analytical response proportional to the analyte amount. The contribution of interfering substances that could be oxidized at the elevated potential necessary for hydrogen peroxide (product of the enzyme reaction) was minimized, as the detection potential was lowered, +0.30 V, given the exploitation of the electrochemical activity of the mediator. Also, the biocatalyst diminished the charge transfer resistance imparted by molybdenum disulfide [151]. In some cases, gold nanoparticle modification can lead to an increase in the charge transfer resistance, as proved by a comparative study involving gold/polyaminothiophenol–nanogold-modified and gold/polyaminothiophenol electrodes; the observation was correlated to an electron transfer obstruction in the presence of the coating monolayer. However, it was reported that the gold/polyaminothiophenol–nanogold-modified electrode had improved analytical parameters like sensitivity, stability, reproducibility, and repeatability [117].
When preparing nanocomposites, the use of toxic chemicals and energy-consuming steps should be lowered. Environmental concerns have led to the application of green synthesis, which employs plant extracts or microorganisms. Cellulose nanostructures (nanofibers or nanocrystals) can be combined with quantum dots [237] or with carbon-based nanomaterials [238] to obtain sensitive, biodegradable, selective sensors, lowering the impact on the environment. Biogenic synthesis employs microorganisms like bacteria and fungi to obtain nanocomposites. Metal ions can be bioaccumulated by microorganisms that are able to synthesize nanoparticles inside the cell, providing a green, biocompatible pathway for nanocomposite manufacture, with the possibility to recycle waste materials [237].
Advanced materials like gold nanoparticles and carbon nanotubes are pivotal in increasing performance, but it was opined that this may involve production expenses, hindering accessibility in resource-limited environments. To solve these issues, research into cost-effective alternatives and additional synthesis techniques can lower material expenditure and facilitate manufacturing processes. Moreover, the impact of environmental conditions should be considered from all standpoints; nanosensor degradation may be affected by various conditions such as moisture, light, or temperature. It is thought that novel strategies should focus on using more resilient coatings and packaging to ensure stability without affecting the analytical performance in different matrices. Another issue is constituted by standardization and scalability. Maintaining analytical parameters to ensure reproducibility during the transition from laboratory- to industrial-scale production may constitute a challenge. Standardization and production on a large scale may be hindered by inconsistency in material characteristics such as dimensions and surface chemistry [21].
The analytical parameters depend on the method chosen, the transducer type, and the biorecognition element, as well as on the features of the analyzed matrix. Expenses and environmental concerns should be taken into account when developing nanosensors, as well as the possibility for large-scale application.
In a novel study, a green nanocomposite based on reduced graphene oxide and magnetite was prepared using corn silk extract as a reductant for graphene oxide. The nitrate reductase/green magnetite–reduced graphene oxide/indium tin oxide nanosensor exhibited outstanding analytical performance such as an excellent sensitivity of 122.1 Ohm/log (mg/L)/cm2 and a low detection limit of 0.076 mg/L for nitrate detection in leafy vegetables. The results obtained using the green nanosensor showed consistency with those provided by HPLC, proving market compliance and nanosensor efficacy in food quality and safety monitoring [239].
Green sensors diminish the environmental impact via the restricted use of hazardous chemicals and reduced waste amounts and energy expenditure. It is believed that the application of standardized green assays linked to environmental friendly technology should be encouraged [240]. Biomaterials are related to the development of portable, miniaturized, and green sensors, applied to the analysis of complex matrixes, under conditions that can be difficult for conventional sensing platforms [241]. The integration of green and microfluidic technologies enables low-volume sample assays, accurate control, and the possibility to simultaneously perform multiple analyses. The main challenges, nonetheless, related to the electrochemical assay of complex samples are considered to be electrode fouling and interference occurrence [242].
Performance improvement can be achieved by using appropriate modifiers to confer stability and a larger surface area, promoting electrocatalytic features. Ligands or different capping agents may be required for the transducer’s specificity and selectivity [243]. Commonly incorporated modifiers include metal nanomaterials and composites, carbon-based compounds, and conductive polymers, lowering interference effects and enhancing selectivity and sensitivity [244].
Before application in various food samples, validation using certified reference materials and comparison with established (reference) methodologies is recommended [245].
Lab-on-a-chip techniques integrate microfluidics and microchips to promote functionality by improving mass transport, lowering sample volume and interferences. The incorporation of microfluidics in electrochemical sensors allows for the sensitive and rapid detection of analytes like heavy metal ions, providing accurate and reliable results [246]. Nevertheless, a comprehensive evaluation of nanosensors’ viability in industrial applications is needed, mainly related to costs and scalability [224].
Metal nanoparticle-based sensors are prone to selectivity and sensitivity improvement via individualized signal amplifications. The interest in nanosensor development is mainly focused on metal nanoparticles, bio-functionalized nanoparticles, and nanocomposites [230].
Electrochemical sensors incorporating biopolymers like cellulose, alginate, chitosan, gelatin, and keratin represent environmentally friendly tools, promoting characteristics like efficacy and sustainability in sensing platforms, including food assays. Biopolymer-based nanocomposites synthesized via physical, chemical, or green methods target the enhancement in sensor performance by integrating conductive materials like metal oxides, graphene, or nanoparticles. Although biopolymers impart benefits, some issues need to be overcome in electrochemical sensors. Analytical performance such as stability can be influenced by their sensitivity to environmental conditions, including moisture and temperature. To preserve the performance and biocompatibility, smart synthesis and functionalization methods would be required, taking advantage of their tunability and flexibility. Biopolymers can be easily combined with carbon nanotubes, graphene, or metal nanoparticles, leading to composite sensors with increased performance [247].
Future trends encompass the integration of nanosensors with digital analytical platforms appliable for fast on-site analysis, exploiting the functionalization strategies, versatility, and transformative capabilities of nanosensors, related to ensuring health and food safety. Advanced electrode development techniques, encompassing electrochemical deposition and 3D printing, can promote enhanced scalability and reproducibility. Their portability can further promote their applicability in regular food safety control. Adapting the surface chemistry and morphologic features of nanomaterials enables the promotion of sensor performance in sensitivity, signal response, and stability. The use of multifunctional sensing platforms, machine learning, and AI integration with electrochemical assays can increase accuracy and versatility, automate signal processing, and promote flexible real-time sensor recalibration [87].
It is thought that integration within miniaturized microfluidic instruments that use on-chip sample handling, as well as electronic, control, and analysis systems, can further enhance sensing properties. Simplicity, portability, cost-effectiveness, a lack of toxicity, disposability, and adaptability are expected. Advanced technology that encompasses the Internet of Things, deep learning, cloud computing, cyber–physical systems, data analysis, and AI can lead to the development of commercial products, as the emphasis on integration and miniaturization can extend the applicability of nanosensors [86].
Author Contributions
A.M.P.: Conceptualization, Validation, Writing—Original Draft, Writing—Review and Editing, and Supervision. F.I.: Conceptualization, Validation, and Writing—Original Draft. L.S.: Conceptualization, Supervision, and Writing—Original Draft. P.M.R.: Supervision, Visualization, and Writing—Review and Editing. N.C.: Supervision, Validation, and Writing—Review and Editing. O.I.G.: Supervision, Visualization, and Writing—Review and Editing. L.B.: Validation, Supervision, and Writing—Review and Editing. A.I.S.: Conceptualization, Validation, Supervision, and Writing—Original Draft. All authors have read and agreed to the published version of the manuscript.
Funding
No funding supported this paper.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare the absence of conflicts of interest.
