A Disposable SWCNTs/AuNPs-Based Screen-Printed ISE at Different Temperatures to Monitor Ca2+ for Hypocalcemia Diagnosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Apparatus and Measurements
2.3. Electrode Preparation Process
2.4. Sample Measurement
3. Results and Discussion
3.1. Characterization of Electrode Preparation Process
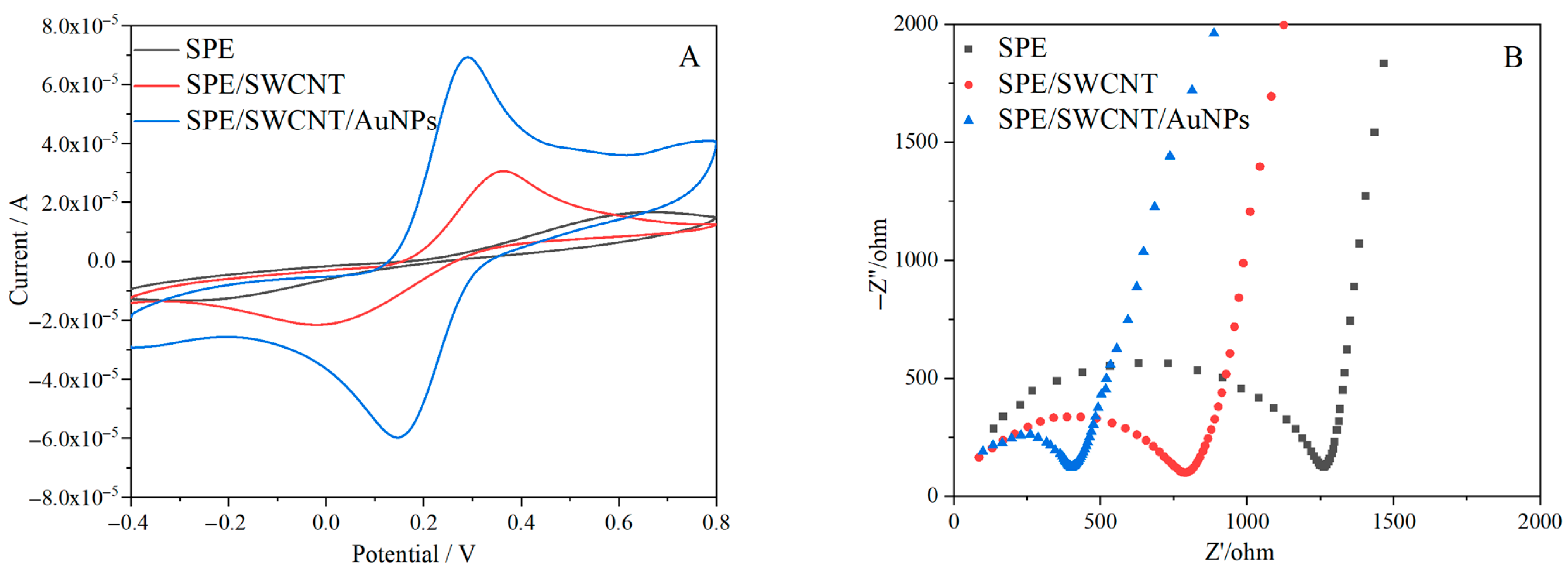
3.2. Electrochemical Characterization of the Fabricated Sensor
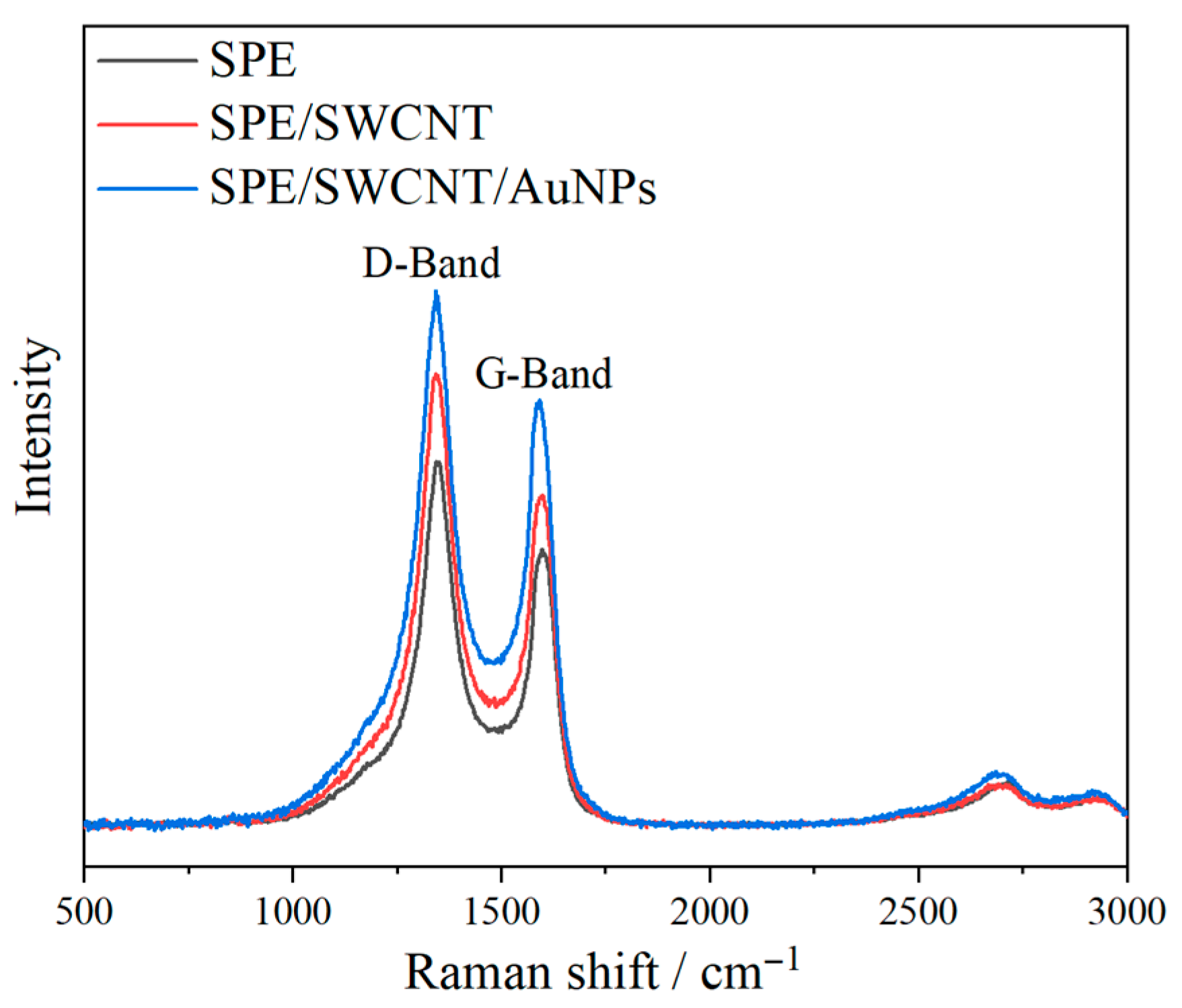
3.3. Raman Spectroscopy
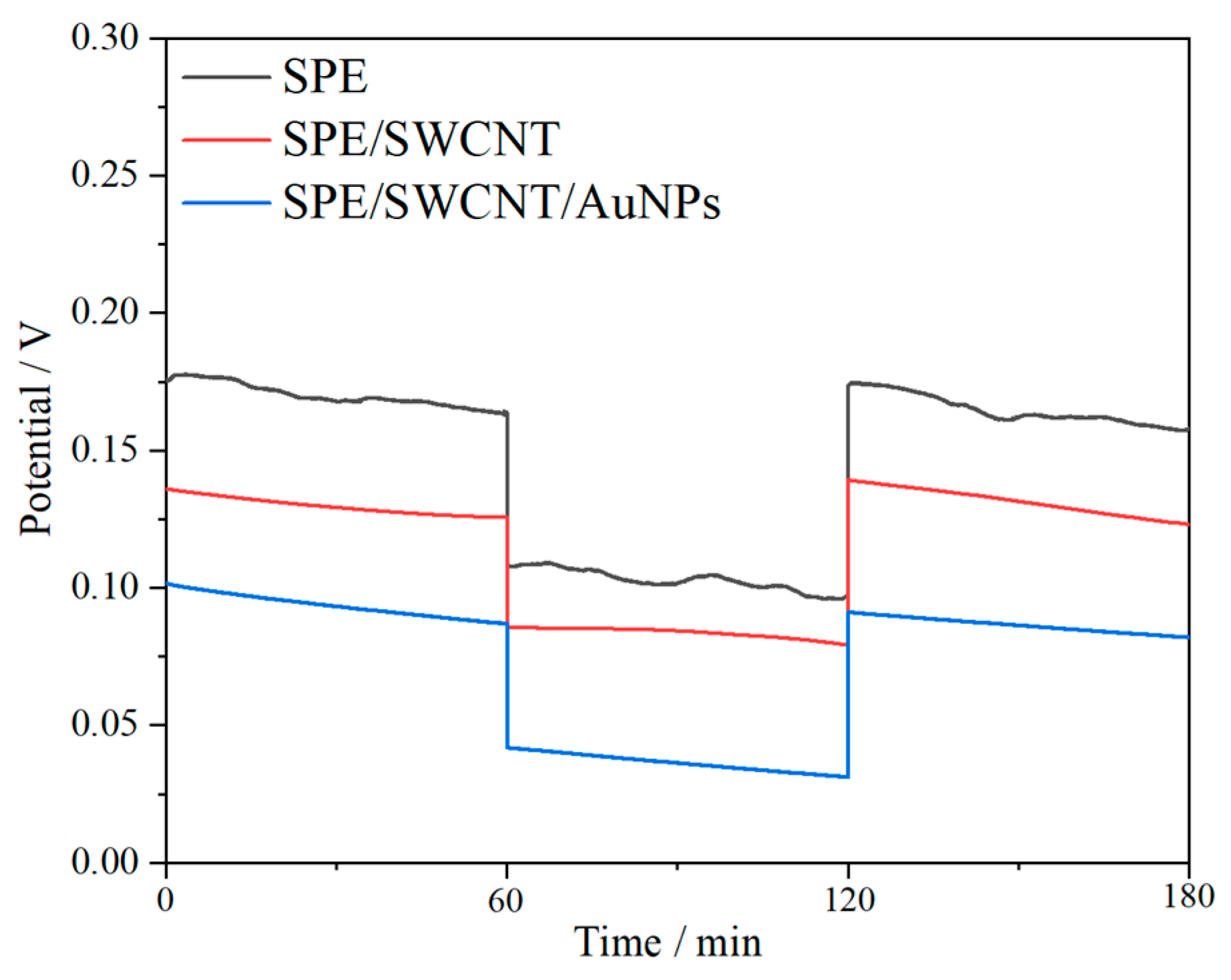
3.4. Water Layer Test
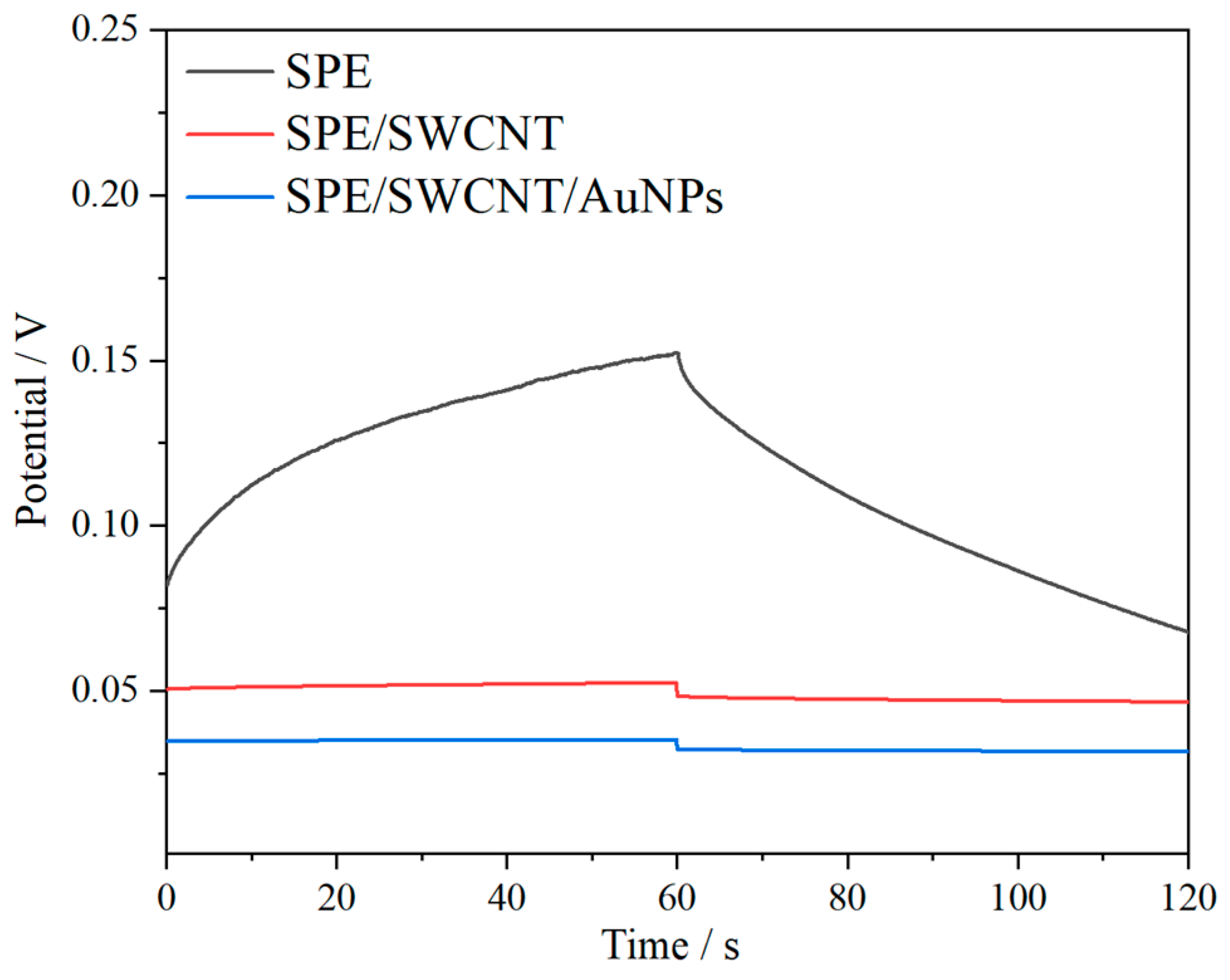
3.5. Chronopotentiometry
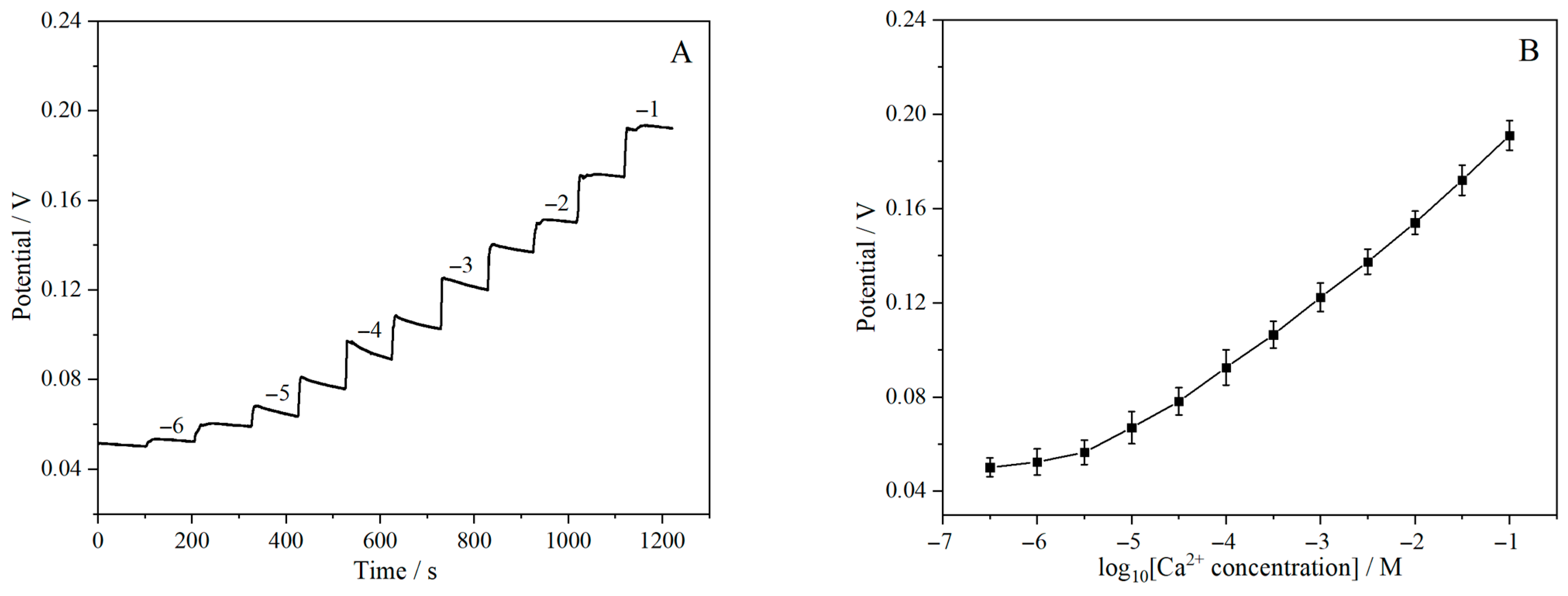
3.6. Potential Response
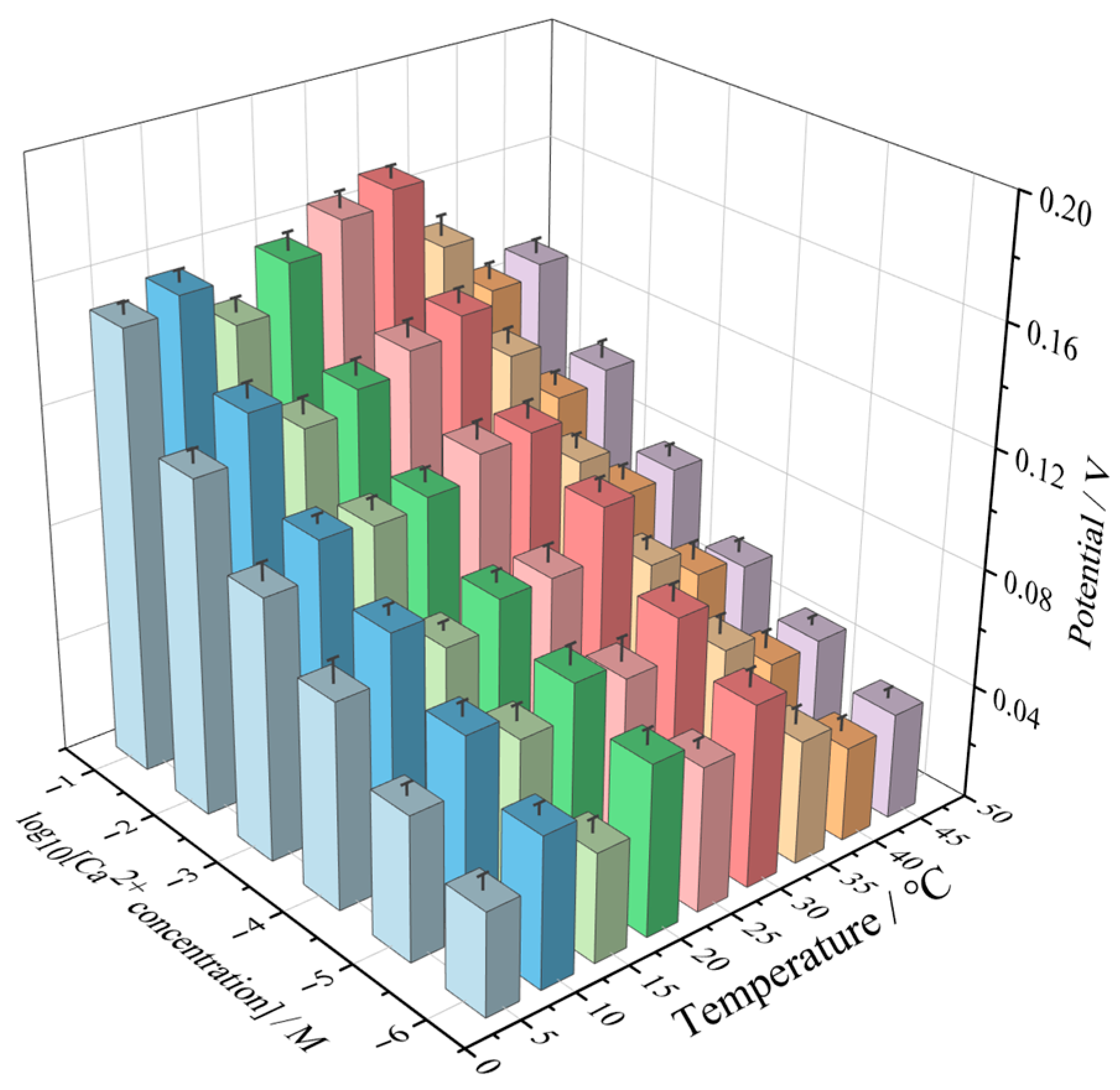
3.7. Effect of Temperature and Ca2+ Concentration on Electrical Signal
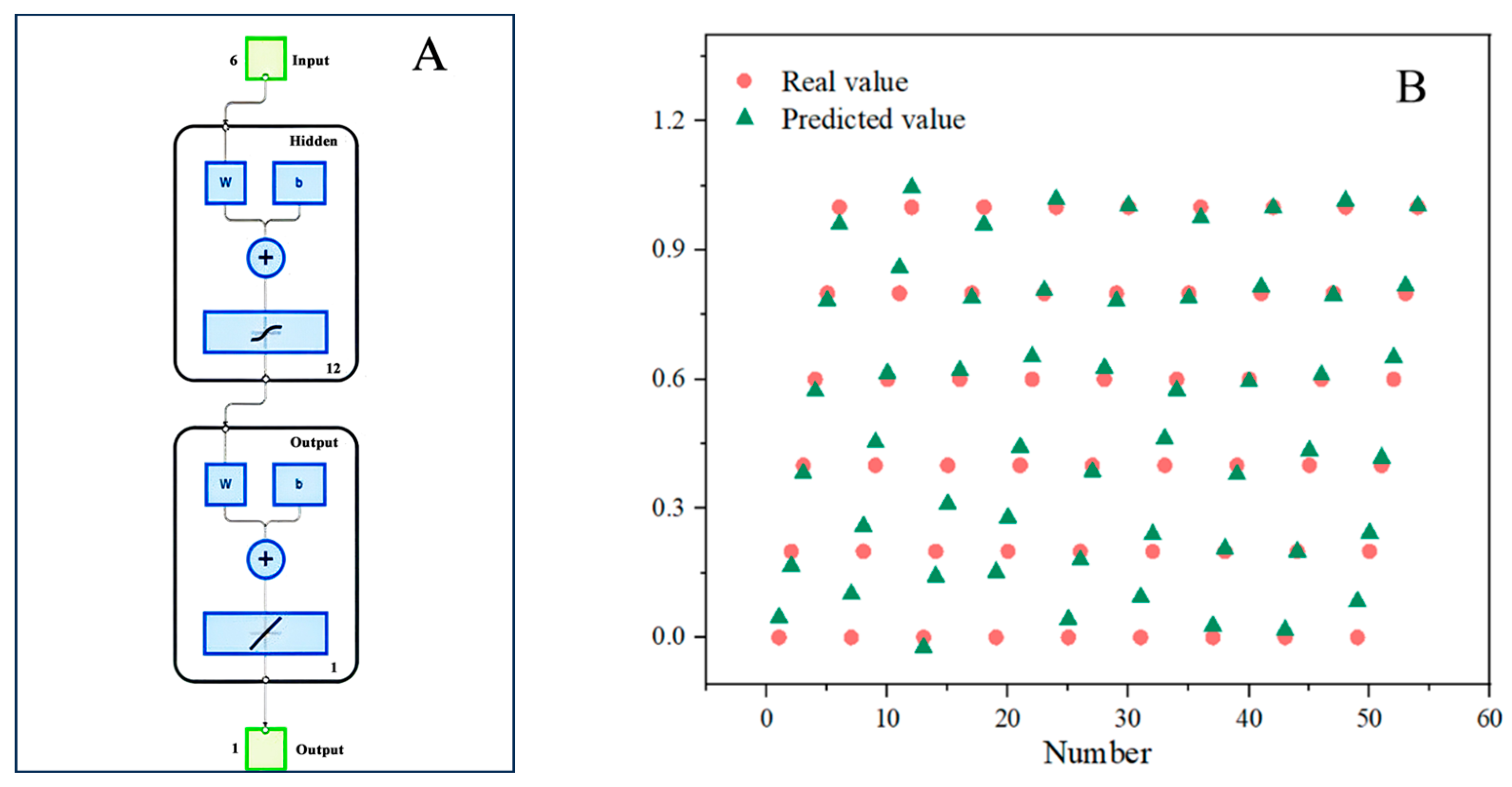
3.8. Data Preprocessing
3.9. Artificial Neural Network Model
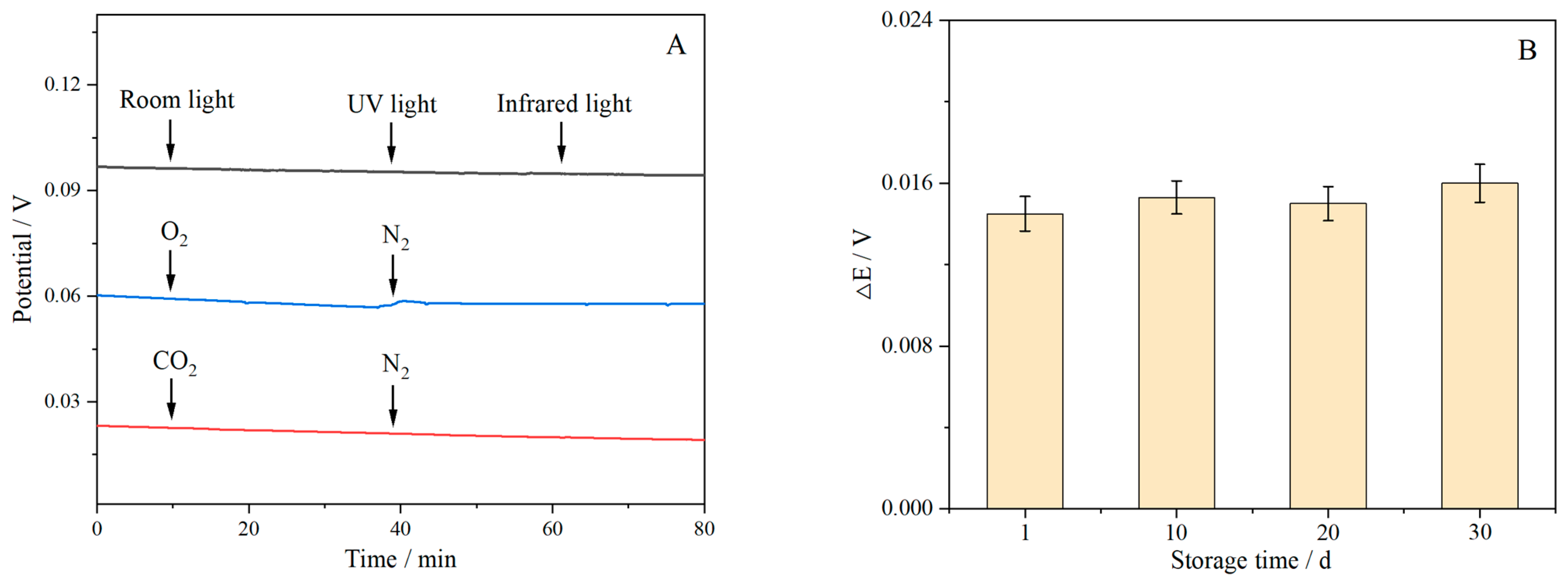
3.10. Interference Test and Stability
3.11. Comparison of Different Ca-ISEs
3.12. Determination of Ca2+ in Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arechiga-Flores, C.F.; Cortés-Vidauri, Z.; Hernández-Briano, P.; Lozano-Domínguez, R.R.; López-Carlos, M.A.; Macías-Cruz, U.; Avendaño-Reyes, L. Hypocalcemia in the dairy cow. Review. Rev. Mex. Cienc. Pecu. 2022, 13, 1025–1054. [Google Scholar] [CrossRef]
- Quiroz-Rocha, G.F.; LeBlanc, S.J.; Duffield, T.F.; Wood, D.; Leslie, K.E.; Jacobs, R.M. Reference limits for biochemical and hematological analytes of dairy cows one week before and one week after parturition. Can. Vet. J.-Rev. Vet. Can. 2009, 50, 383–388. [Google Scholar]
- Doehring, C.; Sundrum, A. The informative value of an overview on antibiotic consumption, treatment efficacy and cost of clinical mastitis at farm level. Prev. Vet. Med. 2019, 165, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Martinez, N.; Risco, C.A.; Lima, F.S.; Bisinotto, R.S.; Greco, L.F.; Ribeiro, E.S.; Maunsell, F.; Galvão, K.; Santos, J.E.P. Evaluation of peripartal calcium status, energetic profile, and neutrophil function in dairy cows at low or high risk of developing uterine disease. J. Dairy Sci. 2012, 95, 7158–7172. [Google Scholar] [CrossRef]
