Recent Advances in Aggregation-Induced Emission (AIE) Fluorescent Sensors for Biomolecule Detection
Abstract
1. Introduction
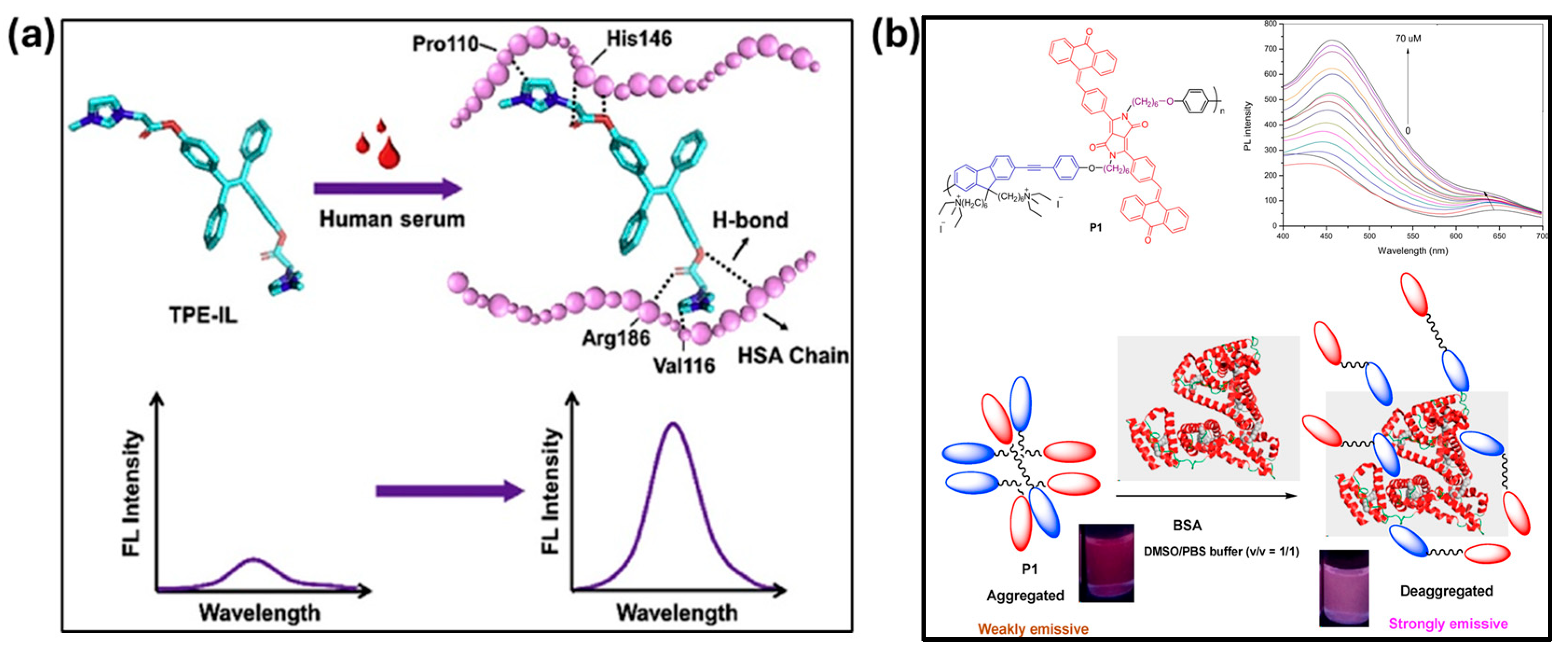
2. General Mechanism
3. AIE Sensors for Biomolecule Detection
3.1. Nucleic Acids
3.2. Detection of Enzyme
3.3. Detection of Amino Acids
3.4. Detection of Biogenic Amines
3.5. Detection of Other Biological Molecules
3.5.1. Saccharides
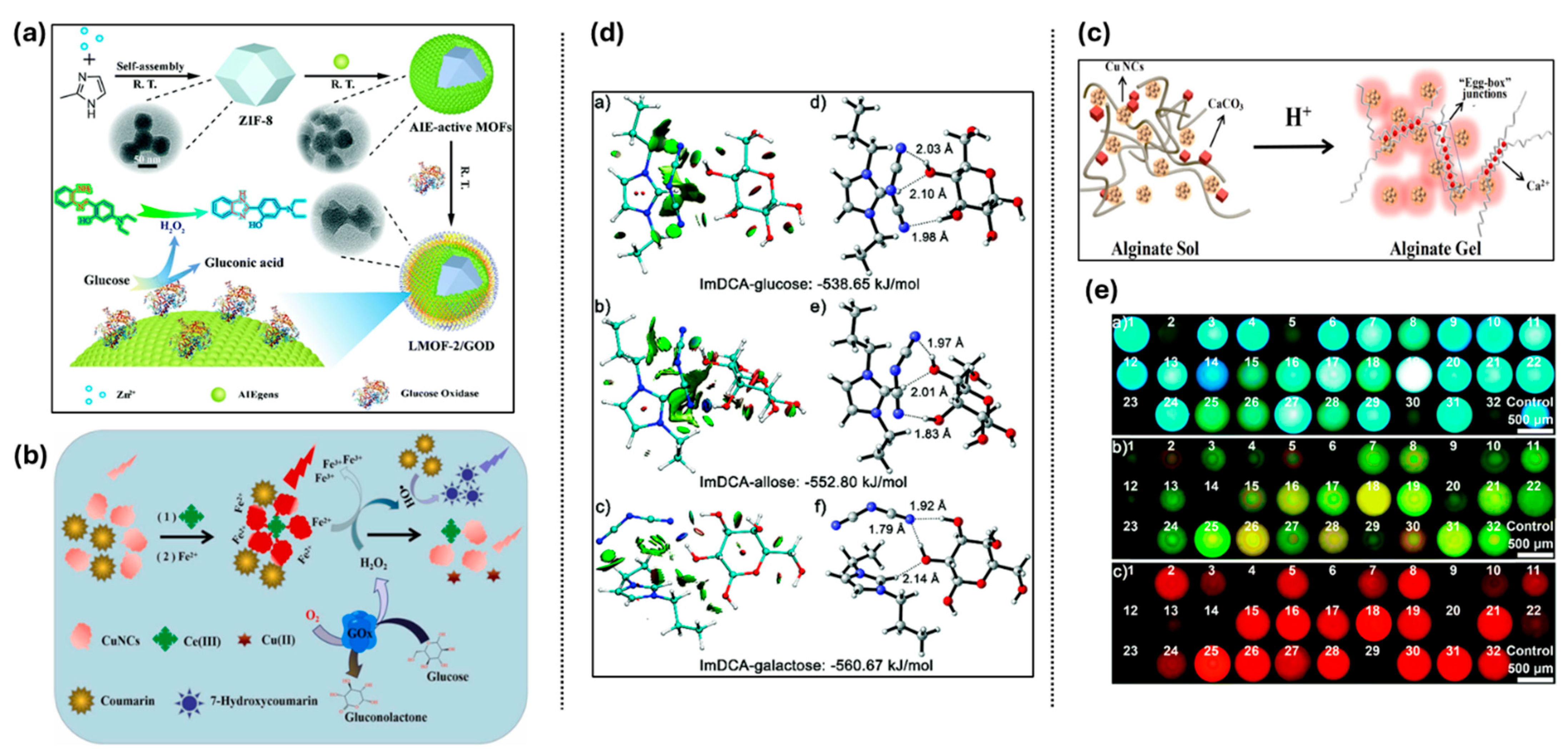
3.5.2. Adenosine Triphosphate (ATP)
3.5.3. Vitamins
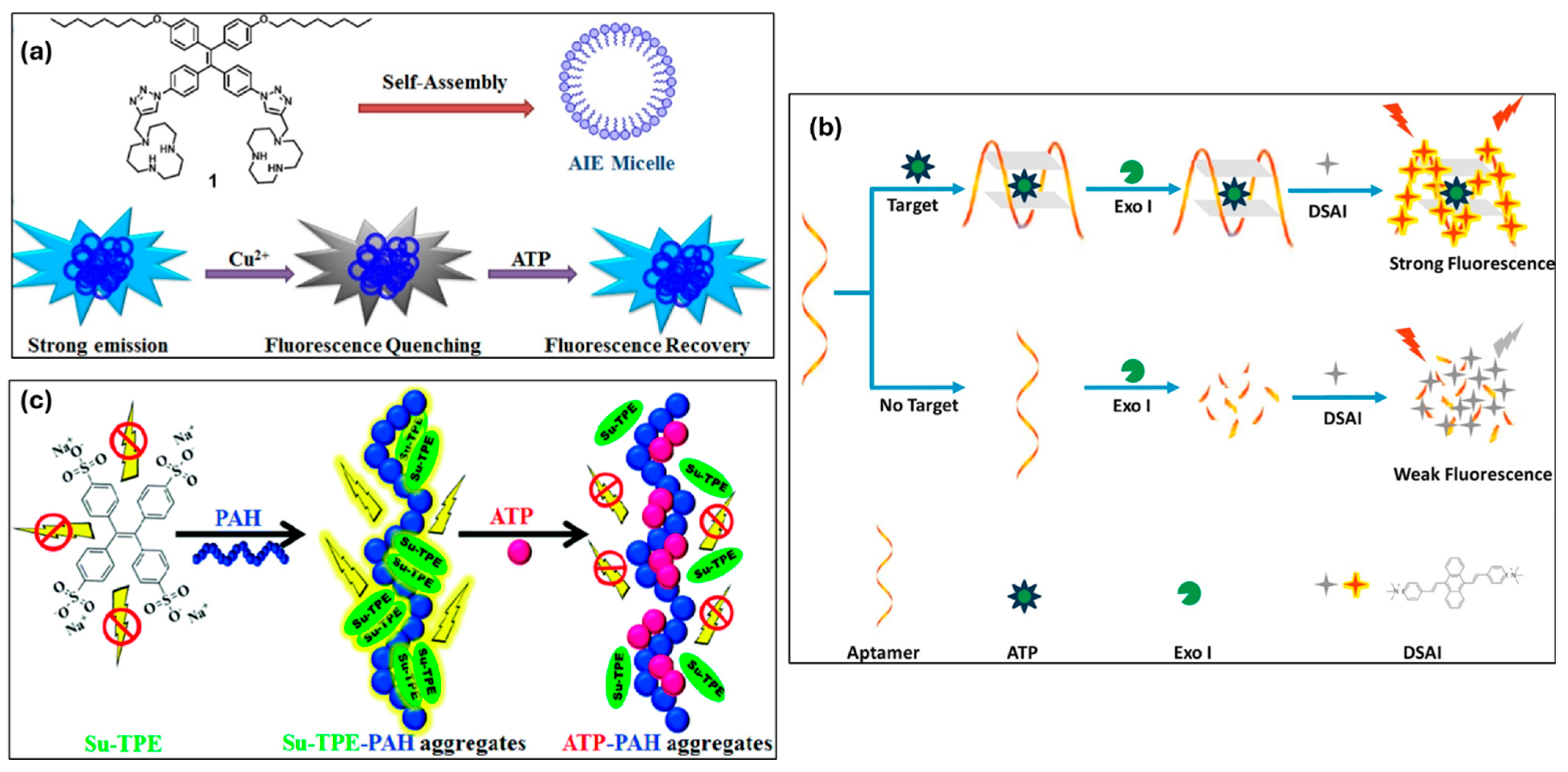
3.5.4. HSA (Human Serum Albumin), BSA (Bovine Serum Albumin), and Collagen
4. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, Y.; Zhang, W.; Xue, Y.; Zhang, J. Fluorescent Sensors for Detecting and Imaging Metal Ions in Biological Systems: Recent Advances and Future Perspectives. Chemosensors 2023, 11, 226. [Google Scholar] [CrossRef]
- Manna, A.S.; Ghosh, S.; Ghosh, T.; Karchaudhuri, N.; Das, S.; Roy, A.; Maiti, D.K. Smart Luminescent Materials for Emerging Sensors: Fundamentals and Advances. Chem. Asian J. 2025, 2, e202401328. [Google Scholar] [CrossRef] [PubMed]
- Zhai, R.; Fang, B.; Lai, Y.; Peng, B.; Bai, H.; Liu, X.; Li, L.; Huang, W. Small-Molecule Fluorogenic Probes for Mitochondrial Nanoscale Imaging. Chem. Soc. Rev. 2022, 52, 942–972. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.Y.; Mei, L.J.; Tian, R.; Li, C.; Wang, Y.L.; Xiang, S.L.; Zhu, M.Q.; Tang, B.Z. Recent Advances in Super-Resolution Optical Imaging Based on Aggregation-Induced Emission. Chem. Soc. Rev. 2024, 53, 3350–3383. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Yoon, J. Recent Advances in Development of Chiral Fluorescent and Colorimetric Sensors. Chem. Rev. 2014, 114, 4918–4959. [Google Scholar] [CrossRef]
- Afrin, A.; Jayaraj, A.; Gayathri, M.S.; Chinna Ayya Swamy, P. An Overview of Schiff Base-Based Fluorescent Turn-on Probes: A Potential Candidate for Tracking Live Cell Imaging of Biologically Active Metal Ions. Sens. Diagn. 2023, 2, 988–1076. [Google Scholar] [CrossRef]
- Fang, X.; Zheng, Y.; Duan, Y.; Liu, Y.; Zhong, W. Recent Advances in Design of Fluorescence-Based Assays for High-Throughput Screening. Anal. Chem. 2019, 91, 482–504. [Google Scholar] [CrossRef]
- Sadananda, D.; Mallikarjunaswamy, A.M.M.; Prashantha, C.N.; Mala, R.; Gouthami, K.; Lakshminarayana, L.; Ferreira, L.F.R.; Bilal, M.; Rahdar, A.; Mulla, S.I. Recent Development in Chemosensor Probes for the Detection and Imaging of Zinc Ions: A Systematic Review. Chem. Pap. 2022, 76, 5997–6015. [Google Scholar] [CrossRef]
- Ritu; Narang, U.; Kumar, V. Heavy Metal Detection with Organic Moiety-Based Sensors: Recent Advances and Future Directions. ChemBioChem 2024, 25, e202400191. [Google Scholar] [CrossRef]
- Sargazi, S.; Fatima, I.; Hassan Kiani, M.; Mohammadzadeh, V.; Arshad, R.; Bilal, M.; Rahdar, A.; Díez-Pascual, A.M.; Behzadmehr, R. Fluorescent-Based Nanosensors for Selective Detection of a Wide Range of Biological Macromolecules: A Comprehensive Review. Int. J. Biol. Macromol. 2022, 206, 115–147. [Google Scholar] [CrossRef]
- Fakayode, S.O.; Lisse, C.; Medawala, W.; Brady, P.N.; Bwambok, D.K.; Anum, D.; Alonge, T.; Taylor, M.E.; Baker, G.A.; Mehari, T.F.; et al. Fluorescent Chemical Sensors: Applications in Analytical, Environmental, Forensic, Pharmaceutical, Biological, and Biomedical Sample Measurement, and Clinical Diagnosis. Appl. Spectrosc. Rev. 2024, 59, 1–89. [Google Scholar] [CrossRef]
- Immanuel David, C.; Lee, H. Cutting-Edge Advances in Colorimetric and Fluorescent Chemosensors for Detecting Lethal Cyanide Ion: A Comprehensive Review. Microchem. J. 2024, 200, 110359. [Google Scholar] [CrossRef]
- AbhijnaKrishna, R.; Velmathi, S. Exploring Fluorescent and Colorimetric Probes for Analyte Detection: Utilizing It in Live Cell Imaging and Real-Time Sample Analysis. Inorganica Chim. Acta 2024, 567, 122039. [Google Scholar] [CrossRef]
- Wang, W.-J.; Xin, Z.-Y.; Su, X.; Hao, L.; Qiu, Z.; Li, K.; Luo, Y.; Cai, X.-M.; Zhang, J.; Alam, P.; et al. Aggregation-Induced Emission Luminogens Realizing High-Contrast Bioimaging. ACS Nano 2025, 19, 281–306. [Google Scholar] [CrossRef]
- Gawade, V.K.; Jadhav, R.W.; Bhosale, S.V. AIE-Based & Organic Luminescent Materials: Nanoarchitectonics and Advanced Applications. Chem. Asian J. 2024, 19, e202400682. [Google Scholar] [CrossRef]
- Arshad, F.; Pal, A.; Sk, M.P. Review—Aggregation-Induced Emission in Carbon Dots for Potential Applications. ECS J. Solid. State Sci. Technol. 2021, 10, 021001. [Google Scholar] [CrossRef]
- Joseph, E.; Ciocca, M.; Wu, H.; Keremane, K.; Poudel, B.; Priya, S.; Brown, T.M. Photovoltaic bioelectronics merging biology with new generation semiconductors and light in biophotovoltaics photobiomodulation and biosensing. Npj Biosensing 2024, 1, 15. [Google Scholar] [CrossRef]
- Debnath, S.; Mohanty, A.; Naik, P.; Dasgupta, J.; Patil, S. Deciphering intramolecular charge transfer in fluoranthene derivatives. J. Mater. Chem. C 2024, 12, 9200–9209. [Google Scholar] [CrossRef]
- Chua, M.H.; Chin, K.L.O.; Loh, X.J.; Zhu, Q.; Xu, J. Aggregation-Induced Emission-Active Nanostructures: Beyond Biomedical Applications. ACS Nano 2023, 17, 1845–1878. [Google Scholar] [CrossRef]
- Qin, A.; Lam, J.W.Y.; Tang, B.Z. Luminogenic Polymers with Aggregation-Induced Emission Characteristics. Prog. Polym. Sci. 2012, 37, 182–209. [Google Scholar] [CrossRef]
- Hu, R.; Qin, A.; Tang, B.Z. AIE Polymers: Synthesis and Applications. Prog. Polym. Sci. 2020, 100, 101176. [Google Scholar] [CrossRef]
- Hu, R.; Leung, N.L.C.; Tang, B.Z. AIE Macromolecules: Syntheses, Structures and Functionalities. Chem. Soc. Rev. 2014, 43, 4494–4562. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhu, B.; Luo, J.; Liu, B. Design and Property Modulation of Metal–Organic Frameworks with Aggregation-Induced Emission. ACS Mater. Lett. 2021, 3, 77–89. [Google Scholar] [CrossRef]
- Shustova, N.B.; Ong, T.-C.; Cozzolino, A.F.; Michaelis, V.K.; Griffin, R.G.; Dincă, M. Phenyl Ring Dynamics in a Tetraphenylethylene-Bridged Metal–Organic Framework: Implications for the Mechanism of Aggregation-Induced Emission. J. Am. Chem. Soc. 2012, 134, 15061–15070. [Google Scholar] [CrossRef]
- Zhai, X.; Cui, Z.; Shen, W. Mechanism, Structural Design, Modulation and Applications of Aggregation-Induced Emission-Based Metal-Organic Framework. Inorg. Chem. Commun. 2022, 146, 110038. [Google Scholar] [CrossRef]
- Dalapati, S.; Jin, E.; Addicoat, M.; Heine, T.; Jiang, D. Highly Emissive Covalent Organic Frameworks. J. Am. Chem. Soc. 2016, 138, 5797–5800. [Google Scholar] [CrossRef]
