Investigation of the Synergistic Effect of Layer-by-Layer Films of Carbon Nanotubes and Polypyrrole on a Flexible Electrochemical Device for Paraquat Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CNT Synthesis and Functionalization of the gPBAT
2.3. Physical Characterizations
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Study of Architecture and Bilayer
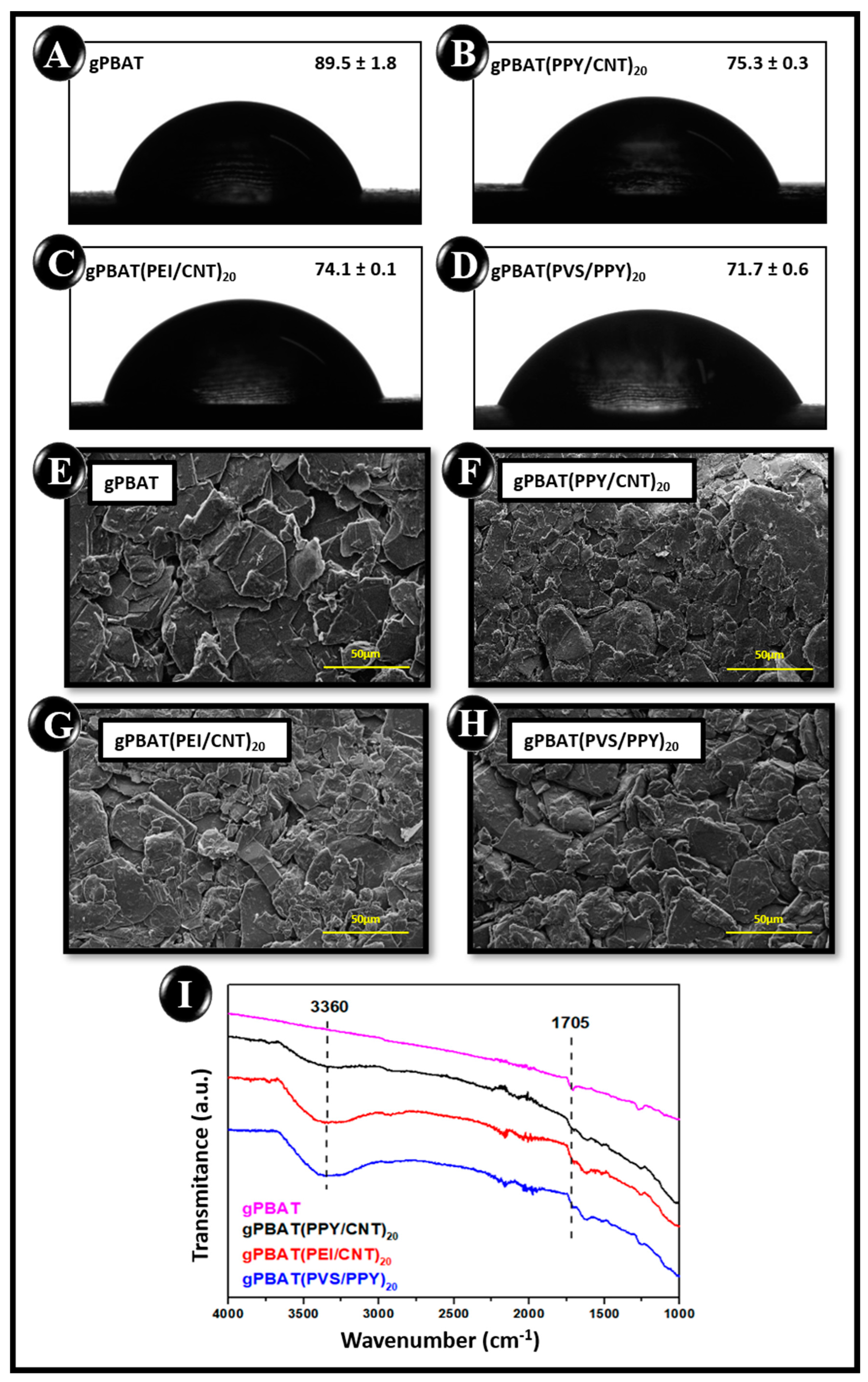
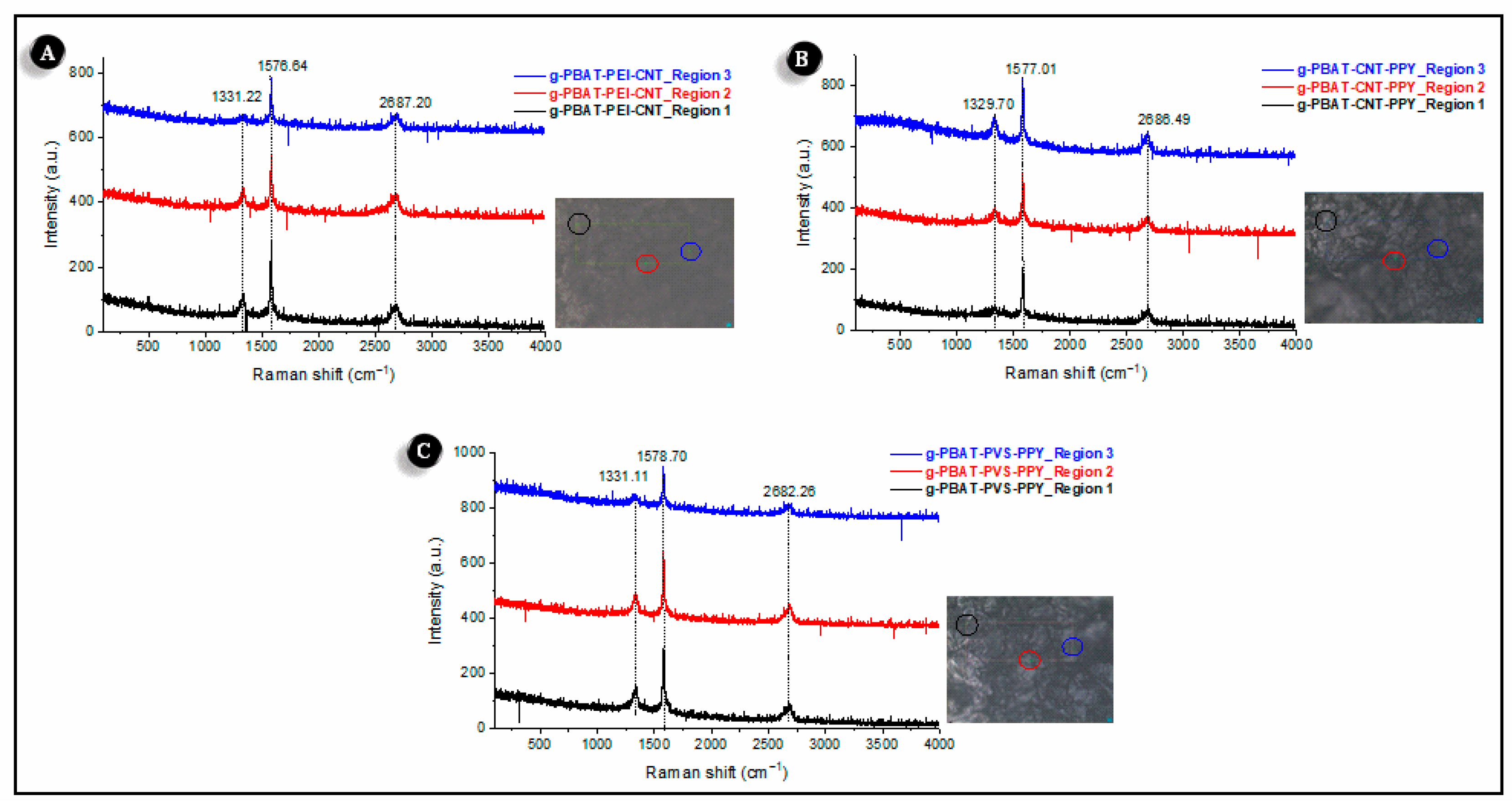
3.2. Physical Characterization
3.3. Zeta Potential of Nanostructured Films
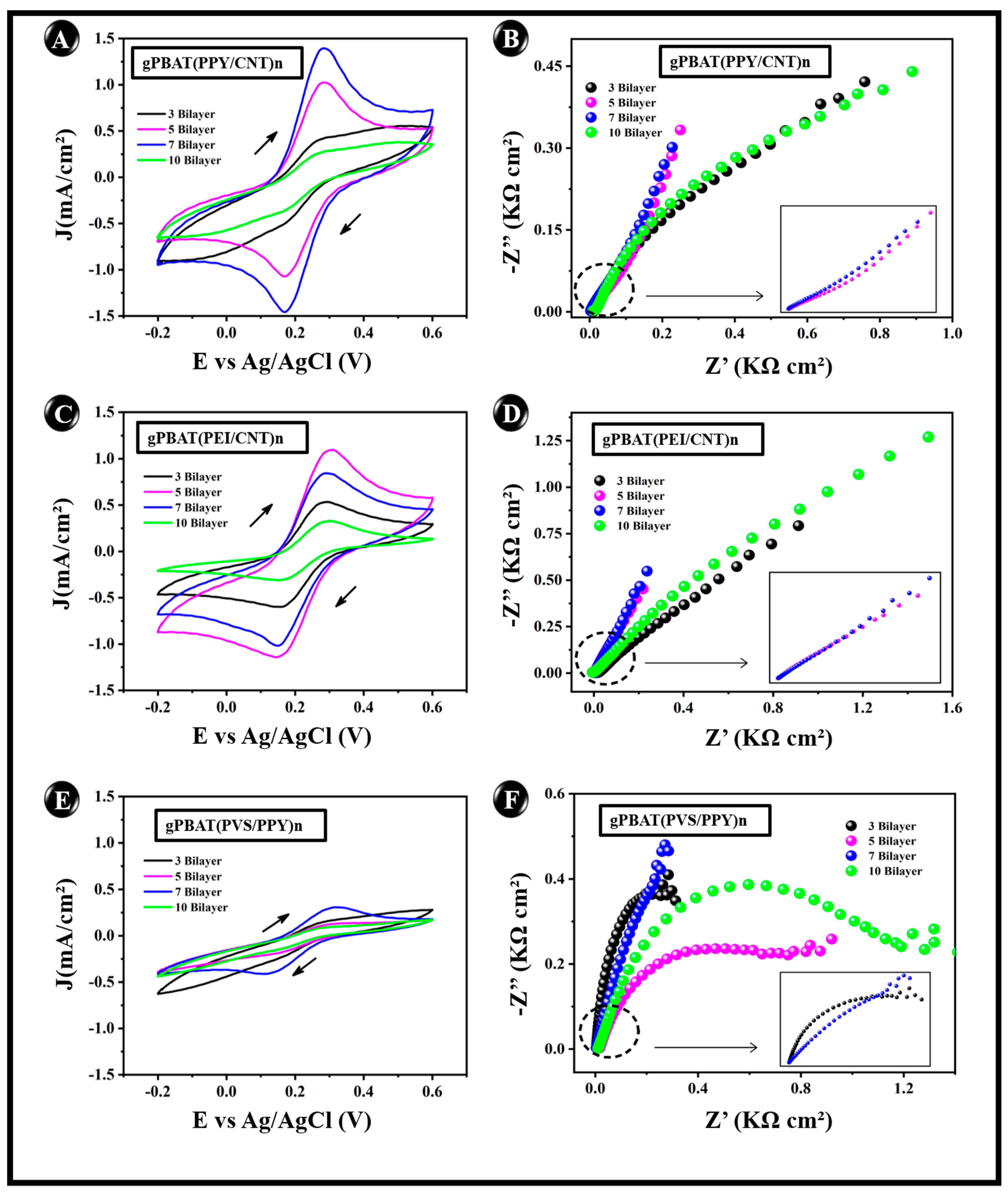
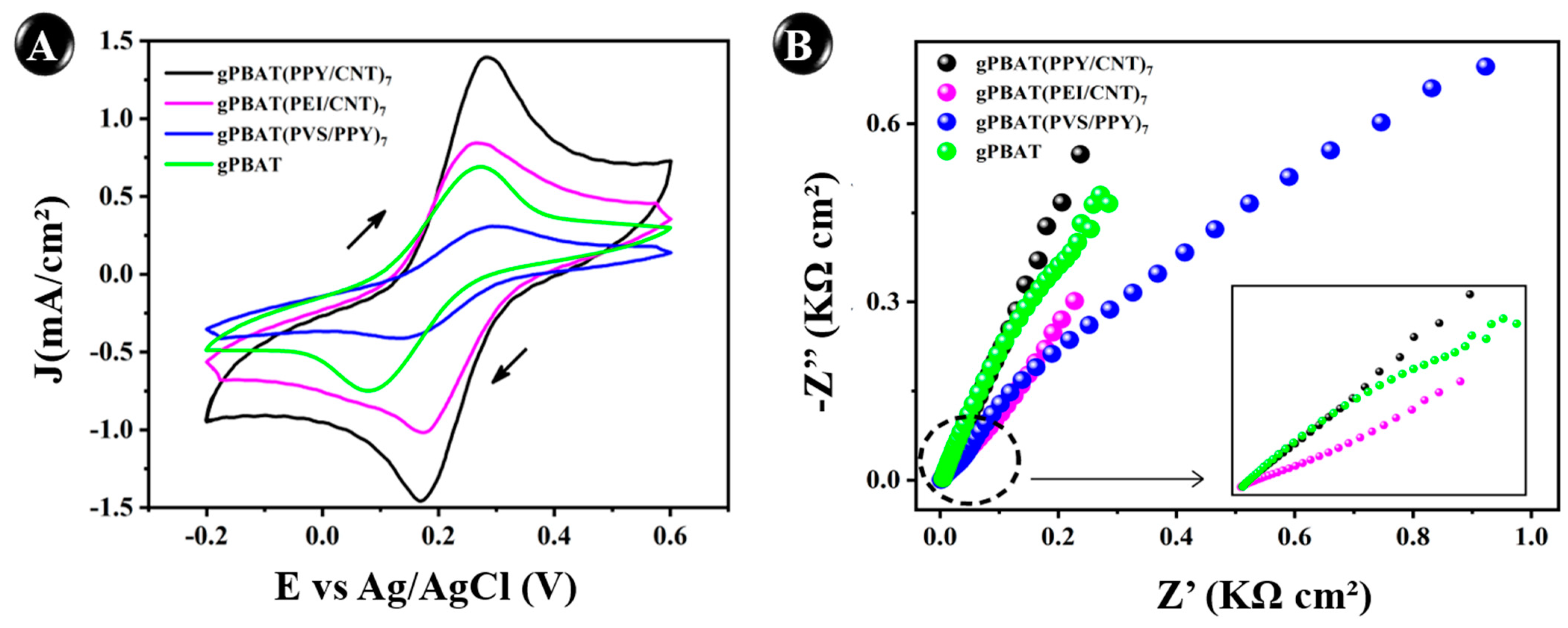
3.4. Electrochemical Behavior
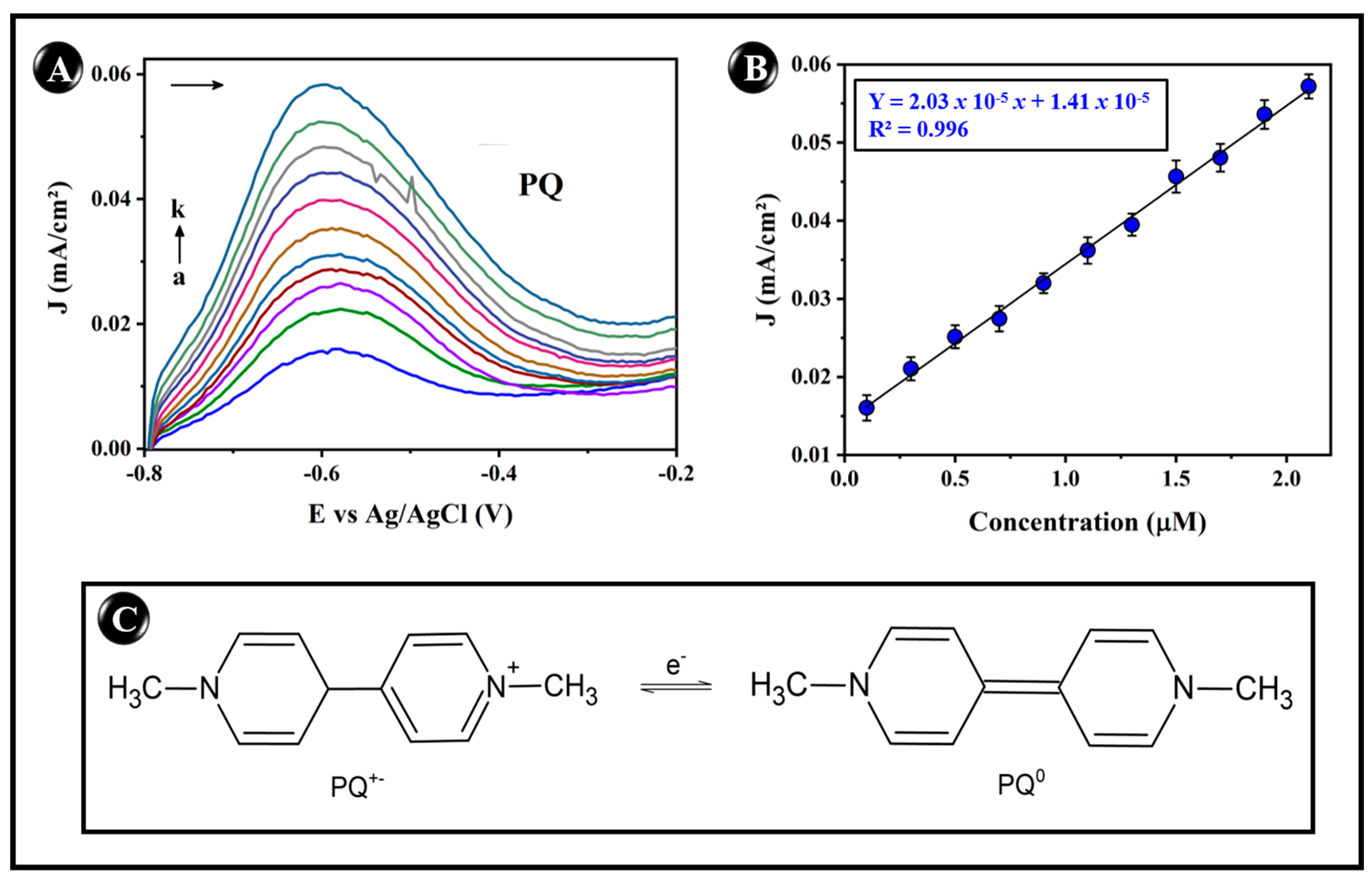
3.5. Electroanalytical Performance for the Detection of PQ
3.5.1. pH Study
3.5.2. Determination of PQ on Modified gPBAT(PPY/CNT)7 Sensors Using DPV
3.5.3. Analysis of Real Water Samples
3.5.4. Reproducibility, Repeatability, Stability, and Selectivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Massumi, S.; Ahmadi, E.; Akbari, A.; Gholivand, M.B. Highly sensitive and selective sensor based on molecularly imprinted polymer for voltammetric determination of Nevirapine in biological samples. J. Electroanal. Chem. 2020, 876, 114508. [Google Scholar] [CrossRef]
- Zhao, L.; Xia, W.; Zhang, P. Economical conductive graphite-filled polymer composites via adjustable segregated structures: Construction, low percolation threshold, and positive temperature coefficient effect. J. Appl. Polym. Sci. 2021, 138, e50295. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Tonda-Turo, C.; Mancuso, E.; Gentile, P. Multilayer nanoscale functionalization to treat disorders and enhance regeneration of bone tissue. Nanomed. Nanotechnol. Biol. Med. 2019, 19, 22–38. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Zhang, X.; Wu, H.; Shen, J.; Chen, R.; Xiong, Y.; Li, J.; Guo, S. Progress on the layer-by-layer assembly of multilayered polymer composites: Strategy, structural control and applications. Prog. Polym. Sci. 2019, 89, 76–107. [Google Scholar] [CrossRef]
