Giant Chemo-Resistive Response of POSS Nano-Spacers in PS- and PMMA-Based Quantum Resistive Vapour Sensors (vQRS) Used for Cancer Biomarker Analysis
Abstract
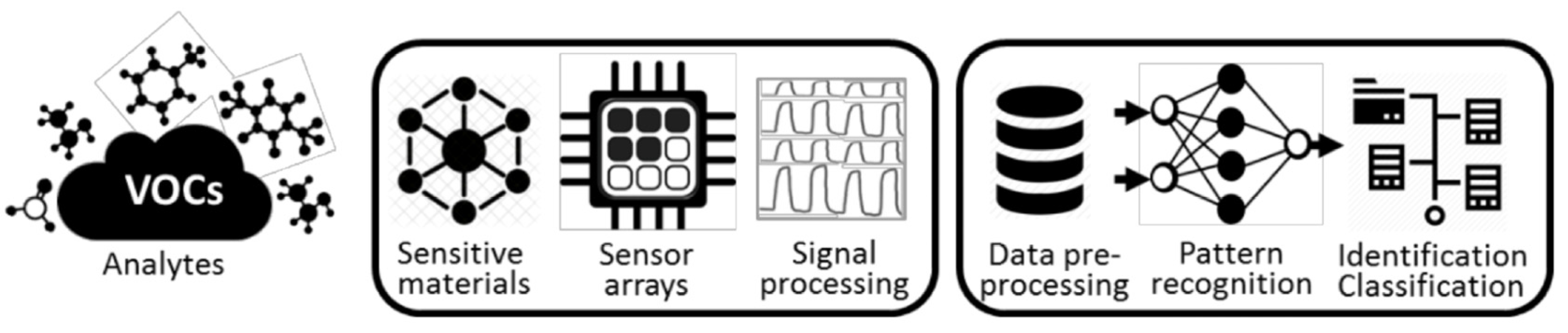
1. Introduction
2. Materials and Methods
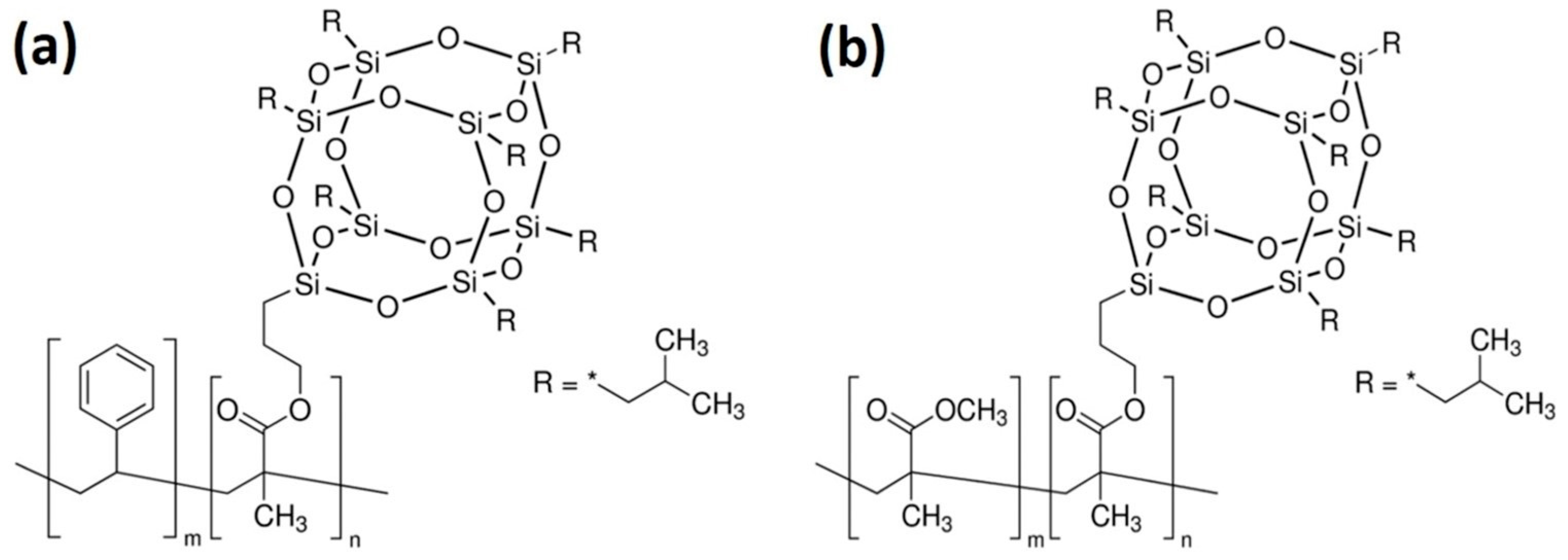
2.1. Materials
2.2. Fabrication of Sensors
2.3. Techniques Used for the Morphological Characterization
2.4. Characterization of the Chemo-Resistive Properties by Dynamic Vapour Sensing (DVS)
3. Results and Discussion
3.1. Morphological Characterization of vQRS Transducers
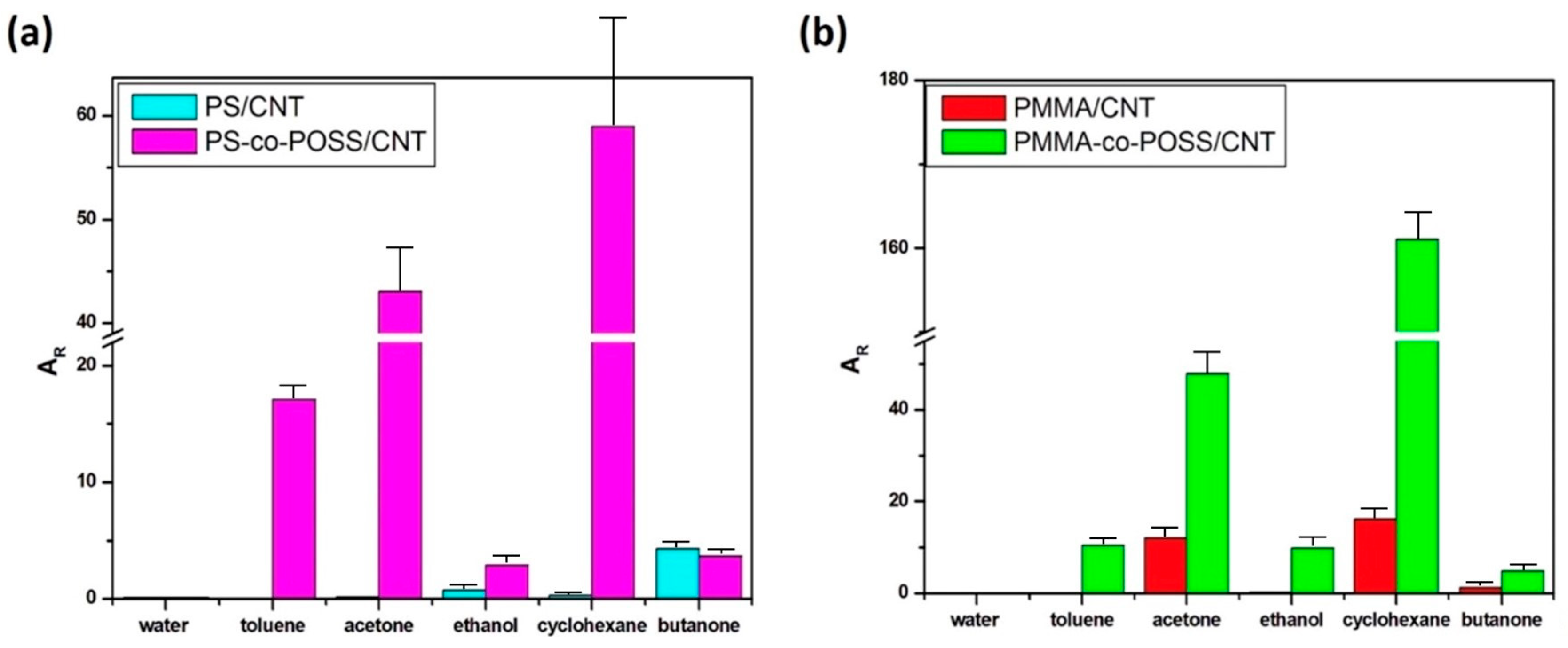
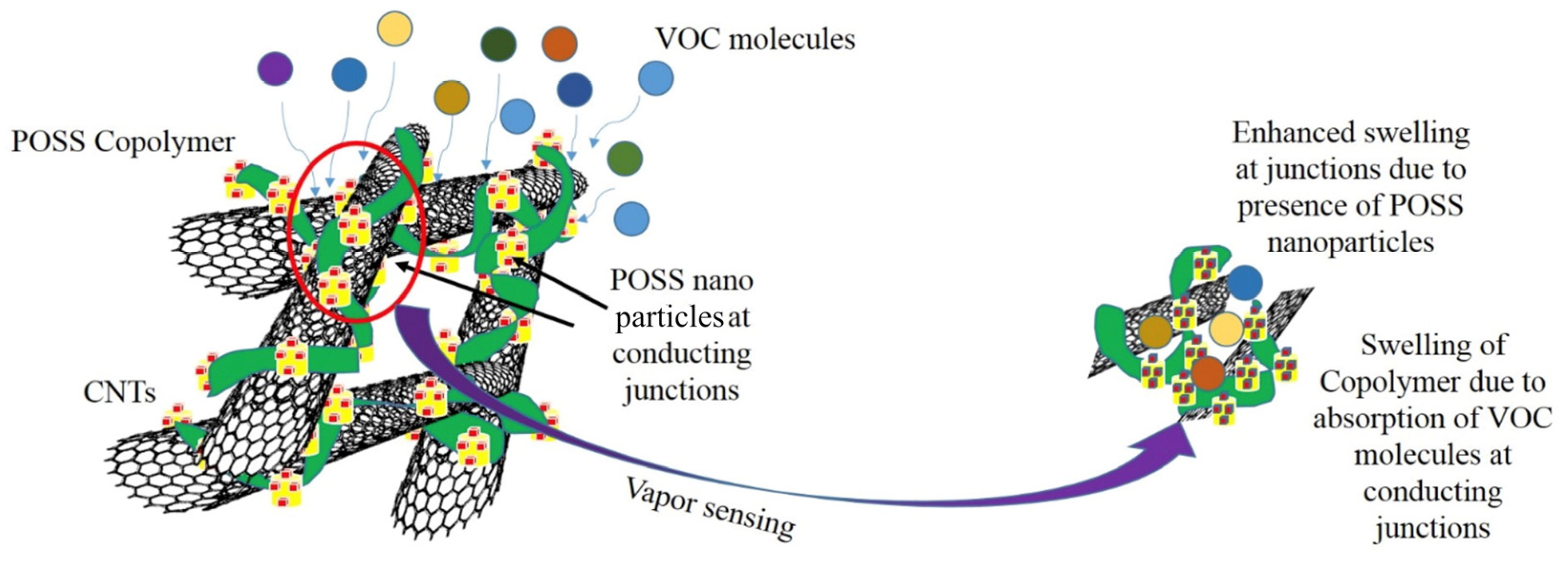
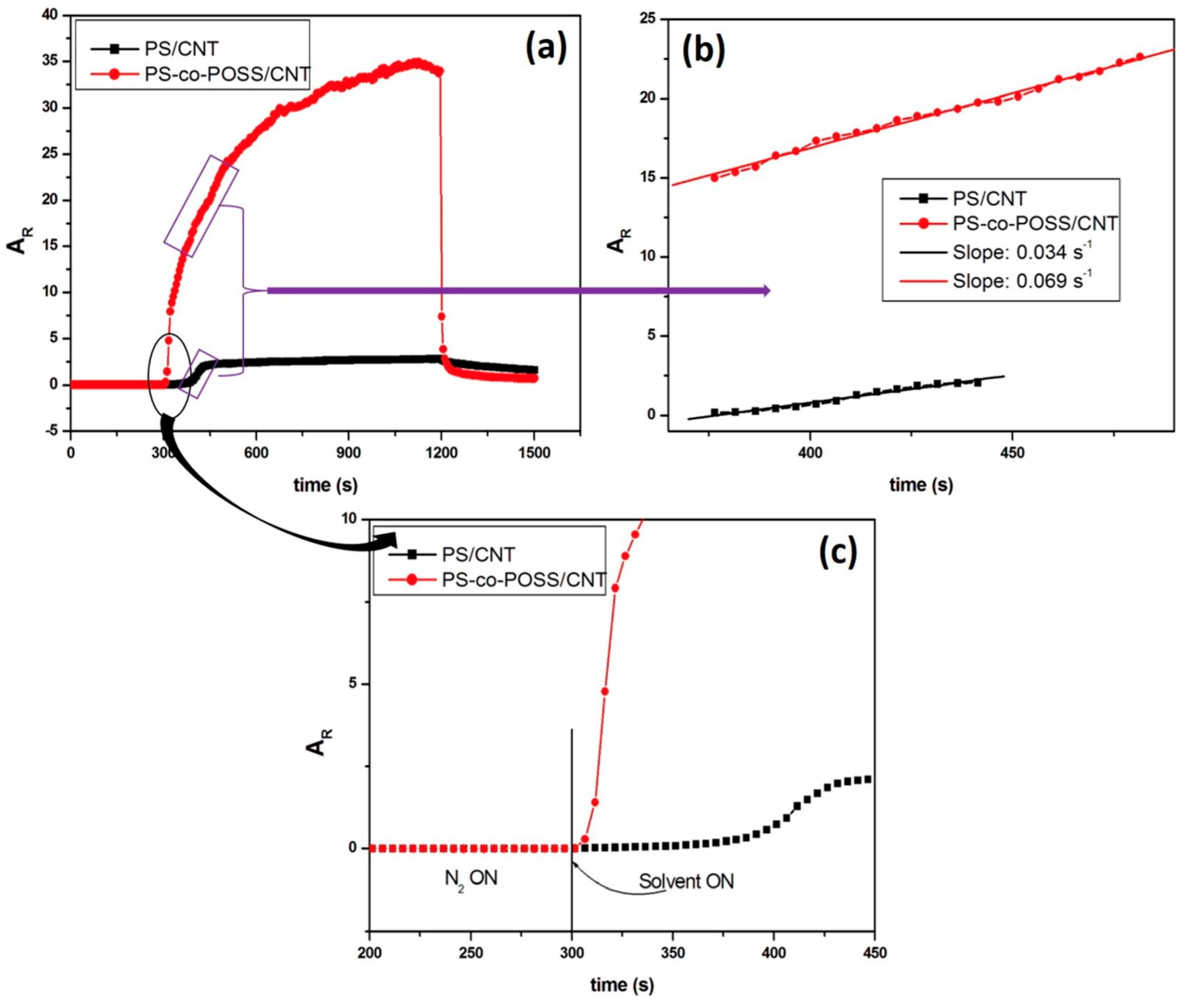
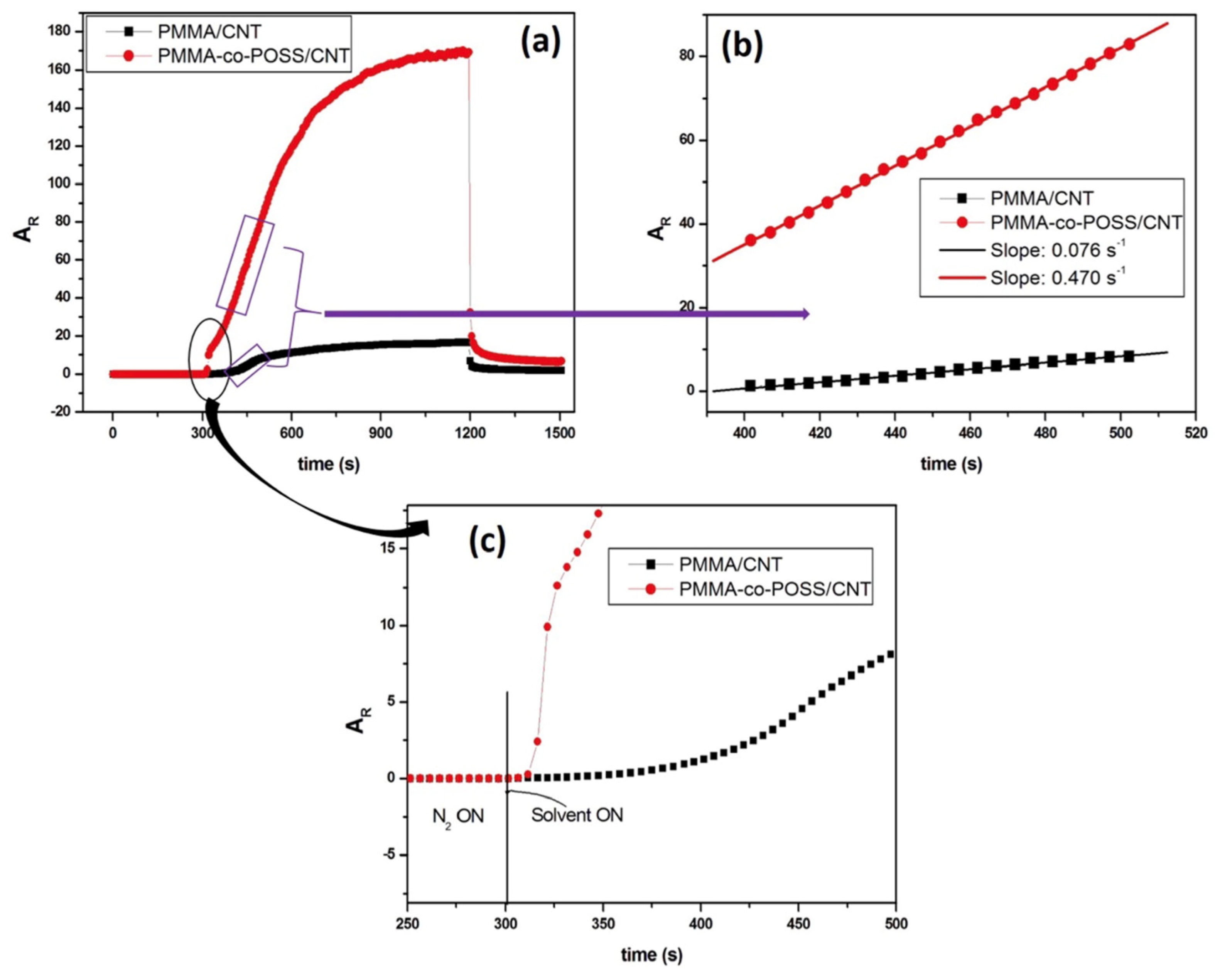
3.2. Effects of POSS on the Chemo-Resistive Behaviour of vQRS
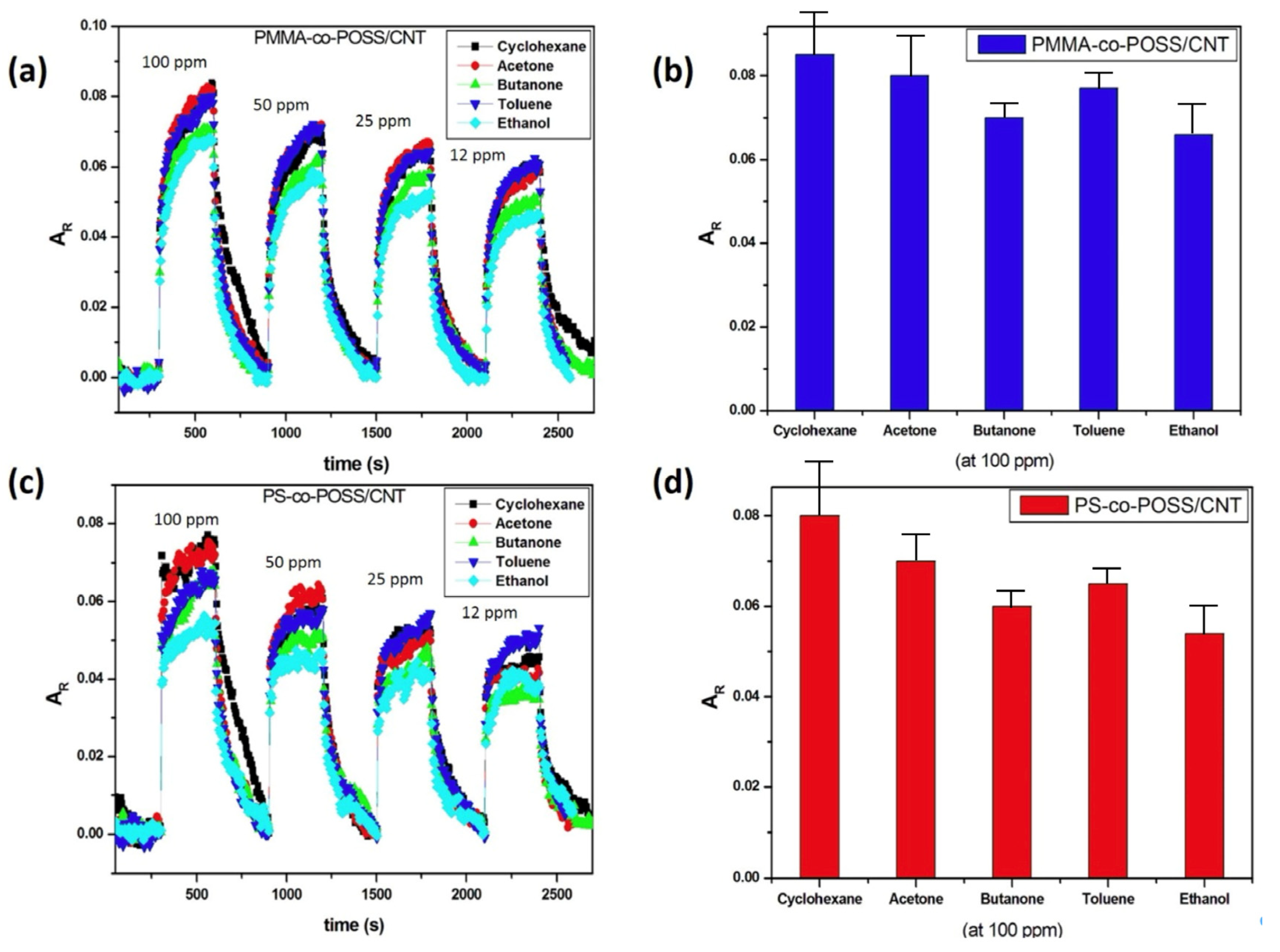
3.3. Vapour Sensing of Some Lung Cancer VOC Biomarkers with POSS-Based vQRS
3.4. Sensitivity of vQRS in the ppm Range of Concentration and Determination of the Limit of Detection
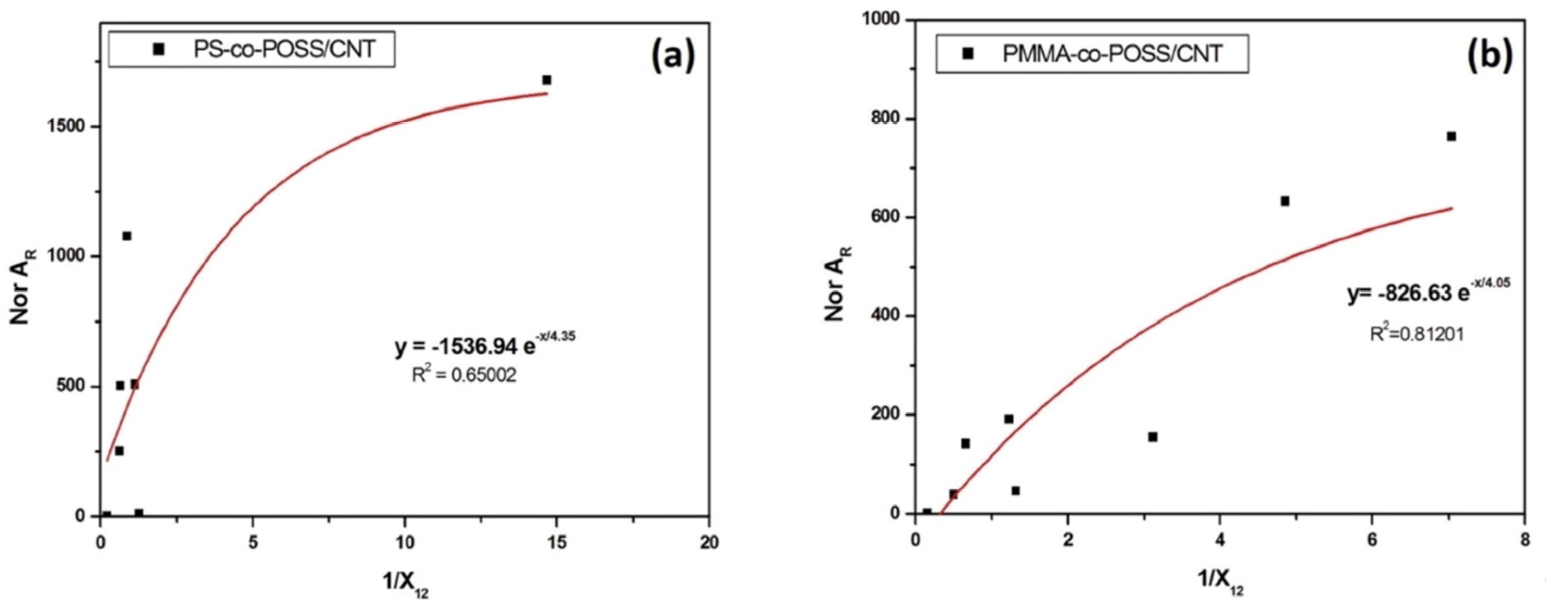
3.5. Origin of Sensors’ Selectivity
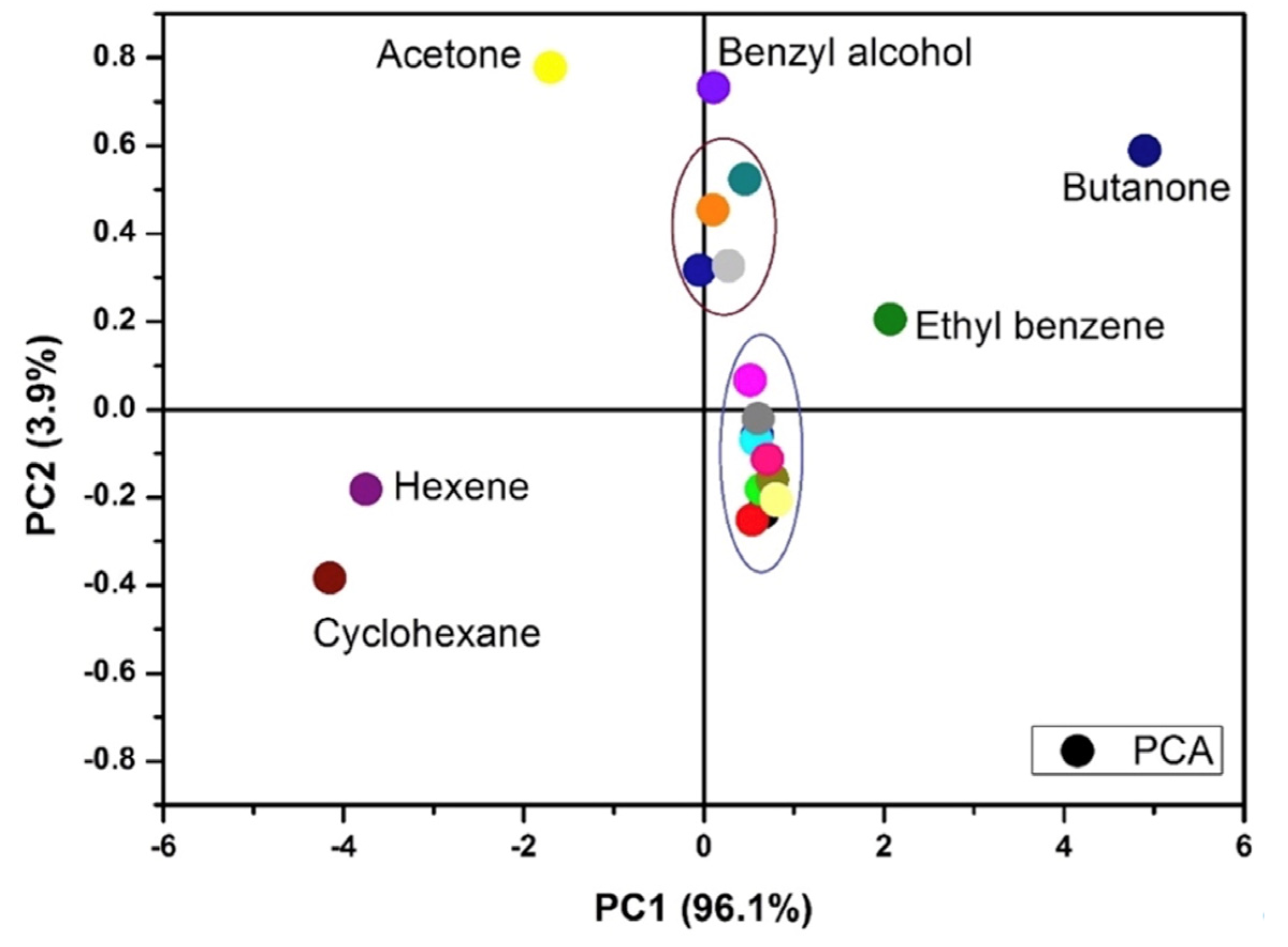
3.6. Analysis of vQRS Responses by PCA Mapping
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasiri, N.; Clarke, C. Nanostructured Chemiresistive Gas Sensors for Medical Applications. Sensors 2019, 19, 462. [Google Scholar] [CrossRef] [PubMed]
- Sachan, A.; Castro, M.; Feller, J.F. Volatolomics for Anticipated Diagnosis of Cancers with Chemoresistive Vapour Sensors: A Review. Chemosensors 2025, 13, 15. [Google Scholar] [CrossRef]
- Kaloumenou, M.; Skotadis, E.; Lagopati, N.; Efstathopoulos, E.; Tsoukalas, D. Breath Analysis: A Promising Tool for Disease Diagnosis—The Role of Sensors. Sensors 2022, 22, 1238. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.; Wang, S.; Duan, Z.; Jiang, Y. Evolution of Breath Analysis Based on Humidity and Gas Sensors: Potential and Challenges. Sens. Actuators B Chem. 2020, 318, 128104. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Zuri, L.; Haick, H. Combined Volatolomics for Monitoring of Human Body Chemistry. Sci. Rep. 2014, 4, 4611. [Google Scholar] [CrossRef]
- Li, W.; Jia, Z.; Xie, D.; Chen, K.; Cui, J.; Liu, H. Recognizing Lung Cancer Using a Homemade E-Nose: A Comprehensive Study. Comput. Biol. Med. 2020, 120, 103706. [Google Scholar] [CrossRef]
- Lee, T.; Yu, J.; Lee, S.W.; Oh, S.H.; Kang, D.; Son, H.; Hwang, H.J.; You, J.H.; Lee, G. Machine Learning-Integrated Biomimetic Electronic Noses: Future Perspectives. Microchem. J. 2025, 213, 113638. [Google Scholar] [CrossRef]
