Chiral Trapped-Headspace GC-QMS-IMS: Boosting Untargeted Benchtop Volatilomics to the Next Level
Abstract
1. Introduction
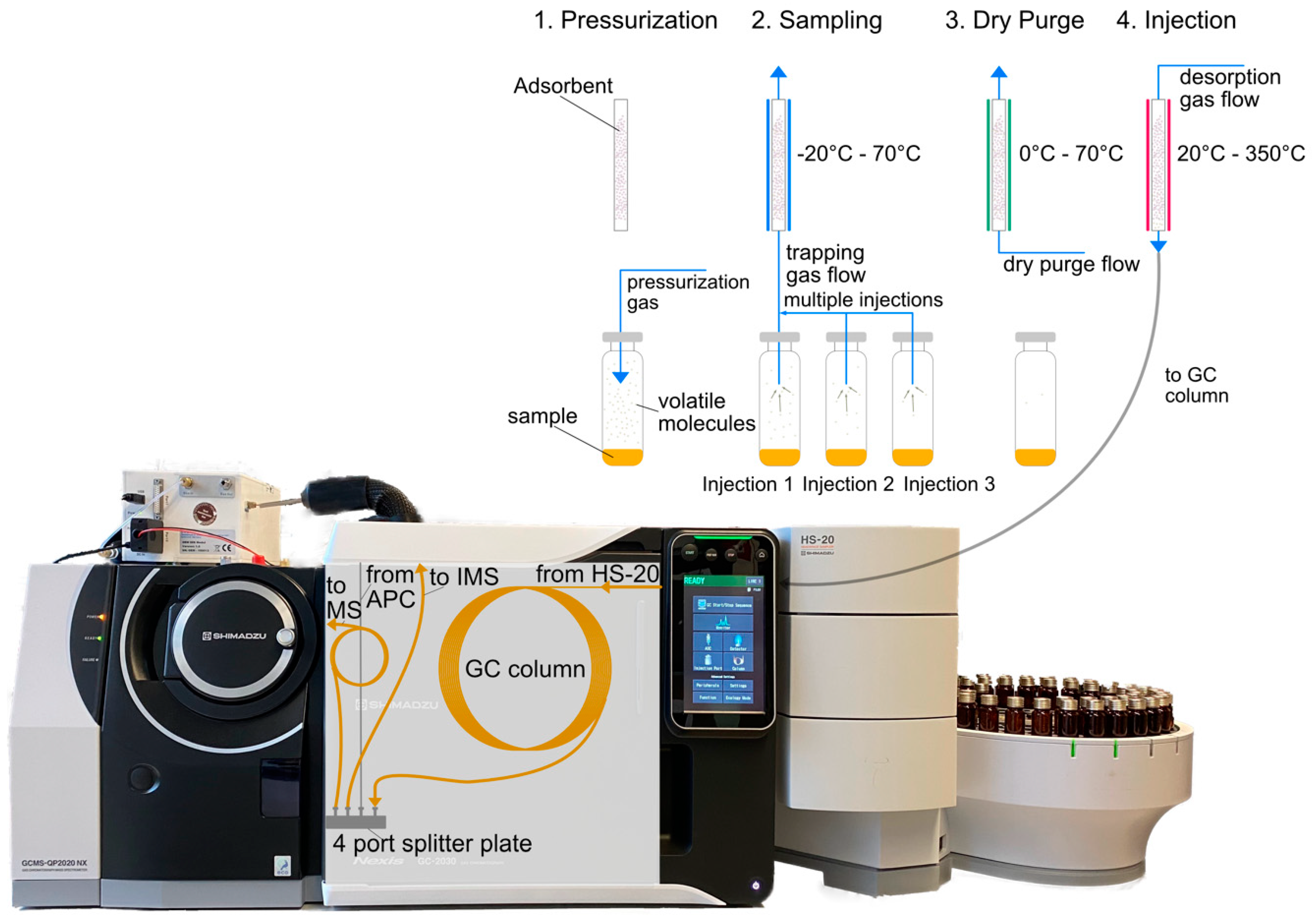
1.1. Trapped-Headspace Sampling
1.2. Ion Mobility Spectrometry in VOC Analysis
1.3. VOC Analysis of Mangos
2. Materials and Methods
2.1. Reagents and Mango Samples
2.2. Instrumentation (HS-GC-MS-IMS)
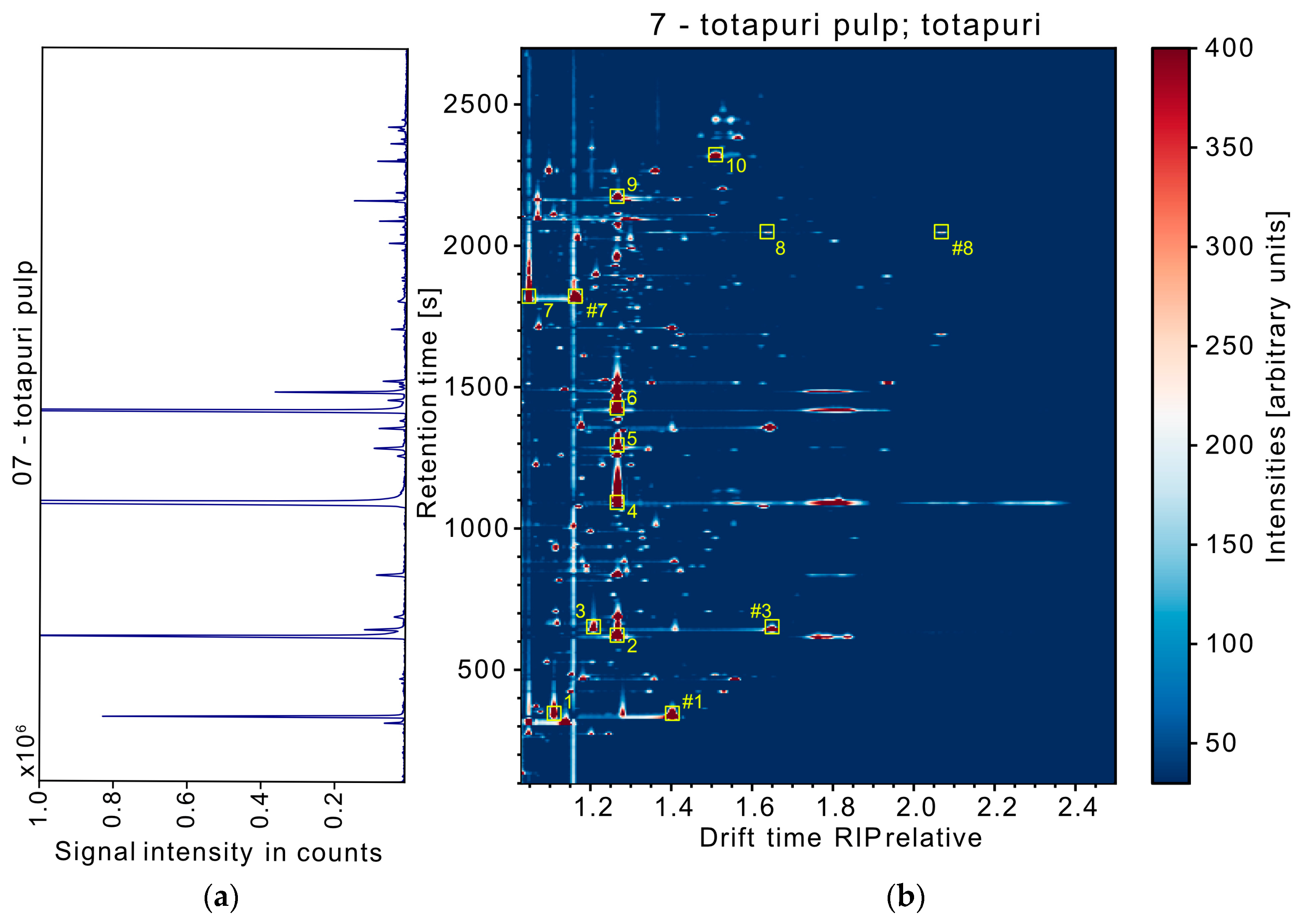
2.3. Data Processing and Evaluation
3. Results and Discussion
3.1. Performance of the THS-GC-MS-IMS System
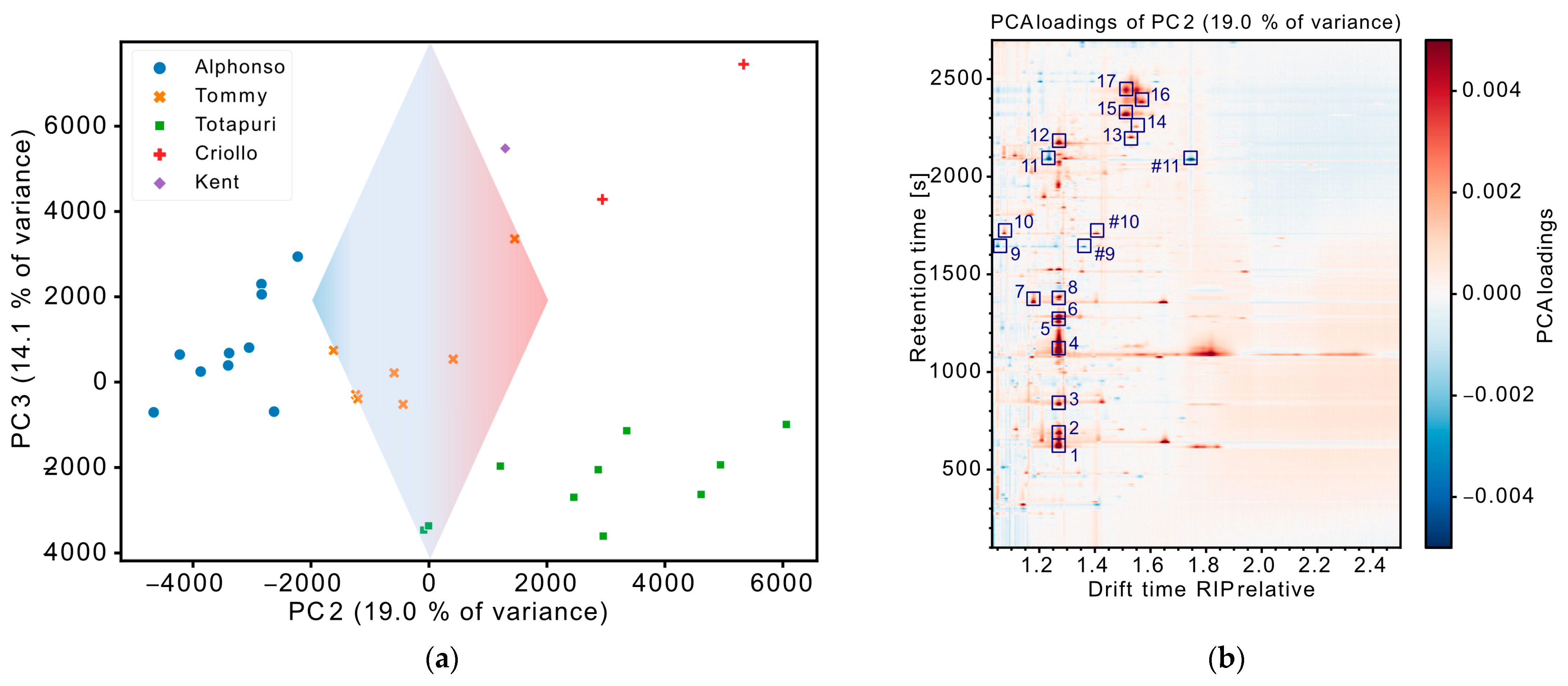
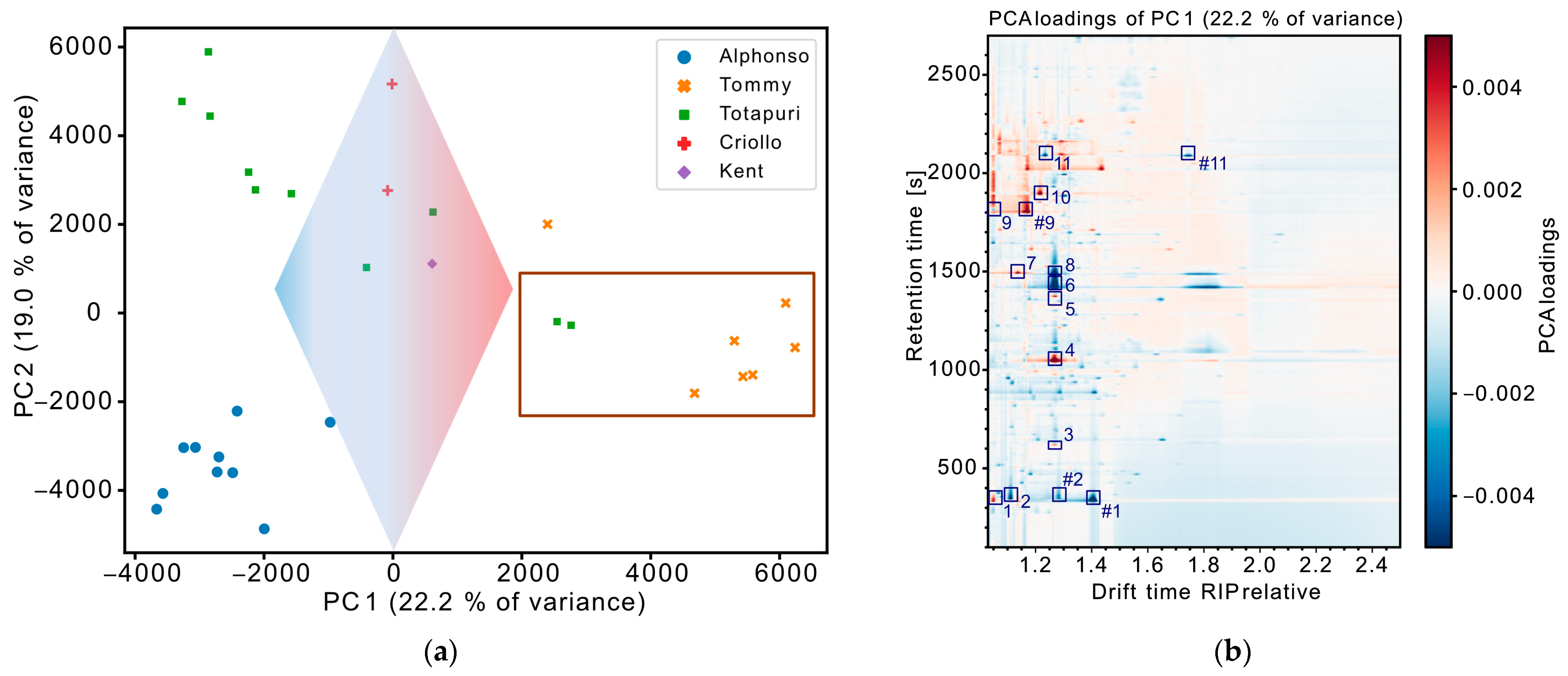
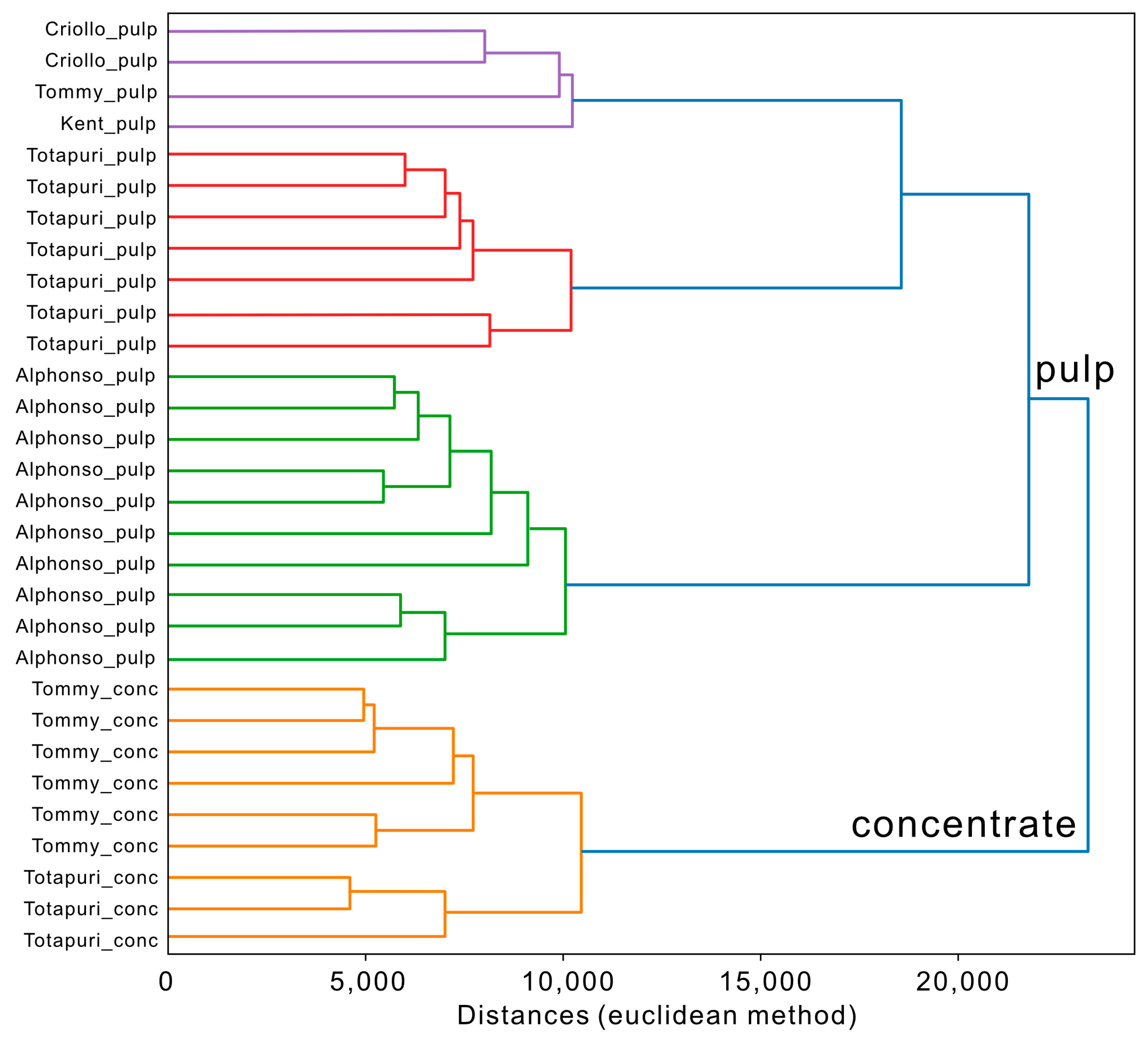
3.2. Exploratory Data Evaluation of Non-Targeted IMS Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernández-Mesa, M.; Ropartz, D.; García-Campaña, A.M.; Rogniaux, H.; Dervilly-Pinel, G.; Le Bizec, B. Ion Mobility Spectrometry in Food Analysis: Principles, Current Applications and Future Trends. Molecules 2019, 24, 2706. [Google Scholar] [CrossRef]
- Brendel, R.; Schwolow, S.; Rohn, S.; Weller, P. Volatilomic Profiling of Citrus Juices by Dual-Detection HS-GC-MS-IMS and Machine Learning-An Alternative Authentication Approach. J. Agric. Food Chem. 2021, 69, 1727–1738. [Google Scholar] [CrossRef]
- Capitain, C.; Weller, P. Non-Targeted Screening Approaches for Profiling of Volatile Organic Compounds Based on Gas Chromatography-Ion Mobility Spectroscopy (GC-IMS) and Machine Learning. Molecules 2021, 26, 5457. [Google Scholar] [CrossRef]
- Karpas, Z. Applications of ion mobility spectrometry (IMS) in the field of foodomics. Food Res. Int. 2013, 54, 1146–1151. [Google Scholar] [CrossRef]
- Parastar, H.; Weller, P. Towards greener volatilomics: Is GC-IMS the new Swiss army knife of gas phase analysis? TrAC Trends Anal. Chem. 2024, 170, 117438. [Google Scholar] [CrossRef]
- Borsdorf, H.; Eiceman, G.A. Ion Mobility Spectrometry: Principles and Applications. Appl. Spectrosc. Rev. 2006, 41, 323–375. [Google Scholar] [CrossRef]
- Schanzmann, H.; Ruzsanyi, V.; Ahmad-Nejad, P.; Telgheder, U.; Sielemann, S. A novel coupling technique based on thermal desorption gas chromatography with mass spectrometry and ion mobility spectrometry for breath analysis. J. Breath Res. 2023, 18, 016009. [Google Scholar] [CrossRef] [PubMed]
