Voltammetric Sensor Based on Titania Nanoparticles Synthesized with Aloe vera Extract for the Quantification of Dithiophosphates in Industrial and Environmental Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Methodology
2.2.1. Synthesis and Characterization of Nanoparticles and Electrode Surfaces
2.2.2. Preparation of Carbon Paste Electrodes
2.2.3. Study of the Electrochemical Response to Dithiophosphate
2.2.4. Electrochemical Study of the Electrodes
2.2.5. Linear Range, LOD, and LOQ
2.2.6. Repeatability and Reproducibility
2.2.7. Trueness
2.2.8. Analytical Interferences
3. Results and Discussion
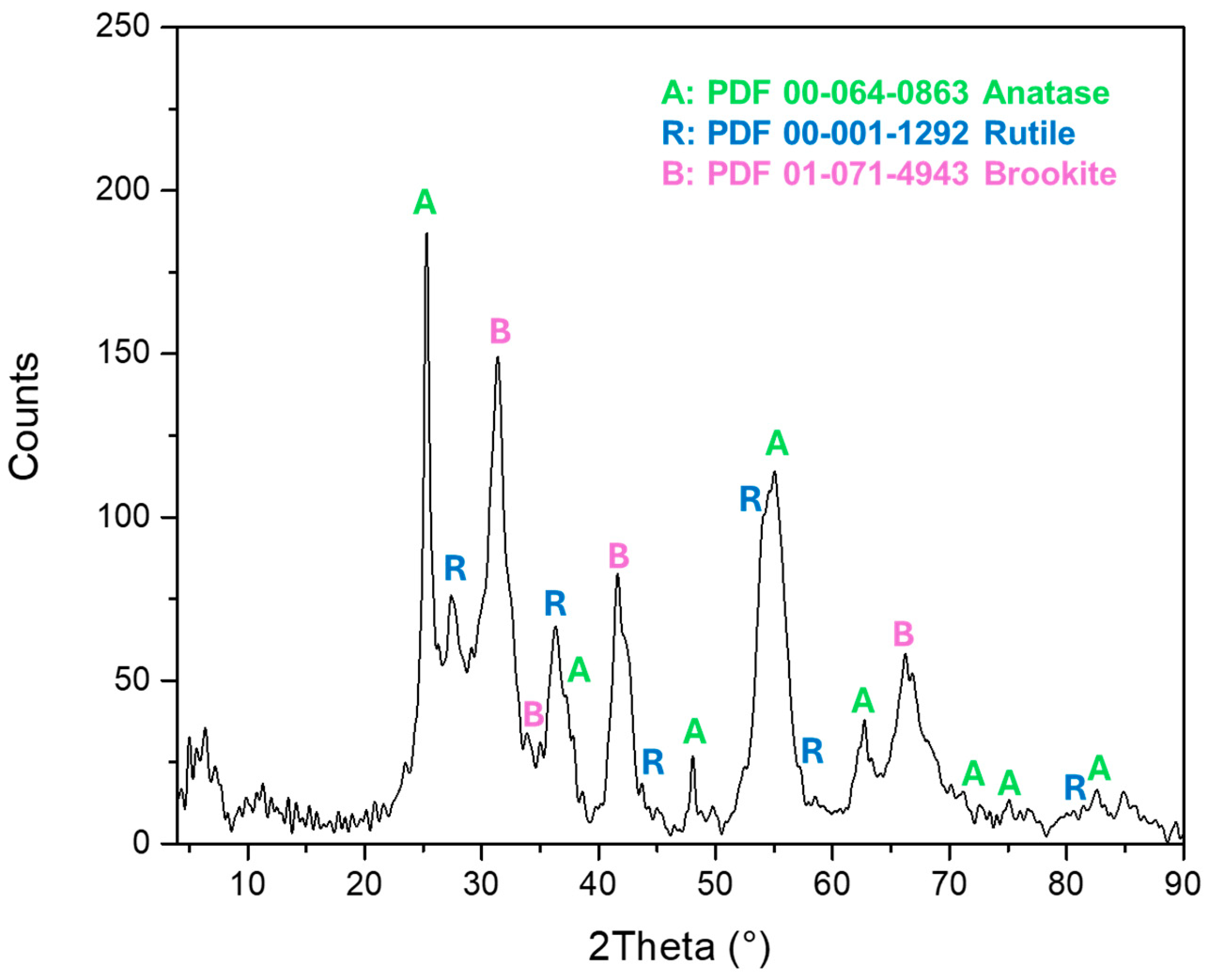
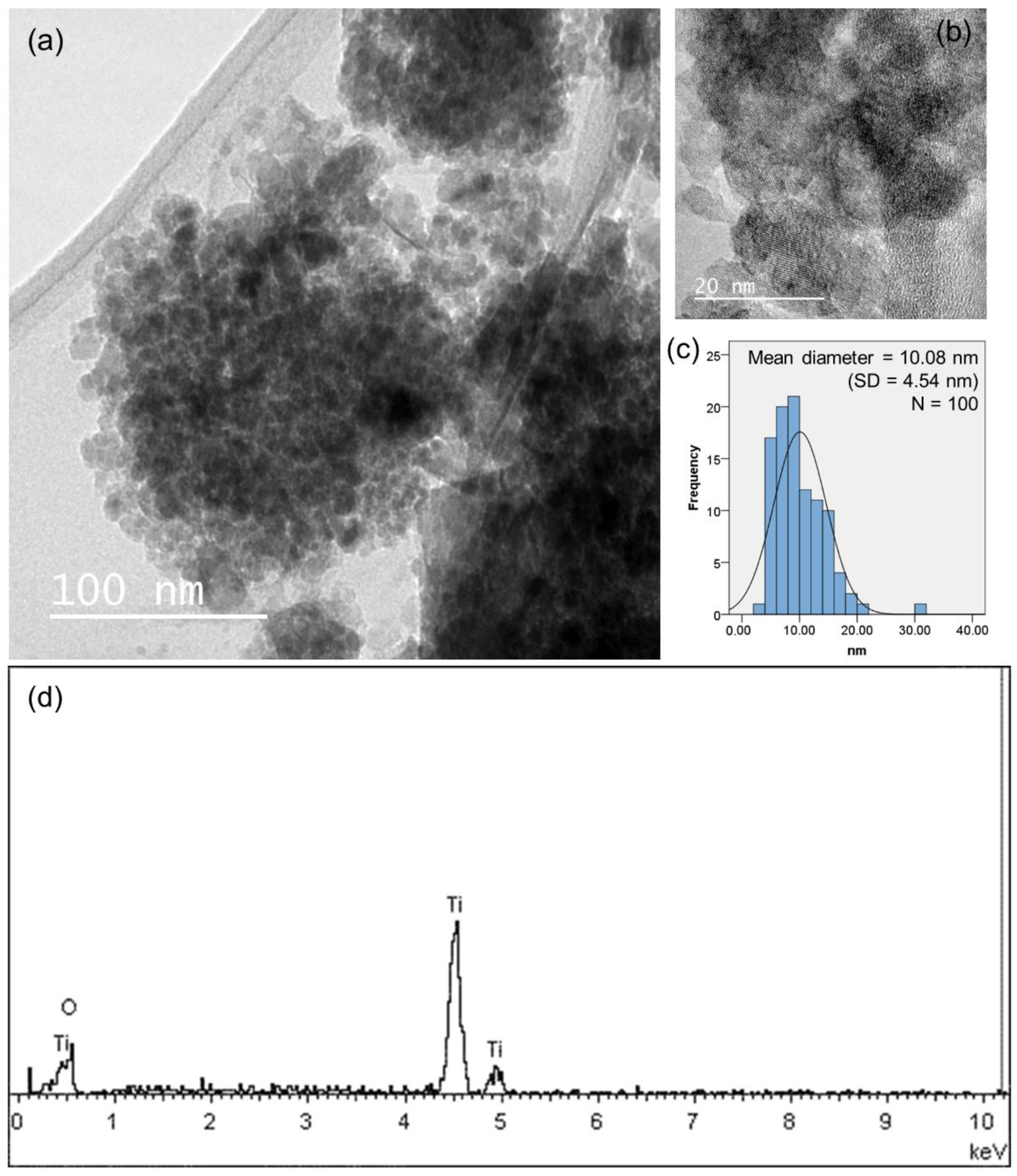
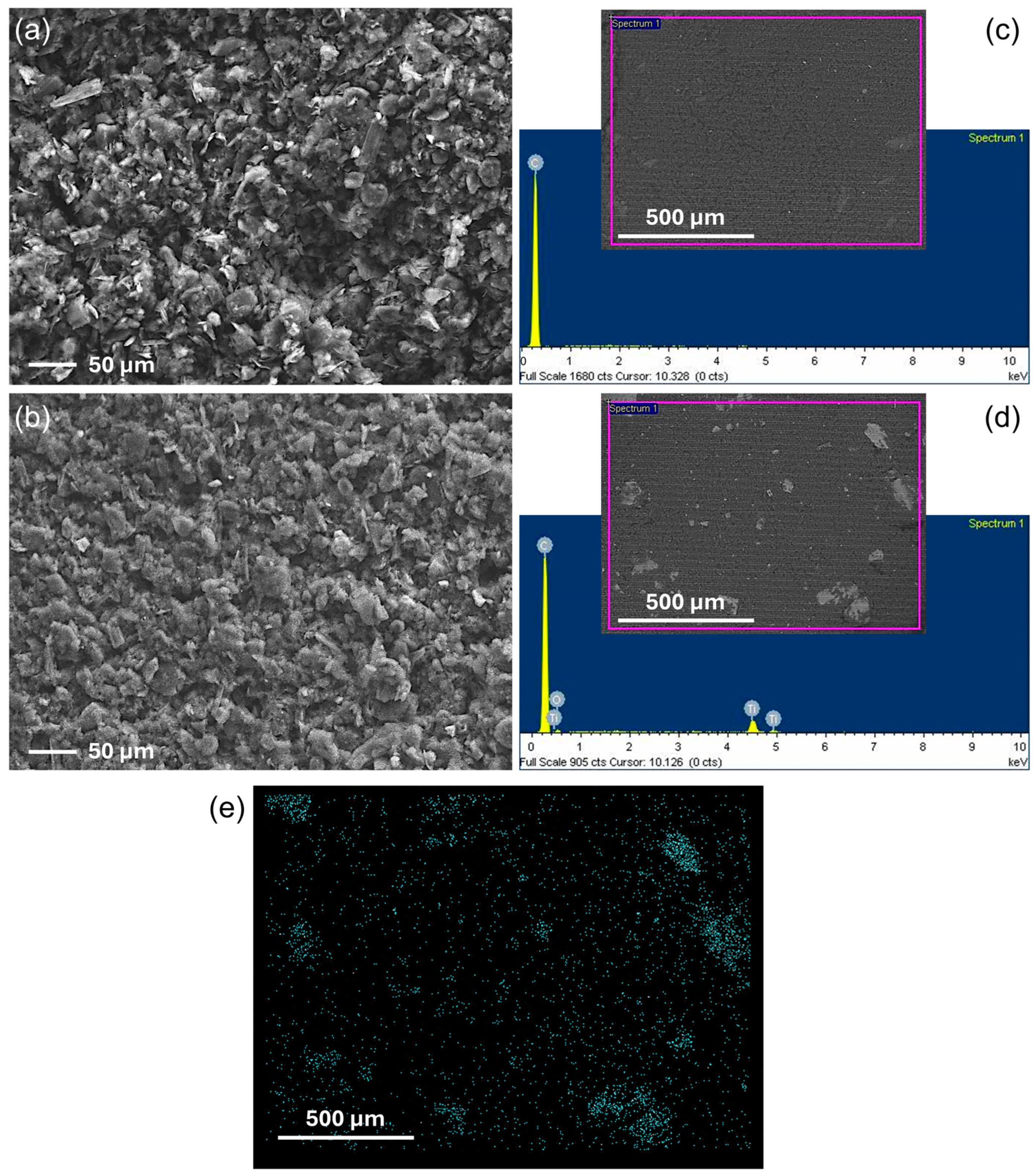
3.1. Characterization of Nanoparticles and Electrode Surfaces
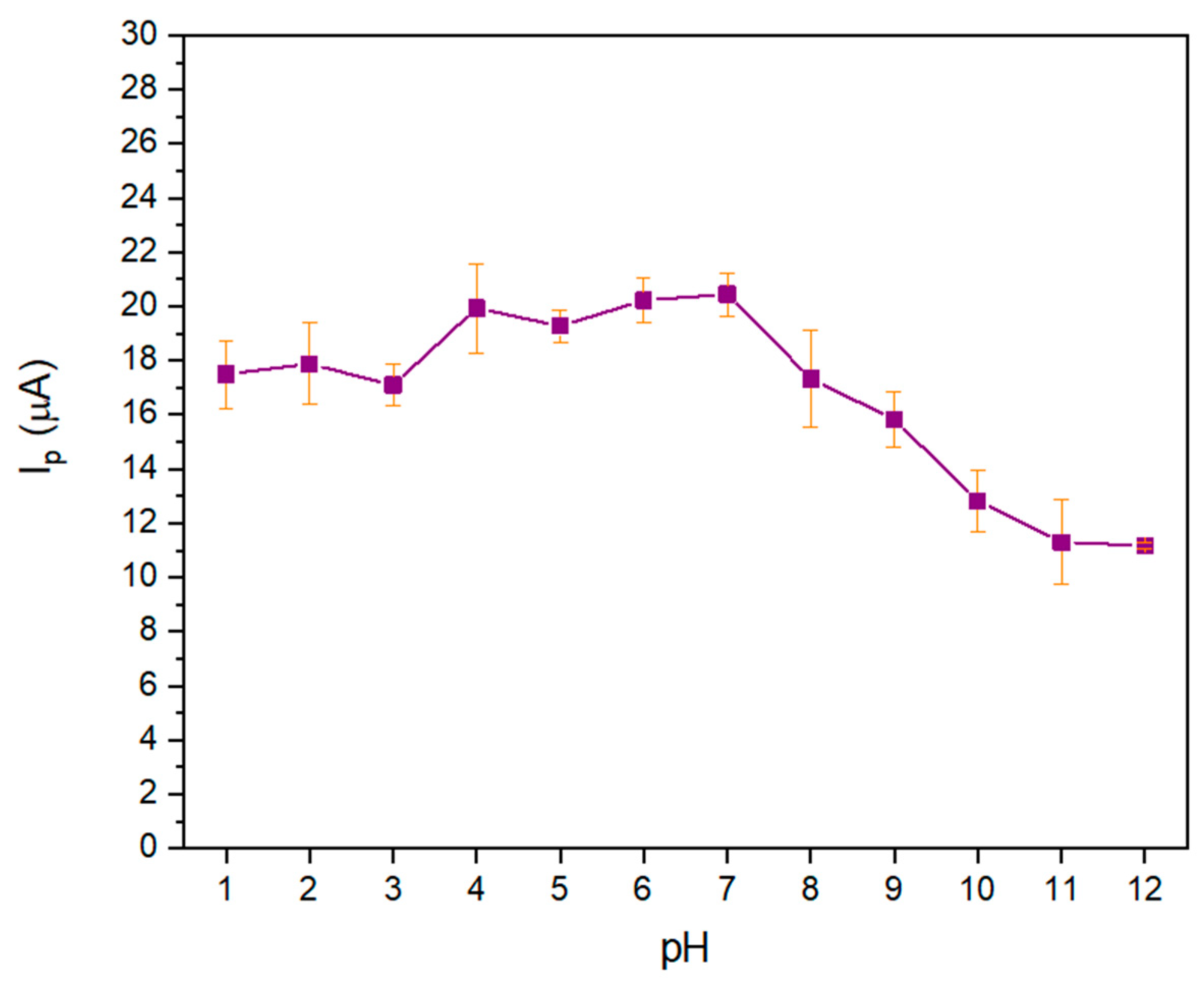
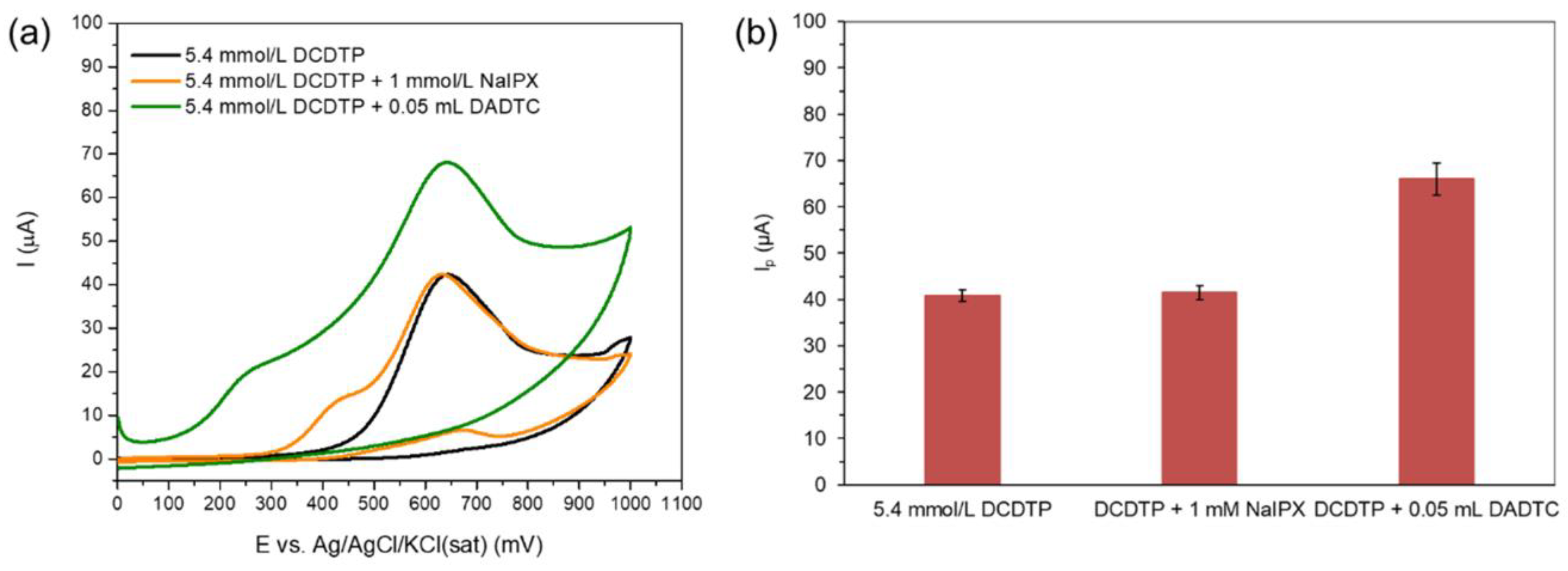
3.2. Study of the Electrochemical Response to Dithiophosphate
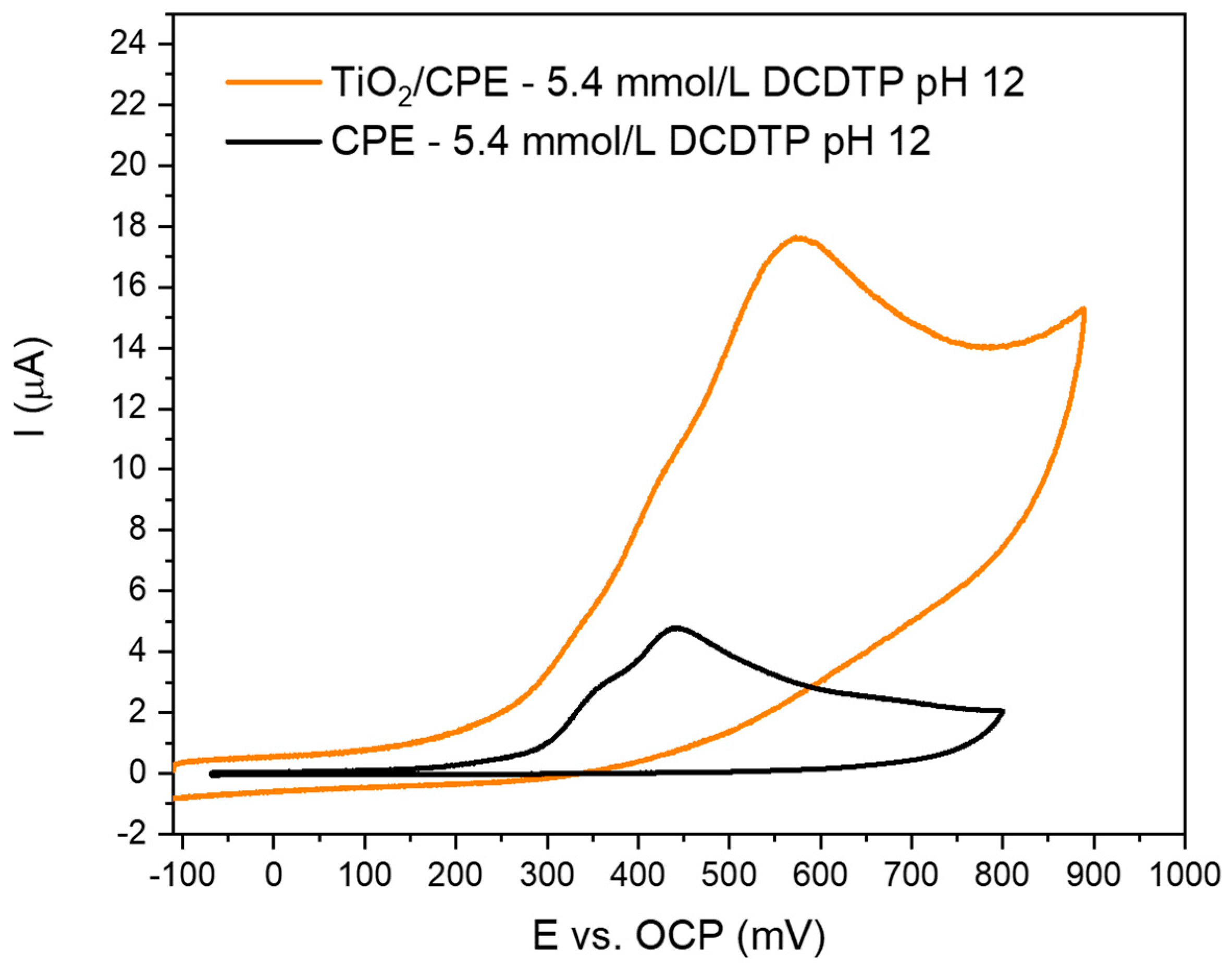
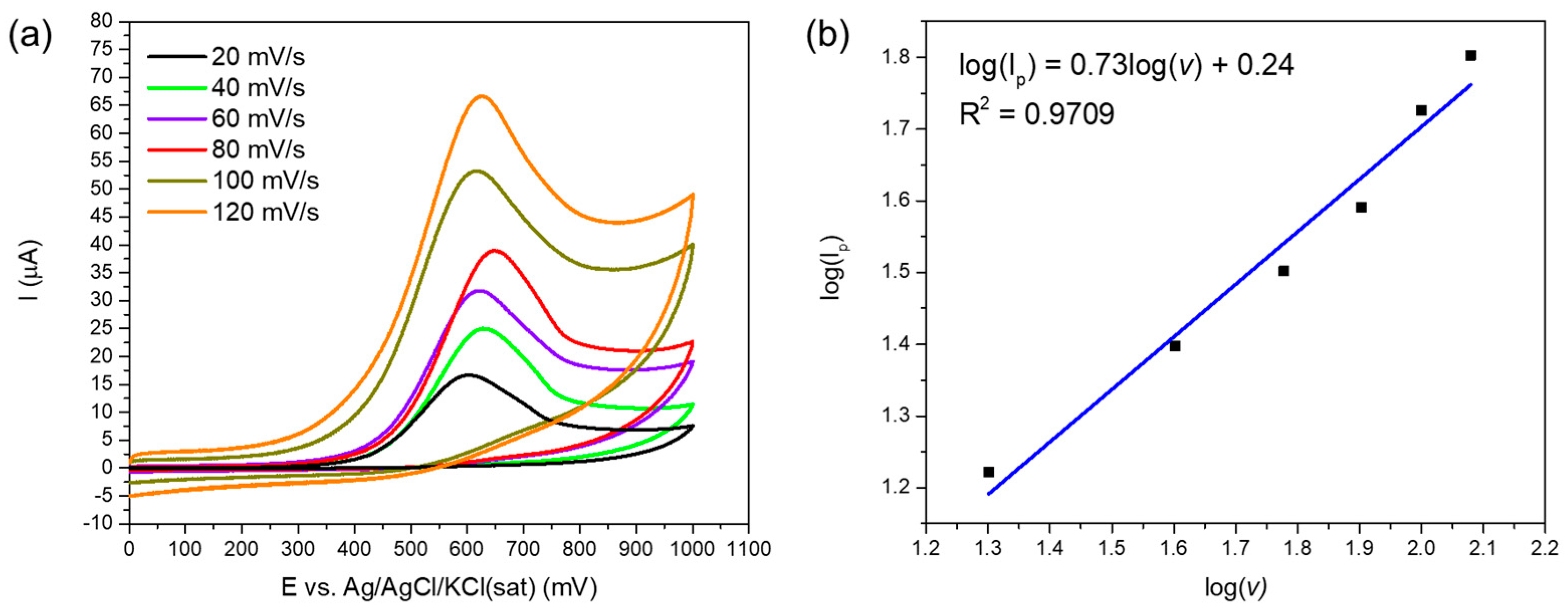
3.3. Electrochemical Study of the Electrodes
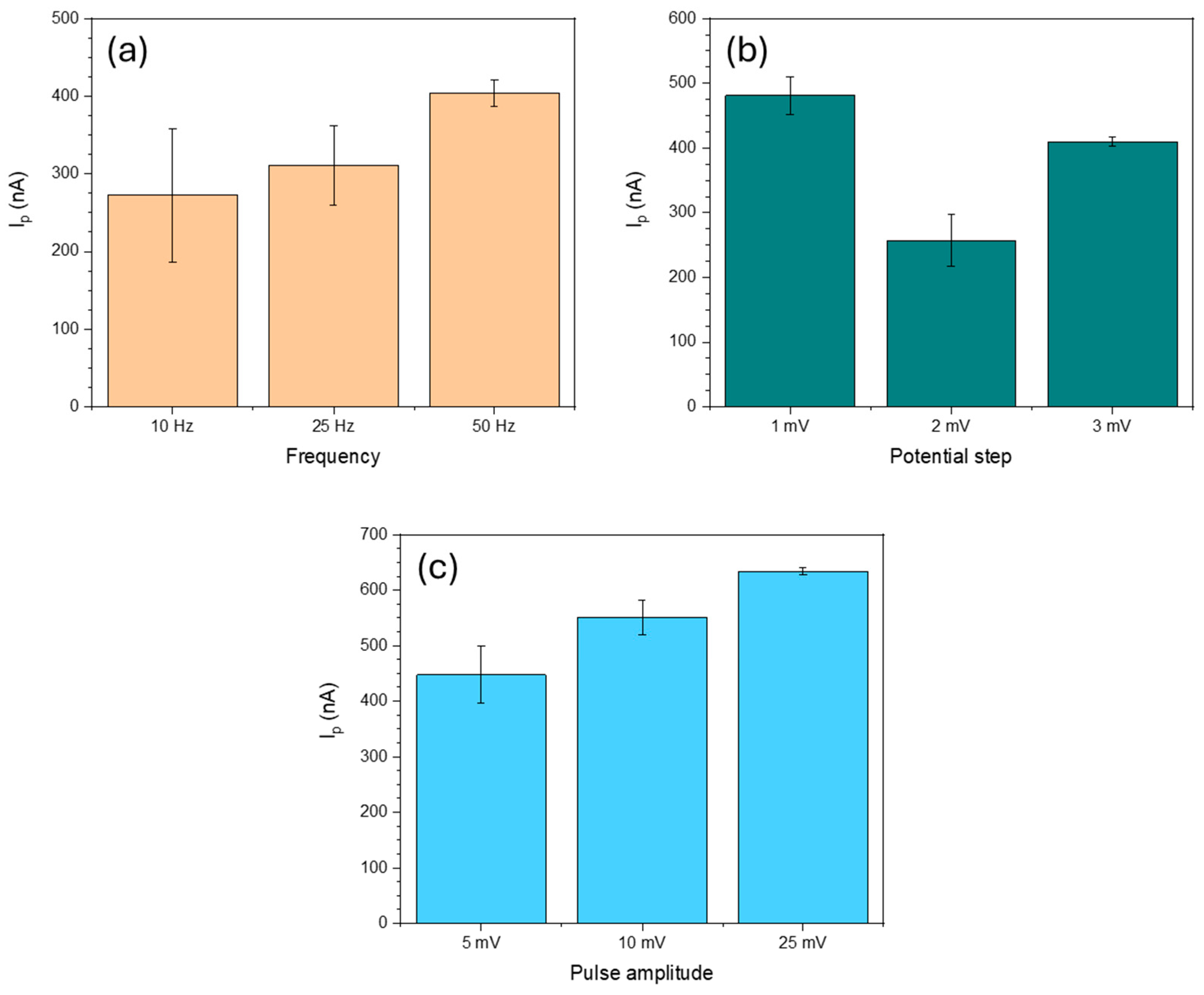
3.4. Analytical Performance of the Sensor
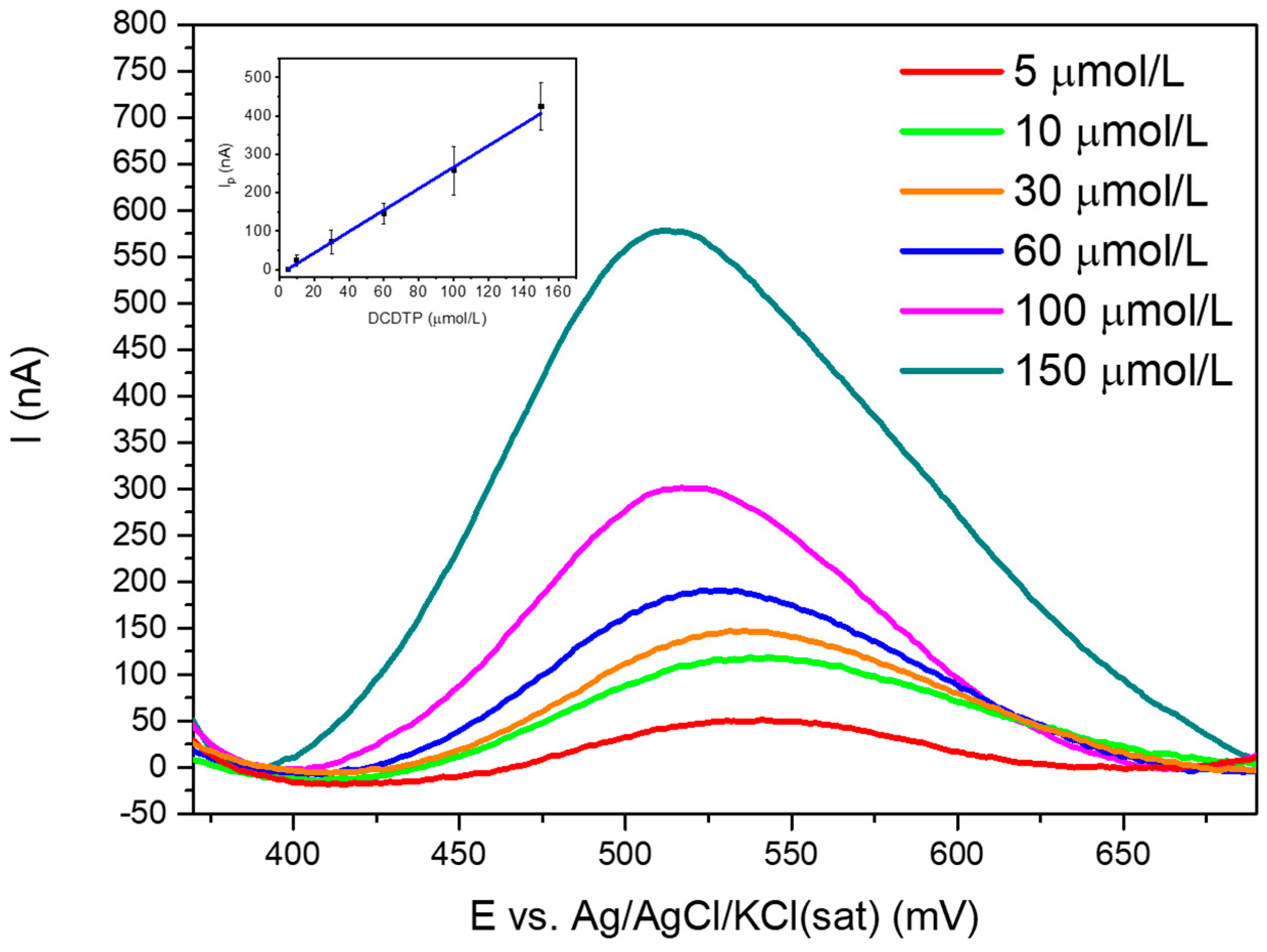
3.4.1. Linear Range, LOD and LOQ
3.4.2. Repeatability and Reproducibility
3.4.3. Trueness
3.4.4. Analytical Interferences
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pienaar, D.; Jordaan, T.; McFadzean, B.; O’Connor, C.T. The Synergistic Interaction between Dithiophosphate Collectors and Frothers at the Air-Water and Sulphide Mineral Interface. Miner. Eng. 2019, 138, 125–132. [Google Scholar] [CrossRef]
- Herrera Urbina, R. Recent Developments and Advances in Formulations and Applications of Chemical Reagents Used in Froth Flotation. Miner. Process. Extr. Metall. Rev. 2003, 24, 139–182. [Google Scholar] [CrossRef]
