Abstract
Lactic acid is a vital molecule for health and food quality control. Its detection, typically via L-lactate, is a valuable indicator for conditions like disease, product spoilage, and stress. Electrochemical biosensors offer a promising, user-friendly solution for lactate detection. These versatile devices allow for tailored surfaces, adapting to sample characteristics, detection mechanisms, and end-user needs. Despite the variety of existing electrochemical biosensor architectures, including microfluidic, wearable, paper-based, carbon-based, and glassy carbon electrode types, routine lactate analysis with these devices remains a significant challenge. This work will explore diverse electrochemical lactate biosensors, detailing their designs, modifications, common transducers, analyzed samples, and validation. We will also survey commercially available options. Finally, this review assesses the current commercialization status and future perspectives of these biosensors, highlighting their growing importance in clinical and industrial applications.
1. Introduction
Lactate is a crucial intermediate in cell metabolism and a vital biomarker in clinical diagnostics, food industry, and sports medicine. Its concentration serves as a key molecular marker for assessing patient health. While often used interchangeably, lactic acid is a weak acid, and lactate is its conjugate base [1].
Lactic acid, also known as 2-hydroxypropanoic acid (C3H6O3), is a chiral molecule that exists in two enantiomeric forms: L-lactic acid and D-lactic acid. Depending on the production process, it may exhibit optical activity in either the L (+) or D (−) form, or as a racemic mixture containing both forms [1,2].
Blood lactate levels range from 0.5 to 1.5 mmol. L−1 but can rise to 25.0 mmol. L−1 during intense exercise [3,4]. The production of this organic acid derives from sugar metabolism, and in anaerobic conditions results in the production of L-lactate by the action of lactate dehydrogenase (LDH, Lactate dehydrogenase) [5].
In human physiology, L-lactate constitutes the predominant form of lactate produced. Its concentration in the bloodstream is approximately 100 times greater than that of D-lactate [6,7]. Therefore, its pathological relevance is primarily linked to the presence of L-lactate. In certain cases, D-lactate accumulation is typically linked to pathological conditions such as gastrointestinal disorders and dysbiosis [8].
Lactate monitoring is valuable for patients experiencing high stress, as it aids in detecting conditions such as tissue hypoxia, septic shock, lactic acidosis, and heart failure [9,10]. Its detection offers real-time, in situ assessment for embryonic cell culture selection. Therefore, early detection of these conditions enables healthcare professionals to take timely action, thereby enhancing patient outcomes [11].
Biosensors have emerged and offer a fast response, multiplex detection, affordability, ease of use, and low cost [12]. Natural enzymes are crucial as recognition elements in lactate biosensor construction, catalyzing reactions to generate detectable signals. However, they have drawbacks: high production cost and low stability, limiting their function under extreme pH and temperature conditions [13,14].
There are two main types of lactate biosensors: lactate dehydrogenase (LDH) and lactate oxidase (LOx). These two natural oxidoreductase enzymes are commonly used for L-lactate detection [15]. The LDH and LOx facilitate the oxidation of lactate within cells, with the LDH being well-known for this function in the human body [16]. To a lesser extent, the lactate monooxygenase is a flavoprotein enzyme that has also been used in lactate biosensors [17,18]. Lactate concentration in blood is related to the kinetics of metabolic processes, as measuring the maximal lactate steady state in blood requires understanding lactate’s appearance and disappearance rates, typically expressed as mg·mL−1 or mg·kg−1·min−1 when normalized to body mass (3–4 mg·kg−1·min−1, equivalent to 4–8 mmol·L−1) [19].
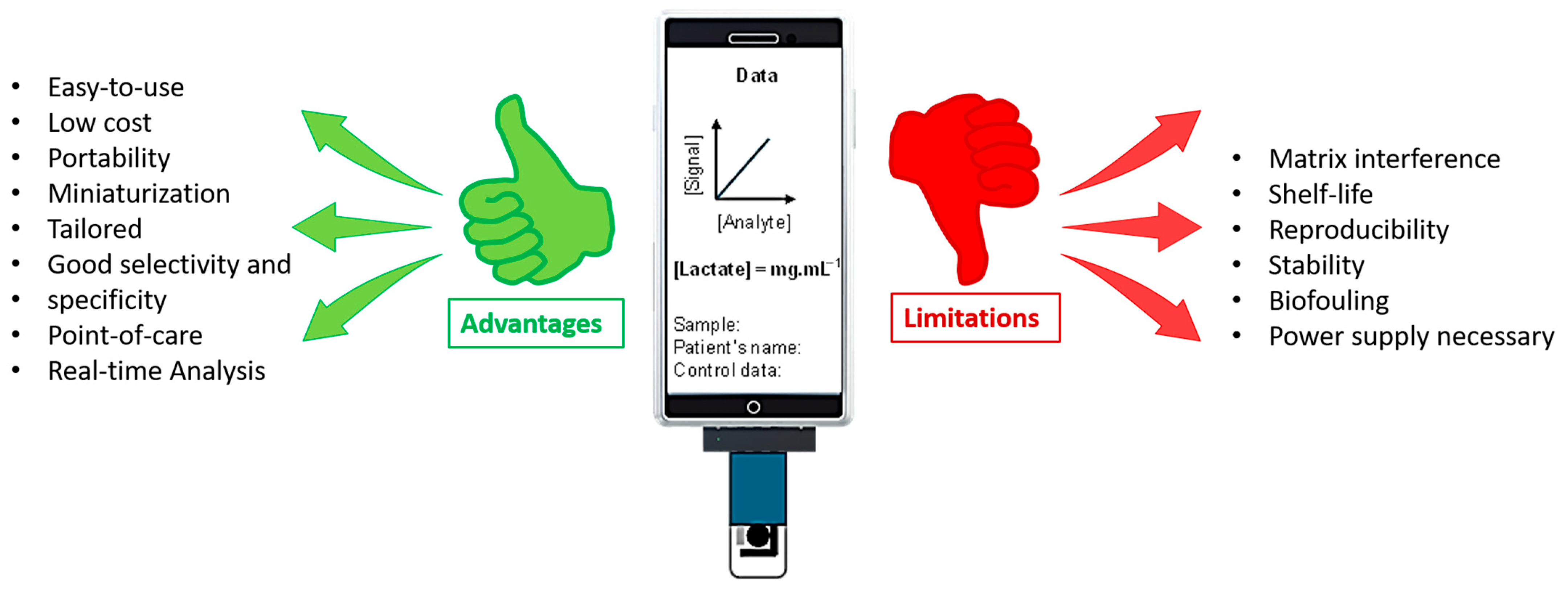
This is where biosensors offer a significant advantage over conventional healthcare systems, which often rely on time-consuming and expensive sample collection and complex laboratory analysis. Electrochemical biosensors excel at efficiently detecting low concentrations of analytes [20]. Unlike other L-lactate detection methods, biosensing is simple, direct, and real-time, requires no complex sample preparation, offers rapid response with high specificity, and is generally affordable and user-friendly (Figure 1).
Figure 1.
Electrochemical lactate sensors provide rapid analysis and portability, which are important for monitoring in various applications. However, they also require frequent calibration and are susceptible to interference, necessitating careful consideration to ensure accurate results.
Various supports have been developed for lactate biosensors, including polymer matrices, membranes, gel matrices, screen-printed electrodes, hydrogel supports, and nanoparticles [21]. It is crucial, however, that the application of these biosensors is considered within specific contexts, such as the patient’s physical activities or medical history.
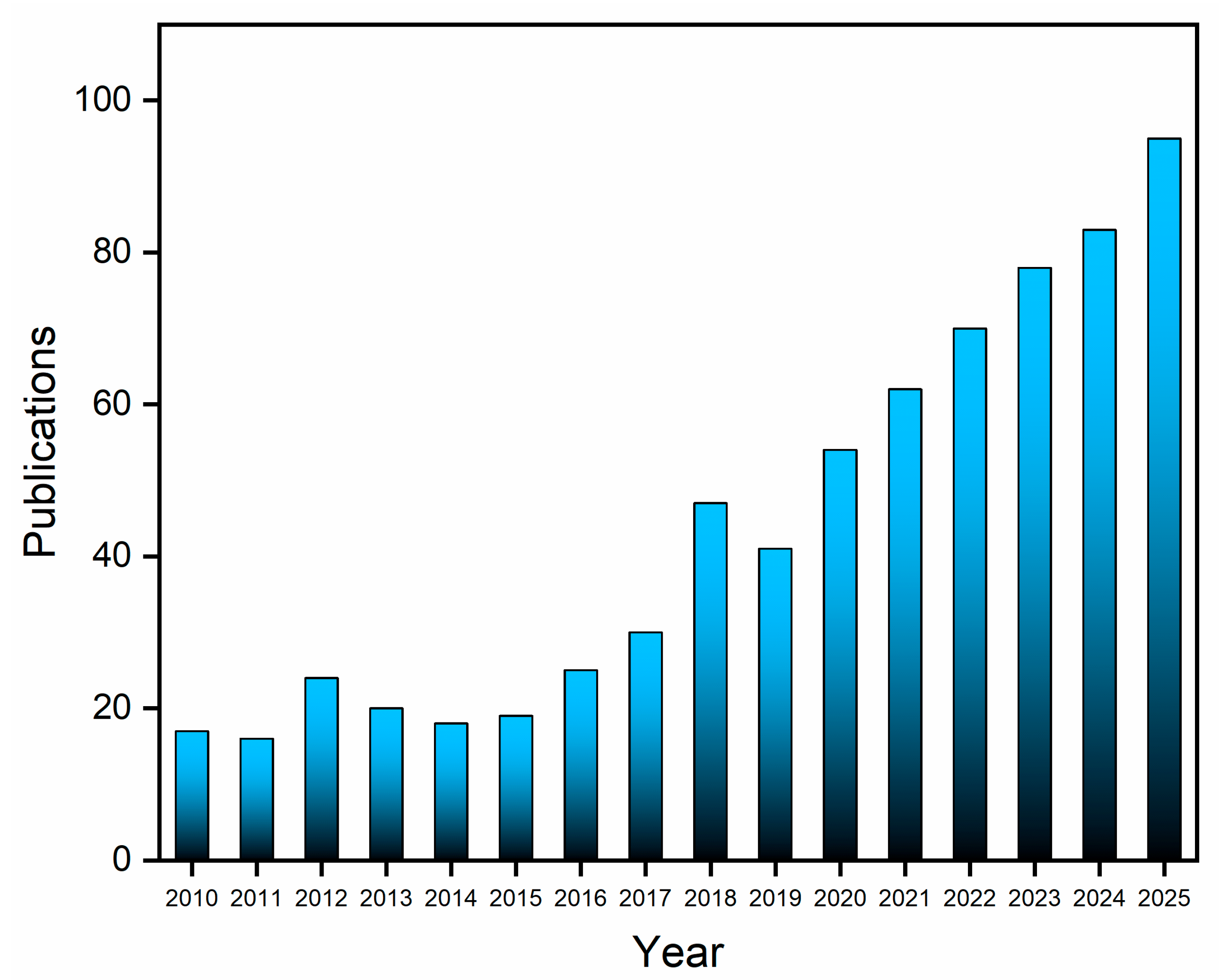
Bibliographic data indicate a sustained academic interest in lactate biosensors. A search conducted across the Web of Science and Scopus databases for “lactate biosensors” or “electrochemical lactate sensor”, filtered to include only articles and reviews published within the last 15 years and excluding duplicates, yielded 707 relevant records (Figure 2). This significant volume of publications underscores the continuing research activity in lactate detection and highlights the substantial potential for advancements in sensing technologies.
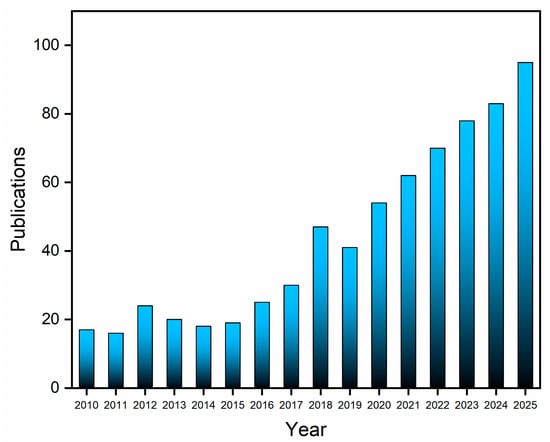
Figure 2.
Data on the publication of articles and reviews per year (2010–2025) collected from the Web of Science and Scopus databases using the search terms “lactate biosensor” or “electrochemical lactate sensor”.
Annual publication numbers remained stable until 2010, but a notable increase began in 2016, culminating in a peak in 2018. As of October 2025, 95 articles have already been published, indicating sustained growth in the field. An analysis of the literature, specifically from a materials technology perspective, shows that up until mid-2018, research predominantly focused on conventional electrochemical sensors [22,23,24] and screen-printed electrodes, often modified with nanomaterials [25,26,27].
Simultaneously, studies explored the use of flexible materials, such as hydrogels and conductive polymers [28,29,30]. These efforts primarily targeted lactate detection in food and blood samples, often including simultaneous glucose monitoring [31,32]. From approximately 2018 onwards, emerging trends began to emphasize the application of graphene-based materials, such as reduced graphene oxide [33,34,35], alongside the development of wearable sensors for continuous lactate monitoring in sweat, particularly for athletic performance analysis [36,37,38].
Since 2022, the research has increasingly concentrated on wearable, point-of-care devices capable of real-time and continuous lactate monitoring [39,40,41,42]. This is possible considering the levels of lactate relatively high in sweat, with values varying from 2.0 to 115 mmol L−1 depending on body region [39,43]. Current studies emphasize the noninvasive, simultaneous detection of lactate and other biomarkers in sweat, underscoring a significant shift toward integrated biosensing platforms with multifunctional capabilities.
This comprehensive review presents an analysis that extends beyond traditional academic reviews. It examines the future market impact of biosensors, evaluates Technology Readiness Level (TRL), explores the electrochemical biosensors marketplace, and analyzes production costs for lactate biosensor manufacturing and Key Performance Indicators (KPIs) by combining scientific rigor with commercial and market viability, significantly differentiating this work, contributing to the specialized literature for a better understanding of the commercial landscape of L-lactate electrochemical sensors.
2. Fundamentals of Lactate Biosensing
Enzyme Immobilization Strategies
Enzymatic biosensors are important analytical tools, valued for their sensitivity and selectivity. These characteristics enable their widespread application across various fields, including clinical, environmental and industrial [44,45]. As anticipated, the surface modification and immobilization of bioreceptors are a crucial step for generating good performance since they affect the charge of the surface, bioactivity, and electron transfer kinetics [44,46,47]. Five principal methods are employed for the immobilization of biomolecules: adsorption, the formation of covalent bonds, entrapment, cross-linking (including affinity-based approaches), and integrated strategies [46,48,49,50,51,52,53].
Adsorption is based on the interaction of the enzyme and material surface through intermolecular forces, such as van der Waals and hydrogen bonds, and electrostatic interactions. The method is considered easiest, not needing modification or linkers, and as a non-destructive process for enzyme activity, it is simple, economic, fast, and reusable [46,48,49,50,51,53]. A disadvantage is the low stability of the binding [46,52,53]. The enzyme can easily desorb from the support due to changes in pH, ionic strength, or temperature, or the presence of other competitors in the medium [48,50]. For this, it is rarely the ideal choice for high-performance lactate biosensors [25]. This method is used with organic and synthetic materials that adsorb enzymes, including cellulose, collagen, silica gel, polyaniline, and polypyrrole [51].
Entrapment is a physical method for enzymatic immobilization that uses three-dimensional matrices. It involves capturing and stabilizing enzymes within polymeric films, membranes on electrode surfaces, or gel materials. Crucially, the biomolecule is not chemically attached to the support in this technique [25,46,48,49,50,51,53]. This method efficiently allows substrates and products to pass through the polymer network while retaining the enzyme [53]. It is particularly useful for biosensors that require high enzyme loading and some protection from the external environment. However, diffusion restrictions can be a bottleneck for sensor response time and sensitivity, especially at low lactate concentrations.