- Qin, W.; Ding, D.; Liu, J.; Yuan, W.Z.; Hu, Y.; Liu, B.; Tang, B.Z. Biocompatible Nanoparticles with Aggregation-Induced Emission Characteristics as Far-Red/near-Infrared Fluorescent Bioprobes for in Vitro and in Vivo Imaging Applications. Adv. Funct. Mater. 2012, 22, 771–779. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, Y.; Xu, B.; Tian, W. Fluorescent Nanoparticles Based on AIE Fluorogens for Bioimaging. Nanoscale 2016, 8, 2471–2487. [Google Scholar] [CrossRef]
- Wan, Q.; Huang, Q.; Liu, M.; Xu, D.; Huang, H.; Zhang, X.; Wei, Y. Aggregation-Induced Emission Active Luminescent Polymeric Nanoparticles: Non-Covalent Fabrication Methodologies and Biomedical Applications. Appl. Mater. Today 2017, 9, 145–160. [Google Scholar] [CrossRef]
- Patel, C.K.; Kant, K.; Naik, P.; Sharma, V.; Keremane, K.; Singh, V.; Malakar, C.C. Reductive C−N Bond Formation of Nitroarenes Using Pd@rGO-CuFe2O4 Magnetic Nanoparticles in Water towards the Synthesis of N-Aryl Formamide and Azole Derivatives. Asian J. Org. Chem. 2024, 13, e202400255. [Google Scholar] [CrossRef]
- Bai, J.; Qin, F.; He, P.; Wu, S.; Zhu, Y.; Yuan, G.; Wang, X.; Yu, X.; Ren, L. Carbon Dots-Based Luminescent Materials with Aggregation-Induced Emission and Solvent Crystallization-Induced Emission Behaviors. Inorg. Chem. Commun. 2023, 151, 110614. [Google Scholar] [CrossRef]
- Ru, Y.; Waterhouse, G.I.N.; Lu, S. Aggregation in Carbon Dots: Special Issue: Emerging Investigators. Aggregate 2022, 3, e296. [Google Scholar] [CrossRef]
- Wan, Z.; Li, Y.; Zhou, Y.; Peng, D.; Zhang, X.; Zhuang, J.; Lei, B.; Liu, Y.; Hu, C. High-Efficiency Solid-State Luminescence from Hydrophilic Carbon Dots with Aggregation-Induced Emission Characteristics. Adv. Funct. Mater. 2023, 33, 2207296. [Google Scholar] [CrossRef]
- Shi, Y.E.; Ma, J.; Feng, A.; Wang, Z.; Rogach, A.L. Aggregation-Induced Emission of Copper Nanoclusters. Aggregate 2021, 2, e112. [Google Scholar] [CrossRef]
- Wang, J.; Lin, X.; Shu, T.; Su, L.; Liang, F.; Zhang, X. Self-Assembly of Metal Nanoclusters for Aggregation-Induced Emission. Int. J. Mol. Sci. 2019, 20, 1891. [Google Scholar] [CrossRef]
- Goswami, N.; Yao, Q.; Luo, Z.; Li, J.; Chen, T.; Xie, J. Luminescent Metal Nanoclusters with Aggregation-Induced Emission. J. Phys. Chem. Lett. 2016, 7, 962–975. [Google Scholar] [CrossRef]
- Jia, X.; Yang, X.; Li, J.; Li, D.; Wang, E. Stable Cu Nanoclusters: From an Aggregation-Induced Emission Mechanism to Biosensing and Catalytic Applications. Chem. Commun. 2014, 50, 237–239. [Google Scholar] [CrossRef]
- Li, Z.; Ji, X.; Xie, H.; Tang, B.Z. Aggregation-Induced Emission-Active Gels: Fabrications, Functions, and Applications. Adv. Mater. 2021, 33, 2100021. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Li, H.; Song, N.; Wang, D.; Tang, B.Z. Supramolecular Materials Based on AIE Luminogens (AIEgens): Construction and Applications. Chem. Soc. Rev. 2020, 49, 1144–1172. [Google Scholar] [CrossRef]
- Liow, S.S.; Zhou, H.; Sugiarto, S.; Guo, S.; Chalasani, M.L.S.; Verma, N.K.; Xu, J.; Loh, X.J. Highly Efficient Supramolecular Aggregation-Induced Emission-Active Pseudorotaxane Luminogen for Functional Bioimaging. Biomacromolecules 2017, 18, 886–897. [Google Scholar] [CrossRef]
- Li, Z.; Liu, P.; Ji, X.; Gong, J.; Hu, Y.; Wu, W.; Wang, X.; Peng, H.Q.; Kwok, R.T.K.; Lam, J.W.Y.; et al. Bioinspired Simultaneous Changes in Fluorescence Color, Brightness, and Shape of Hydrogels Enabled by AIEgens. Adv. Mater. 2020, 32, 1906493. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Hong, Y.; Dong, Y.; Häußler, M.; Lam, J.W.Y.; Li, Z.; Guo, Z.; Guo, Z.; Tang, B.Z. Fluorescent “Light-up” Bioprobes Based on Tetraphenylethylene Derivatives with Aggregation-Induced Emission Characteristics. Chem. Commun. 2006, 35, 3705–3707. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Law, C.C.W.; Lam, J.W.Y.; Dong, Y.; Lo, S.M.F.; Williams, I.D.; Zhu, D.; Tang, B.Z. Synthesis, Light Emission, Nanoaggregation, and Restricted Intramolecular Rotation of 1,1-Substituted 2,3,4,5-Tetraphenylsiloles. Chem. Mater. 2003, 15, 1535–1546. [Google Scholar] [CrossRef]
- Wang, Z.; Miao, Y.; Ou, Q.; Niu, R.-X.; Jiang, Y.; Zhang, C. Full-Color-Tunable Nanohydrogels as High-Stability Intracellular Nanothermometers. ACS Appl. Mater. Interfaces 2022, 14, 55423–55430. [Google Scholar] [CrossRef]
- Duangkamol, C.; Wangngae, S.; Wet-osot, S.; Khaikate, O.; Chansaenpak, K.; Lai, R.Y.; Kamkaew, A. Quinoline-Malononitrile-Based Aggregation-Induced Emission Probe for Monoamine Oxidase Detection in Living Cells. Molecules 2023, 28, 2655. [Google Scholar] [CrossRef]
- Chen, M.; Li, L.; Nie, H.; Tong, J.; Yan, L.; Xu, B.; Sun, J.Z.; Tian, W.; Zhao, Z.; Qin, A.; et al. Tetraphenylpyrazine-Based AIEgens: Facile Preparation and Tunable Light Emission. Chem. Sci. 2015, 6, 1932–1937. [Google Scholar] [CrossRef]
- Tao, W.; He, W.; Feng, X.; Liu, G.; Shi, Q.; Tan, J.; Hu, J.; Yang, S.; Liu, G.; Yang, R. Cationic Single-Unit Monomer Insertion (CSUMI): From Discrete Oligomers to the α-/ω-End and In-Chain Sequence-Regulated Polymers. J. Am. Chem. Soc. 2023, 145, 3636–3646. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Mao, X.; Wang, Q.; Mu, Z.; An, L.; Zhang, W.; Feng, X.; Redshaw, C.; Cao, C.; et al. Pyrene-Based Aggregation-Induced Emission Luminogens (AIEgens) with Less Colour Migration for Anti-Counterfeiting Applications. J. Mater. Chem. C Mater. 2021, 9, 12828–12838. [Google Scholar] [CrossRef]
- Gu, H.; Liu, W.; Li, H.; Sun, W.; Du, J.; Fan, J.; Peng, X. 2,1,3-Benzothiadiazole Derivative AIEgens for Smart Phototheranostics. Coord. Chem. Rev. 2022, 473, 214803. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, W.; Guo, H.; Liang, J.; Huang, D.; Xiao, H. Two Spirobifluorene-Based Fluorescent Probes with Aggregation-Induced Emission Properties: Synthesis and Application in the Detection of Zn2+ and Cell Imaging. J. Mater. Chem. C Mater. 2019, 7, 2240–2249. [Google Scholar] [CrossRef]
- Fowler, W.C. Intrinsic Fluorescence in Peptide Amphiphile Micelles with Protein-Inspired Phosphate Sensing. Biomacromolecules 2022, 23, 4804–4813. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Phenomenon, Mechanism and Applications. Chem. Commun. 2009, 29, 4332–4353. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Li, Q.; Wang, Z.; Shen, J.; Yu, W. AIEgens: Next Generation Signaling Source for Immunoassays? ACS Sens. 2022, 7, 3243–3257. [Google Scholar] [CrossRef]
