Revolutionizing Electrochemical Sensing with Nanomaterial-Modified Boron-Doped Diamond Electrodes
Abstract
1. Introduction
1.1. Boron-Doped Diamond (BDD) Electrodes
1.2. Significance of BDD Electrodes in Electrochemical Applications
1.3. Tunability and Structural Considerations
1.4. Synthesis Techniques and Versatility in Applications

1.5. Motivation for Nanomaterial-Modifying BDD Electrodes
1.6. Objectives of This Review Article
1.7. Overview of Nanomaterials and Their Role in BDD Electrode Enhancement
1.8. Methods Used to Fabricate Nanomaterial-Modified BDD Electrodes
- Physical vapor deposition (PVD): In PVD, nanomaterials are vaporized through physical means, such as evaporation or sputtering, and then condensed onto the BDD electrode. These techniques enable the modification of BDD electrodes with nanomaterials to enhance their performance in various applications. The sputtering method is a versatile technique for depositing nanomaterials onto BDD electrodes [96,97,98,99,100,101,102,103,104]. In this technique, nanomaterials are employed as a target material, and high-energy ions are used to dislodge and deposit atoms or molecules onto the BDD surface. Sputtering has advantages such as great adhesion, minimal contamination, and the ability to deal with a wide range of materials such as metals, metal oxides, and ceramics.
1.9. Factors Affecting Properties of Nanomaterial-Modified BDD Electrodes
1.10. Biosensors Based on Carbon Nanomaterial-Modified BDD Electrodes
1.11. Biosensors Based on Metallic Nanostructure-Modified BDD Electrodes
1.12. Biosensors Utilizing Alloy, Composite, and Hybrid Nanomaterial-Modified BDD Electrodes
1.13. Other Nano-Material/-Structure Innovations for Modified BDD-Based Biosensors
2. Current Challenges and Limitations
3. Potential Future Directions and Areas of Development
4. Conclusions
Funding
Conflicts of Interest
Abbreviations
| BDD | Boron-doped diamond |
| MWCNTs | Microwave plasma chemical vapor deposition |
| HFCVD | Hot filament chemical vapor deposition |
| CV | Cyclic voltammetry |
| EIS | Electrochemical impedance spectroscopy |
| HPHT | High-pressure high-temperature |
| EPD | Electrophoretic deposition |
| MWCNTs | Multi-walled carbon nanotubes |
| SWCNTs | Single-walled carbon nanotubes |
| HNTs | Half-nanotubes |
| CVD | Chemical vapor deposition |
| PVD | Physical vapor deposition |
| SEM | Scanning Electron Microscopy |
| XRD | X-ray Diffraction |
| EDX | Energy-dispersive X-ray spectroscopy |
| XPS | X-ray Photoelectron Spectroscopy |
| FTIR | Fourier-transform infrared spectroscopy |
| AChE | Acetylcholinesterase |
| NG | Nanometer-sized graphite |
| MCR-ALS | Multivariate curve resolution-alternating least squares |
| CNts | Carbon nanotubes |
| BPA | Bisphenol A |
| BDUNCD | Boron-doped Ultrananocrystalline diamond |
| MCH | 6-mercapto-1-hexanol |
| N-BDD | Nitrogen-terminated BDD |
| LOD | Limit of detection |
| Pt-BDD | Platinum-deposited BDD |
| Hb | Hemoglobin |
| ChOx | Cholesterol oxidase |
| PTFE | Polytetrafluoroethylene |
| GA2 | Glutardialdehyde |
| OPs | Organophosphate pesticides |
| CSs | Carbon spheres |
| NDs | Nanodiamonds |
| NBDD | Nanocrystalline boron-doped diamond |
| BDD HRs | Free-standing BDD hollow nanorods |
| WOx NRs | Tungsten oxide nanorods |
| DA | Dopamine |
| TNIL | Thermal nanoimprint lithography |
| ssDNA | Single standard DNA |
| FSCV | Fast scan cyclic voltammetry |
References
- Balogun, M.-S.; Luo, Y.; Qiu, W.; Liu, P.; Tong, Y. A Review of Carbon Materials and Their Composites with Alloy Metals for Sodium Ion Battery Anodes. Carbon 2016, 98, 162–178. [Google Scholar] [CrossRef]
- Zhai, Z.; Zhang, L.; Du, T.; Ren, B.; Xu, Y.; Wang, S.; Miao, J.; Liu, Z. A Review of Carbon Materials for Supercapacitors. Mater. Des. 2022, 221, 111017. [Google Scholar] [CrossRef]
- Speranza, G. Carbon Nanomaterials: Synthesis, Functionalization and Sensing Applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef]
- Zaytseva, O.; Neumann, G. Carbon Nanomaterials: Production, Impact on Plant Development, Agricultural and Environmental Applications. Chem. Biol. Technol. Agric. 2016, 3, 17. [Google Scholar] [CrossRef]
- Park, S.-J.; Deshmukh, M.A.; Kang, B.-C.; Jeon, J.-Y.; Chen, C.; Ha, T.-J. Review—A Review of Advanced Electronic Applications Based on Carbon Nanomaterials. ECS J. Solid State Sci. Technol. 2020, 9, 71002. [Google Scholar] [CrossRef]
- Kothandam, G.; Singh, G.; Guan, X.; Lee, J.M.; Ramadass, K.; Joseph, S.; Benzigar, M.; Karakoti, A.; Yi, J.; Kumar, P.; et al. Recent Advances in Carbon-Based Electrodes for Energy Storage and Conversion. Adv. Sci. 2023, 10, 2301045. [Google Scholar] [CrossRef]
- Testa, C.; Zammataro, A.; Pappalardo, A.; Trusso Sfrazzetto, G. Catalysis with Carbon Nanoparticles. RSC Adv. 2019, 9, 27659–27664. [Google Scholar] [CrossRef]
- Olivero, P.; Rubanov, S.; Reichart, P.; Gibson, B.C.; Huntington, S.T.; Rabeau, J.R.; Greentree, A.D.; Salzman, J.; Moore, D.; Jamieson, D.N.; et al. Characterization of Three-Dimensional Microstructures in Single-Crystal Diamond. Diam. Relat. Mater. 2006, 15, 1614–1621. [Google Scholar] [CrossRef]
- Kondrin, M.V.; Brazhkin, V.V. Diamond Monohydride: The Most Stable Three-Dimensional Hydrocarbon. Phys. Chem. Chem. Phys. 2015, 17, 17739–17744. [Google Scholar] [CrossRef]
- Xu, B.; Tian, Y. Diamond Gets Harder, Tougher, and More Deformable. Matter Radiat. Extrem. 2020, 5, 68103. [Google Scholar] [CrossRef]
- Dunlap, R.A. Diamond. In Novel Microstructures for Solids; Morgan & Claypool Publishers: San Rafael, CA, USA, 2018; pp. 7–21. [Google Scholar]
- Ullah, M.; Ahmed, E.; Hussain, F.; Rana, A.M.; Raza, R. Electrical Conductivity Enhancement by Boron-Doping in Diamond Using First Principle Calculations. Appl. Surf. Sci. 2015, 334, 40–44. [Google Scholar] [CrossRef]
- Poferl, D.J.; Gardner, N.C.; Angus, J.C. Growth of Boron-doped Diamond Seed Crystals by Vapor Deposition. J. Appl. Phys. 2003, 44, 1428–1434. [Google Scholar] [CrossRef]
- Einaga, Y. Boron-Doped Diamond Electrodes: Fundamentals for Electrochemical Applications. Acc. Chem. Res. 2022, 55, 3605–3615. [Google Scholar] [CrossRef]
