Abstract
The purpose of this study was to investigate the use of an experimental electronic nose (E-nose) as a predictive tool for detecting the formation of chemical process contaminants in roasted almonds. Whole and ground almonds were subjected to different thermal treatments, and the levels of acrylamide, hydroxymethylfurfural (HMF) and furfural were analysed. Subsequently, the aromas were detected by using the electronic device. Roasted almonds were classified as positive or negative sensory attributes by a tasting panel. Positive aromas were related to the intensity of the almond odour and the roasted aroma, whereas negative ones were linked to a burnt smell resulting from high-intensity thermal treatments. The electronic signals obtained by the E-nose were correlated with the content of acrylamide, HMF, and furfural ( > 0.83; > 0.76 in whole roasted almonds; > 0.88; > 0.95 in ground roasted almonds). This suggest that the E-nose can predict the presence of these contaminants in roasted almonds. In conclusion, the E-nose may be a useful device to evaluate the quality of roasted foods based on their sensory characteristics but also their safety in terms of the content of harmful compounds, making it a useful predictive chemometric tool for assessing the formation of contaminants during almond processing.
1. Introduction
The temperatures applied during the production and cooking of foods can have a significant impact on the quality and nutritional aspects of these products. Such changes are related to the development of the Maillard reaction, which leads to the formation of compounds responsible for the taste, aroma, and appearance of food products [], as well as desirable compounds with potential health benefits []. However, concurrently, other harmful and undesirable compounds may also be generated, such as acrylamide and some emerging process contaminants, including hydroxymethylfurfural (HMF) and furfural [,]. Acrylamide is considered a potential carcinogen for humans by the International Agency for Research on Cancer []. It is formed when free asparagine and reducing sugars react at temperatures above 120 °C. The European Food Safety Agency (EFSA) has identified the presence of acrylamide in food as a public health concern []. HMF and furfural are intermediate products of the Maillard reaction, and HMF can also be produced by the caramelization of sugars at high temperatures [,]. These compounds have been evaluated as emerging processing contaminants due to the potential genotoxic and mutagenic effects of HMF [,,] and the association of furfural with hepatotoxicity outcomes [].
Normalized analytical protocols are applied to monitor the occurrence of regulated chemical contaminants in processed foods. These methods involve robust, highly specialized instrumentation, time-consuming and expensive procedures, and predominating chromatographic techniques, such as GC–MS, GC–MS–MS, HPLC–MS, and LC–MS–MS []. To facilitate the determination, there is an increasing demand for rapid and precise alternative methods that allow a fast screening of these harmful compounds in foods. In this regard, the European regulation for acrylamide allows its analysis to be replaced by measuring product attributes or process parameters as long as a statistical correlation can be demonstrated between them and the acrylamide level []. Most of these methodologies rely on linking the colour of processed foods to the acrylamide levels [,]. However, few studies have established a connection between the formation of chemical process contaminants and the development of aromatic compounds [].
The electronic nose (E-nose) is a cost-effective and powerful electronic device able to conduct fast measurements to discriminate flavours. It is widely used in many disciplines as a commodity, such as environmental, medicinal and food sciences, mainly with the aim of evaluating the quality of the products and detecting off-flavours that can be attributed to food deterioration [,]. Since molecules responsible for the aromas released during food processing are recognizable by the human nose, it may be possible to use the E-nose to detect changes that occur in processed food products. Moreover, due to the relationship between the formation of aromatic compounds and the generation of chemical contaminants during processing, the E-nose could also be used as a chemometric tool to predict the formation of the toxic compounds. In a previous study, Martín-Tornero et al. [] used an E-nose to analyse the aroma of table olives, demonstrating the effectivity of this device to indirectly quantify the acrylamide levels in these foods. Based on these results, it would be worthwhile to verify the effectiveness of the E-nose in other food matrices.
Almonds are among the most consumed tree nuts in the world [], and they are recommended as a healthy food and a source of beneficial constituents for human health []. Nevertheless, they contain amino acids and reducing sugars in appreciable amounts, and therefore, the roasting temperature and physical form (whole, sliced, and cut) of the almond kernel strongly promote the development of the Maillard reaction, leading then to the formation of aromatic compounds but also to the generation of chemical process contaminants []. Previous studies have examined the development of acrylamide and furanic compounds during the roasting of almonds at various temperature and time settings [,,,]. However, none of them have explored the possible correlation between these compounds and the resulting aroma, which could be measured with chemometric tools such as the electronic nose. In this context, the objective of this work was to evaluate the feasibility of using an E-nose as a predictive tool for the formation of acrylamide and furanic compounds in almonds that were processed under different roasting conditions and in different structural formats (whole and ground). The findings would provide a straightforward and efficient approach to manage the development of contaminants during the processing of this particular food matrix.
