A DFT Study on Adsorption of SF6 Decomposition Products on Zr-MOF-808
Abstract
1. Introduction
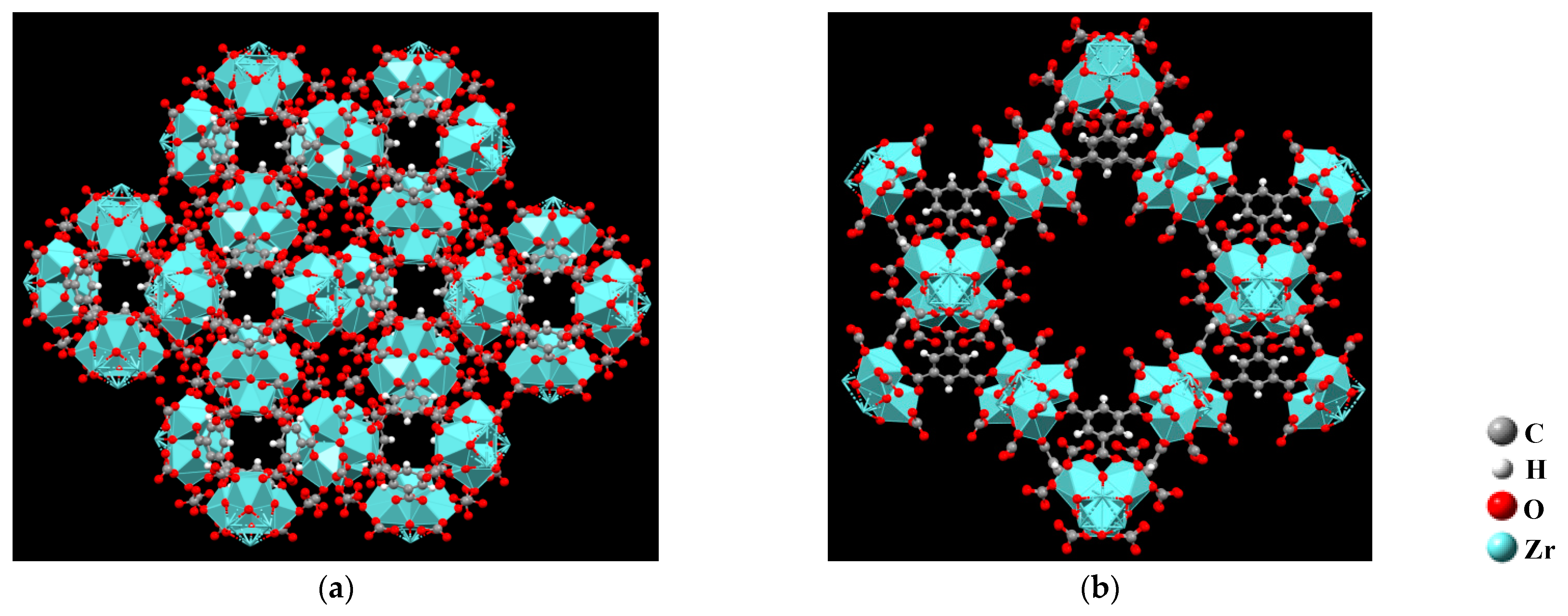
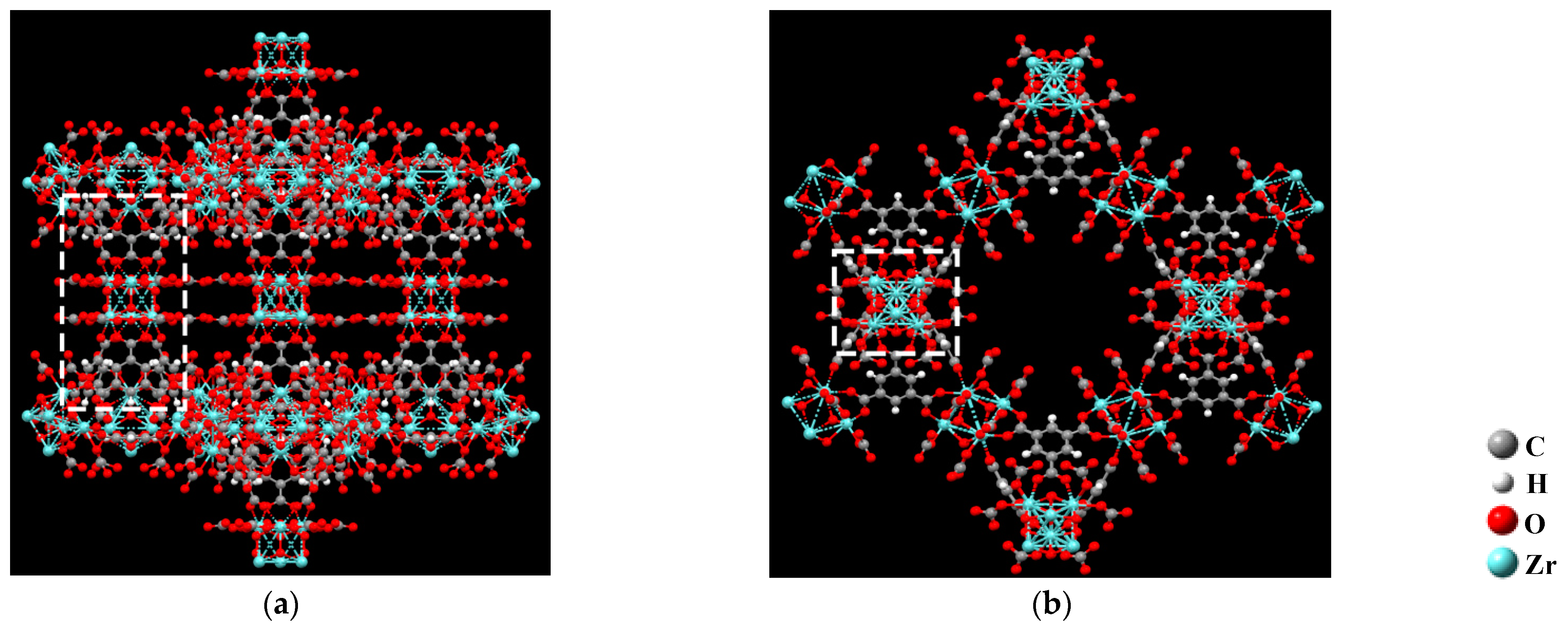
2. Calculation Methods
3. Results and Discussion
