Abstract
Alzheimer’s disease (AD) affects a significant portion of humanity’s elderly population across the globe. Recent studies have identified Amyloid-Beta 42 (Aβ42) as a key biomarker for AD. In this research, we examined the feasibility of using string-shaped electrodes to develop a potentially wearable biosensor for the early detection of AD. Two types of flexible electrochemical electrodes were fabricated using a commercial thread (25% cotton-75% polyester) and an electrospun nanofiber-based string. Decorating the strings with either gold or SiC nanoparticles, several different electrodes were tested to explore their responses to Aβ42. Our results show that the nanofiber-based electrode decorated with gold nanoparticles had the highest sensitivity of 1.71 µA/pg.cm and the best limit of detection (LoD) of 8.36 pg/mL. These findings highlight the importance of the string structure in designing highly sensitive sensors.
1. Introduction
Alzheimer’s disease (AD) is a well-known and currently incurable condition that primarily affects individuals over the age of 65 [1]. The most notable characteristic of Alzheimer’s disease is cognitive decline, making early detection crucial for effectively managing the disease in its asymptomatic stage. This early intervention can help mitigate the impact of AD on both patients and their caregivers [2]. Given the importance of early diagnosis, there is a significant need for a rapid and cost-effective testing method that allows for timely treatment before cognitive decline progresses further. Recent studies indicate that the Amyloid-Beta 42 (Aβ42) peptide is a reliable indicator of AD [3,4]. A key feature of this biomarker is its presence in cerebrospinal fluid and blood; however, these invasive techniques limit the possibility of at-home detection. In contrast, noninvasive methods present a more significant challenge. Recent research has shown that saliva and sweat contain sufficient Aβ42 to distinguish between individuals with and without AD [5]. Therefore, this study aims to evaluate the feasibility of developing a string-shaped electrode for the electrochemical detection of Aβ42, with potential use in a wearable sensor. One of the main challenges is the low concentration of this peptide in saliva and sweat, which ranges from 50 to 70 pg/mL [6]. This necessitates the design of highly sensitive sensors capable of detecting Aβ42 within this concentration range.
Several studies have shown the promising detection of Aβ42 at the picoscale level for biosensors with varying levels of success [7,8,9]. Chen et al. designed a field-effect transistor-based sensor that utilizes functionalized carbon nanotubes [7]. Dr. Kemiklioglu and her colleagues developed a biosensor using liquid crystals [8]. An international team led by Dr. Lai fabricated an electrochemical sensor featuring a planar gold electrode, achieving a limit of detection (LoD) of 10.4 fg/mL [9]. A critical factor in achieving high selectivity and sensitivity is the effective immobilization of the Aβ antibody on electrode surfaces. In this context, planar electrodes made of gold or glassy carbon have shown promising results [10,11,12]. However, the rigid structure of these devices makes them unsuitable for wearable sensor applications. One promising solution for immobilizing antibodies on flexible electrodes is the incorporation of nanoparticles (NPs) into the electrode structure [13]. For instance, several researchers successfully utilized gold nanoparticles or nanowires to create sensors with reduced graphene oxide (rGO) [14], hydrogel [15], polymer-based mesh [16], and indium-tin-oxide (ITO) electrodes [17].
Gold has been commonly used as an electrode material in many biosensors. However, silicon carbide (SiC) presents a promising alternative due to its nontoxic and biocompatible properties. In our lab, we previously conducted research on detecting Aβ42 using both gold (Au) and silicon carbide (SiC) planar electrodes [18]. In this study, we considered both gold and SiC NPs to fabricate string-shaped electrodes for the electrochemical detection of Aβ42. The focus of this research is on the effects of the type of string and the impact of the type of NPs on their responses when being exposed to Aβ42 in a buffer electrolyte solution. We tested two types of strings: the electrospun fibers of a polymer rolled into nanofiber felt (NF-Felt) strings using a devised method [19], as well as a commercially available thread. By decorating the electrodes with NPs, we studied various string-shaped electrodes for their ability to detect Aβ42 in a buffer solution.
2. Materials and Methods
2.1. Materials and Equipment
Phosphate-buffer solution (PBS), 3-Mercaptopropionic acid (MPA), N-(3-Dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), dimethyl sulfoxide (DMSO), Au NP ink in citrate buffer solution from Sigma (5 nm diameter), SiC NP (<50 nm), methanol, PEDOT:PSS (4% in H2O), potassium ferricyanide (329.26 g/mol), N,N-dimethylformamide (DMF), dodecylbenzenesulfonic acid (DBSA), ethylene glycol (EG), and polyvinylidene fluoride-co-hexafluoropropylene (PVDF-HFP) were all purchased from Sigma Aldrich. Additionally, 1 mg of IgG in 0.1 mL (1 mg/mL) of PBS pH 7.4 with 0.09% sodium azide Amyloid Beta [1–12,24–39] rabbit polyclonal antibody and 1 mg of lyophilized solid packaged Amyloid Beta [1–42] peptide (Aβ42) were obtained from Abbiotec. Per Abbiotec’s recommendation, the Amyloid Beta [1–42] peptide was dissolved in 100% DMSO, and then the Aβ [1–12,24–39] antibody was dissolved in 0.1 M PBS to achieve the desired concentration. The use of antibodies was to serve as a proof of concept for the antibody-based electrochemical biosensor being implemented.
All electrochemical experiments were conducted in a 3-probe setup (20 mL beaker) using the string electrodes as the working electrode, and a Pt wire and an Ag/AgCl electrode as the counter and reference electrodes, respectively. Following a procedure recommended in other publications [18,20], an equimolar (5 mM) solution of K4Fe(CN)6 and K3Fe(CN)6 in a PBS buffer (0.1 M, pH = 7.4) was used as the electrolyte for all the electrochemical experiments. A VersaSTAT 4 Potentiostat (Princeton Applied Research, PAR, Oak Ridge, TN, USA) was used for all the electrochemical experiments. Five cycles of the experiments with a scan rate of 50 mV/s were applied to perform cyclic voltammetry (CV). The results of the third loop are presented in this work. Electrochemical impedance spectroscopy (EIS) was conducted from 0.1 Hz to 10 kHz. To assess the response of the electrodes to the concentrations of Aβ42, a constant voltage of 0.5 V was applied (vs. reference) and the current was monitored (chronoamperometry, CA, experiment), and in a titration process, Aβ42 solution was added every 30 s to the electrolyte (increasing the concentration by 15 pg/mL at each step). A Hitachi SU-70 (Hitachi America, Santa Clara, CA, USA) and Quanta 200 3D Dual Beam Electron Microscope (Thermo Fisher Scientific, Waltham, MA, USA) were used for conducting the scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) studies.
