Preclinical Validation of an Electrochemical Sensor for Alcohol Consumption Monitoring in a Polydrug Self-Administration Animal Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instruments
2.3. Procedures
2.3.1. Labeling of Transferrin with Os(VI)-Based Electrochemical Tag
2.3.2. Immunoextraction Using Anti-Tf Magnetic Beads
2.3.3. Electrochemical Sensing
2.3.4. Animals, Experimental Design, and Plasma Preparation
Animals
Surgical Procedures
Self-Administration Protocol
Sample Collection and Analysis
2.3.5. Statistical Studies
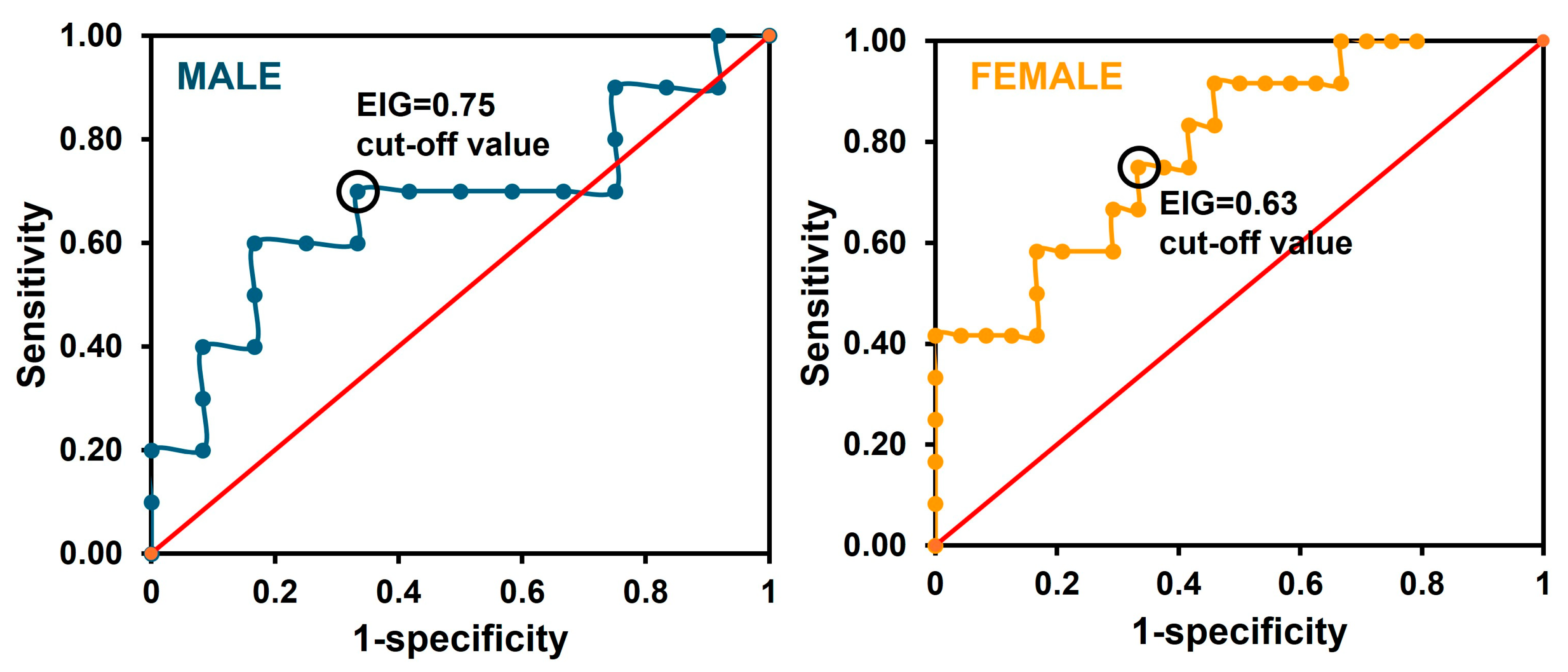
2.3.6. Clinical Sensitivity and Specificity
3. Results and Discussion
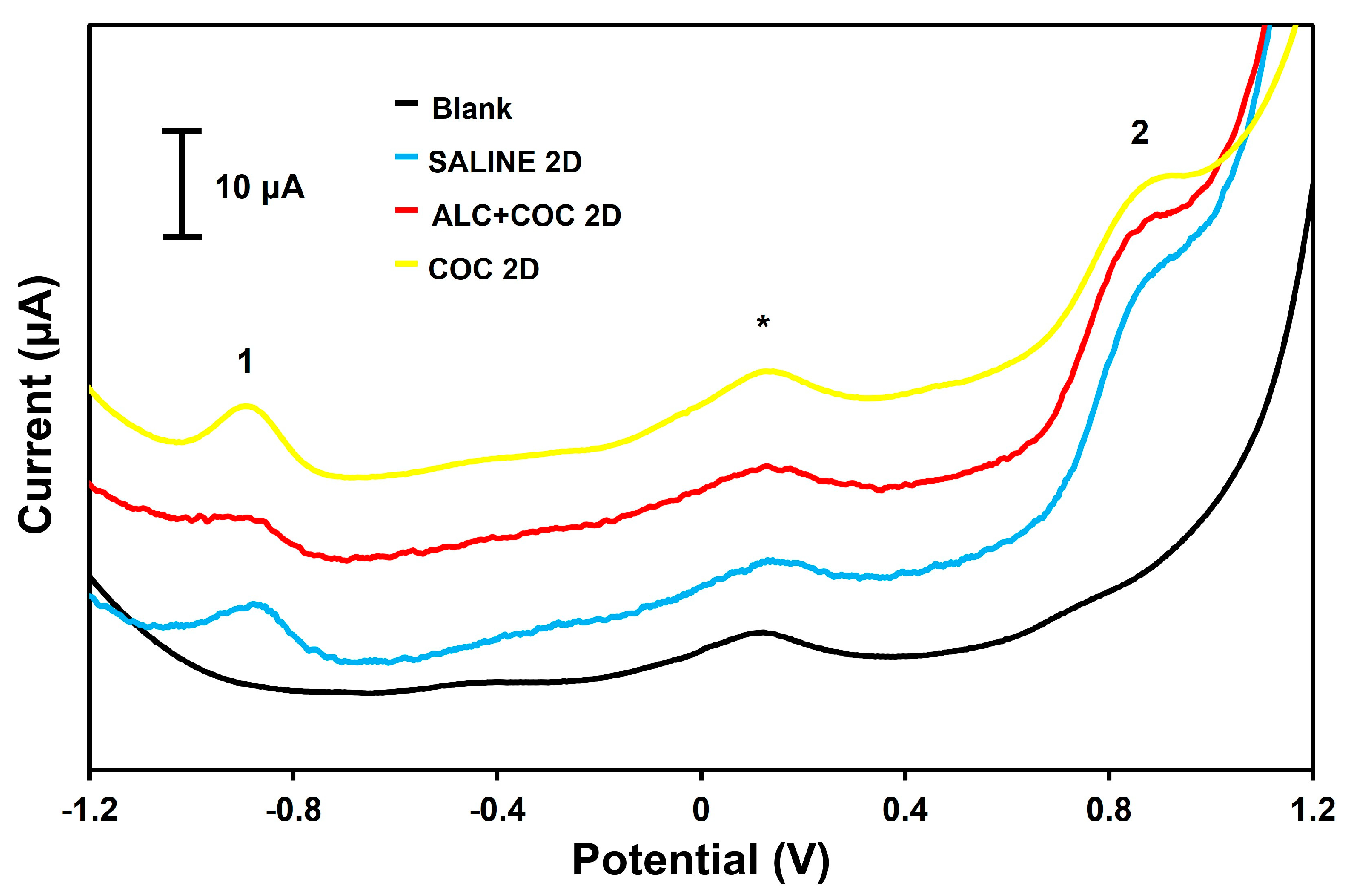
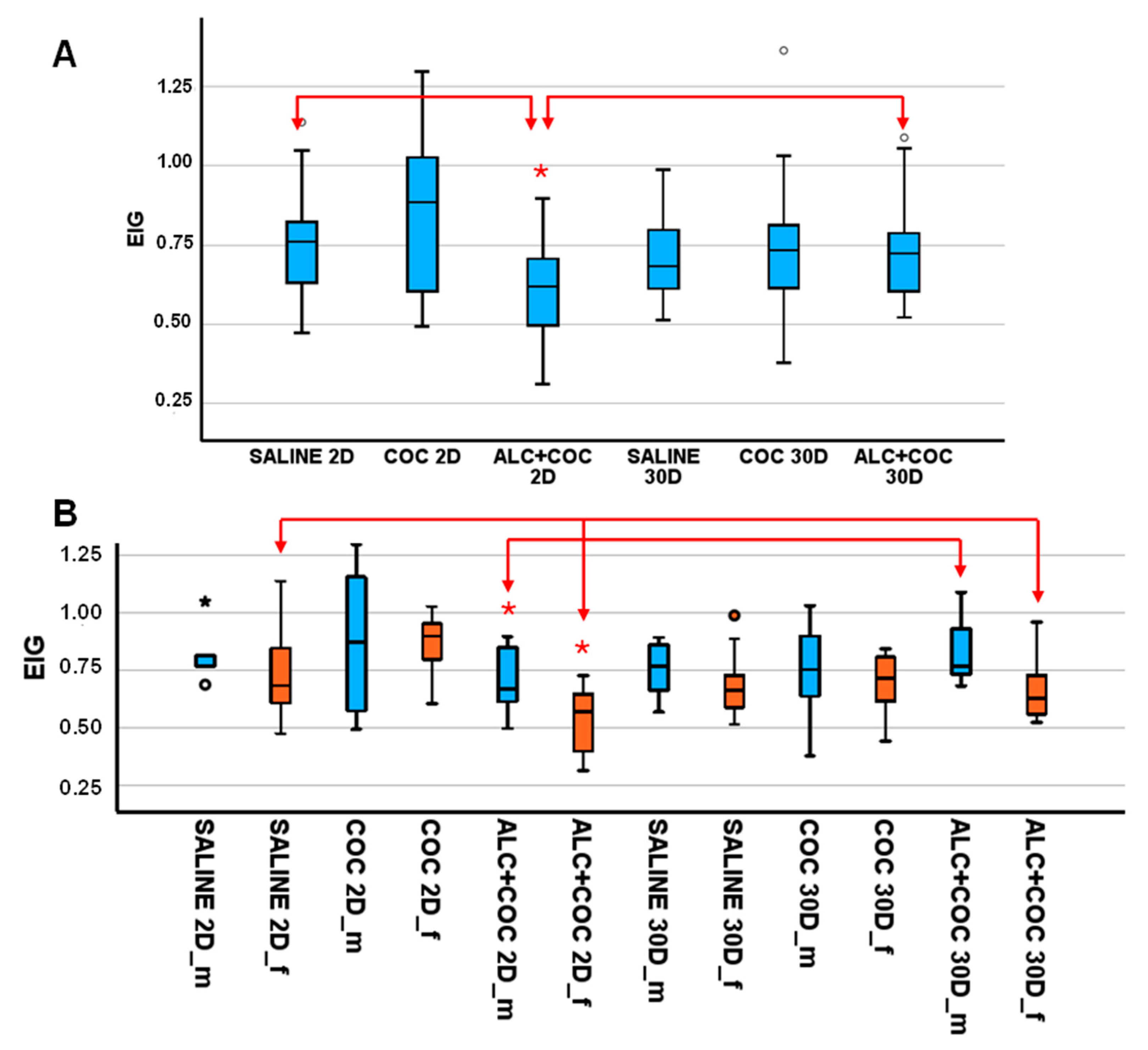
3.1. EIG Level in Rat Plasma from Polydrug-Consuming Rats
3.2. Preclinical Validation of the Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AdTSWV | adsorptive transfer square wave voltammetry |
| ALC | Alcohol |
| AUD | Alcohol use disorders |
| CDT | Carbohydrate-deficient transferrin |
| COC | Cocaine |
| EIG | Electrochemical index of glycosylation |
| POCT | Point-of-care testing |
| ROC | Receiver operating characteristic |
| SWV | Square wave voltammetry |
| Tf | Transferrin |
| TEMED | N,N,N’,N’-tetramethylethylenediamine |
References
- Feltenstein, M.W.; See, R.E.; Fuchs, R.A. Neural Substrates and Circuits of Drug Addiction. Cold Spring Harb. Perspect. Med. 2021, 11, a039628. [Google Scholar] [CrossRef] [PubMed]
- Fleurya, M.-J.; Djouini, A.; Huỳnh, C.; Tremblay, J.; Ferland, F.; Ménard, J.-M.; Belleville, G. Remission from substance use disorders: A systematic review and meta-analysis. Drug Alcohol Depend. 2016, 168, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Anderson, P.; Barry, J.; Dimitrov, P.; Elekes, Z.; Feijão, F.; Frick, U.; Gual, A.; Gmel, J.G.; Kraus, L.; et al. Prevalence of and Potential Influencing Factors for Alcohol Dependence in Europe. Eur. Addict. Res. 2014, 21, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.F.; Goldstein, R.B.; Saha, T.D.; Chou, S.P.; Jung, J.; Zhang, H.; Pickering, R.P.; Ruan, W.J.; Smith, S.M.; Huang, B.; et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 2015, 72, 757–766. [Google Scholar] [CrossRef]
