Interaction of the Polymeric Layer Derived from 3-(4-Trifluoromethyl)-phenyl)-thiophene with Synthetic Stimulants on the Phase Boundary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Modification of Electrode Surface
2.3. Raman Spectroscopy
2.4. Electrochemical Impedance Spectroscopy
2.5. Square Wave Voltammetry
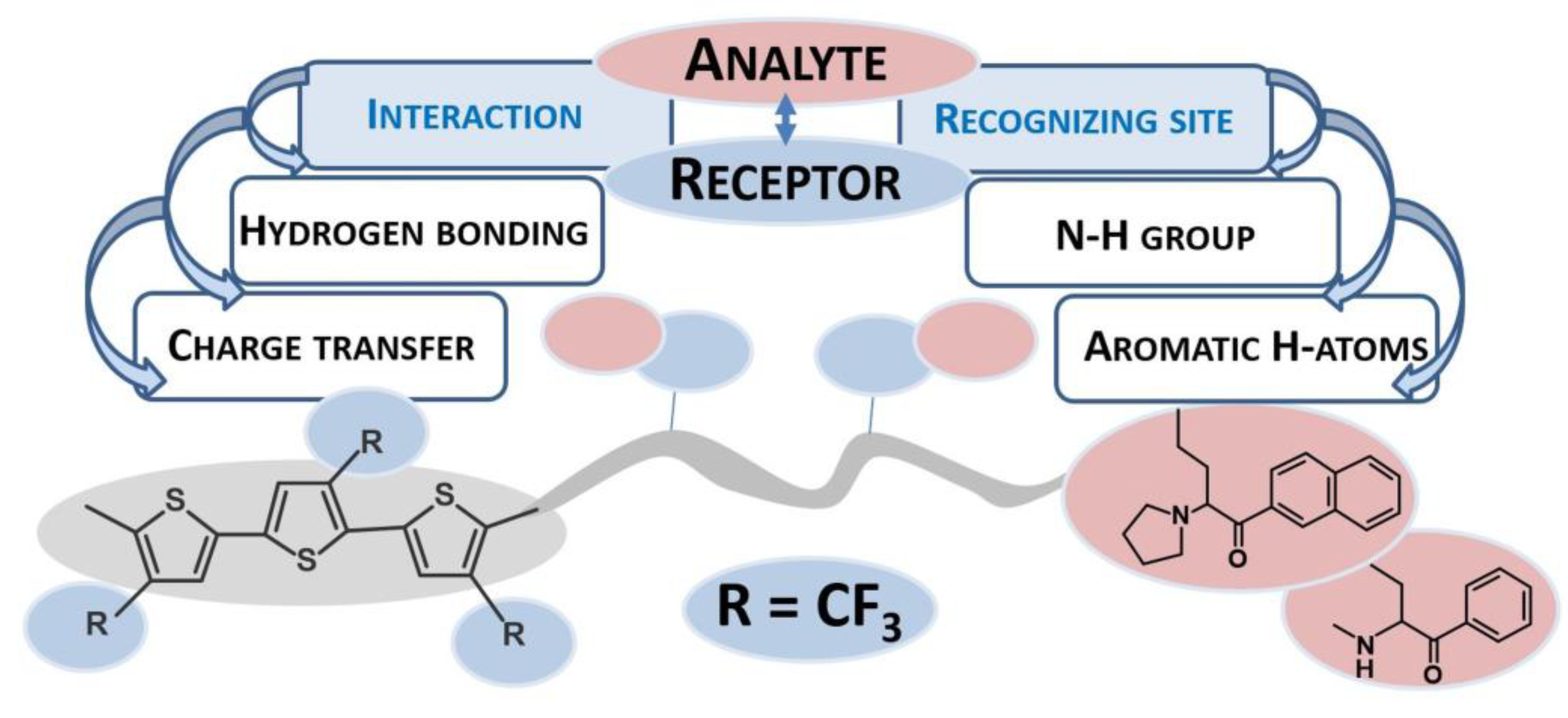
3. Results and Discussion
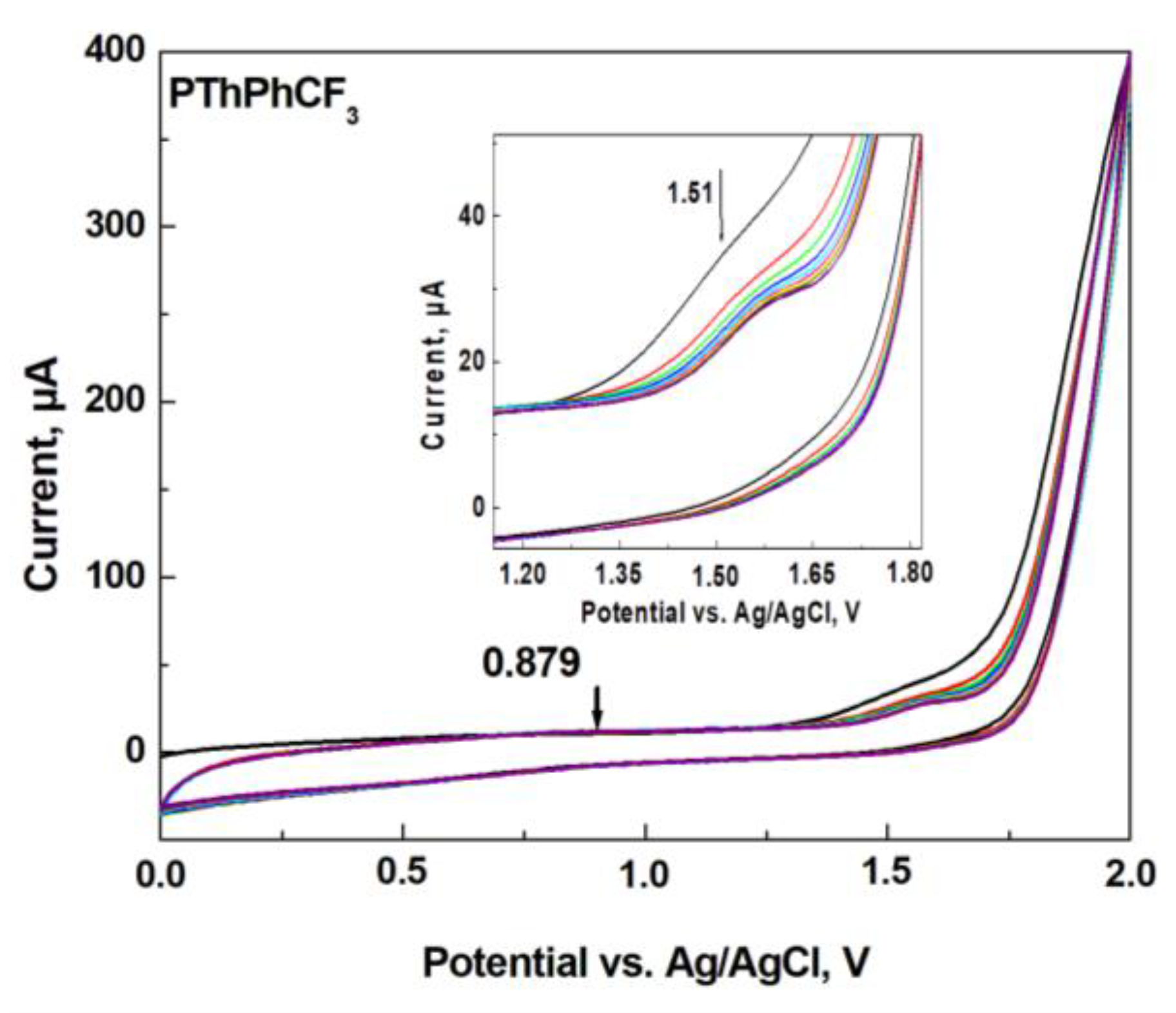
3.1. Cyclic Voltammetry
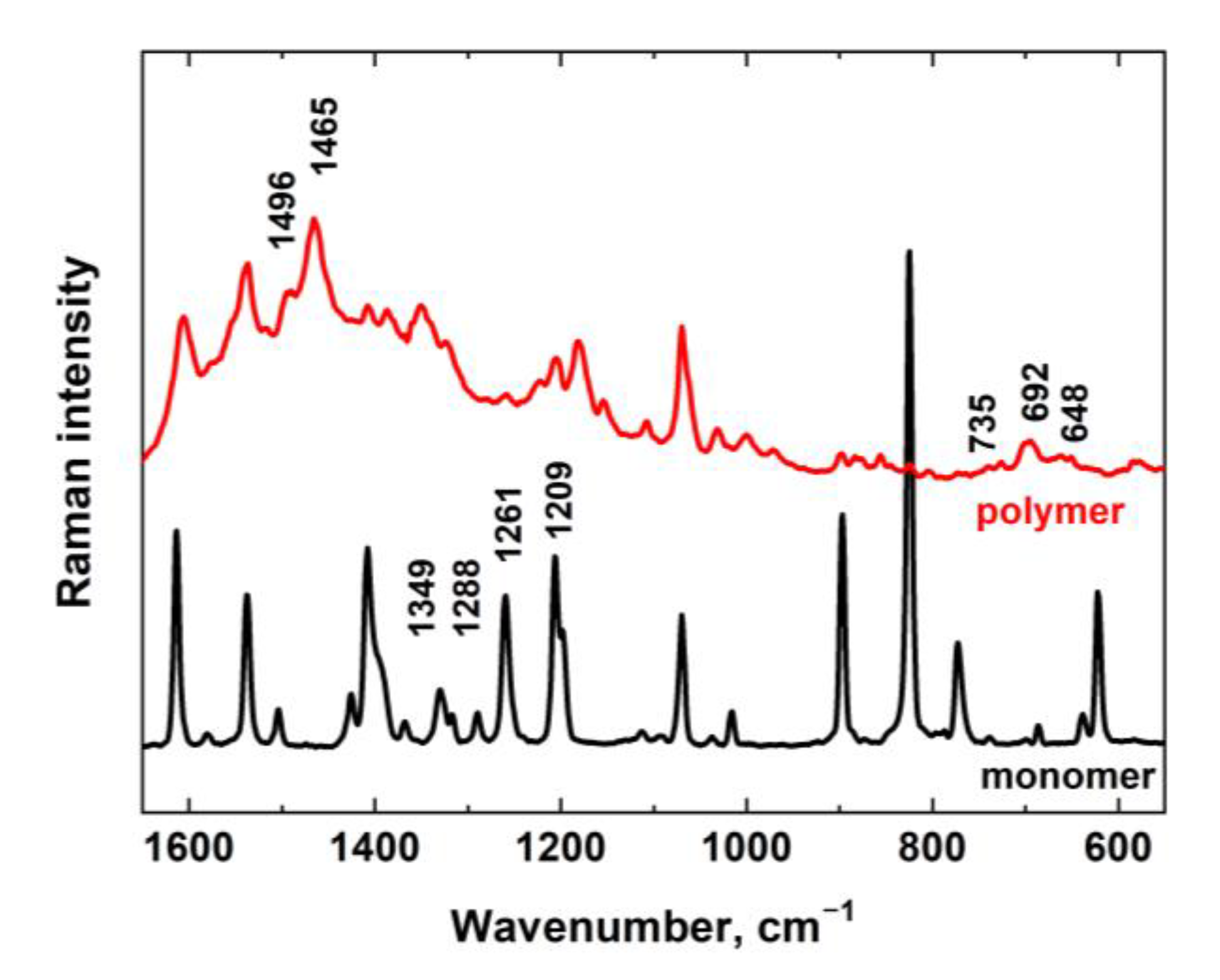
3.2. Raman Spectroscopy
3.3. Affinity Properties of the Modified Electrodes
3.4. Square Wave Voltammetry
3.5. Analytical Parameters and Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porada, R.; Jedlińska, K.; Lipińska, J.; Baś, B. Review—Voltammetric sensors with laterally placed working electrodes: A review. J. Electrochem. Soc. 2020, 167, 037536. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Nguyen, M.T.N.; Lee, J.S. Carbon-based materials and their applications in sensing by electrochemical voltammetry. Inorganics 2023, 11, 81. [Google Scholar] [CrossRef]
- John, A.; Benny, L.; Cherian, A.R.; Narahari, S.Y.; Varghese, A.; Hegde, G. Electrochemical sensors using conducting polymer/noble metal nanoparticle nanocomposites for the detection of various analytes: A review. J. Nanostruct. Chem. 2021, 11, 1–31. [Google Scholar] [CrossRef]
- Melo, L.M.A.; de Faria, L.V.; Arantes, L.C.; Vojs, M.; Marton, M.; Brocenschi, R.F.; Richter, E.M.; Munoz, R.A.A.; dos Santos, W.T.P. Use of a lab-made screen-printed sensor with chemically deposited boron-doped diamond for simple and selective electrochemical detection of the synthetic cathinone N-ethylpentylone in forensic samples. Electrochim. Acta 2023, 465, 142996. [Google Scholar] [CrossRef]
- Araújo, D.S.; Arantes, L.C.; Faria, L.V.; Souza, K.A.O.; Pimentel, D.M.; Barbosa, S.L.; Richter, E.M.; Muñoz, R.A.A.; dos Santos, W.T.P. Electrochemistry of 5F-MDMB-PICA synthetic cannabinoid using a boron-doped diamond electrode with short anodic-cathodic pretreatment: A simple screening method for application in forensic analysis. Electrochim. Acta 2023, 454, 142356. [Google Scholar] [CrossRef]
