Sensitive Detection of Fungicide Folpet by Surface-Enhanced Raman Scattering: Experimental and Theoretical Approach
Abstract
1. Introduction
2. Experimental and Theoretical Methods
2.1. Materials
2.2. Preparation of Ag Nanoparticles
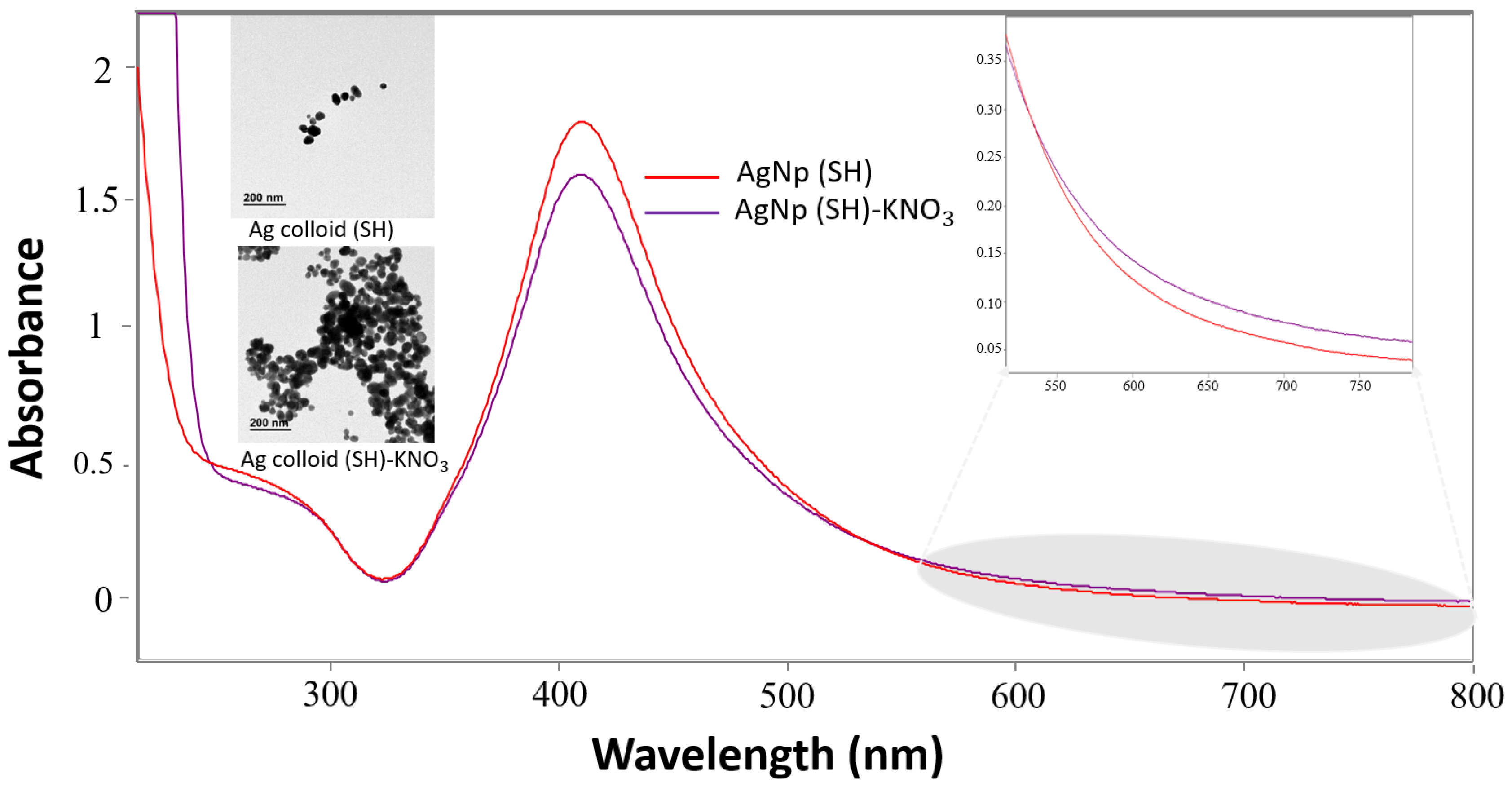
2.3. Characterization of AgNPs: UV-Vis and TEM Images
2.4. Preparation of Samples
2.5. Instrumentation
2.6. DFT Calculations
3. Results and Discussion
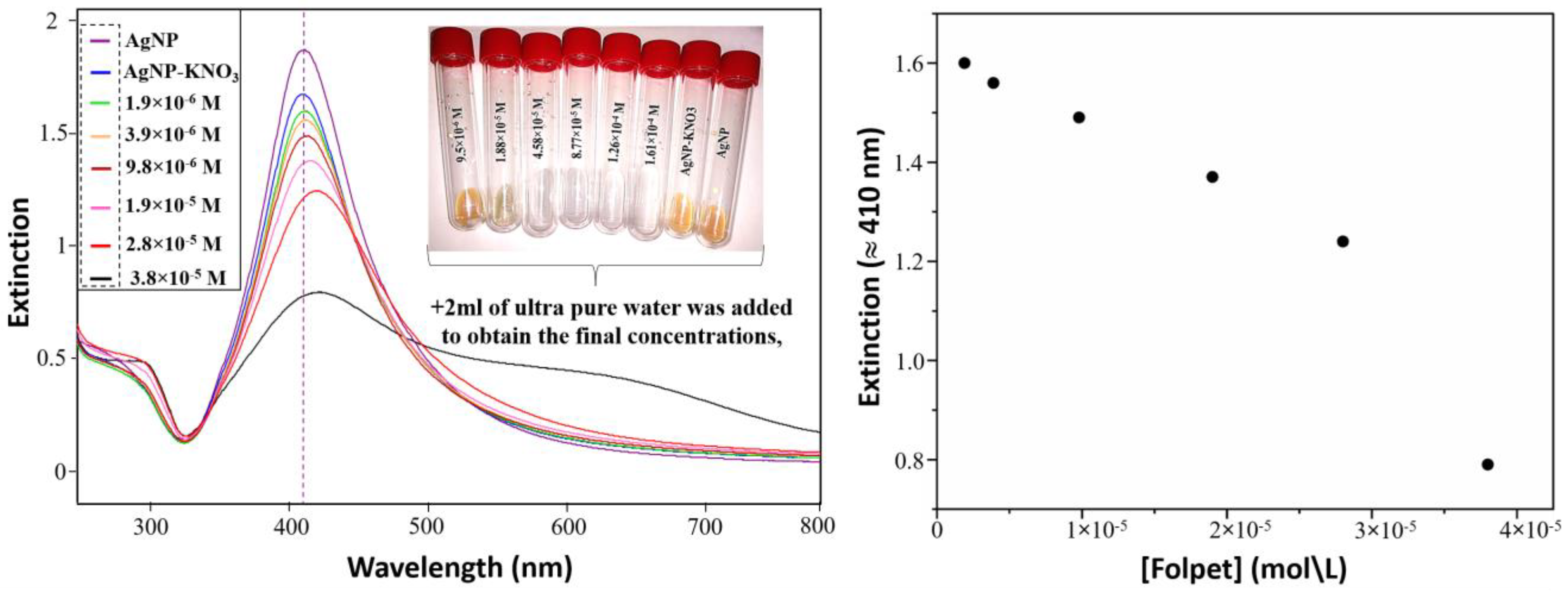
3.1. Extinction Measurements (External Standard Method)
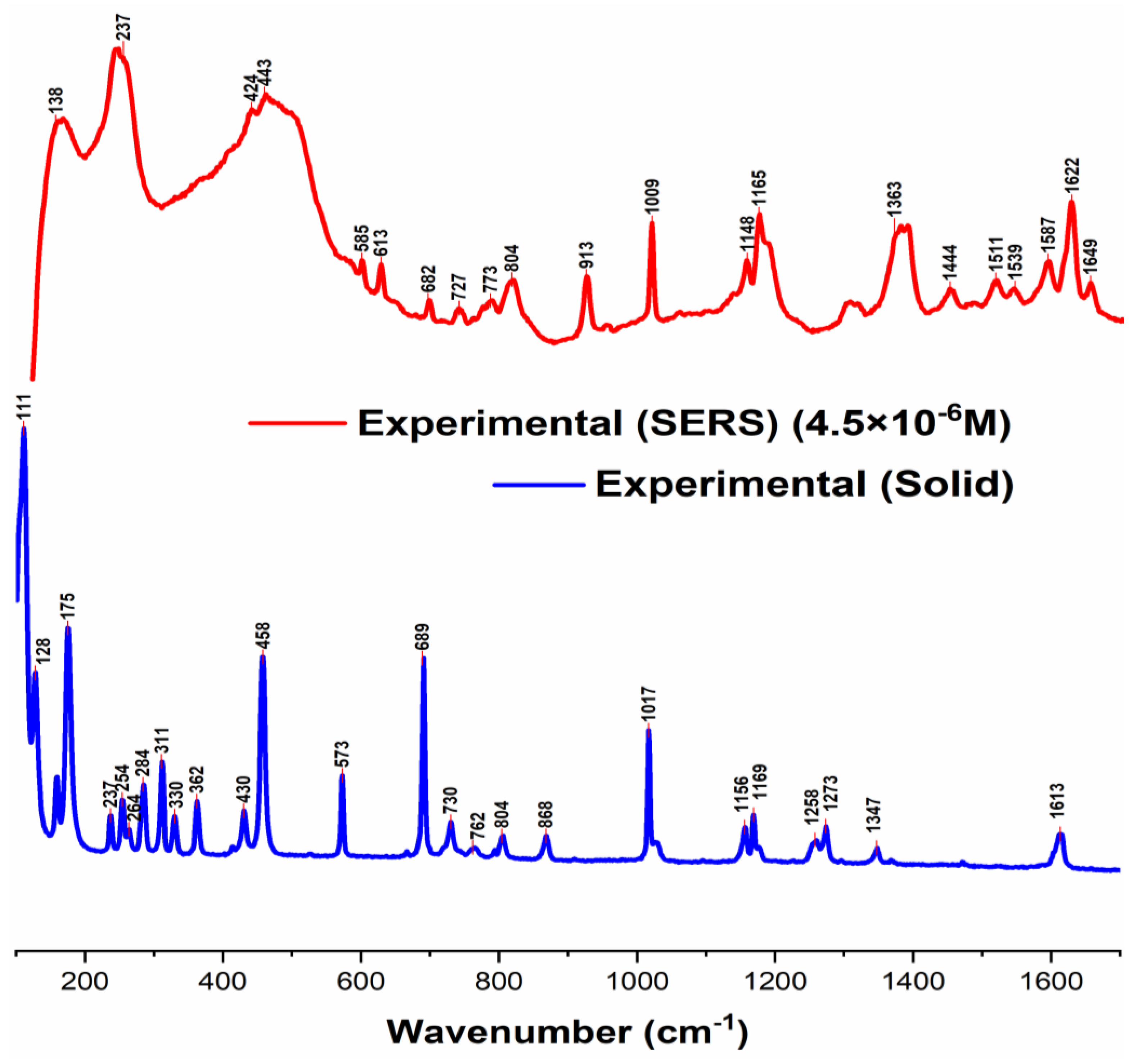
3.2. Raman and SERS Measurements of Folpet in AgH Colloid
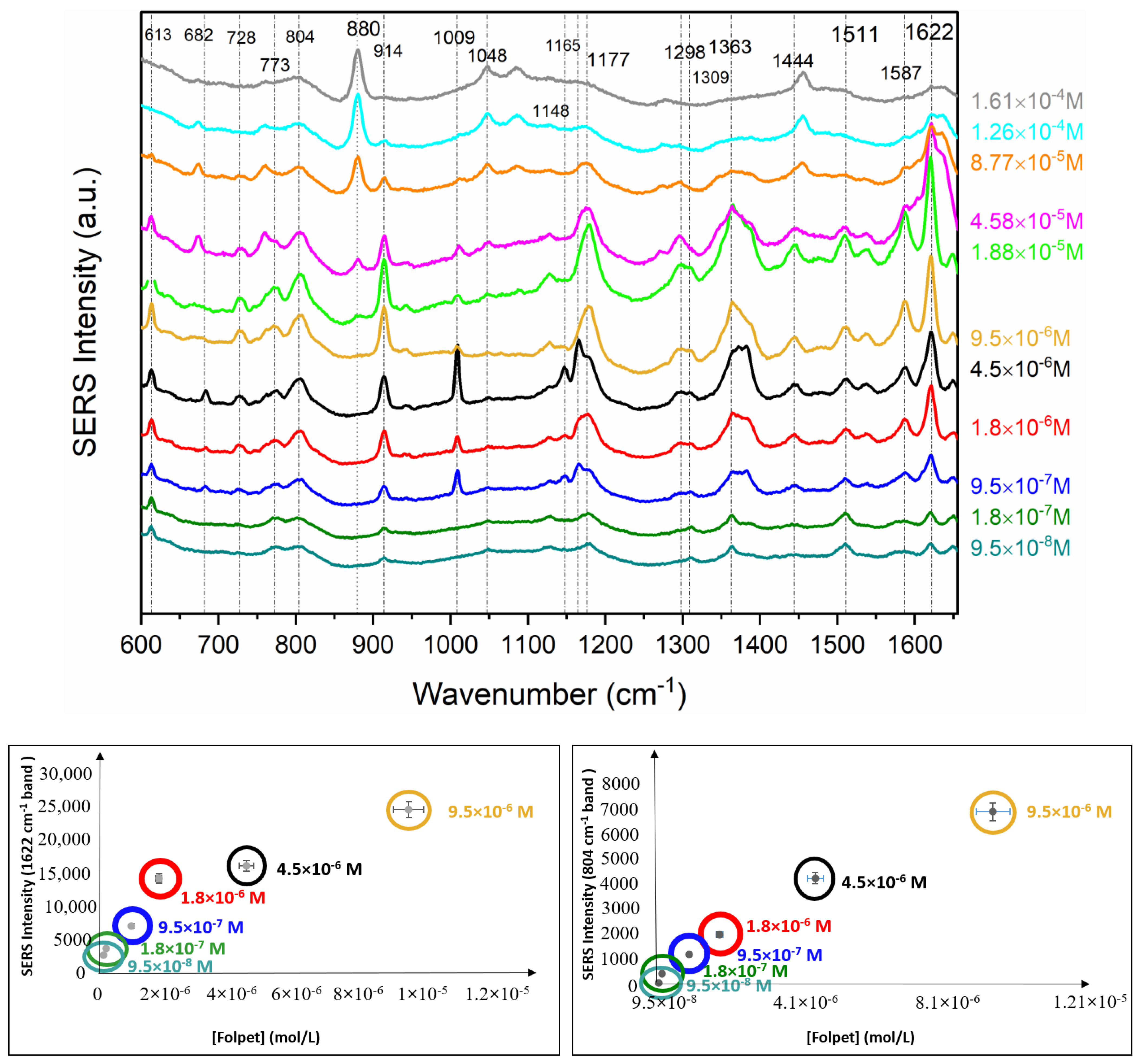
3.3. Quantitative Analysis
3.4. Degradation of Folpet Revealed by SERS Spectra

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority (EFSA). Conclusion Regarding the Peer Review of the Pesticide Risk Assessment of the Active Substance Folpet. EFSA J. 2009, 7, 297r. [Google Scholar] [CrossRef]
- Leepheng, P.; Limthin, D.; Homchan, W.; Suramitr, S.; Phromyothin, D. An Experimental and Theoretical Study of Molecularly Imprinted Electrode Based on Methyl Methacrylate Polymer for Pesticide Detection. Jpn. J. Appl. Phys. 2020, 59, SIIJ09. [Google Scholar] [CrossRef]
