High-Performance MEMS Oxygen Sensors Based on Au/TiO2 Films
Abstract
:1. Introduction
2. Materials and Methods
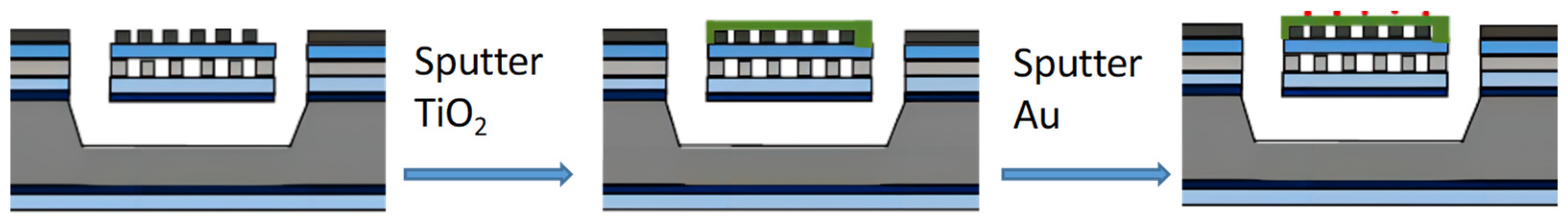
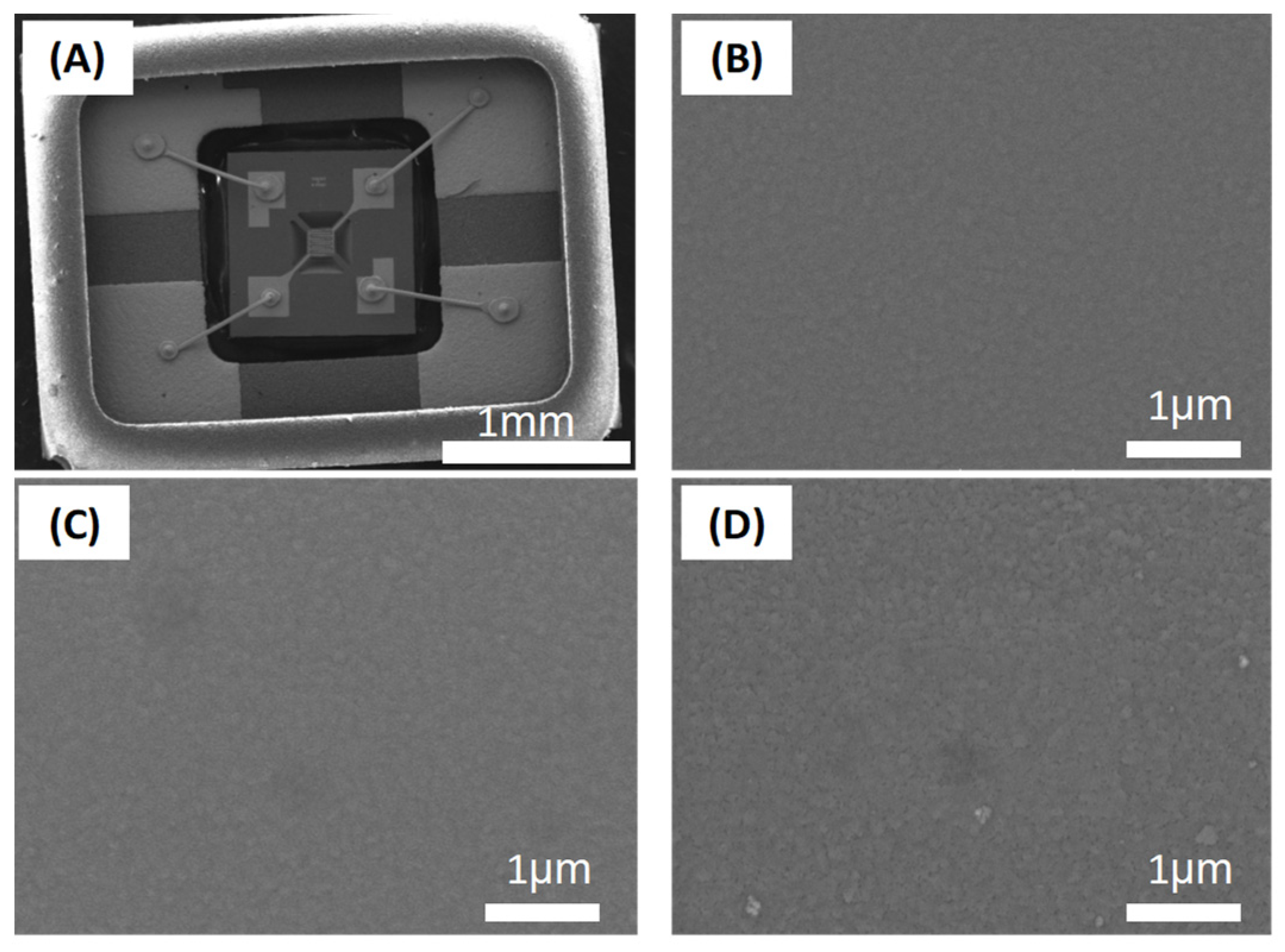
2.1. Film Deposition and Characterization
2.2. Device Measurement
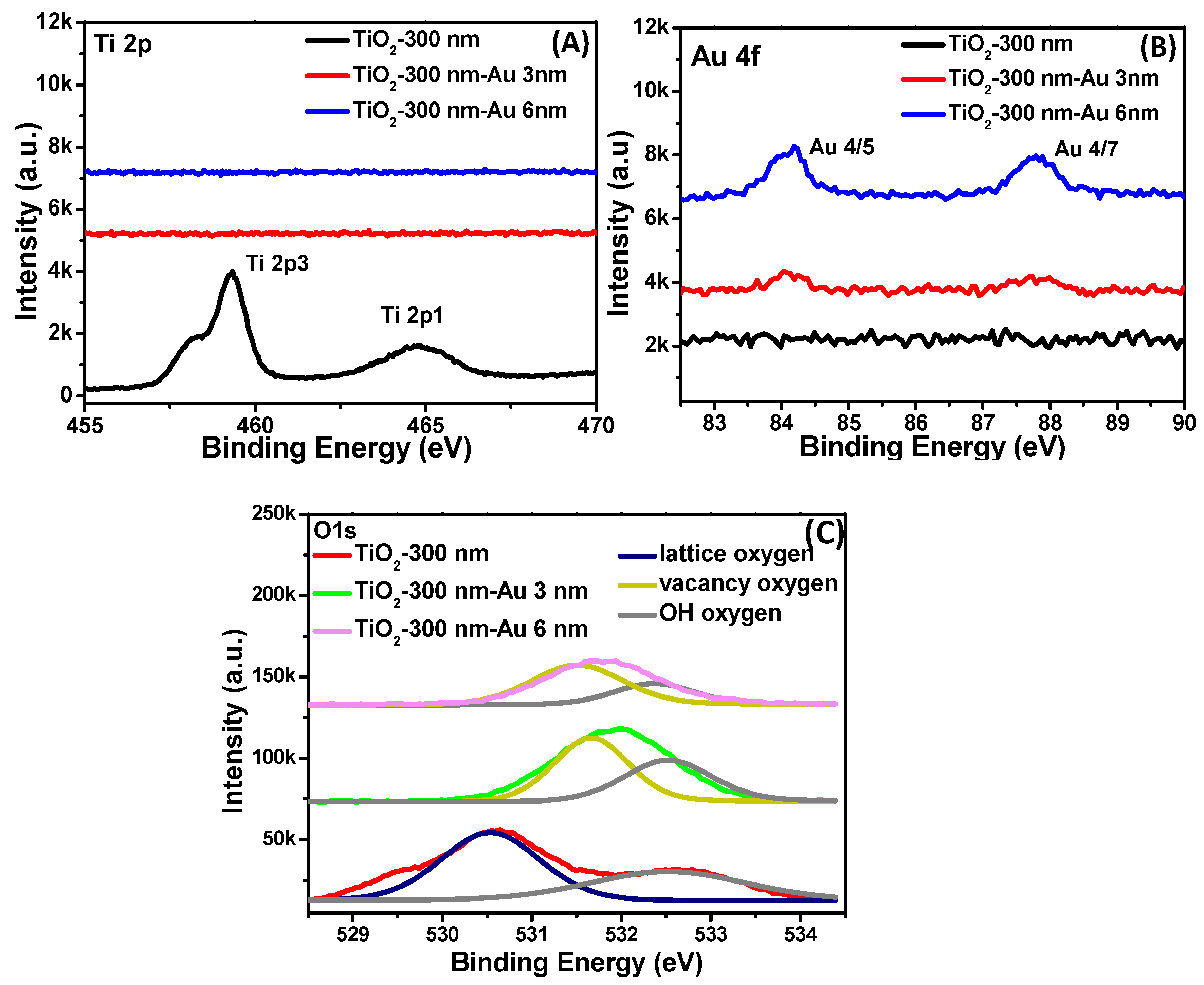
3. Results
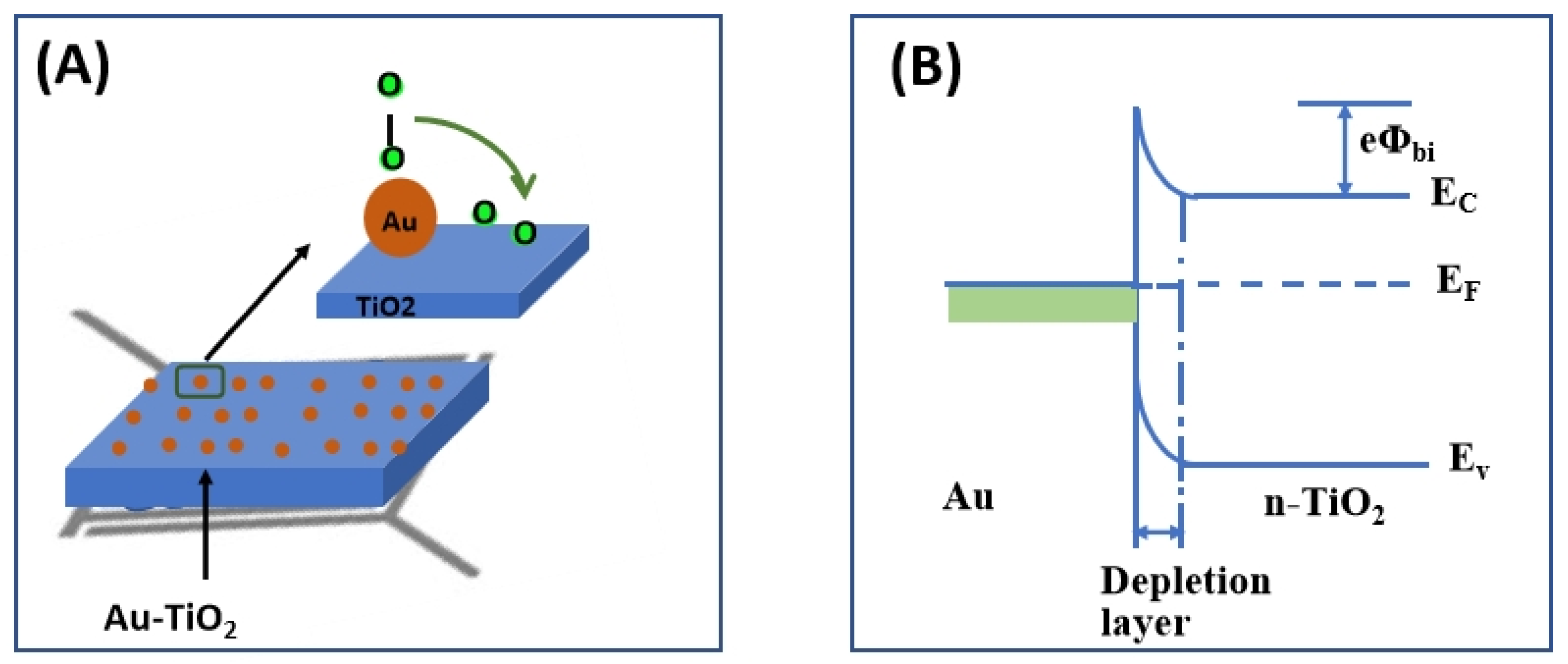
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiao, M.; Van Duy, N.; Chien, N.V.; Hoa, N.D.; Van Hieu, N.; Hjort, K.; Nguyen, H. On-Chip Growth of Patterned ZnO Nanorod Sensors with PdO Decoration for Enhancement of Hydrogen-Sensing Performance. Int. J. Hydrogen Energy 2017, 42, 16294–16304. [Google Scholar] [CrossRef]
- Jiao, M.; Chen, X.Y.; Hu, K.X.; Qian, D.Y.; Zhao, X.H.; Ding, E.J. Recent Developments of Nanomaterials-Based Conductive Type Methane Sensors. Rare Met. 2021, 40, 1515–1527. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Dutta, P.K.; Akbar, S.A. Oxygen Sensors: Materials, Methods, Designs and Applications. J. Mater. Sci. 2003, 38, 4271–4282. [Google Scholar] [CrossRef]
- Phan, T.T.; Tosa, T.; Majima, Y. 20-nm-Nanogap Oxygen Gas Sensor with Solution-Processed Cerium Oxide. Sens. Actuators B Chem. 2021, 343, 130098. [Google Scholar] [CrossRef]
- Ramshanker, N.; Ganapathi, K.L.; Bhat, M.S.; Mohan, S. RF Sputtered CeO2 Thin Films-Based Oxygen Sensors. IEEE Sens. J. 2019, 19, 10821–10828. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Simonenko, E.P.; Simonenko, N.P.; Bukunov, K.A.; Sevastyanov, V.G.; Kuznetsov, N.T. Gas-Sensing Properties of Nanostructured CeO2-XZrO2 Thin Films Obtained by the Sol-Gel Method. J. Alloys Compd. 2019, 773, 1023–1032. [Google Scholar] [CrossRef]
