Quantitative Detection of the Influenza a Virus by an EGOFET-Based Portable Device
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Biosensor Fabrication Technique
3.2. Electrical Characteristics of Three Types of EGOFETs with a Biorecognition Layer
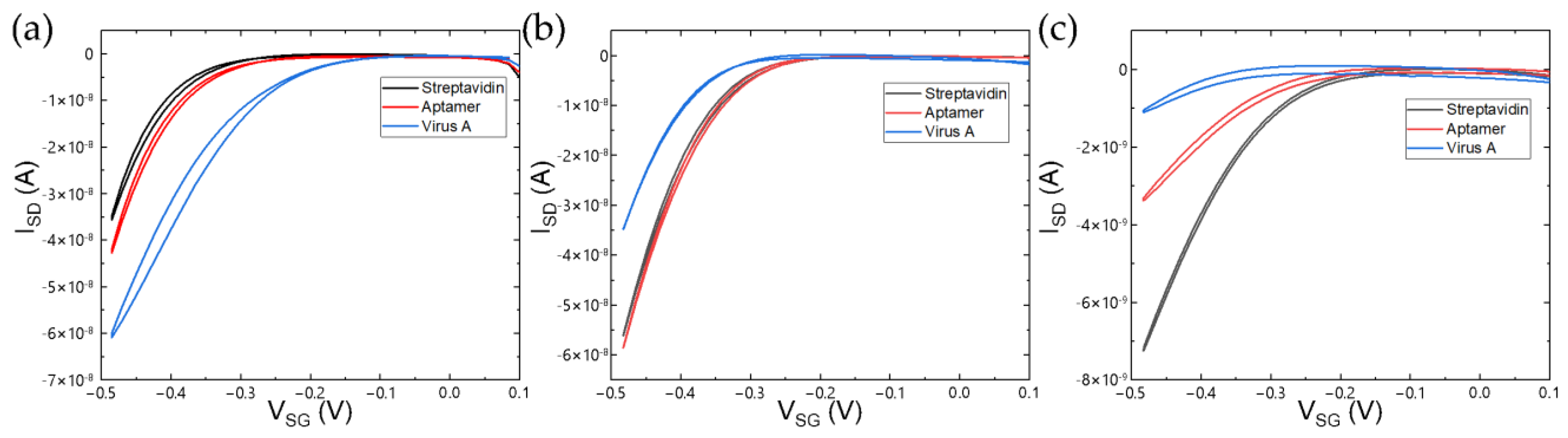
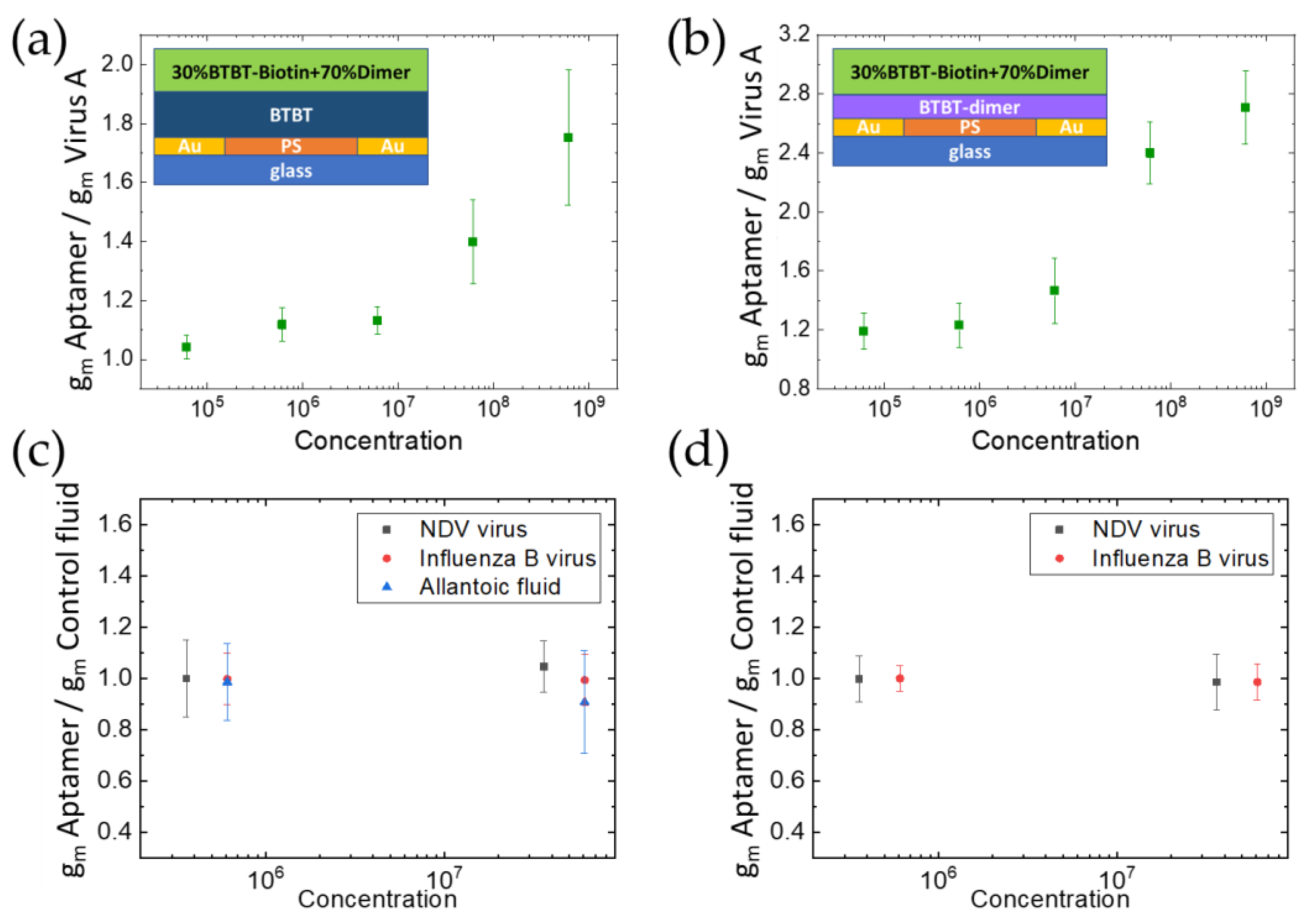
3.3. Detection of Specific and Non-Specific Interactions with Virus Particles
3.4. Effect of the Active Layer Organic Semiconductor Material and LOD of the Devices
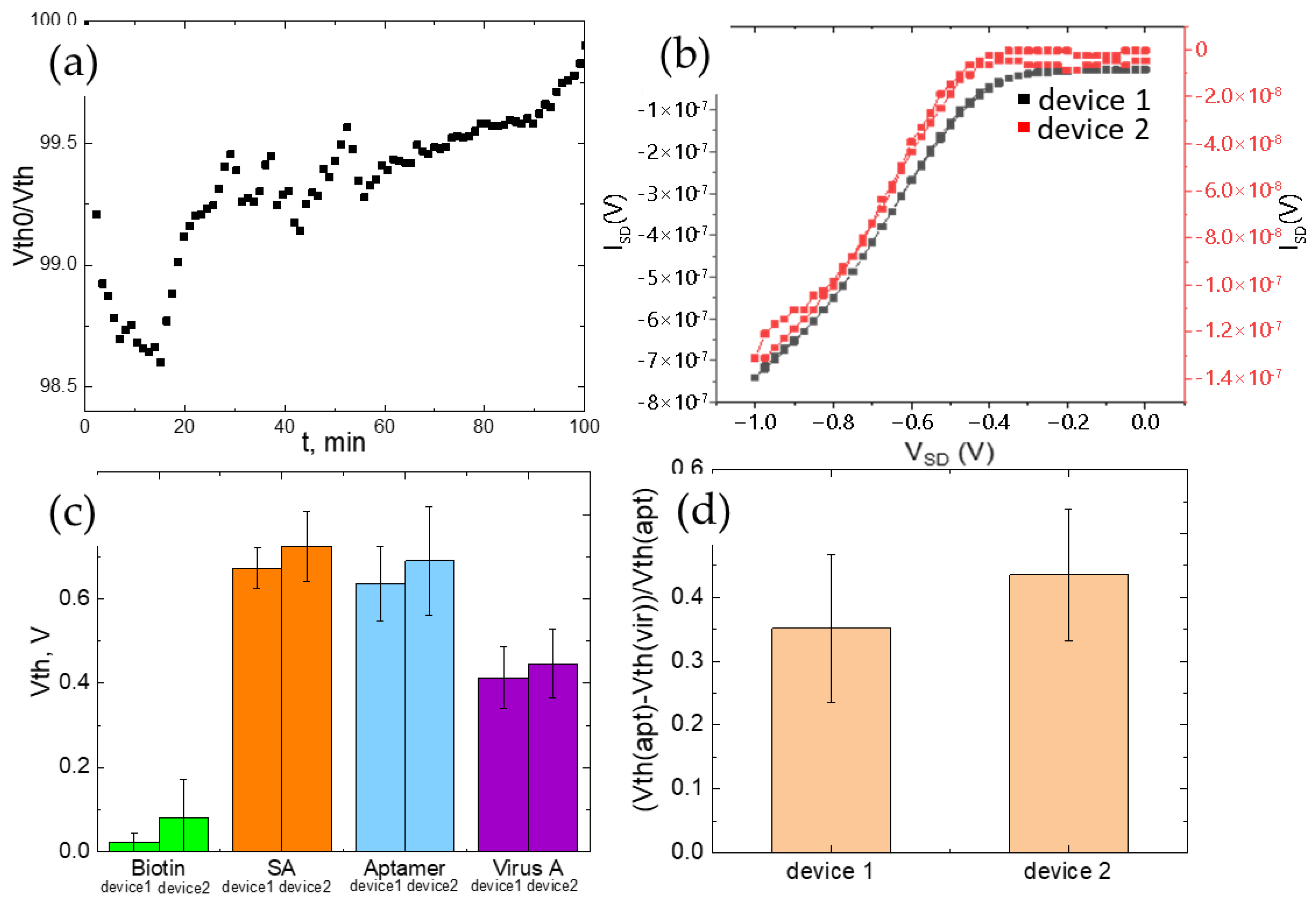
3.5. Portable Device Fabrication and Virus Detection with Multi-Flow Chamber
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torricelli, F.; Manoli, K.; Macchia, E.; Torsi, L.; Magliulo, M. Electrolyte-gated organic transistors for biosensing applications. Org. Sen. Mat. Appl. 2016, 71–105. [Google Scholar] [CrossRef]
- Courtney, S.J.; Stromberg, Z.R.; Myers y Gutiérrez, A.; Jacobsen, D.; Stromberg, L.R.; Lenz, K.D.; Theiler, J.; Foley, B.T.; Gans, J.; Yusim, K.; et al. Optical Biosensor Platforms Display Varying Sensitivity for the Direct Detection of Influenza RNA. Biosensors 2021, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Boukraa, R.; Mattana, G.; Battaglini, N.; Piro, B. A Complementary Reduced Graphene Oxide-Based Inverter for Ion Sensing. Eng. Proc. 2022, 16, 2. [Google Scholar] [CrossRef]
- Ramalingam, M.; Jaisankar, A.; Cheng, L.; Krishnan, S.; Lan, L.; Hassan, A.; Sasmazel, H.T.; Kaji, H.; Deigner, H.-P.; Pedraz, J.L.; et al. Impact of nanotechnology on conventional and artificial intelligence-based biosensing strategies for the detection of viruses. Discov. Nano 2023, 18, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Macchia, E.; Torsi, L. Biochemical Sensing, in Introduction to Bioelectronics: Materials, devices, applications. AIP Publ. 2022, 5-1–5-22. [Google Scholar] [CrossRef]
