Abstract
Vespa velutina is an invasive species introduced by accident into Europe. Since its entry, it has spread rapidly and become a threat to biodiversity and beekeeping. Chemical communication between hornets is one of the main reasons for the proper functioning of hornet colonies. These signals can be from endogenous and/or exogenous chemical compounds. In this work, the volatile organic compound profile of Vespa velutina larvae was obtained by GC-MS previous solvent extraction procedures. A total of 99 volatile compounds were identified in the larvae volatile profile, of which 33 were common to adult hornets, suggesting a possible endogenous origin and a functional role in physiological processes and chemical communication within the colony. A total of 42 compounds were detected exclusively in the larvae, belonging to aldehydes, alcohols, alkanes, alkenes, amines, ketones, piperidines, pyrrolidines, furanones, fatty acid esters, ethers, and pyridines chemical families. The detection of these compounds in larval stages, before environmental exposure, supports the hypothesis of their endogenous origin. The findings of this work can contribute to widening the knowledge of the biology and chemical composition of V. velutina and could help identify potential semiochemicals useful for the design of more selective and sustainable strategies for its control.
1. Introduction
The invasive species Vespa velutina nigrithorax (Lepeletier, 1836) (V. velutina), also known as the yellow-legged hornet, has become an ecological, economic, and health problem in many regions, mainly in Europe, where it has expanded rapidly since its accidental entry in goods from Asia [1,2,3]. This hymenopteran is a predator of local insects, especially honeybees, causes serious damage to beekeeping production, and affects the biodiversity of local ecosystems [4,5,6,7]. Given the problems caused since its arrival, various strategies have been developed to control it, from traps with attractants to capture nests to the application of biocides to eliminate the nest [8,9,10,11,12]. However, expanding knowledge about its biology, especially communication between individuals in a colony, remains a key challenge for the design of more effective and selective control methods.
Communication between social insects, such as the V. velutina, plays a fundamental role in the proper functioning of the colony [13,14]. This allows them to control interactions such as the recognition of individuals in the colony who are not members, defense, alarm, or the attraction of conspecifics in mating seasons, among other things [15,16]. Interactions between insects can be carried out by means of different types of messages, such as acoustic, vibrational, visual, and tactile messages, or by means of chemical signals (pheromones) [17,18,19]. The latter is one of the most widely used types of communication and is based on the secretion/release of different compounds or groups of chemical compounds by the different glands that hornets have (mouth, legs, abdomen, antennae, etc.) [18,19].
Hydrocarbons (HCs) are used as alarm or recognition signals between members of the colony and non-members of the colony [20,21]. When hydrocarbons are on the cuticle, they are denominated as cuticular hydrocarbons (CHCs), and their main function is to prevent insects from desiccation, although they are also used for recognition [22,23,24,25]. Most compounds present in the cuticle are endogenous, some of which are produced during the larval stage and persist into the adult phase, while others change chemically throughout the insect’s development [26]. These endogenous compounds can be transferred by contact between workers, as well as from workers to the nest during its construction. Nevertheless, it is possible that exogenous compounds can be absorbed by the cuticle from the nest building material or food, thus providing a characteristic odor to the colony [21,23,27].
Compounds exclusive to hornets may represent chemical signals specific to the species that are essential for its communication and social organization [28]. In the specific case of V. velutina, the differentiation between exogenous and endogenous compounds could be important both for a better understanding of its biology and for the development of more selective attractant control methods for this species.
The profile of volatile organic compounds (VOCs) in V. velutina larvae and its possible involvement in chemical communication or in other functional aspects of colony development has not been studied to date. However, general characterization of the composition of V. velutina larvae and their saliva, focusing on the analysis of their nutritional value, has been reported. [29,30]. This lack of information highlights the relevance of the present study, which provides new data on the larval chemistry of this invasive species.
In a previous study carried out by this research group, volatile organic compounds present in hornets and in the outer covering of the nests of V. velutina were identified [31]. Therefore, with the aim of expanding knowledge about the differences between exogenous and endogenous compounds in V. velutina, in this work, the profile of volatile organic compounds (VOCs) of the larvae of this species was obtained. For this purpose, gas chromatography coupled with mass spectrometry (GC-MS) was selected as the analytical technique.
The analysis of V. velutina larvae before the individuals are exposed to external environmental factors could offer a more precise approach to identifying potentially endogenous compounds. The volatile compounds detected in these larvae could contribute to a better understanding of the chemical profile of this species. Furthermore, comparing these compounds with those previously identified in adult hornets and the nest structures would allow us to differentiate between compounds that originate intrinsically in the insect and those acquired from the environment, providing relevant information on chemical communication and the development of V. velutina.
