Water-Soluble Photoluminescent Ag Nanoclusters Stabilized by Amphiphilic Copolymers as Nanoprobe for Hypochlorite Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
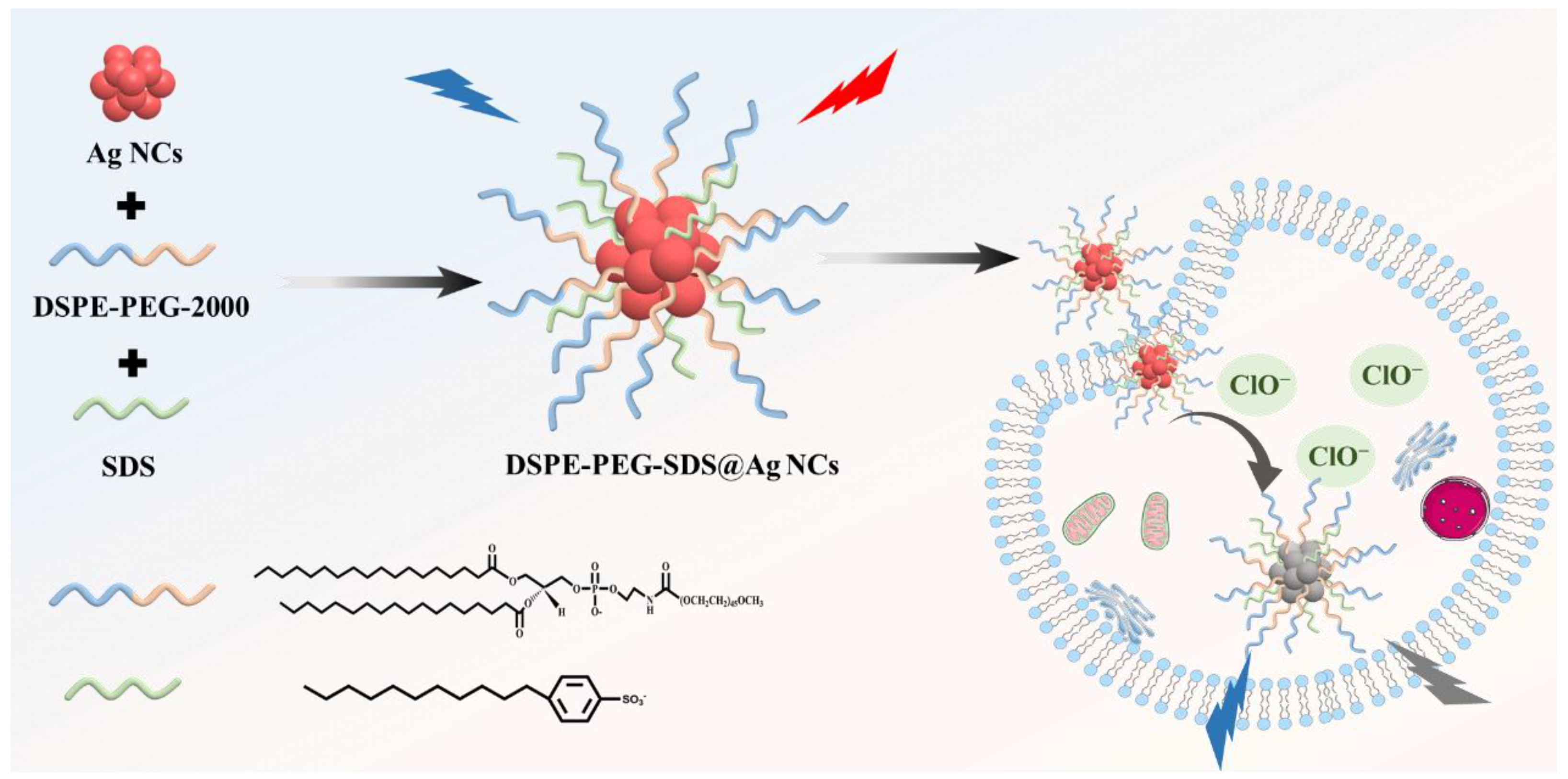
2.2. Synthesis of Luminescent Ultrasmall Ag NCs and Phase Transfer
2.3. Detection of ClO−
2.4. Evaluation of Cytotoxicity of the As-Prepared DSPE-PEG-SDS@Ag NCs
2.5. Cellular Imaging
2.6. Instrumentation
3. Results
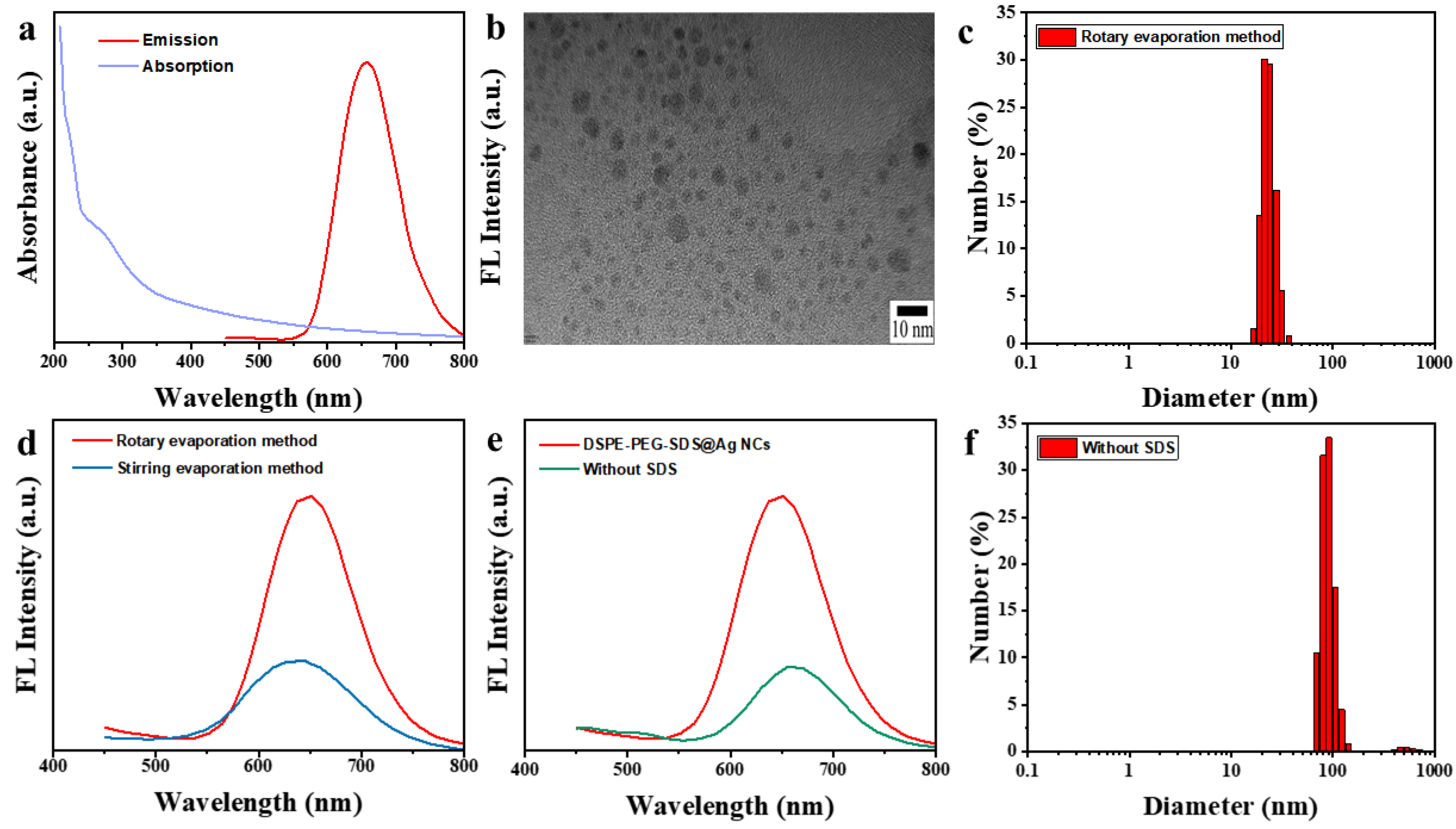
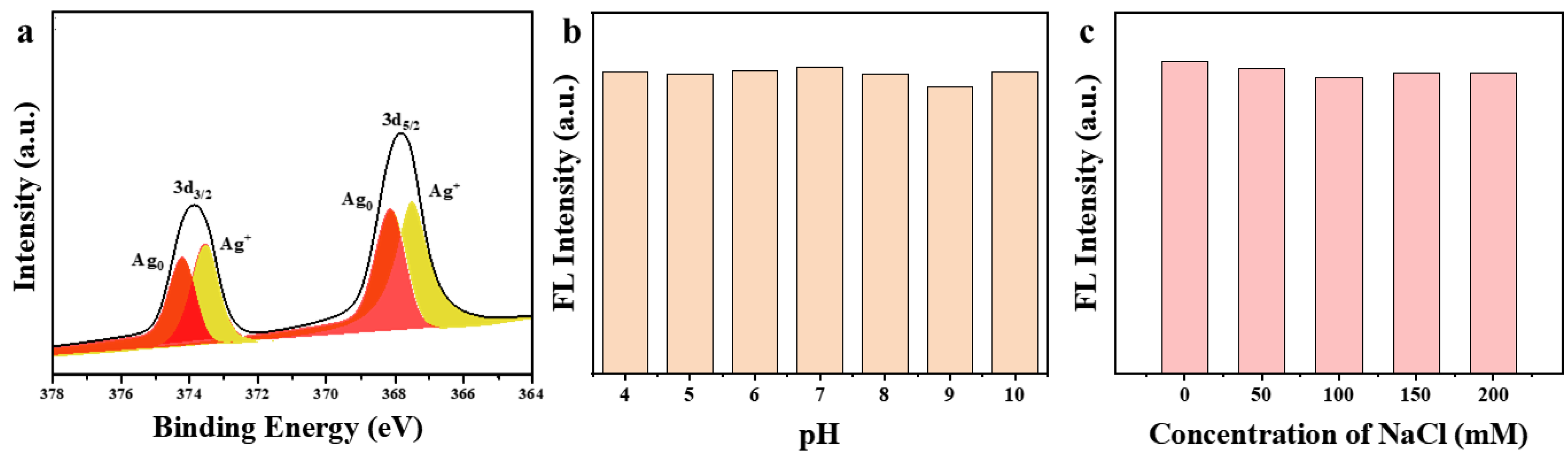
3.1. Characterizations of the DSPE-PEG-SDS@Ag NCs
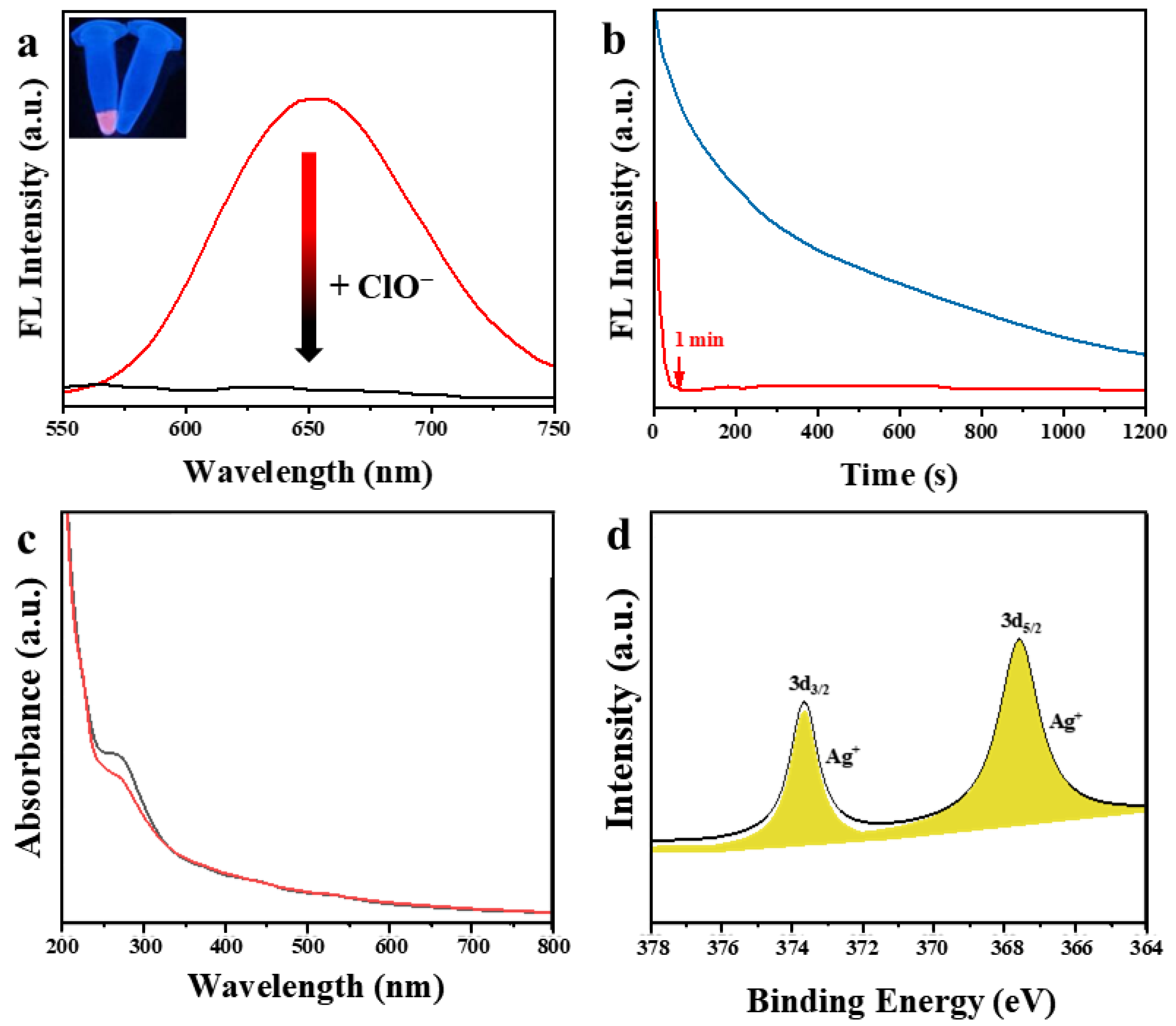
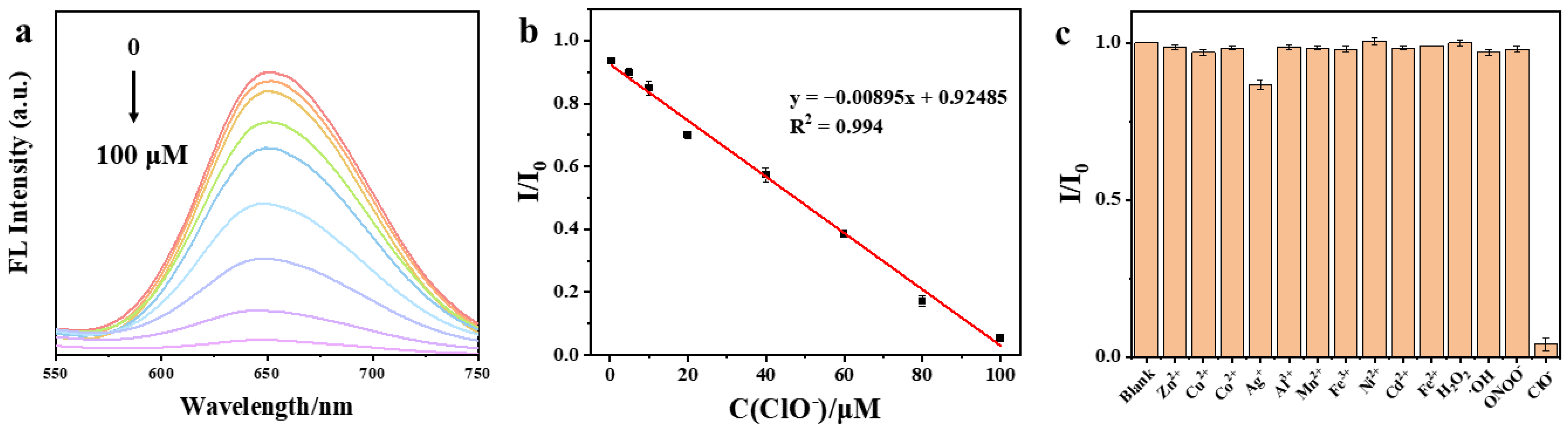
3.2. Response of the DSPE-PEG-SDS@Ag NCs to ClO−
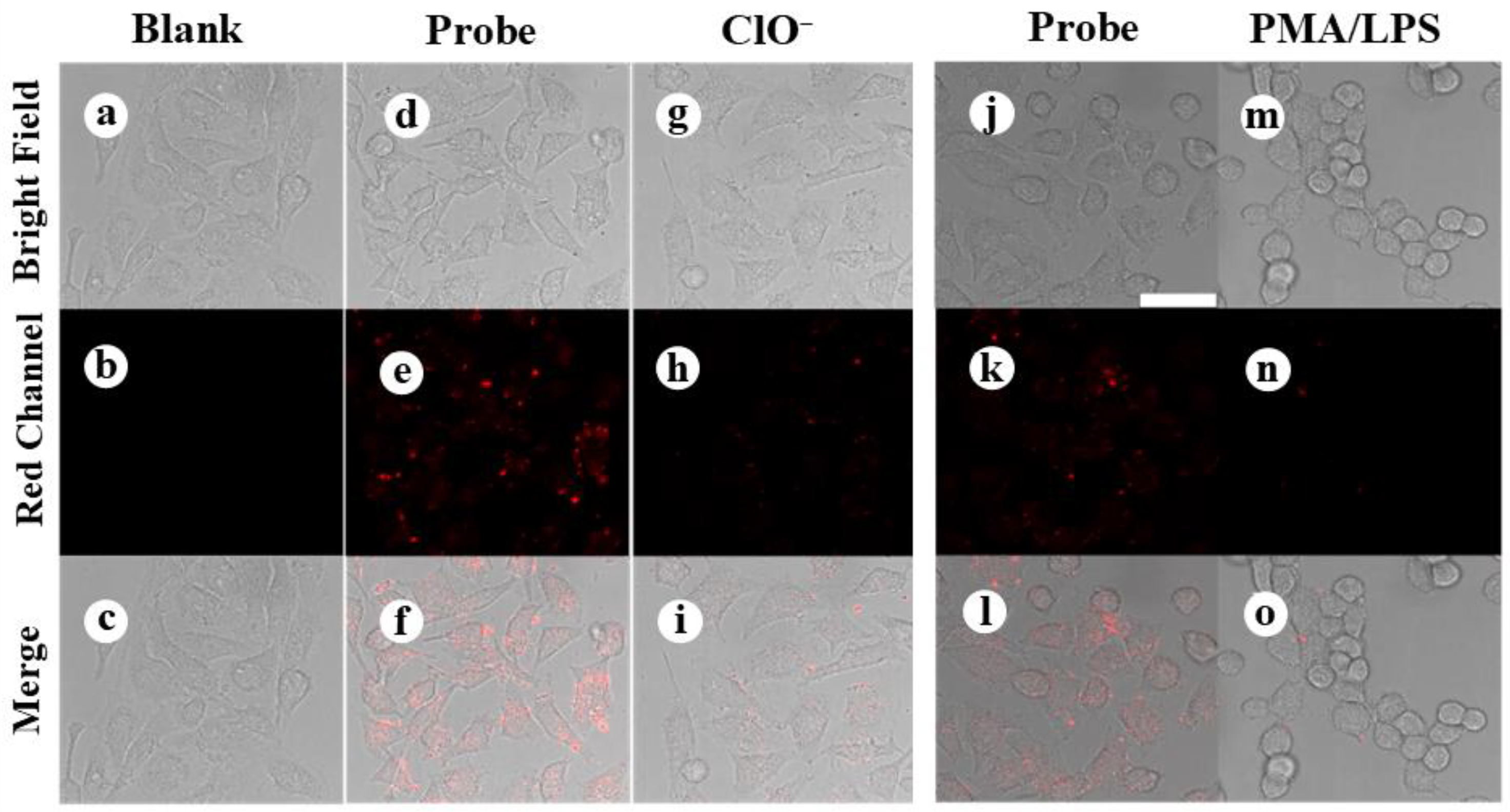
3.3. Cellular Imaging Using the DSPE-PEG-SDS@Ag NCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mutze, S.; Hebling, U.; Stremmel, W.; Wang, J.; Arnhold, J.; Pantopoulos, K.; Mueller, S. Myeloperoxidase-derived hypochlorous acid antagonizes the oxidative stress-mediated activation of iron regulatory protein 1. J. Biol. Chem. 2003, 278, 40542–40549. [Google Scholar] [CrossRef]
- Yang, Y.T.; Whiteman, M.; Gieseg, S.P. HOCl causes necrotic cell death in human monocyte derived macrophages through calcium dependent calpain activation. Biochim. Biophys. Acta 2012, 1823, 420–429. [Google Scholar] [CrossRef][Green Version]
