Near-Infrared Multiwavelength Raman Anti-Stokes/Stokes Thermometry of Titanium Dioxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Techniques
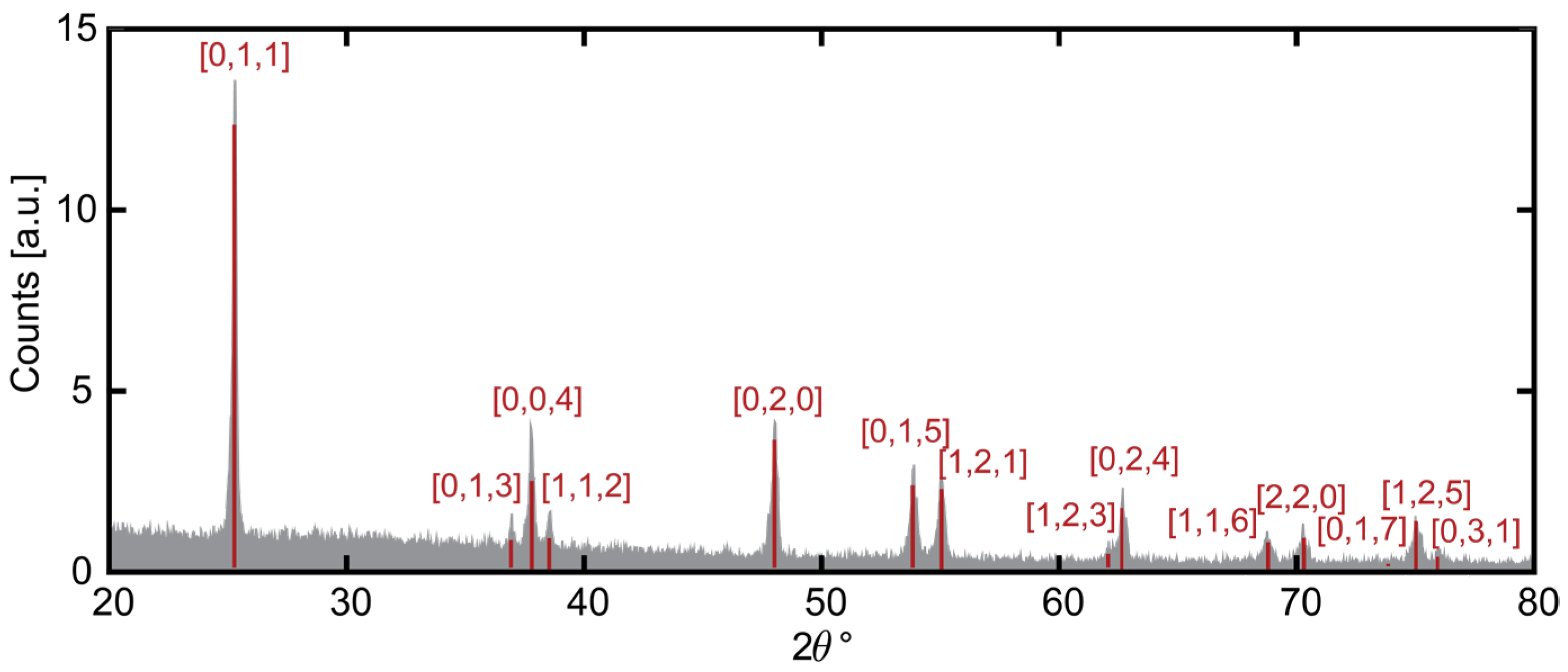
2.2.1. Structural Characterization—XRD
2.2.2. Diffuse Reflectance
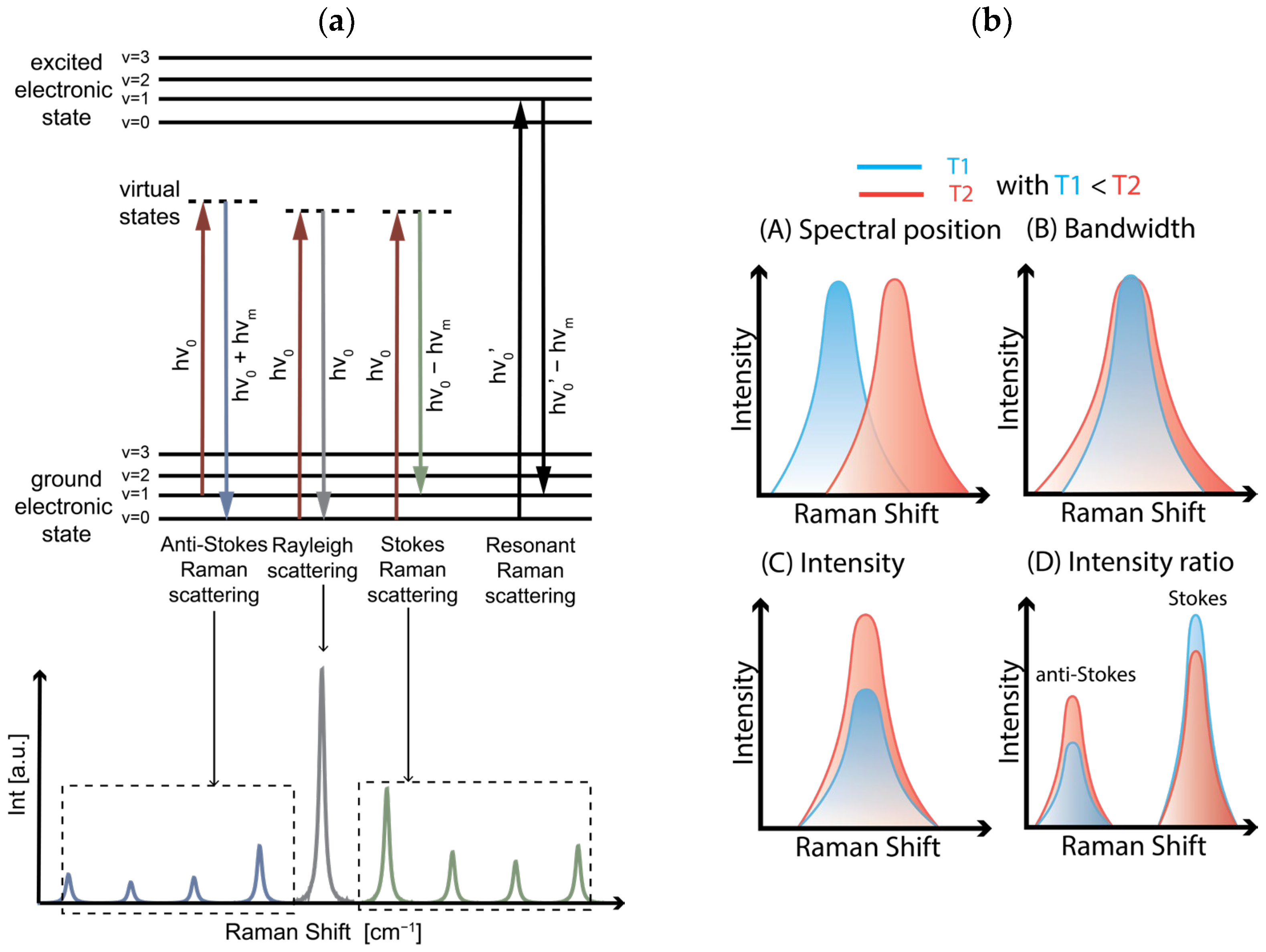
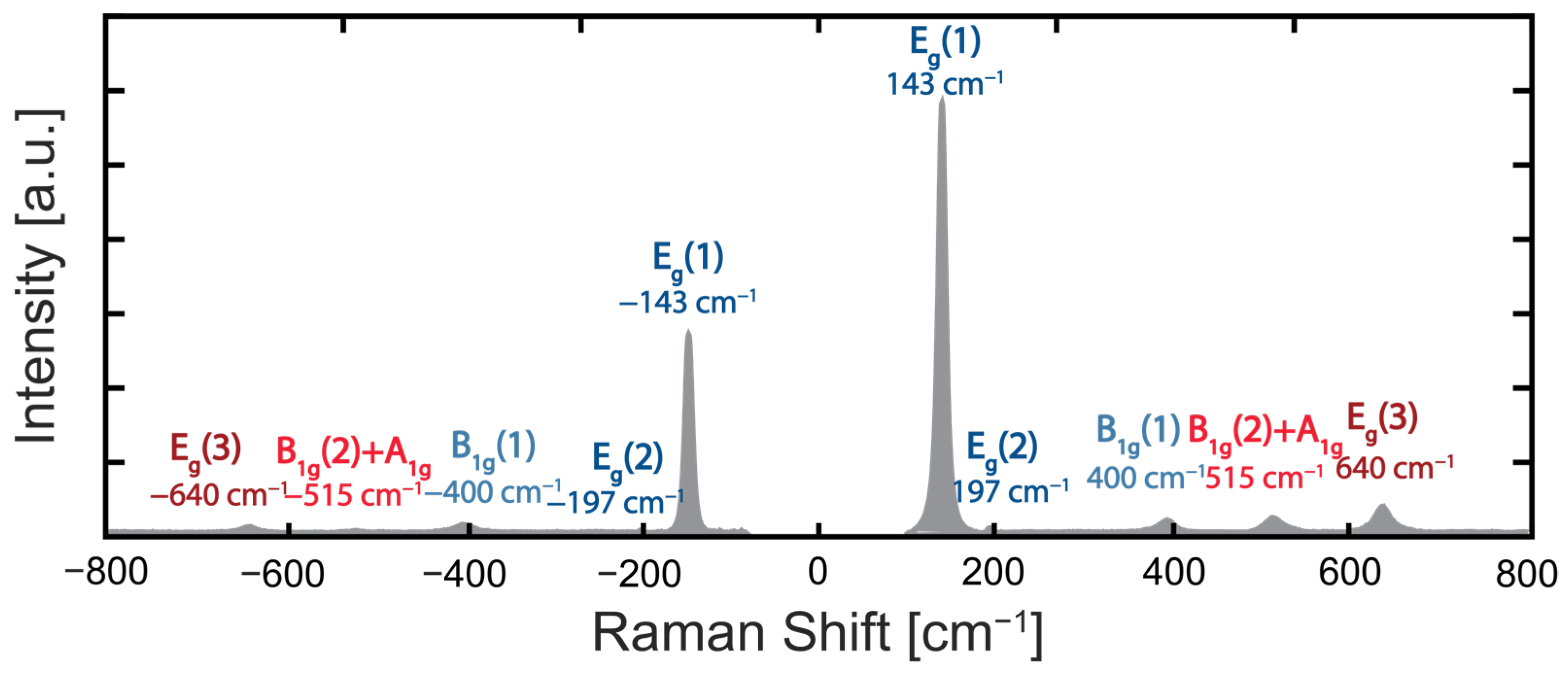
2.2.3. Optical Characterization—Raman Spectroscopy Set-Up
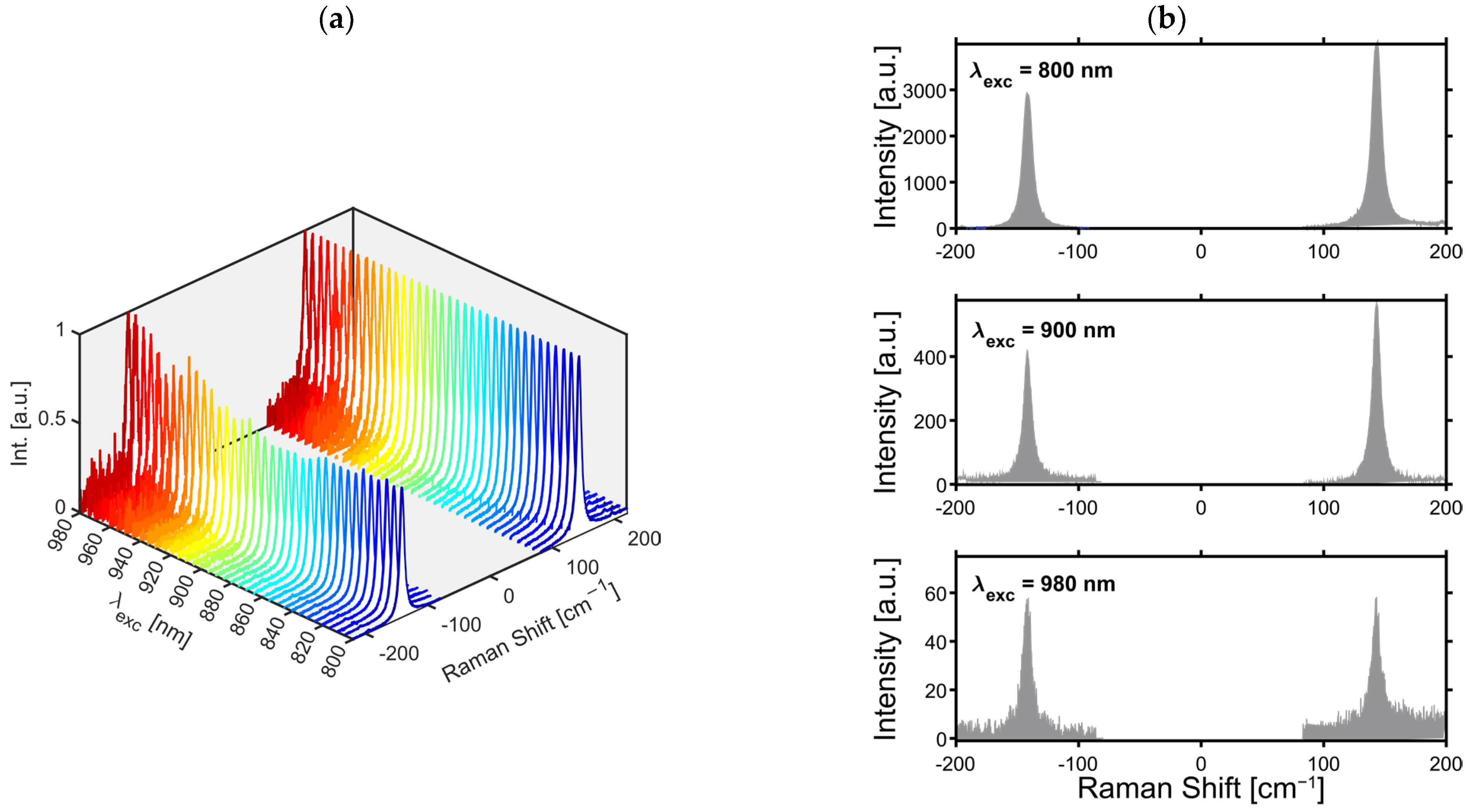
2.2.4. Excitation Wavelength Measurements
2.2.5. Temperature Detection
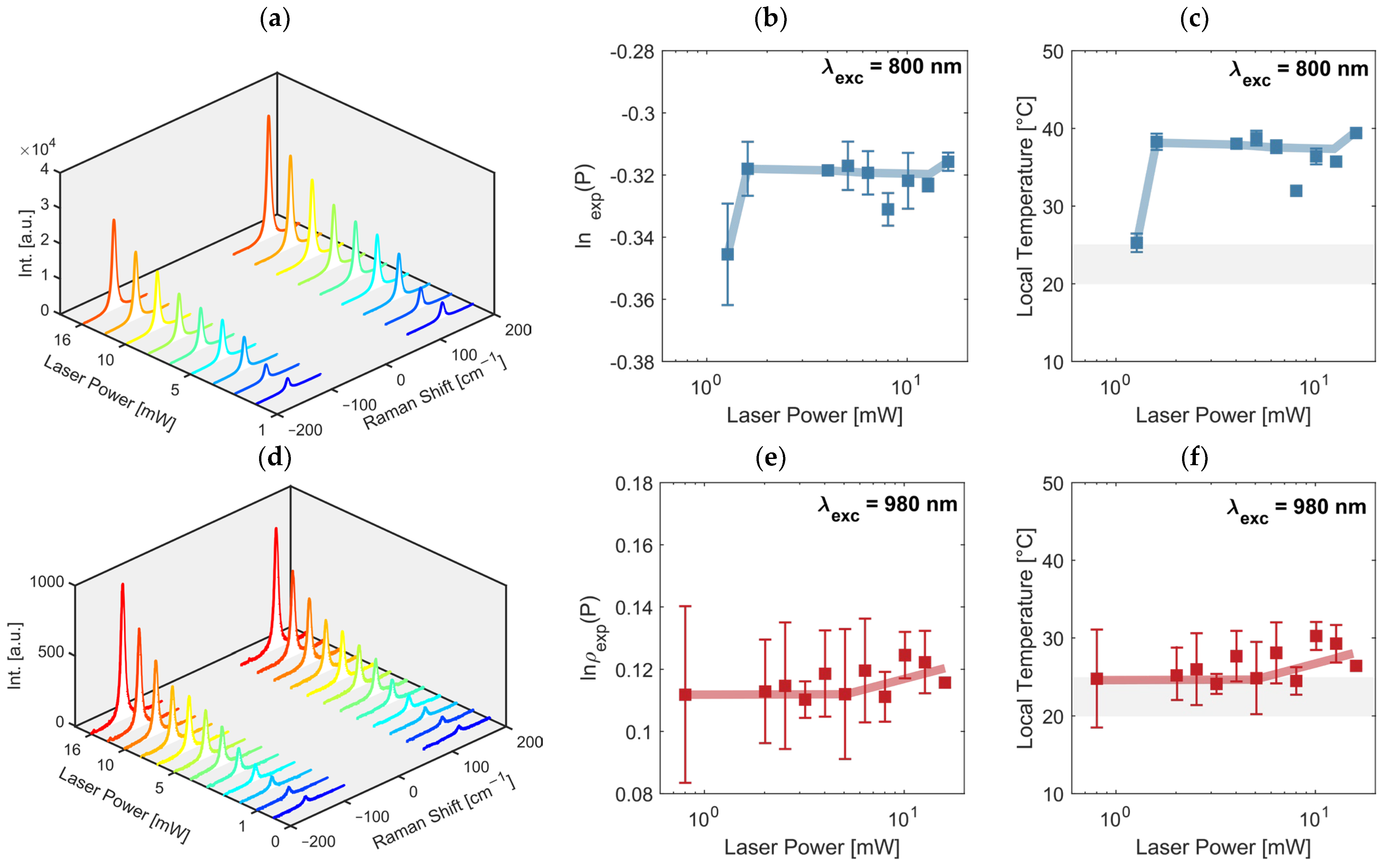
2.2.6. Laser Power Effect on the Local Temperature
2.3. Methods
2.3.1. Raman Nanothermometry
2.3.2. Use of the Proposed Method to Determine the Increase in the Local Temperature of the Sample
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brites, C.D.S.; Lima, P.P.; Silva, N.J.O.; Millan, A.; Amaral, V.S.; Palacio, F.; Carlos, L.D. Thermometry at the nanoscale. Nanoscale 2012, 4, 4799–4829. [Google Scholar] [CrossRef] [PubMed]
- Del Rosal, B.; Ximendes, E.; Rocha, U.; Jaque, D. In Vivo Luminescence Nanothermometry: From Materials to Applications. Adv. Opt. Mater. 2017, 5, 1600508. [Google Scholar] [CrossRef]
- Gota, C.; Okabe, K.; Funatsu, T.; Harada, Y.; Uchiyama, S. Hydrophilic Fluorescent Nanogel Thermometer for Intracellular Thermometry. J. Am. Chem. Soc. 2009, 131, 2766–2767. [Google Scholar] [CrossRef]