- da Silva, J.L.; Buffon, E.; Beluomini, M.A.; Pradela-Filho, L.A.; Gouveia Araujo, D.A.; Santos, A.L.; Takeuchi, R.M.; Stradiotto, N.R. Non-enzymatic lactose molecularly imprinted sensor based on disposable graphite paper electrode. Anal. Chim. Acta 2021, 1143, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.; George, P. Conductive polymers to modulate the post-stroke neural environment. Brain Res. Bull. 2019, 148, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Beluomini, M.A.; da Silva, J.L.; de Sá, A.C.; Buffon, E.; Pereira, T.C.; Stradiotto, N.R. Electrochemical sensors based on molecularly imprinted polymer on nanostructured carbon materials: A review. J. Electroanal. Chem. 2019, 840, 343–366. [Google Scholar] [CrossRef]
- Jang, D.; Farooq, S.Z.; Yoon, H.N.; Khalid, H.R. Design of a highly flexible and sensitive multi-functional polymeric sensor incorporating CNTs and carbonyl iron powder. Compos. Sci. Technol. 2021, 207, 108725. [Google Scholar] [CrossRef]
- Zubkiewicz, A.; Szymczyk, A.; Franciszczak, P.; Kochmanska, A.; Janowska, I.; Paszkiewicz, S. Comparing multi-walled carbon nanotubes and halloysite nanotubes as reinforcements in EVA nanocomposites. Materials 2020, 13, 3809. [Google Scholar] [CrossRef]
- Ariga, K.; Ito, M.; Mori, T.; Watanabe, S.; Takeya, J. Atom/molecular nanoarchitectonics for devices and related applications. Nano Today 2019, 28, 100762. [Google Scholar] [CrossRef]
- Maciel, C.C.; Freitas, A.; Medrades, J.; Ferreira, M. Simultaneous Determination of Catechol and Paraquat Using a Flexible Electrode of PBAT and Graphite Modified with Gold Nanoparticles and Copper Phthalocyanine (g-PBAT/AuNP/CuTsPc) LbL Film. J. Electrochem. Soc. 2022, 169, 27505. [Google Scholar] [CrossRef]
