Abstract
This review is a comprehensive overview of recent advancements in underwater in situ heavy metal voltammetric analyzers (UIHVAs). It explores various types of in situ voltammetric analyzers, including the voltammetric in situ profiling system, submersible integrated multi-channel trace metal sensing probes, vibrating gold microwire electrode voltammetric analyzers, and electrochemical analyzers designed for on-site flow measurements. It also covers electrochemical sensors based on flexible liquid crystal polymers, deep-sea mercury sensors, and other in situ electrochemical analyzers. This review systematically examines the research and development progress of microelectrode arrays, screen-printed, carbon, bismuth, antimony, and lab-on-a-chip electrodes. The final section looks at key trends in the research and development of voltammetric analyzers, highlighting the exploration of novel working electrodes, the integration of smart monitoring and data analysis technologies, and the promotion of interdisciplinary collaboration and innovation. From a global perspective, in situ heavy metal voltammetric analysis technology has demonstrated significant applicability in various fields, such as environmental monitoring, marine science, and biogeochemistry. This technology holds considerable potential for further development. However, extensive research and continuous improvement are required to improve detection performance. We are convinced that with continued technological advances and dedicated research efforts, these challenges can be overcome and will pave the way for the widespread application of UIHVAs.
1. Introduction
The issue of heavy metal pollution in aquatic environments has become a significant global environmental concern [1,2,3]. Industrialization and urbanization have accelerated the discharge of heavy metal pollutants into water bodies through various pathways, including wastewater discharges, atmospheric deposition, and land-use change, significantly impacting water quality [4,5,6,7]. Heavy metals pose significant threats to ecosystems and human health due to their high toxicity, potential for bioaccumulation, and persistence in the environment [8,9,10,11,12,13,14].
Among the various techniques for detecting heavy metals, voltammetric analysis in electrochemistry has attracted significant attention due to its distinct advantages in analyzing heavy metal ions within complex matrices. This method enables high-precision in situ determination of heavy metals and allows for the simultaneous detection of multiple elements [15,16]. It offers rapid analysis, outstanding sensitivity and accuracy, good selectivity, minimal sample requirements, and a wide detection range. Additionally, its simplicity of operation and portability make it widely applicable in environmental monitoring and analysis [17].
Traditional heavy metal environmental monitoring primarily relies on centralized laboratory analysis, where samples are susceptible to various interferences during collection, preservation, transportation, and processing, potentially affecting the accuracy of the results [18,19]. To more accurately reflect in situ heavy metal concentrations, the development of in situ heavy metal sensors is crucial. Underwater in situ voltammetric analyzers have emerged to address this need, inheriting the advantages of voltammetric analysis while offering real-time, continuous, and highly sensitive underwater in situ analysis capabilities [20,21]. Compared to traditional laboratory methods, this technology provides faster and more accurate data on the heavy metal content in water bodies [22,23].
Underwater in situ voltammetric analyzers achieve rapid and accurate detection of heavy metal ions by directly measuring their voltammetric characteristics underwater, demonstrating broad application prospects [24]. In the field of water environment monitoring, they can be utilized for real-time monitoring and assessment of heavy metal pollution in rivers, lakes, oceans, and other aquatic environments, aiding in the timely detection of, and response to, pollution issues. Additionally, in biological and oceanographic research, this technology provides an important means of monitoring heavy metal content in organisms, with profound implications for biogeochemical cycling studies [25,26,27]. However, the development of underwater in situ voltammetric analyzers faces numerous challenges, including the selection and design of electrode materials, optimization of signal acquisition and processing systems, and improvement of data transmission and storage technologies [28]. Future advancements in this technology will focus on enhancing detection sensitivity, reducing costs, simplifying operation processes, and meeting the needs of environmental protection and scientific research [29]. Meanwhile, ex situ electrochemical heavy metal detection techniques remain in a stage of continuous evolution and development. It is particularly noteworthy that the ongoing innovations in environmentally friendly electrode designs, including bismuth electrodes, antimony electrodes, and lab-on-a-chip systems, have infused new vitality into the field of heavy metal detection. When it comes to performing large-scale rapid measurement tasks, ex situ measurement techniques currently possess irreplaceable advantages due to their efficiency and convenience. With the continuous updating and improvement of technology, ex situ measurement will continue to play a significant role in the field of electrochemical heavy metal detection.
Recent advancements in the voltammetric analysis of heavy metals have demonstrated a significant increase in research output. This surge is evident in the number of publications indexed in the Web of Science over the past decade (2014–2024), where 2013 scientific articles include the keyword “Trace Metal, Voltammetric”, and 276 of these also feature the keyword “in situ”. This underscores the growing importance and research intensity surrounding in situ voltammetric analysis techniques. These studies encompass both fundamental theoretical research on heavy metal voltammetry and the practical application of this method in underwater in situ environments. Particularly in the domain of in situ detection technology, there is a focused effort on developing robust instruments capable of reliably performing heavy metal analysis in underwater settings. The advancement of such technologies holds substantial significance for environmental monitoring and marine ecological protection.
This review outlines the principles of in situ heavy metal voltammetric analysis technology, its main research directions, and the advantages of in situ measurements. It provides detailed introductions and comparisons of various in situ voltammetric analyzers, followed by a systematic analysis of electrode development. Finally, the paper examines the main development trends of current voltammetric analyzers, summarizing the research status, achievements, and existing challenges in this field (Figure 1). To date, only the voltammetric in situ profiling system has achieved commercial application in this field. Our review aims to provide a valuable reference for researchers by summarizing the current state of technology, highlighting the critical challenges, and offering insights into future research directions.
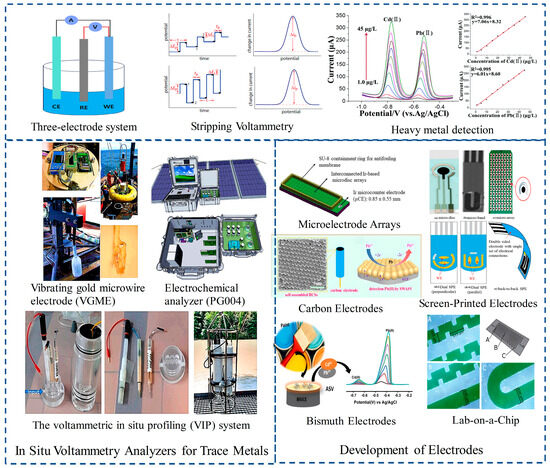
Figure 1.
Instruments that have been developed include VIP/VGME/PG004. Advancements in electrode technology are of paramount importance for enhancing detection accuracy and efficiency. The main types of electrodes currently include microelectrode arrays, screen-printed, carbon, bismuth, antimony, and lab-on-a-chip electrodes.
2. Research Directions and Advantages of Voltammetry
Voltammetry is a pivotal technique for real-time, on-site, and in situ monitoring of trace metals, and there is increasing recognition of its importance in heavy metal ion analysis [30,31,32,33,34]. Research in this field is guided by the “6S” principle: Sensitivity, Selectivity, Size, Speed, Stability, and Safety [35]. Sensitivity, crucial for sensor performance, is enhanced by stripping voltammetry, which pre-concentrates target ions on the electrode surface, achieving detection levels in the parts-per-billion (ppb) range [36,37]. Advances focus on electrode modifications to improve electrochemical properties. Selectivity, essential for distinguishing different metal ions, is improved by modifying electrode surfaces with sensitive materials and using machine learning to analyze voltammograms [38]. Size affects instrument portability, with technologies like microelectrodes, screen-printed electrodes, and lab-on-a-chip enabling miniaturization and real-time field monitoring [39]. Speed, both an advantage and challenge, is advanced with techniques like Fast Scan Cyclic Voltammetry, although improvements in sensitivity and selectivity are needed [40]. Stability, challenged by natural water compositions, is maintained using disposable sensors and renewable surface electrodes [41]. Safety concerns over mercury electrodes have led to the development of mercury-free materials such as carbon, bismuth, and antimony, which enhance sensitivity and selectivity while meeting environmental standards.
Compared to traditional analytical methods, underwater in situ heavy metal voltammetry analyzers offer the following significant technical advantages.
Real-time monitoring enables immediate detection of heavy metals on-site, avoiding delays associated with laboratory analysis. This capability allows researchers to track dynamic fluctuations of heavy metals in water bodies accurately, providing a detailed understanding of pollutant dispersion patterns [42].
Portability is a major highlight; with compact and lightweight designs, these analyzers facilitate easy setup and use in various aquatic environments. This enhances the applicability of the instrument in field studies, making it a powerful tool for environmental monitoring and scientific exploration [43].
Economic efficiency is achieved by reducing labor, material, and time costs, as sample transport and processing in the laboratory are eliminated. Additionally, this minimizes the risk of sample contamination during transit and storage [44].
3. In Situ Voltammetry Analyzer
3.1. From Voltage In Situ Profiling System to Submersible Integrated Multi-Channel Trace Metal Sensing Probe
In the 1990s, the Tercier-Waeber research group pioneered the development of on-chip micro-electrochemical sensors (GIMEs) [45], marking a significant advancement in electrochemical sensor technology. In 2005, these sensors, comprising an array of iridium-based microdisks coated with mercury film, were termed mercury film micro-electrochemical sensors (Hg-GIMEs) [46]. They enabled efficient in situ dynamic analysis of Cu(II), Pb(II), Cd(II), and Zn(II) at sub-nanomolar levels (2009, 2011, 2015). Subsequently, these Hg-GIMEs were integrated into the voltammetric in situ profiling (VIP) system, which demonstrated high-resolution in situ monitoring across various aquatic environments [47,48,49].
Recent advancements include the development of a new generation of GIME electrodes that further enhance sensing performance (2021). These electrodes employ more interconnected iridium-based microdisk arrays and utilize mercury or gold nanoparticles/filaments as the electrochemical coating, enhancing both sensitivity and detection range. Recent studies indicate that these new electrodes can accurately quantify inorganic arsenic (III) and mercury (II) in environmental samples with minimal pretreatment [50].
Creffield et al. (2023) effectively employed VIP systems with GIME electrodes to conduct in situ high-resolution quantification of the bioavailable nickel fraction in natural waters (Figure 2) [51].
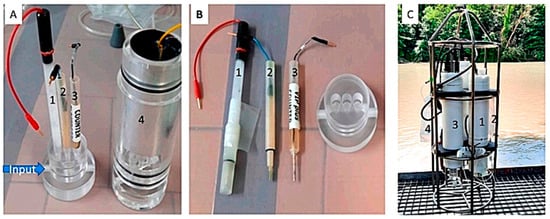
Figure 2.
Picture of the (A) in-house flowthrough plexiglass cell with the mini-electrodes (1, 2, and 3) and the shielded Plexiglas holder (4) enabling incorporation of the flowthrough cell into the bottom of the VIP electronic housing; (B) mini-reference electrode (1), the working microelectrode (2), and the mini-counter electrode (3); and (C) the VIP system made up of the peristaltic pump (1), the chirurgical bag containing the nioxime and buffer solutions (2), the voltammetric probe with the in-house flowthrough plexiglass cell at the bottom (3), and the chirurgical bag to collect the waste (4) [51].
The latest development from the Tercier-Waeber team, TracMetal (2021), is an advanced multichannel in situ electrochemical sensor. This compact, low-power sensor integrates the newly designed Hg-GIME and AuNF-GIME into a three-channel flow cell connected to a multichannel peristaltic pump, allowing for the automatic, real-time, simultaneous monitoring of multiple harmful metals in situ [52].
