Detection of Xylene Using Ni(OH)2-Enhanced Co3O4 Nanoplate via p–n Junctions
Abstract
:1. Introduction
| Materials | Concentration (ppm) | Temperature (°C) | Response (Rg/Ra) | Lower Detection Limit (ppm) | Reference |
|---|---|---|---|---|---|
| C/Co3O4 | 100 | 183 | 17.62 | 1 | [32] |
| W-doped NiO | 200 | 375 | 8.74 | 15 | [34] |
| NiCo2O4/WO3 | 100 | 300 | 15.69 | 5 | [35] |
| CuO/WO3 | 50 | 260 | 6.36 | 0.3 | [36] |
| Ag-Co3O4 | 50 | 250 | 2.47 | 0.14 | [11] |
| Co3O4@NiMoO4 | 100 | 255 | 24.6 | 0.42 | [12] |
| Ni(OH)2/Co3O4 | 100 | 175 | 14.1 | 0.1 | This work |
2. Materials and Experimental Details
2.1. Experimental Materials Overview
2.2. Synthesis of Ni(OH)2 Nanosheets
2.3. Synthesis of Co3O4 Nanoplates and Ni(OH)2/Co3O4 Nanomaterials
2.4. Material Characterization Methods
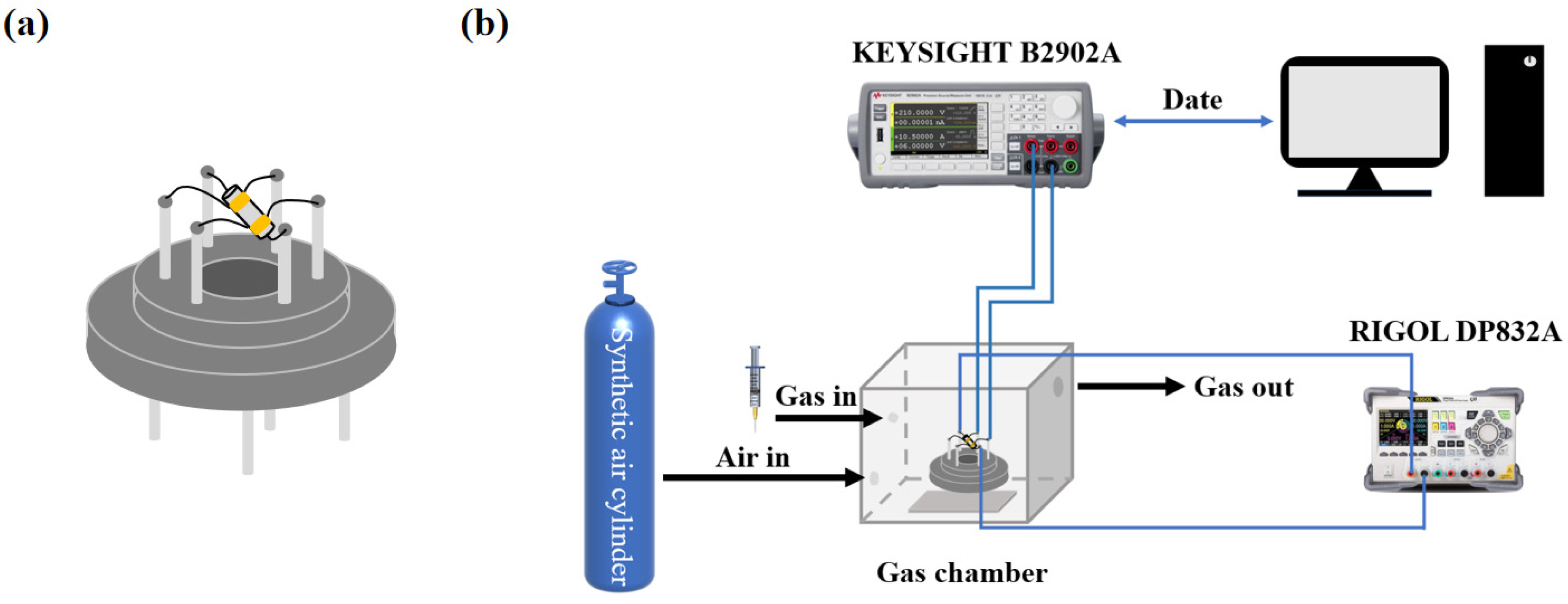
2.5. Gas Sensor Fabrication and Gas Sensing Performance Test
3. Results and Discussion
3.1. Characterization of Material Structure and Morphology
3.2. Xylene Gas-Sensing Properties
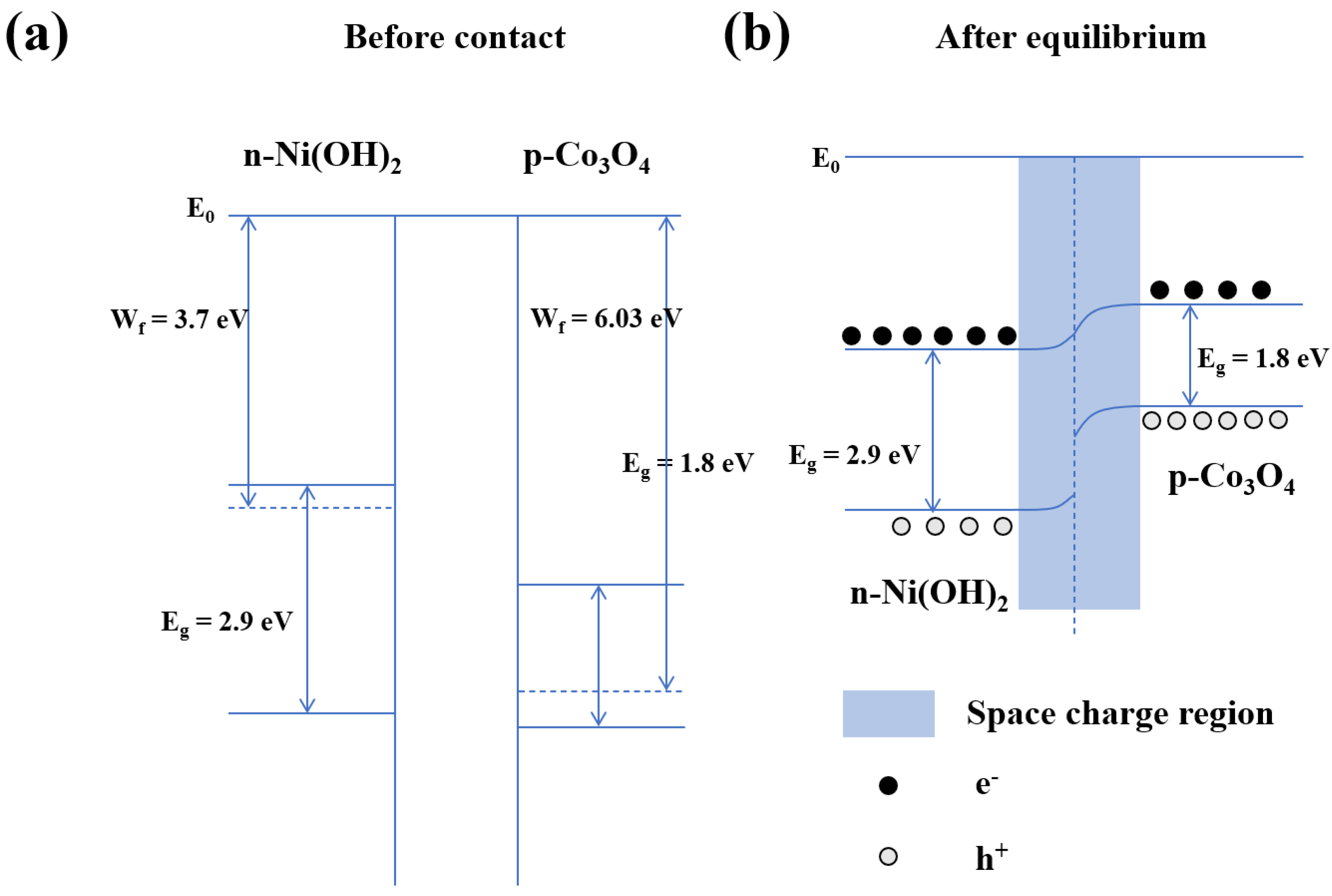
4. Xylene Sensing Mechanism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yassaa, N.; Brancaleoni, E.; Frattoni, M.; Ciccioli, P. Isomeric analysis of BTEXs in the atmosphere using β-cyclodextrin capillary chromatography coupled with thermal desorption and mass spectrometry. Chemosphere 2006, 63, 502–508. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, K.; Liu, S.; Liang, X.; Zhang, M. Reasonable construction of 2D porous NiO/Co3O4 nanosheets for efficient detection of xylene. Sens. Actuators B Chem. 2023, 377, 133002. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. Resistive-based gas sensors for detection of benzene, toluene and xylene (BTX) gases: A review. J. Mater. Chem. C 2018, 6, 4342–4370. [Google Scholar] [CrossRef]
- Moon, Y.K.; Kim, K.B.; Jeong, S.-Y.; Lee, J.-H. Designing oxide chemiresistors for detecting volatile aromatic compounds: Recent progresses and future perspectives. Chem. Commun. 2022, 58, 5439–5454. [Google Scholar] [CrossRef]
- He, T.; Wang, W.; He, B.-G.; Chen, J. Review on Optical Fiber Sensors for Hazardous-Gas Monitoring in Mines and Tunnels. IEEE Trans. Instrum. Meas. 2023, 72, 7003722. [Google Scholar] [CrossRef]
- Zhang, Y.; Nizamidin, P.; Abudukeremu, H.; Yimit, A. Optical waveguide xylene gas sensor based on sodium dodecylbenzene sulfonate (SDBS)–TiO2 film for detection at room temperature. Opt. Mater. Express 2020, 10, 2212. [Google Scholar] [CrossRef]