Covalent bonding creates strong, irreversible links between enzyme and support surface. The binding happens via functional groups shared between them, such as amino, carboxyl, hydroxyl, and phenolic, are often non-essential for catalytic activity [25,46,49,50,51,52]. This method requires activation steps and strict control of reaction conditions. However, covalent bonding is often preferred when long-term stability and robustness, such as in biosensors for in vivo monitoring or reusable devices. The challenge lies in optimizing the process to minimize loss of enzymatic activity; besides that, the higher manufacturing costs and complexity are also limiting factors [46]. Normally, covalent immobilization requires activation of the support using reagents such as glutaraldehyde, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide, and N-hydroxysuccinimide (EDC/NHS) to facilitate bonding with the enzyme [46,51,53]. This method has advantages, such as not causing diffusion barriers, stability, preventing enzyme desorption, and having a short response time for the enzymatic reaction, and is the most widely used [25,46,48,51].
The cross-linking method utilizes bifunctional reagents to create a network structure, immobilizing the enzyme within the support [46]. These crosslinkers possess free reactive ends that form strong covalent bonds, anchoring the enzyme onto the support surface [46,48,49,51,53]. While glutaraldehyde is a common crosslinker, its use can create surface gaps leading to undesirable reactions and impaired device performance [46,51]. To mitigate this, inert proteins like bovine serum albumin (BSA) are often co-applied to block interference [46]. Although this method provides strong chemical binding and is generally simple, stable, and functional for biocatalysis, it can reduce enzymatic activity by altering active sites and lead to poor reusability [25,46,53]. Affinity immobilization uses specific, non-covalent bonds between a protein’s active sites and an activated support. This strategy allows a controlled and oriented immobilization, which helps prevent enzyme deactivation and active site blocking. Common examples of these interactions include the avidin-biotin system, lectin-carbohydrate and metal cation-chelator interactions [25,46,51]. For accurate and durable electrochemical lactate biosensors, covalent binding and entrapment strategies are most promising. Current research used nanomaterials to combine the benefits of different immobilization methods, enhancing performance and applicability.
3. Electrochemical L-Lactate Biosensor Design and Fabrication: Structured Overview
3.1. Modification Strategies on the Working Electrode Surface
In the architecture of electrochemical biosensors, the working electrode is the component where the electrochemical reaction takes place, and the analytical signal is transduced. Its surface characteristics are paramount for dictating the sensor’s performance, including its sensitivity, selectivity, and stability. Consequently, to maximize their analytical capabilities, a broad spectrum of modifications is applied [52]. This allows for the application of various materials, including nanomaterials, carbonaceous compounds, polymers, and sol–gels, to modify these biosensors [52,54].
To obtain a biosensor with specific features depending on the intended application, it is necessary to consider different indicators to achieve the intended objectives, with this being addressed in Table 1, which presents some key performance indicators for lactate biosensors. As can be seen, the improvement of lactate biosensors is a scientific process directly linked to the optimization of their KPIs. Table 1 illustrates the cause-and-effect relationship, where each KPI, such as sensitivity and the limit of detection, is enhanced through the application of specific strategies and materials. Selectivity, another crucial KPI, ensures the biosensor identifies only lactate amidst a complex mixture of biological substances.

Table 1.
Key Performance Indicators for Lactate Biosensors, adapted from [55].
In this context, several strategies have been used to improve the performance de biosensors thought modifications on surface. Developing a suitable substrate requires balancing factors such as enzyme immobilization, electron transfer, non-specific adsorption, and scalability in manufacturing. Such substrates support the sensitivity, reliability, and durability of electrochemical biosensors for various applications [52,54].
Selecting materials for immobilizing LOx and LDH requires careful consideration due to their complexity. Besides substrate choice, the use of different materials aims to enhance biocompatibility, electrochemical stability, and surface area, like nanomaterials, sol–gels, polymers, screen-printed electrodes, and membranes.
3.1.1. Nanomaterials
Nanomaterials are key in electrochemical biosensors because of their strong affinity for biomolecules and their ability to significantly boost the analytical signal. Various metallic and carbon-based nanoparticles are commonly used to anchor sensing elements to the working electrode. For instance, gold (AuNPs) and silver (AgNPs) nanoparticles are highly effective in lactate detection due to their advantageous electrochemical properties [16,56]. Carbon nanotubes and other nanostructures serve as interfaces between the electrode and the biochemical reactions. Nanomaterials are advantageous for preconditioning in biomolecule adsorption [19]. Such modifications can increase biosensor sensitivity by enhancing the stability of lactate-nanomaterial interactions.
Conventional electrodes can undergo these modifications. Tamborelli and colleagues modified GCE with avidin-functionalized multi-walled carbon nanotubes (MWCNT-Av), enabling the anchoring of recombinant biotinylated lactatee. The biosensor was successfully used to determine the concentration of L-lactate in food samples. This biosensor was crucial in the industry for monitoring the quality and freshness of products, since the presence of lactate can indicate bacterial fermentation [57].
Another approach involves combining nanozymes, such as hemin-functionalized carbon microfibers and platinum or gold nanoparticles, with LOx immobilized on a graphite rod electrode (GE) for amperometric detection. These modified biosensors are applicable in both food and drink industries and clinical diagnostics [58].
In electrochemical lactate sensors, nanomaterials (like AuNPs, CNTs, graphene, MOFs, and MXene) offer significant advantages. They typically provide high surface area, excellent conductivity, and enhanced catalytic activity, greatly improving response time, sensitivity and signal-to-noise ratio. However, their main drawbacks relate to dispersion issues (e.g., AuNPs aggregating, CNTs unevenly dispersing), given that, due to their high surface energy, nanoparticles are susceptible to aggregation in solution or within the sensor matrix, which decreases their effective surface area and compromises the intended enhancements. This aggregation, coupled with potential leaching or degradation in complex biological fluids (such as sweat or blood), restricts the long-term operational stability and shelf-life of the biosensor which can affect performance. Additionally, their high cost and complex fabrication are key limitations [59,60].
Although many nanomaterials are generally regarded as safe, their interactions with biological components at the nanoscale are not fully understood, raising concerns about their long-term effects, especially for materials that might leach or accumulate within tissues [9].
Additionally, the large surface area, while advantageous for enzyme loading, can also inadvertently increase non-specific adsorption (biofouling) of unwanted proteins and other biomolecules, resulting in signal drift, reduced sensitivity, and eventual sensor failure over time. Finally, the complexity of surface functionalization required to effectively immobilize enzymes onto nanomaterials can increase fabrication costs and complexity, potentially altering the native conformation and activity of the enzyme, thereby undermining the sensor’s performance [61].
3.1.2. Sol–Gel
Sol–gel matrices, characterized as translucent, porous gels, are widely employed for enzyme immobilization. They effectively encapsulate enzymes within their intricate three-dimensional network, which exhibits characteristics of both solid and liquid phases. This versatility allows for precise control over the porosity and the fixation of the enzyme, offering a stable and protective environment that can enhance enzyme activity and stability [62].
The method uses colloidal suspensions of solid particles in a liquid to synthesize nanostructured and amorphous silica-based materials. For example, TiO2 composite types can be deposited on silicon wafers via this process, forming an anatase crystalline structure [21]. Enzymes can be immobilized via sol–gel processes on conventional electrodes, such as GCE. Nanoparticles can also be incorporated to enhance electroanalytical response [22].
Hydrogel materials such as polyvinyl alcohol (PVA), polyacrylamide (PAM), and sodium alginate (SA) hydrogels are commonly used in lactate electrochemical sensors [9]. Their high-water content and outstanding biocompatibility provide stable, conductive environments, enhancing sensor sensitivity and stability [59].
Park et al. developed an amperometric biosensor for lactate detection by integrating LOx and an osmium redox polymer on a glassy carbon electrode. The surface was coated with a sol–gel film from methyltriethoxysilane. The electrode showed a diffusion-controlled electrooxidation current, and the addition of lactate produced a distinct electrocatalytic oxidation wave, allowing for precise amperometric quantification [63]. Notably, Kim et al. reported the development of a porous nickel oxide-based sensor for lactate detection utilizing the reverse micelle sol–gel technique. Their research also focused on the critical aspect of maintaining structural stability [64]
A stable bioelectrochemical sensor was developed by Zhao et al., using a glassy carbon electrode (GCE). The sensor, constructed with LDH and gold nanoparticles modified with oxidized graphene in a sol–gel of tetraethyl orthosilicate (TEOS). The biosensor exhibited pyruvate detection and strong reproducibility, regenerability, storage stability, and superior anti-interference capabilities against common biological species [65].
Sol–gel matrices, despite their benefits for enzyme stabilization, have certain limitations. One of them is their non-conductive nature, which affects efficient electron transfer between the enzyme and the electrode surface. This characteristic leads to lower biosensor sensitivity, often requiring the incorporation of conductive materials [66]. The addition of conductive additives (like carbon nanotubes, graphene, or metallic nanoparticles) into the sol–gel matrix adds complexity to the fabrication process and potentially compromises the homogeneity and mechanical integrity of the film. However, without these additives, sensitivity is limited, and higher operating potential might be required, increasing the risk of interference [66].
Moreover, mass transport limitations within the porous network of the sol–gel can delay response time and restrict the sensor’s linear detection range, as diffusion of the analyte and reaction products may be affected. Lastly, the mechanical fragility or cracking upon drying of some sol–gel formulations can impact on the long-term durability and stability of the biosensor, which are essential for practical applications [67].
The diffusion rate of lactate into the matrix to reach the immobilized enzyme, as well as the subsequent diffusion of reaction products (such as H2O2 or NADH) out to the electrode surface, can influence both the response time and the linear operational range of the sensor. Excessively tortuous or restrictive pore structures may result in delayed responses and lead to saturation at lower analyte concentrations [65,68].
3.1.3. Polymers
Polymers are extensively utilized in the development of electrochemical lactate biosensors, primarily owing to their ease of preparation and inherent biocompatibility. Their versatility allows them to effectively coat electrode surfaces, which can help mitigate interference from undesirable molecules in complex biological samples. Common polymers are readily synthesized through their carbon chain structures, offering a broad range of tunable properties. While a significant number of polymers are non-conductive, limiting direct electron transfer, they can be modified or doped with conductive species (like carbon nanomaterials or metallic nanoparticles) to enhance their electrical properties [26].
Examples of polymers employed in this field include chitosan, polyvinyl alcohol, polyaniline, and polyimide, each offering distinct advantages in terms of mechanical stability, film formation, and chemical properties. Molecularly Imprinted Polymers (MIPs) are artificial receptor substrates that provide highly selective binding sites for lactate; as such, MIPs may contribute to the development of more robust and specific biosensors [26].
Madden et al. developed a chronoamperometric sensor using a laser-scribed graphene (LSG) electrode with polyimide. They developed a flexible and low-cost lactate biosensor, with platinum, chitosan, and LOx, which demonstrates excellent analytical performance and potential for practical applications in various biological fluids [69]. Xuan et al. created a biosensor with LOx immobilized in a polymer layer containing tetradodecylammonium tetrakis(4-chlorophenyl)borate. The biosensor’s effectiveness for on-body sweat lactate monitoring was shown with an epidermal microfluidic patch, tested at three body locations during cycling and with iontophoresis-induced sweat [70]. The analytical strategy is used to limit the amount of lactate that reaches the LOx, which is immobilized in the biosensor’s core. This was achieved through an outer plasticized polymeric layer. The layer not only controls lactate flux but also helps to reduce the influence of pH and temperature variations on the sensor’s performance.
Shitanda et al. developed a screen-printed biosensor incorporating magnesium oxide, graft-polymerized LOx, and 1,2-naphthoquinone, integrated with polydimethylsiloxane, for continuous lactate monitoring via sweat delivery in a microfluidic system [71].
Polymer materials like polypyrrole (PPy), polyaniline (PANI), and polyvinyl alcohol (PVA) offer good conductivity and tunable chemical properties, leading to exceptional electrochemical activity and biocompatibility in sensors. However, polymers often have poor mechanical strength, becoming brittle or deforming under stress or long-term use. Some are also prone to swelling or instability, degrading performance [72]. For electron transfer applications, conductive polymers are the optimal choice. Nonetheless, biocompatibility is essential, particularly in contexts involving biological samples or human contact, to prevent adverse reactions.
The polymer must allow lactate to reach the enzyme and reaction products to access the electrode, while blocking interferents that could affect the signal. Polymer film thickness affects response time and sensitivity, and its chemical and mechanical stability is key for sensor durability [72]. The polymer matrix must not only shield the enzyme but also preserve its activity and ensure proper orientation. The alignment facilitates lactate interaction with the enzyme’s active site and promotes efficient electron transfer. Additionally, the quantity of immobilized enzymes must be balanced to achieve adequate sensitivity without creating a layer so thick that it hinders diffusion or causes enzyme leaching [73].
3.1.4. Membranes
Membranes are employed in the development of electrochemical lactate biosensors due to their ease of preparation and inherent biocompatibility. This approach precisely controls the enzyme’s microenvironment, enhancing its activity and stability. Additionally, it facilitates regulated diffusion of lactate from the sample to the active site of the enzyme, followed by efficient electron transfer to the electrochemical transducer [9].
The membrane-based design addresses challenges such as interference from other molecules in complex samples and extends lifespan of the biosensor, making it suitable for applications that require high selectivity and reliable performance. Materials used for membranes include cellulose acetate, Nafion, and Teflon, which provide physical stability and enhance the longevity of the sensors [9].
Berketa et al. developed an amperometric biosensor that can rapidly and accurately measure the amount of l-lactate in human serum. LOx was immobilized in bovine albumin matrix within a platinum electrode. The semipermeable polyphenylenediamine membrane that coats the electrode minimizes interference from other substances present in the blood, such as salts and proteins, ensuring that the analysis is as clean and dependable as possible [74].
Vokhmyanina et al. immobilized LOx into a membrane based on alkoxysilane monomers (3-aminopropyl)trimethoxysilane and trimethoxyl [3-(methylamino)propyl]silane for human blood serum analysis [75]. Liu et al. developed a flexible electrochemical biosensor capable of simultaneously detecting glucose and lactate in saliva. Made on a polyethylene terephthalate glycol substrate via screen-printing, the biosensor uses ferrocene to connect enzymes to electrodes, avoiding signal interference. It performs well at low polarization potential and accurately measures physiological levels of glucose and lactate in saliva [76].
Membrane materials such as Nafion and polycarbonate enhance sensor performance. They offer selective permeability and protect enzyme activity. Nafion membranes are popular for their high ionic conductivity and selective permeability, though their high cost and sometimes poor conductivity are downsides. In contrast, polycarbonate membranes provide good mechanical strength and stability, but they struggle to fully block small molecule interferents [55].
For membrane-based electrochemical lactate biosensors, effectiveness and reliability hinge on several critical points. Firstly, the properties of the membrane are fundamental. It must allow for precise control over the enzyme’s microenvironment, optimizing the activity and stability of the enzyme, such as LOx. The membrane must control the diffusion of lactate from the sample to the enzyme and of reaction products to the transducer, ensuring an efficient response, while also acting as a selective barrier against interferents.
For biological or in vivo applications, the biocompatibility of the membrane material is indispensable to prevent adverse reactions, and its chemical and mechanical stability, including good adhesion to the electrode, is vital for sensor durability. Furthermore, resistance to biofouling is a significant challenge in biological environments, directly impacting long-term performance.
LOx or LDH enzymes easily integrate onto SPE surfaces. Their design simplifies surface modification, cutting complex mass production and reducing manufacturing costs. SPEs are portable, enabling in situ analysis, and are readily disposable. They offer high sensitivity compared to commercial electrodes, and their disposable nature mitigates cross-contamination while simplifying analysis [77,78]. This makes SPE devices potentially modifiable using the methods mentioned above for the development of biosensors for lactate, despite the limitations like less sensitivity and homogenous surface than traditional electrodes.
