Biochar for Water Pollution Control: From Sensing to Decontamination
Abstract
1. Introduction
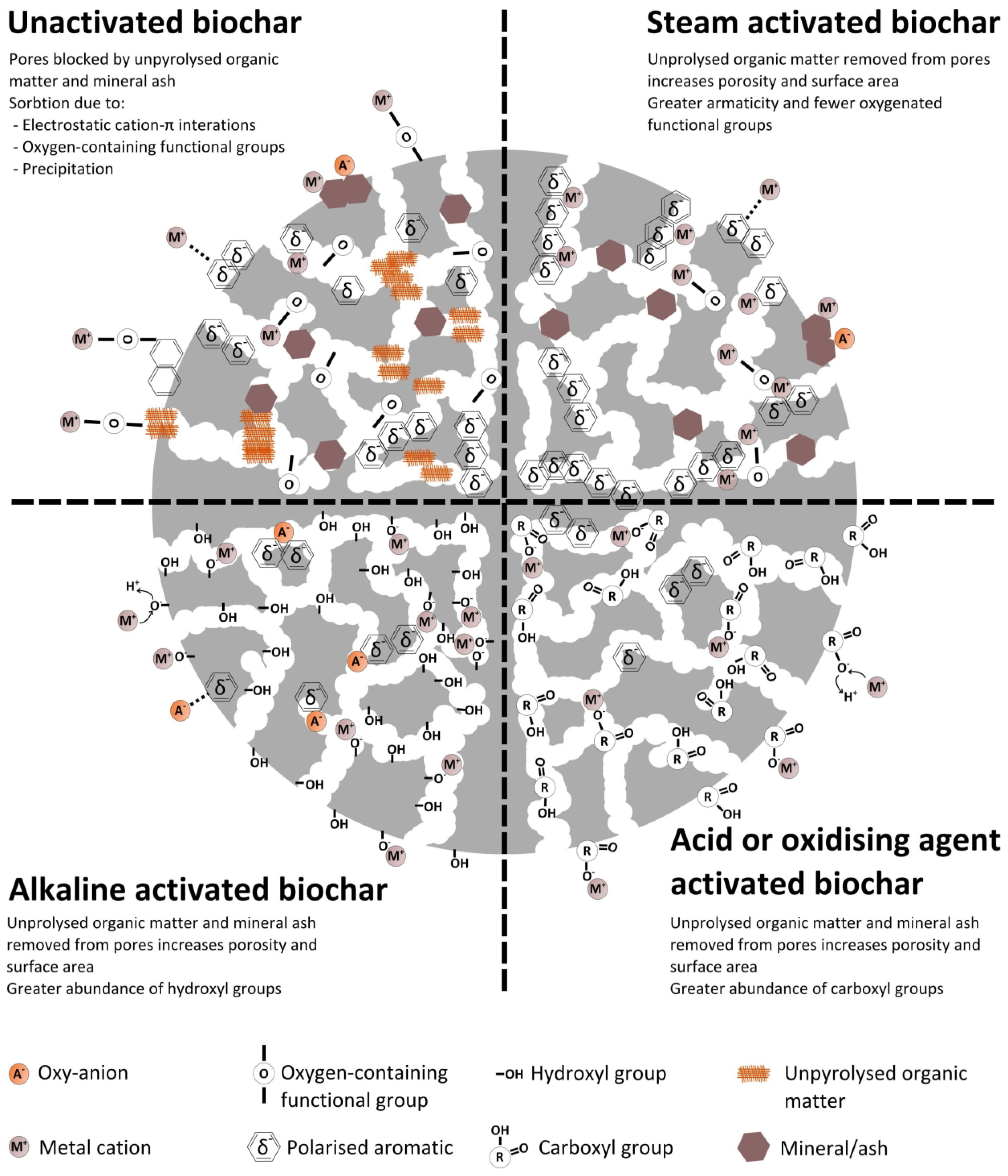
2. Treatment-Dependent Sorption Capacity of Biochar
2.1. Acid Modification
2.2. Alkaline Modification
2.3. Modification with Metal Salts or Oxides
2.4. Steam Modification
| Analyte | Biochar Feedstock, Pyrolysis Temperature | Driving Adsorption Mechanism | Adsorption Capacity [mg/g] | pH | Ref. |
|---|---|---|---|---|---|
| Paracetamol | Glucose, 900 °C | Pore-filling, H-bonding, n–π, π–π interactions | 286 | 2–11 | [18] |
| Paracetamol | Softwood, 550 °C | Van der Waals forces and H-bonding | 40 | 6–9 | [19] |
| Paracetamol | Municipal waste, 500 °C | Chemical reactions with oxygenated functional groups | 33.3 | 2 | [20] |
| Paracetamol | Pine chips, 300 °C | π–π interactions | N/S | 6.5 | [21] |
| Diclofenac | Waste sludge and leaves, 200 °C | Electrostatic interaction | 877 | 6.5 | [22] |
| Diclofenac | Plant waste, 600 °C | Pore-filling, van der Waals forces, π–π interactions | 23.3 | 6 | [23] |
| Diclofenac | Fish scales, 600 °C | Interactions with N, P, sp2 C, and C=O surface groups | 967.1 | 1.5–4.1 | [24] |
| Diclofenac | Sewage sludge, 600 °C | π–π interactions, H-bonding | 92.7 | 3–4 | [25] |
| Naproxen | Peanut shells, 800 °C | Pore-filling, π–π interactions | 324 | 5 | [26] |
| Naproxen | Sewage sludge, 600 °C | π–π interactions, H-bonding | 127 | 2–11 | [25] |
| Ibuprofen | Tamarind seeds, N/S | Chemical reactions with oxygenated functional groups | 10.5 | 2 | [27] |
| Ibuprofen | Pepper stems, 700 °C | Pore-filling, π–π interactions, H-bonding | 569 | 4 | [28] |
| Ibuprofen | Walnut shells, 450 °C | Pore-filling, π–π interactions, H-bonding | 69.7 | 4 | [29] |
| Ibuprofen | Alligator weed, 600 °C | Chemical and physical adsorption, not specified further | 172 | 4 | [30] |
| Caffeine | Tea waste, 700 °C | Electrostatic and nucleophilic interactions | 15.4 | 3.5 | [31] |
| Caffeine | Macrophytes, 750 °C | Chemisorption, not specified further | 117.8 | 2–11 | [32] |
| Caffeine | Pine needles, 650 °C | Electrostatic interactions | 6.54 | 4 | [33] |
| Caffeine | Sugarcane pulp, 850 °C | Pore-filling, π–π interactions, H-bonding | 4.72 | 2–11 | [34] |
| Caffeine | Pine needles, 900 °C | Chemical and physical adsorption, not specified further | 11.85 | N/S | [35] |
| Malachite Green | Crab shells, 800 °C | π–π interactions, H-bonding | 28140 | 6–8 | [36] |
| Malachite Green | Chinese fan palm, 500 °C | N/S | 21.4 | 7 | [37] |
| Basic Red 46 | Chrysanthemum flowers, 200 °C | Electrostatic and functional groups interactions, π–π interactions, H-bonding | 53.19 | 7 | [38] |
| Acid Orange 7 | Mandarin peels, 150 °C | Electrostatic interactions | 312.5 | 2 | [39] |
| Acid Orange 7 | Pea peels, 105 °C | Electrostatic interactions | 523.1 | 2 | [40] |
| Reactive Yellow 145 | Nutshells, 400 °C | N/S | 7.33 | 2 | [41] |
| Indosol Black | Wood waste, N/S | Electrostatic interactions | 185 | 2 | [42] |
| Reactive Red 120 | Microalgae, 600 °C | Electrostatic and surface groups interactions | 331.9 | <4 | [43] |
| Acidic Blue 7, 120 | Sewage sludge, 200 °C | N/S | 99% removal | 2 | [44] |
| Eriochrome Black | Rice husk waste, 600 °C | N/S | 94% removal | 2 | [45] |
| Congo Red | Orange peel waste, 700 °C | Electrostatic interactions | 136 | 2–3 | [46] |
| Congo Red | Leather shavings, 900 °C | Surface groups interactions | 1916 | 7 | [47] |
| Methylene Blue | Cardboard, 525 °C | Chemisorption, not specified further | 25.1 | 6.5 | [48] |
| Methylene Blue | Municipal waste, 300 °C | π–π interactions | 7.2 | 5 | [14] |
| Methylene Blue | Municipal waste, 500 °C | Electrostatic interactions | 35 | Unaffected | [20] |
| As (V) | Municipal waste, 600 °C | Electrostatic interactions, precipitation | 28 | 6 | [12] |