- Bogdanowicz, R.; Ryl, J. Structural and Electrochemical Heterogeneities of Boron-Doped Diamond Surfaces. Curr. Opin. Electrochem. 2022, 31, 100876. [Google Scholar] [CrossRef]
- Luong, J.H.T.; Male, K.B.; Glennon, J.D. Boron-Doped Diamond Electrode: Synthesis, Characterization, Functionalization and Analytical Applications. Analyst 2009, 134, 1965–1979. [Google Scholar] [CrossRef] [PubMed]
- Cobb, S.J.; Ayres, Z.J.; Macpherson, J.V. Boron Doped Diamond: A Designer Electrode Material for the Twenty-First Century. Annu. Rev. Anal. Chem. 2018, 11, 463–484. [Google Scholar] [CrossRef]
- Kondo, T. Recent Electroanalytical Applications of Boron-Doped Diamond Electrodes. Curr. Opin. Electrochem. 2022, 32, 100891. [Google Scholar] [CrossRef]
- Freitas, J.M.; Oliveira, T.D.C.; Munoz, R.A.A.; Richter, E.M. Boron Doped Diamond Electrodes in Flow-Based Systems. Front. Chem. 2019, 7, 190. [Google Scholar] [CrossRef]
- Joshi, P.; Riley, P.; Goud, K.Y.; Mishra, R.K.; Narayan, R. Recent Advances of Boron-Doped Diamond Electrochemical Sensors toward Environmental Applications. Curr. Opin. Electrochem. 2022, 32, 100920. [Google Scholar] [CrossRef]
- Chakrabarty, K. Microwave Plasma CVD Synthesis of Superhard CNOB Materials. Ph.D. Thesis, The University of Alabama at Birmingham, Birmingham, AL, USA, 2023. [Google Scholar]
- Sharma, A. Diamond as a Precision Cutting Tool; David, G., Ed.; IntechOpen: Rijeka, Croatia, 2023; p. Ch. 1. ISBN 978-1-80356-318-3. [Google Scholar]
- Purcell, E.K.; Becker, M.F.; Guo, Y.; Hara, S.A.; Ludwig, K.A.; McKinney, C.J.; Monroe, E.M.; Rechenberg, R.; Rusinek, C.A.; Saxena, A.; et al. Next-Generation Diamond Electrodes for Neurochemical Sensing: Challenges and Opportunities. Micromachines 2021, 12, 128. [Google Scholar] [CrossRef]
- Piret, G.; Hébert, C.; Mazellier, J.-P.; Rousseau, L.; Scorsone, E.; Cottance, M.; Lissorgues, G.; Heuschkel, M.O.; Picaud, S.; Bergonzo, P.; et al. 3D-Nanostructured Boron-Doped Diamond for Microelectrode Array Neural Interfacing. Biomaterials 2015, 53, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, M.; Taylor, A.; Fjorback, M.; Zachar, V.; Pennisi, C.P. Boron-Doped Nanocrystalline Diamond Electrodes for Neural Interfaces: In Vivo Biocompatibility Evaluation. Front. Neurosci. 2016, 10, 87. [Google Scholar] [CrossRef]
- Macpherson, J.V. A Practical Guide to Using Boron Doped Diamond in Electrochemical Research. Phys. Chem. Chem. Phys. 2015, 17, 2935–2949. [Google Scholar] [CrossRef] [PubMed]
- Jiwanti, P.K.; Hendri, M.; Wafiroh, S.; Einaga, Y. Development of Ofloxacin Electrochemical Sensor in Milk Sample Using Boron-Doped Diamond Electrode Decorated by Zinc Nanoparticles. Anal. Bioanal. Electrochem. 2023, 15, 280–293. [Google Scholar]
- Bates, A.M. Aqueous Redox Flow Batteries with Boron Doped Diamond as an Electrode. Master’s Thesis, University of Louisville, Louisville, KY, USA, 2020. [Google Scholar]
- Gong, Y.; Jia, W.; Zhou, B.; Zheng, K.; Ma, D.; Li, Z.; Gao, J.; Ma, Y.; Hei, H.; Yu, S.; et al. Effect of Boron Doping Levels on the Microstructure and Characteristics of High-Quality Boron-Doped Diamond Electrodes Prepared by MPCVD. Diam. Relat. Mater. 2023, 139, 110377. [Google Scholar] [CrossRef]
- Sotelo-Gil, J.; Cuevas-Yañez, E.; Frontana-Uribe, B.A. Recent Advances on Boron Doped Diamond (BDD) Electrode as Cathode in Organic and Inorganic Preparative Electrotransformations. Curr. Opin. Electrochem. 2022, 34, 101004. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, Y.; Song, P.-K.; Yoon, J.-H. Effect of Boron Doping Level on BDD Electrode for Heavy Metal Ion Sensor. In Electrochemical Society Meeting Abstracts Prime2020; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2020; p. MA2020-02, 3343. [Google Scholar]
- Kim, S.; Jeong, Y.; Park, M.-O.; Jang, Y.; Bae, J.-S.; Hong, K.-S.; Kim, S.; Song, P.; Yoon, J.-H. Development of Boron Doped Diamond Electrodes Material for Heavy Metal Ion Sensor with High Sensitivity and Durability. J. Mater. Res. Technol. 2023, 23, 1375–1385. [Google Scholar] [CrossRef]
- Zeng, H.; Konicek, A.R.; Moldovan, N.; Mangolini, F.; Jacobs, T.; Wylie, I.; Arumugam, P.U.; Siddiqui, S.; Carpick, R.W.; Carlisle, J.A. Boron-Doped Ultrananocrystalline Diamond Synthesized with an H-Rich/Ar-Lean Gas System. Carbon 2015, 84, 103–117. [Google Scholar] [CrossRef]
- Watanabe, T.; Honda, Y.; Kanda, K.; Einaga, Y. Tailored Design of Boron-Doped Diamond Electrodes for Various Electrochemical Applications with Boron-Doping Level and Sp2-Bonded Carbon Impurities. Phys. Status Solidi 2014, 211, 2709–2717. [Google Scholar] [CrossRef]
- Ivandini, T.A.; Watanabe, T.; Matsui, T.; Ootani, Y.; Iizuka, S.; Toyoshima, R.; Kodama, H.; Kondoh, H.; Tateyama, Y.; Einaga, Y. Influence of Surface Orientation on Electrochemical Properties of Boron-Doped Diamond. J. Phys. Chem. C 2019, 123, 5336–5344. [Google Scholar] [CrossRef]
- Long, H.; Hu, H.; Wen, K.; Liu, X.; Liu, S.; Zhang, Q.; Chen, T. Thickness Effects on Boron Doping and Electrochemical Properties of Boron-Doped Diamond Film. Molecules 2023, 28, 2829. [Google Scholar] [CrossRef] [PubMed]
- Rehacek, V.; Hotovy, I.; Marton, M.; Mikolasek, M.; Michniak, P.; Vincze, A.; Kromka, A.; Vojs, M. Voltammetric Characterization of Boron-Doped Diamond Electrodes for Electroanalytical Applications. J. Electroanal. Chem. 2020, 862, 114020. [Google Scholar] [CrossRef]
- Brosler, P.; Neto, M.Â.; Silva, R.F.; Tedim, J.; Oliveira, F.J. Customized Boron-Doped Diamond Electrodes for Efficient Water Treatment via HF-CVD Parameter Optimization. Diam. Relat. Mater. 2024, 141, 110595. [Google Scholar] [CrossRef]
- Xu, J.; Yokota, Y.; Wong, R.A.; Kim, Y.; Einaga, Y. Unusual Electrochemical Properties of Low-Doped Boron-Doped Diamond Electrodes Containing Sp2 Carbon. J. Am. Chem. Soc. 2020, 142, 2310–2316. [Google Scholar] [CrossRef]
- Einaga, Y.; Foord, J.S.; Swain, G.M. Diamond Electrodes: Diversity and Maturity. MRS Bull. 2014, 39, 525–532. [Google Scholar] [CrossRef]
- Cięciwa, A.; Wüthrich, R.; Comninellis, C. Electrochemical Characterization of Mechanically Implanted Boron-Doped Diamond Electrodes. Electrochem. Commun. 2006, 8, 375–382. [Google Scholar] [CrossRef]