- Glöckler, J.; Mitrovics, J.; Beeken, S.; Leja, M.; Welearegay, T.; Österlund, L.; Haick, H.; Shani, G.; Di Natale, C.; Murillo, R.; et al. Infrared Spectroscopic Electronic Noses: An Innovative Approach for Exhaled Breath Sensing. ACS Sens. 2025, 10, 427–438. [Google Scholar] [CrossRef]
- Dawson, J.; Green, K.; Lazarowicz, H.; Cornford, P.; Probert, C. Analysis of Urinary Volatile Organic Compounds for Prostate Cancer Diagnosis: A Systematic Review. BJUI Compass 2024, 5, 936–947. [Google Scholar] [CrossRef]
- Bernabei, M.; Pennazza, G.; Santonico, M.; Corsi, C.; Roscioni, C.; Paolesse, R.; Di Natale, C.; D’Amico, A. A Preliminary Study on the Possibility to Diagnose Urinary Tract Cancers by an Electronic Nose. Sens. Actuators B Chem. 2008, 131, 1–4. [Google Scholar] [CrossRef]
- Velumani, M.; Prasanth, A.; Narasimman, S.; Chandrasekhar, A.; Sampson, A.; Meher, S.R.; Rajalingam, S.; Rufus, E.; Alex, Z.C. Nanomaterial-Based Sensors for Exhaled Breath Analysis: A Review. Coatings 2022, 12, 1989. [Google Scholar] [CrossRef]
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of Lung, Breast, Colorectal, and Prostate Cancers from Exhaled Breath Using a Single Array of Nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.; Bono, R.; Pennazza, G.; Santonico, M.; Mantini, G.; Bernabei, M.; Zarlenga, M.; Roscioni, C.; Martinelli, E.; Paolesse, R.; et al. Identification of Melanoma with a Gas Sensor Array. Ski. Res. Technol. 2008, 14, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.; Gallagher, M.; Ozdener, M.H.; Wysocki, C.J.; Goldsmith, B.R.; Isamah, A.; Faranda, A.; Fakharzadeh, S.S.; Herlyn, M.; Johnson, A.T.C.; et al. Volatile Biomarkers from Human Melanoma Cells. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 931, 90–96. [Google Scholar] [CrossRef]
- Shurmer, H.V.; Gardner, J.W.; Corcoran, P. Intelligent Vapour Discrimination Using a Composite 12-Element Sensor Array. Sens. Actuators B Chem. 1990, 1, 256–260. [Google Scholar] [CrossRef]
- Freund, M.S.; Lewis, N.S. A Chemically Diverse Conducting Polymer-Based “Electronic Nose”. Proc. Natl. Acad. Sci. USA 1995, 92, 2652–2656. [Google Scholar] [CrossRef]
- Persaud, K.; Dodd, G. Analysis of Discrimination Mechanisms in the Mammalian Olfactory System Using a Model Nose. Nature 1982, 299, 352–355. [Google Scholar] [CrossRef]
- D’Amico, A.; Di Natale, C.; Macagnano, A.; Davide, F.; Mantini, A.; Tarizzo, E.; Paolesse, R.; Boschi, T. Technologies and Tools for Mimicking Olfaction: Status of the Rome “Tor Vergata” Electronic Nose. Biosens. Bioelectron. 1998, 13, 711–721. [Google Scholar] [CrossRef]