2. Materials and Methods
2.1. Reagents and Chemicals
Acrylamide standard (99%) [CAS. 79-06-01], potassium hexacyanoferrate (II) trihydrate (98%, Carrez-I) [CAS. 14459-95-1], and zinc acetate dihydrate (>99%, Carrez-II) [CAS. 5970-45-6] were purchased from Sigma (St. Louis, MO, USA). Formic acid (98%) [CAS. 64-18-6], methanol (99.5%) [CAS. 67-56-1] and hexane [CAS. 110-54-3] were obtained from Panreac (Barcelona, Spain). [13C3]-acrylamide (isotopic purity 99%) [CAS.287399-26-2] was obtained from Cambridge Isotope Labs (Andover, MA, USA). The Milli-Q water used was produced using an Elix 3 Millipore water purification system coupled to a Milli-Q module (model Advantage A10) (Millipore, Molsheim, France). All other chemicals, solvents, and reagents were of analytical grade.
2.2. Samples and Roasting Treatments
Two formats (whole and ground) of raw almonds (Prunus dulcis) from the Marcona cultivar were purchased from Spanish markets between March/2022 and April/2022. Fifteen bags of the same batch were acquired for each format to ensure that there was a sufficient quantity of the same sample to conduct the complete kinetic study of the roasting experiments.
Roasting experiments were carried out in a convective and air-forced oven (Memmert UFE400, Scwabach, Germany). Two batches of whole almonds (60 g) and ground almonds (30 g) were roasted simultaneously in aluminium trays (21.9 × 15.8 × 3.8 cm; length × wide × height). Samples were placed on the tray laying in a uniform layer. A calibrated K-type thermocouple data logger (Delta Ohm, Caselle di Selvazzano, Italy) was used to monitor and register the temperature of hot air within and above the almonds (°C/s). Samples were roasted at 120, 135, 150, 165, 180, and 200 °C for 20 min. Temperature/time combinations would resemble two levels at moderate and three at more intense heat load above a reference. The temperature/time combination at 150 °C/20 min was selected as reference. After roasting, samples were cooled until the temperature within the tray stayed below 30 °C. A portion was placed in a sealed bottle and kept under refrigeration (4–6 °C) until analysis.
2.3. Analysis
2.3.1. Sensory Analysis
Sensory analysis was carried out by a tasting committee made up of eight tasters in the tasting room of the Research Centre of Extremadura (CICYTEX). The panel members were trained to evaluate agri-food products. The samples, consisting of 3 g of whole and ground roasted almonds, were placed in a standard glass on a heating plate at 25 °C. The tasting panel identified almond and roasted aromas as positive attributes, while burnt attributes were classified as negative in both whole and ground almond samples. The roasted aroma was evaluated on a scale of 0 to 10 points based on its intensity, with scores categorized as either positive or negative. The test was considered valid only if the coefficient of variation was less than 20% [].
2.3.2. LC-ESI-MS/MS Determination of Acrylamide
Liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) was used to determine the levels of acrylamide, following the method described by González-Mulero et al. []. Acrylamide standard was added to the samples to determine the recovery rate, obtaining values between 95% and 107%. Relative standard deviations (RSD) for precision (2.8%), repeatability (1.2%), and reproducibility (2.5%) were calculated to confirm the precision of the analysis. Analytical determination was checked through the analysis of different food matrices provided as proficiency tests by the Food Analysis Performance Assessment Scheme (FAPAS) program. The z-scores obtained from these tests demonstrated the accuracy of the results: −0.1 for crispbread (test 30,118, January 2022) and 0.4 for potato crisps (test 30,120, April 2022). The limit of quantitation (LOQ) was set at 15 μg/kg, and the results were expressed as μg/kg sample.
2.3.3. HPLC Determination of Hydroxymethylfurfural and Furfural
HMF and furfural were determined by HPLC according to Mesias et al. []. The LOQ for HMF and furfural were estimated to be 0.6 mg/kg and 0.3 mg/kg, respectively, and the results were expressed as mg/kg sample.