- Liu, Y.; Williamson, V.G.; Setlow, B.; Cottler, L.B.; Knackstedt, L.A. The importance of considering polysubstance use: Lessons from cocaine research. Drug Alcohol Depend. 2018, 192, 16–28. [Google Scholar] [CrossRef]
- Cabezas, J.; Lucey, M.R.; Bataller, R. Biomarkers for Monitoring Alcohol Use. Clin. Liver Dis. 2016, 8, 59–63. [Google Scholar] [CrossRef]
- Liu, J.; Man, K. Biomarkers for monitoring alcohol sobriety after liver transplantation for alcoholic liver disease. J. Gastroenterol. Hepatol. 2023, 38, 1227–1232. [Google Scholar] [CrossRef]
- Andresen-Streichert, H.; Müller, A.; Glahn, A.; Skopp, G.; Sterneck, M. Alcohol Biomarkers in Clinical and Forensic Contexts. Dtsch. Arztebl. Int. 2018, 115, 309–315. [Google Scholar] [CrossRef]
- Volkow, N.D.; Koob, G.; Baler, R. Biomarkers in substance use disorders. ACS Chem. Neurosci. 2015, 6, 522–525. [Google Scholar] [CrossRef]
- Van Uytfanghe, K.; De Boosere, E.; Stove, C.P. Monitoring the use of alcohol—A critical overview of the state-of-the-art biomarkers. WIREs Forensic. Sci. 2022, 4, e1457. [Google Scholar] [CrossRef]
- Bortolotti, F.; Sorio, D.; Bertaso, A.; Tagliaro, F. Analytical and diagnostic aspects of carbohydrate deficient transferrin (CDT): A critical review over years 2007–2017. J. Pharm. Biomed. Anal. 2018, 147, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Caslavska, J.; Thormann, W. Monitoring of alcohol markers by capillary electrophoresis. J. Sep. Sci. 2013, 36, 75–95. [Google Scholar] [CrossRef]
- Delanghe, J.R.; Helander, A.; Wielders, J.P.; Pekelharing, J.M.; Roth, H.J.; Schellenberg, F.; Born, C.; Yagmur, E.; Gentzer, W.; Althaus, H. Development and multicenter evaluation of the N Latex CDT direct immunonephelometric assay for serum carbohydrate-deficient transferrin. Clin. Chem. 2007, 53, 1115–1121. [Google Scholar] [CrossRef]