- Singh, A.; Ahmed, E.; Rather, M.D.; Sundararajan, A.; Sharma, A.; Choudhary, F.S.; Sundramoorthy, A.K.; Dixit, S.; Vatin, N.I.; Arya, S. Marketing Strategies in Nanomaterials for Sensor Applications: Bridging Lab to Market. Glob. Chall. 2025, 9, 2400294. [Google Scholar] [CrossRef]
- Tomić, M.; Šetka, M.; Vojkůvka, L.; Vallejos, S. VOC Sensing by Metal Oxides, Conductive Polymers, and Carbon-Based Materials. Nanomaterials 2021, 11, 552. [Google Scholar] [CrossRef]
- Amann, A.; Corradi, M.; Mazzone, P.; Mutti, A. Lung Cancer Biomarkers in Exhaled Breath. Expert Rev. Mol. Diagn. 2011, 11, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile Organic Compounds of Lung Cancer and Possible Biochemical Pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Sun, Q.; Xue, F.; Lin, D. Adsorption of Volatile Organic Compounds by Metal–Organic Frameworks MIL-101: Influence of Molecular Size and Shape. J. Hazard. Mater. 2011, 195, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.A.; Alexis, N.; Bacchus, H.; Bernstein, I.L.; Fritz, P.; Horner, E.; Li, N.; Mason, S.; Nel, A.; Oullette, J.; et al. The Health Effects of Nonindustrial Indoor Air Pollution. J. Allergy Clin. Immunol. 2008, 121, 585–591. [Google Scholar] [CrossRef]
- Hopkins, A.R.; Lewis, N.S. Detection and Classification Characteristics of Arrays of Carbon Black/Organic Polymer Composite Chemiresistive Vapor Detectors for the Nerve Agent Simulants Dimethylmethylphosphonate and Diisopropylmethylphosponate. Anal. Chem. 2001, 73, 884–892. [Google Scholar] [CrossRef][Green Version]
- Chan, H.P.; Lewis, C.; Thomas, P.S. Exhaled Breath Analysis: Novel Approach for Early Detection of Lung Cancer. Lung Cancer 2009, 63, 164–168. [Google Scholar] [CrossRef]
- Tisch, U.; Haick, H. Nanomaterials for Cross-Reactive Sensor Arrays. MRS Bull. 2011, 35, 797–803. [Google Scholar] [CrossRef]
- Peng, G.; Tisch, U.; Haick, H. Detection of Nonpolar Molecules by Means of Carrier Scattering in Random Networks of Carbon Nanotubes: Toward Diagnosis of Diseases via Breath Samples. Nano Lett. 2009, 9, 1362–1368. [Google Scholar] [CrossRef]
- Wilson, A. Advances in Electronic-Nose Technologies for the Detection of Volatile Biomarker Metabolites in the Human Breath. Metabolites 2015, 5, 140–163. [Google Scholar] [CrossRef]
