Abstract
The importance of phosphates has sparked researchers’ considerable interest in the electrochemical detection of phosphates within aqueous solutions in recent years. In this study, we present a novel all-solid-state phosphate ion-selective electrode (ISE) that integrates copper, copper nanoparticles, and copper phosphate. By modifying the copper substrate of the electrode with a copper nanoparticle film and creating a lamellar copper phosphate film through electrochemical treatment, we significantly enhanced the electrode’s electron transfer efficiency. This microstructure with large specific surface area markedly improved the electrode’s responsiveness to the targeted ions by accelerating the achievement of chemical equilibrium on the electrode surface, thereby boosting its sensitivity and stability. The newly developed electrode was capable of detecting phosphate ions in solutions with a pH range from 6 to 11 and performed optimally in neutral solutions at pH 7, following Nernst principle, with a detection limit of 1 × M. The electrode exhibited a short response time of less than 10 s with significant reproducibility, stability, longevity—maintaining functionality for more than two months. It also displayed good selectivity as the electrochemical equilibrium was not influenced by up to 1 mM of potential competing species like , , and . We compared the detection results of current phosphate ion sensor and conventional determination methods for phosphate content in natural lake and aquaculture water samples, with a detection discrepancy of about 10% (RSD). Considering all feasible performance characteristics combined with its low cost, simple manufacture and portability, the sensor provides a new possibility for rapid, reliable, and long-term real-time in situ detection of phosphates.
1. Introduction
Phosphorus is widely distributed in the biological ecosystem and in environmental systems and is of great significance to the biogeochemistry cycle and healthy habitat. Phosphorus usually occurs in phosphate and phosphate is the key substance in inorganic chemistry, biochemistry and biogeochemistry. In recent years, the rising global population and substantial growth of industrial and agricultural activities resulted in the release of a significant amount of phosphorus-containing pollutants into nature waters. An excessive accumulation of phosphate in lakes, oceans and other natural water bodies induces eutrophication—a phenomenon followed by algal blooms, anoxia, acidification, and a biodiversity crisis. Eventually, a series of water quality and environmental problems are triggered [1,2,3]. Therefore, phosphates act as both a crucial nutrient and a growth inhibitor in aquatic ecosystems; real-time detection of phosphates is of significant importance as it serves to reflect the water quality and health status of natural water bodies and water used in hydroponic and aquaculture systems [1,3]. In addition, phosphorus is one of the important constituent elements of living cells, and phosphate excessiveness or deficiency in the human body will cause a series of physiological problems, such as hyperphosphatemia, which will harm the kidneys, thyroid gland and so on [4,5,6]. Additionally, phosphates are widely used in food processing as well as food additives and ingredients. Therefore, effective determination of phosphate concentration is important in various fields such as chemistry, biomedicine, agriculture, industry and environmental pollution prevention and control [7,8,9]. In a word, it is of great value to design and develop a low-cost, portable, efficient, accurate and real-time on-line sensor for phosphate ion detection.
Over the past few decades, environmental agencies in many countries have specified recommended range values for total phosphorus or phosphate concentrations and standard procedures for their detection [10]. The phosphor molybdenum blue spectrophotometric method is currently the most widely used phosphate detection method [11,12]. With the advancement of instrumentation, ion chromatography has gradually become a common method for determining phosphates [13,14]. The methods mentioned above possess good sensitivity and reliability. However, they necessitate sample pretreatment, are complex, time-consuming, and costly. Additionally, they cannot meet the demands of long-term real-time online monitoring. These limitations to some extent restrict the application of these methods [15,16]. In recent years, electrochemical analysis techniques, especially ion-selective electrodes (ISEs), have been widely studied and applied due to their capabilities of rapid and efficient measurement, easy operation, high sensitivity, and strong anti-interference ability [17,18].
At present, there is no commercial phosphate ion sensor in the electrochemical ion sensor market. The development of ion-selective sensors for phosphates is more challenging compared to other ions. The large tetrahedral structure of the phosphate ions results in high hydration energy and a tendency to remain in aqueous solutions, meaning that it is not easy for the ion-selective electrodes to exhibit excellent selectivity for phosphates [19]. Furthermore, phosphates in aqueous solutions undergo three levels of dissociation, and the valence state and relative distribution ratio of phosphates vary under different pH conditions, which brings great difficulty to the quantitative detection of phosphates. In recent years, some researchers have reported a lot of research progress on phosphate ion sensors. Many have developed a series of spectrophotometric phosphate sensors based on the spectral characteristics and fluorescent response of phosphates [20,21,22,23,24,25,26,27,28]. In terms of electrochemical sensors, cobalt metal is most commonly used in the research of phosphate sensors due to its unique responsiveness and selectivity to phosphate ions. Xiao et al. proposed a novel all-solid-state cobalt-based phosphate ion sensor, which presented a selective response to by modifying the surface of metallic cobalt with a cobalt phosphate-sensitive membrane [29]. Meruva et al. confirmed that the selective response of this cobalt-based phosphate electrode to was a mixed potential response mechanism and the response potential was affected by dissolved oxygen [30]. Xu et al. made further improvements to this cobalt-based electrode by coating cobalt with cobalt phosphate on the surface and conducted a further analysis of the electrode’s mechanism and performance [31]. To improve the sensitivity and stability of cobalt-based phosphate sensors, polymers and electron transfer media were used to fix cobalt powder and improve the electron transport efficiency. Zhao et al. proposed a new type of phosphate ISE composed of polypyrrole, cobalt, and ordered mesoporous carbon, which had good sensitivity and stability in response to and performed well in actual water sample detection [32]. Since cobalt-based sensors can only respond to under weakly acidic conditions, Xu et al. used the method of surface doping with phosphate complexes to develop a new type of tungsten-based phosphate ISE. This electrode showed a selective response to under neutral to weakly alkaline conditions, with a detection limit of 10−6 M under neutral conditions [33].
In addition, other metals, such as silver [34] and nickel [35], played a role in the research of phosphate ion sensors. While there has been extensive research on inorganic metals and their phosphates, some researchers have embarked on exploring the construction of polymer-sensitive membranes on an all-solid-state phosphate ISE. Jeong et al. prepared an effective phosphate ionophore using the special affinity of niacinamide and phosphate through hydrogen bonding and charge interactions; the sensor modified by this ionophore can determine phosphate with a relatively lower limit of 0.85 × 10−6 M compared with most reported electrodes [36]. Ben et al. reported a novel PE-based phosphate sensor in which they embedded octamolybdate anions with periodic mesoporous organosilica nanosphere, which was suitable for detecting extremely low concentrations of phosphates with a detection limit of 0.16 nM [37].