3.1. Parameters of Adsorption
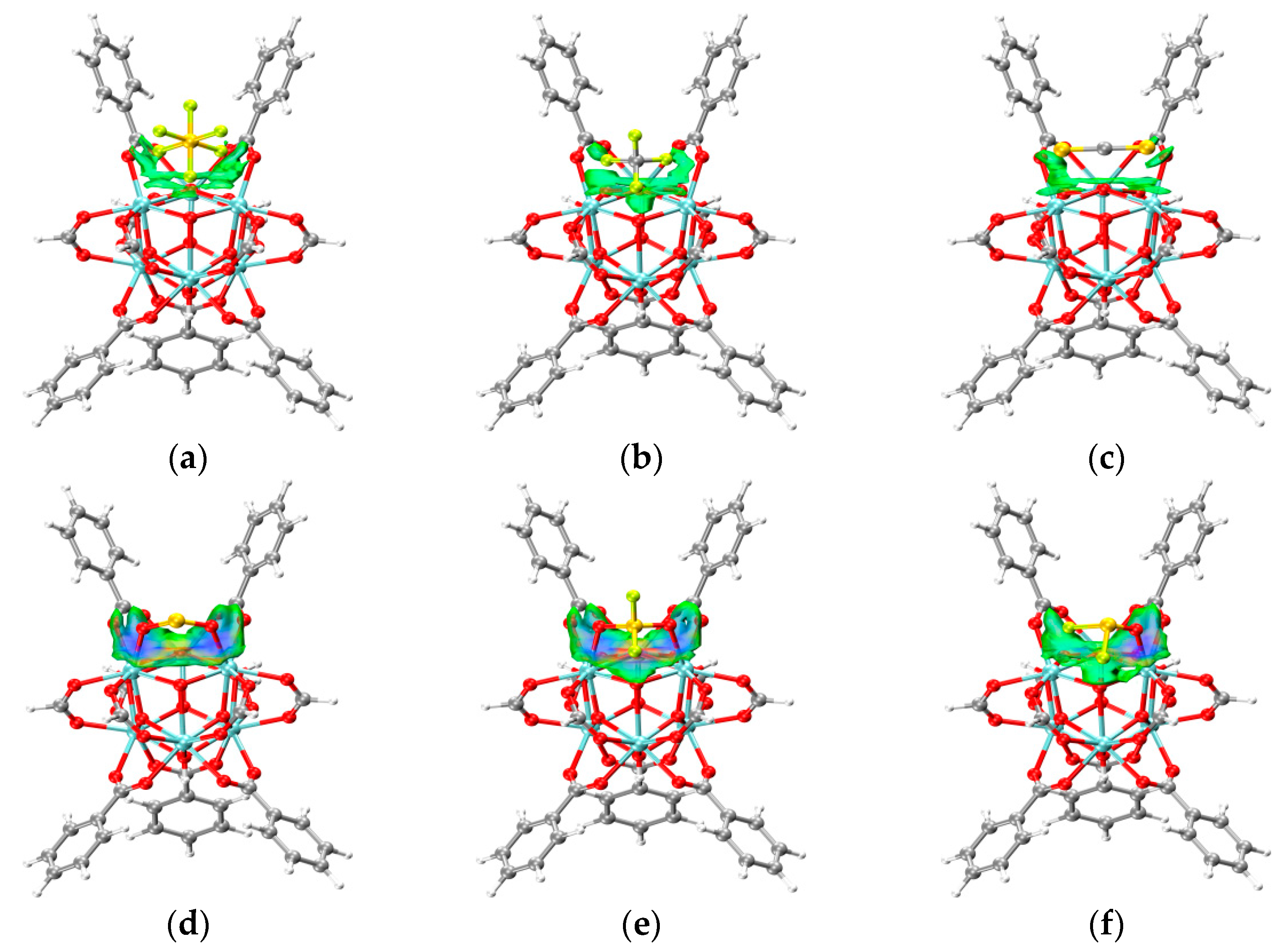
3.2. IGMH Model of the System after Adsorption
3.3. Changes in Bond Length and Bond Angle after the Adsorption of Gas Molecules
