Contribution of Gas Chromatography—Mass Spectrometry (GC-MS) to the Volatile Organic Compound Profile of Vespa velutina nigrithorax Larvae
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Instrumentation
2.3. Larvae Treatment Procedure and GC-MS Analysis
2.4. Data Treatment
2.4.1. Volatile Organic Compound Identification
2.4.2. Univariate and Multivariate Analysis
3. Results and Discussion
3.1. Data Treatment
3.1.1. Volatile Organic Compound Identification
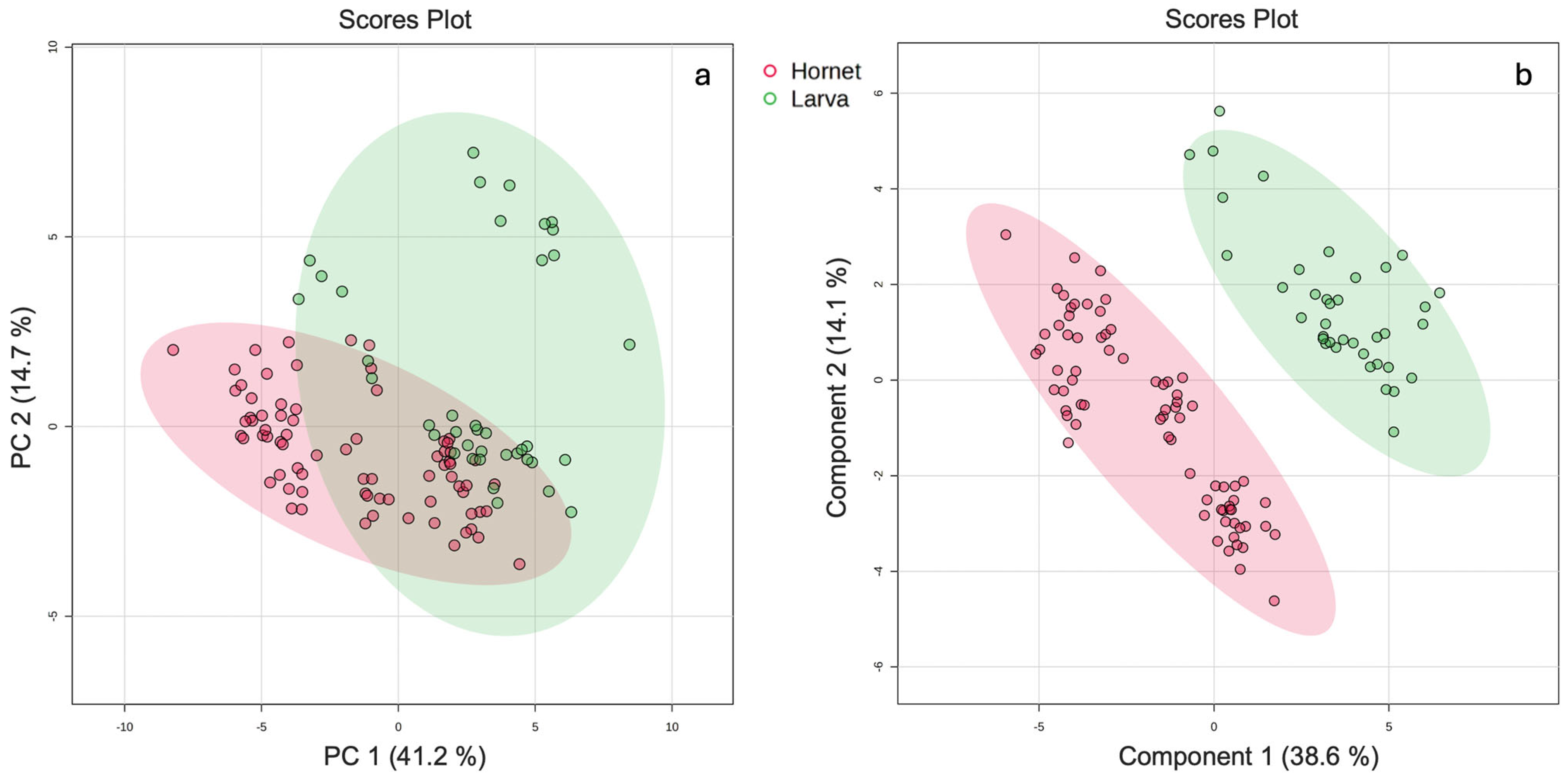
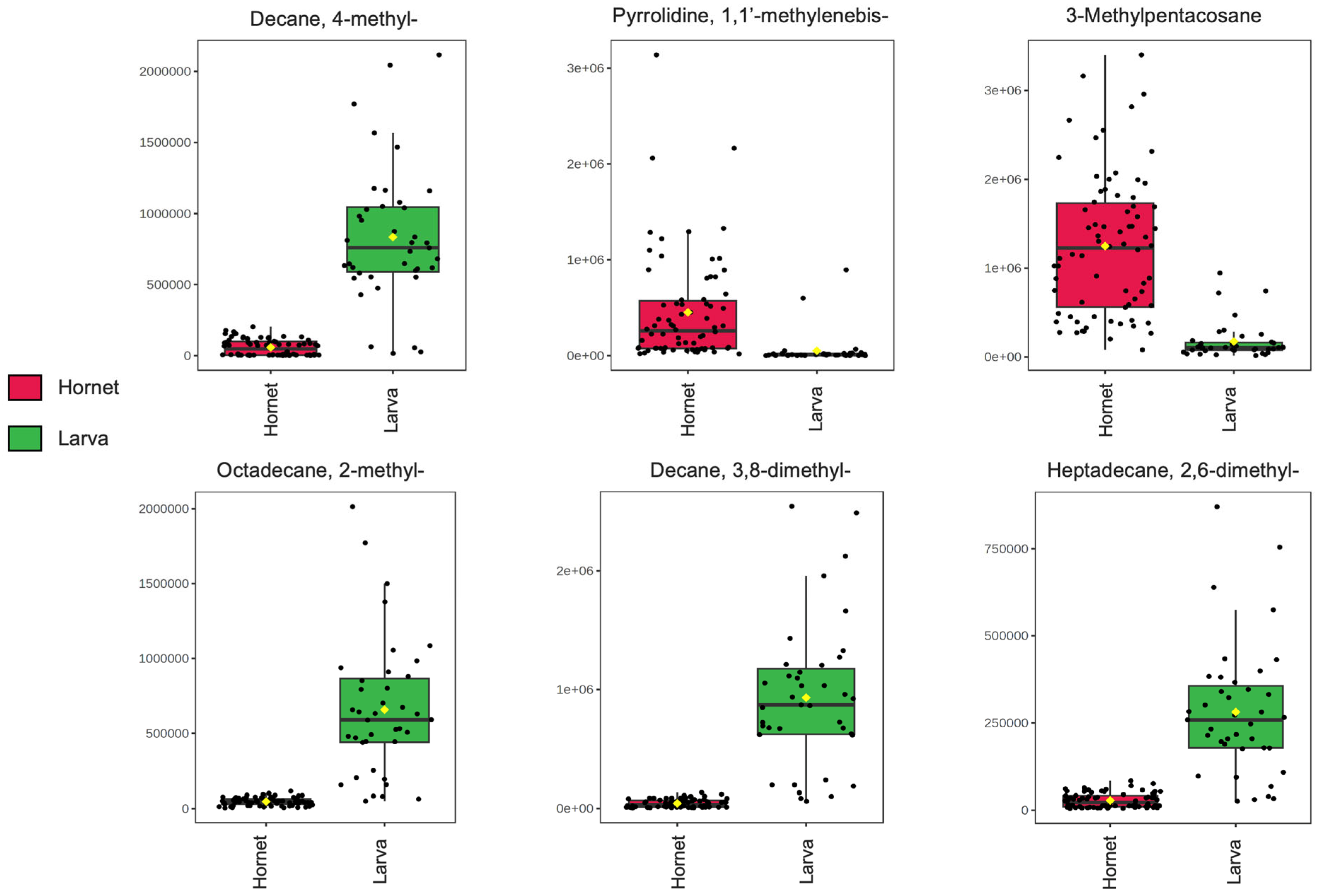
3.1.2. Univariate and Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leza, M.; Miranda, M.Á.; Colomar, V. First detection of Vespa velutina nigrithorax (Hymenoptera:Vespidae) in the balearic islands (Western Mediterranean): A challenging study case. Biol. Invasions 2018, 20, 1643–1649. [Google Scholar] [CrossRef]
- Laurino, D.; Lioy, S.; Carisio, L.; Manino, A.; Porporato, M. Vespa velutina: An alien driver of honey bee colony losses. Diversity 2020, 12, 5. [Google Scholar] [CrossRef]
- Castro, L.; Pagola-Carte, S. Vespa velutina Lepeletier, 1836 (Hymenoptera:Vespidae), recolectada en la Península Ibérica. Heteropterus Rev. Entomol. 2010, 10, 193–196. [Google Scholar]
- Chauzat, M.-P.; Martin, S. A foreigner in France: The Asian hornet. Biologist 2009, 2, 86–91. [Google Scholar]
- Leza, M.; Herrera, C.; Marques, A.; Roca, P.; Sastre-Serra, J.; Pons, D. The impact of the invasive species Vespa velutina on honeybees: A new approach based on oxidative stress. Sci. Total Environ. 2019, 689, 709–715. [Google Scholar] [CrossRef]
- Monceau, K.; Bonnard, O.; Thiéry, D. Vespa velutina: A new invasive predator of honeybees in Europe. J. Pest Sci. 2013, 87, 1–16. [Google Scholar] [CrossRef]
- Martin, S.J. The Asian Hornet: Threats, Biology & Expansion; IBRA and Northern Bee Books: Mytholmroyd, UK, 2017. [Google Scholar]
- Puebla, H.; Roy, P.K.; Velasco-Perez, A.; Gonzalez-Brambila, M.M. Biological pest control using a model-based robust feedback. IET Syst. Biol. 2018, 12, 233–240. [Google Scholar] [CrossRef]
- Edwards, E.; Toft, R.; Joice, N.; Westbrooke, I. The efficacy of Vespex® wasp bait to control Vespula species (Hymenoptera:Vespidae) in New Zealand. Int. J. Pest Manag. 2017, 63, 266–272. [Google Scholar] [CrossRef]