In this study, we proposed a novel all-solid-state phosphate ISE that is efficient, accurate, low cost, easy to operate, and has a long lifetime. Our approach involved enhancing the surface electrochemical activity of the copper-based electrode through the modification of a copper nanoparticle film. Subsequently, the copper nanoparticle film created a mesh-like film structure with a significantly larger specific surface area by electrochemical modification. This microstructure not only enhanced electron transfer efficiency within the electrode but also improved the electrode surface membrane’s response to target ions, with excellent sensitivity and stability. We analyzed and studied the response mechanism of the electrode by cyclic voltammetry, scanning electron microscopy, and energy spectrum analysis. Our systematic characterization of the prepared phosphate ISE covered aspects such as its detection limit, response time, selectivity, reproducibility, stability, and lifespan. Experimental results evidenced that the current phosphate ISE was capable of detecting phosphate ions across a broad pH range, from neutral to weakly alkaline.
2. Materials and Methods
The preparation process of the electrochemically modified all-solid-state copper electrode, designated as the copper phosphate electrode, is briefly described as follows. Firstly, we prepared a uniform copper nanoparticle film on the surface of copper wire through electrochemical deposition. Then, we fabricated a uniform copper phosphate-sensitive membrane on the surface of the above electrochemically treated copper wire through electrochemical synthesis. The schematic diagram is shown in Figure 1.
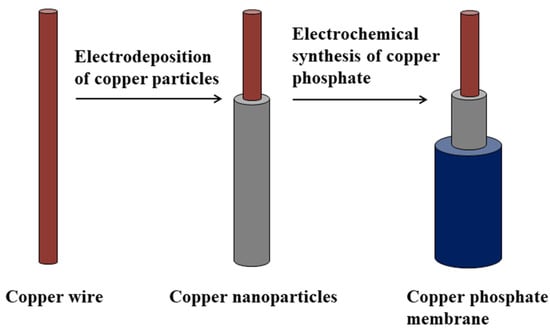
Figure 1.
Schematic diagram of electrode fabrication process.
2.1. Reagents and Apparatus
The copper sulfate (Cu2SO4), sodium chloride (NaCl), sodium hydroxide (NaOH), nitric acid (HNO3), disodium hydrogen phosphate (Na2HPO4), sodium dihydrogen phosphate (NaH2PO4), sodium sulfate (Na2SO4), sodium bicarbonate (NaHCO3), sodium acetate (CH3COONa), sodium silicate (Na2SiO3), sodium nitrate (NaNO3), and copper wire (99.9%, 0.5 mm in diameter) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All chemical reagents were of analytical grade and used as received without further purification. All solutions used in the experiment were prepared with ultrapure water with a resistivity of 18.2 MΩ·cm.
We applied a SG1020A function generator (Rhett, Huaian, China) to carry out the nanoparticles’ electrodeposition process by connecting a 1N5819 Schottky diode (OnSemi, Scottsdale, AZ, USA). The electrochemical synthesis and the electrochemical performance testing of the electrode were implemented using a CHI760D electrochemical workstation (CHI, Shanghai, China). We purchased the Ag/AgCl reference electrode (3.8 mm) from Jinghong Electronic Technology Development Co., Ltd. (Shanghai, China). We acquired the platinum electrode (3.8 mm) from Gaoss Union Electronic Technology Co., Ltd. (Chengdu, China).
The SEM analysis and the EDS measurements were performed with a Zeiss Sigma 500 field emission scanning electron microscope (Carl Zeiss, Oberkochen, Germany). The pH of the solutions was measured with a E-201F composite pH electrode (INESA, Shanghai, China). The phosphomolybdenum blue spectrophotometric method was performed with a CleverChem 380 Plus auto discrete analyzer (DeChem-Tech, Hamburg, Germany).
2.2. Electrode Pretreatment and Fabrication
We polished a copper wire (φ0.5 mm × 50 mm) by using abrasive paper of #1000, #3000, #5000 and #7000 in turns for 2 min. It was then ultrasonically washed with 0.1 M nitric acid, acetone and deionized water in sequence for 15 min, and finally dried for later use.
In order to form a copper nanoparticle film, we applied a SG1020A function generator with sine wave parameters of an amplitude of 1.5 V and frequency of 50 Hz. The cathode was connected to the pretreated copper wire, and the anode was attached to a Schottky diode and contributing copper wire. Two copper wires were simultaneously immersed into a 0.05 M Cu2SO4 solution and were electroplated for 60 s. In the process of electrochemical deposition, the metallic luster on the surface of the copper electrode gradually disappeared, and the color became darker. Ultimately, a layer of copper nanoparticles was uniformly fabricated on the surface of the cathodic copper wire.
We carried out the electrochemical deposition process of copper phosphate by using a three-electrode system on the CHI760D workstation. A saturated Ag/AgCl electrode served as a reference electrode, a copper electrode modified with a copper nanoparticle film functioned as a working electrode, and a platinum electrode acted as a counter electrode. We placed the abovementioned electrodes simultaneously in 0.1 M Na2HPO4 solution at pH 9.0 and the electrochemical synthesis process of the copper phosphate-sensitive membrane was accomplished through a chronoamperometry method for 300 s at a potential of 0.8 V. In the process of constant potential electroplating, the surface of the copper electrode was gradually covered with a blue crystal film. After 300 s of electroplating, the electroplating was stopped when the surface morphology of the electrode was no longer changed. Then, the copper phosphate electrode dried naturally for the characterization and performance evaluation.
2.3. Electrode Performance Characterization
The SEM analysis was carried out to observe the surface morphology of the electrode and its elemental distribution was determined by the EDS measurement. All potential measurements were carried out with a two-electrode system on a CHI760D electrochemical workstation at room temperature using an Ag/AgCl electrode as a reference electrode and the prepared copper phosphate electrode as a working electrode. We recorded the potential values instantaneously until it was stable and evaluated the performance of the electrode according to the measurement results.