| As (III) | Rice husk, 700 °C | Formation of complexes between As and biochar surface groups | 19.3 | 8 | [49] |
| As (III) | Pine bark, 400 °C | Ion-exchange | 13.1 | 5 | [50] |
| Cd (II) | Sewage sludge, 900 °C | Ion exchange and surface precipitation | 40 | 2 | [51] |
| Cd (II) | Coconut shells, 400 °C | Ion exchange and surface precipitation | 205 | 5 | [52] |
| Pb (II) | Pinewood, 300 °C | N/S | 4.25 | 5 | [53] |
| Pb (II) | Cotton stalk, 650 °C | Chemisorption | 147 | 2 | [54] |
| Hg (II) | Wood chips, 600 °C | Formation of Hg–C(π) bonds, Hg interaction with carboxyl groups | 107.5 | 6 | [55] |
| Hg (0) | Municipal waste, 600 °C | Chemisorption and subsequent transformation of Hg0 to HgCl2 by Cl–Cl bonds | 0.16 | N/S | [56] |
3. Biochar-Integrated Sensing
3.1. Conventional Carbon-Based Sensors
3.2. Carbon Paste Sensors
3.3. Screen-Printed Sensors
3.4. Innovative Biochar-Based Sensors
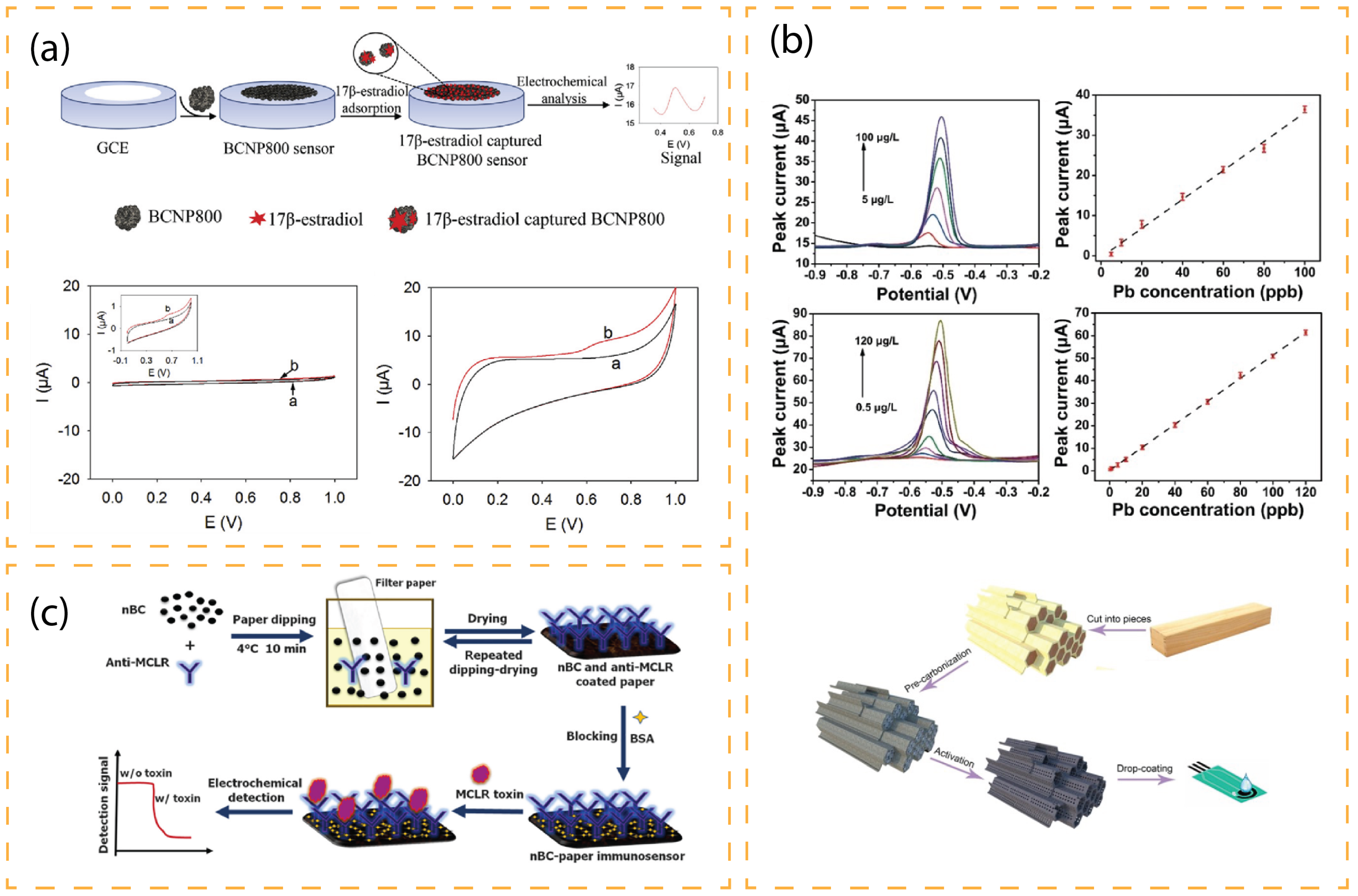
| Analyte | Matrix | Electrode | Treatment | Method | LOD [nM] | Ref. |
|---|---|---|---|---|---|---|
| 17β-estradiol | Water | GCE | HCl for BC NPs dispersion | CV, DPV | 11.3 | [57] |
| Bisphenol-A | Water | GCE | BC NPs | CV | 3.18 | [62] |
| Bisphenol-A | Tap and Ground Water | GCE | HCl for BC NPs and ZnO dispersion | DPV | 100 | [67] |
| Catechol | Tap Water | GCE | AuNPs decorated BC | CV, DPV | 9 | [68] |
| Catechol | Tap Water | GCE | AuNPs decorated BC | CV, DPV | 4 | [68] |
| Glyphosate | River Water | CPE | CuHPc integrated BC | SWV | 20 | [63] |
| Hydroquinone | Tap Water | GCE | AuNPs decorated BC | CV, DPV | 3.4 | [68] |
| Hydroquinone | Tap Water | GCE | AuNPs decorated BC | CV, DPV | 2 | [68] |
| Methyl Parathion | Potable Water | CPE | HNO3 oxidized 10% (v/v) BC | DPAdSV | 39 | [60] |
| Microcystin-LR | Tap, Lake, and River water | paper | Atb MCLR coated BC NPs | CA | 0.017 | [66] |
| Paraquat | Water | CPE | 20% (v/v) BC | DPAdSV | 7.5 | [69] |
| Paraquat | Wastewater | CPE | HNO3 oxidized BC and rGO | DPAdSV | 20 | [70] |
| Cd (II) | River Water | GCE | Nanodiamonds and Chitosan modified BC | SWASV | 110 | [71] |
| Cd (II) | Industrial Wastewater | CPE | 0–30% (v/v) BC | DPAdSV | 69 | [72] |
| Hg (II) | Tap Water | GCE | KOH activated BC on MOF film | DPAdSV | 1 | [73] |
| Pb (II) | Potable Water | SPE | Hierarchical porous tubular BC | SWASV | 20 | [64] |
| Pb (II) | River Water | GCE | Nanodiamonds and Chitosan modified BC | SWASV | 56 | [71] |
| Pb (II) | Industrial Wastewater | CPE | 0–30% BC | DPAdSV | 9.8 | [72] |
| Pb (II) | Tap Water | GCE | KOH activated BC on MOF film | DPAdSV | 1 | [73] |
| Ni (II) | Discharge Water | CPE | HNO3 oxidized 15% BC | CV | 250 | [61] |
| Nitrite | Mineral and Tap Water | GCE | Eggshell membrane and copper ions modified BC | CV, DPV, CA | 630 | [74] |
4. Use of Biochar in Water Decontamination
4.1. Organic Pollutants
4.1.1. Non-Steroidal Anti-Inflammatory Drugs
4.1.2. Stimulants
4.1.3. Organic Dyes
4.2. Heavy Metals
5. Perspectives in Real-Time Monitoring of Decontamination
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Margot, J.; Rossi, L.; Barry, D.A.; Holliger, C. A Review of the Fate of Micropollutants in Wastewater Treatment Plants. Wiley Interdiscip. Rev. Water 2015, 2, 457–487. [Google Scholar] [CrossRef]
- Lehmann, J.; Cowie, A.; Masiello, C.A.; Kammann, C.; Woolf, D.; Amonette, J.E.; Cayuela, M.L.; Camps-Arbestain, M.; Whitman, T. Biochar in Climate Change Mitigation. Nat. Geosci. 2021, 14, 883–892. [Google Scholar] [CrossRef]
- Monticelli, D.; Binda, G.; Spanu, D.; Cancelliere, R.; Cianciaruso, M.; Carbone, K.; Micheli, L. Biochar: A Sustainable Alternative in the Development of Electrochemical Printed Platforms. Chemosensors 2022, 10, 344. [Google Scholar] [CrossRef]