TracMetal has been successfully deployed for high-resolution monitoring in Arcachon Bay, an ecologically and economically significant area on the southwestern Atlantic coast of Europe. Comprehensive water sample analysis provides fundamental data on the dynamic concentrations and temporal variations of the bioavailable fractions of specific harmful metals, offering solid technical support for environmental impact studies (2021) [53,54]. However, the instrument’s underwater in situ continuous operation duration has not exceeded 10 days, indicating a need for improved stability and reliability for long-term operation.
3.2. Vibrating Gold Microwire Electrode Voltammetric Analyzer
Gibbon-Walsh et al. (2011) introduced an electrochemical method for determining manganese and zinc concentrations in coastal waters using a vibrating gold microwire electrode (VGME) (Figure 3) [55]. Chapman et al. (2012) further explored the use of VGME by designing an apparatus for in situ copper monitoring in coastal waters [56]. Additionally, Domingos et al. (2016) applied VGME in the AGNES technique to quantify free copper concentrations directly [57]. This method facilitates metal detection in seawater without the need for reagents, making it ideal for in situ monitoring applications. The device integrates a VGME, an energy supply module, a potentiostat, and an advanced data acquisition system, enabling effective detection in areas up to 40 m deep.
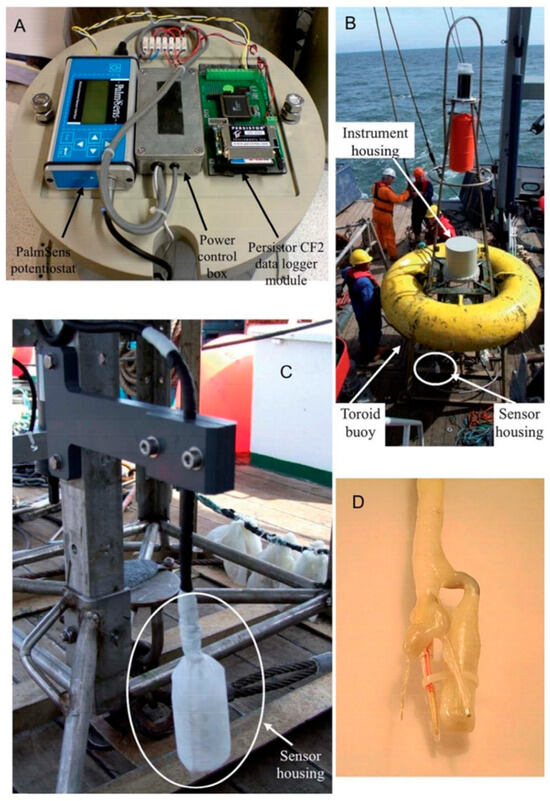
Figure 3.
Photographs of the instrumentation. (A) The electronics of the instrument. (B) Instrument and sensor on the buoy just before deployment. (C) The sensor’s protective housing. (D) The electrodes and the vibrator [56]. Copyright 2012 Elsevier.
For the quantitative analysis of copper, the system employs square-wave anodic stripping voltammetry to ensure high-precision measurements. To monitor dissolved oxygen (DO) levels in real time, the system utilizes negative potential linear sweep voltammetry. The system maintains electrode activity and measurement accuracy by reactivating the working electrode with a specific potential sequence during measurement intervals.
The VGME measurement system’s introduction into environmental monitoring, specifically for copper detection, is notable. This system efficiently detects low copper concentrations without requiring pumping equipment or chemical reagents. The introduction of a new potential sequence significantly enhances the stability and reliability of long-term measurements. With a continuous measurement cycle of up to six weeks and automatic data collection every 12 h, the device provides consistent and efficient data support for environmental monitoring.
To verify the system’s stability and suitability for long-term copper monitoring, a several-week autonomous buoy deployment test was conducted in the Irish Sea, yielding preliminary data. Although the buoy’s vertical movement introduced approximately 15% measurement error, normalizing the copper response to the DO response effectively reduced the long-term variability of the electrode [57]. The system successfully detected active copper concentrations ranging from 1.5 to 4 nM, with a total copper concentration of approximately 10 nM, aligning closely with laboratory voltammetry measurements [57].
3.3. An Electrochemical Analyzer for On-Site Flow Measurement
Bezerra Dos Santos et al. (2014, 2015) developed an electrochemical analyzer utilizing a potentiostat/galvanostat (PG004) as its core technology, integrated with online data transmission and global positioning systems (GPS). This integration allows for accurate geolocation of monitoring sites (Figure 4). The analyzer employs square-wave anodic stripping voltammetry (SWASV), supplemented by a flow batch analysis (FBA) system and a thermally stabilized electrochemical flow cell (EFC). Additionally, the use of a boron-doped diamond electrode (BDD) enables high-precision on-site quantitative analysis of Pb2+ and Cd2+ ions in water samples [58,59].
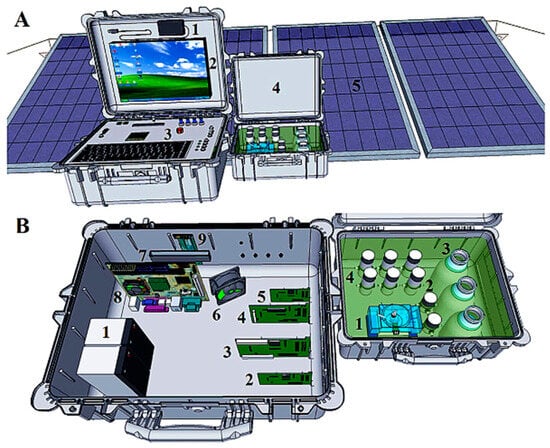
Figure 4.
The PG004. (A) 1: GPS, 2: touchscreen, 3: panel with an optional keyboard, keys, and command buttons, 4: flow module, and 5: solar boards. The internal view of the PG004. (B) 1: 12 V batteries, 2: actuator of the flow system, 3: galvanostat board, 4: potentiostat board, 5: thermostatted control, 6: fan, 7: USB hub, 8: CPU, and 9: microcontrolled board to control the batteries. The Wi-Fi and Bluetooth board is inserted in the CPU. Flow module, with 1: EFC, 2: SVs, 3: solutions compartment, and 4: mPs [58]. Copyright 2015 Elsevier.
The team systematically explored the influence of temperature on the field analysis results of Pb2+ and Cd2+ ions and conducted a comprehensive performance evaluation of the PG004 analyzer. The experimental results show that the analyzer’s detection limits for Pb2+ and Cd2+ are as low as 0.08 and 0.18 mg L−1, respectively, indicating high sensitivity and the capability to analyze both ions simultaneously.
In field applications for environmental monitoring, the PG004 analyzer has been successfully deployed for on-site analysis of lake water samples, producing high-resolution voltammetric spectra with minimal noise interference. The recovery rate stabilizes within the range of 93.3% to 109%, with the waste generated from a single measurement at merely 700 mL, significantly reducing the environmental impact.
To further verify the analyzer’s accuracy, a t-test (n = 3) was conducted by measuring the Cd2+ and Pb2+ contents in standard certified water (NIST). The results showed that at a 95% confidence level, the measured data were highly consistent with the standard values, validating the analyzer’s accuracy. The instrument, equipped with a GPS receiver and solar panels for sustainable energy, facilitates fast, online, and environmentally friendly monitoring of Pb2+ and Cd2+.
3.4. Electrochemical Sensors Based on Flexible Liquid Crystal Polymers
Wang et al. developed an electrochemical sensor based on a flexible liquid crystal polymer (LCP). The core component is a bismuth (Bi) thin film electrode on an LCP substrate, designed for direct in situ measurement of zinc (II) ions. The choice of LCP as the substrate material enhances the sensor’s operational stability, durability, and flexibility, making it adaptable to various installation environments. Through square-wave anodic stripping voltammetry experiments, the sensor achieved a detection limit of 1.22 nM for Zn(II) within a deposition time of 180 s [60,61].
This sensor exhibits several technical advantages, including high analytical sensitivity (1.55 nA·nM−1·mm−2), a wide linear detection range (4.59 to 1071 nM), and low relative standard deviations for repeated measurements. Additionally, the sensor’s efficacy in real-time in situ detection applications has been validated by monitoring Zn(II) concentrations in seawater.
To explore diverse applications, the research team integrated a flexible array comprising four LCP-based sensors into the hull of an autonomous kayak, enabling remote operation and control. This sensor array successfully captured significant fluctuations in zinc (II) concentrations in seawater, corroborated by inductively coupled plasma mass spectrometry (ICP-MS) analysis. This study supports the potential application of flexible LCP electrochemical sensors in on-site environmental monitoring.
3.5. Deep-Sea Mercury Sensor
Yamamoto et al. developed a deep-sea mercury sensor based on anodic stripping voltammetry [62]. This sensor utilizes a large gold annular disk electrode with a surface area of 402 mm2, significantly enhancing its sensitivity to mercury in seawater. To improve electrodeposition efficiency, a propeller screw is installed in front of the working electrode, generating stable water flow and enhancing mercury electrodeposition efficiency.
This sensor accurately captures the peak current signal corresponding to the mercury concentration in water samples. In a 0.6 M NaCl solution, following a 20 min deposition process, the sensor achieves a minimum detection limit of 0.94 ng L−1 (ppt), surpassing previous detection limits. The sensor has been effectively deployed for in situ measurements of ppt-level mercury concentrations in the marine environment, corroborated by comparative analysis with cold vapor atomic fluorescence spectrometry.
However, precise calibration remains challenging under strict laboratory conditions. Future research should focus on developing novel calibration techniques to reduce reliance on large volumes of standard solutions. Additionally, in environments with high hydrogen sulfide content (e.g., near hydrothermal vents), the sensor may experience interference, necessitating performance improvements. Nonetheless, this sensor presents significant potential for monitoring mercury pollution in seawater on a broad scale.
3.6. In Situ Electrochemical Analyzer
Luther III et al. developed an in situ electrochemical analyzer (ISEA) utilizing solid microelectrode technology [63]. This analyzer enables simultaneous monitoring of various redox species and trace metals across diverse environments, including sediments, microbial mats, cultures, and hydrothermal vent-rich water columns. The ISEA can perform continuous environmental monitoring in both crewed and uncrewed modes, making it suitable for probing intricate heterogeneous environments like salt marsh sediment root zones. Data collected by the system demonstrate minimal overlap between the distribution of O2 and Mn2+ in marine sediment pore water and microbial biofilms on metal surfaces, indicating that O₂ is not a direct oxidant of Mn2+. During the analysis of hydrothermal vent water samples, the ISEA detected Fe2+, H₂S, and soluble FeS clusters (FeSaq), providing evidence for the roles of H₂S and FeSaq in pyrite formation. Utilizing fixed-position electrodes, a three-day continuous data collection in the Riftia pachyptila habitat revealed no significant correlation between O₂ and H₂S but a general correlation between H₂S and temperature.
