Phthalocyanine-Modified Electrodes Used in the Electroanalysis of Monoamine Neurotransmitters
Abstract
1. Introduction
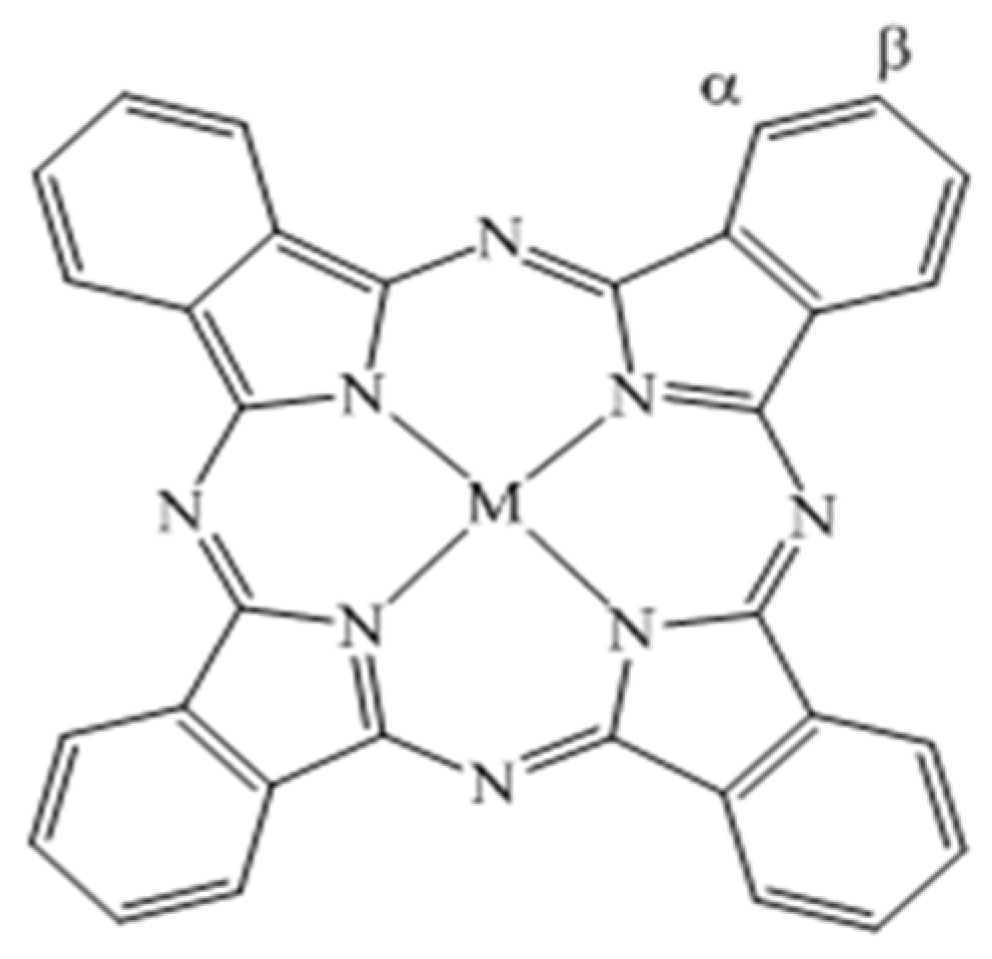
2. Metallo-Phthalocyanines
2.1. Metallo-Phthalocyanine Properties
2.2. Electrocatalysis
3. Metallo-Phthalocyanine-Modified Electrodes
3.1. Carbonaceous Materials for Electrode Modification
3.1.1. Carbon Nanotubes
Carbon Nanotubes as Electrode Material
Carbon Nanotube Paste Electrodes
3.1.2. Graphene
3.2. Methods of Electrode Modification
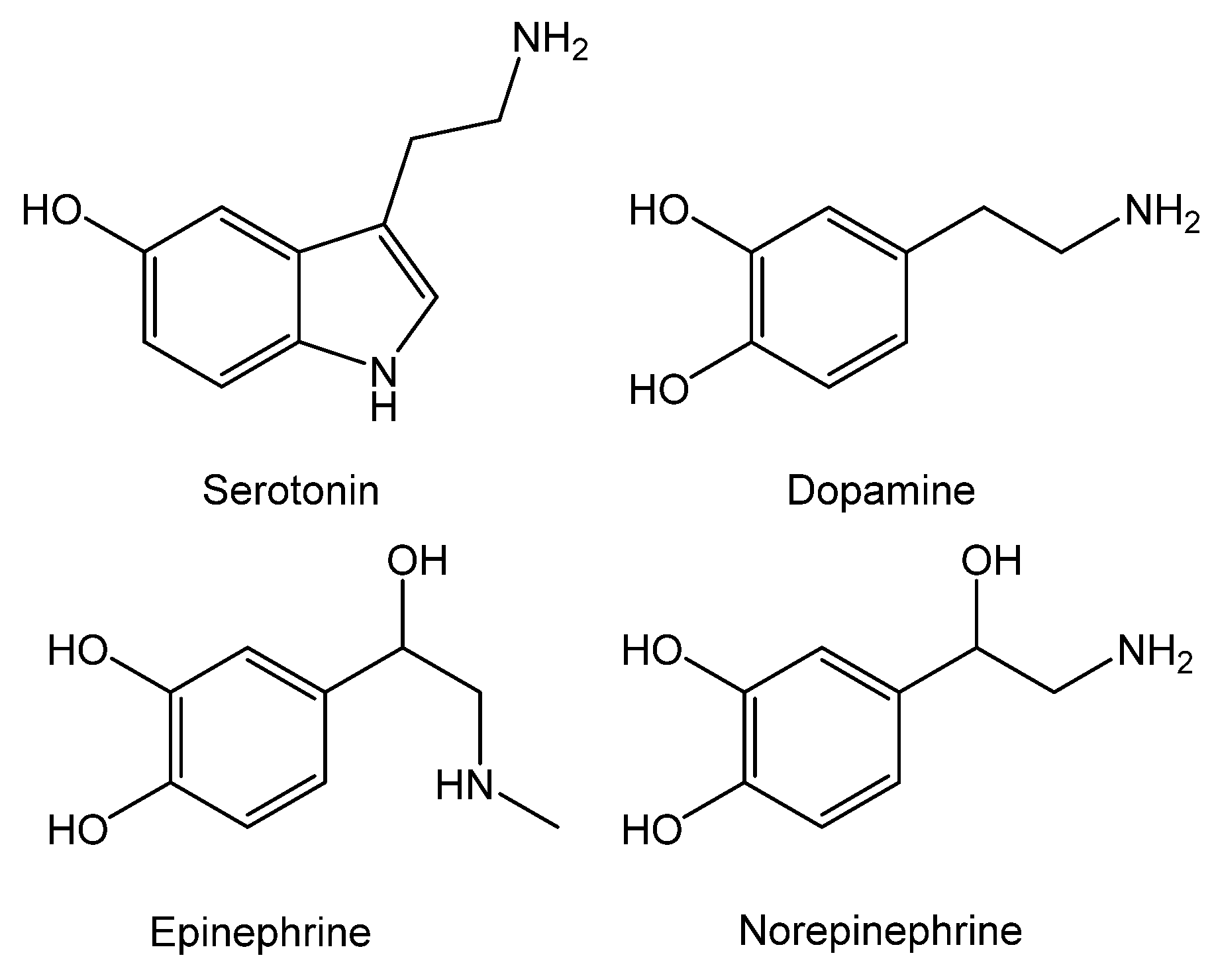
4. Analytical Applications of MPc-Modified Electrodes for Detection of Monoamine Neurotransmitters
4.1. Determination of Dopamine
4.2. Determination of Epinephrine and Norepinephrine
4.3. Determination of Serotonin
5. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 29H,31H-Pc | 29H,31H-Phthalocyanine |
| 3N-CoPc | non-peripheral cobalt phthalocyanine |
| 3N-CuPc | non-peripheral copper phthalocyanine |
| 5-HT | serotonin |
| ABS | acetate buffer solution |
| AET | aminoethanethiol |
| AmGQDs | aminated graphene quantum dots |
| AGCE | activated glassy carbon electrode |
| AgNPs | silver nanoparticles |
| Amp | amperometry |
| AA | ascorbic acid |
| BRB | Britton–Robinson buffer |
| CA | chronoamperometry |
| Car | carbazole |
| CCE | carbon ceramic electrode |
| CFE | carbon fiber electrode |
| Clicked-α-CoPc-flav3 | clicked film of an asymmetric A3B (A = 3-oxyflavone, B = α-(ethynyl)benzyl alcohol) CoPc complex |
| CNP | carbon nanoparticles |
| CoOCAPc | cobalt(II) octa acyl chloride phthalocyanine |
| CoPc | cobalt phthalocyanine |
| CoTAPc | cobalt tetra-amino phthalocyanine |
| CoTBuPc | Co(III) tetrakis-(tert-butyl)-phthalocyanine |
| CoTCPhOPc | cobalt(II) tetra-(3-carboxyphenoxy) phthalocyanine |
| CoTfurNH2Pc | cobalt (II) tetra furfurylamide phthalocyanine |
| CoTGPc | ganciclovir-cobalt (II) phthalocyanine |
| CoTMBANAPc | tetra8[(E)(4methoxybenzylidene)amino] naphthalene1amine cobalt (II) phthalocyanine |
| CoTNBAPc | cobalt (II) tetra[β-N-(4-nitrophenyl) benzamide] phthalocyanine |
| CPE | carbon paste electrode |
| CTAB | cetyltrimethylammonium bromide |
| Cu-MAPA | copper monoamino-phthalocyanine-acrylate |
| CuNPs | copper nanoparticles |
| CuTsPc | copper(II) tetrasulfophthalocyanine |
| CV | cyclic voltammetry |
| DA | dopamine |
| Db | cationic 1,4-diazoniabicyclo [2.2.2]octane group of silsesquioxane |
| DMZ | dimetridazole |
| DPV | differential pulse voltammetry |
| DPSV | differential pulse stripping voltammetry |
| DS01 | antimicrobial peptide dermaseptin 01 |
| ED | electrodeposition |
| EP | epinephrine |
| ERGO | electrochemically reduced graphene oxide |
| FeOCAPc | iron octa carboxylic acid phthalocyanine |
| FePc | iron phthalocyanine |
| FeTAPc | iron(II) tetraaminophthalocyanine |
| FeTBImPc | iron tetrabenzimidazole phthalocyanine |
| FeTsPc | iron(II) tetrasulfonated phthalocyanine |
| FIA | flow injection analysis |
| f-MWCNTs | functionalized multi-walled carbon nanotubes |
| G | graphite |
| GCE | glassy carbon electrode |
| GO | graphene oxide |
| GPE | graphite paste carbon |
| Gr | graphene |
| GQDs | graphene quantum dots |
| IPA | isophthalic acid |
| ITOE | indium tin oxide electrode |
| L-dopa | levodopa |
| LSV | linear scan voltammetry |
| MPCs | metallo-phthalocyanines |
| MnPc | manganese phthalocyanine |
| Mn-TPP | manganese tetraphenylporphyrin |
| MWCNTs | multiwalled carbon nanotubes |
| Na+MMT | sodium montmorillonite clay |
| N-G | nitrogen-doped graphene |
| NGCSs | N-doped graphitic carbon nanosheets |
| NGQDs | nitrogen-doped graphene quantum dots |
| NiTAPc | nickel(II) tetraaminophthalocyanine |
| NiTsPc | nickel tetrasulfonated phthalocyanine |
| NP | norepinephrine |
| OSWV | Osteryoung square wave voltammetry |
| P8BT | poly[(9,9-di-n-octylfluorenyl-2,7-diyl)-alt-(benzothiadia-zol-4,8-diyl)] |
| PAHs | poly allylamine hydrocarbons |
| PAMAM | poly (amidoamine) |
| PANI | polyaniline |
| PAR | paracetamol |
| Pdots | polymer dots |
| PEC | photoelectrochemical cell |
| PBS | phosphate buffer solution |
| PdTAPc | palladium(II) tetraaminophthalocyanine |
| PEA | phenylethylamine |
| PEI | polyelectrolyte |
| PGE | pencil graphite electrode |
| PPO | polyphenol oxidase |
| rGO | reduced graphene oxide |
| SAM | self-assembled monolayer |
| SPCE | screen-printed carbon electrode |
| SPGE | screen-printed graphite electrode |
| SWCNTs | single-wall carbon nanotubes |
| SWV | square wave voltammetry |
| TRIS | tris[hydroxymethyl]aminomethane hydrochloride |
| UA | uric acid |
| XO | xanthine |
| ZnONPs | zinc oxide nanoparticles |
| ZnPc | zinc phthalocyanine |
| ZnTPEBIPc | zinc tetra [4-{2-[(E)-2-phenylethenyl]-1H-benzimidazol-1-yl}]phthalocyanine |
References
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2001. [Google Scholar]
- Ciucu, A.A. Chemically modified electrodes in biosensing. J. Biosens. Bioelectron. 2014, 5, 3. [Google Scholar]
- Elliot, C.M.; Murray, R.W. Chemically modified carbon electrodes. Anal. Chem. 1976, 48, 1247–1254. [Google Scholar] [CrossRef]
- Demir, E.; Silah, H.; Uslu, B. Phthalocyanine modified electrodes in electrochemical analysis. Crit. Rev. Anal. Chem. 2020, 52, 425–461. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, M.A. The phthalocyanines a new class of synthetic pigments and dyes. Ind. Eng. Chem. 1939, 31, 839–847. [Google Scholar] [CrossRef]
- Velloso, N.V.; Muehlmann, L.A.; Longo, J.P.F.; da Silva, J.R.; Zancanela, D.C.; Tedesco, A.C.; de Azevedo, R.B. Aluminum-phthalocyanine chloride-based photodynamic therapy inhibits PI3K/Akt/Mtor pathway in oral squamous cell carcinoma cells in vitro. Chemotherapy 2012, 1, 107. [Google Scholar]
- Basova, T.; Plyashkevich, V.; Hassan, A.; Gürek, A.G.; Gümüş, G.; Ahsen, V. Phthalocyanine films as active layers of optical sensors for pentachlorophenol detection. Sens. Actuat. B Chem. 2009, 139, 557–562. [Google Scholar] [CrossRef]
- Ao, R.; Kummert, L.; Haarer, D. Present limits of data storage using dye molecules in solid matrices. Adv. Mater. 1995, 5, 495–499. [Google Scholar] [CrossRef]
- Ng, D.K.P.; Yeung, Y.O.; Chan, W.K.; Yu, S.C. Columnar liquid crystals based on 2,3-naphthalocyanine core. Tet. Lett. 1997, 38, 6701–6704. [Google Scholar] [CrossRef]
- Wörhle, D.; Meissener, D. Organic solar cells. Adv. Mater. 1991, 3, 129–138. [Google Scholar]
- Jahnke, H.; Schonborn, M.; Zimmermann, G. Organic dyestuffs as catalysts for fuel cells. Top. Curr. Chem. 1976, 61, 133–181. [Google Scholar]
- Iwamoto, M. Nanometric electrostatic interfacial phenomena in organic semiconducting thin films. J. Mater. Chem. 2000, 10, 99–106. [Google Scholar] [CrossRef]
- Sajjan, V.A.; Mohammed, I.; Nemakal, M.; Aralekallu, S.; Kumar, H.K.R.; Swamy, S.; Sannegowda, L.K. Synthesis and electropolymerization of cobalt tetraaminebenzamide phthalocyanine macrocycle for the amperometric sensing of dopamine. J. Electroanal. Chem. 2019, 838, 33–40. [Google Scholar] [CrossRef]
- Zeng, Z.; Fang, X.; Miao, W.; Liu, Y.; Maiyalagan, T.; Mao, S. Electrochemically sensing of trichloroacetic acid with Iron(II) phthalocyanine and Zn-based metal organic framework nanocomposites. ACS Sens. 2019, 4, 1934–1941. [Google Scholar] [CrossRef]
- Diab, N.; Morales, D.M.; Andronescu, C.; Masoud, M.; Schuhmann, W. A sensitive and selective graphene/cobalt tetrasulfonated phthalocyanine sensor for detection of dopamine. Sens. Actuat. B Chem. 2019, 285, 17–23. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, H.; Cai, J.; Peng, Y.; Niu, Y.; Chen, H.; Bai, L.; Zhang, S.; Jin, J.; Liu, L.; et al. A novel oxidation-reduction method for highly selective detection of cysteine over reduced glutathione based on synergistic effect of fully fluorinated cobalt phthalocyanine and ordered mesoporous carbon. Sens. Actuat. B Chem. 2019, 288, 180–187. [Google Scholar] [CrossRef]
