Enhanced Gas Sensing Performance of CuO-ZnO Composite Nanostructures for Low-Concentration NO2 Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Powder
2.1.1. Preparation of CuO and ZnO Structures
- i.
- A total of 0.05 g of copper acetate (Cu(CH3COO)2, Sigma-Aldrich, Steinheim, Germany) was dissolved in 25 mL of ethylene glycol (C2H6O2, Sigma-Alderich) and stirred for 30 min at room temperature. Then, the resultant solution was heated at 150 °C for 40 min. After 20 min, the clear solution turned cloudy due to the formation of an organocopper precursor.
- ii.
- A total of 2 g of zinc acetate (Zn(CH3COO)2, Sigma-Aldrich) was added to 25 mL of ethylene glycol. Then, the mixture was stirred and heated at 150 °C for 6 h.
2.1.2. Synthesis of the CuO-ZnO Composite
2.2. Characterization
2.3. Fabrication of the Sensor Device
3. Results and Discussion
3.1. Morphological Properties
3.2. Structural Properties
3.3. Optical Properties
3.4. Gas Sensing Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faiz, A. Automotive emissions in developing countries-relative implications for global warming, acidification and urban air quality. Transp. Res. Part A Policy Pract. 1993, 27, 167–186. [Google Scholar] [CrossRef]
- Le, D.T.T.; Long, N.D.H.; Xuan, C.T.; Van Toan, N.; Hung, C.M.; Van Duy, N.; Theu, L.T.; Dinh, V.A.; Hoa, N.D. Porous CoFe2O4 nanorods: VOC gas-sensing characteristics and DFT calculation. Sens. Actuators B Chem. 2023, 379, 133286. [Google Scholar]
- Deng, S.; Liu, X.; Chen, N.; Deng, D.; Xiao, X.; Wang, Y. A highly sensitive VOC gas sensor using p-type mesoporous Co3O4 nanosheets prepared by a facile chemical coprecipitation method. Sens. Actuators B Chem. 2016, 233, 615–623. [Google Scholar] [CrossRef]
- Zyryanov, G.V.; Kang, Y.; Rudkevich, D.M. Sensing and Fixation of NO2/N2O4 by Calix[4]Arenes. J. Am. Chem. Soc. 2003, 125, 2997–3007. [Google Scholar] [CrossRef] [PubMed]
- Tamvakos, A.; Korir, K.; Tamvakos, D.; Calestani, D.; Cicero, G.; Pullini, D. NO2 gas sensing mechanism of ZnO thin-film transducers: Physical experiment and theoretical correlation study. ACS Sens. 2016, 1, 406–412. [Google Scholar] [CrossRef]
- Frampton, M.W.; Boscia, J.; Roberts, N.J., Jr.; Azadniv, M.; Torres, A.; Cox, C.; Morrow, P.E.; Nichols, J.; Chalupa, D.; Frasier, L.M. Nitrogen dioxide exposure: Effects on airway and blood cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L155–L165. [Google Scholar] [CrossRef] [PubMed]