- Corin, K.C.; Bezuidenhout, J.C.; O’connor, C.T. The Role of Dithiophosphate as a Co-Collector in the Flotation of a Platinum Group Mineral Ore. Miner. Eng. 2012, 36, 100–104. [Google Scholar] [CrossRef]
- Ramos, J.C.; Curtius, A.J.; Borges, D.L.G. Diethyldithiophosphate (DDTP): A Review on Properties, General Applications, and Use in Analytical Spectrometry. Appl. Spectrosc. Rev. 2012, 47, 583–619. [Google Scholar] [CrossRef]
- Okibe, N.; Johnson, D.B. Toxicity of Flotation Reagents to Moderately Thermophilic Bioleaching Microorganisms. Biotechnol. Lett. 2002, 24, 2011–2016. [Google Scholar] [CrossRef]
- Jones, M.H.; Woodcock, J.T. Determination of Diethyl Dithiophosphate in Flotation Liquors by Solvent Extraction and Ultraviolet Spectrometry. Anal. Chem. 1986, 58, 1845–1848. [Google Scholar] [CrossRef]
- Muzinda, I.; Schreithofer, N. Water Quality Effects on Flotation: Impacts and Control of Residual Xanthates. Miner. Eng. 2018, 125, 34–41. [Google Scholar] [CrossRef]
- Leppinen, J.; Vahtila, S. Differential Pulse Polarographic Determination of Thiol Flotation Collectors and Sulphide in Waters. Talanta 1986, 33, 795–799. [Google Scholar] [CrossRef]
- Ivaska, A.; Leppinen, J. Determination of Trace Amounts of the Flotation Collectors Ethyl Xanthate and Diethyl Dithiophosphate in Aqueous Solutions by Cathodic Stripping Voltammetry. Talanta 1986, 33, 801–806. [Google Scholar] [CrossRef]
- Tang, Y.; Cheng, W. Metallic Nanoparticles as Advanced Electrocatalysts. Sci. Adv. Mater. 2012, 4, 784–797. [Google Scholar] [CrossRef]
- Sahoo, S.; Wickramathilaka, K.Y.; Njeri, E.; Silva, D.; Suib, S.L. A Review on Transition Metal Oxides in Catalysis. Front. Chem. 2024, 12, 1374878. [Google Scholar] [CrossRef]