- Jilani, B.S.; Mruthyunjayachari, C.D.; Malathesh, P.; Mounesh, M.N.; Sharakumar, T.M.; Reddy, V.K.R. Electrochemical sensing based MWCNT-cobalt tetra substituted sorbaamide phthalocyanine onto the glassy carbon electrode towards the determination of 2-amino phenol: A voltammetric study. Sens. Actuat. B Chem. 2019, 301, 127078. [Google Scholar] [CrossRef]
- Martin, C.S.; Alessio, P.; Crespilho, F.N.; Brett, C.M.A.; Constantino, C.J.L. Influence of the supramolecular arrangement of iron phthalocyanine thin films on catecholamine oxidation. J. Electroanal. Chem. 2019, 836, 7–15. [Google Scholar] [CrossRef]
- Ciucu, A.; Negulescu, C.; Baldwin, R.P. Detection of pesticides using an amperometric biosensor based on ferrophthalocyanine chemically modified carbon paste electrode and immobilized bi-enzymatic system. Biosens. Bioelectron. 2003, 18, 293–300. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, L.; Zhang, S.; Lou, Y.; Zhao, S.; Tan, Q.; He, L.; Du, M. A copper(II) phthalocyanine-based metallo-covalent organic framework decorated with silver nanoparticle for sensitively detecting nitric oxide released from cancer cells. Sens. Actuat. B Chem. 2021, 338, 129826. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, J.; Liu, B.; Griveau, S.; Bedioui, F. Enhanced electrochemical sensing of thiols based on cobalt phthalocyanine immobilized on nitrogen-doped graphene. Biosens. Bioelectron. 2015, 66, 438–444. [Google Scholar] [CrossRef]
- Mpeta, L.S.; Sen, P.; Nyokong, T. Development of manganese phthalocyanine decorated with silver nanoparticles nanocomposite for improved electrocatalytic oxidation of hydrazine. J. Electroanal. Chem. 2020, 866, 114173. [Google Scholar] [CrossRef]
- Yin, H.-S.; Zhou, Y.-L.; Ai, S.-Y. Preparation and characteristic of cobalt phthalocyanine modified carbon paste electrode for bisphenol A detection. J. Electroanal. Chem. 2009, 626, 80–88. [Google Scholar] [CrossRef]
- Myers, J.F.; Canham, R.G.W.; Lever, A.B.P. Higher oxidation level phthalocyanine complexes of chromium, iron, cobalt and zinc. Phthalocyanine radical species. Inorg. Chem. 1975, 14, 461–468. [Google Scholar] [CrossRef]
- Clack, D.W.; Hush, N.S.; Woosley, I.S. Reduction potentials of some metal phthalocyanines. Inorg. Chim. Acta 1976, 19, 129–132. [Google Scholar] [CrossRef]
- Louati, A.; Meray, M.E.I.; Andre, J.J.; Simon, J.; Kadish, K.M.; Gross, M.; Giraurdeau, A. Electrochemical reduction of new, good electron acceptors: The metallooctacyanophthalocyanines. Inorg. Chem. 1985, 24, 1175–1179. [Google Scholar] [CrossRef]
- Lever, A.B.P.; Pickens, S.R.; Minor, P.C.; Licoccia, L.; Ramaswamy, B.S.; Magnell, K. Charge-transfer spectra of metallophthalocyanines: Correlation with electrode potentials. J. Am. Chem. Soc. 1981, 103, 6800–6806. [Google Scholar] [CrossRef]
- Manassen, J. Metal complexes of porphirin like compounds as heterogeneous catalysts. Catal. Rev. Sci. Eng. 1974, 9, 223–243. [Google Scholar] [CrossRef]
- Santos, L.M.; Baldwin, R.P. Electrocatalytic response of cobalt phthalocyanine chemically modified electrodes toward oxalic acid and alpha-keto acids. Anal. Chem. 1986, 58, 848–852. [Google Scholar] [CrossRef]
- Ozoemena, K.I.; Nyokong, T. Novel amperometric glucose biosensor based on an ether-linked cobalt(II) phthalocyanine–cobalt(II) tetraphenylporphyrin pentamer as a redox mediator. Electrochim. Acta 2006, 51, 5131–5136. [Google Scholar] [CrossRef]
- Ozoemena, K.I.; Zhao, Z.; Nyokong, T. Immobilized cobalt(II) phthalocyanine–cobalt(II) porphyrin pentamer at a glassy carbon electrode: Applications to efficient amperometric sensing of hydrogen peroxide in neutral and basic media. Electrochem. Commun. 2005, 7, 679–684. [Google Scholar] [CrossRef]
- Geraldo, D.A.; Togo, C.A.; Limson, J.; Nyokong, T. Electrooxidation of hydrazine catalyzed by noncovalently functionalized single-walled carbon nanotubes with CoPc. Electrochim. Acta 2008, 53, 8051–8057. [Google Scholar] [CrossRef]
- Sehlotho, N.; Griveau, S.; Ruille, N.; Boujtita, M.; Nyokong, T.; Bedioui, F. Electro-catalyzed oxidation of reduced glutathione and 2-mercaptoethanol by cobalt phthalocyanine-containing screen printed graphite electrodes. Mater. Sci. Eng. C 2008, 28, 606–612. [Google Scholar] [CrossRef]
- Halbert, M.K.; Baldwin, R.P. Electrocatalytic and analytical response of cobalt phthalocyanine containing carbon paste electrodes toward sulfhydryl compounds. Anal. Chem. 1985, 57, 591–595. [Google Scholar] [CrossRef]
- Zhang, J.; Tse, Y.-H.; Pietro, W.J.; Lever, A.B.P. Electrocatalytic activity of N,N′,N″,N‴-tetramethyl-tetra-3,4-pyridoporphyrazinocobalt(II) adsorbed on a graphite electrode towards the oxidation of hydrazine and hydroxylamine. J. Electroanal. Chem. 1996, 406, 203–211. [Google Scholar] [CrossRef]
- Moraes, F.C.; Cabral, M.F.; Machado, S.A.S.; Mascaro, L.H. Electrocatalytic behavior of glassy carbon electrodes modified with multiwalled carbon nanotubes and cobalt phthalocyanine for selective analysis of dopamine in presence of ascorbic acid. Electroanalysis 2008, 20, 851–857. [Google Scholar] [CrossRef]
- Goux, A.; Bedioui, F.; Robbiola, L.; Pontie, M. Nickel tetraaminophthalocyanine based films for the electrocatalytic activation of dopamine. Electroanalysis 2003, 15, 969–974. [Google Scholar] [CrossRef]
- Kang, T.F.; Shen, G.L.; Yu, R.Q. Voltammetric behaviour of dopamine at nickel phthalocyanine polymer modified electrodes and analytical applications. Anal. Chim. Acta 1997, 354, 343–349. [Google Scholar] [CrossRef]
- Ureta-Zañartu, M.S.; Berrios, C.; Pavez, J.; Zagal, J.; Gutierrez, C.; Marco, J.F. Electrooxidation of 2-chlorophenol on polyNiTSPc-modified glassy carbon electrodes. J. Electroanal. Chem. 2003, 553, 147–156. [Google Scholar] [CrossRef]
- Obirai, J.; Bedioui, F.; Nyokong, T. Electro-oxidation of phenol and its derivatives on poly-Ni(OH)TPhPyPc modified vitreous carbon electrodes. J. Electroanal. Chem. 2005, 576, 323–332. [Google Scholar] [CrossRef]
- Jiang, R.; Dong, S. Study on the electrocatalytic reduction of H2O2 at iron protoporphyrin modified electrode with a rapid rotation-scan method. Electrochim. Acta 1990, 35, 1227–1232. [Google Scholar] [CrossRef]
- Elliot, C.M.; Marrese, C.A. Catalytic reduction of some alkyl halides by iron porphyrin modified carbon electrodes. J. Electroanal. Chem. 1981, 119, 395–401. [Google Scholar] [CrossRef]
- Wang, Z.; Pang, D. Electrocatalysis of metalloporphyrins: Part 9. Catalytic electroreduction of cystine using water-soluble cobalt porphyrins. J. Electroanal. Chem. 1990, 283, 349–358. [Google Scholar] [CrossRef]
- Sorokin, A.; Meunier, B. Efficient H2O2 oxidation of chlorinated phenols catalysed by supported iron phthalocyanines. J. Chem. Soc. Chem. Commun. 1994, 15, 1799–1800. [Google Scholar] [CrossRef]
- Hadasch, A.; Sorokin, A.; Rabion, A.; Meunier, B. Sequential addition of H2O2, pH and solvent effects as key factors in the oxidation of 2,4,6-trichlorophenol catalyzed by iron tetrasulfophthalocyanine. New J. Chem. 1998, 22, 45–51. [Google Scholar] [CrossRef]
- Sanchez, M.; Hadasch, A.; Fell, R.T.; Meunier, B. Key role of the phosphate buffer in the H2O2 oxidation of aromatic pollutants catalyzed by iron tetrasulfophthalocyanine. J. Catal. 2001, 202, 177–186. [Google Scholar] [CrossRef]
- Oni, J.; Nyokong, T. Simultaneous voltammetric determination of dopamine and serotonin on carbon paste electrodes modified with iron(II) phthalocyanine complexes. Anal. Chim. Acta 2001, 432, 9–21. [Google Scholar] [CrossRef]
- Zagal, J.H. Metallophthalocyanines as catalysts in electrochemical reactions. Coord. Chem. Rev. 1992, 119, 89–136. [Google Scholar] [CrossRef]
- Grootboom, N.; Nyokong, T. Electrooxidation of cresols on carbon electrodes modified with phthalocyaninato and octabutoxyphthalocyaninato cobalt(II) complexes. Anal. Chim. Acta 2001, 432, 49–57. [Google Scholar] [CrossRef]