- Ren, S.; Zeng, J.; Zheng, Z.; Shi, H. Perspective and application of modified electrode material technology in electrochemical voltammetric sensors for analysis and detection of illicit drugs. Sens. Actuator A Phys. 2021, 329, 112821. [Google Scholar] [CrossRef]
- Brownson, D.A.C.; Foster, C.W.; Banks, C.E. The electrochemical performance of graphene modified electrodes: An analytical perspective. Analyst 2012, 137, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Fakude, C.T.; Modise, R.P.; Haruna, A.B.; Pillay, J.; Ozoemena, K.I. Advances in the application of nanomaterials for the electrocatalytic detection of drugs of abuse. Adv. Sens. Energy Mater. 2023, 2, 100056. [Google Scholar] [CrossRef]
- Poddar, A.K.; Patel, S.S.; Patel, H.D. Synthesis, characterization and applications of conductive polymers: A brief review. Polym. Adv. Technol. 2021, 32, 4616–4641. [Google Scholar] [CrossRef]
- AL-Refai, H.H.; Ganash, A.A.; Hussein, M.A. Polythiophene and its derivatives—Based nanocomposites in electrochemical sensing: A mini review. Mater. Today Commun. 2021, 26, 101935. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Karagözler, A.E.; Russell, G.C.; Zimmer, H.; Mark, H.B., Jr. Electrochemistry and detection of some organic and biological molecules at conducting poly(3-methylthiophene) electrodes. Biosens. Bioelectron. 1991, 6, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, R. Highly stable voltammetric measurements of phenolic compounds at poly(3-methylthiophene)-coated glassy carbon electrodes. Anal. Chem. 1989, 61, 2809–2811. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.C.; Yeh, W.M.; Tung, T.S.; Liao, J.Y. Amperometric detection of morphine based on poly(3,4-ethylenedioxythiophene) immobilized molecularly imprinted polymer particles prepared by precipitation polymerization. Anal. Chim. Acta 2005, 542, 90–96. [Google Scholar] [CrossRef]
- Weng, C.-H.; Yeh, W.-M.; Ho, K.-C.; Lee, G.-B. A microfluidic system utilizing molecularly imprinted polymer films for amperometric detection of morphine. Sensor. Actuator. B Chem. 2007, 121, 576–582. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Ahmed, R.A. Direct and simple electrochemical determination of morphine at PEDOT modified Pt electrode. Electroanalysis 2011, 23, 737–746. [Google Scholar] [CrossRef]