- Shaposhnik, P.A.; Poimanova, E.Y.; Abramov, A.A.; Trul, A.A.; Anisimov, D.S.; Kretova, E.A.; Agina, E.V.; Ponomarenko, S.A. Applying of C8-BTBT-Based EGOFETs at Different pH Values of the Electrolyte. Chemosensors 2023, 11, 74. [Google Scholar] [CrossRef]
- Liu, N.; Chen, R.; Wan, Q. Recent Advances in Electric-Double-Layer Transistors for Bio-Chemical Sensing Applications. Sensors 2019, 19, 3425. [Google Scholar] [CrossRef]
- Cramer, T.; Kyndiah, A.; Murgia, M.; Leonardi, F.; Casalini, S.; Biscarini, F. Double layer capacitance measured by organic field effect transistor operated in water. Appl. Phys. Lett. 2012, 100, 143302. [Google Scholar] [CrossRef]
- Macchia, E.; Torricelli, F.; Bollella, P.; Sarcina, L.; Tricase, A.; Di Franco, C.; Österbacka, R.; Kovács-Vajna, Z.M.; Scamarcio, G.; Torsi, L. Large-Area Interfaces for Single-Molecule Label-free Bioelectronic Detection. Chem. Rev. 2022, 122, 4636–4699. [Google Scholar] [CrossRef]
- Poimanova, E.Y.; Shaposhnik, P.A.; Anisimov, D.S.; Zavyalova, E.G.; Trul, A.A.; Skorotetcky, M.S.; Borshchev, O.V.; Vinnitskiy, D.Z.; Polinskaya, M.S.; Krylov, V.B.; et al. Biorecognition Layer Based On Biotin-Containing [1]Benzothieno[3,2-b][1]benzothiophene Derivative for Biosensing by Electrolyte-Gated Organic Field-Effect Transistors. ACS Appl. Mater. Interfaces 2022, 14, 16462–16476. [Google Scholar] [CrossRef]
- Arlett, J.L.; Myers, E.B.; Roukes, M.L. Comparative advantages of mechanical biosensors. Nat. Nanotechnol. 2011, 6, 203–215. [Google Scholar] [CrossRef]
- Samuel, V.R.; Rao, K.J. A review on label free biosensors. Biosens. Bioelectron. X 2022, 11, 100216. [Google Scholar] [CrossRef]
- Macchia, E.; Giordano, F.; Magliulo, M.; Palazzo, G.; Torsi, L. An analytical model for bio-electronic organic field-effect transistor sensors. Appl. Phys. Lett. 2013, 103, 103301. [Google Scholar] [CrossRef]
- Ni, S.; Zhuo, Z.; Pan, Y.; Yu, Y.; Li, F.; Liu, J.; Wang, L.; Wu, X.; Li, D.; Wan, Y.; et al. Recent progress in aptamer discoveries and modifications for therapeutic applications. ACS Appl. Mater. Interfaces 2021, 13, 9500–9519. [Google Scholar] [CrossRef] [PubMed]