2.3.4. E-Nose Analysis
Researchers from the School of Industrial Engineering at the University of Extremadura were the designers of the handmade electronic device (E-nose). This device contains four electronic chips with a total of eleven sensors that emit different signals related to the aroma of the sample headspace []. Samples (~3 g) were placed in the tasting glasses in the same way as the tasting panel and E-nose was placed on the top of the cup. In a first phase, known as the adsorption phase, the E-nose was allowed to adsorb the headspace of the sample for 60 s. Once this time elapsed, the electronic device was moved to an empty cup to carry out the desorption phase for 30 s to obtain the baseline. The electronic nose received data in one-second intervals, and eight measurements were carried out for each roasted almond sample. Data analysed were transferred from the E-nose to a smart device via Bluetooth, and a multivariate analysis was performed.
2.4. Chemometric Analysis
Different methods were performed using MATLAB software and PLS_Toolbox: (i) Principal component analysis (PCA); (ii) Partial least squares (PLS). First, a PCA was performed to show the discrimination between the different samples based on the profile of volatile components []. The original variables were autoscaled because the study variables were measured in different units. Finally, for the detection of acrylamide, furfural, and hydroxymethylfurfural with the E-nose data, a PLS model was carried out [].
2.5. Statistical Analysis
Statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as mean ± standard deviation (SD). One-way ANOVA followed by Tukey’s method, or Student’s t-test, was used to identify the overall significance of differences. The homogeneity of variances was determined using Levene’s test. All statistical parameters were evaluated at p < 0.05 significance level.
3. Results and Discussion
3.1. Sensory Analysis
A tasting committee made up of eight tasters conducted a sensory analysis for the discrimination of the aromas of the roasted almond samples (Table 1). The samples were classified as having positive or negative attributes. Positive aroma was associated with the intensity of the almond odour and the roasted aroma caused by the thermal treatment. Conversely, the negative aroma was related to the burnt smell resulting from the over-roasting of the samples. It should be noted that the aroma of almond in both whole and ground samples was more intense when roasted at 150 °C, with a score of 3.8 for whole almonds and 3.2 for ground almonds. From this treatment onwards, the almond aroma started to decrease until it was undetectable in samples roasted at 200 °C. Regarding the roasted odour, it was not detected until Treatment 3 (heating at 150 °C), being of lower intensity in ground almonds. In this case, both type of samples showed the highest intensity at temperatures of 165 °C, with 3.5 points for whole almonds and 2.0 for ground almonds, decreasing with more intense treatments. Again, samples roasted at 200 °C did not exhibit a roasted aroma. In contrast to the positive attributes, burnt aroma was not present in samples heated at lower temperatures, but it was detected when the temperature that was applied was 180 °C, and it reached the maximum value at 200 °C (4.5 points in whole almonds and 4.0 points in ground samples). In conclusion, it can be inferred that most of the whole and ground almond samples roasted up to 165 °C exhibited the most desirable aromas, without any burnt notes.

Table 1.
Descriptive sensory olfactory evaluation of roasted whole and ground almond aroma evaluated by the tasting panel. All samples were roasted at different temperatures for 20 min.
In a similar study, Schlörmann et al. [] conducted a sensory evaluation of roasted nuts assigning points ranging from five (no deviation of expected quality) to one (clear defects) for attributes such as appearance, texture, odour, and taste. Nuts roasted at low (120–140 °C) or middle temperatures (140–160 °C) obtained the highest scores (4.8 and 4.7, respectively), followed by those heated at temperatures between 160–180 °C (4.4). In contrast, a hedonic analysis established points between one (excellent) and five (highly disliked). In this case, nuts roasted at low or middle temperatures received the best scores (1.8 and 2.1, respectively), whereas higher temperatures resulted in lower scores (2.8). Based on these results, temperatures of up to 160 °C were recommended for obtaining almond products suitable for consumption, as higher roasting conditions may result in bitter and over-roasted flavours. Amrein et al. [] also noted that 165 °C is the upper limit for almond roasting, due to the formation of a bitter and over-roasted flavour, in agreement with findings of the present study and with those reported by other authors [].