- Granado, V.L.V.; Gutiérrez-Capitán, M.; Fernández-Sánchez, C.; Gomes, M.T.S.R.; Rudnitskaya, A.; Jimenez-Jorquera, C. Thin-film electrochemical sensor for diphenylamine detection using molecularly imprinted polymers. Anal. Chim. Acta 2014, 809, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, B.E.; Branger, C.; Iordache, T.-V.; Iovu, H.; Vitrik, O.B.; Dyshlyuk, A.V.; Sarbu, A.; Brisset, H. Application of unusual on/off electrochemical properties of a molecularly imprinted polymer based on an EDOT–thiophene precursor for the detection of ephedrine. Electrochem. Commun. 2018, 94, 45–48. [Google Scholar] [CrossRef]
- Hui, Y.; Bian, C.; Xia, S.; Tong, J.; Wang, J. Synthesis and electrochemical sensing application of poly(3,4-ethylenedioxythiophene)-based materials: A review. Anal. Chim. Acta 2018, 1022, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Granado, V.L.V.; Rudnitskaya, A.; Oliveira, J.A.B.P.; Gomes, M.T.S.R. Design of molecularly imprinted polymers for diphenylamine sensing. Talanta 2012, 94, 133–139. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime. The Synthetic Drug Phenomenon. Available online: https://www.unodc.org/res/WDR-2023/WDR23_B3_CH1_Synthetic_drugs.pdf (accessed on 5 July 2023).
- Ribeiro, M.F.M.; da Cruz Júnior, J.W.; Dockal, E.R.; McCord, B.R.; de Oliveira, M.F. Voltammetric determination of cocaine using carbon screen printed electrodes chemically modified with uranyl Schiff base films. Electroanalysis 2016, 28, 320–326. [Google Scholar] [CrossRef]
- Castro, A.S.; de Menezes, M.M.T.; Alves, G.M.; de Oliveira, M.F. Voltammetric analysis of cocaine hydrochloride at carbon paste electrode chemically modified with N,N′-ethylene-bis-(salicylideneiminato) manganese(II) Schiff base complex. Microchem. J. 2020, 153, 104399. [Google Scholar] [CrossRef]