- Yamada, T.; Kubota, Y.; Makinose, Y.; Suzuki, N.; Nakata, K.; Terashima, C.; Matsushita, N.; Okada, K.; Fujishima, A.; Katsumata, K.I. Single Crystal ZrO2 Nanosheets Formed by Thermal Transformation for Solid Oxide Fuel Cells and Oxygen Sensors. ACS Appl. Nano Mater. 2019, 2, 6866–6873. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, M.; Xu, T. TiO2−x Thin Films as Oxygen Sensor. Sens. Actuators B Chem. 2020, 66, 28–30. [Google Scholar] [CrossRef]
- Lu, H.F.; Li, F.; Liu, G.; Chen, Z.G.; Wang, D.W.; Fang, H.T.; Lu, G.Q.; Jiang, Z.H.; Cheng, H.M. Amorphous TiO2 Nanotube Arrays for Low-Temperature Oxygen Sensors. Nanotechnology 2008, 19, 405504. [Google Scholar] [CrossRef]
- Sharma, R.K.; Bhatnagar, M.C.; Sharma, G.L. Influence of Doping on Sensitivity and Response Time of TiO2 Oxygen Gas Sensor. Rev. Sci. Instrum. 2000, 71, 1500–1504. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Simonenko, E.P.; Simonenko, N.P.; Akkuleva, K.T.; Antipov, V.V.; Zaharova, N.V.; Malygin, A.A.; Bukunov, K.A.; Sevastyanov, V.G.; Kuznetsov, N.T. Oxygen Detection Using Nanostructured TiO2 Thin Films Obtained by the Molecular Layering Method. Appl. Surf. Sci. 2019, 463, 197–202. [Google Scholar] [CrossRef]