References
- Darwish, M.A.; Abd-Elaziem, W.; Elsheikh, A.; Zayed, A.A. Advancements in nanomaterials for nanosensors: A comprehensive review. Nanoscale Adv. 2024, 6, 4015–4046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, I.; Ezaz, G.; Ammara, N.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 223–245. [Google Scholar] [CrossRef]
- Khan, Y.; Sadia, H.; Ali Shah, S.Z.; Khan, M.N.; Shah, A.A.; Ullah, N.; Ullah, M.F.; Bibi, H.; Bafakeeh, O.T.; Khedher, N.B.; et al. Classification, Synthetic, and Characterization Approaches to Nanoparticles, and Their Applications in Various Fields of Nanotechnology: A Review. Catalysts 2022, 12, 1386. [Google Scholar] [CrossRef]
- Mekuye, B.; Abera, B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Sel. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Ghazizadeh, E.; Nasery, Z. The viewpoint of nanolipid vesicles (liposomes, exosomes, and microvesicles) as biosensors in medical health advances. Front. Nanotechnol. 2023, 5, 1230407. [Google Scholar] [CrossRef]
- Biswas, N.G.; Das, M.K. Chapter 1—Introduction to nanotechnology in personal care products. In Nanocosmeceuticals; Das, M.K., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 3–29. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Turcuş, V.; Predoi, G.; Iordache, F. Nanoencapsulation techniques for compounds and products with antioxidant and antimicrobial activity—A critical view. Eur. J. Med. Chem. 2018, 157, 1326–1345. [Google Scholar] [CrossRef] [PubMed]
- Musielak, E.; Feliczak-Guzik, A.; Nowak, I. Synthesis and Potential Applications of Lipid Nanoparticles in Medicine. Materials 2022, 15, 682. [Google Scholar] [CrossRef] [PubMed]
- Lippacher, A.; Müller, R.H.; Mäder, K. Investigation on the viscoelastic properties of lipid based colloidal drug carriers. Int. J. Pharm. 2000, 196, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Chirio, D.; Peira, E.; Dianzani, C.; Muntoni, E.; Gigliotti, C.L.; Ferrara, B.; Sapino, S.; Chindamo, G.; Gallarate, M. Development of Solid Lipid Nanoparticles by Cold Dilution of Microemulsions: Curcumin Loading, Preliminary In Vitro Studies, and Biodistribution. Nanomaterials 2019, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.; Jain, K. Dermal and Transdermal Drug Delivery through Vesicles and Particles: Preparation and Applications. Adv. Pharm. Bull. 2022, 12, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Eksin, E.; Kesici, E.; Yaralı, E. Chapter 10—Dendrimers Integrated Biosensors for Healthcare Applications. In Nanotechnology and Biosensors; Nikolelis, D.P., Nikoleli, G.-P., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 307–317. [Google Scholar] [CrossRef]
- Mu, R.; Zhu, D.; Wei, G. Ti3C2 Nanosheets Functionalized with Ferritin–Biomimetic Platinum Nanoparticles for Electrochemical Biosensors of Nitrite. Biosensors 2024, 14, 258. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Impact of Nanoparticles on Brain Health: An Up to Date Overview. J. Clin. Med. 2018, 7, 490. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Anjum, S.; Hano, C.; Anjum, I.; Abbasi, B.H. An Overview of the Applications of Nanomaterials and Nanodevices in the Food Industry. Foods 2020, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.J.; Abedin, M.J.; Rahman, M.Z. 7.15—Nanoparticles, nanocomposites, green/eco-composites, and hybrid composites and their applications in energy sectors. In Comprehensive Materials Processing (Second Edition); Hashmi, S., Ed.; Elsevier: Oxford, UK, 2024; pp. 321–339. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Gutierrez, J.A.; Silber, J.J.; Falcone, R.D.; Correa, N.M. Modified reverse micelle method as facile way to obtain several gold nanoparticle morphologies. J. Mol. Liq. 2021, 331, 115709. [Google Scholar] [CrossRef]
- Khalil, M.; Jan, B.M.; Tong, C.W.; Berawi, M.A. Advanced nanomaterials in oil and gas industry: Design, application and challenges. Appl. Energy 2017, 191, 287–310. [Google Scholar] [CrossRef]
- Awlqadr, F.H.; Altemimi, A.B.; Qadir, S.A.; Hama Salih, T.A.; Alkanan, Z.T.; AlKaisy, Q.H.; Mohammed, O.A.; Hesarinejad, M.A. Emerging trends in nano-sensors: A new frontier in food safety and quality assurance. Heliyon 2025, 11, e41181. [Google Scholar] [CrossRef] [PubMed]
- Schiopu, A.-G.; Iordache, D.M.; Oproescu, M.; Cursaru, L.M.; Ioța, A.-M. Tailoring the Synthesis Method of Metal Oxide Nanoparticles for Desired Properties. Crystals 2024, 14, 899. [Google Scholar] [CrossRef]
- Negrescu, A.M.; Killian, M.S.; Raghu, S.N.V.; Schmuki, P.; Mazare, A.; Cimpean, A. Metal Oxide Nanoparticles: Review of Synthesis, Characterization and Biological Effects. J. Funct. Biomater. 2022, 13, 274. [Google Scholar] [CrossRef] [PubMed]
- Alsaiari, N.S.; Alzahrani, F.M.; Amari, A.; Osman, H.; Harharah, H.N.; Elboughdiri, N.; Tahoon, M.A. Plant and Microbial Approaches as Green Methods for the Synthesis of Nanomaterials: Synthesis, Applications, and Future Perspectives. Molecules 2023, 28, 463. [Google Scholar] [CrossRef] [PubMed]
- Letchumanan, D.; Sok, S.P.M.; Ibrahim, S.; Nagoor, N.H.; Arshad, N.M. Plant-Based Biosynthesis of Copper/Copper Oxide Nanoparticles: An Update on Their Applications in Biomedicine, Mechanisms, and Toxicity. Biomolecules 2021, 11, 564. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Singh, B.; Behera, S.P. Chapter 7—Green approaches for nanoparticle synthesis: Emerging trends. In Nanomaterials; Kumar, R.P., Bharathiraja, B., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 167–193. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, A.; Chandel, M.; Pathania, D. Chapter 9—Modern applications and current status of green nanotechnology in environmental industry. In Green Functionalized Nanomaterials for Environmental Applications; Shanker, U., Hussain, C.M., Rani, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 259–281. [Google Scholar] [CrossRef]
- Arbain, R.; Othman, M.; Palaniandy, S. Preparation of iron oxide nanoparticles by mechanical milling. Miner. Eng. 2011, 24, 1–9. [Google Scholar] [CrossRef]
- Nayak, M.K.; Singh, J.; Singh, B.; Soni, S.; Pandey, V.S.; Tyagi, S. Chapter 1—Introduction to semiconductor nanomaterial and its optical and electronics properties. In Metal Semiconductor Core-Shell Nanostructures for Energy and Environmental Applications; Gupta, R.K., Misra, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–33. [Google Scholar] [CrossRef]
- Almeida, G.; van der Poll, L.; Evers, W.H.; Szoboszlai, E.; Vonk, S.J.W.; Rabouw, F.T.; Houtepen, A.J. Size-Dependent Optical Properties of InP Colloidal Quantum Dots. Nano Lett. 2023, 23, 8697–8703. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Beimborn, J.C., II; Kirkwood, N.; Russo, S.P.; Weber, J.M.; Mulvaney, P. Size-Dependent Response of CdSe Quantum Dots to Hydrostatic Pressure. J. Phys. Chem. C 2023, 127, 8657–8669. [Google Scholar] [CrossRef]
- Uprety, B.; Abrahamse, H. Semiconductor quantum dots for photodynamic therapy: Recent advances. Front. Chem. 2022, 10, 946574. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Dev, A.; Karmakar, S. Nanosensors and nanobiosensors in food and agriculture. Environ. Chem. Lett. 2018, 16, 161–182. [Google Scholar] [CrossRef]
- Cheong, I.T.; Yang Szepesvari, L.; Ni, C.; Butler, C.; O’Connor, K.M.; Hooper, R.; Meldrum, A.; Veinot, J.G.C. Not all silicon quantum dots are equal: Photostability of silicon quantum dots with and without a thick amorphous shell. Nanoscale 2024, 16, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Hosamo, H.; Ying, C.; Nie, R. A Comprehensive Review and Analysis of Nanosensors for Structural Health Monitoring in Bridge Maintenance: Innovations, Challenges, and Future Perspectives. Appl. Sci. 2023, 13, 11149. [Google Scholar] [CrossRef]
- Jaleh, B.; Nasrollahzadeh, M.; Eslamipanah, M.; Nasri, A.; Shabanlou, E.; Manwar, N.R.; Zboril, R.; Fornasiero, P.; Gawande, M.B. The Role of Carbon-Based Materials for Fuel Cells Performance. Carbon 2022, 198, 301–352. [Google Scholar] [CrossRef]
- Chung, S.-H.; Kim, H.; Jeong, S.W. Improved thermal conductivity of carbon-based thermal interface materials by high-magnetic-field alignment. Carbon 2018, 140, 24–29. [Google Scholar] [CrossRef]
- Abd-Elaziem, W.; Elkatatny, S.; Sebaey, T.A.; Darwish, M.A.; Abd El-Baky, M.A.; Hamada, A. Machine learning for advancing laser powder bed fusion of stainless steel. J. Mater. Res. Technol. 2024, 30, 4986–5016. [Google Scholar] [CrossRef]
- Ray, S.C.; Jana, N.R. Chapter 5—Application of Carbon-Based Nanomaterials as Drug and Gene Delivery Carrier. In Carbon Nanomaterials for Biological and Medical Applications; Ray, S.C., Jana, N.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 163–203. [Google Scholar] [CrossRef]
