Abstract
In this study, a simple and effective approach was developed for the quantitative detection of serotonin. Hexagonal copper selenide nanostructures (CuSe) were employed to modify a disposable screen-printed carbon electrode (SPCE), and their ability to electrochemically detect serotonin in serum samples was investigated. The fabricated CuSe nanostructures exhibited an interconnected, cluster-like morphology composed of irregularly shaped particles with a distinct hexagonal crystal structure. The electrochemical results revealed that the CuSe/SPCE sensor showed better electrochemical activity and good analytical sensing performance towards serotonin detection. The sensor exhibited a linear response in the concentration range of 10 to 1000 nM, with an excellent correlation coefficient (R2 = 0.9998) and a low detection limit of 3 nM. Furthermore, the CuSe/SPCE showed better selectivity, impressive sensitivity (12.45 µM/µA cm−2), and good reproducibility toward serotonin detection, making it a promising electrochemical biosensor for serotonin detection in various real biological samples.
1. Introduction
Serotonin (5-Hydroxytryptamine, 5-HT) belongs to a biogenic amine that remains in biological systems, as a neurotransmitter in the central nervous system and as hormones in the gastrointestinal tract [1,2]. Serotonin plays a preliminary role in regulating neuronal activities and mood-related behaviors, indirectly regulating mood, sleep, appetite, and sexual desires. Serotonin deficiency causes depression, migraines, sexual disorders, carcinoid syndrome, mood-related disorders, Parkinson’s, Alzheimer’s, Huntington’s, and infant death syndrome [3,4,5,6,7]. Serotonin level diagnoses are linked to understanding neuron roles and neuronal disorders [8]. The typical range of serotonin concentration in body fluids was found to be 500–1200 nM in whole blood, 295–687 nM in urine, and <0.0591 nM in cerebrospinal fluid, limiting sample treatment and making it a time-consuming and tedious process [9,10,11]. There is a necessity to fabricate a promising technique for accomplishing facile, rapid, susceptible, and point-of-care analysis for serotonin recognition. Presently, electrochemical sensor-based approaches are receiving enormous attention since they exhibit higher sensitivity and an exceptional detection limit. Major challenges in serotonin detection that co-exist via complex matrices are eliminated by modifying the electrode surface in serum or plasma samples since they possess similar oxidation potential on pristine electrode surfaces [12,13,14,15].
In recent decades, various analytical techniques have been employed to detect serotonin across different biological systems, including liquid chromatography (LC), fast-scan cyclic voltammetry (FSCV), and high-performance liquid chromatography (HPLC) [16,17]. These chromatographic approaches are often coupled with advanced detection tools such as mass spectrometry to enhance sensitivity and specificity. Moreover, FSCV has indeed emerged as a gold standard technique for in vivo serotonin detection due to its high temporal resolution and sensitivity [18]. While effective in identifying serotonin, these methods come with notable limitations, as they typically require large, complex instrumentation and skilled personnel, making them less suitable for routine screening or point-of-care diagnostics [19,20]. These constraints highlight the need for alternative strategies that are more practical and accessible. In response, a range of sensing and biosensing technologies has been developed, with electrochemical methods emerging as particularly promising. Electrochemical sensors offer a reliable, rapid, and cost-efficient solution for detecting electroactive neurotransmitters like serotonin, making them well suited for real-time monitoring in clinical and research settings [21,22,23,24].
SPCE boosts the analyte sensitivity of the target molecules because of the large surface-area-to-volume ratio of catalytic materials. Initially, carbon nanomaterials such as graphene oxide, carbon nanotubes, reduced graphene oxide, carbon nanospheres, and carbon quantum dots had been used as electroactive catalytic materials for sensor applications [13,25,26,27,28,29]. Carbonaceous materials were employed, as they enhance electrochemical behavior and electron transport, increasing active areas, which indisputably increases the active adsorption sites, exhibiting the greater improvement in sensing capabilities. Currently, semiconductor nanoparticles are utilized for electrochemical sensors by enhancing the electron mass transport. Majorly, noble semiconductors and transition metal-based halide materials were employed for sensing target molecules due to their electrocatalytic activity by transducing the immobilization of biomolecules [30,31,32]. The presence of halide moieties on the edge of the fabricated catalysts aids in biosensing because of their high intrinsic electrical conductivity, faster heterogeneous electron transfer, high surface energy, and interlayer van der Waals interface instigated by layer stacking and agglomeration [33,34,35]. Chaudhary et al. have fabricated monophasic molybdenum tetraselenide on the surface of reduced graphene oxide (mn Mo3Se4@rGO) to sense serotonin with an ultrasensitivity of 18.4 µA nM−1 cm−2 [36]. Selvam et al. developed self-assembled silver selenide (Ag2Se) nanoparticles on the surface of rGO sheets, and the nano-catalysts attained were utilized for serotonin detection with real-time application by spiking in Alzheimer’s patient serum samples [37]. Umapathi et al. utilized CuSe nanostructures fabricated on the carbon cloth electrode surface using hydrothermal synthesis and electrodeposition approaches for sensing dopamine at a potential <0.18 V vs. Ag/AgCl with a sensitivity of 8.8 µA µM−1 cm−2 (HT) and 26.8 µA µM−1 cm−2 (ED) [38]. Murtada et al. fabricated CuSe nanoparticles doped with Al on the surface of SPCE to detect L-tyrosine over a linear range of 0.15 µM–10 µM, possessing a lower LOD of 0.04 µM with an application extending to pharmaceutical samples [39]. Umapathi et al. developed CuSe nanostructures to sense glucose using non-enzymatic approach selectively at 0.15 V vs. Ag/AgCl exhibiting a sensitivity of 19.4 mA mM−1 cm−2 over a linear range of 100 nM–40 µM with an LOD of 0.196 µM [40]. Yaseen et al. synthesized nanocomposites of CuSe/PVP/GO and CuSe/MWCNTs to sense glucose non-enzymatically, displaying a sensitivity of 2328 µA mM−1 cm−2 and 4157 µA mM−1 cm−2 along with an LOD of 0.2 µM and 0.3 µM [41].
In this study, CuSe nanostructures were employed for the selective and sensitive detection of serotonin. To the best of our knowledge, this is the first report on the fabrication of sensors based on CuSe for serotonin detection. The developed biosensor efficiently detected serotonin over a wide linear range of 10–1000 nM, with a notably low detection limit of 3 nM and a high sensitivity of 12.45 µM/µA cm−2.
2. Experimental Section
2.1. Materials
Analytical-grade copper (II) acetate monohydrate (Cu(C2H3O2)2·H2O), sodium selenite (Na2SeO3), serotonin hydrochloride (C10H12N2O·HCl), electrolyte salts, commercial serum samples, dopamine, ascorbic acid (C6H8O6), uric acid, various other analytes, and hydrazine hydrates were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification.
2.2. Materials Characterization and Instrumentation
The structural characteristics of the synthesized CuSe nanostructures were analyzed using X-ray diffractometry (XRD, D8-Advance, and Bruker, Billerica, MA, USA). The surface morphology and the chemical composition of the CuSe nanostructures were examined via field-emission scanning electron microscopy (FE-SEM) and in situ energy-dispersive X-ray spectroscopy (EDX, Clara LMH electron microscope, Tescan, Brno, Czech Republic), respectively. All electrochemical measurements were carried out using a CHI 660B electrochemical analyzer (CH Instruments, Austin, TX, USA). SPCE (TE-100, ø = 0.071 cm2) was purchased from Zensor Tech. (Taiwan, China) [42]. The carbon working surface of the SPCE was modified with a dispersed CuSe suspension. The integrated carbon and pseudo-Ag/AgCl electrodes served as the counter and reference electrodes, respectively. All measurements were performed at room temperature. Prior to the experiments, inert argon gas was purged through the supporting electrolyte for 5 min to remove dissolved oxygen. Additionally, the electrochemical impedance spectroscopy (EIS) measurement was conducted with a frequency range of 1 Hz to 100 kHz with a bias potential of 0.18 V vs. Ag/AgCl in a standard redox probe of ferricyanide as a supporting electrolyte. Differential pulse voltammetry (DPV) measurements were employed to evaluate the analytical performance of the fabricated sensor under optimized conditions, using the following parameters: pulse amplitude of 100 mV, pulse width of 2 ms, and pulse period of 1000 ms. All glassware was thoroughly washed with diluted nitric acid (0.7 M) solution followed by rinsing with deionized (DI) water and ethanol.
2.3. Microwave-Assisted Synthesis of CuSe Nanoparticles
CuSe nanoparticles were prepared by rapid microwave-assisted method [43]. Typically, the equal concentrations (1 mmol) of copper acetate monohydrate and sodium selenite were prepared using DI water and mixed under continuous stirring at 500 rpm for 40 min at room temperature. Subsequently, 1 mL of hydrazine hydrate and 1 mL of liquid ammonia were added dropwise to the reaction mixture to maintain an alkaline pH. Afterward, the reaction mixture was transferred into a glass container and placed in a microwave reactor (Samsung, Suwon, Republic of Korea) at 400 W for 10 min. A mass of black product was collected after reaching room temperature. The product was collected, thoroughly washed multiple times with DI water until a neutral pH was achieved, and then vacuum filtered and dried in a vacuum oven at 90 °C for 12 h. Next, the obtained dry product was dispersed in ethanol (2 mg/mL) and used for electrochemical biosensor fabrication. About 3 µL of the dispersed CuSe suspensions was drop-casted onto the SPCE working electrode surface and allowed to dry at room temperature and used for further electrochemical measurements.
2.4. Electrochemical Studies
Electrochemical characterization of the fabricated CuSe-modified SPCE (CuSe/SPCE) for serotonin detection was carried out under neutral pH (0.1 M PBS at 7.4) conditions. In addition, the different concentrations of serotonin standard solutions (~30 µL) were dropped onto the miniaturized SPCE surface, and their signal responses were measured. Cyclic voltammetry (CV) measurements were performed at a potential range of −0.5 to 0.5 V at a scan rate of 50 mV/s. Preceding each measurement, the CuSe/SPCE was dipped in 0.1 M PBS solution (blank), and CV was measured to confirm the absence of serotonin anodic signals. After each measurement, the modified SPCE surface was covered with a few µL of DI water for 120 s to remove the serotonin for reclaiming and to restore the active surface.