Even so, the most common and promising approach for electrochemical lactate biosensors is the combination of nanomaterials with screen-printed electrodes. SPEs have become the standard for disposable and portable biosensors due to their low manufacturing cost and ease of use. To enhance sensitivity and stability, these electrodes are routinely modified. This is where the nanomaterial-based approach excels. Table 2 details the core principles, advantages, and disadvantages of each method to help in selecting the most suitable approach for a specific biosensor application.
Iula et al. developed a low-cost, wearable lactate biosensor. It utilizes custom SPEs, modified with a bio-hybrid probe comprising Prussian blue, carbon black, and LOx. Validated with real sweat, in biosensor was incorporated a filter paper-based strip, and was integrated into a 3D-printed armband for comfort and efficient sweat collection. This system represents an advance for continuous and non-invasive health monitoring [79].

Table 2.
Analysis of electrode modification strategies for electrochemical lactate biosensors.
Table 2.
Analysis of electrode modification strategies for electrochemical lactate biosensors.
| Modification Strategy | Advantages | Disadvantages | Materials | Linear Range | Lod | Ref. |
|---|---|---|---|---|---|---|
| Nanomaterial-Based | High surface area for immobilization, sensitivity, mechanical stability, and supports multiple immobilization methods. | High material costs, difficulty uniform dispersion, and possible toxicity in some uses. | MWCNT-Av with LOx | 100–700 µmol L−1 | 33 µmol L−1 | [57] |
| Peroxidase-Mimetic Nanozyme with AuNPs and LOx | 5.0–140 µmol L−1 | 2.0 µmol L−1 | [58] | |||
| Sol–Gel-Based | Use of a silicate or oxide matrix, chemically inert and porous. The matrix maintains enzyme stability and permits substrate diffusion. | Limit substrate and product diffusion, reduce sensitivity and response time, mechanical stability challenges in the sol–gel matrix, and risk enzyme inactivation during curing. | Porous nickel oxide by sol–gel based on P133 in inverse micelle method | 10–7750 µmol L−1 | 27 µmol L−1 | [64] |
| Polymer-Based | Use polymer films, such as chitosan and polyaniline. Capable of immobilizing enzymes by entrapment or covalent bonding. | Regulate the permeability and density of the polymer to avoid diffusion limitations. Selecting polymer is a complex process, searching the compatibility with enzyme and the electrode. | Prussian blue (PB), LOx and a membrane with tetradodecylammonium tetrakis(4-chlorophenyl) borate (ETH 500), polyvinyl chloride (PVC), and bis(2-ethylhexyl) sebacate (DOS) | 1.0–50 µmol L−1 | 0.11 µmol L−1 | [70] |
| MgO, LOx and, 2-naphthoquinone, integrated with polydimethylsiloxane (PDMS) | 0–10 mmol L−1 | 0.3 mmol L−1 | [71] | |||
| Membrane-Based | Selective barrier, shields enzymes from interferents, and regulation of substrate diffusion to the electrode. | Slow response time and reduce sensitivity for diffusion limits; membrane choice is crucial, and application adds a fabrication step. | Polyphenylenediamine semi-permeable membrane with LOx in bovine serum albumin | 7.0–1000 μmol L−1 | 7.0 µmol L−1 | [74] |
| LOx in membrane based on alkoxysilane monomers (3-aminopropyl)trimethoxysilane (APTMS) and trimethoxyl [3-(methylamino)propyl]silane (MAPS) | 1.0–1000 μmol L−1 | 0.5 µmol L−1 | [75] |
Adapted from [3,9,14,59,80,81].
Furthermore, it is worth highlighting the importance and possibility of these types of modifications on the surfaces of traditional electrodes. The GCE is a popular choice for electrochemical lactate biosensors due to its excellent electrical conductivity, broad working potential, and stable, inert surface. The historical importance of GCE for lactate detection is deeply tied to the evolution of electrochemistry and biosensors [82].
For lactate detection, the GCE surface is typically modified to facilitate the immobilization of LOx or alternative biorecognition elements. These modifications can include adsorption, nanomaterial deposition, or polymer coatings, enhancing enzyme anchoring, electron transfer, and selectivity. The GCE’s versatility supports the creation of sensitive sensors for accurate lactate measurement in various samples [9].
Promsuwan et al. proposed a fused LOx enzyme modification adapted to a multiplexed flexible thin-film electrode. The device was electrochemically characterized as a transducer, an organic-inorganic nanocatalyst of poly(acrylic acid), poly(3,4-ethylenedioxythiophene), and Prussian Blue, using a GCE. After LOx immobilization, this biosensor was transferred to an integrated SPE platform for lactate detection in synthetic sweat and skin models [83].
Using GCE in lactate biosensors presents challenges, including achieving surface modification for reliable immobilization of LOx. Ensuring enzyme stability is also difficult, as lactate oxidase can lose activity over time or under changing conditions. In general, these electrodes have contributed to developments in analytical electrochemistry by facilitating the creation of more reliable and sensitive biosensors. Although advanced materials such as nanomaterials are now available, they remain commonly used as a base electrode for additional modifications. This could be overcome by the development of new electrochemical biosensors for lactate using SPEs.
4. Emerging and Alternative Lactate Biosensor Platforms
4.1. Other Lactate Biosensors
Beyond GCEs, other simpler carbon electrode architectures have also been explored for electrochemical lactate determination. The graphite rod electrode has also been a device of choice for enzymatic lactate detection in several reports [84,85,86]. Demkiv et al. described L-Lactate biosensors for food quality monitoring, utilizing flavocytochrome b2 as the biorecognition element and electroactive nanoparticles for enzyme immobilization. The sensor demonstrated efficient direct electron transfer and enhanced electrochemical communication via redox nanomediators and was successfully applied for L-Lactate analysis in yogurt samples, showing high correlation with reference methods, highlighting their promise for food control [87].
A polycrystalline platinum electrode lactate biosensor was developed by immobilizing lactate oxidase by Pagán et al. onto single-walled carbon nanotube electrodes, previously affixed to platinum modified with 4-aminothiophenol. Two immobilization methods were compared: covalent bonding (via EDC) and physical adsorption. While adsorption showed slightly better initial sensitivity, covalent immobilization proved superior in long-term stability, retaining 40% activity after 25 days compared to 20% for adsorbed protein, particularly under thermal stress [88].
Uzunoglu & Stanciu proposed a novel lactate biosensor using ceria-copper oxide mixed metal oxide nanoparticles as electrode material and for lactate oxidase immobilization. This design effectively addressed the oxygen dependence of first-generation amperometric biosensors due to the high oxygen storage capacity of ceria-copper oxide mixed metal oxide nanoparticles, proving promising for continuous lactate detection in human serum [89].
To address the need for noninvasive sweat lactate detection, Uzunoglu introduced a novel reagent-free electrochemical biosensor containing zinc/cerium oxide nanoparticles for enzymatic lactate detection in low-oxygen environments. It leverages molecularly imprinted polymers on screen-printed carbon electrodes with an embedded Prussian blue nanoparticle layer serving as an internal redox probe, eliminating the need for external mediators. The sensor demonstrated a fast and selective response to lactate, along with excellent reproducibility, reusability, and a 10-month shelf life [90].
Hashemzadeh et al. proposed a sensitive amperometric lactate biosensor platinum electrode, modified with a reduced graphene oxide/carbon nanotube composite and in situ reduced gold nanoparticles, with immobilized lactate oxidase. This composite enhances enzyme loading and electron transfer for immobilized lactate oxidase. The sensor detects lactate via H2O2 oxidation, showing a wide linear range, high sensitivity, and a low detection limit. It is stable, repeatable, and promising for lactate detection in biological samples under varying oxygen conditions [33].
Different configurations and detection methods for lactate using electrochemical biosensors impact measurement sensitivity, selectivity, and stability. Electrode material choices like GCE, platinum, or screen-printed electrodes affect electron transfer properties. Incorporating nanomaterials such as carbon nanotubes, graphene, or metallic nanoparticles enhances signal amplification and conductivity while providing ideal environments for enzyme immobilization.
The method of immobilizing lactate oxidase influences its stability and activity. Techniques include adsorption, covalent bonding, encapsulation within membranes, or sol–gel films. Using redox mediators or leveraging metal oxides’ oxygen storage capacity helps optimize responses under various conditions. Each approach has pros and cons: covalent immobilization offers greater stability but might reduce initial sensitivity, while adsorption-based sensors are easy to fabricate and sensitive initially but have lower stability.
Materials such as MIPs provide high stability and lower cost, though their selectivity needs validation in complex samples. Choosing the best configuration depends on the analyte characteristics (lactate) and sample type, whether human serum, sweat, food products, or other biological fluids.
4.2. Lactate Microfluidic Devices
These devices are particularly beneficial for wearable sensors and point-of-care (POC) diagnostics due to their small footprint, portability, and potential for real-time, non-invasive monitoring of lactate in biofluids like sweat [89]. Miniaturization of analytical systems started with the need for devices to dispense controlled small liquid volumes (range of nanoliters to picoliters) for analysis [89,90].
In this context, microfluidic devices are gaining attention in electroanalytical literature. When integrated with (bio)sensors, they become powerful platforms for real-time detection of reduced analyte quantities [91]. For biodevices, microfluidic platforms offer additional benefits like portability, lower production costs, minimal sample/reagent consumption, and agile analyses [92].
A range of materials has been used in the fabrication of these devices, including silicon, glass, paper, hydrogels, elastomers, and textile fibers [93,94]. For lactate detection in biological fluids, both invasive and non-invasive approaches were developed. Saliva or sweat detection is non-invasive, while blood detection is an invasive approach and unsuitable for continuous monitoring [92,95].
A lactate sensor with a microchannel was developed by Shitanda et al. to enable continuous sweat lactate monitoring by overcoming air bubble interference. The design uses a microchannel for sweat supply and drainage, featuring a specific area to trap air bubbles, preventing electrode contact. Evaluated during exercise, the sensor effectively prevented interference, showing a 1 to 50 mM correlation range and demonstrating a strong correlation between sweat and blood lactate levels. This microchannel lactate sensor is highly promising for extended wear and continuous monitoring in medicine and sports [40].
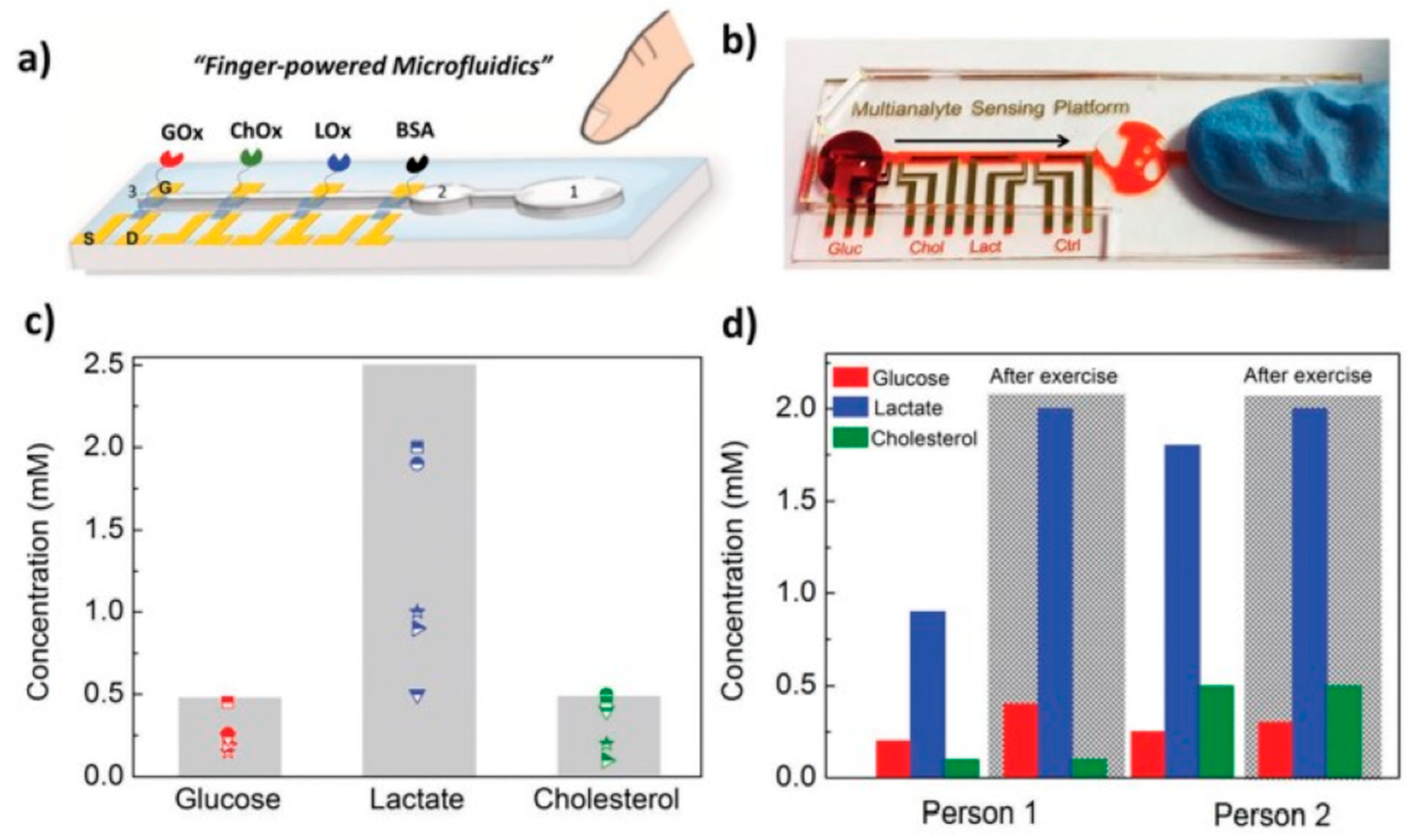
A compact biosensing platform was developed by Pappa et al. using multiplexed organic electrochemical transistors for real-time and simultaneous detection of glucose, lactate, and cholesterol in human saliva. This system was integrated with a simple pumpless poly(dimethylsiloxane) microfluidic device (Figure 3). The device showed excellent analytical performance, covering physiological lactate levels found in saliva [96].
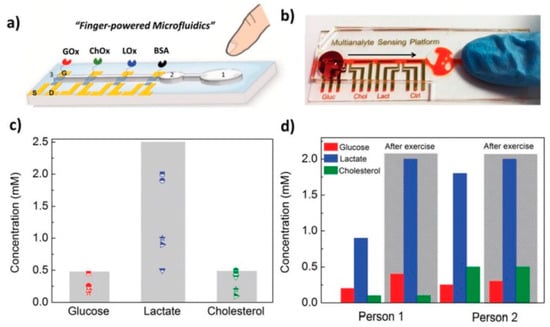
Figure 3.
Selective multianalyte detection in complex media using the OECT array. (a) Schematic illustration of the biosensing multiplatform with the embedded “finger-powered” PDMS microfluidic showing the (1) activation “button,” (2) the liquid reservoir, and (3) the punched inlet. (b) Photograph of the actual device used for the measurements, showing a red-colored solution that was pressure-driven from the inlet through the sensing areas as indicated by the arrow. (c) Salivary metabolite levels of five healthy volunteers as measured with our setup (the marked areas represent the physiological ranges of concentrations for each analyte) and (d) relatively metabolite variations of two healthy volunteers before and after intense physical exercise [96]. Reproduced with permission from Wiley.