- Turchi, L.; Derijard, B. Options for the biological and physical control of Vespa velutina nigrithorax (Hym.: Vespidae) in Europe: A review. J. Appl. Entomol. 2018, 142, 553–562. [Google Scholar] [CrossRef]
- Ruiz-Cristi, I.; Berville, L.; Darrouzet, E. Characterizing thermal tolerance in the invasive yellow-legged hornet (Vespa velutina nigrithorax): The first step toward a green control method. PLoS ONE 2020, 15, e0239742. [Google Scholar] [CrossRef]
- Rojas-Nossa, S.V.; Dasilva-Martins, D.; Mato, S.; Bartolomé, C.; Maside, X.; Garrido, J. Effectiveness of electric harps in reducing Vespa velutina predation pressure and consequences for honey bee colony development. Pest Manag. Sci. 2022, 78, 5142–5149. [Google Scholar] [CrossRef]
- Zablotny, J.E. Encyclopedia of Insects. In Sociality; Elsevier: Amsterdam, The Netherlands, 2009; pp. 928–935. [Google Scholar] [CrossRef]
- Grüter, C.; Czaczkes, T.J. Communication in social insects and how it is shaped by individual experience. Anim. Behav. 2019, 151, 207–215. [Google Scholar] [CrossRef]
- Billen, J.; Morgan, E.D. Pheromone Communication in Social Insects: Sources and Secretions. In Pheromone Communication in Social Insects, 1st ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 3–33. [Google Scholar]
- Bruschini, C.; Cervo, R.; Stefano, T. Pheromones in Social Wasps. Vitam. Horm. 2010, 83, 447–492. [Google Scholar] [CrossRef]
- Karlson, P.; Lüscher, M.; L, M. Pheromones: A New Term for a Class of Biologically Active Substances. Nature 1959, 183, 55–56. [Google Scholar] [CrossRef]
- Billen, J. Signal variety and communication in social insects. Proc. Neth. Entomol. Soc. Meet. 2006, 17, 9–25. [Google Scholar]
- Lamprecht, I.; Schmolz, E.; Schricker, B. Pheromones in the life of insects. Eur. Biophys. J. 2008, 37, 1253–1260. [Google Scholar] [CrossRef]
- Singer, T.L. Roles of Hydrocarbons in the Recognition Systems of Insects. Amer Zool. 1998, 38, 394–405. [Google Scholar] [CrossRef]
- Rodríguez-Flores, M.S.; Falcão, S.I.; Escuredo, O.; Seijo, M.C.; Vilas-Boas, M. Chemical profile from the head of Vespa velutina and V. crabro. Apidologie 2021, 52, 548–560. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Pirk, C.W.W.; Crewe, R.M.; Njagi, P.G.N.; Gordon, I.; Torto, B. Nestmate recognition and the role of cuticular hydrocarbons in the African termite raiding ant Pachycondyla analis. J. Chem. Ecol. 2010, 36, 441–448. [Google Scholar] [CrossRef][Green Version]