3. Results and Discussion
3.1. Electrochemical Characteristics of the Copper Electrode Modified with a Copper Nanoparticle Film in a Na2HPO4 Solution
We compared the electrochemical properties of a copper electrode modified with a copper nanoparticle film, designated as Cu-MCN, in systems with and without phosphate ions using cyclic voltammetry. The aim of this experiment was to ascertain whether Cu-MCN could undergo a specific electrochemical reaction with phosphate ions in a phosphoric acid-containing system, leading to the formation of an insoluble phosphate film. We analyzed the cyclic voltammetry (CV) curve to identify the appropriate reaction potential of Cu-MCN with phosphate ions. As depicted in Figure 2a, in a sodium chloride solution at pH 9, Cu-MCN exhibited an anodic peak around 0.3 V, and two distinct anodic peaks at 0.3 V and 0.75 V in a hydrogen phosphate disodium solution at the same pH. Comparing systems with and without phosphate ions revealed that the anodic wave at approximately 0.3 V occurred in both, attributable to the oxidation of Cu nanoparticles. This suggested that the dual peaks at 0.3 V and 0.75 V may stem from the electrochemical interaction between Cu-MCN and phosphate ions.
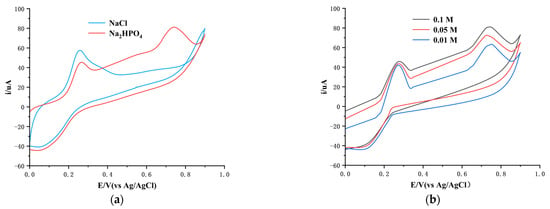
Figure 2.
Cyclic voltammetry curves of the Cu-MCN in different solutions at pH 9.0: (a) in 0.1 M Na2HPO4 and 0.1 M NaCl; (b) in Na2HPO4 of various concentrations.
To delve deeper into the characteristics of these two anodic peaks, we assessed the concentration dependency of Cu-MCN in various phosphate solutions using cyclic voltammetry. There is a corresponding increase in the CV peak intensity as the concentration of Na2HPO4 solution increased from 0.01 M to 0.1 M (see Figure 2b), which confirms that the electrochemical activity at 0.75 V is indeed due to the reaction between Cu-MCN and phosphate ions.
We proceeded with the electrodeposition of the copper phosphate-sensitive membrane on the surface of the Cu-MCN, following the cyclic voltammetry experiments, which established the optimal reaction conditions for Cu-MCN with phosphate ions. This process utilized Cu-MCN and employed a chronoamperometry method at a potential of 0.8 V in a 0.1 M Na2HPO4 solution at pH 9. As illustrated in Figure 3a, the initial electrolytic current was around 2 mA. The current then surged as the copper nanoparticles dissociated from the solid metal to form copper ions. The high electrochemical activity of the copper nanoparticles on the copper wire’s surface caused the current to quickly peak at approximately 3 mA after around 30 s. Subsequently, the current gradually decreased due to the formation of copper phosphate compounds through the reaction of copper ions with phosphate ions on the electrode’s surface. As the electrodeposition continued, these phosphate compounds accumulated, covering the copper electrode’s surface. Approximately 5 min later, the current stabilized near zero and remained constant.
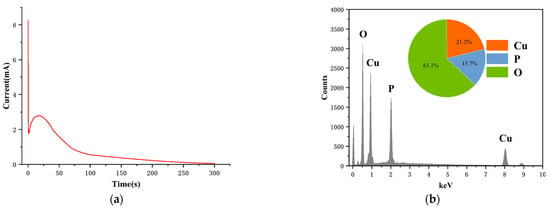
Figure 3.
(a) Current–time curve of the electrodeposition of the copper phosphate membrane in 0.1 M Na2HPO4 solution (pH 9.0) at the constant potential of 0.8 V. (b) Energy dispersive spectrometry (EDS) of the copper phosphate membrane; insert figure: the atomic number distribution of various elements.
3.2. SEM and EDS Analysis of the Copper Phosphate Electrode
Observations from Figure 4a indicate that the surface of the unmodified copper wire showed no significant morphological features, aside from minor scratches. Through the process of copper electrodeposition, the copper particles with tens of nanometers in size uniformly coated on the surface of copper wire (Figure 4b), enhancing its specific surface area and electrochemical activity. In addition, this nanoparticle film tightly attached to the substrate surface can also strengthen the bond between the substrate and the sensitive membrane. Utilizing nanomaterials—recognized for their large specific surface area and exceptional electronic transport capabilities in microstructures—to modify electrodes can significantly boost electrochemical performance metrics such as sensitivity and response range, as cited in references [38,39]. As shown in Figure 4c, dense copper phosphate-sensitive film coated uniformly on the electrode surface after the electrochemical modification of the copper nanoparticle film, which indicated the electroplating was perfect. When enlarged amplification times, it can be observed that the copper phosphate adheres closely to the surface of the electrode substrate, forming a sheet-like network interwoven across the surface (Figure 4d). This thin film structure with a large specific surface area formed on the basis of copper nanoparticles not only enhances its responsiveness to target ions, but also has a low internal resistance [38].
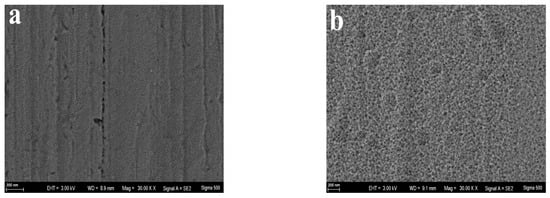
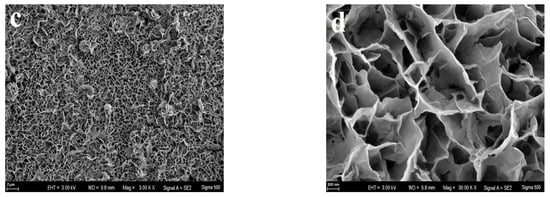
Figure 4.
SEM images of the surface of pure Cu wire (a), Cu nanoparticle layer (b), copper phosphate membrane layer (c), viewed at 3000× magnification; (d), viewed at 30,000× magnification.
Figure 3b illustrates the elemental composition and relative distribution ratios within the copper phosphate film, revealing an atomic ratio of phosphorus to oxygen nearing 1:4. This ratio is consistent with the molecular structure of phosphates, indicating copper nanoparticles successfully react with phosphate ions to form insoluble phosphate solid films. The copper phosphate electrode is a crystalline membrane electrode with metal insoluble salt as ion-sensitive membrane, and this ISE produces equilibrium potential and specific response to the anions of membrane components by precipitation dissolution equilibrium. CuHPO4 and Cu3(PO4)2 are the two main forms of insoluble phosphate salts of phosphate ion and copper ion (Cu(H2PO4)2 is a water-soluble phosphate). For comparison, the copper to phosphorus atomic ratios in CuHPO4 and Cu3(PO4)2 are 1:1 and 3:2, respectively. The copper to phosphorus atomic ratio in the copper phosphate film is approximately 4:3, which falls between the ratios of the two referenced compounds. This indicates that the combined film is possibly composed mainly of Cu3(PO4)2, with a smaller proportion of CuHPO4. Therefore, the reaction described below Equations (1)–(7) [40] seems to occur on the electrode surface. Copper phosphate-crystalline film selectively respond to in solution by achieving chemical equilibrium with it.