- Ferreira, S.L.; dos Santos, A.M.; de Souza, G.R.; Polito, W.L. Analysis of the emissions of volatile organic compounds from the compression ignition engine fueled by diesel–biodiesel blend and diesel oil using gas chromatography. Energy 2008, 33, 1801–1806. [Google Scholar] [CrossRef]
- Šutka, A.; Gross, K.A. Spinel ferrite oxide semiconductor gas sensors. Sens. Actuators B Chem. 2016, 222, 95–105. [Google Scholar] [CrossRef]
- Batra, A.K.; Chilvery, A.K.; Guggilla, P.; Aggarwal, M.; Currie, J.R. Micro- and Nano-Structured Metal Oxides Based Chemical Sensors: An Overview. J. Nanosci. Nanotechnol. 2014, 14, 2065–2085. [Google Scholar] [CrossRef]
- Souri, M.; Salar Amoli, H. Gas sensing mechanisms in ABO3 perovskite materials at room temperature: A review. Mater. Sci. Semicond. Process. 2023, 156, 107271. [Google Scholar] [CrossRef]
- Shin, K.Y.; Mirzaei, A.; Lee, H.Y.; Bang, J.H.; Oum, W.; Kim, E.B.; Kim, H.M.; Majhi, S.M.; Kim, S.S.; Kim, H.W. Formation of nanograined Ag-Co3O4 core@shell structure to achieve enhanced xylene sensing characteristics. Sens. Actuators B Chem. 2023, 392, 134049. [Google Scholar] [CrossRef]
- Qu, F.; Zhang, S.; Zhang, B.; Zhou, X.; Du, S.; Lin, C.-T.; Ruan, S.; Yang, M. Hierarchical Co3O4@NiMoO4 core-shell nanowires for chemiresistive sensing of xylene vapor. Microchim. Acta 2019, 186, 222. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, Y.; Peng, Y.; Huang, Z.; Ma, Q.; Zhang, H. Two-dimensional metal–organic framework nanosheets: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 6267–6295. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, D.; Wang, H.; Huang, W.; Hu, L.; Tang, Y.; Guo, Z.; Ouyang, Z.; Zhang, H. Recent advances in two-dimensional-material-based sensing technology toward health and environmental monitoring applications. Nanoscale 2020, 12, 3535–3559. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liao, T.; Kou, L. Strategies for designing metal oxide nanostructures. Sci. China Mater. 2016, 60, 1–24. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, M.; Yin, X.; Gao, Q.; Jiang, S.; Cheng, J.; Kong, X.; Liu, B.; Peng, H.-Q. Recent advances in two-dimensional nanomaterials as bifunctional electrocatalysts for full water splitting. J. Mater. Chem. A 2023, 11, 18502–18529. [Google Scholar] [CrossRef]