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R.; Khan, A.u. Graphene synthesis, characterization and its applications: A review. Results Chem. 2021, 3, 100163. [Google Scholar] [CrossRef]
- Kurbanoglu, S.; Ozkan, S.A. Electrochemical carbon based nanosensors: A promising tool in pharmaceutical and biomedical analysis. J. Pharm. Biomed. Anal. 2018, 147, 439–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yi, L.; Huang, F.; Huang, Q.; Zhou, T. Facile synthesis of graphene nanosheets on wastewater sediments for high efficient adsorption of methylene blue. Sep. Purif. Technol. 2024, 337, 126366. [Google Scholar] [CrossRef]
- Paramasivan, T.; Sivarajasekar, N.; Muthusaravanan, S.; Subashini, R.; Prakashmaran, J.; Sivamani, S.; Ajmal Koya, P. Graphene Family Materials for the Removal of Pesticides from Water. In A New Generation Material Graphene: Applications in Water Technology; Naushad, M., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 309–327. [Google Scholar] [CrossRef]
- Huang, R.; Guo, H.; Gu, Z.; Ling, Y. Advances in laser processed material of soft sensing and soft actuation. Mater. Today Commun. 2023, 37, 107187. [Google Scholar] [CrossRef]
- First, P.N.; de Heer, W.A.; Seyller, T.; Berger, C.; Stroscio, J.A.; Moon, J.-S. Epitaxial Graphenes on Silicon Carbide. MRS Bull. 2010, 35, 296–305. [Google Scholar] [CrossRef]
- Hong, X.; Yu, W.; Wang, A.; Chung, D.D.L. Graphite oxide paper as a polarizable electrical conductor in the through-thickness direction. Carbon 2016, 109, 874–882. [Google Scholar] [CrossRef]
- Iijima, S. Synthesis of Carbon Nanotubes. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Hata, K.; Futaba, D.N.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Water-Assisted Highly Efficient Synthesis of Impurity-Free Single-Walled Carbon Nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef] [PubMed]
- Epelle, E.I.; Desongu, K.S.; Obande, W.; Adeleke, A.A.; Ikubanni, P.P.; Okolie, J.A.; Gunes, B. A comprehensive review of hydrogen production and storage: A focus on the role of nanomaterials. Int. J. Hydrog. Energy 2022, 47, 20398–20431. [Google Scholar] [CrossRef]
- Carlsson, J.-O.; Martin, P.M. Chapter 7—Chemical Vapor Deposition. In Handbook of Deposition Technologies for Films and Coatings (Third Edition); Martin, P.M., Ed.; William Andrew Publishing: Boston, MA, USA, 2010; pp. 314–363. [Google Scholar] [CrossRef]
- Kumar Jagadeesan, A.; Thangavelu, K.; Dhanancheyan, V. Carbon Nanotubes: Synthesis, Properties and Applications. In 21st Century Surface Science—A Handbook; Pham, P.V., Goel, P., Kumar, S., Yadav, K., Eds.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Mansuriya, B.D.; Altintas, Z. 14—Carbon nanomaterials for sensing applications. In Fundamentals of Sensor Technology; Barhoum, A., Altintas, Z., Eds.; Woodhead Publishing: Sawston, UK, 2023; pp. 367–400. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-Dimensional Nanostructures: Synthesis, Characterization, and Applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Zou, G.; Zhang, D.; Dong, C.; Li, H.; Xiong, K.; Fei, L.; Qian, Y. Carbon nanofibers: Synthesis, characterization, and electrochemical properties. Carbon 2006, 44, 828–832. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Lee, J.S. Electrospinning-Based Carbon Nanofibers for Energy and Sensor Applications. Appl. Sci. 2022, 12, 6048. [Google Scholar] [CrossRef]
- Dong, Y.; Du, X.-q.; Liang, P.; Man, X.-l. One-pot solvothermal method to fabricate 1D-VS4 nanowires as anode materials for lithium ion batteries. Inorg. Chem. Commun. 2020, 115, 107883. [Google Scholar] [CrossRef]
- Jiang, Y.; Peng, Z.; Zhang, S.; Li, F.; Liu, Z.; Zhang, J.; Liu, Y.; Wang, K. Facile in-situ Solvothermal Method to synthesize double shell ZnIn2S4 nanosheets/TiO2 hollow nanosphere with enhanced photocatalytic activities. Ceram. Int. 2018, 44, 6115–6126. [Google Scholar] [CrossRef]
- Chai, B.; Xu, M.; Yan, J.; Ren, Z. Remarkably enhanced photocatalytic hydrogen evolution over MoS2 nanosheets loaded on uniform CdS nanospheres. Appl. Surf. Sci. 2018, 430, 523–530. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Venkatesan, S.; Visvalingam, B.; Mannathusamy, G.; Viswanathan, V.; Rao, A.G. In situ synthesis of multi-walled carbon nanorings by catalytic chemical vapor deposition process. Int. Nano Lett. 2019, 9, 119–126. [Google Scholar] [CrossRef]
- Elugoke, S.E.; Adekunle, A.S.; Fayemi, O.E.; Mamba, B.B.; Sherif, E.-S.M.; Ebenso, E.E. Carbon-Based Quantum Dots for Electrochemical Detection of Monoamine Neurotransmitters—Review. Biosensors 2020, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Jorns, M.; Pappas, D. A Review of Fluorescent Carbon Dots, Their Synthesis, Physical and Chemical Characteristics, and Applications. Nanomaterials 2021, 11, 1448. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Liu, J.; Meng, X.; Wei, J.; Liu, T.; Tang, F. Synthesis of Ultra-Stable Fluorescent Carbon Dots from Polyvinylpyrrolidone and Their Application in the Detection of Hydroxyl Radicals. Chem.—An Asian J. 2014, 9, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Liu, Y.; Wang, S.; Ullah, M.W.; Chen, Q.; Zhang, D.; Kang, Z.; Mao, B. Advancements in the green synthesis of carbon dots for sustainable development. Sustain. Mater. Technol. 2024, 41, e01004. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Biasi, A.d.l.M.; Takara, E.A.; Scala-Benuzzi, M.L.; Valverde, A.M.; Gómez, N.N.; Messina, G.A. Modification of electrodes with polymer nanocomposites: Application to the simultaneous determination of Zn(II), Cd(II), and Cu(II) in water samples. Anal. Chim. Acta 2023, 1273, 341499. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Malode, S.J.; Alodhayb, A.N.; Mondal, K.; Shetti, N.P. Conducting polymer-based electrochemical sensors: Progress, challenges, and future perspectives. Talanta Open 2025, 11, 100395. [Google Scholar] [CrossRef]
- Sohrabi, H.; Salahshour Sani, P.; Zolfaghari, R.; Majidi, M.R.; Yoon, Y.; Khataee, A. MOF-Based Mycotoxin Nanosensors for Food Quality and Safety Assessment through Electrochemical and Optical Methods. Molecules 2022, 27, 7511. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Liang, K.; Reboul, J.; Ibarra, I.A.; Furukawa, S.; Falcaro, P. Sol–Gel Processing of Metal–Organic Frameworks. Chem. Mater. 2017, 29, 2626–2645. [Google Scholar] [CrossRef]
- Marimuthu, M.; Arumugam, S.S.; Jiao, T.; Sabarinathan, D.; Li, H.; Chen, Q. Metal organic framework based sensors for the detection of food contaminants. TrAC Trends Anal. Chem. 2022, 154, 116642. [Google Scholar] [CrossRef]
- Cheng, W.; Tang, X.; Zhang, Y.; Wu, D.; Yang, W. Applications of metal-organic framework (MOF)-based sensors for food safety: Enhancing mechanisms and recent advances. Trends Food Sci. Technol. 2021, 112, 268–282. [Google Scholar] [CrossRef]
- Ling, P.; Lei, J.; Zhang, L.; Ju, H. Porphyrin-Encapsulated Metal–Organic Frameworks as Mimetic Catalysts for Electrochemical DNA Sensing via Allosteric Switch of Hairpin DNA. Anal. Chem. 2015, 87, 3957–3963. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Li, M.; Gao, J.; Zhang, Z.; Ma, S.; Chen, Y. Incorporation of biomolecules in Metal-Organic Frameworks for advanced applications. Coord. Chem. Rev. 2019, 384, 90–106. [Google Scholar] [CrossRef]
- Byakodi, M.; Shrikrishna, N.S.; Sharma, R.; Bhansali, S.; Mishra, Y.; Kaushik, A.; Gandhi, S. Emerging 0D, 1D, 2D, and 3D nanostructures for efficient point-of-care biosensing. Biosens. Bioelectron. X 2022, 12, 100284. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, R.S.; Jerman, I.; Heath, D.; Bohm, S.; Gandhi, S.; Sadhu, V.; Baker, S.; Horvat, M. Emerging tri-s-triazine-based graphitic carbon nitride: A potential signal-transducing nanostructured material for sensor applications. Nano Sel. 2021, 2, 712–743. [Google Scholar] [CrossRef]
- Pandit, S.; Behera, P.; Sahoo, J.; De, M. In Situ Synthesis of Amino Acid Functionalized Carbon Dots with Tunable Properties and Their Biological Applications. ACS Appl. Bio Mater. 2019, 2, 3393–3403. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Gong, J.; Chen, J.; Zeng, Z.; Huang, W.; Pu, K.; Liu, J.; Chen, P. Recent Advances on Graphene Quantum Dots: From Chemistry and Physics to Applications. Adv. Mater. 2019, 31, 1808283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, J.; Zhang, Y.; Chen, J.; Zhao, Y.; Niu, Y.; Yu, C. Cerium dioxide-doped carboxyl fullerene as novel nanoprobe and catalyst in electrochemical biosensor for amperometric detection of the CYP2C19*2 allele in human serum. Biosens. Bioelectron. 2018, 102, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Tan, H.; Chen, Z.; Chen, X. FRET-Based Semiconducting Polymer Dots for pH Sensing. Sensors 2019, 19, 1455. [Google Scholar] [CrossRef] [PubMed]