- Kim, C.; Kang, M.S.; Raja, I.S.; Joung, Y.K.; Han, D.-W. Advancements in nanobiosensor technologies for in-vitro diagnostics to point of care testing. Heliyon 2024, 10, e40306. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, A.; Luong, J.H.T. Emerging Technologies for Next-Generation Point-of-Care Testing. Trends Biotechnol. 2015, 33, 692–705. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, J.F.; Rojas, D.; Escarpa, A. Electrochemical Sensing Directions for Next-Generation Healthcare: Trends, Challenges, and Frontiers. Anal. Chem. 2020, 93, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Liu, C.C. Recent Advances on Electrochemical Biosensing Strategies toward Universal Point-of-Care Systems. Angew Chem. Int. Ed. Engl. 2019, 58, 12355–12368. [Google Scholar] [CrossRef]
- Paimard, G.; Ghasali, E.; Baeza, M. Screen-Printed Electrodes: Fabrication, Modification, and Biosensing Applications. Chemosensors 2023, 11, 113. [Google Scholar] [CrossRef]
- Campbell, A.S.; Kim, J.; Wang, J. Wearable electrochemical alcohol biosensors. Cur. Op. Electrochem. 2018, 10, 126–135. [Google Scholar] [CrossRef]
- Costa, N.G.; Antunes, J.C.; Paleo, A.J.; Rocha, A.M. A Review on Flexible Electrochemical Biosensors to Monitor Alcohol in Sweat. Biosensors 2022, 12, 252. [Google Scholar] [CrossRef]
- Lin, K.-C.; Kinnamon, D.; Sankhala, D.; Muthukumar, S.; Prasad, S. AWARE: A Wearable Awareness with Real-time Exposure, for monitoring alcohol consumption impact through ethyl glucuronide detection. Alcohol 2019, 81, 93–99. [Google Scholar] [CrossRef]