- Shaban, S.M.; Kim, D.-H. Recent advances in aptamer sensors. Sensors 2021, 21, 979. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, B.; Das, S.; Gupta, A.; Xiong, Y.T.-V.V.; Kizer, M.E.; Duan, J.; Chandrasekaran, A.R.; Wang, X. Aptamers for Viral Detection and Inhibition. ACS Infect. Dis. 2022, 8, 667–692. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Noh, S.; Park, J.A.; Park, S.-C.; Park, S.J.; Lee, J.-H.; Ahn, J.-H.; Lee, T. Recent Advances in Aptasensor for Cytokine Detection: A Review. Sensors 2021, 21, 8491. [Google Scholar] [CrossRef] [PubMed]
- Ambartsumyan, O.; Gribanyov, D.; Kukushkin, V.; Kopylov, A.; Zavyalova, E. SERS-based biosensors for virus determination with oligonucleotides as recognition elements. Int. J. Mol. Sci. 2020, 21, 3373. [Google Scholar] [CrossRef]
- Lou, B.; Liu, Y.; Shi, M.; Chen, J.; Li, K.; Tan, Y.; Chen, L.; Wu, Y.; Wang, T.; Liu, X.; et al. Aptamer-based biosensors for virus protein detection. Trends Analyt. Chem. 2022, 157, 116738. [Google Scholar] [CrossRef]
- Kukushkin, V.; Kristavchuk, O.; Andreev, E.; Meshcheryakova, N.; Zaborova, O.; Gambaryan, A.; Nechaev, A.; Zavyalova, E. Aptamer-coated track-etched membranes with a nanostructured silver layer for single virus detection in biological fluids. Front. Bioeng. Biotechnol. 2023, 10, 1076749. [Google Scholar] [CrossRef]
- Yuan, Y.; Giri, G.; Ayzner, A.L.; Zoombelt, A.P.; Mannsfeld, S.C.B.; Chen, J.; Nordlund, D.; Toney, M.F.; Huang, J.; Bao, Z. Ultra-high mobility transparent organic thin film transistors grown by an off-centre spin-coating method. Nat. Commun. 2014, 5, 3005. [Google Scholar] [CrossRef]