- Caro, C.A.; Bedioui, F.; Páez, M.A.; Cárdenas-Jirón, G.I.; Zagal, J.H. Experimental and theoretical study of the activity of substituted metallophthalocyanines for nitrite electro-oxidation. J. Electrochem. Soc. 2004, 151, E32. [Google Scholar] [CrossRef]
- Agboola, B.O. Catalytic Activities of Metallophthalocyanines Towards Detection and Transformation of Pollutants. Ph.D. Thesis, Rhodes University, Makhanda, South Africa, 2007. Available online: https://commons.ru.ac.za/vital/access/services/Download/vital:4427/SOURCEPDF (accessed on 15 March 2025).
- Sajid, M.; Nazal, M.K.; Mansha, M.; Alsharaa, A.; Jillani, S.M.S.; Basheer, C. Chemically modified electrodes for electrochemical detection of dopamine in the presence of uric acid and ascorbic acid: A review. Trends Anal. Chem. 2016, 76, 15–29. [Google Scholar] [CrossRef]
- Fan, F.R.; Faulkner, L.R. Phthalocyanine thin films as semiconductor electrodes. J. Am. Chem. Soc. 1979, 101, 4779–4787. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, R.; Luo, K.; Zhang, W.; Zhao, J. Electrocatalytic performances of multi-walled carbon nanotubes chemically modified by metal phthalocyanines in Li/SOCl2 batteries. RSC Adv. 2016, 6, 75632–75639. [Google Scholar] [CrossRef]
- Foster, C.W.; Pillay, J.; Metters, J.P.; Banks, C.E. Cobalt phthalocyanine modified electrodes utilised in electroanalysis: Nano-structured modified electrodes vs. bulk modified screen-printed electrodes. Sensors 2014, 14, 21905–21922. [Google Scholar] [CrossRef] [PubMed]
- Griveau, S.; Gulppi, M.; Pavez, J.; Zagal, J.H.; Bedioui, F. Cobalt phthalocyanine-based molecular materials for the electrocatalysis and electroanalysis of 2-mercaptoethanol, 2-mercaptoethanesulfonic acid, reduced glutathione and L-cysteine. Electroanalysis 2003, 15, 779–785. [Google Scholar] [CrossRef]
- Mphuthi, N.G.; Adekunle, A.S.; Ebenso, E.E. Electrocatalytic oxidation of epinephrine and norepinephrine at metal oxide doped phthalocyanine/MWCNT Composite Sensor. Sci. Rep. 2016, 6, 26938. [Google Scholar] [CrossRef]
- Mounesh; Malathesh, P.; Kumara, N.Y.P.; Jilani, B.S.; Mruthyunjayachari, C.D.; Reddy, K.R.V. Synthesis and characterization of tetra-ganciclovir cobalt (II) phthalocyanine for electroanalytical applications of AA/DA/UA. Heliyon 2019, 5, e01946. [Google Scholar] [CrossRef]
- Lei, P.; Zhou, Y.; Zhu, R.; Liu, Y.; Dong, C.; Shuang, S. Facile synthesis of iron phthalocyanine functionalized n, b–doped reduced graphene oxide nanocomposites and sensitive electrochemical detection for glutathione. Sens. Actuat. B Chem. 2019, 297, 126756. [Google Scholar] [CrossRef]
- Porto, L.S.; da Silva, D.N.; Silva, M.C.; Pereira, A.C. Electrochemical sensor based on multi-walled carbon nanotubes and cobalt phthalocyanine composite for pyridoxine determination. Electroanalysis 2019, 31, 820–828. [Google Scholar] [CrossRef]
- Pari, M.; Ramareddy, K.; Reddy, V. Electrochemical investigation of uric acid using MWCNTs-decorated novel substituted cobalt (II) phthalocyanine modified GCE. Anal. Bioanal. Electrochem. 2019, 11, 1383–1397. [Google Scholar]
- Ozoemena, K.I. Anodic oxidation and amperometric sensing of hydrazine at a glassy carbon electrode modified with cobalt (II) phthalocyanine-cobalt (II) tetraphenylporphyrin (Copc-(Cotpp)(4)) supramolecular complex. Sensors 2006, 6, 874–891. [Google Scholar] [CrossRef]
- Lopes, I.C.; De Souza, D.; Machado, S.A.S.; Tanaka, A.A. Voltammetric detection of paraquat pesticide on a phthalocyanine-based pyrolitic graphite electrode. Anal. Bioanal. Chem. 2007, 388, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Ozoemena, K.I.; Stefan, R.I.; Nyokong, T. Determination of 2’, 3-dideoxyinosine using iron(II) phthalocyanine modified carbon paste electrode. Anal. Lett. 2004, 37, 2641–2648. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Maschmann, M.R.; Amama, P.B.; Goyal, A.; Iqbal, Z.; Fisher, T.S. Freestanding vertically oriented single-walled carbon nanotubes synthesized using microwave plasma-enhanced CVD. Carbon 2006, 44, 2758–2763. [Google Scholar] [CrossRef]
- Wang, G.; Wang, B.; Park, J.; Yang, J.; Shen, X.; Yao, J. Synthesis of enhanced hydrophilic and hydrophobic graphene oxide nanosheets by a solvothermal method. Carbon 2009, 47, 68–72. [Google Scholar] [CrossRef]
- Takehara, H.; Fujiwara, M.; Arikawa, M.; Diener, M.D.; Alford, J.M. Experimental study of industrial scale fullerene production by combustion synthesis. Carbon 2005, 43, 311–319. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Rivas, G.A.; Rubianes, M.D.; Rodríguez, M.C.; Ferreyra, N.F.; Luque, G.L.; Pedano, M.L.; Miscoria, S.A.; Parrado, C. Carbon nanotubes for electrochemical biosensing. Talanta 2007, 74, 291–307. [Google Scholar] [CrossRef]
- Wu, K.; Fei, J.; Hu, S. Simultaneous determination of dopamine and serotonin on a glassy carbon electrode coated with a film of carbon nanotubes. Anal. Biochem. 2003, 318, 100–106. [Google Scholar] [CrossRef]
- Ajayan, P.M. Nanotubes from carbon. Chem. Rev. 1999, 99, 1787–1800. [Google Scholar] [CrossRef]
- Gullapalli, S.; Wong, M.S. Nanotechnology: A guide to nano-objects. Chem. Engin. Prog. 2011, 107, 28–32. [Google Scholar]
- Merkoçi, A.; Pumera, M.; Llopis, X.; Pérez, B.; del Valle, M.; Alegret, S. New materials for electrochemical sensing VI: Carbon nanotubes. Trends Anal. Chem. 2005, 24, 826–838. [Google Scholar] [CrossRef]
- Yu, M.F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.F.; Rouff, R.S. Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.; de Heer, W.A. Carbon nanotubes—The route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef]
- Nugent, J.M.; Santhanam, K.S.V.; Rubio, A.; Ajayan, P.M. Fast electron transfer kinetics on multiwalled carbon nanotube microbundle electrodes. Nano Lett. 2001, 1, 87–91. [Google Scholar] [CrossRef]
- Wang, J. Carbon-nanotube based electrochemical biosensors: A review. Electroanalysis 2005, 17, 7–14. [Google Scholar] [CrossRef]
- Luo, H.; Shi, Z.; Li, N.; Gu, Z.; Zhuang, Q. Investigation of the electrochemical and electrocatalytic behavior of single-wall carbon nanotube film on a glassy carbon electrode. Anal. Chem. 2001, 73, 915–920. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Shi, Z.; Li, N.; Gu, Z. Direct Electrochemistry of cytochrome c at a glassy carbon electrode modified with single-wall carbon nanotubes. Anal. Chem. 2002, 74, 1993–1997. [Google Scholar] [CrossRef]
- Musameh, M.; Wang, J.; Merkoçi, A.; Lin, Y. Low-potential stable NADH detection at carbon-nanotube-modified glassy carbon electrodes. Electrochem. Comm. 2002, 4, 743–746. [Google Scholar] [CrossRef]
- Wu, F.H.; Zhao, G.-C.; Wei, X.-W. Electrocatalytic oxidation of nitric oxide at multi-walled carbon nanotubes modified electrode. Electrochem. Comm. 2002, 4, 690–694. [Google Scholar] [CrossRef]
- Guo, M.; Chen, J.; Li, J.; Tao, B.; Yao, S. Fabrication of polyaniline/carbon nanotube composite modified electrode and its electrocatalytic property to the reduction of nitrite. Anal. Chim. Acta 2005, 532, 71–77. [Google Scholar] [CrossRef]
- Ji, X.B.; Kadara, R.O.; Krussma, J.; Chen, Q.Y.; Banks, C.E. Understanding the physicoelectrochemical properties of carbon nanotubes: Current state of the art. Electroanalysis 2010, 22, 7–19. [Google Scholar] [CrossRef]
- Banks, C.E.; Moore, R.R.; Davies, T.J.; Compton, R.G. Investigation of modified basal plane pyrolytic graphite electrodes: Definitive evidence for the electrocatalytic properties of the ends of carbon nanotubes. Chem. Comm. 2004, 16, 1804–1805. [Google Scholar] [CrossRef] [PubMed]
- McCreery, L. Electroanalytical Chemistry; Bard, A.J., Ed.; Marcel Dekker: New York, NY, USA, 1991; Volume 17. [Google Scholar]
- Britto, P.J.; Santhanam, K.S.V.; Alonso, V.; Rubio, A.; Ajayan, P.M. Improved charge transfer at carbon nanotube electrodes. Adv. Mater. 1999, 11, 154–157. [Google Scholar] [CrossRef]
