Investigation of WO3 and BiVO4 Photoanodes for Photoelectrochemical Sensing of Xylene, Toluene and Methanol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Tungsten Oxide and Bismuth Vanadate
2.3. Structural and Morphological Analysis of WO3 and BiVO4
2.4. Photoelectrochemical Investigations
3. Results and Discussion
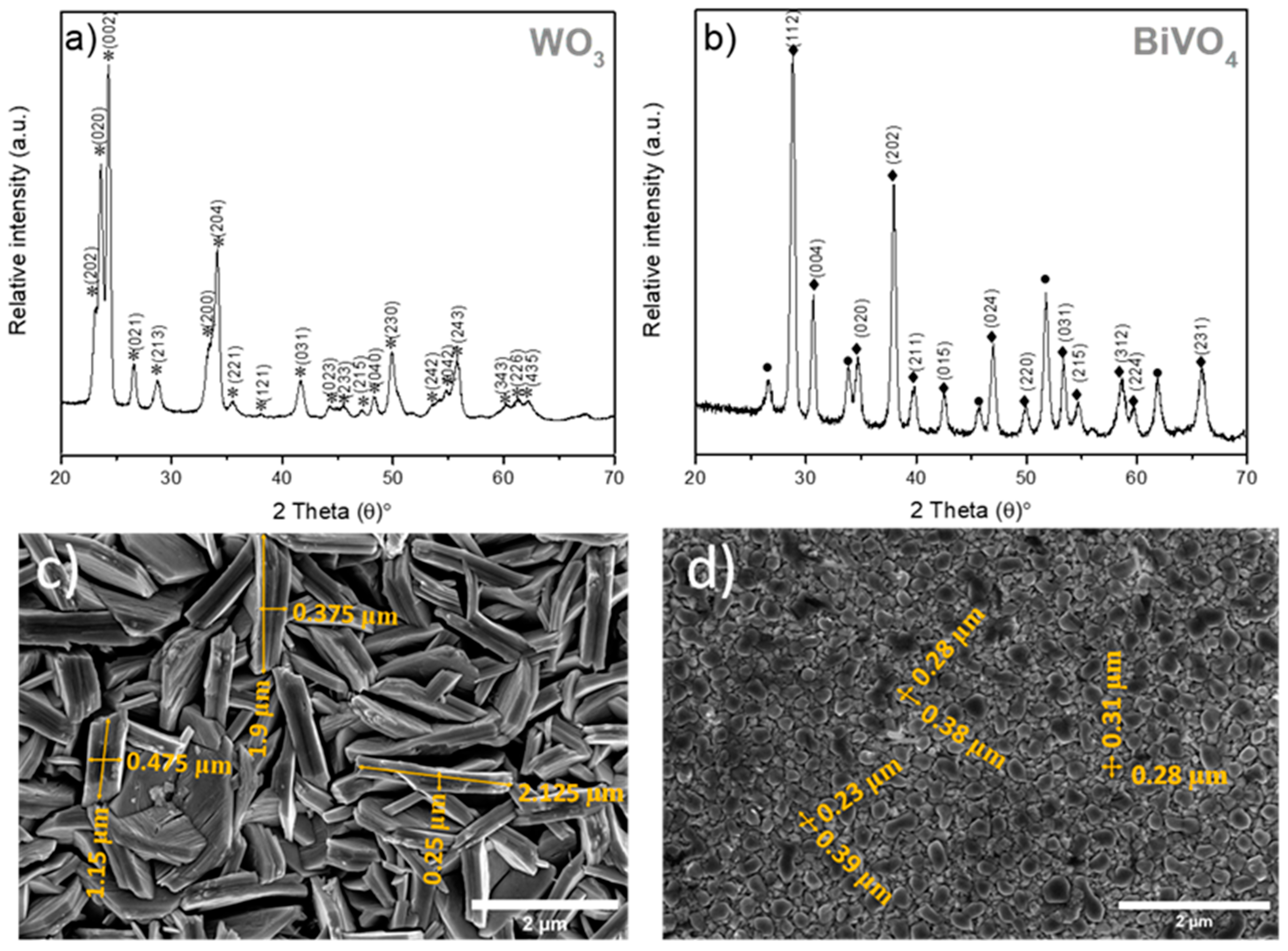
3.1. Structural and Morphological Characterisation of WO3 and BiVO4 Coatings
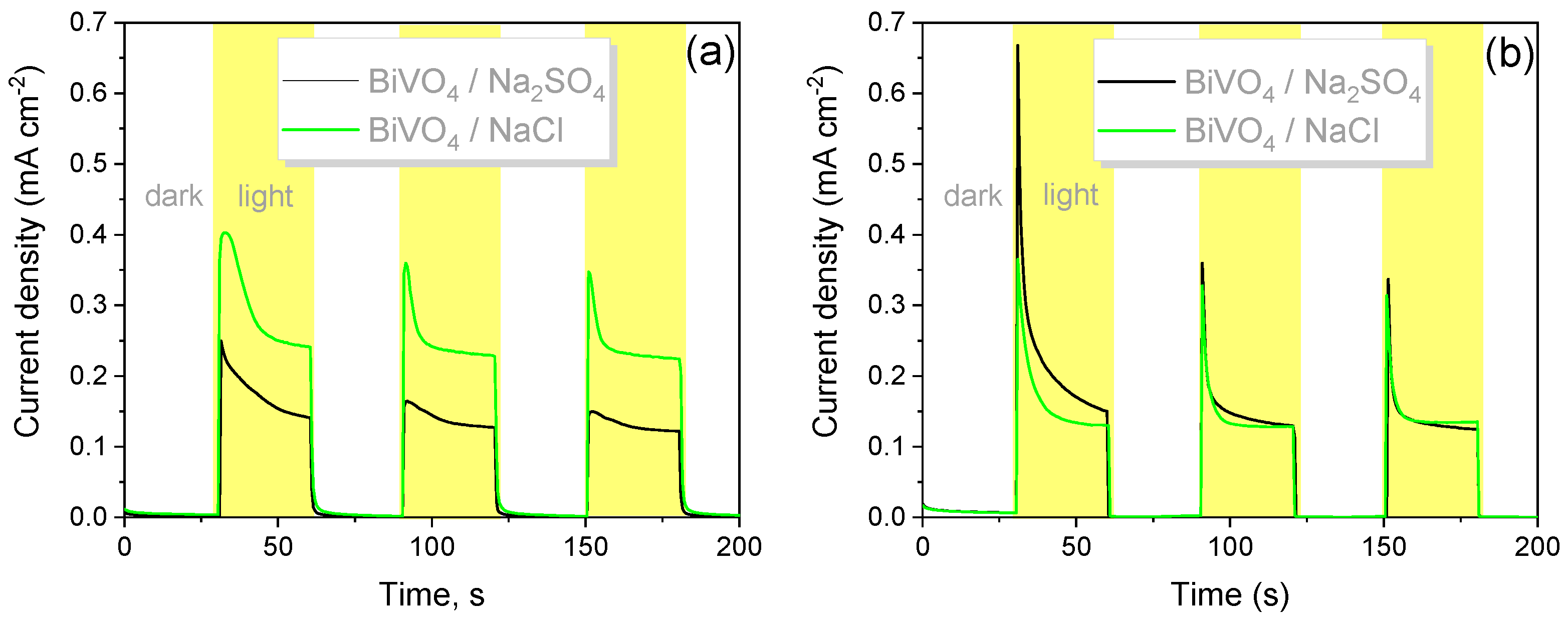
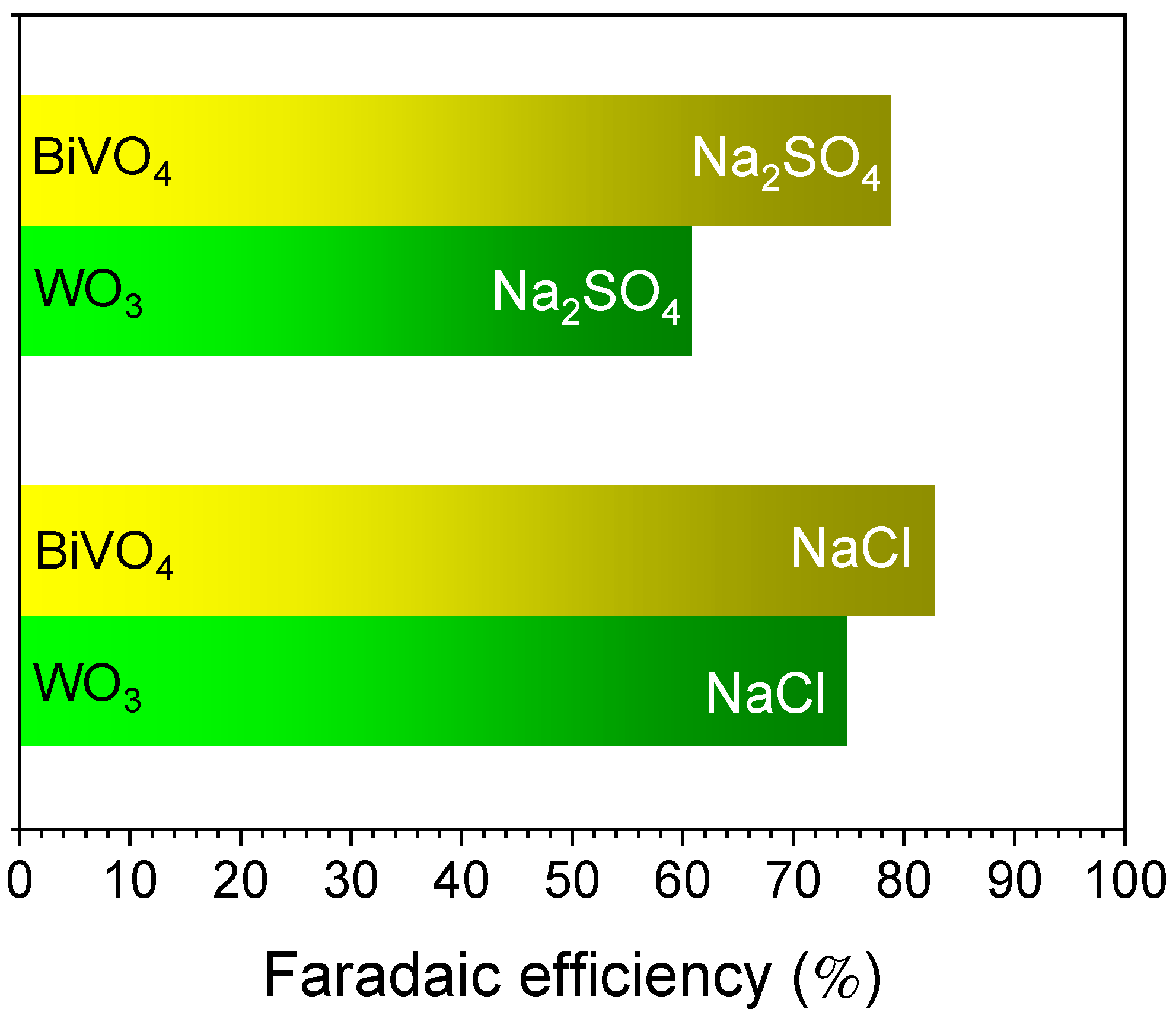
3.2. Photoelectrochemical Characterization of WO3 and BiVO4 Coatings
3.3. PEC Detection of Xylene with WO3 and BiVO4 Photoanodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Golfinopoulos, S.K.; Lekkas, T.D.; Nikolaou, A.D. Comparison of methods for determination of volatile organic compounds in drinking water. Chemosphere 2001, 45, 275–284. [Google Scholar] [CrossRef]
- Lloyd, A.C.; Cackette, T.A. Diesel Engines: Environmental Impact and Control. J. Air Waste Manag. Assoc. 2001, 51, 809–847. [Google Scholar] [CrossRef]
- Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various natural and anthropogenic factors responsible for water quality degradation: A review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, X.; Wang, X.; Mahmood, A.; Qiu, H.; Sun, J. Difference of photodegradation characteristics between single and mixed VOC pollutants under simulated sunlight irradiation. J. Photochem. Photobiol. A Chem. 2019, 384, 112029. [Google Scholar] [CrossRef]
- Morin, J.; Brochard, G.; Bergé, V.; Rosset, A.; Artous, S.; Clavaguera, S.; Strekowski, R.S.; Wortham, H. Uptake of m-xylene and VOC emissions by mineral photocatalytic paints of indoor air building interest. Environ. Sci. Nano 2023, 10, 1704–1714. [Google Scholar] [CrossRef]
- Beauchet, R.; Mijoin, J.; Batonneau-Gener, I.; Magnoux, P. Catalytic oxidation of VOCs on NaX zeolite: Mixture effect with isopropanol and o-xylene. Appl. Catal. B Environ. 2010, 100, 91–96. [Google Scholar] [CrossRef]
- Jayawardhana, Y.; Gunatilake, S.R.; Mahatantila, K.; Ginige, M.P.; Vithanage, M. Sorptive removal of toluene and m-xylene by municipal solid waste biochar: Simultaneous municipal solid waste management and remediation of volatile organic compounds. J. Environ. Manag. 2019, 238, 323–330. [Google Scholar] [CrossRef]
- Chary, N.S.; Fernandez-Alba, A.R. Determination of volatile organic compounds in drinking and environmental waters. TrAC Trends Anal. Chem. 2012, 32, 60–75. [Google Scholar] [CrossRef]