- Zhang, R.; Song, B.; Yuan, J. Bioanalytical methods for hypochlorous acid detection: Recent advances and challenges. Trends Anal. Chem. 2018, 99, 1–33. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Z.; Yang, Y.; Zheng, B.; Zhu, Y.; Wu, F.; Xiong, H. Visualization of endogenous hypochlorite in drug-induced liver injury mice via a bioluminescent probe combined with firefly luciferase mRNA-loaded lipid nanoparticles. Anal. Chem. 2024, 96, 6978–6985. [Google Scholar] [CrossRef]
- Davies, M.J. Myeloperoxidase-derived oxidation mechanisms of biological damage and its prevention. J. Clin. Biochem. Nutr. 2010, 48, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Jeitner, T.M.; Kalogiannis, M.; Krasnikov, B.F.; Gomolin, I.; Peltier, M.R.; Moran, G.R. Linking inflammation and parkinson disease: Hypochlorous acid generates parkinsonian poisons. Toxicol. Sci. 2016, 151, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tan, H.S.; Wang, A.J.; Li, S.S.; Feng, J.J. Fluorescent metal nanoclusters: From luminescence mechanism to applications in enzyme activity assays. Biosens. Bioelectron. 2024, 257, 116323. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wu, Z.; Yao, Q.; Xie, J. Luminescent metal nanoclusters: Biosensing strategies and bioimaging applications. Aggregate 2021, 2, 114–132. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Li, M.; Ma, K.; Wang, D.; Su, L.; Zhang, X.; Tang, B.Z. Nanolab in a cell: Crystallization-induced in situ self-assembly for cancer theranostic amplification. J. Am. Chem. Soc. 2022, 144, 14388–14395. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhu, L.; Yang, W.; Xu, W. Nucleic acid-templated silver nanoclusters: A review of structures, properties, and biosensing applications. Coordin. Chem. Rev. 2023, 491, 215247. [Google Scholar] [CrossRef]