3.3. Response Characteristics of the Copper Phosphate Electrode
In order to maintain a constant ionic strength in the standard calibration solution, we chose 1 mM NaNO3 (nitrate is an inert electrolyte for the electrode) solution as the supporting electrolyte, which can maintain stable background currents and ionic strength. Prior to measurement, we must activate the electrode by immersing it into 10−3 M Na2HPO4 solution for 1 h at room temperature.
3.3.1. Response Slope, Detection Linear Range, Detection Limit and Reproducibility
The response potential of the electrode was measured in Na2HPO4 solutions (pH = 8) of varying concentrations, from 10−8 M to 10−1 M, and plotted to construct a calibration curve as depicted in Figure 5. This figure delineates two fitted lines across the concentration span. According to IUPAC guidelines [41], the segment with the steeper slope, corresponding to higher concentrations, is considered the detection range. The detection limit is defined by the intersection point on the x-axis of the two lines. The calibration curve showed that the electrode responded linearly to phosphate ion concentrations ranging from 10−5 to 10−1 M, with a response slope of −27.8 mV·dec−1 and an of 0.9985, characteristic of Nernst behavior [41]. The detection limit was calculated to be 4 × 10−6 M.
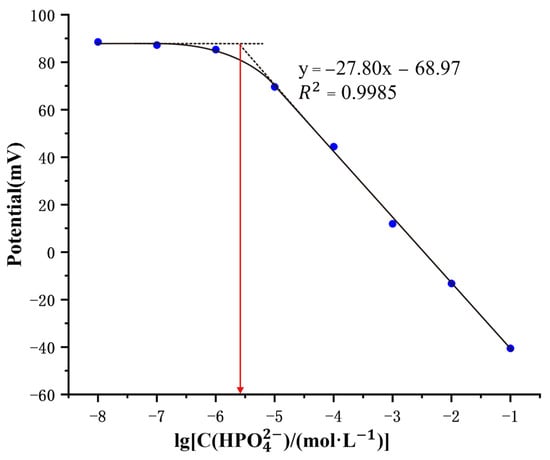
Figure 5.
Response characteristics of the copper phosphate electrode in 10−8–10−1 M Na2HPO4 standard solution at pH 8.
We test the repeatability of the electrode through five repetitive measurements of a 10−3 M Na2HPO4 solution at pH 8, yielding response potentials of 14.67 mV, 14.28 mV, 13.95 mV, 14.42 mV, and 15.03 mV. The standard deviation of these measurements was under 1 mV. For further testing of the reproducibility of the electrode, we measured five simultaneously fabricated phosphate ISEs by using 10−6 to 10−1 M Na2HPO4 solutions at pH 8 and recorded the results. The response slopes of the five electrodes were −27.80, −27.45, −27.93, −28.12, and −27.68 mV·dec−1, respectively. The average value was −27.8 (±0.4) mV·dec−1, with a relative standard deviation (RSD) under 0.5%, which affirmed the electrodes’ great reproducibility.
3.3.2. Response Time
The response time is a crucial characteristic for analytical applications, impacting the accuracy of real-time monitoring and the sensor’s throughput. We evaluate the electrode’s response time by which is the time required for the potential value to reach more than 95% of its final value according to the definition by IUPAC [41]. We immersed the prepared electrode into Na2HPO4 solutions ranging from 10−5–10−1 M at pH 8, with continuous and repeated testing, and each measurement lasted for 300 s. Figure 6 shows that the electrode’s response time is less than 3 s from higher to lower Na2HPO4 concentration, while the average response time is approximately 10 s when measured in the reverse order. The swift response time of the prepared phosphate ISE can be ascribed to the efficient ion-to-electron transfer facilitated by the copper nanoparticle solid contact layer, which hastens the attainment of chemical equilibrium on the electrode surface.
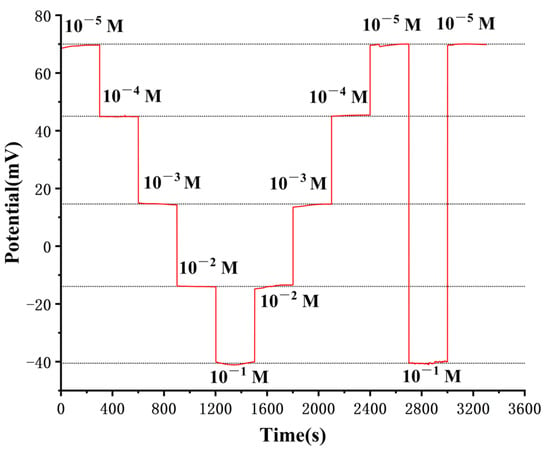
Figure 6.
Dynamic response time curve of the electrode in 10−5–10−1 M Na2HPO4 solutions at pH 8 using an open circuit technique.
Figure 6 also presents the final potential values at each stage as 69.59, 44.84, 14.67, −13.96, −40.52, −13.41, 14.60, 45.42, 69.97, −40.07, and 70.02 mV, respectively. Notably, the potential difference did not exceed 1 mV at the same concentration, indicating that the response behavior of the developed phosphate ISE remained consistent when the potential values were measured across concentrations in either direction. Furthermore, this experiment corroborated the absence of any memory effect in the electrode, as the results displayed no evidence of such an effect.
3.3.3. Electrode Stability and Lifetime
The stability of the ISE is another concern for its practical application. To assess the copper phosphate electrode’s capability for long-term real-time monitoring of low-concentration phosphate ions, we put the prepared electrode into a Na2HPO4 solution of various concentration (10−5 M, 10−4 M, and 10−3 M) for real-time monitoring for 5 h and continuously recorded its response potential. As shown in Figure 7, the average potential fluctuation was less than ±2 mV. Notably, the potential fluctuated significantly at a 10−5 M concentration, possibly because of the solution instability at such low concentrations or that the concentration approached the electrode’s lower detection limit. At a 10−3 M concentration, the potential gradually increased which may attribute to temperature changes.