- Rosenberger, A.G.; Dragunski, D.C.; Muniz, E.C.; Módenes, A.N.; Alves, H.J.; Tarley, C.R.T.; Machado, S.A.S.; Caetano, J. Electrospinning in the preparation of an electrochemical sensor based on carbon nanotubes. J. Mol. Liq. 2020, 298, 112068. [Google Scholar] [CrossRef]
- Maciel, C.C.; de Lima, L.F.; Ferreira, A.L.; de Araujo, W.R.; Ferreira, M. Development of a flexible and disposable electrochemical sensor based on poly (butylene adipate-co-terephthalate) and graphite for hydroquinone sensing. Sens. Actuators Rep. 2022, 4, 100091. [Google Scholar] [CrossRef]
- Nasir, T.; Herzog, G.; Hébrant, M.; Despas, C.; Liu, L.; Walcarius, A. Mesoporous Silica Thin Films for Improved Electrochemical Detection of Paraquat. ACS Sens. 2018, 3, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Sayyahmanesh, M.; Asgari, S.; Meibodi, A.S.E.; Ahooyi, T.M. Voltammetric Determination of Paraquat Using Graphite Pencil Electrode Modified with Doped Polypyrrole. Chem. Phys. 2016, 1, 1–11. [Google Scholar]
- Rashidipour, M.; Rasoulian, B.; Maleki, A.; Davari, B.; Pajouhi, N.; Mohammadi, E. Pectin/chitosan/tripolyphosphate encapsulation protects the rat lung from fibrosis and apoptosis induced by paraquat inhalation. Pestic. Biochem. Physiol. 2021, 178, 104919. [Google Scholar] [CrossRef]
- Maciel, C.C.; Barros, A.; Mazali, I.O.; Ferreira, M. Flexible biodegradable electrochemical sensor of PBAT and CNDs composite for the detection of emerging pollutants. J. Electroanal. Chem. 2023, 940, 117491. [Google Scholar] [CrossRef]
- Silva, J.S.; De Barros, A.; Constantino, C.J.L.; Simões, F.R.; Ferreira, M. Layer-by-layer films based on carbon nanotubes and polyaniline for detecting 2-chlorophenol. J. Nanosci. Nanotechnol. 2014, 14, 6586–6592. [Google Scholar] [CrossRef] [PubMed]
- Klunder, K.J.; Nilsson, Z.; Sambur, J.B.; Henry, C.S. Patternable Solvent-Processed Thermoplastic Graphite Electrodes. J. Am. Chem. Soc. 2017, 139, 12623–12631. [Google Scholar] [CrossRef]
- Perween, M.; Parmar, D.B.; Bhadu, G.R.; Srivastava, D.N. Polymer–graphite composite: A versatile use and throw plastic chip electrode. Analyst 2014, 139, 5919–5926. [Google Scholar] [CrossRef]