2.2. Fabrication Process of the String-Shaped Electrodes
2.2.1. Fabrication Process of Nanofiber-Felt (NF-Felt) Strings
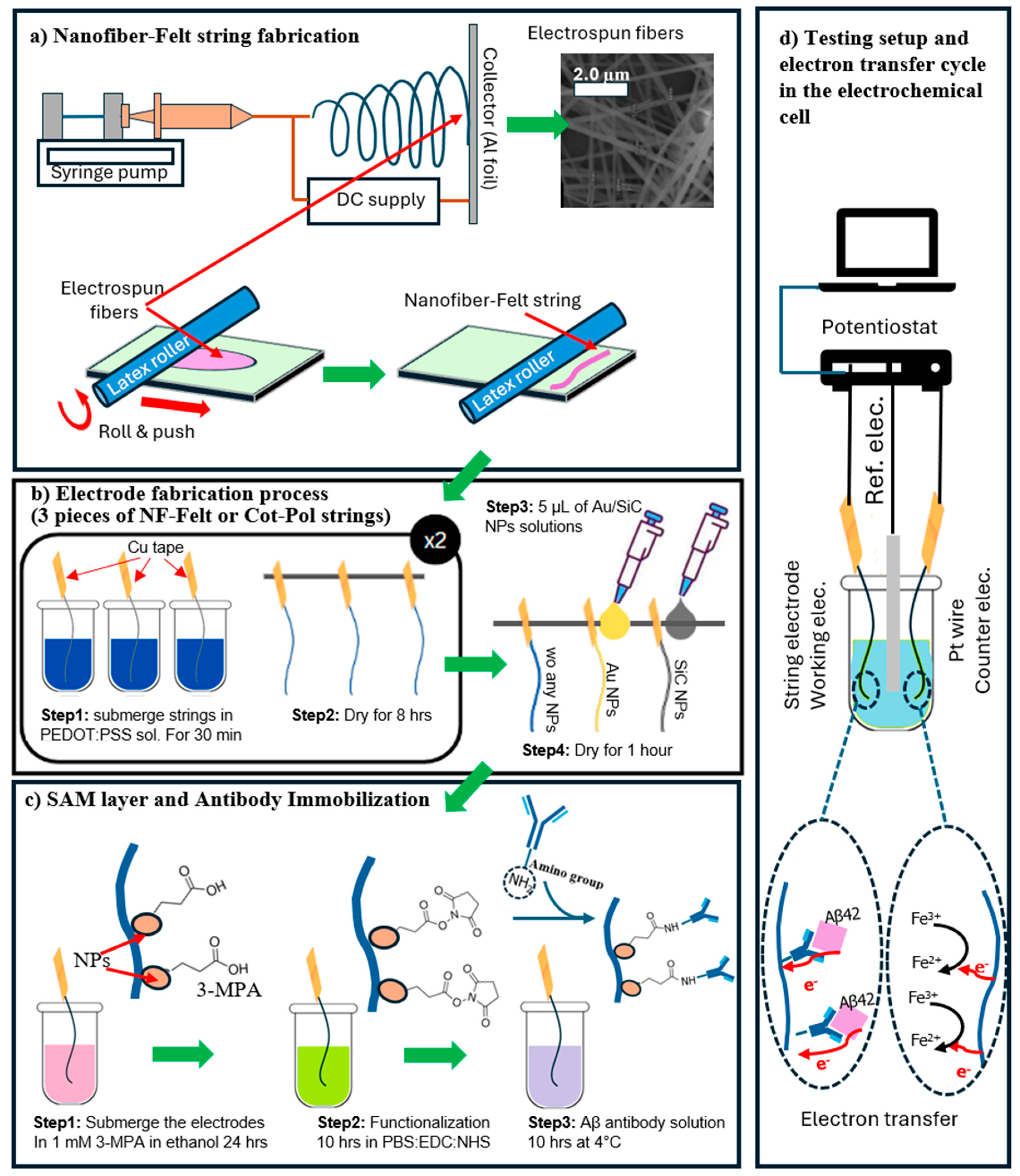
The process of fabricating NF-Felt strings was explained in our previous publication [19]. Briefly, due to insolubility in water, PVDF-HFP was selected. An in-house-designed electrospinning system was used to produce PVDF-HFP NFs (15 wt.% solution of PVDF-HFP in DMF). A 22 G needle and a high-voltage power supply (22 kV) were used for electrospinning. The distance between the needle and an aluminum foil substrate was set at 20 cm, and the syringe pump rate was 0.2 mL/h. After 5 min of running, the fibers were collected as a mesh with a diameter of ~5 cm. The mesh was placed on a rubber mat and a latex-based roller was used to form the NF-Felt strings by rotating the roller upward when it was moved forward on the mat. The process of making the NF-Felt strings is shown in Figure 1a. To remove the excess solvent, the string was soaked in an isopropyl bath for 10 min, rinsed with deionized (DI) water, and dried.
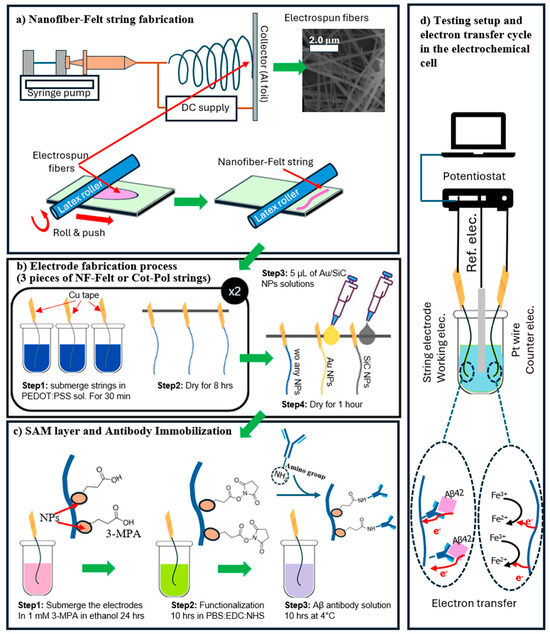
Figure 1.
(a) The process of fabricating NF-Felt strings. Electrospun fibers form a mesh. Using a roller in a devised approach, the mesh is converted to a string with a felt structure. (b) The NF-Felt and Cot-Pol strings are coated with PEDOT:PSS. A droplet of the Au NP or SiC NP solution is applied to the surface of the strings. (c) After applying a layer of SAM, the electrodes are functionalized, and then the antibody is immobilized. (d) The fabricated string-shaped electrodes are tested in an electrochemical cell. The natural docking mechanism between Aβ42 and its antibody allows electron transfer from the electroactive part of Aβ42 to the string electrode. The K4Fe(CN)6/K3Fe(CN)6 redox couple participates in the charge transfer at the counter electrode.
2.2.2. PEDOT:PSS Coating
Two different types of string were tested in this work: a fabricated NF-Felt and a commercially available 25% cotton–75% polyester (called Cot-Pol) thread (from Walmart, Bentonville, AR, USA). The Cot-Pol thread was selected after testing half a dozen different off-the-shelf threads [21]. The criteria for the selection of the thread were based on the conductivity of the string after being coated with PEDOT:PSS. Both NF-Felt and Cot-Pol strings are naturally non-conductive. To fabricate the electrodes (to convert non-conductive strings to conductive electrodes), they were coated with PEDOT:PSS. The Cot-Pol and the NF-Felt strings were cut into 3 cm long pieces. Three pieces of each were dipped into a solution of PEDOT:PSS, DBSA, and EG with a 75:20:5 mass ratio [17]. To use them as electrodes, copper tape was used to make electric contacts at one end of each piece. The strings were removed after 30 min and dried at room temperature for 8 h. The process was repeated twice for each string.