- Cho, S.Y.; Yoo, H.W.; Kim, J.Y.; Jung, W.B.; Jin, M.L.; Kim, J.S.; Jeon, H.J.; Jung, H.T. High-Resolution p-Type Metal Oxide Semiconductor Nanowire Array as an Ultrasensitive Sensor for Volatile Organic Compounds. Nano Lett. 2016, 16, 4508–4515. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, C.; Li, P.; Sun, Y. Layer-by-Layer Self-Assembly of Co3O4 Nanorod-Decorated MoS2 Nanosheet-Based Nanocomposite toward High-Performance Ammonia Detection. ACS Appl. Mater. Interfaces 2017, 9, 6462–6471. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Wang, J.; Wang, B.; Zhu, L.; Hong, X. Ripeness Prediction of Post Harvest Kiwifruit Using a MOS E-Nose Combined with Chemometrics. Sensors 2019, 19, 419. [Google Scholar] [CrossRef] [PubMed]
- Bochenkov, V.E.; Sergeev, G.B. Sensitivity, Selectivity, and Stability of Gas-Sensitive Metal-Oxide Nanostructures. In Metal Oxide Nanostructures & Their Applications; Umar, A., Hahn, Y.B., Eds.; American Scientific Publishers: Valencia, CA, USA, 2010; Volume 3, pp. 31–52. ISBN 1588831760. [Google Scholar]
- Zhang, L.; Tian, J.; Wang, Y.; Wang, T.; Wei, M.; Li, F.; Li, D.; Yang, Y.; Yu, H.; Dong, X. Polyoxometalates Electron Acceptor-Intercalated In2O3@SnO2 Nanofibers for Chemiresistive Ethanol Gas Sensors. Sens. Actuators B Chem. 2024, 410, 135728. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Zhao, T.; Li, H.; Jiang, J.; Ye, J. Electronic Nose for the Detection and Discrimination of Volatile Organic Compounds: Application, Challenges, and Perspectives. TrAC Trends Anal. Chem. 2024, 180, 117958. [Google Scholar] [CrossRef]
- Li, J.; Qiao, J.; Liu, C.; Zhou, Z.; Kong, C.; Chang, Z.; Weng, X.; Zhang, S. Study on the Geographic Traceability and Growth Age of Panax Ginseng C. A. Meyer Base on an Electronic Nose and Fourier Infrared Spectroscopy. Chemosensors 2025, 13, 176. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef]
- Tung, T.T.; Castro, M.; Feller, J.F.; Kim, T.Y.; Suh, K.S. Hybrid Film of Chemically Modified Graphene and Vapor-Phase-Polymerized PEDOT for Electronic Nose Applications. Org. Electron. 2013, 14, 2789–2794. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, S.; Yin, H.; Weisbecker, H.; Tran, H.T.; Guo, Z.; Han, T.; Wang, Y.; Liu, Y.; Wu, Y.; et al. Skin-Inspired, Sensory Robots for Electronic Implants. Nat. Commun. 2024, 15, 4777. [Google Scholar] [CrossRef]
- Chen, S.; Xu, S.; Fan, X.; Xiao, X.; Duan, Z.; Zhao, X.; Chen, G.; Zhou, Y.; Chen, J. Advances in 2D Materials for Wearable Biomonitoring. Mater. Sci. Eng. R Rep. 2025, 164, 100971. [Google Scholar] [CrossRef]
- Wang, S.; Yang, T.; Zhang, D.; Hua, Q.; Zhao, Y. Unveiling Gating Behavior in Piezoionic Effect: Toward Neuromimetic Tactile Sensing. Adv. Mater. 2024, 36, 2405391. [Google Scholar] [CrossRef]