- Wei, Z.; Cai, C.; Huang, Y.; Wang, P.; Song, J.; Deng, L.; Fu, Y. Strong biodegradable cellulose materials with improved crystallinity via hydrogen bonding tailoring strategy for UV blocking and antioxidant activity. Int. J. Biol. Macromol. 2020, 164, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Michalska, M.; Andrzejczuk, M.; Krawczyńska, A.; Roguska, A.; Nikiforow, K.; Żurek, P.; Sikora, A.; Peikertová, P. The effect of MWCNT modification on structural and morphological properties of Li4Ti5O12. Diam. Relat. Mater. 2021, 113, 108276. [Google Scholar] [CrossRef]
- Krajewski, M.; Liao, P.Y.; Michalska, M.; Tokarczyk, M.; Lin, J.Y. Hybrid electrode composed of multiwall carbon nanotubes decorated with magnetite nanoparticles for aqueous supercapacitors. J. Energy Storage 2019, 26, 101020. [Google Scholar] [CrossRef]
- Kim, G.M.; Kil, T.; Lee, H.K. A novel physicomechanical approach to dispersion of carbon nanotubes in polypropylene composites. Compos. Struct. 2021, 258, 113377. [Google Scholar] [CrossRef]
- Zaman, A.C.; Kaya, F.; Kaya, C. A study on optimum surfactant to multiwalled carbon nanotube ratio in alcoholic stable suspensions via UV–Vis absorption spectroscopy and zeta potential analysis. Ceram. Int. 2020, 46, 29120–29129. [Google Scholar] [CrossRef]
- MacLeod, A.J. A note on the Randles-Sevcik function from electrochemistry. Appl. Math. Comput. 1993, 57, 305–310. [Google Scholar] [CrossRef]
- Henstridge, M.C.; Laborda, E.; Dickinson, E.J.F.; Compton, R.G. Redox systems obeying Marcus–Hush–Chidsey electrode kinetics do not obey the Randles–Ševčík equation for linear sweep voltammetry. J. Electroanal. Chem. 2012, 664, 73–79. [Google Scholar] [CrossRef]
- Hernández-Pérez, L.; Carrillo-Abad, J.; Ortega, E.M.; Pérez-Herranz, V.; Montañés, M.T.; Martí-Calatayud, M.C. Voltammetric and electrodeposition study for the recovery of antimony from effluents generated in the copper electrorefining process. J. Environ. Chem. Eng. 2023, 11, 109139. [Google Scholar] [CrossRef]
