Abstract
Approximately 4% of women of reproductive age are estimated to suffer from premenstrual dysphoric disorder (PMDD), a condition likely underdiagnosed due to various biases, suggesting that actual prevalence may be higher. Addressing this, a novel electrochemical sensor was developed using a screen-printed electrode of reduced graphene oxide modified with a Cu(II) triazole complex, Cu(LNO2)2/rGO/SPCE. This sensor aims to determine levels of serotonin and 17β-estradiol rapidly, and simultaneously, key analytes implicated in PMDD. The method demonstrated high sensitivity for both analytes, achieving sensitivity levels of 0.064 μA/μmol L−1 for serotonin and 0.055 μA/μmol L−1 for 17β-estradiol, with a linear detection range of 2 to 42 μmol L−1. Detection limits were 42 nmol L−1 for serotonin and 53 nmol L−1 for estrogen. The sensor also exhibited high stability and selectivity against common interferents found in biological fluids. It was successfully used to measure serotonin and 17β-estradiol in human serum and urine, with recovery percentages within the expected ranges. This demonstrates that the sensor proposed in this work holds significant potential to contribute not only to the accurate diagnosis of such disorders but also to their treatment. We hope that this research will pave the way for the development of devices that have a positive impact on the quality of life of women suffering from multisystem diseases caused by hormonal malfunctions.
1. Introduction
Premenstrual disorders consist of a set of somatic and psychiatric symptoms that occur in the luteal phase of the menstrual cycle [1]. The International Society for the Study of Premenstrual Disorders (ISPMD) classifies premenstrual symptoms into 3 groups: (i) women who have presented occasional psychiatric and somatic symptoms during the luteal phase; (ii) women who present Premenstrual Syndrome (PMS) where the symptoms can affect daily life; and (iii) Premenstrual Dysphoric Disorder (PMDD), where severe and disabling symptoms occur, significantly affecting quality of life and female productivity [1,2,3]. It has been reported that 80% of women of reproductive age have experienced minor symptoms associated with PMS, 12% have been diagnosed with PMS and 4% suffer from PMDD, the latter comes with much uncertainty because the symptoms are similar to those of bipolar affective disorder (BAD), confusing its diagnosis [2,3,4]. PMS is associated with somatic symptoms such as headaches, muscle pain, a feeling of fullness, weight gain, change in appetite, lack of energy, insomnia or hypersomnia, breast tenderness, pelvic pain, and abdominal distention [5]. The diagnostic criteria for PMDD, as defined by the American Psychiatric Association (APA) in the DSM-V, include somatic symptoms described and psychiatric symptoms such as anger outbursts, anxiety, mental confusion, depression, irritability, social withdrawal, difficulty concentrating, and subjective feeling of being overwhelmed [5].
The affective symptoms related to these disorders represent a considerable and worrying burden, as they can make women particularly susceptible to suicidal tendencies, including ideation, planning, or even attempts. In this context, several studies have successfully associated suicide attempts in women with specific phases of their menstrual cycle and with levels of steroid hormones such as estrogen and progesterone [6,7,8,9,10,11,12]. Papadopoulou et al. [9] found that 59% of suicide attempts by women occurred during the last four days of the luteal phase and the first four days of menstruation. On the other hand, Leenaraars et al. [8] carried out a study to evaluate the influence of the menstrual cycle on suicide. They performed autopsies on 56 women who had completed suicides and compared the findings with 44 women who died from other causes. The results indicated that 25% of the women who had committed suicide were menstruating at the time of death, compared to 4.5% of the control group. This led to the statistical conclusion that there might be an association between menstruation and suicide in women.
The etiology of PMDD is still an area of active research. Among the potential causes under investigation are genetic factors and stress-related mechanisms [13]. Identified mechanisms include involvement of the following: steroid hormones such as progesterone, estradiol [14], and cortisol [15]; neurosteroids like allopregnanolone [16]; and neurotransmitters including GABAA receptors and serotonin [17]. The progesterone metabolite involved in PMDD is allopregnanolone (APα). This neuroactive steroid acts as a positive allosteric modulator of the GABAA receptor, exhibiting anxiolytic, anesthetic, and sedative properties. On the other hand, estradiol exerts potent effects on multiple neurotransmitter systems, playing a crucial role in regulating mood, cognition, sleep, eating, and various other behavioral aspects. Research has established that ovarian steroids alter the expression of the 5-HT2A receptor and the serotonin transporter (SERT). Clinically, women with PMDD exhibit specific abnormalities in serotonin (5-HT) levels, particularly evident in the late luteal phase when estrogen levels have decreased. These abnormalities manifest as low mood, cravings for specific foods, and poor cognitive performance [1,6,18,19].
Although previous studies have linked PMS or PMDD with suicidal tendencies and have identified the involved analytes, research on this condition remains limited. Of the few studies conducted, most focus on the psychiatric aspect, often overlooking physiological investigations in both diagnosis and treatment. This limitation can be attributed to the use of analytical techniques such as liquid chromatography, fluorescence and gas chromatography, as well as enzyme-linked immunoassay techniques for detecting estrogen, progesterone, and serotonin [20,21,22,23,24,25,26,27]. Despite their excellent analytical capabilities, these methods pose challenges, such as cost and analysis time, for conducting studies that accurately profile the variation of these analytes in the menstrual cycle. In this field, electrochemical sensors are in a unique position to allow the miniaturization of a clinical laboratory.
Currently, no electrochemical device capable of simultaneously determining estradiol and serotonin with a focus on PMDD detection and control has been developed. Regarding serotonin, several analytical methodologies have been reported for its detection in human serum and urine samples using electrodes modified with various materials. Yilmaz et al. [28] reported ultrasensitive electrochemical sensors for the simultaneous determination of dopamine and serotonin, using a SPCE modified with TiO2NP, achieving detection limits of 2.47 nmol L−1 for serotonin. In contrast, Gholivand et al. [29] developed a method for the simultaneous determination of dopamine, serotonin, and tryptophan using a carbon paste electrode modified with ZrO2–CuO co-doped with CeO2, reaching detection limits of 3.49 nmol L−1. Both methodologies exhibit excellent detection limits; however, they do not include estradiol in their determinations. Regarding estradiol determination, Honeychurch et al. [30] developed a methodology using SPCE modified with rGO-AuNPs/CNT, reporting a detection limit of 3 nmol L−1. However, this methodology was used for water samples. Conversely, Lingling et al. [31] reported a method applied to human serum samples. They modified a glassy carbon electrode with a wrinkled mesoporous carbon nanomaterial, obtaining a detection limit of 8.3 nmol L−1.