- Ayyub, M.M.; Singh, R.; Rao, C.N.R. Hydrogen Generation by Solar Water Splitting Using 2D Nanomaterials. Sol. RRL 2020, 4, 2000050. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, J.; Qin, C.; Zhang, B.; Sun, G.; Zhang, Z. Synthesis and Enhanced Ethanol Gas Sensing Properties of the g-C3N4 Nanosheets-Decorated Tin Oxide Flower-Like Nanorods Composite. Nanomaterials 2017, 7, 285. [Google Scholar] [CrossRef]
- Hu, J.; Chen, M.; Rong, Q.; Zhang, Y.; Wang, H.; Zhang, D.; Zhao, X.; Zhou, S.; Zi, B.; Zhao, J.; et al. Formaldehyde sensing performance of reduced graphene oxide-wrapped hollow SnO2 nanospheres composites. Sens. Actuators B Chem. 2020, 307, 127584. [Google Scholar] [CrossRef]
- Parichenko, A.; Huang, S.; Pang, J.; Ibarlucea, B.; Cuniberti, G. Recent advances in technologies toward the development of 2D materials-based electronic noses. TrAC Trends Anal. Chem. 2023, 166, 117185. [Google Scholar] [CrossRef]
- Munusami, V.; Arutselvan, K.; Vadivel, S.; Govindasamy, S. High sensitivity LPG and H2 gas sensing behavior of MoS2/graphene hybrid sensors prepared by facile hydrothermal method. Ceram. Int. 2022, 48, 29322–29331. [Google Scholar] [CrossRef]
- Meng, F.; Li, X.; Yuan, Z.; Lei, Y.; Qi, T.; Li, J. Ppb-Level Xylene Gas Sensors Based on Co3O4 Nanoparticle-Coated Reduced Graphene Oxide(rGO) Nanosheets Operating at Low Temperature. IEEE Trans. Instrum. Meas. 2021, 70, 9511510. [Google Scholar] [CrossRef]
- Wang, Z.; Bu, M.; Hu, N.; Zhao, L. An overview on room-temperature chemiresistor gas sensors based on 2D materials: Research status and challenge. Compos. Part B Eng. 2023, 248, 110378. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N.; Mokhtarzadeh, A.; Ramezani, M. Two dimension (2-D) graphene-based nanomaterials as signal amplification elements in electrochemical microfluidic immune-devices: Recent advances. Mater. Sci. Eng. C 2016, 68, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Kröner, A.; Hirsch, T. Current Trends in the Optical Characterization of Two-Dimensional Carbon Nanomaterials. Front. Chem. 2020, 7, 927. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Yu, Y.; Yin, X.; Wang, X. A wafer-scale 1 nm Ni(OH)2 nanosheet with superior electrocatalytic activity for the oxygen evolution reaction. Nanoscale 2018, 10, 5054–5059. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, J.; Shi, G.; Yue, D. Different crystallographic Ni(OH)2 as highly efficient Fenton-like catalysts for sulfate radical activation. Chem. Commun. 2023, 59, 1341–1344. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Wang, Q.; Yao, X.; Zhou, Y.; Yu, X.-Y. Phase-engineering of nickel hydroxide in the Ni/Ni(OH)2 interface for efficient hydrogen evolution and hydrazine-assisted water splitting in seawater. J. Mater. Chem. A 2022, 10, 21848–21855. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Hu, J.; Jia, Y.-B.; Liu, R.-T.; Cai, T.; Xu, Z.P. Two-dimensional layered double hydroxide nanoadjuvant: Recent progress and future direction. Nanoscale 2021, 13, 7533–7549. [Google Scholar] [CrossRef]
- Jeon, S.S.; Lim, J.; Kang, P.W.; Lee, J.W.; Kang, G.; Lee, H. Design Principles of NiFe-Layered Double Hydroxide Anode Catalysts for Anion Exchange Membrane Water Electrolyzers. ACS Appl. Mater. Interfaces 2021, 13, 37179–37186. [Google Scholar] [CrossRef]
- Li, Y.; Ma, X.; Guo, S.; Wang, B.; Sun, D.; Zhang, X.; Ruan, S. Hydrothermal synthesis and enhanced xylene-sensing properties of pompon-like Cr-doped Co3O4hierarchical nanostructures. RSC Adv. 2016, 6, 22889–22895. [Google Scholar] [CrossRef]
- Wang, W.; Fan, Y.; Sun, D.; Guan, H.; Wang, Q.; Chen, Y.; Ruan, S. Detection of xylene from C/Co3O4 nanocomposites synthesized from double-template using g-C3N4/ZIF-67. Chem. Eng. J. 2023, 466, 143363. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, H.; Yuan, Z.; Meng, F. Conductometric butanone gas sensor based on Co3O4 modified SnO2 hollow spheres with ppb-level detection limit. Sens. Actuators B Chem. 2023, 374, 132787. [Google Scholar] [CrossRef]
- Feng, C.; Wang, C.; Zhang, H.; Li, X.; Wang, C.; Cheng, P.; Ma, J.; Sun, P.; Gao, Y.; Zhang, H.; et al. Enhanced sensitive and selective xylene sensors using W-doped NiO nanotubes. Sens. Actuators B Chem. 2015, 221, 1475–1482. [Google Scholar] [CrossRef]
- Xu, K.; Yang, Y.; Yu, T.; Yuan, C. WO3 nanofibers anchored by porous NiCo2O4 nanosheets for xylene detection. Ceram. Int. 2018, 44, 21717–21724. [Google Scholar] [CrossRef]
- Guo, M.; Luo, N.; Chen, Y.; Fan, Y.; Wang, X.; Xu, J. Fast-response MEMS xylene gas sensor based on CuO/WO3 hierarchical structure. J. Hazard. Mater. 2022, 429, 127471. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Cai, Y.; Li, H.; Cai, X.; Djerdj, I.; Wang, Y. A facial method to synthesize Ni(OH)2 nanosheets for improving the adsorption properties of Congo red in aqueous solution. Powder Technol. 2013, 235, 121–125. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, D.; Liu, X.; Cao, D.; Wang, G. Influence of CTAB on morphology, structure, and supercapacitance of β-Ni(OH)2. Ionics 2014, 21, 533–540. [Google Scholar] [CrossRef]