- Kremser, A.; Jochmann, M.A.; Schmidt, T.C. Systematic comparison of static and dynamic headspace sampling techniques for gas chromatography. Anal. Bioanal. Chem. 2016, 408, 6567–6579. [Google Scholar] [CrossRef]
- Poole, C.F. (Ed.) Gas Chromatography, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128206751. [Google Scholar]
- Soria, A.C.; García-Sarrió, M.J.; Sanz, M.L. Volatile sampling by headspace techniques. TrAC Trends Anal. Chem. 2015, 71, 85–99. [Google Scholar] [CrossRef]
- Costa Freitas, A.M.; Gomes da Silva, M.D.R.; Cabrita, M.J. Sampling Techniques for the Determination of Volatile Components in Grape Juice, Wine and Alcoholic Beverages. In Comprehensive Sampling and Sample Preparation: Analytical Techniques for Scientists; Academic Press: Amsterdam, The Netherlands, 2012; pp. 27–41. ISBN 9780123813749. [Google Scholar]
- Ikem, A. Measurement of volatile organic compounds in bottled and tap waters by purge and trap GC–MS: Are drinking water types different? J. Food Compos. Anal. 2010, 23, 70–77. [Google Scholar] [CrossRef]
- Schulz, K.; Dressler, J.; Sohnius, E.-M.; Lachenmeier, D.W. Determination of volatile constituents in spirits using headspace trap technology. J. Chromatogr. A 2007, 1145, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Manzini, S.; Durante, C.; Baschieri, C.; Cocchi, M.; Sighinolfi, S.; Totaro, S.; Marchetti, A. Optimization of a Dynamic Headspace-Thermal Desorption-Gas Chromatography/Mass Spectrometry procedure for the determination of furfurals in vinegars. Talanta 2011, 85, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Jeleń, H.; Gracka, A.; Myśków, B. Static Headspace Extraction with Compounds Trapping for the Analysis of Volatile Lipid Oxidation Products. Food Anal. Methods 2017, 10, 2729–2734. [Google Scholar] [CrossRef][Green Version]
- Soria, A.C.; García-Sarrió, M.J.; Ruiz-Matute, A.I.; Sanz, M.L. Headspace Techniques for Volatile Sampling. In Green Extraction Techniques—Principles, Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 255–278. ISBN 9780128110829. [Google Scholar]
- Christmann, J.; Weber, M.; Rohn, S.; Weller, P. Nontargeted Volatile Metabolite Screening and Microbial Contamination Detection in Fermentation Processes by Headspace GC-IMS. Anal. Chem. 2024, 96, 3794–3801. [Google Scholar] [CrossRef]