- Rajesh; Ahuja, T.; Kumar, D. Recent Progress in the Development of Nano-Structured Conducting Polymers/Nanocomposites for Sensor Applications. Sens. Actuators B Chem. 2009, 136, 275–286. [Google Scholar] [CrossRef]
- Castro, M.; Kumar, B.; Feller, J.F.; Haddi, Z.; Amari, A.; Bouchikhi, B. Novel E-Nose for the Discrimination of Volatile Organic Biomarkers with an Array of Carbon Nanotubes (CNT) Conductive Polymer Nanocomposites (CPC) Sensors. Sens. Actuators B Chem. 2011, 159, 213–219. [Google Scholar] [CrossRef]
- Michael, E.K.; Robert, H.G.; Lewis, N.S. Properties of Vapor Detector Arrays Formed through Plasticization of Carbon Black−Organic Polymer Composites. Anal. Chem. 2002, 76, 1307–1315. [Google Scholar] [CrossRef]
- Mondal, I.; Zoabi, A.; Haick, H. Biodegradable, Humidity-insensitive Mask-Integrated E-nose for Sustainable and Non-invasive Continuous Breath Analysis. Adv. Funct. Mater. 2025, 2425193. [Google Scholar] [CrossRef]
- Chatterjee, S.; Castro, M.; Feller, J.F. An E-Nose Made of Carbon Nanotube Based Quantum Resistive Sensors for the Detection of Eighteen Polar/Nonpolar VOC Biomarkers of Lung Cancer. J. Mater. Chem. B 2013, 1, 4563. [Google Scholar] [CrossRef]
- Kumar, B.; Castro, M.; Feller, J.F. Tailoring the Chemo-Resistive Response of Self-Assembled Polysaccharide-CNT Sensors by Chain Conformation at Tunnel Junctions. Carbon 2012, 50, 3627–3634. [Google Scholar] [CrossRef]
- Zhang, W.; Müller, A.H.E. Architecture, Self-Assembly and Properties of Well-Defined Hybrid Polymers Based on Polyhedral Oligomeric Silsequioxane (POSS). Prog. Polym. Sci. 2013, 38, 1121–1162. [Google Scholar] [CrossRef]
- Pielichowski, K.; Njuguna, J.; Janowski, B.; Pielichowski, J. Polyhedral Oligomeric Silsesquioxanes (POSS)-Containing Nanohybrid Polymers. In Supramolecular Polymers Polymeric Betains Oligomers; Springer: Berlin/Heidelberg, Germany, 2006; pp. 225–296. ISBN 978-3-540-31924-5. [Google Scholar]
- Gnanasekaran, D.; Madhavpan, K.; Reddy, R.S.R. Developments of Polyhedral Oligomeric Silsesquioxanes (POSS), POSS Nanocomposites and Their Applications: A Review. J. Sci. Ind. Res. 2009, 68, 437–464. [Google Scholar]
- Franchini, E.; Galy, J.; Gérard, J.F.; Tabuani, D.; Medici, A. Influence of POSS Structure on the Fire Retardant Properties of Epoxy Hybrid Networks. Polym. Degrad. Stab. 2009, 94, 1728–1736. [Google Scholar] [CrossRef]
- Raftopoulos, K.N.; Pielichowski, K. Segmental Dynamics in Hybrid Polymer/POSS Nanomaterials. Prog. Polym. Sci. 2016, 52, 136–187. [Google Scholar] [CrossRef]