- Mulligan, F.T.; O’Grady, L.; Rice, D.A.; Doherty, M.L. A herd health approach to dairy cow nutrition and production diseases of the transition cow. Anim. Reprod. Sci. 2006, 96, 331–353. [Google Scholar] [CrossRef]
- Kimura, K.; Reinhardt, T.A.; Goff, J.P. Parturition and hypocalcemia blunts calcium signals in immune cells of dairy cattle. J. Dairy Sci. 2006, 89, 2588–2595. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Bariya, M.; Kivimäki, L.; Uusitalo, S.; Liaw, T.S.; Jansson, E.; Ahn, C.H.; Hangasky, J.A.; Zhao, J.Q.; Lin, Y.J.; et al. Regional and correlative sweat analysis using high-throughput microfluidic sensing patches toward decoding sweat. Sci. Adv. 2019, 5, eaaw9906. [Google Scholar] [CrossRef]
- Hu, J.B.; Stein, A.; Bühlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. Trac-Trend Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Lewenstam, A. Routines and Challenges in Clinical Application of Electrochemical Ion-Sensors. Electroanalsis 2014, 26, 1171–1181. [Google Scholar] [CrossRef]
- Zuliani, C.; Diamond, D. Opportunities and challenges of using ion-selective electrodes in environmental monitoring and wearable sensors. Electrochim. Acta 2012, 84, 29–34. [Google Scholar] [CrossRef]
- Shao, Y.Z.; Ying, Y.B.; Ping, J.F. Recent advances in solid-contact ion-selective electrodes: Functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 2020, 49, 4405–4465. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yin, T.; Li, Z.; Wang, J. Potentiometric/time resolved chronopotentiometric sensing for an all-solid-state ion-selective electrode based on MXene/MWCNTs as solid contact. Sens. Actuators B Chem. 2024, 405, 135269. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, Q.Y.; Xiang, F.F.; Hu, Y.; Zhang, L.J.; Xu, X.J.; Liu, N.N.; Huang, S.M. Applying AuNPs/SWCNT to fabricate electrical nanogap device for DNA hybridization detection. Carbon 2020, 157, 40–46. [Google Scholar] [CrossRef]
