A Minimal Electronic Nose Based on Graphene Functionalized with Metalated Pyrazinoporphyrazines and Phthalocyanines for Ammonia, Benzene, and Hydrogen Sulfide Discrimination
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnPR and CuPR
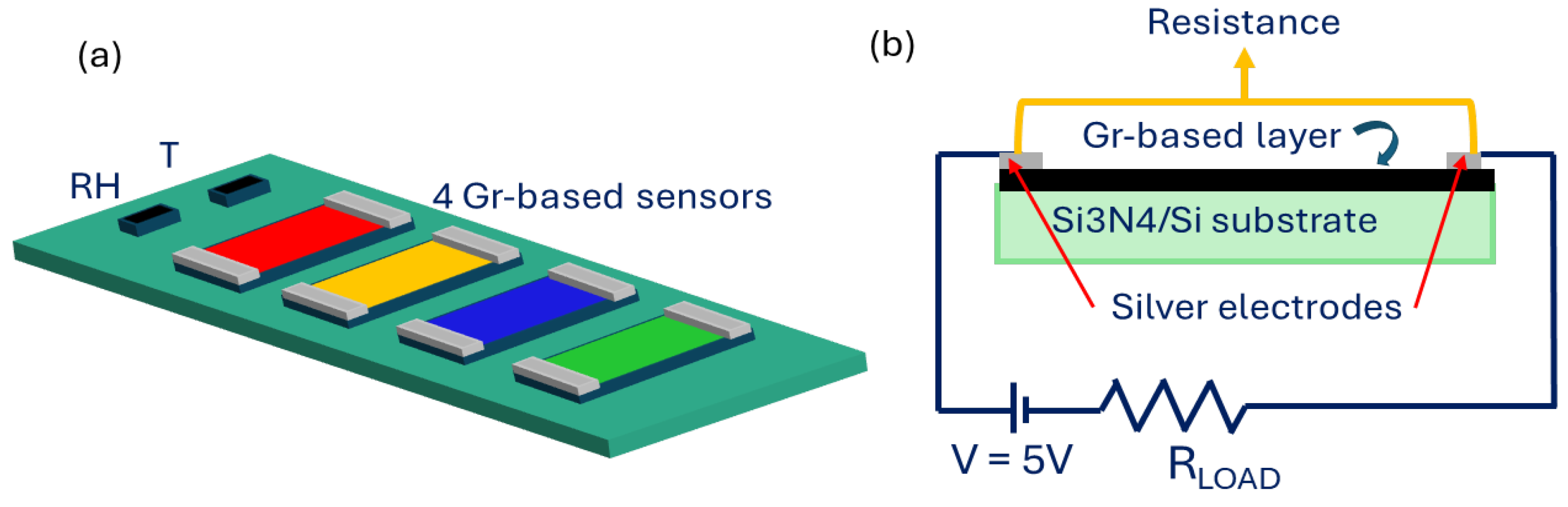
2.2. Sample Preparation
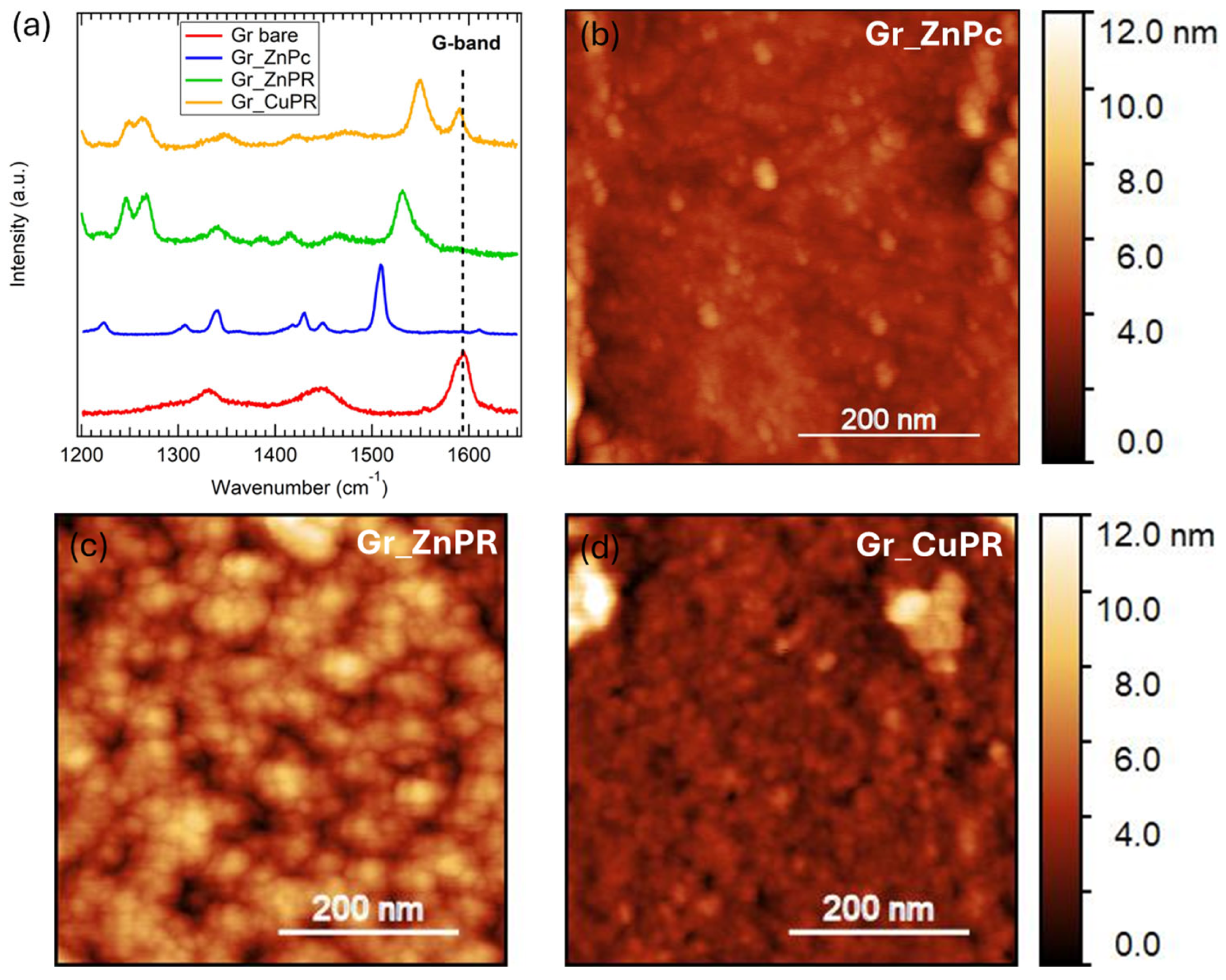
2.3. Characterization
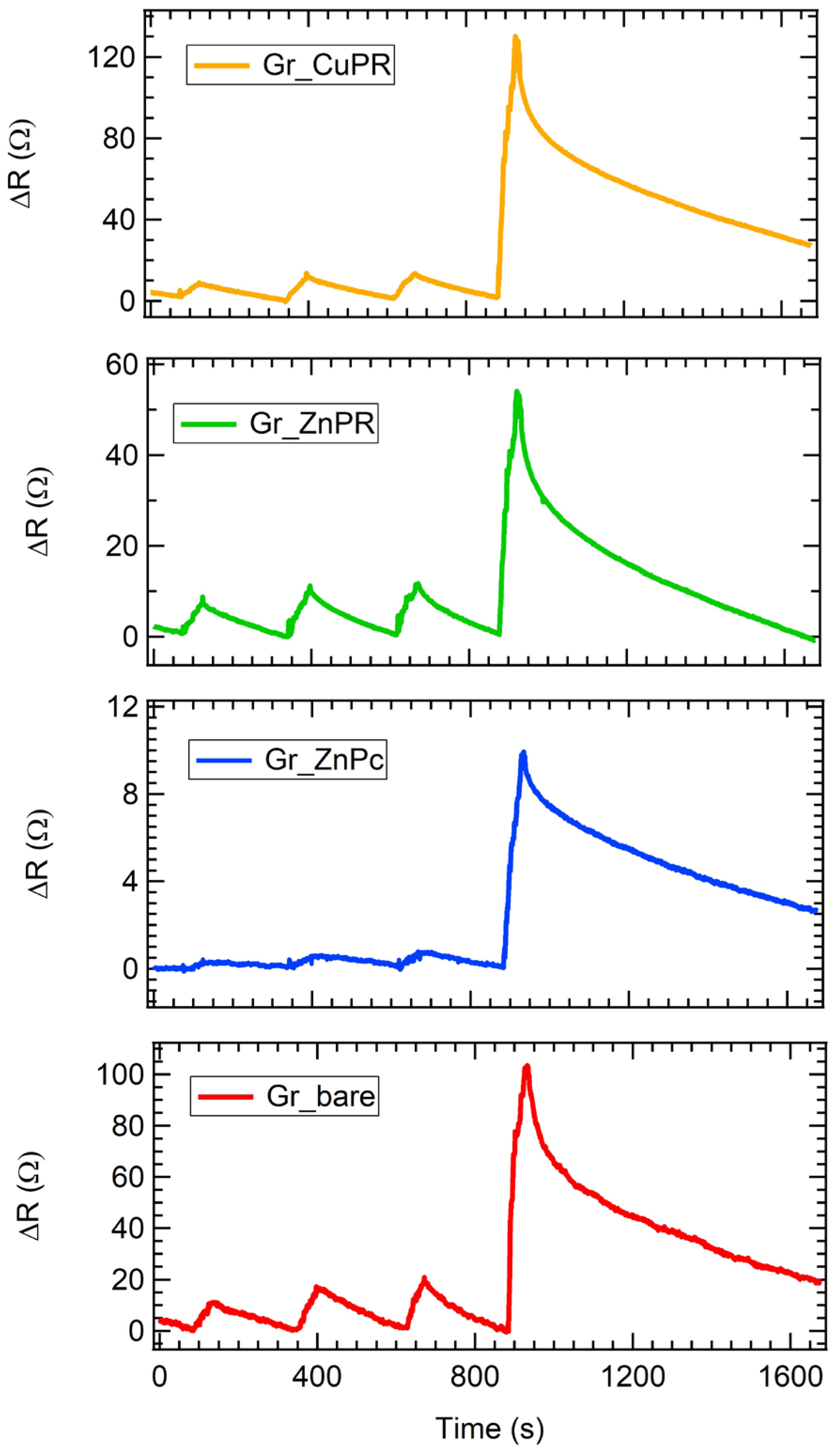
2.4. Gas Sensing Measurements
2.5. Statistical Analysis
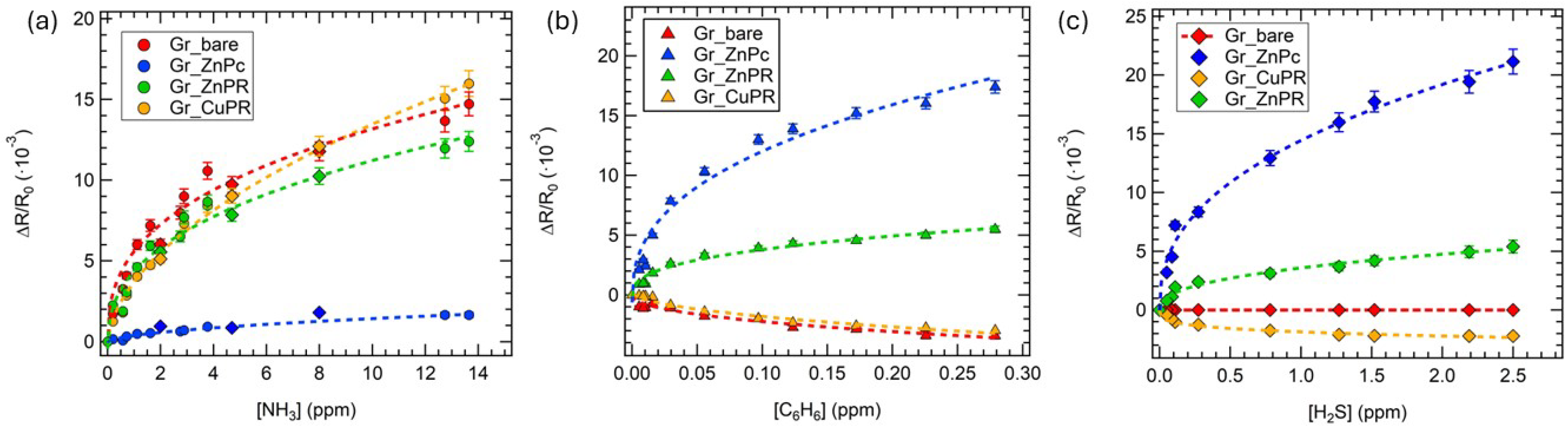
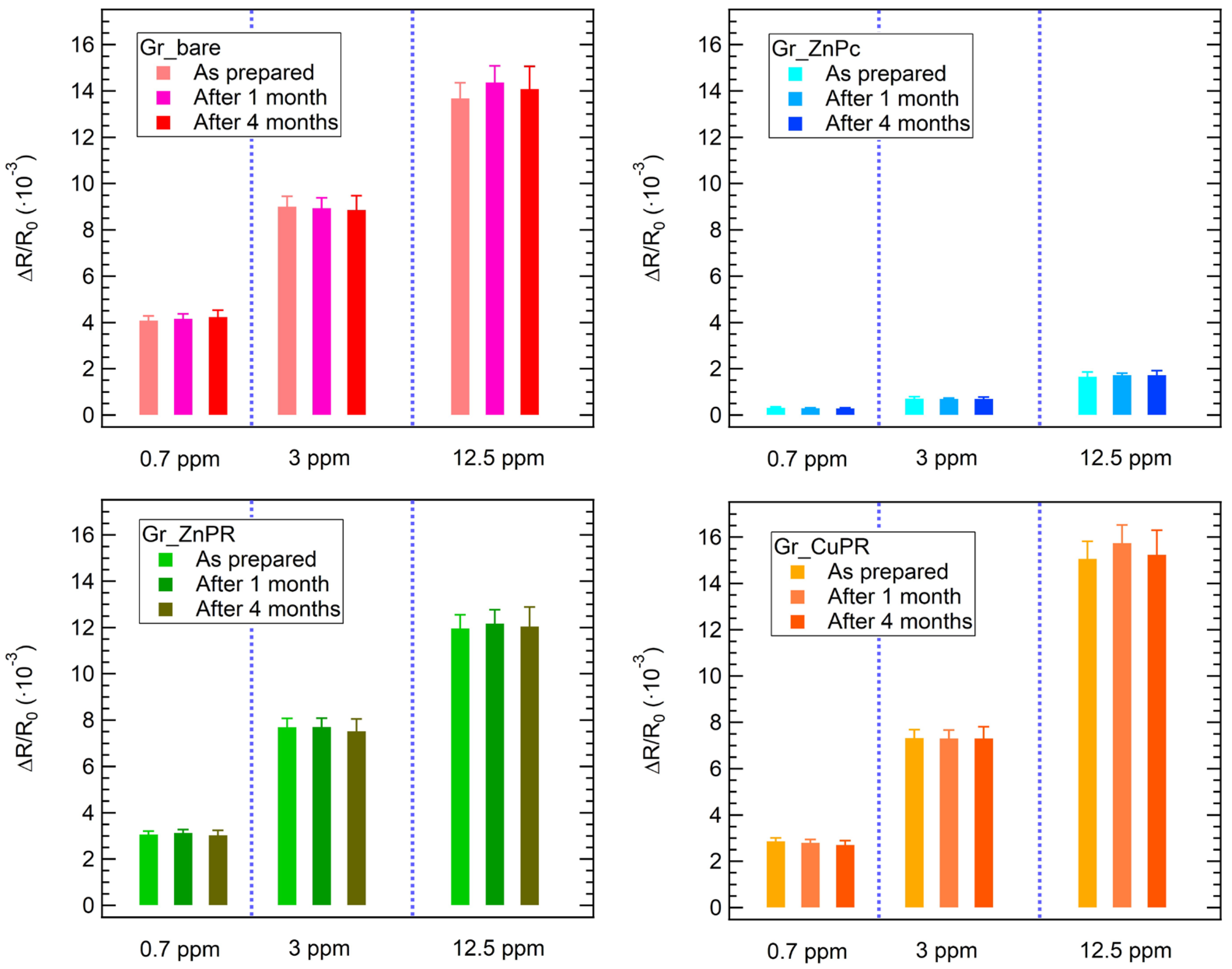
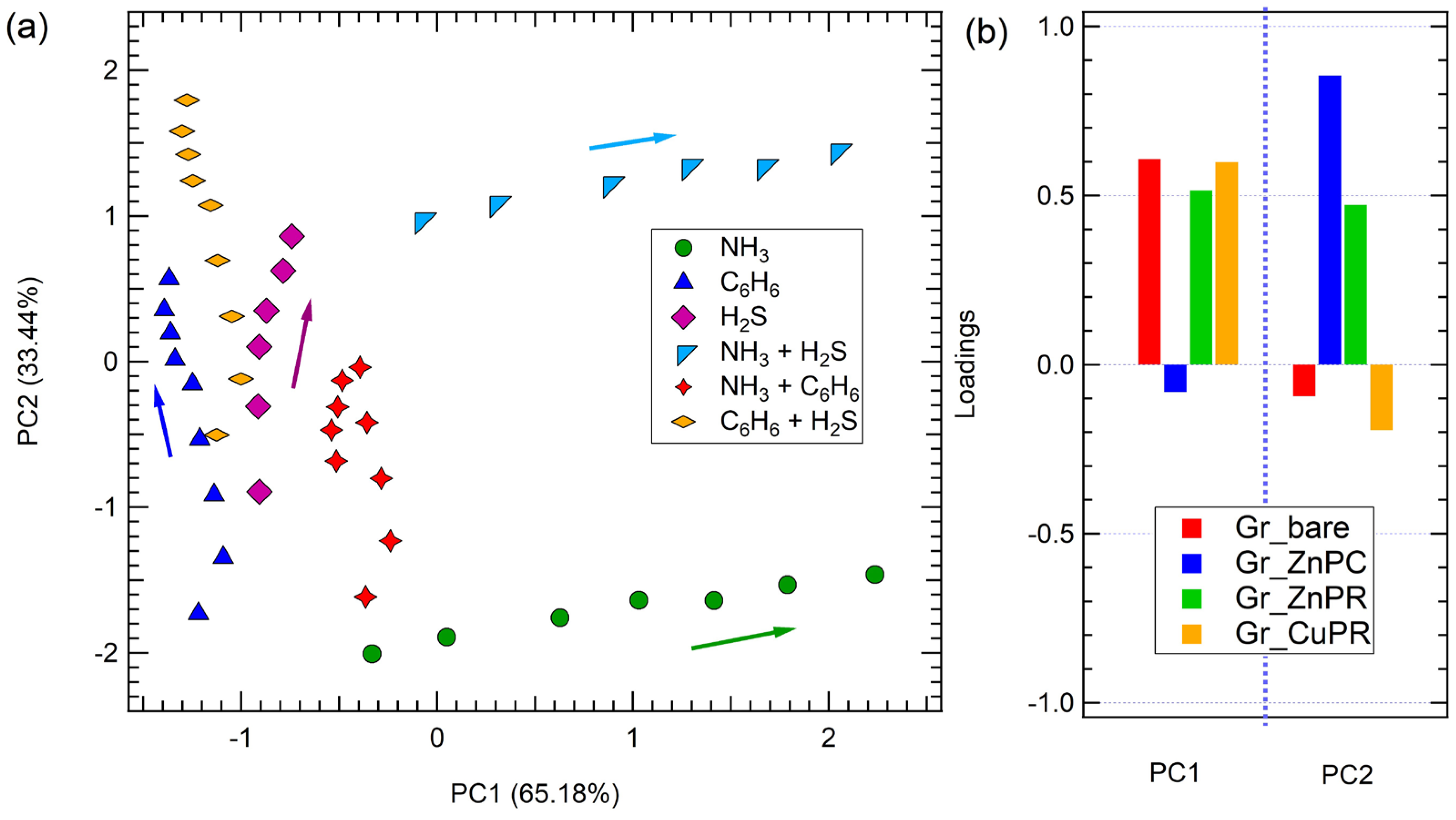
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bag, A.; Lee, N.E. Recent advancements in development of wearable gas sensors. Adv. Mater. Technol. 2021, 6, 2000883. [Google Scholar] [CrossRef]
- Freddi, S.; Sangaletti, L. Trends in the Development of Electronic Noses Based on Carbon Nanotubes Chemiresistors for Breathomics. Nanomaterials 2022, 12, 2992. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Leishman, M.; Bagnall, D.; Nasiri, N. Nanostructured gas sensors: From air quality and environmental monitoring to healthcare and medical applications. Nanomaterials 2021, 11, 1927. [Google Scholar] [CrossRef] [PubMed]