- Harada, Y.; Hishinuma, R.; Spătaru, N.; Sakurai, Y.; Miyasaka, K.; Terashima, C.; Uetsuka, H.; Suzuki, N.; Fujishima, A.; Kondo, T.; et al. High-Speed Synthesis of Heavily Boron-Doped Diamond Films by in-Liquid Microwave Plasma CVD. Diam. Relat. Mater. 2019, 92, 41–46. [Google Scholar] [CrossRef]
- Yence, M.; Cetinkaya, A.; Ozcelikay, G.; Kaya, S.I.; Ozkan, S.A. Boron-Doped Diamond Electrodes: Recent Developments and Advances in View of Electrochemical Drug Sensors. Crit. Rev. Anal. Chem. 2022, 52, 1122–1138. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Toghill, K.E.; Compton, R.G. Metal Nanoparticle Modified Boron Doped Diamond Electrodes for Use in Electroanalysis. Electroanalysis 2010, 22, 1947–1956. [Google Scholar] [CrossRef]
- Jiwanti, P.K.; Ichzan, A.M.; Dewandaru, R.K.P.; Atriardi, S.R.; Einaga, Y.; Ivandini, T.A. Improving the CO2 Electrochemical Reduction to Formic Acid Using Iridium-Oxide-Modified Boron-Doped Diamond Electrodes. Diam. Relat. Mater. 2020, 106, 107874. [Google Scholar] [CrossRef]
- Jiwanti, P.K.; Einaga, Y. Electrochemical Reduction of CO2 Using Palladium Modified Boron-Doped Diamond Electrodes: Enhancing the Production of CO. Phys. Chem. Chem. Phys. 2019, 21, 15297–15301. [Google Scholar] [CrossRef] [PubMed]
- Hrdlička, V.; Matvieiev, O.; Navrátil, T.; Šelešovská, R. Recent Advances in Modified Boron-Doped Diamond Electrodes: A Review. Electrochim. Acta 2023, 456, 142435. [Google Scholar] [CrossRef]
- Bansal, R.; Verduzco, R.; Wong, M.S.; Westerhoff, P.; Garcia-Segura, S. Development of Nano Boron-Doped Diamond Electrodes for Environmental Applications. J. Electroanal. Chem. 2022, 907, 116028. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Diamond-Based Electrodes for Detection of Metal Ions and Anions. Nanomaterials 2022, 12, 64. [Google Scholar] [CrossRef]
- Ivandini, T.A.; Luhur, M.S.P.; Khalil, M.; Einaga, Y. Modification of Boron-Doped Diamond Electrodes with Gold–Palladium Nanoparticles for an Oxygen Sensor. Analyst 2021, 146, 2842–2850. [Google Scholar] [CrossRef] [PubMed]
- Asha, A.B.; Narain, R. Chapter 15—Nanomaterials Properties. In Polymer Science and Nanotechnology; Narain, R., Asha, A.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 343–359. ISBN 978-0-12-816806-6. [Google Scholar]
- Saleh, T.A. Nanomaterials: Classification, Properties, and Environmental Toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Prakash Sharma, V.; Sharma, U.; Chattopadhyay, M.; Shukla, V.N. Advance Applications of Nanomaterials: A Review. Mater. Today Proc. 2018, 5, 6376–6380. [Google Scholar] [CrossRef]
- Lieber, C.M. Semiconductor Nanowires: A Platform for Nanoscience and Nanotechnology. MRS Bull. 2011, 36, 1052–1063. [Google Scholar] [CrossRef]
- Li, Y.; Somorjai, G.A. Nanoscale Advances in Catalysis and Energy Applications. Nano Lett. 2010, 10, 2289–2295. [Google Scholar] [CrossRef]
- Lee, S.J.; Jang, H.; Lee, D.N. Recent Advances in Nanoflowers: Compositional and Structural Diversification for Potential Applications. Nanoscale Adv. 2023, 5, 5165–5213. [Google Scholar] [CrossRef] [PubMed]
- Escorcia-Díaz, D.; García-Mora, S.; Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C. Advancements in Nanoparticle Deposition Techniques for Diverse Substrates: A Review. Nanomaterials 2023, 13, 2586. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Y.; Ren, D.; Sun, L.; Zhuang, Y.; Yi, L.; Wang, S. Recent Advances in Nanomaterial-Assisted Electrochemical Sensors for Food Safety Analysis. Food Front. 2022, 3, 453–479. [Google Scholar] [CrossRef]
- Kant, T.; Shrivas, K.; Dewangan, K.; Kumar, A.; Jaiswal, N.K.; Deb, M.K.; Pervez, S. Design and Development of Conductive Nanomaterials for Electrochemical Sensors: A Modern Approach. Mater. Today Chem. 2022, 24, 100769. [Google Scholar] [CrossRef]
- Yuan, F.; Xia, Y.; Lu, Q.; Xu, Q.; Shu, Y.; Hu, X. Recent Advances in Inorganic Functional Nanomaterials Based Flexible Electrochemical Sensors. Talanta 2022, 244, 123419. [Google Scholar] [CrossRef] [PubMed]
- Caratelli, V.; Di Meo, E.; Colozza, N.; Fabiani, L.; Fiore, L.; Moscone, D.; Arduini, F. Nanomaterials and Paper-Based Electrochemical Devices: Merging Strategies for Fostering Sustainable Detection of Biomarkers. J. Mater. Chem. B 2022, 10, 9021–9039. [Google Scholar] [CrossRef]
- Gupta, P.K.; Tiwari, S.; Khan, Z.H.; Solanki, P.R. Amino Acid Functionalized ZrO2 Nanoparticles Decorated Reduced Graphene Oxide Based Immunosensor. J. Mater. Chem. B 2017, 5, 2019–2033. [Google Scholar] [CrossRef]
- Chauhan, D.; Gupta, P.K.; Solanki, P.R. Electrochemical Immunosensor Based on Magnetite Nanoparticles Incorporated Electrospun Polyacrylonitrile Nanofibers for Vitamin-D3 Detection. Mater. Sci. Eng. C 2018, 93, 145–156. [Google Scholar] [CrossRef]
- Gupta, P.K.; Pachauri, N.; Khan, Z.H.; Solanki, P.R. One Pot Synthesized Zirconia Nanoparticles Embedded in Amino Functionalized Amorphous Carbon for Electrochemical Immunosensor. J. Electroanal. Chem. 2017, 807, 59–69. [Google Scholar] [CrossRef]
- Gupta, P.K.; Khan, Z.H.; Solanki, P.R. One-Step Electrodeposited Porous ZnO Thin Film Based Immunosensor for Detection of Vibrio cholerae Toxin. J. Electrochem. Soc. 2016, 163, B309–B318. [Google Scholar] [CrossRef]
- Gupta, P.K.; Gupta, A.; Dhakate, S.R.; Khan, Z.H.; Solanki, P.R. Functionalized Polyacrylonitrile-nanofiber Based Immunosensor for Vibrio cholerae Detection. J. Appl. Polym. Sci. 2016, 133, 44170. [Google Scholar] [CrossRef]
- Cornejo, O.M.; Murrieta, M.F.; Castañeda, L.F.; Nava, J.L. Electrochemical Reactors Equipped with BDD Electrodes: Geometrical Aspects and Applications in Water Treatment. Curr. Opin. Solid State Mater. Sci. 2021, 25, 100935. [Google Scholar] [CrossRef]
- Souza, F.L.; Lopes, O.F.; Santos, E.V.; Ribeiro, C. Promoting CO2 Electroreduction on Boron-Doped Diamond Electrodes: Challenges and Trends. Curr. Opin. Electrochem. 2022, 32, 100890. [Google Scholar] [CrossRef]
- Triana, Y.; Ogata, G.; Einaga, Y. Application of Boron Doped Diamond Electrodes to Electrochemical Gas Sensor. Curr. Opin. Electrochem. 2022, 36, 101113. [Google Scholar] [CrossRef]
- Losey, M.W.; Kelly, J.J.; Badgayan, N.D.; Sahu, S.K.; Sreekanth, P.R. Electrodeposition. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-803581-8. [Google Scholar]