- de Almeida, L.S.; Oreste, E.Q.; Maciel, J.V.; Heinemann, M.G.; Dias, D. Electrochemical Devices Obtained from Biochar: Advances in Renewable and Environmentally-Friendly Technologies Applied to Analytical Chemistry. Trends Environ. Anal. Chem. 2020, 26, e00089. [Google Scholar] [CrossRef]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar Modification to Enhance Sorption of Inorganics from Water. Bioresour. Technol. 2017, 246, 34–47. [Google Scholar] [CrossRef]
- Peng, H.; Gao, P.; Chu, G.; Pan, B.; Peng, J.; Xing, B. Enhanced Adsorption of Cu(II) and Cd(II) by Phosphoric Acid-Modified Biochars. Environ. Pollut. 2017, 229, 846–853. [Google Scholar] [CrossRef]
- Peng, P.; Lang, Y.H.; Wang, X.M. Adsorption Behavior and Mechanism of Pentachlorophenol on Reed Biochars: PH Effect, Pyrolysis Temperature, Hydrochloric Acid Treatment and Isotherms. Ecol. Eng. 2016, 90, 225–233. [Google Scholar] [CrossRef]
- Huff, M.D.; Lee, J.W. Biochar-Surface Oxygenation with Hydrogen Peroxide. J. Environ. Manag. 2016, 165, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cao, X.; Zhao, L. Comparison of Rice Husk- and Dairy Manure-Derived Biochars for Simultaneously Removing Heavy Metals from Aqueous Solutions: Role of Mineral Components in Biochars. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef]
- Petrović, J.T.; Stojanović, M.D.; Milojković, J.V.; Petrović, M.S.; Šoštarić, T.D.; Laušević, M.D.; Mihajlović, M.L. Alkali Modified Hydrochar of Grape Pomace as a Perspective Adsorbent of Pb2+ from Aqueous Solution. J. Environ. Manag. 2016, 182, 292–300. [Google Scholar] [CrossRef]
- Sun, K.; Tang, J.; Gong, Y.; Zhang, H. Characterization of Potassium Hydroxide (KOH) Modified Hydrochars from Different Feedstocks for Enhanced Removal of Heavy Metals from Water. Environ. Sci. Pollut. Res. 2015, 22, 16640–16651. [Google Scholar] [CrossRef]
- Jin, H.; Capareda, S.; Chang, Z.; Gao, J.; Xu, Y.; Zhang, J. Biochar Pyrolytically Produced from Municipal Solid Wastes for Aqueous As(V) Removal: Adsorption Property and Its Improvement with KOH Activation. Bioresour. Technol. 2014, 169, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Preparation, Modification and Environmental Application of Biochar: A Review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Hoslett, J.; Ghazal, H.; Mohamad, N.; Jouhara, H. Removal of Methylene Blue from Aqueous Solutions by Biochar Prepared from the Pyrolysis of Mixed Municipal Discarded Material. Sci. Total Environ. 2020, 714, 136832. [Google Scholar] [CrossRef] [PubMed]
- Micháleková-Richveisová, B.; Frišták, V.; Pipíška, M.; Ďuriška, L.; Moreno-Jimenez, E.; Soja, G. Iron-Impregnated Biochars as Effective Phosphate Sorption Materials. Environ. Sci. Pollut. Res. Int. 2017, 24, 463–475. [Google Scholar] [CrossRef]
- Frišták, V.; Micháleková-Richveisová, B.; Víglašová, E.; Ďuriška, L.; Galamboš, M.; Moreno-Jimenéz, E.; Pipíška, M.; Soja, G. Sorption Separation of Eu and As from Single-Component Systems by Fe-Modified Biochar: Kinetic and Equilibrium Study. J. Iran. Chem. Soc. 2017, 14, 521–530. [Google Scholar] [CrossRef]
- Shim, T.; Yoo, J.; Ryu, C.; Park, Y.K.; Jung, J. Effect of Steam Activation of Biochar Produced from a Giant Miscanthus on Copper Sorption and Toxicity. Bioresour. Technol. 2015, 197, 85–90. [Google Scholar] [CrossRef]
- Tran, H.N.; Tomul, F.; Thi Hoang Ha, N.; Nguyen, D.T.; Lima, E.C.; Le, G.T.; Chang, C.T.; Masindi, V.; Woo, S.H. Innovative Spherical Biochar for Pharmaceutical Removal from Water: Insight into Adsorption Mechanism. J. Hazard. Mater. 2020, 394, 122255. [Google Scholar] [CrossRef]
- Solanki, A.; Boyer, T.H. Physical-Chemical Interactions between Pharmaceuticals and Biochar in Synthetic and Real Urine. Chemosphere 2019, 218, 818–826. [Google Scholar] [CrossRef]