- Nikolaou, A.D.; Golfinopoulos, S.K.; Kostopoulou, M.N.; Kolokythas, G.A.; Lekkas, T.D. Determination of volatile organic compounds in surface waters and treated wastewater in Greece. Water Res. 2002, 36, 2883–2890. [Google Scholar] [CrossRef]
- Sumitsawan, S.; Cho, J.; Sattler, M.L.; Timmons, R.B. Plasma surface modified TiO2 nanoparticles: Improved photocatalytic oxidation of gaseous m-xylene. Environ. Sci. Technol. 2011, 45, 6970–6977. [Google Scholar] [CrossRef]
- Liu, W.; Wei, D.; Zhao, X.; Xiao, F.; Yang, C. Enhanced xylene-sensing property of hierarchical NiO/montmorillonite hetero-structures via in doping. Appl. Surf. Sci. 2022, 602, 154301. [Google Scholar] [CrossRef]
- Li, F.; Qin, Q.; Zhang, N.; Chen, C.; Sun, L.; Liu, X.; Chen, Y.; Li, C.; Ruan, S. Improved gas sensing performance with Pd-doped WO3·H2O nanomaterials for the detection of xylene. Sens. Actuators B Chem. 2017, 244, 837–848. [Google Scholar] [CrossRef]
- Gao, H.; Yu, Q.; Chen, K.; Sun, P.; Liu, F.; Yan, X.; Liu, F.; Lu, G. Ultrasensitive gas sensor based on hollow tungsten trioxide-nickel oxide (WO3-NiO) nanoflowers for fast and selective xylene detection. J. Colloid Interface Sci. 2019, 535, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Kim, K.; Cho, B.; Kwak, Y.; Kim, J. Highly Sensitive Detection of Benzene, Toluene, and Xylene Based on CoPP-Functionalized TiO2 Nanoparticles with Low Power Consumption. ACS Sens. 2020, 5, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Deng, L.; Ding, X.; Zeng, D.; Zhang, S.; Xie, C. High sensitivity and selectivity of C-doped WO3 gas sensors toward toluene and xylene. IEEE Sens. J. 2012, 12, 2209–2214. [Google Scholar] [CrossRef]
- Kim, T.H.; Jeong, S.Y.; Moon, Y.K.; Lee, J.H. Dual-mode gas sensor for ultrasensitive and highly selective detection of xylene and toluene using Nb-doped NiO hollow spheres. Sens. Actuators B Chem. 2019, 301, 127140. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, Y.; Zou, C.; Xu, K.; Luo, X.; Luo, T.; Li, J.; Yang, Q.; Shi, P.; Yuan, C. 2D ultra-thin WO3 nanosheets with dominant {002} crystal facets for high-performance xylene sensing and methyl orange photocatalytic degradation. J. Alloys Compd. 2019, 783, 848–854. [Google Scholar] [CrossRef]
- Sheng, S.; Zhang, Z.; Wang, M.; He, X.; Jiang, C.; Wang, Y. Synthesis of MIL-125(Ti) derived TiO2 for selective photoelectrochemical sensing and photocatalytic degradation of tetracycline. Electrochim. Acta 2022, 420, 140441. [Google Scholar] [CrossRef]
