Improved Microelectrode Array Electrode Design for Heavy Metal Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Design Calculations for Electrodes
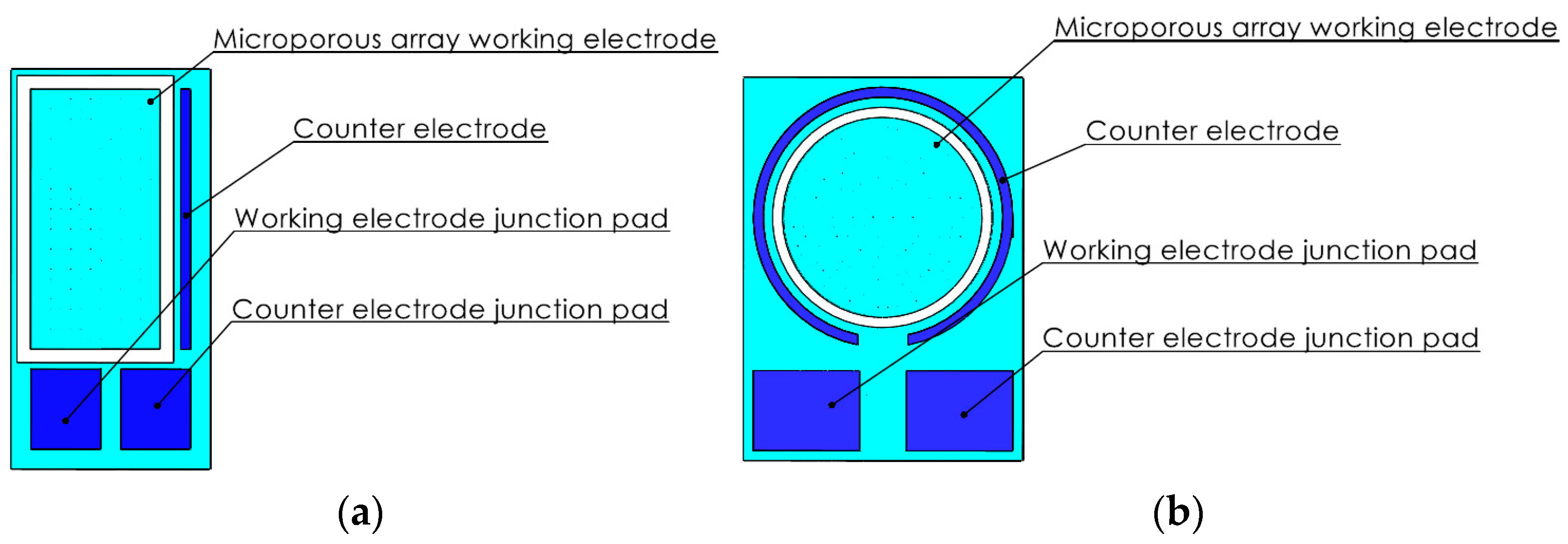
2.2. Detailed Structural Design of the Microelectrode Array Electrode
2.3. Processing of Composite Electrodes for Microelectrode Arrays
- (1)
- A silicon wafer was selected as the substrate and cleaned until it reached the processing standard.
- (2)
- The SiO2 insulating layer was deposited using plasma-enhanced chemical vapor deposition as the underlying insulating layer for the sensor.
- (3)
- Using an ion sputtering apparatus, a 20 nm Ti adhesion layer and a 200 nm Ir electrode layer were sequentially percolated on the substrate, using the substrate as a specimen.
- (4)
- A SiO2 insulating layer was deposited using plasma-enhanced chemical vapor deposition as an insulating layer for the sensor.
- (5)
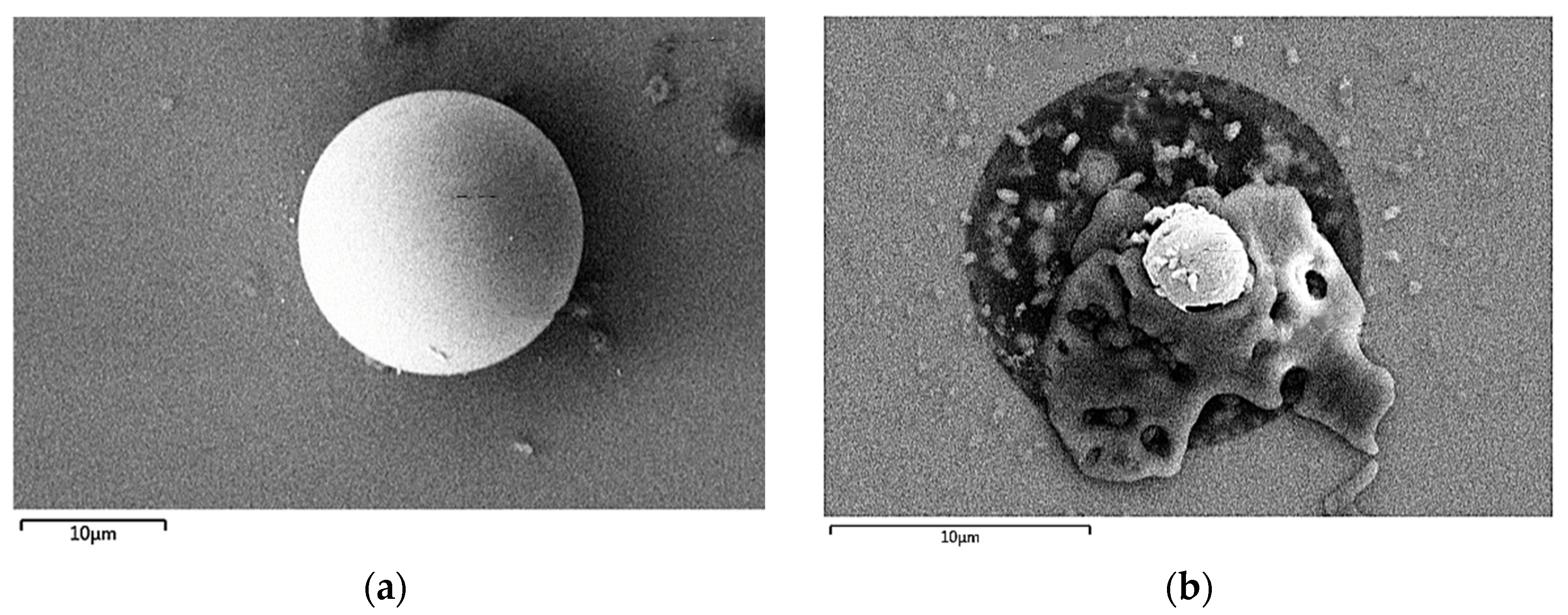
- A 150-μm-thick SU-8 photoresist ring was printed around the periphery of the microporous array working electrode as a protective ring for the gel plating layer (Figure 2).
- (6)
- Using a silicon knife, the wafer was cut to obtain individual electrode chips (Figure 3a).
- (7)
- The gold wire was led out from the chip pad, press-soldered to the pad on the PCB, and sealed with epoxy resin to complete the sensor package (Figure 3b).

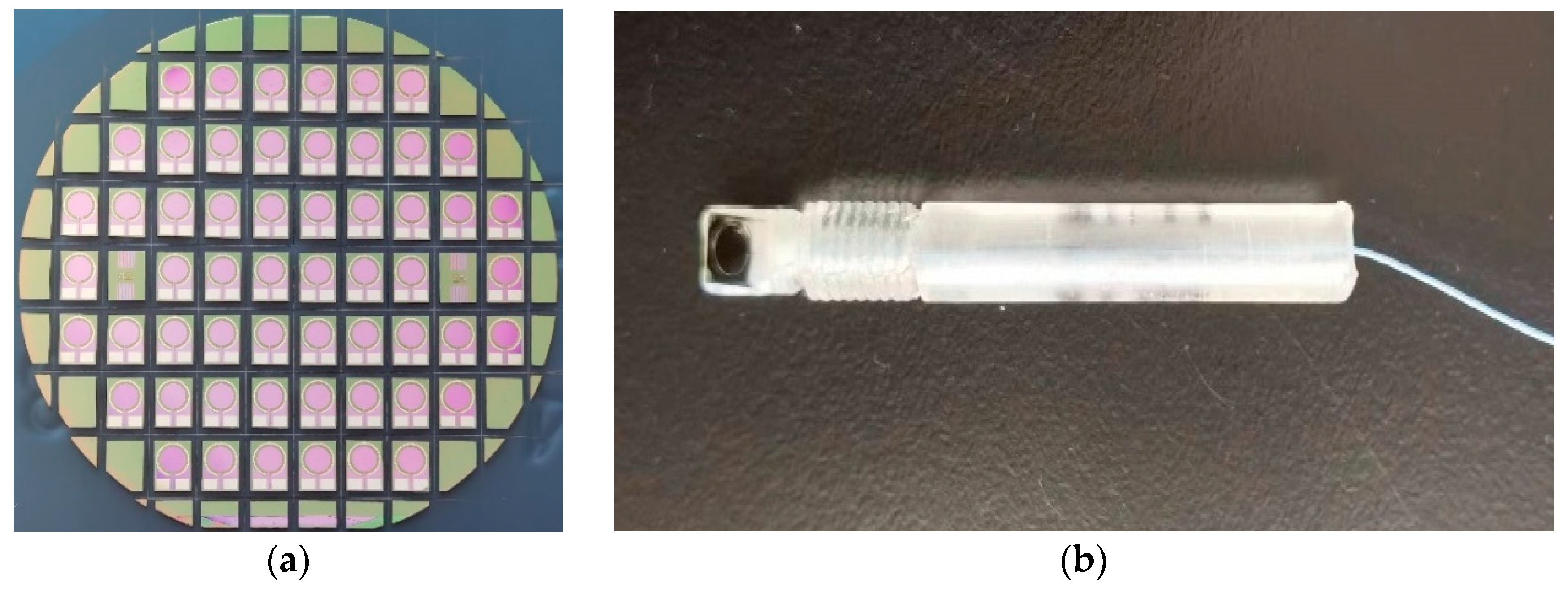
2.4. Instruments and Reagents
2.5. Selection of Measurement Method
2.6. Electrode Modification
2.7. Sample Collection and Processing
3. Results and Discussion
3.1. Electrochemical Characterization
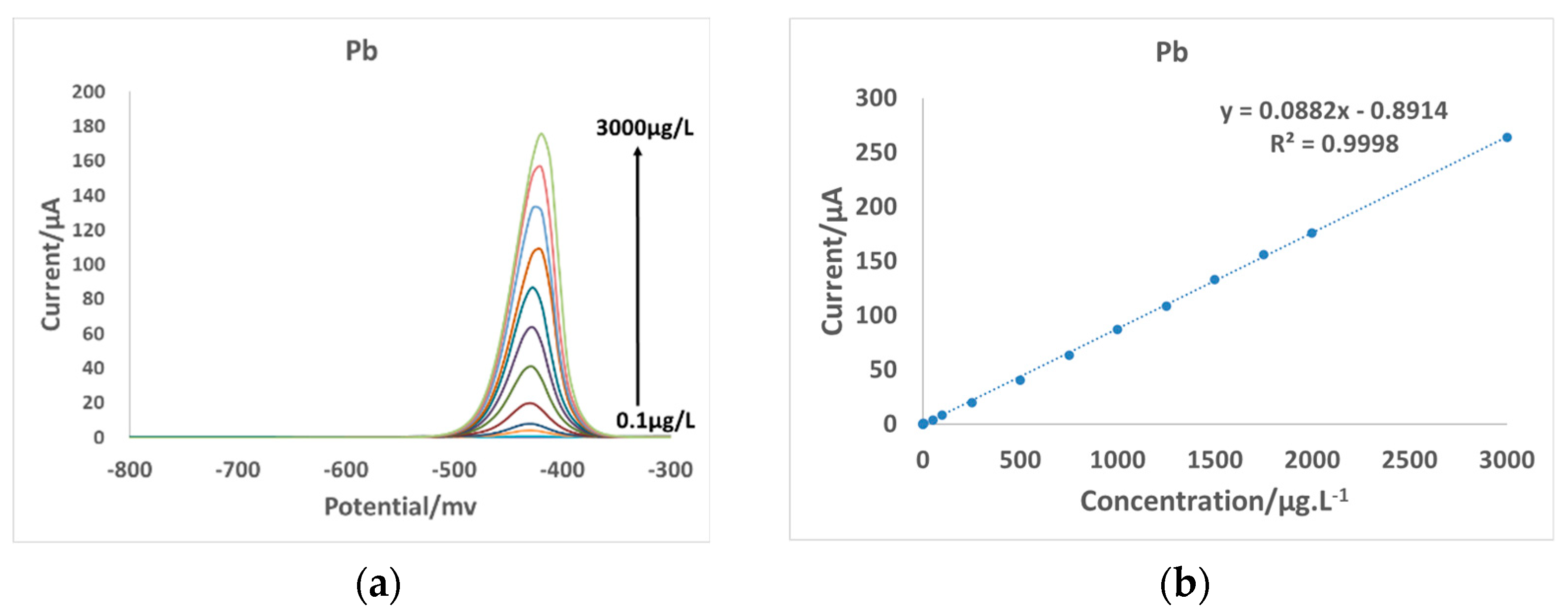
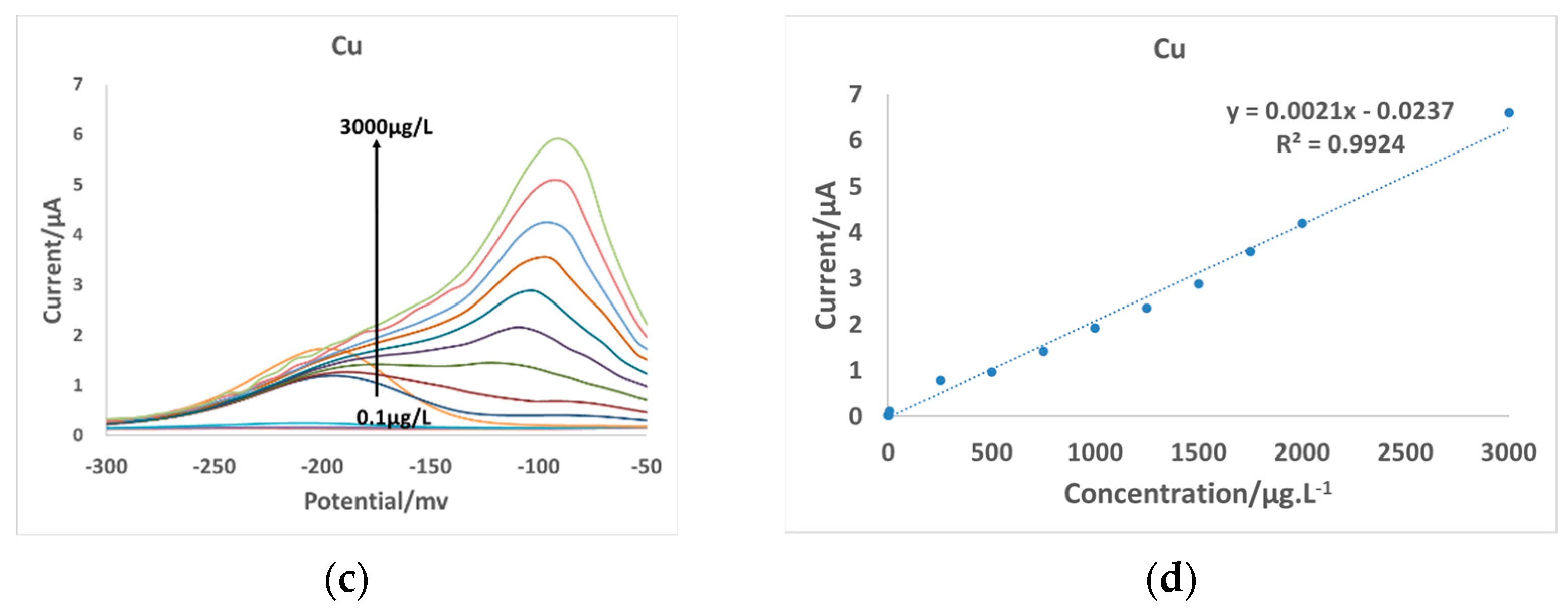
3.2. Electrode Detection Range
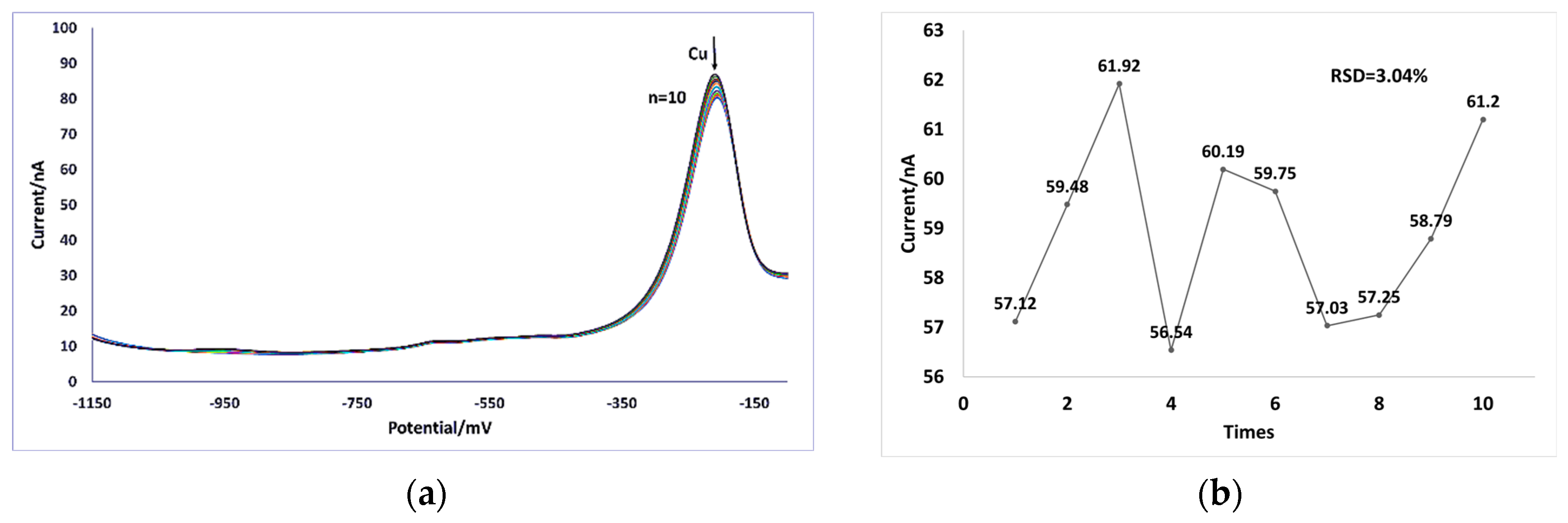