- Steinmetz, I.; Schmolz, E.; Ruther, J. Cuticular lipids as trail pheromone in a social wasp. Proc. R. Soc. B Biol. Sci. 2003, 270, 385–391. [Google Scholar] [CrossRef]
- Cvačka, J.; Jiroš, P.; Šobotník, J.; Hanus, R.; Svatoš, A. Analysis of insect cuticular hydrocarbons using matrix-assisted laser desorption/ionization mass spectrometry. J. Chem. Ecol. 2006, 32, 409–434. [Google Scholar] [CrossRef]
- Espelie, K.E.; Gamboa, G.J.; Grudzien, T.A.; Bura, E.A. Cuticular hydrocarbons of the paper wasp Polistes Fuscatus: A search for recognition pheromones. J. Chem. Ecol. 1994, 20, 1677–1687. [Google Scholar] [CrossRef]
- Frederickx, C.; Dekeirsschieter, J.; Brostaux, Y.; Wathelet, J.-P.; Verheggen, F.; Haubruge, E. Volatile organic compounds released by blowfly larvae and pupae: New perspectives in forensic entomology. Forensic Sci. Int. 2012, 219, 215–220. [Google Scholar] [CrossRef]
- Ruther, J.; Sieben, S.; Schricker, B. Nestmate recognition in social wasps: Manipulation of hydrocarbon profiles induces aggression in the European hornet. Sci. Nat. 2002, 89, 111–114. [Google Scholar] [CrossRef]
- Rodríguez-Flores, M.S.; Falcão, S.I.; Escuredo, O.; Queijo, L.; Seijo, M.C.; Vilas-Boas, M. Assessment of the in vivo and in vitro release of chemical compounds from vespa velutina. Molecules 2021, 26, 6769. [Google Scholar] [CrossRef]
- Ghosh, S.; Namin, S.M.; Meyer-Rochow, V.B.; Jung, C. Chemical composition and nutritional value of different species of Vespa hornets. Foods 2021, 10, 418. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, J.M.; Kim, B.; Nam, J.-O.; Hahn, D.; Choi, M.B. Nutritional Value of the Larvae of the Alien Invasive Wasp Vespa velutina nigrithorax and Amino Acid Composition of the Larval Saliva. Foods 2020, 9, 885. [Google Scholar] [CrossRef]
- de la Hera, O.; Alonso, R.M. Differentiation of Vespa velutina nigrithorax Colonies Using Volatile Organic Compound Profiles of Hornets and Nests. Insects 2024, 15, 811. [Google Scholar] [CrossRef]
- Quintanilla-Casas, B.; Bro, R.; Hinrich, J.L.; Davie-Martin, C.L. Tutorial on PARADISe: PARAFAC2-based Deconvolution and Identification System for processing GC–MS data. In Protoc. Exch.; 2023. [Google Scholar] [CrossRef]
- Makhuvele, R.; Gbashi, S.; Njobeh, P.B. GC-HRTOF-MS metabolite profiling and antioxidant activity of methanolic extracts of Tulbaghia violacea Harv. J. King Saud Univ. Sci. 2022, 34, 102278. [Google Scholar] [CrossRef]