- Robidillo, C.J.T.; Wandelt, S.; Dalangin, R.; Zhang, L.; Yu, H.; Meldrum, A.; Campbell, R.E.; Veinot, J.G.C. Ratiometric Detection of Nerve Agents by Coupling Complementary Properties of Silicon-Based Quantum Dots and Green Fluorescent Protein. ACS Appl. Mater. Interfaces 2019, 11, 33478–33488. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, N.; Khataee, A.; Habibi, B.; Hassanzadeh, J. Mimetic Ag nanoparticle/Zn-based MOF nanocomposite (AgNPs@ZnMOF) capped with molecularly imprinted polymer for the selective detection of patulin. Talanta 2018, 179, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Paras; Yadav, K.; Kumar, P.; Teja, D.R.; Chakraborty, S.; Chakraborty, M.; Mohapatra, S.S.; Sahoo, A.; Chou, M.M.C.; Liang, C.-T.; et al. A Review on Low-Dimensional Nanomaterials: Nanofabrication, Characterization and Applications. Nanomaterials 2023, 13, 160. [Google Scholar]
- Fatima, J.; Shah, A.N.; Tahir, M.B.; Mehmood, T.; Shah, A.A.; Tanveer, M.; Nazir, R.; Jan, B.L.; Alansi, S. Tunable 2D Nanomaterials; Their Key Roles and Mechanisms in Water Purification and Monitoring. Front. Environ. Sci. 2022, 10, 766743. [Google Scholar] [CrossRef]
- Chhowalla, M.; Jena, D.; Zhang, H. Two-dimensional semiconductors for transistors. Nat. Rev. Mater. 2016, 1, 16052. [Google Scholar] [CrossRef]
- Paramasivam, G.; Palem, V.V.; Sundaram, T.; Sundaram, V.; Kishore, S.C.; Bellucci, S. Nanomaterials: Synthesis and Applications in Theranostics. Nanomaterials 2021, 11, 3228. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Recent Advancements in Nanobiosensors: Current Trends, Challenges, Applications, and Future Scope. Biosensors 2022, 12, 892. [Google Scholar] [CrossRef] [PubMed]
- Harun-Ur-Rashid, M.; Imran, A.B.; Foyez, T. Voltammetric sensors modified with nanomaterials: Applications in rapid detection of bioactive compounds for health and safety monitoring. Discov. Electrochem. 2025, 2, 14. [Google Scholar] [CrossRef]
- Tripathi, A.; Gupta, P.; Mittal, A.K.; Mistri, T.K. Outlooks of Nanosensors in Medical and Smart Devices, Agricultural and Food Technology. Lett. Appl. NanoBioSci. 2024, 13, 142. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Serban, A.I.; Negulescu, G.P. Ethanol determination by an amperometric bienzyme sensor based on a Clark-type transducer. J. Electroanal. Chem. 2012, 671, 85–91. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Negulescu, G.-P.; Pisoschi, A. Ascorbic acid determination by an amperometric ascorbate oxidase-based biosensor. Rev. Chim. 2010, 61, 339–344. [Google Scholar]
- Dodevska, T.; LazarovaIvan, Y.; Shterev, S. Amperometric Biosensors for Glucose and Lactate with Applications in Food Analysis: A Brief Review. Acta Chim. Slov. 2019, 66, 762–776. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A. Nanotechnology in Food and Plant Science: Challenges and Future Prospects. Plants 2023, 12, 2565. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Chajanovsky, I.; Suckeveriene, R.Y. Recent Developments in Enzyme-Free PANI-Based Electrochemical Nanosensors for Pollutant Detection in Aqueous Environments. Polymers 2025, 17, 1320. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lin, X.; Zhang, M.; Li, Y.; Luo, C.; Wu, J. Review of Electrochemical Biosensors for Food Safety Detection. Biosensors 2022, 12, 959. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, F.T.T.; Falcão, I.R.d.A.; Souza, J.E.d.S.; Rocha, T.G.; de Sousa, I.G.; Cavalcante, A.L.G.; de Oliveira, A.L.B.; de Sousa, M.C.M.; dos Santos, J.C.S. Designing of Nanomaterials-Based Enzymatic Biosensors: Synthesis, Properties, and Applications. Electrochem 2021, 2, 149–184. [Google Scholar] [CrossRef]
- Vasile, C. Chapter 1—Polymeric Nanomaterials: Recent Developments, Properties and Medical Applications. In Polymeric Nanomaterials in Nanotherapeutics; Vasile, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–66. [Google Scholar] [CrossRef]
- Chang, T.M.S. Chapter II.5.4—Artificial Cells. In Biomaterials Science (Third Edition); Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 811–827. [Google Scholar] [CrossRef]
- Lipskikh, O.I.; Korotkova, E.I.; Khristunova, Y.P.; Barek, J.; Kratochvil, B. Sensors for voltammetric determination of food azo dyes—A critical review. Electrochim. Acta 2018, 260, 974–985. [Google Scholar] [CrossRef]
- Norocel, L.; Gutt, G. Method and Electrochemical Biosensor for Detection of Copper in Wine. Rev. Chim. 2018, 69, 3010–3012. [Google Scholar] [CrossRef]
- Laborda, E.; González, J.; Molina, Á. Recent advances on the theory of pulse techniques: A mini review. Electrochem. Commun. 2014, 43, 25–30. [Google Scholar] [CrossRef]
- Venton, B.J.; DiScenza, D.J. Chapter 3—Voltammetry. In Electrochemistry for Bioanalysis; Patel, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 27–50. [Google Scholar] [CrossRef]
- Tolun, A.; Altintas, Z. Chapter 16—Chemical sensing of food phenolics and antioxidant capacity. In Advanced Sensor Technology; Barhoum, A., Altintas, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 593–646. [Google Scholar] [CrossRef]
- Fakayode, S.O.; Walgama, C.; Fernand Narcisse, V.E.; Grant, C. Electrochemical and Colorimetric Nanosensors for Detection of Heavy Metal Ions: A Review. Sensors 2023, 23, 9080. [Google Scholar] [CrossRef] [PubMed]
- Anchidin-Norocel, L.; Gutt, G.; Tătăranu, E.; Amariei, S. Electrochemical sensors and biosensors: Effective tools for detecting heavy metals in water and food with possible implications for children’s health. Int. J. Electrochem. Sci. 2024, 19, 100643. [Google Scholar] [CrossRef]
- Upasham, S.; Banga, I.K.; Jagannath, B.; Paul, A.; Lin, K.-C.; Muthukumar, S.; Prasad, S. Electrochemical impedimetric biosensors, featuring the use of Room Temperature Ionic Liquids (RTILs): Special focus on non-faradaic sensing. Biosens. Bioelectron. 2021, 177, 112940. [Google Scholar] [CrossRef] [PubMed]
- Motsaathebe, P.C.; Fayemi, O.E. Electrochemical Detection of Ascorbic Acid in Oranges at MWCNT-AONP Nanocomposite Fabricated Electrode. Nanomaterials 2022, 12, 645. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Kumar, H.; Sharma, R.; Kumar, G.; Tundwal, A.; Dhayal, A.; Yadav, A.; Khatkar, A. Highly efficient and reliable voltammetry food sensor for Tartrazine dye using a nanocomposite reformed electrode. Microchem. J. 2024, 196, 109583. [Google Scholar] [CrossRef]
- Saqib, M.; Dorozhko, E.V.; Barek, J.; Korotkova, E.I.; Semin, V.O.; Kolobova, E.; Erkovich, A.V. Sensitive electrochemical sensing of carbosulfan in food products on laser reduced graphene oxide sensor decorated with silver nanoparticles. Microchem. J. 2024, 207, 112253. [Google Scholar] [CrossRef]
- Cheng, Q.; Xia, S.; Tong, J.; Wu, K. Highly-sensitive electrochemical sensing platforms for food colourants based on the property-tuning of porous carbon. Anal. Chim. Acta 2015, 887, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Švorc, Ľ.; Haššo, M.; Sarakhman, O.; Kianičková, K.; Stanković, D.M.; Otřísal, P. A progressive electrochemical sensor for food quality control: Reliable determination of theobromine in chocolate products using a miniaturized boron-doped diamond electrode. Microchem. J. 2018, 142, 297–304. [Google Scholar] [CrossRef]
- Tajeu, K.; Dongmo, L.; Tonle, I. Fullerene/MWCNT/Nafion Modified Glassy Carbon Electrode for the Electrochemical Determination of Caffeine. Am. J. Anal. Chem. 2020, 11, 114–127. [Google Scholar] [CrossRef]
- Rajendrachari, S.; Basavegowda, N.; Adimule, V.M.; Avar, B.; Somu, P.; Saravana Kumar, R.M.; Baek, K.-H. Assessing the Food Quality Using Carbon Nanomaterial Based Electrodes by Voltammetric Techniques. Biosensors 2022, 12, 1173. [Google Scholar] [CrossRef] [PubMed]
- Jirasirichote, A.; Punrat, E.; Suea-Ngam, A.; Chailapakul, O.; Chuanuwatanakul, S. Voltammetric detection of carbofuran determination using screen-printed carbon electrodes modified with gold nanoparticles and graphene oxide. Talanta 2017, 175, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Tümay, S.O.; Şenocak, A.; Sarı, E.; Şanko, V.; Durmuş, M.; Demirbas, E. A new perspective for electrochemical determination of parathion and chlorantraniliprole pesticides via carbon nanotube-based thiophene-ferrocene appended hybrid nanosensor. Sens. Actuators B Chem. 2021, 345, 130344. [Google Scholar] [CrossRef]