- Zhang, Q.; Leonardi, F.; Casalini, S.; Temiño, I.; Mas-Torrent, M. High performing solution-coated electrolyte-gated organic field-effect transistors for aqueous media operation. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Shaposhnik, P.A.; Anisimov, D.A.; Trul, A.A.; Agina, E.V.; Ponomarenko, S.A. A Simple Approach to Fabrication of Highly Efficient Electrolyte-Gated Organic Transistors by Phase Microsegregation of 2,7-Dioctyl [1]Benzothieno[3,2-b]Benzothiophene and Polystyrene Mixtures. Dokl. Phys. Chem. 2021, 496, 20–24. [Google Scholar] [CrossRef]
- Xie, P.; Liu, T.; Sun, J.; Yang, J. Structures, Properties, and Device Applications for [1]Benzothieno[3,2-b]Benzothiophene Derivatives. Adv. Funct. Mater. 2022, 32, 2200843. [Google Scholar] [CrossRef]
- Anisimov, D.S.; Chekusova, V.P.; Trul, A.A.; Abramov, A.A.; Borshchev, O.V.; Agina, E.V.; Ponomarenko, S.A. Fully Integrated Ultra-Sensitive Electronic Nose Based on Organic Field-Effect Transistors. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bayn, A.; Feng, X.; Müllen, K.; Haick, H. Field-efect transistors based on polycyclic aromatic hydrocarbons for the detection and classifcation of volatile organic compounds. ACS Appl. Mater. 2013, 5, 3431–3440. [Google Scholar] [CrossRef]
- Zhavnerko, G.; Marletta, G. Developing Langmuir–Blodgett strategies towards practical devices. Mater. Sci. Eng. 2010, 169, 43–48. [Google Scholar] [CrossRef]
- Shiratori, I.; Akitomi, J.; Boltz, D.A.; Horii, K.; Furuichi, M.; Waga, I. Selection of DNA aptamers that bind to influenza A viruses with high affinity and broad subtype specificity. Biochem. Biophys. Res. Commun. 2014, 443, 37–41. [Google Scholar] [CrossRef]
- Pang, Y.; Rong, Z.; Wang, J.; Xiao, R.; Wang, S. A fluorescent aptasensor for H5N1 influenza virus detection based-on the core-shell nanoparticles metal-enhanced fluorescence (MEF). Biosens. Bioelectron. 2015, 66, 527–532. [Google Scholar] [CrossRef]
- Zou, X.; Wu, J.; Gu, J.; Shen, L.; Mao, L. Application of Aptamers in Virus Detection and Antiviral Therapy. Front. Microbiol. 2019, 10, 1–20. [Google Scholar] [CrossRef]
