HS-SPME-GC/MS Metabolomic Analysis for the Comparative Evaluation between a Plum–Apricot Hybrid and Its Parents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Headspace Solid-Phase Microextraction (HS-SPME) and Gas Chromatography/Mass Spectrometry Analysis (GC/MS)
2.3. Statistical Analysis
3. Results and Discussion
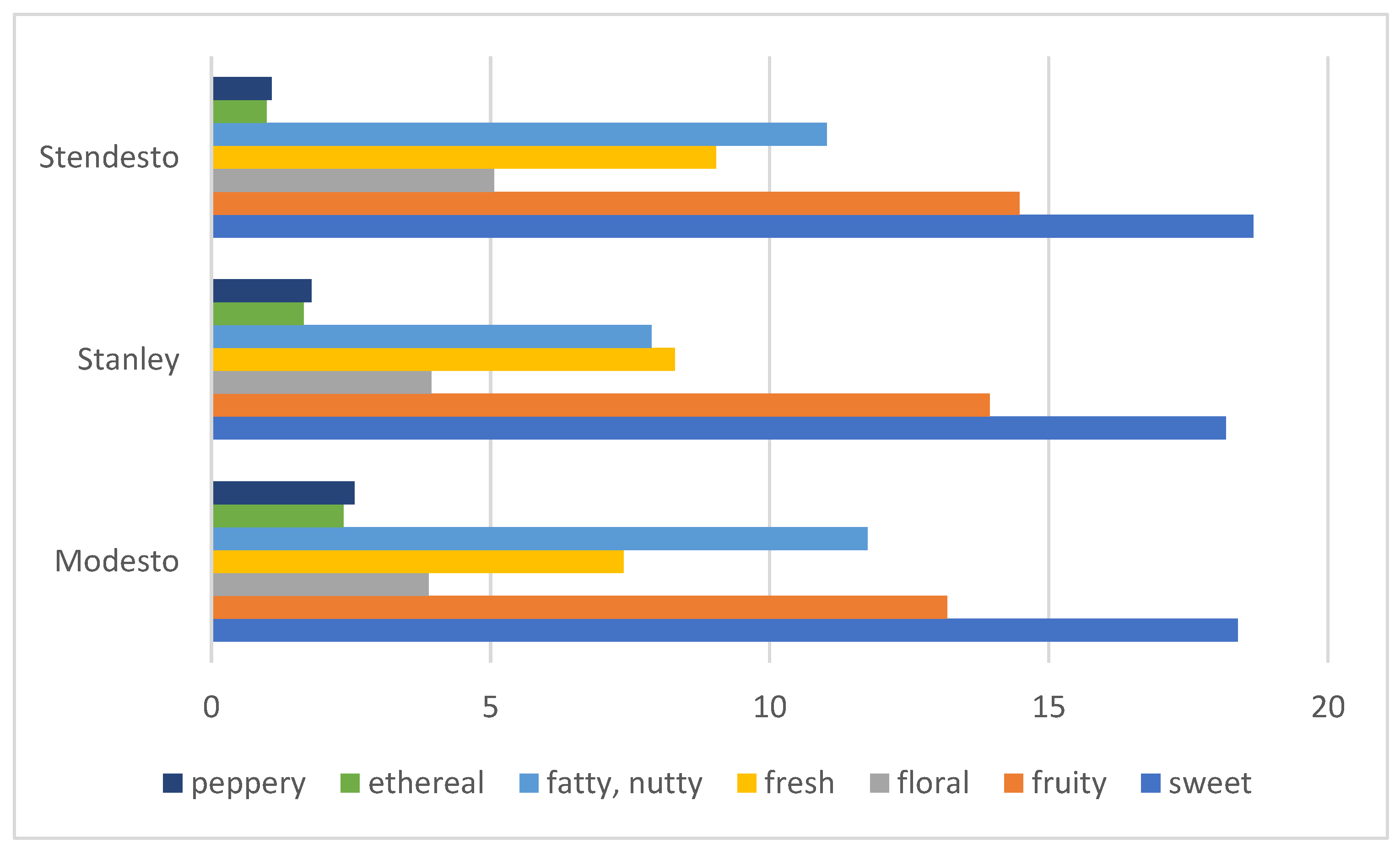
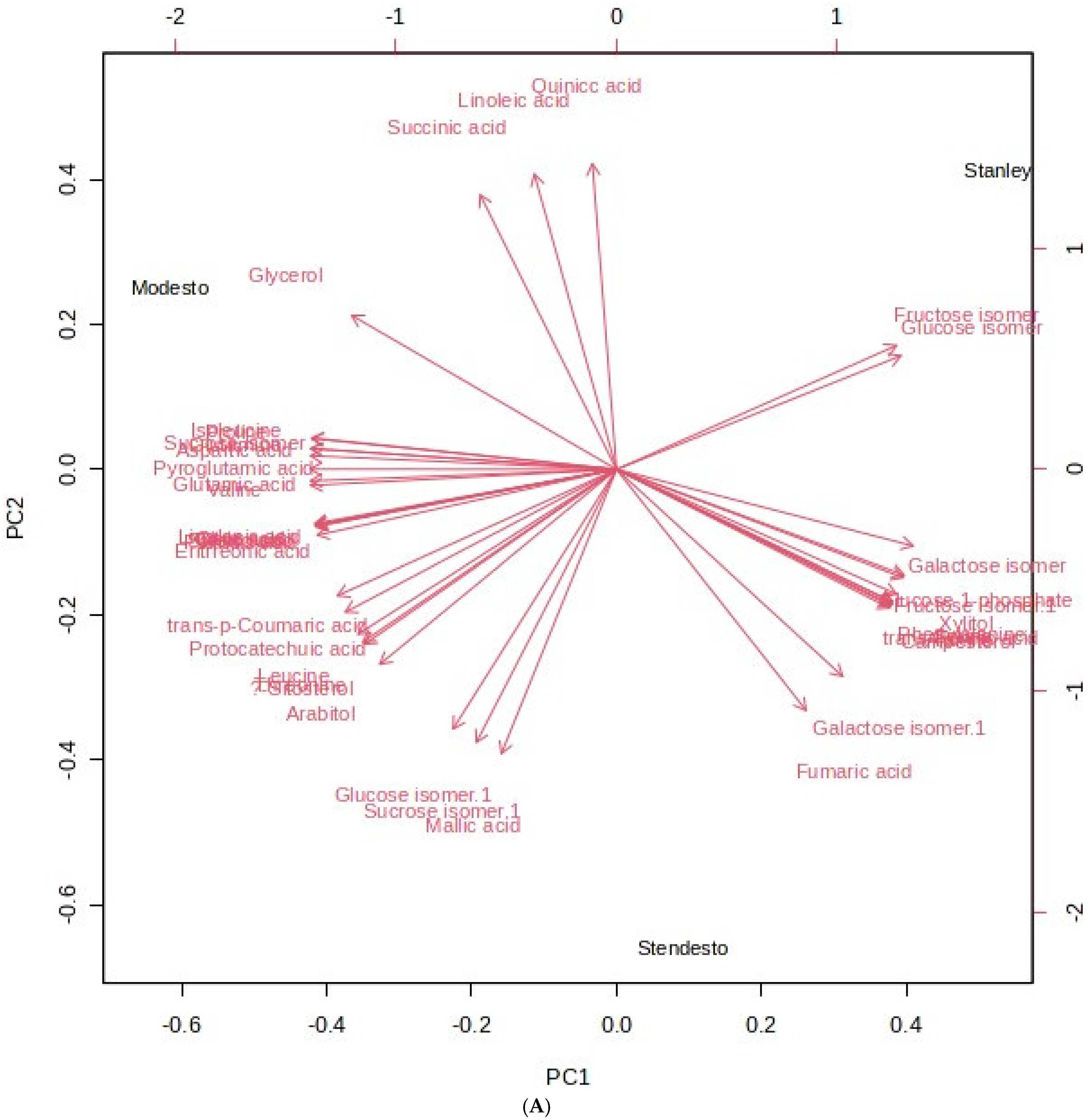
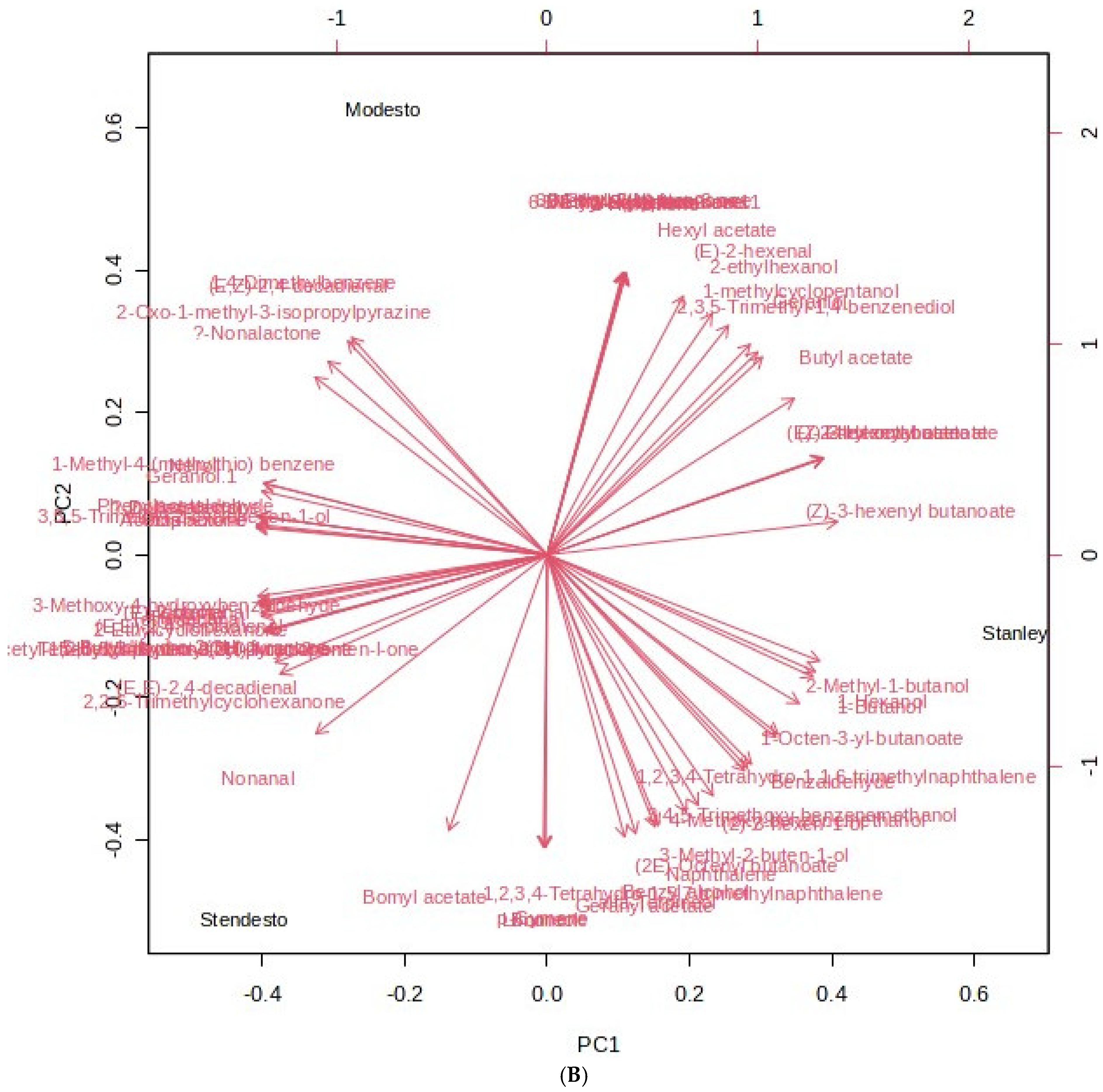
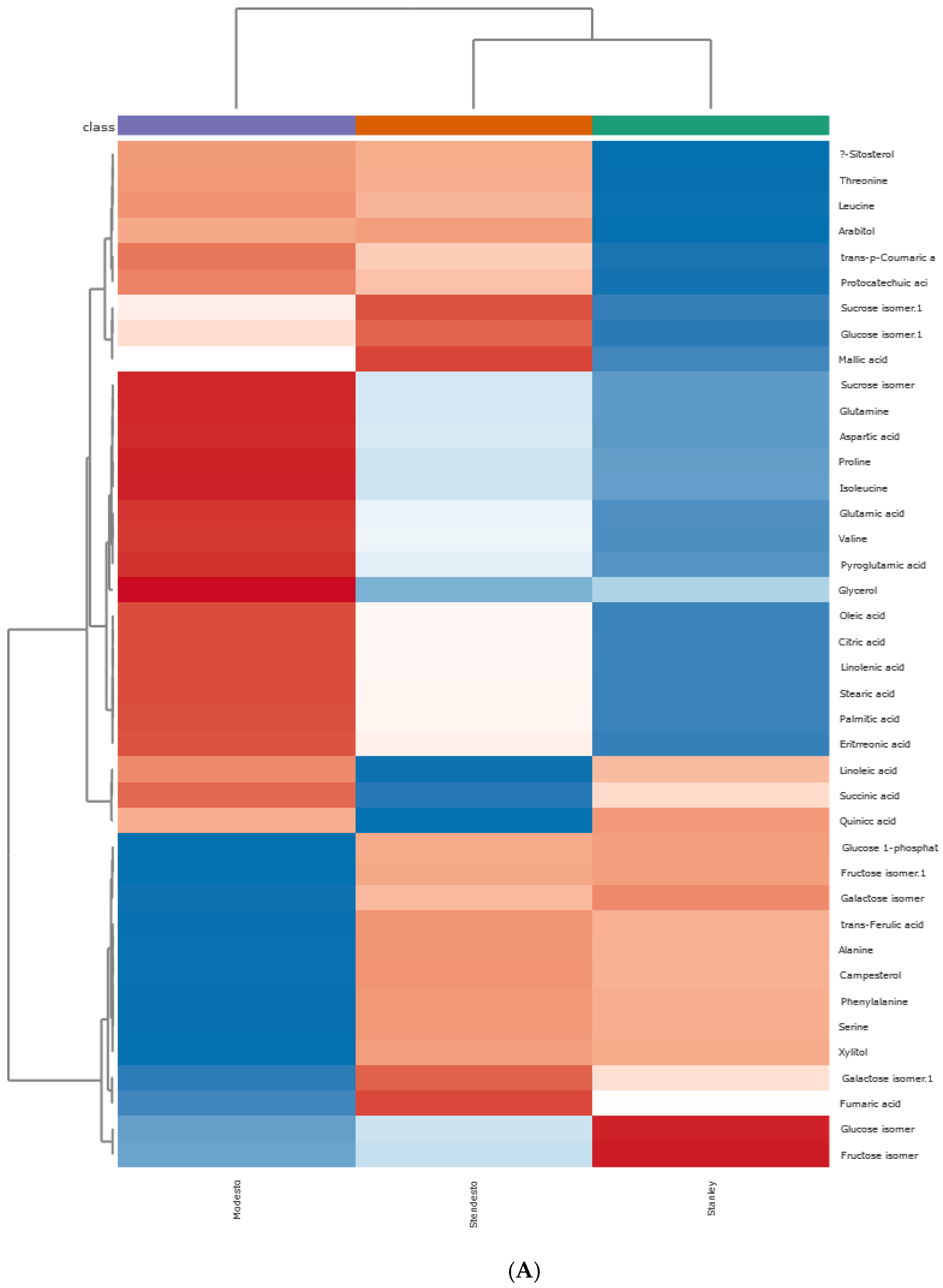
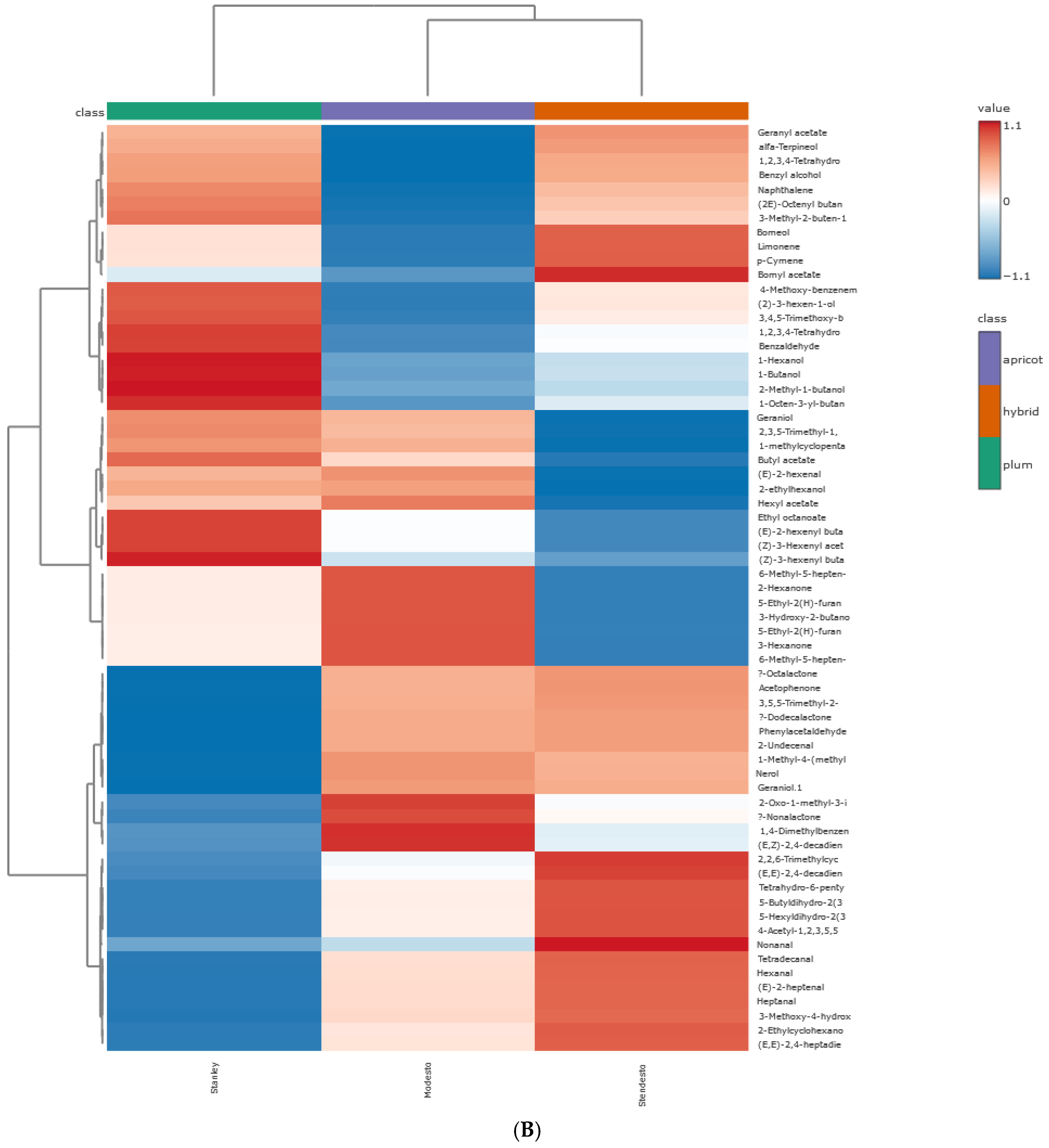
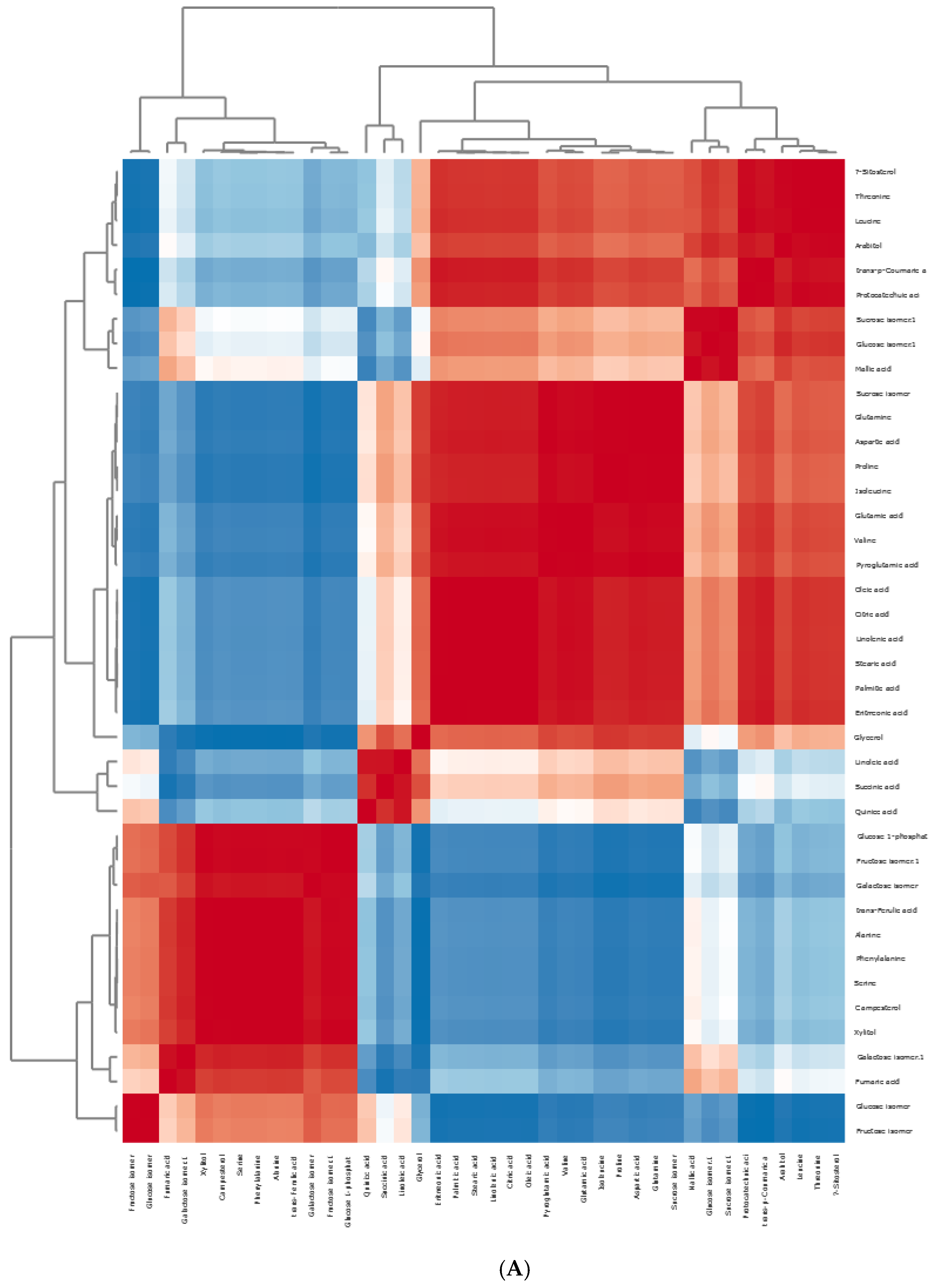
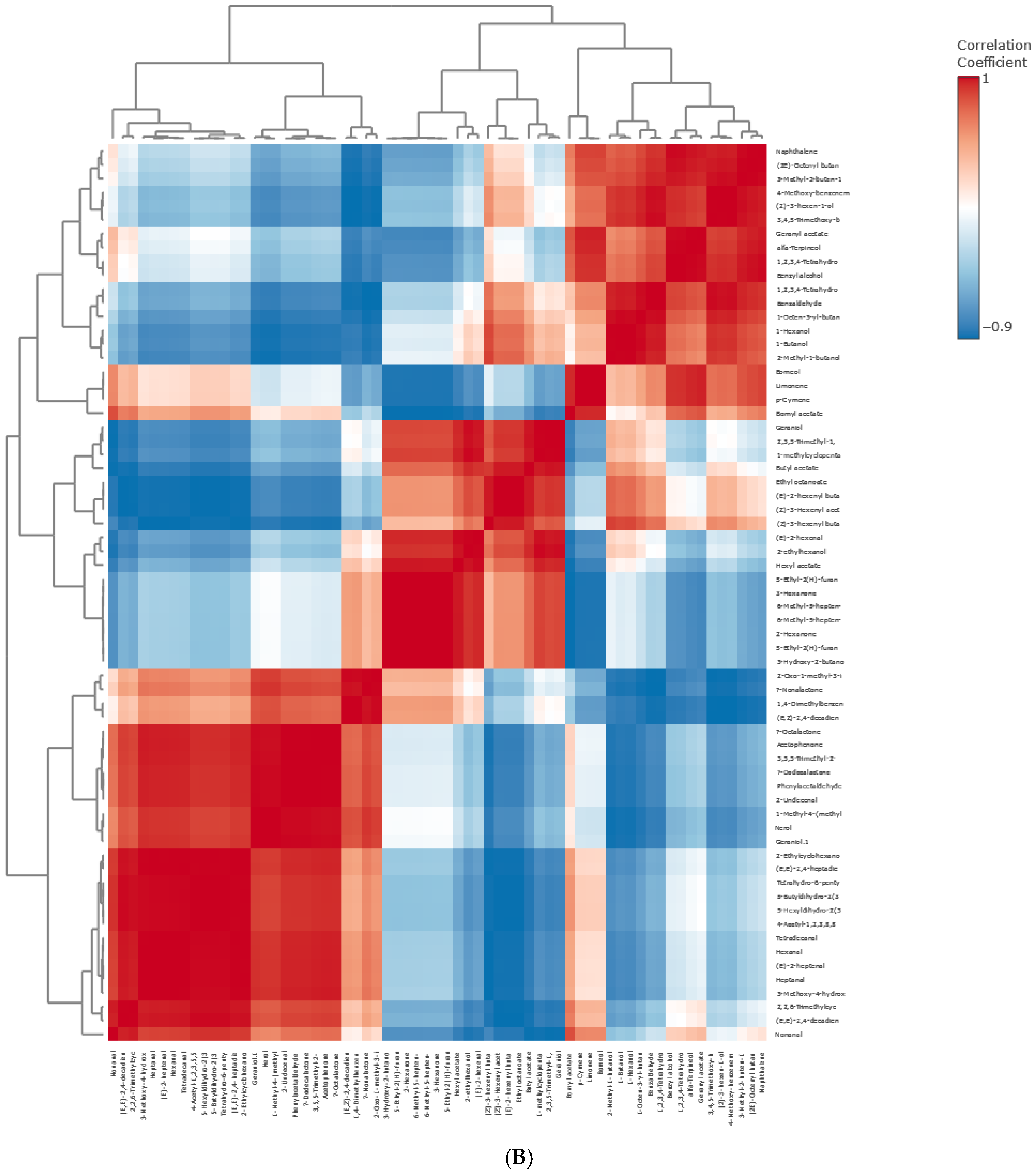
Principal Component Hierarchical Cluster and Correlation Analyses of HS-SPME-GC/MS Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruiz Rodríguez, L.G.; Zamora Gasga, V.M.; Pescuma, M.; Van Nieuwenhove, C.; Mozzi, F.; Sánchez Burgos, J.A. Fruits and Fruit By-Products as Sources of Bioactive Compounds. Benefits and Trends of Lactic Acid Fermentation in the Development of Novel Fruit-Based Functional Beverages. Food Res. Int. 2021, 140, 109854. [Google Scholar] [CrossRef]
- Fratianni, F.; Cozzolino, R.; d’Acierno, A.; Ombra, M.N.; Spigno, P.; Riccardi, R.; Malorni, L.; Stocchero, M.; Nazzaro, F. Biochemical Characterization of Some Varieties of Apricot Present in the Vesuvius Area, Southern Italy. Front. Nutr. 2022, 9, 854868. [Google Scholar] [CrossRef]
- Sarıdaş, M.A.; Ağçam, E.; Ünal, N.; Akyıldız, A.; Kargı, S.P. Comprehensive Quality Analyses of Important Apricot Varieties Produced in Türkiye. J. Food Compos. Anal. 2024, 125, 105791. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, S.; Lin, X.; Peng, J.; Luo, D.; Wan, X.; Zhang, Y.; Dong, X.; Ma, Y. Analysis of Volatile Compounds in Different Varieties of Plum Fruits Based on Headspace Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry Technique. Horticulturae 2023, 9, 1069. [Google Scholar] [CrossRef]
- Zezulová, E.; Ondrášek, I.; Kiss, T.; Nečas, T. Qualitative and Nutritional Characteristics of Plum Cultivars Grown on Different Rootstocks. Horticulturae 2022, 8, 1123. [Google Scholar] [CrossRef]
- Bae, H.; Yun, S.K.; Jun, J.H.; Yoon, I.K.; Nam, E.Y.; Kwon, J.H. Assessment of Organic Acid and Sugar Composition in Apricot, Plumcot, Plum, and Peach during Fruit Development. J. Appl. Bot. Food Qual. 2014, 87, 24–29. [Google Scholar] [CrossRef]
- Lin, Z.; Li, B.; Liao, M.; Liu, J.; Zhou, Y.; Liang, Y.; Yuan, H.; Li, K.; Li, H. The Physicochemical Attributes, Volatile Compounds, and Antioxidant Activities of Five Plum Cultivars in Sichuan. Foods 2023, 12, 3801. [Google Scholar] [CrossRef]