In this context, with the aim of developing a portable, fast, and direct analytical method for the simultaneous determination of serotonin and estrogen, we fabricated a sensor based on the modification of a reduced graphene oxide screen printed electrode (rGO/SPCE) with a Cu(II) triazole complex substituted with nitro groups on the phenyl ring (see Figure 1). This complex was synthesized by Nelson et al., who studied its capacity for hydrogen peroxide and sulfite detection [32,33]. In these studies, the authors reported the excellent electrocatalytic properties of Cu(LNO2)2 complex, attributing them to the resonance effect between the triazole and phenyl rings substituted with electron withdrawing groups. In addition, the nitro group in the phenyl ring enhances the reduction capacity of the complex due to its electron-accepting nature, thereby improving the electroactive capacity in the oxidation of species.
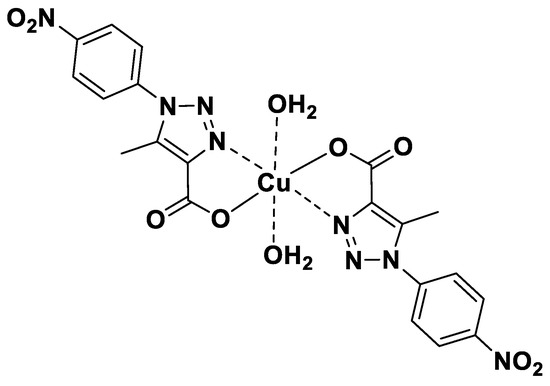
Figure 1.
Chemical structure of copper(II) complexes Cu(LNO2)2 [32].
2. Materials and Methods
2.1. Reagents
Serotonin (CAS 50-67-9), Estrogen 17β-estradiol (CAS 50-28-2), Ethanol (CAS 64-17-5), dihydrogen phosphate (KH2PO4) (CAS 7778–77–0), dipotassium hydrogen phosphate (K2HPO4) (CAS 7758–11–4), dopamine (CAS 62-31-7), uric acid (CAS 69-93-2), ascorbic acid (CAS 50–81–7), potassium chloride, potassium hexacyanoferrate (II) trihydrate, potassium hexacyanoferrate (II) trihydrate, and human serum (P2918) were purchased from Sigma-Aldrich. Synthetic human urine (BR-397) was purchased from Bio-Rad Laboratories. Screen-printed reduced graphene oxide (refs.110-RGPHOX) was purchased from DropSens (Madrid, Spain). Deionized water for sample preparation, dilution of reagents, and rinsing purposes was obtained from the laboratory water system Adrona CB 1901 (resistivity: 18.2 MΩ).
2.2. Apparatus
SEM images were obtained using a Hitachi FE-SEM SU5000 (Hitachinaka, Japan) with XFlash 6I30, Bruker detector. Raman spectra were collected using a Confocal Raman Microscope (Jasco, NRS-4500, Easton, MD, USA), equipped with an air-cooled Peltier CCD detector, and employing a 785 nm wavelength laser. The scanned range was from 50 to 4000 cm−1, at a data interval of 1 cm−1, collecting three accumulations of 90 s each, and using a laser power of 0.3 mW to avoid damage to the samples. Electrochemical measurements were carried out on a Potentiostat/Galvanostat Origaflex OGF05A with an impedance module OGFEIS.
2.3. Fabrication of Cu(LNO2)2/rGO/SPCE
The preparation of the Cu(II) triazole complex was carried out as described by Nelson et al. [32]. Cu(LNO2)2 was diluted to 1 mg mL−1 in DMF reagent and sonicated for 30 min. This solution was homogenized by stirring, and then 2 μL was coated over the working electrode’s surface and dried at a temperature of 60 °C for 10 min. This procedure was repeated successively until reaching a total volume of 10 μL of Cu(II) triazole complex (1 mg mL−1).
2.4. Electrochemical Measurements
The electrochemical cell was composed of a modified Cu(LNO2)2/rGO screen-printed electrode and supporting electrolyte with a 40%/60% (v/v) mixture of ethanol and phosphate buffer solution. PB was prepared by mixing K2HPO4 and KH2PO4 solutions at 0.1 M and pH 7.0, and the pH was adjusted with 4.0 mol L−1 of sodium hydroxide solution. For analyte measurement, a mixture of 10 mmol L−1 serotonin and 10 mmol L−1 17β-estradiol stock solution was prepared in ethanol. A solution of 1 mmol L−1 serotonin and 1 mmol L−1 17β-estradiol was prepared daily from this stock solution and diluted in a mixture of 40% ethanol and 60% 0.1 mol L−1 phosphate buffer solution. Linear sweep voltammetry (LSV) was carried out over a potential range of 0.0 to 1.0 V at a scan rate of 50 mV s−1. Cyclic voltammetry (CV) was performed from −800 to 800 mV with a sweep speed of 50 mV s−1. Impedance measurements were performed with frequency from 0.1 to 400 kHz, pulse amplitude of 10 mV, and open circuit using KCl 0.1 mol L−1/[Fe(CN)6]3−/4−.
3. Results
3.1. Morphological Characterization of Cu(LNO2)2/rGO/SPCE
Morphologically, characterization by field emission scanning electron microscopy (FE-SEM) and elemental mapping of SPCE, rGO/SPCE, and Cu(LNO2)2/rGO/SPCE were performed, as shown in Figure 2a–c. SPCE and rGO/SPCE exhibited typical morphology of commercial electrodes (Figure 2a,b). Elemental mapping corroborated the presence of Cu on the surface of the modified rGO/SPCE and revealed a smooth film, indicating that Cu(LNO2)2 covered the electrode surface with a homogeneous film (Figure 2c). The Raman spectra of pure SPCE, rGO/SPCE, Cu(LNO2)2/rGO/SPCE, and Cu(LNO2)2 pure complexes were analyzed and depicted in Figure 3. The G and D bands, corresponding to the in-plane stretching vibrations of the sp2 carbons and the ring breathing mode from the sp2 carbons ring, respectively, were throughout the electrode preparation process. Furthermore, pure SPCE exhibited the highest intensity of the characteristic G and D bands, indicating the successful dispersion of the complex and rGO on the electrode by comparing the Raman spectra. Additional evidence is obtained by examining the peak shapes, which generally broaden as the bare electrode is prepared with rGO and subsequently with the complex. The latest spectra show the widest peaks, as these signals include the Raman modes present in the same region as the complex.
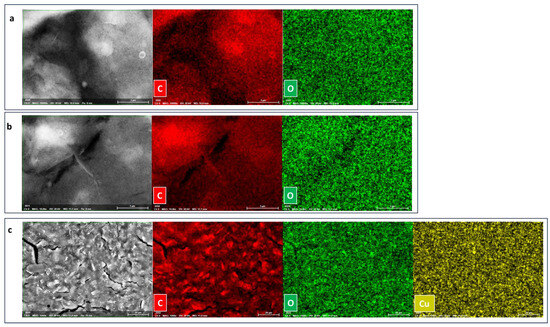
Figure 2.
SEM and EDS element mapping images of (a) SPCE, (b) rGO/SPCE, and (c) Cu(LNO2)2/rGO/SPCE. See EDS spectra in supporting materials (Figure S1).