- Shanmugasundaram, K.; Sai-Anand, G.; Gopalan, A.I.; Lee, H.G.; Yeo, H.K.; Kang, S.W.; Lee, K.P. Direct electrochemistry of cytochrome c with three-dimensional nanoarchitectured multicomponent composite electrode and nitrite biosensing. Sens. Actuators B Chem. 2016, 228, 737–747. [Google Scholar] [CrossRef]
- Kalinke, C.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Carbon Paste Electrode Modified with Biochar for Sensitive Electrochemical Determination of Paraquat. Electroanalysis 2016, 28, 764–769. [Google Scholar] [CrossRef]
- de Souza, D.C.; Orzari, L.O.; de Oliveira, P.R.; Kalinke, C.; Bonacin, J.A.; Malaspina, O.; Nocelli, R.C.F.; Janegitz, B.C. Electrochemical Sensor Based on Beeswax and Carbon Black Thin Biofilms for Determination of Paraquat in Apis mellifera Honey. Food Anal. Methods 2021, 14, 606–615. [Google Scholar] [CrossRef]
- Nematollahi, D.; Shayani-Jam, H.; Alimoradi, M.; Niroomand, S. Electrochemical oxidation of acetaminophen in aqueous solutions: Kinetic evaluation of hydrolysis, hydroxylation and dimerization processes. Electrochim. Acta 2009, 54, 7407–7415. [Google Scholar] [CrossRef]
- Kenne Dedzo, G.; Péguy Nanseu-Njiki, C.; Ngameni, E. Amperometric sensors based on sawdust film modified electrodes: Application to the electroanalysis of paraquat. Talanta 2012, 99, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Gu, Y.; Wang, C. Fabrication of the Cu2O/polyvinyl pyrrolidone-graphene modified glassy carbon-rotating disk electrode and its application for sensitive detection of herbicide paraquat. Sens. Actuators B Chem. 2012, 173, 530–539. [Google Scholar] [CrossRef]
- Garcia, L.L.C.; Figueiredo-Filho, L.C.S.; Oliveira, G.G.; Fatibello-Filho, O.; Banks, C.E. Square-wave voltammetric determination of paraquat using a glassy carbon electrode modified with multiwalled carbon nanotubes within a dihexadecylhydrogenphosphate (DHP) film. Sens. Actuators B Chem. 2013, 181, 306–311. [Google Scholar] [CrossRef]