- Zacometti, C.; Sammarco, G.; Massaro, A.; Lefevre, S.; Frégière-Salomon, A.; Lafeuille, J.-L.; Candalino, I.F.; Piro, R.; Tata, A.; Suman, M. Authenticity assessment of ground black pepper by combining headspace gas-chromatography ion mobility spectrometry and machine learning. Food Res. Int. 2024, 179, 114023. [Google Scholar] [CrossRef] [PubMed]
- Babis, J.S.; Sperline, R.P.; Knight, A.K.; Jones, D.A.; Gresham, C.A.; Denton, M.B. Performance evaluation of a miniature ion mobility spectrometer drift cell for application in hand-held explosives detection ion mobility spectrometers. Anal. Bioanal. Chem. 2009, 395, 411–419. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, X.; Zhong, Y.; Chingin, K.; Chen, H. Rapid and sensitive detection of acetone in exhaled breath through the ambient reaction with water radical cations. Analyst 2021, 146, 5037–5044. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, X.; Chingin, K.; Xu, J.; Yan, X.; Chen, H. Mass spectrometry distinguishing C=C location and cis/trans isomers: A strategy initiated by water radical cations. Anal. Chim. Acta 2020, 1139, 146–154. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Tan, C.; Tian, Y.; Tao, L.; Xie, J.; Wang, M.; Zhang, F.; Yu, Z.; Sheng, J.; Zhao, C. Exploring the Effect of Milk Fat on Fermented Milk Flavor Based on Gas Chromatography–Ion Mobility Spectrometry (GC-IMS) and Multivariate Statistical Analysis. Molecules 2024, 29, 1099. [Google Scholar] [CrossRef]
- Valli, E.; Panni, F.; Casadei, E.; Barbieri, S.; Cevoli, C.; Bendini, A.; García-González, D.L.; Gallina Toschi, T. An HS-GC-IMS Method for the Quality Classification of Virgin Olive Oils as Screening Support for the Panel Test. Foods 2020, 9, 657. [Google Scholar] [CrossRef] [PubMed]
- Brendel, R.; Schwolow, S.; Rohn, S.; Weller, P. Gas-phase volatilomic approaches for quality control of brewing hops based on simultaneous GC-MS-IMS and machine learning. Anal. Bioanal. Chem. 2020, 412, 7085–7097. [Google Scholar] [CrossRef]
- Schanzmann, H.; Augustini, A.L.R.M.; Sanders, D.; Dahlheimer, M.; Wigger, M.; Zech, P.-M.; Sielemann, S. Differentiation of Monofloral Honey Using Volatile Organic Compounds by HS-GCxIMS. Molecules 2022, 27, 7554. [Google Scholar] [CrossRef]
- Lauricella, M.; Emanuele, S.; Calvaruso, G.; Giuliano, M.; D’Anneo, A. Multifaceted Health Benefits of Mangifera indica L. (Mango): The Inestimable Value of Orchards Recently Planted in Sicilian Rural Areas. Nutrients 2017, 9, 525. [Google Scholar] [CrossRef] [PubMed]