- Vannucci, G.; Cañamares, M.V.; Prati, S.; Sanchez-Cortes, S. Analysis of the Tautomeric Equilibrium of Two Red Monoazo Dyes by UV–Visible, Raman and SERS Spectroscopies. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2021, 261, 120007. [Google Scholar] [CrossRef] [PubMed]
- Douass, O.; Samoudi, B.; Bendaou, O.; Sanchez Cortes, S. A Brief Review of Recent Advances in Surface-Enhanced Raman Spectroscopy and Microfluidics Technology for the Ultrasensitive Detection of Pesticides. Egypt. J. Chem. 2022, 65, 133–150. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Etchegoin, P.G. Principles of Surface-Enhanced Raman Spectroscopy: And Related Plasmonic Effects, 1st ed.; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2009; ISBN 978-0-444-52779-0. [Google Scholar]
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Surface-Enhanced Raman Scattering and Biophysics. J. Phys. Condens. Matter 2002, 14, R597–R624. [Google Scholar] [CrossRef]
- Campion, A. Raman Spectroscopy of Molecules Adsorbed on Surfaces: Progress, Issues and Prospects. J. Electron Spectrosc. Relat. Phenom. 1990, 54–55, 877–880. [Google Scholar] [CrossRef]
- Dong, T.; Lin, L.; He, Y.; Nie, P.; Qu, F.; Xiao, S. Density Functional Theory Analysis of Deltamethrin and Its Determination in Strawberry by Surface Enhanced Raman Spectroscopy. Molecules 2018, 23, 1458. [Google Scholar] [CrossRef]