- Chen, M.; Kitiphatpiboon, N.; Feng, C.; Abudula, A.; Ma, Y.; Guan, G. Recent Progress in Transition-Metal-Oxide-Based Electrocatalysts for the Oxygen Evolution Reaction in Natural Seawater Splitting: A Critical Review. eScience 2023, 3, 100111. [Google Scholar] [CrossRef]
- Manjunatha, L.S.; Kumara Swamy, B.E.; Manjunatha, K.G. Cadmium Oxide Nanoparticle Modified Carbon Paste Electrode Sensor for Sulfadiazine: A Voltammetric Study. Inorg. Chem. Commun. 2023, 150, 110534. [Google Scholar] [CrossRef]
- Abumelha, H.M.; Alorabi, A.Q.; Alessa, H.; Alamrani, N.A.; Alharbi, A.; Keshk, A.A.; El-Metwaly, N.M. Novel Iron Oxide Nanoparticle-Fortified Carbon Paste Electrode for the Sensitive Voltammetric Determination of Atomoxetine. ACS Omega 2023, 8, 19006–19015. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsabur, K.M.; Abd-Elsabour, M.; Assaf, F.H.; Hasan, I.M.A. Electrochemical Estimation of Cd and Cu Ions Simultaneously Using a Modified MgO/Fe2O3 Nanocomposite/Carbon Paste Electrode. Electrocatalysis 2023, 14, 875–890. [Google Scholar] [CrossRef]
- Moutcine, A.; Laghlimi, C.; Ifguis, O.; Smaini, M.A.; El Qouatli, S.E.; Hammi, M.; Chtaini, A. A Novel Carbon Paste Electrode Modified by NP-Al2O3 for the Electrochemical Simultaneous Detection of Pb (II) and Hg (II). Diam. Relat. Mater. 2020, 104, 107747. [Google Scholar] [CrossRef]
- Fatoni, A.; Widanarto, W.; Anggraeni, M.D.; Dwiasi, D.W. Glucose Biosensor Based on Activated Carbon–NiFe2O4 Nanoparticles Composite Modified Carbon Paste Electrode. Results Chem. 2022, 4, 100433. [Google Scholar] [CrossRef]
- Mijajlović, A.; Ognjanović, M.; Manojlović, D.; Vlahović, F.; Đurđić, S.; Stanković, V.; Stanković, D. Eu2O3@Cr2O3 Nanoparticles-Modified Carbon Paste Electrode for Efficient Electrochemical Sensing of Neurotransmitters Precursor L-DOPA. Biosensors 2023, 13, 201. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef]
