A Novel Pyrene-Based Fluorescent Probe for the Detection of Cu2+
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
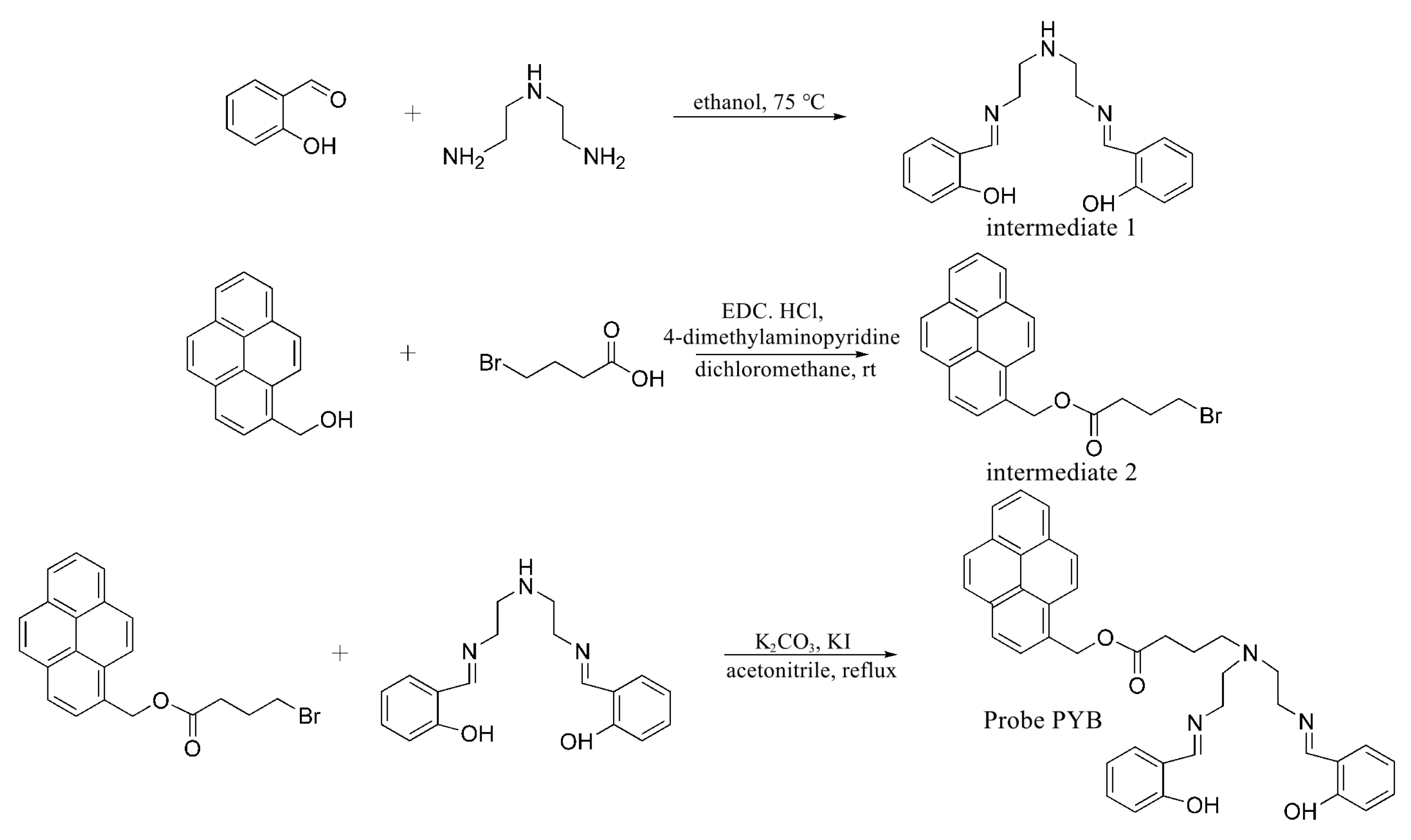
2.2. Synthesis of Probe PYB
3. Results
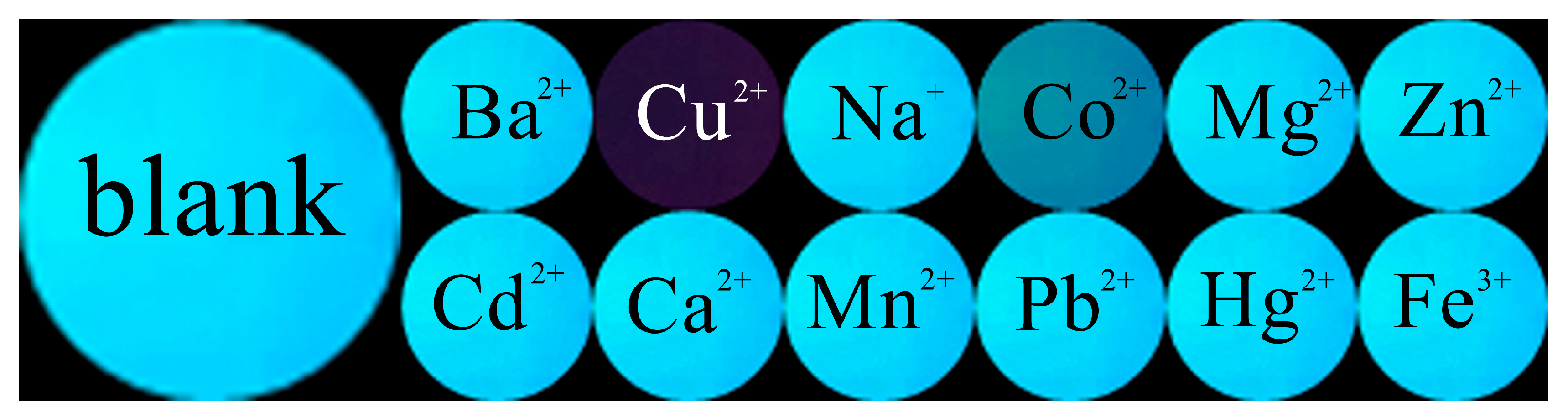
3.1. Absorption Spectral Response
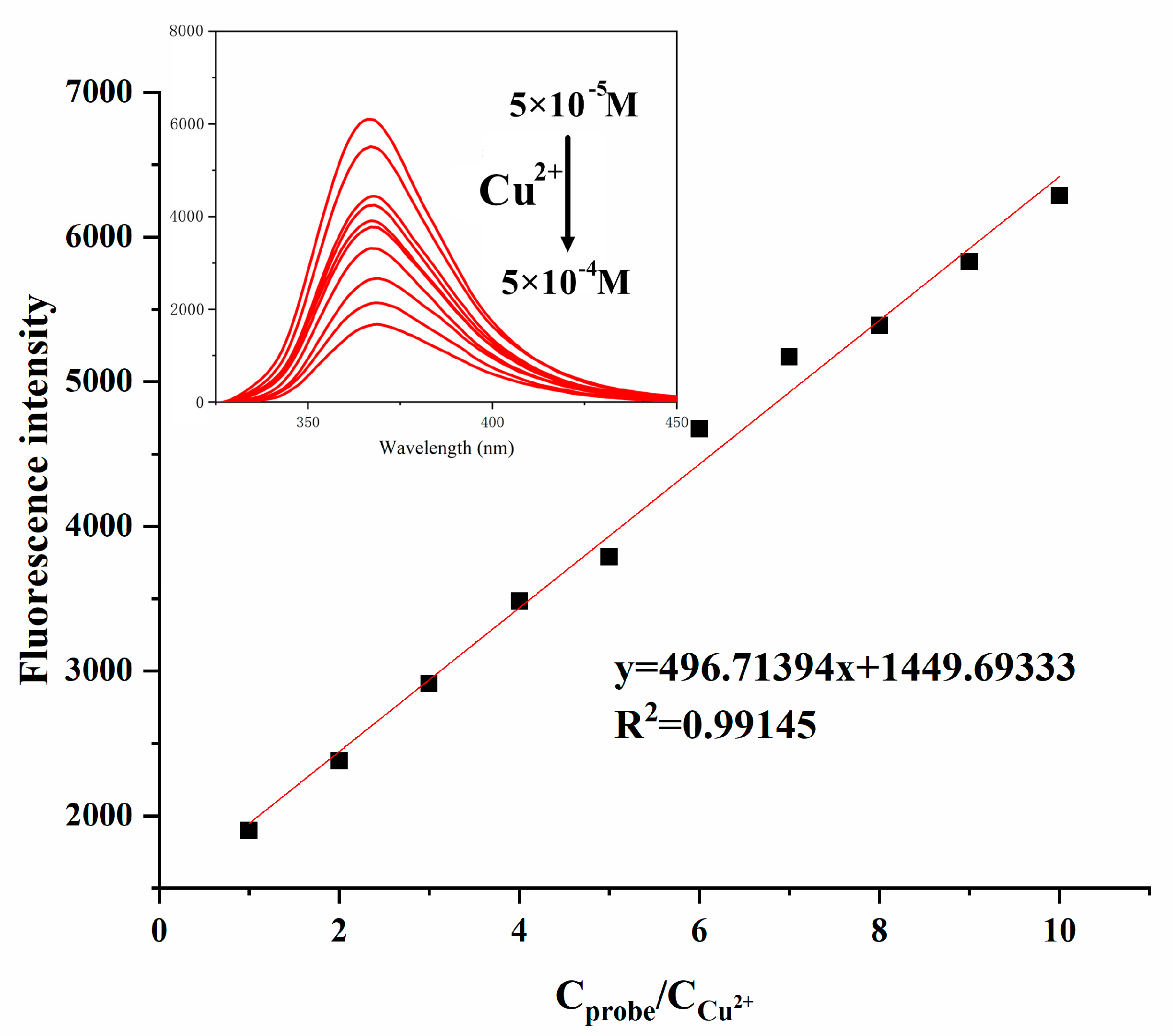
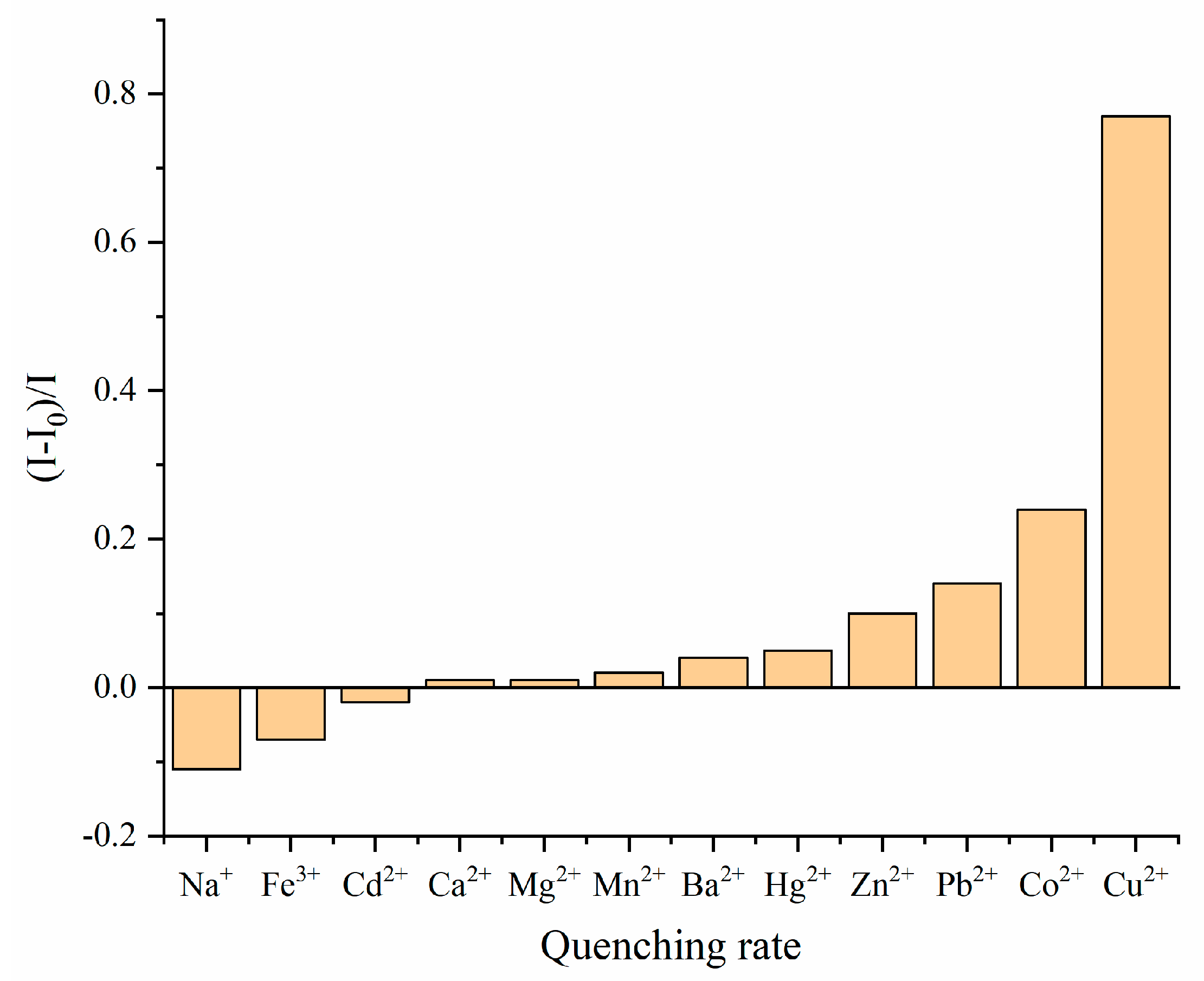
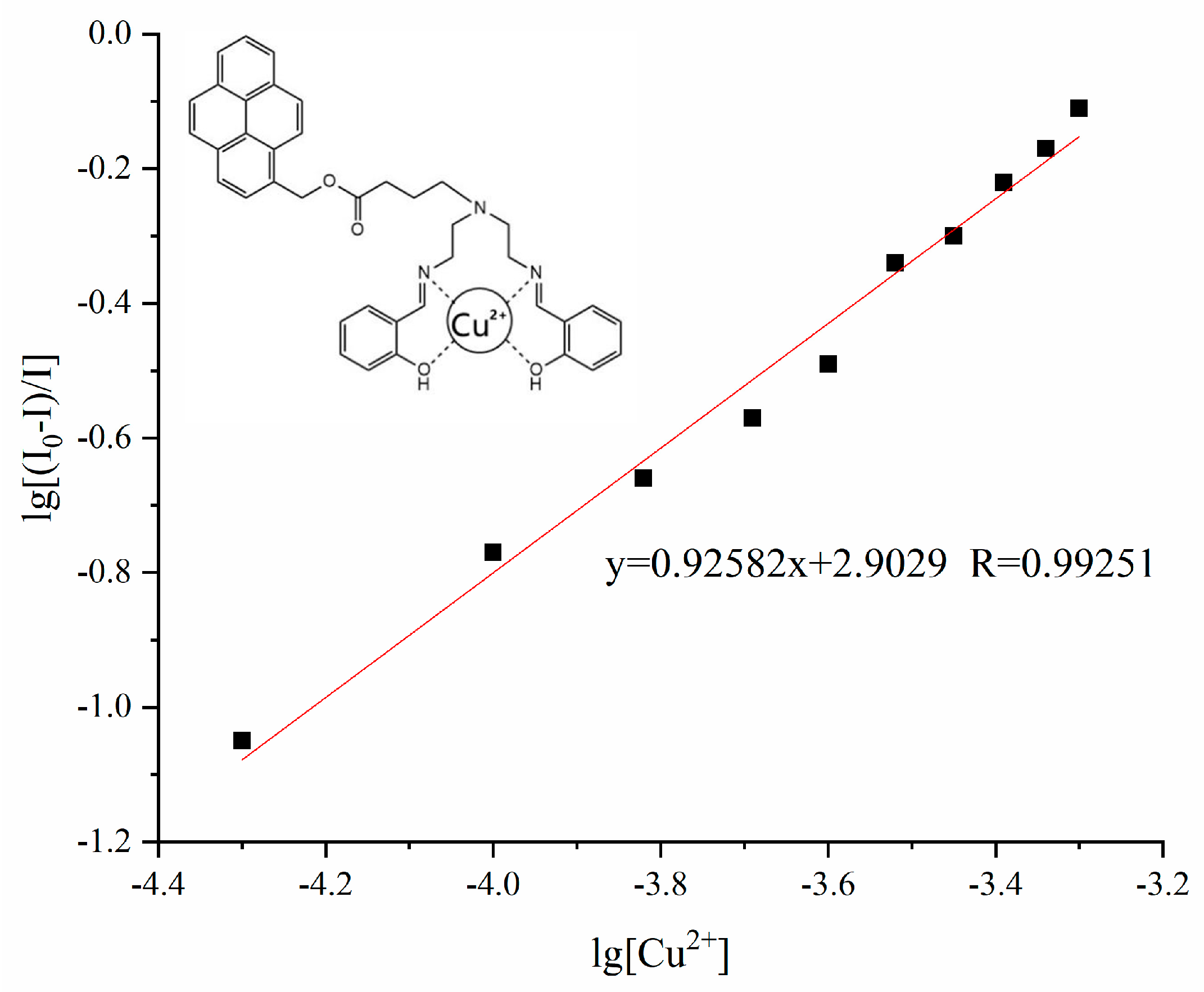
3.2. Fluorescence Spectral Response
3.3. Test Strip Experiment
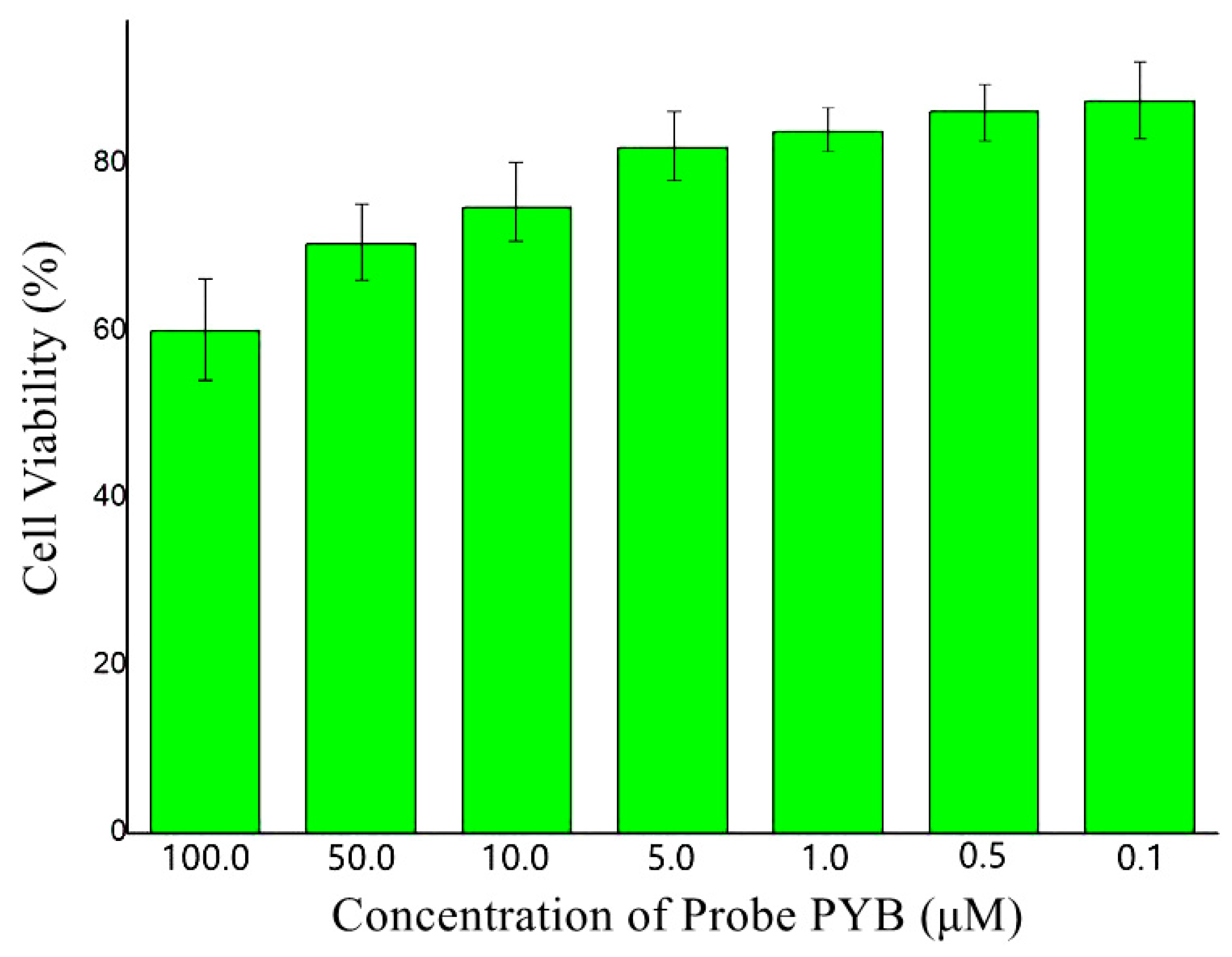
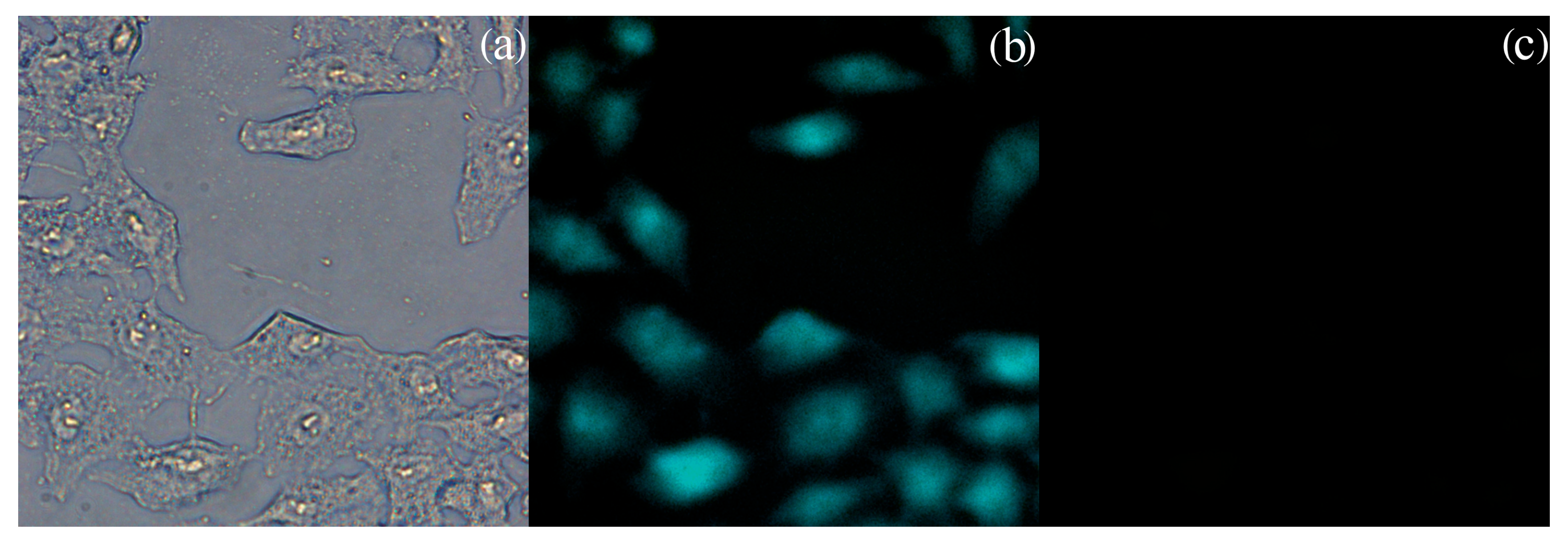
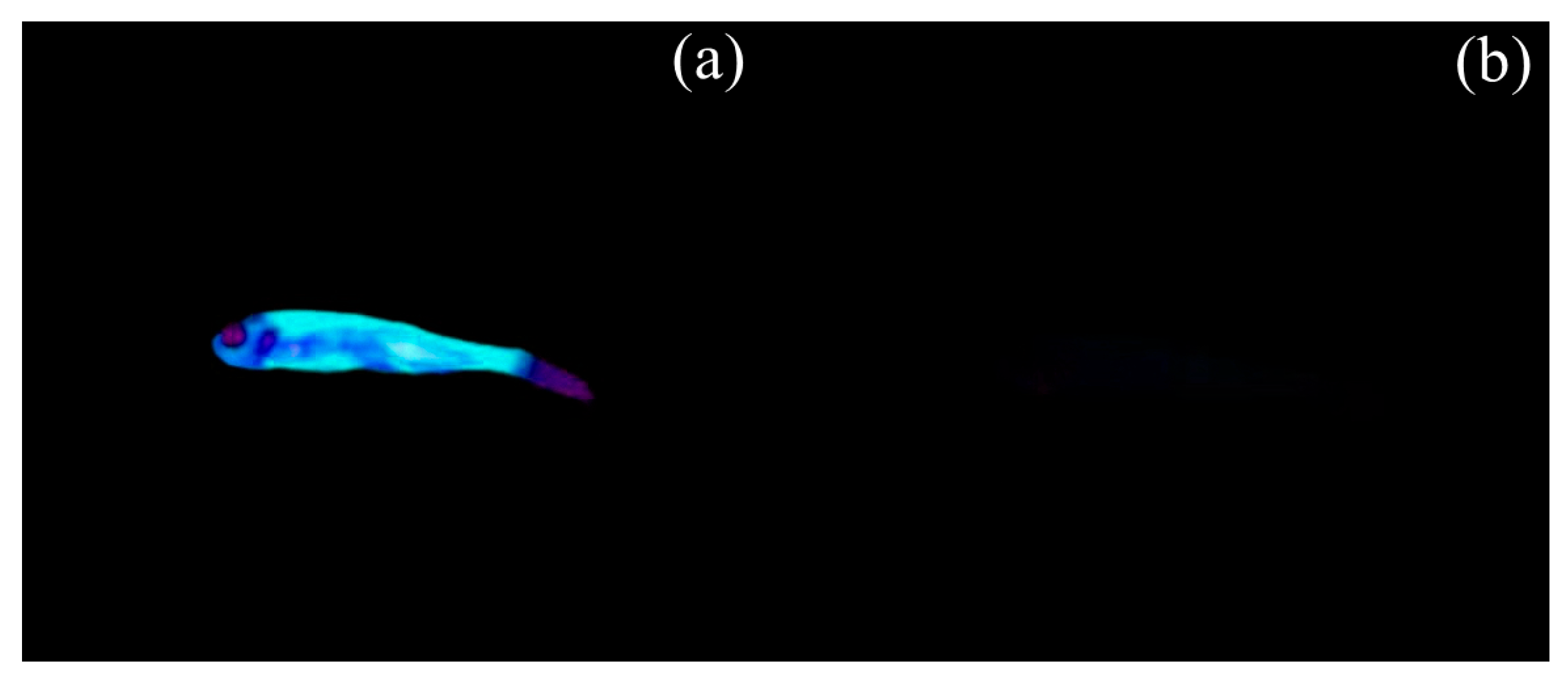
3.4. Biological Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, S.; Yuan, X.; Song, Z.; Lin, Z.; Zhao, F.; Wang, L.; Gao, J. A sequential treatment strategy by copper ion-doped nanofiber dressing for highly efficient biofilm combating and rapid wound healing. Chem. Eng. J. 2024, 500, 156974. [Google Scholar] [CrossRef]