In summary (Table 1), the current in situ heavy metal detection instruments based on electrochemical stripping voltammetry have a solid research foundation and have been deployed in various waters, from offshore to deep-sea environments. These instruments are calibrated using the standard addition method either in the laboratory or on-site, and the chemical cleaning method is employed for instrument maintenance. The reuse of the instrument mainly depends on the renewal of the electrodes; after electrode renewal, these instruments can be reused. However, these instruments still exhibit certain limitations. VIP systems lead in terms of technological maturity, with commercial products already available, yet their technology requires further improvement and optimization to cater to broader applications. For instance, while the VIP and its upgraded version TracMetal can detect elements such as Cu(II), Pb(II), Cd(II), Zn(II), and As(III), their operational depth is limited to 100 m, restricting deeper water applications. The advantage of the VGME lies in its short enrichment time of only 5 min, suitable for detecting Cu(II) with low concentration requirements. However, its operational depth is limited to 40 m, thus restricting its application range. The PG004 can rapidly detect Pb(II) and Cd(II) in surface waters, but its detection limits of 0.39 and 1.6 mM do not meet the requirements for trace metal detection. Both the LCP and the deep-sea mercury sensor currently detect only one element, limiting their functionality. The ISEA can operate at depths of 6000 m in deep-sea environments; however, its detection limits for Mn(II) and Fe(II) are as high as 5 and 10 μM, suitable primarily for hydrothermal/cold spring areas and challenging for detecting trace metals in conventional seawater environments.

Table 1.
Summary of in situ voltammetry analyzers.
4. Development of Electrodes
4.1. Microelectrode Arrays
Microelectrode array technology has become a central focus in electroanalytical chemistry, particularly for detecting trace metals. This technology enhances mass transfer on the electrode surface by leveraging the hemispherical diffusion characteristics of microelectrodes, mimicking a convective environment. This allows for the rapid acquisition of stable non-zero current readings, reducing the need for stirring and making it highly suitable for in situ sensing applications. Additionally, the low iR drop and high signal-to-noise ratio inherent to microelectrode arrays ensure accurate measurements in high-resistance environments, such as low ionic strength media, without the need for additional supporting electrolytes or complex sample pretreatment steps [46,47,48].
The Tercier-Waeber team has been instrumental in advancing microelectrode array (MEA) technology for the detection of heavy metals in natural waters [64]. Over the years, their innovative approaches and applications have significantly contributed to the field of environmental monitoring. Noël et al. (2003) laid the groundwork by integrating complexing gels with MEAs to directly detect free metal ion concentrations in natural waters. This early work highlighted the potential of MEAs in providing accurate and real-time data on metal ion dynamics, establishing a foundation for future innovations [65]. In 2009, Tercier-Waeber, Hezard, and Masson advanced the application of MEAs by monitoring the diurnal evolution of dynamic metal species in the Riou-Mort River and bioavailable inorganic mercury in marine systems. This study demonstrated the capability of MEAs to capture temporal variations in metal concentrations, essential for understanding environmental processes and impacts [47]. Tercier-Waeber et al. (2021) made notable progress in environmental monitoring through their development of a gel-integrated nanostructured gold-based interconnected microelectrode array (MEA) designed for continuous in situ arsenite monitoring in aquatic environments (Figure 5) [50]. This advanced sensor allowed for high-resolution and real-time tracking of arsenite levels, significantly improving the accuracy of measurements in dynamic aquatic systems. Additionally, they applied similar gel-integrated nanostructured gold-based MEAs for the detection of bioavailable inorganic mercury in marine environments. The sensors demonstrated high sensitivity and specificity, crucial for evaluating mercury bioavailability and understanding its ecological risks. These innovations represent a significant leap forward in the monitoring and assessment of hazardous substances in water systems [50,54]. Most recently, Creffield et al. (2023) focused on addressing the challenge of sensor fouling. They designed an on-chip antifouling gel-integrated MEA for high-resolution quantification of the nickel fraction available for bio-uptake in natural waters. This advancement ensures the reliability and accuracy of MEAs in complex and biofouling-prone environments [51].
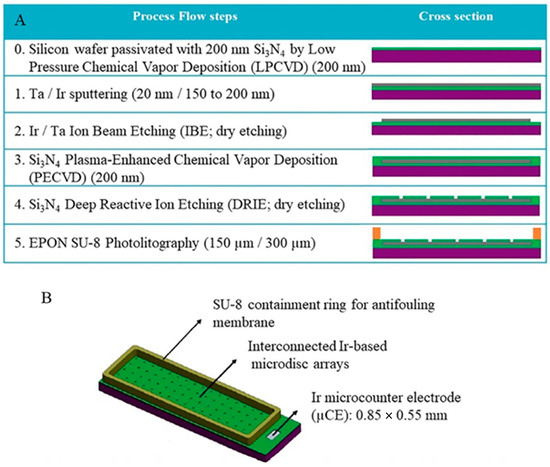
Figure 5.
(A) Thin film process flow steps for the manufacturing of the on-chip interconnected Ir-based microdisk arrays; (B) scheme of a chip incorporating an interconnected Ir microdisk array and an Ir microcounter electrode [50]. Copyright 2021 Elsevier.
Microelectrode arrays (MEAs) are pivotal in heavy metal detection due to their high sensitivity and spatial resolution (Table 2), enabling trace-level detection and localized monitoring in complex environmental matrices. They facilitate real-time, continuous monitoring, crucial for understanding temporal variations and responding promptly to environmental changes. MEA miniaturization allows integration into compact, portable devices, enhancing accessibility and applicability in diverse environmental settings. Advances in electrode materials enable selective detection amidst complex matrices, reducing interference and improving accuracy [64,65]. Challenges include fouling from environmental species, complex and costly fabrication, and the need for improved durability in harsh conditions. Future improvements must focus on robust antifouling strategies, novel materials, nanotechnology applications, integration with data analytics for real-time processing, standardized protocols for reliability, and expanding environmental applications beyond laboratories. Addressing these challenges will enhance the effectiveness of MEAs in environmental monitoring, emphasizing continued research and innovation in sensor technology and data integration.

Table 2.
Typical microelectrode arrays.
4.2. Screen-Printed Electrodes
Screen-printed electrodes (SPEs) have garnered significant attention in electrochemical stripping analysis, particularly for detecting trace heavy metals. This technology is pivotal in environmental monitoring, providing detailed insights into heavy metal ion distribution and enabling in situ analysis [66,67,68,69,70,71,72].
García-González et al. (2014) developed dual screen-printed electrodes featuring elliptic working electrodes arranged either parallel or perpendicular to the strip, demonstrating improved electrochemical performance for various analytes. This innovative design highlights the potential of SPEs in achieving precise and reliable measurements in complex matrices [73]. In a pioneering study, de Souza et al. (2015) introduced back-to-back screen-printed electroanalytical sensors specifically designed for heavy metal ion sensing. These sensors exhibited remarkable performance in the detection of trace metal ions, underscoring the robustness and versatility of SPEs in environmental and industrial applications [74]. Further exploring the potential of SPEs, Jadav et al. (2018) developed a silver/carbon screen-printed electrode for the rapid determination of vitamin C in fruit juices. Although not directly related to heavy metal detection, this study demonstrated the adaptability and broad applicability of SPEs in diverse analytical contexts, which can be translated to heavy metal analysis [75]. Ong et al. (2021) provided a critical review highlighting the electro-reactivity of screen-printed nanocomposite electrodes. Their study emphasized the role of SPEs in safeguarding the environment from trace metals, underscoring the electrode’s capability to detect and quantify metals at low concentrations (Figure 6) [76]. Recent innovations have further enhanced SPE performance. Birara et al. (2023) explored the use of bismuth/poly(bromocresol purple)-modified screen-printed carbon electrodes for quantifying Cd(II) and Pb(II) in wastewater. Their findings underscored the electrode’s applicability in complex matrices, demonstrating robust analytical performance [77]. Furthermore, Pasakon et al. (2023) investigated screen-printed ionic liquid/graphene electrodes for simultaneous electrochemical sensing of Cd2+ and Pb2+. This study highlighted the electrodes’ versatility in different environmental conditions, offering insights into optimizing sensor design for enhanced detection capabilities [78]. Zhang et al. (2024) developed a screen-printed electrode incorporating a bismuth/graphene oxide hybrid, enabling simultaneous detection of cadmium and lead ions. This modification enhances the sensitivity and selectivity, which are crucial for accurate environmental monitoring [79].
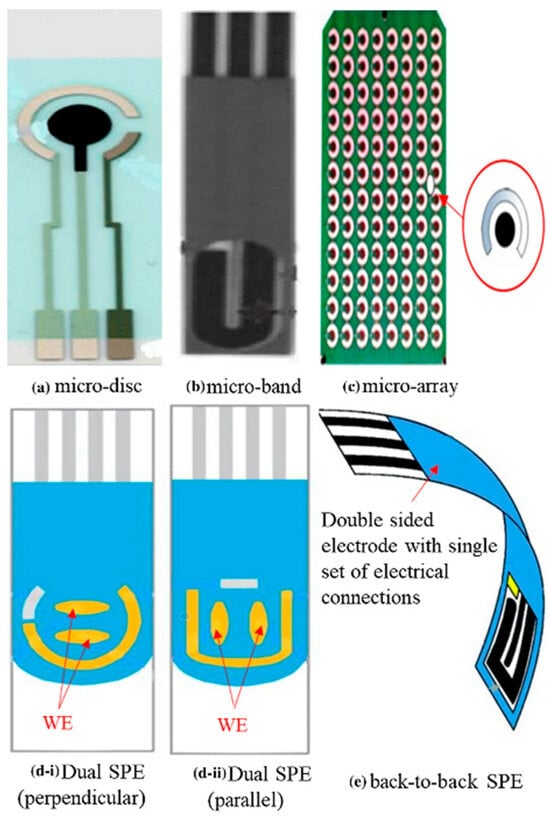
Figure 6.
Common geometries in SPE: (a) micro-disc [75], (b) micro-band [80], (c) micro-array [81], (d) dual SPE in perpendicular [73] (d-i) and parallel (d-ii) and (e) double-sided SPE [74]. Copyright 2021 Elsevier.
However, SPEs face challenges such as batch-to-batch reproducibility (Table 3), limited stability with prolonged use, and sensitivity to environmental conditions, necessitating ongoing refinement efforts. Future research, as suggested by Pasakon et al. (2023), aims to improve SPEs through innovations like ionic liquids and graphene integration to enhance stability and sensitivity in detection. Advancements in nanomaterials and surface modifications offer promising avenues for enhancing SPE performance, including increased sensitivity, selectivity, and operational lifespan [82].

Table 3.
Typical screen-printed electrodes.
4.3. Carbon Electrodes
Modified carbon electrodes, particularly glassy carbon electrodes, and carbon paste electrodes, are pivotal in metal stripping voltammetry for the precise determination of low-concentration metal ions (Figure 2). The choice of electrode material is crucial for measurement accuracy. Carbon electrodes have emerged as versatile tools in electrochemical sensing due to their excellent conductivity, surface area, and compatibility with various modifications aimed at enhancing sensitivity and selectivity [85,86,87,88].