- Fouskaki, M.; Chaniotakis, N. Fullerene-based electrochemical buffer layer for ion-selective electrodes. Analyst 2008, 133, 1072–1075. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Jin, H.L.; Yang, T.; Bao, W.W.; Huang, S.M.; Wang, J.C. Electrochemical detection of hepatitis B and papilloma virus DNAs using SWCNT array coated with gold nanoparticles. Biosens. Bioelectron. 2013, 41, 205–210. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, Y.; Yang, D.G.; Luo, X.J.; Tang, Y.M.; Li, H.M. Sensitivity and selectivity determination of bisphenol A using SWCNT-CD conjugate modified glassy carbon electrode. J. Hazard. Mater. 2012, 199, 111–118. [Google Scholar] [CrossRef]
- Ping, J.F.; Wang, Y.X.; Ying, Y.B.; Wu, J. Application of Electrochemically Reduced Graphene Oxide on Screen-Printed Ion-Selective Electrode. Anal. Chem. 2012, 84, 3473–3479. [Google Scholar] [CrossRef]
- Rizwan, M.; Elma, S.; Lim, S.A.; Ahmed, M.U. AuNPs/CNOs/SWCNTs/chitosan-nanocomposite modified electrochemical sensor for the label-free detection of carcinoembryonic antigen. Biosens. Bioelectron. 2018, 107, 211–217. [Google Scholar] [CrossRef]
- Crespo, G.A.; Macho, S.; Bobacka, J.; Rius, F.X. Transduction Mechanism of Carbon Nanotubes in Solid-Contact Ion-Selective Electrodes. Anal. Chem. 2009, 81, 676–681. [Google Scholar] [CrossRef]
- Gao, Y.S.; Zhu, X.F.; Xu, J.K.; Lu, L.M.; Wang, W.M.; Yang, T.T.; Xing, H.K.; Yu, Y.F. Label-free electrochemical immunosensor based on Nile blue A-reduced graphene oxide nanocomposites for carcinoembryonic antigen detection. Anal. Biochem. 2016, 500, 80–87. [Google Scholar] [CrossRef]
- Lim, S.A.; Yoshikawa, H.; Tamiya, E.; Yasin, H.M.; Ahmed, M.U. A highly sensitive gold nanoparticle bioprobe based electrochemical immunosensor using screen printed graphene biochip. RSC Adv. 2014, 4, 58460–58466. [Google Scholar] [CrossRef]