- Nakajima, H.; Fujimoto, N.; Yamamoto, Y.; Amemiya, T.; Itoh, K. Effect of Cu on the fluorescence of the Cu-hyperaccumulator lichen stereocaulon sorediiferum. Environ. Sci. Pollut. Res. 2019, 26, 36754–36763. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, I.; Lüthen, F.; Prinz, C.; Kreikemeyer, B.; Zietz, C.; Neumann, H.; Rychly, J. A dual function of copper in designing regenerative implants. Biomaterials 2015, 44, 36–44. [Google Scholar] [CrossRef]
- He, T.; Tang, Q.; Ren, Q.; Liu, Y.; He, G.; Pan, Y.; Wang, Z.; Huang, P.; Lin, J. Different Valence States of Copper Ion Delivery against Triple-Negative Breast Cancer. ACS Nano 2024, 18, 5434–5445. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, G.; Zhang, H.; Jiang, S.; Jiang, L.; Li, X.; Tang, K.; Fu, X.; Su, Z.; Jin, L.; et al. Copper ion-chelated chitosan-coated urinary catheters: Synergistic antibacterial and anti-inflammatory effects in clinical applications. Mater. Des. 2025, 258, 114664. [Google Scholar] [CrossRef]
- Gu, G.; Erişen, D.; Yang, K.; Zhang, B.; Shen, M.; Zou, J.; Qi, X.; Chen, S.; Xu, X. Antibacterial and anti-inflammatory activities of chitosan/copper complex coating on medical catheters: In vitro and in vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Kielhorn, J.; Melber, C.; Keller, D.; Mangelsdorf, I. Palladium-A review of exposure and effects to human health. Int. J. Hyg. Environ. Health 2002, 205, 417–432. [Google Scholar] [CrossRef]
- Kolemen, S.; Akkaya, E.U. Reaction-based BODIPY probes for selective bio-imaging. Coord. Chem. Rev. 2018, 354, 121–134. [Google Scholar] [CrossRef]
- Tigreros, A.; Portilla, J. Recent progress in chemosensors based on pyrazole derivatives. RSC Adv. 2020, 10, 19693–19712. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, J.; Han, G.; Liu, Z.; Liu, C.; Zhang, C.; Liu, B.; Jiang, C.; Liu, R.; Zhao, T.; et al. Real-time discrimination and versatile profiling of spontaneous reactive oxygen species in living organisms with a single fluorescent probe. J. Am. Chem. Soc. 2016, 138, 3769–3778. [Google Scholar] [CrossRef]