- Weinmayr, G.; Romeo, E.; De Sario, M.; Weiland, S.K.; Forastiere, F. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: A systematic review and meta-analysis. Environ. Health Perspect. 2010, 118, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, N.; Carel, R.S.; Derazne, E.; Bibi, H.; Shpriz, M.; Tzur, D.; Portnov, B.A. Different effects of long-term exposures to SO2 and NO2 air pollutants on asthma severity in young adults. J. Toxicol. Environ. Health Part A 2016, 79, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Gillespie-Bennett, J.; Pierse, N.; Wickens, K.; Crane, J.; Howden-Chapman, P. The respiratory health effects of nitrogen dioxide in children with asthma. Eur. Respir. J. 2011, 38, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Andersen, Z.J.; Bønnelykke, K.; Hvidberg, M.; Jensen, S.S.; Ketzel, M.; Loft, S.; Sørensen, M.; Tjønneland, A.; Overvad, K.; Raaschou-Nielsen, O. Long-term exposure to air pollution and asthma hospitalisations in older adults: A cohort study. Thorax 2012, 67, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Mele, M.; Magazzino, C.; Schneider, N.; Strezov, V. NO2 levels as a contributing factor to COVID-19 deaths: The first empirical estimate of threshold values. Environ. Res. 2021, 194, 110663. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xie, C.; Zhang, G.; Zhao, J.; Yu, X.; Zeng, D.; Zhang, S. Enhanced response to NO2 with CuO/ZnO laminated heterostructured configuration. Sens. Actuators B Chem. 2014, 195, 500–508. [Google Scholar] [CrossRef]
- Fischer, S.; Pohle, R.; Farber, B.; Proch, R.; Kaniuk, J.; Fleischer, M.; Moos, R. Method for detection of NOx in exhaust gases by pulsed discharge measurements using standard zirconia-based lambda sensors. Sens. Actuators B Chem. 2010, 147, 780–785. [Google Scholar] [CrossRef]
- Geupel, A.; Schönauer, D.; Röder-Roith, U.; Kubinski, D.J.; Mulla, S.; Ballinger, T.H.; Chen, H.-Y.; Visser, J.H.; Moos, R. Integrating nitrogen oxide sensor: A novel concept for measuring low concentrations in the exhaust gas. Sens. Actuators B Chem. 2010, 145, 756–761. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Fierro, J.L.G. Metal Oxides: Chemistry and Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Kumbhakar, P.; Gowda, C.C.; Mahapatra, P.L.; Mukherjee, M.; Malviya, K.D.; Chaker, M.; Chandra, A.; Lahiri, B.; Ajayan, P.; Jariwala, D. Emerging 2D metal oxides and their applications. Mater. Today 2021, 45, 142–168. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, S.; Zhou, P.; Cui, B.; Liu, W.; Wei, D.; Shen, Y. Room-temperature NO2 sensing properties and mechanism of CuO nanorods with Au functionalization. Sens. Actuators B Chem. 2021, 328, 129070. [Google Scholar] [CrossRef]
- Das, A.; Venkataramana, B.; Partheephan, D.; Prasad, A.; Dhara, S.; Tyagi, A. Facile synthesis of nanostructured CuO for low temperature NO2 sensing. Phys. E Low-Dimens. Syst. Nanostruct. 2013, 54, 40–44. [Google Scholar] [CrossRef]