- Tharanathan, R.N.; Yashoda, H.M.; Prabha, T.N. Mango (Mangifera indica L.), “The King of Fruits”—An Overview. Food Rev. Int. 2006, 22, 95–123. [Google Scholar] [CrossRef]
- Shimizu, K.; Matsukawa, T.; Kanematsu, R.; Itoh, K.; Kanzaki, S.; Shigeoka, S.; Kajiyama, S.I. Volatile profiling of fruits of 17 mango cultivars by HS-SPME-GC/MS combined with principal component analysis. Biosci. Biotechnol. Biochem. 2021, 85, 1789–1797. [Google Scholar] [CrossRef]
- Tandel, J.; Tandel, Y.; Kapadia, C.; Singh, S.; Gandhi, K.; Datta, R.; Singh, S.; Yirgu, A. Nontargeted Metabolite Profiling of the Most Prominent Indian Mango (Mangifera indica L.) Cultivars Using Different Extraction Methods. ACS Omega 2023, 8, 40184–40205. [Google Scholar] [CrossRef]
- Musharraf, S.G.; Uddin, J.; Siddiqui, A.J.; Akram, M.I. Quantification of aroma constituents of mango sap from different Pakistan mango cultivars using gas chromatography triple quadrupole mass spectrometry. Food Chem. 2016, 196, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A.; Mesa, J.; Muñoz, Y.; Martí, M.P.; Marbot, R. Volatile components from mango (Mangifera indica L.) cultivars. J. Agric. Food Chem. 2005, 53, 2213–2223. [Google Scholar] [CrossRef]
- Mahattanatawee, K.; Goodner, K.; Baldwin, E.A. Volatile constituents and character impact compounds of selected Florida’s tropical fruit. Proc. Fla. State Hort. Soc. 2005, 118, 414–418. [Google Scholar]
- Pandit, S.S.; Chidley, H.G.; Kulkarni, R.S.; Pujari, K.H.; Giri, A.P.; Gupta, V.S. Cultivar relationships in mango based on fruit volatile profiles. Food Chem. 2009, 114, 363–372. [Google Scholar] [CrossRef]
- Ziegler, H. Flavourings: Production, Composition, Applications, Regulations, 2nd ed.; Wiley: Chichester, UK; Weinheim, Germany, 2007; ISBN 3527611452. [Google Scholar]
- Farag, M.A.; Dokalahy, E.U.; Eissa, T.F.; Kamal, I.M.; Zayed, A. Chemometrics-Based Aroma Discrimination of 14 Egyptian Mango Fruits of Different Cultivars and Origins, and Their Response to Probiotics Analyzed via SPME Coupled to GC-MS. ACS Omega 2022, 7, 2377–2390. [Google Scholar] [CrossRef]
- Indrati, N.; Sumpavapol, P.; Samakradhamrongthai, R.S.; Phonsatta, N.; Poungsombat, P.; Khoomrung, S.; Panya, A. Volatile and non-volatile compound profiles of commercial sweet pickled mango and its correlation with consumer preference. Int. J. Food Sci. Technol. 2022, 57, 3760–3770. [Google Scholar] [CrossRef]