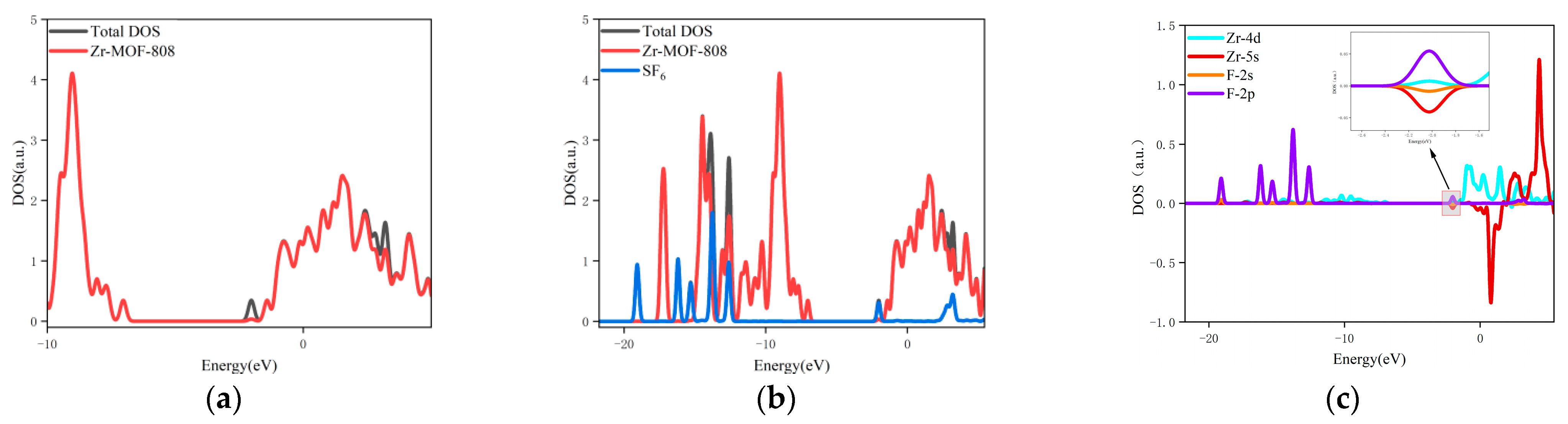
3.4. The Changes in the Orbital Occupation of Each System before and after Gas Adsorption
- The changes in the DOS of Zr-MOF-808 Cluster 1 after SF6 adsorption are shown in Figure 6a–c.
- 2.