- Sierra, T.; Crevillen, A.G.; Escarpa, A. Electrochemical sensor for the assessment of carbohydrate deficient transferrin: Application to diagnosis of congenital disorders of glycosilation. Biosens. Bioelectron. 2021, 179, 113098. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Hidalgo, G.; Sierra, T.; Dortez, S.; Marcos, A.; Ambrosio, E.; Crevillen, A.G.; Escarpa, A. Transferrin analysis in wistar rats plasma: Towards an electrochemical point-of-care approach for the screening of alcohol abuse. Microchem. J. 2022, 181, 107738. [Google Scholar] [CrossRef]
- Trefulka, M.; Paleček, E. Direct chemical modification and voltammetric detection of glycans in glycoproteins. Electrochem. Commun. 2014, 48, 52–55. [Google Scholar] [CrossRef]
- Trefulka, M.; Černocká, H.; Staroňová, T.; Ostatná, V. Voltammetric analysis of glycoproteins containing sialylated and neutral glycans at pyrolytic graphite electrode. Bioelectrochemistry 2024, 163, 108851. [Google Scholar] [CrossRef]
- Marcos, A.; Moreno, M.; Orihuel, J.; Ucha, M.; de Paz, A.M.; Higuera-Matas, A.; Capellán, R.; Crego, A.L.; Martínez-Larrañaga, M.-R.; Ambrosio, E.; et al. The effects of combined intravenous cocaine and ethanol self-administration on the behavioral and amino acid profile of young adult rats. PLoS ONE 2020, 15, e0227044. [Google Scholar] [CrossRef] [PubMed]
- Conigrave, K.M.; Degenhardt, L.J.; Whitfield, J.B.; Saunders, J.B.; Helander, A.; Tabakoff, B. CDT, GGT, and AST as markers of alcohol use: The WHO/ISBRA Collaborative Project. Alcohol. Clin. Exp. Res. 2002, 26, 332–339. [Google Scholar] [CrossRef]
- Ikegami, A.; Olsen, C.M.; Fleming, S.M.; E Guerra, E.; A Bittner, M.; Wagner, J.; Duvauchelle, C.L. Intravenous ethanol/cocaine self-administration initiates high intake of intravenous ethanol alone. Pharmacol. Biochem. Behav. 2002, 72, 787–794. [Google Scholar] [CrossRef]
- Sierra, T.; González, M.C.; Moreno, B.; Crevillen, A.G.; Escarpa, A. Total α1-acid glycoprotein determination in serum samples using disposable screen-printed electrodes and osmium (VI) as electrochemical tag. Talanta 2018, 180, 206–210. [Google Scholar] [CrossRef]