- Sodkrathok, P.; Karuwan, C.; Kamsong, W.; Tuantranont, A.; Amatatongchai, M. Patulin-imprinted origami 3D-ePAD based on graphene screen-printed electrode modified with Mn–ZnS quantum dot coated with a molecularly imprinted polymer. Talanta 2023, 262, 124695. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wei, Q.; Li, H.; Gao, H.; An, J.; Qi, B. Highly Sensitive Electrochemical Sensor based on Carbon Dots Reduced Gold Nanoparticles for Ractopamine Detection in Pork Meat. Int. J. Electrochem. Sci. 2020, 15, 3495–3503. [Google Scholar] [CrossRef]
- Üzer, A.; Sağlam, Ş.; Can, Z.; Erçağ, E.; Apak, R. Electrochemical Determination of Food Preservative Nitrite with Gold Nanoparticles/p-Aminothiophenol-Modified Gold Electrode. Int. J. Mol. Sci. 2016, 17, 1253. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Sun, J.; Meng, W.; Song, L.; Zhang, Y. Electrochemical sensor based on graphene and mesoporous TiO2 for the simultaneous determination of trace colourants in food. Food Chem. 2013, 141, 3731–3737. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Sun, J.; Zhu, H.; Zhu, J.; Liu, D. Synthesis and characterization of graphene and ordered mesoporous TiO2 as electrocatalyst for the determination of azo colorants. J. Solid State Electrochem. 2013, 17, 2193–2201. [Google Scholar] [CrossRef]
- He, J.-L.; Kou, W.; Li, C.; Cai, J.-J.; Kong, F.-Y.; Wang, W. Short Communication: Electrochemical Sensor Based on Single-Walled Carbon Nanotube-TiN Nannocomposites for Detecting Amaranth. Int. J. Electrochem. Sci. 2015, 10, 10074–10082. [Google Scholar] [CrossRef]
- Han, Q.; Wang, X.; Yang, Z.; Zhu, W.; Zhou, X.; Jiang, H. Fe3O4@rGO doped molecularly imprinted polymer membrane based on magnetic field directed self-assembly for the determination of amaranth. Talanta 2014, 123, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Sun, J.; Wu, Q.; Jing, Q.; Yu, S. Graphene Decorated with Nickel Nanoparticles as a Sensitive Substrate for Simultaneous Determination of Sunset Yellow and Tartrazine in Food Samples. Electroanalysis 2013, 25, 1505–1512. [Google Scholar] [CrossRef]
- Gan, T.; Sun, J.; Ji, L.; Shi, Z.; Liu, Y. Electrocatalytic Oxidation and Determination of Synthetic Food Dyes at Iron/Nickel Oxide Nanoparticles–Graphene Based Sensor. Sens. Lett. 2014, 12, 75–83. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Wang, H.; Yang, P.; Du, Y. Highly sensitive electrochemical determination of Sunset Yellow based on gold nanoparticles/graphene electrode. Anal. Chim. Acta 2015, 893, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Palisoc, S.T.; Chua, R.V.M.; Natividad, M.T. Highly sensitive determination of heavy metals in upland and lowland rice using AgNP/BiNP/MWCNT/nafion modified glassy carbon electrode via anodic stripping voltammetry. Mater. Res. Express 2020, 7, 015081. [Google Scholar] [CrossRef]
- Pungjunun, K.; Nantaphol, S.; Praphairaksit, N.; Siangproh, W.; Chaiyo, S.; Chailapakul, O. Enhanced sensitivity and separation for simultaneous determination of tin and lead using paper-based sensors combined with a portable potentiostat. Sens. Actuators B Chem. 2020, 318, 128241. [Google Scholar] [CrossRef]
- Nishino, T.; Hayashi, N.; Bui, P.T. Direct Measurement of Electron Transfer through a Hydrogen Bond between Single Molecules. J. Am. Chem. Soc. 2013, 135, 4592–4595. [Google Scholar] [CrossRef] [PubMed]
- Banks, C.E.; Davies, T.J.; Wildgoose, G.G.; Compton, R.G. Electrocatalysis at graphite and carbon nanotube modified electrodes: Edge-plane sites and tube ends are the reactive sites. Chem. Commun. 2005, 7, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Mnati, F.A.N.; Okman Koçoğlu, İ. Amperometric sensor based on carbon nanotube, ionic liquid, and polyaspartic acid for ascorbic acid determination. J. Chin. Chem. Soc. 2024, 71, 1274–1285. [Google Scholar] [CrossRef]
- Smart, A.; Westmacott, K.L.; Crew, A.; Doran, O.; Hart, J.P. An Electrocatalytic Screen-Printed Amperometric Sensor for the Selective Measurement of Thiamine (Vitamin B1) in Food Supplements. Biosensors 2019, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Fan, Y. Determination of nitrite in food specimens using electrochemical sensor based on polyneutral red modified reduced graphene oxide paste electrode. Int. J. Electrochem. Sci. 2023, 18, 100290. [Google Scholar] [CrossRef]
- Awal, A.; Mia, M.M.; Ferdaus, F.; Hossain, M.A.K.; Nayem, S.M.A.; Shah, S.S.; Shaikh, M.N.; Mazumder, M.A.J.; Aziz, M.A.; Ahammad, A.J.S. Electrochemical detection of sulfite using gold nanoparticles decorated poly[2-(methacryloyloxy) ethyl] trimethylammonium chloride: Kinetic and mechanistic studies. Nano Express 2024, 5, 035017. [Google Scholar] [CrossRef]
- Islam, S.; Awal, A.; Abu Nayem, S.M.; Naher, S.; Delwar Hossain, M.; Ahammad, A.J.S. Electrooxidation of Toxic Sulfite Material Using Ni-Ru-Based Heterometallo Supramolecular Polymer as Electrocatalyst: Synthesis and Kinetics Study. J. Electrochem. Soc. 2023, 170, 097507. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A.; Khan, R. Electrochemical immunosensor based on poly (3,4-ethylenedioxythiophene) modified with gold nanoparticle to detect aflatoxin B1. Mater. Sci. Eng. C 2017, 76, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Du, S.; Wang, X. Amperometric immunosensor for carbofuran detection based on gold nanoparticles and PB-MWCNTs-CTS composite film. Eur. Food Res. Technol. 2012, 235, 469–477. [Google Scholar] [CrossRef]
- Amatatongchai, M.; Sroysee, W.; Jarujamrus, P.; Nacapricha, D.; Lieberzeit, P.A. Selective amperometric flow-injection analysis of carbofuran using a molecularly-imprinted polymer and gold-coated-magnetite modified carbon nanotube-paste electrode. Talanta 2018, 179, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.; El-Beltagy, A.E.; El-Sayed, M.E.A.; Abdel Fattah, A.A.; Kishk, Y.F.M.; Alharthi Salman, S. Novel Potentiometric Liquid Membrane Sensor for Chitosan Determination in Food Supplements. Int. J. Electrochem. Sci. 2021, 16, 211128. [Google Scholar] [CrossRef]
- Gautier, E.A.; Gettar, R.T.; Servant, R.E. Simultaneous determination of lanthanum, strontium and copper in superconductor materials by ion chromatography. Anal. Chim. Acta 1993, 283, 350–353. [Google Scholar] [CrossRef]
- El-Seify, F.A.; Azab, H.A.; Degedy, F.S.; Abdel-Mageed, K.A.; El-Dossoki, F.I. Physico-analytical studies on some heterocyclic azo dyes and their metal complexes with some transition metals. BMC Chem. 2022, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Karimian, F.; Rounaghi, G.H.; Arbab-Zavar, M.H. Construction of a PVC based 15-crown-5 electrochemical sensor for Ag(I) cation. Chin. Chem. Lett. 2014, 25, 809–814. [Google Scholar] [CrossRef]
- Ali, T.A.; Mohamed, G.G.; El-Dessouky, M.M.I.; Abou El Ella, S.M.; Mohamed, R.T.F. Modified Carbon Paste Ion Selective Electrodes for the Determination of Iron (III) in Water, Soil and Fish Tissue Samples. Int. J. Electrochem. Sci. 2013, 8, 1469–1486. [Google Scholar] [CrossRef]
- Umezawa, Y.; Bühlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric Selectivity Coefficients of Ion-Selective Electrodes. Part I. Inorganic Cations (Technical Report). Pure Appl. Chem. 2000, 72, 1851–2082. [Google Scholar] [CrossRef]
- Minami, T.; Sato, T.; Minamiki, T.; Fukuda, K.; Kumaki, D.; Tokito, S. A novel OFET-based biosensor for the selective and sensitive detection of lactate levels. Biosens. Bioelectron. 2015, 74, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Guadarrama-Fernández, L.; Novell, M.; Blondeau, P.; Andrade, F.J. A disposable, simple, fast and low-cost paper-based biosensor and its application to the determination of glucose in commercial orange juices. Food Chem. 2018, 265, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.A.; Rezayi, M.; Manan, N.S.A.; Narimani, L.; Rosli, A.N.B.; Alias, Y. A Novel Potentiometric Sensor Based on 1,2-Bis(N’-benzoylthioureido)benzene and Reduced Graphene Oxide for Determination of Lead (II) Cation in Raw Milk. Electrochim. Acta 2015, 165, 221–231. [Google Scholar] [CrossRef]
- Guo, J.; Chai, Y.; Yuan, R.; Song, Z.; Zou, Z. Lead (II) carbon paste electrode based on derivatized multi-walled carbon nanotubes: Application to lead content determination in environmental samples. Sens. Actuators B Chem. 2011, 155, 639–645. [Google Scholar] [CrossRef]
- Özbek, O.; Gürdere, M.B.; Berkel, C.; Isildak, Ö. Electroanalytical Determination of Copper(II) Ions Using a Polymer Membrane Sensor. J. Electrochem. Sci. Technol. 2023, 14, 66–74. [Google Scholar] [CrossRef]