- Bhattacharyya, B. Chapter 6—Design and Developments of Microtools. In Electrochemical Micromachining for Nanofabrication, MEMS and Nanotechnology; William Andrew Publishing: Norwich, NY, USA, 2015; pp. 101–122. ISBN 978-0-323-32737-4. [Google Scholar]
- Anggraini, L.E.; Saepudin, E.; Ivandini, T.A. Modification of Boron-Doped Diamond with Gold through Wet-Chemical Seeding and Electrodeposition Techniques for the Application of Acrylamide Biosensor. IOP Conf. Ser. Mater. Sci. Eng. 2020, 763, 12019. [Google Scholar] [CrossRef]
- Umam, K.; Saepudin, E.; Ivandini, T.A. Preparation of Hemoglobin-Modified Boron-Doped Diamond for Acrylamide Biosensors. IOP Conf. Ser. Mater. Sci. Eng. 2017, 188, 12006. [Google Scholar] [CrossRef]
- Garcia, E.M.; Cordero, P.A.; Kazemeini, S.; Murillo-Soto, A.; Gonzalez, K.A.; McClement, A.; Rusinek, C.A. Platinum and Palladium Nanoparticles on Boron-Doped Diamond for the Electrochemical Detection of Hydrogen Peroxide: A Comparison Study. Anal. Bioanal. Chem. 2023, 415, 5781–5795. [Google Scholar] [CrossRef]
- Wulandari, R.; Ivandini, T.A.; Saefudin, E. A Boron-Doped Diamond Electrode Decorated with Hemoglobin-Modified Platinum Nanoparticles as a Biosensor for Acrylamide Detection. IOP Conf. Ser. Mater. Sci. Eng. 2019, 496, 12011. [Google Scholar] [CrossRef]
- Nantaphol, S.; Chailapakul, O.; Siangproh, W. A Novel Paper-Based Device Coupled with a Silver Nanoparticle-Modified Boron-Doped Diamond Electrode for Cholesterol Detection. Anal. Chim. Acta 2015, 891, 136–143. [Google Scholar] [CrossRef]
- Agustiany, T.; Khalil, M.; Einaga, Y.; Jiwanti, P.K.; Ivandini, T.A. Stable Iridium-Modified Boron-Doped Diamond Electrode for the Application in Electrochemical Detection of Arsenic (III). Mater. Chem. Phys. 2020, 244, 122723. [Google Scholar] [CrossRef]
- Traipop, S.; Yakoh, A.; Jampasa, S.; Chaiyo, S.; Boonyongmaneerat, Y.; Panpranot, J.; Praserthdam, P.; Chailapakul, O. Sequential Electrodeposition of Cu–Pt Bimetallic Nanocatalysts on Boron-Doped Diamond Electrodes for the Simple and Rapid Detection of Methanol. Sci. Rep. 2021, 11, 14354. [Google Scholar] [CrossRef]
- Kuramochi, S.; Fiorani, A.; Einaga, Y. Molybdenum Oxide Electrodeposition on Boron-Doped Diamond: Investigation of Valence State and Application in Electrochemical Nitrogen Reduction to Ammonia. Diam. Relat. Mater. 2023, 139, 110277. [Google Scholar] [CrossRef]
- Komkova, M.A.; Pasquarelli, A.; Andreev, E.A.; Galushin, A.A.; Karyakin, A.A. Prussian Blue Modified Boron-Doped Diamond Interfaces for Advanced H2O2 Electrochemical Sensors. Electrochim. Acta 2020, 339, 135924. [Google Scholar] [CrossRef]
- Djebbi, M.A.; Boubakri, S.; Braiek, M.; Jaffrezic-Renault, N.; Namour, P.; Ben Haj Amara, A. High Performance Non-Enzymatic Electrochemical Lactate Sensor Based on ZnAl Layered Double Hydroxide Nanosheets Supported Gold Nanoparticles. J. Electrochem. Soc. 2021, 168, 57529. [Google Scholar] [CrossRef]
- Wei, M.; Feng, S. Amperometric Determination of Organophosphate Pesticides Using a Acetylcholinesterase Based Biosensor Made from Nitrogen-Doped Porous Carbon Deposited on a Boron-Doped Diamond Electrode. Microchim. Acta 2017, 184, 3461–3468. [Google Scholar] [CrossRef]
- Zehani, N.; Fortgang, P.; Saddek Lachgar, M.; Baraket, A.; Arab, M.; Dzyadevych, S.V.; Kherrat, R.; Jaffrezic-Renault, N. Highly Sensitive Electrochemical Biosensor for Bisphenol A Detection Based on a Diazonium-Functionalized Boron-Doped Diamond Electrode Modified with a Multi-Walled Carbon Nanotube-Tyrosinase Hybrid Film. Biosens. Bioelectron. 2015, 74, 830–835. [Google Scholar] [CrossRef]
- Wei, M.; Zeng, G.; Lu, Q. Determination of Organophosphate Pesticides Using an Acetylcholinesterase-Based Biosensor Based on a Boron-Doped Diamond Electrode Modified with Gold Nanoparticles and Carbon Spheres. Microchim. Acta 2014, 181, 121–127. [Google Scholar] [CrossRef]
- Wei, M.; Wang, J. A Novel Acetylcholinesterase Biosensor Based on Ionic Liquids-AuNPs-Porous Carbon Composite Matrix for Detection of Organophosphate Pesticides. Sens. Actuators B Chem. 2015, 211, 290–296. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, M. Development of Acetylcholinesterase Biosensor Based on Platinum–Carbon Aerogels Composite for Determination of Organophosphorus Pesticides. Food Control 2014, 36, 49–54. [Google Scholar] [CrossRef]
- Chang, A.-Y.; Arumugam, P. Fabrication and Characterization of Boron-Doped Ultrananocrystalline Diamond Microelectrodes Modified with Multi-Walled Carbon Nanotubes and Nafion. In Proceedings of the SPIE—The International Society for Optical Engineering, San Diego, CA, USA, 5 September 2018; Volume 10728, p. 1072803. [Google Scholar]
- Chang, A.-Y.; Siddiqui, S.; Arumugam, P.U. Nafion and Multiwall Carbon Nanotube Modified Ultrananocrystalline Diamond Microelectrodes for Detection of Dopamine and Serotonin. Micromachines 2021, 12, 523. [Google Scholar] [CrossRef]
- Dai, W.; Li, M.; Gao, S.; Li, H.; Li, C.; Xu, S.; Wu, X.; Yang, B. Fabrication of Nickel/Nanodiamond/Boron-Doped Diamond Electrode for Non-Enzymatic Glucose Biosensor. Electrochim. Acta 2016, 187, 413–421. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Dickerson, J.H. Electrophoretic Deposition: Fundamentals and Applications. J. Phys. Chem. B 2013, 117, 1501. [Google Scholar] [CrossRef] [PubMed]
- Amrollahi, P.; Krasinski, J.S.; Vaidyanathan, R.; Tayebi, L.; Vashaee, D. Electrophoretic Deposition (EPD): Fundamentals and Applications from Nano- to Micro-Scale Structures BT—Handbook of Nanoelectrochemistry: Electrochemical Synthesis Methods, Properties and Characterization Techniques. In Handbook of Nanoelectrochemistry; Aliofkhazraei, M., Makhlouf, A.S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–27. ISBN 978-3-319-15207-3. [Google Scholar]
- Wadley, H.N.G.; Zhou, X.; Johnson, R.A.; Neurock, M. Mechanisms, Models and Methods of Vapor Deposition. Prog. Mater. Sci. 2001, 46, 329–377. [Google Scholar] [CrossRef]
- Wang, P.; Yuan, X.; Cui, Z.; Xu, C.; Sun, Z.; Li, J.; Liu, J.; Tian, Y.; Li, H. A Nanometer-Sized Graphite/Boron-Doped Diamond Electrochemical Sensor for Sensitive Detection of Acetaminophen. ACS Omega 2021, 6, 6326–6334. [Google Scholar] [CrossRef]