2.2.3. Electrode Fabrications
In this study, a total of six electrodes were tested: three samples made from Cot-Pol and three samples made from NF-Felt. Each material included one sample with only PEDOT:PSS, without any additional coatings. The other two samples for each material were coated with either gold nanoparticles (Au NPs) or silicon carbide nanoparticles (SiC NPs). For the Au NP coating, approximately 5 µL of the Au NP solution was applied to a hanging piece of string using a pipette. After applying the nanoparticle solution, the electrode was left to dry for one hour at room temperature. Similarly, 5 µL of SiC NP solution was applied to a piece of string made from both Cot-Pol and NF-Felt. The process of coating the strings with PEDOT:PSS and then creating three types of electrodes (one without nanoparticles, one with Au NPs, and one with SiC NPs) is illustrated in Figure 1b. We refer to the electrodes as follows: Cot-Pol, Cot-Pol-Au, Cot-Pol-SiC, NF-Felt, NF-Felt-Au, and NF-Felt-SiC. To establish baseline responses, all electrodes were tested individually in a three-probe electrochemical cell. After these baseline measurements, a self-assembled monolayer (SAM) of 3-MPA was formed on the electrodes, which were then functionalized prior to incubating the Aβ antibody (as shown in Figure 1c). The steps for antibody immobilization are similar to the process used for planar gold and SiC electrodes [18]. Initially, the electrodes were immersed in a 1 mM solution of 3-MPA in ethanol for 24 h. The functionalization process involved submerging the string electrodes in a solution containing PBS (0.1 M), EDC (0.25 M), and NHS (0.05 M) for 10 h.
After functionalization, the antibody (Aβ [1–28]) was incubated at 4 °C while the electrodes were submerged in PBS containing the antibody (18.75 µg/mL) for 20 h. Following this incubation, the electrodes were electrochemically characterized again to assess the antibody response. The CV and EIS responses of the electrodes to the peptide were further investigated by adding Aβ42 at a concentration of 120 pg/mL to the electrolyte. Chronoamperometry was performed by applying a constant voltage of 0.5 V to the working electrode vs. reference electrode and monitoring the current while the concentration of Aβ42 was increased by 15 pg/mL every 30 s. A schematic of the electrochemical setup with the expected electron transfer path in the cell is shown in Figure 1d.
3. Results and Discussion
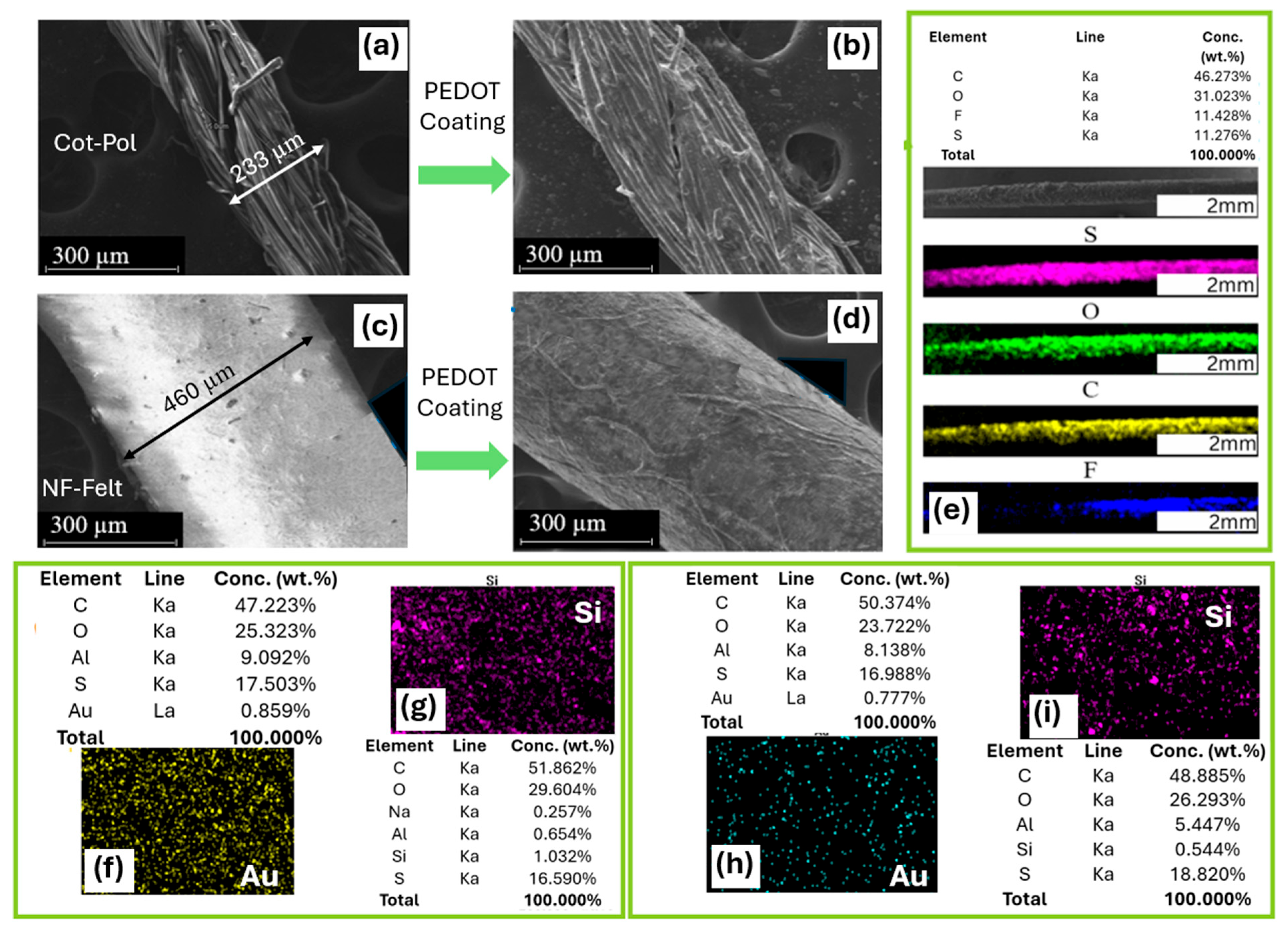
SEM and EDS methods were utilized to examine the structure of the string electrodes and to confirm the presence of nanoparticles (NPs) on these electrodes. As illustrated in Figure 2a,c, the diameter of the Cot-Pol thread, which has a dense twisted fiber structure, is nearly half that of the fabricated nanofiber-based string with a felt structure. Figure 2b,d demonstrate that both threads were uniformly coated with PEDOT:PSS. The EDS images and analysis of the NF-Felt string after being coated with PEDOT:PSS confirm the presence of sulfur (S), oxygen (O), and carbon (C) from the conducting polymer, as well as fluorine (F), likely due to the PVDF used in the electrospinning of the fibers (Figure 2e). The analysis of the threads coated with gold nanoparticles (Au NPs) reveals that the percentages of gold on Cot-Pol (0.859%) and NF-Felt (0.777%) are comparable (Figure 2f,h). However, the monitoring of silicon (Si) on the SiC-coated threads (Figure 2g,i) indicates that the Cot-Pol thread contains almost double the amount of silicon (1.032%) compared to the NF-Felt thread (0.554%).