- Shu, T.; Su, L.; Wang, J.; Lu, X.; Liang, F.; Li, C.; Zhang, X. Value of the debris of reduction sculpture: Thiol etching of Au nanoclusters for preparing water-soluble and aggregation-induced emission-active Au(I) Complexes as phosphorescent copper ion sensor. Anal. Chem. 2016, 88, 6071–6077. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, X.; Chen, W.; He, M.; Yu, Y.; Gao, G.; Sun, T. Advance of gold nanoclusters for bioimaging. iScience 2022, 25, 105022. [Google Scholar] [CrossRef]
- Kang, X.; Zhu, M. Tailoring the photoluminescence of atomically precise nanoclusters. Chem. Soc. Rev. 2019, 48, 2422–2457. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Song, X.; Chai, O.J.H.; Yao, Q.; Yang, H.; Xie, J. Photoluminescent characterization of metal nanoclusters: Basic parameters, methods, and applications. Adv. Mater. 2024, 36, e2401002. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, R. On the ligand’s role in the fluorescence of gold nanoclusters. Nano Lett. 2010, 10, 2568–2573. [Google Scholar] [CrossRef]
- Deng, G.; Malola, S.; Yuan, P.; Liu, X.; Teo, B.K.; Hakkinen, H.; Zheng, N. Enhanced surface ligands reactivity of metal clusters by bulky ligands for controlling optical and chiral properties. Angew. Chem. Int. Ed. 2021, 60, 12897–12903. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Xin, J.; Li, J.; Li, H.; Kang, X.; Pei, Y.; Zhu, M. Fluorescence or phosphorescence? The metallic composition of the nanocluster kernel does matter. Angew. Chem. Int. Ed. 2022, 61, e202205947. [Google Scholar] [CrossRef] [PubMed]