An initial homogenization step of the V. velutina larva, before the two extraction methods with hexane and a mixture of acetone/methanol (50:50 v/v), was applied. After that, extracts were injected into the GC-MS system. The detected volatile compounds were identified based on their mass spectra, using spectral databases. Univariate and multivariate analyses were applied to the VOCs identified as common in larvae and hornets of V. velutina. Thereafter, obtained data were compared with volatile compounds previously identified in adult hornets and in the nest structure, with the aim of identifying those compounds shared by hornets and larvae. At the same time, those compounds existing exclusively in larvae were also considered.
2. Materials and Methods
2.1. Sample Collection
Several secondary nests from different locations in Basque Country (Spain) were collected and supplied by nest removal specialized personnel from Basalan (Biscay Provincial Council, Spain). These nests were supplied in plastic boxes with holes in their lids that allowed the hornets to breathe. In the laboratory, to manipulate these nests safely the hornets were asleep, with diethyl ether (99.7%) (Panreac Applichem, Barcelona, Spain) as an anaesthetic. Next, hornets and the external covers of their nests were removed, and the combs that contained larvae were separated in plastic boxes and frozen (−20 °C) until their analysis by GC-MS. A total of 86 larvae of Vespa velutina were extracted from these nests.
2.2. Instrumentation
A gas chromatography system, the Agilent 6890N Network (Santa Clara, CA, USA), coupled to an autosampler, a CTC-PAL 120 (Zwingen, Switzerland), was employed. An HP-5MS UI column (30 m × 0.25 mm ID × 0.25 µm) from Agilent Technologies was used for chromatographic separation. An Agilent 5973-N mass spectrometric detector (Santa Clara, CA, USA) coupled to the chromatographic system was used. An analytical balance (precision 0.0001 g), a Sartorius CP224S (Madrid, Spain), was used to weigh samples.
2.3. Larvae Treatment Procedure and GC-MS Analysis
Each weighed whole larva (568 mg) was placed in a 2 mL vial containing seven zirconium balls (3 mm in diameter) and 1 mL of corresponding extraction solvent (hexane or acetone-to-methanol 50:50) was added. These mixtures were homogenised using a Tissuelyser Precellys 24 homogenizer from Bertin Instruments (Montigny-le-Bretonneus, France) at 4000 rpm for 1 min (3 cycles of 20 s, with 30 s of rest). Each mixture obtained was measured to a final volume of 3 mL and centrifuged (5000 rpm, 10 min), and then the supernatant was transferred to test tubes. The extract was dried at 40 °C with a nitrogen gas stream. Finally, the residue was reconstituted in 1 mL of the corresponding extraction solvent, filtered (0.22 µm), transferred to a 2 mL vial, and injected into GC-MS. Blank samples were prepared following the same extraction procedures as used for larva samples. Table 1 shows the GC-MS analysis conditions.

Table 1.
GC-MS analysis conditions for the extraction of volatile organic compounds in Vespa velutina hornets and nests’ external covers.
HPLC-grade solvents hexane (Scharlau, Sentmenat, Spain) and a 50:50 (v/v) mixture of acetone (Merck, Darmstadt, Germany) and methanol (Scharlau, Sentmenat, Spain) (AcMeOH) were used for non-polar and polar extraction, respectively. These solvents were considered as blank for the GC-MS system.
2.4. Data Treatment
2.4.1. Volatile Organic Compound Identification
Volatile organic compounds (VOCs) in V. velutina larvae were identified using PARADISe software (6.0.1) developed by the University of Copenhagen [32]. This tool, based on PARAllel FACtor 2 (PARAFAC2) analysis, was used for GC-MS data alignment, deconvolution, and identification (performed with the NIST14 library database from Agilent Technologies). Only those compounds that exceeded a 70% match factor with the NIST14 library were considered.
2.4.2. Univariate and Multivariate Analysis
The area of the chromatographic peaks of the total compounds identified in V. velutina larvae by GC-MS were used for multivariate analysis. Before analysis, to avoid bias in different samples, obtained data were normalized by weight. Next, to stabilize the variance, the areas obtained were transformed into logarithms to base 10 (log 10) and scaled using autoscaling, a method that centers data around zero and divides them by the standard deviation, facilitating a comparison between variables and the interpretation of results in different multivariate analyses.