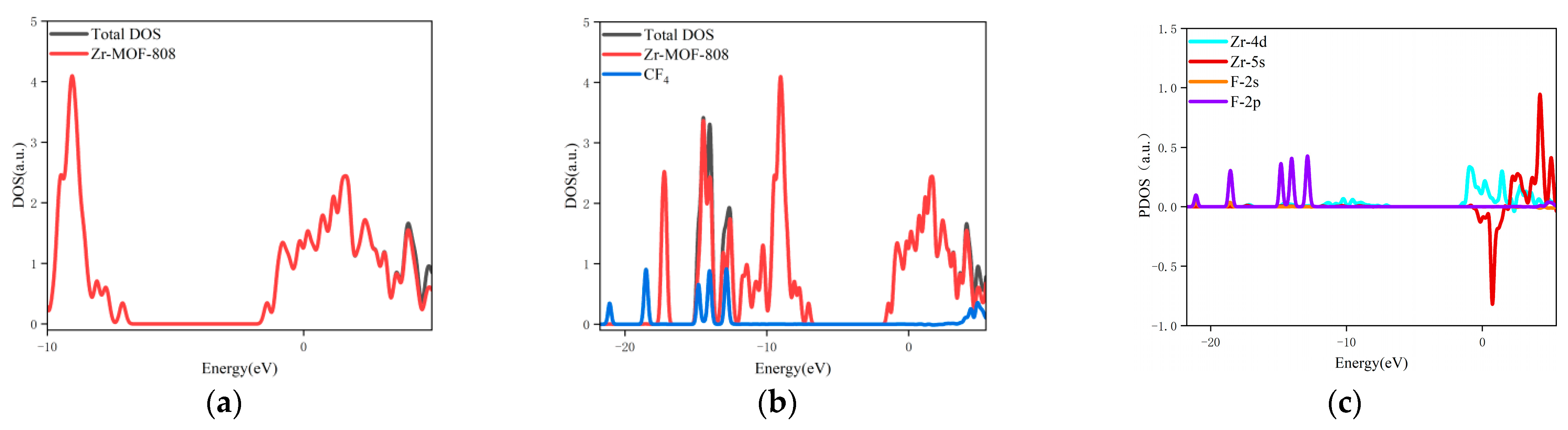
- The changes in the DOS of Zr-MOF-808 Cluster 1 after CF4 adsorption are shown in Figure 7a–c.
- 3.
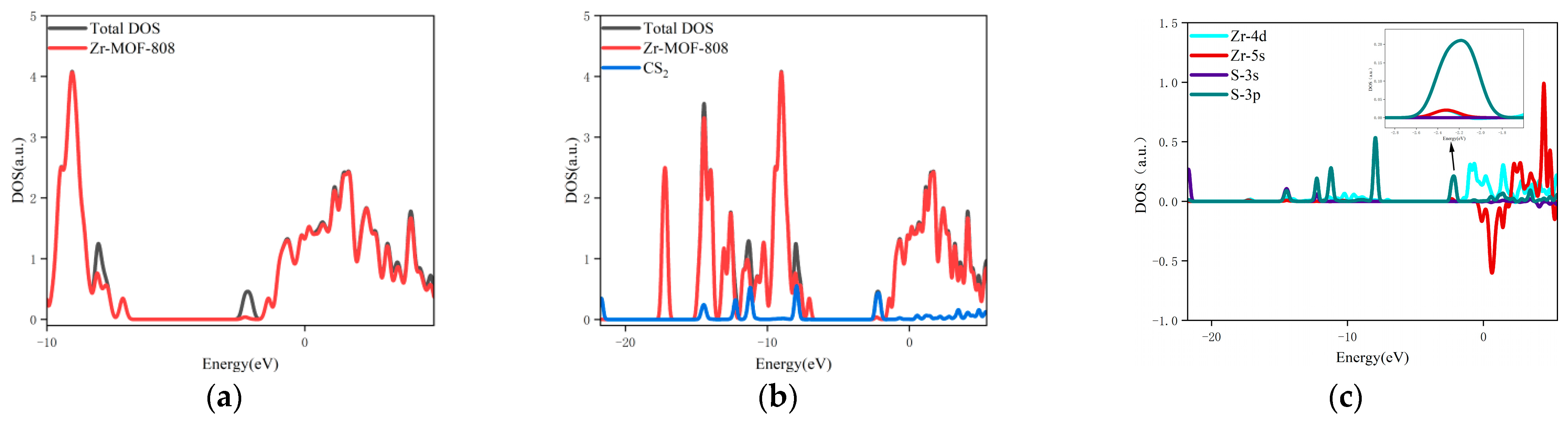
- The changes in the DOS of Zr-MOF-808 Cluster 1 after CS2 adsorption are shown in Figure 8a–c.
- 4.
- The changes in the DOS of Zr-MOF-808 Cluster 1 after SO2 adsorption are shown in Figure 9a–c.
- 5.