- Bonneau, A.; Boulanger, R.; Lebrun, M.; Maraval, I.; Gunata, Z. Aroma compounds in fresh and dried mango fruit (Mangifera indica L. cv. K ent): Impact of drying on volatile composition. Int. J. Food Sci. Technol. 2016, 51, 789–800. [Google Scholar] [CrossRef]
- Li, L.; Yi, P.; Sun, J.; Tang, J.; Liu, G.; Bi, J.; Teng, J.; Hu, M.; Yuan, F.; He, X.; et al. Genome-wide transcriptome analysis uncovers gene networks regulating fruit quality and volatile compounds in mango cultivar ‘Tainong’ during postharvest. Food Res. Int. 2023, 165, 112531. [Google Scholar] [CrossRef]
- Xie, H.; Meng, L.; Guo, Y.; Xiao, H.; Jiang, L.; Zhang, Z.; Song, H.; Shi, X. Effects of Volatile Flavour Compound Variations on the Varying Aroma of Mangoes ‘Tainong’ and ‘Hongyu’ during Storage. Molecules 2023, 28, 3693. [Google Scholar] [CrossRef] [PubMed]
- Christmann, J.; Rohn, S.; Weller, P. gc-ims-tools—A new Python package for chemometric analysis of GC–IMS data. Food Chem. 2022, 224, 133476. [Google Scholar] [CrossRef]
- Parastar, H.; Christmann, J.; Weller, P. Automated 2D peak detection in gas chromatography-ion mobility spectrometry through persistent homology. Anal. Chim. Acta 2024, 1289, 342204. [Google Scholar] [CrossRef]
- Capitain, C.C.; Zischka, M.; Sirkeci, C.; Weller, P. Evaluation of IMS drift tube temperature on the peak shape of high boiling fragrance compounds towards allergen detection in complex cosmetic products and essential oils. Talanta 2023, 257, 124397. [Google Scholar] [CrossRef]
- D’Orazio, G.; Fanali, C.; Asensio-Ramos, M.; Fanali, S. Chiral separations in food analysis. TrAC Trends Anal. Chem. 2017, 96, 151–171. [Google Scholar] [CrossRef]
- Christmann, J.; Rohn, S.; Weller, P. Finding features—Variable extraction strategies for dimensionality reduction and marker compounds identification in GC-IMS data. Food Res. Int. 2022, 161, 111779. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.; Chidley, H.; Deshpande, A.; Schmidt, A.; Pujari, K.; Giri, A.; Gershenzon, J.; Gupta, V. An oxidoreductase from ‘Alphonso’ mango catalyzing biosynthesis of furaneol and reduction of reactive carbonyls. Springerplus 2013, 2, 494. [Google Scholar] [CrossRef] [PubMed]