- Navale, Y.; Navale, S.; Galluzzi, M.; Stadler, F.; Debnath, A.; Ramgir, N.; Gadkari, S.; Gupta, S.; Aswal, D.; Patil, V. Rapid synthesis strategy of CuO nanocubes for sensitive and selective detection of NO2. J. Alloys Compd. 2017, 708, 456–463. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, W.; Li, Y. Metal oxide gas sensors for detecting NO2 in industrial exhaust gas: Recent developments. Sens. Actuators B Chem. 2022, 359, 131579. [Google Scholar] [CrossRef]
- Huang, J.; Dai, Y.; Gu, C.; Sun, Y.; Liu, J. Preparation of porous flower-like CuO/ZnO nanostructures and analysis of their gas-sensing property. J. Alloys Compd. 2013, 575, 115–122. [Google Scholar] [CrossRef]
- Sankaran, A.; Kumaraguru, K. The novel two step synthesis of CuO/ZnO and CuO/CdO nanocatalysts for enhancement of catalytic activity. J. Mol. Struct. 2020, 1221, 128772. [Google Scholar] [CrossRef]
- Figlarz, M.; Fievet, F.; Lagier, J.-P. Process for the Reduction of Metallic Compounds by Polyols, and Metallic Powders Obtained by This Process. U.S. Patent 4539041, 3 September 1985. [Google Scholar]
- Sun, Y.; Xia, Y. Large-scale synthesis of uniform silver nanowires through a soft, self-seeding, polyol process. Adv. Mater. 2002, 14, 833–837. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Y.-C.; Feldmann, C. Polyol synthesis of nanoparticles: Status and options regarding metals, oxides, chalcogenides, and non-metal elements. Green Chem. 2015, 17, 4107–4132. [Google Scholar] [CrossRef]
- Barreca, D.; Gasparotto, A.; Maccato, C.; Maragno, C.; Tondello, E.; Comini, E.; Sberveglieri, G. Columnar CeO2 nanostructures for sensor application. Nanotechnology 2007, 18, 125502. [Google Scholar] [CrossRef]
- Cao, A.-M.; Hu, J.-S.; Liang, H.-P.; Song, W.-G.; Wan, L.-J.; He, X.-L.; Gao, X.-G.; Xia, S.-H. Hierarchically structured cobalt oxide (Co3O4): The morphology control and its potential in sensors. J. Phys. Chem. B 2006, 110, 15858–15863. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, X.; Li, Y.; Sulieman, K.; He, X.; Sun, F. Hierarchical nanostructures of cupric oxide on a copper substrate: Controllable morphology and wettability. J. Mater. Chem. 2006, 16, 4427–4434. [Google Scholar] [CrossRef]
- Suber, L.; Sondi, I.; Matijević, E.; Goia, D.V. Preparation and the mechanisms of formation of silver particles of different morphologies in homogeneous solutions. J. Colloid Interface Sci. 2005, 288, 489–495. [Google Scholar] [CrossRef]