Zhang et al. (2016) investigated size-dependent electrochemical detection of trace heavy metal ions using nano-patterned carbon sphere electrodes. Their study demonstrated that the size and surface properties of carbon spheres significantly influence the sensitivity and detection limits of heavy metals, emphasizing the importance of electrode nanostructuring in enhancing analytical performance (Figure 7) [89]. Xhanari et al. (2018) presented the validation and optimization of an in situ copper-modified glassy carbon electrode. This work highlighted the enhanced electrochemical performance for heavy metal detection achieved through modification with copper, which facilitated better electron transfer kinetics and improved detection limits [90]. Tüzün and Atun (2023) investigated the use of carbon paste electrodes modified with titania nanoparticles for individual and simultaneous determination of heavy metal ions. Their study emphasized the role of nanostructured materials in improving electrode performance, offering insights into the optimization of detection methods for environmental monitoring [91]. Finšgar and Rajh (2023) employed a factorial design and simplex optimization approach to develop a bismuth film glassy carbon electrode for Cd2+ and Pb 2+ determination. Their work underscored the importance of electrode surface modification and optimization strategies in achieving reliable and accurate heavy metal sensing [92]. Roushani et al. (2024) explored an aminoclay-based porous covalent organic polymer/multi-walled carbon nanotube-modified glassy carbon electrode for the detection of Pb2+, Cu2+, and Hg2+. Their research highlighted the use of novel polymer–nanotube composites to enhance the electrode’s sensitivity and stability, which are crucial for multi-metal ion detection [93].
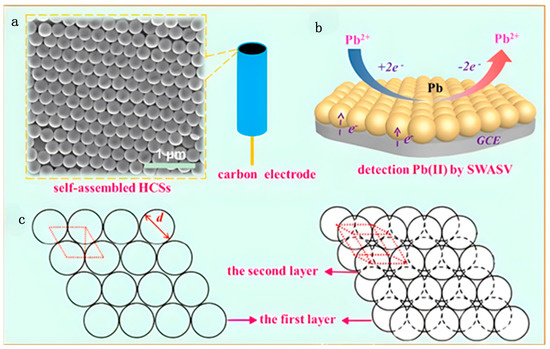
Figure 7.
(a) SEM image of the self-assembled carbon sphere electrodes. (b) The scheme of the first layer (left) and ordered structures (right) of the hexagonal close-packed structures model. (c) The process of the electrochemical detection of trace Pb(II) in an aqueous solution using SWASV [89]. Copyright 2016 Elsevier.
Carbon electrodes are widely used in heavy metal detection due to their excellent conductivity and chemical stability, ensuring reliable electrochemical responses (Table 4). Their high surface area allows effective modification with nanomaterials and polymers, enhancing selectivity and sensitivity for specific analytical needs. Additionally, carbon materials are cost-effective and easy to prepare, facilitating scalable deployment in environmental monitoring and industrial applications. However, challenges such as sensitivity limitations for trace heavy metal ions require advanced modification strategies to improve detection limits. Issues like electrode lifespan and stability under varying chemical conditions also impact long-term reliability. Complex sample matrices in environmental samples pose challenges to accurate real-time monitoring using carbon-based sensors. Future research should focus on enhancing sensitivity through optimized surface modifications with graphene, metal nanoparticles, or conducting polymers to improve signal-to-noise ratios and lower detection limits. Improving electrode stability and lifespan can be achieved by exploring new carbon composites or protective coatings resistant to harsh conditions. Integrating advanced signal processing techniques and selective recognition elements can mitigate matrix effects and enhance specificity in heavy metal detection. These advancements are crucial for expanding the application of carbon electrodes in environmental monitoring and industrial quality control, reinforcing their pivotal role in electrochemical sensing [94,95,96,97,98].

Table 4.
Typical carbon electrodes.
4.4. Bismuth Electrodes
Bismuth electrodes are increasingly utilized in electrochemical sensing due to their unique attributes, such as low toxicity, high hydrogen evolution overpotential, and wide potential window [99].
Promsuwan et al. (2024) developed a novel single-drop electrodeposition method to fabricate nanoneedle-like bismuth on disposable graphene electrodes. Their work demonstrated efficient on-site electrochemical detection of cadmium and lead, leveraging the enhanced electrocatalytic properties of bismuth nanoneedles for sensitive and selective detection [100]. Muluneh et al. (2023) investigated the use of a bismuth-modified glassy carbon electrode for the determination of lead (II) and cadmium (II) in water-based paints. This highlighted the applicability of anodic stripping voltammetry for accurate quantification of heavy metal ions, showcasing the electrode’s effectiveness in complex sample matrices (Figure 8) [101]. In a different application, Liu et al. (2024) developed integrated equipment utilizing smartphone control and machine-learning algorithms for the automated detection of bioavailable heavy metals in soils. Although not directly focused on bismuth electrodes, their innovative approach underscores the evolving technologies in environmental monitoring and heavy metal detection [102]. Martynov et al. (2024) explored the determination of indium using adsorptive stripping voltammetry at a bismuth film electrode with a combined electrode system facilitating medium exchange. Published in Talanta, their study highlighted advancements in electrode design and methodology to achieve sensitive and reliable analysis of heavy metals [103].
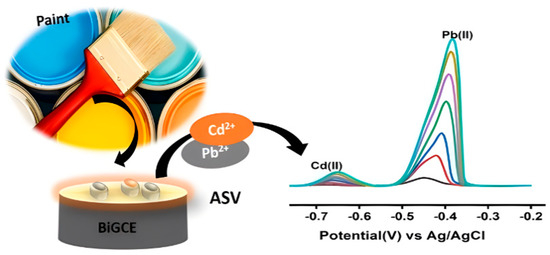
Figure 8.
Simultaneous determination of lead (II) and cadmium (II) in water paint using a bismuth-modified gassy carbon electrode with anodic stripping voltammetry [101]. Copyright 2014 Elsevier. Copyright 2023 Elsevier.
The bismuth electrode represents an ideal alternative to traditional mercury electrodes due to its outstanding electrocatalytic performance, particularly in the realm of heavy metal detection (Table 5). It enables the sensitive and selective detection of ions like cadmium and lead, facilitated by nanostructured forms such as nanoneedles that enhance surface area and electron transfer efficiency. Moreover, bismuth electrodes demonstrate robust chemical stability across varying conditions, ensuring reliable performance in prolonged analytical use. However, challenges include susceptibility to fouling and surface passivation in complex sample matrices, compromising long-term stability and sensor effectiveness. Achieving consistent reproducibility in electrode fabrication remains critical for reliable analytical results. While sensitivity to trace levels of heavy metals has improved, further enhancements are needed for ultra-trace detection applications. Future research should focus on innovative approaches to overcome these challenges. These include refining the bismuth electrode design through advanced nanostructuring techniques like controlled deposition of bismuth nanomaterials to enhance surface morphology and electrochemical activity. The integration of novel materials or hybrid structures can mitigate fouling effects and enhance stability in challenging environmental conditions. Optimizing surface modification strategies with functional nanomaterials or conducting polymers holds promise for enhancing sensitivity and selectivity, enabling real-time and precise monitoring of heavy metals in diverse environmental samples [104,105,106,107,108,109].

Table 5.
Typical bismuth electrodes.
4.5. Lab-on-a-Chip (LOC)
Lab-on-a-chip (LOC) technology has emerged as a revolutionary approach in chemical and biological analysis, providing miniaturized, automated, and highly efficient platforms for diverse applications. This review synthesizes recent advancements and seminal contributions in LOC technology, drawing insights from key references [110,111,112,113,114,115,116,117].
Jung et al. (2011) developed a polymer-based LOC sensor with microfabricated planar silver electrodes for continuous and on-site heavy metal measurement. Their research focused on achieving real-time monitoring capabilities, emphasizing the scalability and cost-effectiveness of polymer-based microfluidic platforms [118]. Zhao et al. (2014) introduced a portable LOC system for gold-nanoparticle-based colorimetric detection of metal ions in water. Their work demonstrated the integration of nanoparticle-based assays for rapid and sensitive detection, suitable for field deployment and environmental monitoring [119]. Chałupniak and Merkoçi (2017) presented a graphene oxide-poly(dimethylsiloxane)-based LOC platform for heavy metal preconcentration and electrochemical detection. Their study highlighted the advantages of graphene oxide in enhancing preconcentration efficiency and sensitivity in complex environmental samples (Figure 9) [120]. Wang et al. (2021) developed an electrochemical paper-based microfluidic device for online isolation of proteins and direct detection of lead in urine. Their work demonstrated the feasibility of integrating sample preparation and detection in a portable format, emphasizing the potential for point-of-care applications [121].
Figure 9.
(a) Microfluidic channels of the graphene oxide–polydimethylsiloxane (GO-PDMS) chip. A—inlet section of the chip, B—middle section, C—outlet section. (b) GO-PDMS chip device. Despite the high content of GO, the composite maintains typical physical properties of PDMS like mechanical durability and elasticity [120]. Copyright 2017 Elsevier.
Lab-on-a-chip (LOC) electrodes revolutionize heavy metal detection with their compact integration of multiple analytical functions, reducing sample volume and analysis time (Table 6). This miniaturization enhances efficiency and portability, catering to on-site and point-of-care applications. Optimized microfluidic channels and electrode configurations enable LOC electrodes to achieve high sensitivity and selectivity, crucial for accurately detecting trace levels of heavy metals. However, despite these strengths, LOC technology faces significant challenges that limit its widespread adoption. Complex fabrication processes and high initial costs remain major barriers, impeding accessibility for many potential users and applications. Moreover, integrating complex sample matrices into microfluidic systems without interference poses a substantial hurdle, affecting the reliability and accuracy required for real-world applications. Addressing these challenges requires continuous improvement of microfabrication techniques to streamline production and reduce costs. Exploring novel materials and enhancing microfluidic control systems are essential steps towards enhancing the reliability, scalability, and versatility of LOC devices. These advancements are crucial for expanding the capabilities of LOC electrodes in environmental monitoring, healthcare diagnostics, and industrial quality control, ensuring they can effectively meet the demands of diverse analytical challenges in the field of heavy metal detection [122,123,124].

Table 6.
Typical lab-on-a-chip devices.
In summary (Table 7), MEAs offer high sensitivity and low detection limits, making them suitable for in situ measurements, but they are complex to fabricate, expensive, and are typically reusable. SPEs are low-cost, easy to produce, portable, and disposable, though they generally have lower sensitivity and shorter lifespans. Carbon Electrodes are stable, have a wide potential window, and can be enhanced with nanomaterials, but they may require surface modification, have higher detection limits, and are reusable. Bismuth electrodes are an environmentally friendly, non-toxic alternative to mercury electrodes, offering good sensitivity and low detection limits, but they can be less stable, and are also reusable. Lab-on-a-Chip (LOC) devices integrate multiple functions and require small sample volumes, making them portable and suitable for on-site analysis, but they involve complex and costly fabrication processes and can be either single-use or reusable depending on the design.

Table 7.
Comparison table of different types of electrodes.
5. Trends and Future Directions
5.1. Advancements in Electrode Research
Extensive research is dedicated to advancing electrode materials to enhance analyzer performance. Carbon nanomaterials, including carbon nanotubes and graphene, are at the forefront of current research due to their expansive specific surface area and superior conductivity [125]. Composite materials that combine metal oxides with conductive polymers also exhibit significant electrochemical activity and stability [126,127]. Recent years have seen the emergence of biosensor materials exhibiting heightened selectivity and sensitivity through various biomolecule applications. Moreover, emerging nanomaterials like metal–organic frameworks (MOFs) and quantum dots garner attention for their distinctive advantages as electrode materials [128,129,130,131,132,133]. This underscores the need for continued in-depth research into high-performance electrode materials to advance the innovation and application of underwater in situ heavy metal monitoring technology.