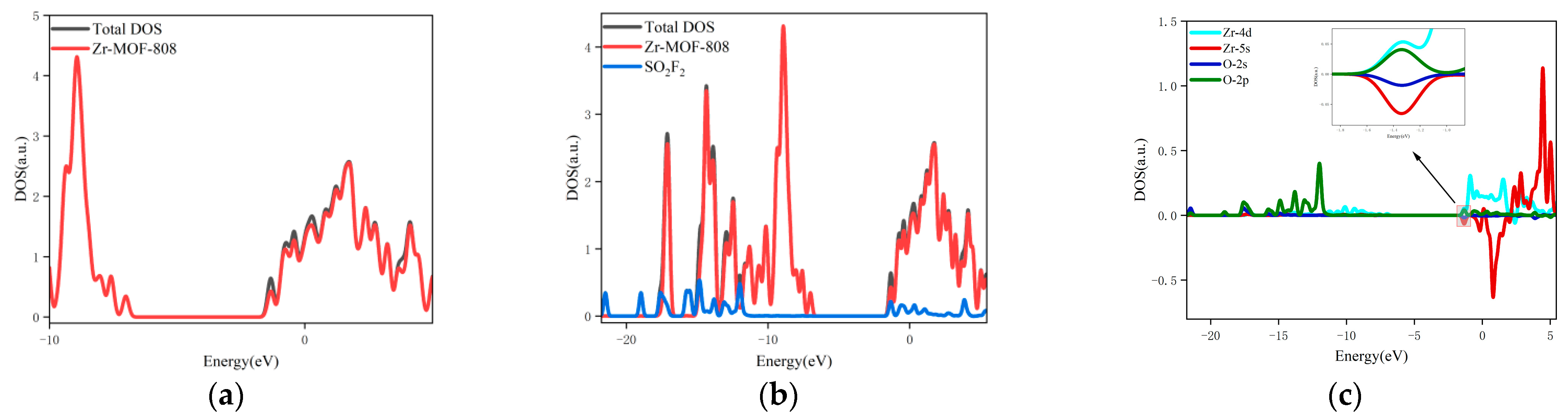
- The changes in the DOS of Zr-MOF-808 Cluster 1 after SO2F2 adsorption are shown in Figure 10a–c.
- 6.
- The changes in the DOS of Zr-MOF-808 Cluster 1 after SOF2 adsorption are shown in Figure 11a–c.
3.5. Conductivity Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaghi, O.M.; Li, H. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Kalaj, M.; Barcus, K.S.; Cohen, S.M. Self-Assembly of MOF Nanoparticle Monolayer sand Free-Standing Multilayers. J. Am. Chem. Soc. 2019, 141, 20000–20003. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Banda, H.; Chen, M.; Niu, L.; Chen, M.; Wu, T.; Wang, J.; Wang, R.; Feng, J.; Chen, T.; et al. Molecular understanding of charge storage and charging dynamics in supercapacitors with MOF electrodes and ionic liquid electrolytes. Nat. Mater. 2020, 19, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.A.; Zhou, H.-C. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef]
- Hosono, N.; Uemura, T. Development of Functional Materials via Polymer Encapsulation into Meta-Organic Frameworks. B. Chem. Soc. JPN. 2021, 94, 2139–2148. [Google Scholar] [CrossRef]
- Gonzalez, M.I.; Turkiewicz, A.B.; Darago, L.E.; Oktawiec, J.; Bustillo, K.; Grandjean, F.; Long, G.J.; Long, J.R. Confinement of atomically defined-metal halide sheets in a metal-organic framework. Nature 2020, 577, 64–68. [Google Scholar] [CrossRef]
- Reddy, R.C.K.; Lin, J.; Chen, Y.; Zeng, C.; Lin, X.; Cai, Y.; Su, C.-Y. Progress of nanostructured metal oxides derived from metal-organic frameworks as anode materials for lithium-ion Batteries. Coordin. Chem. Rev. 2020, 420, 213434. [Google Scholar] [CrossRef]
- Luo, L.; Lo, W.-S.; Si, X.; Li, H.; Wu, Y.; An, Y.; Zhu, Q.; Chou, L.-Y.; Li, T.; Tsung, C.-K. Directional Engraving within SingleCrystalline Metal-Organic Framework Particles via Oxidative Linker Cleaving. J. Am. Chem. Soc. 2019, 141, 20365–20370. [Google Scholar] [CrossRef]
- Feng, L.; Yuan, S.; Zhang, L.-L.; Tan, K.; Li, J.-L.; Kirchon, A.; Liu, L.-M.; Zhang, P.; Han, Y.; Chabal, Y.J.; et al. Creating Hierarchical Pores by Controlled Linker Thermolysis in Multivariate Metal-Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 2363–2372. [Google Scholar] [CrossRef]
- Karadeniz, B.; Žili, D.; Huskic, I.; Germann, L.S.; Fidelli, A.M.; Muratović, S.; Lončarić, I.; Etter, M.; Dinnebier, R.E.; Barišić, D.; et al. Controlling the polymorphism and topology transformation in porphyrinic zirconium metal-organic frameworks via mechanochemistry. J. Am. Chem. Soc. 2019, 141, 19214–19220. [Google Scholar] [CrossRef]
- Han, Y.; Chen, Y.; Ma, Y.; Bailey, J.; Wang, Z.; Lee, D.; Sheveleva, A.M.; Tuna, F.; McInnes, E.J.L.; Frogley, M.D.; et al. Control of the pore chemistry in metal-organic frameworks for efficient adsorption of benzene and separation of benzene/cyclohexane. Chem 2023, 9, 739–754. [Google Scholar] [CrossRef]
- Xu, W.; Thapa, K.B.; Ju, Q.; Fang, Z.; Huang, W. Heterogeneous catalysts based on mesoporous metal-organic frameworks. Coordin. Chem. Rev. 2018, 373, 199–232. [Google Scholar]
- Chen, B.; Eddaoudi, M.; Hyde, S.T.; O’Keeffe, M.; Yaghi, O.M. Interwoven metal-organic framework on a periodic minimal surface with extra-large pores. Science 2001, 291, 1021–1023. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, J.; Xu, Q.; Wu, X.-T.; Zhu, Q.-L. Pore surface engineering of metal-organic frameworks for heterogeneous catalysis. Coordin. Chem. Rev. 2018, 376, 248–276. [Google Scholar] [CrossRef]