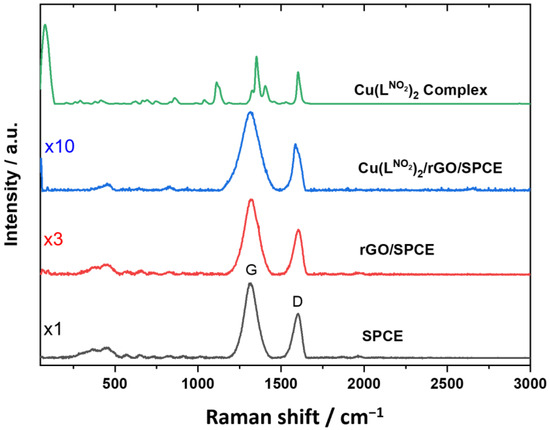
Figure 3.
Raman spectra of a SPCE, rGO/SPCE, Cu(LNO2)2/rGO/SPCE, and Cu(LNO2)2 pure complex.
3.2. Electrochemical Characterization of Cu(LNO2)2/rGO/SPCE
The electrochemical performance of unmodified SPCE and modified rGO/SPCE and Cu(LNO2)2/rGO/SPCE was studied using cyclic voltammetry in the presence of 5 mmol L−1 [Fe(CN)6]3−/4− in 0.1 M KCl. Electrochemical impedance spectroscopy (EIS) was utilized to evaluate the interfacial properties of the Cu(LNO2)2 modified electrode. Cyclic voltammograms are shown in Figure 4a. A well-defined redox peak appears at SPCE and rGO/SPCE; however, when the electrode was modified with Cu(LNO2)2, the reversibility of the model system [Fe(CN)6]3−/4− decreased, shifting the signals to less positive potentials. The current intensity of the redox couple peaks increased when Cu(LNO2)2 was deposited on the electrode surface, demonstrating that its conductive characteristics facilitate electron transfer. The Nyquist diagram and equivalent circuit model used for the three electrodes are represented in Figure 4b, where Rs indicates the resistance to the electrolyte, Rct is the resistance to charge transfer and Wo is the Warburg resistance. The results obtained showed that the Rs for the rGO electrode was 2.24 Ω which is lower than that for Cu(LNO2)2/rGO and SPCE, with resistance values 2.75 and 2.31 Ω, respectively, indicating that rGO improves the electrode wettability. However, the Cu(LNO2)2 addition increased the resistance of the electrolyte probably due to the polar properties of 4-nitrophenyl-1,2,3-triazole groups in the complex structure (see Table S2 in supporting materials). On the other hand, the charge transfer resistance (Rct) was determined, showing a lowest semicircle in the Nyquist diagram for Cu(LNO2)2/rGO/SPCE (see inset of Figure 4b). The determined resistance values were 767.40, 836.70, and 869.40 Ω for Cu(LNO2)2/rGO/SPCE, rGO/SPCE, and SPCE, respectively, demonstrating that the addition of the Cu(LNO2)2 complex on the rGO electrode surface improves the charge transfer attributable to the conjugated character of Cu(LNO2)2 structure. This property allows greater charge transfer [32]. Finally, the electron-withdrawing properties of the -NO2 group caused a shift of the copper redox couple towards more cathodic potentials, allowing a rapid charge transfer and a higher electron flow at a lower energy cost than electrodes. This can also be demonstrated with the Warburg resistance (Wo), which is related to the diffusion processes at the electrode/electrolyte interface. The values obtained for Cu(LNO2)2/rGO/SPCE/ was 5.34 Ω, while that for the electrode of rGO/SPCE was 6.57 Ω and SPCE was 10.34 Ω, indicating that the complex improves electronic transfer processes and shows less dependence on controlled processes by diffusion and mass transport.
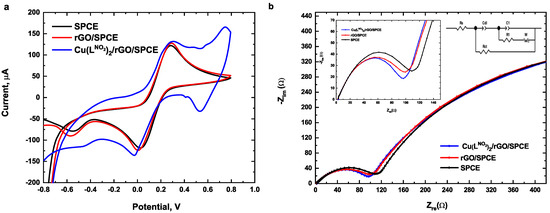
Figure 4.
(a) Cyclic voltammograms (b) Nyquist plot of SPCE, rGO/SPCE, and Cu(LNO2)2/rGO/SPCE in 5 mmol L−1 [Fe(CN)6]3−/4− 0.1 mol L −1 KCl.
3.3. Electrochemical Performance of Cu(LNO2)2/rGO/SPCE
Initially, the electrochemical oxidation of serotonin and 17β-estradiol were studied by linear sweep voltammetry (LSV) on four different electrochemical platforms: (i) SPCE; (ii) nAu/SPCE; (iii) nPEDOT/SPCE; and (iv) rGO/SPCE, all modified with 1 mg mL−1 of Cu(LNO2)2 (Figure 5a,b). The analytes signals were monitored in a potential range from 0.0 to 1.0 V and at a scan rate of 50 mV/s. The results showed that when the Cu(LNO2)2 complex was added to the SPCE surface, a very small signal current was observed for 17β-estradiol (0.31 μA). Similarly, for nAu/SPCE, the signal current intensity was low for both analytes (0.38 μA for serotonin and 0.51 μA for 17β-estradiol). Better results were obtained with nPEDOT; however, the increase in the current response was significantly higher with rGO/SPCE. This enhancement is attributed to the wrinkled surface of rGO, which increases the effective electrode area [34].
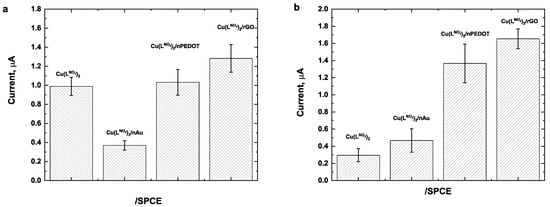
Figure 5.
Electrochemical response of 25 μmol L−1 (a) serotonin and (b) 17β-estradiol obtained with different electrochemical platforms modified with 1 mg mL−1 of Cu(LNO2)2. Conditions: 40/60 EtOH/PB 0.1 mol L−1, pH 7.0, Cu(LNO2)2 1 mg/mL scan rate of 50 mV/s.
The electrochemical processes of serotonin and 17β-estradiol were investigated simultaneously by cyclic voltammetry (CV) using the Cu(LNO2)2/rGO/SPCE in a potential range from 0.0 to 1.2 V and at a scan rate of 50 mV/s. As shown in Figure 6, two peaks were observed with modified Cu(LNO2)2/rGO/SPCE, indicating the simultaneous oxidation of serotonin and 17β-estradiol (Figure 6, red line). In contrast, the unmodified rGO/SPCE shows a peak corresponding to the serotonin and a very weak oxidation peak for 17β-estradiol (see inset of Figure 6). The signal enhancement observed when using Cu(LNO2)2/rGO/SPCE can be ascribed to the synergistic effect between Cu(LNO2)2 and rGO which improves the electrocatalytic ability for the oxidation of 17β-estradiol. On the other hand, no reduction peak was observed for any of the analytes, indicating that the electrochemical process of serotonin and 17β-estradiol are irreversible. The oxidation peak of serotonin and 17β-estradiol was +0.20 and +0.42 V (vs. Ag/AgCl), respectively.