- Sumalinog, D.A.G.; Capareda, S.C.; de Luna, M.D.G. Evaluation of the Effectiveness and Mechanisms of Acetaminophen and Methylene Blue Dye Adsorption on Activated Biochar Derived from Municipal Solid Wastes. J. Environ. Manag. 2018, 210, 255–262. [Google Scholar] [CrossRef]
- Jung, C.; Oh, J.; Yoon, Y. Removal of Acetaminophen and Naproxen by Combined Coagulation and Adsorption Using Biochar: Influence of Combined Sewer Overflow Components. Environ. Sci. Pollut. Res. Int. 2015, 22, 10058–10069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tu, Y.J.; Duan, Y.P.; Liu, J.; Zhi, W.; Tang, Y.; Xiao, L.S.; Meng, L. Production of Biochar from Waste Sludge/Leaf for Fast and Efficient Removal of Diclofenac. J. Mol. Liq. 2020, 299, 112193. [Google Scholar] [CrossRef]
- Xu, D.; Li, Z.; Wang, P.; Bai, W.; Wang, H. Aquatic Plant-Derived Biochars Produced in Different Pyrolytic Conditions: Spectroscopic Studies and Adsorption Behavior of Diclofenac Sodium in Water Media. Sustain. Chem. Pharm. 2020, 17, 100275. [Google Scholar] [CrossRef]
- Xie, J.; Liu, M.; He, M.; Liu, Y.; Li, J.; Yu, F.; Lv, Y.; Lin, C.; Ye, X. Ultra-Efficient Adsorption of Diclofenac Sodium on Fish-Scale Biochar Functionalized with H3PO4 via Synergistic Mechanisms. Environ. Pollut. 2023, 322, 121226. [Google Scholar] [CrossRef]
- Czech, B.; Kończak, M.; Rakowska, M.; Oleszczuk, P. Engineered Biochars from Organic Wastes for the Adsorption of Diclofenac, Naproxen and Triclosan from Water Systems. J. Clean. Prod. 2021, 288, 125686. [Google Scholar] [CrossRef]
- Tomul, F.; Arslan, Y.; Kabak, B.; Trak, D.; Kendüzler, E.; Lima, E.C.; Tran, H.N. Peanut Shells-Derived Biochars Prepared from Different Carbonization Processes: Comparison of Characterization and Mechanism of Naproxen Adsorption in Water. Sci. Total Environ. 2020, 726, 137828. [Google Scholar] [CrossRef]
- Show, S.; Karmakar, B.; Halder, G. Sorptive Uptake of Anti-Inflammatory Drug Ibuprofen by Waste Biomass–Derived Biochar: Experimental and Statistical Analysis. Biomass Convers. Biorefin. 2022, 12, 3955–3973. [Google Scholar] [CrossRef]
- Naima, A.; Ammar, F.; Abdelkader, O.; Rachid, C.; Lynda, H.; Syafiuddin, A.; Boopathy, R. Development of a Novel and Efficient Biochar Produced from Pepper Stem for Effective Ibuprofen Removal. Bioresour. Technol. 2022, 347, 126685. [Google Scholar] [CrossRef]
- Patel, M.; Chaubey, A.K.; Pittman, C.; Mohan, D. Aqueous Ibuprofen Sorption by Using Activated Walnut Shell Biochar: Process Optimization and Cost Estimation. Environ. Sci. Adv. 2022, 1, 530–545. [Google Scholar] [CrossRef]
- Du, Y.-D.; Zhang, X.-Q.; Shu, L.; Feng, Y.; Lv, C.; Liu, H.-Q.; Xu, F.; Wang, Q.; Zhao, C.-C.; Kong, Q. Safety Evaluation and Ibuprofen Removal via an Alternanthera Philoxeroides-Based Biochar. Environ. Sci. Pollut. Res. 2020, 28, 40568–40586. [Google Scholar] [CrossRef]
- Keerthanan, S.; Bhatnagar, A.; Mahatantila, K.; Jayasinghe, C.; Ok, Y.S.; Vithanage, M. Engineered Tea-Waste Biochar for the Removal of Caffeine, a Model Compound in Pharmaceuticals and Personal Care Products (PPCPs), from Aqueous Media. Environ. Technol. Innov. 2020, 19, 100847. [Google Scholar] [CrossRef]
- Zanella, H.G.; Spessato, L.; Lopes, G.K.P.; Yokoyama, J.T.C.; Silva, M.C.; Souza, P.S.C.; Ronix, A.; Cazetta, A.L.; Almeida, V.C. Caffeine Adsorption on Activated Biochar Derived from Macrophytes (Eichornia Crassipes). J. Mol. Liq. 2021, 340, 117206. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Katsouromalli, A.; Pashalidis, I. Oxidized Biochar Obtained from Pine Needles as a Novel Adsorbent to Remove Caffeine from Aqueous Solutions. J. Mol. Liq. 2020, 304, 112661. [Google Scholar] [CrossRef]
- Correa-Navarro, Y.M.; Giraldo, L.; Moreno-Piraján, J.C. Biochar from Fique Bagasse for Remotion of Caffeine and Diclofenac from Aqueous Solution. Molecules 2020, 25, 1849. [Google Scholar] [CrossRef]