- Hou, C.-C.; Xu, Q. Metal-Organic Frameworks for Energy. Adv. Energy Mater. 2018, 9, 1801307. [Google Scholar] [CrossRef]
- Fu, X.-P.; Wang, Y.-L.; Zhang, X.-F.; Krishna, R.; He, C.-T.; Liu, Q.-Y.; Chen, B. Collaborative pore partition and pore surface fluorination within a metal-organic framework for high-performance C2H2/CO2 separation. Chem. Eng. J. 2022, 432, 134433. [Google Scholar] [CrossRef]
- Tu, T.N.; Nguyen, H.T.D.; Tran, N.T. Tailoring the pore size and shape of the one-dimensional channels in iron-based MOFs for enhancing the methane storage capacity. Inorg. Chem. Front. 2019, 6, 2441–2447. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, X.; Huang, H.; Zhang, Z.; Yildirim, T.; Zhou, W.; Xiang, S.; Chen, B. A microporous aluminum-based metal-organic framework for high methane, hydrogen, and carbon dioxide storage. Nano Res. 2021, 14, 507–511. [Google Scholar] [CrossRef]
- Li, J.; Han, X.; Zhang, X.; Sheveleva, A.M.; Cheng, Y.; Tuna, F.; McInnes, E.J.L.; McCormick McPherson, L.J.; Teat, S.J.; Daemen, L.L.; et al. Capture of nitrogen dioxide and conversion to nitric acid in a porous metal-organic framework. Nat. Chem. 2019, 11, 1085–1090. [Google Scholar] [CrossRef]
- Peng, S.; Bie, B.; Sun, Y.; Liu, M.; Cong, H.; Zhou, W.; Xia, Y.; Tang, H.; Deng, H.; Zhou, X. Metal-organic frameworks for precise inclusion of single-stranded DNA and transfection in immune cells. Nat. Commun. 2018, 9, 1293. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lv, N.; Xue, L.; Liu, B.; Feng, J.; Ren, X.; Guo, T.; Chen, D.; Stoddart, J.F.; Gref, R.; et al. Composite CD-MOF nanocrystals-containing micro-spheres for sustained drug delivery. Nanoscale 2017, 9, 7454–7463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhuang, S.; Liao, L.; Wang, C.; Xia, N.; Gan, Z.; Gu, W.; Li, J.; Deng, H.; Wu, Z. A Dual Purpose Strategy to Endow Gold Nanoclusters with Both Catalysis Activity and Water Solubility. J. Am. Chem. Soc. 2020, 142, 973–977. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, Z.; Wang, Y.; Wang, J.; Guo, Y.; Hu, H.; He, X.; Wang, C.; Lin, W. Photo-Activation of Cu Centers in Metal-Organic Frame- works for Selective CO2 Conversion to Ethanol. J. Am. Chem. Soc. 2020, 142, 75–79. [Google Scholar] [CrossRef]
- Liang, W.; Bhatt, P.M.; Shkurenko, A.; Adil, K.; Mouchaham, G.; Aggarwal, H.; Mallick, A.; Jamal, A.; Belmabkhout, Y.; Eddaoudi, M. A Tailor-Made Interpenetrated MOF with Exceptional Carbon-Capture Performance from Flue Gas. Chem 2019, 5, 950–963. [Google Scholar] [CrossRef]
- Gupta, N.K.; López-Olvera, A.; González-Zamora, E.; Martínez-Ahumada, E.; Ibarra, I.A. Sulfur Dioxide Capture in Metal-Organic Frameworks, Metal-Organic Cages, and Porous Organic Cages. ChemPlusChem 2022, 87, e202200131. [Google Scholar] [CrossRef] [PubMed]
- Brandt, P.; Nuhnen, A.; Lange, M.; Möllmer, J.; Weingart, O.; Janiak, C. Metal-Organic Frameworks with Potential Application for SO2 Separation and Flue Gas Desulfurization. ACS Appl. Mater. Inter. 2019, 11, 17350–17358. [Google Scholar] [CrossRef] [PubMed]
- Belmabkhout, Y.; Bhatt, P.M.; Adil, K.; Pillai, R.S.; Cadiau, A.; Shkurenko, A.; Maurin, G.; Liu, G.; Koros, W.J.; Eddaoudi, M. Natural gas upgrading using a fluorinated MOF with tuned H2S and CO2 adsorption selectivity. Nat. Energy 2018, 3, 1059–1066. [Google Scholar] [CrossRef]
- Zhuang, Y.; Hu, X.; Tang, B.; Wang, S.; Cui, A.; Hou, K.; He, Y.; Zhu, L.; Li, W.; Chu, J. Effects of SF6 decomposition components and concentrations on the discharge faults and insulation defects in GIS equipment. Sci. Rep. 2020, 10, 15039. [Google Scholar] [CrossRef]