- Guo, S.; Wang, E. Synthesis and electrochemical applications of gold nanoparticles. Anal. Chim. Acta 2007, 598, 181–192. [Google Scholar] [CrossRef]
- Curulli, A.; Bianchini, C.; Zane, D. Electrochemical Sensing Platform Based on Single-Walled Carbon Nanotubes (SWCNTs)/Gold Nanoparticles (AuNps) Nanocomposite. Electrocatalsis 2012, 3, 30–38. [Google Scholar] [CrossRef]
- Eder, D. Carbon nanotube-inorganic hybrids. Chem. Rev. 2010, 110, 1348–1385. [Google Scholar] [CrossRef]
- Zhu, J.W.; Li, X.; Qin, Y.; Zhang, Y.H. Single-piece solid-contact ion-selective electrodes with polymer-carbon nanotube composites. Sens. Actuat B-Chem. 2010, 148, 166–172. [Google Scholar] [CrossRef]
- Ping, J.F.; Wang, Y.X.; Fan, K.; Wu, J.; Ying, Y.B. Direct electrochemical reduction of graphene oxide on ionic liquid doped screen-printed electrode and its electrochemical biosensing application. Biosens. Bioelectron. 2011, 28, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, D.W.; Xiu, G.L.; Long, Y.T. Applications of screen-printed electrodes in current environmental analysis. Curr. Opin. Electrochem. 2017, 3, 137–143. [Google Scholar] [CrossRef]
- Schwarz, J.; Trommer, K.; Gerlach, F.; Mertig, M. All-solid-state screen-printed sensors for potentiometric calcium (II) determinations in environmental samples. Am. J. Anal. Chem. 2018, 9, 113–123. [Google Scholar] [CrossRef]
- Silva, L.R.; Rodrigues, J.G.; Franco, J.P.; Santos, L.P.; D’Elia, E.; Romão, W.; Ferreira, R.D.Q. Development of a portable electroanalytical method using nickel modified screen-printed carbon electrode for ethinylestradiol determination in organic fertilizers. Ecotoxicol. Environ. Saf. 2021, 208, 111430. [Google Scholar] [CrossRef]
- Andreescu, S.; Noguer, T.; Magearu, V.; Marty, J.-L. Screen-printed electrode based on AChE for the detection of pesticides in presence of organic solvents. Talanta 2002, 57, 169–176. [Google Scholar] [CrossRef]
