Electrochemical Sweat Sensors
Abstract
1. Introduction
1.1. Electrochemical Biosensing of Sweat Composition as a Diagnostic Tool
| Target Analyte | Concentration in Sweat | Disease Correlation | Sensing Modality | Ref. | |
|---|---|---|---|---|---|
| Ions | Na+ | 10–100 mM | Dehydration, hyponatremia, electrolyte imbalances | Ion-selective potentiometry | [7,8] |
| Cl− | 10–100 mM | Dehydration, cystic fibrosis | [7,9] | ||
| K+ | 1–18.5 mM | Hypokalaemia, muscle cramps | [7] | ||
| Ca2+ | 0.41–12.4 mM | Myeloma, cirrhosis, renal failure, acid–base balance disorder | [9] | ||
| pH | 3–8 | Pathogenesis of skin diseases, wound healing | [10] | ||
| NH4+ | 0.1–1 mM | Shift from aerobic to anaerobic metabolic conditions | [10] | ||
| Metabolites | Glucose | 10–200 μM | Diabetes | Amperometric enzymatic biosensors | [7] |
| Lactate | 5–20 mM | Cystic fibrosis, stress ischaemia, lactic acidosis | [11] | ||
| Ethanol | 2–30 mM | Alcoholism, hepatitis B, diabetes, drunk driving | [12] | ||
| Uric acid | 2–10 mM | Hyperuricemia, gout, kidney disease | [13] | ||
| Ascorbic acid | 10–50 μM | Tumours, cancer, kidney disease, thrombosis, stones | [13] | ||
| Hormones | Cortisol | 22–390 nM | Stress | Voltammetry, electrochemical impedance spectroscopy | [14] |