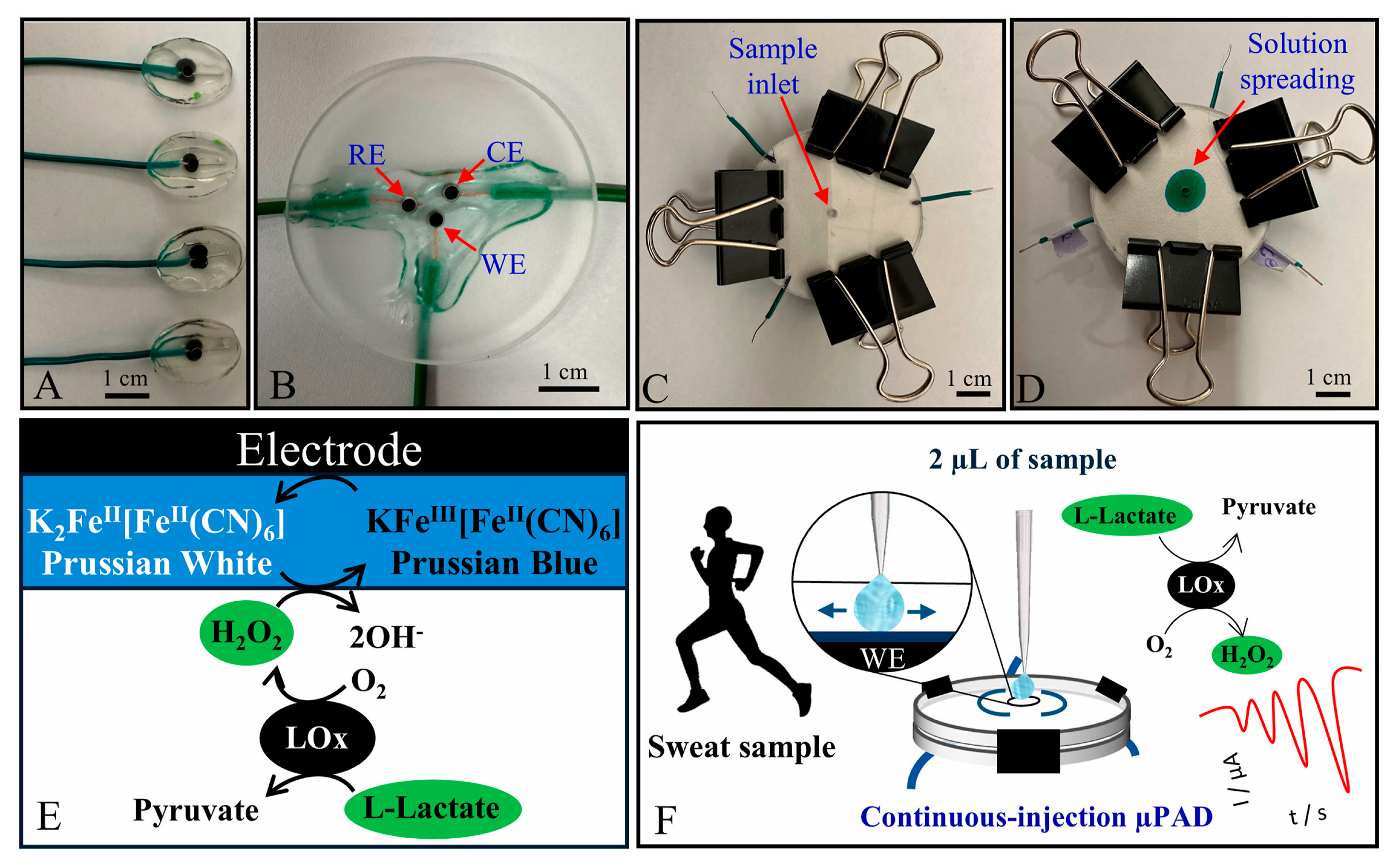
Berkheimer et al. developed an electrode fabricated using a 3D printing pen and carbon black filament, supported by Poly(methyl methacrylate), for lactate detection. These electrodes were modified with specific materials, including Prussian Blue, lactate oxidase, chitosan, and Nafion. Integrated into microfluidic paper-based analytical devices (μPADs), the system allowed pumpless radial sample flow, enabling repeated injections and rapid responses [97].
Other devices for lactate biosensing in sweat have also been reported in recent literature [97,98]. In addition, platforms capable of simultaneously detecting glutamate and lactate are being developed [99,100]. Despite the many advantages associated with enzyme-based sensors, their performance is often affected by factors such as temperature and pH [101]. As an alternative, non-enzymatic sensors are being explored. However, studies that do not rely on enzymes for lactate detection remain scarce in the current literature (Figure 4).
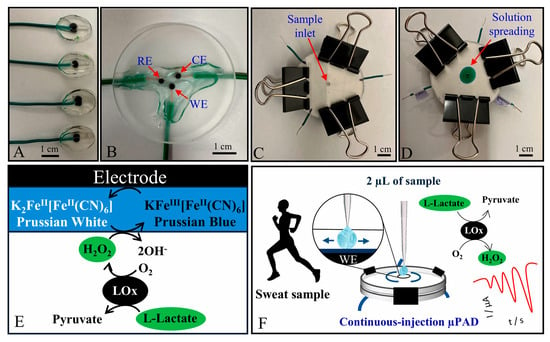
Figure 4.
Photographs of (A) working electrodes; (B) 3-electrode chip made of extruded carbon black filament; and paper-based microfluidic device (C) before and (D) after injecting a food dye. Scheme of the (E) sensing mechanism for lactate quantification and (F) μPAD applicability in human sweat analyses [97]. Reproduced with permission from Elsevier.
Non-invasive lactate monitoring in sweat presents complexities; the correlation between sweat and blood lactate levels is inconsistent, influenced by factors such as sweat rate and hydration. Biofouling further impacts sensitivity and accuracy over time. To address these issues, innovations in enzyme immobilization, electrode material science, and signal processing are needed to enhance the accuracy and reliability of these sensors [89].
The studies briefly described above are a promising approach for real-time and non-invasive lactate detection; their widespread implementation encounters several challenges. One of the primary obstacles is the collection and handling of samples. For sweat biosensors, ensuring the acquisition of adequate and consistent sweat volumes for analysis is problematic due to variable and irregular perspiration rates [89].
Additionally, maintaining sensor stability and performance in complex biological environments, such as sweat or serum, is tough. Biofouling, where proteins and other molecules adsorb onto the electrode surface, can lead to sensor passivation over time, thereby reducing its sensitivity and lifespan. Minimizing interference from other electroactive species in the sample is also necessary to ensure the selectivity of lactate detection. Finally, the integration and miniaturization of all necessary components pose substantial obstacles [102].
The combination of electrochemical sensing elements, microfluidic channels for fluid transport, power sources (particularly for self-powered devices like biofuel cells), and electronics for data processing and wireless communication into a compact, flexible, and wearable format requires significant advancements. The precise fabrication of these micro-scale structures and ensuring their robustness in real-world conditions are ongoing challenges that research aims to overcome to enable the next generation of lactate biosensors [103].
4.3. Lactate Paper Devices
Aligned with Green Analytical Chemistry, paper-based electrochemical devices offer sustainable, miniaturized, portable, and cost-effective alternatives to plastic systems. In electroanalytical applications, they are not only substitutes for conventional electrodes but also enhance platform functionality, leading to their widespread exploration as (bio)sensors in environmental and biomedical fields [104,105].
In this context, Dungchai et al. introduced the first electrochemical detection on paper-based microfluidic devices, fabricated using photolithography and screen-printing. The devices successfully quantify glucose, lactate, and uric acid in biological samples using a single electrode type, leveraging oxidase enzyme reactions that produce H2O2. Selectivity for H2O2 was enhanced with Prussian Blue. Measurements in control serum samples correlated well with traditional tests, highlighting this platform as an easy-to-use, inexpensive, and portable alternative for point-of-care monitoring [106].
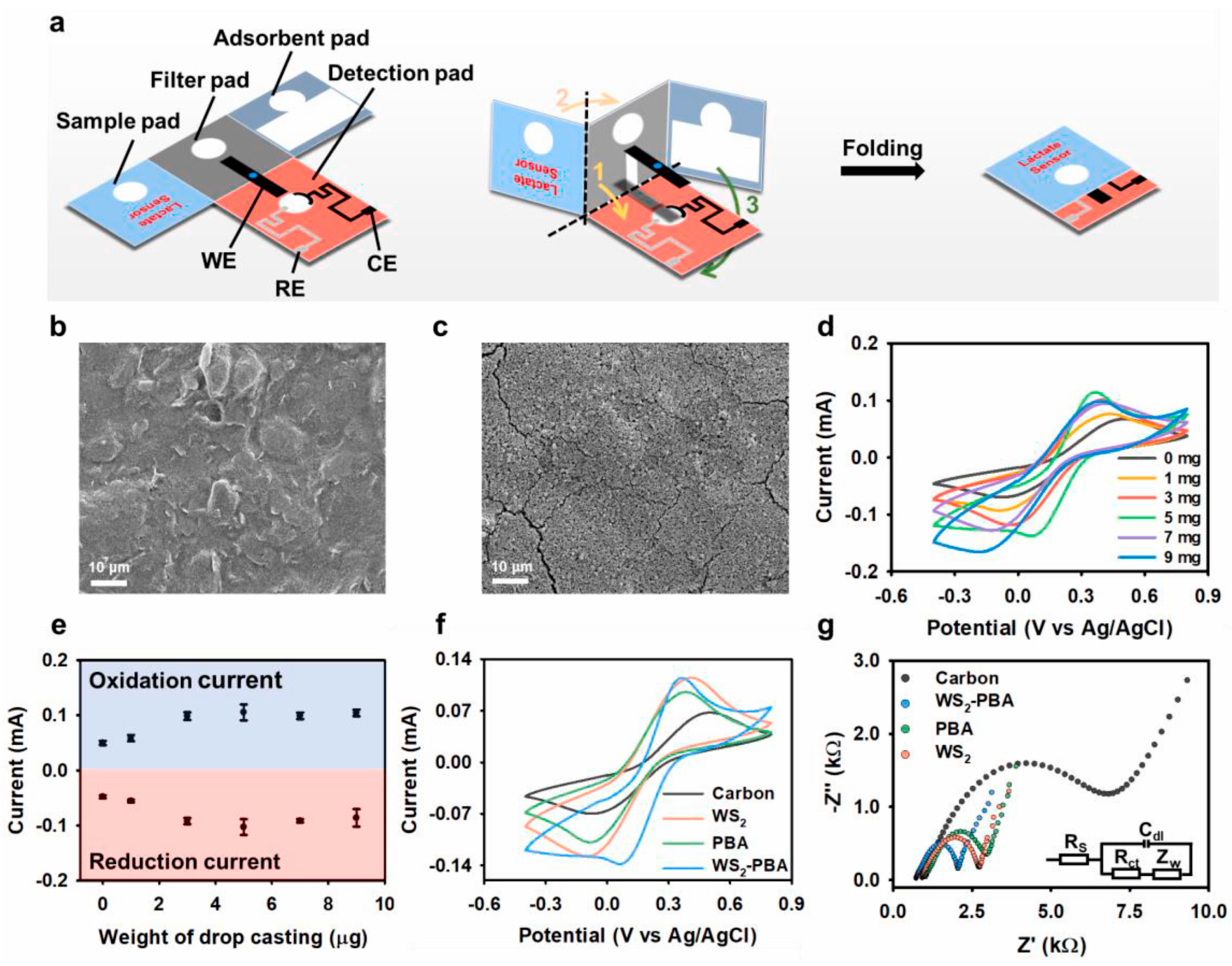
Chen et al. proposed a three-dimensional origami paper-based electrochemical platform that was developed for lactate detection in sweat and evaluated for its potential in biomedical applications. The biosensor, modified by drop-casting with tungsten-disulfide, Prussian blue analog, and LOx, showed amplified current signals and decreased impedance. Key electrochemical results, alongside a scanning electron microscopy image, are provided in Figure 5. This device demonstrated a low limit of detection and achieved nearly 100% lactate recovery in clinical sweat samples [107].

Figure 5.
Characterization and optimization of paper-based electrochemical platforms. (a) Conceptual illustration of pop-up paper-based electrochemical platform. (b) SEM image of bare working electrode. (c) SEM image of the modified electrode of the WS2-PBA. (d) CV of various modified weights of the WS2-PBA. (e) Graph displaying oxidation and reduction current values for the WS2-PBA of various weights. (f) CV of different materials modified electrodes. (g) Nyquist diagrams of different materials modified working electrodes [107]. Reproduced with permission from Elsevier.
A dual-channel electrochemical sensor capable of simultaneously detecting glucose and lactate in sweat using a low-cost, free-standing, and disposable highly integrated sensing paper device (named as HIS paper) was developed [99]. The HIS paper integrates hydrophobic protecting wax, conducting electrodes, and the incorporated MXene/metylene blue (Ti3C2Tx/MB) active materials. Outside the context of microfluidics, few studies in the literature report the use of paper-based electrochemical devices for lactate detection.
In summary, paper-based devices for lactate measurement hold immense potential to democratize access to rapid and accessible diagnostics. Their affordability, simplicity, portability, and ease of use make them particularly attractive for point-of-care applications and resource-limited settings. However, the route to their implementation needs continuous research and development. Overcoming current challenges in sensitivity, accuracy, stability of bioreceptors, and robustness against external interferences remains critical. Finally, continued advancements and rigorous real-world validation are essential to fully harness their promise, ensuring these innovative devices can consistently contribute to improved health monitoring and diagnostic capabilities globally.
5. Commercial and Translational Aspects (Revised for Coherence)
5.1. Cost of Production of Lactate Biosensor Production Can Be Volatile
The feasibility and widespread accessibility of lactate biosensors, especially for point-of-care and mass production applications, hinge on a complex ecosystem of interconnected factors. The initial production cost is only one piece of the puzzle. The price is significantly impacted by technological choices, such as selecting inexpensive substrates like paper or polymers over silicon, which can drastically reduce the per-unit cost. The choice of electrode components, including graphene, noble metals, or carbon-based materials, and the necessity for performance-enhancing nanoparticles, also contribute to the final budget. In enzymatic biosensors, high-purity enzymes like Lactate Oxidase (LOx) are among the most expensive components, and their stabilization within the sensor adds both complexity and cost. Non-enzymatic biosensors, conversely, can avoid this expense.
Manufacturing methodologies are equally influential. Techniques such as screen-printing, inkjet printing, or roll-to-roll printing are scalable and low-cost, making these devices suitable for disposable, single-use applications. Screen-printing is a cost-efficient method with high reproducibility, making it ideal for fabricating printed electrodes [108,109,110,111]. While sensor miniaturization allows for multiple units on a single chip, optimizing material usage and lowering the per-sensor cost, the complexity of bioreceptor immobilization and purification steps can, conversely, increase total costs. For instance, the first commercialized biosensor, the blood glucose meter, was successful partly because its core enzyme (glucose oxidase) is relatively stable and low-cost.
The implications of these costs for development and application are considerable. Low-cost biosensors enhance accessibility, facilitating lactate detection in resource-limited settings or for personal monitoring, and enable new, economically feasible applications like large-scale, continuous industrial monitoring [112]. However, an inexpensive sensor that fails to meet performance requirements is useless. For clinical applications, additional costs associated with rigorous validation and compliance with Good Manufacturing Practice are substantial [113,114]. Beyond manufacturing, operational costs are also significant. Biosensors that simplify or eliminate the need for complex sample pre-treatment can lower these overall operational costs, increasing their viability in various contexts.
A final, significant challenge lies in the infrastructure and logistics required for biosensor construction and distribution. Not all regions possess the advanced microfabrication facilities necessary for production, and the rapid logistics of shipping inputs, reagents, and finished devices can be a bottleneck. Moreover, environmental factors like extreme humidity and temperature can affect a biosensor’s sensitive components during storage and transport, influencing the final price due to the need for special packaging or temperature control. The production of lactate biosensors, therefore, requires a constant compromise between technological innovation for high performance and maintaining costs that enable widespread adoption and a positive impact across different fields.