- Han, G.; Kim, D.; Park, Y.; Bouffard, J.; Kim, Y. Excimers Beyond Pyrene: A Far-Red Optical Proximity Reporter and Its Application to the Label-Free Detection of DNA. Angew. Chem. 2015, 127, 3984–3988. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Bu, L.; Li, J.; Yang, C.; Li, X.; Tao, Y.; Yang, W. Aqueous Nanoaggregation-Enhanced One- and Two-Photon Fluorescence, Crystalline J-Aggregation-Induced Red Shift, and Amplified Spontaneous Emission of 9,10-Bis(p-Dimethylaminostyryl)anthracene. J. Phys. Chem. C 2012, 116, 15576–15583. [Google Scholar] [CrossRef]
- An, B.-K.; Kwon, S.-K.; Jung, S.-D.; Park, S.Y. Enhanced Emission and Its Switching in Fluorescent Organic Nanoparticles. J. Am. Chem. Soc. 2002, 124, 14410–14415. [Google Scholar] [CrossRef]
- Hu, R.; Lager, E.; Aguilar-Aguilar, A.; Liu, J.; Lam, J.W.Y.; Sung, H.H.Y.; Williams, I.D.; Zhong, Y.; Wong, K.S.; Peña-Cabrera, E.; et al. Twisted Intramolecular Charge Transfer and Aggregation-Induced Emission of BODIPY Derivatives. J. Phys. Chem. C 2009, 113, 15845–15853. [Google Scholar] [CrossRef]
- Hu, R.; Li, S.; Zeng, Y.; Chen, J.; Wang, S.; Li, Y.; Yang, G. Understanding the Aggregation Induced Emission Enhancement for a Compound with Excited State Intramolecular Proton Transfer Character. Phys. Chem. Chem. Phys. 2011, 13, 2044–2051. [Google Scholar] [CrossRef]
- Chen, Y.; Lam, J.W.Y.; Kwok, R.T.K.; Liu, B.; Tang, B.Z. Aggregation-Induced Emission: Fundamental Understanding and Future Developments. Mater. Horiz. 2019, 6, 428–433. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-Induced Emission of 1-Methyl-1,2,3,4,5-Pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, H.; Niu, Y.; Li, W.; Wang, D.; Peng, Q.; Shuai, Z.; Liang, W. Spectroscopic Signature of the Aggregation-Induced Emission Phenomena Caused by Restricted Nonradiative Decay: A Theoretical Proposal. J. Phys. Chem. C 2015, 119, 5040–5047. [Google Scholar] [CrossRef]
- Keremane, K.; Pellegrin, Y.; Planchat, A.; Odobel, F.; Adhikari, A.V. New carbazole-based sensitizers for p-type DSSCs: Impact of the position of acceptor units on device performance. J. Phys. Chem. C 2022, 126, 12383–12390. [Google Scholar] [CrossRef]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef]
- Ding, D.; Li, K.; Liu, B.; Tang, B.Z. Bioprobes Based on AIE Fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Qian, X.; Xu, Z. Fluorescence Imaging of Metal Ions Implicated in Diseases. Chem. Soc. Rev. 2015, 44, 4487–4493. [Google Scholar] [CrossRef]
- Liang, J.; Tang, B.Z.; Liu, B. Specific Light-up Bioprobes Based on AIEgen Conjugates. Chem. Soc. Rev. 2015, 44, 2798–2811. [Google Scholar] [CrossRef]
- Kwok, R.T.K.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. Biosensing by Luminogens with Aggregation-Induced Emission Characteristics. Chem. Soc. Rev. 2015, 44, 4228–4238. [Google Scholar] [CrossRef]
- Choi, H.; Kim, S.; Lee, S.; Kim, C.; Ryu, J.-H. Array-Based Protein Sensing Using an Aggregation-Induced Emission (AIE) Light-Up Probe. ACS Omega 2018, 3, 9276–9281. [Google Scholar] [CrossRef]
- Nguyen, P.Q.; Soenksen, L.R.; Donghia, N.M.; Angenent-Mari, N.M.; de Puig, H.; Huang, A.; Lee, R.; Slomovic, S.; Galbersanini, T.; Lansberry, G.; et al. Wearable Materials with Embedded Synthetic Biology Sensors for Biomolecule Detection. Nat. Biotechnol. 2021, 39, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kim, H.; Han, J.; Nguyen, V.N.; Peng, X.; Yoon, J. Activity-Based Smart AIEgens for Detection, Bioimaging, and Therapeutics: Recent Progress and Outlook. Aggregate 2021, 2, e51. [Google Scholar] [CrossRef]
- Mai, D.K.; Badon, I.W.; Lim, J.M.; Vales, T.P.; Lee, J.; Kim, H.J. Triphenylphosphonium-functionalized BODIPY derivatives for mitochondria-targeted cell imaging and fluorescence turn-on sensing with protein selectivity. Dyes Pigm. 2023, 208, 110856. [Google Scholar] [CrossRef]
- Khan, I.M.; Niazi, S.; Iqbal Khan, M.K.; Pasha, I.; Mohsin, A.; Haider, J.; Iqbal, M.W.; Rehman, A.; Yue, L.; Wang, Z. Recent Advances and Perspectives of Aggregation-Induced Emission as an Emerging Platform for Detection and Bioimaging. TrAC Trends Anal. Chem. 2019, 119, 115637. [Google Scholar] [CrossRef]
- Chua, M.H.; Shah, K.W.; Zhou, H.; Xu, J. Recent Advances in Aggregation-Induced Emission Chemosensors for Anion Sensing. Molecules 2019, 24, 2711. [Google Scholar] [CrossRef]
- Zhang, G.-F.; Aldred, M.P.; Gong, W.-L.; Li, C.; Zhu, M.-Q. Utilising Tetraphenylethene as a Dual Activator for Intramolecular Charge Transfer and Aggregation Induced Emission. Chem. Commun. 2012, 48, 7711–7713. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, Y.; Ye, H.; Huang, K.; Lv, Z.; Ke, Y. Different Effects of Titanium Dioxide Nanoparticles Instillation in Young and Adult Mice on DNA Methylation Related with Lung Inflammation and Fibrosis. Ecotoxicol. Environ. Saf. 2019, 176, 1–10. [Google Scholar] [CrossRef]
- Yang, C.; Li, Y.; Wang, J.; He, J.; Hou, H.; Li, K. Fast and Highly Selective Detection of Acetaldehyde in Liquor and Spirits by Forming Aggregation-Induced Emission Luminogen. Sens. Actuators B Chem. 2019, 285, 617–624. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Tian, X.; Yang, C.; Lu, L.; Zhou, Z.; Huang, Y.; Nie, Y. Construction of Salicylaldehyde Analogues as Turn-on Fluorescence Probes and Their Electronic Effect on Sensitive and Selective Detection of As(v) in Groundwater. Anal. Methods 2019, 11, 955–964. [Google Scholar] [CrossRef]
- Niu, Q.; Sun, T.; Li, T.; Guo, Z.; Pang, H. Highly Sensitive and Selective Colorimetric/Fluorescent Probe with Aggregation Induced Emission Characteristics for Multiple Targets of Copper, Zinc and Cyanide Ions Sensing and Its Practical Application in Water and Food Samples. Sens. Actuators B Chem. 2018, 266, 730–743. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Cao, H.; Li, Q.; Li, Y.; Han, M.; Wang, H.; Li, J. Photodynamic Therapy with Liposomes Encapsulating Photosensitizers with Aggregation-Induced Emission. Nano Lett. 2019, 19, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Jalili, R.; Khataee, A. Aluminum(III) Triggered Aggregation-Induced Emission of Glutathione-Capped Copper Nanoclusters as a Fluorescent Probe for Creatinine. Microchim. Acta 2019, 186, 29. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nie, J.; Niu, J.; Wang, W.; Lin, W. An AIE + ESIPT Ratiometric Fluorescent Probe for Monitoring Sulfur Dioxide with Distinct Ratiometric Fluorescence Signals in Mammalian Cells, Mouse Embryonic Fibroblast and Zebrafish. J. Mater. Chem. B 2018, 6, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Duan, Q.; Zheng, G.; Yang, L.; Zhang, J.; Wang, Y.; Zhang, H.; He, J.; Sun, H.; Ho, D. An Ultra-Sensitive and Ratiometric Fluorescent Probe Based on the DTBET Process for Hg2+ Detection and Imaging Applications. Analyst 2019, 144, 1353–1360. [Google Scholar] [CrossRef]