| Macromolecules | Peptides Proteins (antibodies, antigens, enzymes) | - | Square wave voltammetry, electrochemical impedance spectroscopy | ||
1.2. Key Health-Related Examples of Sweat Biochemical Analysis
2. Sampling Techniques
2.1. Traditional Methods of Sampling Sweat
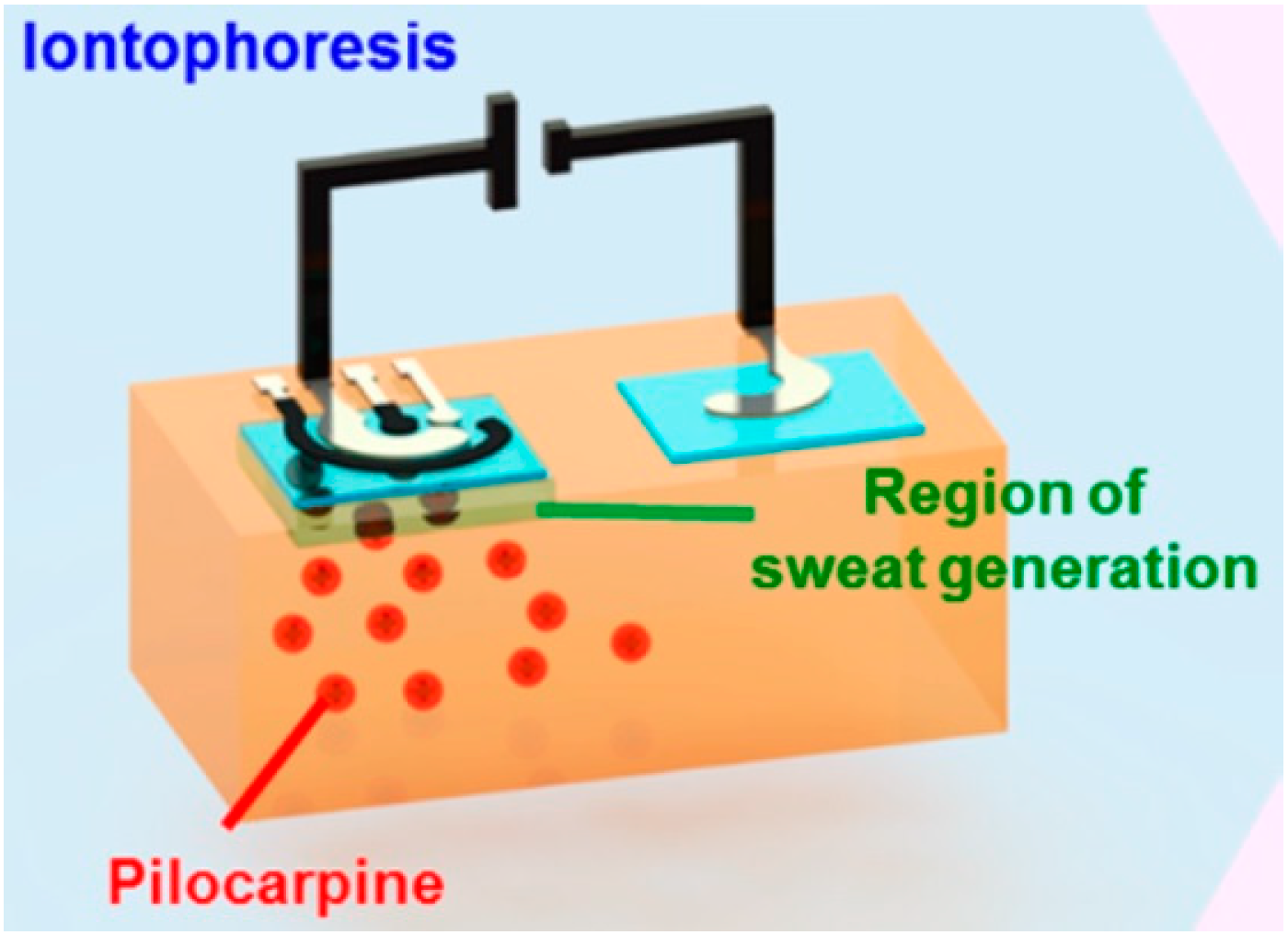
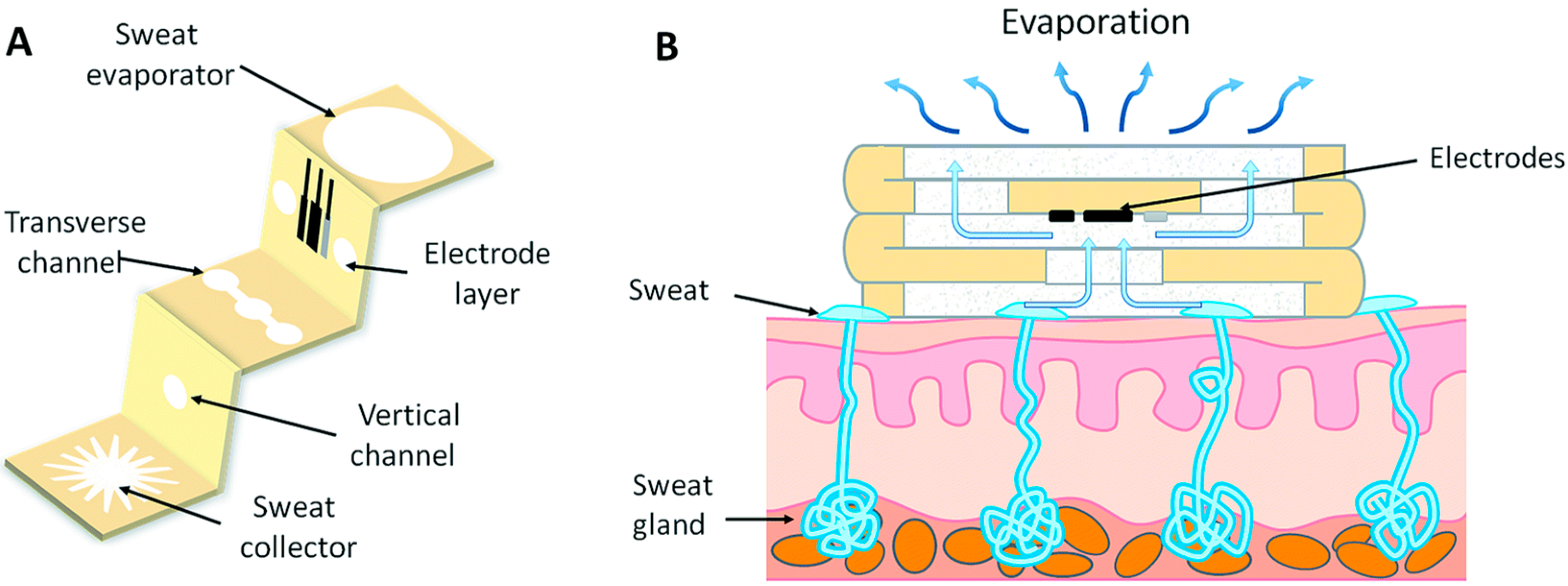
2.2. Sampling Methods for Wearable Systems: Sweat Generation and Collection
3. Sensing Electrolytes in Sweat with Potentiometric Ion-Selective Electrodes
3.1. Reference Electrode
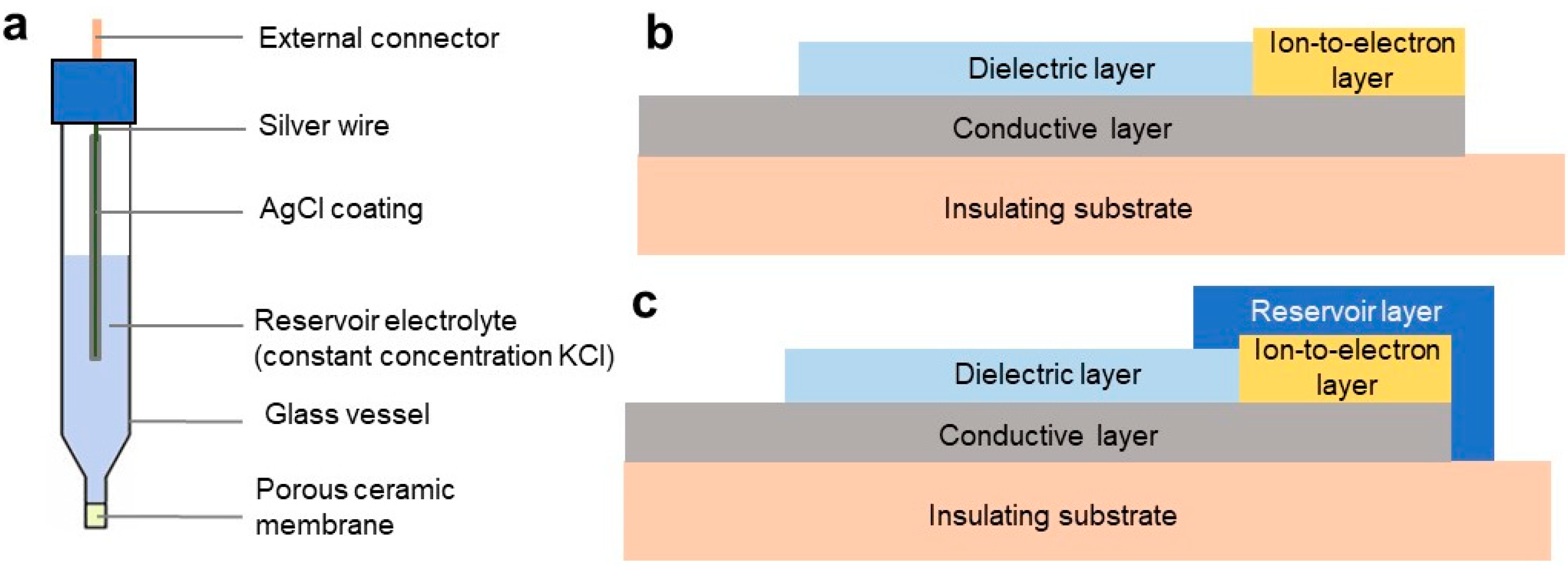