- Banks, C.E.; Davies, T.J.; Wildgoose, G.G.; Compton, R.G. Electrocatalysis at graphite and carbon nanotube modified electrodes: Edge-plane sites and tube ends are the reactive sites. Chem. Commun. 2005, 7, 829–841. [Google Scholar] [CrossRef]
- Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J.M. Role of carbon nanotubes in electroanalytical chemistry: A review. Anal. Chim. Acta 2008, 622, 11–47. [Google Scholar] [CrossRef]
- Chen, J.; Hamon, M.A.; Hu, H.; Chen, Y.; Rao, A.M.; Eklund, P.C.; Haddon, R.C. Solution properties of single-walled carbon nanotubes. Science 1998, 282, 95–98. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Georgakilas, V.; Prato, M. Soluble carbon nanotubes. Chem. Eur. J. 2003, 9, 4000–4008. [Google Scholar] [CrossRef]
- Islam, M.F.; Rojas, E.; Bergey, D.M.; Johnson, A.T.; Yodh, A.G. High weight fraction surfactant solubilization of single-wall carbon nanotubes in water. Nano Lett. 2003, 3, 269–273. [Google Scholar] [CrossRef]
- Star, A.; Stoddart, J.F.; Steuerman, D.; Diehl, M.; Boukai, A.; Wong, E.W.; Yang, X.; Chung, S.W.; Choi, H.; Heath, J.R. Preparation and properties of polymer-wrapped single-walled carbon nanotubes. Angew. Chem. Int. Ed. 2001, 40, 1721–1725. [Google Scholar] [CrossRef]
- Ramesh, S.; Ericson, L.M.; Davis, V.A.; Saini, R.K.; Pasquali, C.K.M.; Billups, W.E.; Adams, W.; Hauge, R.H.; Smalley, R.E. Dissolution of pristine single walled carbon nanotubes in superacids by direct protonation. J. Phys. Chem. B 2004, 108, 8794–9798. [Google Scholar] [CrossRef]
- Wang, J.; Musameh, M.; Lin, Y. Solubilization of carbon nanotubes by Nafion toward the preparation of amperometric biosensors. J. Am. Chem. Soc. 2003, 125, 2408–2409. [Google Scholar] [CrossRef]
- Hu, C.G.; Wang, W.L.; Liao, K.J.; Liu, G.B.; Wang, Y.T. Systematic investigation on the properties of carbon nanotube electrodes with different chemical treatments. J. Phys. Chem. Solids 2004, 65, 1731–1736. [Google Scholar] [CrossRef]
- Wang, Z.H.; Liang, Q.-L.; Wang, Y.-M.; Luo, G.-A. Carbon nanotube-intercalated graphite electrodes for simultaneous determination of dopamine and serotonin in the presence of ascorbic acid. J. Electroanal. Chem. 2003, 540, 129–134. [Google Scholar] [CrossRef]
- Valentini, F.; Orlanducci, S.; Terranova, M.L.; Amine, A.; Palleschi, G. Carbon nanotubes as electrode materials for the assembling of new electrochemical biosensors. Sens. Actuat. B Chem. 2004, 100, 117–125. [Google Scholar] [CrossRef]
- Jacobs, C.B.; Peairs, M.J.; Venton, B.J. Review: Carbon nanotube based electrochemical sensors for biomolecules. Anal. Chim. Acta 2010, 662, 105–127. [Google Scholar] [CrossRef]
- Luong, J.H.T.; Hrapovic, S.; Wang, D.; Bensebaa, F.; Simard, B. Solubilization of multiwall carbon nanotubes by 3-aminopropyltriethoxysilane towards the fabrication of electrochemical biosensors with promoted electron transfer. Electroanalysis 2004, 16, 132–139. [Google Scholar] [CrossRef]
- Hirsch, A. Functionalization of single-walled carbon nanotubes. Angew. Chemie Int. Ed. 2002, 41, 1853–1859. [Google Scholar] [CrossRef]
- Wong, S.S.; Joselevich, E.; Woolley, A.T.; Cheung, C.L.; Lieber, C.M. Covalently functionalized nanotubes as nanometre- sized probes in chemistry and biology. Nature 1998, 394, 52–55. [Google Scholar] [CrossRef]
- Dai, L.; He, P.; Li, S. Functionalized surfaces based on polymers and carbon nanotubes for some biomedical and optoelectronic applications. Nanotechnology 2003, 14, 1081–1097. [Google Scholar] [CrossRef][Green Version]
- Merkoci, A. Carbon nanotubes in analytical sciences. Microchim. Acta 2006, 152, 157–174. [Google Scholar] [CrossRef]
- Banks, C.E.; Crossley, A.; Salter, C.; Wilkins, S.J.; Compton, R.G. Carbon nanotubes contain metal impurities which are responsible for the “electrocatalysis” seen at some nanotube-modified electrodes. Angew. Chem. Int. Ed. 2006, 45, 2533–2537. [Google Scholar] [CrossRef] [PubMed]
- Gooding, J.J. Nanostructuring electrodes with carbon nanotubes: A review on electrochemistry and applications for sensing. Electrochim. Acta 2005, 50, 3049–3060. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Biomolecule-functionalized carbon nanotubes: Applications in nanobioelectronics. Chem. Phys. Chem. 2004, 5, 1084–1104. [Google Scholar] [CrossRef]
- Davis, J.J.; Coles, R.J.; Hill, A.O. Protein electrochemistry at carbon nanotube electrodes. J. Electroanal Chem. 1997, 440, 279–282. [Google Scholar]
- Britto, P.J.; Santhanam, K.S.V.; Ajayan, P.M. Carbon nanotube electrode for oxidation of dopamine. Bioelectrochem. Bioenerg. 1996, 41, 121–125. [Google Scholar] [CrossRef]
- Rubianes, M.D.; Rivas, G.A. Enzymatic biosensors based on carbon nanotubes paste electrodes. Electroanalysis 2005, 17, 73–78. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Cano-Márquez, A.; Rodríguez-Macías, F.; Campos-Delgado, J.; Espinosa-González, C.; Tristán-López, F.; Ramírez-González, D.; Cullen, D.; Smith, D.; Terrones, M.; Vega-Cantú, Y. Ex-mwnts: Graphene sheets and ribbons produced by lithium intercalation and exfoliation of carbon nanotubes. Nano Lett. 2009, 9, 1527–1533. [Google Scholar] [CrossRef]
- Fitzer, E.; Köchling, K.-H.; Boehm, H.P.; Marsh, H. Recommended terminology for the description of carbon as a solid (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67, 473–506. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y. Synthesis of graphene and its applications: A review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Geim, A.; Novoselov, K. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Meyer, J.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.; Roth, S. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 644–648. [Google Scholar] [CrossRef]
- Ostrovsky, P.M.; Gornyi, I.V.; Mirlin, A.D. Electron transport in disordered graphene. Phys. Rev. B 2006, 74, 235443. [Google Scholar] [CrossRef]
- Lawal, A.T. Synthesis and utilization of grapheme for fabrication of electrochemical sensors. Talanta 2015, 131, 424–443. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.M.; Tang, L.H.; Lu, J.; Li, J.H. Application of graphene-modified electrode for selective detection of dopamine. Electrochem. Commun. 2009, 11, 889–892. [Google Scholar] [CrossRef]
- Alwarappan, S.; Erdem, A.; Liu, C.; Li, C.Z. Probing the electrochemical properties of graphene nanosheets for biosensing applications. J. Phys. Chem. C 2009, 113, 8853–8857. [Google Scholar] [CrossRef]
- Pumera, M. Electrochemistry of graphene: New horizons for sensing and energy storage. Chem. Rec. 2009, 9, 211–223. [Google Scholar] [CrossRef]
- Pumera, M. Graphene-based nanomaterials and their electrochemistry. Chem. Soc. Rev. 2010, 39, 4146–4157. [Google Scholar] [CrossRef]
- Kuila, T.; Bosea, S.; Khanra, P.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Recent advances in graphene-based biosensors. Biosens. Bioelectron. 2011, 26, 4637–4648. [Google Scholar] [CrossRef] [PubMed]
- Segal, M. Selling graphene by the ton. Nat. Nanotechnol. 2009, 4, 612–614. [Google Scholar] [CrossRef]
- de la Torre, G.; Blau, W.; Torres, T. A survey on the functionalization of single-walled nanotubes. The chemical attachment of phthalocyanine moieties. Nanotechnology 2003, 14, 765–771. [Google Scholar] [CrossRef]
- Oyama, N.; Anson, F.C. Facile attachment of transition metal complexes to graphite electrodes coated with polymeric ligands. Observation and control of metal-ligand coordination among reactants confined to electrode surfaces. J. Am. Chem. Soc. 1979, 101, 739–741. [Google Scholar] [CrossRef]
- Caro, C.A.; Bedioui, F.; Zagal, J.H. Electrocatalytic oxidation of nitrite on a vitreous carbon electrode modified with cobalt phthalocyanine. Electrochim. Acta 2002, 47, 1489–1494. [Google Scholar] [CrossRef]
- Cook, M.J. Thin film formulations of substituted phthalocyanines. J. Mater. Chem. 1996, 6, 677–689. [Google Scholar] [CrossRef]