3.3. Electrode Repeatability Tests
3.4. Comparison of This Study with Other Literature
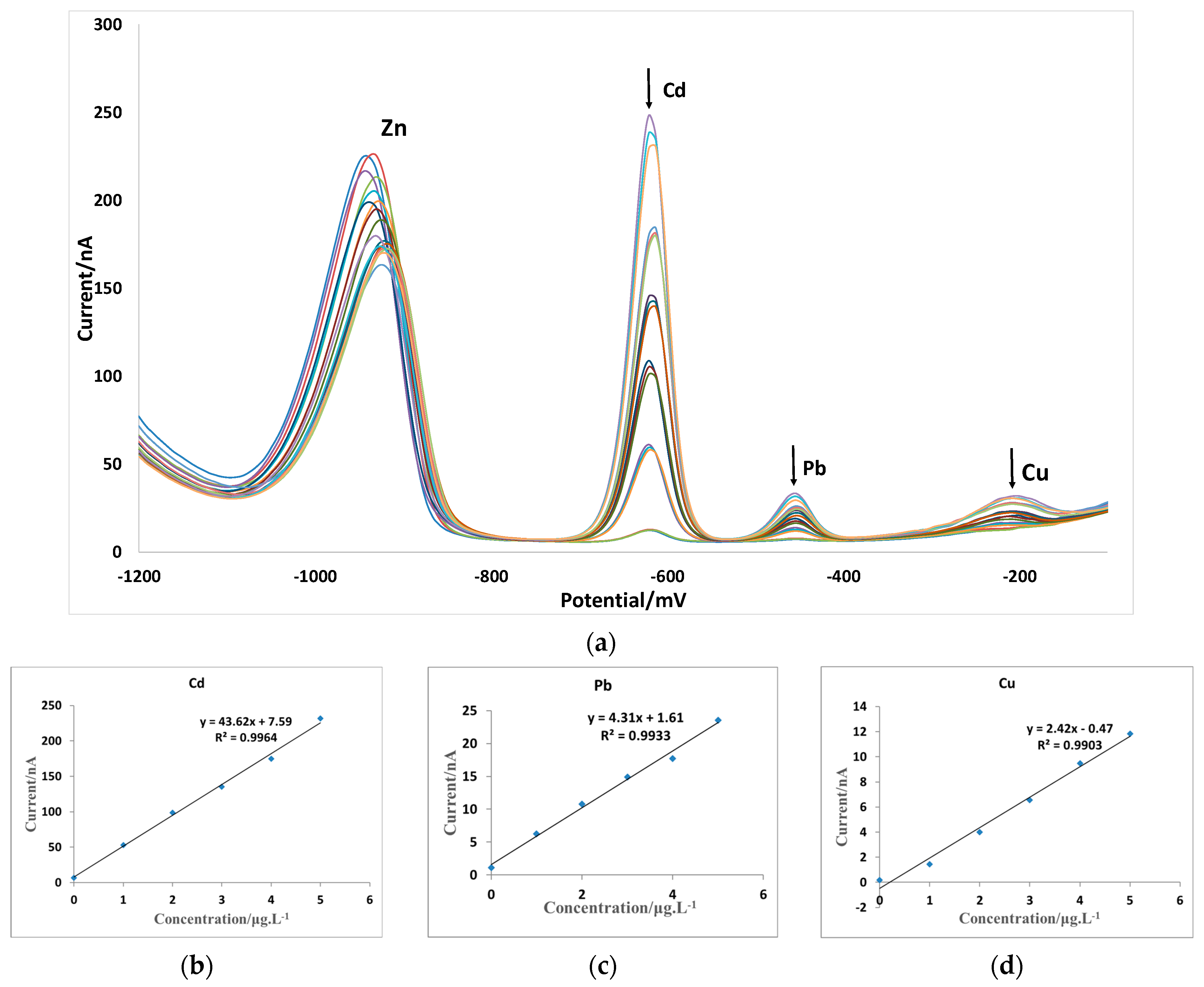
3.5. Electrode Detection Performance
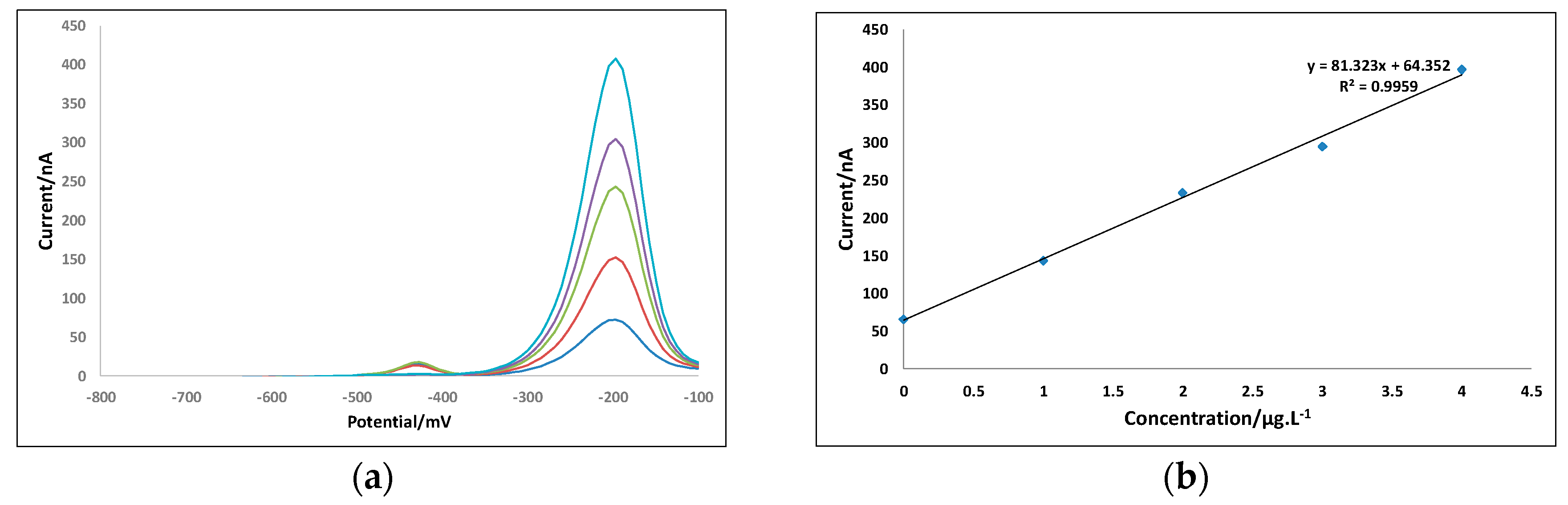
3.6. Measurement of Lead and Copper Ion Concentrations in Sanya River Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, F.; Zhang, H.; Xie, S.; Wei, C.; Yang, X. Concentrations of heavy metals in water, sediments and aquatic organisms from a closed realgar mine. Environ. Sci. Pollut. Res. Int. 2023, 30, 4959–4971. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Han, D.; Song, X.; Liu, S. Sources and migration of heavy metals in a karst water system under the threats of an abandoned Pb-Zn mine, Southwest China. Environ. Pollut. 2021, 277, 116774. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Martorell, J.J.; Galindo-Riaño, M.D.; García-Vargas, M.; Granado-Castro, M.D. Bioavailability of heavy metals monitoring water, sediments and fish species from a polluted estuary. J. Hazard. Mater. 2009, 162, 823. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.Q.; Wang, J.; Xu, M.; Zhao, L.; Wang, Z. Spatial distribution, source apportionment and ecological risk assessment of heavy metals in the sediments of Haizhou Bay national ocean park, China. Mar. Pollut. Bull. 2019, 149, 110651. [Google Scholar] [CrossRef]
- Han, J.L.; Pan, X.D.; Chen, Q.; Huang, B.F. Health risk assessment of heavy metals in marine fish to the population in Zhejiang, China. Sci. Rep. 2021, 11, 11079. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Bhattacharya, T.; Singh, G.; Maity, J.P. Benthic macroalgae as biological indicators of heavy metal pollution in the marine environments: A biomonitoring approach for pollution assessment. Ecotoxicol. Environ. Saf. 2014, 100, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.X.; Chao, S.H.; Liu, J.W.; Yang, Y.; Chen, Y.; Zhang, A.; Cao, H. Source apportionment and health risk assessment of heavy metals in soil for a township in Jiangsu Province, China. Chemosphere 2017, 168, 1658–1668. [Google Scholar] [CrossRef]
- Wang, C.L.; Zou, X.Q.; Feng, Z.Y.; Hao, Z.; Gao, J. Distribution and transport of heavy metals in estuarine-inner shelf regions of the East China Sea. Sci. Total Environ. 2018, 644, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, S.; Andersson, A.; Nilsson, M.B.; Skyllberg, U.; Lundberg, E.; Schaefer, J.K.; Åkerblom, S.; Björn, E. Terrestrial discharges mediate trophic shifts and enhance methylmercury accumulation in estuarine biota. Sci. Adv. 2017, 3, e1601239. [Google Scholar] [CrossRef]
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Sidhu, G.P.S.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y.; et al. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef]
- Buffle, J.; Tercier-Waeber, M.L. Voltammetric environmental trace-metal analysis and speciation: From laboratory to in situ measurements. TrAC Trends Anal. Chem. 2005, 24, 172–191. [Google Scholar] [CrossRef]
- Tercier-Waeber, M.L.; Buffle, J. Submersible online oxygen removal system coupled to an in situ voltammetric probe for trace element monitoring in freshwater. Environ. Sci. Technol. 2000, 34, 4018–4024. [Google Scholar] [CrossRef]
- Morais, P.V.; Suman, P.H.; Schöning, M.J.; Siqueira, J.R., Jr.; Orlandi, M.O. Layer-by-Layer Film Based on Sn3O4 Nanobelts as Sensing Units to Detect Heavy Metals Using a Capacitive Field-Effect Sensor Platform. Chemosensors 2023, 11, 436. [Google Scholar] [CrossRef]