3.2. Effect of Thermal Treatments on Chemical Process Contaminant Content in Roasted Almonds
The temperature-dependent formation of acrylamide, HMF, and furfural in roasted almond is shown in Table 2. As expected, an increase in temperature promoted the formation of the chemical process contaminants during roasting. Several studies have suggested that the formation of acrylamide in almonds is primarily influenced by the roasting temperature rather than the duration of the thermal treatment [,,]. In that sense, an increase of 15 °C resulted in an almost 3-fold increase in acrylamide content in whole almonds and up to 2.5-fold in ground almonds. Whole and ground almonds exhibited different profiles for the acrylamide levels, ranging from 25 to 466 μg/kg in whole samples and from 19 to 397 µg/kg in ground nuts. Acrylamide concentration in whole almonds reached the highest level when roasted at 165 °C and drastically (p < 0.05) decreased to 285 and 170 μg/kg when the temperature was raised to 180 and 200 °C, respectively. In the case of ground almonds, the maximum formation was observed at 180 °C, decreasing to 301 µg/kg with roasting at 200 °C. This type of kinetic pattern was similar to that observed in roasted coffee, sesame seeds or chia seeds, as reported in previous studies [,,]. As is known, during the thermal treatment of foods, both the formation and the elimination of acrylamide occur simultaneously. Acrylamide formation will predominate in the presence of its precursors (asparagine and carbonyl compounds), while its elimination becomes dominant in the absence of any precursor. Almonds contain acrylamide precursors in high quantity in the range of 98–641 mg/100 g for free asparagine [], 3100–4680 mg/100 g for sucrose, and 70–134 mg/100 g for the total content of glucose and fructose []. Among them, strong correlations between asparagine levels and acrylamide formation in almonds have been reported, suggesting that asparagine may be the limiting factor in this food [,,]. Based on the results, it can be inferred that at temperatures of 165 °C (for whole almonds) and 180 °C (for ground almonds), the precursor content (particularly asparagine) would be significantly reduced, leading to a higher prevalence of elimination over the formation of acrylamide, resulting in decreased levels of the contaminant in the roasted samples.

Table 2.
Acrylamide (µg/kg), HMF (mg/kg) and furfural (mg/kg) content of whole and ground almonds roasted under different temperatures for 20 min. Descriptive sensory olfactory evaluation of roasted whole and ground almonds aromas evaluated by the tasting panel. All samples were roasted at different temperatures (range 120–200 °C) for 20 min.
The acrylamide content reported by other authors has shown high variability, probably due to the different content of the precursor associated with the almond variety or even with the growing region and harvest year in addition to the processing conditions. Similar vales have been described in almonds experimentally roasted for 22.5 min at 130 °C (94 µg/kg), but higher after roasting for 20 min at 145 °C (around 500 µg/kg) and 165 °C (more than 1500 µg/kg). Industrial samples heated for 22 min at 150 °C also exhibited greater values (885 µg/kg) []. In contrast, Lasekan and Abbas [] presented results between 42 and 65 µg/kg in almonds roasted at 150 °C for 15 and 25 min, respectively, increasing only up to 60 and 86 µg/kg at 200 °C. Regardless of the levels reached, all the studies found in the literature indicate that acrylamide formation begins when the temperature exceeds 130 °C [], with low levels at 145 °C, and reaching a maximum in the range of 165–180 °C, decreasing afterward [], and leading to unacceptable bitter off-flavours [], in agreement with observations of this research.
The formation of HMF and furfural increased during roasting at all temperatures, except for whole almonds roasted at 200 °C, where it slightly decreased. At the lowest temperatures (120 °C), the levels of these compounds were relatively low, but they significantly increased as the temperature rose to 200 °C. In whole almonds, HMF concentrations ranged between 0.99 and 100.14 mg/kg, with maximum formation at 180 °C, and decreased to 98.92 mg/kg at 200 °C. For ground almonds, HMF levels increased from 0.75 to 149.77 mg/kg, with maximum values at the maximum temperature. Furfural concentrations in whole samples increased from 0.35 to 23.85 mg/kg, and in ground samples from 0.42 to 13.32 mg/kg. The results for HMF were within the range reported for roasted commercial samples (10.52–49.37 mg/kg) [].
Comparing the two sample formats, ground almonds promoted a greater formation of HMF, reaching maximum levels 200 times higher than those at 120 °C. In contrast, the largest increase for furfural was observed in the whole almonds, with concentrations almost 70 times higher than those at 120 °C. Previous studies have reported that the grinding increases the accessibility of constituents of the inner part of chia seeds, and consequently, their reactivity to promote the formation of acrylamide []. This may explain why roasted ground almonds showed the highest levels of furanic compounds compared to whole almonds. However, this was not observed in the acrylamide results. In this regard, it is necessary to point out that the raw materials were not the same, and therefore, they could present a different composition, with differences in the levels of precursors, leading to varying levels of acrylamide. To analyse the content of precursors in the raw material was not the objective of this work; however, it is known that the levels of free asparagine in almonds can vary considerably and to a larger extent than those of the sugars [], which could explain the variability in the acrylamide levels and the highest concentrations for furanic compounds in ground samples.