- Pathan, S.; Jalal, M.; Prasad, S.; Bose, S. Aggregation-Induced Enhanced Photoluminescence in Magnetic Graphene Oxide Quantum Dots as a Fluorescence Probe for As(Iii) Sensing. J. Mater. Chem. A Mater. 2019, 7, 8510–8520. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, K.; Xiao, K.; Xu, Q.; Wang, L.; Li, P.; Zhou, J.; Zhao, D.; Bai, L.; Cheng, Y.; et al. Biomolecule Sensors Based on Organic Electrochemical Transistors. npj Flex. Electron. 2025, 9, 9. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z. AIE Probes towards Biomolecules: The Improved Selectivity with the Aid of Graphene Oxide. Sci. China Chem. 2015, 58, 1800–1809. [Google Scholar] [CrossRef]
- Alam, P.; Leung, N.L.C.; Zhang, J.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. AIE-Based Luminescence Probes for Metal Ion Detection. Coord. Chem. Rev. 2021, 429, 213693. [Google Scholar] [CrossRef]
- Singh, V. Advances in Synthetic Biology; Springer: Singapore, 2020; ISBN 9789811500817. [Google Scholar]
- Christensen, T.M.; Jama, M.; Ponek, V.; Lyon, E.; Wilson, J.A.; Hoffmann, M.L.; Bejjani, B.A. Design, Development, Validation, and Use of Synthetic Nucleic Acid Controls for Diagnostic Purposes and Application to Cystic Fibrosis Testing. J. Mol. Diagn. 2007, 9, 315–319. [Google Scholar] [CrossRef]
- Erickson, R.P. Somatic Gene Mutation and Human Disease Other than Cancer: An Update. Mutat. Res./Rev. Mutat. Res. 2010, 705, 96–106. [Google Scholar] [CrossRef]
- Chang, Y.; Jin, L.; Duan, J.; Lu, Y. New conjugated poly(pyridinium salt) derivative: AIE characteristics, the interaction with DNA and selective fluorescence enhancement induced by dsDNA. RSC Adv. 2015, 5, 103358–103364. [Google Scholar] [CrossRef]
- Maurya, R.; Bhattacharjee, G.; Gohil, N.; Alzahrani, K.J.; Singh, V. Chapter Nine—Aggregation Induced Emission Molecules for Detection of Nucleic Acids. In Progress in Molecular Biology and Translational Science; Bhosale, R.S., Singh, V., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 184, pp. 219–227. ISSN 1877-1173. [Google Scholar]
- Ligasová, A.; Koberna, K. Dna Dyes—Highly Sensitive Reporters of Cell Quantification: Comparison with Other Cell Quantification Methods. Molecules 2021, 26, 5515. [Google Scholar] [CrossRef] [PubMed]
- Dragan, A.I.; Pavlovic, R.; McGivney, J.B.; Casas-Finet, J.R.; Bishop, E.S.; Strouse, R.J.; Schenerman, M.A.; Geddes, C.D. SYBR Green I: Fluorescence Properties and Interaction with DNA. J. Fluoresc. 2012, 22, 1189–1199. [Google Scholar] [CrossRef]
- Tuma, R.S.; Beaudet, M.P.; Jin, X.; Jones, L.J.; Cheung, C.-Y.; Yue, S.; Singer, V.L. Characterization of SYBR Gold Nucleic Acid Gel Stain: A Dye Optimized for Use with 300-Nm Ultraviolet Transilluminators. Anal. Biochem. 1999, 268, 278–288. [Google Scholar] [CrossRef]
- Dragan, A.I.; Casas-Finet, J.R.; Bishop, E.S.; Strouse, R.J.; Schenerman, M.A.; Geddes, C.D. Characterization of PicoGreen Interaction with DsDNA and the Origin of Its Fluorescence Enhancement upon Binding. Biophys. J. 2010, 99, 3010–3019. [Google Scholar] [CrossRef]
- Xu, X.; Yan, S.; Zhou, Y.; Huang, R.; Chen, Y.; Wang, J.; Weng, X.; Zhou, X. A Novel Aggregation-Induced Emission Fluorescent Probe for Nucleic Acid Detection and Its Applications in Cell Imaging. Bioorg Med. Chem. Lett. 2014, 24, 1654–1656. [Google Scholar] [CrossRef]
- Li, Y.; Kwok, R.T.K.; Tang, B.Z.; Liu, B. Specific Nucleic Acid Detection Based on Fluorescent Light-up Probe from Fluorogens with Aggregation-Induced Emission Characteristics. RSC Adv. 2013, 3, 10135–10138. [Google Scholar] [CrossRef]
- Xu, J.-P.; Song, Z.-G.; Fang, Y.; Mei, J.; Jia, L.; Qin, A.J.; Sun, J.Z.; Ji, J.; Tang, B.Z. Label-Free Fluorescence Detection of Mercury(Ii) and Glutathione Based on Hg2+-DNA Complexes Stimulating Aggregation-Induced Emission of a Tetraphenylethene Derivative. Analyst 2010, 135, 3002–3007. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, S.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. Water-Soluble Tetraphenylethene Derivatives as Fluorescent “Light-up” Probes for Nucleic Acid Detection and Their Applications in Cell Imaging. Chemistry 2013, 8, 1806–1812. [Google Scholar] [CrossRef]
- Hu, Y.; Cao, X.; Guo, Y.; Zheng, X.; Li, D.; Chen, S.K.; Chen, G.; You, J. An Aggregation-Induced Emission Fluorogen/DNA Probe Carrying an Endosome Escaping Pass for Tracking Reduced Thiol Compounds in Cells. Anal. Bioanal. Chem. 2020, 412, 7811–7817. [Google Scholar] [CrossRef]
- Kawamura, K.; Matsumoto, A.; Murashima, T. Facile DNA Detection Based on Fluorescence Switching of A Hydrophobic AIE Dye-Labeled Peptide Nucleic Acid Probe by Aggregation/Disaggregation. Int. J. Med. Nano Res. 2015, 2, 11. [Google Scholar] [CrossRef]
- Gao, Y.; He, Z.; He, X.; Zhang, H.; Weng, J.; Yang, X.; Meng, F.; Luo, L.; Tang, B.Z. Dual-Color Emissive AIEgen for Specific and Label-Free Double-Stranded DNA Recognition and Single-Nucleotide Polymorphisms Detection. J. Am. Chem. Soc. 2019, 141, 20097–20106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Y.; Sun, X.; Bai, J.; Peng, Y.; Ning, B.; Gao, Z.; Liu, B. Ultrasensitive Competitive Detection of Patulin Toxin by Using Strand Displacement Amplification and DNA G-Quadruplex with Aggregation-Induced Emission. Anal. Chim. Acta 2020, 1106, 161–167. [Google Scholar] [CrossRef]
- Niu, S.; Bi, C.; Song, W. Detection of DNA Methyltransferase Activity Using Template-Free DNA Polymerization Amplification Based on Aggregation-Induced Emission. Anal. Biochem. 2020, 590, 113532. [Google Scholar] [CrossRef]
- Zhang, Q.; Yin, B.; Huang, Y.; Gu, Y.; Yan, J.; Chen, J.; Li, C.; Zhang, Y.; Wong, S.H.D.; Yang, M. A Dual “Turn-on” Biosensor Based on AIE Effect and FRET for in Situ Detection of MiR-125b Biomarker in Early Alzheimer’s Disease. Biosens. Bioelectron. 2023, 230, 115270. [Google Scholar] [CrossRef]
- Duo, Y.; Xiang, Z.; Gao, G.; Luo, G.; Tang, B.Z. Biomedical Application of Aggregation-Induced Emission Luminogen-Based Fluorescent Sensors. TrAC Trends Anal. Chem. 2023, 167, 117252. [Google Scholar] [CrossRef]
- Chen, Y.; Min, X.; Zhang, X.; Zhang, F.; Lu, S.; Xu, L.P.; Lou, X.; Xia, F.; Zhang, X.; Wang, S. AIE-Based Superwettable Microchips for Evaporation and Aggregation Induced Fluorescence Enhancement Biosensing. Biosens. Bioelectron. 2018, 111, 124–130. [Google Scholar] [CrossRef]
- Zhao, E.; Lai, P.; Xu, Y.; Zhang, G.; Chen, S. Fluorescent Materials with Aggregation-Induced Emission Characteristics for Array-Based Sensing Assay. Front. Chem. 2020, 8, 288. [Google Scholar] [CrossRef]
- Zhang, J.; Chai, X.; He, X.-P.; Kim, H.-J.; Yoon, J.; Tian, H. Fluorogenic Probes for Disease-Relevant Enzymes. Chem. Soc. Rev. 2019, 48, 683–722. [Google Scholar] [CrossRef]