- Yang, X.; He, L.; Xu, K.; Yang, Y.; Lin, W. A turn-on fluorescent formaldehyde probe regulated by combinational PET and ICT mechanisms for bioimaging applications. Anal. Methods 2018, 10, 2963–2967. [Google Scholar] [CrossRef]
- Nootem, J.; Daengngern, R.; Sattayanon, C.; Wattanathana, W.; Wannapaiboon, S. The synergy of CHEF and ICT toward fluorescence ‘turn-on’ probes based on push-pull benzothiazoles for selective detection of Cu2+ in acetonitrile/water mixture. J. Photochem. Photobiol. A Chem. 2021, 415, 113318. [Google Scholar] [CrossRef]
- Sedgwick, A.C.; Wu, L.; Han, H.; Bull, S.; He, X.; James, T.; Sessler, J.; Tang, B.; Tian, H.; Yoon, J. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef]
- Jiang, D.; Zheng, M.; Yan, X.; Huang, H.; Huang, H.; Gong, T.; Liu, K.; Liu, J. A “turn-on” ESIPT fluorescence probe of 2-(aminocarbonyl)phenylboronic acid for the selective detection of Cu(ii). RSC Adv. 2022, 12, 31186–31191. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Song, F.; Xiong, X.; Peng, X. Fluorescent nanosensors based on fluorescence resonance energy transfer (FRET). Ind. Eng. Chem. Res. 2013, 52, 11228–11245. [Google Scholar] [CrossRef]
- He, C.; Zhou, H.; Yang, N.; Niu, N.; Hussain, E.; Li, Y.; Yu, C. A turn-on fluorescent BOPHY probe for Cu2+ ion detection. New J. Chem. 2018, 42, 2520–2525. [Google Scholar] [CrossRef]
- Qi, J.; Li, B.; Wang, X.; Zhang, Z.; Wang, Z.; Han, J.; Chen, L. Three-dimensional paper-based microfluidic chip device for multiplexed fluorescence detection of Cu2+ and Hg2+ ions based on ion imprinting technology. Sens. Actuators B-Chem. 2017, 251, 224–233. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef]
- Ma, X.; Qian, K.; Kandawa-Schulz, M.; Miao, W.; Wang, Y. Direct determination of Cu2+ based on the electrochemical catalytic reaction of Fe3+/Cu2+. Electroanalysis 2020, 32, 620–625. [Google Scholar] [CrossRef]