3.3. Discrimination of Roasted Almonds by Using E-Nose
Roasted almonds submitted to different thermal treatments were analysed by an electronic devise. The following algorithm feature was used to obtain the raw data for the E-nose: maximum signal value minus minimum, plus 100, and minus one. Then, Principal Component Analysis (PCA) was carried out to observe the clusters formed in the different thermal treatments on both roasted whole and ground almonds (Figure 1). In the two groups of samples, more than 90% of the total variance was explained by PC1. The clustering of the results in both PCA showed that the E-nose is able to discriminate the different thermal treatments. In ground almonds the clusters are better differentiated. However, in the PCA of whole almonds, samples roasted at 200 °C (Treatment 6) are far behind the rest of the treatments, in the negative region. Almonds heated at 165 and 180 °C (Treatments 4 and 5) are found in the quadrant of PC1 positive and PC2 positive, whereas those roasted at the lowest temperatures (120 and 135 °C, Treatments 1 and 2) are in PC1 positive and PC2 negative. It should be noted that these analyses support the tasting panel as neither the almond odour nor the roasted one was detected in Treatment 6 (200 °C), in which the burnt aroma appears with a high intensity. These results demonstrate the effectiveness of the electronic nose to differentiate the quality of the almonds according to the degree of roasting, allowing an adequate classification of these products. Previous studies have also reported the successful use of the electronic olfaction in food quality control, such as in the analysis of edible oils to detect adulteration and deterioration caused by external factors [,,] or in roasted coffee samples to differentiate the degree of roasting [,].
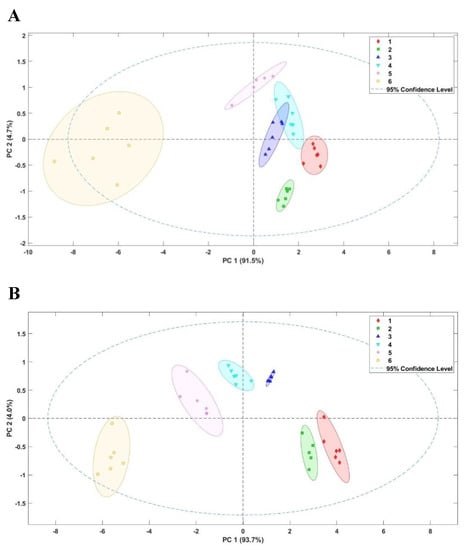
Figure 1.
Score plot of the Principal Component Analysis (PCA) for roasted whole (A) and ground (B) almond aroma submitted to different thermal treatments (1–6), at different temperatures for 20 min. Treatment 1, 120 °C; Treatment 2, 135 °C; Treatment 3, 150 °C; Treatment 4; 165 °C; Treatment 5, 180 °C; Treatment 6, 200 °C.
3.4. Prediction of the Presence of Chemical Process Contaminants in Roasted Almonds by E-Nose
A PLS model was stablished to correlate the data obtained by E-nose and the presence of chemical process contaminants in the roasted almonds (Figure 2). A total of 70% of the sample data were used for calibration and cross-validation of the models, whereas the remaining 30% were used for testing the strength and precision of the generated models. Three distinct models were built for both whole and ground roasted almonds, considering the three compounds evaluated in the present study (acrylamide, HMF, and furfural). In the case of whole roasted almonds, the value was 0.89 for the model developed for acrylamide and furfural and 0.83 for HMF. Higher values were found for ground roasted almonds, with results of 0.99 for acrylamide, 0.98 for HMF, and 0.88 for furfural. The RMSECV values were also estimated, being 61.1, 18.4, and 3.0, for acrylamide, HMF, and furfural, respectively, in whole roasted almonds, and 14.0, 9.8, and 1.8 for ground roasted samples. and RMSEP are parameters related to the validation methods. Regarding the values, they were 0.76 for acrylamide, 0.85 for HMF and 0.94 for furfural in whole roasted almonds, increasing up to 0.99, 0.97, and 0.95, respectively, in ground roasted almonds. Finally, the RMSEP values for whole almonds were 78.8, 18.6, and 2.6 for ground almonds and 17.7, 11.3, and 1.2 for the three compounds, respectively. The results show a good correlation between the experimental and the predicted values, suggesting that this model is suitable for prediction and allows the quantification of the chemical process contaminants in roasted almonds.
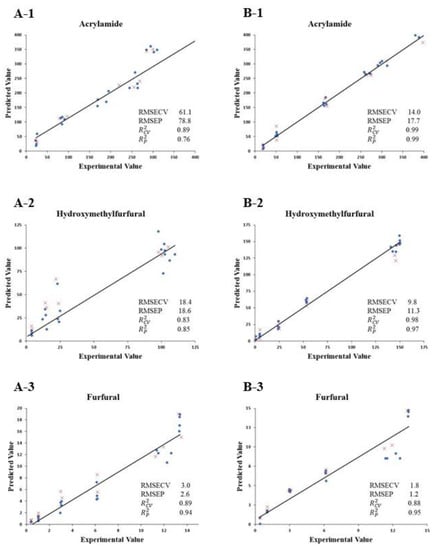
Figure 2.