- Filer, J.E.; Channon, R.B.; Henry, C.S.; Geiss, B.J. A Nuclease Protection ELISA Assay for Colorimetric and Electrochemical Detection of Nucleic Acids. Anal. Methods 2019, 11, 1027–1034. [Google Scholar] [CrossRef]
- de Poulpiquet, A.; Diez-Buitrago, B.; Dumont Milutinovic, M.; Sentic, M.; Arbault, S.; Bouffier, L.; Kuhn, A.; Sojic, N. Dual Enzymatic Detection by Bulk Electrogenerated Chemiluminescence. Anal. Chem. 2016, 88, 6585–6592. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Pan, W.; Li, N.; Tang, B. Fluorescent Probes for Organelle-Targeted Bioactive Species Imaging. Chem. Sci. 2019, 10, 6035–6071. [Google Scholar] [CrossRef] [PubMed]
- Keremane, K.; Rao, R.; Adhikari, A.V. Simple 3, 6-disubstituted Carbazoles as Potential Hole Transport Materials: Photophysical, Electrochemical and Theoretical Studies. Photochem. Photobiol. 2021, 97, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Tang, B.Z. Fluorescent Sensors Based on Aggregation-Induced Emission: Recent Advances and Perspectives. ACS Sens. 2017, 2, 1382–1399. [Google Scholar] [CrossRef]
- Sun, H.; Wen, J.; Chen, S.; Han, Y.; Ogaji, O.D.; Biu, A.M.; Cui, H.; Meng, X.; Li, J.; Du, K.; et al. Review of Advancement in Aggregation-Induced Emission-Based Fluorescent Biosensors for Enzyme Detection: Mechanisms and Biomedical Applications. Anal. Chim. Acta 2025, 1346, 343716. [Google Scholar] [CrossRef]
- Liang, J.; Kwok, R.T.K.; Shi, H.; Tang, B.Z.; Liu, B. Fluorescent Light-up Probe with Aggregation-Induced Emission Characteristics for Alkaline Phosphatase Sensing and Activity Study. ACS Appl. Mater. Interfaces 2013, 5, 8784–8789. [Google Scholar] [CrossRef]
- Li, Y.; Xie, R.; Pang, X.; Zhou, Z.; Xu, H.; Gu, B.; Wu, C.; Li, H.; Zhang, Y. Aggregation-Induced Emission Fluorescent Probe for Monitoring Endogenous Alkaline Phosphatase in Living Cells. Talanta 2019, 205, 120143. [Google Scholar] [CrossRef]
- Song, Z.; Kwok, R.T.K.; Zhao, E.; He, Z.; Hong, Y.; Lam, J.W.Y.; Liu, B.; Tang, B.Z. A Ratiometric Fluorescent Probe Based on ESIPT and AIE Processes for Alkaline Phosphatase Activity Assay and Visualization in Living Cells. ACS Appl. Mater. Interfaces 2014, 6, 17245–17254. [Google Scholar] [CrossRef]
- Qu, F.; Meng, L.; Zi, Y.; You, J. Ratiometric Detection of Alkaline Phosphatase Based on Aggregation-Induced Emission Enhancement. Anal. Bioanal. Chem. 2019, 411, 7431–7440. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, R.; Cheng, X.; Xu, S.; Liu, B. A FRET Probe with AIEgen as the Energy Quencher: Dual Signal Turn-on for Self-Validated Caspase Detection. Chem. Sci. 2016, 7, 4245–4250. [Google Scholar] [CrossRef]
- Han, A.; Wang, H.; Kwok, R.T.K.; Ji, S.; Li, J.; Kong, D.; Tang, B.Z.; Liu, B.; Yang, Z.; Ding, D. Peptide-Induced AIEgen Self-Assembly: A New Strategy to Realize Highly Sensitive Fluorescent Light-Up Probes. Anal. Chem. 2016, 88, 3872–3878. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.N.; Jiang, Y.F.; Ru, J.N.; Lu, J.H.; Ding, B.; Wu, J. Amino Acid Metabolism in Health and Disease. Signal Transduct. Target Ther. 2023, 8, 345. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.-H.; Han, X.; Shao, Y.; Fang, J.; Zhang, Z.-H.; Wang, Y.-W.; Peng, Y. Fluorescein-Based Chromogenic and Ratiometric Fluorescence Probe for Highly Selective Detection of Cysteine and Its Application in Bioimaging. Anal. Chem. 2017, 89, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Xue, X.; Zhang, F.; Zhang, B.; Li, T.; Peng, L.; Cho, D.-H.; Chen, H.; Fang, J.; Chen, X. A Novel AIEgen-Based Probe for Detecting Cysteine in Lipid Droplets. Anal. Chim. Acta 2020, 1127, 20–28. [Google Scholar] [CrossRef]
- Zhang, M.; Saha, M.L.; Wang, M.; Zhou, Z.; Song, B.; Lu, C.; Yan, X.; Li, X.; Huang, F.; Yin, S.; et al. Multicomponent Platinum(II) Cages with Tunable Emission and Amino Acid Sensing. J. Am. Chem. Soc. 2017, 139, 5067–5074. [Google Scholar] [CrossRef]
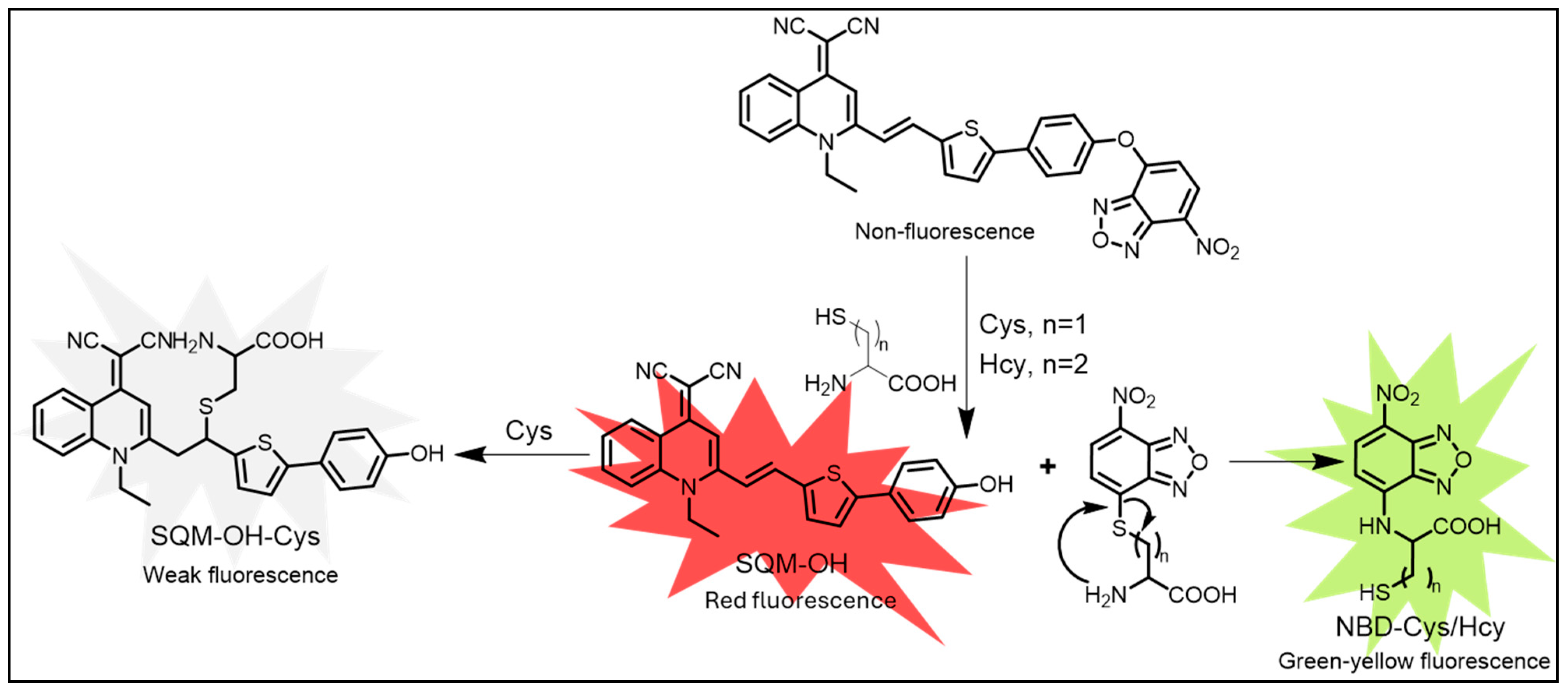
- Song, H.; Zhou, Y.; Qu, H.; Xu, C.; Wang, X.; Liu, X.; Zhang, Q.; Peng, X. A Novel AIE Plus ESIPT Fluorescent Probe with a Large Stokes Shift for Cysteine and Homocysteine: Application in Cell Imaging and Portable Kit. Ind. Eng. Chem. Res. 2018, 57, 15216–15223. [Google Scholar] [CrossRef]
- Chua, M.H.; Zhou, H.; Lin, T.T.; Wu, J.; Xu, J. Triphenylethylene- and Tetraphenylethylene-Functionalized 1,3-Bis(Pyrrol-2-Yl)Squaraine Dyes: Synthesis, Aggregation-Caused Quenching to Aggregation-Induced Emission, and Thiol Detection. ACS Omega 2018, 3, 16424–16435. [Google Scholar] [CrossRef]
- Jiang, R.; Liu, N.; Li, F.; Fu, W.; Zhou, Y.; Zhang, Y. Novel PSMA-Coated on-off-On Fluorescent Chemosensor Based on Organic Dots with AIEgens for Detection of Copper (II), Iron (III) and Cysteine. Polymers 2018, 10, 786. [Google Scholar] [CrossRef]
- Zhou, J.; Xiao, Y.; Zhang, X.; Wang, S. A Novel Ratiometric Fluorescence Nanoprobe Based on Aggregation-Induced Emission of Silver Nanoclusters for the Label-Free Detection of Biothiols. Talanta 2018, 188, 623–629. [Google Scholar] [CrossRef]
- Li, N.-N.; Shi, N.-N.; Yang, D.; Wu, R.-X.; Xu, C.-G.; Zhu, B.; Shao, F.; Zhang, X.; Bi, S.-Y.; Fan, Y.-H. Solid-State Fluorescent Switch Based on the Intercoversion of J-Aggregation and Dimer and Aggregation Pattern-Dependent Fluorescence Colorimetric Sensing of GSH/Zn2+/Cd2+. J. Mol. Liq. 2021, 342, 116946. [Google Scholar] [CrossRef]
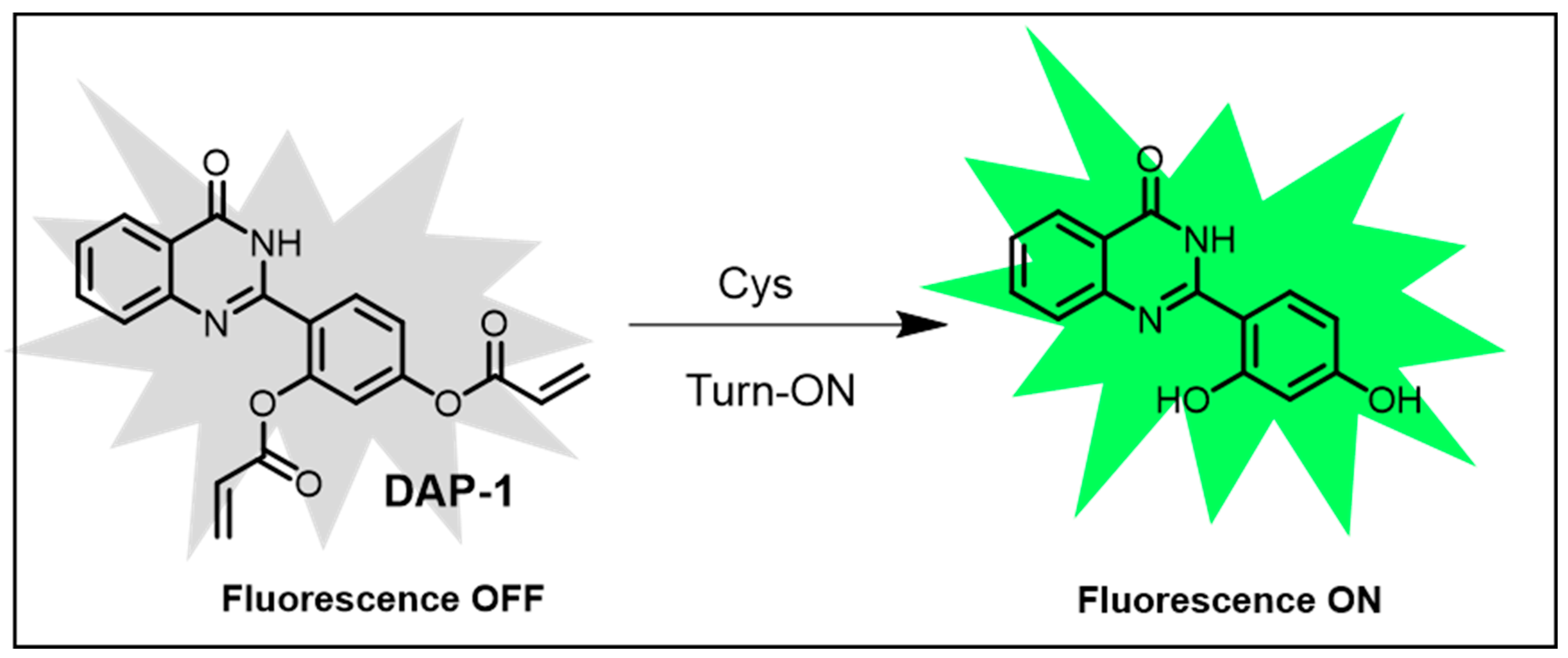
- Sheng, H.; Hu, Y.; Zhou, Y.; Fan, S.; Cao, Y.; Zhao, X.; Yang, W. A Hydroxyphenylquinazolinone-Based Fluorescent Probe for Turn-on Detection of Cysteine with a Large Stokes Shift and Its Application in Living Cells. J. Photochem. Photobiol. A Chem. 2018, 364, 750–757. [Google Scholar] [CrossRef]
- Shu, T.; Lin, X.; Zhou, Z.; Zhao, D.; Xue, F.; Zeng, F.; Wang, J.; Wang, C.; Su, L.; Zhang, X. Understanding Stimuli-Responsive Oligomer Shell of Silver Nanoclusters with Aggregation-Induced Emission via Chemical Etching and Their Use as Sensors. Sens. Actuators B Chem. 2019, 286, 198–205. [Google Scholar] [CrossRef]
- Zhang, X.; Kong, R.; Tan, Q.; Qu, F.; Qu, F. A Label-Free Fluorescence Turn-on Assay for Glutathione Detection by Using MnO2 Nanosheets Assisted Aggregation-Induced Emission-Silica Nanospheres. Talanta 2017, 169, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Peng, Z.; Ji, M.; Chen, J.; Wang, P. Highly Selective Fluorescent Probe Based on AIE for Identifying Cysteine/Homocysteine. Bioorg Chem. 2022, 126, 105902. [Google Scholar] [CrossRef]
- Costantini, A.; Vaudano, E.; Pulcini, L.; Carafa, T.; Garcia-Moruno, E. An Overview on Biogenic Amines in Wine. Beverages 2019, 5, 19. [Google Scholar] [CrossRef]