- Monti, M.; Brandt, L.; Ikomi-Kumm, J.; Olsson, H. Microcalorimetric investigation of cell metabolism in tumour cells from patients with non-Hodgkin lymphoma (NHL). Scand. J. Haematol. 1986, 36, 353–357. [Google Scholar] [CrossRef]
- Quintanilla, M.; Henriksen-Lacey, M.; Renero-Lecuna, C.; Liz-Marzán, L.M. Challenges for optical nanothermometry in biological environments. Chem. Soc. Rev. 2022, 51, 4223–4242. [Google Scholar] [CrossRef]
- Zhou, H.; Sharma, M.; Berezin, O.; Zuckerman, D.; Berezin, M.Y. Nanothermometry: From Microscopy to Thermal Treatments. Chem. Phys. Chem. 2016, 17, 27–36. [Google Scholar] [CrossRef]
- Wang, M.; Skripka, A.; Zhang, Y.; Cheng, T.; Ng, M.; Wong, S.Y.; Zhao, Y.; Sun, X.; Li, X.; Bhakoo, K.K.; et al. Theranostic Nanocapsules: Heating, Imaging, and Luminescence Nanothermometry. Chem. Mater. 2024, 36, 3285–3295. [Google Scholar] [CrossRef]
- Debasu, M.L.; Brites, C.D.S.; Balabhadra, S.; Oliveira, H.; Rocha, J.; Carlos, L.D. Nanoplatforms for Plasmon-Induced Heating and Thermometry. Chem. Nano Mat. 2016, 2, 520–527. [Google Scholar] [CrossRef]
- Jaque, D.; Maestro, L.M.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Rodríguez, E.M.; Solé, J.G. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.; Cheng, Z.; Hou, Z.; Huang, S.; Lin, J. Functional nanomaterials for near-infrared-triggered cancer therapy. Biomater. Sci. 2016, 4, 890–909. [Google Scholar] [CrossRef]