With the aim of evaluating the existence of possible groupings between these data, a multiblock modelling of principal component analysis (MB-PCA) was applied to the volatile organic compounds obtained by GC-MS common to larvae and hornets of V. velutina using different extraction solvents. Multiblock modelling of partial least squares-discriminant analysis (MB-PLS-DA) was also applied. In addition, a volcano plot was constructed to observe significant features identified in the larvae and in adult hornets. For multivariate analysis, the analysis software MetaboAnalyst 6.0 was used (https://www.metaboanalyst.ca/, accessed on 22 March 2025).
The VOCs identified in the hornets were obtained in a previous study in which both V. velutina hornets and the external covers of their nests were analyzed by GC-MS using two extraction procedures with hexane and acetone/methanol, respectively [31].
3. Results and Discussion
3.1. Data Treatment
3.1.1. Volatile Organic Compound Identification
Areas of chromatographic peaks of each of the VOCs identified in larva samples were compared with areas obtained in the blank. Those compounds that showed a value area in the blank greater than 10% were eliminated, leaving a total of 99 compounds (56 in AcMeOH and 45 in hexane), with two of them in common in both extraction solvents. The most abundant families of compounds were fatty acid esters (20), ketones (5), piperidines (5), amines (4), and pyrrolidines (4) for the AcMeOH extraction, whereas in the hexane extraction they were mainly alkanes (28), alkenes (6), and alcohols (4).
Compounds identified in the larvae in both extractants were compared with the VOCs previously identified in hornets and in the outer covers of Vespa velutina nests [31]. Of the total number of compounds identified, 33 were in common between the larvae and the V. velutina hornets for both extractants, and were not found in the nests. Table 2 and Table 3 show the volatile organic compounds identified in common in the larvae and Vespa velutina hornets for both extractant solvents, respectively. Those compounds in italics were reported in the literature in plant species, and those in bold in different insect species [22,26,31,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52].

Table 2.
Volatile organic compounds identified in both Vespa velutina larvae and hornets after extraction with AcMeOH, retention time (RT), match factor (MF), reverse match factor (RMF), and mean normalized areas.

Table 3.
Volatile organic compounds identified in both Vespa velutina larvae and hornets after extraction with hexane, retention time (RT), match factor (MF), reverse match factor (RMF), and mean normalized areas.
Of the total compounds existing in the profile of larvae, 42 were identified exclusively in larvae and are indicated in bold in Tables S1 and S2 of the Supplementary Material. Most of these compounds belong to the alkanes (10), fatty acid esters (8), and piperidines (4) chemical families. However, the compounds that showed the highest abundance considering the area of the chromatographic peaks were 3-Hexen-2-one, (S)-(+)-2-Pyrrolidinemethanol, and 1,3-Dioxolane-4-carboxaldehyde, 2,2-dimethyl-, (R)- representing 19%, 14%, and 13%, respectively. Tables S1 and S2 of the Supplementary Material show the total identified compounds in larvae with their corresponding families, retention times, mean normalized areas, match factors (MFs), and reverse match factors (RMFs).
As indicated above, the VOCs highlighted in bold in Table 2 and Table 3 are those that have been reported in the literature in different insect species, including ants, termites, coleoptera, and various social hornets such as Vespa crabro, Vespa orientalis, and Vespa velutina. These compounds have been found in various parts of insects, such as the cuticle, the venom, and Dufour’s glands. The coincidence of V. velutina larvae having chemical profiles similar to those obtained from other insects could support the possibility that these compounds are currently associated with entomological organisms.
3.1.2. Univariate and Multivariate Analysis
MB-PCA was applied to the VOCs identified in both larvae and hornets extracted by the two solvents, with the aim of evaluating possible groupings as well as the presence of outliers. Figure 1a shows the scores plots for the first two components, which represent 55.9% of the total variance.
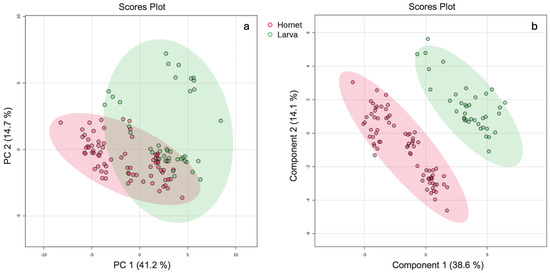
Figure 1.
Scores plots PC1 and PC2 of (a) MB-PCA and (b) MB-PLD-DA, built with the volatile organic compounds in common in larvae and hornets of Vespa velutina.