- González-Cebrino, F.; García-Parra, J.; Ramírez, R. Aroma Profile of a Red Plum Purée Processed by High Hydrostatic Pressure and Analysed by SPME–GC/MS. Innov. Food Sci. Emerg. Technol. 2016, 33, 108–114. [Google Scholar] [CrossRef]
- Gong, D.; Bi, Y.; Zong, Y.; Li, Y.; Sionov, E.; Prusky, D. Characterization and Sources of Volatile Organic Compounds Produced by Postharvest Pathogenic Fungi Colonized Fruit. Postharvest Biol. Technol. 2022, 188, 111903. [Google Scholar] [CrossRef]
- El Hadi, M.A.M.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in Fruit Aroma Volatile Research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Li, Y.; He, L.; Song, Y.; Zhang, P.; Chen, D.; Guan, L.; Liu, S. Comprehensive Study of Volatile Compounds and Transcriptome Data Providing Genes for Grape Aroma. BMC Plant Biol. 2023, 23, 171. [Google Scholar] [CrossRef]
- Lancioni, C.; Castells, C.; Candal, R.; Tascon, M. Headspace Solid-Phase Microextraction: Fundamentals and Recent Advances. Adv. Sample Prep. 2022, 3, 100035. [Google Scholar] [CrossRef]
- Razo-Belman, R.; Ozuna, C. Volatile Organic Compounds: A Review of Their Current Applications as Pest Biocontrol and Disease Management. Horticulturae 2023, 9, 441. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Manríquez, D.; Luengwilai, K.; González-Agüero, M. Chapter 1 Aroma Volatiles: Biosynthesis and Mechanisms of Modulation during Fruit Ripening. Adv. Bot. Res. 2009, 50, 1–37. [Google Scholar] [CrossRef]
- Mihaylova, D.; Popova, A.; Vrancheva, R.; Dincheva, I. HS-SPME-GC–MS Volatile Profile Characterization of Peach (Prunus persica L. Batsch) Varieties Grown in the Eastern Balkan Peninsula. Plants 2022, 11, 166. [Google Scholar] [CrossRef]
- Assaad, H.I.; Zhou, L.; Carroll, R.J.; Wu, G. Rapid Publication-Ready MS-Word Tables for One-Way ANOVA. SpringerPlus 2014, 3, 1–8. [Google Scholar] [CrossRef]
- Holeček, M. Aspartic Acid in Health and Disease. Nutrients 2023, 15, 4023. [Google Scholar] [CrossRef]
- Young Park, S.; Kim, J.; Il Son, J.; Youl Rhee, S.; Kim, D.-Y.; Chon, S.; Lim, H.; Woo, J.-T. Dietary Glutamic Acid and Aspartic Acid as Biomarkers for Predicting Diabetic Retinopathy. Sci. Rep. 2021, 11, 7244. [Google Scholar] [CrossRef]
- Famiani, F.; Battistelli, A.; Moscatello, S.; Cruz-Castillo, J.G.; Walker, R.P. The Organic Acids That Are Accumulated in the Flesh of Fruits: Occurrence, Metabolism and Factors Affecting Their Contents—A Review. Rev. Chapingo Ser. Hortic. 2015, 21, 97–128. [Google Scholar] [CrossRef]