- Tanaka, K.; Adachi, S.; Chujo, Y. Structure–Property Relationship of Octa-Substituted POSS in Thermal and Mechanical Reinforcements of Conventional Polymers. J. Polym. Sci. A Polym. Chem. 2009, 47, 5690–5697. [Google Scholar] [CrossRef]
- Li, S.; Simon, G.P.; Matisons, J.G. Morphology of Blends Containing High Concentrations of POSS Nanoparticles in Different Polymer Matrices. Polym. Eng. Sci. 2010, 50, 991–999. [Google Scholar] [CrossRef]
- Fina, A.; Monticelli, O.; Camino, G. POSS-Based Hybrids by Melt/Reactive Blending. J. Mater. Chem. 2010, 20, 9297–9305. [Google Scholar] [CrossRef]
- Thakur, V.K.; Ding, G.; Ma, J.; Lee, P.S.; Lu, X. Hybrid Materials and Polymer Electrolytes for Electrochromic Device Applications. Adv. Mater. 2012, 24, 4071–4096. [Google Scholar] [CrossRef]
- Maitra, P.; Wunder, S.L. POSS Based Electrolytes for Rechargeable Lithium Batteries. Electrochem. Solid-State Lett. 2004, 7, A88. [Google Scholar] [CrossRef]
- Nguyen, T.-P. Polymer-Based Nanocomposites for Organic Optoelectronic Devices. A Review. Surf. Coat. Technol. 2011, 206, 742–752. [Google Scholar] [CrossRef]
- Ghanbari, H.; Cousins, B.G.; Seifalian, A.M. A Nanocage for Nanomedicine: Polyhedral Oligomeric Silsesquioxane (POSS). Macromol. Rapid Commun. 2011, 32, 1032–1046. [Google Scholar] [CrossRef]
- Wu, J.; Mather, P.T. POSS Polymers: Physical Properties and Biomaterials Applications. Polym. Rev. 2009, 49, 25–63. [Google Scholar] [CrossRef]
- Nag, S.; Sachan, A.; Castro, M.; Choudhary, V.; Feller, J.F. Spray Layer-by-Layer Assembly of POSS Functionalized CNT Quantum Chemo-Resistive Sensors with Tuneable Selectivity and Ppm Resolution to VOC Biomarkers. Sens. Actuators B Chem. 2016, 222, 362–373. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A.; Vollebregt, S. Effect of Humidity on Gas Sensing Performance of Carbon Nanotube Gas Sensors Operated at Room Temperature. IEEE Sens. J. 2021, 21, 5763–5770. [Google Scholar] [CrossRef]
- Sachan, A.; Castro, M.; Choudhary, V.; Feller, J.F. Influence of Water Molecules on the Detection of Volatile Organic Compounds (VOC) Cancer Biomarkers by Nanocomposite Quantum Resistive Vapour Sensors VQRS. Chemosensors 2018, 6, 64. [Google Scholar] [CrossRef]
- Sachan, A.; Castro, M.; Choudhary, V.; Feller, J.F. VQRS Based on Hybrids of CNT with PMMA-POSS and PS-POSS Copolymers to Reach the Sub-Ppm Detection of Ammonia and Formaldehyde at Room Temperature despite Moisture. Chemosensors 2017, 5, 22. [Google Scholar] [CrossRef]
- Kumar, B.; Castro, M.; Feller, J.F. Quantum Resistive Vapour Sensors Made of Polymer Coated Carbon Nanotubes Random Networks for Biomarkers Detection. Chem. Sens. 2013, 3, 1–7. [Google Scholar]