- Castro, A.S.; Rodrigues, C.H.P.; de Menezes, M.M.T.; da Silva, A.B.D.; Bruni, A.T.; de Oliveira, M.F. Fe(II), Ni(II), Cu(II), and Co(II) salen Schiff base complexes: Proposal for a voltammetric sensor to analyze cocaine hydrochloride and its interferents. Forensic Chem. 2021, 25, 100347. [Google Scholar] [CrossRef]
- Sengel, T.Y.; Guler, E.; Gumus, Z.P.; Aldemir, E.; Coskunol, H.; Akbulut, H.; Colak, D.G.; Cianga, I.; Yamada, S.; Timur, S.; et al. An immunoelectrochemical platform for the biosensing of ‘Cocaine use’. Sens. Actuators B Chem. 2017, 246, 310–318. [Google Scholar] [CrossRef]
- Florea, A.; Cowen, T.; Piletsky, S.; De Wael, K. Polymer platforms for selective detection of cocaine in street samples adulterated with levamisole. Talanta 2018, 186, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. New psychoactive substances: A review and updates. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320967197. [Google Scholar] [CrossRef] [PubMed]
- Couto, R.A.S.; Costa, S.S.; Mounssef, B.; Pacheco, J.G.; Fernandes, E.; Carvalho, F.; Rodrigues, C.M.P.; Delerue-Matos, C.; Braga, A.A.C.; Moreira Gonçalves, L.; et al. Electrochemical sensing of ecstasy with electropolymerized molecularly imprinted poly(o-phenylenediamine) polymer on the surface of disposable screen-printed carbon electrodes. Sens. Actuators B Chem. 2019, 290, 378–386. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, F.; Huang, X.; He, S.; Han, Q.; Zhang, Z.; Liu, W. Fabrication of a molecularly-imprinted-polymer-based graphene oxide nanocomposite for electrochemical sensing of new psychoactive substances. Nanomaterials 2023, 13, 751. [Google Scholar] [CrossRef] [PubMed]
- Merli, D.; Lio, E.; Protti, S.; Coccia, R.; Profumo, A.; Alberti, G. Molecularly imprinted polymer-based voltammetric sensor for amino acids/indazole derivatives synthetic cannabinoids detection. Anal. Chim. Acta 2024, 1288, 342151. [Google Scholar] [CrossRef]
- Florea, A.; de Jong, M.; de Wael, K. Electrochemical strategies for the detection of forensic drugs. Curr. Opin. Electrochem. 2018, 11, 34–40. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Perspectives of Polymers in Forensic Analysis. Macromol 2023, 3, 108–119. [Google Scholar] [CrossRef]