This section avoids detailed pricing analysis for a fundamental reason: such data is inherently volatile and often proprietary. Price estimates can vary widely based on the final application, materials used, production scale, and company confidentiality. Any specific, fixed values would quickly become outdated and misleading.
The cost of a biosensor is a function of technological and logistical choices, not a fixed number. The final conductivity and performance of the electrode are affected by the presence of various materials, including metallic and non-metallic nanoparticles, polymers, and carbonaceous materials [115]. Furthermore, the final price of the device is significantly increased by the need for miniaturized equipment, such as potentiostats, connectors, and external devices. These are crucial components for a complete solution.
From a broader perspective, the global market for electrochemical biosensors is indeed projected to grow from USD 21.76 billion in 2024 to USD 37.51 billion by 2032 [116]. However, despite this positive forecast, several restraining factors can profoundly impact the final profitability. These include the challenges of reproducibility, which affects standardized products, and strict regulations that vary by region. Because of these variables and the confidential nature of production data, overall market estimates remain uncertain. Ultimately, the goal of this section is to provide a comprehensive strategic overview of the key cost drivers and market factors, rather than a specific and therefore limited financial report.
5.2. Technology Readiness Levels (TRLs) and Market Entry for a Lactate Biosensor
KPIs serve as a critical measure of a biosensor’s success in the market, highlighting the commercial viability, enabling applications in precise clinical diagnostics and the analysis of small-volume samples, such as sweat and saliva, which are integral to the expansion of wearable and non-invasive device markets. Besides selectivity, operational and shelf stability significantly influence a biosensor’s overall value proposition; extended shelf-life not only reduces user costs but also minimizes waste, thereby enhancing sustainability, appeal, and reliability in the market.
Reproducibility and repeatability are fundamental for large-scale manufacturing, ensuring consistent product quality and meeting regulatory requirements set by agencies such as the Food and Drug Administration (FDA-USA), Conformité Européenne—In Vitro Diagnostic (CE-IVD—Europe), or Agência Nacional de Vigilância Sanitária (Anvisa-Brazil), which are essential for establishing a reputable brand.
The progression of a lactate biosensor from laboratory innovation to commercial deployment can be systematically analyzed using Technology Readiness Levels (TRLs). This framework, which spans from TRL 1 (basic principles observed) to TRL 9 (system proven in operational environments), provides an essential structure for evaluating technological maturity. In the context of electrochemical lactate biosensors, the initial phases (TRLs 1–3) are characterized by foundational scientific research and early proof-of-concept efforts, frequently involving advancements in electrode materials or enzymatic immobilization strategies [117].
Transitioning into TRLs 4–6, the focus shifts toward engineering application, where prototype devices are assessed in more representative settings, such as simulated biological fluids or controlled clinical samples, evaluating sensitivity, selectivity, and stability, to determine suitability for practical use. Despite promising advances, technologies encounter obstacles as interference in real-world conditions, long-term stability, and manufacturability often impede further progress. This stage determines whether a scientific breakthrough evolves into a robust, market-ready product or remains confined to academic interest. Achieving the uppermost TRLs (7–9) differentiates commercially viable products from ongoing research endeavors. Now, the biosensor undergoes full-scale development, comprehensive validation through clinical trials, and demonstration of large-scale manufacturability. The commercial success of electrochemical biosensors, exemplified by glucometers, highlights the necessity of advancing to higher TRLs [117].
To compete in the marketplace, a biosensor must not only exhibit high accuracy but also deliver user-friendliness, cost efficiency, and robustness suitable for mass production. Regulatory compliance requires superior reliability, specifically repeatability and reproducibility. The commercialization of a lactate biosensor hinges on its capacity to perform dependably outside controlled laboratory settings and demonstrates tangible value to end-users, including clinicians and athletes [117,118,119].
Lactate biosensors, upon achieving advanced TRL, serve as valuable tools across multiple domains. A prominent example is Nova Biomedical’s StatStrip Xpress LAC/Hb/Hct, a disposable electrochemical biosensor regarded as indispensable in hospital and ambulance settings. This device enables rapid diagnosis of conditions such as sepsis and shock by delivering results within 13 s from a minimal blood sample, reflecting its high technological maturity [120].
In sports, companies like Zimmer & Peacock have introduced lactate sensors designed for sweat monitoring, including wearable models, which support the optimization of athletic performance [121]. Within biotechnology, solutions from IST AG and Shenzhen Sieman Technology illustrate industry innovation. IST AG offers enzymatic biosensors compatible with integration into large-scale systems for continuous monitoring during clinical trials and bioprocesses, notable for their stability and longevity.
Meanwhile, Sieman specializes in glucose and lactate analyzers tailored for cell culture and bioreactor monitoring, essential for quality assurance in biopharmaceutical manufacturing [122]. These cases highlight the substantial influence of lactate biosensors in healthcare, sports, and biotechnology once intermediate TRL challenges are addressed.
Although lactate monitoring holds significant promise in emergency medicine (as an indicator of sepsis and shock), elite sports, and numerous other applications, the market continues to lack low-cost, widely accessible electrochemical lactate biosensors. This gap primarily arises from the difficulty in translating laboratory-level accuracy to the operational stability and user-friendliness necessary for practical implementation.
In contrast to glucose, which remains constant in the blood, lactate is a dynamic metabolite whose concentrations may fluctuate rapidly in response to physiological stress. As a result, biosensors must meet more stringent requirements for response time and stability. Although some prototype lactate biosensors show good sensitivity and selectivity in isolation, their accuracy drops when exposed to interfering substances in biological samples like blood or sweat.
There are several factors that limit commercialization at both global and local levels. Securing regulatory approval are responsible for establishing safety for use. These regulatory pathways are extremely demanding and aim to ensure the safety, efficacy, and reliability of the device to protect the patient. A lactate biosensor intended for sepsis diagnosis, for example, is classified as a high-risk device, requiring detailed documentation of its design, preclinical testing, and data from extensive clinical trials.
The time and cost associated with these processes are significant barriers that can take years to overcome. Manufacturers must demonstrate not only that the biosensor is accurate and selective, but also that it is manufacturable at scale, that consistency between batches is maintained, and that the product has a stable shelf life.
Validating a biosensor for wearable use in athletes, for example, involves demonstrating that sweat lactate measurement is a reliable representation of blood lactate levels, which requires correlation with reference methods. The complexity of these steps and the need to adhere to rigorous protocols from the early stages of the project are key factors that influence the maturity of a technology and its ability to move from research to mass production.
Thus, changes in lactate levels can be associated with diseases such as sepsis and hypoxia. However, lactate levels in the human body are dynamic, making it difficult to obtain commercially available devices that provide a rapid analysis response. Furthermore, these dynamic concentration changes necessitate the use of a device focused on long-term patient monitoring, adding another barrier to development beyond stability: obtaining accurate and convenient wearable systems. Another factor potentially contributing to the low number of biosensors on the market could be the shift in focus from curative to preventive medicine, depending on patients’ geographic location, as well as related economic considerations.
5.3. Optimizing Lactate Biosensors for Commercialization: The Role of Electrode Surface Modification
The functionalization of electrode surfaces is a central aspect of electrochemical lactate biosensor development, enabling the transformation of a basic inert electrode into a highly selective and responsive detection platform. This process is critical for three main reasons: it enhances sensitivity, allowing for the detection of low lactate concentrations; it improves selectivity by providing a barrier against interference from other substances; and it increases operational stability, thereby protecting immobilized enzymes and extending the sensor’s lifespan.
Although fundamental to performance, functionalization is also the main factor that increases the final cost and complicates the biosensor’s production, and this is where the critical relationship with TRLs becomes evident. In the initial TRLs (1–3), the focus is on proof-of-concept and the pursuit of maximum performance, without considering the costs, which can be extremely high. The transition to intermediate TRLs (4–6) shifts the focus from determining if a technology works to evaluating whether it can be manufactured consistently and at a reasonable cost. At this stage, some research methods may not be suitable for large-scale production, which can result in reproducibility challenges for the project.
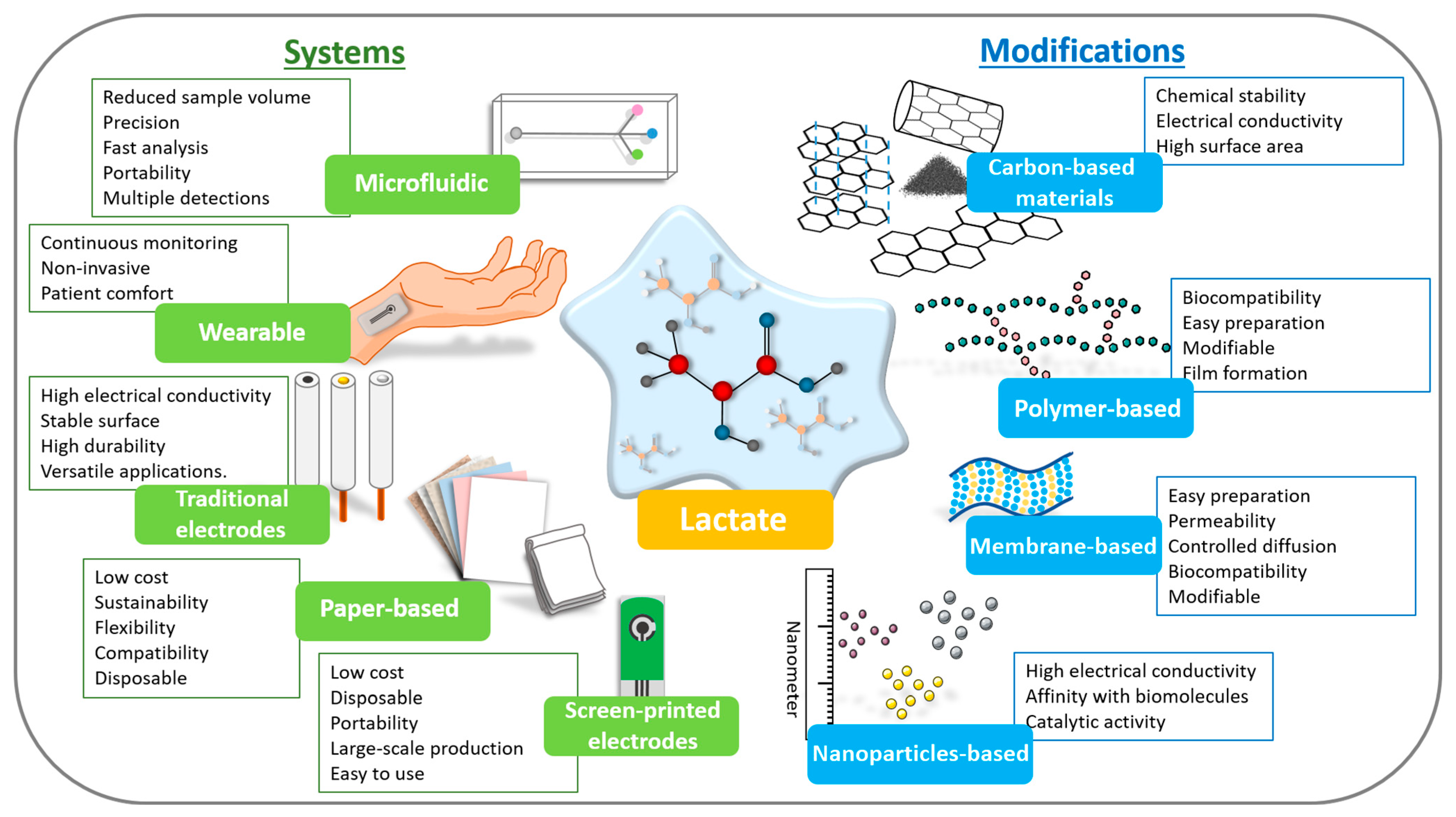
Finally, to reach the highest TRLs (7–9) and commercial success, functionalization must be radically simplified. Complex methods give way to robust, low-cost processes that can be automated on an industrial scale. The choice of materials is then based not only on performance but also on availability and cost. The critical insight is that market success depends on a balanced approach, where functionalization is optimized not just for sensitivity but to be reproducible, scalable, and economically viable for mass production. Considering this, Figure 6 schematically illustrates the available systems and types of modifying materials that can be used in the development of lactate biosensors until they reach the final TRL levels and are commercialized. It is, ultimately, a game of trade-offs, where simplicity and reliability trump complexity, and extreme performance is important. Table 3 compiles the most recent lactate biosensors, detailing modifications employed for effective detection across different sample types.
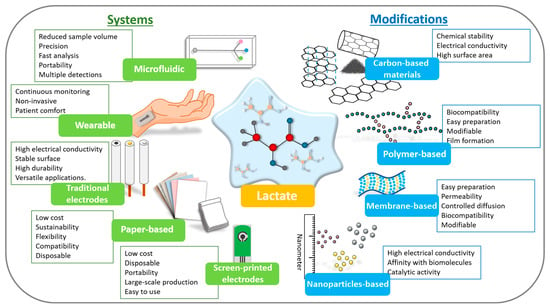
Figure 6.
Scheme highlighting the types of devices and modifications that can be used in lactate biosensors with the main points of each.

Table 3.
Lactate biosensors published in last 15 years detailing WE surface modification, electrochemical technique, linear range, LOD, and analyzed sample.
Parameters such as the Limit of Detection (LOD) and linear range are fundamental to both clinical and sports applications; however, transitioning these metrics from low to high Technology Readiness Levels (TRLs) presents significant technical and economic challenges. In laboratory settings (TRL 1–3), achieving a very low LOD and broad linear range is prioritized. Conversely, at the commercialization stage (TRL 7–9), maintaining the stability of these parameters amid matrix interference, such as molecules found in complex biological samples like blood or sweat, becomes the primary technological obstacle.
The selection of the electrochemical technique plays a pivotal role in a biosensor’s journey through the TRLs and its eventual daily applicability. In early TRLs (1–3), techniques like Cyclic Voltammetry (CV), Differential Pulse Voltammetry (DPV), and Square Wave Voltammetry (SWV) are invaluable. They provide rich mechanistic information, allowing researchers to deeply understand redox processes, optimize reaction conditions, and achieve the lowest possible LODs in controlled environments.
However, these methods often require complex instrumentation, specialized software, and trained personnel, making them unsuitable for routine, point-of-care use. The inherent technological difficulties and the need for a sophisticated infrastructure limit their accessibility and contribute significantly to the final cost. As a biosensor progresses to higher TRLs (7–9) for real-world application, simplicity and robustness become paramount. Here, amperometry (especially constant potential amperometry) emerges as the preferred technique.
Its straightforward nature, measuring current at a fixed potential translates into simpler, more compact, and significantly cheaper instrumentation. This directly addresses the need for cost accessibility and ease of use for the end-user, whether it is a patient monitoring lactate at home or a coach on the field.
While amperometry might offer less mechanistic detail than CV or DPV, its ability to provide rapid, reliable, and quantifiable results, even in the presence of matrix interference (often managed through careful sensor design and selective membranes), makes it ideal for mass-produced devices. The trade-off is often a slight compromise in the ultimate LOD, or linear range, compared to research-grade methods, but this is a necessary step to ensure the technology is practical and affordable for widespread adoption (Table 3).
The journey from a lab-based discovery to a market-ready product is not linear and is significantly influenced by a multitude of factors that are beyond the control of an individual researcher. These include the following:
The regulatory landscape varies significantly from country to country. Each region has its own regulatory bodies, such as the FDA in the United States, ANVISA in Brazil, or the EMA in the European Union, and each agency operates with distinct approval processes, documentation requirements, and timelines. As a result, a roadmap designed for one country cannot be directly applied to another.
The commercialization process also depends heavily on the institutional ecosystem. Researchers often do not have the specialized expertise needed for tasks such as patenting, securing intellectual property, or managing licensing agreements. Universities play a critical role in this context, particularly through their technology transfer offices and legal advisors, who help navigate bureaucratic hurdles while providing the necessary legal and financial guidance. Without this type of institutional support, commercialization efforts frequently stall.