- Seman, N.; Tarmizi, Z.I.; Ali, R.R.; Taib, S.H.M.; Salleh, M.S.N.; Zhe, J.C.; Mohamad Sukri, S.N.A. Preparation Method of Titanium Dioxide Nanoparticles and Its Application: An Update. In Proceedings of the 9th AUN/SEED-Net Regional Conference on Natural Disaster, Virtual, 1 November 2022; IOP Publishing: Bristol, UK, 2022; Volume 1091, p. 012064. [Google Scholar]
- Mironyuk, I.F.; Soltys, L.M.; Tatarchuk, T.R.; Savka, K.O. Methods of Titanium Dioxide Synthesis (Review). Phys. Chem. Solid State 2020, 21, 462–477. [Google Scholar] [CrossRef]
- Nyamukamba, P.; Okoh, O.; Mungondori, H.; Taziwa, R.; Zinya, S.; Nyamukamba, P.; Okoh, O.; Mungondori, H.; Taziwa, R.; Zinya, S. Synthetic Methods for Titanium Dioxide Nanoparticles: A Review. In Titanium Dioxide-Material for a Sustainable Environment; Yang, D., Ed.; IntechOpen: London, UK, 2018; pp. 152–175. ISBN 978-1-78923-327-8. [Google Scholar]
- Olcay, R.H.; Reyes, I.A.; Palacios, E.G.; García, L.; Ramírez, P.A.; Guzmán, L.; Flores, M.U. Synthesis and Characterization of TiO2 Nanoparticles by Green Chemistry, Using Aloe Vera. In Proceedings of the Characterization of Minerals, Metals, and Materials 2024; Springer: Cham, Switzerland, 2024; pp. 685–692. [Google Scholar]
- Alarif, W.M.; Shaban, Y.A.; Orif, M.I.; Ghandourah, M.A.; Turki, A.J.; Alorfi, H.S.; Tadros, H.R.Z. Green Synthesis of TiO2 Nanoparticles Using Natural Marine Extracts for Antifouling Activity. Mar. Drugs 2023, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Shameli, K.; Abd Hamid, S.B. Synthesis and Characterization of Anatase Titanium Dioxide Nanoparticles Using Egg White Solution via Sol-Gel Method. J. Chem. 2013, 1, 848205. [Google Scholar] [CrossRef]