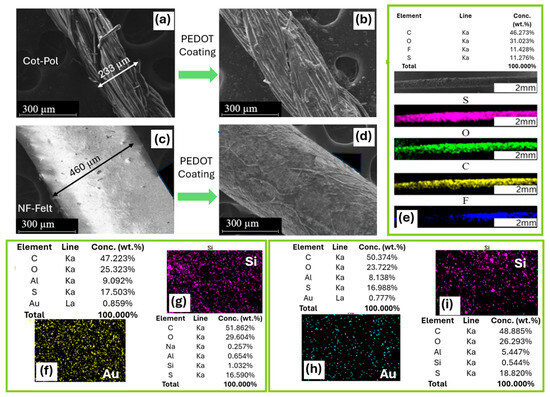
Figure 2.
SEM images of (a) bare and (b) PEDOT:PSS-coated Cot-Pol thread, and of the fabricated NF-Felt string (c) before and (d) after coating with PEDOT:PSS. (e) EDS analysis of the conductive NF-Felt string. EDS analysis of (f) Cot-Pol-Au, (g) Cot-Pol-SiC, (h) NF-Felt-Au, and (i) NF-Felt-SiC electrodes. (f,h) The density of Au and (g,i) the density of Si.
The performance of six different strings was evaluated at various stages: before and after antibody immobilization (baseline and antibody), as well as after the addition of Aβ42 to the electrolyte. Figure S1 in the Supplementary Materials displays the cyclic voltammetry (CV) results from the baseline measurements. The difference in current scale between the NF-Felt and Cot-Pol electrodes indicates that the conductivity of the NF-Felt electrodes is one order of magnitude higher than that of the Cot-Pol electrodes. Additionally, the width of the CV loop around 0.0 V for the NF-Felt electrodes is nearly twice that of the Cot-Pol electrodes, suggesting that the effective surface area of the NF-Felt electrodes is two times larger than that of the Cot-Pol strings. This can be attributed to the larger diameter and distinct structure of the NF-Felt electrodes, as shown in the SEM images. When comparing the results from the strings with and without a nanoparticle (NP) coating, it is evident that decorating PEDOT:PSS with NPs altered the baseline loops. Likewise, the CVs of the electrodes with immobilized antibodies exhibit significant differences between those with and without NPs (see Figure S2).
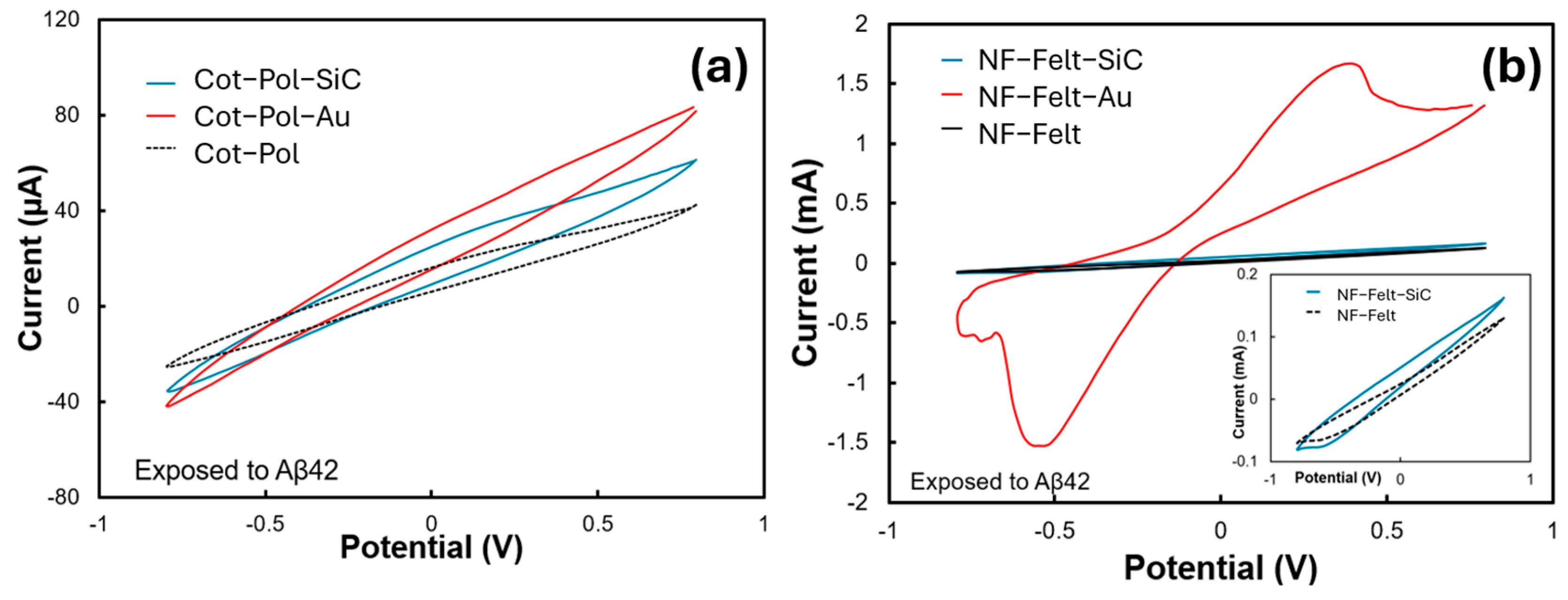
Figure 3a,b show the CV results from all six electrodes when they were exposed to Aβ42 (120 pg/mL). Among them, the NF-Felt electrode decorated with gold (Au) displayed the strongest response, characterized by a distinct peak at 0.4 V and a current reaching 1.67 mA. No redox peak was observed in the Cot-Pol electrode. However, the CV loop for the Cot-Pol-Au electrode was the largest compared to the other two electrodes in Figure 3a. The absence of a redox peak in all electrodes, except for NF-Felt-Au, may be attributed to a much weaker redox current in comparison to the double-layer current observed in the CV responses. Notably, no redox peak was detected prior to the addition of Aβ42 into the electrolyte, indicating that the peak shown in Figure 3b is a direct result of the redox reaction involving Aβ42, rather than ferro/ferricyanide interactions. As explained by Zhang et al. [22], there are electroactive residues within the structure of Aβ (such as tyrosine, methionine, and histidine) that can enhance the intermolecular electron transfer rate once a voltage is applied to the electrode, leading to an increase in the current level. The mechanism of binding between Aβ42 and its antibody is assumed to be a natural docking (antibody–antigen) mechanism. The presence of K4Fe(CN)6/K3Fe(CN)6 in the electrolyte allows a reduction reaction at the counter electrode, which completes the cycle of charge transfer in the electrochemical cell (Figure 1d). It is important to note that the response of each electrode largely depends on the effective immobilization of the antibody on the electrode surface. Therefore, a low redox current (in comparison with the double-layer current) may indicate poor coverage of the electrode surface with immobilized antibodies.