- Griveau, S.; Pavez, J.; Zagal, J.H.; Bedioui, F. Electro-oxidation of 2-mercaptoethanol on adsorbed monomeric and electropolymerized cobalt tetra-aminophthalocyanine films. Effect of film thickness. J. Electroanal. Chem. 2001, 497, 75. [Google Scholar] [CrossRef]
- Cook, M.J. Phthalocyanine thin films. Pure Appl. Chem. 1999, 71, 2145–2151. [Google Scholar] [CrossRef]
- Vukusic, P.S.; Sambles, J.R. Cobalt phthalocyanine as a basis for the optical sensing of nitrogen dioxide using surface plasmon resonance. Thin Solid Film. 1992, 221, 311–317. [Google Scholar] [CrossRef]
- Chambrier, I.; Cook, M.J.; Russell, D.A. Synthesis and characterisation of functionalized phthalocyanine compounds for fabrication of self-assembled monolayers. Synthesis 1995, 10, 1283–1286. [Google Scholar] [CrossRef]
- Fomo, G.; Nwaji, N.; Nyokong, T. Low symmetric metallophthalocyanine modified electrode via click chemistry for simultaneous detection of heavy metals. J. Electroanal. Chem. 2018, 813, 58–66. [Google Scholar] [CrossRef]
- Bélanger, D.; Pinson, J. Electrografting: A powerful method for surface modification. Chem. Soc. Rev. 2011, 40, 3995–4048. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Qiu, W.; Zhu, D. Immobilization of tetra-tert-butylphthalocyanines on carbon nanotubes: A first step towards the development of new nanomaterials. J. Mater. Chem. 2002, 12, 1636–1639. [Google Scholar] [CrossRef]
- Ciucu, A.; Baldwin, R.P. Determination of 2-thiothiazolidine-4-carboxylic acid in urine by liquid chromatography with electrochemical detection. Electroanalysis 1992, 4, 515–519. [Google Scholar] [CrossRef]
- Cooper, J.R.; Bloom, F.E.; Roth, R.H. The Biochemical Basis of Neuropharmacology, 8th ed.; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Kovács, G.L. The Endocrine Brain: Pathophysiological Role of Neuropeptide-Neurotransmitter Interactions. EJIFCC 2004, 15, 107–112. [Google Scholar]
- Lövheim, H. A new three-dimensional model for emotions and monoamine neurotransmitters. Med. Hypotheses 2012, 78, 341–348. [Google Scholar] [CrossRef]
- Day, M.; Wang, Z.; Ding, J.; An, X.; Ingham, C.A.; Shering, A.F.; Wokosin, D.; Ilijic, E.; Sun, Z.; Sampson, A.R.; et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat. Neurosci. 2006, 9, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.S.; Trachtenberg, F.L.; Thompson, E.G.; Belliveau, R.A.; Beggs, A.H.; Darnall, R. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA 2006, 296, 2124–2132. [Google Scholar] [CrossRef]
- Pluto, R.; Bürger, P. Normal values of catecholamines in blood plasma determined by high-performance liquid chromatography with amperometric detection. Int. J. Sports Med. 1988, 9, 75–78. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Primo, E.N.; Eguílaz, M.; Parrado, C.; Rubianes, M.D.; Rivas, G.A. Quantification of neurotransmitters and metabolically related compounds at glassy carbon electrodes modified with bamboo-like carbon nanotubes dispersed in double stranded DNA. Microchem. J. 2017, 130, 40–46. [Google Scholar] [CrossRef]
- Yu, D.; Zeng, Y.; Qi, Y.; Zhou, T.; Shi, G. A novel electrochemical sensor for determination of dopamine based on AuNPs@SiO2 core-shell imprinted composite. Biosens. Bioelectron. 2012, 38, 270–277. [Google Scholar] [CrossRef]
- Babaei, A.; Sohrabi, M.; Afrasiabi, M. A Sensitive simultaneous determination of epinephrine and piroxicam using a glassy carbon electrode modified with a nickel hydroxide nanoparticles/multiwalled carbon nanotubes composite. Electroanalysis 2012, 24, 2387–2394. [Google Scholar] [CrossRef]
- Chiara, G.D. The principles of nerve cell communication. Alcohol Health Res. World 1997, 21, 108–114. [Google Scholar]
- Babaei, A.; Taheri, A. Nafion/Ni(OH)2 nanoparticles-carbon nanotube composite modified glassy carbon electrode as a sensor for simultaneous determination of dopamine and serotonin in the presence of ascorbic acid. Sens. Actuators B Chem. 2013, 176, 543–555. [Google Scholar] [CrossRef]
- Venton, B.J.; Wightman, R.M. Psychoanalytical electrochemistry: Dopamine and behavior. Anal. Chem. 2003, 75, 414A–421A. [Google Scholar] [CrossRef]
- Wightman, R.M.; Amatore, C.; Engstrom, R.C.; Hale, P.D.; Kristensen, E.W.; Kubr, W.G.; May, L.J. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience 1988, 25, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Kawagoe, T.K.; Wightman, R.M. Characterization of amperometry for in vivo measurement of dopamine dynamics in the rat brain. Talanta 1994, 41, 865–874. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Shao, X.; Hu, X. Voltammetric determination of dopamine in human serum and urine at a glassy carbon electrode modified by cysteic acid based on electrochemical oxidation of L-cysteine. Anal. Lett. 2007, 40, 689–704. [Google Scholar] [CrossRef]
- Sarkar, C.; Basu, B.; Chakroborty, D.; Dasgupta, P.S.; Basu, S. The immunoregulatory role of dopamine: An update. Brain Behav. Immunol. 2010, 24, 525–528. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Agboola, B.O.; Pillay, J.; Ozoemena, K.I. Electrocatalytic detection of dopamine at single-walled carbon nanotubes–iron (III) oxide nanoparticles platform. Sens. Actuators B Chem. 2010, 148, 93–102. [Google Scholar] [CrossRef]
- Pecina, M.; Mickey, B.J.; Love, T.; Wang, H.; Langenecker, S.A.; Hodgkinson, C.; Shen, P.H.; Villafuerte, S.; Hsu, D.; Weisenbach, S.L.; et al. DRD2 polymorphisms modulate reward and emotion processing, dopamine neurotransmission and openness to experience. Cortex 2013, 49, 877–890. [Google Scholar] [CrossRef]
- Siqueira, J.R., Jr.; Gasparotto, L.H.S.; Oliveira, O.N., Jr.; Zucolotto, V. Processing of electroactive nanostructured films incorporating carbon nanotubes and phthalocyanines for sensing. J. Phys. Chem. C 2008, 112, 9050–9055. [Google Scholar] [CrossRef]
- Fashedemi, O.O.; Ozoemena, K.I. A facile approach to the synthesis of hydrophobic iron tetrasulfophthalocyanine (FeTSPc) nano-aggregates on multi-walled carbon nanotubes: A potential electrocatalyst for the detection of dopamine. Sens. Actuat. B. 2011, 160, 7–14. [Google Scholar] [CrossRef]
- Yang, J.; Mu, D.; Gao, Y.; Tan, J.; Lu, A.; Ma, D. Cobalt phthalocyanine-graphene complex for electro-catalytic oxidation of dopamine. J. Nat. Gas Chem. 2012, 21, 265–269. [Google Scholar] [CrossRef]
- Shaidarova, L.G.; Gedmina, A.V.; Artamonova, M.L.; Chelnokova, I.A.; Budnikov, H.C. Voltammetry determination of dopamine by the electrocatalytic response of an electrode modified by a polyaniline film with an inclusion of copper(II) tetrasulfophthalocyanine. J. Anal. Chem. 2013, 68, 516–524. [Google Scholar] [CrossRef]
- Karuppiah, C.; Devasenathipathy, R.; Chen, S.M.; Arulraj, D.; Palanisamy, S.; Mani, V.; Vasantha, V.S. Fabrication of nickel tetrasulfonated phthalocyanine functionalized multiwalled carbon nanotubes on activated glassy carbon electrode for the detection of dopamine. Electroanalysis 2015, 27, 485–493. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, J.; Yan, L.; Zhu, L.; Liu, B. An electrochemical sensor for selective detection of dopamine based on nickel tetrasulfonated phthalocyanine functionalized nitrogen-doped graphene nanocomposites. J. Electroanal. Chem. 2016, 779, 92–98. [Google Scholar] [CrossRef]
- Sakthinathan, S.; Lee, H.F.; Chen, S.M.; Tamizhdurai, P. Electrocatalytic oxidation of dopamine based on non-covalent functionalization of manganese tetraphenylporphyrin/reduced graphene oxide nanocomposite. J. Colloid Interface Sci. 2016, 468, 120–127. [Google Scholar] [CrossRef]
- Nemakal, M.; Aralekallu, S.; Imadadulla, M.; Prabhu, C.P.K.; Lokesh, K.S. Chemisorbed palladium phthalocyanine for simultaneous determination of biomolecules. Microchem. J. 2018, 143, 82–91. [Google Scholar] [CrossRef]