- Seesaard, T.; Kamjornkittikoon, K.; Wongchoosuk, C. A comprehensive review on advancements in sensors for air pollution applications. Sci. Total Environ. 2024, 951, 175696. [Google Scholar] [CrossRef]
- Weston, M.; Geng, S.; Chandrawati, R. Food sensors: Challenges and opportunities. Adv. Mater. Technol. 2021, 6, 2001242. [Google Scholar] [CrossRef]
- Kumar, A.; Castro, M.; Feller, J.F. Review on sensor array-based analytical technologies for quality control of food and beverages. Sensors 2023, 23, 4017. [Google Scholar] [CrossRef]
- Reis, T.; Moura, P.C.; Gonçalves, D.; Ribeiro, P.A.; Vassilenko, V.; Fino, M.H.; Raposo, M. Ammonia Detection by Electronic Noses for a Safer Work Environment. Sensors 2024, 24, 3152. [Google Scholar] [CrossRef]
- Tan, L.; Feng, Z.; Zheng, H.; Yao, Z.; Weng, X.; Wang, F.; Chang, Z. Development trend of electronic nose technology in closed cabins gas detection: A review. Appl. Sci. 2022, 12, 9326. [Google Scholar] [CrossRef]
- Groysman, A.; Groysman, A. Process units in oil refineries and petrochemical plants. In Corrosion Problems and Solutions in Oil Refining and Petrochemical Industry; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–7. [Google Scholar]
- Torres, W.; Pansare, S.S.; Goodwin Jr, J.G. Hot gas removal of tars, ammonia, and hydrogen sulfide from biomass gasification gas. Catal. Rev. 2007, 49, 407–456. [Google Scholar] [CrossRef]
- Marcantonio, V.; Bocci, E.; Ouweltjes, J.P.; Del Zotto, L.; Monarca, D. Evaluation of sorbents for high temperature removal of tars, hydrogen sulphide, hydrogen chloride and ammonia from biomass-derived syngas by using Aspen Plus. Int. J. Hydrogen Energy 2020, 45, 6651–6662. [Google Scholar] [CrossRef]
- Cronkite, E.P.; Drew, R.T.; Inoue, T.; Hirabayashi, Y.; Bullis, J.E. Hematotoxicity and carcinogenicity of inhaled benzene. Environ. Health Perspect. 