- Wójcik, W.; Łukasiewicz, M.; Puppel, K. Biogenic Amines: Formation, Action and Toxicity—A Review. J. Sci. Food Agric. 2021, 101, 2634–2640. [Google Scholar] [CrossRef]
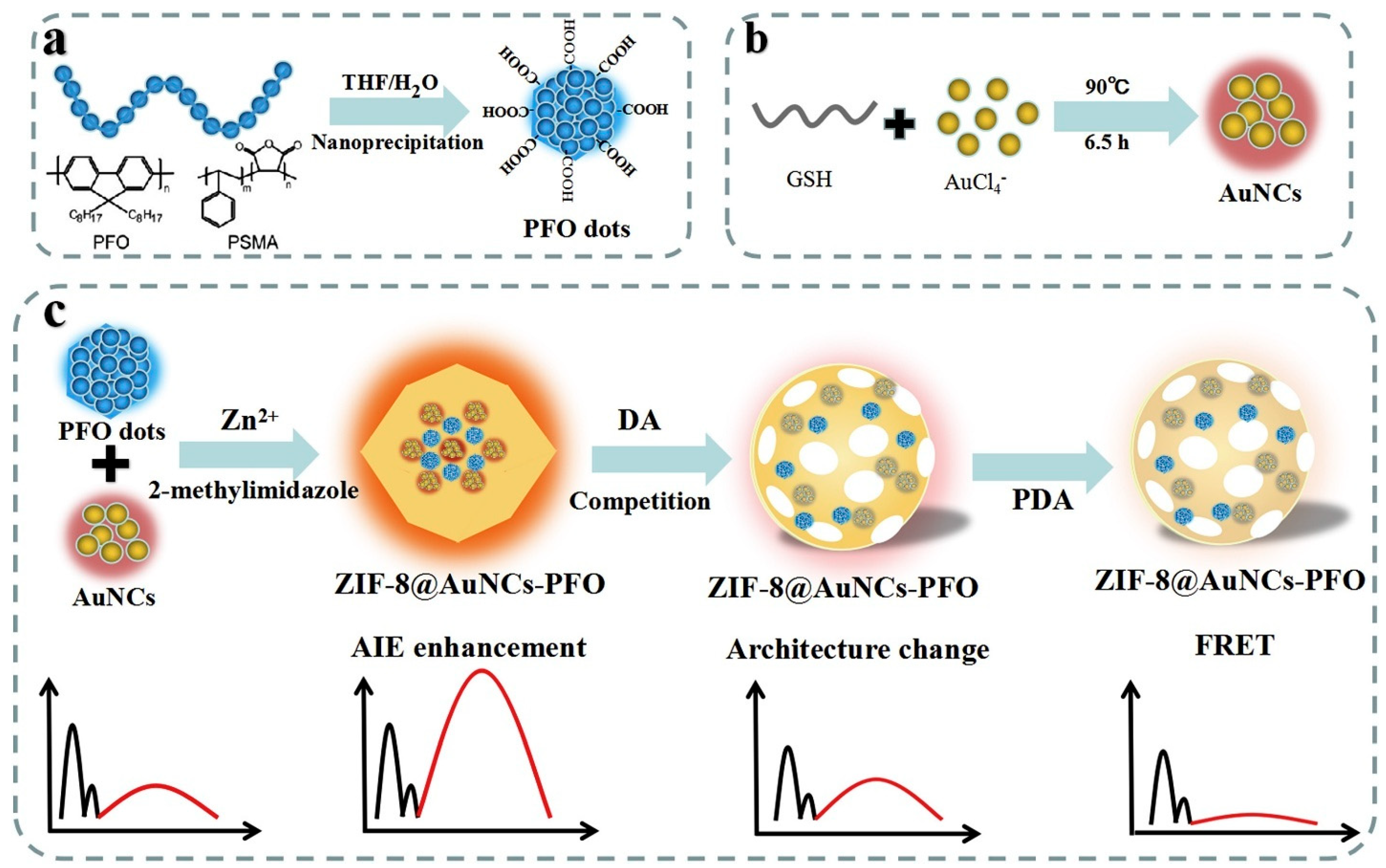
- Shao, K.; You, J.; Ye, S.; Gu, D.; Wang, T.; Teng, Y.; Shen, Z.; Pan, Z. Gold Nanoclusters-Poly(9,9-Dioctylfluorenyl-2,7-Diyl) Dots@zeolitic Imidazolate Framework-8 (ZIF-8) Nanohybrid Based Probe for Ratiometric Analysis of Dopamine. Anal. Chim. Acta 2020, 1098, 102–109. [Google Scholar] [CrossRef]
- Qu, F.; Fa, Q.; Yin, T.; Jiang, D.; Zhao, X. Aggregation-Induced Emission of Lead Halide Perovskites Modulated by Dispersed/Aggregated Gold Nanoparticles for Application in the Detection of Dopamine. Mater. Res. Bull. 2021, 142, 111405. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, L.; Zhang, X.Y.; Wang, X.H.; Zhou, J.; Sun, Z.; Li, N.B.; Luo, H.Q. Ratiometric Fluorescence Detection of Dopamine Based on Effect of Ligand on the Emission of Ag Nanoclusters and Aggregation-Induced Emission Enhancement. Sens. Actuators B Chem. 2020, 310, 127858. [Google Scholar] [CrossRef]
- Jiang, G.; Zhu, W.; Chen, Q.; Li, X.; Zhang, G.; Li, Y.; Fan, X.; Wang, J. Selective Fluorescent Probes for Spermine and 1-Adamantanamine Based on the Supramolecular Structure Formed between AIE-Active Molecule and Cucurbit[n]Urils. Sens. Actuators B Chem. 2018, 261, 602–607. [Google Scholar] [CrossRef]
- Naik, V.G.; Kumar, V.; Bhasikuttan, A.C.; Kadu, K.; Ramanan, S.R.; Bhosle, A.A.; Banerjee, M.; Chatterjee, A. Solid-Supported Amplification of Aggregation Emission: A Tetraphenylethylene–Cucurbit[6]Uril@Hydroxyapatite-Based Supramolecular Sensing Assembly for the Detection of Spermine and Spermidine in Human Urine and Blood. ACS Appl. Bio Mater. 2021, 4, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
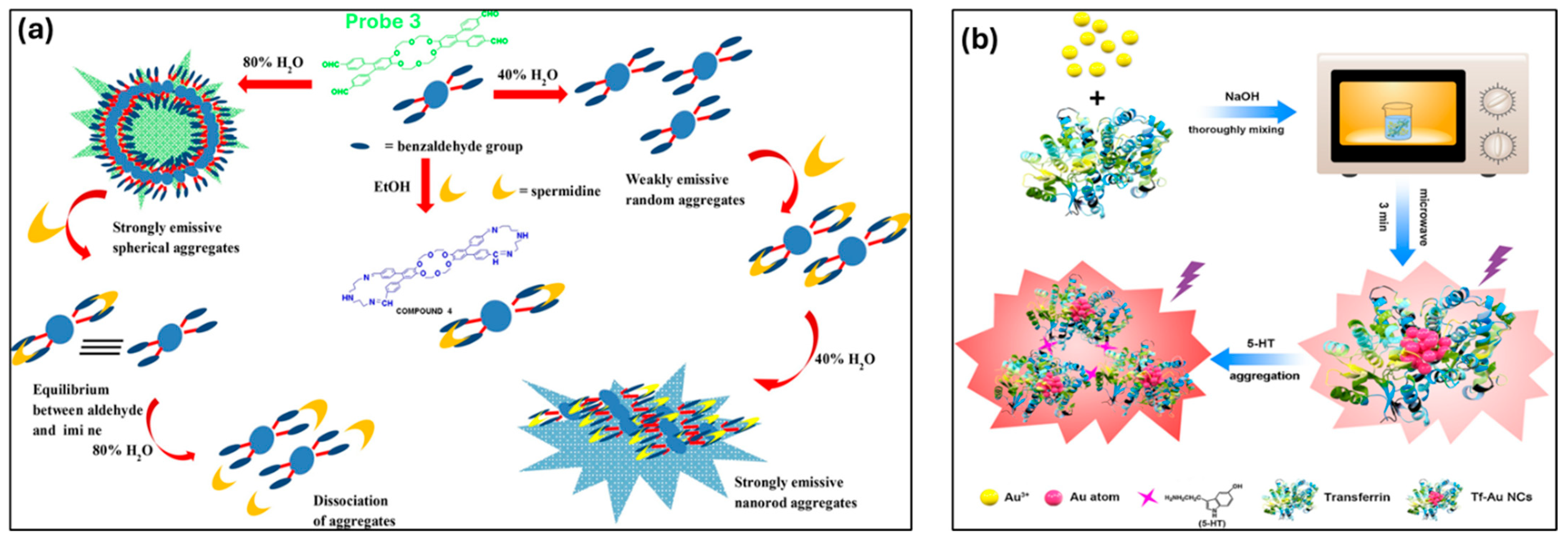
- Tejpal, R.; Kumar, M.; Bhalla, V. Spermidine Induced Aggregation of Terphenyl Derivative: An Efficient Probe for Detection of Spermidine in Living Cells. Sens. Actuators B Chem. 2018, 258, 841–849. [Google Scholar] [CrossRef]
- Sha, Q.; Sun, B.; Yi, C.; Guan, R.; Fei, J.; Hu, Z.; Liu, B.; Liu, X. A Fluorescence Turn-on Biosensor Based on Transferrin Encapsulated Gold Nanoclusters for 5-Hydroxytryptamine Detection. Sens. Actuators B Chem. 2019, 294, 177–184. [Google Scholar] [CrossRef]
- Zang, T.; Xie, Y.; Su, S.; Liu, F.; Chen, Q.; Jing, J.; Zhang, R.; Niu, G.; Zhang, X. In Vitro Light-Up Visualization of a Subunit-Specific Enzyme by an AIE Probe via Restriction of Single Molecular Motion. Angew. Chem. Int. Ed. 2020, 59, 10003–10007. [Google Scholar] [CrossRef]
- Yan, L.; Raj, P.; Yao, W.; Ying, H. Glucose Metabolism in Pancreatic Cancer. Cancers 2019, 11, 1460. [Google Scholar] [CrossRef]
- Ahmed, I.; Jiang, N.; Shao, X.; Elsherif, M.; Alam, F.; Salih, A.; Butta, H.; Yetisen, A.K. Recent Advances in Optical Sensors for Continuous Glucose Monitoring. Sens. Diagn. 2022, 1, 1098–1125. [Google Scholar] [CrossRef]
- Wu, X.; Wu, P.; Gu, M.; Xue, J. Ratiometric Fluorescent Probe Based on AuNCs Induced AIE for Quantification and Visual Sensing of Glucose. Anal. Chim. Acta 2020, 1104, 140–146. [Google Scholar] [CrossRef]
- Sun, Y.; Shu, T.; Ma, J.; Dai, Q.; Peng, P.; Zhou, Z.; Zhou, X.; Su, L.; Zhang, X. Rational Design of ZIF-8 for Constructing Luminescent Biosensors with Glucose Oxidase and AIE-Type Gold Nanoclusters. Anal. Chem. 2022, 94, 3408–3417. [Google Scholar] [CrossRef]
- Xie, S.; Liu, Q.; Zhu, F.; Chen, M.; Wang, L.; Xiong, Y.; Zhu, Y.; Zheng, Y.; Chen, X. AIE-Active Metal–Organic Frameworks: Facile Preparation, Tunable Light Emission, Ultrasensitive Sensing of Copper(Ii) and Visual Fluorescence Detection of Glucose. J. Mater. Chem. C Mater. 2020, 8, 10408–10415. [Google Scholar] [CrossRef]
- Mei, H.; Wang, J.; Zhu, X.; Sun, J.; Shi, W.; Wang, H.; Qu, S.; Wang, X. Ce3+ and Fe2+ Co-Enhanced Ratiometric Fluorescence Probe Utilizing Copper Nanoclusters and Coumarin for Sensitive Assay of Hydrogen Peroxide and Glucose. Ecotoxicol. Environ. Saf. 2022, 245, 114117. [Google Scholar] [CrossRef]
- Gou, S.; Shi, Y.; Li, P.; Wang, H.; Li, T.; Zhuang, X.; Li, W.; Wang, Z. Stimuli-Responsive Luminescent Copper Nanoclusters in Alginate and Their Sensing Ability for Glucose. ACS Appl. Mater. Interfaces 2019, 11, 6561–6567. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wang, J.; Qileng, A.; Chen, S.; Zhang, Q.; Liu, W.; Liu, Y. “Two-in-One” Portable Strategy for Non-GOx Glucose Monitoring: Electrochromic and Fluorescent Assay Using Reversible Hydrogel. Sens. Actuators B Chem. 2021, 349, 130744. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Liang, Y.; Gao, N.; Liu, C.; Wang, S.; Yin, X.; Li, G. Poly(Ionic Liquid)s as a Distinct Receptor Material to Create a Highly-Integrated Sensing Platform for Efficiently Identifying Numerous Saccharides. Chem. Sci. 2019, 10, 6617–6623. [Google Scholar] [CrossRef]
- Deng, J.; Walther, A. ATP-Responsive and ATP-Fueled Self-Assembling Systems and Materials. Adv. Mater. 2020, 32, 2002629. [Google Scholar] [CrossRef]
- Geng, L.-Y.; Zhao, Y.; Kamya, E.; Guo, J.-T.; Sun, B.; Feng, Y.-K.; Zhu, M.-F.; Ren, X.-K. Turn-off/on Fluorescent Sensors for Cu2+ and ATP in Aqueous Solution Based on a Tetraphenylethylene Derivative. J. Mater. Chem. C Mater. 2019, 7, 2640–2645. [Google Scholar] [CrossRef]
- Ding, A.-X.; Shi, Y.-D.; Zhang, K.-X.; Sun, W.; Tan, Z.-L.; Lu, Z.-L.; He, L. Self-Assembled Aggregation-Induced Emission Micelle (AIE Micelle) as Interfacial Fluorescence Probe for Sequential Recognition of Cu2+ and ATP in Water. Sens. Actuators B Chem. 2018, 255, 440–447. [Google Scholar] [CrossRef]
- Li, H.; Guo, Z.; Xie, W.; Sun, W.; Ji, S.; Tian, J.; Lv, L. A Label-Free Fluorometric Aptasensor for Adenosine Triphosphate (ATP) Detection Based on Aggregation-Induced Emission Probe. Anal. Biochem. 2019, 578, 60–65. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Yin, J.; Li, M.; Luo, H.; Yang, Y.; Xu, X.; Yong, Q.; Wang, S. An Easily Available Camphor-Derived Ratiometric Fluorescent Probe with AIE Feature for Sequential Ga3+ and ATP Sensing in a near-Perfect Aqueous Media and Its Bio-Imaging in Living Cells and Mice. Sens. Actuators B Chem. 2020, 320, 128249. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, B.W.; Moon, H.; Yoo, H.; Kang, R.H.; Hur, J.K.; Oh, Y.; Kim, B.M.; Kim, D. AIEgen-Based Nanoprobe for the ATP Sensing and Imaging in Cancer Cells and Embryonic Stem Cells. Anal. Chim. Acta 2021, 1152, 338269. [Google Scholar] [CrossRef]