- Finšgar, M.; Rajh, B. A Factorial Design and Simplex Optimization of a Bismuth Film Glassy Carbon Electrode for Cd(II) and Pb(II) Determination. Chemosensors 2023, 11, 129. [Google Scholar] [CrossRef]
- Prontera, C.T.; Sciurti, E.; De Pascali, C.; Giampetruzzi, L.; Biscaglia, F.; Blasi, L.; Esposito, V.; Casino, F.; Siciliano, P.A.; Francioso, L.N. Anodic Stripping Voltammetric Determination of Copper Ions in Cell Culture Media: From Transwell® to Organ-on-Chip Systems. Chemosensors 2023, 11, 466. [Google Scholar] [CrossRef]
- Hirbodvash, Z.; Houache, M.S.E.; Krupin, O.; Khodami, M.; Northfield, H.; Olivieri, A.; Baranova, E.A.; Berini, P. Electrochemical Performance of Lithographically-Defined Micro-Electrodes for Integration and Device Applications. Chemosensors 2021, 9, 277. [Google Scholar] [CrossRef]
- Silva, P.R.M.; Khakani, M.A.E.; Chaker, M.; Champagne, G.Y.; Chevalet, J.; Gastonguay, L.; Lacasse, R.; Ladouceur, M. Development of Hg-electroplated-iridium based microelectrode arrays for heavy metal traces analysis. Anal. Chim. Acta 1999, 385, 249–255. [Google Scholar] [CrossRef]
- Wightman, R.M. Voltammetry with microscopic electrodes in new domains. Science 1988, 240, 415–420. [Google Scholar] [CrossRef]
- Uhlig, A.; Schnakemberg, U.; Hintsche, R. Highly sensitive heavy metal analysis on platinum- and gold-ultramicroelectrode arrays. Electroanalysis 1997, 9, 125–129. [Google Scholar] [CrossRef]
- Tercier-Waeber, M.L.; Fighera, M.; Abdou, M.; Bakker, E.; van der Wal, P. Newly designed gel-integrated nanostructured gold-based interconnected microelectrode arrays for continuous in situ arsenite monitoring in aquatic systems. Sens. Actuators B Chem. 2021, 328, 128996. [Google Scholar] [CrossRef]