- Forlani, L. Catalysis in nucleophilic aromatic substitution reactions. The presence of molecular complexes on the pathway of reactions between 1-fluoro- and 1-chloro-2,4-dinitrobenzene and aliphatic amines. J. Chem. Soc. Perkin Trans. 1993, 8, 1525–1530. [Google Scholar] [CrossRef]
- Forlani, L.; Mezzina, E. Interactions between amines and aromatic fluoro derivatives. 19F NMR Investigation in [2H8]toluene. J. Chem. Soc. Perkin Trans. 1995, 11, 2019–2021. [Google Scholar] [CrossRef]
- Boitsov, S.; Songstad, J.; Törnroos, K.W. [4-(Trifluoromethyl)phenyl]aceto-nitrile. Acta Cryst. C 2002, 58, o145–o147. [Google Scholar] [CrossRef]
- McCullough, R.D. The chemistry of conducting polythiophenes. Adv. Mater. 1998, 10, 93–116. [Google Scholar] [CrossRef]
- Kim, D.-M.; Shim, K.-B.; Son, J.I.; Reddy, S.S.; Shima, Y.-B. Spectroelectrochemical and electrochromic behaviors of newly synthesized poly[3′-(2-aminopyrimidyl)-2,2′:5′,2″-terthiophene]. Electrochim. Acta 2013, 104, 322–329. [Google Scholar] [CrossRef]
- Bauerle, P.; Scheib, S. Synthesis and characterization of thiophenes, oligothiophenes and polythiophenes with crown ether units in direct n-conjugation. Acta Polym. 1995, 46, 124–129. [Google Scholar] [CrossRef]
- Tutuncu, E.; Ozkut, M.I.; Balci, B.; Berk, H.; Cihaner, A. Electrochemical and optical characterization of a multielectrochromic copolymer based on 3,4-ethylenedioxythiophene and functionalized dithienylpyrrole derivative. Eur. Polym. J. 2019, 110, 233–239. [Google Scholar] [CrossRef]
- Ahumada, J.C.; Soto, J.P.; Alemán, C.; Torras, J. Synthesis and characterization of a new benzobisoxazole/thiophene derivative polymer and the effect of the substituent on the push/pull properties. J. Polym. Sci. 2021, 59, 3167–3180. [Google Scholar] [CrossRef]
- Tanaka, K.; Shichiri, T.; Wang, S.; Yamabe, T. A study of the electropolymerization of thiophene. Synth. Met. 1988, 24, 203–215. [Google Scholar] [CrossRef]
- Furukawa, Y.; Akimoto, M.; Harada, I. Vibrational key bands and electrical conductivity of polythiophene. Synth. Met. 1987, 18, 151–156. [Google Scholar] [CrossRef]