5.2. Intelligent Monitoring and Data Analytics
Technological advancements are enabling intelligent monitoring and data analytics in underwater in situ heavy metal voltammetry analyzers. The advent of Internet of Things (IoT) technology facilitates real-time, precise monitoring of underwater heavy metal concentrations alongside remote data transmission. This advancement streamlines operational processes, ensuring heightened measurement accuracy and operational efficiency. Furthermore, coupling data mining with artificial intelligence (AI) analysis techniques enables profound data exploration, unveiling pollution sources and trends while enhancing analysis accuracy and efficiency. Integration with geographic information systems (GIS) enables comprehensive three-dimensional visualization of underwater environmental pollution. These transformative technologies propel ongoing research, promising more efficient and precise support for environmental protection and scientific inquiry [26,134].
5.3. Interdisciplinary Collaboration and Technological Innovation
The fusion of environmental science and engineering technology is crucial for advancing underwater in situ heavy metal voltammetry analyzers. Environmental science elucidates the mechanisms underlying heavy metal pollution, furnishing crucial insights for instrument development and material selection. Engineering technologies, encompassing sensing, micro/nanotechnology, and the IoT, fuel the optimization of analyzer performance. Notably, IoT technology enables remote real-time monitoring, amplifying monitoring efficiency. Interdisciplinary collaboration fosters technological innovation, exemplified by biosensors inspired by biological systems. This collaborative framework drives technological progress, facilitating more efficient and precise monitoring of heavy metal pollution, thereby amplifying contributions to environmental conservation and human health [43,135,136,137].
6. Conclusions
This review systematically examines recent advancements in underwater in situ trace metal analysis technology. Specifically, it is the first to comprehensively cover nearly all types of underwater in-situ trace metals voltammetry analyzers currently available, providing significant reference value for research in the related field.
Despite significant advancements, current in situ trace metals detection instruments utilizing electrochemical stripping voltammetry have established a robust research foundation and are deployed in diverse aquatic environments, ranging from offshore to deep-sea settings. However, these instruments still possess inherent limitations. Presently, no instruments, including the commercial VIP system, are capable of operating effectively at depths exceeding 1000 m while achieving accuracy at the parts-per-trillion (ppt) level. Furthermore, besides the VIP system’s ability to detect multiple metal elements, other instruments are constrained to identifying no more than three trace metals.
In current electrode technology, microelectrode arrays (MEAs) enable trace detection and localized monitoring in complex environmental matrices. However, they face challenges such as environmental species contamination, complex and expensive fabrication processes, and durability under harsh conditions. Carbon electrodes are widely used for trace metals detection due to their excellent conductivity and chemical stability. Future research should focus on optimizing surface modifications using graphene, metal nanoparticles, or conductive polymers to enhance the signal-to-noise ratio and lower the detection limit. Bismuth electrodes, as ideal substitutes for mercury electrodes, benefit from nanostructures such as nanoneedles that enhance surface area and electron transport efficiency. However, challenges include contamination and surface passivation in complex samples. Future research should explore advanced nanostructuring techniques and the integration of novel materials to overcome these challenges and improve the sensitivity and selectivity of bismuth electrodes in trace metals detection. Lab-on-a-chip (LOC) electrodes have revolutionized trace metals detection through their compact multifunctional integration. Nonetheless, complex fabrication processes and high costs limit their widespread application. Future research should aim to improve microfabrication technologies, explore new materials, and enhance microfluidic control systems to expand the applicability of LOC electrodes in environmental monitoring, medical diagnostics, and industrial quality control.
In summary, despite the significant potential of electrochemical sensors for in situ detection of trace metals in seawater, there is still a lack of sufficient in situ experimental data to validate their practical application. Although there have been relevant research and development advancements in this field, most achievements remain at the laboratory stage and have not been widely applied in real-world environments. Currently, only the VIP system has successfully achieved commercialization, but its market penetration and application scope are still limited. Electrochemical sensors hold remarkable potential for driving innovations in seawater analysis technology. However, to realize this goal, it is essential to clearly define the research and development pathways and conduct specialized in-depth studies.
Author Contributions
F.Z., S.W., Z.W. and J.Z. conceived and designed the study; J.Z., S.W., Z.W., F.Z., B.J. and C.Y. analyzed and summarized the relevant articles; J.Z., S.W., F.Z., B.J. and C.Y. wrote the manuscript; and F.Z. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by 2024 Zhejiang Province “Jianbing” Plan Project ‘Research and Product Development of In situ Analysis Instrument Technology for Heavy Metals Based on Marine Ecological Environment Observation’ (2024C03035).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Ahmad, A.; Kamaruddin, M.A.; Abdul Khalil, H.P.S.; Yahya, E.B.; Muhammad, S.; Rizal, S.; Ahmad, M.I.; Surya, I.; Abdullah, C.K. Recent Advances in Nanocellulose Aerogels for Efficient Heavy Metal and Dye Removal. Gels 2023, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Le, T.V.; Nguyen, B.T. Heavy Metal Pollution in Surface Water Bodies in Provincial Khanh Hoa, Vietnam: Pollution and Human Health Risk Assessment, Source Quantification, and Implications for Sustainable Management and Development. Environ. Pollut. 2024, 343, 123216. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.W.; Liu, J.M.; Li, C.Y.; Zhao, N.; Wang, Z.H.; Wang, S. A Novel and Universal Metal–Organic Frameworks Sensing Platform for Selective Detection and Efficient Removal of Heavy Metal Ions. Chem. Eng. J. 2019, 375, 122111. [Google Scholar] [CrossRef]
- Rastogi, M.; Nandal, M. Toxic Metals in Industrial Wastewaters and Phytoremediation Using Aquatic Macrophytes for Environmental Pollution Control: An Eco-Remedial Approach. In Bioremediation Industrial Waste Environmental Safety; Springer: Singapore, 2019; pp. 257–282. [Google Scholar]
- Rajoria, S.; Vashishtha, M.; Sangal, V.K. Treatment of Electroplating Industry Wastewater: A Review on the Various Techniques. Environ. Sci. Pollut. Res. Int. 2022, 29, 72196–72246. [Google Scholar] [CrossRef] [PubMed]
- Chukwu, K.B.; Abafe, O.A.; Amoako, D.G.; Essack, S.Y.; Abia, A.L.K. Antibiotic, Heavy Metal, and Biocide Concentrations in a Wastewater Treatment Plant and Its Receiving Water Body Exceed PNEC Limits: Potential for Antimicrobial Resistance Selective Pressure. Antibiotics 2023, 12, 1166. [Google Scholar] [CrossRef] [PubMed]
- Sheraz, N.; Shah, A.; Haleem, A.; Iftikhar, F.J. Comprehensive Assessment of Carbon-, Biomaterial- and Inorganic-Based Adsorbents for the Removal of the Most Hazardous Heavy Metal Ions from Wastewater. RSC Adv. 2024, 14, 11284–11310. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, V.; Venkatrayulu, C. Bioaccumulation of Heavy Metals in Edible Marine Fish from Coastal Areas of Nellore, Andhra Pradesh, India. GSC Biol. Pharm. Sci. 2020, 10, 18–24. [Google Scholar] [CrossRef]
- Garai, P.; Banerjee, P.; Mondal, P.; Saha, R. Effect of Heavy Metals on Fishes: Toxicity and Bioaccumulation. J. Clin. Toxicol. 2021, S18, 001. [Google Scholar]
- Torbati, S.; Atashbar Kangarloei, B.; Khataee, A. Bioconcentration of Heavy Metals by Three Plant Species Growing in Golmarz Wetland, in Northwestern Iran: The Plants’ Antioxidant Responses to Metal Pollutions. Environ. Technol. Innov. 2021, 24, 101804. [Google Scholar] [CrossRef]
- Chen, Y.G.; He, X.L.S.; Huang, J.H.; Luo, R.; Ge, H.Z.; Wołowicz, A.; Wawrzkiewicz, M.; Gładysz-Płaska, A.; Li, B.; Yu, Q.X.; et al. Impacts of Heavy Metals and Medicinal Crops on Ecological Systems, Environmental Pollution, Cultivation, and Production Processes in China. Ecotoxicol. Environ. Saf. 2021, 219, 112336. [Google Scholar] [CrossRef]
- Kong, M.; Zhu, Y.; Han, T.; Zhang, S.; Li, J.L.; Xu, X.; Chao, J.; Zhang, Y.; Gao, Y. Interactions of Heavy Metal Elements Across Sediment-Water Interface in Lake Jiaogang. Environ. Pollut. 2021, 286, 117578. [Google Scholar] [CrossRef] [PubMed]
- Baharuddin, N.; Mustaffa, N.K.; Ong, M.C. Faunus Ater (Mollusca, Gastropoda) as a Potential Bioindicator Contamination by Heavy Metals. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2023; Volume 1251, p. 012006. [Google Scholar]
- Shen, M.; Hu, Y.; Zhao, K.; Li, C.; Liu, B.; Li, M.; Lyu, C.; Sun, L.; Zhong, S. Occurrence, Bioaccumulation, Metabolism and Ecotoxicity of Fluoroquinolones in the Aquatic Environment: A Review. Toxics 2023, 11, 966. [Google Scholar] [CrossRef] [PubMed]
- Keramari, V.; Karastogianni, S.; Girousi, S. New Prospects in the Electroanalysis of Heavy Metal Ions (Cd, Pb, Zn, Cu): Development and Application of Novel Electrode Surfaces. Methods Protoc. 2023, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Shirsat, M.D.; Hianik, T. Electrochemical Detection of Heavy Metal Ions Based on Nanocomposite Materials. J. Compos. Sci. 2023, 7, 473. [Google Scholar] [CrossRef]
- Martínez-Paredes, G.; González-García, M.B.; Costa-García, A. Determination of Lead with Electrodes Nanostructured with Gold Nanoparticles. In Nanostructured Electrodes for Chemical Analysis; Academic Press: New York, NY, USA, 2020; Chapter 23; pp. 300–325. [Google Scholar]
- Li, D.; Deng, Y.; Liu, L.; Wang, J.; Huang, Z.; Zhang, X. Analysis of Heavy Metal and Polycyclic Aromatic Hydrocarbon Pollution Characteristics of A Typical Metal Rolling Industrial Site Based on Data Mining. Environ. Geochem. Health 2024, 46, 146. [Google Scholar] [CrossRef] [PubMed]
- Tercier-Waeber, M.-L.; Hezard, T.; Masson, M.; Schäfer, J. In Situ Monitoring of the Diurnal Cycling of Dynamic Metal Species in A Stream Under Contrasting Photobenthic Biofilm Activity and Hydrological Conditions. Environ. Sci. Technol. 2009, 43, 7237–7244. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Gao, J.; Tu, Y.; Zhang, Y.; Gao, J. Estimating Low Concentration Heavy Metals in Water Through Hyperspectral Analysis and Genetic Algorithm-Partial Least Squares Regression. Sci. Total Environ. 2024, 916, 170225. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, S.N.; Rahim, H.A.; Lau, W.J. Detection of Contaminants in Water Supply: A Review on State-of-the-Art Monitoring Technologies and Their Applications. Sens. Actuators B Chem. 2018, 255, 2657–2689. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yao, Y.; Ying, Y.; Ping, J. Recent Advances in Nanomaterial-Enabled Screen-Printed Electrochemical Sensors for Heavy Metal Detection. Trends Anal. Chem. 2019, 115, 187–202. [Google Scholar] [CrossRef]