- Guo, J.; Lindner, E. Cyclic voltammograms at coplanar and shallow recessed microdisk electrode arrays: Guidelines for design and experiment. Anal. Chem. 2009, 81, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Stueben, D.; Berner, Z. The application of microelectrodes for the measurements of trace metals in water. Anal. Lett. 2005, 38, 2281–2300. [Google Scholar] [CrossRef]
- Kiani, H.; Beheshti, B.; Borghei, A.M.; Rahmati, M.H. Detection of olive oil contaminated with heavy metals using a three-electrode system based on cyclic voltammetry. J. Electroanal. Chem. 2021, 116, 293–303. [Google Scholar] [CrossRef]
- Pitsou, M.; Kokkinos, C.; Economou, A.; Fielden, P.R.; Baldock, S.J.; Goddard, N.J. “Green” Three-Electrode Sensors Fabricated by Injection-Moulding for On-Site Stripping Voltammetric Determination of Trace In(III) and Tl(I). Chemosensors 2021, 9, 310. [Google Scholar] [CrossRef]
- Davis, F.; Higson, S.P.J. Arrays of microelectrodes: Technologies for environmental investigations. Environ. Sci. Process. Impacts 2013, 15, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Tercier-Waeber, M.L.; Abdou, M.; Fighera, M.; Kowal, J.; Bakker, E.; van der Wal, P. In situ voltammetric sensor of potentially bioavailable inorganic mercury in marine aquatic systems based on gel-integrated nanostructured gold-based microelectrode arrays. ACS Sens. 2021, 6, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Abdou, M.; Tercier-Waeber, M.L.; Dutruch, L.; Bossy, C.; Pougnet, F.; Coynel, A.; Bakker, E.; Blanc, G.; Schäfer, J. Estuarine dissolved speciation and partitioning of trace metals: A novel approach to study biogeochemical processes. Environ. Res. 2022, 208, 112596. [Google Scholar] [CrossRef] [PubMed]