As can be seen in Figure 1a, extracts of V. velutina larvae and hornets show an overlap of samples of both matrixes. This fact supports the hypothesis that some volatile compounds could be of endogenous origin. In particular, compounds identified in V. velutina hornets in our previous study, and whose presence has been reported in other insect species, but not specifically in V. velutina, offer new information about the chemical composition of this species. Their identification in larvae indicates that these compounds do not come from the environment and are probably generated internally from the earliest stages of development.
MB-PLS-DA was applied to the identified VOCs, using the type of matrix as the discriminating variable, in order to find possible clusters between larvae and hornets. In Figure 1b, the scores plots for the first two components, which represent 52.7% of the total variance, are shown. The discriminant analysis was able to cluster the samples based on larvae and hornets groups. To evaluate whether or not the predictive ability of the model and the statistical significance were fortuitous, the model was validated by cross-validation (CV) (5-fold) and a permutation test (1000 iterations), as shown in Figures S1 and S2 of the Supplementary Material, respectively.
As a complement to multivariate analysis and to deepen the comparison between V. velutina larvae’s and hornets’ volatile profiles, a volcano plot was built with the area of their chromatographic peaks. As can be seen in Figure 2, several compounds show statistically significant differences. Among them, Decane, 4-methyl-, Heptadecane, 2,6-dimethyl-, Pyrrolidine, 1,1′-Methylene bis-, Octadecane, 2-methyl, Decane, 3,8-dimethyl-, and 3-Methylpentacosane showed the highest values of –log10 (p-value) and log2 (fold change). These VOCs belong to the hydrocarbons family except for Pyrrolidine, 1,1′-Methylene bis-.
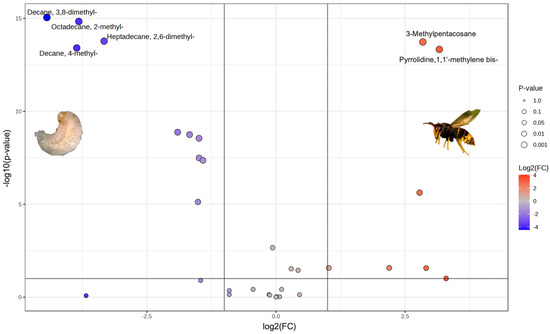
Figure 2.
Volcano plot for VOCs identified in common in larvae and hornets of V. velutina.
These compounds could reflect processes associated with the development of the insect from the larval stage to adulthood, giving rise to variations in their abundance.
Box plots were generated for the VOCs that presented the highest significance values in the volcano plot to visualize the increasing or decreasing trends of evolution in the different development stages of this species.
Compounds such as 3-Methylpentacosane and Pyrrolidine, 1,1′-methylenebis- showed a significantly increasing trend from larva to adult hornet, as seen in Figure 3. This could be related to specific functions in chemical communication and/or cuticular protection of already developed individuals [23,27,42]. It is important to note that in the literature, only the first compound has been reported in insects such as ants, termites, and Vespa velutina hornets [24,31,47], while Pyrrolidine, 1,1′-methylenebis- has been identified as a secondary metabolite of plants [49].
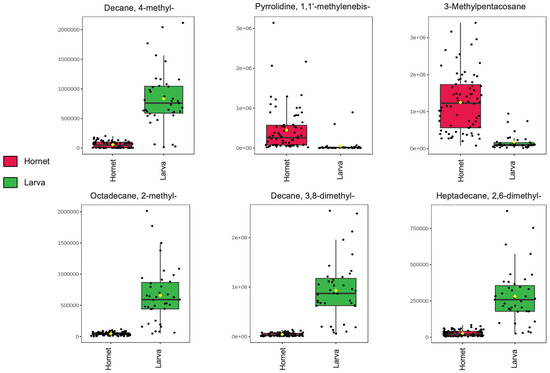
Figure 3.
Variations in the normalized areas of the chromatographic peaks for the six compounds with the most statistically significant differences.
For the rest of the hydrocarbons, a decreasing tendency was observed from larva to adult. This suggests a possible relationship with the physiological functions of larval development, such as the formation of initial cuticular structures, which could decrease or transform in the metamorphosis stage [26]. Within this group of compounds, Decane, 3,8-dimethyl- and Octadecane, 2-methyl- have been reported in the literature in different insect species, including ants, coleoptera, and, in our previous work, Vespa velutina hornets [31,50,51]. However, the remaining two compounds, heptadecane, 2,6-dimethyl- and decane, 4-methyl-, have been found in plants [52], while in our analysis of nests and Vespa velutina hornets they were only found in hornets. Therefore, the presence of these compounds in larvae and hornets and not in their nests could be due not only to their possible endogenous origin, but also to their introduction through feeding.