- Prabhu, C.P.K.; Nemakal, M.; Aralekallu, S.; Imadadulla, M.; Palanna, M.; Sajjan, V.A.; Akshitha, D.; Sannegowda, L.K. A comparative study of carboxylic acid and benzimidazole phthalocyanines and their surface modification for dopamine sensing. J. Electroanal. Chem. 2019, 847, 113262. [Google Scholar] [CrossRef]
- Demir, F.; Yenilmez, H.Y.; Koca, A.; Bayir, Z.A. Metallo-phthalocyanines containing thiazole moieties: Synthesis, characterization, electrochemical and spectroelectrochemical properties and sensor applications. J. Electroanal. Chem. 2019, 832, 254–265. [Google Scholar] [CrossRef]
- Mounesh; Jilani, B.S.; Pari, M.; Reddy, K.R.V.; Lokesh, K.S. Simultaneous and sensitive detection of ascorbic acid in presence of dopamine using MWCNTs-decorated cobalt (II) phthalocyanine modified GCE. Microchem. J. 2019, 147, 755–763. [Google Scholar] [CrossRef]
- Jilani, B.S.; Mounesh, P.M.; Reddy, K.R.V. Tetrafurfurylamine anchored N4-macrocycle as potential catalyst for electrochemical redox reactions of biomolecules. Anal. Bioanal. Electrochem. 2019, 11, 892–912. [Google Scholar]
- Chen, Y.; Zhang, X.F.; Wang, A.J.; Zahng, Q.L.; Huang, H.; Feng, J.J. Ultrafine Fe3C nanoparticles embedded in N-doped graphitic carbon sheets for simultaneous determination of ascorbic acid, dopamine, uric acid and xanthine. Microchim. Acta 2019, 186, 651–660. [Google Scholar] [CrossRef]
- Pari, M.; Reddy, K.R.V.; Fasiulla; Chandrakala, K.B. Amperometric determination of dopamine based on an interface platform comprising tetra-substituted Zn2+ phthalocyanine film layer with embedment of reduced graphene oxide. Sens. Actuat. A 2020, 316, 112377. [Google Scholar] [CrossRef]
- Fredj, Z.; Ali, M.B.; Abbas, M.N.; Dempsey, E. Simultaneous determination of ascorbic acid, uric acid and dopamine using silver nanoparticles and copper monoamino-phthalocyanine functionalized acrylate polymer. Anal. Methods 2020, 12, 3883–3891. [Google Scholar] [CrossRef]
- Ndebele, N.; Sen, P.; Nyokong, T. Electrochemical detection of dopamine using phthalocyanine-nitrogen-doped graphene quantum dot conjugates. J. Electroanal. Chem. 2021, 886, 115111. [Google Scholar] [CrossRef]
- Sariogullari, H.; Sengul, I.F.; Gurek, A.G. Comparative study on sensing and optical properties of carbazole linked novel zinc (II) and cobalt (II) phthalocyanines. Polyhedron 2022, 227, 116139. [Google Scholar] [CrossRef]
- Sudhakara, S.M.; Kotresh, H.M.N.; Devendrachari, M.C.; Khan, F. Synthesis and electrochemical investigation of tetra amino cobalt (II) phthalocyanine functionalized polyaniline nanofiber for the selective detection of dopamine. Electroanalysis 2020, 32, 1807–1817. [Google Scholar] [CrossRef]
- Akyuz, D.; Demirbas, U. Sensor performances of novel piperidine substituted cobalt (II) and copper (II) phthalocyanines for detection of dopamine, ascorbic acid and uric acid. J. Organomet. Chem. 2022, 982, 122537. [Google Scholar] [CrossRef]
- Wu, B.; Li, M.; Ramachandran, R.; Niu, G.; Zhang, M.; Zhao, C.; Xu, Z.; Wang, F. GQDs incorporated CoPc nanorods for electrochemical detection of dopamine and uric acid. Adv. Mater. Interfaces 2023, 10, 2200738. [Google Scholar] [CrossRef]
- Luhana, C.; Mashazi, P. Simultaneous detection of dopamine and paracetamol on electroreduced graphene oxide–cobalt phthalocyanine polymer nanocomposite electrode. Electrocatalysis 2023, 14, 406–417. [Google Scholar] [CrossRef]
- Shoba, S.; Mambanda, A.; Booysen, I.N. Electrocatalytic effects of a clicked film of an asymmetric A3B (A = 3-oxyflavone, B = α-(ethynyl)benzyl alcohol) CoPc complex on a glassy carbon electrode for the detection of dopamine. J. Electroanal. Chem. 2024, 956, 118086. [Google Scholar] [CrossRef]
- Ozsoz, M.; Erdern, A.; Kilinc, E.; Gokgunnec, L. Mushroom-based cobalt phthalocyanine dispersed amperometric biosensor for the determination of phenolic compounds. Electroanalysis 1996, 8, 147–150. [Google Scholar] [CrossRef]
- Oni, J.; Westbroek, P.; Nyokong, T. Electrochemical behavior and detection of dopamine and ascorbic acid at an iron(II)-tetrasulfo-phthalocyanine modified carbon paste microelectrode. Electroanalysis 2003, 15, 848–954. [Google Scholar] [CrossRef]
- Yang, G.J.; Xu, J.J.; Wang, K.; Chen, H.Y. Electrocatalytic oxidation of dopamine and ascorbic acid on carbon paste electrode modified with nanosized cobalt phthalocyanine particles: Simultaneous determination in the presence of CTAB. Electroanalysis 2006, 18, 282–290. [Google Scholar] [CrossRef]
- Patrascu, D.; David, I.; David, V.; Mihailciuc, C.; Stamatin, I.; Ciurea, J.; Nagy, L.; Nagy, G.; Ciucu, A.A. Selective voltammetric determination of electroactive neuromodulating species in biological samples using iron(II) phthalocyanine modified multi-wall carbon nanotubes paste electrode. Sens. Actuat. B 2011, 156, 731–736. [Google Scholar] [CrossRef]
- Deon, M.; Caldas, E.M.; da Rosa, D.S.; de Menezes, E.W.; Dias, S.L.P.; Pereira, M.B.; Costa, T.M.H.; Arenas, L.T.; Benvenutti, E.V. Mesoporous silica xerogel modified with bridged ionic silsesquioxane used to immobilize copper tetrasulfonated phthalocyanine applied to electrochemical determination of dopamine. J. Solid State Electrochem. 2015, 19, 2095–2105. [Google Scholar] [CrossRef]
- Zampa, F.M.; de Brito, A.C.; Kitagawa, I.L.; Constantino, J.L.C.; Oliviera, O.N., Jr.; Da Cunha, N.H.; Zucolotto, V.; Jose, R.D.S., Jr.; Eiras, C. Natural gum-assisted phthalocyanine immobilization in electroactive nanocomposites: Physicochemical characterization and sensing applications. Biomacromolecules 2007, 8, 3408–3413. [Google Scholar] [CrossRef]
- Alessio, P.; Rodriguez-Mendez, M.L.; De Saja Saez, J.A.; Constantino, C.J.L. Iron phthalocyanine in non-aqueous medium forming layer-by-layer films: Growth mechanism, molecular architecture and applications. Phys. Chem. Chem. Phys. 2010, 12, 3972–3983. [Google Scholar] [CrossRef]
- Zampa, M.F.; Araujo, I.M.S.; dos Santos, J.R., Jr.; Zucolotto, V.; Leite, J.R.S.A.; Eiras, C. Development of a novel biosensor using cationic antimicrobial peptide and nickel phthalocyanine ultrathin films for electrochemical detection of dopamine. Int. J. Anal. Chem. 2012, 2012, 850969. [Google Scholar] [CrossRef] [PubMed]
- de Lucena, N.C.; Miyazaki, C.M.; Shimizu, F.M.; Constantino, C.J.L.; Ferreira, M. Layer-by-layer composite film of nickel phthalocyanine and montmorillonite clay for synergistic effect on electrochemical detection of dopamine. Appl. Surf. Sci. 2018, 436, 957–966. [Google Scholar] [CrossRef]
- Peng, J.; Zhuge, W.; Liu, Y.; Zhang, C.; Yang, W.; Huang, Y. Photoelectrochemical dopamine sensor based on Cu-doped Bi2WO6 micro-flowers sensitized cobalt tetraaminophthalocyanine functionalized graphene oxide. J. Electrochem. Soc. 2019, 166, B1612–B1619. [Google Scholar] [CrossRef]
- Peng, J.; Li, X.; Liu, Y.; Zhuge, W.; Zhang, C.; Huang, Y. Photoelectrochemical sensor based on zinc phthalocyanine semiconducting polymer dots for ultrasensitive detection of dopamine. Sens. Actuat. B 2022, 360, 131619. [Google Scholar] [CrossRef]
- Chernyshov, D.V.; Shvedene, N.V.; Antipova, E.R.; Pletnev, I.V. Ionic liquid-based miniature electrochemical sensors for the voltammetric determination of catecholamines. Anal. Chim. Acta 2008, 621, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.B.A.; Rahim, A.; Tanaka, A.A.; Arenas, L.T.; Landers, R.; Gushikem, Y. In situ immobilization of nickel(II) phthalocyanine on mesoporous SiO2/C carbon ceramic matrices prepared by the sol–gel method: Use in the simultaneous voltammetric determination of ascorbic acid and dopamine. Electrochim. Acta 2013, 87, 140–147. [Google Scholar] [CrossRef]
- Rahim, A.; Barros, S.B.A.; Kubota, L.T.; Gushikem, Y. SiO2/C/Cu(II)phthalocyanine as a biomimetic catalyst for dopamine monooxygenase in the development of an amperometric sensor. Electrochim. Acta 2011, 56, 10116–10121. [Google Scholar] [CrossRef]