Experimental values for acrylamide (1), HMF (2) and furfural (3) against PLS cross-validation predictions (●) and validation set predictions (×) for roasted whole (A) and ground (B) almond aromas submitted to different thermal treatments (1–6), at different temperatures for 20 min. Treatment 1, 120 °C; Treatment 2, 135 °C; Treatment 3, 150 °C; Treatment 4; 165 °C; Treatment 5, 180 °C; Treatment 6, 200 °C.
Until now, the use of the electronic nose as a predictive device for the formation of chemical contaminants during processing has been very rare. In a previous study, Martín-Tornero et al. [] characterized the polyphenol and volatile fractions of Californian-style black olives and demonstrated the innovative application of the E-nose in determining the acrylamide content in these foods. These positive results together with those found in the present study encourage the continuation of the usage of the E-nose not only to control the quality of food but also as a quick and effective chemometric tool to predict the formation of contaminants during processing.
4. Conclusions
The processing of foods promotes the development of the Maillard reaction, leading to the formation of compounds that contribute to the sensory properties of foods, including taste, aroma, and the appearance of foods. However, this reaction can also produce undesirable compounds, known as chemical process contaminants. The determination of compounds such as acrylamide, HMF and furfural requires robust, highly specialized instrumentation, and time-consuming and expensive procedures. Therefore, there is a need to develop other rapid and precise alternative methods that allow a fast screening of these harmful compounds in foods. In the present study, an experimental electronic nose was tested to detect and discriminate between the content of acrylamide, HMF, and furfural in whole and ground almonds roasted under different thermal treatments. The results showed that the E-nose was able to discriminate the positive and negative aromas of different samples and to predict the levels of chemical process contaminants in the roasted almonds. In conclusion, the use of this electronic device, together with a suitable statistical model, allows for the assessment of the quality of roasted foods according to their sensory characteristics, as well as their safety regarding harmful compounds. Therefore, it can be used as a predictive chemometric tool for the formation of contaminants during almond processing.
Author Contributions
Formal analysis, M.M., F.J.M., J.D.B.-R., J.L. and D.M.-V.; investigation, M.M., F.J.M. and D.M.-V.; supervision, M.M., F.J.M. and D.M.-V., writing—original draft, M.M., J.D.B.-R. and D.M.-V.; writing—review and editing, F.J.M., J.L. and D.M.-V.; funding acquisition, F.J.M. and D.M.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was supported by the Community of Madrid and European funding from FSE and FEDER programs (project S2018/BAA-4393, AVANSECAL-II-CM) and by GR21121 and GR21045 Projects, co-funded by FEDER and Junta de Extremadura.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and the raw data that support the findings are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Starowicz, M.; Zielińsk, H. How Maillard Reaction Influences Sensorial Properties (Color, Flavor and Texture) of Food Products? Food Rev. Int. 2019, 35, 707–725. [Google Scholar] [CrossRef]
- Nooshkama, M.; Varidia, M.; Vermac, D.K. Functional and biological properties of Maillard conjugates and their potential application in medical and food: A review. Food Res. Int. 2020, 131, 109003. [Google Scholar] [CrossRef]
- Stadler, R.H.; Blank, I.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A.; Robert, M.C.; Riediker, S. Acrylamide from Maillard reaction products. Nature 2002, 419, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Morales, F.J. Hydroxymethylfurfural (HMF) and related compounds. In Process-Induced Food Toxicants: Occurrence, Formation, Mitigation and Health Risks; Stadler, R.H., Lineback, D.R., Eds.; John Wiley & Sons, Inc., Publications: Hoboken, NJ, USA, 2009; pp. 135–174. [Google Scholar]