- Nahm, F.S. Nonparametric statistical tests for the continuous data: The basic concept and the practical use. Korean J. Anesthesiol. 2016, 69, 8–14. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Szajda, S.D.; Zalewska, A.; Szulc, A.; Kępka, A.; Minarowska, A.; Wojewódzka-Żelezniakowicz, M.; Konarzewska, B.; Chojnowska, S.; Ładny, J.R.; et al. Alcohol abuse and glycoconjugate metabolism. Folia Histochem. Cytobiol. 2012, 50, 1–11. [Google Scholar] [CrossRef]
- Ersche, K.D.; Acosta-Cabronero, J.; Jones, P.S.; Ziauddeen, H.; van Swelm, R.P.L.; Laarakkers, C.M.M.; Raha-Chowdhury, R.; Williams, G.B. Disrupted iron regulation in the brain and periphery in cocaine addiction. Transl. Psychiatry 2017, 7, e1040. [Google Scholar] [CrossRef] [PubMed]
- Trbojević-Akmačić, I.; Vučković, F.; Pribić, T.; Vilaj, M.; Černigoj, U.; Vidič, J.; Šimunović, J.; Kępka, A.; Kolčić, I.; Klarić, L.; et al. Comparative analysis of transferrin and IgG N-glycosylation in two human populations. Commun. Biol. 2023, 6, 312. [Google Scholar] [CrossRef]
- Cargiulo, T. Understanding the health impact of alcohol dependence. Am. J. Health Pharm. 2007, 64, S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Nanau, R.M.; Neuman, M.G. Biomolecules and Biomarkers Used in Diagnosis of Alcohol Drinking and in Monitoring Therapeutic Interventions. Biomolecules 2015, 5, 1339–1385. [Google Scholar] [CrossRef]
- Suzuki, T.; Eguchi, A.; Shigefuku, R.; Nagao, S.; Morikawa, M.; Sugimoto, K.; Iwasa, M.; Takei, Y. Accuracy of carbohydrate-deficient transferrin as a biomarker of chronic alcohol abuse during treatment for alcoholism. Hepatol. Res. 2021, 52, 120–127. [Google Scholar] [CrossRef]
- Chrostek, L.; Cylwik, B.; Gruszewska, E.; Tobolczyk, J. The diagnostic power of direct carbohydrate-deficient transferrin immunoassay in alcoholics. Absolute or relative values? Alcohol 2012, 46, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Ireland, J.; Cheng, D.M.; Samet, J.H.; Bridden, C.; Quinn, E.; Saitz, R. Operating characteristics of carbohydrate-deficient transferrin (CDT) for identifying unhealthy alcohol use in adults with HIV infection. AIDS Care 2011, 23, 1483–1491. [Google Scholar] [CrossRef]