- Famiani, F.; Cultrera, N.G.M.; Battistelli, A.; Casulli, V.; Proietti, P.; Standardi, A.; Chen, Z.H.; Leegood, R.C.; Walker, R.P. Phosphoenolpyruvate Carboxykinase and Its Potential Role in the Catabolism of Organic Acids in the Flesh of Soft Fruit during Ripening. J. Exp. Bot. 2005, 56, 2959–2969. [Google Scholar] [CrossRef]
- Li, Y.; He, Z.; Zou, P.; Ning, Y.; Zhu, X. Determination of Seventeen Sugars and Sugar Alcohols in Fruit Juice Samples Using Hydrophilic Interaction Liquid Chromatography-Tandem Mass Spectrometry Combining Response Surface Methodology Design. Microchem. J. 2023, 193, 109136. [Google Scholar] [CrossRef]
- Bawazeer, S.; Muhsen Ali, A.; Alhawiti, A.; Khalaf, A.; Gibson, C.; Tusiimire, J.; Watson, D.G. A Method for the Analysis of Sugars in Biological Systems Using Reductive Amination in Combination with Hydrophilic Interaction Chromatography and High Resolution Mass Spectrometry. Talanta 2017, 166, 75–80. [Google Scholar] [CrossRef]
- Liu, X.; Ji, D.; Cui, X.; Zhang, Z.; Li, B.; Xu, Y.; Chen, T.; Tian, S. P-Coumaric Acid Induces Antioxidant Capacity and Defense Responses of Sweet Cherry Fruit to Fungal Pathogens. Postharvest Biol. Technol. 2020, 169, 111297. [Google Scholar] [CrossRef]
- Kondrashev, S.; Nesterova, N.; Luzin, A.; Kochanov, V.; Luzina, A.; Matyushin, A. Qualitative and Quantitative Assay of Hydroxycinnamates of Prunus spinosa L. Pharmacogn. J. 2020, 12, 157–161. [Google Scholar] [CrossRef]
- Rey-Serra, P.; Mnejja, M.; Monfort, A. Inheritance of Esters and Other Volatile Compounds Responsible for the Fruity Aroma in Strawberry. Front. Plant Sci. 2022, 13, 959155. [Google Scholar] [CrossRef]
- Bajramova, A.; Spégel, P. A Comparative Study of the Fatty Acid Profile of Common Fruits and Fruits Claimed to Confer Health Benefits. J. Food Compos. Anal. 2022, 112, 104657. [Google Scholar] [CrossRef]
- Chanioti, S.; Katsouli, M.; Tzia, C. β-Sitosterol as a Functional Bioactive. In A Centum of Valuable Plant Bioactives; Academic Press: Cambridge, MA, USA, 2021; pp. 193–212. [Google Scholar] [CrossRef]
- Choi, J.M.; Lee, E.O.; Lee, H.J.; Kim, K.H.; Ahn, K.S.; Shim, B.S.; Kim, N.I.; Song, M.C.; Baek, N.I.; Kim, S.H. Identification of Campesterol from Chrysanthemum coronarium L. and Its Antiangiogenic Activities. Phytother. Res. 2007, 21, 954–959. [Google Scholar] [CrossRef]