- Piermarini, S.; Migliorelli, D.; Volpe, G.; Massoud, R.; Pierantozzi, A.; Cortese, C.; Palleschi, G. Uricase biosensor based on a screen-printed electrode modified with Prussian blue for detection of uric acid in human blood serum. Sens. Actuat B-Chem. 2013, 179, 170–174. [Google Scholar] [CrossRef]
- Gören, A.; Mendes, J.; Rodrigues, H.M.; Sousa, R.E.; Oliveira, J.; Hilliou, L.; Costa, C.M.; Silva, M.M.; Lanceros-Méndez, S. High performance screen-printed electrodes prepared by a green solvent approach for lithium-ion batteries. J. Power Sources 2016, 334, 65–77. [Google Scholar] [CrossRef]
- Kim, J.; Duraisamy, N.; Lee, T.M.; Kim, I.; Choi, K.H. Screen printed silver top electrode for efficient inverted organic solar cells. Mater. Res. Bull. 2015, 70, 412–415. [Google Scholar] [CrossRef]
- Thakur, B.; Bernalte, E.; Smith, J.P.; Foster, C.W.; Linton, P.E.; Sawant, S.N.; Banks, C.E. Utilising copper screen-printed electrodes (CuSPE) for the electroanalytical sensing of sulfide. Analyst 2016, 141, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Viet, N.X.; Ukita, Y.; Chikae, M.; Ohno, Y.; Maehashi, K.; Matsumoto, K.; Viet, P.H.; Takamura, Y. Fabrication of new single-walled carbon nanotubes microelectrode for electrochemical sensors application. Talanta 2012, 91, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Zhang, H.Y.; Li, Y.; Wang, X.A.; Zhao, L.; Xu, J.H.; Zhan, Z.K.; Zhang, G.L.; Li, W.J. Glycated Hemoglobin Electrochemical Immunosensor Based on Screen-Printed Electrode. Biosensors 2022, 12, 902. [Google Scholar] [CrossRef]
- Shokry, A.; Karim, M.; Khalil, M.; Ebrahim, S.; El Nady, J. Supercapacitor based on polymeric binary composite of polythiophene and single-walled carbon nanotubes. Sci. Rep. 2022, 12, 11278. [Google Scholar] [CrossRef]
- Li, T.; Zhu, W.; Shen, R.; Wang, H.Y.; Chen, W.; Hao, S.J.; Li, Y.X.; Gu, Z.G.; Li, Z.J. Three-dimensional conductive porous organic polymers based on tetrahedral polythiophene for high-performance supercapacitors. New J. Chem. 2018, 42, 6247–6255. [Google Scholar] [CrossRef]