- IARC (International Agency for Research on Cancer). Some industrial chemicals. In IARC Monographs on the Evaluation for Carcinogenic Risk of Chemicals to Humans; IARC: Lyon, France, 1994; Volume 60, pp. 435–453. [Google Scholar]
- EFSA (European Food Safety Agency). Scientific Opinion on acrylamide in food. EFSA J. 2015, 13, 4104. [Google Scholar]
- Ames, J.M. The Maillard reaction. In Biochemistry of Food Proteins; Hudson, B.J.F., Ed.; Elsevier Science Publishers: London, UK, 1992; pp. 99–153. [Google Scholar]
- Kroh, L.W. Caramelisation in food and beverages. Food Chem. 1994, 51, 373–379. [Google Scholar] [CrossRef]
- Glatt, H.; Schneider, H.; Liu, Y. V79-hCYP2E1-hSULT1A1, a cell line for the sensitive detection of genotoxic effects induced by carbohydrate pyrolysis products and other food-borne chemicals. Mut. Res. Genet. Toxicol. Environ. Mutagen. 2005, 580, 41–52. [Google Scholar] [CrossRef]
- Høie, A.H.; Svendsen, C.; Brunborg, G.; Glatt, H.; Alexander, J.; Meinl, W.; Husøy, T. Genotoxicity of three food processing contaminants in transgenic mice expressing human sulfotransferases 1A1 and 1A2 as assessed by the in vivo alkaline single cell gel electrophoresis assay. Environ. Mol. Mutagen. 2015, 56, 709–714. [Google Scholar] [CrossRef]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Agency). Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Flavouring Group Evaluation 13 (FGE.13); Furfuryl and furan derivatives with and without additional side-chain substituents and heteroatoms from chemical group 14. EFSA J. 2005, 215, 1–73. [Google Scholar]
- Batra, B.; Pundir, C.S. Detection of Acrylamide by Biosensors. In Acrylamide in Food. Analysis, Content and Potential Health Effects; Gökmen, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 497–505. [Google Scholar]
- EC (European Commission). Commission regulation (EU) 2017/2158 of 20 November 2017 establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. OJ 2017, L304, 24–44. [Google Scholar]
- Aykas, D.P.; Urtubia, A.; Wong, K.; Ren, L.; López-Lira, C.; Rodriguez-Saona, L.E. Screening of Acrylamide of Par-Fried Frozen French Fries Using Portable FT-IR Spectroscopy. Molecules 2022, 27, 1161. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Hernández, R.; Ruiz, P.; Mauri-Aucejo, A.R.; Yusa, V.; Cerver, M.L. Determination of acrylamide in toasts using digital image colorimetry by smartphone. Food Control 2022, 141, 109163. [Google Scholar] [CrossRef]
- Martín-Tornero, E.; Sánchez, R.; Lozano, J.; Martínez, M.; Arroyo, P.; Martín-Vertedor, D. Characterization of Polyphenol and Volatile Fractions of Californian-Style Black Olives and Innovative Application of E-nose for Acrylamide Determination. Foods 2021, 10, 2973. [Google Scholar] [CrossRef] [PubMed]
- Vinaixa, M.; Vergara, A.; Duran, C.; Llobet, E.; Badia, C.; Brezmes, J.; Vilanova, X.; Correig, X. Fast detection of rancidity in potato crisps using e-noses based on mass spectrometry or gas sensors. Sens. Actuators B 2005, 106, 67–75. [Google Scholar] [CrossRef]
- Sánchez, R.; Martín-Tornero, E.; Lozano, J.; Boselli, E.; Arroyo, P.; Meléndez, F.; Martín-Vertedor, D. E-Nose discrimination of abnormal fermentations in Spanish-Style Green Olives. Molecules 2021, 26, 5353. [Google Scholar] [CrossRef]
- Karimi, Z.; Firouzi, M.; Dadmehr, M.; Javad-Mousavi, S.A.; Bagheriani, N.; Sadeghpour, O. Almond as a nutraceutical and therapeutic agent in Persian medicine and modern phytotherapy: A narrative review. Phytother. Res. 2021, 35, 2997–3012. [Google Scholar] [CrossRef]
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Sanches Silva, A.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, I.; et al. Almonds (Prunus dulcis Mill. D. A. Webb): A Source of Nutrients and Health-Promoting Compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef]
- Amrein, T.M.; Lukac, H.; Andres, L.; Perren, R.; Escher, F.; Amadò, R. Acrylamide in roasted almonds and hazelnuts. J. Agric. Food Chem. 2005, 53, 7819–7825. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, G.; Xiao, L.; Seiber, J.; Mitchell, A.E. Acrylamide Formation in Almonds (Prunus dulcis): Influences of Roasting Time and Temperature, Precursors, Varietal Selection, and Storage. J. Agric. Food Chem. 2011, 59, 8225–8232. [Google Scholar] [CrossRef]