- Chan, K.-H.; To, K.K.W.; Chan, J.F.W.; Li, C.P.Y.; Chen, H.; Yuen, K.-Y. Analytical Sensitivity of Seven Point-of-Care Influenza Virus Detection Tests and Two Molecular Tests for Detection of Avian Origin H7N9 and Swine Origin H3N2 Variant Influenza A Viruses. J. Clin. Microbiol. 2013, 51, 3160–3161. [Google Scholar] [CrossRef]
- Herrmann, B.; Larsson, C.; Zweygberg, B.W. Simultaneous Detection and Typing of Influenza Viruses A and B by a Nested Reverse Transcription-PCR: Comparison to Virus Isolation and Antigen Detection by Immunofluorescence and Optical Immunoassay (FLU OIA). J. Clin. Microbiol. 2001, 39, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Jian, M.J.; Chang, C.K.; Lin, J.C.; Yeh, K.M.; Chen, C.W.; Chiu, S.K.; Wang, Y.H.; Liao, S.J.; Li, S.Y.; et al. Novel dual multiplex real-time RT-PCR assays for the rapid detection of SARS-CoV-2, influenza A/B, and respiratory syncytial virus using the BD MAX open system. Emerg. Microbes Infect. 2021, 10, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Xie, Z.; Xie, L.; Liu, J.; Xie, Z.; Deng, X.; Huang, L.; Huang, J.; Zeng, T.; Khan, M.I. Reverse-transcription, loop-mediated isothermal amplification assay for the sensitive and rapid detection of H10 subtype avian influenza viruses. Virol. J. 2015, 12, 1–7. [Google Scholar] [CrossRef]
- Tsang, T.K.; Cowling, B.J.; Fang, V.J.; Chan, K.H.; Ip, D.K.; Leung, G.M.; Peiris, J.S.; Cauchemez, S. Influenza A Virus Shedding and Infectivity in Households. J. Infect. Dis. 2015, 212, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Doumbia, A.; Webb, M.; Behrendt, M.; Wilson, R.; Turner, M. Robust Microfluidic Integrated Electrolyte-Gated Organic Field-Effect Transistor Sensors for Rapid, In Situ and Label-Free Monitoring of DNA Hybridization. Adv. Electron. Mater. 2022, 8, 2200142. [Google Scholar] [CrossRef]