- Singh, V.R.; Malegaonkar, J.N.; Bhosale, S.V.; Singh, P.K. An ATP Responsive Fluorescent Supramolecular Assembly Based on a Polyelectrolyte and an AIE Active Tetraphenylethylene Derivative. Org. Biomol. Chem. 2020, 18, 8414–8423. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, W.E.; Yan, J.Q.; Liu, M.; Zhou, Y.; Shen, X.; Ma, Y.L.; Feng, X.S.; Yang, J.; Li, G.H. A Review of the Extraction and Determination Methods of Thirteen Essential Vitamins to the Human Body: An Update from 2010. Molecules 2018, 23, 1484. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.N.; Seabrook, L.J.; Nguyen, S.T.; Leonard, J.T.; Albrecht, L.V. Simplifying the B Complex: How Vitamins B6 and B9 Modulate One Carbon Metabolism in Cancer and Beyond. Metabolites 2022, 12, 961. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.-T.; Chen, Y.; Wang, Y. Hyperbranched Poly(Amido Amine) Entrapped Tetraphenylethene as a Fluorescence Probe for Sequential Quadruple-Target Detection and Its Potential as a Chemical Logic Gate. Anal. Chem. 2020, 92, 9755–9763. [Google Scholar] [CrossRef] [PubMed]
- Ungor, D.; Szilágyi, I.; Csapó, E. Yellow-Emitting Au/Ag Bimetallic Nanoclusters with High Photostability for Detection of Folic Acid. J. Mol. Liq. 2021, 338, 116695. [Google Scholar] [CrossRef]
- Li, X.; Lai, Z.; Gu, J.; Liu, W.; Gao, J.; Wang, Q. Sequential Determination of Cerium (IV) Ion and Ascorbic Acid via a Novel Organic Framework: A Subtle Interplay between Intramolecular Charge Transfer (ICT) and Aggregated-Induced-Emission (AIE). J. Mol. Liq. 2020, 304, 112705. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, X.; Qiu, J.; Guo, H.; Yang, F. A Fluorescent Sensor for Folic Acid Based on Crown Ether-Bridged Bis-Tetraphenylethylene. Analyst 2019, 144, 2662–2669. [Google Scholar] [CrossRef]
- Naghibi, S.; Chen, T.; Jamshidi Ghahfarokhi, A.; Tang, Y. AIEgen-Enhanced Protein Imaging: Probe Design and Sensing Mechanisms. Aggregate 2021, 2, e41. [Google Scholar]
- Gao, L.; Lin, X.; Chen, X. Ionic Liquid Decorated AIE Luminogen for Selective Detection of HSA in Biofluids and Early Disease Screening. Talanta 2020, 212, 120763. [Google Scholar] [CrossRef]
- Li, Z.; Guan, W.; Lu, C.; Zhou, X.-R.; Luo, S.-Z.; You, Y.; Ouyang, J. Hydrophobicity-Induced Prestaining for Protein Detection in Polyacrylamide Gel Electrophoresis. Chem. Commun. 2016, 52, 2807–2810. [Google Scholar] [CrossRef]
- Sun, J.; Cao, J.; Wang, J.; Wang, J.; Wang, S. Selective and Sensitive Fluorescent Enhancement Detection of Keratin in Aqueous Media with Aggregation-Induced Emission Characteristics. Sens. Actuators B Chem. 2019, 293, 159–165. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.; Zhu, L.; Cao, D.; Li, L. Synthesis, Characterization and Fluorescence “Turn-on” Detection of BSA Based on the Cationic Poly(Diketopyrrolopyrrole-Co-Ethynylfluorene) through Deaggregating Process. Sens. Actuators B Chem. 2016, 231, 733–743. [Google Scholar] [CrossRef]
- Panova, I.G.; Sharova, N.P.; Dmitrieva, S.B.; Poltavtseva, R.A.; Sukhikh, G.T.; Tatikolov, A.S. Use of a Cyanine Dye as a Probe for Albumin and Collagen in the Extracellular Matrix. Anal. Biochem. 2007, 361, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Tatikolov, A.S. Polymethine Dyes as Spectral-Fluorescent Probes for Biomacromolecules. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 55–90. [Google Scholar] [CrossRef]
- Sun, X.; Qiao, Y.; Li, W.; Sui, Y.; Ruan, Y.; Xiao, J. A Graphene Oxide-Aided Triple Helical Aggregation-Induced Emission Biosensor for Highly Specific Detection of Charged Collagen Peptides. J. Mater. Chem. B 2020, 8, 6027–6033. [Google Scholar] [CrossRef]
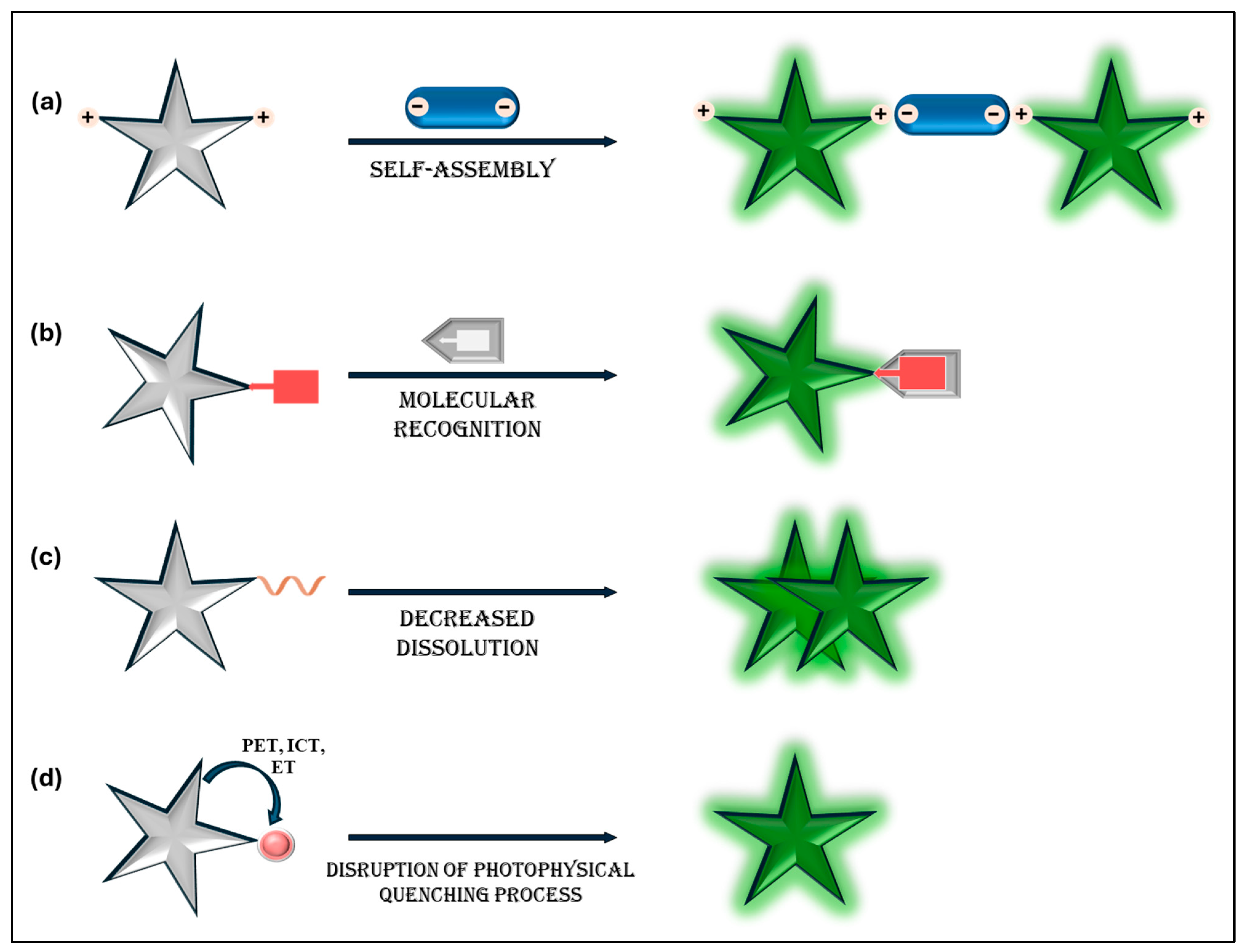
| AIE-Based Fluorescent Sensors | SYBR Green I | SYBR Gold | PicoGreen | |
|---|---|---|---|---|
| Fluorescence mechanism | Non-emissive in solution; restriction of intramolecular motions (RIM) causes it to become highly fluorescent upon aggregation or specific binding to DNA. | Enhances fluorescence upon intercalation by binding to the minor groove of double-stranded DNA (dsDNA). | Intercalate into dsDNA, leading to significant fluorescence enhancement. | Binds to dsDNA, forming a highly luminescent complex compared to the free dye. |
| Signal-to-noise ratio | Very high, due to turn-on mechanism and suppressed background fluorescence. | Moderate to high, depending on experimental conditions. | High, optimized for higher sensitivity. | High, optimized for DNA quantitation assays. |
| Binding specificity | Very high, it can be designed for specific DNA sequences or structures. | Prefers dsDNA over single-stranded DNA (ssDNA); limited sequence specificity. | Similarly to SYBR Green I, but with broader spectrum. | Binds strongly to dsDNA; limited discrimination capability. |
| Sensitivity | Very high; detection limits in the picomolar (pM) to nanomolar (nM) range | High; detection limits in the low nanogram per milliliter (ng/mL) range. | Very high; lower detection limits compared to SYBR Green I. | Very high; detection limits as low as 25 pg/mL. |
| Cytotoxicity | Low; many AIE probes exhibit excellent biocompatibility. | Moderate; not ideal for live-cell imaging. | Moderate to high; limited use in live-cell imaging. | Moderate; limited use in live-cell imaging. |
| Analyte(s) | LOD | Comments | Ref | |
|---|---|---|---|---|
| Nucleic acids | DNA/RNA (TTAPE) | N.A. | Fluorescence activated upon DNA/RNA binding, suitable for gel staining. | [101] |
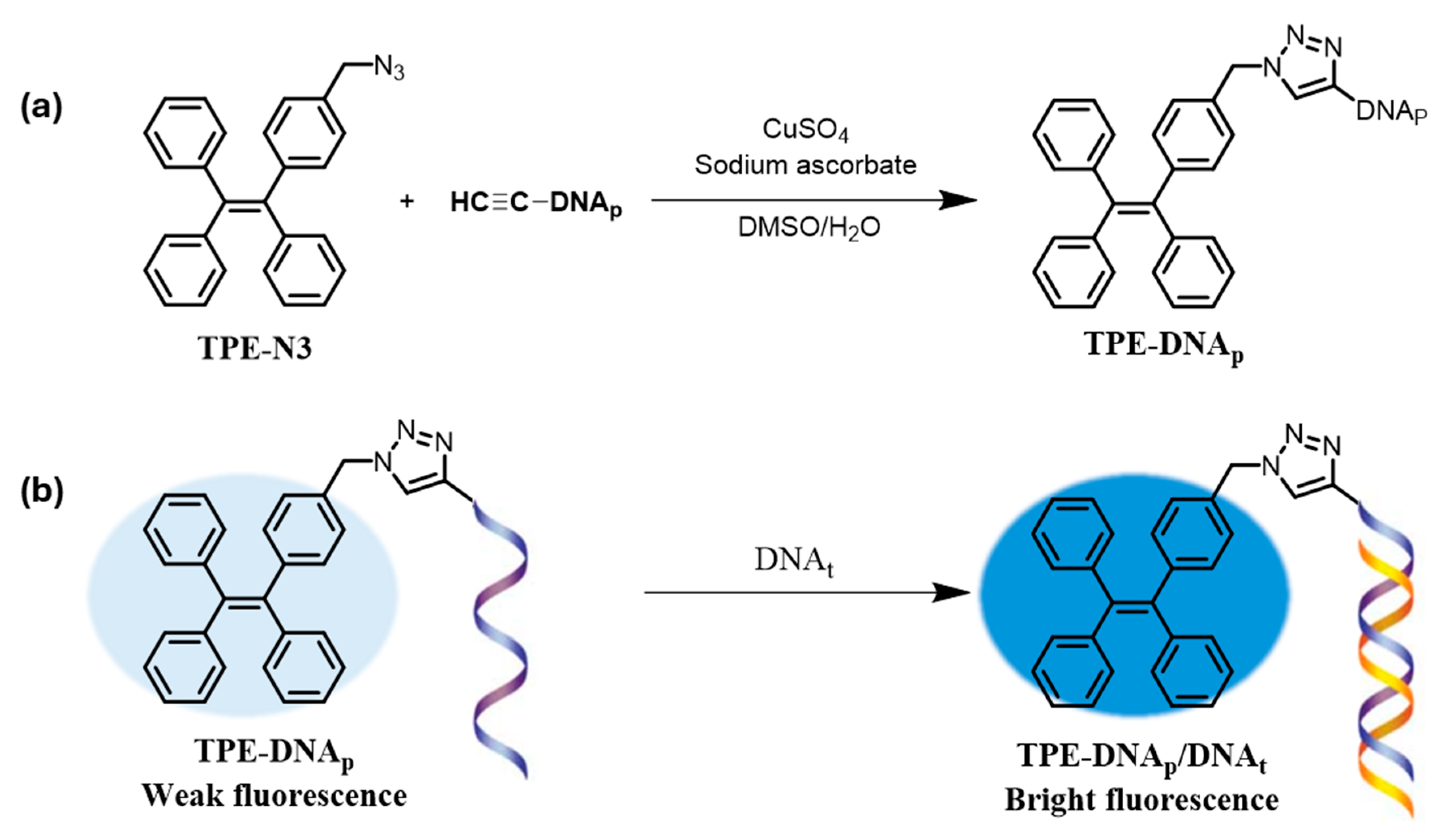
| DNA (MB/DNA/HA/TPE) | 1.0 × 10−9 M | Bio-conjugated AIE-DNA on magnetic beads enables enhanced signal. | [102] | |
| DNA (PNA-labeled dye) | N.A. | Label-free, high selectivity for structural variants. | [103] | |
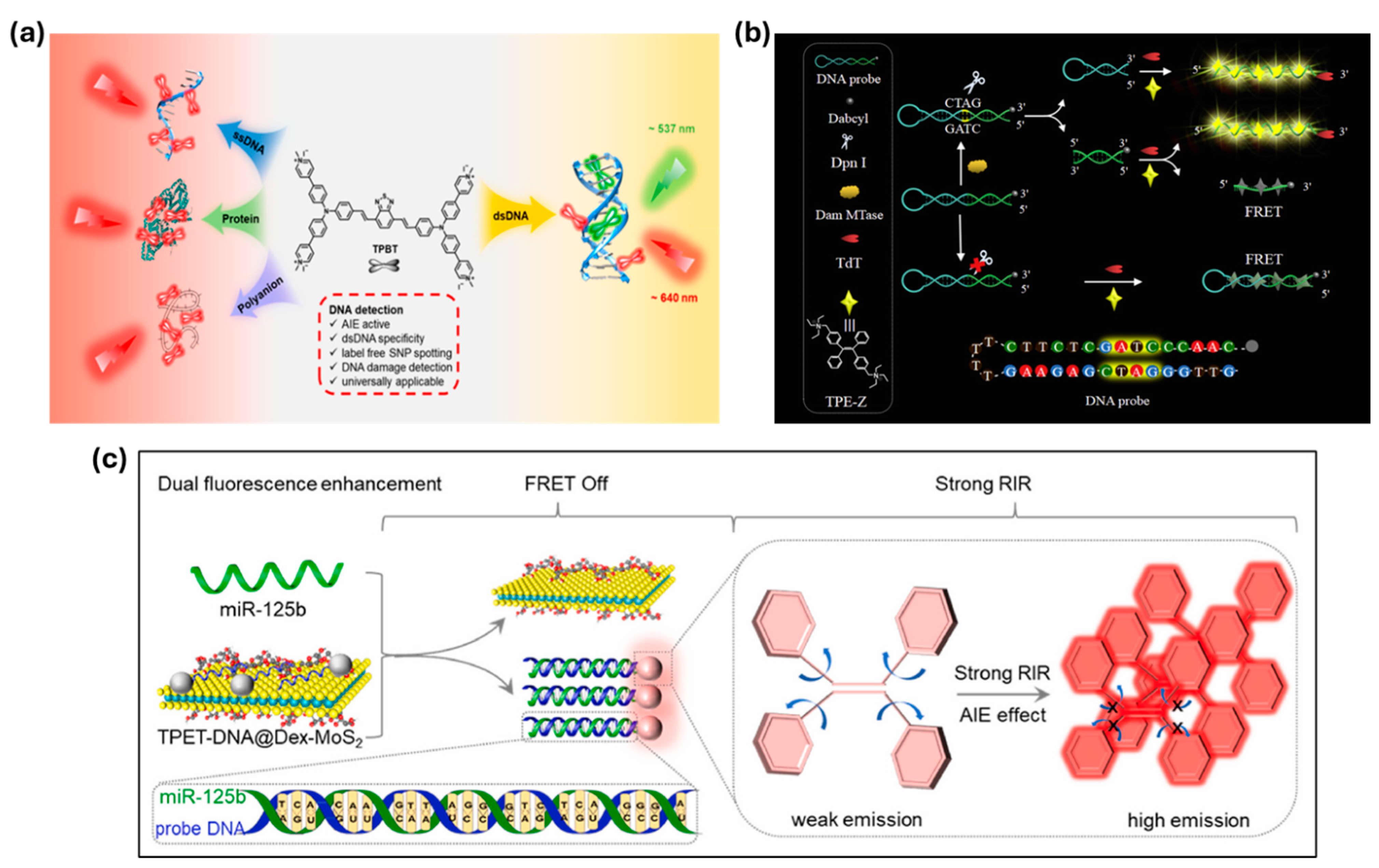
| dsDNA/SNPs (TPBT) | N.A. | Dual-color probe enables SNP and UV-damage detection. | [104] | |
| DNA G-quadruplex | 0.42 pg/mL | Based on strand displacement amplification and G-quadruplex formation. | [105] | |
| DNA Methyltransferase (MTase) | 0.16 U/mL | Hairpin probe and TdT-catalyzed polymerization triggers fluorescence. | [106] | |
| miR-125b (TPET-DNA@Dex-MoS2) | 20.82 pM | miRNA recognition detaches TPET-DNA from MoS2, enabling emission. | [107] | |
| DNA/RNA | N.A. | Probes distinguish ssDNA/dsDNA, with potential for nucleus staining. | [98] | |
| miRNA (TPE-DNA microchip) | N.A. | Evaporation-based miRNA enrichment in microwells amplifies the signal. | [109,110] | |
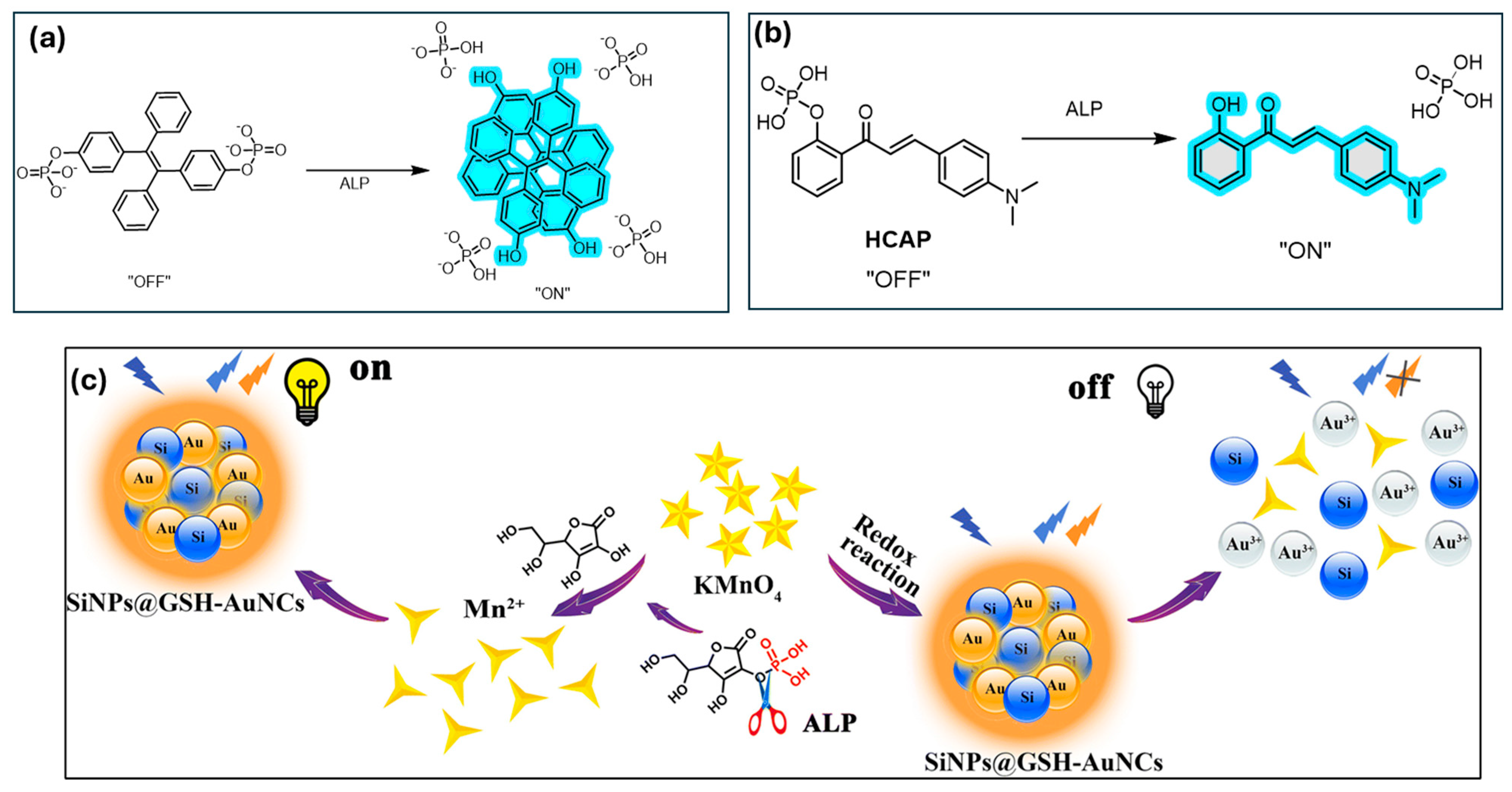
| Enzymes | Alkaline Phosphatase (ALP) | 0.2 U/L | TPE-phosphate probe; fluorescence restored upon enzymatic hydrolysis. | [118] |
| Alkaline Phosphatase (ALP) | 0.6 U/L | FAS-P probe based on ESIPT; water-soluble with lipid droplet targeting. | [119] | |