- Amrein, T.M.; Andres, L.; Schönbächler, B.; Conde-Petit, B.; Escher, F.; Amadò, R. Acrylamide in almond products. Eur. Food Res. Technol. 2005, 221, 14–18. [Google Scholar] [CrossRef]
- Lukac, H.; Amrein, T.M.; Perren, R.; Conde-Petit, B.; Amadò, R.; Escher, F. Influence of roasting conditions on the acrylamide content and the color of roasted almonds. J. Food Sci. 2007, 72, C33–C38. [Google Scholar] [CrossRef] [PubMed]
- González-Mulero, L.; Mesías, M.; Morales, F.J.; Delgado-Andrade, C. Acrylamide Exposure from Common Culinary Preparations in Spain, in Household, Catering and Industrial Settings. Foods 2021, 10, 2008. [Google Scholar] [CrossRef] [PubMed]
- Mesías, M.; Holgado, F.; Márquez-Ruiz, G.; Morales, F.J. Effect of sodium replacement in cookies on the formation of process contaminants and lipid oxidation. LWT Food Sci. Technol. 2015, 62, 633–639. [Google Scholar] [CrossRef]
- Barea-Ramos, J.D.; Cascos, G.; Mesías, M.; Lozano, J.; Martín-Vertedor, D. Evaluation of the Olfactory Quality of Roasted Coffee Beans Using a Digital Nose. Sensors 2022, 22, 8654. [Google Scholar] [CrossRef] [PubMed]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Geladi, P.; Kowalski, B.R. Partial least-squares regression: A tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Schlörmann, W.; Birringer, M.; Böhmc, V.; Löber, K.; Jahreis, G.; Lorkowski, S.; Müller, A.K.; Schöne, F.; Glei, M. Influence of roasting conditions on health-related compounds in different nuts. Food Chem. 2015, 180, 77–85. [Google Scholar] [CrossRef]
- Kocadagli, T.; Göncüoglu, N.; Hamzalioglu, A.; Gökmen, V. In Depth Study of Acrylamide Formation in Coffee during Roasting: Role of Sucrose Decomposition and Lipid Oxidation. Food Funct. 2012, 3, 970–975. [Google Scholar] [CrossRef]
- Berk, E.; Hamzalıoğlu, A.; Gökmen, V. Investigations on the Maillard Reaction in Sesame (Sesamum indicum L.) Seeds Induced by Roasting. J. Agric. Food Chem. 2019, 67, 4923–4930. [Google Scholar] [CrossRef]
- Mesias, M.; Gómez, P.; Olombrada, E.; Morales, F.J. Formation of acrylamide during the roasting of chia seeds (Salvia hispanica L.). Food Chem. 2023, 401, 134169. [Google Scholar] [CrossRef]
- Seron, L.H.; Poveda, E.G.; Moya, M.S.P.; Carratalá, M.L.M.; Berenguer-Navarro, V.; Grané-Teruel, N. Characterisation of 19 almond cultivars on the basis of their free amino acids composition. Food Chem. 1998, 61, 455–459. [Google Scholar] [CrossRef]
- Fourie, P.C.; Basson, D.S. Sugar content of almond, pecan and macadamia nuts. J. Agric. Food Chem. 1990, 38, 101–104. [Google Scholar] [CrossRef]
- Lasekan, O.; Abbas, K. Analysis of volatile flavour compounds and acrylamide in roasted Malaysian tropical almond (Terminalia catappa) nuts using supercritical fluid extraction. Food Chem. Toxicol. 2010, 48, 2212–2216. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.; Xu, D.; Hu, H.; Huang, Y.; Liu, Y.; Nie, S.; Li, C.; Xie, M. Investigation on the contents of heat-induced hazards in commercial nuts. Food Res. Int. 2023, 163, 112041. [Google Scholar] [CrossRef]
- Loutfi, A.; Coradeschi, S.; Mani, G.K.; Shankar, P.; Rayappan, J.B.B. Electronic noses for food quality: A review. J. Food Eng. 2015, 144, 103–111. [Google Scholar] [CrossRef]
- Majchrzak, T.; Wojnowski, W.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Electronic noses in classification and quality control of edible oils: A review. Food Chem. 2018, 246, 192–201. [Google Scholar] [CrossRef]
- Martín-Torres, S.; Ruiz-Castro, L.; Jiménez-Carvelo, A.M.; Cuadros-Rodríguez, L. Applications of multivariate data analysis in shelf life studies of edible vegetal oils–A review of the few past years. Food Packag. Shelf Life 2022, 31, 100790. [Google Scholar] [CrossRef]
- Rodríguez, J.; Durán, C.; Reyes, A. Electronic nose for quality control of Colombian coffee through the detection of defects in “Cup Tests”. Sensors 2009, 10, 36–46. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).