3. Results and Discussion
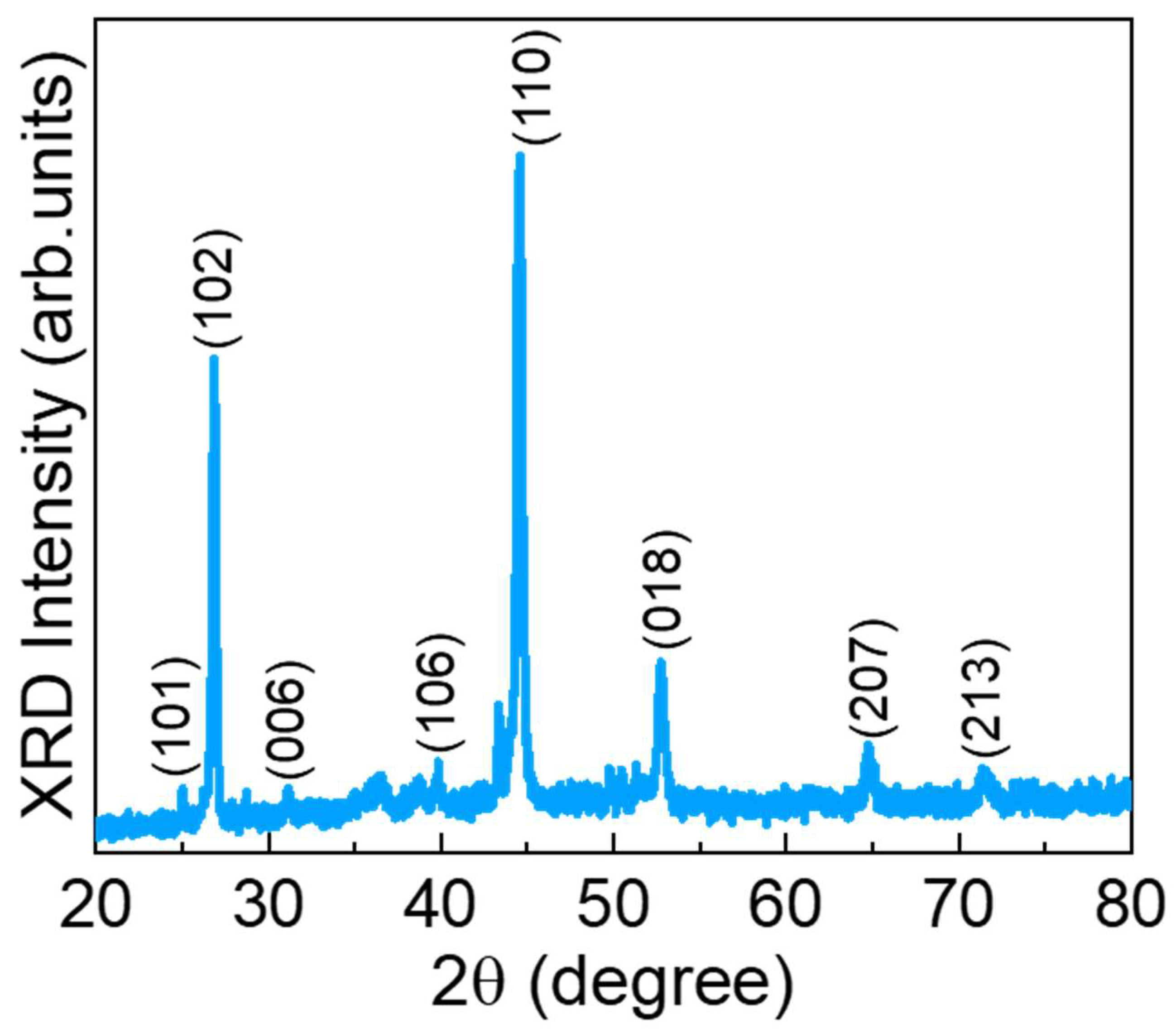
Figure 1 shows the XRD pattern of the CuSe nanostructures. The CuSe showed the various diffraction angles at 25.17, 26.89, 31.17, 40.06, 44.58, 52.72, 64.85, and 71.39° corresponding to the crystal planes of (101), (102), (006), (106), (110), (018), (207), and (213) for the hexagonal klockmannite phase of CuSe (JCPDS card no: 00-006-0427), respectively [44,45,46,47]. Furthermore, no other peaks were observed in the prepared material, demonstrating the high purity of the material.
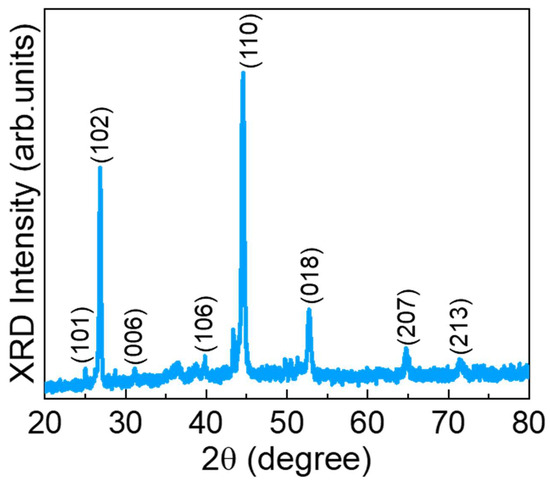
Figure 1.
XRD pattern of the CuSe nanoparticles.
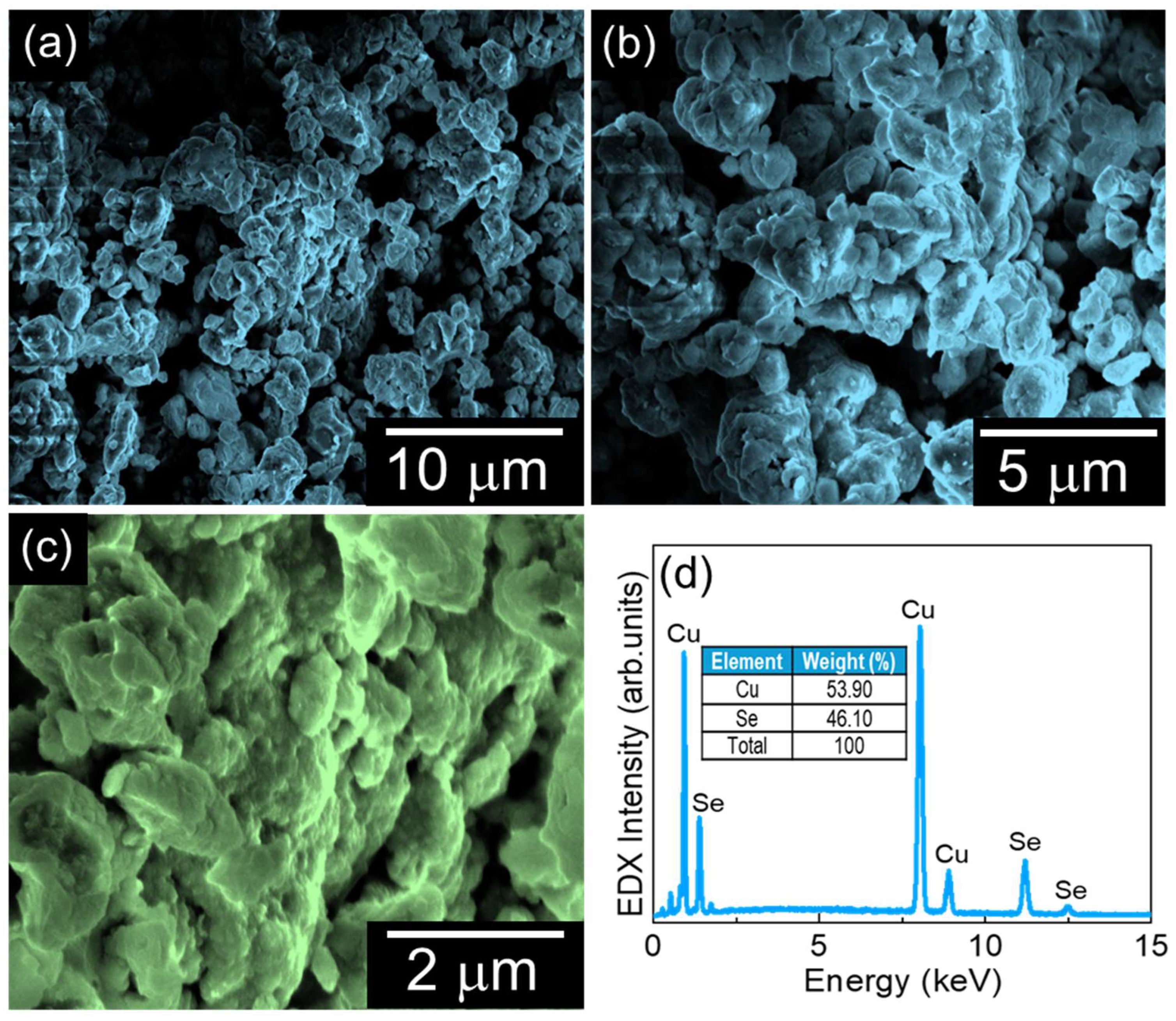
The surface morphology of the prepared CuSe material was examined using FE-SEM measurements. Figure 2a–c display the different magnification FE-SEM images of the CuSe material. The CuSe exhibited an agglomerated, interconnected cluster-like morphology composed of irregularly shaped nanoparticles. The chemical composition of the CuSe nanoparticles was characterized by in situ EDX measurement. Figure 2d shows the EDX spectra of the nanostructures. The CuSe nanostructures revealed their own intrinsic Cu and Se constituents, indicating that the fabricated materials have high purity with no other impurities.