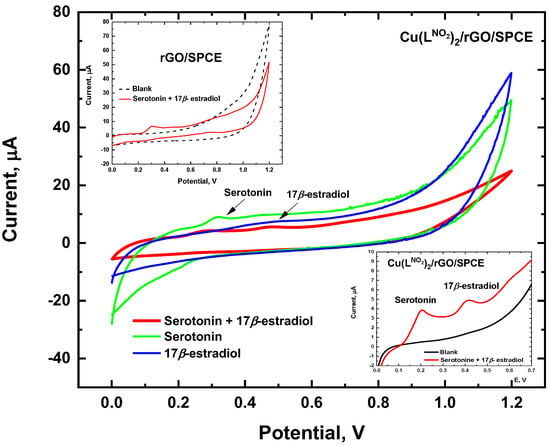
Figure 6.
Electrochemical response simultaneous oxidation of 25 μmol L −1 serotonin and 17β-estradiol. Conditions: 40/60 EtOH/PB 0.1 mol L −1, pH 7.0, Cu(LNO2)2 1 mg/mL scan rate of 50 mV/s.
For a better understanding of the mechanism behind the electrochemical behavior of serotonin and 17β-estradiol on the Cu(LNO2)2/rGO/SPCE and the possible role of atmospheric oxygen in the oxidation process, we carried out electrochemical measurement without oxygen, by continuous bubbling of N2 into the solution, observing that the oxidation peaks of serotonin and 17β-estradiol remained unchanged at +0.20 and +0.42 V (vs. Ag/AgCl), respectively, which was indicative that the process electrochemical oxidation is independent of the presence of oxygen (see Figure S2 in supporting materials). The proposed mechanism for simultaneous serotonin and 17β-estradiol oxidation on the surface of Cu(LNO2)2/rGO/SPCE is presented in Figure 7. According to the experimental results, the oxidation of serotonin is generated by a process via two electrons, similar to processes described for serotonin and dopamine [35,36,37,38], while the oxidation of 17β-estradiol corresponds to a process via one electron, similar to process previously described for 17β-estradiol [39,40,41], estriol [42], and bisphenol A [43].
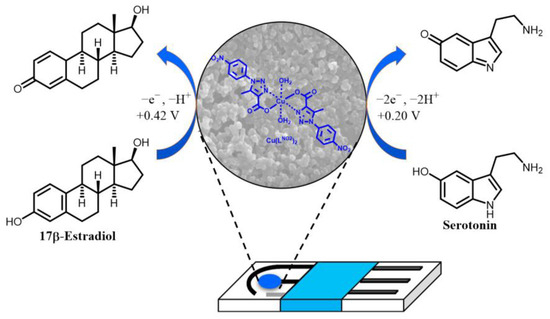
Figure 7.
Proposed mechanism of serotonin and 17-β-estradiol oxidation at Cu(LNO2)2/rGO/SPCE.
3.4. Optimization of Parameters for LSV Serotonin and 17β-Estradiol Simultaneous Detection
The effects of the concentration of Cu(LNO2)2 and pH of HPO42−/PO43 solution on the oxidation current response of a mixture of serotonin and 17β-estradiol were investigated using linear sweep voltammetry (LSV). As shown in Figure 8a, the serotonin oxidation peak current increased between pH 5.0 and pH 7.0, followed by a decrease at pH 8.0 and pH 10.0 which is attributable to the possible decomposition of the Cu(LNO2)2 complex as suggested by Nelson et al. [32]. Moreover, 17β-estradiol maximal current response was observed at pH 6.0. On the other hand, for both analytes, a progressive shift of the Ep to less positive potentials were observed, indicating a pH-dependent behavior. The slope of the linear relationship between the Ep and pH for serotonin and 17β-estradiol were −51 and −54 mV, respectively, close to the theoretical value of −59 mV. This confirms that an equal number of protons and electrons are involved in the electro-oxidation process of serotonin and 17β-estradiol, as reported by Yu et. al. [34] and Brett et al. [44] (see Figure S3 in Supporting Materials). The concentration of Cu(LNO2)2 deposited over the electrode surface was evaluated in a range of 0.4 to 1.2 mg m L−1 (Figure 8b). The maximal current response for both analytes was obtained at complex concentration of 1.0 mg mL−1. Adding higher concentrations of the complex led to a decrease in the signal current, which can be ascribed to the fact that at greater concentrations, 4-nitrophenyltriazole groups in the complex structure overlap each other, hindering electron transfer. Consequently, taking into account the equilibrium of the current response for serotonin and 17β-estradiol, the optimum conditions selected for future studies were as follows: pH 7.0 for the HPO42−/PO43 solution and a Cu(LNO2)2 concentration of 1.0 mg mL−1.
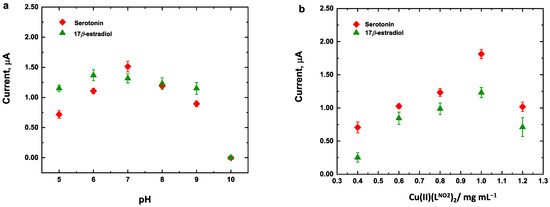
Figure 8.
Optimization of experimental conditions of (a) pH and (b) Cu(LNO2)2 concentration for LSV electrochemical response of Cu(LNO2)2/rGO/SPCE for simultaneous analysis of 25 μmol L−1 serotonin and 17β-estradiol.
3.5. Analytical Performance of Cu(LNO2)2/rGO/SPCE
Linear sweep voltammetry (LSV) was used for the simultaneous detection of serotonin and 17β-estradiol under the optimum conditions previously described. Figure 9a shows the oxidation curves at Cu(LNO2)2/rGO/SPCE and Figure 9b the linear relationships between the current response and concentrations for both analytes. The oxidation peak current for serotonin and 17β-estradiol increases linearly with the concentration in a range of 2.0–42 μmol L−1 (R2 = 0.9981 for serotonin and R2 = 0.9978 for 17β-estradiol). For serotonin, the sensitivity was 0.064 ± 0.001 μA/μmol L−1 and intercept was 0.069 ± 0.02 μA/μmol L−1. For 17-β-estradiol, the sensitivity was 0.055 ± 0.002 μA/μmol L−1 and the intercept was −0.087 ± 0.005 μA/μmol L−1. The LOD was calculated (S/N = 3) as described by Shrivastava et al. [45] for six measurements of blank. The values determined were 42 and 53 nmol L−1 for serotonin and 17β-estradiol, respectively.