1989, 82, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Davidson, R.; Courage, C.; Rushton, L.; Levy, L. Benzene in the environment: An assessment of the potential risks to the health of the population. Occup. Environ. Med. 2001, 58, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Van Aalst, J.A.; Isakov, R.; Polk, J.D.; Van Antwerp, A.D.; Yang, M.; Fratianne, R.B. Hydrogen sulfide inhalation injury. J. Burn Care Rehabil. 2020, 21, 248–253. [Google Scholar] [CrossRef]
- Guidotti, T.L. Hydrogen sulfide intoxication. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 131, pp. 111–133. [Google Scholar]
- Leduc, D.; Gris, P.; Lheureux, P.; Gevenois, P.A.; De Vuyst, P.; Yernault, J.C. Acute and long term respiratory damage following inhalation of ammonia. Thorax 1992, 47, 755–757. [Google Scholar] [CrossRef]
- Vadysinghe, A.N.; Attygalle, U.; Ekanayake, E.M.K.B.; Dharmasena, E.G.I.A. Ammonia exposure: A review of six cases. Am. J. Forensic Med. Pathol. 2021, 42, 373–378. [Google Scholar] [CrossRef]
- Regulations (Standards—29 CFR). Available online: https://www.osha.gov/laws-regs/regulations/standardnumber/1910 (accessed on 16 January 2025).