- Gómez García-Carpintero, E.; Gómez Gallego, M.A.; Sánchez-Palomo, E.; González Viñas, M.A. Impact of Alternative Technique to Ageing Using Oak Chips in Alcoholic or in Malolactic Fermentation on Volatile and Sensory Composition of Red Wines. Food Chem. 2012, 134, 851–863. [Google Scholar] [CrossRef]
- Plotto, A.; Bai, J.; Baldwin, E. Fruits. In Springer Handbook of Odor; Springer: Berlin/Heidelberg, Germany, 2017; pp. 27–28. [Google Scholar] [CrossRef]
- Güler, Z.; Karaca, F.; Yetisir, H. Volatile Compounds and Sensory Properties in Various Melons, Which Were Chosen from Different Species and Different Locations, Grown in Turkey. Int. J. Food Prop. 2013, 16, 168–179. [Google Scholar] [CrossRef]
- Manríquez, D.; El-Sharkawy, I.; Flores, F.B.; El-Yahyaoui, F.; Regad, F.; Bouzayen, M.; Latché, A.; Pech, J.C. Two Highly Divergent Alcohol Dehydrogenases of Melon Exhibit Fruit Ripening-Specific Expression and Distinct Biochemical Characteristics. Plant Mol. Biol. 2006, 61, 675–685. [Google Scholar] [CrossRef]
- Chambers IV, E.; Koppel, K. Associations of Volatile Compounds with Sensory Aroma and Flavor: The Complex Nature of Flavor. Molecules 2013, 18, 4887–4905. [Google Scholar] [CrossRef]
- Pino, J.A.; Quijano, C.E. Study of the Volatile Compounds from Plum (Prunus domestica L. Cv. Horvin) and Estimation of Their Contribution to the Fruit Aroma. Ciênc. Tecnol. Aliment. 2012, 32, 76–83. [Google Scholar] [CrossRef]
- Guo, J.; Yue, T.; Yuan, Y.; Sun, N.; Liu, P. Characterization of Volatile and Sensory Profiles of Apple Juices to Trace Fruit Origins and Investigation of the Relationship between the Aroma Properties and Volatile Constituents. LWT 2020, 124, 109203. [Google Scholar] [CrossRef]
- Zhang, W.; Lao, F.; Bi, S.; Pan, X.; Pang, X.; Hu, X.; Liao, X.; Wu, J. Insights into the Major Aroma-Active Compounds in Clear Red Raspberry Juice (Rubus idaeus L. Cv. Heritage) by Molecular Sensory Science Approaches. Food Chem. 2021, 336, 127721. [Google Scholar] [CrossRef] [PubMed]
- İmrak, B.; Küden, A.B.; Tanriver, E.; Kafkas, E. Volatile and Some Fruit Quality Characteristics of New Promising Peach Genotypes. Acta Sci. Pol. Hortorum Cultus 2015, 14, 3–12. [Google Scholar]
- De Sousa Galvao, M.; Nunes, M.L.; Constant, P.B.L.; Narain, N. Identification of Volatile Compounds in Cultivars Barker, Collinson, Fortuna and Geada of Avocado (Persea americana, Mill.) Fruit. Food Sci. Technol. 2016, 36, 439–447. [Google Scholar] [CrossRef]