- Li, Y.K.; Yang, T.; Chen, M.L.; Wang, J.H. Recent Advances in Nanomaterials for Analysis of Trace Heavy Metals. Crit. Rev. Anal. Chem. 2021, 51, 353–372. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bhattacharyya, S.; Ghosh, K.; Pal, S.; Halder, A.; Naseri, M.; Mohammadniaei, M.; Sarkar, S.; Ghosh, A.; Sun, Y.; et al. Sensory Development for Heavy Metal Detection: A Review on Translation from Conventional Analysis to Field-Portable Sensor. Trends Food Sci. Technol. 2021, 109, 674–689. [Google Scholar] [CrossRef]
- Wang, X.; Kong, L.; Zhou, S.; Ma, C.; Lin, W.; Sun, X.; Kirsanov, D.; Legin, A.; Wan, H.; Wang, P. Development of QDs-Based Nanosensors for Heavy Metal Detection: A Review on Transducer Principles and In-Situ Detection. Talanta 2022, 239, 122903. [Google Scholar] [CrossRef] [PubMed]
- Yaroshenko, I.; Kirsanov, D.; Marjanovic, M.; Lieberzeit, P.A.; Korostynska, O.; Mason, A.; Frau, I.; Legin, A. Real-Time Water Quality Monitoring with Chemical Sensors. Sensors 2020, 20, 3432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silva, G.M.; Campos, D.F.; Brasil, J.A.T.; Tremblay, M.; Mendiondo, E.M.; Ghiglieno, F. Advances in Technological Research for Online and In Situ Water Quality Monitoring—A Review. Sustainability 2022, 14, 5059. [Google Scholar] [CrossRef]
- Yang, J.; Ding, A.; Zhou, J.L.; Yan, B.Y.; Gu, Z.; Wang, H.F. A Floating Capsule Electrochemical System for In Situ and Multichannel Ion-Selective Sensing. Biosensors 2023, 13, 914. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, C.; Zhang, L.; Liu, T.; Wang, Z.; Song, Z.; Cai, H.; Fang, Z.; Chen, J.; Wang, J.; et al. Wearable and Flexible Electrochemical Sensors for Sweat Analysis: A Review. Microsyst. Nanoeng. 2023, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W. Beginner’s Guide to Raman Spectroelectrochemistry for Electrocatalysis Study. Chem. Methods 2023, 3, e202200042. [Google Scholar] [CrossRef]
- Mehta, D.; White, H.S. Finite-Element Analysis of Magnetic Field Driven Transport at Inlaid Platinum Microdisk Electrodes. ChemPhysChem 2003, 4, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Talawar, M.B.; Venugopalan, S.; Narasimhan, V.L. Synthesis, Characterization and Thermolysis Studies on 3,7-Dinitro-1,3,5,7-Tetraazabicyclo [3.3.1]Nonane (DPT): A Key Precursor in the Synthesis of Most Powerful Benchmark Energetic Materials (RDX/HMX) of Today. J. Hazard. Mater. 2008, 152, 1317–1324. [Google Scholar] [CrossRef]
- Brøgger, A.L.; Andreasen, S.Z.; Bosco, F.G.; Andersen, K.B.; Kwasny, D.; Svendsen, W.E.; Boisen, A. Centrifugal Microfluidic Platform with Real-Time Electrochemical Detection. Meet. Abstr. 2013, MA2013-02, 2768. [Google Scholar] [CrossRef]
- Swain, G.M. Damien W. M. Arrigan (Ed.): Electrochemical Strategies in Detection Science. Anal. Bioanal. Chem. 2016, 408, 6245–6246. [Google Scholar] [CrossRef]
- Holmes, J.; Pathirathna, P.; Hashemi, P. Novel Frontiers in Voltammetric Trace Metal Analysis: Towards Real Time, On-Site, In Situ Measurements. TrAC Trends Anal. Chem. 2019, 111, 206–219. [Google Scholar] [CrossRef]
- Hong, Y.S.; Kinney, K.A.; Reible, D.D. Effects of Cyclic Changes in pH and Salinity on Metals Release from Sediments. Environ. Toxicol. Chem. 2011, 30, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.A.; Benoit, G. Temporal Variability in Physical Speciation of Metals During a Winter Rain-On-Snow Event. J. Environ. Qual. 2005, 34, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.H.; Guo, X.Y.; Yin, S.; Tian, C.H.; Li, Y.Y.; Shen, Z.Y. Heavy Metal Partitioning of Suspended Particulate Matter-Water and Sediment-Water in the Yangtze Estuary. Chemosphere 2017, 185, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Matar, Z.; Soares Pereira, C.S.; Chebbo, G.; Uher, E.; Troupel, M.; Boudahmane, L.; Saad, M.; Gourlay-France, C.; Rocher, V.; Varrault, G. Influence of Effluent Organic Matter on Copper Speciation and Bioavailability in Rivers Under Strong Urban Pressure. Environ. Sci. Pollut. Res. Int. 2015, 22, 19461–19472. [Google Scholar] [CrossRef]
- Lam, K.P.; Furness, A. K-AVE+ GNN+ Sobel= An Effective, Highly Parallel Edge Detector Approach. Parallel Distrib. Methods Image Process. 1997, 3166, 190–198. [Google Scholar]
- Reyes, C.; Schneider, D.; Thurmer, A.; Kulkarni, A.; Lipka, M.; Sztejrenszus, S.Y.; Bottcher, R.D.; Friedrich, M.W. Potentially active iron, sulfur, and sulfate reducing bacteria in Skagerrak and Bothnian bay sediments. Geomicrobiol. J. 2017, 34, 840–850. [Google Scholar] [CrossRef]
- Hyun, J.H.; Kim, S.H.; Mok, J.S.; Cho, H.; Lee, T.; Vandieken, V.; Thamdrup, B. Manganese and iron reduction dominate organic carbon oxidation in surface sediments of the deep Ulleung Basin, East Sea. Biogeosciences 2017, 14, 941–958. [Google Scholar] [CrossRef]
- Cuartero, M. Electrochemical sensors for in-situ measurement of ions in seawater. Sens. Actuators B Chem. 2021, 334, 129635. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, S.; Zhang, F.; Jin, B.; Yang, C. Improved microelectrode array electrode design for heavy metal detection. Chemosensors 2024, 12, 51. [Google Scholar] [CrossRef]
- Belmont-Hébert, C.; Tercier, M.L.; Buffle, J.; Fiaccabrino, G.C.; De Rooij, N.F.; Koudelka-Hep, M. Gel-integrated microelectrode arrays for direct voltammetric measurements of heavy metals in natural waters and other complex media. Anal. Chem. 1998, 70, 2949–2956. [Google Scholar] [CrossRef]
- Buffle, J.; Tercier-Waeber, M.L. Voltammetric environmental trace-metal analysis and speciation: From laboratory to in situ measurements. TrAC Trends Anal. Chem. 2005, 24, 172–191. [Google Scholar] [CrossRef]
- Tercier-Waeber, M.L.; Hezard, T.; Masson, M. In situ monitoring of the diurnal evolution of the dynamic metal species in the Riou-Mort river. Geochim. Cosmochim. Acta 2009, 73, A1321. [Google Scholar]
- Money, C.; Braungardt, C.B.; Jha, A.N.; Worsfold, P.J.; Achterberg, E.P. Metal speciation and toxicity of Tamar Estuary water to larvae of the Pacific oyster, Crassostrea gigas. Mar. Environ. Res. 2011, 72, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Braungardt, C.B.; Achterberg, E.P.; Axelsson, B.; Buffle, J.; Graziottin, F.; Howell, K.A.; Illuminati, S.; Scarponi, G.; Tappin, A.D.; Tercier-Waeber, M.-L.; et al. Analysis of dissolved metal fractions in coastal waters: An inter-comparison of five voltammetric in situ profiling (VIP) systems. Mar. Chem. 2009, 114, 47–55. [Google Scholar] [CrossRef]
- Tercier-Waeber, M.-L.; Fighera, M.; Abdou, M.; Bakker, E.; van Der Wal, P. Newly designed gel-integrated nanostructured gold-based interconnected microelectrode arrays for continuous in situ arsenite monitoring in aquatic systems. Sens. Actuators B Chem. 2021, 328, 128996. [Google Scholar] [CrossRef]
- Creffield, S.; Tercier-Waeber, M.-L.; Gressard, T.; Bakker, E.; Layglon, N. On-Chip Antifouling Gel-Integrated Microelectrode Arrays for In Situ High-Resolution Quantification of the Nickel Fraction Available for Bio-Uptake in Natural Waters. Molecules 2023, 28, 1346. [Google Scholar] [CrossRef]
- Liu, R.; Cao, H.; Nie, Z.; Si, S.; Zhao, X.; Zeng, X. A disposable expanded graphite paper electrode with self-doped sulfonated polyaniline/antimony for stripping voltammetric determination of trace Cd and Pb. Anal. Methods 2016, 8, 1618–1625. [Google Scholar] [CrossRef]
- Tercier-Waeber, M.-L.; Confalonieri, F.; Abdou, M.; Dutruch, L.; Bossy, C.; Fighera, M.; Bakker, E.; Graziottin, F.; van der Wal, P.; Schäfer, J. Advanced multichannel submersible probe for autonomous high-resolution in situ monitoring of the cycling of the potentially bioavailable fraction of a range of trace metals. Chemosphere 2021, 282, 131014. [Google Scholar] [CrossRef]
- Tercier-Waeber, M.-L.; Abdou, M.; Fighera, M.; Kowal, J.; Bakker, E.; van der Wal, P. In situ voltammetric sensor of potentially bioavailable inorganic mercury in marine aquatic systems based on gel-integrated nanostructured gold-based microelectrode arrays. ACS Sens. 2021, 6, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Gibbon-Walsh, K.; Salaün, P.; van den Berg, C.M.G. Determination of manganese and zinc in coastal waters by anodic stripping voltammetry with a vibrating gold microwire electrode. Environ. Chem. 2011, 8, 475–484. [Google Scholar] [CrossRef]
- Chapman, C.S.; Cooke, R.D.; Salaün, P.; van den Berg, C.M.G. Apparatus for in situ monitoring of copper in coastal waters. J. Environ. Monit. 2012, 14, 2793–2802. [Google Scholar] [CrossRef] [PubMed]
- Domingos, R.F.; Carreira, S.; Galceran, J.; Salaün, P.; Pinheiro, J.P. AGNES at vibrated gold microwire electrode for the direct quantification of free copper concentrations. Anal. Chim. Acta 2016, 920, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Bezerra Dos Santos, V.; Fava, E.L.; Sá de Miranda Curi, N.S.; Faria, R.C.; Guerreiro, T.B.; Fatibello-Filho, O. An electrochemical analyzer for in situ flow determination of Pb(II) and Cd(II) in lake water with on-line data transmission and a global positioning system. Anal. Methods 2015, 7, 3105–3112. [Google Scholar] [CrossRef]
- Bezerra Dos Santos, V.; Fava, E.L.; Pessoa-Neto, O.D.; Bianchi, S.R.; Faria, R.C.; Fatibello-Filho, O. A versatile and robust electrochemical flow cell with a boron-doped diamond electrode for simultaneous determination of Zn2+ and Pb2+ ions in water samples. Anal. Methods 2014, 6, 8526–8534. [Google Scholar] [CrossRef]
- Wang, N.; Kanhere, E.; Kottapalli, A.G.P.; Miao, J.; Triantafyllou, M.S. Flexible liquid crystal polymer-based electrochemical sensor for in-situ detection of zinc (II) in seawater. Microchim. Acta 2017, 184, 3007–3015. [Google Scholar] [CrossRef]
- Wang, N.; Kanhere, E.; Miao, J.; Triantafyllou, M.S. Nanoparticles-modified chemical sensor fabricated on a flexible polymer substrate for cadmium(II) detection. Polymers 2018, 10, 694. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kodamatani, H.; Kono, Y.; Takeuchi, A.; Takai, K.; Tomiyasu, T.; Marumo, K. Development of a deep-sea mercury sensor using in situ anodic strip voltammetry. Geochem. J. 2015, 49, 613–620. [Google Scholar] [CrossRef]
- Luther, G.W., III; Glazer, B.T.; Ma, S.; Trouwborst, R.E.; Moore, T.S.; Metzger, E.; Kraiya, C.; Waite, T.J.; Druschel, G.; Sundby, B.; et al. Use of voltammetric solid-state (micro)electrodes for studying biogeochemical processes: Laboratory measurements to real-time measurements with an in situ electrochemical analyzer (ISEA). Mar. Chem. 2008, 108, 221–235. [Google Scholar] [CrossRef]
- Silva, P.R.M.; El Khakani, M.A.; Chaker, M.; Dufresne, A.; Courchesne, F. Simultaneous determination of Cd, Pb, and Cu metal trace concentrations in water certified samples and soil extracts by means of Hg-electroplated-Ir microelectrode array based sensors. Sens. Actuators B Chem. 2001, 76, 250–257. [Google Scholar] [CrossRef]
- Noël, S.; Tercier-Waeber, M.-L.; Lin, L.; Buffle, J. Complexing gel integrated microelectrode arrays for direct detection of free metal ion concentrations in natural waters. J. Phys. IV 2003, 107, 965–968. [Google Scholar] [CrossRef]
- Kadara, R.O.; Tothill, I.E. Stripping chronopotentiometric measurements of lead(II) and cadmium(II) in soils extracts and wastewaters using a bismuth film screen-printed electrode assembly. Anal. Bioanal. Chem. 2004, 378, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Stripping-based electrochemical metal sensors for environmental monitoring. Compr. Anal. Chem. 2007, 49, 131–141. [Google Scholar]
- Wang, Z.; Liu, G.; Yang, X. On-Site Determination of Heavy Metal in Soil Using Electrochemical Stripping Analysis. In Proceedings of the 2012 ASABE Annual International Meeting, Dallas, TX, USA, 29 July–1 August 2012; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2012; p. 1. Available online: https://elibrary.asabe.org/abstract.asp?aid=42215 (accessed on 26 June 2024).