- Cao, A.-M.; Monnell, J.D.; Matranga, C.; Wu, J.-M.; Cao, L.-L.; Gao, D. Hierarchical nanostructured copper oxide and its application in arsenic removal. J. Phys. Chem. C 2007, 111, 18624–18628. [Google Scholar] [CrossRef]
- Ghoshal, T.; Kar, S.; Chaudhuri, S. ZnO doughnuts: Controlled synthesis, growth mechanism, and optical properties. Cryst. Growth Des. 2007, 7, 136–141. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Mashock, M.; Yu, K.; Cui, S.; Mao, S.; Lu, G.; Chen, J. Modulating gas sensing properties of CuO nanowires through creation of discrete nanosized p–n junctions on their surfaces. ACS Appl. Mater. Interfaces 2012, 4, 4192–4199. [Google Scholar] [CrossRef]
- Marotti, R.; Giorgi, P.; Machado, G.; Dalchiele, E. Crystallite size dependence of band gap energy for electrodeposited ZnO grown at different temperatures. Sol. Energy Mater. Sol. Cells 2006, 90, 2356–2361. [Google Scholar] [CrossRef]
- Shan, F.; Yu, Y. Band gap energy of pure and Al-doped ZnO thin films. J. Eur. Ceram. Soc. 2004, 24, 1869–1872. [Google Scholar] [CrossRef]
- Datta, N.; Ramgir, N.S.; Kumar, S.; Veerender, P.; Kaur, M.; Kailasaganapathi, S.; Debnath, A.; Aswal, D.; Gupta, S. Role of various interfaces of CuO/ZnO random nanowire networks in H2S sensing: An impedance and Kelvin probe analysis. Sens. Actuators B Chem. 2014, 202, 1270–1280. [Google Scholar] [CrossRef]
- Morales-Mendoza, J.E.; Herrera-Pérez, G.; Fuentes-Cobas, L.; Hermida-Montero, L.A.; Pariona, N.; Paraguay-Delgado, F. Synthesis, structural and optical properties of Cu doped ZnO and CuO–ZnO composite nanoparticles. Nano-Struct. Nano-Objects 2023, 34, 100967. [Google Scholar] [CrossRef]
- Harish, S.; Archana, J.; Sabarinathan, M.; Navaneethan, M.; Nisha, K.D.; Ponnusamy, S.; Muthamizhchelvan, C.; Ikeda, H.; Aswal, D.K.; Hayakawa, Y. Controlled structural and compositional characteristic of visible light active ZnO/CuO photocatalyst for the degradation of organic pollutant. Appl. Surf. Sci. 2017, 418, 103–112. [Google Scholar] [CrossRef]
- Musa, A.M.M.; Rasadujjaman, M.; Gafur, M.A.; Jamil, A.T.M.K. Synthesis and characterization of dip-coated ZnO–CuO composite thin film for room-temperature CO2 gas sensing. Thin Solid Film. 2023, 773, 139838. [Google Scholar] [CrossRef]
- Krishna, K.G.; Parne, S.R.; Nagaraju, P. An optical study of heterojunction n-ZnO/p-CuO nanosheets and detection of n-butanol vapour at room temperature. J. Mater. Sci. 2023, 58, 15660–15675. [Google Scholar] [CrossRef]
- Aqeel, T.; Galstyan, V.; Comini, E.; Bumajdad, A. Efficient one-pot synthesis of antimony-containing mesoporous tin dioxide nanostructures for gas-sensing applications. Arab. J. Chem. 2023, 16, 104797. [Google Scholar] [CrossRef]