- Acevedo Peña, P.; Pedraza Avella, J.A.; Pedraza Rosas, J.E. Utilización de Electrodos de Pasta de Carbono Para La Evaluación Fotoelectroquímica de Materiales Semiconductores: TiO2 En Soluciones Cianuradas. Sci. Et Tech. 2007, 4, 701–706. [Google Scholar]
- Zhu, P.; Zhao, Y. Cyclic Voltammetry Measurements of Electroactive Surface Area of Porous Nickel: Peak Current and Peak Charge Methods and Diffusion Layer Effect. Mater. Chem. Phys. 2019, 233, 60–67. [Google Scholar] [CrossRef]
- Pamuk, D.; Taşdemir, I.H.; Ece, A.; Canel, E.; Kiliç, E. Redox Pathways of Aliskiren Based on Experimental and Computational Approach and Its Voltammetric Determination. J. Braz. Chem. Soc. 2013, 24, 1276–1286. [Google Scholar] [CrossRef]
- Suliborska, K.; Baranowska, M.; Bartoszek, A.; Chrzanowski, W.; Namieśnik, J. Determination of Antioxidant Activity of Vitamin C by Voltammetric Methods. Proc. West Mark. Ed. Assoc. Conf. 2019, 11, 23. [Google Scholar] [CrossRef]
- Magnusson, B.; Örnemark, U. Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; Eurachem, European Union, 2014. [Google Scholar]
- Hamdan, S.A.; Ibrahim, I.M.; Ali, I.M. Comparison of Anatase and Rutile TiO2 Nanostructure for Gas Sensing Application. Dig. J. Nanomater. Biostruct. 2020, 15, 1001–1008. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why Is Anatase a Better Photocatalyst than Rutile?-Model Studies on Epitaxial TiO2 Films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [PubMed]
- Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Titanium Dioxide (Anatase and Rutile): Surface Chemistry, Liquid-Solid Interface Chemistry, and Scientific Synthesis of Supported Catalysts. Chem. Rev. 2014, 114, 9754–9823. [Google Scholar] [CrossRef]
- Woods, R. The Oxidation of Ethyl Xanthate on Platinum, Gold, Copper, and Galena Electrodes. Relation to the Mechanism of Mineral Flotation. J. Phys. Chem. 1971, 75, 354–362. [Google Scholar] [CrossRef]