These results could indicate that the compounds shared by larvae and hornets have functions that vary in relevance throughout the development of V. velutina. Although the exact role of each substance is not clearly defined, their presence in both stages suggests that they could be involved in internal mechanisms related to development or communication within the colony.
4. Conclusions
In this study, the profile of volatile organic compounds (VOCs) in Vespa velutina larvae was obtained using the GC-MS previous solvent extraction procedure.
A total of 99 volatile compounds were identified in larvae, of which 33 were common to adult hornets, suggesting a possible endogenous origin and a functional role in physiological processes and chemical communication within the colony. The application of MB-PLS-DA to the different features identified in common in larvae and hornets allowed them to cluster.
Decane, 4-methyl-, Heptadecane, 2,6-dimethyl-, Octadecane, 2-methyl, Decane, 3,8-dimethyl-, Pyrrolidine, 1,1′-Methylene bis-, and 3-Methylpentacosane are the compounds with the highest significant differences between larvae and adults. The first four hydrocarbons showed a decreasing tendency from larvae to hornets, while for the rest, the tendency was to increase. The different functions of each compound in the development of this species could justify the differences observed.
A total of 42 compounds were detected exclusively in larvae, belonging to aldehydes, alcohols, alkanes, alkenes, amines, ketones, piperidines, pyrrolidines, furanones, fatty acid esters, ethers, and pyridines chemical families. The detection of these compounds in larval stages, before environmental exposure, supports the hypothesis of their endogeneous origin. Results obtained from this work contribute to expanding the knowledge about the biology and chemical composition of V. velutina, and could help identify potential semiochemicals useful for the design of more selective and sustainable control strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors13050175/s1, Table S1: Mean normalized chromatographic peak areas, retention times (RT), match factor (MF) and reverse match factor (RMF), family of compounds, for the volatile organic compounds identified in Vespa velutina larvae extracted in the mixture actone:methanol (50:50). Table S2: Mean normalized chromatographic peak areas, retention times (RT), match factor (MF) and reverse match factor (RMF), family of compounds, for the volatile organic compounds identified in Vespa velutina larvae extracted in hexane. Figure S1: MB-PLS-DA validation: Comparison of the values for accuracy, model fit (R2) and predictive capacity (Q2) according to the number of latent components. The optimal model is indicated by the red asterisk. Figure S2: MB-PLS-DA validation: permutation test performed with 1000 random iterations that shows the observed value of the original model (red line) and the range generated by chance (p < 0.001).
Author Contributions
Conceptualization: O.d.l.H. and R.M.A.; methodology: O.d.l.H. and R.M.A.; formal analysis and investigation: O.d.l.H. and R.M.A.; writing—original draft preparation: O.d.l.H. and R.M.A.; writing—review and editing: O.d.l.H. and R.M.A.; funding acquisition: R.M.A.; resources: R.M.A.; supervision: R.M.A. All authors have read and agreed to the published version of this manuscript.
Funding
Omaira de la Hera thanks the University of the Basque Country (UPV/EHU) for her predoctoral grant. The authors thank the Department of Education of the Basque Country Government (projects IT1673-22, PUE 2018_1_0007, and PUE 2021_1_008) and the University of the Basque Country (UPV/EHU) (project US21/35) for financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented in this study are available on request from the corresponding author due to privacy reasons.
Acknowledgments
The authors thank the public company Basalan (Bizkaia, Spain) for providing nests for this study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of this manuscript; or in the decision to publish these results.