- Madou, M.J.; Morrison, S.R. Chemical Sensing with Solid State Devices; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Bai, H.; Guo, H.; Wang, J.; Dong, Y.; Liu, B.; Xie, Z.; Guo, F.; Chen, D.; Zhang, R.; Zheng, Y. A room-temperature NO2 gas sensor based on CuO nanoflakes modified with rGO nanosheets. Sens. Actuators B Chem. 2021, 337, 129783. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, W.; Zhao, X.; Jiang, X.; Lin, S.; Zhen, Z.; Chen, W.; Xie, D.; Zhu, H. High-response room-temperature NO2 sensor and ultrafast humidity sensor based on SnO2 with rich oxygen vacancy. ACS Appl. Mater. Interfaces 2019, 11, 13441–13449. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Kumbhar, S.; Vanalakar, S.; Tarwal, N.; Mali, S.; Kim, J.; Patil, P. Gas sensing properties of 3D mesoporous nanostructured ZnO thin films. New J. Chem. 2018, 42, 13573–13580. [Google Scholar] [CrossRef]
- Govind, A.; Bharathi, P.; Mohan, M.K.; Archana, J.; Harish, S.; Navaneethan, M. Highly sensitive near room temperature operable NO2 gas-sensor for enhanced selectivity via nanoporous CuO@ZnO heterostructures. J. Environ. Chem. Eng. 2023, 11, 110056. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Xie, L.; Li, X.; Lin, D.; Zhu, Z. Low-temperature and highly sensitivity H2S gas sensor based on ZnO/CuO composite derived from bimetal metal-organic frameworks. Ceram. Int. 2020, 46, 15858–15866. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, Z.; Jia, Q.; Zhao, J.; Zhao, Z.; Ji, H. A CuO–ZnO nanostructured p–n junction sensor for enhanced N-butanol detection. RSC Adv. 2016, 6, 2504–2511. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Hao, F.; Kang, C.; Cui, B.; Wei, D.; Meng, F. Pn junctions based on CuO-decorated ZnO nanowires for ethanol sensing application. Appl. Surf. Sci. 2021, 538, 148140. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Yin, J.; Li, L.; Zhang, L.-X.; Bie, L.-J. Enhanced ethanol gas-sensing properties of flower-like p-CuO/n-ZnO heterojunction nanorods. Sens. Actuators B Chem. 2014, 202, 500–507. [Google Scholar] [CrossRef]
- Navale, Y.H.; Navale, S.T.; Stadler, F.J.; Ramgir, N.S.; Patil, V.B. Enhanced NO2 sensing aptness of ZnO nanowire/CuO nanoparticle heterostructure-based gas sensors. Ceram. Int. 2019, 45, 1513–1522. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Kang, S.Y.; Mirzaei, A.; Choi, M.S.; Bang, J.H.; Kim, S.S.; Kim, H.W. Enhancement of gas sensing properties by the functionalization of ZnO-branched SnO2 nanowires with Cr2O3 nanoparticles. Sens. Actuators B Chem. 2017, 249, 656–666. [Google Scholar] [CrossRef]