- Adhikari, S.; Selvaraj, S.; Kim, D.H. Construction of heterojunction photoelectrode via atomic layer deposition of Fe2O3 on Bi2WO6 for highly efficient photoelectrochemical sensing and degradation of tetracycline. Appl. Catal. B Environ. 2019, 244, 11–24. [Google Scholar] [CrossRef]
- Han, Q.; Zhao, X.; Na, N.; Ouyang, J. Integrating Near-Infrared Visual Fluorescence with a Photoelectrochemical Sensing System for Dual Readout Detection of Biomolecules. Anal. Chem. 2021, 93, 3486–3492. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, M. Detection of pollutants in water bodies: Electrochemical detection or photo-electrochemical detection? Chem. Commun. 2020, 56, 14541–14552. [Google Scholar] [CrossRef] [PubMed]
- Najaf, Z.; Nguyen, D.L.T.; Chae, S.Y.; Joo, O.S.; Shah, A.U.H.A.; Vo, D.V.N.; Nguyen, V.-H.; Le, Q.V.; Rahman, G. Recent trends in development of hematite (α-Fe2O3) as an efficient photoanode for enhancement of photoelectrochemical hydrogen production by solar water splitting. Int. J. Hydrog. Energy 2021, 46, 23334–23357. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-Segura, S. Recent progress of applied TiO2 photoelectrocatalysis for the degradation of organic pollutants in wastewaters. J. Environ. Chem. Eng. 2023, 11, 109635. [Google Scholar] [CrossRef]
- Bai, Z.; Yan, X.; Kang, Z.; Hu, Y.; Zhang, X.; Zhang, Y. Photoelectrochemical performance enhancement of ZnO photoanodes from ZnIn2S4 nanosheets coating. Nano Energy 2015, 14, 392–400. [Google Scholar] [CrossRef]
- Raj, C.J.; Prabakar, K.; Karthick, S.N.; Hemalatha, K.V.; Son, M.K.; Kim, H.J. Banyan root structured Mg-Doped ZnO photoanode dye-sensitized solar cells. J. Phys. Chem. C 2013, 117, 2600–2607. [Google Scholar] [CrossRef]
- Breuhaus-Alvarez, A.G.; Cheek, Q.; Cooper, J.J.; Maldonado, S.; Bartlett, B.M. Chloride Oxidation as an Alternative to the Oxygen-Evolution Reaction on HxWO3 Photoelectrodes. J. Phys. Chem. C 2021, 125, 8543–8550. [Google Scholar] [CrossRef]
- Cao, X.; Zang, X.; Zhou, X.; Chen, M.; Ding, Y. Rationally designed/constructed MnOx/WO3 anode for photoelectrochemical water oxidation. Chin. Chem. Lett. 2018, 29, 811–814. [Google Scholar] [CrossRef]
- Grigioni, I.; Ganzer, L.; VA Camargo, F.; Bozzini, B.; Cerullo, G.; Selli, E. In Operando Photoelectrochemical Femtosecond Transient Absorption Spectroscopy of WO3/BiVO4 Heterojunctions. ACS Energy Lett. 2019, 4, 2213–2219. [Google Scholar] [CrossRef]