- Oginni, O.; Singh, K. Effect of Carbonization Temperature on Fuel and Caffeine Adsorption Characteristics of White Pine and Norway Spruce Needle Derived Biochars. Ind. Crops Prod. 2021, 162, 113261. [Google Scholar] [CrossRef]
- Wu, J.; Yang, J.; Feng, P.; Wen, L.; Huang, G.; Xu, C.; Lin, B. Highly Efficient and Ultra-Rapid Adsorption of Malachite Green by Recyclable Crab Shell Biochar. J. Ind. Eng. Chem. 2022, 113, 206–214. [Google Scholar] [CrossRef]
- Giri, B.S.; Sonwani, R.K.; Varjani, S.; Chaurasia, D.; Varadavenkatesan, T.; Chaturvedi, P.; Yadav, S.; Katiyar, V.; Singh, R.S.; Pandey, A. Highly Efficient Bio-Adsorption of Malachite Green Using Chinese Fan-Palm Biochar (Livistona Chinensis). Chemosphere 2022, 287, 132282. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, W.; Song, Y.; Zhuang, H.; Tang, H. Removal of Cationic Dye BR46 by Biochar Prepared from Chrysanthemum Morifolium Ramat Straw: A Study on Adsorption Equilibrium, Kinetics and Isotherm. J. Mol. Liq. 2021, 340, 116617. [Google Scholar] [CrossRef]
- Eleryan, A.; Hassaan, M.A.; Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; El-Nemr, M.A.; Ragab, S.; Hossain, I.; El Nemr, A. Kinetic and Isotherm Studies of Acid Orange 7 Dye Absorption Using Sulphonated Mandarin Biochar Treated with TETA. Biomass Convers. Biorefin. 2023, 24, 4552–4561. [Google Scholar] [CrossRef]
- El-Nemr, M.A.; Abdelmonem, N.M.; Ismail, I.M.A.; Ragab, S.; El Nemr, A. The Efficient Removal of the Hazardous Azo Dye Acid Orange 7 from Water Using Modified Biochar from Pea-Peels. Desalination Water Treat. 2020, 203, 327–355. [Google Scholar] [CrossRef]
- Krishnasamy, S.; Saiatchyuth, B.A.; Ravindiran, G.; Subramaniyan, B.; Ramalingam, M.; Sai Vamsi, J.U.B.; Ramesh, B.; Razack, N.A. Effective Removal of Reactive Yellow 145 (RY145) Using Biochar Derived from Groundnut Shell. Adv. Mater. Sci. Eng. 2022, 2022, 8715669. [Google Scholar] [CrossRef]
- Kelm, M.A.P.; da Silva Júnior, M.J.; de Barros Holanda, S.H.; de Araujo, C.M.B.; de Assis Filho, R.B.; Freitas, E.J.; dos Santos, D.R.; da Motta Sobrinho, M.A. Removal of Azo Dye from Water via Adsorption on Biochar Produced by the Gasification of Wood Wastes. Environ. Sci. Pollut. Res. Int. 2019, 26, 28558–28573. [Google Scholar] [CrossRef]
- Gurav, R.; Bhatia, S.K.; Choi, T.R.; Choi, Y.K.; Kim, H.J.; Song, H.S.; Lee, S.M.; Lee Park, S.; Lee, H.S.; Koh, J.; et al. Application of Macroalgal Biomass Derived Biochar and Bioelectrochemical System with Shewanella for the Adsorptive Removal and Biodegradation of Toxic Azo Dye. Chemosphere 2021, 264, 128539. [Google Scholar] [CrossRef]
- Ravindiran, G.; Sundaram, H.; Rajendran, E.M.; Ramasamy, S.; Nabil, A.Z.; Ahmed, B. Removal of Azo Dyes from Synthetic Wastewater Using Biochar Derived from Sewage Sludge to Prevent Groundwater Contamination. Urban. Clim. 2023, 49, 101502. [Google Scholar] [CrossRef]
- Sudan, S.; Khajuria, A.; Kaushal, J. Adsorption Potential of Pristine Biochar Synthesized from Rice Husk Waste for the Removal of Eriochrome Black Azo Dye. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Yek, P.N.Y.; Peng, W.; Wong, C.C.; Liew, R.K.; Ho, Y.L.; Wan Mahari, W.A.; Azwar, E.; Yuan, T.Q.; Tabatabaei, M.; Aghbashlo, M.; et al. Engineered Biochar via Microwave CO2 and Steam Pyrolysis to Treat Carcinogenic Congo Red Dye. J. Hazard. Mater. 2020, 395, 122636. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yu, F.; Peng, Q.; Huang, Y. Superb Adsorption Capacity of Biochar Derived from Leather Shavings for Congo Red. RSC Adv. 2018, 8, 29781–29788. [Google Scholar] [CrossRef] [PubMed]
- Lonappan, L.; Rouissi, T.; Das, R.K.; Brar, S.K.; Ramirez, A.A.; Verma, M.; Surampalli, R.Y.; Valero, J.R. Adsorption of Methylene Blue on Biochar Microparticles Derived from Different Waste Materials. Waste Manag. 2016, 49, 537–544. [Google Scholar] [CrossRef]