- Lim, Y.-K.; Song, M.-J.; Lim, D.-S. Non-Enzymatic Glucose Detection Using Free Standing Hollow Boron-Doped Diamond Nanorod Electrodes. J. Electrochem. Soc. 2019, 166, B576. [Google Scholar] [CrossRef]
- Gong, Z.; Hu, N.; Ye, W.; Zheng, K.; Li, C.; Ma, L.; Wei, Q.; Yu, Z.; Zhou, K.; Huang, N.; et al. High-Performance Non-Enzymatic Glucose Sensor Based on Ni/Cu/Boron-Doped Diamond Electrode. J. Electroanal. Chem. 2019, 841, 135–141. [Google Scholar] [CrossRef]
- Gunlazuardi, J.; Kurniawan, A.D.; Jiwanti, P.K.; Einaga, Y.; Ivandini, T.A. Core–Shell Copper-Gold Nanoparticles Modified at the Boron-Doped Diamond Electrode for Oxygen Sensors. Anal. Methods 2022, 14, 726–733. [Google Scholar] [CrossRef]
- Li, C.; Zhao, T.; Wei, Q.; Deng, Z.; Long, H.; Zheng, K.; Li, H.; Guo, Y.; Yu, Z.; Ma, L.; et al. The Effect of Heat Treatment Time on the Carbon-Coated Nickel Nanoparticles Modified Boron-Doped Diamond Composite Electrode for Non-Enzymatic Glucose Sensing. J. Electroanal. Chem. 2019, 841, 148–157. [Google Scholar] [CrossRef]
- Feng, Z.; Gao, N.; Liu, J.; Li, H. Boron-Doped Diamond Electrochemical Aptasensors for Trace Aflatoxin B1 Detection. Anal. Chim. Acta 2020, 1122, 70–75. [Google Scholar] [CrossRef]
- Yao, K.; Dai, B.; Tan, X.; Ralchenko, V.; Yang, L.; Liu, B.; Su, Z.; Zhao, J.; Liu, K.; Han, J.; et al. Fabrication of Au/Ni/Boron-Doped Diamond Electrodes via Hydrogen Plasma Etching Graphite and Amorphous Boron for Efficient Non-Enzymatic Sensing of Glucose. J. Electroanal. Chem. 2020, 871, 114264. [Google Scholar] [CrossRef]
- Long, H.; Liu, X.; Xie, Y.; Hu, N.; Deng, Z.; Jiang, Y.; Wei, Q.; Yu, Z.; Zhang, S. Thickness Effects of Ni on the Modified Boron Doped Diamond by Thermal Catalytic Etching for Non-Enzymatic Glucose Sensing. J. Electroanal. Chem. 2019, 832, 353–360. [Google Scholar] [CrossRef]
- Yuan, X.; Jiang, Z.; Wang, Q.; Gao, N.; Li, H.; Ma, Y. Polychlorinated Biphenyl Electrochemical Aptasensor Based on a Diamond–Gold Nanocomposite to Realize a Sub-Femtomolar Detection Limit. ACS Omega 2020, 5, 22402–22410. [Google Scholar] [CrossRef]
- Deng, Z.; Long, H.; Wei, Q.; Yu, Z.; Zhou, B.; Wang, Y.; Zhang, L.; Li, S.; Ma, L.; Xie, Y.; et al. High-Performance Non-Enzymatic Glucose Sensor Based on Nickel-Microcrystalline Graphite-Boron Doped Diamond Complex Electrode. Sens. Actuators B Chem. 2017, 242, 825–834. [Google Scholar] [CrossRef]
- Zribi, B.; Scorsone, E. Detection of Nitrate/Nitrite Using BDD Electrodes Coated with Metal Nano-Catalysts. Proceedings 2017, 1, 452. [Google Scholar] [CrossRef]
- Bogdanowicz, R.; Fabiańska, A.; Golunski, L.; Sobaszek, M.; Gnyba, M.; Ryl, J.; Darowicki, K.; Ossowski, T.; Janssens, S.D.; Haenen, K.; et al. Influence of the Boron Doping Level on the Electrochemical Oxidation of the Azo Dyes at Si/BDD Thin Film Electrodes. Diam. Relat. Mater. 2013, 39, 82–88. [Google Scholar] [CrossRef]
- Liu, Z.; Baluchová, S.; Sartori, A.F.; Li, Z.; Gonzalez-Garcia, Y.; Schreck, M.; Buijnsters, J.G. Heavily Boron-Doped Diamond Grown on Scalable Heteroepitaxial Quasi-Substrates: A Promising Single Crystal Material for Electrochemical Sensing Applications. Carbon 2023, 201, 1229–1240. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, X.; Zhao, Z.; Zhang, Z.; Pei, J.; Zhang, Z.; Cui, G. Adjusting Surface Morphology of Substrate to Improve the Capacitive Performance for the Formed Boron-Doped Diamond Electrode. Appl. Surf. Sci. 2019, 491, 814–822. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Zhang, Z.; Cong, Y.; Huang, Y.; Du, X. Nanomaterials-Based Electrochemical Immunosensors. Micromachines 2019, 10, 397. [Google Scholar] [CrossRef]
- Baig, N.; Sajid, M.; Saleh, T.A. Recent Trends in Nanomaterial-Modified Electrodes for Electroanalytical Applications. TrAC Trends Anal. Chem. 2019, 111, 47–61. [Google Scholar] [CrossRef]
- Chen, W.; Li, W.; Liu, F.; Miao, D.; Ma, L.; Gao, X.; Wei, Q.; Zhou, K.; Yu, Z.; Yu, Y. Microstructure of Boron Doped Diamond Electrodes and Studies on Its Basic Electrochemical Characteristics and Applicability of Dye Degradation. J. Environ. Chem. Eng. 2020, 8, 104348. [Google Scholar] [CrossRef]
- Navya, P.N.; Daima, H.K. Rational Engineering of Physicochemical Properties of Nanomaterials for Biomedical Applications with Nanotoxicological Perspectives. Nano Converg. 2016, 3, 1. [Google Scholar] [CrossRef]
- Rabbani, M.; Hoque, M.E.; Mahbub, Z.B. Chapter 7—Nanosensors in Biomedical and Environmental Applications: Perspectives and Prospects. In Nanofabrication for Smart Nanosensor Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 163–186. ISBN 978-0-12-820702-4. [Google Scholar]
- Fieber, L.; Evans, J.D.; Huang, C.; Grant, P.S. Single-Operation, Multi-Phase Additive Manufacture of Electro-Chemical Double Layer Capacitor Devices. Addit. Manuf. 2019, 28, 344–353. [Google Scholar] [CrossRef]
- Li, D.; Lv, Q.; Zhang, C.; Zhou, W.; Guo, H.; Jiang, S.; Li, Z. The Effect of Electrode Thickness on the High-Current Discharge and Long-Term Cycle Performance of a Lithium-Ion Battery. Batteries 2022, 8, 101. [Google Scholar] [CrossRef]
- Curulli, A. Nanomaterials in Electrochemical Sensing Area: Applications and Challenges in Food Analysis. Molecules 2020, 25, 5759. [Google Scholar] [CrossRef]
- Ahmad, R.; Wolfbeis, O.S.; Hahn, Y.-B.; Alshareef, H.N.; Torsi, L.; Salama, K.N. Deposition of Nanomaterials: A Crucial Step in Biosensor Fabrication. Mater. Today Commun. 2018, 17, 289–321. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Staszak, K.; Woźniak-Budych, M.J.; Litowczenko, J.; Maciejewska, B.M.; Jurga, S. Surface Functionalization—The Way for Advanced Applications of Smart Materials. Coord. Chem. Rev. 2021, 436, 213846. [Google Scholar] [CrossRef]
- Tiwari, S.; Gupta, P.K.; Bagbi, Y.; Sarkar, T.; Solanki, P.R. L-Cysteine Capped Lanthanum Hydroxide Nanostructures for Non-Invasive Detection of Oral Cancer Biomarker. Biosens. Bioelectron. 2017, 89, 1042–1052. [Google Scholar] [CrossRef]
- Önal, G. Investigation of the Electrochemical Properties of Vinblastine on Boron-Doped Diamond Electrode Treated with Anodic Pre-Treatment in Anionic Surfactant Medium. Diam. Relat. Mater. 2023, 133, 109699. [Google Scholar] [CrossRef]