- Zhang, X.; An, L.; Tian, Q.; Lin, J.; Yang, S. Tumor microenvironment-activated NIR-II reagents for tumor imaging and therapy. J. Mater. Chem. B 2020, 8, 4738–4747. [Google Scholar] [CrossRef] [PubMed]
- Childs, P.R.N.; Greenwood, J.R.; Long, C.A. Review of temperature measurement. Rev. Sci. Instrum. 2000, 71, 2959–2978. [Google Scholar] [CrossRef]
- Quintanilla, M.; Liz-Marzan, L.M. Guiding Rules for Selecting a Nanothermometer. Nano Today 2018, 19, 126–145. [Google Scholar] [CrossRef]
- Jaque, D.; Vetrone, F. Luminescence nanothermometry. Nanoscale 2012, 4, 4301–4326. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Jiang, X.; Sun, Z.; Gui, R. Phosphorescence-based ratiometric probes: Design, preparation and applications in sensing, imaging and biomedicine therapy. Coord. Chem. Rev. 2021, 431, 213694. [Google Scholar] [CrossRef]
- Wetzl, C.; Renero-Lecuna, C.; Cardo, L.; Liz-Marzán, L.M.; Prato, M. Temperature-Dependent Luminescence of Nd3+-Doped Carbon Nanodots for Nanothermometry. ACS Appl. Mater. Interfaces 2024, 16, 35484–35493. [Google Scholar] [CrossRef]