| Alkaline Phosphatase (ALP) | 0.15 mU/mL | Phosphorylated chalcone derivative; ratiometric probe with ESIPT feature. | [120] | |
| Alkaline Phosphatase (ALP) | 0.23 U/L | SiNPs@GSH-AuNCs; fluorescence recovery via redox-triggered mechanism. | [121] | |
| Caspase-3 | N.A. | Coumarin–TPETP FRET system; dual fluorescence turn-on after enzymatic cleavage. | [122] | |
| Caspase-3 | 0.54 pM | TPE-GFFYK peptide-based self-assembly; enhanced fluorescence via structural ordering. | [123] | |
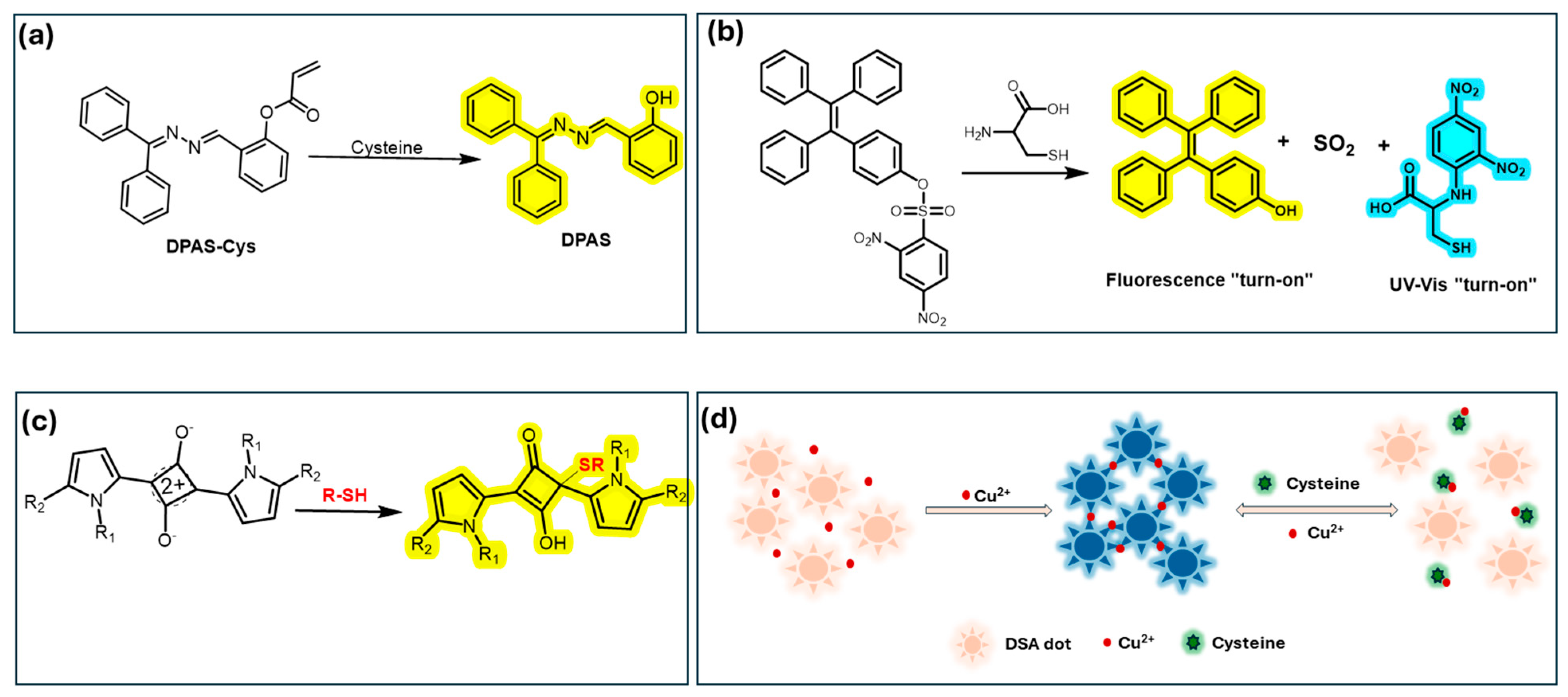
| Amino acids | Cysteine (Cys) | 2.4 μM | DPAS-Cys probe; LD targeting and large Stokes shift. | [126] |
| Cys and GSH | Cys: 2.78 × 10−7 M, GSH: 1.89 × 10−7 M | Disruption of metallacage leading to AIE enhancement. | [127] | |
| Cys/Hcy | 2.84 mM | Colorimetric and fluorescent turn-on probe using ESIPT. | [128] | |
| Cys | N.A. | Squaraine-based device shows color change for Cys/GSH. | [129] | |
| Cu2+, Fe3+, Cys | Cu2+: 107 nM, Fe3+: 120 nM, Cys: 78 nM | On-off-on sensor using AIE dots and metal ion detection. | [130] | |
| GSH | 0.8 μM | Ratiometric probe based on Ag NCs; good serum performance. | [131] | |
| GSH | N.A. | TP derivative; versatile sensing via dimerization and J-aggregation. | [132] | |
| Cys | 0.03 μM | Hydroxyphenylquinazolinone probe; highly selective for Cys. | [133] | |
| Cysteamine (CSH) | 15.7 nM | Selective shell inhibition enables fast and specific CSH detection. | [134] | |
| GSH | 200 nM | MnO2 nanosheets and AIE-silica NPs; applied in human serum. | [135] | |
| Cys, Hcy | Cys: 54 nM, Hcy: 72 nM | SQM-NBD probe enables selective Cys/Hcy detection in cells. | [136] | |
| Biogenic amines | Dopamine (DA) | 4.8 nM | Dual-emission ratiometric probe using ZIF-8@AuNCs-PFO with enhanced AIE and asynchronous response. | [139] |
| Dopamine (DA) | 0.12 µM | Restored fluorescence by DA-induced AuNP aggregation in lead halide perovskites (LHPs) with AIE. | [140] | |
| Dopamine (DA) | 10 nM | Ratiometric probe with three emission peaks and high sensitivity. | [141] | |
| Spermine (SPM) | 1.0 µM | Supramolecular probe 1@CB[7] designed for early cancer diagnostics. | [142] | |
| Spermine (SPM), Spermidine (SPD) | 1.4 × 10−8 M (SP), 3.6 × 10−8 M (SPD) | Solid-supported AIE probe for sensitive detection well below clinical cutoff. | [143] | |
| Spermidine (SPD) | 46 nM | Terphenyl-based AIE-active probe for nanomolar SPD detection. | [144] | |
| 5-Hydroxytryptamine (5-HT) | 0.049 µM | Tf-Au NCs-based AIE biosensor is effective for 5-HT detection in serum. | [145] | |
| CK-B subunit of creatine kinase | 93 U L−1 | Water-soluble AIE fluorescent probe with high selectivity for CK-B. | [146] | |
| Saccharides | Glucose | 4.7 μM | ZIF-8 with GOx and AIE-AuNCs; validated in human serum. | [150] |
| Glucose | 550 pM | AIE-MOF with ESIPT and visual glucose test strip. | [151] | |
| Glucose via H2O2 | 0.96 μM | Ratiometric probe using CuNCs and coumarin oxidation. | [152] | |
| Glucose via pH change | 3.2 × 10−5 M | Gel-based system with Ca2+ and alginate; eye glucose sensing potential. | [153] | |
| Glucose (non-GOx) | 0.018 mM | ALP-PVB hydrogel with AIEE-AuNCs; dual-mode sensing. | [154] | |
| 23 Saccharides | N.A. | PIL-based dynamic AIE system with broad saccharide detection capabilities | [155] | |
| Adenosine triphosphate (ATP) | ATP & Cu2+ | N.A. | TPE-COOH fluorescence turn-on by ATP via competitive binding with Cu2+; test strip format for practical use. | [157] |
| Cu2+ & ATP | Cu2+: 1.0 × 10−7 M, ATP: 1.5 × 10−6 M | TPE amphiphile forms AIE micelles; sequential recognition of Cu2+ and ATP by fluorescence quenching and recovery. | [158] | |
| ATP | ATP: 32.8 nM | Label-free aptamer sensor based on G-quadruplex formation and AIE dye aggregation; effective in serum. | [159] | |
| ATP & Ga3+ | ATP: 32.1 nM | Natural camphor-derived AIE probe; detects ATP via fluorescence shift from CPD-Ga3+ complex. | [160] | |
| ATP | ATP: 0.275 ppb | AAP-1 probe with TPE and triamine; ultra-fast response and high selectivity in aqueous media. | [161] | |
| ATP | ATP: 0.37 μM | Self-assembled Su-TPE-PAH complex; ATP displaces Su-TPE, causing a spectral shift; applicable in serum. | [162] | |
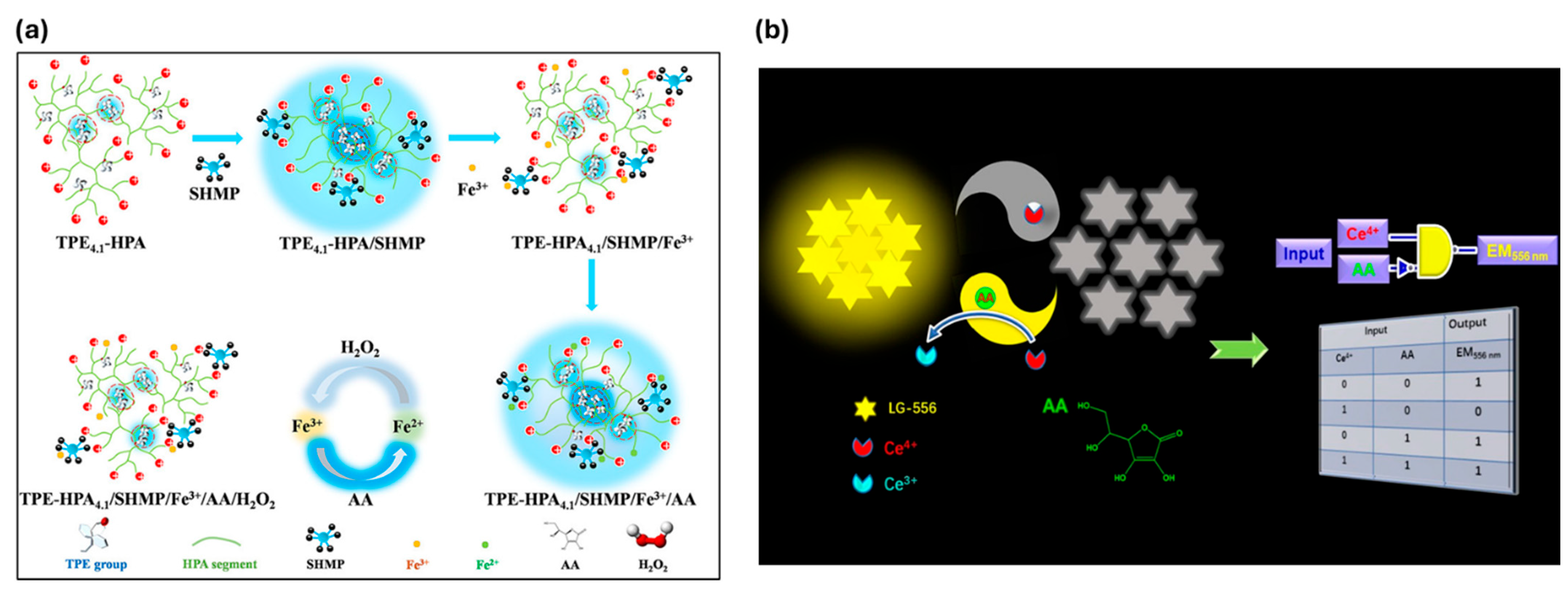
| Vitamins | Ascorbic acid (AA) | 0.66 μM | Quadruple-target sensor with sequential fluorescence response (off–on–off–on–off); detects AA alongside SHMP, Fe3+, and H2O2. | [165] |
| Folic acid (FA) | 0.109 μM | AMP-Au/Ag NCs with pH-dependent AIE, paper-based, and liquid detection formats. | [166] | |
| Ascorbic acid (AA) | 39.2 nM | Fluorescence ‘on-off-on’ system with reversible detection of AA and Ce4+; high specificity and sensitivity. | [167] | |
| Folic acid (FA) | 6.36 × 10−7 M | Crown ether-bridged AIE sensor; selective and interference-free detection of FA. | [168] | |
| Albumin and collagen | HSA | 0.007 μg/mL | The interactions between HSA and TPE-IL lead to form a conjugation and restrict the probe, resulting in remarkable fluorescence emission. | [170] |
| BSA | 0.1 μg/mL | The formation of the TPE-SDS aggregates inside the hydrophobic pocket of BSA leads to a boost as well as a blue shift in the emission. | [171] | |
| BSA | 11 μM | The long chains of the probe penetrate the cavities of BSA, forming a large complex with enhanced AIE characteristics, resulting in strong fluorescence emission. | [172] | |
| BSA | 20 μM | The ammonium group enhances the water solubility of the probe and provides positively charged binding sites, facilitating electrostatic adsorption onto BSA, which in turn induces fluorescence emission. | [173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keremane, K.S.; Acharya, M.G.; Naik, P.; Malakar, C.C.; Wang, K.; Poudel, B. Recent Advances in Aggregation-Induced Emission (AIE) Fluorescent Sensors for Biomolecule Detection. Chemosensors 2025, 13, 174. https://doi.org/10.3390/chemosensors13050174
Keremane KS, Acharya MG, Naik P, Malakar CC, Wang K, Poudel B. Recent Advances in Aggregation-Induced Emission (AIE) Fluorescent Sensors for Biomolecule Detection. Chemosensors. 2025; 13(5):174. https://doi.org/10.3390/chemosensors13050174
Chicago/Turabian StyleKeremane, Kavya S., M. Gururaj Acharya, Praveen Naik, Chandi C. Malakar, Kai Wang, and Bed Poudel. 2025. "Recent Advances in Aggregation-Induced Emission (AIE) Fluorescent Sensors for Biomolecule Detection" Chemosensors 13, no. 5: 174. https://doi.org/10.3390/chemosensors13050174
APA StyleKeremane, K. S., Acharya, M. G., Naik, P., Malakar, C. C., Wang, K., & Poudel, B. (2025). Recent Advances in Aggregation-Induced Emission (AIE) Fluorescent Sensors for Biomolecule Detection. Chemosensors, 13(5), 174. https://doi.org/10.3390/chemosensors13050174