- Joksovic, S.; Kundacina, I.; Milosevic, I.; Stanojev, J.; Radonic, V.; Bajac, B. Single-Walled Carbon Nanotube-Modified Gold Leaf Immunosensor for Detection. ACS Omega 2024, 9, 22277–22284. [Google Scholar] [CrossRef]
- Jorio, A.; Pimenta, M.A.; Souza, A.G.; Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Characterizing carbon nanotube samples with resonance Raman scattering. New J. Phys. 2003, 5, 139. [Google Scholar] [CrossRef]
- Araujo, P.T.; Terrones, M.; Dresselhaus, M.S. Defects and impurities in graphene- like materials. Mater. Today 2012, 15, 98–109. [Google Scholar] [CrossRef]
- Maluta, J.R.; Canevari, T.C.; Machado, S.A.S. Sensitive determination of nitric oxide using an electrochemical sensor based on MWCNTs decorated with spherical Au nanoparticles. J. Solid. State Electr. 2014, 18, 2497–2504. [Google Scholar] [CrossRef]
- Ondera, T.J.; Hamme, A.T. A gold nanopopcorn attached single-walled carbon nanotube hybrid for rapid detection and killing of bacteria. J. Mater. Chem. B 2014, 2, 7534–7543. [Google Scholar] [CrossRef] [PubMed]
- Fibbioli, M.; Morf, W.E.; Badertscher, M.; de Rooij, N.F.; Pretsch, E. Potential drifts of solid-contacted ion-selective electrodes due to zero-current ion fluxes through the sensor membrane. Electroanalsis 2000, 12, 1286–1292. [Google Scholar] [CrossRef]
- Michalska, A.; Dumańska, J.; Maksymiuk, K. Lowering the detection limit of ion-selective plastic membrane electrodes with conducting polymer solid contact and conducting polymer potentiometric sensors. Anal. Chem. 2003, 75, 4964–4974. [Google Scholar] [CrossRef]
- Hernández, R.; Riu, J.; Rius, F.X. Determination of calcium ion in sap using carbon nanotube-based ion-selective electrodes. Analyst 2010, 135, 1979–1985. [Google Scholar] [CrossRef]
- Ping, J.F.; Wang, Y.X.; Wu, J.; Ying, Y.B. Development of an all-solid-state potassium ion-selective electrode using graphene as the solid-contact transducer. Electrochem. Commun. 2011, 13, 1529–1532. [Google Scholar] [CrossRef]
- Bobacka, J. Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef]