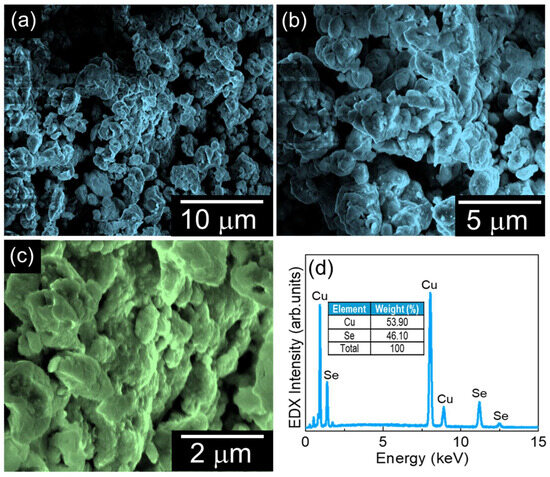
Figure 2.
(a–c) Different magnification FE-SEM images of CuSe nanoparticles and (d) EDX spectra of CuSe nanoparticles.
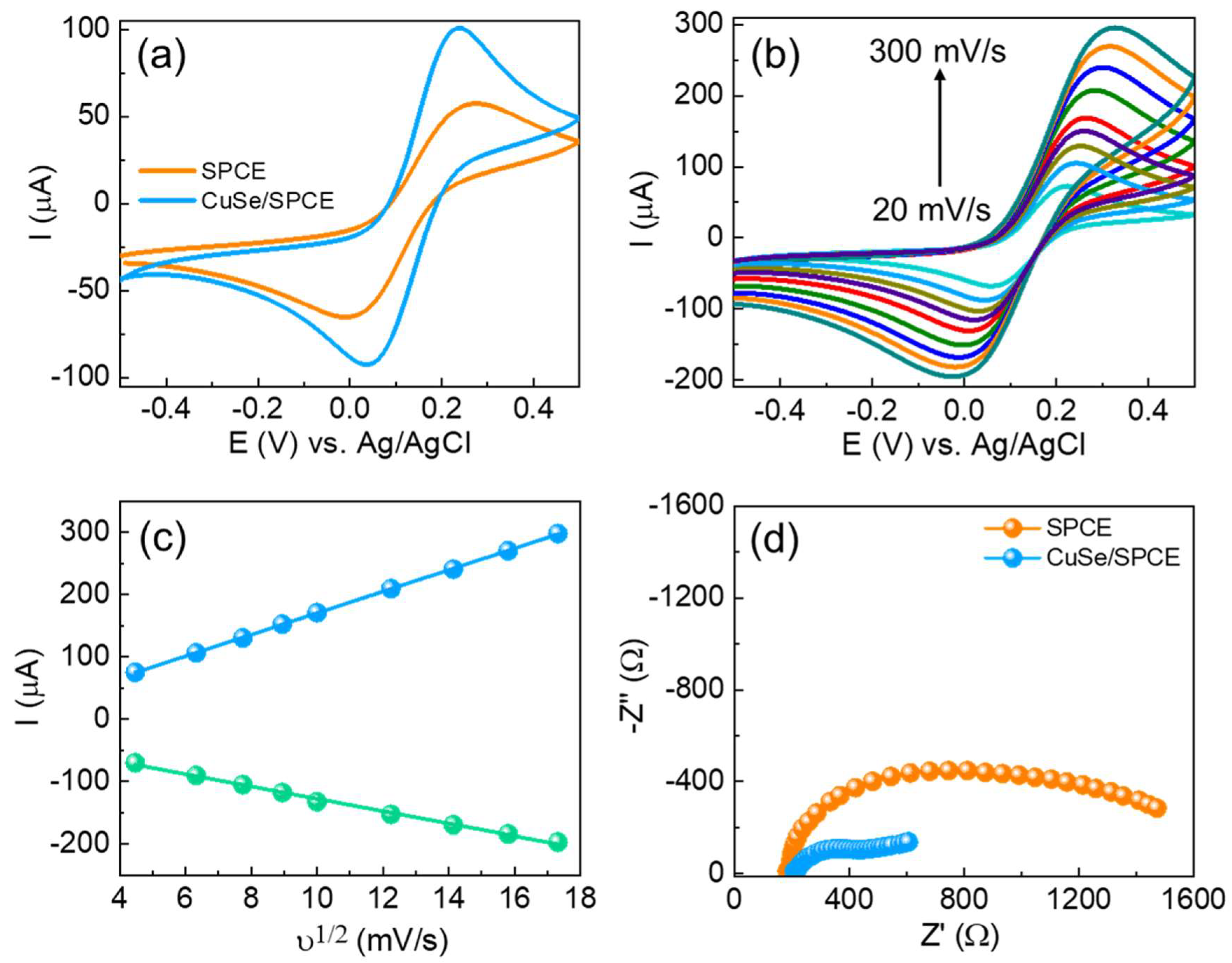
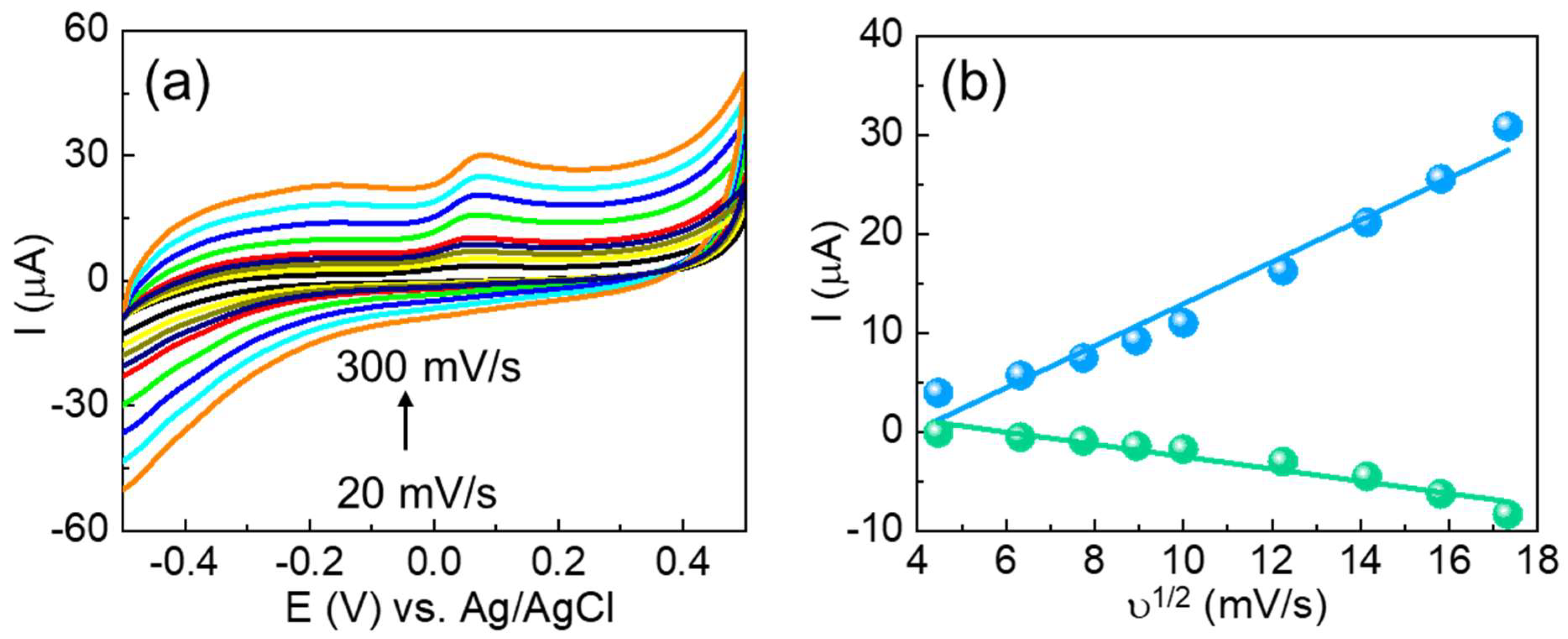
The electrochemical behavior and electrode–electrolyte interfacial properties of the fabricated CuSe/SPCE were initially examined by using CV measurements. CV is a widely utilized technique for preliminary assessment of electrode surfaces in half-cell systems, as it provides critical information about both capacitive and Faradaic processes over a broad potential window. Figure 3a shows the CV results of both unmodified and CuSe-coated SPCEs in 5 mM of [Fe(CN)6]3−/4− in 0.1 M KCl solution with a sweeping potential of −0.5 to 0.5 V vs. Ag/AgCl at a scan rate of 50 mV/s. Based on the CV results, the unmodified SPCE showed a minimal peak of Epa: 0.27 V, Ipa: 58.57 µA, Epc: −0.008 V, and Ipc: −66.54 µA with a peak-to-peak separation (∆Ep) of 0.27 mV. The CuSe/SPCE showed enhanced redox performance with Epa: 0.23 V, Ipa: 102.1 µA, Epc: 0.03 V, and Ipc: −93.34 µA with a low ∆Ep of 0.19 mV attributed to a fast electron transfer nature with an enhanced active surface area. In addition, one of the main reasons for the redox current enhancement on the CuSe/SPCE is due to the enhanced electroactive surface, which results in enhancing the electrocatalytic performance towards serotonin oxidation. To verify the electrochemical active surface area, CV with different scan rates was examined in standard redox (5 mM of [Fe(CN)6]3−/4− in 0.1 M KCl) solution with both electrodes, and the results obtained are shown in Figure 3b. The CV results revealed that the redox peak current was linearly increased while increasing scan rates from 20 to 300 mV/s. By using the Randles–Sevcik formula, the electrochemically active surface was estimated from the redox current vs. square root of scan rates (Figure 3c) as per our earlier reports [48,49], and the estimated active surface area of the unmodified SPCE 0.0632 and CuSe/SPCE of 0.1759 cm2 is enhanced 2.7-fold. These results revealed that the enhanced electrochemical active surface area during SPCE modification and the redox current response increased.