- Xia, L.; Bai, J.; Li, J.; Zeng, Q.; Li, L.; Zhou, B. High-performance BiVO4 photoanodes cocatalyzed with an ultrathin α-Fe2O3 layer for photoelectrochemical application. Appl. Catal. B Environ. 2017, 204, 127–133. [Google Scholar] [CrossRef]
- Rong, Y.Q.; Yang, X.F.; Zhang, W.D.; Yu, Y.X. Porous ultrathin WO3 nanoflake arrays as highly efficient photoanode for water splitting. Mater. Lett. 2019, 246, 161–164. [Google Scholar] [CrossRef]
- Lee, B.R.; Lee, M.G.; Park, H.; Lee, T.H.; Lee, S.A.; Bhat, S.S.M.; Kim, C.; Lee, S.; Jang, H.W. All-Solution-Processed WO3/BiVO4 Core-Shell Nanorod Arrays for Highly Stable Photoanodes. ACS Appl. Mater. Interfaces 2019, 11, 20004–20012. [Google Scholar] [CrossRef] [PubMed]
- Petruleviciene, M.; Juodkazyte, J.; Parvin, M.; Tereschenko, A. Tuning the Photo-Luminescence Properties of WO3 Layers by the Adjustment of Layer Formation Conditions. Materials 2020, 13, 2814. [Google Scholar] [CrossRef] [PubMed]
- Petruleviciene, M.; Parvin, M.; Savickaja, I.; Gece, G.; Naujokaitis, A.; Pakstas, V.; Pilipavicius, J.; Gegeckas, A.; Gaigalas, G.; Juodkazyte, J. WO3 coatings for photoelectrochemical synthesis of persulfate: Efficiency, stability and applicability. J. Solid State Electrochem. 2022, 26, 1021–1035. [Google Scholar] [CrossRef]
- Petruleviciene, M.; Savickaja, I.; Juodkazyte, J.; Grinciene, G.; Ramanavicius, A. Investigation of BiVO4-based advanced oxidation system for decomposition of organic compounds and production of reactive sulfate species. Sci. Total Environ. 2023, 875, 162574. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.C.; Choi, K.S. Effect of electrolytes on the selectivity and stability of n-type WO3 photoelectrodes for use in solar water oxidation. J. Phys. Chem. C 2012, 116, 7612–7620. [Google Scholar] [CrossRef]
- Petruleviciene, M.; Savickaja, I.; Griguceviciene, A.; Naujokaitis, A.; Ramanauskas, R.; Juodkazyte, J. Optimization of BiVO4 coatings for high yield photoelectrochemical production of reactive chlorine species. J. Photochem. Photobiol. A Chem. 2023, 443, 114842. [Google Scholar] [CrossRef]
- Parvin, M.; Petrulevičienė, M.; Savickaja, I.; Šebeka, B.; Karpicz, R.; Grigucevičienė, A.; Ramanauskas, R.; Juodkazytė, J. Influence of morphology on photoanodic behaviour of WO3 films in chloride and sulphate electrolytes. Electrochim. Acta 2022, 403, 139710. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, J.; Liu, H.; Murugadoss, V.; Zu, G.; Che, H.; Lai, C.; Li, H.; Ding, T.; Gao, Q.; et al. Tungsten oxide nanostructures and nanocomposites for photoelectrochemical water splitting. Nanoscale 2019, 11, 18968–18994. [Google Scholar] [CrossRef]