- Jyothilal, H.; Shukla, G.; Walia, S.; Bharath, S.P.; Angappane, S. UV Assisted Room Temperature Oxygen Sensors Using Titanium Dioxide Nanostructures. Mater. Res. Bull. 2021, 140, 111324. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, X.; Liu, B.; Chen, Y.; Lu, Y.; Wang, F.; Zhang, L. UV-Induced Desorption of Oxygen at the TiO2 Surface for Highly Sensitive Room Temperature O2 Sensing. J. Alloys Compd. 2019, 793, 583–589. [Google Scholar] [CrossRef]
- Raghu, A.V.; Karuppanan, K.K.; Pullithadathil, B. Highly Sensitive, Temperature-Independent Oxygen Gas Sensor Based on Anatase TiO2 Nanoparticle Grafted, 2D Mixed Valent VOx Nanoflakelets. ACS Sens. 2018, 3, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Buono, C.; Desimone, M.; Schipani, F.; Aldao, C.M.; Vignatti, C.I.; Morgade, C.I.N.; Cabeza, G.F.; Garetto, T.F. N-Doping Effects on the Oxygen Sensing of TiO2 Films. J. Electroceramics 2018, 40, 72–77. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Chen, L.; Yao, Y.; Sun, Q.; Qunming, Z. Integrated Microoxygen Sensor Based on Nanostructured TiO2 Thin Films. Micro Nano Lett. 2015, 10, 597–602. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Q.; Yao, Y.; Li, Y.; Wang, J.; Chen, L. A Micro Sensor Based on TiO2 Nanorod Arrays for the Detection of Oxygen at Room Temperature. Ceram. Int. 2016, 42, 8565–8571. [Google Scholar] [CrossRef]
- Liang, L.; Yin, J.; Bao, J.; Cong, L.; Huang, W.; Lin, H.; Shi, Z. Preparation of Au Nanoparticles Modified TiO2 Nanotube Array Sensor and Its Application as Chemical Oxygen Demand Sensor. Chin. Chem. Lett. 2019, 30, 167–170. [Google Scholar] [CrossRef]