Another essential factor is access to investment. The financial resources required for clinical trials, manufacturing, and market entry are substantial and vary greatly by region. The availability of grants, venture capital, and institutional funding depends on geographical context and cannot be generalized into a single roadmap.
For example, the journey of a biosensor from the laboratory bench to the Brazilian market is a complex process that involves technical, legal, and commercial stages. This roadmap serves as a strategic guide to the main phases, although each project has its own unique characteristics.
In the first phase, focused on research, proof of concept, and pre-clinical validation, the aim is to demonstrate that the technology works on a laboratory scale and has the potential for practical application. At this stage, the scientific concept of the biosensor must be validated, while sensitivity, selectivity, and stability are tested. Feasibility studies and pre-clinical validation are conducted when required by the product’s risk classification, and the robustness and reproducibility of the method are also assessed.
The second phase concerns intellectual property protection, which is essential to safeguard the invention and guarantee product exclusivity. This step involves consulting the university’s Technology Transfer Office or a specialized law firm, performing a prior art search to confirm originality, and filing a patent application with the National Institute of Industrial Property (INPI). The patent becomes the main asset for attracting investors, and confidentiality agreements must also be established with potential partners.
In the third phase, the focus shifts to prototype development and production engineering. The laboratory concept is transformed into a functional prototype that can be scaled for production. This requires developing an optimized prototype for large-scale manufacturing; defining materials, components, and processes; performing engineering tests to ensure durability and robustness; and preparing a complete technical dossier containing all design, testing, and validation data.
The fourth phase is the regulation and approval process with ANVISA, which represents the most critical stage for placing the biosensor on the Brazilian market. The biosensor’s risk must be classified by ANVISA, and this classification (I, II, III, or IV) determines the registration requirements. Devices related to health generally fall into higher classes, demanding more documentation and testing. A technical dossier and testing plan must be submitted to ANVISA, proving safety and effectiveness, and clinical trial data may be required. Additionally, the Company Operating Authorization (AFE) for the manufacturing facility must be obtained, clinical trials must be conducted when applicable, and the registration process must be followed until final approval is granted.
The fifth phase is dedicated to the business plan, funding, and marketing strategy. At this point, it is necessary to develop a solid business plan, including market analysis, competition, target audience, and revenue model. Funding sources such as development programs, incubators, accelerators, or angel investors should be identified. The marketing and communication strategy must be defined, and a pitch prepared for presentation to investors and potential partners.
Finally, the sixth phase is production and commercialization. This step involves setting up the production structure or contracting a specialized company, establishing distribution and sales networks, and officially launching the product on the market. Once commercialization begins, monitoring the product’s performance and collecting feedback becomes essential to guide future improvements.
The process of developing a biosensor in Brazil, including phases such as Research, Intellectual Property, Prototype, Regulation, Business, and Production, typically spans five to ten years. This pathway is not strictly linear; it is common for development to return to earlier stages to address performance issues or comply with new regulatory requirements. These cycles of adjustment highlight the value of a multidisciplinary team. Researchers may not possess all the skills required for navigating legal, financial, or marketing considerations. Similar to how companies comprise teams with expertise in sales, finance, administration, and human resources, the successful commercialization of advanced technology requires integrated efforts from professionals across multiple fields.
6. Conclusions and Future Directions
Bringing a lactate biosensor to market is a complex journey, requiring meticulous planning and execution across multiple fronts: technical development, rigorous validation, stringent regulatory compliance, scalable manufacturing, and strategic commercialization, all supported by continuous post-market surveillance. This multi-year process is iterative, emphasizing robust design controls and a comprehensive Quality Management System (QMS).
The current market offers a strong opportunity for lactate biosensors due to increasing demand for real-time, non-invasive monitoring in critical care, sports performance, and chronic disease management. This market need, combined with advancements in electrochemical biosensing and wearable technology, positions lactate biosensors for significant growth. However, successfully launching such a product demands key strategic pillars, and the path is challenging, which explains why few companies achieve large-scale production.
Success hinges on integrated development through the TRLs, rigorous real-world validation, an initiative-taking regulatory strategy, scalable manufacturing with quality control, and strategic commercialization backed by continuous post-market surveillance. This highlights the substantial hurdles advanced technologies face when entering a broad market. Bringing a lactate biosensor to market requires a complete and integrated approach.
Success depends on a deep understanding of scientific and engineering challenges, a clear vision for clinical utility, an adaptive regulatory strategy, efficient and quality-driven manufacturing, and a comprehensive commercialization plan that uses post-market insights for sustained innovation and market leadership. Not to mention that regulatory compliance is paramount and varies significantly by region, necessitating meticulous documentation and strict adherence to Quality Management System (QMS) standards.
Recent advancements in emerging technologies suggest a promising trajectory for the development of more robust, efficient, and sustainable lactate biosensors. Non-enzymatic lactate sensors, utilizing conductive nanomaterials such as metal oxides and carbon-based structures, have demonstrated enhanced operational stability and extended shelf life by circumventing the inherent limitations of enzyme-based systems.
The integration of artificial intelligence (AI) into biosensing platforms enables real-time analysis of complex biological signals, facilitating more accurate and personalized data interpretation. Another rapidly evolving area involves self-powered biosensing systems, particularly those based on biofuel cells that utilize lactate as an energy source, allowing for portable, battery-free operation.
Additionally, there is growing interest in green and low-cost fabrication strategies, including the use of biodegradable materials, scalable printing techniques (e.g., screen printing), and recyclable substrates aimed at producing environmentally friendly and disposable devices. Collectively, these innovations are paving the way for a new generation of lactate biosensors with broader applicability in biomedical, athletic, and environmental monitoring contexts.
Finally, estimating the final cost of an electrochemical lactate biosensor is an exceptionally complex undertaking, far removed from simply summing material expenses. This intricacy stems from a multitude of interconnected factors that evolve significantly across the TRLs.
Key among these are labor costs, encompassing not only the highly specialized scientific and engineering talent required for development but also the skilled workforce needed for manufacturing, quality control, and assembly. The production infrastructure itself represents a substantial investment, demanding specialized clean rooms, precise machinery, and rigorous quality assurance systems, all of which must meet stringent regulatory standards.
Furthermore, the ease of device scalability is a critical determinant; a method that works for a few prototypes might be prohibitively expensive or technically impossible for millions of units. Finally, distribution networks and profit margins must be factored in, adding layers of complexity related to logistics, marketing, sales, and the financial viability required to sustain commercial operations. These combined elements create a challenging economic landscape that often dictates whether a promising biosensor technology can truly transition from laboratory success to widespread market availability.
Author Contributions
Writing—original draft preparation: K.C. and B.C.J.; Writing—review and editing: K.C., R.M., A.C., R.C.F., A.M.d.O., T.A.S. and B.C.J.; Investigation: K.C., R.M., R.M., R.C.F., A.M.d.O., T.A.S. and B.C.J.; Visualization: K.C., R.M., R.M., R.C.F., A.M.d.O., T.A.S. and B.C.J.; Supervision: K.C., R.M., R.M., R.C.F., T.A.S. and B.C.J.; Conceptualization: K.C., R.M., R.M., R.C.F., A.M.d.O. and T.A.S.; Resources: B.C.J.; Funding acquisition: B.C.J.; Project administration: B.C.J.; Conceptualization: B.C.J. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the financial support provided by Fundação de Amparo à Pesquisa do Estado de São Paulo (2023/06793–4 and 2025/15000-3), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES 001), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 401977/2023–4), Financiadora de Estudos e Projetos (FINEP, MARTMA, #01.22.0179.00).
Data Availability Statement
Data derived from public domain resources.
Conflicts of Interest
The authors declare no conflict of financial interests or relationships that could to influence the work reported in this paper.
Glossary
| AI | artificial intelligence |
| ANVISA | National Health Surveillance Agency |
| BSA | Bovine serum albumin |
| CE | Counter electrode |
| CE-IVD | Conformité Européenne—In Vitro Diagnostic |
| CNTs | Carbon nanotubes |
| CPE | Carbon paste electrodes |
| CV | Cyclic voltammetry |
| DET | Direct electron transfer |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| ELB | Lactic acid biosensor |
| FADH2 | Flavin adenine dinucleotide |
| FDA | Food and Drug Administration |
| GCE | Glassy carbon electrodes |
| GMP | Good Manufacturing Practice |
| IP | Intellectual property |
| KPIs | Key Performance Indicators |
| LA | Lactate acid |
| LDH | Lactate dehydrogenase |
| LOD | Limit of Detection |
| LOx | Lactate Oxidase |
| LSG | Laser-scribed graphene |
| MIPs | Molecularly Imprinted Polymers |
| MOFs | Metal-organic frameworks |
| NAD+ | oxidized form of nicotinamide Adenine Dinucleotide |
| NADH | Nicotinamide Adenine Dinucleotide |
| NHS | N-hydroxysuccinimide |
| PAM | Polyacrylamide |
| PANI | Polyaniline |
| pH | Potential of hydrogen |
| POC | point-of-care |
| PPy | Polypyrrole |
| PVA | Polyvinyl alcohol |
| QMS | Quality Management System |
| RE | Reference electrode |
| SA | Sodium alginate |
| SPEs | Screen-printed electrodes |
| TRL | Technology Readiness Level |
| WE | Working electrode |
References
- Ojo, A.O.; de Smidt, O. Lactic Acid: A Comprehensive Review of Production to Purification. Processes 2023, 11, 688. [Google Scholar] [CrossRef]
- Moradi, S.; Firoozbakhtian, A.; Hosseini, M.; Karaman, O.; Kalikeri, S.; Raja, G.G.; Karimi-Maleh, H. Advancements in wearable technology for monitoring lactate levels using lactate oxidase enzyme and free enzyme as analytical approaches: A review. Int. J. Biol. Macromol. 2024, 254, 127577. [Google Scholar] [CrossRef]
- Rathee, K.; Dhull, V.; Dhull, R.; Singh, S. Biosensors based on electrochemical lactate detection: A comprehensive review. Biochem. Biophys. Rep. 2016, 5, 35–54. [Google Scholar] [CrossRef] [PubMed]
- García-Guzmán, J.J.; Sierra-Padilla, A.; Palacios-Santander, J.M.; Fernández-Alba, J.J.; Macías, C.G.; Cubillana-Aguilera, L. What is left for real-life lactate monitoring? Current advances in electrochemical lactate (bio) sensors for agrifood and biomedical applications. Biosensors 2022, 12, 919. [Google Scholar] [CrossRef] [PubMed]
- Pundir, C.S.; Narwal, V.; Batra, B. Determination of lactic acid with special emphasis on biosensing methods: A review. Biosens. Bioelectron. 2016, 86, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Monroe, G.R.; van Eerde, A.M.; Tessadori, F.; Duran, K.J.; Savelberg, S.M.C.; van Alfen, J.C.; Terhal, P.A.; van der Crabben, S.N.; Lichtenbelt, K.D.; Fuchs, S.A.; et al. Identification of human D lactate dehydrogenase deficiency. Nat. Commun. 2019, 10, 1477. [Google Scholar] [CrossRef]
- Lafuente, J.-L.; González, S.; Aibar, C.; Rivera, D.; Avilés, E.; Beunza, J.-J. Continuous and non-invasive lactate monitoring techniques in critical care patients. Biosensors 2024, 14, 148. [Google Scholar] [CrossRef]
- Li, J.; Ma, P.; Liu, Z.; Xie, J. L- and D-Lactate: Unveiling their hidden functions in disease and health. Cell Commun. Signal. 2025, 23, 134. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, L.; Wen, J.; Ma, Y.; Dai, G.; Mo, F.; Wang, J. A Comprehensive Review of Advanced Lactate Biosensor Materials, Methods, and Applications in Modern Healthcare. Sensors 2025, 25, 1045. [Google Scholar] [CrossRef]
- Rattu, G.; Khansili, N.; Maurya, V.K.; Krishna, P.M. Lactate detection sensors for food, clinical and biological applications: A review. Environ. Chem. Lett. 2021, 19, 1135–1152. [Google Scholar] [CrossRef]
- Hernández-Ibáñez, N.; García-Cruz, L.; Montiel, V.; Foster, C.W.; Banks, C.E.; Iniesta, J. Electrochemical lactate biosensor based upon chitosan/carbon nanotubes modified screen-printed graphite electrodes for the determination of lactate in embryonic cell cultures. Biosens. Bioelectron. 2016, 77, 1168–1174. [Google Scholar] [CrossRef]
- Brazaca, L.C.; Sempionatto, J.R. Biosensors in Precision Medicine: From Fundamentals to Future Trends; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Prabhakar, T.; Giaretta, J.; Zulli, R.; Rath, R.J.; Farajikhah, S.; Talebian, S.; Dehghani, F. Covalent immobilization: A review from an enzyme perspective. Chem. Eng. J. 2025, 503, 158054. [Google Scholar] [CrossRef]