- Jurga, N.; Runowski, M.; Grzyb, T. Lanthanide-based nanothermometers for bioapplications: Excitation and temperature sensing in optical transparency windows. J. Mater. Chem. C 2024, 12, 12218–12248. [Google Scholar] [CrossRef]
- Borisov, E.V.; Kalinichev, A.A.; Kolesnikov, I.E. ZnTe Crystal Multimode Cryogenic Thermometry Using Raman and Luminescence Spectroscopy. Materials 2023, 16, 1311. [Google Scholar] [CrossRef]
- Xie, X.; Wang, W.; Chen, H.; Yang, R.; Wu, H.; Gan, D.; Li, B.; Kong, X.; Li, Q.; Chang, Y. CaGdF5 based heterogeneous core@shell upconversion nanoparticles for sensitive temperature measurement. RSC Adv. 2023, 13, 8535–8539. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, X.; Liu, W.; Gao, Z.; Zhong, L.; Qin, Y.; Li, B.; Li, J. Semiconductor Plasmon Enhanced Upconversion toward a Flexible Temperature Sensor. ACS Appl. Mater. Interfaces 2023, 15, 4469–4476. [Google Scholar] [CrossRef]
- Jia, M.; Chen, X.; Sun, R.; Wu, D.; Li, X.; Shi, Z.; Chen, G.; Shan, C. Lanthanide-based ratiometric luminescence nanothermometry. NanoResearch 2023, 16, 2949–2967. [Google Scholar] [CrossRef]
- Beechem, T.E.; Serrano, J.R. Raman Thermometry of Microdevices: Choosing a Method to Minimize Error. Spectroscopy 2011, 26, 36–44. [Google Scholar]