- Gao, W.; W, H.; Pu, S. A highly selective fluorescent probe for Cu2+ based on a diarylethene with a benzo [1,2,5] oxadiazol-4-ylamine Schiff base unit. J. Photochem. Photobiol. A Chem. 2018, 364, 208–218. [Google Scholar] [CrossRef]
- Dayanidhi, K.; Vadivel, P.; Jothi, S.; Eusuff, N. White Eggshells: A potential biowaste material for synergetic adsorption and naked-eye colorimetric detection of heavy metal ions from aqueous solution. ACS Appl. Mater. Interfaces 2020, 12, 1746–1756. [Google Scholar] [CrossRef]
- Li, T.; Xiao, X.; Zhou, C.; Luo, M. Design and application of Cu2+ fluorescent sensor based on carbazole derivatives. J. Fluoresc. 2025, 35, 2993–3001. [Google Scholar] [CrossRef]
- Li, F.; Zhou, C.; Hou Zhang, H.; Hou, Y.; Pan, Q.; Sun, J.; Li, X. A novel colorimetric and ‘‘turn-on” fluorescent sensor for selective detection of Cu2+. Arab. J. Chem. 2022, 15, 104176–104183. [Google Scholar] [CrossRef]
- Yin, J.; Wang, Z.; Zhao, F.; Yang, H.; Li, M.; Yang, Y. A novel dual functional pyrene-based turn-on fluorescent probe for hypochlorite and copper (II) ion detection and bioimaging applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118470. [Google Scholar] [CrossRef]
- Wu, C.; Yu, Y.; You, J.; Chen, S.; Wu, W.; Liang, Y. Synthesis and application of pyrene-based fluorescent probe for the continuous detection of Cu2+ and picric acid. Luminescence 2025, 40, 70300. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yang, M.; Zhang, J. A pyrene-based multifunctional fluorescent probe for the detection of Cu2+ and Al3+. Microchem. J. 2025, 208, 112531. [Google Scholar] [CrossRef]
- Wang, Y.; Di, H. A pyrene-based fluorescent probe for the fluorescent recognition of copper ions. J. Phys. Conf. Ser. 2025, 3021, 012034. [Google Scholar] [CrossRef]

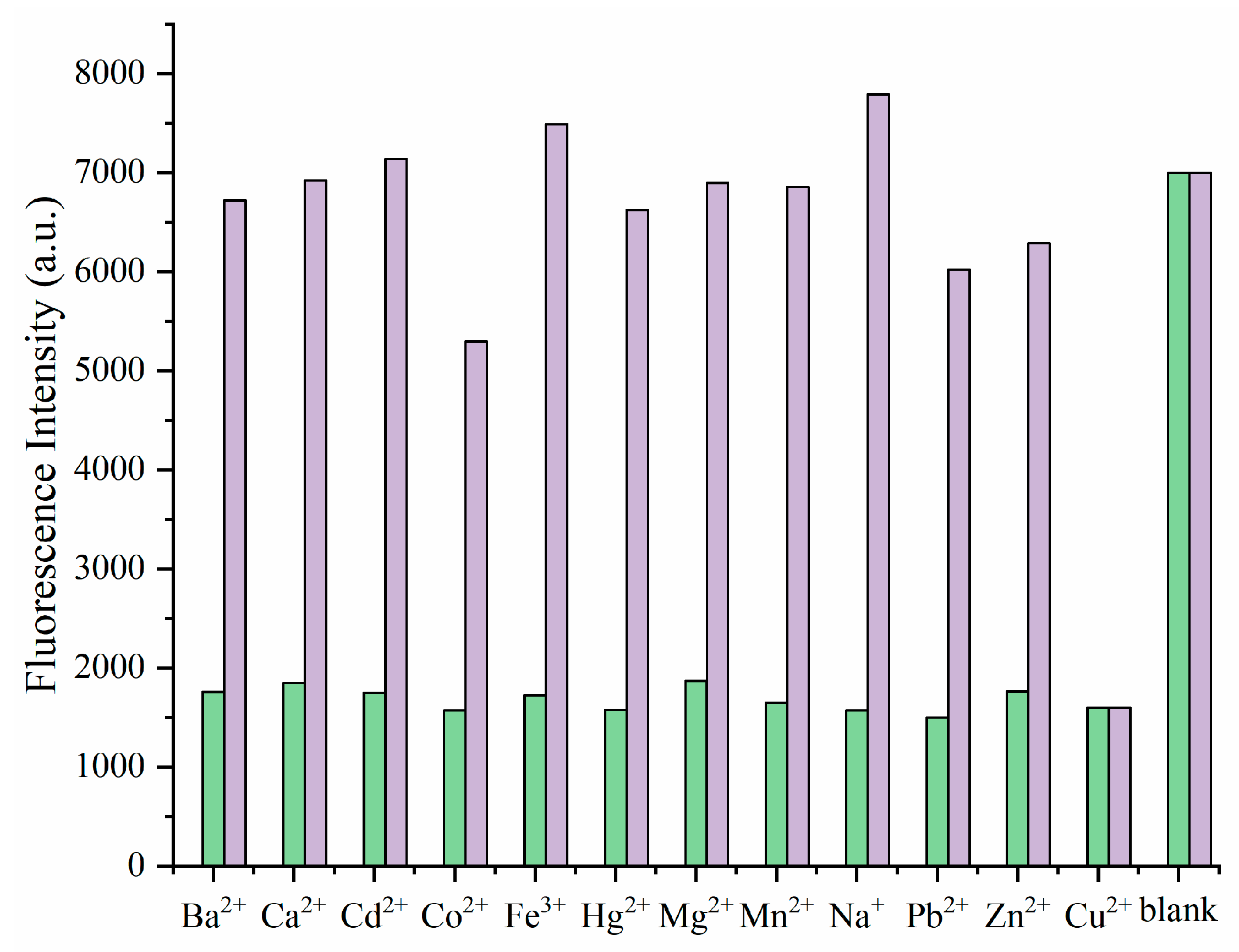