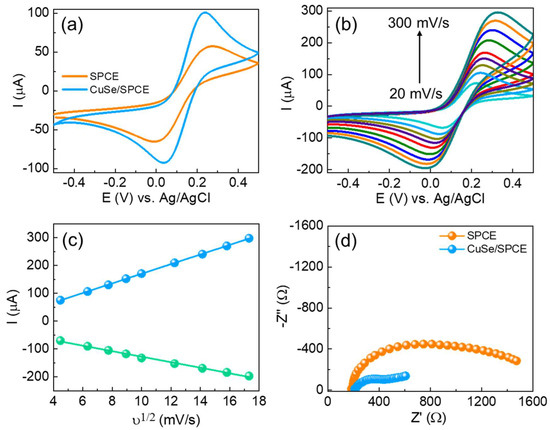
Figure 3.
(a) CV results of unmodified and CuSe-modified SPCEs in 5 mM of [Fe(CN)6]3−/4− in 0.1 M KCl solution; (b) effect of different scan rates (20 to 300 mV/s) in similar experimental conditions of CuSe; (c) relationship between ν1/2 vs. redox current of CuSe; (d) EIS results of different electrodes in 5 mM of [Fe(CN)6]3−/4− in 0.1 M KCl solution with an applied potential of 0.18 V vs. Ag/AgCl.
EIS techniques are a significant technique for analyzing electrode–electrolyte interfacial changes in the modified surfaces. The impedance measurements showed how surface structure modification significantly influences electrochemical performance. Figure 3d shows the Nyquist graphs of both unmodified and CuSe/SPCE sensors in 5 mM of [Fe(CN)6]3−/4− in 0.1 M KCl solution. The EIS results were further fitted with the Randles equivalent circuit model. The results indicate that the semicircle part corresponds to the charge transfer resistance (Rct) because of the electron transfer kinetics in the electrode–electrolyte interface. The unmodified SPCE showed Rct of 224.1 Ω, while the CuSe/SPCE showed a smaller semicircle Rct of 145.2 Ω. Based on the EIS, the CuSe/SPCE exhibited enhanced electrical conductivity with minimal charge transfer resistance. From the EIS and Rct values, the exchanged current density (jo) was estimated, based on our previous reports [50,51] and the noted jo values, to be 0.0574 and 0.3541 cm−2 for the unmodified and CuSe/SPCE sensors, respectively. Moreover, the CuSe/SPCE exhibited 6.1 times the enhanced current density compared with unmodified SPCE, and these results revealed outstanding electrochemical performance related to other electrodes.
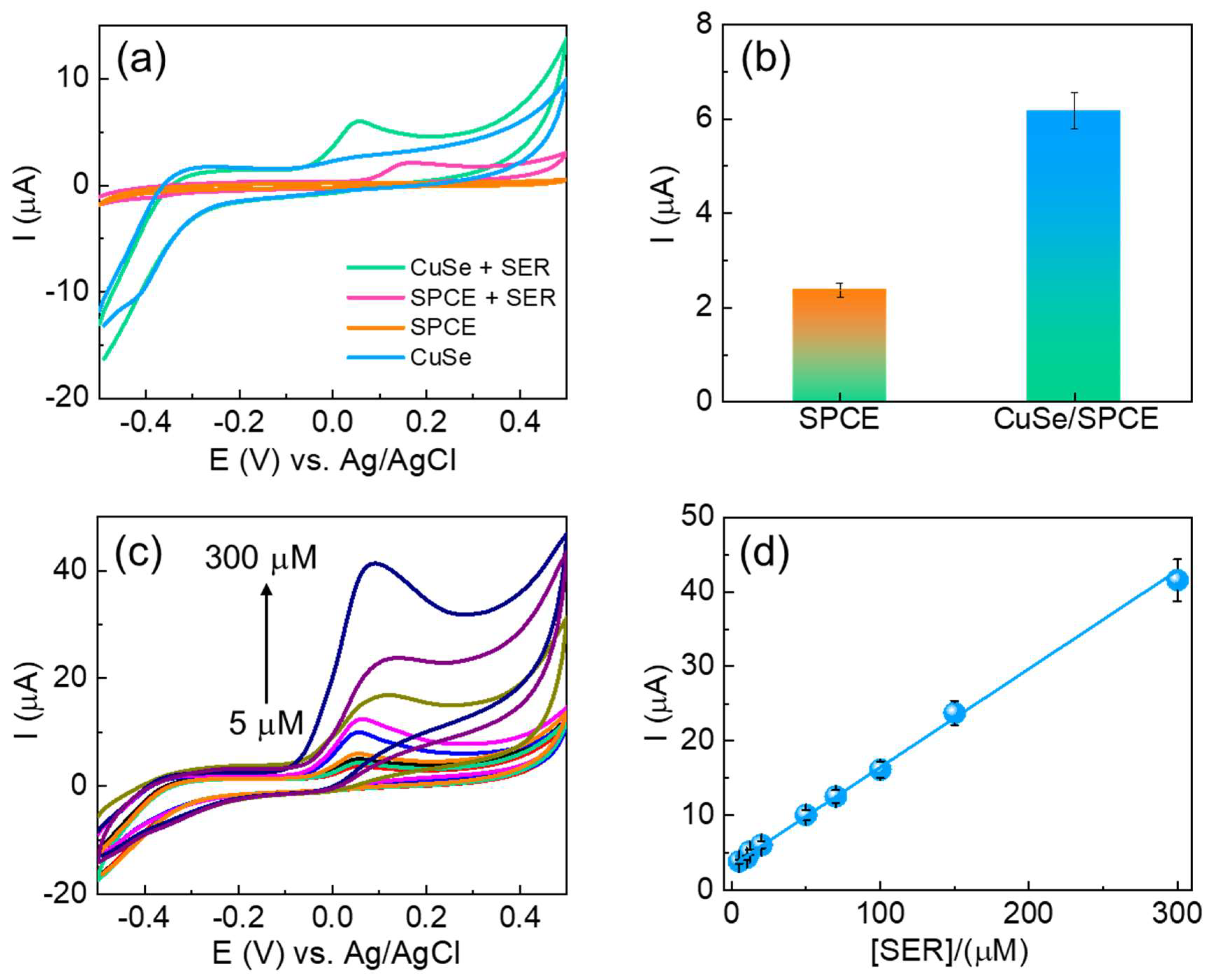
The electroanalytical studies of the fabricated CuSe/SPCE were examined for serotonin detection using CV techniques. The unmodified and CuSe/SPCE sensors were used to detect 20 µM of serotonin in 0.1 M PBS (pH-7.4), and the results are shown in Figure 4a. The unmodified SPCE showed poor oxidation (sensing) performance at an oxidation potential of 0.16 V with a lower current of 2.23 µA. The CuSe/SPCE showed a lower oxidation potential of 0.07 V with an enhanced peak current of 6.15 µA (Figure 4b). This greater electrochemical sensing performance of the CuSe/SPCE towards serotonin detection is attributed to an enhanced electrochemical active surface area with rapid electron transfer kinetics and the synergistic effect of the developed nanostructures. Under optimized experimental conditions, CV techniques were applied for serotonin detection with different concentrations (Figure 4c). From the CV results, as serotonin concentrations increased, their corresponding oxidation peak current increased linearly in the concentrations from 5 to 300 µM (Figure 4d), with a good correlation coefficient of 0.9964. The CV results suggest that the fabricated CuSe nanostructures based electrochemical biosensors were highly applicable towards the detection of serotonin.
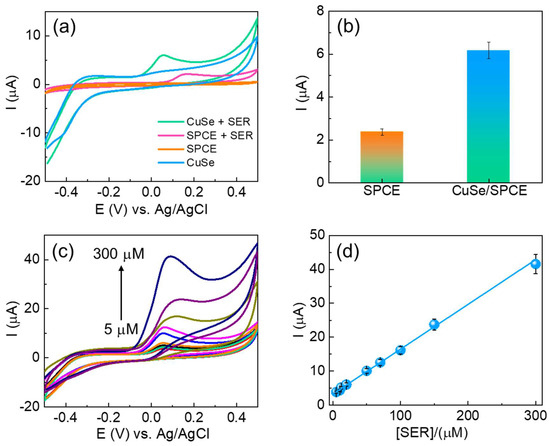
Figure 4.
(a) CV results of unmodified and CuSe-modified SPCEs towards serotonin detection; (b) bar chart of serotonin oxidation current obtained from CV results; (c) CV results of different concentrations of serotonin detection of (5–300 µM) in 0.1 M PBS (pH-7.4) as supporting electrolytes at a 50 mV/s scan rate of CuSe/SPCE (d) corresponding linear concentration graph (5–300 µM).
In addition, the fabricated CuSe/SPCE used for the detection of serotonin was optimized with different experimental parameters including supporting electrolyte pH and concentrations of supporting electrolytes, and different scan rate influences and stabilities were examined to better determine analytical detection performance. The effect of supporting electrolytes (buffer solutions) was examined using 20 µM of serotonin. Different electrolytes including acetate buffer, phosphate buffer (pH range of 5.5 to 8.5), and KCl and NaCl (0.1 M concentrations) were tested using DPV techniques. Among the different electrolytes, the phosphate buffer (pH-7.4) showed an enhanced oxidation peak current. Moreover, most neurotransmitters showed a better oxidation response under a neutral pH condition because the serotonin pKa of 9.97 can easily form the protonated amine group as a result of the effective electrostatic interaction with the modified electrode surface and the effective electrochemical oxidation process, which are more favorable in the neutral medium [42,51,52]. Thus, 0.1 M PBS (pH-7.4) was used as a supporting electrolyte towards effective detection of serotonin in the electrochemical measurements. The viability of the electron transfer kinetics for serotonin detection was examined using CuSe/SPCE with different scan rates (20 to 300 mV/s), and the results obtained are shown in Figure 5a. Here, with enhancing the scan rate, the serotonin oxidation peak current increased linearly with a linear relationship between the different scan rates and their corresponding oxidation and reduction peak current, given by the following: Ipa = 2.1205ν + 2.2275, R2 = 0.9843 and Ipc = 3.6711ν − 0.6205, R2 = 0.9619 (Figure 5b). These results suggested that the serotonin oxidation process on the CuSe/SPCE surface is basically ruled by a quasi-reversible adsorption process [51]. Hereby, only the oxidation peak potential is observed, indicating the ability of electrochemical oxidation of serotonin to form quinone-like species, whilst the reduction kinetics is obscured, which typically indicates that the sensing mechanism is less reversible.