- Pozzi, E.A.; Zrimsek, A.B.; Lethiec, C.M.; Schatz, G.C.; Hersam, M.C.; Van Duyne, R.P. Evaluating Single-Molecule Stokes and Anti-Stokes SERS for Nanoscale Thermometry. J. Phys. Chem. C 2015, 119, 21116–21124. [Google Scholar] [CrossRef]
- Kumar, K.; Stefanczyk, O.; Chorazy, S.; Nakabayashi, K.; Ohkoshi, S. Ratiometric Raman and Luminescent Thermometers Constructed from Dysprosium Thiocyanidometallate Molecular Magnets. Adv. Optical Mater. 2022, 10, 2201675. [Google Scholar] [CrossRef]
- Tuschel, D.; Adar, F. Molecular Spectroscopy Workbench Raman Thermometry. Spectroscopy 2016, 31, 8–13. [Google Scholar]
- Freitag, M.; Steiner, M.; Martin, Y.; Perebeinos, V.; Chen, Z.; Tsang, J.C.; Avouris, P. Energy Dissipation in Graphene Field-Effect Transistors. Nano Lett. 2009, 9, 1883–1888. [Google Scholar] [CrossRef]
- Smith, J.D.; Cappa, C.D.; Drisdell, W.S.; Cohen, R.C.; Saykally, R.J. Raman Thermometry Measurements of Free Evaporation from Liquid Water Droplets. J. Am. Chem. Soc. 2006, 128, 12892–12898. [Google Scholar] [CrossRef]
- Sugiyama, T.; Tsuji, H.; Furukawa, Y. Temperature measurements of the PEDOT-PSS layer in a polymer light-emitting diode by Stokes and anti-Stokes Raman scattering. Chem. Phys. Lett. 2008, 453, 238–241. [Google Scholar] [CrossRef]
- Zani, V.; Pedron, D.; Pilot, R.; Signorini, R. Contactless Temperature Sensing at the Microscale Based on Titanium Dioxide Raman Thermometry. Biosensors 2021, 11, 102. [Google Scholar] [CrossRef]