- Tercier-Waeber, M.L.; Confalonieri, F.; Koudelka-Hep, M.; Dessureault-Rompré, J.; Graziottin, F.; Buffle, J. Gel-integrated voltammetric microsensors and submersible probes as reliable tools for environmental trace metal analysis and speciation. Electroanalysis 2008, 20, 240–258. [Google Scholar] [CrossRef]
- Wang, J. Stripping analysis at bismuth electrodes: A review. Electroanalysis 2005, 17, 1341–1346. [Google Scholar] [CrossRef]
- Injang, U.; Noyrod, P.; Siangproh, W.; Dungchai, W.; Motomizu, S.; Chailapakul, O. Determination of trace heavy metals in herbs by sequential injection analysis-anodic stripping voltammetry using screen-printed carbon nanotubes electrodes. Anal. Chim. Acta 2010, 668, 54–60. [Google Scholar] [CrossRef]
- Kachoosangi, R.T.; Banks, C.E.; Ji, X.; Compton, R.G. Electroanalytical determination of cadmium (II) and lead (II) using an in-situ bismuth film modified edge plane pyrolytic graphite electrode. Anal. Sci. 2007, 23, 283–289. [Google Scholar] [CrossRef]
- Herzog, G.; Arrigan, D.W.M. Determination of trace metals by underpotential deposition–stripping voltammetry at solid electrodes. Trends Anal. Chem. 2005, 24, 208–217. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, J.; Dai, W.; Lin, X.; Ye, J.; Ye, J. A screen-printed carbon electrode modified with a bismuth film and gold nanoparticles for simultaneous stripping voltammetric determination of Zn(II), Pb(II) and Cu(II). Microchim. Acta 2017, 184, 4731–4740. [Google Scholar] [CrossRef]
- Guo, Z.; Li, D.D.; Luo, X.K.; Li, Y.H.; Zhao, Q.N.; Li, M.M.; Zhao, Y.T.; Sun, T.S.; Ma, C. Simultaneous determination of trace Cd(II), Pb(II) and Cu(II) by differential pulse anodic stripping voltammetry using a reduced graphene oxide-chitosan/poly-l-lysine nanocomposite modified glassy carbon electrode. J. Colloid Interface Sci. 2017, 490, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Meng, Z.; Zhang, H.; Zheng, J. Fullerene-based anodic stripping voltammetry for simultaneous determination of Hg(II), Cu(II), Pb(II) and Cd(II) in foodstuff. Microchim. Acta 2018, 185, 274. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Jia, M.; Wang, Z.; Zhang, W.; Zhang, Q.; Liu, G.; Zhang, Z.; Li, P. Simultaneous voltammetric determination of cadmium (II), lead (II), mercury (II), zinc (II), and copper (II) using a glassy carbon electrode modified with magnetite (Fe3O4) nanoparticles and fluorinated multiwalled carbon nanotubes. Mikrochim. Acta 2019, 186, 97. [Google Scholar] [CrossRef] [PubMed]
- Mourya, A.; Sinha, S.K.; Mazumdar, B. Glassy carbon electrode modified with blast furnace slag for electrochemical investigation of Cu2+ and Pb2+ metal ions. Microchem. J. 2019, 147, 707. [Google Scholar] [CrossRef]
- Fan, C.; Chen, L.; Jiang, R.; Ye, J.; Li, H.; Shi, Y.; Luo, Y.; Wang, G.; Hou, J.; Guo, X. ZnFe2O4 nanoparticles for electrochemical determination of trace Hg (II), Pb (II), Cu (II), and glucose. ACS Appl. Nano Mater. 2021, 4, 4026. [Google Scholar] [CrossRef]
- Xiong, S.; Xu, J.; Xie, F.; Hu, X.; Gong, G.; Wu, Z.; Yao, L. Stripping analysis of Pb(II), Cd(II), Hg(II) and Cu(II) based on irradiated attapulgite/Ionic liquid composites. Chem. Eng. J. 2017, 316, 383–392. [Google Scholar] [CrossRef]
- Suteerapataranon, S.; Jakmunee, J.; Vaneesorn, Y.; Grudpan, K. Exploiting flow injection and sequential injection anodic stripping voltammetric systems for simultaneous determination of some metals. Talanta 2002, 58, 1235. [Google Scholar] [CrossRef]
- GB 17378.4-2007; The Specification for Marine Monitoring—Part 4: Seawater Analysis. Standards Press of China: Beijing, China, 2007.