- Chen, C.; Niu, X.; Chai, Y.; Zhao, H.; Lan, M. Bismuth-based porous screen-printed carbon electrode with enhanced sensitivity for trace heavy metal detection by stripping voltammetry. Sens. Actuators B Chem. 2013, 178, 339–342. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, H.; Zhou, S.; Song, T.; Wang, H.; Li, S.; Gan, W.; Yuan, Q. Simultaneous detection of Cd(II) and Pb(II) by differential pulse anodic stripping voltammetry at a nitrogen-doped microporous carbon/nafion/bismuth-film electrode. Electrochim. Acta 2014, 143, 143–151. [Google Scholar] [CrossRef]
- Sánchez-Calvo, A.; Blanco-López, M.C.; Costa-García, A. Based Working Electrodes Coated with Mercury or Bismuth Films for Heavy Metals Determination. Biosensors 2020, 10, 52. [Google Scholar] [CrossRef]
- Economou, A. Screen-printed electrodes modified with “green” metals for electrochemical stripping analysis of toxic elements. Sensors 2018, 18, 1032. [Google Scholar] [CrossRef]
- García-González, R.; Costa-García, A.; Fernández-Abedul, M.T. Dual screen-printed electrodes with elliptic working electrodes arranged in parallel or perpendicular to the strip. Sens. Actuators B Chem. 2014, 198, 302–308. [Google Scholar] [CrossRef]
- de Souza, A.P.R.; Foster, C.W.; Kolliopoulos, A.V.; Bertotti, M.; Banks, C.E. Screen-printed back-to-back electroanalytical sensors: Heavy metal ion sensing. Analyst 2015, 140, 4130–4136. [Google Scholar] [CrossRef]
- Jadav, J.K.; Umrania, V.V.; Rathod, K.J.; Golakiya, B.A. Development of silver/carbon screen-printed electrode for rapid determination of vitamin C from fruit juices. LWT 2018, 88, 152–158. [Google Scholar] [CrossRef]
- Ong, C.S.; Ng, Q.H.; Low, S.C. Critical reviews of electro-reactivity of screen-printed nanocomposite electrode to safeguard the environment from trace metals. Monatshefte Chem.-Chem. Mon. 2021, 152, 705–723. [Google Scholar] [CrossRef]
- Birara, A.; Washe, A.P.; Bayeh, Y.; Ashebr, T.G. Simultaneous Quantification of Cd(II) and Pb(II) by Bismuth/Poly(bromocresol purple) Modified Screen-Printed Carbon-Electrode in Wastewater. Int. J. Electrochem. Sci. 2023, 19, 100431. [Google Scholar] [CrossRef]
- Pasakon, P.; Kamsong, W.; Primpray, V.; Wisitsoraat, A.; Lomas, T.; Tuantranont, A.; Karuwan, C. Simultaneous Electrochemical Sensing of Cd2+ and Pb2+ Using Screen-Printed Ionic Liquid/Graphene Electrodes. Int. J. Environ. Anal. Chem. 2023, 1–16. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Zhai, S.; Mu, W.; Li, C.; Han, X. Screen-Printed Electrode Containing Bismuth/Graphene Oxide Hybrid for Simultaneous Detection of Cadmium and Lead Ions. J. Electroanal. Chem. 2024, 961, 118222. [Google Scholar]
- Metters, J.P.; Kadara, R.O.; Banks, C.E. Electroanalytical properties of screen printed graphite microband electrodes. Sens. Actuators B Chem. 2012, 169, 136–143. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, S.; Guo, L.H. Development of microplate-based photoelectrochemical DNA biosensor array for high throughput detection of DNA damage. Sens. Actuators B Chem. 2012, 161, 334–340. [Google Scholar] [CrossRef]
- Huang, H.; Wang, J.; Zheng, Y.; Bai, W.; Ma, Y.; Zhao, A. A Screen-Printed Carbon Electrode Modified with Bismuth Nanoparticles and Conjugated Mesoporous Polymer for Simultaneous Determination of Pb(II) and Cd(II) in Seafood Samples. J. Food Compos. Anal. 2024, 125, 105837. [Google Scholar] [CrossRef]
- Tiwari, M.S.; Kadu, A.K. Thiol-Based Chemically Modified Carbon Screen-Printed Electrode for Simultaneous Quantification of Trace Level Pb(II) and Cd(II). Anal. Sci. 2024, 40, 1449–1457. [Google Scholar] [CrossRef]
- Chen, X.; Yi, Z.; Peng, G.; Yuan, Z.; Wang, R.; Li, Y. In-Situ Deposition of Gold Nanoparticles on Screen-Printed Carbon Electrode for Rapid Determination of Hg2+ in Water Samples. Int. J. Electrochem. Sci. 2024, 19, 100544. [Google Scholar] [CrossRef]
- Djane, N.K.; Armalis, S.; Ndung’u, K.; Johansson, G.; Mathiasson, L. Supported liquid membrane coupled on-line to potentiometric stripping analysis at a mercury-coated reticulated vitreous carbon electrode for trace metal determinations in urine. Analyst 1998, 123, 393–396. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, W. Simultaneous determination of Cd2+ and Pb2+ using a chemically modified electrode. Anal. Sci. 2001, 17, 1015–1018. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, W.; Zhang, S. Determination of mercury at a dithizone-modified glassy carbon electrode by anodic stripping voltammetry. Anal. Sci. 2002, 18, 83–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ngassa, G.B.P.; Tchoffo, R.; Boutianala, M.; Nintedem, L.; Yameni Tchonguang, S.A.; Leuga Fogang, H.F.; Kenfack Tonle, I. Electrochemical sensor based on a thin film of organokaolinite material modified glassy carbon electrode (GCE) and application to the simultaneous sensitive detection of Pb2+ and Cu2+ ions in contaminated water. J. Appl. Electrochem. 2024, 1–21. [Google Scholar] [CrossRef]
- Zhang, L.-H.; Li, W.-C.; Yan, D.; Wang, H.; Lu, A.-H. Size Dependent Electrochemical Detection of Trace Heavy Metal Ions Based on Nano-Patterned Carbon Sphere Electrodes. Nanoscale 2016, 8, 13695–13700. [Google Scholar] [CrossRef] [PubMed]
- Xhanari, K.; Šašek, Ž.; Petovar, B.; Finšgar, M. Validation and Optimization of an In-Situ Copper-Modified Glassy Carbon Electrode. In Proceedings of the International Conference on Advanced Materials and Systems (ICAMS), Bucharest, Romania, 18–20 October 2018; The National Research & Development Institute for Textiles and Leather—INCDTP: Bucharest, Romania, 2018; pp. 489–494. [Google Scholar]
- Tüzün, E.; Atun, G. Individual and Simultaneous Determination of Heavy Metal Ions Using Carbon Paste Electrode Modified with Titania Nanoparticles. Electrocatalysis 2023, 14, 636–647. [Google Scholar] [CrossRef]
- Finšgar, M.; Rajh, B. A Factorial Design and Simplex Optimization of a Bismuth Film Glassy Carbon Electrode for Cd (II) and Pb (II) Determination. Chemosensors 2023, 11, 129. [Google Scholar] [CrossRef]
- Roushani, M.; Ali, N.M.; Karazan, Z.M.; Nasibipour, M.; Hoseini, S.J. Electrochemical Sensing of Pb2+, Cu2+, and Hg2+ by an Aminoclay-Based Porous Covalent Organic Polymer/Multi-Walled Carbon Nanotubes Modified Glassy Carbon Electrode. J. Mol. Struct. 2024, 1312, 138602. [Google Scholar] [CrossRef]
- Švancara, I.; Vytřas, K.; Kalcher, K.; Walcarius, A.; Wang, J. Carbon paste electrodes in facts, numbers, and notes: A review on the occasion of the 50-years jubilee of carbon paste in electrochemistry and electroanalysis. Electroanalysis 2009, 21, 7–28. [Google Scholar] [CrossRef]
- Devnani, H.; Satsangee, S.P. Anthocyanin modified carbon paste electrode for determination of copper ions at trace levels. Meet. Abstr. 2013, MA2013-01, 46. [Google Scholar] [CrossRef]
- Gong, Z.; Chan, H.T.; Chen, Q.; Chen, H. Application of Nanotechnology in Analysis and Removal of Heavy Metals in Food and Water Resources. Nanomaterials 2021, 11, 1792. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Teng, Z.; Wang, S.; Feng, K.; Wang, X.; Wang, C.; Wang, G. Voltammetric simultaneous ion flux measurements platform for Cu2+, Pb2+ and Hg2+ near rice root surface: Utilizing carbon nitride heterojunction film modified carbon fiber microelectrode. Sens. Actuators B Chem. 2018, 256, 98–106. [Google Scholar] [CrossRef]
- Pang, Y.-H.; Yang, Q.-Y.; Jiang, R.; Wang, Y.-Y.; Shen, X.-F. A stack-up electrochemical device based on metal–organic framework modified carbon paper for ultra-trace lead and cadmium ions detection. Food Chem. 2023, 398, 133822. [Google Scholar] [CrossRef]
- Petovar, B.; Xhanari, K.; Finšgar, M. A detailed electrochemical impedance spectroscopy study of a bismuth-film glassy carbon electrode for trace metal analysis. Anal. Chim. Acta 2018, 1004, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Promsuwan, K.; Sanguarnsak, C.; Samoson, K.; Saichanapan, J.; Soleh, A.; Saisahas, K.; Wangchuk, S.; Limbut, W. Single-drop electrodeposition of nanoneedle-like bismuth on disposable graphene electrode for on-site electrochemical detection of cadmium and lead. Talanta 2024, 276, 126179. [Google Scholar] [CrossRef]
- Muluneh, E.Y.; Mekonnen, M.L.; Debebe, S.E. Determination of Lead (II) and Cadmium (II) in Selected Commercially Available Water-Based Paints of Ethiopia by Anodic Stripping Voltammetry Using a Bismuth-Modified Glassy Carbon Electrode. ChemistrySelect 2023, 8, e202300963. [Google Scholar] [CrossRef]