- Wu, G.; Xue, J.; Li, X.; Ren, K.; Leng, Z.; Bi, Q.; Tang, C. Excellent Sensitive Temperature Sensing Performance Based on Fluorescence Intensity Ratio Technique of Yb3+/Er3+ Codoped Gd2(WO4)3. Phys. Status Solidi B 2023, 260, 2200471. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, D. Lanthanide-functionalized metal–organic frameworks as ratiometric luminescent sensors. J. Mater. Chem. C 2020, 8, 12739–12754. [Google Scholar] [CrossRef]
- Carbone, R.; Marangi, I.; Zanardi, A.; Giorgetti, L.; Chierici, E.; Berlanda, G.; Podestà, A.; Fiorentini, F.; Bongiorno, G.; Piseri, P.; et al. Biocompatibility of cluster-assembled nanostructured TiO2 with primary and cancer cells. Biomaterials 2006, 27, 3221–3229. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wen, C.; Hodgson, P.; Li, Y. Biocompatibility of TiO2 nanotubes with different topographies. J. Biomed. Mater. Res. A 2014, 102, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Long, D.A. The Raman Effect; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002. [Google Scholar] [CrossRef]
- Alencar, M.A.R.C.; Maciel, G.S.; de Araújo, C.B.; Patra, A. Er3+-doped BaTiO3 nanocrystals for thermometry: Influence of nanoenvironment on the sensitivity of a fluorescence based temperature sensor. Appl. Phys. Lett. 2004, 84, 4753–4755. [Google Scholar] [CrossRef]