- Matatagui, D.; Lopez-Sanchez, J.; Pena, A.; Serrano, A.; Del Campo, A.; de la Fuente, O.R.; Carmona, N.; Navarro, E.; Marin, P.; del Carmen Horrillo, M. Ultrasensitive NO2 gas sensor with insignificant NH3-interference based on a few-layered mesoporous graphene. Sens. Actuators B Chem. 2021, 335, 129657. [Google Scholar] [CrossRef]
- Galstyan, V.; Poli, N.; D’Arco, A.; Macis, S.; Lupi, S.; Comini, E. A novel approach for green synthesis of WO3 nanomaterials and their highly selective chemical sensing properties. J. Mater. Chem. A 2020, 8, 20373–20385. [Google Scholar] [CrossRef]
- Agmon, N. The grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Pakdel, H.; Galstyan, V.; D’Arco, A.; Mancini, T.; Lupi, S.; Moumen, A.; Borsi, M.; Comini, E. Synthesis of WO3 nanopowder using a green surfactant for efficient gas sensing applications. Ceram. Int. 2023, 49, 30501–30509. [Google Scholar] [CrossRef]
- Boyle, J.; Jones, K. The effects of CO, water vapor and surface temperature on the conductivity of a SnO2 gas sensor. J. Electron. Mater. 1977, 6, 717–733. [Google Scholar] [CrossRef]
- Mali, S.M.; Narwade, S.S.; Navale, Y.H.; Tayade, S.B.; Digraskar, R.V.; Patil, V.B.; Kumbhar, A.S.; Sathe, B.R. Heterostructural CuO–ZnO Nanocomposites: A Highly Selective Chemical and Electrochemical NO2 Sensor. ACS Omega 2019, 4, 20129–20141. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.H.; Choi, M.S.; Mirzaei, A.; Kwon, Y.J.; Kim, S.S.; Kim, T.W.; Kim, H.W. Selective NO2 sensor based on Bi2O3 branched SnO2 nanowires. Sens. Actuators B Chem. 2018, 274, 356–369. [Google Scholar] [CrossRef]
- Shi, J.; Cheng, Z.; Gao, L.; Zhang, Y.; Xu, J.; Zhao, H. Facile synthesis of reduced graphene oxide/hexagonal WO3 nanosheets composites with enhanced H2S sensing properties. Sens. Actuators B Chem. 2016, 230, 736–745. [Google Scholar] [CrossRef]
- Zhang, D. Morphology Genetic Materials Templated from Nature Species; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Karthik, T.; Martínez-García, H.; Ortiz-Chi, F.; Espinosa-González, C.; Torres-Torres, J.; Hernandez, A.; Godavarthi, S.; Kesarla, M. CO2 gas sensing properties of graphitic carbon nitride (g-C3N4) thin films. Diam. Relat. Mater. 2023, 133, 109736. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, L.; Guo, F.; Liu, S.; Qi, L.; Shan, M.; Fan, X. Facial development of high performance room temperature NO2 gas sensors based on ZnO nanowalls decorated rGO nanosheets. Appl. Surf. Sci. 2017, 423, 721–727. [Google Scholar] [CrossRef]
- Compton, R.; Reinhardt, P.; Cooper, C. Collisional ionization of Na, K, and Cs by CO2, COS, and CS2: Molecular electron affinities. J. Chem. Phys. 1975, 63, 3821–3827. [Google Scholar] [CrossRef]