- Tang, L.; Li, X.; Cammarata, R.C.; Friesen, C.; Sieradzki, K. Electrochemical Stability of Elemental Metal Nanoparticles. J. Am. Chem. Soc. 2010, 132, 11722–11726. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Zribi, B.; Dragoe, D.; Scorsone, E. BDD Electrodes Modified with Metal Nano-Catalysts for Coffee Discrimination in Real Samples. Sens. Actuators B Chem. 2019, 290, 147–154. [Google Scholar] [CrossRef]
- Kuang, P.; Natsui, K.; Einaga, Y.; Feng, C.; Cui, Y.; Zhang, W.; Deng, Y. Annealing Enhancement in Stability and Performance of Copper Modified Boron-Doped Diamond (Cu-BDD) Electrode for Electrochemical Nitrate Reduction. Diam. Relat. Mater. 2021, 114, 108310. [Google Scholar] [CrossRef]
- Hwang, H.S.; Jeong, J.W.; Kim, Y.A.; Chang, M. Carbon Nanomaterials as Versatile Platforms for Biosensing Applications. Micromachines 2020, 11, 814. [Google Scholar] [CrossRef]
- Rajendrachari, S.; Basavegowda, N.; Adimule, V.M.; Avar, B.; Somu, P.; RM, S.K.; Baek, K.-H. Assessing the Food Quality Using Carbon Nanomaterial Based Electrodes by Voltammetric Techniques. Biosensors 2022, 12, 1173. [Google Scholar] [CrossRef]
- Deng, Z.; Long, H.; Wang, Y.; Yu, Z.; Ma, L.; Zhou, K.; Wei, Q. Template-Free Synthesis of Millimeter-Scale Carbon Nanorod Arrays on Boron-Doped Diamond with Superior Glucose Sensing Performance. Appl. Surf. Sci. 2022, 572, 151468. [Google Scholar] [CrossRef]
- Li, H.; Zhou, K.; Cao, J.; Wei, Q.; Lin, C.-T.; Pei, S.E.; Ma, L.; Hu, N.; Guo, Y.; Deng, Z.; et al. A Novel Modification to Boron-Doped Diamond Electrode for Enhanced, Selective Detection of Dopamine in Human Serum. Carbon 2021, 171, 16–28. [Google Scholar] [CrossRef]
- Casanova, A.; Iniesta, J.; Gomis-Berenguer, A. Recent Progress in the Development of Porous Carbon-Based Electrodes for Sensing Applications. Analyst 2022, 147, 767–783. [Google Scholar] [CrossRef]
- Kumar, Y.; Nirbhaya, V.; Chauhan, D.; Shankar, S.; Chandra, R.; Kumar, S. Nanostructured Zirconia Embedded Porous Carbon Based Ultrasensitive Electrochemical Biosensor for SAA Biomarker Detection. Mater. Chem. Phys. 2023, 294, 126983. [Google Scholar] [CrossRef]
- Rezaei, M.; Jalalvand, A.R.; Saeed, D.W.M.; Farzaei, M.H.; Arkan, E. An Intelligent Biosensing Procedure Based on Three-Way Calibrations and Generation of Second-Order Hydrodynamic Square Wave Voltammetric Data for Detection of DNA Damage and Simultaneous Biosensing of Three Polycyclic Aromatic Hydrocarbons. Chemom. Intell. Lab. Syst. 2023, 235, 104781. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, T.; Zhang, X. Graphene-Based Biosensors for Detection of Biomarkers. Micromachines 2020, 11, 60. [Google Scholar] [CrossRef]
- Rivas, G.A.; Rubianes, M.D.; Rodríguez, M.C.; Ferreyra, N.F.; Luque, G.L.; Pedano, M.L.; Miscoria, S.A.; Parrado, C. Carbon Nanotubes for Electrochemical Biosensing. Talanta 2007, 74, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Ajayan, P.M. Nanotubes from Carbon. Chem. Rev. 1999, 99, 1787–1800. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-K.; Song, M.-J.; Kim, J.-H.; Lim, Y.-K.; Chun, Y.-S.; Lim, D.-S. Selective Growth of Carbon Nanotubes on Boron-Doped Diamond for Electrochemical Biosensor Application. RSC Adv. 2015, 5, 23395–23400. [Google Scholar] [CrossRef]
- Ke, H.; Liu, M.; Zhuang, L.; Li, Z.; Fan, L.; Zhao, G. A Fetomolar Level 17β-Estradiol Electrochemical Aptasensor Constructed On Hierachical Dendritic Gold Modified Boron-Doped Diamond Electrode. Electrochim. Acta 2014, 137, 146–153. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, J.; Li, H. Diamond-Based Electrochemical Aptasensor Realizing a Femtomolar Detection Limit of Bisphenol A. Biosens. Bioelectron. 2017, 92, 21–25. [Google Scholar] [CrossRef]
- Annisa, T.N.; Saepudin, E.; Ivandini, T.A. Modification of Nitrogen-Terminated Boron-Doped Diamond Electrodes with Gold Nanoparticles and Hemoglobin for Acrylamide Biosensors. AIP Conf. Proc. 2018, 2023, 20108. [Google Scholar]
- Rismetov, B.; Ivandini, T.A.; Saepudin, E.; Einaga, Y. Electrochemical Detection of Hydrogen Peroxide at Platinum-Modified Diamond Electrodes for an Application in Melamine Strip Tests. Diam. Relat. Mater. 2014, 48, 88–95. [Google Scholar] [CrossRef]
- Wulandari, R.; Ivandini, T.A.; Saepudin, E.; Einaga, Y. Modification of Boron-Doped Diamond Electrodes with Platinum to Increase the Stability and Sensitivity of Haemoglobin-Based Acrylamide Sensors. Sens. Mater. 2019, 31, 1105–1117. [Google Scholar] [CrossRef]
- Henderson, S.; Bhardwaj, K.; Perugachi, V.; Espinoza-Montero, P.; Galligan, J.J.; Swain, G.M. In Vitro Monitoring of Nitric Oxide Release in the Mouse Colon Using a Boron-Doped Diamond Microelectrode Modified with Platinum Nanoparticles and Nafion. Anal. Chem. 2023, 95, 1027–1037. [Google Scholar] [CrossRef]
- Franceschini, F.; Taurino, I. Nickel-Based Catalysts for Non-Enzymatic Electrochemical Sensing of Glucose: A Review. Phys. Med. 2022, 14, 100054. [Google Scholar] [CrossRef]
- Dai, W.; Li, M.; Li, H.; Yang, B. Amperometric Biosensor Based on Nanoporous Nickel/Boron-Doped Diamond Film for Electroanalysis of l-Alanine. Sens. Actuators B Chem. 2014, 201, 31–36. [Google Scholar] [CrossRef]
- George, J.M.; Antony, A.; Mathew, B. Metal Oxide Nanoparticles in Electrochemical Sensing and Biosensing: A Review. Microchim. Acta 2018, 185, 358. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Metal Oxide Nanoparticles as Biomedical Materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Chauhan, D.; Khan, Z.H.; Solanki, P.R. ZrO2 Nanoflowers Decorated with Graphene Quantum Dots for Electrochemical Immunosensing. ACS Appl. Nano Mater. 2020, 3, 2506–2516. [Google Scholar] [CrossRef]
- Gupta, P.K.; Khan, Z.H.; Solanki, P.R. Improved Electrochemical Performance of Metal Doped Zirconia Nanoparticles for Detection of Ochratoxin-A. J. Electroanal. Chem. 2018, 829, 69–80. [Google Scholar] [CrossRef]
- Dai, W.; Li, H.; Li, M.; Li, C.; Wu, X.; Yang, B. Electrochemical Imprinted Polycrystalline Nickel–Nickel Oxide Half-Nanotube-Modified Boron-Doped Diamond Electrode for the Detection of l-Serine. ACS Appl. Mater. Interfaces 2015, 7, 22858–22867. [Google Scholar] [CrossRef]