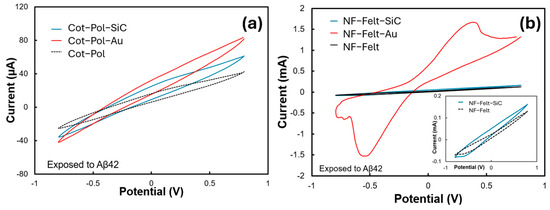
Figure 3.
CV results of the (a) Cot-Pol-based and (b) NF-Felt-based electrodes when exposed to Aβ42. (inset) The data for the NF-Felt and NF-Felt-SiC are presented at a different scale. The experiments were conducted at a 50 mV/s scan rate in a 3-probe setup.
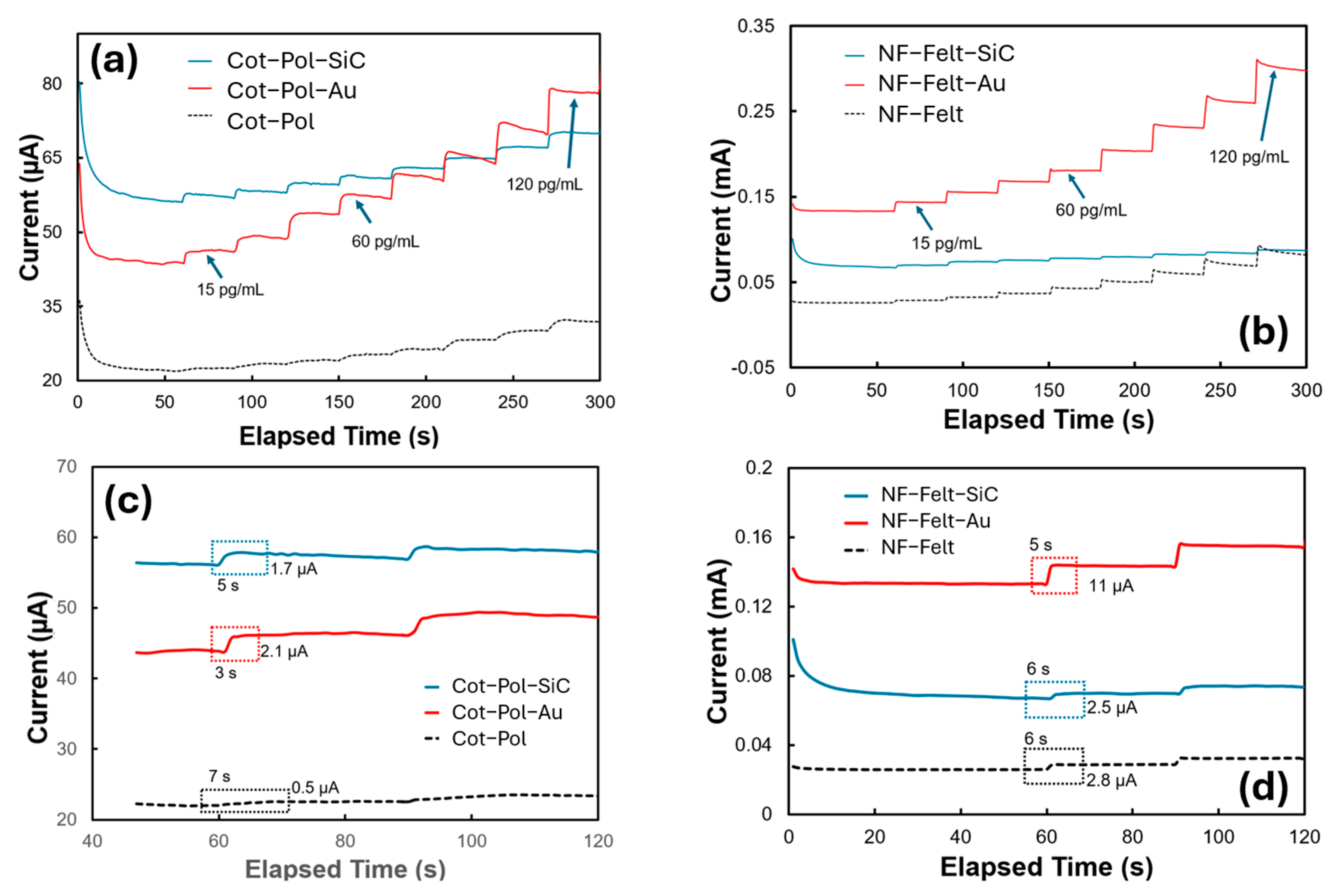
Considering the potential application of the sensor in a wearable device, a simple method such as chronoamperometric (CA) may be used to measure the concentration of Aβ42 in sweat/saliva. Hence, we studied the response of the electrodes to different concentrations of Aβ42 in 5 mL of a PBS-based electrolyte containing K4Fe(CN)6 and K3Fe(CN)6 at a concentration of 5 mM each. In the CA test, we applied a voltage of 0.5 V and monitored the current. Since the redox peak in the NF-Felt-Au electrode occurred at 0.4 V (see Figure 3b), we selected 0.5 V to ensure that the potential exceeded the redox peak. However, using higher voltages is not advisable for wearable electronics, where optimizing the sensor’s power consumption is crucial. The given range tested was from 15 pg/mL to 120 pg/mL. This range was selected as it was reported to be the threshold window in saliva and sweat for determining whether or not a patient has early-stage Alzheimer’s [6]. A concentration of 20 pg/mL is considered typical for a normal patient, while levels greater than 70 pg/mL indicate stages 1–2 of early-onset Alzheimer’s [2,6]. As demonstrated in Figure 4a,b, the presence of NPs enhanced the signals significantly, with gold nanoparticles having a particularly notable impact. Consistent with the CV results, the NF-Felt-Au electrode exhibited the greatest variation in response to the addition of 15 pg/mL of Aβ42 at each step. Figure 4c,d provide details of these step responses. The electrodes without NPs produced the smallest signals, with a settling time of 6 to 7 s. In contrast, the NF-Felt-Au electrode showed the largest response, with an increase of 11 µA in current and a time constant of 5 s. Although the step response in the Cot-Pol-Au electrode was only 2.1 µA, it demonstrated a faster response time of approximately 3 s.
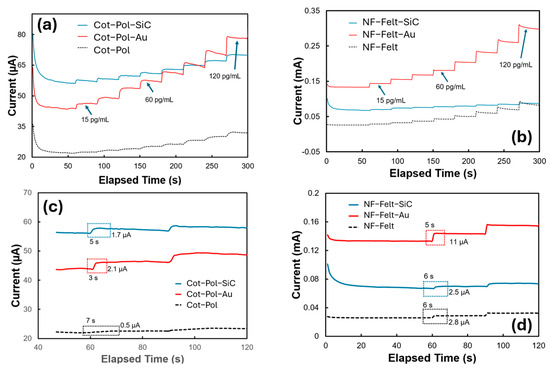
Figure 4.