- Armstrong, D.A.; Huie, R.E.; Koppenol, W.H.; Lymar, S.V.; Merényi, G.; Neta, P.; Ruscic, B.; Stanbury, D.M.; Steenken, S.; Wardman, P. Standard electrode potentials involving radicals in aqueous solution: Inorganic radicals (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1139–1150. [Google Scholar] [CrossRef]
- Karlsson, R.K.B.; Cornell, A. Selectivity between Oxygen and Chlorine Evolution in the Chlor-Alkali and Chlorate Processes. Chem. Rev. 2016, 116, 2982–3028. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Huang, C.; Chen, Y. Potential for activated persulfate degradation of BTEX contamination. Water Res. 2008, 42, 4091–4100. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Guo, N.; Xu, X.; Yu, Y.; Wang, Q.; Wang, N.; Han, X.; Yu, H. Degradation of bisphenol a using peroxymonosulfate activated by WO3@MoS2/Ag hollow nanotubes photocatalyst. Chemosphere 2019, 227, 589–597. [Google Scholar] [CrossRef]
- Ye, Q.; Xu, H.; Zhang, J.; Wang, Q.; Zhou, P.; Wang, Y.; Huang, X.; Huo, X.; Liu, C.; Lu, J. Enhancement of peroxymonosulfate activation for antibiotics removal by nano zero valent tungsten induced Cu(II)/Cu(I) redox cycles. Chem. Eng. J. 2020, 382, 123054. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Dong, S.; Qiao, J.; Zeng, Z.; Shao, S. A visible-light-driven photoelectrochemical sensing platform based on the BiVO4/FeOOH photoanode for dopamine detection. Electrochim. Acta 2022, 414, 140207. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Ohkubo, K. Organic synthetic transformations using organic dyes as photoredox catalysts. Org. Biomol. Chem. 2014, 12, 6059–6071. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, X.; Xie, X.; Mahmood, A.; Lu, G.; Wang, Y.; Sun, J. Band bending of TiO2 induced by O-xylene and acetaldehyde adsorption and its effect on the generation of active radicals. J. Colloid Interface Sci. 2020, 572, 374–383. [Google Scholar] [CrossRef]
- Mureithi, A.W.; Sun, Y.; Mani, T.; Howell, A.R.; Zhao, J. Impact of hole scavengers on photocatalytic reduction of nitrobenzene using cadmium sulfide quantum dots. Cell Rep. Phys. Sci. 2022, 3, 100889. [Google Scholar] [CrossRef]
- Mesa, C.A.; Kafizas, A.; Francàs, L.; Pendlebury, S.R.; Pastor, E.; Ma, Y.; Le Formal, F.; Mayer, M.T.; Grätzel, M.; Durrant, J.R. Kinetics of Photoelectrochemical Oxidation of Methanol on Hematite Photoanodes. J. Am. Chem. Soc. 2017, 139, 11537–11543. [Google Scholar] [CrossRef]