- Pniakowska, A.; Kumaranchira Ramankutty, K.; Obstarczyk, P.; Peric Bakulic, M.; Sanader Marsic, Z.; Bonacic-Koutecky, V.; Burgi, T.; Olesiak-Banska, J. Gold-doping effect on two-photon absorption and luminescence of atomically precise silver ligated nanoclusters. Angew. Chem. Int. Ed. 2022, 61, e202209645. [Google Scholar] [CrossRef] [PubMed]
- Ishii, W.; Okayasu, Y.; Kobayashi, Y.; Tanaka, R.; Katao, S.; Nishikawa, Y.; Kawai, T.; Nakashima, T. Excited state engineering in Ag29 nanocluster through peripheral modification with silver(I) complexes for bright near-infrared photoluminescence. J. Am. Chem. Soc. 2023, 145, 11236–11244. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Cheng, S.; You, Y.; Zhang, S.; Xian, Y. Sensitive monitoring and bioimaging intracellular highly reactive oxygen species based on gold nanoclusters@nanoscale metal-organic frameworks. Anal. Chim. Acta. 2019, 1092, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yi, S.; Lei, Z.; Xiao, Y. Amphiphilic polymer-encapsulated Au nanoclusters with enhanced emission and stability for highly selective detection of hypochlorous acid. RSC Adv. 2021, 11, 14678–14685. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Mi, W.; Guo, S.; Yang, Q.Z.; Jin, Y.; Shao, N. Peptide-capped functionalized Ag/Au bimetal nanoclusters with enhanced red fluorescence for lysosome-targeted imaging of hypochlorite in living cells. Talanta 2020, 216, 120926. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Tian, M.; Cao, C.; Shu, T.; Wang, J.; Wen, Y.; Su, L.; Zhang, X. Strongly phosphorescent and water-soluble gold(I)-silver(I)-cysteine nanoplatelets via versatile small biomolecule cysteine-assisted synthesis for intracellular hypochlorite detection. Biosens. Bioelectron. 2021, 193, 113571. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, Y.; Ge, Y.; Song, G.; Zhou, J. Smartphone-assisted visual ratio-fluorescence detection of hypochlorite based on copper nanoclusters. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 255, 119740. [Google Scholar] [CrossRef] [PubMed]
- Kastantin, M.; Missirlis, D.; Black, M.; Ananthanarayanan, B.; Peters, D.; Tirrell, M. Thermodynamic and kinetic stability of dspe-peg 2000 micelles in the presence of bovine serum albumin. J. Phys. Chem. B 2010, 114, 12632–12640. [Google Scholar] [CrossRef] [PubMed]
- Kastantin, M.; Ananthanarayanan, B.; Karmali, P.; Ruoslahti, E.; Tirrell, M. Effect of the lipid chain melting transition on the stability of DSPE-PEG(2000) micelles. Langmuir 2009, 25, 7279–7286. [Google Scholar] [CrossRef]
- Chen, Z.; Walsh, A.G.; Wei, X.; Zhu, M.; Zhang, P. Site-specific electronic properties of [Ag25SR18]− nanoclusters by X-ray spectroscopy. Small 2021, 17, e2005162. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, Q.; Yue, N.; Wang, H. Study of fluorescence quenching mechanism between quercetin and tyrosine-H2O2-enzyme catalyzed product. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 72, 572–576. [Google Scholar] [CrossRef]