- Recommendations of the SCOEL. Available online: https://echa.europa.eu/recommendations-of-the-scoel (accessed on 16 January 2025).
- Freddi, S.; Emelianov, A.V.; Bobrinetskiy, I.I.; Drera, G.; Pagliara, S.; Kopylova, D.S.; Chiesa, M.; Santini, G.; Mores, N.; Moscato, U.; et al. Development of a sensing array for human breath analysis based on swcnt layers functionalized with semiconductor organic molecules. Adv. Healthc. Mater. 2020, 9, 2000377. [Google Scholar] [CrossRef]
- Popa, C.; Dutu, D.C.; Cernat, R.; Matei, C.; Bratu, A.M.; Banita, S.; Dumitras, D.C. Ethylene and ammonia traces measurements from the patients’ breath with renal failure via LPAS method. Appl. Phys. B 2011, 105, 669. [Google Scholar] [CrossRef]
- Chatterjee, S.; Castro, M.; Feller, J.F. An e-nose made of carbon nanotube based quantum resistive sensors for the detection of eighteen polar/nonpolar VOC biomarkers of lung cancer. J. Mater. Chem. B 2013, 1, 4563. [Google Scholar] [CrossRef]
- Suzuki, Y.; Saito, J.; Uematsu, M.; Fukuhara, A.; Sato, S.; Togawa, R.; Sato, Y.; Misa, K.; Nikaido, T.; Fukuhara, N.; et al. Clinical application of hydrogen sulfide (H2S) as a marker of asthma management. Eur. Respir. J. 2016, 48, PA3379. [Google Scholar]
- Pham, Y.L.; Beauchamp, J. Breath biomarkers in diagnostic applications. Molecules 2021, 26, 5514. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Zhao, T.; Li, H.; Jiang, J.; Ye, J. Electronic nose for the detection and discrimination of volatile organic compounds: Application, challenges, and perspectives. TrAC Trends Anal. Chem. 2024, 180, 117958. [Google Scholar] [CrossRef]
- Varghese, S.S.; Lonkar, S.; Singh, K.K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sens. Actuators B 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Freddi, S.; Marzuoli, C.; Pagliara, S.; Drera, G.; Sangaletti, L. Targeting biomarkers in the gas phase through a chemoresistive electronic nose based on graphene functionalized with metal phthalocyanines. RSC Adv. 2023, 13, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, M.; Freddi, S.; Sangaletti, L. Physical Virtualization of a GFET for a Versatile, High-Throughput, and Highly Discriminating Detection of Target Gas Molecules at Room Temperature. Adv. Mater. Technol. 2025, 10, 2400985. [Google Scholar] [CrossRef]
- Capman, N.S.; Zhen, X.V.; Nelson, J.T.; Chaganti, V.S.K.; Finc, R.C.; Lyden, M.J.; Williams, T.L.; Freking, M.; Sherwood, G.J.; Bühlmann, P.; et al. Machine learning-based rapid detection of volatile organic compounds in a graphene electronic nose. ACS Nano 2022, 16, 19567–19583. [Google Scholar] [CrossRef]
- Alzate-Carvajal, N.; Park, J.; Bargaoui, I.; Rautela, R.; Comeau, Z.J.; Scarfe, L.; Menard, J.-M.; Darling, S.B.; Luican-Mayer, A. Arrays of functionalized graphene chemiresistors for selective sensing of volatile organic compounds. ACS Appl. Electron. Mater. 2023, 5, 1514–1520. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Wang, Y. Strategies for the performance enhancement of graphene-based gas sensors: A review. Talanta 2021, 235, 122745. [Google Scholar] [CrossRef]
- Recum, P.; Hirsch, T. Graphene-based chemiresistive gas sensors. Nanoscale Adv. 2024, 6, 11–31. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Ghanbari, R.; Safaiee, R.; Sheikhi, M.H.; Golshan, M.M.; Horastani, Z.K. Graphene decorated with silver nanoparticles as a low-temperature methane gas sensor. ACS Appl. Mater. Interfaces 2019, 11, 21795–21806. [Google Scholar] [CrossRef] [PubMed]
- Freddi, S.; Rodriguez Gonzalez, M.C.; Casotto, A.; Sangaletti, L.; Feyter, S. Machine learning-aided NO2 discrimination with an array of graphene chemiresistors covalently functionalized by diazonium chemistry. Chem. A Eur. J. 2023, 29, e202302154. [Google Scholar] [CrossRef] [PubMed]
- Calmeiro, J.M.; Tomé, J.P.; Lourenço, L.M. Supramolecular graphene–phthalocyanine assemblies for technological breakthroughs. J. Mater. Chem. C 2020, 8, 8344–8361. [Google Scholar] [CrossRef]