- Hernández-Estrada, M.G.; De Muñoz, F.L.; Hurtado-Sil, G.; Lanz-Mendoza, H.; Rodríguez, M.H.; Hernández-Hernández, F.C. Regulación por Dexametasona y Ácido Araquidónico sobre la expresión de una Aldoceto Reductasa/Di-hidrodiol Deshi-drogenasa (DDH) en estómagos del mosquito Aedes aegypti, principal vector del dengue en México. Investig. Univ. Multidiscip. Rev. Investig. Univ. Simón Bolívar 2007, 6, 2. [Google Scholar]
- Rodrigues, D.P.; Ameixa, O.M.C.C.; Vázquez, J.A.; Calado, R. Improving the Lipid Profile of Black Soldier Fly (Hermetia illucens) Larvae for Marine Aquafeeds: Current State of Knowledge. Sustainability 2022, 14, 6472. [Google Scholar] [CrossRef]
- Gupta, V.; Tyagi, S.; Tripathi, R. Hexadecanoic acid methyl ester, a potent hepatoprotective compound in leaves of Pistia stratiotes L. Appl. Biol. Chem. J. 2023, 4, 118–120. [Google Scholar] [CrossRef]
- Baeshen, R.S.; Baz, M.M. Efficacy of Acacia nilotica, Eucalyptus camaldulensis, and Salix safsafs on the mortality and development of two vector-borne mosquito species, Culex pipiens and Aedes aegypti, in the laboratory and field. Heliyon 2023, 9, e16378. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, S.-T.; Wu, X.-M.; Pang, X.-Q.; Ni, L.-L.; Yuan, S.-M.; Yang, Z.-B.; Li, Y.-H.; Xiao, H. GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro. Open Chem. 2022, 20, 602–610. [Google Scholar] [CrossRef]
- Schulz, S.; Francke, W.; Edgar, J.; Schneider, D. Volatile Compounds from Androconial Organs of Danaine and Ithomiine Butterflies. Z. Für Naturforschung 1988, 43, 99–104. [Google Scholar] [CrossRef]
- Esteves, F.G.; dos Santos-Pinto, J.R.A.; Saidemberg, D.M.; Palma, M.S. Using a proteometabolomic approach to investigate the role of Dufour’s gland in pheromone biosynthesis in the social wasp Polybia paulista. J. Proteom. 2017, 151, 122–130. [Google Scholar] [CrossRef]
- Oladele, J.O.; Adewole, T.S.; Ogundepo, G.E.; Oyeleke, O.M.; Kuku, A. Efficacy of selected Nigerian tropical plants in the treatment of COVID-19: In silico and in vitro investigations. Environ. Sci. Pollut. Res. 2022, 29, 89295–89339. [Google Scholar] [CrossRef]
- Butts, D.P.; Camann, M.A.; Espelie, K.E. Workers and queens of the European hornet Vespa crabro L. have colony-specific cuticular hydrocarbon profiles (Hymenoptera:Vespidae). Insectes Sociaux 1995, 42, 45–55. [Google Scholar] [CrossRef]