- Gil, B.; Hall, T.A.G.; Freeman, D.M.E.; Ming, D.; Kechagias, S.; Nabilla, S.; Cegla, F.; van Arkel, R.J. Wireless implantable bioelectronics with a direct electron transfer lactate enzyme for detection of surgical site infection in orthopaedics. Biosens. Bioelectron. 2024, 263, 116571. [Google Scholar] [CrossRef] [PubMed]
- Saputra, H.A.; Karim, M.M. Enzymatic and Enzyme-Free Electrochemical Lactate Sensors: A Review of the Recent Developments. Electrochem. Sci. Adv. 2025, 5, e202400021. [Google Scholar] [CrossRef]
- Tsvik, L.; Steiner, B.; Herzog, P.; Haltrich, D.; Sützl, L. Flavin mononucleotide-dependent L-lactate dehydrogenases: Expanding the toolbox of enzymes for L-lactate biosensors. ACS Omega 2022, 7, 41480–41492. [Google Scholar] [CrossRef]
- Rassaei, L.; Olthuis, W.; Tsujimura, S.; Sudhölter, E.J.R.; van den Berg, A. Lactate biosensors: Current status and outlook. Anal. Bioanal. Chem. 2014, 406, 123–137. [Google Scholar] [CrossRef]
- Maeda-Yorita, K.; Aki, K.; Sagai, H.; Misaki, H.; Massey, V. L-lactate oxidase and L-lactate monooxygenase: Mechanistic variations on a common structural theme. Biochimie 1995, 77, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Billat, V.L.; Sirvent, P.; Py, G.; Koralsztein, J.-P.; Mercier, J. The concept of maximal lactate steady state: A bridge between biochemistry, physiology and sport science. Sports Med. 2003, 33, 407–426. [Google Scholar] [CrossRef] [PubMed]
- Orzari, L.O.; Kalinke, C.; Silva-Neto, H.A.; Rocha, D.S.; Camargo, J.R.; Coltro, W.K.T.; Janegitz, B.C. Screen-printing vs additive manufacturing approaches: Recent aspects and trends involving the fabrication of electrochemical sensors. Anal. Chem. 2025, 97, 1482–1494. [Google Scholar] [CrossRef]
- Castrovilli, M.C.; Scognamiglio, V.; Tempesta, E.; Chiarinelli, J.; Parracino, M.; Frisulli, V.; Giardi, M.T.; Avaldi, L.; Rossi, D.; Cartoni, A. Improved reuse and storage performances at room temperature of a new environmentally friendly lactate oxidase biosensor prepared by ambient electrospray immobilization. Green Chem. 2023, 25, 5257–5266. [Google Scholar] [CrossRef]
- He, X.; Yu, J.; Ge, S.; Zhang, X.; Lin, Q.; Zhu, H.; Feng, S.; Yuan, L.; Huang, J. Amperometric L-lactate biosensor based on sol-gel film and multi-walled carbon nanotubes/platinum nanoparticles enhancement. Chin. J. Anal. Chem. 2010, 38, 57–61. (In Chinese) [Google Scholar] [CrossRef]
- Haghighi, B.; Bozorgzadeh, S. Fabrication of a highly sensitive electrochemiluminescence lactate biosensor using ZnO nanoparticles decorated multiwalled carbon nanotubes. Talanta 2011, 85, 2189–2193. [Google Scholar] [CrossRef]
- Kucherenko, D.Y.; Kucherenko, I.S.; Soldatkin, O.O.; Topolnikova, Y.V.; Dzyadevych, S.V.; Soldatkin, A.P. A highly selective amperometric biosensor array for the simultaneous determination of glutamate, glucose, choline, acetylcholine, lactate and pyruvate. Bioelectrochemistry 2019, 128, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; Sánchez, S.; Fabregas, E. Enzymatic strategies to construct L-lactate biosensors based on polysulfone/carbon nanotubes membranes. Electroanalysis 2012, 24, 967–974. [Google Scholar] [CrossRef]
- Lamas-Ardisana, P.J.; Loaiza, O.A.; Añorga, L.; Jubete, E.; Borghei, M.; Ruiz, V.; Ochoteco, E.; Cabañero, G.; Grande, H.J. Disposable amperometric biosensor based on lactate oxidase immobilised on platinum nanoparticle-decorated carbon nanofiber and poly (diallyldimethylammonium chloride) films. Biosens. Bioelectron. 2014, 56, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Azzouzi, S.; Rotariu, L.; Benito, A.M.; Maser, W.K.; Ali, M.B.; Bala, C. A novel amperometric biosensor based on gold nanoparticles anchored on reduced graphene oxide for sensitive detection of l-lactate tumor biomarker. Biosens. Bioelectron. 2015, 69, 280–286. [Google Scholar] [CrossRef]
- Shimomura, T.; Sumiya, T.; Ono, M.; Ito, T.; Hanaoka, T.-a. Amperometric L-lactate biosensor based on screen-printed carbon electrode containing cobalt phthalocyanine, coated with lactate oxidase-mesoporous silica conjugate layer. Anal. Chim. Acta 2012, 714, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Hirst, N.A.; Hazelwood, L.D.; Jayne, D.G.; Millner, P.A. An amperometric lactate biosensor using H2O2 reduction via a Prussian Blue impregnated poly (ethyleneimine) surface on screen printed carbon electrodes to detect anastomotic leak and sepsis. Sens. Actuators B Chem. 2013, 186, 674–680. [Google Scholar] [CrossRef]
- Minami, T.; Sato, T.; Minamiki, T.; Fukuda, K.; Kumaki, D.; Tokito, S. A novel OFET-based biosensor for the selective and sensitive detection of lactate levels. Biosens. Bioelectron. 2015, 74, 45–48. [Google Scholar] [CrossRef]
- Briones, M.; Casero, E.; Vázquez, L.; Pariente, F.; Lorenzo, E.; Petit-Domínguez, M.D. Diamond nanoparticles as a way to improve electron transfer in sol–gel l-lactate biosensing platforms. Anal. Chim. Acta 2016, 908, 141–149. [Google Scholar] [CrossRef]
- Shkotova, L.V.; Piechniakova, N.Y.; Kukla, O.L.; Dzyadevych, S.V. Thin-film amperometric multibiosensor for simultaneous determination of lactate and glucose in wine. Food Chem. 2016, 197, 972–978. [Google Scholar] [CrossRef]
- Hashemzadeh, S.; Omidi, Y.; Rafii-Tabar, H. Amperometric lactate nanobiosensor based on reduced graphene oxide, carbon nanotube and gold nanoparticle nanocomposite. Microchim. Acta 2019, 186, 680. [Google Scholar] [CrossRef]
- Reza, M.S.; Seonu, S.; Zahed, M.A.; Asaduzzaman, M.; Song, H.; Jeong, S.H.; Park, J.Y. Reduced graphene oxide-functionalized polymer microneedle for continuous and wide-range monitoring of lactate in interstitial fluid. Talanta 2024, 270, 125582. [Google Scholar] [CrossRef]
- Phamonpon, W.; Promphet, N.; Saengkiettiyut, K.; Boonyongmaneerat, Y.; Rattanawaleedirojn, P.; Hinestroza, J.P.; Rodthongkum, N. Novel bioelectrode for sweat lactate sensor based on platinum nanoparticles/reduced graphene oxide modified carbonized silk cocoon. Sens. Actuators B Chem. 2025, 423, 136717. [Google Scholar] [CrossRef]
- Konno, S.; Suzuki, Y.; Suzuki, M.; Kudo, H. Evaluation of exercise intensity by real-time skin lactate monitoring system. Electron. Commun. Jpn. 2020, 103, 97–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Qing, X.; Wang, Y.; Zhong, W.; Wang, W.; Chen, Y.; Liu, Q.; Li, M.; Wang, D. Fiber organic electrochemical transistors based on multi-walled carbon nanotube and polypyrrole composites for noninvasive lactate sensing. Anal. Bioanal. Chem. 2020, 412, 7515–7524. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, X.; Wu, Q.; Liu, X. A thread-based wearable sweat nanobiosensor. Biosens. Bioelectron. 2021, 188, 113270. [Google Scholar] [CrossRef]
- Saha, T.; Songkakul, T.; Knisely, C.T.; Yokus, M.A.; Daniele, M.A.; Dickey, M.D.; Bozkurt, A.; Velev, O.D. Wireless wearable electrochemical sensing platform with zero-power osmotic sweat extraction for continuous lactate monitoring. ACS Sens. 2022, 7, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Shitanda, I.; Ozone, Y.; Morishita, Y.; Matsui, H.; Loew, N.; Motosuke, M.; Mukaimoto, T.; Kobayashi, M.; Mitsuhara, T.; Sugita, Y. Air-bubble-insensitive microfluidic lactate biosensor for continuous monitoring of lactate in sweat. ACS Sens. 2023, 8, 2368–2374. [Google Scholar] [CrossRef]
- Weng, X.; Li, M.; Chen, L.; Peng, B.; Jiang, H. A wearable nanozyme–enzyme electrochemical biosensor for sweat lactate monitoring. Talanta 2024, 279, 126675. [Google Scholar] [CrossRef]
- Asadian, E.; Hekmat, F.; Kahnamouei, M.H.; Mohammadpour, R.; Shahrokhian, S.; Sasanpour, P. Supercapacitor-powered wearable biosensor for continuous lactate monitoring from sweat. Biosens. Bioelectron. 2025, 275, 117226. [Google Scholar] [CrossRef]
- Derbyshire, P.J.; Barr, H.; Davis, F.; Higson, S.P.J. Lactate in human sweat: A critical review of research to the present day. J. Physiol. Sci. 2012, 62, 429–440. [Google Scholar] [CrossRef]
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical sensors and biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent advances in electrochemical biosensors: Applications, challenges, and future scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef]
- Asal, M.; Özen, Ö.; Şahinler, M.; Baysal, H.T.; Polatoğlu, İ. An overview of biomolecules, immobilization methods and support materials of biosensors. Sens. Rev. 2019, 39, 377–386. [Google Scholar] [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V. Enzyme biosensors for biomedical applications: Strategies for safeguarding analytical performances in biological fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef] [PubMed]
- Mohidem, N.A.; Mohamad, M.; Rashid, M.U.; Norizan, M.N.; Hamzah, F.; Mat, H.b. Recent advances in enzyme immobilisation strategies: An overview of techniques and composite carriers. J. Compos. Sci. 2023, 7, 488. [Google Scholar] [CrossRef]
- Vicentini, F.C.; Silva, L.R.G.; Stefano, J.S.; Lima, A.R.F.; Prakash, J.; Bonacin, J.A.; Janegitz, B.C. Starch-based electrochemical sensors and biosensors: A review. Biomed. Mater. Devices 2023, 1, 319–338. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, C.; He, H.; Zhang, M.; Wang, H.; Ji, K.; Wei, L.; Mao, X.; Sun, R.; Zhou, F. Recent advances in wearable biosensors for non-invasive detection of human lactate. Biosensors 2022, 12, 1164. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, J.; Wang, L.; Zhang, J.; Ding, L.; Liu, H.; Yu, X. Nanozyme-enhanced electrochemical biosensors: Mechanisms and applications. Small 2024, 20, 2307815. [Google Scholar] [CrossRef]
- Tamborelli, A.; Mujica, M.L.; Amaranto, M.; Barra, J.L.; Rivas, G.; Godino, A.; Dalmasso, P. L-Lactate Electrochemical Biosensor Based on an Integrated Supramolecular Architecture of Multiwalled Carbon Nanotubes Functionalized with Avidin and a Recombinant Biotinylated Lactate Oxidase. Biosensors 2024, 14, 196. [Google Scholar] [CrossRef]
- Smutok, O.; Kavetskyy, T.; Prokopiv, T.; Serkiz, R.; Šauša, O.; Novák, I.; Švajdlenková, H.; Maťko, I.; Gonchar, M.; Katz, E. Biosensor based on peroxidase-mimetic nanozyme and lactate oxidase for accurate L-lactate analysis in beverages. Biosensors 2022, 12, 1042. [Google Scholar] [CrossRef]
- Jannath, K.A.; Karim, M.M.; Saputra, H.A.; Seo, K.D.; Kim, K.B.; Shim, Y.B. A review on the recent advancements in nanomaterials for nonenzymatic lactate sensing. Bull. Korean Chem. Soc. 2023, 44, 407–419. [Google Scholar] [CrossRef]
- Ramesh, M.; Janani, R.; Deepa, C.; Rajeshkumar, L. Nanotechnology-enabled biosensors: A review of fundamentals, design principles, materials, and applications. Biosensors 2022, 13, 40. [Google Scholar] [CrossRef]
- Rusling, J.F. Nanomaterials-based electrochemical immunosensors for proteins. Chem. Rec. 2012, 12, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Ferrag, C.; Kerman, K. Grand challenges in nanomaterial-based electrochemical sensors. Front. Sens. 2020, 1, 583822. [Google Scholar] [CrossRef]
- Park, T.-M.; Iwuoha, E.I.; Smyth, M.R.; Freaney, R.; McShane, A.J. Sol-gel based amperometric biosensor incorporating an osmium redox polymer as mediator for detection of L-lactate. Talanta 1997, 44, 973–978. [Google Scholar] [CrossRef]
- Kim, S.; Yang, W.S.; Kim, H.-J.; Lee, H.-N.; Park, T.J.; Seo, S.-J.; Park, Y.M. Highly sensitive non-enzymatic lactate biosensor driven by porous nanostructured nickel oxide. Ceram. Int. 2019, 45, 23370–23376. [Google Scholar] [CrossRef]
- Zhao, Y.; Geng, X.; Zhou, X.; Xu, L.; Li, S.; Li, Z.; Guo, Y.; Li, C. A novel high-stability bioelectrochemical sensor based on sol-gel immobilization of lactate dehydrogenase and AuNPs-rGO signal enhancement for serum pyruvate detection. Anal. Chim. Acta 2023, 1265, 341335. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S. Sol-Gel Synthesis Strategies for Tailored Catalytic Materials; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Preda, G.; Bizerea, O.; Vlad-Oros, B. Sol-gel technology in enzymatic electrochemical biosensors for clinical analysis. In Biosensors for Health, Environment and Biosecurity; Serra, P.A., Ed.; IntechOpen: London, UK, 2011; pp. 363–368. [Google Scholar]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Madden, J.; Vaughan, E.; Thompson, M.; O’Riordan, A.; Galvin, P.; Iacopino, D.; Teixeira, S.R. Electrochemical sensor for enzymatic lactate detection based on laser-scribed graphitic carbon modified with platinum, chitosan and lactate oxidase. Talanta 2022, 246, 123492. [Google Scholar] [CrossRef]
- Xuan, X.; Perez-Rafols, C.; Chen, C.; Cuartero, M.; Crespo, G.A. Lactate biosensing for reliable on-body sweat analysis. ACS Sens. 2021, 6, 2763–2771. [Google Scholar] [CrossRef]
- Shitanda, I.; Mitsumoto, M.; Loew, N.; Yoshihara, Y.; Watanabe, H.; Mikawa, T.; Tsujimura, S.; Itagaki, M.; Motosuke, M. Continuous sweat lactate monitoring system with integrated screen-printed MgO-templated carbon-lactate oxidase biosensor and microfluidic sweat collector. Electrochim. Acta 2021, 368, 137620. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Molina, B.G. Polymers and plastics modified electrodes for biosensors: A review. Molecules 2020, 25, 2446. [Google Scholar] [CrossRef] [PubMed]
- Freigang, M.; Wurster, C.D.; Hagenacker, T.; Stolte, B.; Weiler, M.; Kamm, C.; Schreiber-Katz, O.; Osmanovic, A.; Petri, S.; Kowski, A.; et al. Serum creatine kinase and creatinine in adult spinal muscular atrophy under nusinersen treatment. Ann. Clin. Transl. Neurol. 2021, 8, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Berketa, K.; Buzhak, A.; Vakhovskyi, Y.; Mruga, D.; Sverstiuk, A.; Soldatkina, O.; Lyubovych, O.; Marchuk, O.; Dzyadevych, S.; Soldatkin, O. Amperometric Biosensor Based on a Semipermeable Poly-Meta-Phenylenediamine Membrane and Immobilized Lactate Oxidase for Highly Accurate l-Lactate Determination in Blood Serum. Electroanalysis 2025, 37, e12011. [Google Scholar] [CrossRef]
- Vokhmyanina, D.V.; Sharapova, O.E.; Buryanovataya, K.E.; Karyakin, A.A. Novel siloxane derivatives as membrane precursors for lactate oxidase immobilization. Sensors 2023, 23, 4014. [Google Scholar] [CrossRef]
- Liu, M.; Yang, M.; Wang, M.; Wang, H.; Cheng, J. A flexible dual-analyte electrochemical biosensor for salivary glucose and lactate detection. Biosensors 2022, 12, 210. [Google Scholar] [CrossRef]
- Khan, I.; Lee, J.H.; Park, J.; Wooh, S. Nano/micro-structural engineering of Nafion membranes for advanced electrochemical applications. J. Saudi Chem. Soc. 2022, 26, 101511. [Google Scholar] [CrossRef]