- Samsuri, A.W.; Sadegh-Zadeh, F.; Seh-Bardan, B.J. Adsorption of As(III) and As(V) by Fe Coated Biochars and Biochars Produced from Empty Fruit Bunch and Rice Husk. J. Environ. Chem. Eng. 2013, 1, 981–988. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Bricka, M.; Smith, F.; Yancey, B.; Mohammad, J.; Steele, P.H.; Alexandre-Franco, M.F.; Gómez-Serrano, V.; Gong, H. Sorption of Arsenic, Cadmium, and Lead by Chars Produced from Fast Pyrolysis of Wood and Bark during Bio-Oil Production. J. Colloid. Interface Sci. 2007, 310, 57–73. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Z.; Han, R.; Meng, R.; Wang, H.; Lu, W. Adsorption of Cadmium by Biochar Derived from Municipal Sewage Sludge: Impact Factors and Adsorption Mechanism. Chemosphere 2015, 134, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, T.; Wang, J.; Zhang, Y.; Pan, W.P. A Novel Modified Method for the Efficient Removal of Pb and Cd from Wastewater by Biochar: Enhanced the Ion Exchange and Precipitation Capacity. Sci. Total Environ. 2021, 754, 142150. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, F.S. Removal of Lead from Water Using Biochars Prepared from Hydrothermal Liquefaction of Biomass. J. Hazard. Mater. 2009, 167, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, Z.; Yi, W.; Li, Y.; Zhang, P.; Zhang, A.; Wang, L. Impacts of Pyrolysis Temperature on Lead Adsorption by Cotton Stalk-Derived Biochar and Related Mechanisms. J. Environ. Chem. Eng. 2021, 9, 105602. [Google Scholar] [CrossRef]
- Park, J.H.; Wang, J.J.; Zhou, B.; Mikhael, J.E.R.; DeLaune, R.D. Removing Mercury from Aqueous Solution Using Sulfurized Biochar and Associated Mechanisms. Environ. Pollut. 2019, 244, 627–635. [Google Scholar] [CrossRef]
- Li, G.; Shen, B.; Li, F.; Tian, L.; Singh, S.; Wang, F. Elemental Mercury Removal Using Biochar Pyrolyzed from Municipal Solid Waste. Fuel Process. Technol. 2015, 133, 43–50. [Google Scholar] [CrossRef]
- Dong, X.; He, L.; Liu, Y.; Piao, Y. Preparation of Highly Conductive Biochar Nanoparticles for Rapid and Sensitive Detection of 17β-Estradiol in Water. Electrochim. Acta 2018, 292, 55–62. [Google Scholar] [CrossRef]
- Liu, G.; Li, L.; Zhang, K.; Wang, X.; Chang, J.; Sheng, Y.; Bai, L.; Wen, Y. Facile Preparation of Water-Processable Biochar Based on Pitch Pine and Its Electrochemical Application for Cadmium Ion Sensing. Int. J. Electrochem. Sci. 2016, 11, 1041–1054. [Google Scholar] [CrossRef]
- Sfragano, P.S.; Laschi, S.; Renai, L.; Fichera, M.; Del Bubba, M.; Palchetti, I. Electrochemical Sensors Based on Sewage Sludge–Derived Biochar for the Analysis of Anthocyanins in Berry Fruits. Anal. Bioanal. Chem. 2022, 414, 6295–6307. [Google Scholar] [CrossRef]
- de Oliveira, P.R.; Kalinke, C.; Gogola, J.L.; Mangrich, A.S.; Junior, L.H.M.; Bergamini, M.F. The Use of Activated Biochar for Development of a Sensitive Electrochemical Sensor for Determination of Methyl Parathion. J. Electroanal. Chem. 2017, 799, 602–608. [Google Scholar] [CrossRef]
- Kalinke, C.; Oliveira, P.R.; Oliveira, G.A.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Activated Biochar: Preparation, Characterization and Electroanalytical Application in an Alternative Strategy of Nickel Determination. Anal. Chim. Acta 2017, 983, 103–111. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, L.; He, L.; Liu, N.; Piao, Y. Electrochemical Enzyme Biosensor Bearing Biochar Nanoparticle as Signal Enhancer for Bisphenol A Detection in Water. Sensors 2019, 19, 1619. [Google Scholar] [CrossRef]