- Cecotti, R.; Carpana, E.; Falchero, L.; Paoletti, R.; Tava, A. Determination of the volatile fraction of Polygonum bistorta L. at different growing stages and evaluation of its antimicrobial activity against two major honeybee (Apis mellifera) pathogens. Chem. Biodivers. 2012, 9, 359–369. [Google Scholar] [CrossRef]
- Yarazari, S.B.; Jayaraj, M. GC–MS Analysis of Bioactive Compounds of Flower Extracts of Calycopteris floribunda Lam.: A Multi Potent Medicinal Plant. Appl. Biochem. Biotechnol. 2022, 194, 5083–5099. [Google Scholar] [CrossRef]
- Mitra, A.; Gadagkar, R. The Dufour’s gland and the cuticle in the social wasp Ropalidia marginata contain the same hydrocarbons in similar proportions. J. Insect Sci. 2014, 14, 1–18. [Google Scholar] [CrossRef]
- Abdalla, F.C.; Jones, G.R.; Morgan, D.; da Cruz-Landim, C. Chemical Composition of the Dufour Gland Secretion in Queens of Melipona bicolor (Hymenoptera, Meliponini). J. Braz. Chem. Soc. 2004, 15, 621–625. [Google Scholar] [CrossRef]
- Löfqvist, J.; Bergström, G. Volatile communication substances in dufour’s gland of virgin females and old queens of the ant Formica polyctena. J. Chem. Ecol. 1980, 6, 309–320. [Google Scholar] [CrossRef]
- Gévar, J.; Bagnères, A.-G.; Christidès, J.-P.; Darrouzet, E. Chemical Heterogeneity in Inbred European Population of the Invasive Hornet Vespa velutina nigrithorax. J. Chem. Ecol. 2017, 43, 763–777. [Google Scholar] [CrossRef]
- Fanokh Al-Owaidi, M.; Naser, E.H.; Ahmed Abed, S.; Abo Khthr, M.F.; Abed, S.A. Cardaria drabra L.: A review of Phytochemistry and Therapeutic Uses Phytochemistry and Therapeutic Uses of Cardaria draba L.: A Review. Plant Arch. 2019, 19, 118–125. [Google Scholar]
- Jurenka, R.A.; Subchev, M. Identification of Cuticular Hydrocarbons and the Alkene Precursor to the Pheromone in Hemolymph of the Female Gypsy Moth, Lymantria dispar. Arch. Insect Biochem. Physiol. 2000, 43, 108–115. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, M.; Tang, L.; Ma, R.; He, H. Chemical Components of Dufour’s and Venom Glands in Camponotus japonicus (Hymenoptera, Formicidae). Insects 2023, 14, 664. [Google Scholar] [CrossRef]