- Louarn, G.; Buisson, J.P.; Lefrant, S.; Fichou, D. Vibrational studies of a series of. alpha.-oligothiophenes as model systems of polythiophene. J. Phys. Chem. 1995, 99, 11399–11404. [Google Scholar] [CrossRef]
- Chen, R.; Chen, S.; Zhou, Y.; Wei, Z.; Wang, H.; Zheng, Y.; Li, M.; Sun, K.; Li, Y. Unsubstituted polythiophene film deposited via in-situ sequential solution polymerization for chemo-/electrochromism. Macromolecules 2020, 53, 4247–4254. [Google Scholar] [CrossRef]
- Kinno, Y.; Omachi, H.; Nakanishi, Y.; Shinohara, H. Synthesis of long-chain polythiophene inside carbon nanotubes. Chem. Lett. 2018, 47, 1022–1025. [Google Scholar] [CrossRef]
- Bouabdallaoui, M.; Aouzal, Z.; Ben Jadi, S.; El Jaouhari, A.; Bazzaoui, M.; Lévi, G.; Aubard, J.; Bazzaoui, E.A. X-ray photoelectron and in situ and ex situ resonance Raman spectroscopic investigations of polythiophene overoxidation. J. Solid State Electrochem. 2017, 21, 3519–3532. [Google Scholar] [CrossRef]
- Fu, M.; Shi, G.; Chen, F.; Hong, X. Doping level change of polythiophene film during its electrochemical growth process. Phys. Chem. Chem. Phys. 2002, 4, 2685–2690. [Google Scholar] [CrossRef]
- Subbulakshmi, R.R.; Palanichamy, E.; Arivazhagan, M.; Manivel, S. Theoretical studies on molecular structure and vibrational spectra of 2,4-difluoro-1-methoxy benzene and 1-chloro-3-methoxy benzene. Int. J. Sci. Res. Phys. Appl. Sci. 2019, 7, 34–48. [Google Scholar] [CrossRef]
- Jo, S.G.; Park, D.H.; Kim, B.-G.; Seo, S.; Lee, S.J.; Kim, J.; Kim, J.; Joo, J. Dual-mode waveguiding of Raman and luminescence signals in a crystalline organic microplate. J. Mater. Chem. C 2014, 2, 6077–6083. [Google Scholar] [CrossRef]
- Zhang, G.; He, W.; Chen, D. On difference of properties between organic fluorine hydrogen bond C-H F-C and conventional hydrogen bond. Mol. Phys. 2014, 112, 1736–1744. [Google Scholar] [CrossRef]