- Zhang, Y.; Demokritou, P.; Ryan, D.K.; Bello, D. Comprehensive assessment of short-lived ROS and H2O2 in laser printer emissions: Assessing the relative contribution of metal oxides and organic constituents. Environ. Sci. Technol. 2019, 53, 7574–7583. [Google Scholar] [CrossRef]
- Abdul-Baki, A.A. A selected literature review of hypochlorite chemistry and definition of terms. J. Seed Technol. 1979, 1, 43–56. [Google Scholar]
- Rhee, S.G. H2O2-a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef]
- Li, H.; Wu, Y.; Xu, Z.; Wang, Y. In situ anchoring Cu nanoclusters on Cu-MOF: A new strategy for a combination of catalysis and fluorescence toward the detection of H2O2 and 2,4-DNP. Chem. Eng. J. 2024, 479, 147508. [Google Scholar] [CrossRef]
- Mi, W.; Tang, S.; Jin, Y.; Shao, N. Au/Ag bimetallic nanoclusters stabilized by glutathione and lysozyme for ratiometric sensing of H2O2 and hydroxyl radicals. ACS. Appl. Nano Mater. 2021, 4, 1586–1595. [Google Scholar] [CrossRef]
- Yue, G.; Li, S.; Liu, W.; Ding, F.; Zou, P.; Wang, X.; Zhao, Q.; Rao, H. Ratiometric fluorescence based on silver clusters and N, Fe doped carbon dots for determination of H2O2 and UA: N, Fe doped carbon dots as mimetic peroxidase. Sens. Actuat. B-Chem. 2019, 287, 408–415. [Google Scholar] [CrossRef]
- Gui, L.; Yan, J.; Zhao, J.; Wang, S.; Ji, Y.; Liu, J.; Wu, J.; Yuan, K.; Liu, H.; Deng, D.; et al. Hypochlorite activatable ratiometric fluorescent probe based on endoplasmic reticulum stress for imaging of atherosclerosis. Biosens. Bioelectron. 2023, 240, 115660. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Yan, S.; Yu, Y.; Xue, Y.; Yu, Y.; Han, C. Dual-Responsive Ratiometric fluorescent probe for hypochlorite and peroxynitrite detection and imaging in vitro and in vivo. Anal. Chem. 2022, 94, 1415–1424. [Google Scholar] [CrossRef]
- Gopu, C.L.; Shanti Krishna, A.; Sreenivasan, K. Fluorimetric detection of hypochlorite using albumin stabilized gold nanoclusters. Sens. Actuat. B-Chem. 2015, 209, 798–802. [Google Scholar] [CrossRef]
- Zhao, G.; Lv, C.-C.; Yang, X.-K.; Zhao, X.; Xie, F. Levonorgestrel protected Au10 cluster for hypochlorite sensing in living organisms. Anal. Chim. Acta 2024, 1320, 343033. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, Y.; Li, Y.; Yan, C.; Liu, Z.; Wang, H.; Peng, H.; Du, J.; Zheng, B.; Guo, Y. Synthesis of vitamin B1-stabilized gold nanoclusters with high quantum yields for application as sensors. ACS Appl. Nano Mater. 2022, 5, 17234–17242. [Google Scholar] [CrossRef]
- Tang, Q.; Yang, T.; Huang, Y. Copper nanocluster-based fluorescent probe for hypochlorite. Microchim. Acta 2015, 182, 2337–2343. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Z.; Zhao, H.; Jiao, Y.; Li, J.; Shuang, S.; Dong, C. Facile synthesis of multifunctional carbon dots with 54.4% orange emission for label-free detection of morin and endogenous/exogenous hypochlorite. J. Hazard. Mater. 2022, 424, 127289. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, Y.; Zhang, H.; Lu, W.; Jiao, Y.; Shuang, S.; Dong, C. One-pot synthesis of efficient multifunctional nitrogen-doped carbon dots with efficient yellow fluorescence emission for detection of hypochlorite and thiosulfate. J. Mater. Chem. B 2022, 10, 8910–8917. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zheng, X.; Chen, Z.; Teng, R.; Zhang, Y.; Li, H.; Ding, C.; Huang, Y. Fluorescent-colorimetric dual signal ratio sensor with AuNRs@UCNPs superstructure nanoprobe for accurate hypochlorite detection. Sens. Actuat. B-Chem. 2024, 419, 136384. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Dong, Q.; Chang, Y.; Zhang, S.; Shi, P. Water-Soluble Photoluminescent Ag Nanoclusters Stabilized by Amphiphilic Copolymers as Nanoprobe for Hypochlorite Detection. Chemosensors 2024, 12, 166. https://doi.org/10.3390/chemosensors12080166
Lin X, Dong Q, Chang Y, Zhang S, Shi P. Water-Soluble Photoluminescent Ag Nanoclusters Stabilized by Amphiphilic Copolymers as Nanoprobe for Hypochlorite Detection. Chemosensors. 2024; 12(8):166. https://doi.org/10.3390/chemosensors12080166
Chicago/Turabian StyleLin, Xiangfang, Qinhui Dong, Yalin Chang, Shusheng Zhang, and Pengfei Shi. 2024. "Water-Soluble Photoluminescent Ag Nanoclusters Stabilized by Amphiphilic Copolymers as Nanoprobe for Hypochlorite Detection" Chemosensors 12, no. 8: 166. https://doi.org/10.3390/chemosensors12080166
APA StyleLin, X., Dong, Q., Chang, Y., Zhang, S., & Shi, P. (2024). Water-Soluble Photoluminescent Ag Nanoclusters Stabilized by Amphiphilic Copolymers as Nanoprobe for Hypochlorite Detection. Chemosensors, 12(8), 166. https://doi.org/10.3390/chemosensors12080166