- Brandão-Silva, A.C.; Gomes, M.A.; Macedo, Z.S.; Avila, J.F.M.; Rodrigues, J.J.; Alencar, M.A.R.C. Multiwavelength Fluorescence Intensity Ratio Nanothermometry: High Sensitivity over a Broad Temperature Range. J. Phys. Chem. C 2018, 122, 20459–20468. [Google Scholar] [CrossRef]
- Frank, O.; Zukalova, M.; Laskova, B.; Kürti, J.; Koltai, J.; Kavan, L. Raman spectra of titanium dioxide (anatase, rutile) with identified oxygen isotopes (16, 17, 18). Phys. Chem. Chem. Phys. 2012, 14, 14567–14572. [Google Scholar] [CrossRef]
- Giarola, M.; Sanson, A.; Monti, F.; Mariotto, G.; Bettinelli, M.; Speghini, A.; Salviulo, G. Vibrational dynamics of anatase TiO2 Polarized Raman spectroscopy and ab initio calculations. Phys. Rev. B 2010, 81, 174305. [Google Scholar] [CrossRef]
- Swamy, V.; Kuznetsov, A.; Dubrovinsky, L.S.; Caruso, R.A.; Shchukin, D.G.; Muddle, B.C. Finite-size and pressure effects on the Raman spectrum nanocrystalline anatase TiO2. Phys. Rev. B 2005, 71, 184302. [Google Scholar] [CrossRef]
- Divya, S.; Nampoori, V.P.N.; Radhakrishnan, P.; Mujeeb, A. Electronic and optical properties of TiO2 and its polymorphs by Z-scan method. Chin. Phys. B 2014, 23, 084203. [Google Scholar] [CrossRef]