- Adhikari, H.; Ghimire, M.; Ranaweera, C.K.; Bhoyate, S.; Gupta, R.K.; Alam, J.; Mishra, S.R. Synthesis and electrochemical performance of hydrothermally synthesized Co3O4 nanostructured particles in presence of urea. J. Alloys Compd. 2017, 708, 628–638. [Google Scholar] [CrossRef]
- Ma, Y.; Ji, B.; Lv, X.; Xiong, D.; Zhao, X.; Xie, H.; Zhang, Z. Confined heterogeneous catalysis by boron nitride-Co3O4 nanosheet cluster for peroxymonosulfate oxidation toward ranitidine removal. Chem. Eng. J. 2022, 435, 135126. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Sun, J.; Xiu, K.; Hu, N.; Zhao, L.; Ma, Y.; Zhu, Y.; Zhang, R.; Yu, W. Defect engineering of two-dimensional ultrathin Co3O4 nanosheets for wireless and high-performance monitoring of the sensing properties of volatile organic compounds. Mater. Today Chem. 2023, 32, 101639. [Google Scholar] [CrossRef]
- Cao, J.; Qin, C.; Wang, Y.; Zhang, H.; Zhang, B.; Gong, Y.; Wang, X.; Sun, G.; Bala, H.; Zhang, Z. Synthesis of g-C3N4 nanosheet modified SnO2 composites with improved performance for ethanol gas sensing. RSC Adv. 2017, 7, 25504–25511. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Pan, Q.; Pan, K.; Zhang, G. Metal-organic framework (MOF) derived In2O3 and g-C3N4 composite for superior NOx gas-sensing performance at room temperature. Sens. Actuators B Chem. 2022, 352, 131001. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Z.; Zhang, R.; Ji, H.; Xing, C.; Meng, F. MoO3/SnO2 Nanocomposite-Based Gas Sensor for Rapid Detection of Ammonia. IEEE Trans. Instrum. Meas. 2021, 70, 9514209. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, W.; Yuan, Z.; Gao, H.; Meng, F. Highly Sensitive and Selective NH3 Sensor Based on Au Nanoparticle Loaded MoO3 Nanorods. IEEE Sens. J. 2021, 21, 18435–18442. [Google Scholar] [CrossRef]
- Umar, A.; Ibrahim, A.A.; Algadi, H.; Nakate, U.T.; Choudhury, S.P.; Alsuwian, T.; Albargi, H.; Alsaiari, M.A.; Baskoutas, S. Selective ethanol gas sensing performance of flower-shaped CuO composed of thin nanoplates. J. Mater. Sci. Mater. Electron. 2021, 32, 18565–18579. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Z.; Yang, Y.; Xing, X.; Zou, T.; Wang, Z.; Wang, Y. Raspberry-like SnO2 hollow nanostructure as a high response sensing material of gas sensor toward n-butanol gas. J. Phys. Chem. Solids 2018, 120, 173–182. [Google Scholar] [CrossRef]