- Groot, D.R. Electrochemical Investigation of the Reactions of Diethyl Dithiophosphate and Mercaptobenzo-Thiazole on Noble-Metal Electrodes. S. Afr. J. Chem. 1984, 37, 103–108. [Google Scholar]
- Rajaram, P.; Jeice, A.R.; Jayakumar, K. Review of Green Synthesized TiO2 Nanoparticles for Diverse Applications. Surf. Interfaces 2023, 39, 102912. [Google Scholar] [CrossRef]
- Dos Santos, T.A.D.; Barreto, L.N.; Ritta, A.G.S.L.; De Meneses, W.S.; Nunes, R.S.; Semaan, F.S. Cost-Effective Composite Electrode for the Fast Voltammetric Screening and Determination of Riboflavin (B2) and Pyridoxine (B6) in Pharmaceuticals. Rev. Virtual De Quim. 2013, 5, 548–562. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy—A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical Impedance Spectroscopy. Nat. Rev. Methods Primers 2021, 1, 41. [Google Scholar] [CrossRef]
- Bard, A.; Inzelt, G.; Scholz, F. Electrochemical Dictionary, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Bard, A.; Faulkner, L.; White, H. Electrochemical Methods: Fundamentals and Applications, 3rd ed.; John Wiley & Sons Ltd.: New York, NY, USA, 2022. [Google Scholar]
- Ikeuba, A.I.; Faithpraise, F.O.; Nwokolo, K.I.; Umo, F.E.; Echem, O.C.; Ibrahim, A.T.; Edet, H.O.; Ita, B.I.; Okafor, P.C.; Asogwa, F.C.; et al. A Combined Electrochemical and DFT Investigation of Ornidazole as a Benign Anti-Corrosion Agent for Carbon Steel Materials in Acidizing Environments. Results Mater. 2024, 21, 100542. [Google Scholar] [CrossRef]
- Dhar, P.; Thornhill, M.; Kota, H.R. Investigation of Copper Recovery from a New Copper Ore Deposit (Nussir) in Northern Norway: Dithiophosphates and Xanthate-Dithiophosphate Blend as Collectors. Minerals 2019, 9, 146. [Google Scholar] [CrossRef]