- Cho, Y.S.; Hong, T.Y.; Youn, Y.W.; Sun, J.H.; Lee, S.-H. Study on the Correlation between Partial Discharge Energy and SF6 Decomposition Gas Generation. Energies 2020, 13, 4655. [Google Scholar] [CrossRef]
- Zeng, F.; Lei, Z.; Yang, X.; Tang, J.; Yao, Q.; Miao, Y. Evaluating DC Partial Discharge with SF6 Decomposition Characteristics. IEEE Trans. Power Deliv. 2019, 34, 1383–1392. [Google Scholar] [CrossRef]
- Rao, X.; Tang, J.; Zeng, F.; Li, D.; Xia, X.; Su, Y.; Lu, Y. Mechanism of Trace O2 on SF6 Characteristic Decomposed Components Under Spark Discharge. Plasma Chem. Plasma Process. 2020, 40, 469–481. [Google Scholar] [CrossRef]
- Liu, M. Decomposing Mechanism of SF6 under Positive DC Partial Discharge in the Presence of Trace H2O. ACS Omega 2020, 5, 13389–13395. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, F.; Zhang, X.; Pan, J.; Yao, Q.; Hou, X.; Tang, Y. Relationship between Decomposition Gas Ratios and Partial Discharge Energy in GIS, and the Influence of Residual Water and Oxygen. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1226–1234. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, S.; Xiao, S.; Huang, Y.; Liu, F. Partial discharge decomposition characteristics of typical defects in the gas chamber of SF6 insulated ring network cabinet. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1794–1801. [Google Scholar] [CrossRef]
- Beyer, C.; Jenett, H.; Kfockow, D. Influence of Reactive SFx, Gases on Electrode Surfaces After Electrical Discharges under SF6, Atmosphere. IEEE Trans. Dielectr. Electr. Insul. 2000, 7, 234–240. [Google Scholar] [CrossRef]
- Ammar, S.M.; Zulkurnain, A.-M.; Rai, N.A. SF6 Decomposed Component Analysis for Partial Discharge Diagnosis in GIS: A Review. IEEE Access 2022, 10, 27270–27288. [Google Scholar]
- Tang, J.; Yang, X.; Yang, D.; Yao, Q.; Miao, Y.; Zhang, C.; Zeng, F. Using SF6 Decomposed Component Analysis for the Diagnosis of Partial Discharge Severity Initiated by Free Metal Particle Defect. Energies 2017, 10, 1119. [Google Scholar] [CrossRef]
- Zeng, F.; Tang, J.; Zhang, X.; Xie, Y.; Yao, Q.; Miao, Y. Reconstructing and extracting information on SF6 decomposition characteristic components induced by partial overthermal fault in GIE. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 183–193. [Google Scholar] [CrossRef]
- Yang, A.; Li, W.; Chu, J.; Wang, D.; Yuan, H.; Zhu, J.; Wang, X.; Rong, M. Enhanced sensing of sulfur hexafluoride decomposition components based on noble-metal-functionalized cerium oxide. Mater. Design 2020, 187, 08391. [Google Scholar] [CrossRef]
- Wang, Y.; Gui, Y.; Ji, C.; Tang, C.; Zhou, Q.; Li, J.; Zhang, X. Adsorption of SF6 decomposition components on Pt3-TiO2(101) surface: A DFT study. Appl. Surf. Sci. 2018, 459, 242–248. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Hu, K.; Li, T.; Yan, Y.; Li, J. The adsorption and sensing performances of Ir-modified MoS2 monolayer toward SF6 decomposition products: A DFT study. Nanomaterials 2021, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liao, Y. Selective detection of SO2 in SF6 insulation devices by Rh- doped HfSe2 monolayer: A first-principles study. Appl. Phys. A Mater. 2019, 125, 468. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Tang, J.; Cui, H.; Chen, Z.; Li, Y. Different doping of penta-graphene as adsorbent and gas sensing material for scavenging and detecting SF6 decomposed species. Sustain. Mater. Technol. 2019, 21, e00100. [Google Scholar] [CrossRef]