- Siemer, N.; Lüken, A.; Zalibera, M.; Frenzel, J.; Muñoz-Santiburcio, D.; Savitsky, A.; Lubitz, W.; Muhler, M.; Marx, D.; Strunk, J. Atomic-Scale Explanation of O2 Activation at the Au-TiO2 Interface. J. Am. Chem. Soc. 2018, 140, 18082–18092. [Google Scholar] [CrossRef]
- Ren, H.; Weng, H.; Huang, J.; Lu, X.; Joo, S.W. Synthesis of Au Nanoparticle-Modified Porous TiO2 Nano-spheres for Detection of Toxic Volatile Organic Vapors. J. Alloys Compd. 2022, 919, 165843. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, L.; Huang, B.; Li, X. Enhanced Sensing Performance of Au-Decorated TiO2 Nanospheres with Hollow Structure for Formaldehyde Detection at Room Temperature. Sens. Actuators B Chem. 2022, 358, 131465. [Google Scholar] [CrossRef]
- Mintcheva, N.; Srinivasan, P.; Rayappan, J.B.B.; Kuchmizhak, A.A.; Gurbatov, S.; Kulinich, S.A. Room-Temperature Gas Sensing of Laser-Modified Anatase TiO2 Decorated with Au Nanoparticles. Appl. Surf. Sci. 2020, 507, 145169. [Google Scholar] [CrossRef]
- Engelkamp, B.; Schierbaum, K. Oxygen Sensing of Pt/Peo-TiO2 in Humid Atmospheres at Moderate Temperatures. Sensors 2021, 21, 2558. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Mokrushin, A.S.; Simonenko, N.P.; Voronov, V.A.; Kim, V.P.; Tkachev, S.V.; Gubin, S.P.; Sevastyanov, V.G.; Kuznetsov, N.T. Ink-Jet Printing of a TiO2–10%ZrO2 Thin Film for Oxygen Detection Using a Solution of Metal Alkoxoacetylacetonates. Thin Solid Films 2019, 670, 46–53. [Google Scholar] [CrossRef]


| Materials | Preparation Method | Working Temperature/°C | Oxygen Concentration (%) | Sensitivity Rg/R0 | Response Time/s | Recovery Time/s | Reference |