- Seenivasan, N. Phytochemical profiling of burrowing nematode (Radopholus similis) resistant and susceptible banana (Musa spp.) genotypes for detection of marker compounds. Fruits 2018, 73, 48–59. [Google Scholar] [CrossRef]

| Parameter | Conditions | |
|---|---|---|
| GC | Carrier gas Column Injection temperature | Helium 1 mL/min (constant flow) HP-5MS UI (30 m × 0.25 mm ID × 0.25 µm) 270 °C |
| Temperature program | Initial temp.: 50 °C for 1 min Ramp: 10 °C/min to 150 °C; 5 °C/min to 250 °C; 15 °C/min to 300 °C; and hold 2 min | |
| Scan time | 36.3 min | |
| MS | Mode | SCAN |
| m/z range | 40 to 400 | |
| Detector temperature | 300 °C |
| AcMeOH Solvent Compound Name | RT (min) | MF (%) | RMF (%) | Mean Area Larvae | Mean Area Hornets |
|---|---|---|---|---|---|
| Pyrrole, 1-methyl-3-(1,1-dimethylethyl)- | 5.781 | 78.3 | 84.9 | 493655 | 3678596 |
| 1-Butanamine, 2-methyl-N-(2-methylbutylidene)- | 6.757 | 79.1 | 85.7 | 152517 | 529451 |
| Pyrrolidine, 1,1′-methylenebis- | 6.766 | 78.2 | 86.4 | 53285 | 449479 |
| Phorone | 7.937 | 90.4 | 91.8 | 40058 | 658607 |
| Piperidine, 1-(1-methylpentyl)- | 9.806 | 74.1 | 74.5 | 322355 | 2237919 |
| Dodecanoic acid | 14.792 | 94.2 | 94.2 | 357096 | 251819 |
| Methyl tetradecanoate | 17.376 | 95.0 | 95.2 | 1583267 | 513351 |
| Tetradecanoic acid | 18.285 | 95.8 | 95.8 | 716012 | 323360 |
| 7-Hexadecenoic acid, methyl ester, (Z)- | 20.657 | 87.7 | 87.7 | 979162 | 1210963 |
| 9-Hexadecenoic acid, methyl ester, (Z)- | 20.682 | 91.4 | 91.5 | 884859 | 293279 |
| Heptadecanoic acid, methyl ester | 22.826 | 86.5 | 88.3 | 102037 | 86182 |
| 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 24.082 | 93.5 | 93.5 | 2014274 | 3043294 |
| 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)- | 24.294 | 91.1 | 91.3 | 3599951 | 5785947 |
| Methyl stearate | 24.670 | 94.0 | 94.5 | 3065267 | 3590694 |
| 5,8,11,14-Eicosatetraenoic acid, methyl ester, (all-Z)- | 26.928 | 90.4 | 91.9 | 231248 | 110396 |
| 5,8,11,14,17-Eicosapentaenoic acid, methyl ester, (all-Z)- | 27.038 | 92.8 | 93.0 | 382048 | 354834 |
| Octadecanamide | 29.042 | 82.0 | 84.1 | 109889 | 701726 |
| 13-Methylheptacosane | 34.814 | 84.3 | 90.3 | 308667 | 1854433 |
| Hexane Solvent Compound Name | RT (min) | MF (%) | RMF (%) | Mean Area Larvae | Mean Area Hornets |
|---|---|---|---|---|---|
| 2-Methyl-Z-4-tetradecene | 8.246 | 81.1 | 86.7 | 274879 | 278847 |
| Decane, 4-methyl- | 8.804 | 83.6 | 87.5 | 793814 | 56198 |