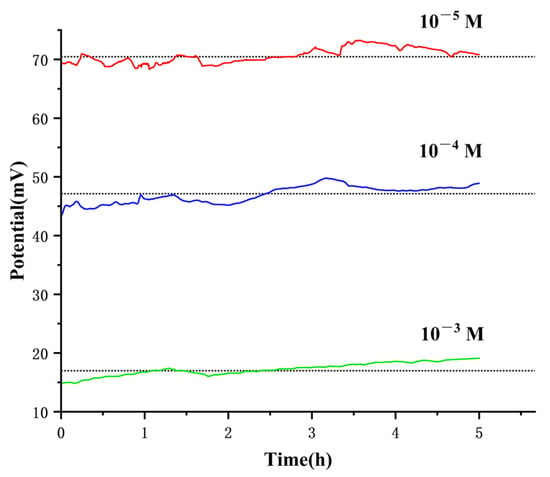
Figure 7.
Response potential measurements of the electrode over 5 h in 10−5–10−3 M Na2HPO4 solutions at pH 8.
The lifetime of an electrode is crucial for its application in long-term in-situ monitoring. We evaluated the lifespan of the copper phosphate electrode by storing it in deionized water for 90 days, conducting periodic performance tests in a series of Na2HPO4 solutions at pH 8. Table 1 shows that for over 75 days, the electrode’s slopes remained stable between −27.01 and −29.42 mV·dec−1, with determination coefficient exceeding 0.99, and the linear response range consistently maintained at 10−5–10−1 M. On the 90th day, there was a significant alteration in response potential with marked declines in both the response slope and linear range, indicating a substantial loss in the electrode’s ability to detect phosphate ions. Thus, the copper phosphate electrode is viable for continuous use in an aqueous environment for up to 75 days, after which it requires modification.

Table 1.
Performance of the electrode in 10−5–10−1 M Na2HPO4 solutions at pH 8 over 90 days.
3.3.4. Electrode Selectivity
The specific selectivity of ISE towards target ion is relative, and it can also respond to coexisting ions in the solution to varying extents. Therefore, the specific response of the electrode to the target ion can be disturbed by other ions with a similar charge in aqueous solution. Given the significantly complex ion environment in real water bodies, the selectivity of an electrode for the common ions is crucial for reliable determination in actual water bodies. In the presence of interfering ions, the Nikolskii–Eisenman equation [42] (Equation (8), the generalized Nernst equation) determines the response potential of the electrode to the target ion. In Equation (8), the selectivity coefficient quantifies the influence of interfering ion j on the response potential of target ion i, indicating the electrode’s preferential ability to selectively respond to the ion i in the presence of ion j.
Here, ion i and ion j are the target ion and the interfering ion, respectively; E is the measured response potential; is the reference potential under standard conditions, which can be regarded as a constant under certain measurement conditions; R is the gas constant (8.314 J/K/mol), T is the absolute temperature (273.15 + t °C), F is the Faraday constant (9.648 C/mol); and and are the charge numbers of the ions i and j, respectively; is the selectivity coefficient of the target ion i toward interfering ion j, and the logarithm of selectivity coefficient log < 0 means that the electrode displayed excellent selectivity for ion i over the ion j; the smaller the value of log, the better the selectivity of the electrode to the target ion [43]; and are the activities of ions i and j, respectively. There is the following equation (Equation (9)) between ion activity and ion concentration in solution:
Here, is the activity of ion i; is the activity coefficient of ion i, which is related to the ionic strength in the solution; is the ion concentration. In general, in a solution with a relatively simple composition and constant ionic strength, the ion activity in the Nernst equation can be replaced by the ion concentration.
The separated solution method (SSM) and the fixed interference method (FIM) are two common analytical methods recommended by IUPAC [44] for determining selectivity coefficient . We analyzed and calculated the selectivity coefficients of the electrode for different interfering ions using the FIM based on Equation (8). In this method, within mixed solutions containing both interfering ion j and target ion i, we maintained the concentration of ion j constant (10−3 M) while varying the concentration of ion i (10−6–10−1 M). We measured the response potential of the electrode in a series of mixed solutions with concentration gradients of ion i, and calculated the selectivity coefficient by the simplified equation (Equation (10)) based on Equation (8):
As shown in Table 2, we established the following selectivity sequence: > > > > > > > . The log values for common anions, aside from the hydroxide ion, are less than −2, indicating that the electrode’s response to the target phosphate ion is unaffected by these interfering ions when their concentration is less than 100 times that of the phosphate ion. However, the hydroxide ion exhibits strong interference compared to other interfering ions, likely because the solubility product of Cu(OH)2 is quite low (Ksp(Cu(OH)2) = 2.2 × 10−20). At relatively high concentrations of hydroxide ions, a chemical equilibrium is established between copper ions and hydroxide ions, which can significantly interfere with the copper phosphate electrode—an crystalline membrane electrode that relies on chemical equilibrium for a Nernst response.

Table 2.
Selectivity coefficients of common anions measured by FIM.
3.3.5. pH Interference on the Phosphate Ions’ Measurement
As phosphoric acid belongs to ternary weak acid, which has a three-stage dissociation in aqueous solution (see Equations (11)–(13)), Equations (14)–(16) (in the equation: = [] = ) show the dissociation equilibrium constant of phosphoric acid at different stages at room temperature. Under different pH conditions, the existing forms of phosphate hydroxyl groups and the distribution proportions of various valence phosphate ions in different dissociation states are different. The morphological distribution diagram (Figure 8) of phosphate ions was plotted through the calculation of Equations (11)–(16). Figure 8 shows that the dominant species of phosphate ions is in the solution from pH 5 to pH 6, and predominates in the solution pH range of 8 to 11.
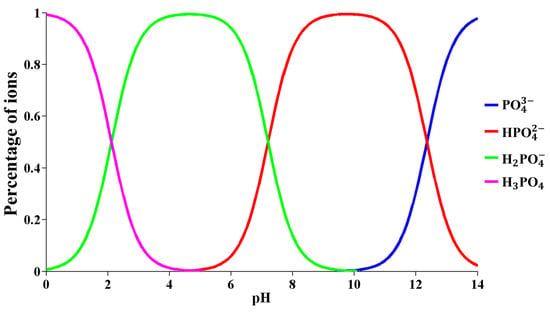
Figure 8.
Distribution proportions of various valence phosphate ions at different pH values.