CA results of (a,c) the Cot-Pol and (b,d) NF-Felt string electrodes. The current was recorded as 0.5 V was applied to the electrodes with respect to the reference electrode while Aβ42 was titrated to the electrolyte (5 mL of PBS), increasing its concentration by 15 pg/mL at each step.
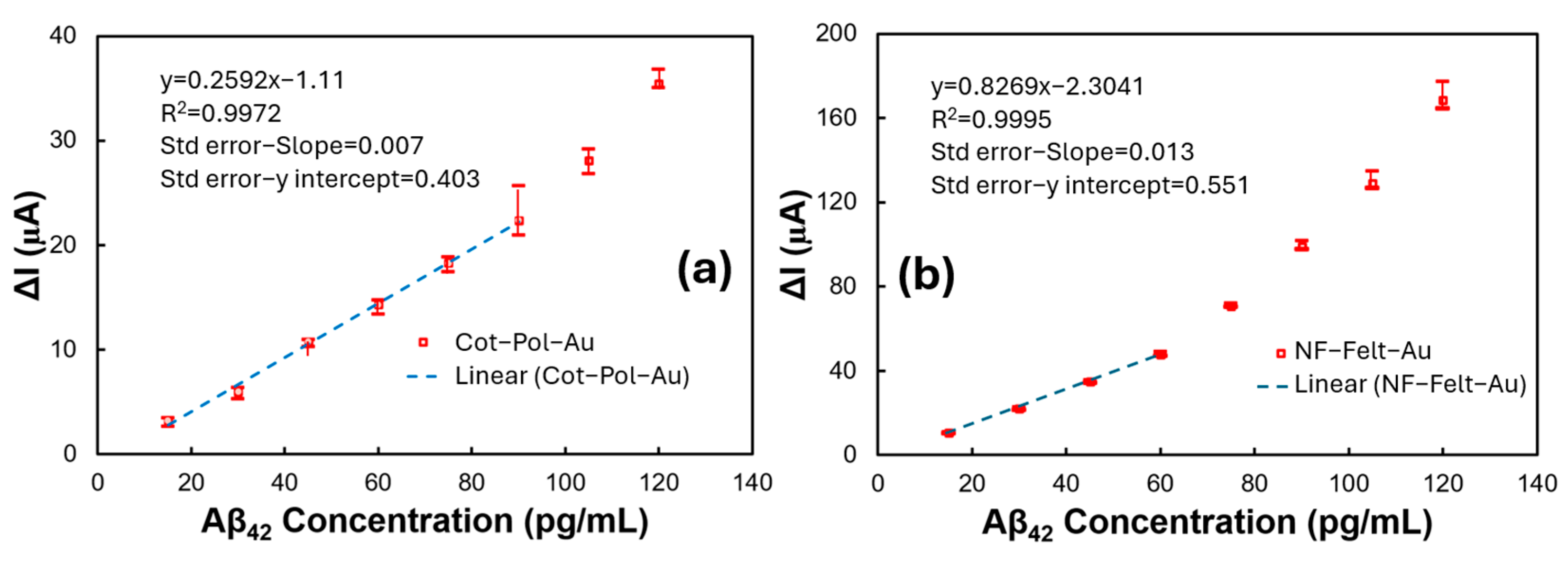
The data from the Cot-Pol-Au and NF-Felt-Au electrodes were further analyzed to better understand the sensitivity, limit of detection (LoD), and linearity of the electrodes. The maximum, minimum, and average responses of the electrodes in relation to the base current (the current measured at a zero concentration of Aβ) were plotted against the concentration, as shown in Figure 5. While the overall responses were not linear, the data at low concentrations could be approximated using linear functions. The calibration equations derived from these linear approximations yielded R2 values of 0.9972 for the Cot-Pol-Au electrode and 0.9995 for the NF-Felt-Au electrode. To normalize the sensitivity to the electrode area, we considered the apparent surface area of the electrodes calculated as L × π × D (where D is the diameter and L is the length of the string inside the electrolyte). Based on this approach, the estimated sensitivity was 1.71 µA/pg·cm for the NF-Felt-Au electrode and 1.02 µA/pg·cm for the Cot-Pol-Au electrode. Using the formula LoD = 3 × (standard deviation)/sensitivity [19], we calculated the limits of detection to be 12.85 pg/mL for the Cot-Pol-Au electrode and 8.36 pg/mL for the NF-Felt-Au electrode. Table 1 presents the data from our sensors alongside results from other studies published in scientific journals. It is noteworthy that some studies did not report the sensitivity of their devices. Nevertheless, considering the limits of detection and the range for testing Aβ42, our NF-Felt-Au device demonstrated a commendable performance.
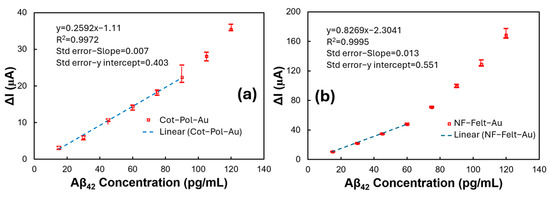
Figure 5.
The current difference between the measured value at different concentrations and the current with a zero concentration of Aβ versus the Aβ42 concentration in devices with (a) the Cot-Pol-Au and (b) NF-Felt-Au electrodes. The low concentration parts of each curve are approximated with linear curves. The calibration equation and R2 value are presented.

Table 1.
Summary of reviewed works in comparison to this work.