- Nouira, W.; Maaref, A.; Elaissari, H.; Vocanson, F.; Siadat, M.; Jaffrezic-Renault, N. Comparative study of conductometric glucose biosensor based on gold and on magnetic nanoparticles. Mater. Sci. Eng. C 2013, 33, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Souiri, M.; Mora-Ponsonnet, L.; Glinel, K.; Othmane, A.; Jouenne, T.; Duncan, A.C. Surface assembly on biofunctional magnetic nanobeads for the study of protein–ligand interactions. Colloids Surf. B Biointerfaces 2009, 68, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Lazanas, A.C.; Prodromidis, M.I. Correction to “Electrochemical Impedance Spectroscopy—A Tutorial”. ACS Meas. Sci. Au 2025, 5, 156. [Google Scholar] [CrossRef] [PubMed]
- Parra-Alfambra, A.M.; Casero, E.; Vázquez, L.; Quintana, C.; del Pozo, M.; Petit-Domínguez, M.D. MoS2 nanosheets for improving analytical performance of lactate biosensors. Sens. Actuators B Chem. 2018, 274, 310–317. [Google Scholar] [CrossRef]
- Štukovnik, Z.; Fuchs-Godec, R.; Bren, U. Nanomaterials and Their Recent Applications in Impedimetric Biosensing. Biosensors 2023, 13, 899. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Su, H.; Qu, X.; Ju, P.; Cui, L.; Ai, S. Acetylcholinesterase biosensor based on 3-carboxyphenylboronic acid/reduced graphene oxide–gold nanocomposites modified electrode for amperometric detection of organophosphorus and carbamate pesticides. Sens. Actuators B Chem. 2011, 160, 1255–1261. [Google Scholar] [CrossRef]
- Caetano, K.d.S.; Lieberknecht, G.; Benvenutti, E.V.; Pereira, M.B.; Hinrichs, R.; Hertz, P.F.; Rodrigues, R.C.; de Menezes, E.W.; Arenas, L.T.; Costa, T.M.H. Sol-gel immobilization of lipase enzyme on FTO modified with silica-zirconia and gold nanoparticles films for design of triglyceride impedimetric biosensor. Electrochim. Acta 2024, 483, 143997. [Google Scholar] [CrossRef]
- Costa, M.P.; Frías, I.A.M.; Andrade, C.A.S.; Oliveira, M.D.L. Impedimetric immunoassay for aflatoxin B1 using a cysteine modified gold electrode with covalently immobilized carbon nanotubes. Microchim. Acta 2017, 184, 3205–3213. [Google Scholar] [CrossRef]
- Wang, Y.; Kumar, A.K.S.; Compton, R.G. Optimising Adsorptive Stripping Voltammetry: Strategies and Limitations. ChemElectroChem 2021, 8, 2343–2352. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Gajaila, I.; Iordache, F.; Dobre, R.; Cazimir, I.; Serban, A.I. Analytical methods applied to the assay of sulfur-containing preserving agents. Microchem. J. 2020, 155, 104681. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Bilteanu, L.; Serban, A.I. Antioxidant Determination with the Use of Carbon-Based Electrodes. Chemosensors 2021, 9, 72. [Google Scholar] [CrossRef]
- Dzyadevych, S.; Jaffrezic-Renault, N. 6—Conductometric biosensors. In Biological Identification; Schaudies, R.P., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 153–193. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Safaei, M.; Zhang, K.; Van Le, Q.; Varma, R.S.; Jang, H.W.; Shokouhimehr, M. Developments and applications of nanomaterial-based carbon paste electrodes. RSC Adv. 2020, 10, 21561–21581. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.-Y.; Wong, M.H.; Lew, T.T.S.; Bisker, G.; Lee, M.A.; Kaplan, A.; Dong, J.; Liu, A.T.; Koman, V.B.; Sinclair, R.; et al. Nanosensor Technology Applied to Living Plant Systems. Annu. Rev. Anal. Chem. 2017, 10, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D.; Nag, S.; Das, D.; Banerjee Roy, R. Electrochemical detection of folic acid in food extracts using molecularly imprinted polyacrylonitrile imbued graphite electrode. Anal. Chim. Acta 2024, 1325, 343120. [Google Scholar] [CrossRef] [PubMed]
- Mohammadnezhad, K.; Ahour, F.; Keshipour, S. Electrochemical determination of ascorbic acid using palladium supported on N-doped graphene quantum dot modified electrode. Sci. Rep. 2024, 14, 5982. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Ruan, C.; Liu, L.; Yan, R.; Zhao, F.; Zeng, B. Single-walled carbon nanotube-ionic liquid paste electrode for the sensitive voltammetric determination of folic acid. Sens. Actuators B Chem. 2008, 134, 895–901. [Google Scholar] [CrossRef]
- Devi, R.; Gogoi, S.; Barua, S.; Sankar Dutta, H.; Bordoloi, M.; Khan, R. Electrochemical detection of monosodium glutamate in foodstuffs based on Au@MoS2/chitosan modified glassy carbon electrode. Food Chem. 2019, 276, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wang, S. Enhanced-oxidation and electrochemical determination of honokiol and magnolol using NMP-exfoliated graphene nanosheets. J. Mol. Liq. 2015, 208, 52–57. [Google Scholar] [CrossRef]
- Manrique Rodriguez, N.A.; Costa, M.; Di Masi, S.; Zaleski, C.; García-Cruz, A.; Mele, G.; Paradiso, V.M.; Piletsky, S.; Malitesta, C.; De Benedetto, G.E. Adsorption Isotherm Analysis for Hybrid Molecularly Imprinted Polymeric Gold-Decorated Nanoparticles Suitable for Reliable Quantification of Gluconic Acid in Wine. Nanomaterials 2025, 15, 211. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.C.; Stradiotto, N.R. Electrochemical sensing of lactate by using an electrode modified with molecularly imprinted polymers, reduced graphene oxide and gold nanoparticles. Microchim. Acta 2019, 186, 764. [Google Scholar] [CrossRef] [PubMed]
- Pardakhty, A.; Ahmadzadeh, S.; Avazpour, S.; Gupta, V.K. Highly sensitive and efficient voltammetric determination of ascorbic acid in food and pharmaceutical samples from aqueous solutions based on nanostructure carbon paste electrode as a sensor. J. Mol. Liq. 2016, 216, 387–391. [Google Scholar] [CrossRef]
- Jamali, T.; Karimi-Maleh, H.; Khalilzadeh, M.A. A novel nanosensor based on Pt:Co nanoalloy ionic liquid carbon paste electrode for voltammetric determination of vitamin B9 in food samples. LWT—Food Sci. Technol. 2014, 57, 679–685. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Moazampour, M.; Yoosefian, M.; Sanati, A.L.; Tahernejad-Javazmi, F.; Mahani, M. An Electrochemical Nanosensor for Simultaneous Voltammetric Determination of Ascorbic Acid and Sudan I in Food Samples. Food Anal. Methods 2014, 7, 2169–2176. [Google Scholar] [CrossRef]
- Duzmen, S.; Baytak, A.K.; Aslanoglu, M. A novel voltammetric platform composed of poly(aminopyrazine), ZrO2 and CNTs for a rapid, sensitive and selective determination of ascorbic acid in pharmaceuticals and food samples. Mater. Chem. Phys. 2020, 252, 123170. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, L.; Chen, G. Preparation of a carbon nanotube-copper nanoparticle hybrid by chemical reduction for use in the electrochemical sensing of carbohydrates. Carbon 2012, 50, 2563–2570. [Google Scholar] [CrossRef]
- Garehbaghi, S.; Adam, V.; Přibyl, J.; Richtera, L.; Ashrafi, A.M. An enzyme cascade biosensor based on multiwalled carbon nanotube-RuO2 nanocomposite for selective amperometric determination of lactose in milk samples. Microchem. J. 2024, 204, 111138. [Google Scholar] [CrossRef]
- Artigues, M.; Abellà, J.; Colominas, S. Analytical Parameters of an Amperometric Glucose Biosensor for Fast Analysis in Food Samples. Sensors 2017, 17, 2620. [Google Scholar] [CrossRef] [PubMed]
- Guzsvány, V.; Anojčić, J.; Vajdle, O.; Radulović, E.; Madarász, D.; Kónya, Z.; Kalcher, K. Amperometric Determination of Glucose in White Grape and in Tablets as Ingredient by Screen-Printed Electrode Modified with Glucose Oxidase and Composite of Platinum and Multiwalled Carbon Nanotubes. Food Anal. Methods 2019, 12, 570–580. [Google Scholar] [CrossRef]
- Tang, L.; Yan, Y.; He, C.; Zhou, J.; Yin, J.; Zhang, Z.; Tu, L.; Liu, Z.; Yang, Q.; He, J.; et al. NiCo layered double hydroxide nanosheet arrays constructed via in-situ plasma etching as non-enzymatic electrochemical sensor for glucose in food. Microchem. J. 2024, 206, 111496. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A.; Khan, R. A highly sensitive amperometric immunosensor probe based on gold nanoparticle functionalized poly (3, 4-ethylenedioxythiophene) doped with graphene oxide for efficient detection of aflatoxin B1. Synth. Met. 2018, 235, 136–144. [Google Scholar] [CrossRef]
- Han, Z.; Tang, Z.; Jiang, K.; Huang, Q.; Meng, J.; Nie, D.; Zhao, Z. Dual-target electrochemical aptasensor based on co-reduced molybdenum disulfide and Au NPs (rMoS2-Au) for multiplex detection of mycotoxins. Biosens. Bioelectron. 2020, 150, 111894. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wen, Y.; Wang, W.; Zhao, Z.; Han, Y.; Tang, K.; Wang, D. Nanobody-based electrochemical competitive immunosensor for the detection of AFB1 through AFB1-HCR as signal amplifier. Microchim. Acta 2020, 187, 352. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Huang, Q.; Nie, D.; Zhao, X.; Cao, H.; Wu, W.; Han, Z. A Multifunctional N-Doped Cu–MOFs (N–Cu–MOF) Nanomaterial-Driven Electrochemical Aptasensor for Sensitive Detection of Deoxynivalenol. Molecules 2021, 26, 2243. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Seenivasan, R.; Wang, Y.-C.; Yu, J.-H.; Gunasekaran, S. An Electrochemical Immunosensor for Rapid and Sensitive Detection of Mycotoxins Fumonisin B1 and Deoxynivalenol. Electrochim. Acta 2016, 213, 89–97. [Google Scholar] [CrossRef]