- Vaughan-Jones, R.D.; Kaila, K. The sensitivity of liquid sensor, ion-selective microelectrodes to changes in temperature and solution level. Pflügers Arch. 1986, 406, 641–644. [Google Scholar] [CrossRef]
- Huang, Z.-Q. The Introduction of Electrolyte Solution Theory; Science Press in Beijing: Beijing, China, 1983. [Google Scholar]
- Zhang, L.; Zhang, M.; Sheng, M. Development of a Multi-Channel Soil Macronutrient Detection System Using Ion-Selective Electrode; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2012; p. 1. [Google Scholar]
- Zeng, X.; Qin, W. A solid-contact potassium-selective electrode with MoO2 microspheres as ion-to-electron transducer. Anal. Chim. Acta 2017, 982, 72–77. [Google Scholar] [CrossRef]
- Hu, J.B.; Zou, X.U.; Stein, A.; Bühlmann, P. Ion-Selective Electrodes with Colloid-Imprinted Mesoporous Carbon as Solid Contact. Anal. Chem. 2014, 86, 7111–7118. [Google Scholar] [CrossRef] [PubMed]
- Jansod, S.; Cherubini, T.; Soda, Y.; Bakker, E. Optical Sensing with a Potentiometric Sensing Array by Prussian Blue Film Integrated Closed Bipolar Electrodes. Anal. Chem. 2020, 92, 9138–9145. [Google Scholar] [CrossRef] [PubMed]
- Teekayupak, K.; Lomae, A.; Agir, I.; Chuaypen, N.; Dissayabutra, T.; Henry, C.S.; Chailapakul, O.; Ozer, T.; Ruecha, N. Large-scale fabrication of ion-selective electrodes for simultaneous detection of Na+, K+, and Ca2+ in biofluids using a smartphone-based potentiometric sensing platform. Microchim. Acta 2023, 190, 237. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Yu, H.; Ding, J.; Qin, W. An integrated screen-printed potentiometric strip for determination of Ca2+ in seawater. J. Electrochem. Soc. 2019, 166, B589. [Google Scholar] [CrossRef]


| Temperature (°C) | Equation | R2 |
|---|---|---|
| 5 | y = 0.0209x + 0.1575 | 0.9654 |
| 10 | y = 0.0195x + 0.1685 | 0.9776 |
| 15 | y = 0.0219x + 0.1603 | 0.9928 |
| 20 | y = 0.0205x + 0.1671 | 0.963 |
| 25 | y = 0.023x + 0.1757 | 0.9806 |
| 30 | y = 0.023x + 0.1944 | 0.9895 |