- Batool, R.; Rhouati, A.; Nawaz, M.H.; Hayat, A.; Marty, J.L. A Review of the Construction of Nano-Hybrids for Electrochemical Biosensing of Glucose. Biosensors 2019, 9, 46. [Google Scholar] [CrossRef]
- Soto, D.; Orozco, J. Hybrid Nanobioengineered Nanomaterial-Based Electrochemical Biosensors. Molecules 2022, 27, 3841. [Google Scholar] [CrossRef]
- Kurc, B.; Pigłowska, M.; Rymaniak, Ł.; Fuć, P. Modern Nanocomposites and Hybrids as Electrode Materials Used in Energy Carriers. Nanomaterials 2021, 11, 538. [Google Scholar] [CrossRef]
- Gupta, P.K.; Son, S.E.; Seong, G.H. Functionalized Ultra-Fine Bimetallic PtRu Alloy Nanoparticle with High Peroxidase-Mimicking Activity for Rapid and Sensitive Colorimetric Quantification of C-Reactive Protein. Microchim. Acta 2021, 188, 119. [Google Scholar] [CrossRef] [PubMed]
- Munonde, T.S.; Nomngongo, P.N. Nanocomposites for Electrochemical Sensors and Their Applications on the Detection of Trace Metals in Environmental Water Samples. Sensors 2021, 21, 131. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A Review on Graphene-Based Nanocomposites for Electrochemical and Fluorescent Biosensors. RSC Adv. 2019, 9, 8778–8881. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Jadon, N.; Jain, R. Next-Generation Polymer Nanocomposite-Based Electrochemical Sensors and Biosensors: A Review. TrAC Trends Anal. Chem. 2016, 82, 55–67. [Google Scholar] [CrossRef]
- Koçak, B.; İpek, Y.; Keçeci, A. A Novel Electrochemical Sensor for Metoprolol Analysis Based on Glutardialdehyde–Zinc Oxide Modified Boron Doped Diamond Electrode. Diam. Relat. Mater. 2023, 131, 109558. [Google Scholar] [CrossRef]
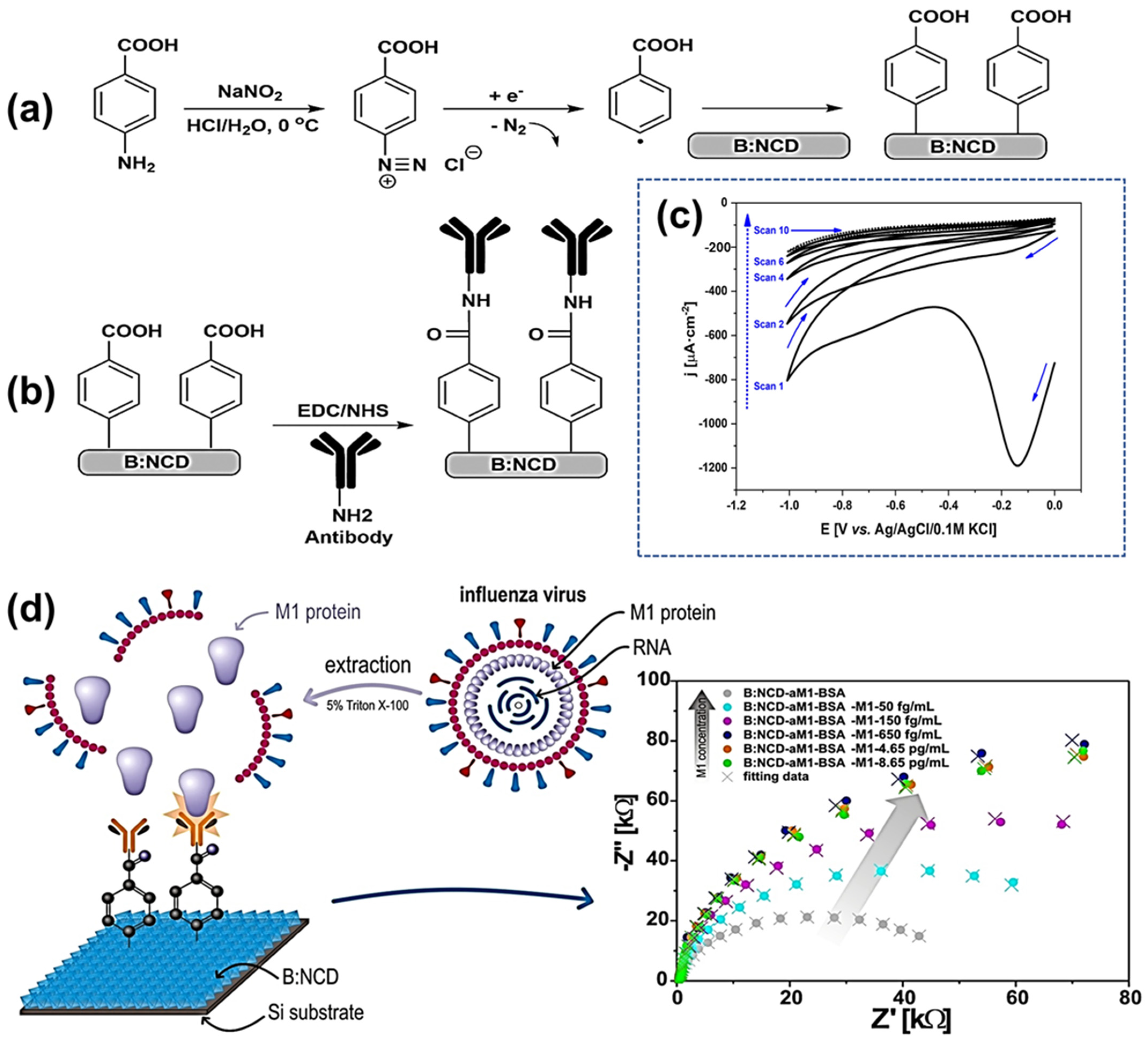
- Siuzdak, K.; Niedziałkowski, P.; Sobaszek, M.; Łęga, T.; Sawczak, M.; Czaczyk, E.; Dziąbowska, K.; Ossowski, T.; Nidzworski, D.; Bogdanowicz, R. Biomolecular Influenza Virus Detection Based on the Electrochemical Impedance Spectroscopy Using the Nanocrystalline Boron-Doped Diamond Electrodes with Covalently Bound Antibodies. Sens. Actuators B Chem. 2019, 280, 263–271. [Google Scholar] [CrossRef]
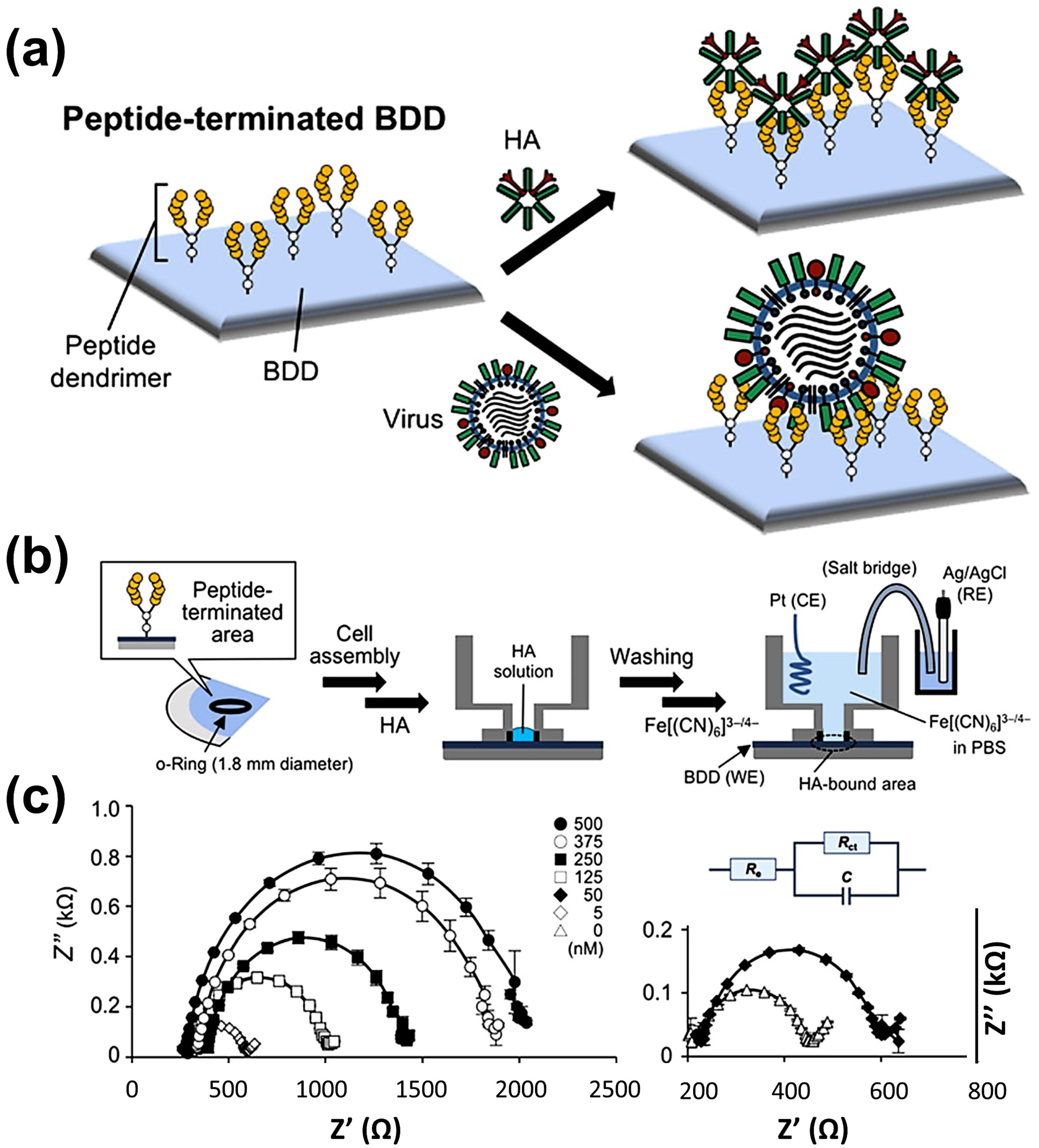
- Matsubara, T.; Ujie, M.; Yamamoto, T.; Einaga, Y.; Daidoji, T.; Nakaya, T.; Sato, T. Avian Influenza Virus Detection by Optimized Peptide Termination on a Boron-Doped Diamond Electrode. ACS Sens. 2020, 5, 431–439. [Google Scholar] [CrossRef]
- Li, T.; Lu, X.-M.; Zhang, M.-R.; Hu, K.; Li, Z. Peptide-Based Nanomaterials: Self-Assembly, Properties and Applications. Bioact. Mater. 2022, 11, 268–282. [Google Scholar] [CrossRef]
- Zanut, A.; Cian, A.; Cefarin, N.; Pozzato, A.; Tormen, M. Nanoelectrode Arrays Fabricated by Thermal Nanoimprint Lithography for Biosensing Application. Biosensors 2020, 10, 90. [Google Scholar] [CrossRef]
- Ito, Y.; Fukusaki, E. DNA as a ‘Nanomaterial’. J. Mol. Catal. B Enzym. 2004, 28, 155–166. [Google Scholar] [CrossRef]
- Niedzialkowski, P.; Slepski, P.; Wysocka, J.; Chamier-Cieminska, J.; Burczyk, L.; Sobaszek, M.; Wcislo, A.; Ossowski, T.; Bogdanowicz, R.; Ryl, J. Multisine Impedimetric Probing of Biocatalytic Reactions for Label-Free Detection of DEFB1 Gene: How to Verify That Your Dog Is Not Human? Sens. Actuators B Chem. 2020, 323, 128664. [Google Scholar] [CrossRef]
- Song, M.-J.; Kim, J.-H.; Lee, S.-K.; Lim, D.-S.; Hwang, S.W.; Whang, D. Analytical Characteristics of Electrochemical Biosensor Using Pt-Dispersed Graphene on Boron Doped Diamond Electrode. Electroanalysis 2011, 23, 2408–2414. [Google Scholar] [CrossRef]
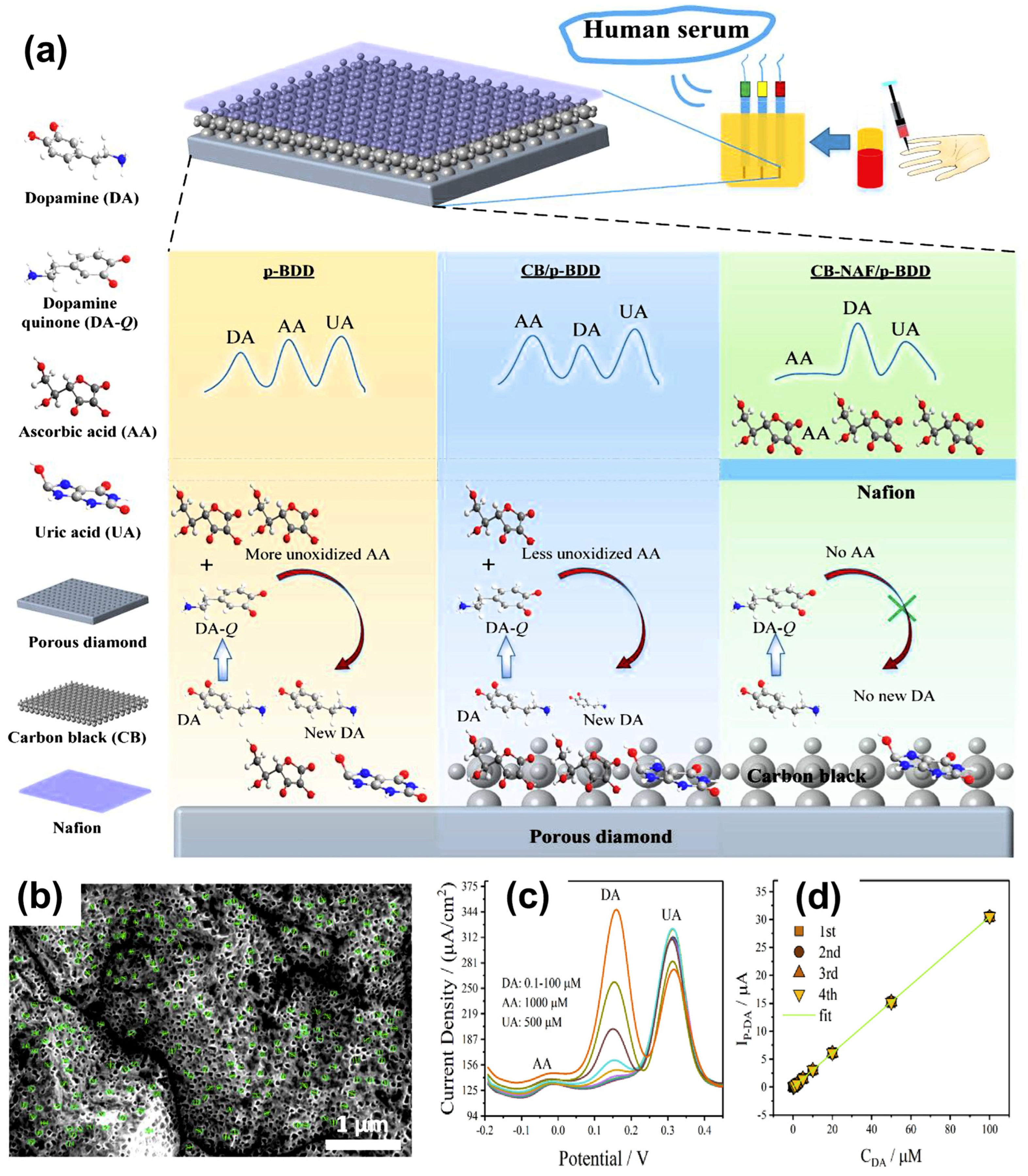
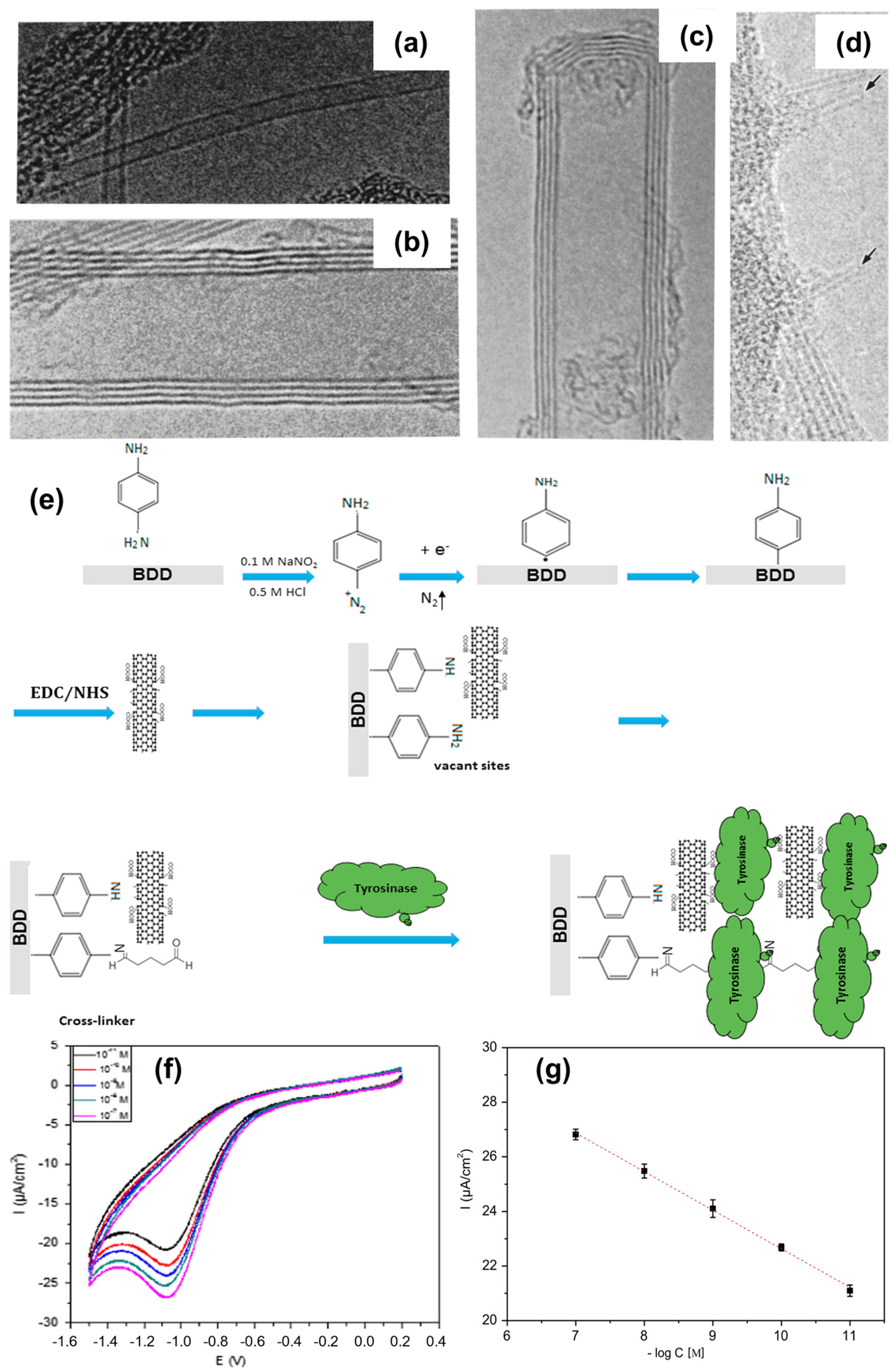
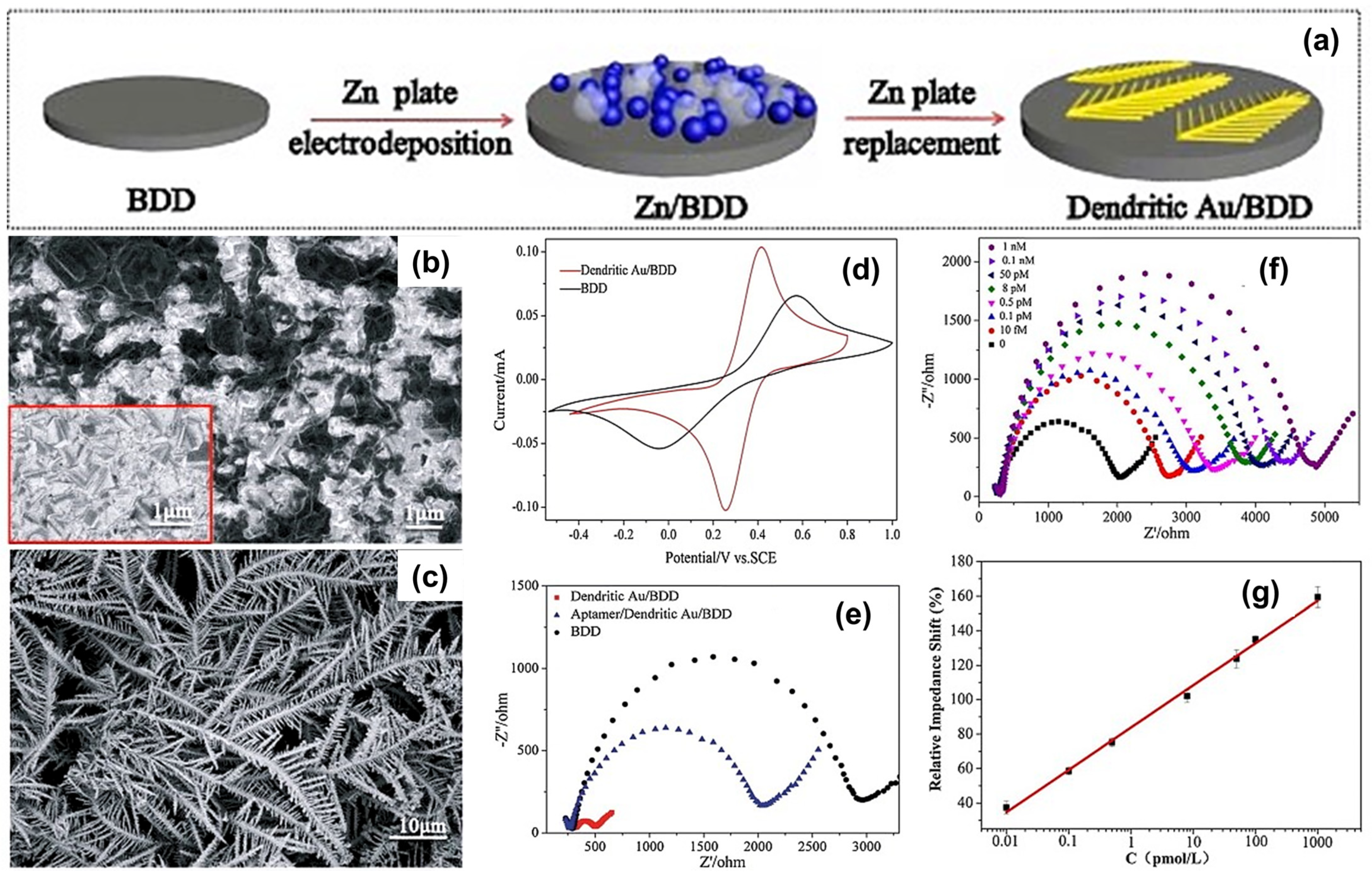
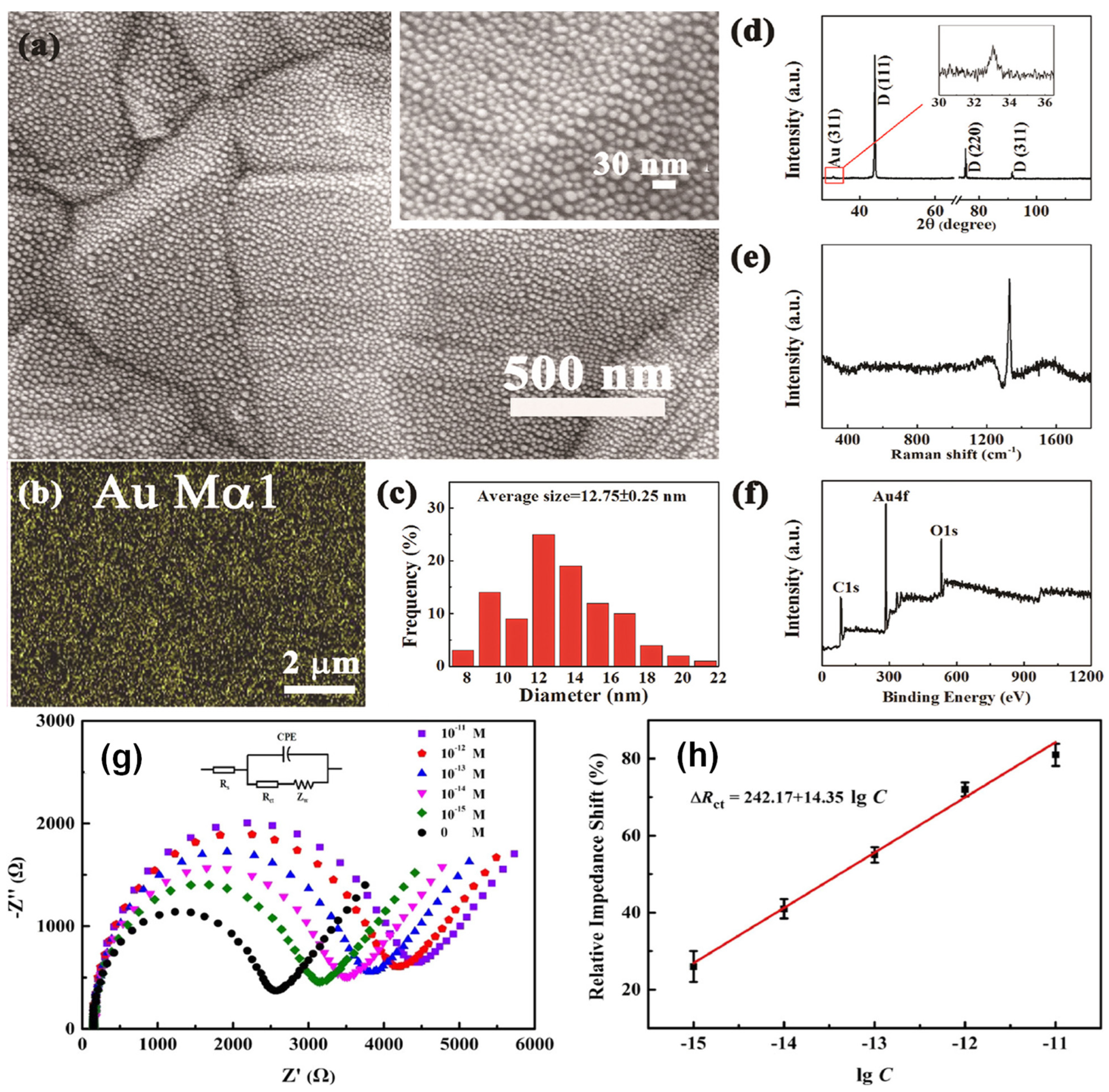
| Modification Strategy | Advantages | Disadvantages |
|---|---|---|
| Electrodeposition |
|
|
| Drop Casting |
|
|
| Electrophoretic Deposition (EPD) |
|
|
| Chemical Vapor Deposition (CVD) |
|
|
| Physical Vapor Deposition (PVD) |
|
|
| Nanomaterial Morphology | Modification Method | Enhanced Properties | Technique | Analyte | Linear Detection Range | Sensitivity | LOD | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Carbon | Carbon Black | Drop casting | Sensitivity, Selectivity, Resistance to Interference | Electrochemical | Dopamine | 1 to 100 μM | 0.305 μA μM−1 | 54 nM | [128] |
| NG Nanoparticles | CVD | Surface Area, Electron Transfer | DPV | Acetaminophen | 0.02–50 μM | -- | 5 nM | [94] | |
| MWCNTs | EPD | Surface Area, Electron Transfer | FSCV | Dopamine | 1 nM to 100 μM | 0.15 μA μM−1 | 1.78 nM | [88] | |
| MWCNTs | EPD | Sensitivity, Selectivity | DPV | Dopamine and serotonin | 1 nM to 100 μM | 6.75 µA µM−1 cm−2 | 5.4 nM | [89] | |
| MWCNT | Drop casting and then exposure to glutaraldehyde vapor (as a cross-linker) | Surface Area, Catalytic Activity | CV | BPA | 0.01 nM to 100 nM | 1.81 µA µM−1 cm−2 | 0.01 nM | [84] | |
| MWCNT | Patterned for selective growth on BDD | Surface Area, Electron Transfer | Amperometric | Glucose | 0.006–1.16 mM | 7.2 μA mM−1 cm−2 | 0.07 μM | [135] | |
| Porous N-doped carbon | Template-assisted synthesis, followed by drop casting on BDD | Conductivity, Catalytic Activity | Chronoamperometric | OPs | 100 pg·L−1 to 10.0 μg·L−1 | -- | 1.50 pg·L−1 | [83] | |
| Metal and metal oxide nanomaterials | Au Nps | Electrodeposition and wet chemical seeding | -- | Electrochemical | Acrylamide | 0.6 to 6 μM | 0.035 µA µM−1 cm−2 | 0.84 μM | [73] |
| Au NPs | In situ synthesis of Au NPs | Electron Transfer, Sensitivity | CV | Acrylamide | 0–30 µM | -- | 13.10 µM | [138] | |
| Au NPs | Sputtering | Sensitivity, Selectivity | EIS | Aflatoxin B1 | 1.0 × 10−13 to 1.0 × 10−8 mol L−1 | -- | 5.5 × 10−14 mol L−1 | [99] | |
| Au NPs | Sputtering | Electron Transfer, Sensitivity, Immobilization | EIS | PCB-77 | 1.0 × 10−15 to 1.0 × 10−11 M | -- | 0.32 fM | [102] | |
| Au NPs | Electrodeposition | Electron Transport, Immobilization | CV | Arylamide | 5–50 μM | -- | 5.14 μM | [74] | |
| Au Hierarchical dendritic microstructure | Double-template method | Surface Area, Electron Transfer | EIS | 17β-estradiol (E2) | 1.0 × 10−14 to 1.0 × 10−9 mol L−1 | -- | 5.0 × 10−15 mol L−1 | [136] | |
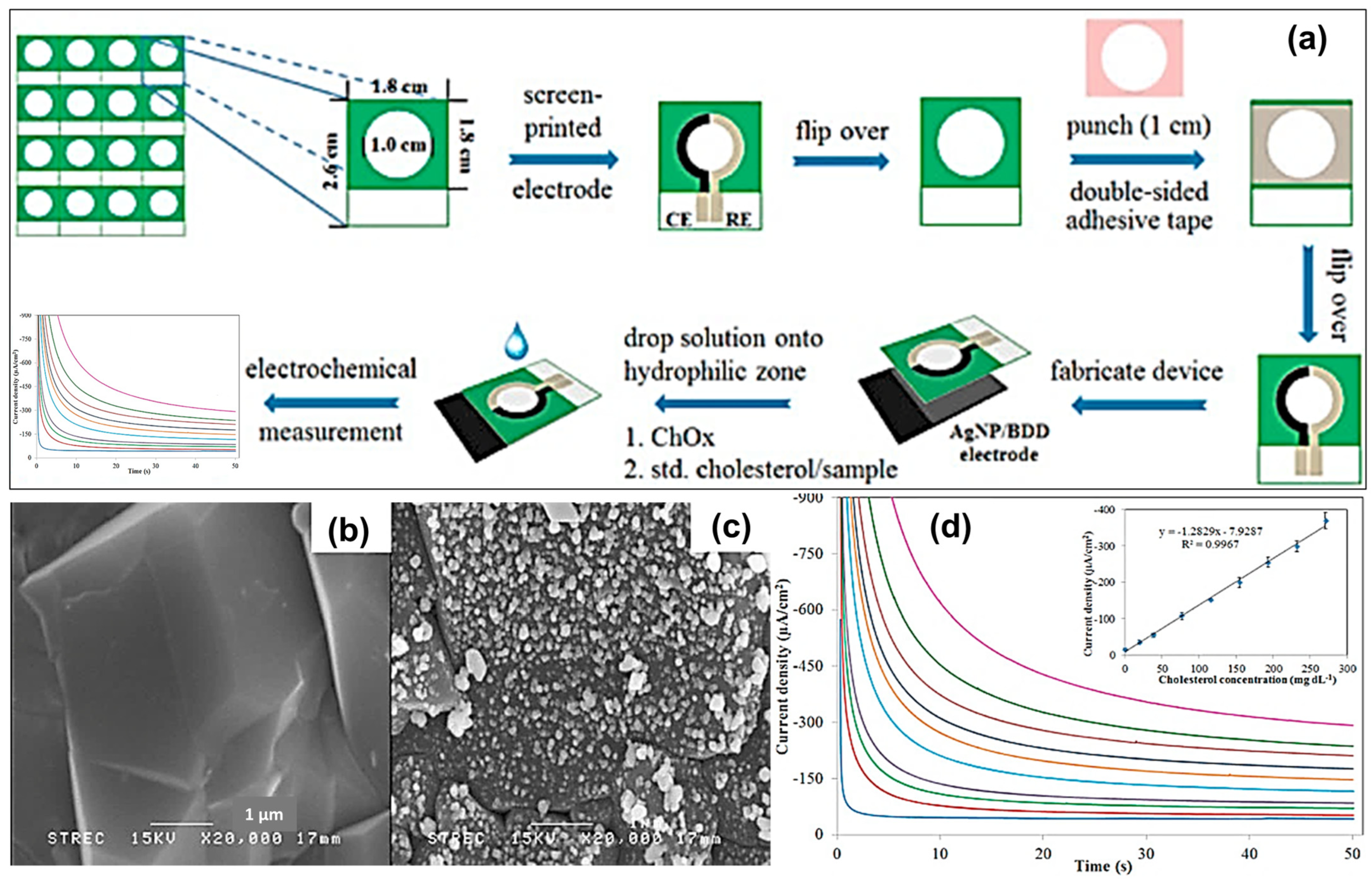