- Liu, J.; Dai, M.; Wang, T.; Sun, P.; Liang, X.; Lu, G.; Shimanoe, K.; Yamazoe, N. Enhanced Gas Sensing Properties of SnO2 Hollow Spheres Decorated with CeO2 Nanoparticles Heterostructure Composite Materials. ACS Appl. Mater. Interfaces 2016, 8, 6669–6677. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.; Yu, Q.; Zhao, L.; Sun, P.; Wang, T.; Liu, F.; Yan, X.; Gao, Y.; Liang, X.; et al. One step synthesis of branched SnO2/ZnO heterostructures and their enhanced gas-sensing properties. Sens. Actuators B Chem. 2019, 281, 415–423. [Google Scholar] [CrossRef]
- Großmann, K.; Wicker, S.; Weimar, U.; Barsan, N. Impact of Pt additives on the surface reactions between SnO2, water vapour, CO and H2; an operando investigation. Phys. Chem. Chem. Phys. 2013, 15, 19151. [Google Scholar] [CrossRef]
- Kim, H.W.; Kwon, Y.J.; Mirzaei, A.; Kang, S.Y.; Choi, M.S.; Bang, J.H.; Kim, S.S. Synthesis of zinc oxide semiconductors-graphene nanocomposites by microwave irradiation for application to gas sensors. Sens. Actuators B Chem. 2017, 249, 590–601. [Google Scholar] [CrossRef]
- Zhao, L.; Jin, R.; Wang, C.; Wang, T.; Sun, Y.; Sun, P.; Lu, G. Flower-like ZnO-Co3O4 heterojunction composites for enhanced acetone sensing. Sens. Actuators B Chem. 2023, 390, 133964. [Google Scholar] [CrossRef]
- Mirzaei, A.; Park, S.; Kheel, H.; Sun, G.-J.; Ko, T.; Lee, S.; Lee, C. Acetone Sensors Based on In2O3–Co3O4 Composite Nanoparticles. J. Nanosci. Nanotechnol. 2017, 17, 4087–4090. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, M.; Yuan, Z.; Zhu, H.; Gao, H.; Meng, F. Detection of Xylene Using Ni(OH)2-Enhanced Co3O4 Nanoplate via p–n Junctions. Chemosensors 2023, 11, 568. https://doi.org/10.3390/chemosensors11110568
Ran M, Yuan Z, Zhu H, Gao H, Meng F. Detection of Xylene Using Ni(OH)2-Enhanced Co3O4 Nanoplate via p–n Junctions. Chemosensors. 2023; 11(11):568. https://doi.org/10.3390/chemosensors11110568
Chicago/Turabian StyleRan, Mengran, Zhenyu Yuan, Hongmin Zhu, Hongliang Gao, and Fanli Meng. 2023. "Detection of Xylene Using Ni(OH)2-Enhanced Co3O4 Nanoplate via p–n Junctions" Chemosensors 11, no. 11: 568. https://doi.org/10.3390/chemosensors11110568
APA StyleRan, M., Yuan, Z., Zhu, H., Gao, H., & Meng, F. (2023). Detection of Xylene Using Ni(OH)2-Enhanced Co3O4 Nanoplate via p–n Junctions. Chemosensors, 11(11), 568. https://doi.org/10.3390/chemosensors11110568