| 35 | y = 0.0218x + 0.1655 | 0.9971 |
| 40 | y = 0.0184x + 0.1387 | 0.9959 |
| 45 | y = 0.0207x + 0.1475 | 0.9942 |
| Sensor | Linear Range (M) | Response Time (s) | Detection Limit (M) | Slope (mV/Decade) | Storage Time (d) | Ref. |
|---|---|---|---|---|---|---|
| Ca/SWCNT/ISM | 10−5~10−2.5 | - | 10−6.2 | 28.7 | 21 | [46] |
| Ca-BPE | 10−4~10−2 | - | - | 29.6 ± 0.6 | 30 | [54] |
| Ca2+-ISE | 10−4~10−1 | 10 | 10−5 | 28.7 ± 0.2 | 30 | [55] |
| SPE-II/Ca2+-ISE | 10−5~10−1 | - | 10−6 | 26.7 ±1.3 | - | [56] |
| SPE/ERGNO/Ca-ISM | 10−5.6~10−1.6 | 10 | 10−5.8 | 29.1 | 90 | [17] |
| SPE/SWCNTs/AuNPs/Ca-ISM | 10−5.5~10−1 | 15 | 10−5.5 | 30 ± 0.3 | 30 | This work |
| No. | Added (mM) | Found (mM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | - | 1.23 | - | 3.3 |
| 2 | 0.10 | 1.35 | 101.50 | 2.9 |
| 3 | 0.20 | 1.41 | 98.60 | 3.5 |
| 4 | 0.30 | 1.48 | 96.73 | 2.7 |
| 5 | 0.40 | 1.54 | 94.48 | 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Wang, H.; He, Y.; Chen, R.; Tang, X.; Xiong, B. A Disposable SWCNTs/AuNPs-Based Screen-Printed ISE at Different Temperatures to Monitor Ca2+ for Hypocalcemia Diagnosis. Chemosensors 2025, 13, 252. https://doi.org/10.3390/chemosensors13070252
Yu Z, Wang H, He Y, Chen R, Tang X, Xiong B. A Disposable SWCNTs/AuNPs-Based Screen-Printed ISE at Different Temperatures to Monitor Ca2+ for Hypocalcemia Diagnosis. Chemosensors. 2025; 13(7):252. https://doi.org/10.3390/chemosensors13070252
Chicago/Turabian StyleYu, Zhixue, Hui Wang, Yue He, Ruipeng Chen, Xiangfang Tang, and Benhai Xiong. 2025. "A Disposable SWCNTs/AuNPs-Based Screen-Printed ISE at Different Temperatures to Monitor Ca2+ for Hypocalcemia Diagnosis" Chemosensors 13, no. 7: 252. https://doi.org/10.3390/chemosensors13070252
APA StyleYu, Z., Wang, H., He, Y., Chen, R., Tang, X., & Xiong, B. (2025). A Disposable SWCNTs/AuNPs-Based Screen-Printed ISE at Different Temperatures to Monitor Ca2+ for Hypocalcemia Diagnosis. Chemosensors, 13(7), 252. https://doi.org/10.3390/chemosensors13070252