| Ag NPs | Electrodeposition | Electron Transfer | Amperometric | Cholesterol | 0.39 to 270.69 mg dL−1 | 49.61 µA µM−1 cm−2 | 0.25 mg dL−1 | [77] | |
| Pt NPs | CV | Electron Transfer, Selectivity | CV | H2O2 and melamine | 0.05–20 mM and 5–100 μg mL−1 | -- | 100 nM and 0.51 μg mL−1 | [139] | |
| Pt NPs | Wet chemical seeding followed by thermal annealing | Sensitivity, Selectivity | CV | Acrylamide | 0.01 to 0.1 nM | -- | 0.008 nM | [140] | |
| Ni nanoporous | Ultrasound-assisted electrodeposition | Electron Transport | Amperometric | L-alanine | 0.5 to 4.5 μM | 0.05 μA μM−1 cm−2 | 0.01 μM | [143] | |
| Pt NPs | Electrochemical deposition | Surface Area, Electron Transfer, Sensitivity | Amperometric | Glucose | 1.98 μM to 1.95 mM | 17.1 μA mM−1 | 0.14 μM | [163] | |
| Ni NPs | Sputtering | Surface Area, Sensitivity | Amperometric | Glucose | 9.9 μM to 5.64 mM | 839.30 μA mM−1 cm−2 | 1.23 μM | [101] | |
| PB NPs | Electrodeposition | Sensitivity, Selectivity | Electrochemical | H2O2 | 0.1 µM to 1 mM | ~0.14 A M−1 cm−2 | 100 nM | [81] | |
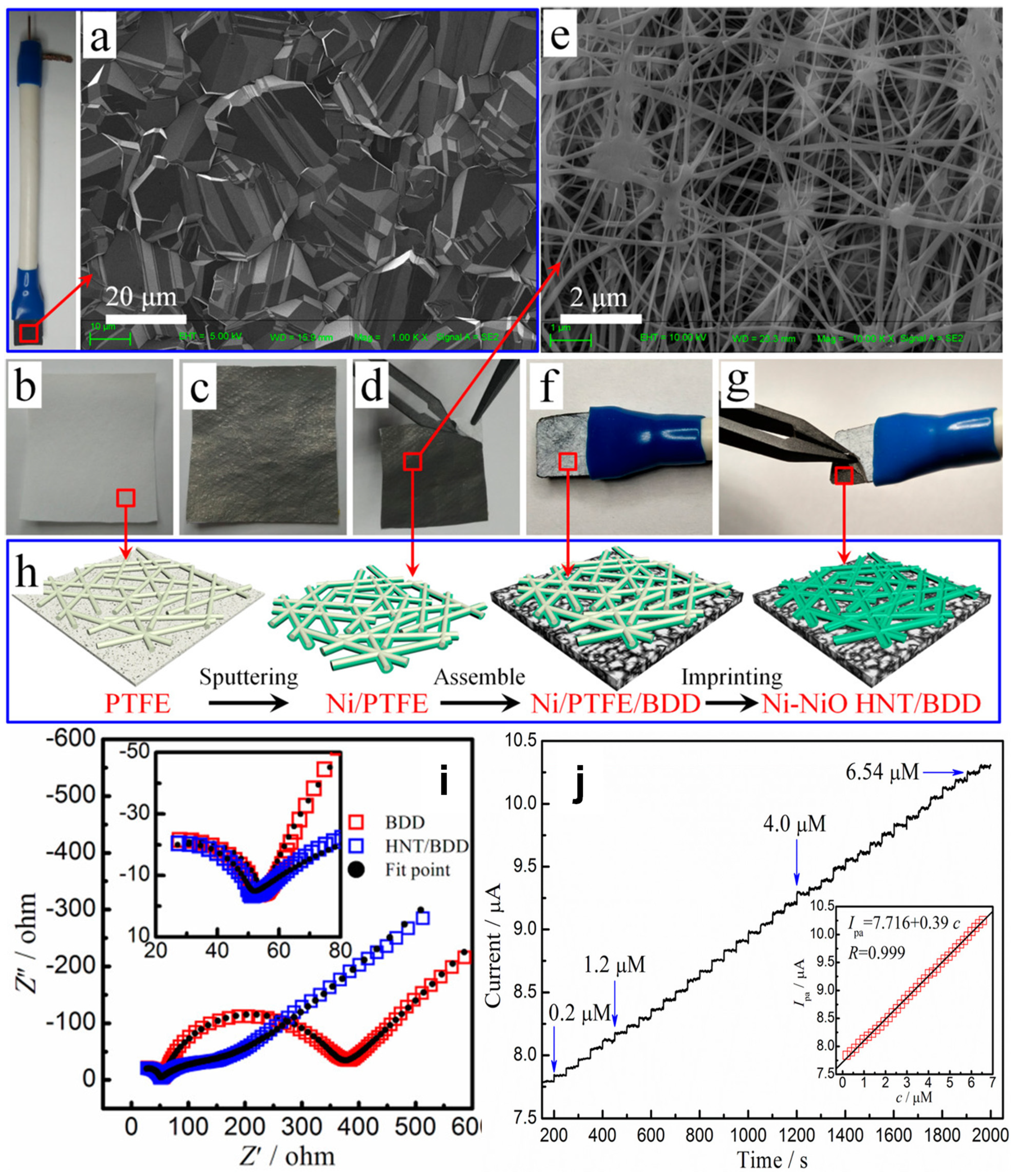
| Ni–NiO half-nanotube | Electrochemical imprinting | Surface Area, Electron Transport, Electrocatalytic Activity | Amperometric | L-serine | 0.2 to 6.54 μM | 0.33 μA μM−1 | 0.1 μM | [148] | |
| Nanocomposites, alloys, nanohybrids | Pt NPs | Wet chemical seeding followed by electrodeposition | Sensitivity, Selectivity | CV and chronoamperometry | H2O2 | 0.1 to 1.0 mM | 40.1 μA mM−1 cm−2 | 1.7 μM | [75] |
| Pd NPs | Wet chemical seeding followed by electrodeposition | Sensitivity, Selectivity | CV and chronoamperometry | H2O2 | 0.1 to 1.0 mM | 80.8 μA mM−1 cm−2 | 0.7 μM | [75] | |
| Au NP-embedded carbon sphere nanocomposite | Nanocomposite synthesized via the Stöber method and drop-cast on BDD | Surface Area, Electron Transfer | Electrochemical | Organophosphate pesticides (OPs) | 10−11 to 10−7 M and 10−12 to 10−6 M | -- | 1.29 × 10−13 M and 4.9 × 10−13 M | [85] | |
| Porous carbon _ Au NP nanocomposite | Drop casting followed by drying | Biocompatibility, Conductivity | Electrochemical | Organophosphate pesticides | 4.5 × 10−13 –4.5 × 10−9 M | -- | 2.99 × 10−13 M | [86] | |
| Platinum–carbon aerogel (Pt–CAs) Composite | Drop casting followed by drying | Surface Area, Conductivity, Sensitivity | Electrochemical | Organophosphorus pesticides | 10−11 to 10−6 M | -- | 3.1 × 10−13 M | [87] | |
| ZnAl nanosheets and Au NP assembly | Drop casting | Surface Area, Electrocatalytic Activity, Electron-Transfer Rate | SWV | Lactate | 0.1 to 30 μM | 13.9 μA mM−1 cm−2 | 0.1 μM | [82] | |
| GA2–ZnO nanocomposite | Drop casting—drying | Electrochemical | Metoprolol | 9.99 × 10−7 to 3.85 × 10−5 mol·L−1 | -- | 7.53 × 10−8 mol·L−1 | [156] | ||
| Cu@Au Core–shell | Allylamine as a binder | Electrochemical | Glucose and BOD | 0.1 to 0.5 mM | 0.017 mA mM−1 | -- | [97] | ||
| Cu@Au Core–shell | Allylamine as a binder | Electrochemical | Glucose and BOD | 1.9–50.0 mg L−1 | 17.4 μA mg−1 L | 1.9 mg L−1 | [97] | ||
| Au/Ni porous | Sputtering followed by heat treatment | Surface Area, Sensitivity | Amperometric | Glucose | 0.02—2.0 mM, and 2–9 mM | 157.5 μA mM−1 cm−2 | 2.60 μM | [100] | |
| C@Ni NPs | Sputtering | Electroactive Surface Area, Mass Transfer Rate | Amperometric | Glucose | 1.0 × 10−3–4.0 mM; and 4.0–10.0 mM | 1130, and 420 μA mM−1 cm−2 | 0.69 μM | [98] | |
| Ni + Nanodiamond NPs | EPD | Conductivity, Electron Transfer, Potential Window | Amperometric | Glucose | 0.2–12; 31.3–1055.4 μM | 120; 35.6 μA mM−1 cm−2 | 0.05 μM | [90] | |
| Graphene + cobalt NPs Nanostructures | Drop cast + drying | Surface Area, Immobilization, Electron Transfer | Electrochemical | Benzo(a)pyrene (BP) | 0.05–5.4 pM | -- | 0.01 pM | [131] | |
| Graphene + Cobalt NP nanostructures | Drop cast + drying | Surface Area, Immobilization, Electron Transfer | Electrochemical | dibenzo(a,e)pyrene (DBP) | 0.5–6.8 pM | -- | 0.12 pM | [131] | |
| Graphene + cobalt NPs nanostructures | Drop cast + drying | Surface Area, Immobilization, Electron Transfer | Electrochemical | indeno(1,2,3-cd)pyrene (IP) | 0.1–8 pM | -- | 0.23 pM | [131] | |
| Ni-microcrystalline graphite nano | Ni by sputtering, followed by thermally catalytic etching for MG | Surface Area, Electrochemical Stability, Electron Transfer | Amperometric | Glucose | 2.0 μM–0.5 mM; and 0.5–1 5.0 mM | 1010.8; and 660.8 μA mM−1 cm−2 | 0.24 μM | [103] | |
| Ni-Cu Alloy NPs | Sputtering | Electrochemical | Glucose | 0.022–18.3 mM | 1007.7 μA mM−1 cm−2 | 5.7 μM | [96] | ||
| Other nano-materials/-structures | Peptide | Click chemistry | Viral Binding and Charge-Transfer Resistance | EIS | Influenza viruses | 3 to 400 pfu | -- | 0.33 pfu | [158] |
| BDD nanorods | HF-CVD followed by Electrochemical oxidation | Surface Area, Electron Transfer | Amperometric | Glucose | 1.12 μM to 0.067 mM | 349.7 μA mM−1 cm−2 | 0.066 μM | [95] | |
| BDD nanocrystalline | Chemical vapor deposition | Electron Transfer, Surface Area, Sensitivity | EIS | Influenza | 50.0 to 650.0 fg mL−1 | -- | 5 × 10−14 g/mL | [157] | |
| DNA | Click chemistry | Selectivity, | EIS | DEFB1 gene | -- | -- | -- | [162] | |
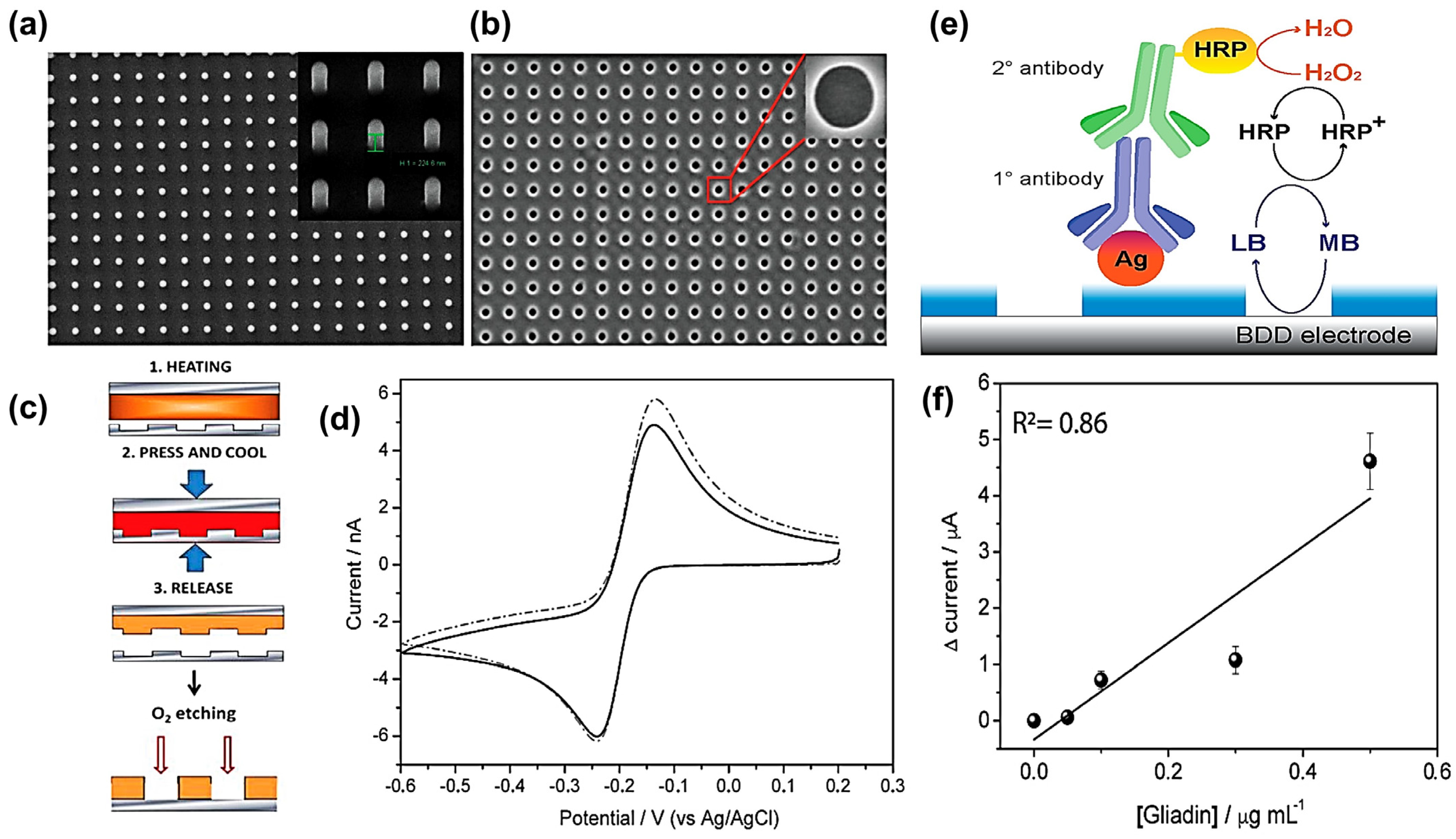
| BDD nanohole arrays | Thermal nanoimprint lithography | Surface Area, Immobilization | CV | Gliadin | 0.1 to 0.5 μg mL−1 | -- | 0.1 μg mL−1 | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, P.K.; Siegenthaler, J.R. Revolutionizing Electrochemical Sensing with Nanomaterial-Modified Boron-Doped Diamond Electrodes. Chemosensors 2025, 13, 183. https://doi.org/10.3390/chemosensors13050183
Gupta PK, Siegenthaler JR. Revolutionizing Electrochemical Sensing with Nanomaterial-Modified Boron-Doped Diamond Electrodes. Chemosensors. 2025; 13(5):183. https://doi.org/10.3390/chemosensors13050183
Chicago/Turabian StyleGupta, Pramod K., and James R. Siegenthaler. 2025. "Revolutionizing Electrochemical Sensing with Nanomaterial-Modified Boron-Doped Diamond Electrodes" Chemosensors 13, no. 5: 183. https://doi.org/10.3390/chemosensors13050183
APA StyleGupta, P. K., & Siegenthaler, J. R. (2025). Revolutionizing Electrochemical Sensing with Nanomaterial-Modified Boron-Doped Diamond Electrodes. Chemosensors, 13(5), 183. https://doi.org/10.3390/chemosensors13050183