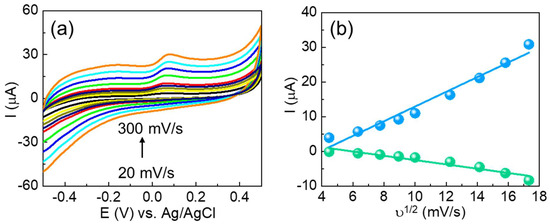
Figure 5.
(a) CV results of different scan rates (20–300 mV/s) for the redox response of CuSe/SPCE in 0.1 M PBS containing serotonin and (b) corresponding current vs. ν1/2 of CuSe/SPCE.
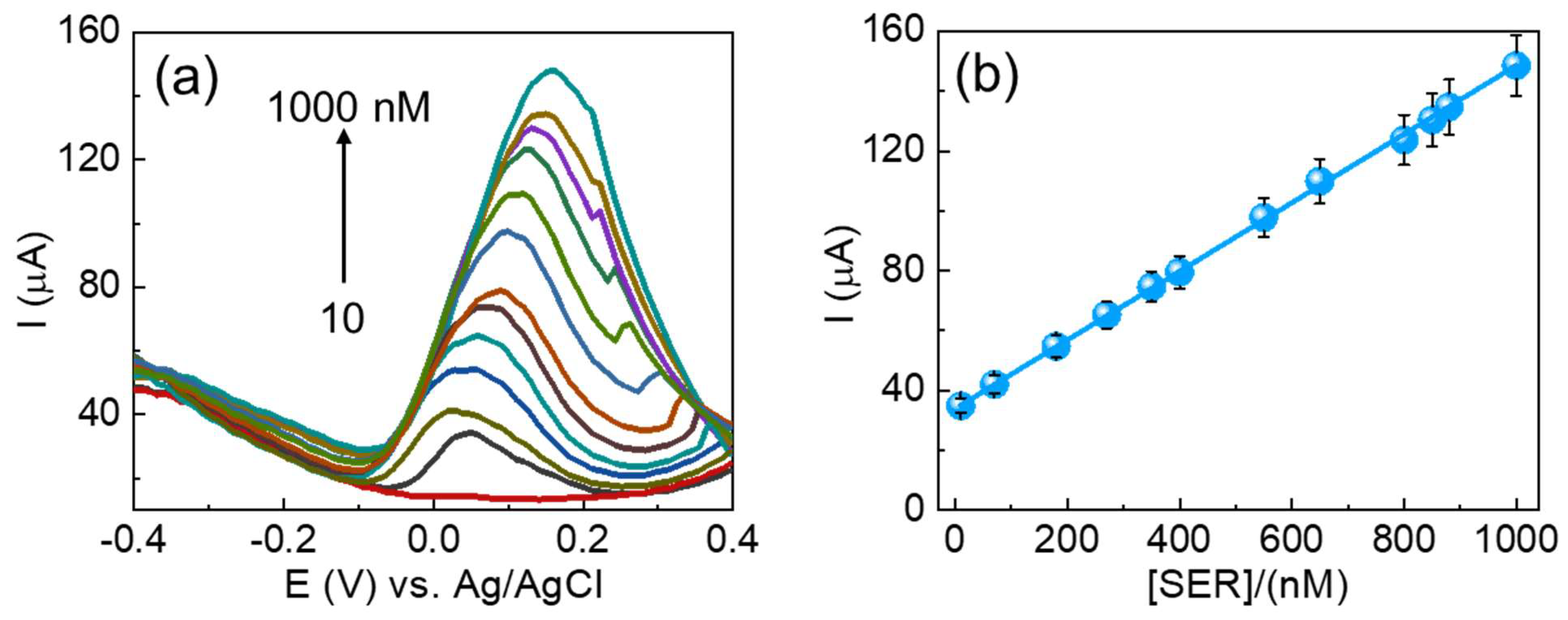
The analytical performance of the fabricated CuSe/SPCE was effectively applied for the DPV technique analysis of different concentrations of serotonin under optimized experimental conditions (Figure 6a). For the DPV results, the oxidation peak gradually increased, while raising the serotonin concentrations from 10 to 1000 nM. Figure 6b shows a linear relationship between the serotonin concentrations’ oxidation current and their corresponding concentrations. The achieved linear regression formula is Ipa (µA) = 3.3930 (serotonin) + 0.1146 with a correlation coefficient of (R2) 0.9997. In addition, the estimated detection limit (S/N = 3) is 3 nM, and these results are highly suitable concentrations for the detection of serotonin in serum samples. Explicitly, patients suffering from mental illness can have overly low concentrations of serotonin or fluctuating concentrations, which are difficult to detect with standard diagnostic sensing techniques. The sensitivity of the fabricated CuSe/SPCE was estimated to be 12.45 µA/µM cm−2. Moreover, the analytical performance results achieved were benchmarked with earlier reports and are shown in Table 1. Based on the benchmarked results, the fabricated CuSe/SPCE could be qualified as a biosensing approach for the detection of serotonin with an enhanced electroactive surface, fast electron transfer kinetics with high conducting nature, and enhanced electrocatalytic as well as synergistic performance. In addition, compared with earlier reports, the CuSe/SPCE showed lower oxidation potential towards serotonin detection.
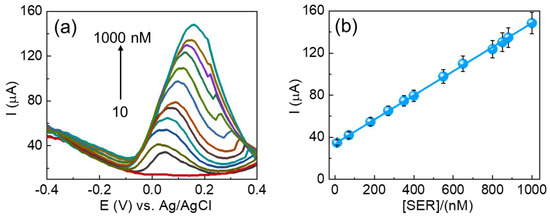
Figure 6.
(a) DPV results of CuSe/SPCE for different concentrations of serotonin detection and (b) corresponding linear concentration vs. current graph (10–1000 nM) of CuSe/SPCE.

Table 1.
Benchmarking the analytical performance of the different electrochemical biosensors for the detection of serotonin.
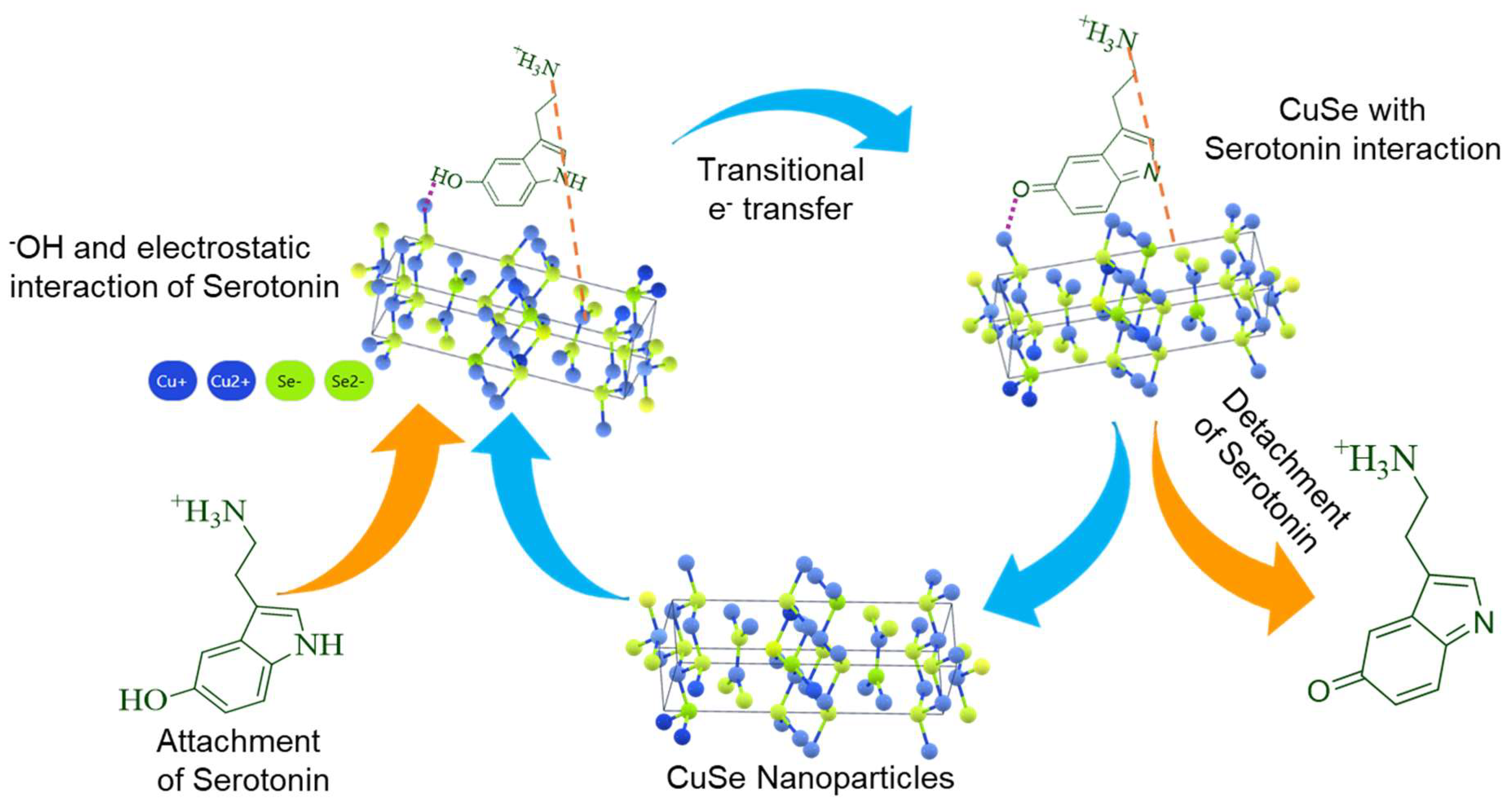
The feasible electrochemical detection mechanism for serotonin using CuSe/SPCE is shown in Figure 7. Evidently, several features are synergistically valuable for highly specific and sensitive detection of serotonin. Initially, the serotonin oxidation reaction process involves the conversion of the hydroxyl (-OH) group to the keto (C=O) group, and this conversion process started from the surface adsorption of serotonin through the coordination of a hydroxyl group containing serotonin with active catalytic metal sites. A Lewis acid (transition metal active sites) participated in the oxidation reaction to accommodate an electron-rich Lewis base; as a result, the redox potential of these transition metal active sites had excessive influence on the ease of hydroxyl group connection on the sensor surface, which is reflected in the bias potential required for serotonin oxidation. Typically, hydroxyl group connections on the active metal sites will occur at lower potential if the neighboring oxidation process occurs at the lowest potential [38]. Earlier, it was suggested that the oxidation process at local active sites followed by the adsorption of hydroxyl groups to the transition metal active sites can be modified by altering the ligand coordination environment near the active sites. Normally, minimizing the electronegativity and enhancing covalent connection around the active sites leads to a reduction in the local site oxidation potential and high facile hydroxyl group attachment