- Wong, A.; de Lima, D.G.; Ferreira, P.A.; Khan, S.; da Silva, R.A.B.; de Faria, J.L.B.; Del Pilar Taboada Sotomayor, M. Voltammetric Sensing of Glyphosate in Different Samples Using Carbon Paste Electrode Modified with Biochar and Copper(II) Hexadecafluoro-29H,31 Phtalocyanine Complex. J. Appl. Electrochem. 2021, 51, 761–768. [Google Scholar] [CrossRef]
- Chen, X.; Lu, K.; Lin, D.; Li, Y.; Yin, S.; Zhang, Z.; Tang, M.; Chen, G. Hierarchical Porous Tubular Biochar Based Sensor for Detection of Trace Lead (II). Electroanalysis 2021, 33, 473–482. [Google Scholar] [CrossRef]
- Nemčeková, K.; Labuda, J. Advanced Materials-Integrated Electrochemical Sensors as Promising Medical Diagnostics Tools: A Review. Mater. Sci. Eng. C 2021, 120, 111751. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; He, L.; Yang, Y.; Zhang, Y.; Liu, Z.; Liang, L.; Piao, Y. Nanobiochar Paper Based Electrochemical Immunosensor for Fast and Ultrasensitive Detection of Microcystin-LR. Sci. Total Environ. 2021, 750, 141692. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Mao, D.; Duan, P.; Li, K.; Lin, Y.; Wang, X.; Piao, Y. Green Synthesis of ZnO/BC Nanohybrid for Fast and Sensitive Detection of Bisphenol A in Water. Chemosensors 2022, 10, 163. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Xu, P.; Liu, H.; Zhang, L.; Zhang, S.; Tian, L. Gold Nanoparticles Decorated Biochar Modified Electrode for the High-Performance Simultaneous Determination of Hydroquinone and Catechol. Sens. Actuators B Chem. 2020, 306, 127590. [Google Scholar] [CrossRef]
- Kalinke, C.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Carbon Paste Electrode Modified with Biochar for Sensitive Electrochemical Determination of Paraquat. Electroanalysis 2016, 28, 764–769. [Google Scholar] [CrossRef]
- Sant’Anna, M.V.S.; Silva, J.d.O.S.; Gevaerd, A.; Lima, L.S.; Monteiro, M.D.S.; Carregosa, I.S.C.; Wisniewski, A.; Marcolino-Junior, L.H.; Bergamini, M.F.; Sussuchi, E.M. Selective Carbonaceous-Based (Nano)Composite Sensors for Electrochemical Determination of Paraquat in Food Samples. Food Chem. 2022, 373, 131521. [Google Scholar] [CrossRef]
- Wong, A.; Ferreira, P.A.; Santos, A.M.; Cincotto, F.H.; Silva, R.A.B.; Sotomayor, M.D.P.T. A New Electrochemical Sensor Based on Eco-Friendly Chemistry for the Simultaneous Determination of Toxic Trace Elements. Microchem. J. 2020, 158, 105292. [Google Scholar] [CrossRef]
- Suguihiro, T.M.; de Oliveira, P.R.; de Rezende, E.I.P.; Mangrich, A.S.; Marcolino Junior, L.H.; Bergamini, M.F. An Electroanalytical Approach for Evaluation of Biochar Adsorption Characteristics and Its Application for Lead and Cadmium Determination. Bioresour. Technol. 2013, 143, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Qian, W.; Li, Y.; Yu, Q.; Yu, Y.; Chen, S.; Qu, F.; Gao, Y.; Lu, L. Multilayer Activated Biochar/UiO-66-NH2 Film as Intelligent Sensing Platform for Ultra-Sensitive Electrochemical Detection of Pb2+ and Hg2+. Appl. Surf. Sci. 2021, 569, 151006. [Google Scholar] [CrossRef]
- Cao, L.; Kang, Z.W.; Ding, Q.; Zhang, X.; Lin, H.; Lin, M.; Yang, D.P. Rapid Pyrolysis of Cu2+-Polluted Eggshell Membrane into a Functional Cu2+-Cu+/Biochar for Ultrasensitive Electrochemical Detection of Nitrite in Water. Sci. Total Environ. 2020, 723, 138008. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.S.; Kumar, P.S.; Show, P.L. A Review on Effective Removal of Emerging Contaminants from Aquatic Systems: Current Trends and Scope for Further Research. J. Hazard. Mater. 2021, 409, 124413. [Google Scholar] [CrossRef]
- Vasilachi, I.C.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Occurrence and Fate of Emerging Pollutants in Water Environment and Options for Their Removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Priya, A.K.; Gnanasekaran, L.; Rajendran, S.; Qin, J.; Vasseghian, Y. Occurrences and Removal of Pharmaceutical and Personal Care Products from Aquatic Systems Using Advanced Treatment-A Review. Environ. Res. 2022, 204, 112298. [Google Scholar] [CrossRef]