- Pietruś, W.; Kafel, R.; Bojarski, A.J.; Kurczab, R. Hydrogen bonds with fluorine in ligand-protein complexes—The PDB analysis and energy calculations. Molecules 2022, 27, 1005. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dragan, A.M.; Feier, B.G.; Tertiș, M.; Bodoki, E.; Truta, F.; Ștefan, M.-G.; Kiss, B.; Van Durme, F.; De Wael, K.; Oprean, R.; et al. Forensic analysis of synthetic cathinones on nanomaterials-based platforms: Chemometric-assisted voltammetric and UPLC-MS/MS investigation. Nanomaterials 2023, 13, 2393. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.; Qian, K. Nanomaterial-based electrochemical sensors: Mechanism, preparation, and application in bio-medicine. Adv. NanoBiomed Res. 2021, 1, 2000104. [Google Scholar] [CrossRef]
- Schram, J.; Parrilla, M.; Sleegers, N.; Van Durme, F.; Van Den, J.; van Nuijs, A.L.N.; De Wael, K. Electrochemical profiling and LC-MS characterization of synthetic cathinones: From methodology to detection in forensic samples. Drug Test. Anal. 2021, 13, 1282–1294. [Google Scholar] [CrossRef]
- Ross, E.A.; Watson, M.; Goldberger, B. “Bath salts” intoxication. N. Engl. J. Med. 2011, 365, 967–968. [Google Scholar] [CrossRef]
- Shishkanova, T.V.; Vatrsková, L.; Spálovská, D.; Králík, F.; Cuřínová, P.; Winkler, M.; Budka, J.; Jurásek, B.; Kuchař, M.; Setnička, V. Complexation of cathinones by 4-tert-butylcalix[4]arene tetraacetate as a possible technique for forensic analysis. Forensic Toxicol. 2020, 38, 70–78. [Google Scholar] [CrossRef]
- Shishkanova, T.V.; Štěpánková, N.; Tlustý, M.; Tobrman, T.; Jurásek, B.; Kuchař, M.; Trchová, M.; Fitl, P.; Vrňata, M. Electro-chemically oxidized 15-crown-5 substituted thiophene and host-guest interaction with new psychoactive substances. Electrochim. Acta 2021, 373, 137862. [Google Scholar] [CrossRef]

| NPS * | Structure | Working Electrode | Eanodic, V | Janodic, μA mm−2 |
|---|---|---|---|---|
| NEH a | 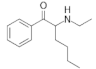 | G/SPE | 1.04 | 0.43 |
| GPH/SPE | 0.85 | 2.50 | ||
| MWCNT/SPE | 0.92 | 1.45 | ||
| PVP a |  | G/SPE | 0.76/0.92 (a split peak) | 1.28/0.29 |
| GPH/SPE | 0.69/0.83 | 0.55/0.71 | ||
| MWCNT/SPE | 0.70/0.86 | 1.32/0.33 | ||
| 2-Aminoindane b |  | |||
| PTh/G | 0.88/1.06 | 2.76 | ||
| PThPhCF3/G | 1.095 | 3.12/– | ||
| Buphedrone (MABP) b |  | |||
| PTh/G | 0.87/1.05 | 2.76 | ||
| PThPhCF3/G | 0.84/1.02 | 3.12/– | ||
| Naphyrone (O-2482) b |  | |||
| PTh/G | 0.87 | 5.98 | ||