- Kallio, H.P. Historical Review on the Identification of Mesifurane, 2,5-Dimethyl-4-methoxy-3(2 H)-furanone, and Its Occurrence in Berries and Fruits. J. Agric. Food Chem. 2018, 66, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, G.; Zhu, G. The imitation and creation of a mango flavor. Food Sci. Technol. 2022, 42, e34622. [Google Scholar] [CrossRef]
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses, 6th ed.; Wiley-VCH: Weinheim, Germany, 2016; ISBN 978-3-527-69317-7. [Google Scholar]
- Burdock, G.A.; Fenaroli, G. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2010; ISBN 9780429150838. [Google Scholar]
| Volatile Compound | Retention Time IMS [min] | Retention Time MS [min] | RT Difference MS-IMS [min] | MS Match Quality [%] |
|---|---|---|---|---|
| Ethyl acetate | 5.53 ± 0.02 | 5.50 ± 0.04 | 0.03 | 97 |
| α-pinene | 10.28 ± 0.01 | 10.23 ± 0.01 | 0.05 | 96 |
| Ethyl butyrate | 10.70 ± 0.02 | 10.66 ± 0.02 | 0.04 | 95 |
| β-myrcene | 18.15 ± 0.02 | 18.12 ± 0.03 | 0.03 | 95 |
| D-limonene | 21.42 ± 0.01 | 21.38 ± 0.01 | 0.04 | 95 |
| Trans-β-ocimene | 23.60 ± 0.03 | 23.56 ± 0.01 | 0.04 | 95 |
| Acetic acid | 30.10 ± 0.04 | 30.05 ± 0.05 | 0.05 | 95 |
| Nonanal | 34.11 ± 0.02 | 34.02 ± 0.01 | 0.07 | 95 |
| α-terpineol | 36.06 ± 0.03 | 36.01 ± 0.01 | 0.05 | 95 |
| β-caryophyllene | 38.51 ± 0.01 | 38.36 ± 0.01 | 0.15 | 96 |
| Mango Cultivar | ||||||
|---|---|---|---|---|---|---|
| No. | Volatile Compound | Alphonso | Totapuri | Criollo | Tommy | Kent |
| 1 | Ethanol | x | x | x | x | x |
| 2 | Ethyl acetate | x | x | x | x | x |
| 3 | Unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 4 | Ethylcyclohexane | x | x | x | x. | x |
| 5 | Ethyl propanoate | x | x (Pulp only) | x | x (Pulp only) | x |
| 6 | Propyl acetate | x | x (Pulp only) | IMS only | n.d. | IMS only |
| 7 | Methyl butyrate | IMS only | x (Pulp only) | x | x (Pulp only) | x |
| 8 | Unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 9 | Unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 10 | α-pinene | x | x | x | x | x |
| 11 | Ethyl butyrate | x | x (Pulp only) | x | x | x |
| 12 | Unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 13 | Camphene | x | x | x | x | x |
| 14 | 2-butenoic acid, methyl ester, (z)- | IMS only | x (Pulp only) | x | x (Pulp only) | x |
| 15 | Butyl acetate | IMS only | x | x | x | x |