- Abreu, N.J.; Valdés, H.; Zaror, C.A.; de Oliveira, T.F.; Azzolina-Jury, F.; Thibault-Starzyk, F. Evidence of Synergy Effects between Zinc and Copper Oxides with Acidic Sites on Natural Zeolite during Photocatalytic Oxidation of Ethylene Using Operando DRIFTS Studies. Catalysts 2023, 13, 610. [Google Scholar] [CrossRef]
- Simon Patrick, D.; Govind, A.; Bharathi, P.; Krishna Mohan, M.; Harish, S.; Archana, J.; Navaneethan, M. Hierarchical ZnO/g-C3N4 nanocomposites for enhanced NO2 gas sensing applications. Appl. Surf. Sci. 2023, 609, 155337. [Google Scholar] [CrossRef]
- Jin, C.; Kim, H.; Park, S.; Choi, S.W.; Kim, S.S.; Lee, C. NO2 gas sensing properties of ZnO sheathed CuO nanorods. Surf. Interface Anal. 2012, 44, 1534–1537. [Google Scholar] [CrossRef]
- Fang, H.; Li, S.; Zhao, H.; Deng, J.; Wang, D.; Li, J. Enhanced NO2 gas sensing performance by hierarchical CuO–Co3O4 spheres. Sens. Actuators B Chem. 2022, 352, 131068. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, G.; Liao, J.; Lv, S.; Zhu, Z.; Li, Z. Vertically aligned MoS2/ZnO nanowires nanostructures with highly enhanced NO2 sensing activities. Appl. Surf. Sci. 2018, 456, 808–816. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Zhang, Y.; Dong, Z.; Zhang, T. Preparation of zinc oxide nanoparticle–reduced graphene oxide–gold nanoparticle hybrids for detection of NO2. RSC Adv. 2015, 5, 91760–91765. [Google Scholar] [CrossRef]
- Han, T.-H.; Bak, S.-Y.; Kim, S.; Lee, S.H.; Han, Y.-J.; Yi, M. Decoration of CuO NWs gas sensor with ZnO NPs for improving NO2 sensing characteristics. Sensors 2021, 21, 2103. [Google Scholar] [CrossRef] [PubMed]
- Kamble, V.S.; Zemase, R.K.; Gupta, R.H.; Aghav, B.D.; Shaikh, S.A.; Pawara, J.M.; Patil, S.K.; Salunkhe, S.T. Improved toxic NO2 gas sensing response of Cu-doped ZnO thin-film sensors derived by simple co-precipitation route. Opt. Mater. 2022, 131, 112706. [Google Scholar] [CrossRef]
- Şahin, Y.; Öztürk, S.; Kılınç, N.; Kösemen, A.; Erkovan, M.; Öztürk, Z.Z. Electrical conduction and NO2 gas sensing properties of ZnO nanorods. Appl. Surf. Sci. 2014, 303, 90–96. [Google Scholar] [CrossRef]
- Sun, K.; Zhan, G.; Zhang, L.; Wang, Z.; Lin, S. Highly sensitive NO2 gas sensor based on ZnO nanoarray modulated by oxygen vacancy with Ce doping. Sens. Actuators B Chem. 2023, 379, 133294. [Google Scholar] [CrossRef]
- Tian, H.; Fan, H.; Ma, J.; Liu, Z.; Ma, L.; Lei, S.; Fang, J.; Long, C. Pt-decorated zinc oxide nanorod arrays with graphitic carbon nitride nanosheets for highly efficient dual-functional gas sensing. J. Hazard. Mater. 2018, 341, 102–111. [Google Scholar] [CrossRef] [PubMed]
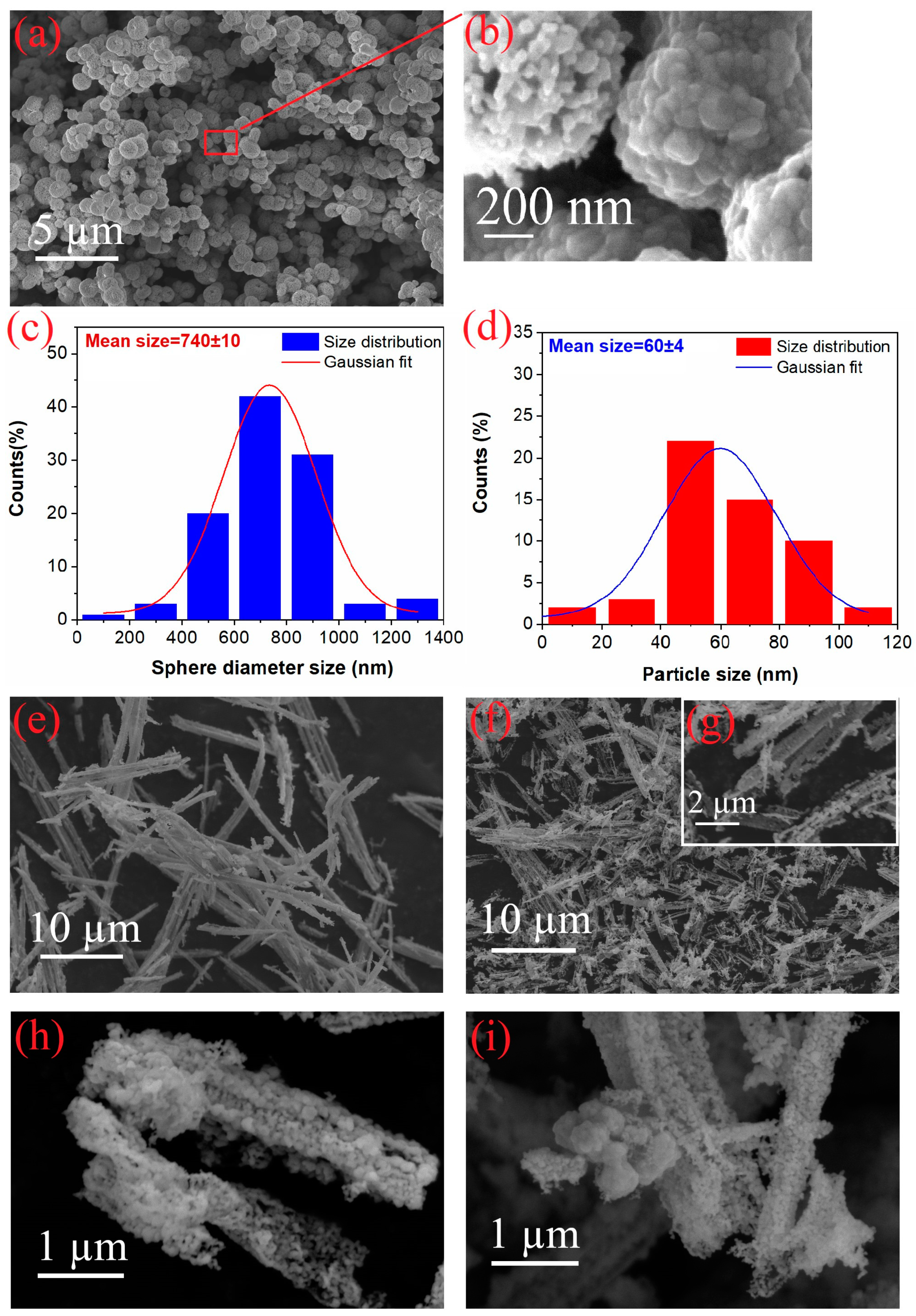


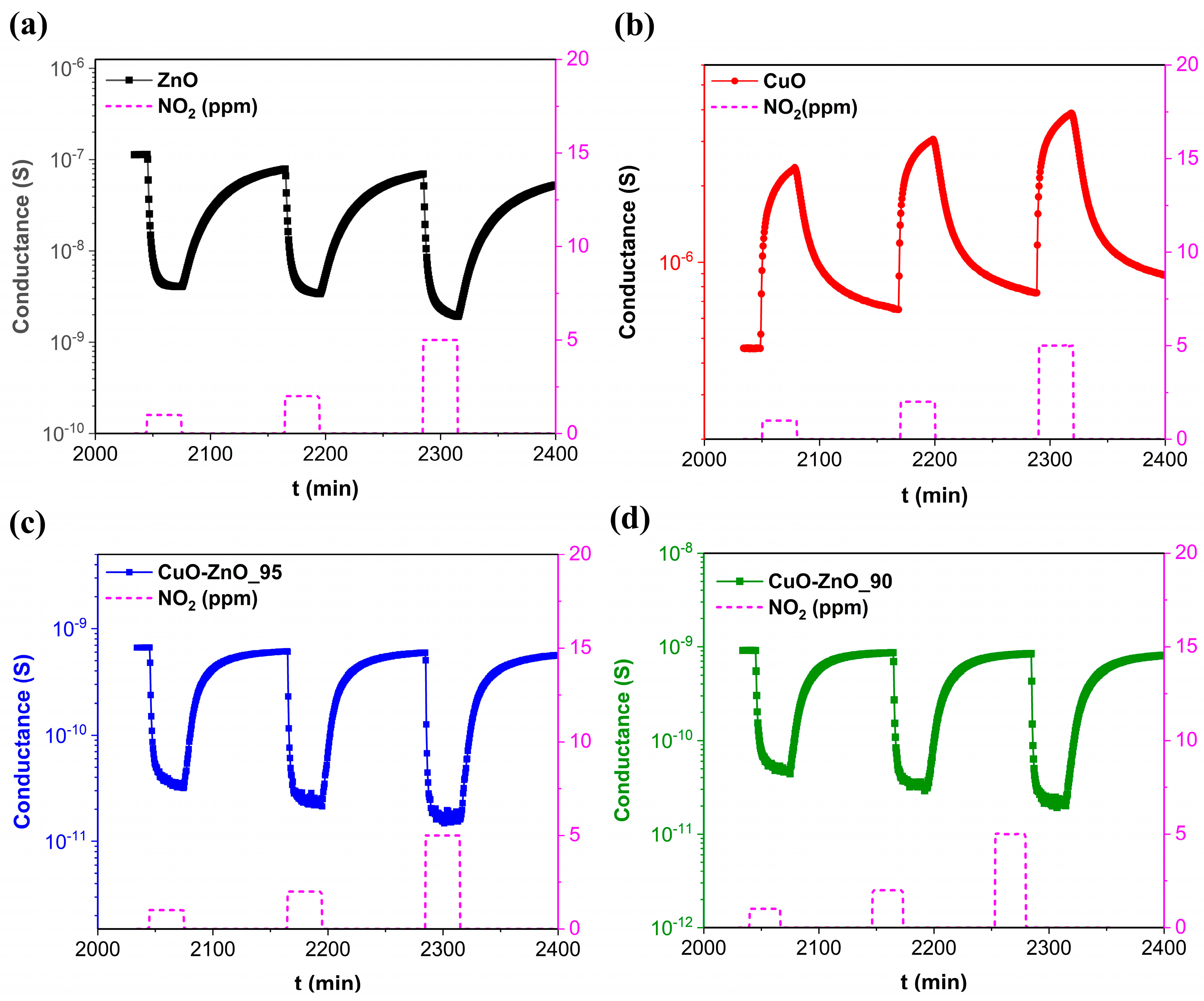