| PThPhCF3/G | 0.84/1.02 | 10.64/– |
| Analyte | Electrode | pH | Sensitivity, μA L μmol−1 | Intercept, μA | Correlation Coefficient | LOD, μmol L−1 | LOQ, μmol L−1 |
|---|---|---|---|---|---|---|---|
| 2-AI | PThPhCF3/G | 7.0 | 0.583 | 10.047 | 0.9833 | 1.8 | 5.4 |
| Buphedrone (MABP) | 1.419 | 1.369 | 0.9851 | 1.7 | 5.0 | ||
| Naphyrone (O-2482) | 1.176 | 27.885 | 0.9806 | 1.9 | 5.5 | ||
| NEH * | GPH-SPE | 12.0 | 0.041 | 1.539 | 0.9700 | 16.67 | – |
| PVP * | 0.155 | 0.831 | 0.8310 | 1.67 | – |
| NPS * | Method | Introduced, mol L−1 | Found, mol L−1 | Sr | Reference |
|---|---|---|---|---|---|
| Buphedrone (MABP) | ISE a | 2.1 × 10−4 (44 μg/mL) | (2.1 ± 0.4) × 10−4 | 0.10 | [55] |
| EIS b | 4.0 × 10−5 (8.5 μg/mL) | (4.0 ± 1.7) × 10−5 | 0.27 | [56] | |
| SWV | 2.0 × 10−6 (0.43 μg/mL) | (2.0 ± 0.6) × 10−6 | 0.12 | Present | |
| Naphyrone (O-2482) | SWV | 2.0 × 10−6 (0.56 μg/mL) | (2.0 ± 0.2) × 10−6 | 0.05 | Present |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shishkanova, T.V.; Štěpánková, N.; Broncová, G.; Vrňata, M. Interaction of the Polymeric Layer Derived from 3-(4-Trifluoromethyl)-phenyl)-thiophene with Synthetic Stimulants on the Phase Boundary. Chemosensors 2024, 12, 99. https://doi.org/10.3390/chemosensors12060099
Shishkanova TV, Štěpánková N, Broncová G, Vrňata M. Interaction of the Polymeric Layer Derived from 3-(4-Trifluoromethyl)-phenyl)-thiophene with Synthetic Stimulants on the Phase Boundary. Chemosensors. 2024; 12(6):99. https://doi.org/10.3390/chemosensors12060099
Chicago/Turabian StyleShishkanova, Tatiana V., Natalie Štěpánková, Gabriela Broncová, and Martin Vrňata. 2024. "Interaction of the Polymeric Layer Derived from 3-(4-Trifluoromethyl)-phenyl)-thiophene with Synthetic Stimulants on the Phase Boundary" Chemosensors 12, no. 6: 99. https://doi.org/10.3390/chemosensors12060099
APA StyleShishkanova, T. V., Štěpánková, N., Broncová, G., & Vrňata, M. (2024). Interaction of the Polymeric Layer Derived from 3-(4-Trifluoromethyl)-phenyl)-thiophene with Synthetic Stimulants on the Phase Boundary. Chemosensors, 12(6), 99. https://doi.org/10.3390/chemosensors12060099