- Popova, A.; Mihaylova, D.; Pandova, S.; Doykina, P. Research-Gap-Spotting in Plum–Apricot Hybrids—Bioactive Compounds, Antioxidant Activities, and Health Beneficial Properties. Horticulturae 2023, 9, 584. [Google Scholar] [CrossRef]
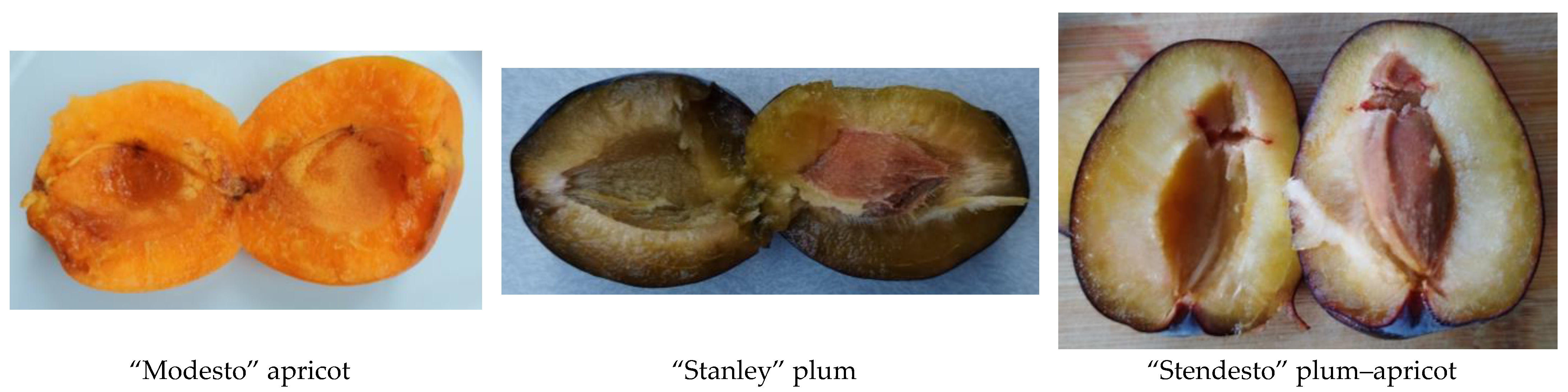
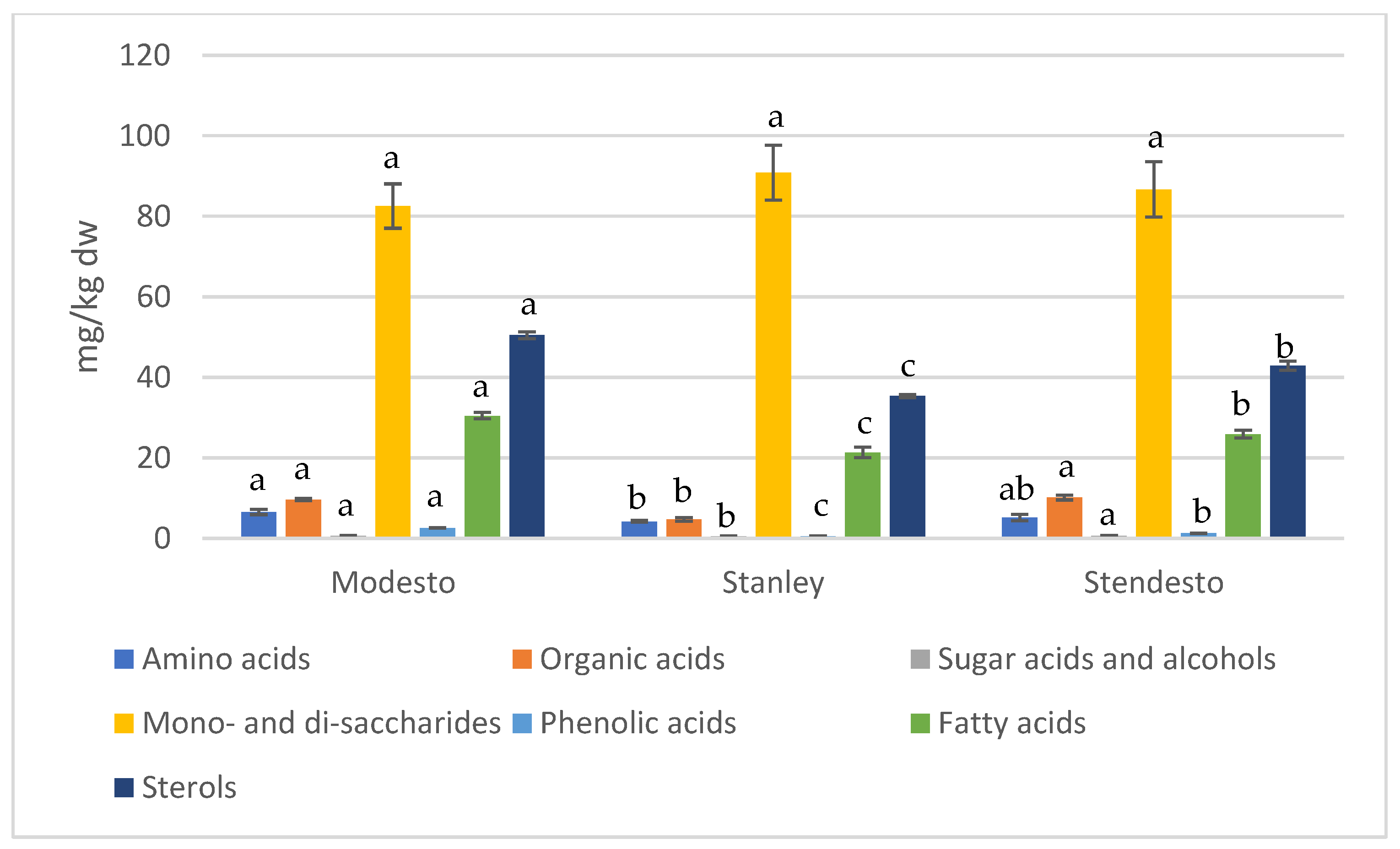
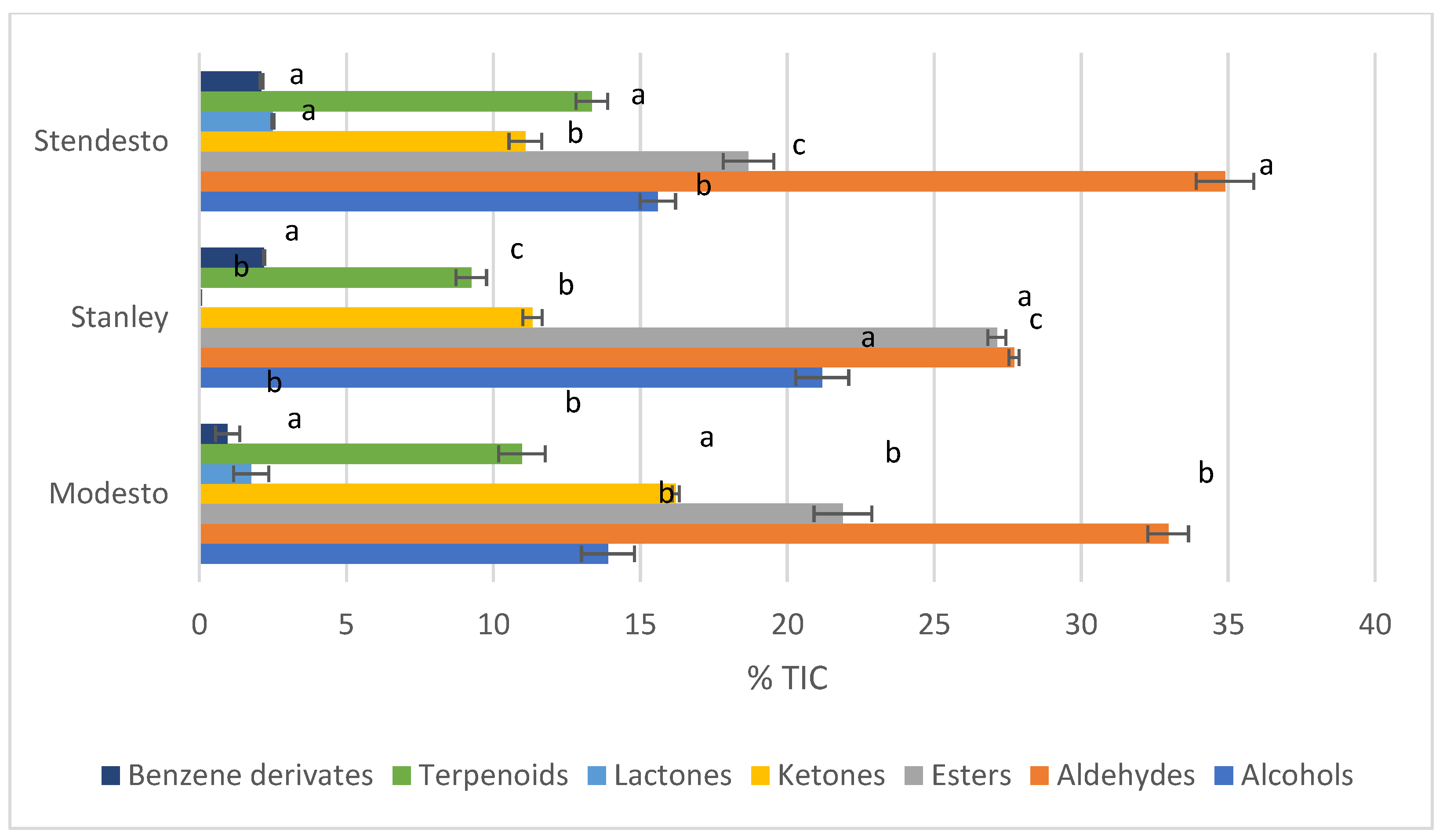
| RT | RI | Class/Name | Modesto | Stanley | Stendesto |
|---|---|---|---|---|---|
| Amino acids | |||||
| 4.28 | 1106 | Alanine | nd | 0.13 ± 0.03 | 0.15 ± 0.03 |
| 5.22 | 1234 | Valine | 0.23 ± 0.05 a | 0.15 ± 0.03 a | 0.18 ± 0.04 a |
| 5.74 | 1261 | Leucine | 0.29 ± 0.06 a | 0.20 ± 0.04 b | 0.22 ± 0.04 b |
| 6.00 | 1298 | Isoleucine | 0.25 ± 0.05 a | 0.17 ± 0.03 ab | 0.19 ± 0.04 b |
| 6.08 | 1303 | Proline | 0.36 ± 0.07 a | 0.24 ± 0.05 b | 0.27 ± 0.05 a |
| 6.62 | 1340 | Serine | 0.40 ± 0.08 a | 0.27 ± 0.05 a | 0.31 ± 0.06 a |
| 6.88 | 1361 | Threonine | 0.46 ± 0.09 | nd | 0.35 ± 0.07 |
| 8.04 | 1502 | Aspartic acid | 3.04 ± 0.61 a | 2.03 ± 0.41 a | 2.33 ± 0.47 a |
| 8.22 | 1517 | Pyroglutamic acid | 0.27 ± 0.05 a | 0.18 ± 0.04 a | 0.21 ± 0.04 a |
| 9.36 | 1616 | Glutamic acid | 0.32 ± 0.06 a | 0.21 ± 0.04 a | 0.25 ± 0.05 a |
| 9.44 | 1625 | Phenylalanine | 0.40 ± 0.08 a | 0.27 ± 0.05 a | 0.31 ± 0.06 a |
| 11.14 | 1775 | Glutamine | 0.54 ± 0.11 a | 0.36 ± 0.07 a | 0.41 ± 0.08 a |
| Organic acids | |||||
| 6.24 | 1314 | Succinic acid | 0.32 ± 0.03 a | 0.13 ± 0.01 c | 0.20 ± 0.02 b |
| 6.56 | 1330 | Fumaric acid | 0.80 ± 0.08 b | 0.37 ± 0.04 c | 1.65 ± 0.16 a |
| 7.83 | 1475 | Mallic acid | 2.53 ± 0.25 b | 1.51 ± 0.15 c | 4.25 ± 0.42 a |
| 8.33 | 1530 | γ-Aminobutyric acid | 0.10 ± 0.01 | nd | nd |
| 11.15 | 1823 | Quinicc acid | 0.22 ± 0.02 a | 0.26 ± 0.03 a | 0.20 ± 0.02 a |
| 12.00 | 1870 | Citric acid | 5.65 ± 0.56 a | 2.46 ± 0.25 c | 3.85 ± 0.38 b |
| Sugar acids and alcohols | |||||
| 5.78 | 1264 | Glycerol | 0.37 ± 0.04 a | 0.21 ± 0.02 b | 0.19 ± 0.02 b |
| 8.52 | 1541 | Eritrreonic acid | 0.14 ± 0.01 a | 0.08 ± 0.01 c | 0.11 ± 0.01 b |
| 10.36 | 1695 | Xylitol | nd | 0.24 ± 0.02 | 0.27 ± 0.03 |
| 10.43 | 1718 | Arabitol | 0.12 ± 0.01 | nd | 0.13 ± 0.01 |
| Mono- and di-saccharides | |||||
| 11.76 | 1856 | Fructose isomer | 3.59 ± 0.36 b | 4.79 ± 0.48 a | 3.86 ± 0.39 ab |
| 11.87 | 1868 | Fructose isomer | 5.20 ± 0.52 a | 6.45 ± 0.64 a | 5.98 ± 0.60 a |
| 12.34 | 1877 | Galactose isomer | nd | 2.14 ± 0.21 | 1.63 ± 0.16 |
| 12.37 | 1879 | Glucose isomer | 1.18 ± 0.12 b | 13.81 ± 1.38 a | 2.38 ± 0.24 b |
| 13.15 | 1898 | Galactose isomer | nd | 1.80 ± 0.18 | 4.30 ± 0.43 |