- Banks, C.E.; Foster, C.W.; Kadara, R.O. Screen-Printing Electrochemical Architectures; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Iula, G.; Miglione, A.; Kalligosfyri, P.M.; Spinelli, M.; Amoresano, A.; Di Natale, C.; Darwish, I.A.; Cinti, S. On-body electrochemical measurement of sweat lactate with the use of paper-based fluidics and 3D-printed flexible wearable biosensor. Anal. Bioanal. Chem. 2025, 417, 3825. [Google Scholar]
- Yashina, E.I.; Borisova, A.V.; Karyakina, E.E.; Shchegolikhina, O.I.; Vagin, M.Y.; Sakharov, D.A.; Tonevitsky, A.G.; Karyakin, A.A. Sol− gel immobilization of lactate oxidase from organic solvent: Toward the advanced lactate biosensor. Anal. Chem. 2010, 82, 1601–1604. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, G.; Chapa, I.; Liu, Y. Reagent-free lactate detection using Prussian blue and electropolymerized-molecularly imprinted polymers-based electrochemical biosensors. ACS Appl. Mater. Interfaces 2024, 16, 66921–66931. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V. A historical review of glassy carbon: Synthesis, structure, properties and applications. Carbon Trends 2021, 5, 100116. [Google Scholar] [CrossRef]
- Promsuwan, K.; Saichanapan, J.; Soleh, A.; Saisahas, K.; Samoson, K.; Wangchuk, S.; Limbut, W. Bio-functionalized conductive poly(acrylic acid):poly(3,4-Ethylenedioxythiophene)-Prussian blue hybrid transducer for biosensors and bioelectronics interfaces. Mater. Today Chem. 2024, 40, 102271. [Google Scholar] [CrossRef]
- Yang, C.; Yu, S.; Yang, Q.; Wang, Q.; Xie, S.; Yang, H. Graphene Supported Platinum Nanoparticles Modified Electrode and Its Enzymatic Biosensing for Lactic Acid. J. Electrochem. Soc. 2018, 165, B665. [Google Scholar] [CrossRef]
- Halpin, G.; Herdman, K.; Dempsey, E. Electrochemical investigations into enzymatic polymerisation of 1,10-phenanthroline-5,6-dione as a redox mediator for lactate sensing. Sens. Actuators Rep. 2021, 3, 100032. [Google Scholar] [CrossRef]
- Fitriana, M.; Hiraka, K.; Ikebukuro, K.; Sode, K.; Tsugawa, W. A Thiol-reactive Phenazine Ethosulfate–A Novel Redox Mediator for Quasi-direct Electron-transfer-type Sensors. Sens. Mater. 2022, 34, 2105–2124. [Google Scholar] [CrossRef]
- Demkiv, O.; Gayda, G.; Stasyuk, N.; Moroz, A.; Serkiz, R.; Kausaite-Minkstimiene, A.; Gonchar, M.; Nisnevitch, M. Flavocytochrome b2-Mediated Electroactive Nanoparticles for Developing Amperometric L-Lactate Biosensors. Biosensors 2023, 13, 587. [Google Scholar] [CrossRef]
- Pagán, M.; Suazo, D.; del Toro, N.; Griebenow, K. A comparative study of different protein immobilization methods for the construction of an efficient nano-structured lactate oxidase-SWCNT-biosensor. Biosens. Bioelectron. 2015, 64, 138–146. [Google Scholar] [CrossRef]
- Uzunoglu, A.; Stanciu, L.A. Novel CeO2–CuO-decorated enzymatic lactate biosensors operating in low oxygen environments. Anal. Chim. Acta 2016, 909, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Uzunoglu, A. CeO2-ZrO2 Nanoparticle-Modified Enzymatic Lactate Biosensors with Reduced Oxygen Susceptibility. J. Electrochem. Soc. 2018, 165, B436. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, C. Wearable electrochemical sensors for noninvasive monitoring of health—A perspective. Curr. Opin. Electrochem. 2020, 23, 42–46. [Google Scholar] [CrossRef]
- Milić, L.; Zambry, N.S.; Ibrahim, F.B.; Petrović, B.; Kojić, S.; Thiha, A.; Joseph, K.; Jamaluddin, N.F.; Stojanović, G.M. Advances in textile-based microfluidics for biomolecule sensing. Biomicrofluidics 2024, 18, 051502. [Google Scholar] [CrossRef]
- Kalkal, A.; Kumar, S.; Kumar, P.; Pradhan, R.; Willander, M.; Packirisamy, G.; Kumar, S.; Malhotra, B.D. Recent advances in 3D printing technologies for wearable (bio)sensors. Addit. Manuf. 2021, 46, 102088. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Miyazaki, M. Enzyme-immobilized microfluidic devices for biomolecule detection. TrAC Trends Anal. Chem. 2023, 159, 116908. [Google Scholar] [CrossRef]
- Zhang, C.; Su, Y.; Liang, Y.; Lai, W. Microfluidic cloth-based analytical devices: Emerging technologies and applications. Biosens. Bioelectron. 2020, 168, 112391. [Google Scholar] [CrossRef] [PubMed]
- Pappa, A.M.; Curto, V.F.; Braendlein, M.; Strakosas, X.; Donahue, M.J.; Fiocchi, M.; Malliaras, G.G.; Owens, R.M. Organic transistor arrays integrated with finger-powered microfluidics for multianalyte saliva testing. Adv. Healthc. Mater. 2016, 5, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Berkheimer, Z.A.; Tahir, A.; Nordin, G.P.; Paixão, T.R.L.C.; Woolley, A.T.; do Nascimento, G.H.M.; de Araujo, W.R.; Pradela-Filho, L.A. Extruded filament electrodes for lactate biosensing in continuous-injection paper-based microfluidic devices. Biosens. Bioelectron. 2025, 278, 117390. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, J.; Liu, Y.; Wong, T.; Su, J.; Yao, K.; Zhou, J.; Huang, Y.; Li, H.; Li, D.; et al. Epidermal self-powered sweat sensors for glucose and lactate monitoring. Bio-Des. Manuf. 2022, 5, 201–209. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Liu, R.; Li, J.; Zhang, Q.; Shi, G.; Li, Y.; Hou, C.; Wang, H. A highly integrated sensing paper for wearable electrochemical sweat analysis. Biosens. Bioelectron. 2021, 174, 112828. [Google Scholar] [CrossRef]
- Vinoth, R.; Nakagawa, T.; Mathiyarasu, J.; Mohan, A.M.V. Fully printed wearable microfluidic devices for high-throughput sweat sampling and multiplexed electrochemical analysis. ACS Sens. 2021, 6, 1174–1186. [Google Scholar] [CrossRef]
- Cicatiello, C.; Gowers, S.A.N.; Smith, G.K.; Pinggera, D.; Orlob, S.; Wallner, B.; Schiefecker, A.; Moser, N.; Georgiou, P.; Helbok, R.; et al. The Neurochemical Signature of Cardiac Arrest: A Multianalyte Online Microdialysis Study. ACS Chem. Neurosci. 2025, 16, 1323–1334. [Google Scholar] [CrossRef]
- Samper, I.C.; Gowers, S.A.N.; Rogers, M.L.; Murray, D.R.K.; Jewell, S.L.; Pahl, C.; Strong, A.J.; Boutelle, M.G. 3D printed microfluidic device for online detection of neurochemical changes with high temporal resolution in human brain microdialysate. Lab Chip 2019, 19, 2038–2048. [Google Scholar] [CrossRef]
- Wei, M.; Qiao, Y.; Zhao, H.; Liang, J.; Li, T.; Luo, Y.; Lu, S.; Shi, X.; Lu, W.; Sun, X. Electrochemical non-enzymatic glucose sensors: Recent progress and perspectives. Chem. Commun. 2020, 56, 14553–14569. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jeerapan, I.; Wang, J. Wearable Chemical Sensors: Present Challenges and Future Prospects. ACS Sens. 2016, 1, 464–482. [Google Scholar] [CrossRef]
- Adam, H.; Gopinath, S.C.B.; Md Arshad, M.K.; Adam, T.; Hashim, U.; Sauli, Z.; Fakhri, M.A.; Subramaniam, S.; Chen, Y.; Sasidharan, S.; et al. Integration of microfluidic channel on electrochemical-based nanobiosensors for monoplex and multiplex analyses: An overview. J. Taiwan Inst. Chem. Eng. 2023, 146, 104814. [Google Scholar] [CrossRef]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. Electrochemical Detection for Paper-Based Microfluidics. Anal. Chem. 2009, 81, 5821–5826. [Google Scholar] [CrossRef]
- Chen, W.-T.; Yan, C.-F.; Yu, C.-J.; Liao, Y.-C.; Chen, C.-F. Highly catalytic Prussian blue analogues and their application on the three-dimensional origami paper-based sweat sensors. Biosens. Bioelectron. 2024, 254, 116188. [Google Scholar] [CrossRef]
- Campos-Arias, L.; Peřinka, N.; Lau, Y.C.; Castro, N.; Pereira, N.; Correia, V.M.G.; Costa, P.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. Improving Definition of Screen-Printed Functional Materials for Sensing Application. ACS Appl. Electron. Mater. 2024, 6, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Abbas, N.; Ali, A. Inkjet Printing: A Viable Technology for Biosensor Fabrication. Chemosensors 2022, 10, 103. [Google Scholar] [CrossRef]
- Huttunen, O.-H.; Happonen, T.; Hiitola-Keinänen, J.; Korhonen, P.; Ollila, J.; Hiltunen, J. Roll-To-Roll Screen-Printed Silver Conductors on a Polydimethyl Siloxane Substrate for Stretchable Electronics. Ind. Eng. Chem. Res. 2019, 58, 19909–19916. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Zheng, S.; Zhang, L.; Shi, X.; He, J.; Chou, X.; Wu, Z.-S. Ink formulation, scalable applications and challenging perspectives of screen printing for emerging printed microelectronics. J. Energy Chem. 2021, 63, 498–513. [Google Scholar] [CrossRef]
- Boček, Ž.; Zubak, M.; Kassal, P. Fully Inkjet-Printed Flexible Graphene–Prussian Blue Platform for Electrochemical Biosensing. Biosensors 2025, 15, 28. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária-ANVISA. Good Manufacturing Practices. Available online: www.gov.br/anvisa/pt-br/english/regulation-of-companies (accessed on 31 May 2025).
- World Health Organization. Good Manufacturing. Available online: www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/norms-and-standards/gmp (accessed on 31 May 2025).
- Qin, Y.; Ouyang, X.; Lv, Y.; Liu, W.; Liu, Q.; Wang, S. A Review of Carbon-Based Conductive Inks and Their Printing Technologies for Integrated Circuits. Coatings 2023, 13, 1769. [Google Scholar] [CrossRef]
- Business Research Insights. Electrochemical Biosensors Market Report Overview. Available online: www.businessresearchinsights.com/market-reports/electrochemical-biosensors-market-103057#:~:text=The%20global%20electrochemical%20biosensors%20market,7.05%25%20during%20the%20forecast%20period (accessed on 1 June 2025).
- U.S. Department of Health & Human Services. Technology Readiness Levels (Trls) for Medical Countermeasure Products (Diagnostics and Medical Devices). Available online: www.medicalcountermeasures.gov/trl/trls-for-medical-devices (accessed on 1 June 2025).
- Ronan, Y. Medical Device Design and Development: Process, Phases and Technologies. Available online: www.3erp.com/blog/medical-device-design-and-development/ (accessed on 1 June 2025).
- Agência Nacional de Vigilância Sanitária-ANVISA. Approval of Medical Devices in Brazil. Available online: www.gov.br/anvisa/pt-br/english/regulation-of-products/medical-devices (accessed on 1 June 2025).
- News Medical Life Sciences. Nova Biomedical Capillary Blood Testing for Lactate, Hb and Hct with the StatStrip® LAC/Hb/Hct*. Available online: www.news-medical.net/suppliers/Nova-Biomedical.aspx (accessed on 31 July 2025).
- Zimmer & Peacock. Lactate in Sweat. Available online: www.zimmerpeacock.com/2024/06/24/lactate-in-sweat/ (accessed on 31 July 2025).
- Shenzhen Sieman Technology Co. M-2000 plus Bioprocess Biochemistry Analyzer. Available online: www.siemanbio.com/ (accessed on 31 July 2025).
- Zhao, Y.; Fang, X.; Gu, Y.; Yan, X.; Kang, Z.; Zheng, X.; Lin, P.; Zhao, L.; Zhang, Y. Gold nanoparticles coated zinc oxide nanorods as the matrix for enhanced l-lactate sensing. Colloids Surf. B Biointerfaces 2015, 126, 476–480. [Google Scholar] [CrossRef]
- Chan, D.; Barsan, M.M.; Korpan, Y.; Brett, C.M.A. L-lactate selective impedimetric bienzymatic biosensor based on lactate dehydrogenase and pyruvate oxidase. Electrochim. Acta 2017, 231, 209–215. [Google Scholar] [CrossRef]
- Tu, D.; He, Y.; Rong, Y.; Wang, Y.; Li, G. Disposable L-lactate biosensor based on a screen-printed carbon electrode enhanced by graphene. Meas. Sci. Technol. 2016, 27, 045108. [Google Scholar] [CrossRef]
- Imani, S.; Bandodkar, A.J.; Mohan, A.M.V.; Kumar, R.; Yu, S.; Wang, J.; Mercier, P.P. A wearable chemical–electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 2016, 7, 11650. [Google Scholar] [CrossRef]
- Lei, Y.; Zhao, W.; Zhang, Y.; Jiang, Q.; He, J.-H.; Baeumner, A.J.; Wolfbeis, O.S.; Wang, Z.L.; Salama, K.N.; Alshareef, H.N. A MXene-Based Wearable Biosensor System for High-Performance In Vitro Perspiration Analysis. Small 2019, 15, 1901190. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Nakagawa, T.; Pavinatto, A.; Mensah, S.T.; Imani, S.; Mercier, P.; Wang, J. Eyeglasses based wireless electrolyte and metabolite sensor platform. Lab Chip 2017, 17, 1834–1842. [Google Scholar] [PubMed]
- Pappa, A.M.; Ohayon, D.; Giovannitti, A.; Maria, I.P.; Savva, A.; Uguz, I.; Rivnay, J.; McCulloch, I.; Owens, R.M.; Inal, S. Direct metabolite detection with an n-type accumulation mode organic electrochemical transistor. Sci. Adv. 2018, 4, eaat0911. [Google Scholar] [CrossRef]
- Wang, R.; Zhai, Q.; An, T.; Gong, S.; Cheng, W. Stretchable gold fiber-based wearable textile electrochemical biosensor for lactate monitoring in sweat. Talanta 2021, 222, 121484. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, D.; Xu, C.; Ge, Y.; Liu, X.; Wei, Q.; Huang, L.; Ren, X.; Wang, C.; Wang, Y. Wearable electrochemical biosensor based on molecularly imprinted Ag nanowires for noninvasive monitoring lactate in human sweat. Sens. Actuators B Chem. 2020, 320, 128325. [Google Scholar] [CrossRef]
- Hickey, D.P.; Reid, R.C.; Milton, R.D.; Minteer, S.D. A self-powered amperometric lactate biosensor based on lactate oxidase immobilized in dimethylferrocene-modified LPEI. Biosens. Bioelectron. 2016, 77, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Parra-Alfambra, A.M.; Casero, E.; Vázquez, L.; Quintana, C.; del Pozo, M.; Petit-Domínguez, M.D. MoS2 nanosheets for improving analytical performance of lactate biosensors. Sens. Actuators B Chem. 2018, 274, 310–317. [Google Scholar] [CrossRef]
- Chang, A.S.; Memon, N.N.; Amin, S.; Chang, F.; Aftab, U.; Abro, M.I.; dad Chandio, A.; Shah, A.A.; Ibupoto, M.H.; Ansari, M.A.; et al. Facile Non-enzymatic Lactic Acid Sensor Based on Cobalt Oxide Nanostructures. Electroanalysis 2019, 31, 1296–1303. [Google Scholar] [CrossRef]
- Zhou, L. Molecularly Imprinted Sensor based on Ag-Au NPs/SPCE for Lactate Determination in Sweat for Healthcare and Sport Monitoring. Int. J. Electrochem. Sci. 2021, 16, 211043. [Google Scholar] [CrossRef]
- Kumar, N.; Lin, Y.-J.; Huang, Y.-C.; Liao, Y.-T.; Lin, S.-P. Detection of lactate in human sweat via surface-modified, screen-printed carbon electrodes. Talanta 2023, 265, 124888. [Google Scholar] [CrossRef]
- Schmitt, R.E.; Molitor, H.R.; Wu, T. Voltammetric Method for the Determination of Lactic Acid Using a Carbon Paste Electrode Modified with Cobalt Phthalocyanine. Int. J. Electrochem. Sci. 2012, 7, 10835–10841. [Google Scholar] [CrossRef]
- Sajna, M.S.; Cabibihan, J.-J.; Malik, R.A.; Kumar Sadasivuni, K.; Geetha, M.; Khalid Alahmad, J.; Anwar Hijazi, D.; Alsaedi, F. Nonenzymatic electrochemical lactic acid sensor using CuO nanocomposite. Mater. Sci. Eng. B 2023, 288, 116217. [Google Scholar] [CrossRef]
- Zhu, C.; Xue, H.; Zhao, H.; Fei, T.; Liu, S.; Chen, Q.; Gao, B.; Zhang, T. A dual-functional polyaniline film-based flexible electrochemical sensor for the detection of pH and lactate in sweat of the human body. Talanta 2022, 242, 123289. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).