- Miller, J.C.; Miller, J.N. Basic Statistical Methods for Analytical Chemistry Part 1. Statistics of Repeated Measurements. A review. Analyst 1988, 113, 1351–1356. [Google Scholar] [CrossRef]
- Ziegel, E.R. Statistics and Chemometrics for Analytical Chemistry. Technometrics 2004, 46, 498–499. [Google Scholar] [CrossRef]
- de Lima, L.F.; Daikuzono, C.M.; Miyazaki, C.M.; Pereira, E.A.; Ferreira, M. Layer-by-Layer nanostructured films of magnetite nanoparticles and polypyrrole towards synergistic effect on methylparaben electrochemical detection. Appl. Surf. Sci. 2020, 505, 144278. [Google Scholar] [CrossRef]
- Miyazaki, C.M.; Pereira, T.P.; Tavares Mascagni, D.B.; de Moraes, M.L.; Ferreira, M. Monoamine oxidase B layer-by-layer film fabrication and characterization toward dopamine detection. Mater. Sci. Eng. C-Mater. Biol. Appl. 2016, 58, 310–315. [Google Scholar] [CrossRef]
- Lettieri, R.; Cardova, L.; Gatto, E.; Mazzuca, C.; Monti, D.; Palleschi, A.; Placidi, E.; Drasar, P.; Venanzi, M. Hierarchical transfer of chiral information from the molecular to the mesoscopic scale by Langmuir-Blodgett deposition of tetrasteroid-porphyrins. New J. Chem. 2017, 41, 639–649. [Google Scholar] [CrossRef]
- Marangoni, V.S.; Martins, M.V.A.; Souza, J.A.; Oliveira, O.N.; Zucolotto, V.; Crespilho, F.N. The processing of polyelectrolyte-covered magnetite nanoparticles in the form of nanostructured thin films. J. Nanopart. Res. 2012, 14, 176. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Carreira, C.A.; Lee, H.J.; Silva, F.; Martins, A.; Pereira, C.M. Voltammetric determination of paraquat at DNA-gold nanoparticle composite electrodes. Electrochim. Acta 2010, 55, 7892–7896. [Google Scholar] [CrossRef]