| Sampling Point | Pretreatment |
|---|---|
| E (initial) | −1200 to −800 mV |
| E (final) | −100 to 0 mV |
| Pulse amplitude | 25 mV |
| Step amplitude | 8 mV |
| Frequency | 15Hz |
| Preconc | −1200 to −800 mV; t = 600 s |
| Sampling Point | Pretreatment |
|---|---|
| Sanya Bay | filtration with acid 1 L × 3; filtration 1 L × 3 |
| Nanbianhai Pier | filtration with acid 1 L × 3; filtration 1 L × 3 |
| Xinfengqiao | filtration with acid 1 L × 3; filtration 1 L × 3 |
| Fenghuangshuicheng | filtration with acid 1 L × 3; filtration 1 L × 3 |
| Electrode | Measurement Method | Pb Detection Range (μg/L) | Pb Sensitivity (nA/μg/L) | Pb Detection Limit (μg/L) | Cu Detection Range (μg/L) | Cu Sensitivity (nA/μg/L) | Cu Detection Limit (μg/L) | Reference |
|---|---|---|---|---|---|---|---|---|
| Bi/AuNP-SPCE | DPV | 1.00–150.00 | 0.48 | 0.03 | 1.00–150.00 | 0.48 | 0.03 | [33] |
| RGO-CSCS/PLL/GCE | DPV | 0.05–2.00 | 4.84 | 0.02 | 0.05–2.00 | 4.84 | 0.02 | [34] |
| C60-CS/GCE | DPV | 1.04–1242.00 | 1.29 | 0.21 | 6.40–384.00 | 1.29 | 0.21 | [35] |
| F-MWCNT | SWV | 5.80–6210.00 | 0.21 | 1.74 | 1.09–2016.00 | 0.21 | 1.74 | [36] |
| GCE-BFS | DPV | 103.50–16,560.00 | 0.15 | 17.39 | 32.00–5120.00 | 0.15 | 17.39 | [37] |
| ZFO/GCE | DPV | 20.70–414.00 | 0.56 | 1.06 | 6.40–64.00 | 0.56 | 1.06 | [38] |
| MAE | SWV | 0.1–3000 | 4.31 | 0.01 | 0.1–3000 | 2.42 | 0.02 | this study |
| Sampling Location | Pb Ion Concentration (μg/L) | Cu Ion Concentration (μg/L) |
|---|---|---|
| Sanya Bay | 0.39 | 0.28 |
| Nanbianhai Pier | 0.61 | 0.45 |
| Xinfengqiao | 0.12 | 0.23 |
| Fenghuangshuicheng | 0.35 | 0.25 |
| Sampling Location | Pb Ion Concentration (μg/L) | Cu Ion Concentration (μg/L) |
|---|---|---|
| Sanya Bay | 0.98 | 0.79 |
| Nanbianhai Pier | 0.78 | 0.70 |
| Xinfengqiao | 0.42 | 0.96 |
| Fenghuangshuicheng | 0.53 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wu, S.; Zhang, F.; Jin, B.; Yang, C. Improved Microelectrode Array Electrode Design for Heavy Metal Detection. Chemosensors 2024, 12, 51. https://doi.org/10.3390/chemosensors12040051
Zhang J, Wu S, Zhang F, Jin B, Yang C. Improved Microelectrode Array Electrode Design for Heavy Metal Detection. Chemosensors. 2024; 12(4):51. https://doi.org/10.3390/chemosensors12040051
Chicago/Turabian StyleZhang, Jian, Shijun Wu, Feng Zhang, Bo Jin, and Canjun Yang. 2024. "Improved Microelectrode Array Electrode Design for Heavy Metal Detection" Chemosensors 12, no. 4: 51. https://doi.org/10.3390/chemosensors12040051
APA StyleZhang, J., Wu, S., Zhang, F., Jin, B., & Yang, C. (2024). Improved Microelectrode Array Electrode Design for Heavy Metal Detection. Chemosensors, 12(4), 51. https://doi.org/10.3390/chemosensors12040051