| 16 | Unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 17 | β-pinene | x | x | x | x | n.d. |
| 18 | 1-butanol | x | x (Pulp only) | x | x | x |
| 19 | Isovaleraldehyd | x | x | x | x | x |
| 20 | Isobutanol | x | x (Pulp only) | x | x | IMS only |
| 21 | Isobutyraldehyde | x | x | x | x | x |
| Unidentified | IMS only | n.d. | n.d. | n.d. | IMS only | |
| 22 | Pentanal | x | x | x | x | x |
| 23 | Isopentyl alcohol | x | x (Pulp only) | x | x | x |
| 24 | 3-carene | n.d. | n.d. | x | x | x |
| 25 | Ethyl cyclopropancarboxylate | n.d. | x (Pulp only) | x | x | x |
| 26 | β-myrcene | x | x | x | x | x |
| 27 | α-phellandrene | IMS only | x | x | x | x |
| 28 | 2-butanone | x | x | x | x | x |
| 29 | α-terpinene | IMS only | x | x | x | x |
| 30 | Isobutyl butyrate | IMS only | x (Pulp only) | n.d. | n.d. | n.d. |
| 31 | D-limonene | x | x | x | x | x |
| 32 | Unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 33 | 2-butenoic acid, ethyl ester, (e) | n.d. | x (Pulp only) | x | x | x |
| 34 | β-phellandrene | n.d. | x | x | x | x |
| 35 | Trans-β-ocimene | x | x | x | n.d. | n.d. |
| 36 | 4-carene | n.d. | n.d. | x | x | x |
| 37 | 2,3-butanedion | x | x (Conc. only) | x | x | x |
| 38 | Cis-β-ocimene | x | x | x | n.d. | n.d. |
| 39 | Butyl butyrate | IMS only | x (Pulp only) | x | x | x |
| 40 | α-terpinolene | n.d. | x | x | x | x |
| 41 | Ethyl hexanoate | n.d. | n.d. | x | x | n.d. |
| 42 | 2-pronanone,1-methoxy | x | n.d. | x | n.d. | IMS only. |
| 43 | 2-methylbutyl butyrate | n.d. | x | x | n.d. | n.d. |
| 44 | Isoamyl butyrate | x | x (Pulp only) | x | x | x |
| 45 | 3-penten-2-one | x | x | x | x | x |
| 46 | Unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 47 | Unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 48 | Acetic acid | x | x | x | n.d. | x |
| 49 | Alloocimene | x | x | x | n.d. | n.d. |
| 50 | Ethyl-3-hydroxbutyrate | x | x | x | x | x |
| 51 | p-1,3,8-menthatriene | x | x | n.d. | n.d. | n.d. |
| 52 | Neo-alloocimene | x | x | x | n.d. | n.d. |
| 53 | Nonanal | x | x | x | x | x |
| 54 | Furfural | x | x | x | x | x |
| 55 | Ethyl octanoate | n.d. | x (Pulp only) | x | x | x |
| 56 | Trans-sabinene hydrate | n.d. | x | n.d. | n.d. | n.d. |
| 57 | 2,5-dimethyl-4-methoxy-3(2h)-furanone | x | n.d. | n.d. | n.d. | n.d. |
| 58 | Acetoin | n.d. | x | x | x | x |
| 59 | β-terpineol | n.d. | x | n.d | x | n.d. |