- Casotto, A.; Drera, G.; Perilli, D.; Freddi, S.; Pagliara, S.; Zanotti, M.; Schio, L.; Verdini, A.; Floreano, L.; Di Valentin, C.; et al. π-Orbital mediated charge transfer channels in a monolayer Gr–NiPc heterointerface unveiled by soft X-ray electron spectroscopies and DFT calculations. Nanoscale 2022, 14, 13166–13177. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, B.; Wang, X.; Li, Y.; Gai, S.; Wu, Y.; Cheng, X.-L. A high-sensitive room temperature gas sensor based on cobalt phthalocyanines and reduced graphene oxide nanohybrids for the ppb-levels of ammonia detection. RSC Adv. 2019, 9, 37518. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Guo, Z.; Gai, S.; Li, Y.; Wu, Y. A highly sensitive ppb-level H2S gas sensor based on fluorophenoxy-substituted phthalocyanine cobalt/rGO hybrids at room temperature. RSC Adv. 2021, 11, 5993–6001. [Google Scholar] [CrossRef]
- Yabaş, E.; Biçer, E.; Altındal, A. Novel reduced graphene oxide/zinc phthalocyanine and reduced graphene oxide/cobalt phthalocyanine hybrids as high sensitivity room temperature volatile organic compound gas sensors. J. Mol. Struct. 2023, 1271, 134076. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A. An electronic nose based on carbon nanotube-titanium dioxide hybrid nanostructures for detection and discrimination of volatile organic compounds. Sens. Acts B Chem. 2022, 357, 131418. [Google Scholar] [CrossRef]
- Verma, G.; Gupta, A. Next-Generation Chemiresistive Wearable Breath Sensors for Non-Invasive Healthcare Monitoring: Advances in Composite and Hybrid Materials. Small 2025, 21, 2411495. [Google Scholar] [CrossRef]
- Tang, X.; Debliquy, M.; Lahem, D.; Yan, Y.; Raskin, J.P. A review on functionalized graphene sensors for detection of ammonia. Sensors 2021, 21, 1443. [Google Scholar] [CrossRef]
- Ghani, F.; Kristen, J.; Riegler, H. Solubility Properties of Unsubstituted Metal Phthalocyanines in Different Types of Solvents. J. Chem. Eng. Data 2012, 57, 439–449. [Google Scholar] [CrossRef]
- Parravicini, M.; Vaghi, L.; Cravotto, G.; Masciocchi, N.; Maspero, A.; Palmisano, G.; Penoni, A. A novel porphyrazine ligand tailored to homogeneous metal catalyzed transformations. Arkivoc 2014, 2014, 72–85. [Google Scholar] [CrossRef]
- Murray, C.; Dozova, N.; McCaffrey, J.G.; FitzGerald, S.; Shafizadeh, N.; Crepin, C. Infra-red and Raman spectroscopy of free-base and zinc phthalocyanines isolated in matrices. Phys. Chem. Chem. Phys. 2010, 12, 10406–10422. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Zhang, Y.; Jiang, J. The molecular, electronic structures and IR and Raman spectra of metal-free, N, N′-dideuterio, and magnesium tetra-2,3-pyrazino-porphyrazines: Density functional calculations. Vib. Spectrosc. 2017, 43, 447–459. [Google Scholar] [CrossRef]
- Zhabanov, Y.A.; Eroshin, A.V.; Ryzhov, I.V.; Kuzmin, I.A.; Finogenov, D.N.; Stuzhin, P.A. Molecular structure, thermodynamic and spectral characteristics of metal-free and nickel complex of tetrakis (1, 2, 5-thiadiazolo) porphyrazine. Molecules 2021, 26, 2945. [Google Scholar] [CrossRef]
- Li, D.; Peng, Z.; Deng, L.; Shen, Y.; Zhou, Y. Theoretical studies on molecular structure and vibrational spectra of copper phthalocyanine. Vib. Spectrosc. 2005, 39, 191–199. [Google Scholar] [CrossRef]
- Gorski, A.; Gawinkowski, S.; Starukhin, A.; Gladkov, L.; Chizhova, N.; Mamardashvili, N.; Scheblykin, I.; Waluk, J. Resonance Raman and FTIR spectra of Mg-porphyrazines. J. Mol. Struct. 2014, 1058, 197–204. [Google Scholar] [CrossRef]
- Angeloni, S.; Bauer, E.M.; Ercolani, C.; Popkova, I.A.; Stuzhin, P.A. Tetrakis (selenodiazole) porphyrazines. 2: Metal complexes with Mn (II), Co (II), Ni (II), and Zn (II). J. Porphyr. Phthalocyanines 2001, 5, 881–888. [Google Scholar] [CrossRef]
- Freddi, S.; Perilli, D.; Vaghi, L.; Monti, M.; Papagni, A.; Di Valentin, C.; Sangaletti, L. Pushing down the limit of NH3 detection of graphene-based chemiresistive sensors through functionalization by thermally activated tetrazoles dimerization. ACS Nano 2022, 16, 10456–10469. [Google Scholar] [CrossRef]
- Liang, T.; Liu, R.; Lei, C.; Wang, K.; Li, Z.; Li, Y. Preparation and test of NH3 gas sensor based on single-layer graphene film. Micromachines 2020, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, M.; Jiang, C.; Song, S.; Li, T.; Sun, M.; Chen, W.; Peng, H. Highly sensitive graphene-based ammonia sensor enhanced by electrophoretic deposition of MXene. Carbon 2023, 202, 561–570. [Google Scholar] [CrossRef]