References
- Leza, M.; Miranda, M.Á.; Colomar, V. First detection of Vespa velutina nigrithorax (Hymenoptera:Vespidae) in the balearic islands (Western Mediterranean): A challenging study case. Biol. Invasions 2018, 20, 1643–1649. [Google Scholar] [CrossRef]
- Laurino, D.; Lioy, S.; Carisio, L.; Manino, A.; Porporato, M. Vespa velutina: An alien driver of honey bee colony losses. Diversity 2020, 12, 5. [Google Scholar] [CrossRef]
- Castro, L.; Pagola-Carte, S. Vespa velutina Lepeletier, 1836 (Hymenoptera:Vespidae), recolectada en la Península Ibérica. Heteropterus Rev. Entomol. 2010, 10, 193–196. [Google Scholar]
- Chauzat, M.-P.; Martin, S. A foreigner in France: The Asian hornet. Biologist 2009, 2, 86–91. [Google Scholar]
- Leza, M.; Herrera, C.; Marques, A.; Roca, P.; Sastre-Serra, J.; Pons, D. The impact of the invasive species Vespa velutina on honeybees: A new approach based on oxidative stress. Sci. Total Environ. 2019, 689, 709–715. [Google Scholar] [CrossRef]
- Monceau, K.; Bonnard, O.; Thiéry, D. Vespa velutina: A new invasive predator of honeybees in Europe. J. Pest Sci. 2013, 87, 1–16. [Google Scholar] [CrossRef]
- Martin, S.J. The Asian Hornet: Threats, Biology & Expansion; IBRA and Northern Bee Books: Mytholmroyd, UK, 2017. [Google Scholar]
- Puebla, H.; Roy, P.K.; Velasco-Perez, A.; Gonzalez-Brambila, M.M. Biological pest control using a model-based robust feedback. IET Syst. Biol. 2018, 12, 233–240. [Google Scholar] [CrossRef]
- Edwards, E.; Toft, R.; Joice, N.; Westbrooke, I. The efficacy of Vespex® wasp bait to control Vespula species (Hymenoptera:Vespidae) in New Zealand. Int. J. Pest Manag. 2017, 63, 266–272. [Google Scholar] [CrossRef]
- Turchi, L.; Derijard, B. Options for the biological and physical control of Vespa velutina nigrithorax (Hym.: Vespidae) in Europe: A review. J. Appl. Entomol. 2018, 142, 553–562. [Google Scholar] [CrossRef]
- Ruiz-Cristi, I.; Berville, L.; Darrouzet, E. Characterizing thermal tolerance in the invasive yellow-legged hornet (Vespa velutina nigrithorax): The first step toward a green control method. PLoS ONE 2020, 15, e0239742. [Google Scholar] [CrossRef]
- Rojas-Nossa, S.V.; Dasilva-Martins, D.; Mato, S.; Bartolomé, C.; Maside, X.; Garrido, J. Effectiveness of electric harps in reducing Vespa velutina predation pressure and consequences for honey bee colony development. Pest Manag. Sci. 2022, 78, 5142–5149. [Google Scholar] [CrossRef]
- Zablotny, J.E. Encyclopedia of Insects. In Sociality; Elsevier: Amsterdam, The Netherlands, 2009; pp. 928–935. [Google Scholar] [CrossRef]
- Grüter, C.; Czaczkes, T.J. Communication in social insects and how it is shaped by individual experience. Anim. Behav. 2019, 151, 207–215. [Google Scholar] [CrossRef]
- Billen, J.; Morgan, E.D. Pheromone Communication in Social Insects: Sources and Secretions. In Pheromone Communication in Social Insects, 1st ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 3–33. [Google Scholar]
- Bruschini, C.; Cervo, R.; Stefano, T. Pheromones in Social Wasps. Vitam. Horm. 2010, 83, 447–492. [Google Scholar] [CrossRef]
- Karlson, P.; Lüscher, M.; L, M. Pheromones: A New Term for a Class of Biologically Active Substances. Nature 1959, 183, 55–56. [Google Scholar] [CrossRef]
- Billen, J. Signal variety and communication in social insects. Proc. Neth. Entomol. Soc. Meet. 2006, 17, 9–25. [Google Scholar]
- Lamprecht, I.; Schmolz, E.; Schricker, B. Pheromones in the life of insects. Eur. Biophys. J. 2008, 37, 1253–1260. [Google Scholar] [CrossRef]
- Singer, T.L. Roles of Hydrocarbons in the Recognition Systems of Insects. Amer Zool. 1998, 38, 394–405. [Google Scholar] [CrossRef]