- Tuschel, D. Practical Group Theory and Raman Spectroscopy, Part i: Normal Vibrational Modes. Spectroscopy 2014, 29, 14. [Google Scholar]
- Cortés, E.; Besteiro, L.V.; Alabastri, A.; Baldi, A.; Tagliabue, G.; Demetriadou, A.; Narang, P. Challenges in Plasmonic Catalysis. ACS Nano 2020, 14, 16202–16219. [Google Scholar] [CrossRef]
- Kim, Y.; Dumett Torres, D.; Jain, P.K. Activation Energies of Plasmonic Catalysts. Nano Lett. 2016, 16, 3399–3407. [Google Scholar] [CrossRef]
- Fuenzalida, F.B.; Slepčíková, P.; Repovská, M.; Jutková, A.; Vega Cañamares, M.; Miškovský, P.; Jurašeková, Z.; Sanchez-Cortes, S. Selective and Ultrasensitive Detection of the Herbicide Glyphosate by Means of Plasmon Catalysis on Ag Nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 323, 124845. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Z.; Li, W.P.; Kang, W.J.; Feng, Y.; Li, Z.; Zhong, X.Y.; Yang, J.; Liu, H.; Dong, C.K.; Yin, P.F.; et al. Silver Clusters with Adatoms as a Catalyst for the Oxygen Reduction Reaction. ACS Catal. 2023, 13, 9181–9189. [Google Scholar] [CrossRef]