on the sensor surface at the lowest applied potential. Thus, it can be expected that the reduced electronegativity and enhanced covalent connection of Se compared with O could create serotonin absorption on the surface of selenide-based catalysts at the lowest oxidation potential compared with metal oxides, thereby achieving the lower serotonin oxidation potential of the proposed CuSe/SPCE. Moreover, interfacial electron transfer (electrode–electrolyte) behavior occurs during serotonin oxidation, and adsorption on the sensor surface results in the oxidation of serotonin-to-serotonin quinoneimine. Notable electron transfer will be assisted by the enhanced conducting nature of the developed nanostructures. In addition, the increased covalent nature of the anionic ligand also enhances conductivity by minimizing the bandgap in the selenides. Serotonin’s oxidized form can willingly detach from the nanostructures’ surface while the electro-catalytic surfaces are restored. Moreover, the coordination geometrical structure near the active catalytic sites of Cu can also participate in the serotonin adsorption process. The CuSe crystal structure has two coordination sites for Cu, which are trigonal and tetragonal coordination structures [60,61]. The lowest coordination numbers with a layered geometrical structure can start to facilitate the connection of serotonin. Finally, the high active surface area of the CuSe nanostructures showed that the ultra-thin edges of the nanoflakes improve the coverage of the metal active sites to the serotonin, leading to the enhanced efficacy of serotonin detection through a direct oxidation process. Hence, the synergistic effect of surface morphology, porosity, coordination of geometrical structure, enhanced conductivity, and enhanced anionic covalent nature leads to serotonin oxidation on the CuSe/SPCE surface occurring at a lower potential with an enhanced oxidation peak current with increased sensitivity, achieving the lowest detection limit.
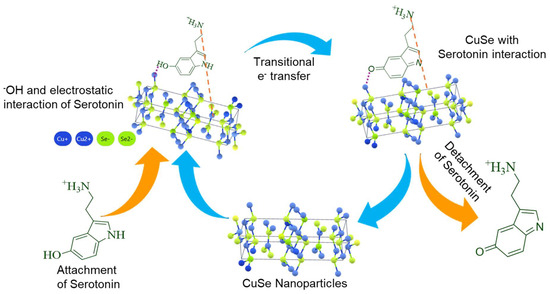
Figure 7.
Possible electrochemical detection mechanism of CuSe/SPCE for serotonin.
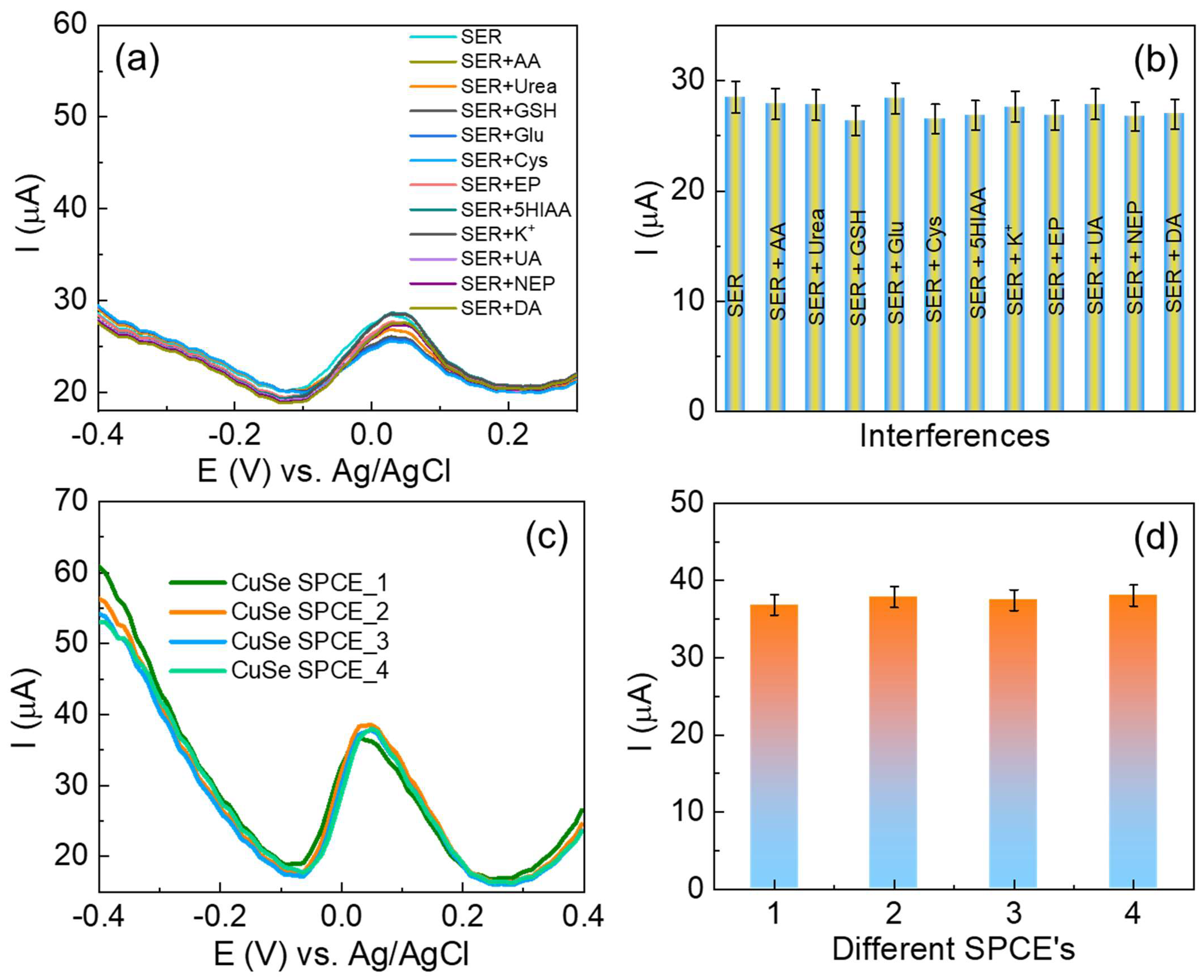
To examine the competence of the fabricated CuSe/SPCE, the interference effect of foreign species during serotonin detection (110 µM) was investigated for several specific interfering species in the samples studied, including ascorbic acid, urea, glutathione, glucose, cysteine, epinephrine, dopamine (5-fold excess concentrations), and Na+ and K+ ions (10-fold excess concentrations), and the DPV results obtained are shown in Figure 8a,b. There were no significant signal changes observed, even when other interfering species present in 5-fold excess concentrations suggested that the fabricated CuSe/SPCE has better selectivity towards serotonin detection. However, 5 µM of dopamine was minimally affected during serotonin oxidation (~2.1 µA current reduced). The possibility of potential interference was studied when an analytical deviation of above 30% compared to the analytical signal achieved with the developed method was present in the absence of interfering species. These interference results revealed that the fabricated CuSe/SPCE-based electrochemical biosensor has better anti-interference ability towards the detection of serotonin.
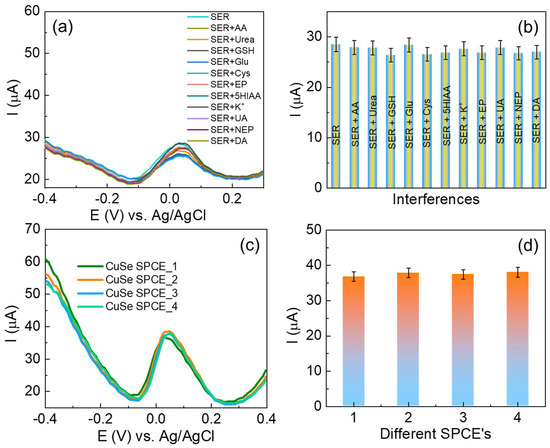
Figure 8.
(a) DPV results for CuSe/SPCE towards selective detection of serotonin in the presence of possible interfering species; (b) bar graphs of different interfering species and their oxidation current response; (c) reproducibility studies of different CuSe/SPCEs for serotonin detection DPV results; (d) serotonin oxidation current obtained from different CuSe/SPCEs.