- Liu, N.; Ye, W.; Zhao, G.; Liu, G. Development of smartphone-controlled and machine-learning-powered integrated equipment for automated detection of bioavailable heavy metals in soils. J. Hazard. Mater. 2024, 465, 133140. [Google Scholar] [CrossRef]
- Martynov, L.Y.; Sadova, M.K.; Sakharov, K.A.; Yashtulov, N.A.; Zaytsev, N.K. Determination of indium by adsorptive stripping voltammetry at the bismuth film electrode using combined electrode system facilitating medium exchange. Talanta 2024, 271, 125680. [Google Scholar] [CrossRef]
- Švancara, I.; Prior, C.; Hočevar, S.B.; Wang, J. A decade with bismuth-based electrodes in electroanalysis. Electroanalysis 2010, 22, 1405–1420. [Google Scholar] [CrossRef]
- Wang, J.; Lu, J.; Hocevar, S.B.; Farias, P.A.M.; Ogorevc, B. Bismuth-coated carbon electrodes for anodic strip voltammetry. Anal. Chem. 2000, 72, 3218–3222. [Google Scholar] [CrossRef]
- Kefala, G.; Economou, A.; Voulgaropoulos, A. A study of nafion-coated bismuth-film electrodes for the determination of trace metals by anodic stripping voltammetry. Analyst 2004, 129, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.H.; Han, W.K.; Park, J.S.; Kang, S.G. Determination of trace metals by anodic stripping voltammetry using a bismuth-modified carbon nanotube electrode. Talanta 2008, 76, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Torma, F.; Kádár, M.; Tóth, K.; Tatár, E. Nafion/2,2′-bipyridyl-modified bismuth film electrode for anodic stripping voltammetry. Anal. Chim. Acta 2008, 619, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-J.; Kim, C.K.; Lee, M.K.; Rhee, C.K. Advanced use of nanobismuth/nafion electrode for trace analyses of zinc, cadmium, and lead. J. Electrochem. Soc. 2010, 157, J241–J244. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Raptis, I. Microfabricated disposable lab-on-a-chip sensors with integrated bismuth microelectrode arrays for voltammetric determination of trace metals. Anal. Chim. Acta 2012, 710, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Bong, S.; Ha, J.; Kwak, M.; Park, S.-K.; Piao, Y. Electrochemical deposition of bismuth on activated graphene-nafion composite for anodic stripping voltammetric determination of trace heavy metals. Sens. Actuators B Chem. 2015, 215, 62–69. [Google Scholar] [CrossRef]
- Lee, S.; Oh, J.; Kim, D.; Piao, Y. A sensitive electrochemical sensor using an iron oxide/graphene composite for the simultaneous detection of heavy metal ions. Talanta 2016, 160, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jing, G.; Cui, T. Highly sensitive micro sensor with nafion coated bismuth for trace lead determination. In Proceedings of the 19th International Conference on Solid-State Sensors, Kaohsiung, Taiwan, 18–22 June 2017. [Google Scholar] [CrossRef]
- Schöning, M.J.; Jacobs, M.; Muck, A.; Knobbe, D.-T.; Wang, J.; Chatrathi, M.P.; Spillmann, S. Amperometric PDMS/glass capillary electrophoresis-based biosensor microchip for catechol and dopamine detection. Sens. Actuators B Chem. 2005, 108, 688–694. [Google Scholar] [CrossRef]
- Xu, T.; Hwang, W.L.; Su, F.; Chakrabarty, K. Automated design of pin-constrained digital microfluidic biochips under droplet-interference constraints. ACM J. Emerg. Technol. Comput. Syst. 2007, 3, 14-es. [Google Scholar] [CrossRef]
- Quang, L.X.; Lim, C.; Seong, G.H.; Choo, J.; Do, K.J.; Yoo, S.-K. A portable surface-enhanced Raman scattering sensor integrated with A lab-on-a-chip for field analysis. Lab Chip 2008, 8, 2214–2219. [Google Scholar] [CrossRef]
- Huang, Y.; Mason, A.J. Lab-on-CMOS integration of microfluidics and electrochemical sensors. Lab Chip 2013, 13, 3929–3934. [Google Scholar] [CrossRef] [PubMed]
- Economou, A.; Kokkinos, C. Advances in Stripping Analysis of Metals. In Electrochemical Strategies in Detection Science; Arrigan, D.W.M., Ed.; The Royal Society of Chemistry: London, UK, 2015. [Google Scholar]
- Wang, W.; Ding, S.; Wang, Z.; Lv, Q.; Zhang, Q. Electrochemical paper-based microfluidic device for on-line isolation of proteins and direct detection of lead in urine. Biosens. Bioelectron. 2021, 187, 113310. [Google Scholar] [CrossRef]
- Chałupniak, A.; Merkoçi, A. Graphene oxide–poly (dimethylsiloxane)-based lab-on-a-chip platform for heavy-metals preconcentration and electrochemical detection. ACS Appl. Mater. Interfaces 2017, 9, 44766–44775. [Google Scholar] [CrossRef]
- Zhao, C.; Zhong, G.; Kim, D.; Liu, J.; Liu, X. A portable lab-on-a-chip system for gold-nanoparticle-based colorimetric detection of metal ions in water. Biomicrofluidics 2014, 8, 052107. [Google Scholar] [CrossRef]
- Zou, Z.; Jang, A.; MacKnight, E.T.; Wu, P.-M.; Do, J.; Shim, J.S.; Bishop, P.L.; Ahn, C.H. An on-site heavy metal analyzer with polymer lab-on-a-chips for continuous sampling and monitoring. IEEE Sens. J. 2009, 9, 586–594. [Google Scholar] [CrossRef]
- Jung, W.; Jang, A.; Bishop, P.L.; Ahn, C.H. A polymer lab chip sensor with microfabricated planar silver electrode for continuous and on-site heavy metal measurement. Sens. Actuators B Chem. 2011, 155, 145–153. [Google Scholar] [CrossRef]
- Jang, A.; Zou, Z.; Lee, K.K.; Ahn, C.H.; Bishop, P.L. Potentiometric and voltammetric polymer lab chip sensors for determination of nitrate, pH and Cd(II) in water. Talanta 2010, 83, 1–8. [Google Scholar] [CrossRef]
- Zhan, S.; Wu, Y.; Wang, L.; Zhan, X.; Zhou, P. A mini-review on functional nucleic acids-based heavy metal ion detection. Biosens. Bioelectron. 2016, 86, 353–368. [Google Scholar] [CrossRef]
- Luan, E.; Shoman, H.; Ratner, D.M.; Cheung, K.C.; Chrostowski, L. Silicon photonic biosensors using label-free detection. Sensors 2018, 18, 3519. [Google Scholar] [CrossRef]
- Yantasee, W.; Lin, Y.; Hongsirikarn, K.; Fryxell, G.E.; Addleman, R.; Timchalk, C. Electrochemical sensors for the detection of lead and other toxic heavy metals: The next generation of personal exposure biomonitors. Environ. Health Perspect. 2007, 115, 1683–1690. [Google Scholar] [CrossRef]
- Xu, R.-X.; Yu, X.-Y.; Gao, C.; Jiang, Y.-J.; Han, D.-D.; Liu, J.-H.; Huang, X.-J. Non-conductive nanomaterial enhanced electrochemical response in stripping voltammetry: The use of nanostructured magnesium silicate hollow spheres for heavy metal ions detection. Anal. Chim. Acta 2013, 790, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-F.; Wang, J.-J.; Gan, L.; Han, X.-J.; Fan, H.-L.; Mei, L.-Y.; Huang, J.; Liu, Y.-Q. Individual and simultaneous electrochemical detection toward heavy metal ions based on L-cysteine modified mesoporous MnFe2O4 nanocrystal clusters. J. Alloys Compd. 2017, 721, 492–500. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2018, 178, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lu, Z.; Dai, W.; Liu, B.; Zhang, Y.; Li, J.; Ye, J. Laser engraved nitrogen-doped graphene sensor for the simultaneous determination of Cd(II) and Pb(II). J. Electroanal. Chem. 2018, 828, 41–49. [Google Scholar] [CrossRef]
- Ramalingam, M.; Ponnusamy, V.K.; Narayanan, S.N. A nanocomposite consisting of porous graphitic carbon nitride nanosheets and oxidized multiwalled carbon nanotubes for simultaneous stripping voltammetric determination of cadmium(II), mercury(II), lead(II) and zinc(II). Mikrochim. Acta 2019, 186, 69. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, T.; Lin, H.; Meng, W.; Zhang, C.; Cai, P.; Hao, T.; Wu, Y.; Guo, Z. Disposable faraday cage-type aptasensor for ultrasensitive determination of sub-picomolar Hg(II) via fast scan voltammetry. Sens. Actuators B Chem. 2020, 320, 128349. [Google Scholar] [CrossRef]
- Eswaran, M.; Tsai, P.-C.; Wu, M.-T.; Ponnusamy, V.K. Novel nano-engineered environmental sensor based on polymelamine/graphitic-carbon nitride nanohybrid material for sensitive and simultaneous monitoring of toxic heavy metals. J. Hazard. Mater. 2021, 418, 126267. [Google Scholar] [CrossRef]
- Guo, C.; Wang, C.; Sun, H.; Dai, D.; Gao, H. A simple electrochemical sensor based on RGO/MoS2/CS modified GCE for highly sensitive detection of Pb(II) in tobacco leaves. RSC Adv. 2021, 11, 29590–29597. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Lai, Q.; Fan, W.; Zhang, Y.; Liu, Z. Advances in portable heavy metal ion sensors. Sensors 2023, 23, 4125. [Google Scholar] [CrossRef]
- Mills, G.; Fones, G. A review of in situ methods and sensors for monitoring the marine environment. Sens. Rev. 2012, 32, 17–28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).