- Rodríguez-Flores, M.S.; Falcão, S.I.; Escuredo, O.; Seijo, M.C.; Vilas-Boas, M. Chemical profile from the head of Vespa velutina and V. crabro. Apidologie 2021, 52, 548–560. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Pirk, C.W.W.; Crewe, R.M.; Njagi, P.G.N.; Gordon, I.; Torto, B. Nestmate recognition and the role of cuticular hydrocarbons in the African termite raiding ant Pachycondyla analis. J. Chem. Ecol. 2010, 36, 441–448. [Google Scholar] [CrossRef][Green Version]
- Steinmetz, I.; Schmolz, E.; Ruther, J. Cuticular lipids as trail pheromone in a social wasp. Proc. R. Soc. B Biol. Sci. 2003, 270, 385–391. [Google Scholar] [CrossRef]
- Cvačka, J.; Jiroš, P.; Šobotník, J.; Hanus, R.; Svatoš, A. Analysis of insect cuticular hydrocarbons using matrix-assisted laser desorption/ionization mass spectrometry. J. Chem. Ecol. 2006, 32, 409–434. [Google Scholar] [CrossRef]
- Espelie, K.E.; Gamboa, G.J.; Grudzien, T.A.; Bura, E.A. Cuticular hydrocarbons of the paper wasp Polistes Fuscatus: A search for recognition pheromones. J. Chem. Ecol. 1994, 20, 1677–1687. [Google Scholar] [CrossRef]
- Frederickx, C.; Dekeirsschieter, J.; Brostaux, Y.; Wathelet, J.-P.; Verheggen, F.; Haubruge, E. Volatile organic compounds released by blowfly larvae and pupae: New perspectives in forensic entomology. Forensic Sci. Int. 2012, 219, 215–220. [Google Scholar] [CrossRef]
- Ruther, J.; Sieben, S.; Schricker, B. Nestmate recognition in social wasps: Manipulation of hydrocarbon profiles induces aggression in the European hornet. Sci. Nat. 2002, 89, 111–114. [Google Scholar] [CrossRef]
- Rodríguez-Flores, M.S.; Falcão, S.I.; Escuredo, O.; Queijo, L.; Seijo, M.C.; Vilas-Boas, M. Assessment of the in vivo and in vitro release of chemical compounds from vespa velutina. Molecules 2021, 26, 6769. [Google Scholar] [CrossRef]
- Ghosh, S.; Namin, S.M.; Meyer-Rochow, V.B.; Jung, C. Chemical composition and nutritional value of different species of Vespa hornets. Foods 2021, 10, 418. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, J.M.; Kim, B.; Nam, J.-O.; Hahn, D.; Choi, M.B. Nutritional Value of the Larvae of the Alien Invasive Wasp Vespa velutina nigrithorax and Amino Acid Composition of the Larval Saliva. Foods 2020, 9, 885. [Google Scholar] [CrossRef]
- de la Hera, O.; Alonso, R.M. Differentiation of Vespa velutina nigrithorax Colonies Using Volatile Organic Compound Profiles of Hornets and Nests. Insects 2024, 15, 811. [Google Scholar] [CrossRef]
- Quintanilla-Casas, B.; Bro, R.; Hinrich, J.L.; Davie-Martin, C.L. Tutorial on PARADISe: PARAFAC2-based Deconvolution and Identification System for processing GC–MS data. In Protoc. Exch.; 2023. [Google Scholar] [CrossRef]
- Makhuvele, R.; Gbashi, S.; Njobeh, P.B. GC-HRTOF-MS metabolite profiling and antioxidant activity of methanolic extracts of Tulbaghia violacea Harv. J. King Saud Univ. Sci. 2022, 34, 102278. [Google Scholar] [CrossRef]
- Hernández-Estrada, M.G.; De Muñoz, F.L.; Hurtado-Sil, G.; Lanz-Mendoza, H.; Rodríguez, M.H.; Hernández-Hernández, F.C. Regulación por Dexametasona y Ácido Araquidónico sobre la expresión de una Aldoceto Reductasa/Di-hidrodiol Deshi-drogenasa (DDH) en estómagos del mosquito Aedes aegypti, principal vector del dengue en México. Investig. Univ. Multidiscip. Rev. Investig. Univ. Simón Bolívar 2007, 6, 2. [Google Scholar]
- Rodrigues, D.P.; Ameixa, O.M.C.C.; Vázquez, J.A.; Calado, R. Improving the Lipid Profile of Black Soldier Fly (Hermetia illucens) Larvae for Marine Aquafeeds: Current State of Knowledge. Sustainability 2022, 14, 6472. [Google Scholar] [CrossRef]
- Gupta, V.; Tyagi, S.; Tripathi, R. Hexadecanoic acid methyl ester, a potent hepatoprotective compound in leaves of Pistia stratiotes L. Appl. Biol. Chem. J. 2023, 4, 118–120. [Google Scholar] [CrossRef]