We studied the effect of solution pH in the range of 5 to 12 on the electrode’s potential response of phosphate ions. The phosphate solutions with different pH values were adjusted by using different concentrations of sodium hydroxide and nitric acid based on Na2HPO4 and NaH2PO4. As shown in Figure 9, the electrode exhibited optimal performance at pH 7 with a detection limit of 10−6 M when the dominant species of phosphate ions gradually transitioned to in the solution pH range of 6 to 11, while the detection limit decreased to about 10−4 M at pH 6 and pH 11 as well as about 10−5 M in the pH range of 8 to 10. The response slopes from pH 6 to pH 11 were −29.4, −29.31, −27.9, −26.7, −25.5 and −22.32 mV·dec−1, respectively. In addition, the electrode’s response to phosphate ions completely deviated from the Nernst behavior at pH 5. We infer that the performance loss and gradual deviation from Nernst response of the electrode in alkaline solutions arise from the interference of a relatively high concentration of , which leads to the precipitation–dissolution equilibrium of insoluble Cu(OH)2 formed by the reaction of hydroxide ions, and copper ions gradually dominate the electrode surface.
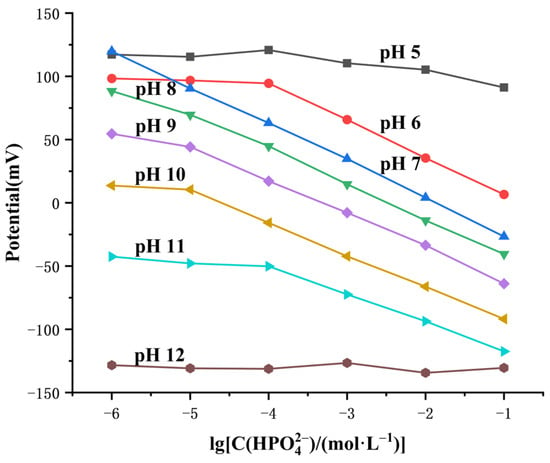
Figure 9.
Influence of pH on the potential response of the electrode.
As a result, the prepared electrode exhibited an excellent selectivity to and can be used for detecting solutions with a pH range from neutral to weakly alkaline. In the practical applications, it is crucial to consider the actual pH of the solutions to ensure accurate phosphate ions detection.
3.3.6. Analysis of Real Samples
The growth of humans, plants, and aquatic organisms depends on receiving an appropriate supply of nutrient phosphate salts, which are prevalent in our water systems [45]. They are found in natural surface waters, contributing to eutrophication when present in excessive amounts, in human drinking water, aquaculture water utilized for aquatic farming, and in soil water systems in the field. Maintaining an appropriate phosphate supply is crucial for preserving a healthy state in these environments [46]. To assess the practical application of the sensors to actual liquid samples in the aforementioned environmental issues, we applied the proposed phosphate ISE to detect phosphate in various real samples, including tap water, laboratory wastewater, drinking natural mineral water, natural lake water (obtained from Zhoushan Campus of Zhejiang University) and aquatic water (obtained from Zhoushan Fenghe Aquaculture Farm). When pH is constant, the ratio of monohydrogen phosphate ions to total phosphate is fixed. Our approach involved directly measuring the concentration of monohydrogen phosphate ions in the samples using the electrode, then calculating the total phosphate concentration based on the pH value. For method validation, we compared the electrode’s detection results with those obtained from the CleverChem 380 Plus auto discrete analyzer, which employs an automated version of the phosphomolybdate blue spectrophotometric method. We adjusted the sample pH to between 7.0 and 9.0 using varying concentrations of nitric acid and sodium hydroxide prior to measurement. As indicated in Table 3, although initially no phosphate was detected in these samples (either these samples may not contain phosphate themselves or the phosphate concentration is less than 10−7 M, and we only need to detect them when their phosphate content exceeds the normal standard or higher), after adding a known quantity of phosphate, the recovery values derived from the electrode’s detection results affirmed the method’s accuracy. Table 4 presents that the total phosphate content detected by the electrode in lake water and aquaculture water samples are close to the results measured by the CleverChem 380 Plus, with a relative standard deviation (RSD) of about 10%. The results showed that the prepared phosphate ISE was capable of application for phosphate detection in real water bodies.

Table 3.
Recovery test in different solutions.

Table 4.
Comparison of the phosphate concentrations detected by the phosphate ISE and CleverChem 380 Plus in real aqueous samples.
3.3.7. Comparison of the Copper Phosphate Electrode and Previously Reported Electrodes
In summary, Table 5 presents a comparative analysis with the relevant literature, showcasing the competitive edge of our copper phosphate ISE. The detection range and the limit of our phosphate ISE are on par with those of similar ISEs reported in prior research. Notably, our phosphate ISE exhibits a wider pH detection range for phosphate ions, with a detection limit in neutral solutions of 1 × 10−6 M. Additionally, it features the fastest response time recorded at 10 s and an impressive service life of 75 days, outperforming most recently reported phosphate ISEs.

Table 5.
Performance comparison of references and current research.
4. Conclusions
In this study, we proposed an innovative all-solid-state ISE for phosphate ions, utilizing a copper/nano-copper/copper phosphate matrix. This sensor is not only economical to produce but also simple in its manufacturing process. We characterized the electrode’s surface morphology and elemental composition with a scanning electron microscope and an energy dispersive spectrometer, respectively. Nanoscale modifications to the electrode have significantly expanded its specific surface area, boosting its efficiency in responding to target ions and enhancing both its sensitivity and stability. The electrode performed remarkable sensitivity and stability, evidenced by a swift response time of less than 10 s and fewer potential fluctuations, staying within ±2 mV over a 5 h continuous testing period. The interference tests indicated the electrode’s excellent selectivity against common anions found in natural water sources. The detection range and limits of the phosphate ISE developed through this research are on par with the existing literature. The advantage of this electrode is its easy fabrication, capacity to detect accurately across a wide pH spectrum from 6 to 11, and a quick response time, combined with a service lifetime of two months. And these qualities hold the potential to mark it as a viable candidate for commercial test instrumentation. Furthermore, the results of this study provide feasible reference information for the design of new chemical sensors tailored for environmental monitoring purposes.