- Tshenkeng, K.; Mashazi, P. Covalent attachment of cobalt (II) tetra-(3-carboxyphenoxy) phthalocyanine onto pre-grafted gold electrode for the determination of catecholamine neurotransmitters. Electrochim. Acta 2020, 360, 137015. [Google Scholar] [CrossRef]
- Luhana, C.; Moyo, I.; Tshenkeng, K.; Mashazi, P. In-sera selectivity detection of catecholamine neurotransmitters using covalent composite of cobalt phthalocyanine and aminated graphene quantum dots. Microchem. J. 2022, 180, 107605. [Google Scholar] [CrossRef]
- Moyo, I.; Mwanza, D.; Mashazi, P. Novel covalent immobilization of cobalt (II) octa acyl chloride phthalocyanines onto phenylethylamine pre-grafted gold via spontaneous amidation. Electrochim. Acta 2022, 422, 140550. [Google Scholar] [CrossRef]
- Moyo, I.; Mwanza, D.; Mashazi, P. pH sensitive thin films of iron phthalocyanines as electrocatalysts for the detection of neurotransmitters. J. Organomet. Chem. 2023, 990, 122662. [Google Scholar] [CrossRef]
- da Silva, V.N.C.; Farias, E.A.O.; Araujo, A.R.; Magalhaes, F.E.X.; Fernandes, J.R.N.; Souza, J.M.T.; Eiras, C.; da Silva, D.A.; do Vale Bastos, V.H.; Teixeira, S.S. Rapid and selective detection of dopamine in human serum using an electrochemical sensor based on zinc oxide nanoparticles, nickel phthalocyanines, and carbon nanotubes. Biosens. Bioelectron. 2022, 210, 114211. [Google Scholar]
- Buleandra, M.; Popa, D.E.; David, I.G.; Ciucu, A.A. A simple and efficient cyclic square wave voltammetric method for simultaneous determination of epinephrine and norepinephrine using an activated pencil graphite electrode. Microchem. J. 2021, 160, 105621. [Google Scholar] [CrossRef]
- Lechin, F.; Van der Dijs, B.; Lechin, A.E. Circulating serotonin, catecholamines and CNS circuitry related to some cardiorespiratory and vascular disorders. J. Appl. Res. 2005, 5, 605–621. [Google Scholar]
- Wood, J.P.; Traub, S.J.; Lipinski, C. Safety of epinephrine for anaphylaxis in the emergency setting. World J. Emerg. Med. 2013, 4, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.; Cahalan, M. Vasopressors and inotropes. In Pharmacology and Physiology for Anesthesia; Hemmings, H.C., Egan, T.D., Eds.; Saunders (Elsevier): Amsterdam, The Netherlands, 2013; pp. 390–404. [Google Scholar]
- Pihel, K.; Schroeder, T.J.; Wightman, R.M. Rapid and selective cyclic voltammetric measurements of epinephrine and norepinephrine as a method to measure secretion from single bovine adrenal medullary cells. Anal. Chem. 1994, 66, 4532–4537. [Google Scholar] [CrossRef]
- Ni, Y.; Gui, Y.I.; Kokot, S. Application of multiway-variate calibration to simultaneous voltammetric determination of three catecholamines. Anal. Methods 2011, 3, 385–392. [Google Scholar] [CrossRef]
- Cho, T.; Wang, J. Selective voltammetric measurements of epinephrine and norepinephrine in presence of common interferences using cyclic square-voltammetry at unmodified carbon electrodes. Electroanalysis 2018, 30, 1028–1032. [Google Scholar] [CrossRef]
- Beitollahi, H.; Karimi-Maleh, H.; Khabazzadeh, H. Nanomolar and selective determination of epinephrine in the presence of norepinephrine using CPE modified with carbon nanotubes and novel 2-(4-Oxo-3-phenyl-3,4-dihydro-quinazolinyl)-N-phenylhydrazinecarbothioamide. Anal. Chem. 2008, 80, 9848–9851. [Google Scholar] [CrossRef]
- Goyal, R.N.; Bishnoi, S. Simultaneous determination of epinephrine and norepinephrine in human blood plasma and urine samples using nanotubes modified edge plane pyrolytic graphite electrode. Talanta 2011, 84, 78–83. [Google Scholar] [CrossRef]
- Lavanya, N.; Sekar, C. Electrochemical sensor for simultaneous determination of epinephrine and norepinephrine based on cetyltrimethylammonium bromide assisted SnO2 nanoparticles. J. Electroanal. Chem. 2017, 801, 503–510. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Ghalkhani, M.; Amini, M.K. Application of carbon-paste electrode modified with iron phthalocyanine for voltammetric determination of epinephrine in the presence of ascorbic acid and uric acid. Sens. Actuat. B 2009, 137, 669–675. [Google Scholar] [CrossRef]
- Moraes, F.C.; Golinelli, D.L.C.; Mascaro, L.H.; Machado, S.A.S. Determination of epinephrine in urine using multi-walled carbon nanotube modified with cobalt phthalocyanine in a paraffin composite electrode. Sens. Actuat. B 2010, 148, 492–497. [Google Scholar] [CrossRef]
- Young, S.N.; Leyton, M. The role of serotonin in human mood and social interaction. Insight from altered tryptophan levels. Pharmacol. Biochem. Behav. 2002, 71, 857–865. [Google Scholar] [CrossRef]
- Isbister, G.K.; Bowe, S.J.; Dawson, A.H.; Whyte, I.M. Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose. J. Toxicol. Clin. Toxicol. 2004, 42, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Seyedabadi, M.; Fakhfouri, G.; Ramezani, V.; Mehr, S.E.; Rahimian, R. The role of serotonin in memory: Interactions with neurotransmitters and downstream signaling. Exp. Brain Res. 2014, 232, 723. [Google Scholar] [CrossRef] [PubMed]
- Kaye, W.H.; Weltzin, T.E.; Hsu, L.G. Serotonin and norepinephrine activity in anorexia and bulimia nervosa: Relationship to nutrition, feeding, and mood. In Biology of Depressive Disorders, Part B: Subtypes of Depression and Comorbid Disorders; Mann, J.J., Kupfe, D.J., Eds.; Plenum Press: New York, NY, USA, 1993; pp. 127–149. [Google Scholar]
- Sharma, S.; Singh, N.; Tomar, V.; Chandra, R. A review on electrochemical detection of serotonin based on surface modified electrodes. Biosens. Bioelectron. 2018, 107, 76–93. [Google Scholar] [CrossRef]
- de Irazu, S.; Unceta, N.; Samedro, M.B.; Goicolea, M.A.; Barrio, B.R. Multimembrane carbon fiber microelectrodes for amperometric determination of serotonin in human urine. Analyst 2001, 126, 495–500. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Amperometric tyrosinase based biosensors for serotonin detection. Rom. Biotechnol. Lett. 2013, 18, 8253–8262. [Google Scholar]
- Staden, J.F.; Georgescu, R.; Staden, R.I.S.; Calinescu, I. Evaluation of amperometric dot microsensors for the analysis of serotonin in urine samples. J. Electrochem. Soc. 2014, 161, B49–B54. [Google Scholar] [CrossRef]
- de Oliveira, M.S.; de Oliveira Farias, E.A.; de Sousa, A.M.S.; Dionisio, N.A.; Teixeira, P.R.S.; Teixeira, A.S.N.M.; da Silva, D.A.; Eiras, C. Composite films based on copper nanoparticles and nickel phthalocyanine as electrochemical sensors for serotonin detection. Surf. Interfaces 2021, 25, 101245. [Google Scholar] [CrossRef]

| Analytes | Electrode and Medium | Detection Technique | Linear Range [×10−6 mol·L−1] | Detection Limit [×10−6 mol·L−1] | Sample | Recovery (%) | Ref. |
|---|---|---|---|---|---|---|---|
| DA | NiTAPc/GCE 0.1 M PBS, pH 7.4 | LSV | 0.2–20 | 0.09 | Pharmaceuticals Plasma Urine | 96.5–103.0 97.3–103.5 95.7–103.6 | [38] |
| DA | MWCNT/CoPc/GCE 0.1 PBS, pH 4.0 | DPV | 3.11–93.25 | 0.256 | - | - | [36] |
| DA | MWCNT/PAMAM/ NiTsPc/GCE 0.1 M H2SO4 | CV | 2.5–240 | 0.54 | - | - | [156] |
| DA | f-MWCNT/Nano-FeTSPc/GCE 0.1 M PBS, pH 7 | SWV | 20–51 | 0.35 | - | - | [157] |
| DA | CoPc/Gr/GCE 0.2 M PBS, pH 4 | SWV | 3–75 | - | - | - | [158] |
| DA | Nafion/CuTsPc/PANI/GCE 0.1 M H2SO4 | FIA | 0.01–1000 | 0.005 | - | - | [159] |
| DA | MWCNT/NiTsPc/AGCE 0.1 M PBS, pH 4.0 | SWV | 0.02–1384 | 0.001 | Human serum | 98.4–100.7 | [160] |
| DA | N-G/NiTsPc/GCE 0.1 M PBS, pH 7.4 | Amp | 0.1–200 | 0.1 | - | - | [161] |
| DA | rGO/Mn-TPP/GCE 0.05 M PBS, pH 7 | Amp | 0.3–188.8 | 0.008 | Pharmaceuticals Human urine | 90.06–92.06 90.6–97.5 | [162] |
| DA + AA + UA | MWCNTs/PdTAPc/GCE 0.25 M H2SO4 | CV | DA: 2–16 AA: 3–24 UA: 5–40 | DA: 0.6 AA: 1.0 UA: 1.5 | Human urine | DA: 102–103.3 AA: 99–100.6 UA: 99.9–101.3 | [163] |
| DA | CNP/FeTCAPc/GCE PBS (pH 7) | CA | 0.05–0.50 | 0.016 | - | - | [164] |
| DA | CNP/polyFeTBImPc/GCE PBS, pH 7 | CA | 0.05–0.50 | 0.01 | Pharmaceuticals | 100.32–102 | [164] |