- Ignatkina, V.A.; Bocharov, V.A.; D’Yachkov, F.G. Collecting Properties of Diisobutyl Dithiophosphinate in Sulfide Minerals Flotation from Sulfide Ore. J. Min. Sci. 2013, 49, 795–802. [Google Scholar] [CrossRef]
- Zhang, T.; Qin, W. qing Floc Flotation of Jamesonite Fines in Aqueous Suspensions Induced by Ammonium Dibutyl Dithiophosphate. J. Cent. South Univ. 2015, 22, 1232–1240. [Google Scholar] [CrossRef]
- Latimer, G.W. (Ed.) AOAC International Guidelines for Standard Method Performance Requirements. In Official Methods of Analysis of AOAC International; Oxford University Press: Oxford, UK, 2023. [Google Scholar]


| Electrode | CPE | TiO2/CPE |
|---|---|---|
| Rs (Ω) | 0.009873 (SD = 1.25·10−6) | 0.1563 (SD = 0.049) |
| Qdl (S·secn) | 3.49·10−6 (SD = 6.12·10−8) | 2.18·10−8 (SD = 0.73) |
| n | 0.70 | 0.73 |
| Rct (Ω) | 4298 (SD = 8.53) | 530.1 (SD = 4.08) |
| ZW S·sec0.5 | 6.53·10−5 (SD = 1.42) | 3.62·10−4 (SD = 0.54) |
| C (F) | 5.77·10−7 | 1.79·10−7 |
| λ2 | 0.003 | 0.00031 |
| Experiment | Ip (nA) | DCDTP (mmol/L) |
|---|---|---|
| 1 | 276 | 0.095 |
| 2 | 310 | 0.107 |
| 3 | 281 | 0.097 |
| 4 | 304 | 0.105 |
| 5 | 278 | 0.096 |
| 6 | 279 | 0.096 |
| 7 | 306 | 0.105 |
| 8 | 279 | 0.096 |
| 9 | 331 | 0.114 |
| 10 | 296 | 0.102 |
| Mean | 0.101 | |
| SD | 0.006 | |
| RSD (%) | 6.08 | |
| DCDTP (mmol/L) | ||
|---|---|---|
| Experiment | UV | Voltammetry |
| 1 | 4.48 | 5.12 |
| 2 | 4.48 | 4.35 |
| 3 | 4.66 | 4.11 |
| 4 | 4.93 | 4.91 |
| 5 | 5.15 | 4.67 |
| Mean | 4.74 | 4.63 |
| SD | 0.29 | 0.41 |
| t-statistic = 0.50 p-value = 0.6440 | ||
| DCDTP (mmol/L) | ||
|---|---|---|
| Experiment | UV | Voltammetry |
| 1 | 2.99 | 2.51 |
| 2 | 3.15 | 1.61 |
| 3 | 2.82 | 1.46 |
| 4 | 2.79 | 3.19 |
| 5 | 2.80 | 2.47 |
| Mean | 2.91 | 2.25 |
| SD | 0.16 | 0.71 |