Author Contributions
Conceptualization, Y.H., Y.Y. and C.T.; methodology, Y.H., Y.Y. and C.T.; software, Y.H. and H.D.; validation, Y.H.; formal analysis, Y.H., C.H. and Y.Y.; investigation, Y.H., H.D. and C.H; resources, C.H., Y.Y. and C.T.; data curation, Y.H., H.D. and C.H.; writing—original draft preparation, Y.H. and H.D.; writing—review and editing, Y.H., C.H. and C.T.; visualization, Y.H.; supervision, C.T. and Y.Y.; project administration, C.T., Y.Y. and C.H.; funding acquisition, C.H. and C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Research and Development Plan of Zhejiang Province [Grant Numbers 2021C03183] and the Scientific Research Fund of Second Institute of Oceanography, MNR [Grant Number JG1521].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. ECOLOGY Controlling Eutrophication: Nitrogen and Phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef]
- Lan, Y.B.; Gai, S.; Cheng, K.; Yang, F. Advances in biomass thermochemical conversion on phosphorus recovery: Water eutrophication prevention and remediation. Environ. Sci. Wat. Res. Technol. 2022, 8, 1173–1187. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef]
- Block, G.A.; Hulbert-Shearon, T.E.; Levin, N.W.; Port, F.K. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am. J. Kidney Dis. 1998, 31, 607–617. [Google Scholar] [CrossRef]
- Bergwitz, C.; Juppner, H. Phosphate Sensing. Adv. Chronic Kidney Dis. 2011, 18, 132–144. [Google Scholar] [CrossRef]
- Jamal, S.A.; Vandermeer, B.; Raggi, P.; Mendelssohn, D.C.; Chatterley, T.; Dorgan, M.; Lok, C.E.; Fitchett, D.; Tsuyuki, R.T. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: An updated systematic review and meta-analysis. Lancet 2013, 382, 1268–1277. [Google Scholar] [CrossRef]
- Cakmak, E.K.; Hartl, M.; Kisser, J.; Cetecioglu, Z. Phosphorus mining from eutrophic marine environment towards a blue economy: The role of bio-based applications. Water Res. 2022, 219, 118505. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Egle, L.; Rechberger, H.; Krampe, J.; Zessner, M. Phosphorus recovery from municipal wastewater: An integrated comparative technological, environmental and economic assessment of P recovery technologies. Sci. Total Environ. 2016, 571, 522–542. [Google Scholar] [CrossRef]
- McDowell, R.W.; Dils, R.M.; Collins, A.L.; Flahive, K.A.; Sharpley, A.N.; Quinn, J. A review of the policies and implementation of practices to decrease water quality impairment by phosphorus in New Zealand, the UK, and the US. Nutr. Cycl. Agroecosyst. 2016, 104, 289–305. [Google Scholar] [CrossRef]
- Hatta, M.; Measures, C.I.; Ruzicka, J. Determination of traces of phosphate in sea water automated by programmable flow injection: Surfactant enhancement of the phosphomolybdenum blue response. Talanta 2019, 191, 333–341. [Google Scholar] [CrossRef]
- Li, Q.Y.; Zhao, W.H.; Miao, H.; Han, X.T. Effects and improvements of different reagents preservation methods on the determination of phosphate in seawater by phosphomolybdenum blue spectrophotometric method. Mar. Pollut. Bull. 2019, 139, 136–140. [Google Scholar] [CrossRef]
- Lopez-Ruiz, B. Advances in the determination of inorganic anions by ion chromatography. J. Chromatogr. A 2000, 881, 607–627. [Google Scholar] [CrossRef]
- Cheong, C.K.; Miura, T.; Nonose, N. Determination of phosphate in seawater by ion chromatography inductively coupled plasma sector field mass spectrometry. Limnol. Oceanogr. Meth. 2021, 19, 682–691. [Google Scholar] [CrossRef]
- Bowden, A.; Diamond, D. The determination of phosphorus in a microfluidic manifold demonstrating long-term reagent lifetime and chemical stability utilising a colorimetric method. Sens. Actuator B Chem. 2003, 90, 170–174. [Google Scholar] [CrossRef]
- Chen, H.W.; Zhao, L.L.; Yu, F.B.; Du, Q.L. Detection of phosphorus species in water: Technology and strategies. Analyst 2019, 144, 7130–7148. [Google Scholar] [CrossRef]
- Hu, J.B.; Stein, A.; Buhlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. Trac Trends Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Privett, B.J.; Shin, J.H.; Schoenfisch, M.H. Electrochemical Sensors. Anal. Chem. 2010, 82, 4723–4741. [Google Scholar] [CrossRef]
- Mahmud, M.A.P.; Ejeian, F.; Azadi, S.; Myers, M.; Pejcic, B.; Abbassi, R.; Razmjou, A.; Asadnia, M. Recent progress in sensing nitrate, nitrite, phosphate, and ammonium in aquatic environment. Chemosphere 2020, 259, 127492. [Google Scholar] [CrossRef]
- Miao, W.J.; Wang, L.; Liu, Q.; Guo, S.; Zhao, L.Z.; Peng, J.J. Rare earth ions-enhanced gold nanoclusters as fluorescent sensor array for the detection and discrimination of phosphate anions. Chem. Asian J. 2021, 16, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Lv, X.X.; Tian, M.; Yu, Q.C.; Mao, Y.Y.; Qiu, W.W.; Wang, H.; Liu, G.D. A terbium(III)-functionalized zinc(II)-organic framework for fluorometric determination of phosphate. Microchim. Acta 2020, 187, 84. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Leng, W.; Lu, Q.W.; Li, K.P.; Zhang, Y.K.; Liu, J.Y.; Xu, L.Q.; Sheng, G.P. Ratiometric fluorescent sensing of phosphate ion in environmental water samples using flavin mononucleotide-functionalized Fe3O4 particles. Sci. Total Environ. 2023, 857, 159249. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Liu, Y.B.; Wang, W.; Zhang, S.Q.; Tang, L.; Ma, P.Y.; Song, D.Q.; Fei, Q. A ratiometric fluorescent sensor for the detection of phosphate. Luminescence 2023, 38, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, X.C.; Zhao, G.; Shi, Y.Y.; Thuy, N.T.D.; Yang, H.L. SERS Determination of Trace Phosphate in Aquaculture Water Based on a Rhodamine 6G Molecular Probe Association Reaction. Biosensors 2022, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Radujevic, A.; Penavic, A.; Pavlovic, R.Z.; Badjic, J.D.; Anzenbacher, P. Cross-reactive binding versus selective phosphate sensing in an imine macrocycle sensor. Chem 2022, 8, 2228–2244. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.Q.; Li, X.Y.; Yang, P.; Yue, J.Y.; Jiang, Y.; Tang, B. Linker-Eliminated Nano Metal-Organic Framework Fluorescent Probe for Highly Selective and Sensitive Phosphate Ratiometric Detection in Water and Body Fluids. Anal. Chem. 2020, 92, 3722–3727. [Google Scholar] [CrossRef]