- Sanchez-Cortes, S.; Garcıa-Ramos, J.V. Influence of Coverage in the Surface-Enhanced Raman Scattering of Cytosine and Its Methyl Derivatives on Metal Colloids: Chloride and pH Effects. Surf. Sci. 2001, 473, 133–142. [Google Scholar] [CrossRef]
- Rubira, R.J.G.; Furini, L.N.; Constantino, C.J.L.; Sanchez-Cortes, S. SERS Detection of Prometryn Herbicide Based on Its Optimized Adsorption on Ag Nanoparticles. Vib. Spectrosc. 2021, 114, 103245. [Google Scholar] [CrossRef]
- Oliveira, M.J.S.; Rubira, R.J.G.; Furini, L.N.; Batagin-Neto, A.; Constantino, C.J.L. Detection of Thiabendazole Fungicide/Parasiticide by SERS: Quantitative Analysis and Adsorption Mechanism. Appl. Surf. Sci. 2020, 517, 145786. [Google Scholar] [CrossRef]
- Oliveira, M.J.S.; Martin, C.S.; Rubira, R.J.G.; Batagin-Neto, A.; Constantino, C.J.L.; Aroca, R.F. Surface-enhanced Raman Scattering of Thiram: Quantitative and Theoretical Analyses. J. Raman Spectrosc. 2021, 52, 2557–2571. [Google Scholar] [CrossRef]
- Chemical Reduction—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/engineering/chemical-reduction (accessed on 6 October 2023).
- Sánchez-Cortés, S.; García-Ramos, J.V.; Morcillo, G. Morphological Study of Metal Colloids Employed as Substrate in the SERS Spectroscopy. J. Colloid Interface Sci. 1994, 167, 428–436. [Google Scholar] [CrossRef]