- Lu, L.; Gunasekaran, S. Dual-channel ITO-microfluidic electrochemical immunosensor for simultaneous detection of two mycotoxins. Talanta 2019, 194, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.; Xie, H.; Sun, R.; Cao, T.; Paudyal, N.; Fang, W.; Song, H. Development of a Magnetic Nanoparticles-Based Screen-Printed Electrodes (MNPs-SPEs) Biosensor for the Quantification of Ochratoxin A in Cereal and Feed Samples. Toxins 2018, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri–Dehaghani, F.; Zare, H.R.; Shekari, Z. Simultaneous measurement of ochratoxin A and aflatoxin B1 using a duplexed-electrochemical aptasensor based on carbon nanodots decorated with gold nanoparticles and two redox probes hemin@HKUST-1 and ferrocene@HKUST-1. Talanta 2024, 266, 124947. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.; Rong, F.; Guo, C.; Chen, K.; Wang, M.; Zhang, Z.; Pettinari, R.; Zhou, L.; Du, M. Electrochemical aptasensing strategy based on a multivariate polymertitanium-metal-organic framework for zearalenone analysis. Food Chem. 2022, 385, 132654. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xia, Y.; Liu, Y.; Chen, Y.; Zeng, B. An effective ratiometric electrochemical sensor for highly selective and reproducible detection of ochratoxin A: Use of magnetic field improved molecularly imprinted polymer. Sens. Actuators B Chem. 2022, 359, 131582. [Google Scholar] [CrossRef]
- Jahangiri-Dehaghani, F.; Zare, H.R.; Shekari, Z. Encapsulation of hemin in Fe-based metal-organic frameworks and its application for the direct determination of aflatoxin M1. World Mycotoxin J. 2021, 14, 111–120. [Google Scholar] [CrossRef]
- Tang, X.; Catanante, G.; Huang, X.; Marty, J.-L.; Wang, H.; Zhang, Q.; Li, P. Screen-printed electrochemical immunosensor based on a novel nanobody for analyzing aflatoxin M1 in milk. Food Chem. 2022, 383, 132598. [Google Scholar] [CrossRef] [PubMed]
- Riberi, W.I.; Tarditto, L.V.; Zon, M.A.; Arévalo, F.J.; Fernández, H. Development of an electrochemical immunosensor to determine zearalenone in maize using carbon screen printed electrodes modified with multi-walled carbon nanotubes/polyethyleneimine dispersions. Sens. Actuators B Chem. 2018, 254, 1271–1277. [Google Scholar] [CrossRef]
- Jahangiri–Dehaghani, F.; Zare, H.R.; Shekari, Z. Measurement of aflatoxin M1 in powder and pasteurized milk samples by using a label–free electrochemical aptasensor based on platinum nanoparticles loaded on Fe–based metal–organic frameworks. Food Chem. 2020, 310, 125820. [Google Scholar] [CrossRef] [PubMed]
- Yugender Goud, K.; Catanante, G.; Hayat, A.; Satyanarayana, M.; Vengatajalabathy Gobi, K.; Marty, J.L. Disposable and portable electrochemical aptasensor for label free detection of aflatoxin B1 in alcoholic beverages. Sens. Actuators B Chem. 2016, 235, 466–473. [Google Scholar] [CrossRef]
- Rivas, L.; Mayorga-Martinez, C.C.; Quesada-González, D.; Zamora-Gálvez, A.; de la Escosura-Muñiz, A.; Merkoçi, A. Label-Free Impedimetric Aptasensor for Ochratoxin-A Detection Using Iridium Oxide Nanoparticles. Anal. Chem. 2015, 87, 5167–5172. [Google Scholar] [CrossRef] [PubMed]
- Evtugyn, G.; Porfireva, A.; Stepanova, V.; Kutyreva, M.; Gataulina, A.; Ulakhovich, N.; Evtugyn, V.; Hianik, T. Impedimetric Aptasensor for Ochratoxin A Determination Based on Au Nanoparticles Stabilized with Hyper-Branched Polymer. Sensors 2013, 13, 16129–16145. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Long, N.; Xu, Q.; Li, Y.; Song, P.; Yang, M.; Wang, J.; Zhou, L.; Sheng, P.; Kong, W. Development of a Nafion-MWCNTs and in-situ generated Au nanopopcorns dual-amplification electrochemical aptasensor for ultrasensitive detection of OTA. Food Chem. 2023, 403, 134375. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Jiang, X.; Li, Y.; Lu, X.; Luan, S.; Wang, Y.; Li, Y.; Gao, F. Metal−organic frameworks-derived MnO2/Mn3O4 microcuboids with hierarchically ordered nanosheets and Ti3C2 MXene/Au NPs composites for electrochemical pesticide detection. J. Hazard. Mater. 2019, 373, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Li, Y.; Lu, X.; Sun, M.; Liu, H.; Yu, G.; Gao, F. Palladium-copper nanowires-based biosensor for the ultrasensitive detection of organophosphate pesticides. Anal. Chim. Acta 2017, 982, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Talan, A.; Mishra, A.; Eremin, S.A.; Narang, J.; Kumar, A.; Gandhi, S. Ultrasensitive electrochemical immuno-sensing platform based on gold nanoparticles triggering chlorpyrifos detection in fruits and vegetables. Biosens. Bioelectron. 2018, 105, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-W.; Chen, C.-L.; Chen, Y.-H.; Chang, Y.-M.; Liu, F.-J.; Tsai, Y.-C. Electrochemical Organophosphorus Pesticide Detection Using Nanostructured Gold-Modified Electrodes. Sensors 2022, 22, 9938. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, Y.; Liang, Y.; Wu, R. CoO NPs/c-CNTs nanocomposite as electrochemical sensor for sensitive and selective determination of the carbofuran pesticide in fruits and vegetables. Int. J. Electrochem. Sci. 2021, 16, 210616. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, R.; Shi, L.; Han, G.; Xiao, Y. Acetylcholinesterase biosensor based on electrochemically inducing 3D graphene oxide network/multi-walled carbon nanotube composites for detection of pesticides. RSC Adv. 2017, 7, 53570–53577. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Xu, Y.; Pan, H.; Guo, K.; Zhang, Y.; Chen, Y.; Liu, D.; Zhang, Y.; Yao, C.; et al. A novel molecularly imprinted polymer composite based on polyaniline nanoparticles as sensitive sensors for parathion detection in the field. Food Control 2022, 133, 108638. [Google Scholar] [CrossRef]
- Della Pelle, F.; Angelini, C.; Sergi, M.; Del Carlo, M.; Pepe, A.; Compagnone, D. Nano carbon black-based screen printed sensor for carbofuran, isoprocarb, carbaryl and fenobucarb detection: Application to grain samples. Talanta 2018, 186, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Kunpatee, K.; Kaewdorn, K.; Duangtong, J.; Chaiyo, S.; Chailapakul, O.; Kalcher, K.; Kerr, M.; Samphao, A. A new disposable electrochemical sensor for the individual and simultaneous determination of carbamate pesticides using a nanocomposite modified screen-printed electrode. Microchem. J. 2022, 177, 107318. [Google Scholar] [CrossRef]
- Tan, R.; Wang, Z.; Gao, N.; Cai, Z.; Jiang, P.; Pan, C.; Pan, J.; Chang, G.; He, Y. UiO-66 derived nanoporous carbons/electrochemically reduced graphene oxide nanocomposites-based non-enzyme electrochemical sensor towards highly efficient determination of methyl parathion in food samples. J. Mater. Sci. 2023, 58, 10635–10650. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, G.; Zhang, Y.; Tang, L.; Huang, D.; Liu, C.; Pang, Y.; Luo, J. Trace detection of picloram using an electrochemical immunosensor based on three-dimensional gold nanoclusters. Anal. Biochem. 2010, 407, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Amatatongchai, M.; Thimoonnee, S.; Jarujamrus, P.; Nacapricha, D.; Lieberzeit, P.A. Novel amino-containing molecularly-imprinted polymer coating on magnetite-gold core for sensitive and selective carbofuran detection in food. Microchem. J. 2020, 158, 105298. [Google Scholar] [CrossRef]
- Chauhan, N.; Pundir, C.S. An amperometric biosensor based on acetylcholinesterase immobilized onto iron oxide nanoparticles/multi-walled carbon nanotubes modified gold electrode for measurement of organophosphorus insecticides. Anal. Chim. Acta 2011, 701, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Anchidin-Norocel, L.; Savage, W.K.; Gutt, G.; Amariei, S. Development, Optimization, Characterization, and Application of Electrochemical Biosensors for Detecting Nickel Ions in Food. Biosensors 2021, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Saenchoopa, A.; Klangphukhiew, S.; Somsub, R.; Talodthaisong, C.; Patramanon, R.; Daduang, J.; Daduang, S.; Kulchat, S. A Disposable Electrochemical Biosensor Based on Screen-Printed Carbon Electrodes Modified with Silver Nanowires/HPMC/Chitosan/Urease for the Detection of Mercury (II) in Water. Biosensors 2021, 11, 351. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.-M.; Wang, H.-R.; Ge, L.-Q.; Zhou, D.-M.; Xu, J.-Z.; Gu, H.-Y.; Bao, N. Gold-coated nanostructured carbon tape for rapid electrochemical detection of cadmium in rice with in situ electrodeposition of bismuth in paper-based analytical devices. Sens. Actuators B Chem. 2018, 260, 475–479. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, X.; Xu, Y.; Wang, X.; Guo, Z.; Huang, X.; Li, Z.; Shi, J.; Zou, X. Single-step electrochemical sensing of ppt-level lead in leaf vegetables based on peroxidase-mimicking metal-organic framework. Biosens. Bioelectron. 2020, 168, 112544. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Qian, S.; Cao, H.; Yu, J.; Ye, T.; Wu, X.; Chen, L.; Xu, F. An ultra-sensitive electrochemical aptasensor for simultaneous quantitative detection of Pb2+ and Cd2+ in fruit and vegetable. Food Chem. 2022, 382, 132173. [Google Scholar] [CrossRef] [PubMed]