| 60 | Unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 61 | α-terpineol | n.d. | x | n.d. | x | n.d. |
| 62 | α-copaene | n.d. | x | n.d. | x | n.d. |
| 63 | α-gurjujene | n.d. | x | x | x | x |
| 64 | Unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 65 | β-caryophyllene | x | x | x | x | x |
| 66 | α-guaiene | n.d. | x | n.d. | n.d. | n.d. |
| 67 | Unidentified | n.d. | IMS only | IMS only | IMS only | IMS only |
| 68 | Ethyl decanoate | n.d. | n.d. | x | n.d. | n.d. |
| 69 | 1,4,7,-cycloundecatriene, 1,5,9,9- Tetramethyl-, z,z,z | x | x | x | x | x |
| 70 | 4,5-di-epi-aristolochene | n.d. | x | x | x | n.d |
| 71 | γ-gurjujene | n.d. | x | x | n.d. | n.d |
| Eremophilene | n.d. | n.d. | QMS only | n.d. | n.d | |
| 72 | β-selinene | n.d. | x | x | x (Pulp only) | n.d. |
| 73 | α-selinene | n.d. | x | x | x (Pulp only) | n.d. |
| 74 | α-bulnesene | n.d. | x | x | n.d. | n.d. |
| 75 | δ-cadinene | n.d. | x | x | n.d. | n.d. |
| 76 | β-cadinene | n.d. | QMS only | n.d. | n.d. | n.d. |
| 77 | γ-butyrolactone | x | n.d. | x | n.d. | n.d. |
| 78 | γ-hexalactone | x | n.d. | x | n.d. | IMS only |
| 79 | Cis-calamenene | n.d. | n.d. | QMS only | n.d. | n.d. |
| 80 | γ-octalactone | x | n.d. | x | n.d. | n.d. |
| 81 | Ethyl dodecanoate | x | n.d. | x | n.d. | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodenbender, L.; Rohn, S.; Sauer, S.; Jungen, M.; Weller, P. Chiral Trapped-Headspace GC-QMS-IMS: Boosting Untargeted Benchtop Volatilomics to the Next Level. Chemosensors 2024, 12, 165. https://doi.org/10.3390/chemosensors12080165
Bodenbender L, Rohn S, Sauer S, Jungen M, Weller P. Chiral Trapped-Headspace GC-QMS-IMS: Boosting Untargeted Benchtop Volatilomics to the Next Level. Chemosensors. 2024; 12(8):165. https://doi.org/10.3390/chemosensors12080165
Chicago/Turabian StyleBodenbender, Lukas, Sascha Rohn, Simeon Sauer, Markus Jungen, and Philipp Weller. 2024. "Chiral Trapped-Headspace GC-QMS-IMS: Boosting Untargeted Benchtop Volatilomics to the Next Level" Chemosensors 12, no. 8: 165. https://doi.org/10.3390/chemosensors12080165
APA StyleBodenbender, L., Rohn, S., Sauer, S., Jungen, M., & Weller, P. (2024). Chiral Trapped-Headspace GC-QMS-IMS: Boosting Untargeted Benchtop Volatilomics to the Next Level. Chemosensors, 12(8), 165. https://doi.org/10.3390/chemosensors12080165