| Undecane, 3-methyl- | 9.527 | 82.3 | 84.2 | 158953 | 148607 |
| Dodecane, 4,6-dimethyl- | 9.844 | 89.6 | 89.7 | 812669 | 260598 |
| Hexadecane | 10.047 | 87.7 | 88.8 | 338080 | 129642 |
| n-Tridecan-1-ol | 10.952 | 82.0 | 83.3 | 1093270 | 297892 |
| Decane, 3,8-dimethyl- | 11.172 | 85.3 | 85.7 | 890689 | 42138 |
| Dodecane, 2,6,10-trimethyl- | 11.248 | 87.0 | 89.4 | 138501 | 287630 |
| Dodecane, 2,2,11,11-tetramethyl- | 11.629 | 84.2 | 85.0 | 226335 | 82900 |
| Dodecanoic acid, methyl ester | 13.933 | 94.7 | 95.0 | 758464 | 54628 |
| Heptadecane, 2,6,10,15-tetramethyl- | 16.517 | 85.2 | 85.5 | 194241 | 198293 |
| Heptadecane, 2,6-dimethyl- | 16.572 | 87.7 | 87.8 | 271108 | 27570 |
| Hexadecane, 2,6,10,14-tetramethyl- | 16.678 | 86.9 | 87.6 | 429236 | 155111 |
| Octadecane, 2-methyl- | 17.904 | 87.8 | 88.8 | 632384 | 45706 |
| 1-Decanol, 2-octyl- | 18.323 | 82.5 | 82.8 | 699145 | 655943 |
| Octyl tetracosyl ether | 26.615 | 80.2 | 82.8 | 292619 | 368618 |
| Heneicosane | 30.776 | 93.6 | 94.4 | 819736 | 124873 |
| 3-Methylpentacosane | 31.812 | 88.0 | 89.7 | 176353 | 1233468 |
| Hexacosane | 33.144 | 93.0 | 93.2 | 1625684 | 1617645 |
| 13-Methylheptacosane | 33.410 | 86.0 | 87.9 | 488967 | 453312 |
| Tetratetracontane | 34.421 | 83.4 | 85.3 | 203336 | 350114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Hera, O.; Alonso, R.M. Contribution of Gas Chromatography—Mass Spectrometry (GC-MS) to the Volatile Organic Compound Profile of Vespa velutina nigrithorax Larvae. Chemosensors 2025, 13, 175. https://doi.org/10.3390/chemosensors13050175
de la Hera O, Alonso RM. Contribution of Gas Chromatography—Mass Spectrometry (GC-MS) to the Volatile Organic Compound Profile of Vespa velutina nigrithorax Larvae. Chemosensors. 2025; 13(5):175. https://doi.org/10.3390/chemosensors13050175
Chicago/Turabian Stylede la Hera, Omaira, and Rosa María Alonso. 2025. "Contribution of Gas Chromatography—Mass Spectrometry (GC-MS) to the Volatile Organic Compound Profile of Vespa velutina nigrithorax Larvae" Chemosensors 13, no. 5: 175. https://doi.org/10.3390/chemosensors13050175
APA Stylede la Hera, O., & Alonso, R. M. (2025). Contribution of Gas Chromatography—Mass Spectrometry (GC-MS) to the Volatile Organic Compound Profile of Vespa velutina nigrithorax Larvae. Chemosensors, 13(5), 175. https://doi.org/10.3390/chemosensors13050175