| DA | CoTSPc/Gr/GCE 0.1 M PBS, pH 7.3 | DPSV | 0.02–220 | 0.00087 | - | - | [15] |
| DA | CoPc/GCE 0.1 M PBS, pH 7.4 | DPV | 0.223–8.513 | 0.0205 | - | - | [165] |
| DA + UA | MnPc/GCE 0.1 M PBS, pH 7.4 | DPV | DA: 0.029–6.239 UA: 1.127–10.977 | DA: 0.0020 UA: 0.0146 | - | - | [165] |
| DA + UA | ZnPc/GCE 0.1 M PBS, pH 7.4 | DPV | DA: 0.021–7.441 UA: 0.014–8.577 | DA: 0.0105 UA: 0.0305 | - | - | [165] |
| DA + AA | MWCNT/CoTMBANAPc/GCE PBS, pH 7 | DPV | DA: 7.5–67.5 AA: 7.5–67.5 | DA: 0.33 AA: 6.66 | - | - | [166] |
| DA + AA + UA | CoTGPc/GCE PBS, pH 7 | DPV | DA: 2–12 AA: 2–12 UA: 2–12 | DA: 1.2 AA: 0.5 UA: 0.5 | Pharmaceuticals Urine | DA: 107.5 AA: 102 UA: 96.7–101.2 | [58] |
| DA | Poly(CoTNBAPc)/GCE 0.1 M PBS, pH 7 | Amp | 0.1–1 | 0.02 | Pharmaceuticals | 102–104 | [13] |
| DA + UA / DMZ | CoTfurNH2Pc/GCE PBS, pH 7 | DPV | DA: 3–21 UA: 2–14 DMZ: 0.3–2.1 | DA: 1 UA: 0.66 DMZ: 0.1 | Milk powder Urine | DA: – UA: 99.8–101.2 DMZ: 97.5–101.0 | [167] |
| DA + AA + UA + XO | Fe3C@NGCSs/GCE PBS, pH 6 | DPV | DA: 1.2–120.8 AA: 54–5491 UA: 4.8–263 XA: 4.8–361 | DA: 0.34 AA: 16.7 UA: 1.4 XA: 1.5 | Serum | DA: 98.8 AA: 103.16 UA: 97.26 XA: 101.22 | [168] |
| DA | rGO/ZnTPEBIPc/GCE PBS, pH 4 | Amp | 0.02–1 | 0.006 | Pharmaceuticals | 96.7–107.5 | [169] |
| DA + AA + UA | AgNPs/Cu-MAPA/GCE 0.1 M PBS, pH 7.4 | DPV | DA: 0.01–10 AA: 0.01–10 UA: 0.01–10 | DA: 0.0007 AA: 0.0025 UA: 0.005 | Articial urine | 94–107 | [170] |
| DA | NGQD/CoPc/GCE 0.1 M PBS | CA | 100–1000 | 0.12 | - | - | [171] |
| DA + UA | Gr/Car-ZnPc/GCE 0.1 M PBS, pH 7 | DPV | DA: 2–16 UA: 20–160 | DA: 0.079 UA: 0.33 | Tap water | UA: 93.33–95 | [172] |
| DA + UA | Gr/Car-CoPc/GCE 0.1 M PBS, pH 7 | DPV | DA: 2–14 UA: 12–84 | DA: 0.206 UA: 0.53 | Tap water | - | [172] |
| DA | TACoPc/PANI/GCE 0.1 M PBS, pH 7 | CA | 20–200 | 0.064 | Pharmaceuticals | 103–103.6 | [173] |
| DA + AA + UA | Poly-3N-CoPc/GCE PBS, pH 7.4 | DPV | DA: 3.83–19 AA: 1.4–60 UA: 3.94–53 | DA: 3.12 AA: 0.49 UA: 0.87 | - | - | [174] |
| DA + AA + UA | Poly-3N-CuPc/GCE PBS, pH 7.4 | DPV | DA: 3.71–19 AA: 3.69–53 UA: 3.55–53 | DA: 2.53 AA: 0.52 UA: 1.31 | - | - | [174] |
| DA + UA | CoPc/GQDs/GCE 0.001 M PBS, pH 7 | DPV | DA: 2.91–16.2 UA: 10.76–3003 | DA: 0.021 UA: 0.145 | Human urine | DA: 91.3–104.5 UA: 91–95.2 | [175] |
| DA + PAR | Poly-CoTAPc/ERGO/GCE 0.1 M PBS, pH 7.4 | DPV | DA: 2–100 PA: 7–90 | DA: 0.095 PA: 0.104 | Synthetic urine | DA: 99.5–103 PA: 96–101 | [176] |
| DA | Clicked-α-CoPc-flav3/GCE PBS, pH 6.3 | SWV | 2–14 | 0.31 | Wastewater | 97.1 | [177] |
| DA | PPO/CoPc/CPE 0.05 M PBS, pH 7.4 | Amp | Up to 1.8 | 7.5 | Urine | - | [178] |
| DA + 5-HT | FePc/CPE FeTSPc/CPE FeTAPc/CPE TRIS, pH 7.4 | OSWV | DA: 1–15 5-HT: 1–15 | DA: 1 5-HT: 1 | - | - | [47] |
| DA + AA | FeTSPc/CPE pH 7.4 | CV | - | DA: 0.45 AA: 0.75 | - | - | [179] |
| DA + AA | Nano-CoPc/CPE 0.025 M PBS, pH 7.4 + 4×-10−4 M CTAB | DPV | DA: 3–100 AA: 5–300 | DA: 1 AA: 1.7 | Pharmaceuticals | DA: 97.5–103.9 AA: 96.9–104.5 | [180] |
| DA | MWCNT/FePc/CPE PBS, pH 7.4 | DPV | 5–25 | 0.205 | Serum | - | [181] |
| DA | Si-Db/CuTsPc/CPE 0.1 M PBS, pH 4.5 | CA | 9.9–107.1 | 0.42 | - | - | [182] |
| DA | (PAH/NiTsPc)5/ITOE 0.05 M H2SO4 | CV | - | 0.089 | - | - | [183] |
| DA | (PAH/Chicha/PAH/NiTsPc)5/ITOE 0.05 M H2SO4 | CV | 0.025–3 | 0.105 | - | - | [183] |
| DA | PAH/FePc/AgNP/ITOE 0.1 M KCl | CV | 2–97 | 0.86 | - | - | [184] |
| DA | DS01/NiTsPc/ITOE 0.05 M H2SO4 | CV | 0–19.6 | 1.665 | - | - | [185] |
| DA | (PEI/Na+MMT/PEI/NiTsPc)10/ITOE 0.1 M PBS, pH 7.0 | DPV | 5–150 | 1 | Human urine | 94–111 | [186] |
| DA + L-Dopa | FePc/ED/ITOE 0.1 M KCl | DPV | DA: 2–80 LD: 2–120 | DA: 0.288 LD: 0.564 | - | - | [18] |
| DA | CoTAPc-GO/Cu-Bi2WO6/ITOE BRB, pH 7.4 | PEC sensor | 0.05–5 5–250 | 0.0072 | Pharmaceuticals | 99.2–106.7 | [187] |
| DA | ZnPc-P8BT-Pdots/ITOE | PEC sensor | 0.0025–125 | 0.00169 | - | - | [188] |
| DA | CoTCPhOPc/PEA/AuE PBS, pH 7.4 | CV | 5–100 | 1.32 | - | - | [189] |
| DA | AmGQDs-CoTCPhOPc/IPA/AuE 0.1 M PBS, pH 7.4 | DPV | 1–50 | 0.20 | Calf serum | 101–106 | [190] |
| DA | CoOCAPc/PEA/AuE 0.1 M PBS, pH 7.4 | DPV | 50 | 0.064 | - | - | [191] |
| DA | FeOCAPc/PEA/AuE 0.1 M PBS, pH 7.4 | DPV | 1–50 | 0.25 | Calf serum | 96.9–105 | [192] |
| DA | CoTBuPc/IL/SPGE 0.1 M H2SO4 + 0.01 M KCl, pH 1 | CV | 3.9–100 | 1.2 | - | - | [193] |
| DA + AA | NiPc/CCE 0.5 M KCl, pH 5 | DPV | DA: 40–1080 AA: 90–2110 | DA: 0.26 AA: 0.45 | Synthetic | DA: 107.25–108.25 AA: 101.25–108.75 | [194] |
| DA | SiO2/C/CuPc disk electrode 0.08 M BRB, pH 6 | Amp | 10–140 | 0.6 | Synthetic | 100 | [195] |
| DA | NiTsPc/ZnONPs/CNT/PGE 0.1 M KH2PO4, pH 3.4 | DPV CA | 0–15 0–7 | 0.007 0.024 | Human serum | 100.83–104 | [196] |
| Analytes | Electrode and Medium | Detection Technique | Linear Range [×10−6 mol·L−1] | Detection Limit [×10−6 mol·L−1] | Sample | Recovery (%) | Ref. |
|---|---|---|---|---|---|---|---|
| EP | FePc/CPE 0.1 M ABS, pH 4 | DPV | 1–300 | 0.5 | Pharmaceuticals | 103.8 | [207] |
| EP | Paraffin-MWCNT-CoPc/CPE 0.1 M PBS, pH 6 | DPV | 1.33–5.5 | 0.0156 | Human urine | 95.7–98.1 | [208] |
| EP | MWCNT/Fe3O4/29H,31H-P/GCE PBS, pH 7.2 | DPV | 7.5–56 | 4.6 | - | - | [57] |
| EP | MWCNT/ZnO/29H,31H-P/GCE PBS, pH 7.2 | DPV | 7.5–56 | 6.5 | - | - | [57] |
| EP | CoTCPhOPc/PEA/AuE PBS, pH 7.4 | CV | 5–100 | 3.08 | - | - | [189] |
| EP | AmGQDs-CoTCPhOPc/IPA/AuE 0.1 M PBS, pH 7.4 | DPV | 1–50 | 0.23 | Calf serum | 89–92 | [190] |
| EP | CoOCAPc/PEA/AuE 0.1 M PBS, pH 7.4 | DPV | 5–50 | 0.22 | - | - | [191] |
| EP | FeOCAPc/PEA/AuE 0.1 M PBS, pH 7.4 | DPV | 1–30 | 0.45 | Calf serum | 101–106 | [192] |
| EP | CoTBuPc/IL/SPGE 0.1 M H2SO4 + 0.01 M KCl, pH 1 | CV | 0.29–100 | 1.13 | - | - | [193] |
| NP | MWCNT/Fe3O4/29H,31H-P/GCE PBS, pH 7.2 | DPV | 7.5–48 | 2.2 | - | - | [57] |
| NP | MWCNT/ZnO/29H,31H-P/GCE PBS, pH 7.2 | DPV | 7.5–48 | 1.7 | - | - | [57] |
| NP | CoTCPhOPc/PEA/AuE PBS, pH 7.4 | CV | 5–100 | 2.11 | - | - | [189] |
| NP | AmGQDs-CoTCPhOPc/IPA/AuE 0.1 M PBS, pH 7.4 | DPV | 1–50 | 0.45 | Calf serum | 85–102 | [190] |
| NP | CoOCAPc/PEA/AuE 0.1 M PBS, pH 7.4 | DPV | 0.5–50 | 0.17 | - | - | [191] |
| NP | FeOCAPc/PEA/AuE 0.1 M PBS, pH 7.4 | DPV | 1–50 | 0.34 | Calf serum | 93.5–100 | [192] |
| Analytes | Electrode and Medium | Detection Technique | Linear Range [×10−6 mol·L−1] | Detection Limit [×10−6 mol·L−1] | Sample | Recovery (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 5-HT | NiTSPc/Nafion/CFE 0.1 M PBS, pH 7.4 | SWV | 0.005–0.09 | 0.0038 | Urine | 92–93 | [214] |
| 5-HT | Tyr-CoPc/CPE 0.1 M PBS, pH 7 | Amp | 4–140 | 0.84 | Walnuts | 97–104 | [215] |
| 5-HT | G-FePc/GPE 0.1 M KCl + PBS, pH 3 | DPV | 1–1000 | 0.76 | Urine | 96.41 | [216] |
| 5-HT | GR-FePc/GPE 0.1 M KCl + PBS, pH 3 | DPV | 1–1000 | 0.734 | Urine | 97.07 | [216] |
| 5-HT | (PAH/NiTsPc/PAH/ CuNPs)/ITOE 0.1 M BRB, pH 2.2 | CV | 0.35–135 | 0.13 | - | - | [217] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciucu, A.A.; Buleandră, M.; Popa, D.E.; Ștefănescu, D.C. Phthalocyanine-Modified Electrodes Used in the Electroanalysis of Monoamine Neurotransmitters. Chemosensors 2025, 13, 243. https://doi.org/10.3390/chemosensors13070243
Ciucu AA, Buleandră M, Popa DE, Ștefănescu DC. Phthalocyanine-Modified Electrodes Used in the Electroanalysis of Monoamine Neurotransmitters. Chemosensors. 2025; 13(7):243. https://doi.org/10.3390/chemosensors13070243
Chicago/Turabian StyleCiucu, Anton Alexandru, Mihaela Buleandră, Dana Elena Popa, and Dragoș Cristian Ștefănescu. 2025. "Phthalocyanine-Modified Electrodes Used in the Electroanalysis of Monoamine Neurotransmitters" Chemosensors 13, no. 7: 243. https://doi.org/10.3390/chemosensors13070243
APA StyleCiucu, A. A., Buleandră, M., Popa, D. E., & Ștefănescu, D. C. (2025). Phthalocyanine-Modified Electrodes Used in the Electroanalysis of Monoamine Neurotransmitters. Chemosensors, 13(7), 243. https://doi.org/10.3390/chemosensors13070243