- Baeshen, R.S.; Baz, M.M. Efficacy of Acacia nilotica, Eucalyptus camaldulensis, and Salix safsafs on the mortality and development of two vector-borne mosquito species, Culex pipiens and Aedes aegypti, in the laboratory and field. Heliyon 2023, 9, e16378. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, S.-T.; Wu, X.-M.; Pang, X.-Q.; Ni, L.-L.; Yuan, S.-M.; Yang, Z.-B.; Li, Y.-H.; Xiao, H. GC-MS analysis of Vespa velutina auraria Smith and its anti-inflammatory and antioxidant activities in vitro. Open Chem. 2022, 20, 602–610. [Google Scholar] [CrossRef]
- Schulz, S.; Francke, W.; Edgar, J.; Schneider, D. Volatile Compounds from Androconial Organs of Danaine and Ithomiine Butterflies. Z. Für Naturforschung 1988, 43, 99–104. [Google Scholar] [CrossRef]
- Esteves, F.G.; dos Santos-Pinto, J.R.A.; Saidemberg, D.M.; Palma, M.S. Using a proteometabolomic approach to investigate the role of Dufour’s gland in pheromone biosynthesis in the social wasp Polybia paulista. J. Proteom. 2017, 151, 122–130. [Google Scholar] [CrossRef]
- Oladele, J.O.; Adewole, T.S.; Ogundepo, G.E.; Oyeleke, O.M.; Kuku, A. Efficacy of selected Nigerian tropical plants in the treatment of COVID-19: In silico and in vitro investigations. Environ. Sci. Pollut. Res. 2022, 29, 89295–89339. [Google Scholar] [CrossRef]
- Butts, D.P.; Camann, M.A.; Espelie, K.E. Workers and queens of the European hornet Vespa crabro L. have colony-specific cuticular hydrocarbon profiles (Hymenoptera:Vespidae). Insectes Sociaux 1995, 42, 45–55. [Google Scholar] [CrossRef]
- Cecotti, R.; Carpana, E.; Falchero, L.; Paoletti, R.; Tava, A. Determination of the volatile fraction of Polygonum bistorta L. at different growing stages and evaluation of its antimicrobial activity against two major honeybee (Apis mellifera) pathogens. Chem. Biodivers. 2012, 9, 359–369. [Google Scholar] [CrossRef]
- Yarazari, S.B.; Jayaraj, M. GC–MS Analysis of Bioactive Compounds of Flower Extracts of Calycopteris floribunda Lam.: A Multi Potent Medicinal Plant. Appl. Biochem. Biotechnol. 2022, 194, 5083–5099. [Google Scholar] [CrossRef]
- Mitra, A.; Gadagkar, R. The Dufour’s gland and the cuticle in the social wasp Ropalidia marginata contain the same hydrocarbons in similar proportions. J. Insect Sci. 2014, 14, 1–18. [Google Scholar] [CrossRef]
- Abdalla, F.C.; Jones, G.R.; Morgan, D.; da Cruz-Landim, C. Chemical Composition of the Dufour Gland Secretion in Queens of Melipona bicolor (Hymenoptera, Meliponini). J. Braz. Chem. Soc. 2004, 15, 621–625. [Google Scholar] [CrossRef]
- Löfqvist, J.; Bergström, G. Volatile communication substances in dufour’s gland of virgin females and old queens of the ant Formica polyctena. J. Chem. Ecol. 1980, 6, 309–320. [Google Scholar] [CrossRef]
- Gévar, J.; Bagnères, A.-G.; Christidès, J.-P.; Darrouzet, E. Chemical Heterogeneity in Inbred European Population of the Invasive Hornet Vespa velutina nigrithorax. J. Chem. Ecol. 2017, 43, 763–777. [Google Scholar] [CrossRef]
- Fanokh Al-Owaidi, M.; Naser, E.H.; Ahmed Abed, S.; Abo Khthr, M.F.; Abed, S.A. Cardaria drabra L.: A review of Phytochemistry and Therapeutic Uses Phytochemistry and Therapeutic Uses of Cardaria draba L.: A Review. Plant Arch. 2019, 19, 118–125. [Google Scholar]
- Jurenka, R.A.; Subchev, M. Identification of Cuticular Hydrocarbons and the Alkene Precursor to the Pheromone in Hemolymph of the Female Gypsy Moth, Lymantria dispar. Arch. Insect Biochem. Physiol. 2000, 43, 108–115. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, M.; Tang, L.; Ma, R.; He, H. Chemical Components of Dufour’s and Venom Glands in Camponotus japonicus (Hymenoptera, Formicidae). Insects 2023, 14, 664. [Google Scholar] [CrossRef]
- Seenivasan, N. Phytochemical profiling of burrowing nematode (Radopholus similis) resistant and susceptible banana (Musa spp.) genotypes for detection of marker compounds. Fruits 2018, 73, 48–59. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).