| 13.21 | 1903 | Glucose isomer | 8.85 ± 0.89 b | 18.70 ± 1.87 a | 20.15 ± 2.02 a |
| 14.50 | 1960 | Glucose 1-phosphate | 10.70 ± 1.07 a | 13.58 ± 1.36 a | 12.27 ± 1.23 a |
| 23.28 | 2620 | Sucrose isomer | 24.83 ± 2.48 a | 11.59 ± 1.16 b | 14.79 ± 1.48 b |
| 24.29 | 2620 | Sucrose isomer | 28.20 ± 2.82 a | 18.00 ± 1.80 b | 21.33 ± 2.13 b |
| Phenolic acids | |||||
| 12.52 | 1835 | Protocatechuic acid | 0.42 ± 0.04 | nd | 0.29 ± 0.03 |
| 13.77 | 1940 | trans-p-Coumaric acid | 2.21 ± 0.22 a | 0.40 ± 0.04 c | 0.83 ± 0.08 b |
| 16.36 | 2106 | trans-Ferulic acid | nd | 0.14 ± 0.01 | 0.16 ± 0.02 |
| Fatty acids | |||||
| 29.16 | 1920 | Palmitic acid | 10.33 ± 1.55 a | 7.23 ± 1.08 a | 8.78 ± 1.32 a |
| 32.11 | 2094 | Linoleic acid | 4.59 ± 0.69 a | 3.21 ± 0.48 a | 3.90 ± 0.59 a |
| 32.22 | 2101 | Oleic acid | 4.17 ± 0.63 a | 2.92 ± 0.44 a | 3.54 ± 0.53 a |
| 32.28 | 2106 | Linolenic acid | 1.61 ± 0.24 a | 1.12 ± 0.17 a | 1.36 ± 0.20 a |
| 32.71 | 2128 | Stearic acid | 9.78 ± 1.47 a | 6.85 ± 1.03 a | 8.31 ± 1.25 a |
| Sterols | |||||
| 37.22 | 3197 | Campesterol | 14.20 ± 2.13 a | 9.94 ± 1.49 a | 12.07 ± 1.81 a |
| 38.19 | 3297 | β-Sitosterol | 36.28 ± 5.44 a | 25.40 ± 3.81 a | 30.84 ± 4.63 a |
| Name/Class | RI | Modesto | Stanley | Stendesto |
|---|---|---|---|---|
| Alcohols | ||||
| 1-Butanol | 655 | 2.03 ± 0.30 b | 3.63 ± 0.39 a | 2.37 ± 0.36 b |
| 3-Methyl-2-buten-1-ol | 719 | 0.80 ± 0.12 b | 1.95 ± 0.14 a | 0.86 ± 0.13 b |
| 2-Methyl-1-butanol | 724 | 1.22 ± 0.18 b | 2.59 ± 0.24 a | 1.43 ± 0.21 b |
| 1-methylcyclopentanol | 796 | 0.37 ± 0.06 a | 0.48 ± 0.07 a | 0.20 ± 0.03 b |
| (2)-3-hexen-1-ol | 849 | 0.56 ± 0.08 a | 0.73 ± 0.11 a | 0.66 ± 0.10 a |
| 1-Hexanol | 852 | 0.72 ± 0.11 b | 1.34 ± 0.14 a | 0.84 ± 0.13 b |
| 2-ethylhexanol | 1028 | 0.81 ± 0.12 a | 0.75 ± 0.11 ab | 0.50 ± 0.07 b |
| Benzyl alcohol | 1035 | 3.20 ± 0.48 a | 4.16 ± 0.62 a | 3.75 ± 0.56 a |
| 3.5,5-Trimethyl-2-cyclohexen-1-ol | 1147 | 0.69 ± 0.10 a | 0.90 ± 0.13 a | 0.81 ± 0.12 a |
| 2.3,5-Trimethyl-1.4-benzenediol | 1210 | 0.95 ± 0.14 a | 1.35 ± 0.20 a | 1.20 ± 0.18 a |
| 4-Methoxy-benzenemethanol | 1249 | 1.13 ± 0.17 a | 1.46 ± 0.22 a | 1.32 ± 0.20 a |
| 3.4,5-Trimethoxy-benzenemethanol | 1426 | 1.42 ± 0.21 a | 1.85 ± 0.28 a | 1.66 ± 0.25 a |
| Aldehydes | ||||
| Hexanal | 802 | 2.15 ± 0.32 a | 1.72 ± 0.26 a | 2.41 ± 0.36 a |
| (E)-2-hexenal | 848 | 3.22 ± 0.48 a | 2.57 ± 0.39 a | 3.60 ± 0.54 a |
| Heptanal | 898 | 1.68 ± 0.25 a | 1.34 ± 0.20 a | 1.88 ± 0.28 a |