- Buleandra, M.; Rabinca, A.A.; Mihailciuc, C.; Balan, A.; Nichita, C.; Stamatin, I.; Ciucu, A.A. Screen-printed Prussian Blue modified electrode for simultaneous detection of hydroquinone and catechol. Sens. Actuators B Chem. 2014, 203, 824–832. [Google Scholar] [CrossRef]
- de Sá, A.C.; Barbosa, S.C.; Raymundo-Pereira, P.A.; Wilson, D.; Shimizu, F.M.; Raposo, M.; Oliveira, O.N. Flexible carbon electrodes for electrochemical detection of bisphenol-a, hydroquinone and catechol in water samples. Chemosensors 2020, 8, 103. [Google Scholar] [CrossRef]
- Della Pelle, F.; Angelini, C.; Sergi, M.; Del Carlo, M.; Pepe, A.; Compagnone, D. Nano carbon black-based screen printed sensor for carbofuran, isoprocarb, carbaryl and fenobucarb detection: Application to grain samples. Talanta 2018, 186, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Hu, Q.; Wu, J.; Li, X.; Li, P.; Yu, H.; Li, X.; Lei, F. Electrochemical sensor based on molecularly imprinted polymer reduced graphene oxide and gold nanoparticles modified electrode for detection of carbofuran. Sens. Actuators B Chem. 2015, 220, 216–221. [Google Scholar] [CrossRef]
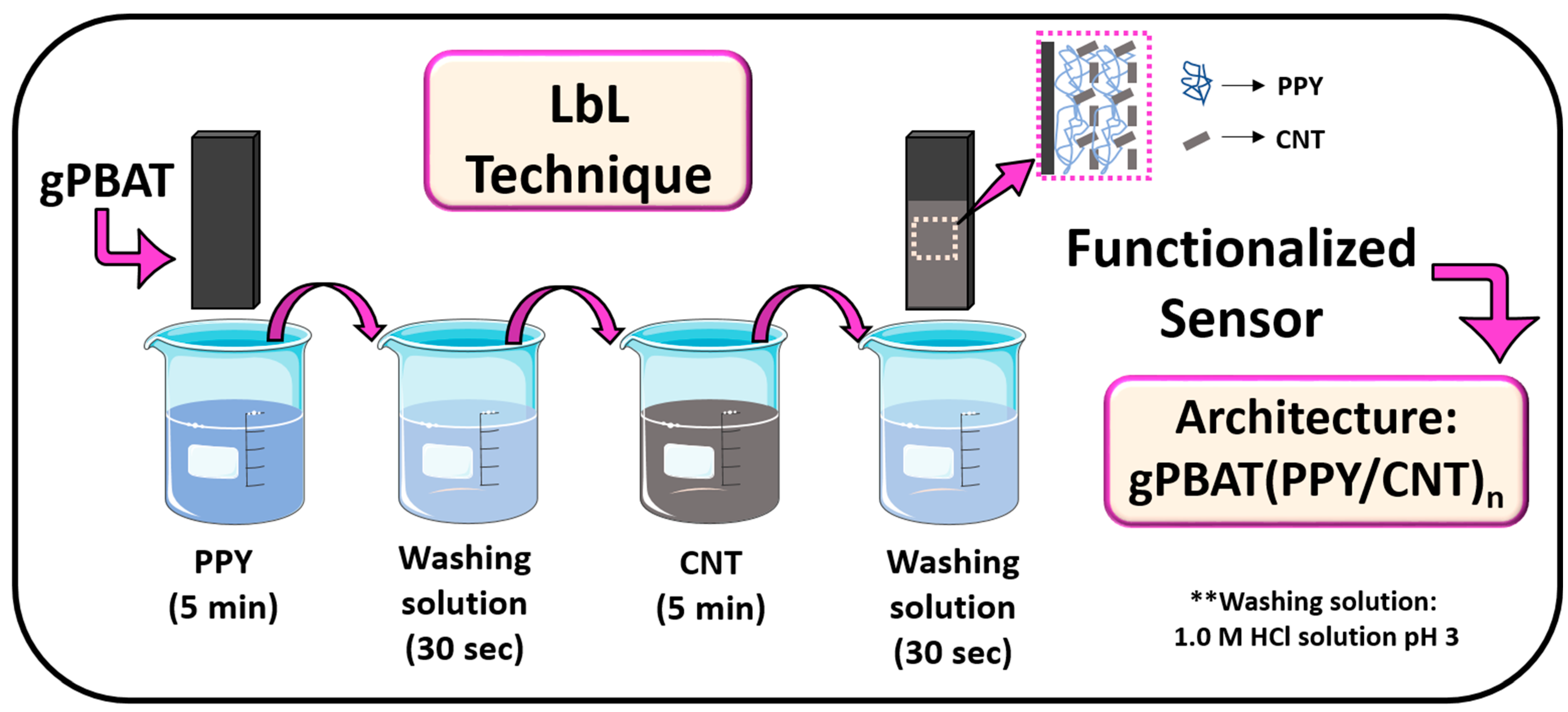

| Architectures | First Layer | Washing | Second Layer |
|---|---|---|---|
| gPBAT(PPY/CNT)n | PPY (5 min) | HCl solution pH 3.0 (30 s) | CNT (5 min) |
| gPBAT(PEI/ CNT)n | PEI (3 min) | HCl solution pH 3.0 (30 s) | CNT (5 min) |
| gPBAT(PVS/PPY)n | PVS (3 min) | HCl solution pH 3.0 (30 s) | PPY (5 min) |
| Film | ζ-Potential (mV) |
|---|---|
| gPBAT(PPY/CNT)7 | −65.19 |
| gPBAT(PEI/CNT)7 | −65.14 |
| gPBAT(PVS/PPY)7 | −50.52 |
| Parameters | ||||
|---|---|---|---|---|
| Electrode Unit | J (mA/cm²) | Rct (KΩ cm2) | A (cm²) | k0 (cm s−1) |
| gPBAT(PPY/CNT)7 | 1.41 ± 7.07 × 10−2 | 0.16 ± 0.02 | 0.84 | 3.96 × 10−4 |
| gPBAT(PEI/CNT)7 | 0.84 ± 4.52 × 10−2 | 0.31 ± 0.04 | 0.70 | 3.60 × 10−4 |
| gPBAT(PVS/PPY)7 | 0.31 ± 1.20 × 10−2 | 0.37 ± 0.08 | 0.36 | 2.16 × 10−4 |
| g-PBAT | 0.69 ± 4.80 × 10−2 | 0.33 ± 0.05 | 0.52 | 3.38 × 10−4 |