The reproducibility measurement of the fabricated CuSe/SPCE was performed by inter- and intra-assay methods. First, the intra-assay examination was carried out by detecting 30 nM serotonin with four separately fabricated SPCEs using the same fabrication protocol, followed by the DPV measurement, and the results are shown in Figure 8c,d with an RSD of 2.58%. In addition, inter-assay examinations were carried out with five successive additions of 30 nM, and their oxidation current responses were observed with four different parallel SPCEs showing an RSD of 9.87%, 8.32%, 6.54%, and 6.74%, respectively. These reproducibility results suggest that the fabricated CuSe/SPCE has better reproducibility for the detection of serotonin. In addition, the long-time sensing stability performance of the fabricated sensor was examined over two weeks, and the sensor exhibited a stable and better oxidation current response towards serotonin detection. After two weeks, the sensor exhibited <4% of the oxidation current due to the surface fouling process or other interference effects on the SPCE. To prevent the sensor surface fouling process and ensure the nanostructure’s stability, the sensor was stored in an air-tight glass container under ambient conditions for future electrochemical detection purposes.
After the successful detection of serotonin in PBS medium, the fabricated CuSe/SPCEs were used for sensing in commercial serum samples using facile standard addition approaches. The commercial serum samples were obtained from Sigma-Aldrich. Serum stock solutions were diluted 100-fold using 0.1 M PBS (pH-7.4). Then, 50 µL of diluted serum was added with different concentrations of serotonin, and the serotonin detection measurements were effectively applied. The DPV method was utilized to measure the serum serotonin concentrations, and the results obtained exhibited recoveries from 96.86% to 102.14% with RSD of 2.48% and 1.79%. The serotonin recovery details are shown in Table 2. These recovery results suggest that the possible interfering species within the serum sample matrix did not effectively affect the serotonin detection process. Hence, the fabricated CuSe/SPCE exhibits better sensing ability towards serotonin in a real serum matrix.

Table 2.
Serotonin detection in commercial serum samples with CuSe/SPCE.
4. Conclusions
CuSe nanostructures were successfully synthesized using a facile and efficient microwave-assisted method. The as-prepared CuSe nanostructures were thoroughly characterized using various spectroscopic, microscopic, and electrochemical techniques to confirm their structural and functional properties. The electroanalytical performance of the fabricated CuSe/SPCE was effectively utilized for serotonin detection and achieved wide linear concentrations (1 to 1000 nM) with a low detection limit (3 nM) and high sensitivity (12.45 µM/µA cm−2). Moreover, the fabricated sensor demonstrated superior specificity in the presence of potential interfering species with highly reproducible results. Further, the real-time application of the fabricated CuSe/SPCE was successfully utilized for serotonin detection in commercial serum samples and obtained excellent recoveries. Since serotonin is a significant neurotransmitter, sensing and diagnosis have become more popular for screening for mental-health-related illnesses. These findings can pave the way toward designing miniaturized serotonin biosensing platforms and will help to realize the correlation between serotonin as a biomarker and the advancement of neurodegenerative illness.
Author Contributions
S.S. (Sankar Sekar): methodology, formal analysis, investigation, and writing—original draft; R.M.: methodology, conceptualization, formal analysis, and investigation; S.K.A.: conceptualization and formal analysis; S.S. (Saravanan Sekar): data curation and formal analysis; Y.L.: data curation and validation; S.-C.C.: data curation, conceptualization, validation, and supervision; S.L.: conceptualization, supervision, investigation, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation (NRF) of Korea through basic science research programs (2021R1I1A1A01049638; RS-2023-NR076644, RS-2022-NR075805) funded by the Korean Government. This study was supported by the Engineering and Academic Research (R&D) Program through the NRF, funded by the Ministry of Education (RS202300249778). This study was also supported by an NRF grant funded by the Korean government (MSIT) (RS-2021-NR060086).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lin, M.; Tsay, H.; Su, W.; Chueh, F. Changes in extracellular serotonin in rat hypothalamus affect thermoregulatory function. Am. J. Physiol. Regul. Integr. Comp. Physio. 1998, 274, R1260–R1267. [Google Scholar] [CrossRef] [PubMed]
- Lacasse, J.R.; Leo, J. Serotonin and depression: A disconnect between the advertisements and the scientific literature. PLoS Med. 2005, 2, e392. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Serotonin in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Canli, T.; Lesch, K.-P. Long story short: The serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 2007, 10, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Markham, C.H.; Clark, W.G. Serotonin (5-HT) metabolism in Huntington’s chorea. Life Sci. 1968, 7, 707–712. [Google Scholar] [CrossRef]
- Bird, E.D. Chemical pathology of Huntington’s disease. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Carver, C.S.; Johnson, S.L.; Joormann, J. Two-mode models of self-regulation as a tool for conceptualizing effects of the serotonin system in normal behavior and diverse disorders. Curr. Dir. Psychol. Sci. 2009, 18, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Tekes, K. HPLC determination of serotonin and its metabolites from human platelet-rich plasma; shift to 5-hydroxytryptophol formation following alcohol consumption. J. Chromatogr. Sci. 2008, 46, 169–173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maurer-Spurej, E.; Dyker, K.; Gahl, W.A.; Devine, D.V. A novel immunocytochemical assay for the detection of serotonin in platelets. Br. J. Haematol. 2002, 116, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevisan, K.; Maleki, H.; Honarvarfard, E.; Baharifar, H.; Gholami, M.; Faridbod, F.; Larijani, B.; Faridi Majidi, R.; Khorramizadeh, M.R. Nanomaterial based electrochemical sensing of the biomarker serotonin: A comprehensive review. Microchim. Acta 2019, 186, 49. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, A.; Noori, A. A cyclodextrin host–guest recognition approach to an electrochemical sensor for simultaneous quantification of serotonin and dopamine. Biosens. Bioelectron. 2011, 26, 4674–4680. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Wang, X.; Zhu, W.; Han, Q.; Zhu, C.; Hong, J.; Zhou, X.; Jiang, H. Electrochemical serotonin sensing interface based on double-layered membrane of reduced graphene oxide/polyaniline nanocomposites and molecularly imprinted polymers embedded with gold nanoparticles. Sens. Actuators B Chem. 2014, 196, 57–63. [Google Scholar] [CrossRef]
- Tertiș, M.; Cernat, A.; Lacatiș, D.; Florea, A.; Bogdan, D.; Suciu, M.; Săndulescu, R.; Cristea, C. Highly selective electrochemical detection of serotonin on polypyrrole and gold nanoparticles-based 3D architecture. Electrochem. Commun. 2017, 75, 43–47. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, N.; Tomar, V.; Chandra, R. A review on electrochemical detection of serotonin based on surface modified electrodes. Biosens. Bioelectron 2018, 107, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Zhao, X.-E.; Zhu, S.; He, Y.; Zheng, L.; Chen, G.; You, J.; Liu, S.; Liu, Z. Determination of dopamine, serotonin, biosynthesis precursors and metabolites in rat brain microdialysates by ultrasonic-assisted in situ derivatization–dispersive liquid–liquid microextraction coupled with UHPLC-MS/MS. Talanta 2016, 161, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Darshan, S.; Baggi, T.R. Recent Advances in Analytical Techniques for Antidepressants Determination in Complex Biological Matrices: A Review. Int. J. Toxicol. 2023, 42, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Dunham, K.E.; Venton, B.J. Improving serotonin fast-scan cyclic voltammetry detection: New waveforms to reduce electrode fouling. Analyst 2020, 145, 7437–7446. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Singh, J.; Saini, K.; Chaudhary, V.; Umar, A.; Ibrahim, A.A.; Akbar, S.; Baskoutas, S. Paper-based sensors: Affordable, versatile, and emerging analyte detection platforms. Anal. Methods 2024, 16, 2777–2809. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Piatkevich, K.D. Techniques for in vivo serotonin detection in the brain: State of the art. J. Neurochem 2023, 166, 453–480. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gupta, R.; Bansal, D.; Bhateria, R.; Sharma, M. A Review on Recent Trends and Future Developments in Electrochemical Sensing. ACS Omega 2024, 9, 7336–7356. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhao, S.; Chen, F.; Lv, Y.; Fu, L. Enhancing Sensitivity and Selectivity: Current Trends in Electrochemical Immunosensors for Organophosphate Analysis. Biosensors 2024, 14, 496. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhao, Y.; Huang, Q.; Huang, J.; Tao, Y.; Chen, J.; Li, H.-Y.; Liu, H. Electrochemical protein biosensors for disease marker detection: Progress and opportunities. Microsyst. Nanoeng. 2024, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Bhalla, V.; Dravid, V.; Shekhawat, G.; Prasad, E.S.; Suri, C.R. Enhancing electrochemical detection on graphene oxide-CNT nanostructured electrodes using magneto-nanobioprobes. Sci. Rep. 2012, 2, 877. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Cui, B.; Liu, J.; Song, Y.; Wang, M.; Peng, D.; Zhang, Z. Novel electrochemical biosensor based on core-shell nanostructured composite of hollow carbon spheres and polyaniline for sensitively detecting malathion. Sens. Actuators B Chem. 2018, 258, 813–821. [Google Scholar] [CrossRef]