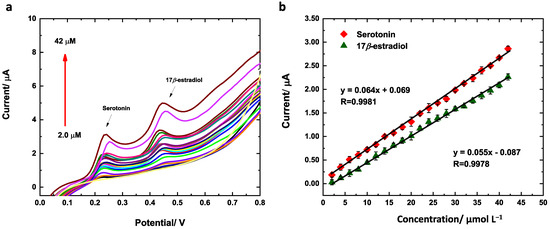
Figure 9.
(a) Calibration curves and (b) LSV responses of Cu(LNO2)2/rGO/SPCE for the simultaneous analysis of 25 μmol L−1 serotonin and 17β-estradiol. Conditions: 40/60 EtOH/PB 0.1 mol L−1, pH 7.0, Cu(LNO2)2 1 mg/mL and scan rate of 50 mV/s.
The methodology developed based on the preparation of Cu(LNO2)2/rGO/SPCE was compared with other electrochemical sensors previously reported since 2020 (Table 1). The LDs for the analytes, serotonin, and estradiol were considerably low. The use of the bidentate triazole complex combined with the characteristics of rGO increased the electrocatalytic capacity and allowed a speciation of these analytes through the difference in their oxidation potential.

Table 1.
Comparison of the Cu(LNO2)2/rGO/SPCE with other electrochemical sensor-modified electrodes for detecting serotonin and 17β-estradiol.
3.6. Repeatability, Stability, and Selectivity of the Cu(LNO2)2/rGO/SPCE
The repeatability of the analytical method was determined from the responses at 15 consecutive measurements in the presence of 25 μmol L−1 of a mixed solution of serotonin and 17β-estradiol. The relative standard deviations obtained were 10.6 and 9.1%, respectively. For stability studies, the electrode was stored at 4 °C for 15 days and the measurements were made every day. Figure 10a,b shows that the fabricated sensor exhibited a relative standard deviation (RSD) for serotonin of 6.1% after 10 days and 15% after 15 days. The RSD for 17β estradiol is 14.6% after 10 days and 15% after 15 days, suggesting satisfactory storage stability. The effect of some electroactive substances with similar oxidation potential of serotonin and 17β-estradiol was investigated utilizing LVS in the potentials range of 0.0 to 1.2 V and at a scan rate of 50 mV/s in 40/60 EtOH/PB 0.1 mol L−1, pH 7.0. Initially, 20 μmol L−1 of uric acid and ascorbic acid were studied, though no current signal was detected (see Figure S4 in supporting materials). Dopamine, which has a catechol group, was investigated at three concentration levels: 5, 10, and 20 μmol L−1. No current signal was detected at 5 and 10 μmol L−1; however, at 20 μmol L−1, a current signal was detected at 0.41 V (see Figure S5 in supporting materials). The effect of 20 μmol L−1 of dopamine on the oxidation peak of 20 μmol L−1 of serotonin and 17β-estradiol was investigated. The percentage values of current change in the presence of dopamine were 16.9% for serotonin and 6.1% for 17β-estradiol and the current signal peaks were shifted to more positive potentials, 0.31 V for serotonin and 0.52 V for 17β-estradiol (see Figure 10c). The similar oxidation potential of dopamine can be explained because the bidentate nature of catechols, such as dopamine, favors interactions with Cu(II) complexes, facilitating the oxidation of dopamine instead of serotonin and 17β-estradiol [55,56,57].
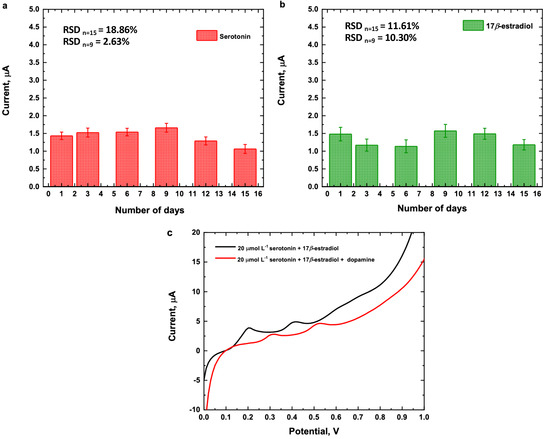
Figure 10.
Current measurements of 25 μmol L−1 (a) serotonin, (b) 17β-estradiol and (c) 20 μmol L−1 of a mixture of serotonin and 17β-estradiol in presence of 20 μmol L−1 of dopamine. Conditions: 40/60 EtOH/PB 0.1 mol L−1, pH 7.0, Cu(LNO2)2 1 mg/mL and scan rate of 50 mV/s.
3.7. Evaluation of the Performance of Cu(LNO2)2/rGO/SPCE for Detection Serotonin and 17β-Estradiol
In order to assess its applicability, the fabricated Cu(LNO2)2/rGO/SPCE and the analytical method were evaluated for measuring the quantity of serotonin and 17β-estradiol in serum and synthetic human urine using the standard addition method. The pH of human serum was measured at 7.3 and that of synthetic human urine at 6.0. Due to the complexity of the matrices, the current of the oxidation signals exhibited a negative shift in both matrices, as illustrated in Figure 11a,b. According to the calibration curve obtained, the recovery concentration values of the spiked samples are shown in Table 2. These values ranged between 85 and 96%, with a RSD% of ≤7.0%, indicating that the electrode fulfills its intended purpose under the optimal parameters.
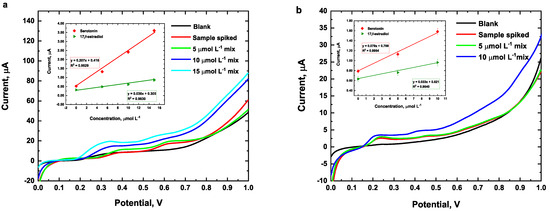
Figure 11.
Electrochemical response of human serum (a) and synthetic human urine (b) spiked with different concentrations of a mixture of serotonin and 17β-estradiol. Conditions: 40/60 EtOH/PB 0.1 mol L−1, pH 7.0, Cu(LNO2)2 1 mg/mL, and scan rate of 50 mV/s.

Table 2.
Determination of serotonin and 17β-estradiol in synthetic serum and human urine samples using Cu(LNO2)2/rGO/.