| Benzaldehyde | 939 | 6.77 ± 0.99 a | 8.05 ± 0.79 a | 7.36 ± 1.10 a |
| (E)-2-heptenal | 953 | 1.18 ± 0.18 a | 0.94 ± 0.14 a | 1.32 ± 0.20 a |
| (E,E)-2.4-heptadienal | 998 | 0.83 ± 0.13 a | 0.67 ± 0.10 a | 0.94 ± 0.14 a |
| Phenylacetaldehyde | 1045 | 1.76 ± 0.26 a | 1.40 ± 0.21 a | 1.97 ± 0.29 a |
| Nonanal | 1105 | 2.04 ± 0.35 a | 1.89 ± 0.28 a | 2.64 ± 0.40 a |
| 2-Undecenal | 1159 | 1.38 ± 0.21 a | 1.10 ± 0.17 a | 1.54 ± 0.23 a |
| Tetradecanal | 1207 | 0.39 ± 0.06 a | 0.31 ± 0.05 a | 0.44 ± 0.07 a |
| (E,E)-2.4-decadienal | 1295 | 6.04 ± 0.96 a | 5.14 ± 0.77 a | 7.19 ± 1.08 a |
| (E,Z)-2.4-decadienal | 1319 | 4.88 ± 0.69 a | 2.07 ± 0.31 b | 2.89 ± 0.43 b |
| 3-Methoxy-4-hydroxybenzaldehyde | 1355 | 0.64 ± 0.10 a | 0.51 ± 0.08 a | 0.71 ± 0.11 a |
| Esters | ||||
| Butyl acetate | 813 | 1.41 ± 0.21 b | 2.83 ± 0.28 a | 3.10 ± 0.17 a |
| (Z)-3-Hexenyl acetate | 1005 | 1.13 ± 0.17 ab | 1.47 ± 0.22 a | 0.88 ± 0.13 b |
| Hexyl acetate | 1010 | 6.02 ± 0.90 a | 5.22 ± 0.78 a | 3.13 ± 0.47 b |
| (Z)-3-hexenyl butanoate | 1183 | 2.04 ± 0.31 b | 2.94 ± 0.44 a | 1.76 ± 0.26 b |
| (E)-2-hexenyl butanoate | 1191 | 3.51 ± 0.53 ab | 4.57 ± 0.69 a | 2.74 ± 0.41 b |
| Ethyl octanoate | 1196 | 1.83 ± 0.28 ab | 2.38 ± 0.36 a | 1.43 ± 0.21 b |
| 1-Octen-3-yl-butanoate | 1280 | 3.15 ± 0.47 a | 4.09 ± 0.61 a | 3.46 ± 0.37 a |
| (2 E)-Octenyl butanoate | 1388 | 2.80 ± 0.42 ab | 3.63 ± 0.55 a | 2.18 ± 0.33 b |
| Ketones | ||||
| 3-Hydroxy-2-butanone | 680 | 2.64 ± 0.40 a | 1.85 ± 0.28 b | 1.11 ± 0.17 b |
| 3-Hexanone | 784 | 1.38 ± 0.21 a | 0.96 ± 0.14 b | 0.58 ± 0.09 b |
| 2-Hexanone | 789 | 2.36 ± 0.35 a | 1.65 ± 0.25 b | 0.99 ± 0.15 c |
| 5-Ethyl-2(H)-furanone | 954 | 1.35 ± 0.20 a | 0.94 ± 0.14 b | 0.57 ± 0.08 b |
| 6-Methyl-5-hepten-2-one | 984 | 1.12 ± 0.17 a | 0.78 ± 0.12 b | 0.47 ± 0.07 b |
| 5-Ethyl-2(H)-furanone | 954 | 1.03 ± 0.15 a | 0.72 ± 0.11 b | 0.43 ± 0.06 c |
| 6-Methyl-5-hepten-2-one | 984 | 1.44 ± 0.22 a | 1.01 ± 0.15 b | 0.61 ± 0.09 b |
| 2.2,6-Trimethylcyclohexanone | 1036 | 0.21 ± 0.03 b | 0.15 ± 0.02 b | 0.32 ± 0.05 a |
| Acetophenone | 1065 | 1.86 ± 0.28 ab | 1.30 ± 0.20 b | 2.38 ± 0.36 a |
| 2-Ethylcyclohexanone | 1158 | 0.67 ± 0.10 ab | 0.47 ± 0.07 b | 0.83 ± 0.12 a |
| 4-Acetyl-1.2,3.5,5-pentamethyl-2-cyclopenten-l-one | 1216 | 0.37 ± 0.06 ab | 0.26 ± 0.04 b | 0.48 ± 0.07 a |
| 5-Butyldihydro-2(3 H)-furanone | 1264 | 0.19 ± 0.03 ab | 0.13 ± 0.02 b | 0.25 ± 0.04 a |
| Tetrahydro-6-pentyl-2 H-pyran-2-one | 1435 | 0.42 ± 0.06 ab | 0.29 ± 0.04 b | 0.55 ± 0.08 a |