- Chen, D.; Tang, J.; Zhang, X.; Fang, J.; Li, Y.; Zhuo, R. Detecting decompositions of sulfur hexafluoride using reduced graphene oxide decorated with Pt nanoparticles. J. Phys. D Appl. Phys. 2018, 51, 185304. [Google Scholar] [CrossRef]
- Furukawa, H.; Gándara, F.; Zhang, Y.-B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water Adsorption in Porous Metal–Organic Frameworks and Related Materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef]
- Sciortino, G.; Lihi, N.; Czine, T.; Maréchal, J.-D.; Lledós, A.; Garribba, E. Accurate prediction of vertical electronic transitions of Ni(II) coordination compounds via time dependent density functional theory. Int. J. Quantum Chem. 2018, 118, e25655. [Google Scholar] [CrossRef]
- Lei, T.X.; Fan, X.Z.; Lv, F.C.; Jiang, B.W. Theoretical Study on Adsorption Behavior of SF6 Decomposition Components on Mg-MOF-74. Nanomaterials 2023, 13, 1705. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Yan, H.; Chen, H.; Mao, W.; Dai, G. Insight into the nature of the noncovalent interactions of furan, pyridine, and pyrazine with AtX. J. Mol. Model. 2023, 29, 13. [Google Scholar] [CrossRef]
- Li, S.; Zhu, S.; Zhou, Q.; Gui, Y.; Wei, X. Adsorption mechanism of decomposition gas of SF6 circuit breaker on MOF-505 analogue. Vacuum 2021, 183, 109816. [Google Scholar] [CrossRef]
- Tian, L.; Qinxue, C. Independent gradient model based on Hirshfeld partition (IGMH): A new method for visual study of interactions in chemical systems. J. Comput. Chem. 2022, 43, 539–555. [Google Scholar]
- Pomogaeva, A.V.; Scheer, M.; Timoshkin, A.Y. Why Do B–P and Al–P Polymers Differ? Structures, Stability, and Electronic Properties of Chain and Ring [H2PEH2]n Oligomers (E=B, Al; n = 1–15). Chem. Eur. J. 2018, 24, 17046–17054. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.Q.; Mai, T.; Pham-Tran, N.-N. Engineering of Band Gap in Metal-Organic Frameworks by Functionalizing Organic Linker: A Systematic Density Functional Theory Investigation. J. Phys. Chem. C 2014, 118, 4567–4577. [Google Scholar] [CrossRef]





| Cluster | Gas | SF6 | CF4 | CS2 | SO2 | SO2F2 | SOF2 |
|---|---|---|---|---|---|---|---|
| Cluster 1 | Adsorption energy (eV) | −0.218 | −0.223 | −0.354 | −0.544 | −0.526 | −0.399 |
| transfer charge (e) | 0.150 | 0.145 | 0.147 | 0.321 | 0.299 | 0.282 | |
| Adsorption distance (Å) | 3.083 | 3.077 | 3.235 | 2.223 | 2.562 | 2.532 | |
| Cluster 2 | Adsorption energy (eV) | −0.225 | −0.218 | −0.338 | −0.599 | −0.518 | −0.451 |
| transfer charge (e) | 0.150 | 0.145 | 0.147 | 0.321 | 0.299 | 0.282 | |
| Adsorption distance (Å) | 3.083 | 3.077 | 3.235 | 2.223 | 2.562 | 2.532 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, T.; Lv, F.; Jiang, B. A DFT Study on Adsorption of SF6 Decomposition Products on Zr-MOF-808. Chemosensors 2023, 11, 402. https://doi.org/10.3390/chemosensors11070402
Lei T, Lv F, Jiang B. A DFT Study on Adsorption of SF6 Decomposition Products on Zr-MOF-808. Chemosensors. 2023; 11(7):402. https://doi.org/10.3390/chemosensors11070402
Chicago/Turabian StyleLei, Tianxiang, Fangcheng Lv, and Bowen Jiang. 2023. "A DFT Study on Adsorption of SF6 Decomposition Products on Zr-MOF-808" Chemosensors 11, no. 7: 402. https://doi.org/10.3390/chemosensors11070402
APA StyleLei, T., Lv, F., & Jiang, B. (2023). A DFT Study on Adsorption of SF6 Decomposition Products on Zr-MOF-808. Chemosensors, 11(7), 402. https://doi.org/10.3390/chemosensors11070402