- Ostroukhova, O.K.; Zenkevich, I.G. A Comparison of the External Standard and Standard Addition Methods for the Quantitative Chromatographic Determination of Pesticide Concentrations in Plant Samples. J. Anal. Chem. 2006, 61, 442–451. [Google Scholar] [CrossRef]
- Gaussian.Com|Expanding the Limits of Computational Chemistry. Available online: https://gaussian.com/ (accessed on 6 October 2023).
- Basis Sets|Gaussian.Com. Available online: https://gaussian.com/basissets/ (accessed on 20 March 2023).
- Cañamares, M.V.; Garcia-Ramos, J.V.; Gómez-Varga, J.D.; Domingo, C.; Sanchez-Cortes, S. Comparative Study of the Morphology, Aggregation, Adherence to Glass, and Surface-Enhanced Raman Scattering Activity of Silver Nanoparticles Prepared by Chemical Reduction of Ag + Using Citrate and Hydroxylamine. Langmuir 2005, 21, 8546–8553. [Google Scholar] [CrossRef]
- Medendorp, N.W.; Bowman, K.J.; Trumble, K.P. Tailoring Particle Size and Morphology of Colloidal Ag Particles via Chemical Precipitation for Ag-BSCCQ Composites. Mater. Sci. Eng. A 1996, 212, 222–227. [Google Scholar] [CrossRef]
- Vrenouf Analyse Phytosanitaire: Levons Le Voile Sur La Notion de Métabolites. Available online: https://labexcell.com/images/smartlink/SL_Analyse_Phytosanitaire (accessed on 5 June 2024).