- Wu, J.; Haddad, T.S.; Mather, P.T. Vertex Group Effects in Entangled Polystyrene-Polyhedral Oligosilsesquioxane (POSS) Copolymers. Macromolecules 2009, 42, 1142–1152. [Google Scholar] [CrossRef]
- Wu, J.; Haddad, T.S.; Kim, G.M.; Mather, P.T. Rheological Behavior of Entangled Polystyren-Polyhedral Oligosilsesquioxane (POSS) Copolymers. Macromolecules 2007, 40, 544–554. [Google Scholar] [CrossRef]
- Ma, X.M.; Wang, B.; Zhang, M.X.; Min, F.F.; He, J. Synthesis and Thermal Characterizations of PMMA Nanocomposite Functionalized by Polyhedral Oligomeric Silsesquioxane. Phosphorus Sulfur Silicon Relat. Elem. 2013, 188, 1819–1826. [Google Scholar] [CrossRef]
- Kotal, A.; Si, S.; Paira, T.K.; Mandal, T.K. Synthesis of Semitelechelic POSS-Polymethacrylate Hybrids by Thiol-Mediated Controlled Radical Polymerization with Unusual Thermal Behaviors. J. Polym. Sci. A Polym. Chem. 2008, 46, 1111–1123. [Google Scholar] [CrossRef]
- Gordon, M.; Taylor, J.S. Ideal Copolymers and the Second-Order Transitions of Synthetic Rubbers. i. Non-Crystalline Copolymers. J. Appl. Chem. 2007, 2, 493–500. [Google Scholar] [CrossRef]
- Kumar, B.; Castro, M.; Feller, J.F. Controlled Conductive Junction Gap for Chitosan-Carbon Nanotube Quantum Resistive Vapour Sensors. J. Mater. Chem. 2012, 22, 10656–10664. [Google Scholar] [CrossRef]
- Feller, J.F.; Lu, J.; Zhang, K.; Kumar, B.; Castro, M.; Gatt, N.; Choi, H.J. Novel Architecture of Carbon Nanotube Decorated Poly(Methyl Methacrylate) Microbead Vapour Sensors Assembled by Spray Layer by Layer. J. Mater. Chem. 2011, 21, 4142–4149. [Google Scholar] [CrossRef]
- Nag, S.; Castro, M.; Choudhary, V.; Feller, J.F. Boosting selectivity and sensitivity to biomarkers of Quantum Resistive vapour Sensors used for volatolomics with nanoarchitectured carbon nanotubes or graphene platelets connected by fullerene junctions. J. Mater. Chem. 2021, 9, 66. [Google Scholar] [CrossRef]
- Chatterjee, S.; Castro, M.; Feller, J.F. Tailoring Selectivity of Sprayed Carbon Nanotube Sensors (CNT) towards Volatile Organic Compounds (VOC) with surfactants. Sens. Actuators B Chem. 2015, 220, 840–849. [Google Scholar] [CrossRef]
- Feller, J.F.; Grohens, Y. Evolution of Electrical Properties of Some Conductive Polymer Composite Textiles with Organic Solvent Vapours Diffusion. Sens. Actuators B Chem. 2004, 97, 231–242. [Google Scholar] [CrossRef]
- Kumar, B.; Feller, J.F.; Castro, M.; Lu, J. Conductive Bio-Polymer Nano-Composites (CPC): Chitosan-Carbon Nanotube Transducers Assembled via Spray Layer-by-Layer for Volatile Organic Compound Sensing. Talanta 2010, 81, 908–915. [Google Scholar] [CrossRef]