- Pourhakkak, P.; Taghizadeh, A.; Taghizadeh, M.; Ghaedi, M.; Haghdoust, S. Fundamentals of Adsorption Technology. Interface Sci. Technol. 2021, 33, 1–70. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of Emerging Contaminants from Water and Wastewater by Modified Biochar: A Review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The Adsorption, Regeneration and Engineering Applications of Biochar for Removal Organic Pollutants: A Review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Han, L.; Wang, J.; Zhu, L.; Zeng, H. Graphene-Based Materials for Adsorptive Removal of Pollutants from Water and Underlying Interaction Mechanism. Adv. Colloid Interface Sci. 2021, 289, 102360. [Google Scholar] [CrossRef] [PubMed]
- Pap, S.; Taggart, M.A.; Shearer, L.; Li, Y.; Radovic, S.; Turk Sekulic, M. Removal Behaviour of NSAIDs from Wastewater Using a P-Functionalised Microporous Carbon. Chemosphere 2021, 264, 128439. [Google Scholar] [CrossRef] [PubMed]
- Bursztyn Fuentes, A.L.; Canevesi, R.L.S.; Gadonneix, P.; Mathieu, S.; Celzard, A.; Fierro, V. Paracetamol Removal by Kon-Tiki Kiln-Derived Biochar and Activated Carbons. Ind. Crops Prod. 2020, 155, 112740. [Google Scholar] [CrossRef]
- Praveen, S.; Jegan, J.; Bhagavathi Pushpa, T.; Gokulan, R.; Bulgariu, L. Biochar for Removal of Dyes in Contaminated Water: An Overview. Biochar 2022, 4, 10. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Sajjad, S.; Saleem, J.; Alherbawi, M.; McKay, G. A Review of the Removal of Dyestuffs from Effluents onto Biochar. Separations 2022, 9, 139. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Hu, X. Removal of Heavy Metals from Soil with Biochar Composite: A Critical Review of the Mechanism. J. Environ. Chem. Eng. 2021, 9, 105830. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a Low-Cost Adsorbent for Aqueous Heavy Metal Removal: A Review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar] [CrossRef]
- Yan, B.; Zheng, J.; Feng, L.; Du, C.; Jian, S.; Yang, W.; Wu, Y.A.; Jiang, S.; He, S.; Chen, W. Wood-Derived Biochar as Thick Electrodes for High-Rate Performance Supercapacitors. Biochar 2022, 4, 50. [Google Scholar] [CrossRef]
- Li, S.; Shao, L.; Zhang, H.; Lu, X.; Lü, F.; He, P. A Nanoscale Observation to Explain the Discrepancy of Electron Exchange Capacities between Biochar Containing Comparable Surface Redox-Active Moieties. Biochar 2022, 4, 41. [Google Scholar] [CrossRef]
- Kane, S.; Ulrich, R.; Harrington, A.; Stadie, N.P.; Ryan, C. Physical and Chemical Mechanisms That Influence the Electrical Conductivity of Lignin-Derived Biochar. Carbon. Trends 2021, 5, 100088. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajčovičová, T.E.; Hatala, M.; Gemeiner, P.; Híveš, J.; Mackuľak, T.; Nemčeková, K.; Svitková, V. Biochar for Water Pollution Control: From Sensing to Decontamination. Chemosensors 2023, 11, 394. https://doi.org/10.3390/chemosensors11070394
Krajčovičová TE, Hatala M, Gemeiner P, Híveš J, Mackuľak T, Nemčeková K, Svitková V. Biochar for Water Pollution Control: From Sensing to Decontamination. Chemosensors. 2023; 11(7):394. https://doi.org/10.3390/chemosensors11070394
Chicago/Turabian StyleKrajčovičová, Timea Ema, Michal Hatala, Pavol Gemeiner, Ján Híveš, Tomáš Mackuľak, Katarína Nemčeková, and Veronika Svitková. 2023. "Biochar for Water Pollution Control: From Sensing to Decontamination" Chemosensors 11, no. 7: 394. https://doi.org/10.3390/chemosensors11070394
APA StyleKrajčovičová, T. E., Hatala, M., Gemeiner, P., Híveš, J., Mackuľak, T., Nemčeková, K., & Svitková, V. (2023). Biochar for Water Pollution Control: From Sensing to Decontamination. Chemosensors, 11(7), 394. https://doi.org/10.3390/chemosensors11070394