- Sun, H.; Zhang, X.; Zuo, W.; Dai, Z.; Zhou, L.; Luo, F.; Yang, M.; Wang, X.; Lou, Z.; Chen, Z. Concentrations, Generation and Risk Characterization of Phthalimide in Tea-Derived from Folpet or Not? Sci. Total Environ. 2022, 852, 158194. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Huang, C.; Jiang, Q.; Chen, L.; Li, Y.; Lou, C.; Lu, H.; Shen, Y. A Method Based on the Conversion and Determination of Folpet and Phthalimide Residues in Tea by GC-MS/MS and GC-TOF-HRMS. Microchem. J. 2022, 179, 107448. [Google Scholar] [CrossRef]
- Beegum, M.F.; Kumari, L.U.; Harikumar, B.; Tresa, H.; Panicker, C.Y. Vibrational wavenumbers and structural determination of dilithium terephthalate. Rasayaa J. Chem. 2008, 1, 117–124. [Google Scholar]
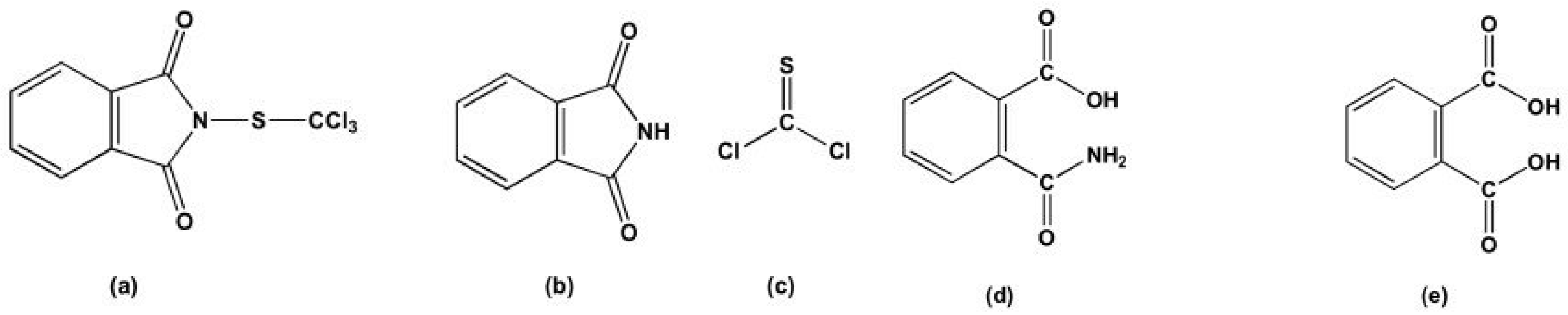
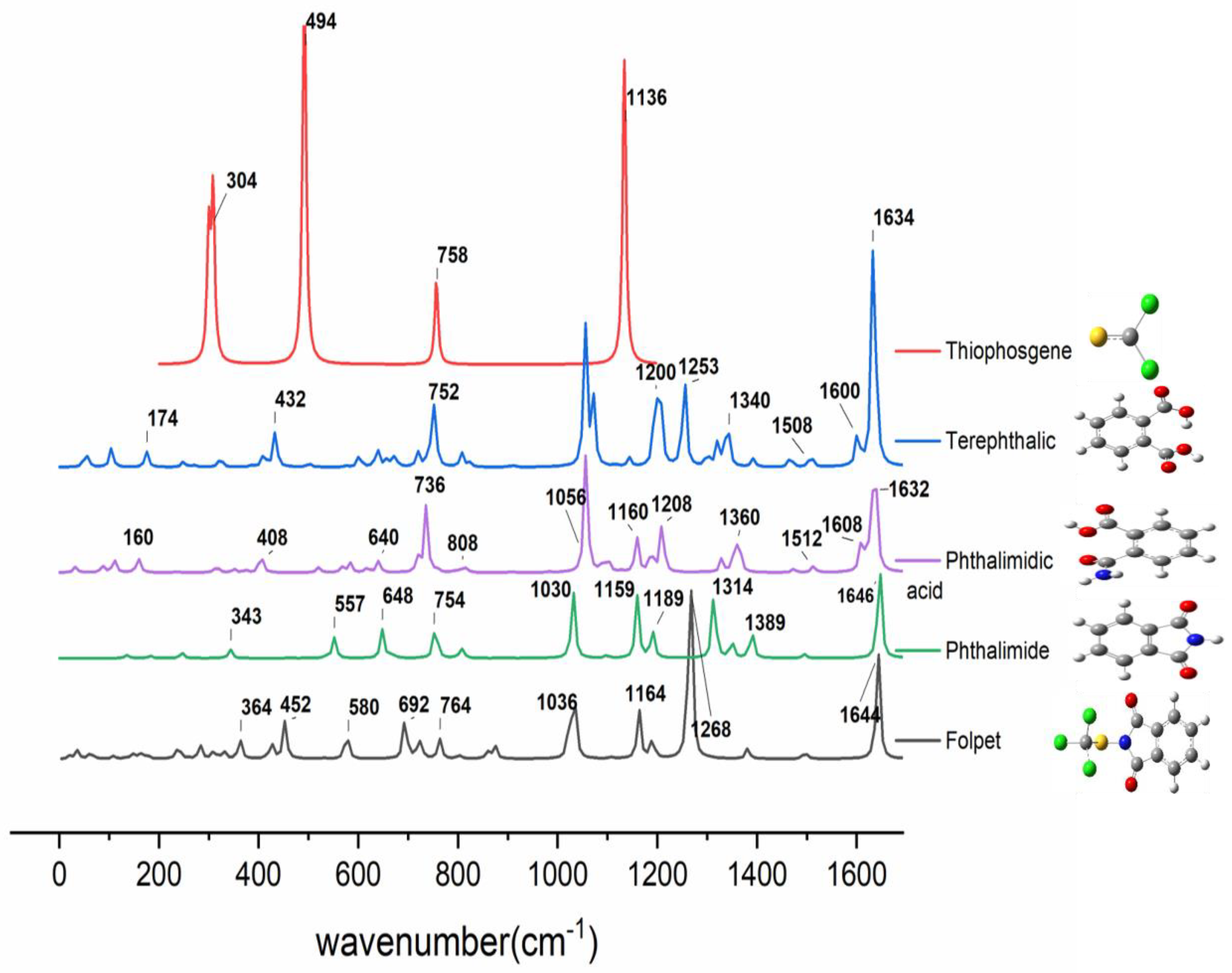
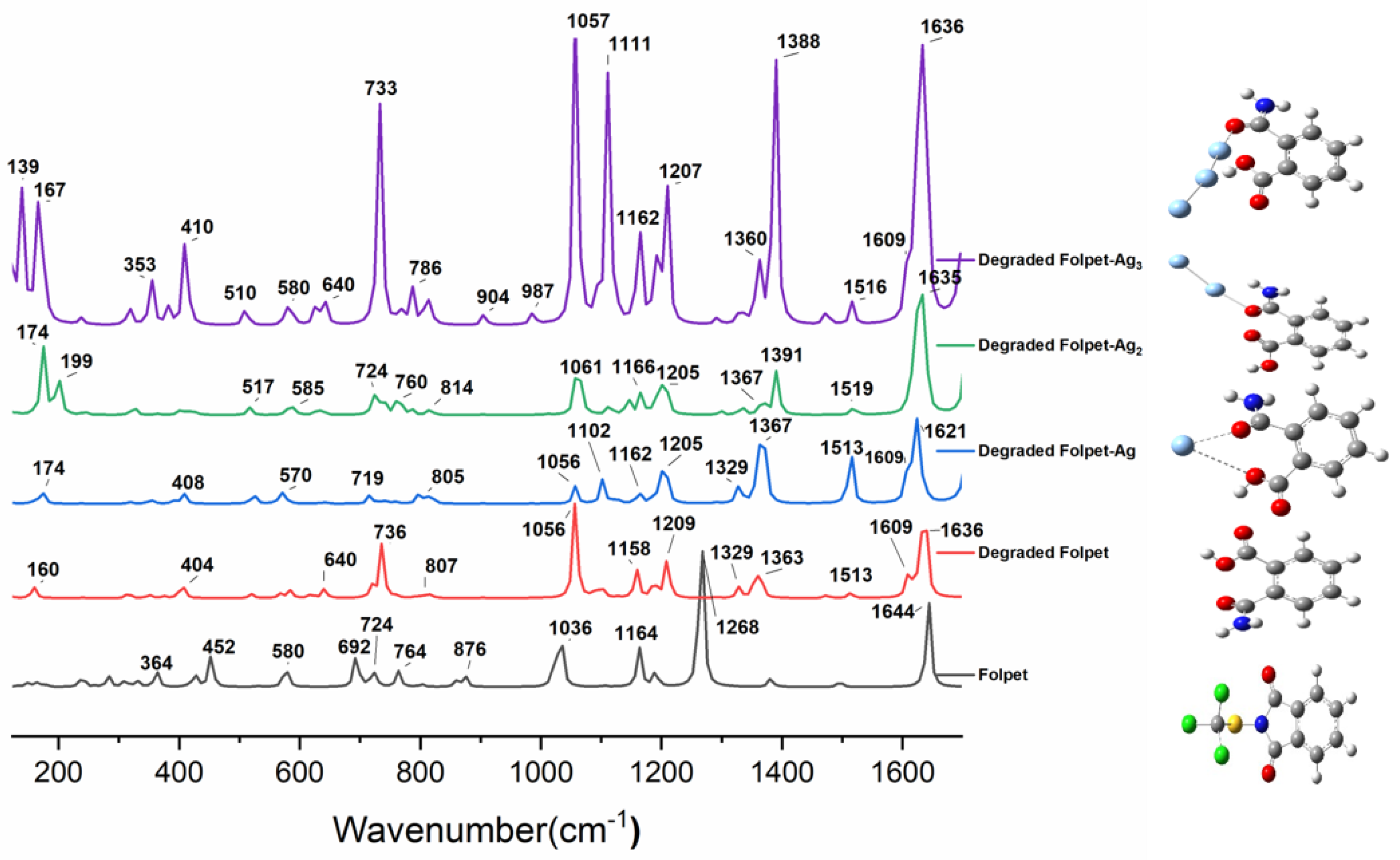
| Experimental Spectra | Theoretical Spectra | Assignments | |||
|---|---|---|---|---|---|
| Folpet SERS (cm−1) | Degraded Folpet | Degraded Folpet-Ag | Degraded Folpet-Ag2 | Degraded Folpet-Ag3 | |
| 20 | 17 | 15 | δ(Ag-NH2) + δ (OH) | ||
| 32 | 31 | 26 | 24 | δ(-CONH2) | |
| 65 | 53 | 54 | δ(Ag-OCNH2) + δ(COOH) | ||
| 86 | 95 | 95 | 96 | δ(NH2) + δ(HOCO) | |