|---|---|---|---|---|---|---|---|
| TiO2-VOx | Thermal deposition and hydrothermal | 500 | 0.01 | 1.32 | ~4 | ~8 | [14] |
| TiO2-Pt | Plasma electrolytic oxidation and sputtering | 30 | 10 | 2 | 150 | 420 | [23] |
| TiO2-10%ZrO2 thin film | Inkjet printing | 400 | 5 | 2.2 | 10 | 28 | [24] |
| TiO2 nanotube | Anodic oxidation | 100 | 4 | 160 | 500 | 1000 | [9] |
| TiO2 nanorods | Glancing angle e-beam evaporation | Room temperature under UV | 0.1 | 74 | 360 | - | [13] |
| TiO2/Pd/TiO2 | Sol–gel and sputtering | 252 | 4 | 1.16 | - | - | [16] |
| TiO2-300 nm- Au-6 nm | Sputtering | 400 | 5 | 61.3 | 37 | 40 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, M.; Zhao, X.; He, X.; Wang, G.; Zhang, W.; Rong, Q.; Nguyen, D. High-Performance MEMS Oxygen Sensors Based on Au/TiO2 Films. Chemosensors 2023, 11, 476. https://doi.org/10.3390/chemosensors11090476
Jiao M, Zhao X, He X, Wang G, Zhang W, Rong Q, Nguyen D. High-Performance MEMS Oxygen Sensors Based on Au/TiO2 Films. Chemosensors. 2023; 11(9):476. https://doi.org/10.3390/chemosensors11090476
Chicago/Turabian StyleJiao, Mingzhi, Xiaohu Zhao, Xinjian He, Gang Wang, Wei Zhang, Qian Rong, and DucHoa Nguyen. 2023. "High-Performance MEMS Oxygen Sensors Based on Au/TiO2 Films" Chemosensors 11, no. 9: 476. https://doi.org/10.3390/chemosensors11090476
APA StyleJiao, M., Zhao, X., He, X., Wang, G., Zhang, W., Rong, Q., & Nguyen, D. (2023). High-Performance MEMS Oxygen Sensors Based on Au/TiO2 Films. Chemosensors, 11(9), 476. https://doi.org/10.3390/chemosensors11090476