- Xiang, C.; Jiang, D.; Zou, Y.; Chu, H.; Qiu, S.; Zhang, H.; Xu, F.; Sun, L.; Zheng, L. Ammonia sensor based on polypyrrole–graphene nanocomposite decorated with titania nanoparticles. Ceram. Int. 2015, 41, 6432–6438. [Google Scholar] [CrossRef]
- Singh, S.; Deb, J.; Kumar, S.; Sarkar, U.; Sharma, S. Selective N, N-dimethylformamide vapor sensing using MoSe2/multiwalled carbon nanotube composites at room temperature. ACS Appl. Nano Mater. 2022, 5, 3913–3924. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Perilli, D.; Freddi, S.; Zanotti, M.; Drera, G.; Casotto, A.; Pagliara, S.; Schio, L.; Sangaletti, L.; Di Valentin, C. Design of highly responsive chemiresistor-based sensors by interfacing NiPc with graphene. Commun. Mater. 2024, 5, 254. [Google Scholar] [CrossRef]
- Polyakov, M.S.; Basova, T.V.; Göksel, M.; Şenocak, A.; Demirbaş, E.; Durmuş, M.; Kadem, B.; Hassan, A. Effect of covalent and non-covalent linking of zinc (II) phthalocyanine functionalised carbon nanomaterials on the sensor response to ammonia. Synth. Met. 2017, 227, 78–86. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Han, L.F.; Xiao, Y.H.; Jia, D.Z.; Guo, Z.H.; Li, F. Understanding dopant and defect effect on H2S sensing performances of graphene: A first-principles study. Comput. Mater. Sci. 2013, 69, 222–228. [Google Scholar] [CrossRef]
- Reshak, A.H.; Auluck, S. Adsorbing H2S onto a single graphene sheet: A possible gas sensor. J. Appl. Phys. 2014, 116, 103702. [Google Scholar] [CrossRef]
- Khodadadi, Z. Evaluation of H2S sensing characteristics of metals–doped graphene and metals-decorated graphene: Insights from DFT study. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 99, 261–268. [Google Scholar] [CrossRef]
- Peng, X.; Liu, D.; Zhao, F.; Tang, C. Gas sensing properties of Mg-doped graphene for H2S, SO2, SOF2, and SO2F2 based on DFT. Int. J. Quantum Chem. 2022, 122, e26989. [Google Scholar] [CrossRef]
- Tsymbalenko, O.; Lee, S.; Lee, Y.M.; Nam, Y.S.; Kim, B.C.; Kim, J.Y.; Lee, K.B. High-sensitivity NH3 gas sensor using pristine graphene doped with CuO nanoparticles. Microchim. Acta 2023, 190, 134. [Google Scholar] [CrossRef] [PubMed]
- Freddi, S. Enhancing the response to ammonia and nitrogen dioxide of bare graphene through NiPc functionalization. Il Nuovo C. 2023, 100, 46. [Google Scholar]
- Freddi, S.; Vergari, M.; Pagliara, S.; Sangaletti, L. A Chemiresistor sensor array based on graphene nanostructures: From the detection of ammonia and possible interfering VOCs to chemometric analysis. Sensors 2023, 23, 882. [Google Scholar] [CrossRef]
- Li, Q.; Chen, W.; Liu, W.; Sun, M.; Xu, M.; Peng, H.; Wu, H.; Song, S.; Li, T.; Tang, S. Highly sensitive graphene ammonia sensor enhanced by concentrated nitric acid treatment. Appl. Surf. Sci. 2022, 586, 152689. [Google Scholar] [CrossRef]
- Jagannathan, M.; Dhinasekaran, D.; Rajendran, A.R.; Subramaniam, B. Selective room temperature ammonia gas sensor using nanostructured ZnO/CuO graphene on paper substrate. Sens. Actuators B Chem. 2022, 350, 130833. [Google Scholar] [CrossRef]
- Freddi, S.; Rodriguez Gonzalez, M.C.; Carro, P.; Sangaletti, L.; De Feyter, S. Chemical defect-driven response on graphene-based chemiresistors for sub-ppm ammonia detection. Angew. Chem. Int. Ed. 2022, 61, e202200115. [Google Scholar] [CrossRef]
- Srivastava, S.; Jain, S.K.; Gupta, G.; Senguttuvan, T.D.; Gupta, B.K. Boron-doped few-layer graphene nanosheet gas sensor for enhanced ammonia sensing at room temperature. RSC Adv. 2020, 10, 1007–1014. [Google Scholar] [CrossRef]
- Wu, D.; Peng, Q.; Wu, S.; Wang, G.; Deng, L.; Tai, H.; Wang, L.; Yang, Y.; Dong, L.; Zhao, Y.; et al. A simple graphene NH3 gas sensor via laser direct writing. Sensors 2018, 18, 4405. [Google Scholar] [CrossRef]
- Lv, R.; Chen, G.; Li, Q.; McCreary, A.; Botello-Méndez, A.; Morozov, S.V.; Liang, L.; Declerck, X.; Perea-López, N.; Cullen, D.A.; et al. Ultrasensitive gas detection of large-area boron-doped graphene. Proc. Natl. Acad. Sci. USA 2015, 112, 14527–14532. [Google Scholar] [CrossRef]
- Seekaew, Y.; Lokavee, S.; Phokharatkul, D.; Wisitsoraat, A.; Kerdcharoen, T.; Wongchoosuk, C. Low-cost and flexible printed graphene–PEDOT: PSS gas sensor for ammonia detection. Org. Electron. 2014, 15, 2971–2981. [Google Scholar] [CrossRef]