| 110 | 115 | 125 | 139 | δ(OCNH2) | |
| 138 | 157 | 162 | 159 | 162 | δ(NH2) + τ(OH) |
| 160 | 174 | 174 | 167 | ν(Ag-Ag) + δ(NH2CO phynel) | |
| 237 | 237 | 239 | 243 | 238 | δ(NH2) + δ(COOH) |
| 350 | 351 | 324 | 353 | δ(CH) + τ(NH2) | |
| 373 | 390 | 366 | 382 | ρ(NH2) | |
| 424 | 404, 407 | 408 | 403, 415, 429 | 410 | δ(NH2) |
| 570 | 570 | 576 | 571 | δ(NH2) + δ(CCC) | |
| 584 | 580 | 585 | 580 | δ(OH) + δ(NH2) | |
| 613 | 618 | 621 | 630 | 626 | δ(NH2CO)+ δ(CCC) |
| 682 | 641 | 639 | 643 | 640 | HOCO def + δ(CCC) |
| 727 | 719 | 717 | 722 | 721 | τ(HOCO) |
| 736 | 737 | 737 | 733 | δ (HOCO) | |
| 757 | 756 | 763 | 766 | δ(CH) + δ(NH2CO) | |
| 773 | 795 | 796 | 785 | 786 | δ(CH) |
| 804 | 807, 817 | 807, 817 | 813, 817 | 809, 816 | δ(CCC) |
| 913 | 906 | 906 | 905 | 905 | ν(C-COO-) |
| 1009 | 1056 | 1056 | 1061 | 1057 | δ(CH) |
| 1165 | 1158 | 1162 | 1166 | 1162 | δ (HCCH) |
| 1177 | 1187 | 1188 | 1190 | 1190 | δ (HCCH) |
| 1209 | 1205 | 1205 | 1207 | δ(HOC) | |
| 1298 | 1292 | 1293 | 1298 | 1292 | δ(HCC) |
| 1329 | 1329 | 1332 | 1331 | ν(CC) | |
| 1363 | 1355, 1363 | 1359, 1367 | 1367 | 1360 | ν(NC) + δ(HOC) |
| 1391 | 1388 | ν(NC) | |||
| 1444 | 1474 | 1474 | 1473 | 1474 | δ(HCC) |