- Haisch, C.; Schneider, J.; Fleisch, M.; Gutzmann, H.; Klassen, T.; Bahnemann, D.W. Cold sprayed WO3 and TiO2 electrodes for photoelectrochemical water and methanol oxidation in renewable energy applications. Dalt. Trans. 2017, 46, 12811–12823. [Google Scholar] [CrossRef]
- Wang, G.; Xie, X.; Cui, X.; Liu, J.; Jiang, L. Photoinduced Pt/BiVO4/Bi2O3Heterostructures for Methanol Oxidation and New Insights on the Photo-/Electrocatalysis Coupling Mechanism. ACS Sustain. Chem. Eng. 2021, 9, 4271–4281. [Google Scholar] [CrossRef]
- Lutze, H.V.; Kerlin, N.; Schmidt, T.C. Sulfate radical-based water treatment in presence of chloride: Formation of chlorate, inter-conversion of sulfate radicals into hydroxyl radicals and influence of bicarbonate. Water Res. 2015, 72, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Monod, A.; Chebbi, A.; Durand-Jolibois, R.; Carlier, P. Oxidation of methanol by hydroxyl radicals in aqueous solution under simulated cloud droplet conditions. Atmos. Environ. 2000, 34, 5283–5294. [Google Scholar] [CrossRef]
- Barroso, J.; Díez-Buitrago, B.; Saa, L.; Möller, M.; Briz, N.; Pavlov, V. Specific bioanalytical optical and photoelectrochemical assays for detection of methanol in alcoholic beverages. Biosens. Bioelectron. 2018, 101, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, Z. A Photoelectrochemical Sensor for Methanol based on Its Oxidation at TiO2 Thin Film Modified Ti Electrode. Anal. Bioanal. Electrochem. 2019, 11, 566–576. [Google Scholar]
- Alam, M.M.; Asiri, A.M.; Uddin, M.T.; Rahman, M.M.; Islam, M.A. An alternative electrochemical approach for toluene detection with ZnO/MgO/Cr2O3 nanofibers on a glassy carbon electrode for environmental monitoring. RSC Adv. 2020, 10, 44641–44653. [Google Scholar] [CrossRef]
- Garcia de Freitas Junior, G.; Florêncio, T.M.; Mendonça, R.J.; Salazar-Banda, G.R.; Oliveira, R.T.S. Simultaneous Voltammetric Determination of Benzene, Toluene and Xylenes. Electroanalysis 2019, 31, 554–559. [Google Scholar] [CrossRef]
- Rao, Y.F.; Chu, W.; Wang, Y.R. Photocatalytic oxidation of carbamazepine in triclinic-WO3 suspension: Role of alcohol and sulfate radicals in the degradation pathway. Appl. Catal. A Gen. 2013, 468, 240–249. [Google Scholar] [CrossRef]