| 5-Hexyldihydro-2(3 H)-furanone | 1461 | 1.17 ± 0.18 ab | 0.82 ± 0.12 b | 1.52 ± 0.23 a |
| Lactones | ||||
| γ-Octalactone | 1255 | 0.59 ± 0.09 | nd | 0.84 ± 0.13 |
| γ-Nonalactone | 1362 | 0.38 ± 0.06 | nd | 0.70 ± 0.10 |
| γ-Dodecalactone | 1413 | 0.79 ± 0.12 | nd | 0.96 ± 0.14 |
| Terpenoids | ||||
| p-Cymene | 1026 | 1.12 ± 0.17 a | 1.45 ± 0.22 a | 1.68 ± 0.25 a |
| Limonene | 1031 | 1.49 ± 0.22 b | 1.93 ± 0.29 ab | 2.23 ± 0.33 a |
| alfa-Terpineol | 1199 | 0.90 ± 0.14 b | 1.18 ± 0.18 ab | 1.36 ± 0.20 a |
| Geraniol | 1221 | 0.87 ± 0.13 a | 1.13 ± 0.17 a | 1.30 ± 0.20 a |
| Bomeol | 1234 | 1.05 ± 0.16 a | 1.36 ± 0.20 a | 1.57 ± 0.24 a |
| Nerol | 1251 | 1.77 ± 0.27 a | 0.30 ± 0.04 b | 1.25 ± 0.40 a |
| Hexyl acetate | 1268 | 2.92 ± 0.44 a | 0.80 ± 0.57 b | 2.39 ± 0.66 a |
| Bomyl acetate | 1287 | 0.35 ± 0.05 b | 0.45 ± 0.07 b | 0.72 ± 0.11 a |
| Geranyl acetate | 1377 | 0.50 ± 0.08 b | 0.65 ± 0.10 ab | 0.85 ± 0.13 a |
| Benzene derivatives | ||||
| 1.4-Dimethylbenzene | 865 | 0.41 ± 0.06 a | 0.29 ± 0.04 a | 0.33 ± 0.05 a |
| Naphthalene | 1186 | nd | 0.51 ± 0.08 | 0.28 ± 0.09 |
| 2-Oxo-1-methyl-3-isopropylpyrazine | 1225 | 0.23 ± 0.03 a | 0.16 ± 0.02 a | 0.19 ± 0.03 a |
| 1.2,3.4-Tetrahydro-1.5,7-trimethylnaphthalene | 1310 | nd | 0.42 ± 0.06 | 0.38 ± 0.07 |
| 1-Methyl-4-(methylthio) benzene | 1316 | 0.29 ± 0.04 a | 0.20 ± 0.03 b | 0.23 ± 0.03 ab |
| 1.2,3.4-Tetrahydro-1.1,6-trimethylnaphthalene | 1349 | nd | 0.62 ± 0.09 | 0.70 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lante, A.; Mihaylova, D.; Dincheva, I.; Popova, A. HS-SPME-GC/MS Metabolomic Analysis for the Comparative Evaluation between a Plum–Apricot Hybrid and Its Parents. Chemosensors 2024, 12, 50. https://doi.org/10.3390/chemosensors12040050
Lante A, Mihaylova D, Dincheva I, Popova A. HS-SPME-GC/MS Metabolomic Analysis for the Comparative Evaluation between a Plum–Apricot Hybrid and Its Parents. Chemosensors. 2024; 12(4):50. https://doi.org/10.3390/chemosensors12040050
Chicago/Turabian StyleLante, Anna, Dasha Mihaylova, Ivayla Dincheva, and Aneta Popova. 2024. "HS-SPME-GC/MS Metabolomic Analysis for the Comparative Evaluation between a Plum–Apricot Hybrid and Its Parents" Chemosensors 12, no. 4: 50. https://doi.org/10.3390/chemosensors12040050
APA StyleLante, A., Mihaylova, D., Dincheva, I., & Popova, A. (2024). HS-SPME-GC/MS Metabolomic Analysis for the Comparative Evaluation between a Plum–Apricot Hybrid and Its Parents. Chemosensors, 12(4), 50. https://doi.org/10.3390/chemosensors12040050