4. Conclusions
As has been described, the rapid and direct effect of 17β-estradiol on brain membranes is capable of modifying the availability of the serotonin receptor, which underscores its importance in the regulation of emotional disorders in women. However, to date, no research has been reported where methodologies are developed that allow the simultaneous determination of these analytes in diseases with a gender focus. In this work, it was possible to achieve significantly low detection limits for Cu(LNO2)2/rGO/SPCE, considering levels reported in previous works (Table 1). It was also possible to simultaneously determine serotonin and 17β-estradiol in enriched samples of human serum and urine with good accuracy and validity. Therefore, it can be concluded that the proposed method has a high potential for monitoring these analytes in samples of human biological fluids. Nevertheless, further research is required to achieve the quantification of 17β-estradiol with the required accuracy. On the other hand, it is important to highlight that the development of these devices could bridge the gap between sophisticated analytical techniques and user demands for convenience, ease of operation, versatility, and prompt results, and contribute to improving the diagnosis and treatment of multisystemic diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors12080164/s1, Figure S1. EDS spectra of Cu(LNO2)2/rGO/SPCE. Table S1. Equivalent circuit element values obtained by full nonlinear least squares fit of the EIS spectra. Figure S2. LSV electrochemical response of Cu(LNO2)2/rGO/SPCE for simultaneous analysis. Figure S3. Effect of pH on the oxidation peak potential of 25 μmol L−1 of serotonin and 17β-estradiol. Figure S4. LVS of 20 μmol L−1 of ascorbic and uric acid. Figure S5. LVS different concentrations of dopamine.
Author Contributions
C.N.: Writing—original draft, project administration, methodology, investigation, data curation, conceptualization, and supervision. R.N.: Investigation, formal analysis, review & editing, and supervision. G.T.: Investigation, formal analysis, review, and data curation. P.P.: Investigation, formal analysis, review, and data curation. R.C.: Investigation, formal analysis, review, and data curation. A.M.: Investigation, formal analysis, review & editing, and data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ANID FONDECYT, Chile (Postdoctoral N°3210269) and 202211010037-VRIDT-UCN.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the Unidad de Equipamiento Científico MAINI-UCN for allowing us to use FE-SEM SU5000 and Confocal Raman Microscope.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Halbreich, U.; Borenstein, J.; Pearlstein, T. The Prevalence, Impairment, Impact, and Burden of Premenstrual Dysphoric Disorder (PMS/PMDD). Psychoneuroendocrinology 2003, 28, 1–23. [Google Scholar] [CrossRef]
- Hofmeister, S.; Bodden, S.; College, M. Premenstrual Syndrome and Premenstrual Dysphoric Disorder. Am. Fam. Physician 2016, 94, 236–240. [Google Scholar]
- Ducasse, D.; Jaussent, I.; Olié, E.; Guillaume, S. Personality Traits of Suicidality Are Associated with Premenstrual Syndrome and Premenstrual Dysphoric Disorder in a Suicidal Women Sample. PLoS ONE 2016, 11, e0148653. [Google Scholar] [CrossRef]
- Slyepchenko, A.; Minuzzi, L.; Frey, B.N. Comorbid Premenstrual Dysphoric Disorder and Bipolar Disorder: A Review. Front. Psychiatry 2021, 12, 719241. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Hantsoo, L.; Epperson, C.N. Premenstrual Dysphoric Disorder: Epidemiology and Treatment. Curr. Psychiatry Rep. 2015, 28, 87. [Google Scholar] [CrossRef] [PubMed]
- Prasad, D.; Wollenhaupt-Aguiar, B.; Kidd, K.N.; Cardoso, T.D.A.; Frey, B.N. Suicidal Risk in Women with Premenstrual Syndrome and Premenstrual Dysphoric Disorder: A Systematic Review and Meta-Analysis. J. Women’s Health 2021, 30, 1693–1707. [Google Scholar] [CrossRef]
- Leenaars, A.A.; Dogra, T.D.; Girdhar, S.; Dattagupta, S.; Leenaars, L. Menstruation and suicide: A histopathological study. Crisis 2009, 30, 202–207. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Efstathiou, V.; Christodoulou, C.; Gournellis, R. Clinical and Psychometric Features of Psychiatric Patients after a Suicide Attempt in Relation with Menstrual Cycle Phases. Arch. Women’s Ment. Health 2019, 22, 605–611. [Google Scholar] [CrossRef]
- Yan, H.; Ding, Y.; Guo, W. Suicidality in Patients with Premenstrual Dysphoric Disorder—A Systematic Review and Meta-Analysis. J. Affect. Disord. 2021, 295, 339–346. [Google Scholar] [CrossRef]
- Osborn, E.; Brooks, J.; Brien, P.M.S.O.; Wittkowski, A. Suicidality in Women with Premenstrual Dysphoric Disorder: A Systematic Literature Review. Arch. Women’s Ment. Health 2021, 24, 173–184. [Google Scholar] [CrossRef]
- Gao, M.; Qiao, M.; An, L.; Wang, G.; Wang, J.; Song, C.; Wei, F.; Yu, Y.; Gong, T.; Gao, D. Brain Reactivity to Emotional Stimuli in Women with Premenstrual Dysphoric Disorder and Related Personality Characteristics. Aging 2021, 13, 19529–19541. [Google Scholar] [CrossRef]
- Critchley, H.O.; Babayev, E.; Bulun, S.E.; Clark, S.; Garcia-Grau, I.; Gregersen, P.K.; Kilcoyne, A.; Kim, J.-Y.; Lavender, M.; Marsh, E.E.; et al. Expert Reviews Menstruation: Science and Society. Am. J. Obstet. Gynecol. 2020, 223, 624–664. [Google Scholar] [CrossRef]
- Nappi, R.E.; Cucinella, L.; Bosoni, D.; Righi, A.; Battista, F.; Molinaro, P.; Stincardini, G.; Piccinino, M.; Rossini, R.; Tiranini, L. Premenstrual Syndrome and Premenstrual Dysphoric Disorder as Centrally Based Disorders. Endocrines 2022, 3, 127–138. [Google Scholar] [CrossRef]
- Nayman, S.; Beddig, T.; Reinhard, I.; Kuehner, C. Effects of Cognitive Emotion Regulation Strategies on Mood and Cortisol in Daily Life in Women with Premenstrual Dysphoric Disorder. Psychol. Med. 2023, 53, 5342–5352. [Google Scholar] [CrossRef] [PubMed]