- Ahmed, N.; Vione, D.; Rivoira, L.; Carena, L.; Castiglioni, M.; Bruzzoniti, M.C. A review on the degradation of pollutants by fenton-like systems based on zero-valent iron and persulfate: Effects of reduction potentials, ph, and anions occurring in waste waters. Molecules 2021, 26, 4584. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Gamal El-Din, M. Insight into in-situ radical and non-radical oxidative degradation of organic compounds in complex real matrix during electrooxidation with boron doped diamond electrode: A case study of oil sands process water treatment. Appl. Catal. B Environ. 2020, 279, 119366. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Takács, E. Rate constants of sulfate radical anion reactions with organic molecules: A review. Chemosphere 2019, 220, 1014–1032. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Ye, T.; Wei, Z.; Luo, S.; Yang, Z.; Spinney, R. Quantitative Structure-Activity Relationship (QSAR) for the Oxidation of Trace Organic Contaminants by Sulfate Radical. Environ. Sci. Technol. 2015, 49, 13394–13402. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Ma, X.; Lu, J.; Zhang, G.; Huo, S.; Gao, M.; Xu, P.; Liu, W. Enhanced visible light photoelectrocatalytic degradation of o-chloronitrobenzene through surface plasmonic Au nanoparticles and g-C3N4 co-modified TiO2 nanotube arrays photoanode. Appl. Catal. B Environ. 2023, 323, 122174. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Y.; Zhou, C.; Bai, J.; Chen, S.; Li, J.; Wang, J.; Guan, X.; Rahim, M.; Zhou, B. Exhaustive denitrification via chlorine oxide radical reactions for urea based on a novel photoelectrochemical cell. Water Res. 2020, 170, 115357. [Google Scholar] [CrossRef] [PubMed]
- Cristino, V.; Pasti, L.; Marchetti, N.; Berardi, S.; Bignozzi, C.A.; Molinari, A.; Passabi, F.; Caramori, S.; Amidani, L.; Orlandi, M.; et al. Photoelectrocatalytic degradation of emerging contaminants at WO3/BiVO4 photoanodes in aqueous solution. Photochem. Photobiol. Sci. 2019, 18, 2150–2163. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Wang, H.; Wu, Y.; Wang, F.; Xia, M.; Chen, Q. Peroxymonosulfate-assisted for facilitating photocatalytic degradation performance of 2D/2D WO3/BiOBr S-scheme heterojunction. Chem. Eng. J. 2022, 430, 132806. [Google Scholar] [CrossRef]
- Rabé, K.; Liu, L.; Nahyoon, N.A.; Zhang, Y.; Idris, A.M. Enhanced Rhodamine B and coking wastewater degradation and simultaneous electricity generation via anodic g-C3N4/Fe 0 (1%)/TiO2 and cathodic WO3 in photocatalytic fuel cell system under visible light irradiation. Electrochim. Acta 2019, 298, 430–439. [Google Scholar] [CrossRef]