| 1511 | 1513 | 1513 | 1519 | 1516 | δ(HCC) |
| 1587 | 1609 | 1609 | 1612 | 1609 | ν(CC) |
| 1622 | 1620 | 1621 | 1626 | 1622 | δ(NH2) |
| 1649 | 1636 | 1635 | 1635 | 1636 | ν(C=O) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douass, O.; Samoudi, B.; Sanchez-Cortes, S. Sensitive Detection of Fungicide Folpet by Surface-Enhanced Raman Scattering: Experimental and Theoretical Approach. Chemosensors 2024, 12, 186. https://doi.org/10.3390/chemosensors12090186
Douass O, Samoudi B, Sanchez-Cortes S. Sensitive Detection of Fungicide Folpet by Surface-Enhanced Raman Scattering: Experimental and Theoretical Approach. Chemosensors. 2024; 12(9):186. https://doi.org/10.3390/chemosensors12090186
Chicago/Turabian StyleDouass, Oumaima, Bousselham Samoudi, and Santiago Sanchez-Cortes. 2024. "Sensitive Detection of Fungicide Folpet by Surface-Enhanced Raman Scattering: Experimental and Theoretical Approach" Chemosensors 12, no. 9: 186. https://doi.org/10.3390/chemosensors12090186
APA StyleDouass, O., Samoudi, B., & Sanchez-Cortes, S. (2024). Sensitive Detection of Fungicide Folpet by Surface-Enhanced Raman Scattering: Experimental and Theoretical Approach. Chemosensors, 12(9), 186. https://doi.org/10.3390/chemosensors12090186