- Zhu, J.M.; Han, G.W.; Hu, X.J.; Zuo, Y.F.; Chen, L.F.; Wang, F.; Yang, Y.; Jiang, F.H.; Sun, C.J.; Zhao, W.H.; et al. A Portable and Accurate Phosphate Sensor Using a Gradient Fabry-Perot Array. ACS Sens. 2020, 5, 1381–1388. [Google Scholar] [CrossRef]
- Pinyorospathum, C.; Rattanarat, P.; Chaiyo, S.; Siangproh, W.; Chailapakul, O. Colorimetric sensor for determination of phosphate ions using anti-aggregation of 2-mercaptoethanesulfonate-modified silver nanoplates and europium ions. Sens. Actuator B-Chem. 2019, 290, 226–232. [Google Scholar] [CrossRef]
- Xiao, D.; Yuan, H.Y.; Li, J.; Yu, R.Q. Surface-modified cobalt-based sensor as a phosphate-sensitive electrode. Anal. Chem. 1995, 67, 288–291. [Google Scholar] [CrossRef]
- Meruva, R.K.; Meyerhoff, M.E. Mixed potential response mechanism of cobalt electrodes toward inorganic phosphate. Anal. Chem. 1996, 68, 2022–2026. [Google Scholar] [CrossRef]
- Xu, K.; Kitazumi, Y.; Kano, K.; Shirai, O. Phosphate ion sensor using a cobalt phosphate coated cobalt electrode. Electrochim. Acta 2018, 282, 242–246. [Google Scholar] [CrossRef]
- Zhao, G.Y.; Fozia; Wen, H.B.; Dai, Z.M.; Nie, Y.H.; Jiang, J.R.; Xu, X.; Ying, M.; Hu, Z.L.; Xu, H. Preparation of a Phosphate Ion-Selective Electrode Using One-Step Process Optimized with Response Surface Method and its Application in Real Sample Detections. Electrocatalysis 2022, 13, 641–652. [Google Scholar] [CrossRef]
- Xu, K.B.; Li, Y.; Li, M. Potentiometric Phosphate Ion Sensor Based on Electrochemical Modified Tungsten Electrode. ACS Omega 2021, 6, 13795–13801. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.F.; Rahman, M.T.; Gurung, A.; Qiao, Q.Q. Electrochemical Phosphate Sensors Using Silver Nanowires Treated Screen Printed Electrodes. IEEE Sens. J. 2018, 18, 3480–3485. [Google Scholar] [CrossRef]
- Xu, K.B.; Wu, B.Y.; Wan, J.L.; Li, Y.; Li, M. A potentiometric phosphate ion sensor based on electrochemically modified nickel electrode. Electrochim. Acta 2022, 412, 140065. [Google Scholar] [CrossRef]
- Jeong, B.; Oh, J.S.; Kim, D.; Kim, D.G.; Kim, Y.I.; Heo, J.; Lee, H.K. Ion-Selective Electrode Based on a Novel Biomimetic Nicotinamide Compound for Phosphate Ion Sensor. Polymers 2022, 14, 3392. [Google Scholar] [CrossRef] [PubMed]
- Ben-Aissa, S.; De Marco, R.; Susmel, S. POM@PMO plastic electrode for phosphate electrochemical detection: A further improvement of the detection limit. Microchim. Acta 2023, 190, 135. [Google Scholar] [CrossRef]
- Wang, F.F.; Tong, J.H.; Li, Y.; Bian, C.; Sun, J.Z.; Xia, S.H. An Electrochemical Microsensor Based on a AuNPs-Modified Microband Array Electrode for Phosphate Determination in Fresh Water Samples. Sensors 2014, 14, 24472–24482. [Google Scholar] [CrossRef]
- Robinson, J.E.; Heineman, W.R.; Sagle, L.B.; Meyyappan, M.; Koehne, J.E. Carbon nanofiber electrode array for the detection of lead. Electrochem. Commun. 2016, 73, 89–93. [Google Scholar] [CrossRef]
- Aksu, S. Electrochemical equilibria of copper in aqueous phosphoric acid solutions. J. Electrochem. Soc. 2009, 156, C387. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, D.M.; Hyun, M.H.; Shim, Y.B. An all-solid-state monohydrogen phosphate sensor based on a macrocyclic ionophore. Talanta 2010, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Zeitoun, R.; Biswas, A. Electrochemical Mechanisms in Potentiometric Phosphate Sensing Using Pure Cobalt, Molybdenum and Their Alloy for Environmental Applications. Electroanalysis 2021, 33, 421–430. [Google Scholar] [CrossRef]
- Debosz, M.; Kozma, J.; Porada, R.; Wieczorek, M.; Paluch, J.; Gyurcsányi, R.E.; Migdalski, J.; Koscielniak, P. 3D-printed manifold integrating solid contact ion-selective electrodes for multiplexed ion concentration measurements in urine. Talanta 2021, 232, 122491. [Google Scholar] [CrossRef] [PubMed]
- Sukesan, R.; Chen, Y.T.; Shahim, S.; Wang, S.L.; Sarangadharan, I.; Wang, Y.L. Instant Mercury Ion Detection in Industrial Waste Water with a Microchip Using Extended Gate Field-Effect Transistors and a Portable Device. Sensors 2019, 19, 2209. [Google Scholar] [CrossRef] [PubMed]
- Jupp, A.R.; Beijer, S.; Narain, G.C.; Schipper, W.; Slootweg, J.C. Phosphorus recovery and recycling—Closing the loop. Chem. Soc. Rev. 2021, 50, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.T.; Chi, L.N.; Wang, X.Z.; Sui, Y.M.; Wang, Y.; Arandiyan, H. Review of metal (hydr)oxide and other adsorptive materials for phosphate removal from water. J. Environ. Chem. Eng. 2018, 6, 5269–5286. [Google Scholar] [CrossRef]
- Xu, K.B.; Kitazumi, Y.; Kano, K.; Sasaki, T.; Shirai, O. Fabrication of a Phosphate Ion Selective Electrode Based on Modified Molybdenum Metal. Anal. Chem. 2020, 36, 201–206. [Google Scholar] [CrossRef]
- Özkütük, E.B.; Yildiz, B.; Gündüz, M.; Hür, E. Phosphate-imprinted polymer as an efficient modifier for the design of ion-selective electrode. J. Chem. Technol. Biotechnol. 2021, 96, 2604–2609. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).