- Hantsoo, L.; Epperson, C.N. Allopregnanolone in Premenstrual Dysphoric Disorder (PMDD): Evidence for Dysregulated Sensitivity to GABA-A Receptor Modulating Neuroactive Steroids across the Menstrual Cycle. Neurobiol. Stress 2020, 12, 100213. [Google Scholar] [CrossRef]
- Dubol, M.; Epperson, C.N.; Lanzenberger, R.; Sundström-poromaa, I. Neuroimaging Premenstrual Dysphoric Disorder: A Systematic and Critical Review. Front. Neuroendocrinol. 2020, 57, 100838. [Google Scholar] [CrossRef]
- Alevizou, F.; Vousoura, E.; Leonardou, A. Premenstrual Dysphoric Disorder: A Critical Review of Its Phenomenology, Etiology, Treatment and Clinical Status. Curr. Women’s Health Rev. 2018, 14, 59–66. [Google Scholar] [CrossRef]
- Andrzej, M.; Diana, J. Premenstrual Syndrome: From Etiology to Treatment. Maturitas 2006, 55S, S47–S54. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, C.; Yang, C.; Zhang, X.; Wu, E. Simultaneous Quantitative Determination of Norgestrel and Progesterone in Human Serum by High-Performance Liquid Chromatography-Tandem Mass Spectrometry with Atmospheric Pressure Chemical Ionization. Analyst 2000, 125, 2201–2205. [Google Scholar] [CrossRef]
- Pucci, V.; Bugamelli, F.; Mandrioli, R.; Luppi, B.; Raggi, M.A. Determination of Progesterone in Commercial Formulations and in Non Conventional Micellar Systems. J. Pharm. Biomed. Anal. 2003, 30, 1549–1559. [Google Scholar] [CrossRef]
- Cao, W.; Gong, P.; Liu, W.; Zhuang, M.; Yang, J. A Sensitive Flow Injection Chemiluminescence Method for the Determination of Progesterone. Drug Test. Anal. 2013, 5, 242–246. [Google Scholar] [CrossRef]
- Mishra, A.; Joy, K.P. HPLC-Electrochemical Detection of Ovarian Estradiol-17β and Catecholestrogens in the catfish Heteropneustes fossilis: Seasonal and Periovulatory Changes. Gen. Comp. Endocrinol. 2006, 145, 84–91. [Google Scholar] [CrossRef]
- Sun, M.; Du, L.; Gao, S.; Bao, Y.; Wang, S. Determination of 17β-Oestradiol by Fluorescence Immunoassay with Streptavidin-Conjugated Quantum Dots as Label. Steroids 2010, 75, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Ying, G.G.; Kookana, R.S.; Chen, Z. On-Line Solid-Phase Extraction and Fluorescence Detection of Selected Endocrine Disrupting Chemicals in Water by High-Performance Liquid Chromatography. J. Environ. Sci. Health Part B 2002, 37, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Ly, D.; Kang, K.; Choi, J.-Y.; Ishihara, A.; Back, K.; Lee, S.-G. HPLC Analysis of Serotonin, Tryptamine, Tyramine, and the Hydroxycinnamic Acid Amides of Serotonin and Tyramine in Food Vegetables. J. Med. Food 2008, 11, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Pussard, E.; Guigueno, N.; Adam, O.; Giudicelli, J.F. Validation of HPLC-Amperometric Detection to Measure Serotonin in Plasma, Platelets, Whole Blood, and Urine. Clin. Chem. 1996, 42, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, A.; Bilgi, M.; Yılmaz, M.; Atıcı, T. Ultra-Sensitive Electrochemical Sensors for Simultaneous Determination of Dopamine and Serotonin Based on Titanium Oxide-Gold Nanoparticles-Poly Nile Blue (in Deep Eutectic Solvent). Electrochim. Acta 2023, 467, 143046. [Google Scholar] [CrossRef]
- Fazl, F.; Gholivand, M.B. High Performance Electrochemical Method for Simultaneous Determination Dopamine, Serotonin, and Tryptophan by ZrO2–CuO Co-Doped CeO2 Modified Carbon Paste Electrode. Talanta 2022, 239, 122982. [Google Scholar] [CrossRef] [PubMed]
- Musa, A.M.; Kiely, J.; Luxton, R.; Honeychurch, K.C. Correction to: An Electrochemical Screen-Printed Sensor Based on Gold-Nanoparticle-Decorated Reduced Graphene Oxide–Carbon Nanotubes Composites for the Determination of 17-β Estradiol. Biosensors 2023, 13, 756. [Google Scholar] [CrossRef]
- Xie, P.; Liu, Z.; Huang, S.; Chen, J.; Yan, Y.; Li, N.; Zhang, M.; Jin, M.; Shui, L. A Sensitive Electrochemical Sensor Based on Wrinkled Mesoporous Carbon Nanomaterials for Rapid and Reliable Assay of 17β-Estradiol. Electrochim. Acta 2022, 408, 139960. [Google Scholar] [CrossRef]
- Cortés, P.; Castroagudín, M.; Kesternich, V.; Pérez-Fehrmann, M.; Carmona, E.; Zaragoza, G.; Vizcarra, A.; Hernández-Saravia, L.P.; Nelson, R. Ligand Influence in Electrocatalytic Properties of Cu(II) Triazole Complexes for Hydrogen Peroxide Detection in Aqueous Media. Dalton Trans. 2023, 52, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Saravia, L.P.; Núñez, C.; Castroagudín, M.; Bertotti, M.; Vizcarra, A.; Arriaza, B.; Nelson, R. Development of a Fast and Simple Electrochemical Sensor for Trace Determination of Sulfite Using Copper(II) Triazole Complexes. Microchem. J. 2024, 201, 110560. [Google Scholar] [CrossRef]
- Su, M.; Lan, H.; Tian, L.; Jiang, M.; Cao, X.; Zhu, C.; Yu, C. Ti3C2Tx-Reduced Graphene Oxide Nanocomposite-Based Electrochemical Sensor for Serotonin in Human Biofluids. Sens. Actuators B Chem. 2022, 367, 132019. [Google Scholar] [CrossRef]
- Nehru, L.; Chinnathambi, S.; Fazio, E.; Neri, F.; Leonardi, S.G.; Bonavita, A.; Neri, G. Electrochemical Sensing of Serotonin by a Modified MnO2-Graphene Electrode. Biosensors 2020, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Dăscălescu, D.; Apetrei, C. Nanomaterials Based Electrochemical Sensors for Serotonin Detection: A Review. Chemosensors 2021, 9, 14. [Google Scholar] [CrossRef]
- Baluta, S.; Zajac, D.; Szyszka, A.; Malecha, K.; Cabaj, J. Enzymatic Platforms for Sensitive Neurotransmitter Detection. Sensors 2020, 20, 423. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Luo, L.; Liu, Z.; Guo, Z.; Huang, X. Highly Sensitive and Selective Serotonin (5-HT) Electrochemical Sensor Based on Ultrafine Fe3O4 Nanoparticles Anchored on Carbon Spheres. Biosens. Bioelectron. 2023, 222, 114990. [Google Scholar] [CrossRef] [PubMed]