- Madhubala, V.; Subhashree, A.R.; Shanthi, B. Serum Carbohydrate Deficient Transferrin as A Sensitive Marker in Diagnosing Alcohol Abuse: A Case—Control Study. J. Clin. Diagn. Res. 2013, 7, 197–200. [Google Scholar] [CrossRef]

| Classification | 2D | 30D | 30D | 2D | ||
| M (SAL) | M (COC) | M (SAL) | M (COC) | M (ALC+COC) | M (ALC+COC) | |
| Non-alcohol | Alcohol | |||||
| Non-alcohol | 5 | 7 | 6 | 7 | 12 | 7 |
| Alcohol | 0 | 4 | 1 | 4 | 0 | 3 |
| %Correct classification | 80 | 30 | ||||
| 71 | ||||||
| Classification | 2D | 30D | 30D | 2D | ||
| F (SAL) | F (COC) | F (SAL) | F (COC) | F (ALC+COC) | F (ALC+COC) | |
| Non-alcohol | Alcohol | |||||
| Non-alcohol | 9 | 6 | 8 | 5 | 8 | 4 |
| Alcohol | 3 | 1 | 4 | 1 | 8 | 8 |
| %Correct classification | 68 | 67 | ||||
| 68 | ||||||
| Classification | 2D | 30D | 30D | 2D | ||
| M (SAL) | M (COC) | M (SAL) | M (COC) | M (ALC+C0C) | M (ALC+COC) | |
| Non-alcohol | Alcohol | |||||
| Non-alcohol | 4 | 6 | 4 | 4 | 8 | 3 |
| Alcohol | 1 | 5 | 3 | 7 | 4 | 7 |
| %Correct classification | 57 | 70 | ||||
| 59 | ||||||
| Classification | 2D | 30D | 30D | 2D | ||
| F (SAL) | F (COC) | F (SAL) | F (COC) | F (ALC+COC) | F (ALC+COC) | |
| Non-alcohol | Alcohol | |||||
| Non-alcohol | 7 | 6 | 7 | 5 | 6 | 2 |
| Alcohol | 5 | 1 | 5 | 1 | 10 | 10 |
| %Correct classification | 58 | 83 | ||||
| 63 | ||||||
| Method | Portability | Studied Group | Biomarker/Cut-Off | Diagnostic Performance | Ref. |
|---|---|---|---|---|---|
| N Latex CDT direct immuno-nephelometric assay | No | 126 Japanese alcohol-dependent patients (107 men/19 women) | %CDT/1.9% | 77.9% sensitivity, 77.1% specificity | [36] |
| N Latex CDT direct immuno-nephelometric assay | No | 127 Polish adult alcoholics (44 men/100 women) | %CDT/2.12% | 91.3% sensitivity, 90.4% specificity | [37] |