- Miao, P.; Tang, Y.; Wang, L. DNA Modified Fe3O4@Au Magnetic Nanoparticles as Selective Probes for Simultaneous Detection of Heavy Metal Ions. ACS Appl. Mater. Interfaces 2017, 9, 3940–3947. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, S.; Erkal, A.; Kariper, İ.A.; Solak, A.O.; Jeon, S.; Mülazımoğlu, İ.E.; Üstündağ, Z. Carbonaceous Materials-12: A Novel Highly Sensitive Graphene Oxide-Based Carbon Electrode: Preparation, Characterization, and Heavy Metal Analysis in Food Samples. Food Anal. Methods 2016, 9, 322–331. [Google Scholar] [CrossRef]
- Soulis, D.; Pagkali, V.; Kokkinos, C.; Economou, A. Plot-on-demand integrated paper-based sensors for drop-volume voltammetric monitoring of Pb(II) and Cd(II) using a bismuth nanoparticle-modified electrode. Microchim. Acta 2022, 189, 240. [Google Scholar] [CrossRef] [PubMed]
- Pungjunun, K.; Chaiyo, S.; Jantrahong, I.; Nantaphol, S.; Siangproh, W.; Chailapakul, O. Anodic stripping voltammetric determination of total arsenic using a gold nanoparticle-modified boron-doped diamond electrode on a paper-based device. Microchim. Acta 2018, 185, 324. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, A.; Moosavi, R.; Madrakian, T.; Keypour, H.; Ramezani-Aktij, A.; Mirzaei-Monsef, M. Construction and Application of an Electrochemical Sensor for Simultaneous Determination of Cd(II), Cu(II) and Hg(II) in Water and Foodstuff Samples. Electroanalysis 2014, 26, 786–795. [Google Scholar] [CrossRef]
- Tan, Z.; Wu, W.; Feng, C.; Wu, H.; Zhang, Z. Simultaneous determination of heavy metals by an electrochemical method based on a nanocomposite consisting of fluorinated graphene and gold nanocage. Microchim. Acta 2020, 187, 414. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-Y.; Lee, J.-W.; Park, D.J.; Lee, J.-Y.; Myung, N.V.; Kwon, S.H.; Lee, K.H. Highly stable potentiometric sensor with reduced graphene oxide aerogel as a solid contact for detection of nitrate and calcium ions. J. Electroanal. Chem. 2021, 897, 115553. [Google Scholar] [CrossRef]
- Zhang, J.; Lovell, J.F.; Shi, J.; Zhang, Y. Nanomaterials for co-immobilization of multiple enzymes. BMEMat 2025, 3, e12080. [Google Scholar] [CrossRef]
- Omanović-Mikličanin, E.; Badnjević, A.; Kazlagić, A.; Hajlovac, M. Nanocomposites: A brief review. Health Technol. 2020, 10, 51–59. [Google Scholar] [CrossRef]
- Ameta, R.; Bhatt, J.P.; Ameta, S.C. Quantum Dots: Fundamentals, Synthesis and Applications; Ameta, R., Bhatt, J.P., Ameta, S.C., Eds.; Elsevier Inc.: New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Baghayeri, M.; Alinezhad, H.; Fayazi, M.; Tarahomi, M.; Ghanei-Motlagh, R.; Maleki, B. A novel electrochemical sensor based on a glassy carbon electrode modified with dendrimer functionalized magnetic graphene oxide for simultaneous determination of trace Pb(II) and Cd(II). Electrochim. Acta 2019, 312, 80–88. [Google Scholar] [CrossRef]
- Singh, R.; Dutt, S.; Sharma, P.; Sundramoorthy, A.K.; Dubey, A.; Singh, A.; Arya, S. Future of Nanotechnology in Food Industry: Challenges in Processing, Packaging, and Food Safety. Glob. Chall. 2023, 7, 2200209. [Google Scholar] [CrossRef] [PubMed]
- del Rosario Herrera-Rivera, M.; Torres-Arellanes, S.P.; Cortés-Martínez, C.I.; Navarro-Ibarra, D.C.; Hernández-Sánchez, L.; Solis-Pomar, F.; Pérez-Tijerina, E.; Román-Doval, R. Nanotechnology in food packaging materials: Role and application of nanoparticles. RSC Adv. 2024, 14, 21832–21858. [Google Scholar] [CrossRef] [PubMed]
- Abedi-Firoozjah, R.; Ebdali, H.; Soltani, M.; Abdolahi-Fard, P.; Heydari, M.; Assadpour, E.; Azizi-Lalabadi, M.; Zhang, F.; Jafari, S.M. Nanomaterial-based sensors for the detection of pathogens and microbial toxins in the food industry; a review on recent progress. Coord. Chem. Rev. 2024, 500, 215545. [Google Scholar] [CrossRef]
- Kumar, V.; Guleria, P.; Mehta, S.K. Nanosensors for food quality and safety assessment. Environ. Chem. Lett. 2017, 15, 165–177. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Elumalai, K.; Manickam, S.; Bakthavatchalam, G.; Tamilselvan, P. Carbon nanomaterials: Revolutionizing biomedical applications with promising potential. Nano Mater. Sci. 2024; in press. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Rab, S.; Suman, R. Exploring the potential of nanosensors: A brief overview. Sens. Int. 2021, 2, 100130. [Google Scholar] [CrossRef]
- Dahlin, A.B. Size Matters: Problems and Advantages Associated with Highly Miniaturized Sensors. Sensors 2012, 12, 3018–3036. [Google Scholar] [CrossRef] [PubMed]
- Eivazzadeh-Keihan, R.; Bahojb Noruzi, E.; Chidar, E.; Jafari, M.; Davoodi, F.; Kashtiaray, A.; Ghafori Gorab, M.; Masoud Hashemi, S.; Javanshir, S.; Ahangari Cohan, R.; et al. Applications of carbon-based conductive nanomaterials in biosensors. Chem. Eng. J. 2022, 442, 136183. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Iordache, F.; Stanca, L.; Mitranescu, E.; Bader Stoica, L.; Geicu, O.I.; Bilteanu, L.; Serban, A.I. Biosensors for Food Mycotoxin Determination: A Comparative and Critical Review. Chemosensors 2024, 12, 92. [Google Scholar] [CrossRef]
- Li, G.; Wu, J.; Jin, H.; Xia, Y.; Liu, J.; He, Q.; Chen, D. Titania/Electro-Reduced Graphene Oxide Nanohybrid as an Efficient Electrochemical Sensor for the Determination of Allura Red. Nanomaterials 2020, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Zhang, J.; Liu, Y.; Wu, J.; Li, G.; Liu, J.; He, Q. A Simple but Efficient Voltammetric Sensor for Simultaneous Detection of Tartrazine and Ponceau 4R Based on TiO2/Electro-Reduced Graphene Oxide Nanocomposite. Chemosensors 2020, 8, 70. [Google Scholar] [CrossRef]
- Bhattacharjee, J.; Bhakta, D.; Roy, S. Characteristics, Applications, and Limitations of Nanocomposites in Biosensing. In Smart and Sustainable Applications of Nanocomposites; IGI Global: Hershey, PA, USA, 2024; pp. 161–191. [Google Scholar] [CrossRef]
- Sheraz, M.; Sun, X.-F.; Siddiqui, A.; Wang, Y.; Hu, S.; Sun, R. Cellulose-Based Electrochemical Sensors. Sensors 2025, 25, 645. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.; Krishnan, P.; Vashist, A.; Sethi, S.; Kumar, R.; Chawla, G.; Dhillon, M.K. Development of the sustainable green nanosensor using corn silk extract for nitrate detection in leafy vegetables. Biosens. Bioelectron. 2024, 260, 116447. [Google Scholar] [CrossRef] [PubMed]
- Berkel, C.; Özbek, O. Green Electrochemical Sensors, Their Applications and Greenness Metrics Used: A Review. Electroanalysis 2024, 36, e202400286. [Google Scholar] [CrossRef]
- Chaudhary, V.; Gaur, P.; Rustagi, S. Sensors, society, and sustainability. Sustain. Mater. Technol. 2024, 40, e00952. [Google Scholar] [CrossRef]
- Ajab, H.; Khan, M.H.; Naveed, P.; Abdullah, M.A. Evolution and recent development of cellulose-modified, nucleic acid-based and green nanosensors for trace heavy metal ion analyses in complex media: A review. Int. J. Biol. Macromol. 2025, 307, 141745. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Jain, V.K.; Nagpal, S. Nanoparticle intervention for heavy metal detection: A review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100667. [Google Scholar] [CrossRef]
- Pakeeza; Draz, M.U.; Yaqub, A.; Jafry, A.T.; Khan, M.; Ajab, H. Electrochemical sensing of B-complex vitamins: Current challenges and future prospects with microfluidic integration. RSC Adv. 2024, 14, 10331–10347. [Google Scholar] [CrossRef] [PubMed]
- Malakootian, M.; Abolghasemi, H.; Mahmoudi-Moghaddam, H. A novel electrochemical sensor based on the modified carbon paste using Eu3+-doped NiO for simultaneous determination of Pb (II) and Cd (II) in food samples. J. Electroanal. Chem. 2020, 876, 114474. [Google Scholar] [CrossRef]
- Li, S.; Zhang, C.; Wang, S.; Liu, Q.; Feng, H.; Ma, X.; Guo, J. Electrochemical microfluidics techniques for heavy metal ion detection. Analyst 2018, 143, 4230–4246. [Google Scholar] [CrossRef] [PubMed]
- Nazreen, A.; Rawat, D.S.; Malik, R.; Meena, J.R. Biopolymers-based Electrochemical Sensors: An Overview of Synthesis and Applications. Acta Biol. Forum 2024, 3, 9–22. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).