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Xiao, N.; Zhou, C.; Kovtunets, E.; Luo, M.; Zou, C.; Wang, Y.; Sun, J. A Novel Pyrene-Based Fluorescent Probe for the Detection of Cu2+. Chemosensors 2025, 13, 403. https://doi.org/10.3390/chemosensors13110403
Wang H, Xiao N, Zhou C, Kovtunets E, Luo M, Zou C, Wang Y, Sun J. A Novel Pyrene-Based Fluorescent Probe for the Detection of Cu2+. Chemosensors. 2025; 13(11):403. https://doi.org/10.3390/chemosensors13110403
Chicago/Turabian StyleWang, Haixia, Ning Xiao, Chen Zhou, Evgeny Kovtunets, Mingxin Luo, Chenyang Zou, Yining Wang, and Jing Sun. 2025. "A Novel Pyrene-Based Fluorescent Probe for the Detection of Cu2+" Chemosensors 13, no. 11: 403. https://doi.org/10.3390/chemosensors13110403
APA StyleWang, H., Xiao, N., Zhou, C., Kovtunets, E., Luo, M., Zou, C., Wang, Y., & Sun, J. (2025). A Novel Pyrene-Based Fluorescent Probe for the Detection of Cu2+. Chemosensors, 13(11), 403. https://doi.org/10.3390/chemosensors13110403