| Working Electrode | Sample | Linear Range (μM) | LOD (µM) | Reference |
|---|---|---|---|---|
| AuNPs/DNA/GE | PQ | 5–1000 | 1.3 | [42] |
| GPE/PPY MICP | PQ | 5–50 | 0.22 | [15] |
| Micro-Cu2O/PVP-GNs/GC-RDE | PQ | 1–200 | 0.26 | [34] |
| gPBAT/AuNP | PQ | 100–2000 | 1.31 | [11] |
| gPBAT(PPY/CNT)7 | PQ | 0.1–2.1 | 0.073 | This work |
| Sample | Added (mM) | Found (mM) | Recovery (%) |
|---|---|---|---|
| PQ | 0.50 | 0.55 | 109.2 |
| 1.00 | 1.03 | 103.4 | |
| 1.70 | 1.69 | 99.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaro, S.F.; Maciel, C.C.; Rodrigues, J.S.; de Freitas, A.d.S.M.; Fré, L.V.B.V.; de Barros, A.; Ferreira, M. Investigation of the Synergistic Effect of Layer-by-Layer Films of Carbon Nanotubes and Polypyrrole on a Flexible Electrochemical Device for Paraquat Sensing. Chemosensors 2023, 11, 420. https://doi.org/10.3390/chemosensors11080420
Amaro SF, Maciel CC, Rodrigues JS, de Freitas AdSM, Fré LVBV, de Barros A, Ferreira M. Investigation of the Synergistic Effect of Layer-by-Layer Films of Carbon Nanotubes and Polypyrrole on a Flexible Electrochemical Device for Paraquat Sensing. Chemosensors. 2023; 11(8):420. https://doi.org/10.3390/chemosensors11080420
Chicago/Turabian StyleAmaro, Stefanny F., Cristiane C. Maciel, Jéssica S. Rodrigues, Amanda de S. M. de Freitas, Lucas V. B. V. Fré, Anerise de Barros, and Marystela Ferreira. 2023. "Investigation of the Synergistic Effect of Layer-by-Layer Films of Carbon Nanotubes and Polypyrrole on a Flexible Electrochemical Device for Paraquat Sensing" Chemosensors 11, no. 8: 420. https://doi.org/10.3390/chemosensors11080420
APA StyleAmaro, S. F., Maciel, C. C., Rodrigues, J. S., de Freitas, A. d. S. M., Fré, L. V. B. V., de Barros, A., & Ferreira, M. (2023). Investigation of the Synergistic Effect of Layer-by-Layer Films of Carbon Nanotubes and Polypyrrole on a Flexible Electrochemical Device for Paraquat Sensing. Chemosensors, 11(8), 420. https://doi.org/10.3390/chemosensors11080420