- Dăscălescu, D.; Apetrei, C. Development of a novel electrochemical biosensor based on organized mesoporous carbon and laccase for the detection of serotonin in food supplements. Chemosensors 2022, 10, 365. [Google Scholar] [CrossRef]
- Sekar, S.; Yun, J.-S.; Lee, S. Metal-free electrocatalytic nanocomposites of poly azovan blue-decorated graphitic carbon nitride for simultaneously sensing paracetamol and 4-aminophenol. Environ. Res. 2023, 239, 117293. [Google Scholar] [CrossRef] [PubMed]
- Liuzhu, Z.; Sekar, S.; Chen, J.; Lee, S.; Kim, D.Y.; Manikandan, R. A polyrutin/AgNPs coated GCE for simultaneous anodic stripping voltammetric determination of Pb(II) and Cd(II)ions in environmental samples. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129082. [Google Scholar] [CrossRef]
- Devi, N.R.; Sasidharan, M.; Sundramoorthy, A.K. Gold nanoparticles-thiol-functionalized reduced graphene oxide coated electrochemical sensor system for selective detection of mercury ion. J. Electrochem. Soc. 2018, 165, B3046–B3053. [Google Scholar] [CrossRef]
- Wang, F.; Hu, S. Electrochemical sensors based on metal and semiconductor nanoparticles. Microchim. Acta 2009, 165, 1–22. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Lin, C.; Zhang, Y.; Hu, S.; Wei, C. A high performance electrochemical biosensor based on Cu2O–carbon dots for selective and sensitive determination of dopamine in human serum. Rsc. Adv. 2015, 5, 54102–54108. [Google Scholar] [CrossRef]
- Chae, S.; Woo, C.; Gu, G.H.; Kim, T.Y.; Jeon, J.; Kwon, H.J.; Oh, S.; Choi, K.H.; Dong, X.; Ahn, J. Vanadium Selenide Nanobelt Electrocatalyst for Dopamine-Selective Detection. ACS Appl. Nano Mater. 2023, 6, 16242–16252. [Google Scholar] [CrossRef]
- Dăscălescu, D.; Apetrei, C. Nanomaterials based electrochemical sensors for serotonin detection: A review. Chemosensors 2021, 9, 14. [Google Scholar] [CrossRef]
- Sekar, S.; Huijun, J.; Liuzhu, Z.; Jin, C.; Lee, S.; Kim, D.Y.; Manikandan, R. Copper phthalocyanine conjugated graphitic carbon nitride nanosheets as an efficient electrocatalyst for simultaneous detection of natural antioxidants. Electrochim. Acta 2022, 413, 140150. [Google Scholar] [CrossRef]
- Chaudhary, C.; Kumar, S.; Chandra, R. Monophasic molybdenum selenide-reduced graphene oxide nanocomposite sheets based immunosensing platform for ultrasensitive serotonin detection. Microchem. J. 2020, 159, 105344. [Google Scholar] [CrossRef]
- Selvam, S.P.; Yun, K. A self-assembled silver chalcogenide electrochemical sensor based on rGO-Ag2Se for highly selective detection of serotonin. Sens. Actuators B Chem. 2020, 302, 127161. [Google Scholar] [CrossRef]
- Umapathi, S.; Masud, J.; Coleman, H.; Nath, M. Electrochemical sensor based on CuSe for determination of dopamine. Microchim. Acta 2020, 187, 440. [Google Scholar] [CrossRef] [PubMed]
- Murtada, K.; Salghi, R.; Ríos, A.; Zougagh, M. A sensitive electrochemical sensor based on aluminium doped copper selenide nanoparticles-modified screen printed carbon electrode for determination of L-tyrosine in pharmaceutical samples. J. Electroanal. Chem. 2020, 874, 114466. [Google Scholar] [CrossRef]
- Umapathi, S.; Singh, H.; Masud, J.; Nath, M. Nanostructured copper selenide as an ultrasensitive and selective non-enzymatic glucose sensor. Mater. Adv. 2021, 2, 927–932. [Google Scholar] [CrossRef]
- Yaseen, J.; Saira, F.; Imran, M.; Fatima, M.; Ahmed, H.E.; Manzoor, M.Z.; Rasheed, M.; Nisa, I.; Mehmood, K.; Batool, Z. Synthesis of CuSe/PVP/GO and CuSe/MWCNTs for their applications as nonenzymatic electrochemical glucose biosensors. RSC Adv. 2024, 14, 6896–6905. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, R.; Kim, M.-J.; Jang, H.-G.; Mugunthan, A.; Kim, C.-S.; Yoon, J.-H.; Lee, J.; Chung, K.W.; Chang, S.-C. Fabrication of bio-mimic nanozyme based on Mxene@AuNPs and molecular imprinted poly(thionine) films for creatinine detection. Biosens. Bioelectron. 2025, 271, 117075. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ji, F.; Wang, Z.; Zhu, Y.; Hu, K.; Ouyang, Y.; Wang, R.; Ma, X.; Cao, C. Microwave-assisted synthesis of CuSe nano-particles as a high -performance cathode for rechargeable magnesium batteries. Electrochim. Acta 2019, 324, 134864. [Google Scholar] [CrossRef]
- Ghobadi, N.; Zamani Meymian, M.-R.; Fallah, M. Exploring secondary optical transitions: A study utilizing the DITM method, and enhanced photocatalytic properties in Ni-doped CuSe. Sci. Rep. 2024, 14, 7754. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.R.; Yeol, H.S.; Hung, C.C.; Jin, L.T.; Ryu, S.O. A Study on Copper Selenide Thin Films for Photovoltaics by a Continuous Flow Microreactor. Mol. Cryst. Liq. Cryst. 2010, 532, 39/[455]–447/[463]. [Google Scholar] [CrossRef]
- Shitu, I.G.; Talib, Z.A.; Chi, J.L.Y.; Kechick, M.M.A.; Baqiah, H. Influence of tartaric acid concentration on structural and optical properties of CuSe nanoparticles synthesized via microwave assisted method. Results Phys. 2020, 17, 103041. [Google Scholar] [CrossRef]
- Rasheed, M.; Saira, F.; Batool, Z.; Khan, H.M.; Yaseen, J.; Arshad, M.; Kalsoom, A.; Ahmed, H.E.; Ashiq, M.N. Facile synthesis of a CuSe/PVP nanocomposite for ultrasensitive non-enzymatic glucose biosensing. RSC Adv. 2023, 13, 26755–26765. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, R.; Kim, J.; Ishigami, A.; Cho, J.Y.; Kim, J.H.; Han, J.T.; Lee, J.; Chang, S.-C. Dispersant-free supra single-walled carbon nanotubes for simultaneous and highly sensitive biomolecule sensing in ex vivo mouse tissues. Carbon 2023, 213, 118275. [Google Scholar] [CrossRef]
- Manikandan, R.; Pugal Mani, S.; Selvan, K.S.; Yoon, J.-H.; Chang, S.-C. Fabrication of S and O-incorporated graphitic carbon nitride linked poly(1,3,4-thiadiazole-2,5-dithiol) film for selective sensing of Hg2+ ions in water, fish, and crab samples. Food Chem. 2023, 425, 136483. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, R.; Pugal Mani, S.; Sangeetha Selvan, K.; Yoon, J.-H.; Chang, S.-C. Anodized screen-printed electrode modified with poly(5-amino-4H-1,2,4-triazole-3-thiol) film for ultrasensitive detection of Hg2+ in fish samples. J. Electroanal. Chem. 2023, 929, 117121. [Google Scholar] [CrossRef]
- Ilanchezhiyan, P.; Manikandan, R.; Sekar, S.; Jin Lee, D.; Chang Jeon, H.; Lee, S.; Chang, S.-C.; Young Kim, D. Two dimensional FeVO4 nanoflakes decorated on Ti3C2 MXene hybrid nanocomposites as a novel effective electrochemical biosensor for ultrasensitive and selective detection of serotonin (5-HT). Appl. Surf. Sci. 2025, 680, 161411. [Google Scholar] [CrossRef]
- Manikandan, R.; Jang, H.-G.; Kim, C.-S.; Yoon, J.-H.; Lee, J.; Paik, H.-j.; Chang, S.-C. Nano-engineered paper-based electrochemical biosensors: Versatile diagnostic tools for biomarker detection. Coord. Chem. Rev. 2025, 523, 216261. [Google Scholar] [CrossRef]
- Wu, B.; Yeasmin, S.; Liu, Y.; Cheng, L.-J. Sensitive and selective electrochemical sensor for serotonin detection based on ferrocene-gold nanoparticles decorated multiwall carbon nanotubes. Sens. Actuators B Chem. 2022, 354, 131216. [Google Scholar] [CrossRef]
- Musuvadhi Babulal, S.; Wu, H.-F.; Chien Wu, C. Two-Dimensional Phosphorus-Doped Tungsten Trioxide Nanosheets for Electrochemical Detection of Serotonin in Biological Fluids. ACS Appl. Nano Mater. 2023, 6, 14552–14562. [Google Scholar] [CrossRef]
- Mufeeda, M.; Ankitha, M.; Rasheed, P.A. Nb2CTx/Protonated Carbon Nitride Nanocomposite for Electrochemical Detection of Serotonin. ACS Appl. Nano Mater. 2023, 6, 21152–21161. [Google Scholar] [CrossRef]
- Xu, Q.-Q.; Luo, L.; Liu, Z.-G.; Guo, Z.; Huang, X.-J. Highly sensitive and selective serotonin (5-HT) electrochemical sensor based on ultrafine Fe3O4 nanoparticles anchored on carbon spheres. Biosens Bioelectron 2023, 222, 114990. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Stine, J.M.; Chapin, A.A.; Ghodssi, R. A portable electrochemical sensing platform for serotonin detection based on surface-modified carbon fiber microelectrodes. Anal. Methods. 2023, 15, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.P.; Prathyusha, V.; Kumar, J.K.K. Eco-friendly surface modification of oxidized carbon nanotubes with curcumin for simultaneous electrochemical detection of dopamine and serotonin. Mater. Chem. Phys. 2022, 287, 126293. [Google Scholar] [CrossRef]
- Motsaathebe, P.C.; Fayemi, O.E. Serotonin electrochemical detection in tomatoes at MWCNT-AONP nanocomposite modified electrode. Mater. Res. Express. 2021, 8, 115004. [Google Scholar] [CrossRef]
- Masud, J.; Ioannou, P.-C.; Levesanos, N.; Kyritsis, P.; Nath, M. A Molecular Ni-complex Containing Tetrahedral Nickel Selenide Core as Highly Efficient Electrocatalyst for Water Oxidation. ChemSusChem 2016, 9, 3128–3132. [Google Scholar] [CrossRef] [PubMed]
- De Silva, U.; Masud, J.; Zhang, N.; Hong, Y.; Liyanage, W.P.R.; Asle Zaeem, M.; Nath, M. Nickel telluride as a bifunctional electrocatalyst for efficient water splitting in alkaline medium. J. Mater. Chem. A. 2018, 6, 7608–7622. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).