- Moraes, F.C.; Rossi, B.; Donatoni, M.C.; De Oliveira, K.T.; Pereira, E.C. Sensitive Determination of 17β-Estradiol in River Water Using a Graphene Based Electrochemical Sensor. Anal. Chim. Acta 2015, 881, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Supchocksoonthorn, P.; Alvior Sinoy, M.C.; de Luna, M.D.G.; Paoprasert, P. Facile Fabrication of 17β-Estradiol Electrochemical Sensor Using Polyaniline/Carbon Dot-Coated Glassy Carbon Electrode with Synergistically Enhanced Electrochemical Stability. Talanta 2021, 235, 122782. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, P.; Tu, X.; Wu, Y.; Zhan, G.; Li, C. A Novel Electrochemical Sensor for Estradiol Based on Nanoporous Polymeric Film Bearing Poly{1-butyl-3-[3-(N-pyrrole)propyl]imidazole dodecyl sulfonate} Moiety. Sens. Actuators B Chem. 2014, 193, 190–197. [Google Scholar] [CrossRef]
- Fu, H.; Wang, Y.; Dong, X.; Liu, Y.; Chen, Z.; Shen, Y.; Yang, C.; Dong, J.; Xu, Z. Application of Nickel Cobalt Oxide Nanoflakes for Electrochemical Sensing of Estriol in Milk. RSC Adv. 2016, 6, 65588–65593. [Google Scholar] [CrossRef]
- Ananthakrishnan, D.; Venkatesvaran, H.; Kannan, A.; Gandhi, S. Simplistic One-Pot Synthesis of an Inorganic–Organic Cubic Caged Material: A New Interface for Detecting Toxic Bisphenol-A Electrochemically. New J. Chem. 2020, 44, 20192–20202. [Google Scholar] [CrossRef]
- Masikini, M.; Ghica, E.; Baker, P.G.L.; Iwuoha, E.I.; Brett, C.M.A. Electrochemical Sensor Based on Multi-Walled Carbon Nanotube/Gold Nanoparticle Modified Glassy Carbon Electrode for Detection of Estradiol in Environmental Samples. Electroanalysis 2019, 31, 1925–1933. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the Determination of Limit of Detection and Limit of Quantitation of the Analytical Methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Wu, B.; Yeasmin, S.; Liu, Y.; Cheng, L. Sensitive and Selective Electrochemical Sensor for Serotonin Detection Based on Ferrocene-Gold Nanoparticles Decorated Multiwall Carbon Nanotubes. Sens. Actuator B Chem. 2022, 354, 131216. [Google Scholar] [CrossRef]
- Boonkaew, S.; Dettlaff, A.; Bogdanowicz, R.; Jönsson-niedzió, M. Electrochemical Determination of Neurotransmitter Serotonin Using Boron/Nitrogen Co-Doped Diamond-Graphene Nanowall-Structured Particles. J. Electroanal. Chem. 2022, 926, 116938. [Google Scholar] [CrossRef]
- Deepa, S.; Kumara Swamy, B.E.; Vasantakumar Pai, K. Electrochemical Sensing Performance of Citicoline Sodium Modified Carbon Paste Electrode for Determination of Dopamine and Serotonin. Mater. Sci. Energy Technol. 2020, 3, 584–592. [Google Scholar] [CrossRef]
- Salova, A.; Mahmud, S.F.; Almasoudie, N.K.A.; Mohammed, N.; Albeer, A.A.; Amer, R.F. CuO-Cu2O Nanostructures as a Sensitive Sensing Platform for Electrochemical Sensing of Dopamine, Serotonin, Acetaminophen, and Caffeine Substances. Inorg. Chem. Commun. 2024, 161, 112065. [Google Scholar] [CrossRef]
- Zeng, C.; Li, Y.; Zhu, M.; Du, Z.; Liang, H.; Chen, Q.; Ye, H.; Li, R.; Liu, W. Simultaneous Detection of Norepinephrine and 5-Hydroxytryptophan Using Poly-Alizarin/Multi-Walled Carbon Nanotubes-Graphene Modified Carbon Fiber Microelectrode Array Sensor. Talanta 2024, 270, 125565. [Google Scholar] [CrossRef] [PubMed]
- Galvão, J.C.R.; Araujo, M.d.S.; Prete, M.C.; Neto, V.L.; Dall’Antonia, L.H.; Matos, R.; Tarley, C.R.T.; Medeiros, R.A. Electrochemical Determination of 17-β-Estradiol Using a Glassy Carbon Electrode Modified with α-Fe2O3 Nanoparticles Supported on Carbon Nanotubes. Molecules 2023, 28, 6372. [Google Scholar] [CrossRef] [PubMed]
- Tanrıkut, E.; Özcan, İ.; Sel, E.; Köytepe, S.; Savan, E.K. Simultaneous Electrochemical Detection of Estradiol and Testosterone Using Nickel Ferrite Oxide Doped Mesoporous Carbon Nanocomposite Modified Sensor. J. Electrochem. Soc. 2020, 167, 124107. [Google Scholar] [CrossRef]
- Souza, M.B.; Santos, J.S.; Pontes, M.S.; Nunes, L.R.; Oliveira, I.P.; Lopez Ayme, A.J.; Santiago, E.F.; Grillo, R.; Fiorucci, A.R.; Arruda, G.J. CeO2 Nanostructured Electrochemical Sensor for the Simultaneous Recognition of Diethylstilbestrol and 17β-Estradiol Hormones. Sci. Total Environ. 2022, 805, 150348. [Google Scholar] [CrossRef]
- Marques, G.L.; Rocha, L.R.; Prete, M.C.; Gorla, F.A.; Moscardi dos Santos, D.; Segatelli, M.G.; Teixeira Tarley, C.R. Development of Electrochemical Platform Based on Molecularly Imprinted Poly(Methacrylic Acid) Grafted on Iniferter-Modified Carbon Nanotubes for 17β-Estradiol Determination in Water Samples. Electroanalysis 2021, 33, 568–578. [Google Scholar] [CrossRef]
- Sandoval-Rojas, A.P.; Ibarra, L.; Cortés, M.T.; Macías, M.A.; Suescun, L.; Hurtado, J. Synthesis and Characterization of Copper(II) Complexes Containing Acetate and N,N-Donor Ligands, and Their Electrochemical Behavior in Dopamine Detection. J. Electroanal. Chem. 2017, 805, 60–67. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Mobin, S.M.; Mathur, P.; Lahiri, G.K.; Srivastava, A.K. Biomimetic Sensor for Certain Catecholamines Employing Copper(II) Complex and Silver Nanoparticle Modified Glassy Carbon Paste Electrode. Biosens. Bioelectron. 2013, 39, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Boulkroune, M.; Chibani, A.; Geneste, F. Monocopper Complex Based on N-Tripodal Ligand Immobilized in a Nafion® Film for Biomimetic Detection of Catechols: Application to Dopamine. Electrochim. Acta 2016, 221, 80–85. [Google Scholar] [CrossRef]
- Kikuchi, H.; Nakatani, Y.; Seki, Y.; Yu, X.; Sekiyama, T.; Sato-Suzuki, I.; Arita, H. Decreased Blood Serotonin in the Premenstrual Phase Enhances Negative Mood in Healthy Women. J. Psychosom. Obstet. Gynecol. 2010, 31, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Carretti, N.; Florio, P.; Bertolin, A.; Costa, C.V.L.; Allegri, G.; Zilli, G. Serum Fluctuations of Total and Free Tryptophan Levels during the Menstrual Cycle Are Related to Gonadotrophins and Reflect Brain Serotonin Utilization. Hum. Reprod. 2005, 20, 1548–1553. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).