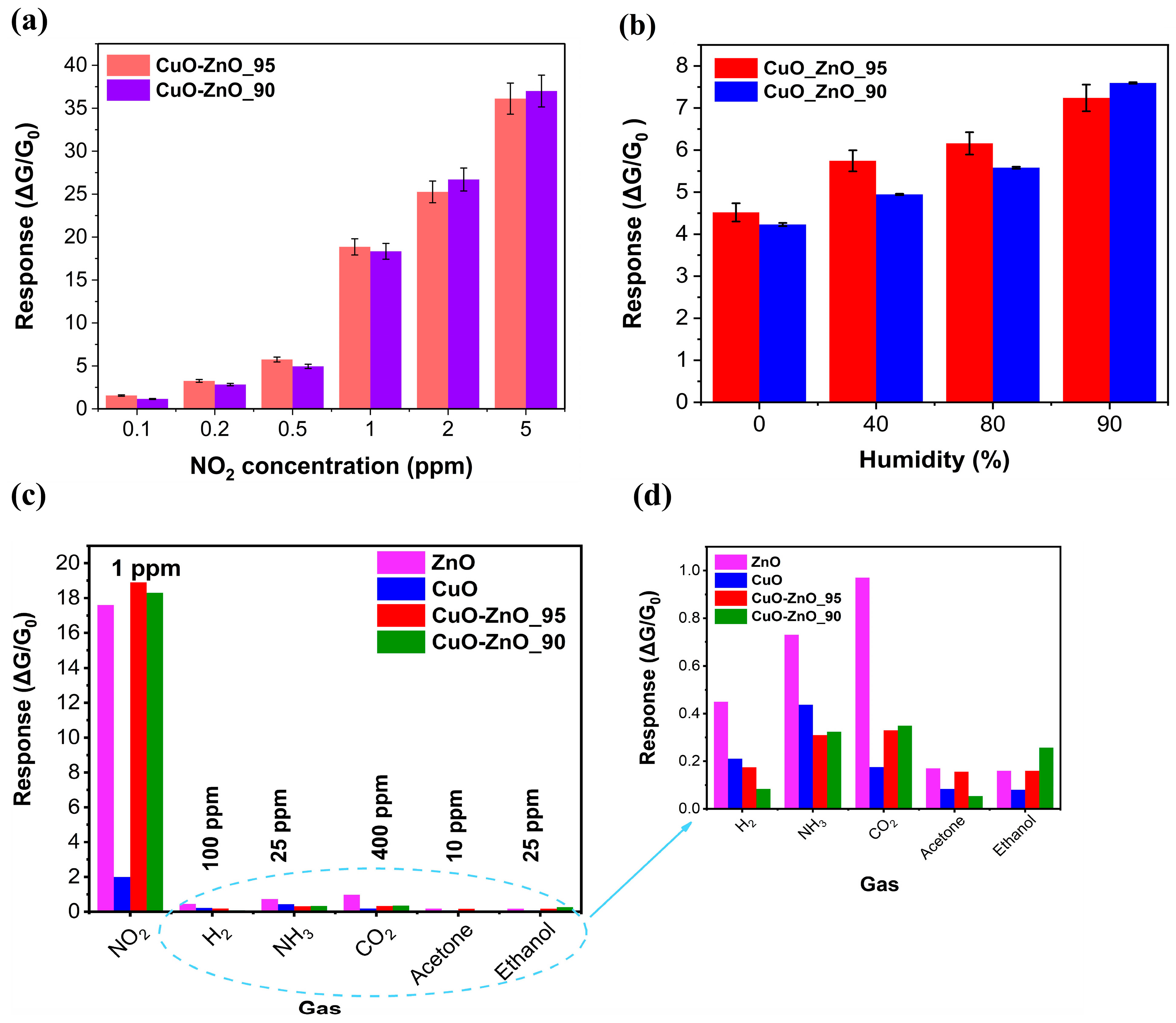
| Samples | Band Gap (eV) |
|---|---|
| ZnO | 3.09 |
| CuO | 1.25 |
| CuO-ZnO_95 | 2.92 |
| CuO-ZnO_90 | 2.81 |
| Gas | NO2 | H2 | NH3 | CO2 | Acetone | Ethanol |
|---|---|---|---|---|---|---|
| Bond | O–NO | H–H | H–NH2 | O–CO | H–CH2COCH3 | H–OC2H5 |
| Bond energy (KJ/mole) | 305.0 | 436.0 | 391.0 | 532.0 | 798.9 | 441.0 |
| Sensing Materials | Operating Temperature (°C) | Concentration (ppm) | Response | Reference |
|---|---|---|---|---|
| CuO NPs | 150 | 40 | 0.03 (R0 − Rf)/Rf | [22] |
| ZnO/g-C3N4 nanocomposite | 180 | 10 | 14.63 (Rf − R0)/R0 | [71] |
| ZnO–CuO core–shell nanorods | 300 | 10 | 0.5 (R0 − Rf)/Rf | [72] |
| ZnO nanowire/CuO nanoparticle | 150 | 100 | 1.75 (Rf − R0)/R0 | [55] |
| CuO-ZnO laminated heterostructure | 350 | 29 | 9.2 (R0/Rf) | [12] |
| CuO-ZnO nanocomposite | 200 | 100 | 0.73 (Rf/R0) | [63] |
| hierarchical CuO-Co3O4 spheres | 160 | 20 | 0.48 (Rf − R0)/R0 | [73] |
| MoS2-ZnO nanowires | 200 | 50 | 0.31 (Rf − R0)/R0 | [74] |
| ZnO/rGO/Au hybrids | 80 | 100 | 0.33 (R0 − Rf)/Rf | [75] |
| nanoporous CuO@ZnO | RT | 5 | 3.37 (R0 − Rf)/Rf | [50] |
| CuO nanowires/ZnO NPs | 250 | 100 | 4.1 (R0/Rf) | [76] |
| Cu:ZnO thin film | 200 | 100 | 3.26 (Rf − R0)/R0 | [77] |
| ZnO nanorods | 200 | 1 | 1.31 (I0 − If)/If | [78] |
| Ce-doped ZnO nanoarray | 250 | 10 | 34.3 (Rf/R0) | [79] |
| Pt/ZnO/g-C3N4 | 150 | 10 | 53 (Rf/R0) | [80] |
| CuO-ZnO_90 | 400 | 1 | 9.2 (G0 – Gf)/Gf | This work |
| CuO-ZnO_90 | 350 | 1 | 18.9 (G0 – Gf)/Gf | This work |
| CuO-ZnO_90 | 300 | 1 | 14.1 (G0 – Gf)/Gf | This work |
| CuO-ZnO_90 | 250 | 1 | 5.6 (G0 – Gf)/Gf | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakdel, H.; Borsi, M.; Ponzoni, M.; Comini, E. Enhanced Gas Sensing Performance of CuO-ZnO Composite Nanostructures for Low-Concentration NO2 Detection. Chemosensors 2024, 12, 54. https://doi.org/10.3390/chemosensors12040054
Pakdel H, Borsi M, Ponzoni M, Comini E. Enhanced Gas Sensing Performance of CuO-ZnO Composite Nanostructures for Low-Concentration NO2 Detection. Chemosensors. 2024; 12(4):54. https://doi.org/10.3390/chemosensors12040054
Chicago/Turabian StylePakdel, Hakimeh, Matteo Borsi, Massimo Ponzoni, and Elisabetta Comini. 2024. "Enhanced Gas Sensing Performance of CuO-ZnO Composite Nanostructures for Low-Concentration NO2 Detection" Chemosensors 12, no. 4: 54. https://doi.org/10.3390/chemosensors12040054
APA StylePakdel, H., Borsi, M., Ponzoni, M., & Comini, E. (2024). Enhanced Gas Sensing Performance of CuO-ZnO Composite Nanostructures for Low-Concentration NO2 Detection. Chemosensors, 12(4), 54. https://doi.org/10.3390/chemosensors12040054