- Rakotomalala, R. TANAGRA: Free Software for Teaching & Research. In Proceedings of the EGC’2005, Amsterdam, The Netherlands, 14–16 February 2005; Volume 2, pp. 697–702. [Google Scholar]



| VOC | Vm (cm3·mol−1) | δt Sol (J1/2·cm−3/2) | χ12 (PS) | χ12 (PMMA) |
|---|---|---|---|---|
| Methanol | 40.7 | 29.6 | 0.7839 | 1.9867 |
| Ethanol | 58.5 | 26.6 | 0.3607 | 1.5103 |
| Propanol | 74.84 | 23.8 | 0.0371 | 0.8163 |
| Isopropanol | 76.4 | 23.57 | 0.0238 | 0.7613 |
| Benzene | 89.7 | 18.5 | 0.6352 | 0.0003 |
| Toluene | 106.3 | 18.16 | 0.8800 | 0.0083 |
| Xylene | 122.8 | 18.2 | 0.9987 | 0.0079 |
| Water | 18.1 | 47.9 | 4.6406 | 6.2685 |
| Acetone | 74 | 19.7 | 0.2668 | 0.0361 |
| Formaldehyde | 36.8 | 24.6 | 0.0541 | 0.5344 |
| Benzyl alcohol | 103.6 | 23.8 | 0.0514 | 1.1301 |
| Pentane | 115.2 | 14.4 | 3.1938 | 0.8197 |
| Cyclohexane | 108.7 | 16.8 | 1.5213 | 0.1420 |
| Butanone | 89.57 | 19.3 | 0.4152 | 0.0177 |
| Ethyl hexanol | 156.6 | 20.1 | 0.4237 | 0.1421 |
| Cyclohexanone | 104 | 19.6 | 0.4005 | 0.0419 |
| Ethyl benzene | 123.1 | 17.9 | 1.1394 | 0.0243 |
| Benzaldehyde | 101.5 | 21.4 | 0.0681 | 0.3210 |
| Carbon tetrachloride | 97.1 | 18.1 | 0.8252 | 0.0097 |
| Hexene | 96.9 | 16.305 | 1.5936 | 0.2058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sachan, A.; Castro, M.; Choudhary, V.; Feller, J.-F. Giant Chemo-Resistive Response of POSS Nano-Spacers in PS- and PMMA-Based Quantum Resistive Vapour Sensors (vQRS) Used for Cancer Biomarker Analysis. Chemosensors 2025, 13, 226. https://doi.org/10.3390/chemosensors13070226
Sachan A, Castro M, Choudhary V, Feller J-F. Giant Chemo-Resistive Response of POSS Nano-Spacers in PS- and PMMA-Based Quantum Resistive Vapour Sensors (vQRS) Used for Cancer Biomarker Analysis. Chemosensors. 2025; 13(7):226. https://doi.org/10.3390/chemosensors13070226
Chicago/Turabian StyleSachan, Abhishek, Mickaël Castro, Veena Choudhary, and Jean-François Feller. 2025. "Giant Chemo-Resistive Response of POSS Nano-Spacers in PS- and PMMA-Based Quantum Resistive Vapour Sensors (vQRS) Used for Cancer Biomarker Analysis" Chemosensors 13, no. 7: 226. https://doi.org/10.3390/chemosensors13070226
APA StyleSachan, A., Castro, M., Choudhary, V., & Feller, J.-F. (2025). Giant Chemo-Resistive Response of POSS Nano-Spacers in PS- and PMMA-Based Quantum Resistive Vapour Sensors (vQRS) Used for Cancer Biomarker Analysis. Chemosensors, 13(7), 226. https://doi.org/10.3390/chemosensors13070226