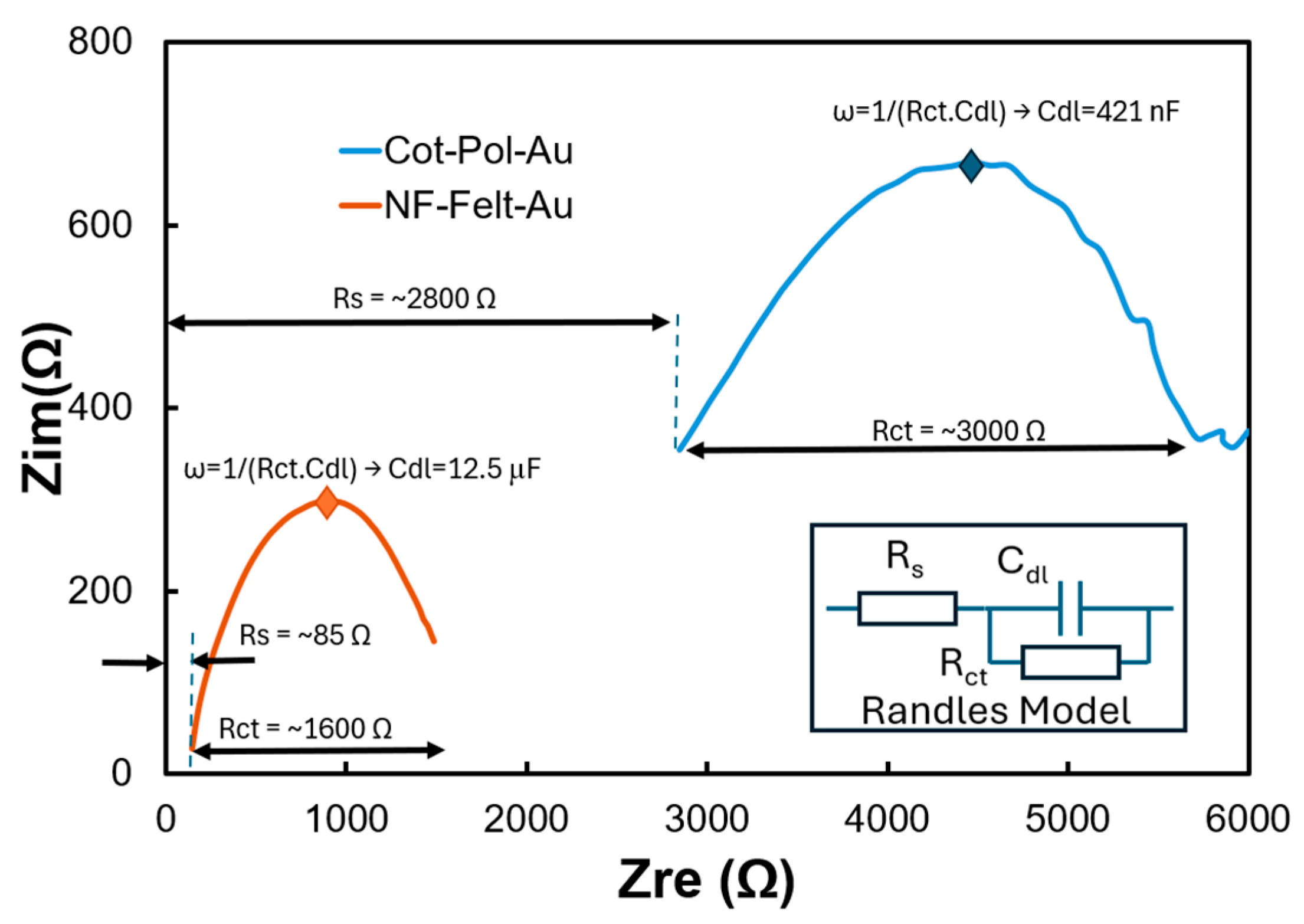
Table 1 presents competitive LODs obtained from gold, glassy carbon, or rGO electrodes. However, unlike the planar and rigid configurations of these electrodes, the string-shaped devices developed in this study are more suitable for integration into fabric, paving the way for wearable sensors. It is important to note that the linker used in this work (i.e., 3-MPA) features a thiol (HS) group at one end, which is effective for binding with gold. While there are reports of utilizing thiols as binding agents for SiC, our findings indicate that the antibody immobilization process on SiC NPs was less effective compared to that on Au NPs. Despite having nearly the same loading of Au NPs on both Cot-Pol-Au and NF-Felt-Au (as shown in the EDS results in Figure 2), a key question arises: why did the CV results for NF-Felt-Au show clear redox peaks, while Cot-Pol-Au did not? To explore the differences between the two electrodes decorated with Au NPs, we conducted EIS tests on both using an electrolyte containing 120 pg/mL of Aβ42. Figure 6 displays the results. The semicircular responses correlate with the Randles electrochemical model (illustrated in the inset of Figure 6). However, evaluating the data at the two ends of the semicircles, along with the frequencies at which the curves peaked, revealed substantial differences in the series resistance (Rs), charge transfer resistance (Rct), and double-layer capacitance (Cdl) between the two electrodes. The larger Rct and Rs values in the Cot-Pol-Au electrode indicate a lower electron transfer rate and reduced electrode conductivity. Conversely, the significantly higher Cdl in NF-Felt-Au suggests a more effective surface area, likely attributed to the structure of the NF-Felt. Thus, it appears that the NF-Felt structure better accommodates Au NPs compared to the twisted fiber structure in Cot-Pol. It is crucial to emphasize that the effectiveness of the NPs is not solely based on their physical presence but also on the electrical contact between the particles and the PEDOT:PSS coatings. Our results (specifically the Rs values) indicate that the electrical contacts between the PEDOT:PSS layer and the Au NPs in NF-Felt are more efficient than those in Cot-Pol.
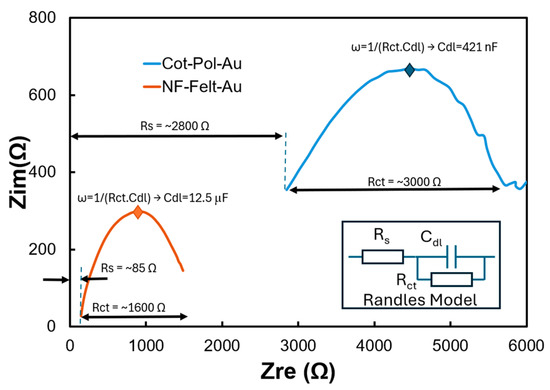
Figure 6.
EIS results from NF-Felt-Au and Cot-Pol-Au electrodes. (Inset) Randle’s model describing semicircle behavior of electrodes.
The outcomes of this study are promising for the application of string-shaped electrodes in developing wearable sensors for monitoring Alzheimer’s disease. To confirm the statistical significance of the electrodes with Au NPs, additional samples should be fabricated and tested to verify repeatability and achieve a more reliable sensitivity and LOD. However, this requires designing an automated rolling machine to produce NF-Felt strings with a consistent density and diameter. Further research is necessary to integrate these fiber-shaped electrodes into a functional wearable system and to evaluate their response with sweat samples. It is likely that the response of the sensor would be affected by the presence of other chemicals in the human serum. Hence, the sensor’s selectivity towards Aβ42 requires validation through an interference study.
4. Conclusions
In conclusion, the NF-Felt string electrodes demonstrated greater sensitivity to the Aβ42 peptide compared to a commercially available Cot-Pol-based electrode. The incorporation of gold nanoparticles into the string electrodes significantly enhanced the signal in both types of threads, making the NF-Felt-Au configuration the most effective among the various structures and materials tested. Overall, the findings of this study are quite intriguing and suggest the potential to create fully flexible electrodes that can perform comparably to traditional rigid substrates.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/chemosensors13060199/s1, Figure S1: CV results of the (a) Cot-Pol and (b) NF-Felt electrodes before antibody immobilization; Figure S2: CV results of the (a) Cot-Pol and (b) NF-Felt electrodes after antibody immobilization.
Author Contributions
Conceptualization, B.S. and A.T.; methodology, B.S.; validation, A.T. and S.T.; formal analysis, B.S.; investigation, B.S.; resources, A.T.; data curation, B.S.; writing—original draft preparation, B.S.; writing—review and editing, A.T. and S.T.; visualization, B.S.; supervision, A.T.; project administration, A.T.; funding acquisition, S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Community Foundation of Tampa Bay (CFTB) and National Science Foundation (NSF), grant number 1953089, as well as the LSAMP BD: University of South Florida Florida-Georgia Louis Stokes Alliance for Minority Participation (FGLSAMP) program, NSF HRD 1906518.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon request.