- Macchia, E.; Kovács-Vajna, Z.M.; Loconsole, D.; Sarcina, L.; Redolfi, M.; Chironna, M.; Torricelli, F.; Torsi, L. A Handheld Intelligent Single-Molecule Binary Bioelectronic System for Fast and Reliable Immunometric Point-of-Care Testing. Sci. Adv. 2022, 8, eabo0881. [Google Scholar] [CrossRef]
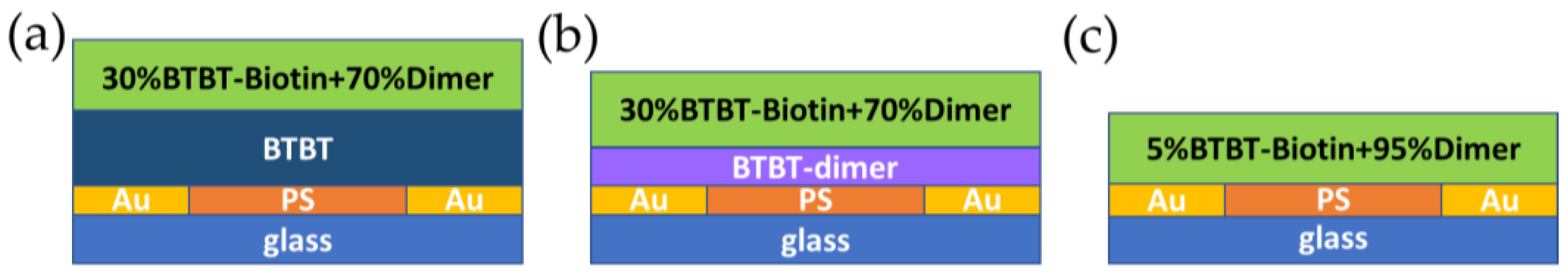



| Device | Ion, µA | Ioff, nA | gm, A1/2 V−1(×106) | Vth, mV |
|---|---|---|---|---|
| C8-BTBT-C8/biorecognition layer with 30%BTBT-biotin | −0.11 ± 0.02 | −0.01 ± 0.004 | −3.5 ± 0.5 | −215 ± 50 |
| BTBT-Dimer/biorecognition layer with 30%BTBT-biotin | −0.35 ± 0.05 | −0.05 ± 0.006 | −6.3 ± 0.7 | −235 ± 30 |
| Biorecognition layer with 5%BTBT-biotin | −0.10 ± 0.03 | −0.01 ± 0.005 | −3.4 ± 0.6 | −112 ± 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poimanova, E.Y.; Zavyalova, E.G.; Kretova, E.A.; Abramov, A.A.; Trul, A.A.; Borshchev, O.V.; Keshek, A.K.; Ponomarenko, S.A.; Agina, E.V. Quantitative Detection of the Influenza a Virus by an EGOFET-Based Portable Device. Chemosensors 2023, 11, 464. https://doi.org/10.3390/chemosensors11080464
Poimanova EY, Zavyalova EG, Kretova EA, Abramov AA, Trul AA, Borshchev OV, Keshek AK, Ponomarenko SA, Agina EV. Quantitative Detection of the Influenza a Virus by an EGOFET-Based Portable Device. Chemosensors. 2023; 11(8):464. https://doi.org/10.3390/chemosensors11080464
Chicago/Turabian StylePoimanova, Elena Y., Elena G. Zavyalova, Elena A. Kretova, Anton A. Abramov, Askold A. Trul, Oleg V. Borshchev, Anna K. Keshek, Sergey A. Ponomarenko, and Elena V. Agina. 2023. "Quantitative Detection of the Influenza a Virus by an EGOFET-Based Portable Device" Chemosensors 11, no. 8: 464. https://doi.org/10.3390/chemosensors11080464
APA StylePoimanova, E. Y., Zavyalova, E. G., Kretova, E. A., Abramov, A. A., Trul, A. A., Borshchev, O. V., Keshek, A. K., Ponomarenko, S. A., & Agina, E. V. (2023). Quantitative Detection of the Influenza a Virus by an EGOFET-Based Portable Device. Chemosensors, 11(8), 464. https://doi.org/10.3390/chemosensors11080464