- Guo, L.H.; Wang, Y.W.; Jiang, Y.Q.; Xiao, S.; He, J. Dependence of Nonlinear Optical Response of Anatase TiO2 on Shape and Excitation Intensity*. Chin. Phys. Lett. 2017, 34, 077803. [Google Scholar] [CrossRef]


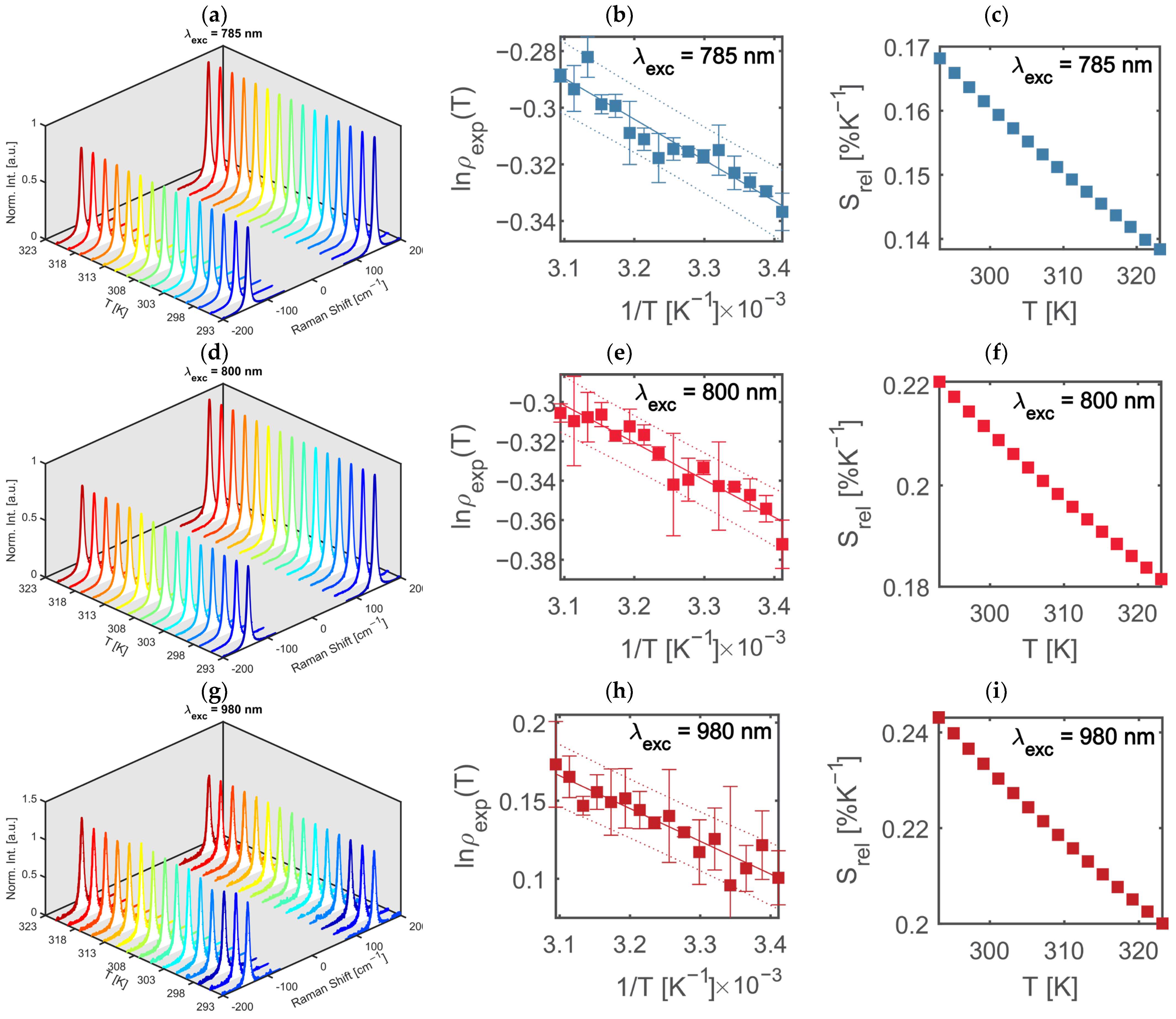
| 514.5 | 0.1229 | 240.5 | 0.13 | 0.27 |
| 785 | 0.1584 | 144.5 | 0.12 | 0.16 |
| 800 | 0.2858 | 189.4 | 0.15 | 0.21 |
| 980 | 0.8161 | 209.7 | 0.26 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zani, V.; Pilot, R.; Pedron, D.; Signorini, R. Near-Infrared Multiwavelength Raman Anti-Stokes/Stokes Thermometry of Titanium Dioxide. Chemosensors 2024, 12, 191. https://doi.org/10.3390/chemosensors12090191
Zani V, Pilot R, Pedron D, Signorini R. Near-Infrared Multiwavelength Raman Anti-Stokes/Stokes Thermometry of Titanium Dioxide. Chemosensors. 2024; 12(9):191. https://doi.org/10.3390/chemosensors12090191
Chicago/Turabian StyleZani, Veronica, Roberto Pilot, Danilo Pedron, and Raffaella Signorini. 2024. "Near-Infrared Multiwavelength Raman Anti-Stokes/Stokes Thermometry of Titanium Dioxide" Chemosensors 12, no. 9: 191. https://doi.org/10.3390/chemosensors12090191
APA StyleZani, V., Pilot, R., Pedron, D., & Signorini, R. (2024). Near-Infrared Multiwavelength Raman Anti-Stokes/Stokes Thermometry of Titanium Dioxide. Chemosensors, 12(9), 191. https://doi.org/10.3390/chemosensors12090191