Acknowledgments
The authors would like to thank the USF Nanotechnology Research and Education Center (NREC).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Alzheimer’s disease |
| Aβ42 | Amyloid-Beta 42 |
| NPs | Nanoparticles |
| ITO | Indium-tin-oxide |
| NF-Felt | Nanofiber-felt |
| PBS | Phosphate-buffer solution |
| MPA | 3-Mercaptopropionic acid |
| EDC | N-(3- Dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride |
| NHS | N-hydroxysuccinimide |
| DMSO | Dimethyl sulfoxide DMSO |
| PEDOT:PSS | Poly(2,3-dihydrothieno-1,4-dioxin)-poly(styrenesulfonate) |
| DMF | N,N-dimethylformamide |
| DBSA | Dodecylben-zenesulfonic acid |
| EG | Ethylene glycol |
| PVDF-HFP | Polyvinylidene fluoride-co-hexafluoropropylene |
| SEM | Scanning electron microscope |
| CV | Cyclic voltammetry |
| EIS | Electrochemical impedance spectroscopy |
| CA | Chronoamperometry |
| EDS | Energy-dispersive X-ray spectroscopy |
| Cot-Pol | 25% cotton-75% polyester |
| SAM | Self-assembled monolayer |
References
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.; Langerman, H. Alzheimer’s Disease—Why We Need Early Diagnosis. Degener. Neurol. Neuromuscul. Dis. 2019, 9, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Yada, Y.; Naoki, H. Few-shot prediction of amyloid beta accumulation from mainly unpaired data on biomarker candidates. NPJ Syst. Biol. Appl. 2023, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Dore, V.; Fowler, C.; Li, Q.X.; Martins, R.; Rowe, C.; et al. High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 2018, 554, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Zurcher, C.; Humpel, C. Saliva: A challenging human fluid to diagnose brain disorders with a focus on Alzheimer’s disease. Neural Regen. Res. 2023, 18, 2606–2610. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, M.N.; Shi, J.; Lee, M.; Arnold, L.; Al-Hasan, Y.; Heim, J.; McGeer, P. Salivary beta amyloid protein levels are detectable and differentiate patients with Alzheimer’s disease dementia from normal controls: Preliminary findings. BMC Neurol. 2018, 18, 155. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiao, M.; He, J.; Zhang, Y.; Liang, Y.; Liu, H.; Zhang, Z. Aptamer-functionalized carbon nanotube field-effect transistor biosensors for Alzheimer’s disease serum biomarker detection. ACS Sens. 2022, 7, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Kemiklioglu, E.; Tuncgovde, E.B.; Ozsarlak-Sozer, G. Development of liquid crystal biosensor for the detection of amyloid beta-42 levels associated with Alzheimer’s disease. J. Biosci. Bioeng. 2021, 132, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Gupta, A.K.; Purwidyantri, A.; Prabowo, B.A.; Chen, C.-H.; Chuang, C.-C.; Tian, Y.-C.; Lu, Y.-J.; Lai, C.-S. Sensing alzheimer’s disease utilizing au electrode by controlling nanorestructuring. Chemosensors 2022, 10, 94. [Google Scholar] [CrossRef]
- Han, J.; Zhang, M.; Chen, G.; Zhang, Y.; Wei, Q.; Zhuo, Y.; Xie, G.; Yuan, R.; Chen, S. Ferrocene covalently confined in porous MOF as signal tag for highly sensitive electrochemical immunoassay of amyloid-β. J. Mater. Chem. B 2017, 5, 8330–8336. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, P.; Loureiro, J.; Delerue-Matos, C.; Morais, S.; do Carmo Pereira, M. Alzheimer’s disease: Development of a sensitive label-free electrochemical immunosensor for detection of amyloid beta peptide. Sens. Actuators B Chem. 2017, 239, 157–165. [Google Scholar] [CrossRef]
- Le, H.; Park, J.; Cho, S. A probeless capacitive biosensor for direct detection of amyloid beta 1–42 in human serum based on an interdigitated chain-shaped electrode. Micromachines 2020, 11, 791. [Google Scholar] [CrossRef] [PubMed]
- Kecili, R.; Hussain, C.M. Mechanism of Adsorption on Nanomaterials. In Nanomaterials in Chromatography; Elsevier: Amsterdam, The Netherlands, 2018; pp. 89–115. [Google Scholar]
- Azimzadeh, M.; Nasirizadeh, N.; Rahaie, M.; Naderi-Manesh, H. Early detection of Alzheimer’s disease using a biosensor based on electrochemically-reduced graphene oxide and gold nanowires for the quantification of serum microRNA-137. RSC Adv. 2017, 7, 55709–55719. [Google Scholar] [CrossRef]
- Sun, L.; Zhong, Y.; Gui, J.; Wang, X.; Zhuang, X.; Weng, J. A hydrogel biosensor for high selective and sensitive detection of amyloid-beta oligomers. Int. J. Nanomed. 2018, 13, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yan, Z.; Jia, L.; Song, P.; Mei, L.; Bai, L.; Liu, Y. Gold nanoparticle decorated electrospun nanofibers: A 3D reproducible and sensitive SERS substrate. Appl. Surf. Sci. 2017, 403, 29–34. [Google Scholar] [CrossRef]
- Tavakkoli, N.; Soltani, N.; Tabar, Z.K.; Jalali, M.R. Determination of dopamine using the indium tin oxide electrode modified with direct electrodeposition of gold–platinum nanoparticles. Chem. Pap. 2019, 73, 1377–1388. [Google Scholar] [CrossRef]
- Montero-Arevalo, B.; Seufert, B.I.; Hossain, M.S.; Bernardin, E.; Takshi, A.; Saddow, S.E.; Schettini, N. SiC electrochemical sensor validation for Alzheimer Aβ42 antigen detection. Micromachines 2023, 14, 1262. [Google Scholar] [CrossRef] [PubMed]
- Seufert, B.; Thomas, S.; Takshi, A. Stretchable Nanofiber-Based Felt as a String Electrode for Potential Use in Wearable Glucose Biosensors. Sensors 2024, 24, 1283. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Molazemhosseini, A.; Liu, C.C. In vitro quantified determination of β-amyloid 42 peptides, a biomarker of neuro-degenerative disorders, in PBS and human serum using a simple, cost-effective thin gold film biosensor. Biosensors 2017, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Rios, N.O.M.; Takshi, A. Stability of fiber-based organic electrochemical transistors with a gel electrolyte for wearable electronics. In Organic and Hybrid Sensors and Bioelectronics XV; SPIE: Bellingham, WA, USA, 2022; pp. 16–21. [Google Scholar]
- Zhang, Y.; Ren, B.; Zhang, D.; Liu, Y.; Zhang, M.; Zhao, C.; Zheng, J. Design principles and fundamental understanding of biosensors for amyloid-β detection. J. Mater. Chem. B 2020, 8, 6179–6196. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).