- Longobucco, G.; Pasti, L.; Molinari, A.; Marchetti, N.; Caramori, S.; Cristino, V.; Boaretto, R.; Bignozzi, C.A. Photoelectrochemical mineralization of emerging contaminants at porous WO3 interfaces. Appl. Catal. B Environ. 2017, 204, 273–282. [Google Scholar] [CrossRef]
- Sun, B.; Li, H.; Wei, Q.; Xue, S.; Zhou, A.; Yue, X. Enhanced quinoline degradation by persulfate-assisted photocatalytic process with WO3-CuFe2O4 Z-scheme system: Properties and mechanism. Sep. Purif. Technol. 2022, 301, 122039. [Google Scholar] [CrossRef]

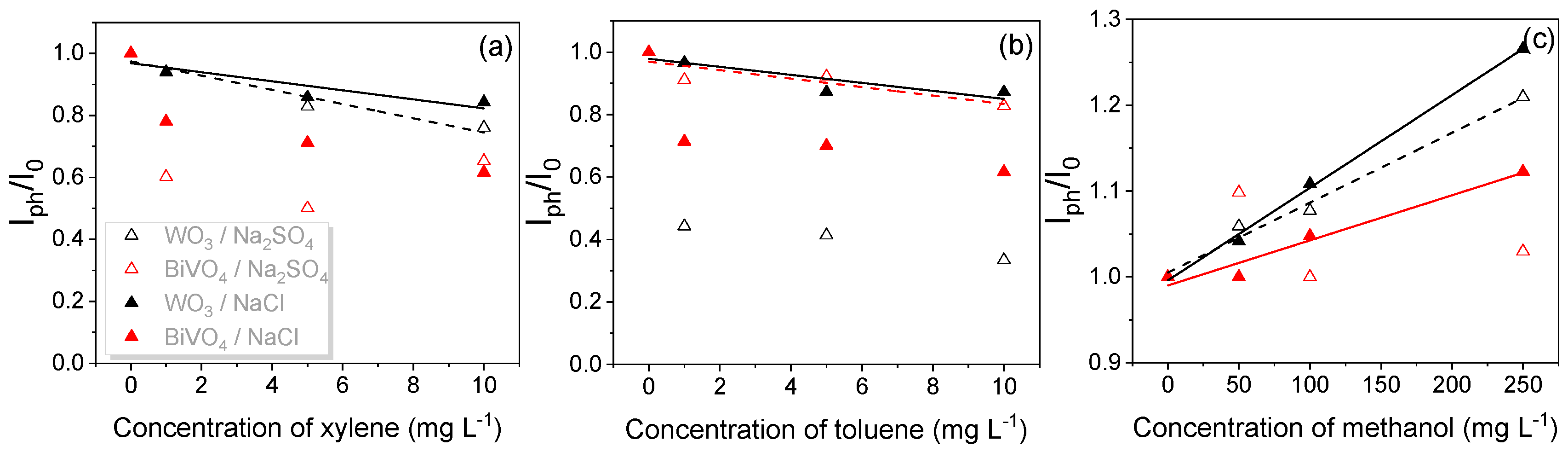

| VOC | PEC System | The Regression Equation Iph/I0 = Slope × C (mg L−1) + Intercept | Correlation Coefficient | LOD mg L−1 |
|---|---|---|---|---|
| Xylene | WO3/NaCl | −0.01284C + 0.9776 | 0.81585 | 6.22 |
| Xylene | WO3/Na2SO4 | −0.0229C + 0.97418 | 0.94192 | 3.25 |
| Xylene | BiVO4/NaCl | −0.03115C + 0.90151 | 0.753 | 7.5 |
| Toluene | WO3/NaCl | −0.01284C + 0.9776 | 0.7885 | 6.77 |
| Toluene | WO3/Na2SO4 | −0.04693C + 0.73532 | 0.48905 | 13.39 |
| Toluene | BiVO4/NaCl | −0.02808C + 0.86992 | 0.58283 | 11.08 |
| Toluene | BiVO4/Na2SO4 | −0.01356C + 0.96963 | 0.77057 | 7.14 |
| Methanol | WO3/NaCl | 0.00108C + 0.99595 | 0.99733 | 16.5 |
| Methanol | WO3/Na2SO4 | 8.14033 × 10−4C + 1.00499 | 0.98764 | 35.76 |
| Methanol | BiVO4/NaCl | 5.2535 × 10−4C + 0.99002 | 0.96099 | 64.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petruleviciene, M.; Savickaja, I.; Juodkazyte, J.; Ramanavicius, A. Investigation of WO3 and BiVO4 Photoanodes for Photoelectrochemical Sensing of Xylene, Toluene and Methanol. Chemosensors 2023, 11, 552. https://doi.org/10.3390/chemosensors11110552
Petruleviciene M, Savickaja I, Juodkazyte J, Ramanavicius A. Investigation of WO3 and BiVO4 Photoanodes for Photoelectrochemical Sensing of Xylene, Toluene and Methanol. Chemosensors. 2023; 11(11):552. https://doi.org/10.3390/chemosensors11110552
Chicago/Turabian StylePetruleviciene, Milda, Irena Savickaja, Jurga Juodkazyte, and Arunas Ramanavicius. 2023. "Investigation of WO3 and BiVO4 Photoanodes for Photoelectrochemical Sensing of Xylene, Toluene and Methanol" Chemosensors 11, no. 11: 552. https://doi.org/10.3390/chemosensors11110552
APA StylePetruleviciene, M., Savickaja, I., Juodkazyte, J., & Ramanavicius, A. (2023). Investigation of WO3 and BiVO4 Photoanodes for Photoelectrochemical Sensing of Xylene, Toluene and Methanol. Chemosensors, 11(11), 552. https://doi.org/10.3390/chemosensors11110552