- Ovsianytskyi, O.; Nam, Y.S.; Tsymbalenko, O.; Lan, P.T.; Moon, M.W.; Lee, K.B. Highly sensitive chemiresistive H2S gas sensor based on graphene decorated with Ag nanoparticles and charged impurities. Sens. Actuators B Chem. 2018, 257, 278–285. [Google Scholar] [CrossRef]
- Zhou, L.; Shen, F.; Tian, X.; Wang, D.; Zhang, T.; Chen, W. Stable Cu2O nanocrystals grown on functionalized graphene sheets and room temperature H2S gas sensing with ultrahigh sensitivity. Nanoscale 2013, 5, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Al-Hartomy, O.A.; Khasim, S.; Roy, A.; Pasha, A. Highly conductive polyaniline/graphene nano-platelet composite sensor towards detection of toluene and benzene gases. Appl. Phys. A 2019, 125, 12. [Google Scholar] [CrossRef]
- Kumar, D.; Chaturvedi, P.; Saho, P.; Jha, P.; Chouksey, A.; Lal, M.; Rawat, J.S.B.S.; Tandon, R.P.; Chaudhury, P.K. Effect of single wall carbon nanotube networks on gas sensor response and detection limit. Sens. Actuators B 2017, 240, 1134–1140. [Google Scholar] [CrossRef]


| Sensor Type | Gas: Dynamic Tested Range (ppm) | Sensitivity Range (%ppm−1) | Detection Limit (ppb) | Reference |
|---|---|---|---|---|
| Gr_bare | NH3: 0.2–14 | 7.75–0.11 | 170 | Present work |
| Gr_ZnPc | NH3: 0.2–14 | 0.75–0.012 | 50 | Present work |
| Gr_ZnPR | NH3: 0.2–14 | 1.124–0.09 | 11 | Present work |
| Gr_CuPR | NH3: 0.2–14 | 0.62–0.12 | 5 | Present work |
| Gr_NiPc | NH3: 0.04–4.64 | 8.6–0.5 | 3.3 | [60] |
| Gr_CuO NPs | NH3: 100 | 0.83 | 41 | [66] |
| MXene deposited Gr | NH3: 0.5–100 | 4–2.5 | 56 | [56] |
| NiPc-Gr | NH3: 5–10 | 0.47–0.19 | - | [67] |
| Gr_CoPt | NH3: 2.2–36.0 | 0.8–0.24 | 0.1 | [68] |
| Gr_NiPc | NH3: 0.5–13.6 | 0.64–0.11 | 50 | [27] |
| Nitric acid treated Gr | NH3: 20–100 | 0.7–0.4 | 27 | [69] |
| S-ZnO@Gr | NH3: 5–15 | 1.9–1.1 | - | [70] |
| Gr_NBD | NH3: 0.05–8.4 | 10.78–0.45 | - | [71] |
| Gr-TCN | NH3: 0.85–22.5 | 6.3–1.5 | 4.2 | [54] |
| B-doped Gr | NH3: 16–256 | 0.12–0.09 | - | [72] |
| CVD graphene | NH3: 100–800 | 0.05–0.01 | - | [55] |
| Laser written Gr | NH3: 75–400 | 0.04–0.075 | - | [73] |
| B-doped Gr | NH3: 1–20 | 0.04–0.042 | 59.9 | [74] |
| TiO2@PPy–GN | NH3: 10–200 | 2.4–1.3 | 1000 | [57] |
| graphene–PEDOT:PSS | NH3: 25–1000 | 0.2–0.019 | 10,000 | [75] |
| Gr_ZnPc | H2S: 0.05–2.5 | 6.41–0.85 | 12.6 | Present work |
| Gr_ZnPR | H2S: 0.05–2.5 | 0.86–0.21 | 23.0 | Present work |
| Gr_CuPR | H2S: 0.05–2.5 | 1.57–0.09 | 29.3 | Present work |
| Gr and AgNPs | H2S: 0.5–50 | 8–2.6 | 100 | [76] |
| CuO2 NCs on Gr | H2S: 0.05–0.1 | 15–35 | 5 | [77] |
| Gr_bare | C6H6: 0.005–0.3 | 22.10–1.22 | 5 | Present work |
| Gr_ZnPc | C6H6: 0.005–0.3 | 44.00–6.22 | 2.7 | Present work |
| Gr_ZnPR | C6H6: 0.005–0.3 | 17.8–1.97 | 2.8 | Present work |
| Gr_CuPR | C6H6: 0.005–0.3 | 1.11–0.93 | 2.2 | Present work |
| Polyaniline–Gr nanoplatelets | C6H6: 1000–22,000 | 0.1–0.8 | - | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freddi, S.; Vaghi, L.; Penoni, A.; Scapinello, L.; Sangaletti, L. A Minimal Electronic Nose Based on Graphene Functionalized with Metalated Pyrazinoporphyrazines and Phthalocyanines for Ammonia, Benzene, and Hydrogen Sulfide Discrimination. Chemosensors 2025, 13, 165. https://doi.org/10.3390/chemosensors13050165
Freddi S, Vaghi L, Penoni A, Scapinello L, Sangaletti L. A Minimal Electronic Nose Based on Graphene Functionalized with Metalated Pyrazinoporphyrazines and Phthalocyanines for Ammonia, Benzene, and Hydrogen Sulfide Discrimination. Chemosensors. 2025; 13(5):165. https://doi.org/10.3390/chemosensors13050165
Chicago/Turabian StyleFreddi, Sonia, Luca Vaghi, Andrea Penoni, Luca Scapinello, and Luigi Sangaletti. 2025. "A Minimal Electronic Nose Based on Graphene Functionalized with Metalated Pyrazinoporphyrazines and Phthalocyanines for Ammonia, Benzene, and Hydrogen Sulfide Discrimination" Chemosensors 13, no. 5: 165. https://doi.org/10.3390/chemosensors13050165
APA StyleFreddi, S., Vaghi, L., Penoni, A., Scapinello, L., & Sangaletti, L. (2025). A Minimal Electronic Nose Based on Graphene Functionalized with Metalated Pyrazinoporphyrazines and Phthalocyanines for Ammonia, Benzene, and Hydrogen Sulfide Discrimination. Chemosensors, 13(5), 165. https://doi.org/10.3390/chemosensors13050165