| N Latex CDT direct immuno-nephelometric assay | No | 47 HIV-positive heavy drinkers with self-reported alcohol consumption (32 men/15 women) | %CDT/2.6% | 36% sensitivity, 88% specificity | [38] |
| Turbidimetric immunoassay, ELISA method (Biorad) | No | 25 chronic alcoholic men | %CDT/2.6% | 84% sensitivity, 92% specificity | [39] |
| Immuno-magnetic beads and electrochemical sensor | Yes | 121 alcohol and cocaine self-administered rats (56 male/65 female) | EIG/0.75 for male 0.63 for female | 70% sensitivity, 67% specificity for male. 75% sensitivity, 67% specificity for female. | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido-Matilla, L.; María-Hormigos, R.; Monago-Maraña, O.; Marcos, A.; Ambrosio, E.; Crevillen, A.G. Preclinical Validation of an Electrochemical Sensor for Alcohol Consumption Monitoring in a Polydrug Self-Administration Animal Model. Chemosensors 2025, 13, 97. https://doi.org/10.3390/chemosensors13030097
Garrido-Matilla L, María-Hormigos R, Monago-Maraña O, Marcos A, Ambrosio E, Crevillen AG. Preclinical Validation of an Electrochemical Sensor for Alcohol Consumption Monitoring in a Polydrug Self-Administration Animal Model. Chemosensors. 2025; 13(3):97. https://doi.org/10.3390/chemosensors13030097
Chicago/Turabian StyleGarrido-Matilla, Lucía, Roberto María-Hormigos, Olga Monago-Maraña, Alberto Marcos, Emilio Ambrosio, and Agustin G. Crevillen. 2025. "Preclinical Validation of an Electrochemical Sensor for Alcohol Consumption Monitoring in a Polydrug Self-Administration Animal Model" Chemosensors 13, no. 3: 97. https://doi.org/10.3390/chemosensors13030097
APA StyleGarrido-Matilla, L., María-Hormigos, R., Monago-Maraña, O., Marcos, A., Ambrosio, E., & Crevillen, A. G. (2025). Preclinical Validation of an Electrochemical Sensor for Alcohol Consumption Monitoring in a Polydrug Self-Administration Animal Model. Chemosensors, 13(3), 97. https://doi.org/10.3390/chemosensors13030097