| t-statistic = 1.86 p-value = 0.1362 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilasó-Cadre, J.E.; Ramírez-Rodríguez, A.; Hidalgo, J.; Reyes-Domínguez, I.A.; Cruz, R.; Flores, M.U.; Rodríguez-Torres, I.; Briones-Gallardo, R.; Hidalgo, L.; Piña Leyte-Vidal, J.J. Voltammetric Sensor Based on Titania Nanoparticles Synthesized with Aloe vera Extract for the Quantification of Dithiophosphates in Industrial and Environmental Samples. Chemosensors 2024, 12, 195. https://doi.org/10.3390/chemosensors12090195
Vilasó-Cadre JE, Ramírez-Rodríguez A, Hidalgo J, Reyes-Domínguez IA, Cruz R, Flores MU, Rodríguez-Torres I, Briones-Gallardo R, Hidalgo L, Piña Leyte-Vidal JJ. Voltammetric Sensor Based on Titania Nanoparticles Synthesized with Aloe vera Extract for the Quantification of Dithiophosphates in Industrial and Environmental Samples. Chemosensors. 2024; 12(9):195. https://doi.org/10.3390/chemosensors12090195
Chicago/Turabian StyleVilasó-Cadre, Javier E., Alondra Ramírez-Rodríguez, Juan Hidalgo, Iván A. Reyes-Domínguez, Roel Cruz, Mizraim U. Flores, Israel Rodríguez-Torres, Roberto Briones-Gallardo, Luis Hidalgo, and Juan Jesús Piña Leyte-Vidal. 2024. "Voltammetric Sensor Based on Titania Nanoparticles Synthesized with Aloe vera Extract for the Quantification of Dithiophosphates in Industrial and Environmental Samples" Chemosensors 12, no. 9: 195. https://doi.org/10.3390/chemosensors12090195
APA StyleVilasó-Cadre, J. E., Ramírez-Rodríguez, A., Hidalgo, J., Reyes-Domínguez, I. A., Cruz, R., Flores, M. U., Rodríguez-Torres, I., Briones-Gallardo, R., Hidalgo, L., & Piña Leyte-Vidal, J. J. (2024). Voltammetric Sensor Based on Titania Nanoparticles Synthesized with Aloe vera Extract for the Quantification of Dithiophosphates in Industrial and Environmental Samples. Chemosensors, 12(9), 195. https://doi.org/10.3390/chemosensors12090195