3.1.1. Reference Electrodes: Structural Overview
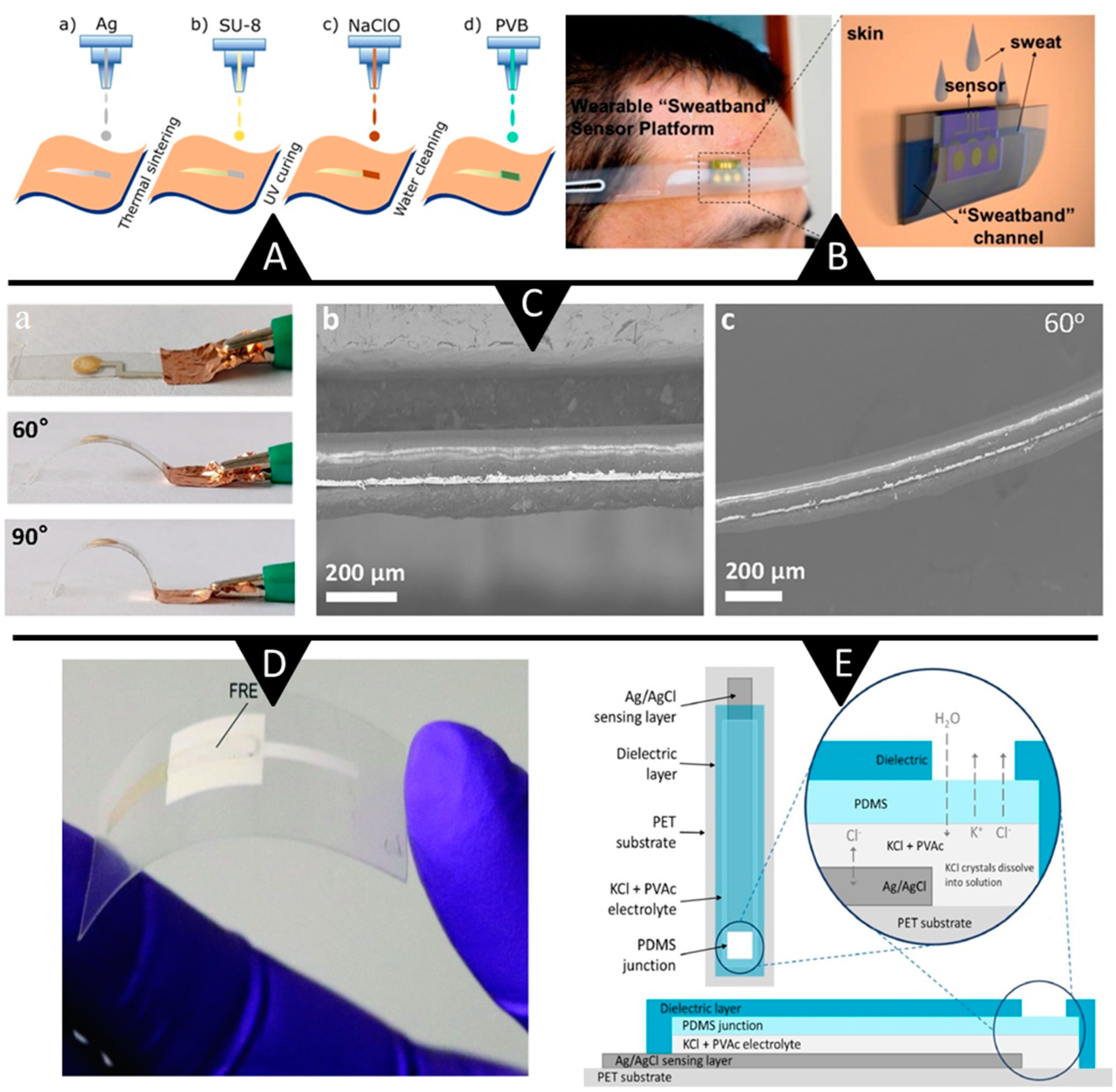
3.1.2. Reference Electrodes: Ongoing Advances
3.1.3. Reference Electrodes: Lingering Challenges
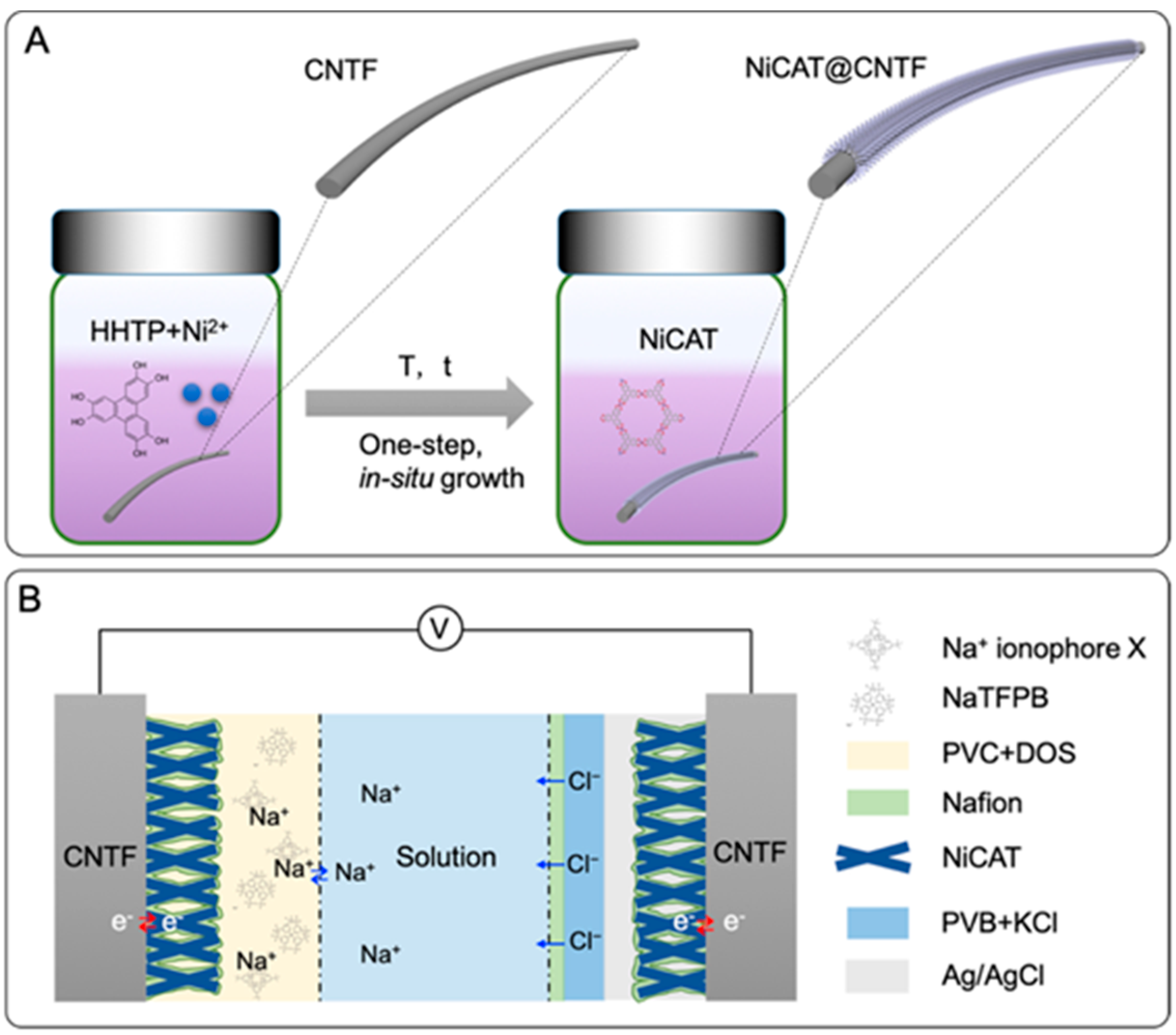
3.2. Ion-Selective Electrodes
3.2.1. ISEs Structural Overview
3.2.2. ISEs: Ongoing Advances
3.2.3. Ion-Selective Electrodes: Lingering Challenges
4. Sensing Metabolites in Sweat with Amperometric Enzyme Electrodes
| Analyte | Relative Content in Sweat | Enzyme | Redox Mediator | Electrode Material | Electrode Substrate | Linear Range; Sensitivity | Detection Limit | Sample | Data Acquisition | Response Time | Operation Stability; Storage Stability | Disease Correlation | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | 10–200 mM [2] | GOx | Prussian Blue | CNT fibber electrode (CE, WE), Ag/AgCl fibre (RE) | Fabric | 0–200 μM; 2.15 nA µm−1 | NA | Sweat after 10 min of exercise | Bluetooth | 30 s | NA | Diabetes | [67] |
| GOx | Prussian Blue | Prussian Blue/graphite ink (WE), SP graphite ink (CE) Ag/AgCl (RE) | Paper (3D-PMED) | 0–1.9 mM; 35.7 mAmM−1 cm−2 | 5 mM | Sweat from forehead during cycling | Wires | 60 s | NA | [33] | |||
| GOx | Mediator-free | PPD/PtNP/Au/ACA (WE), Ag/AgCl (RE) | ACA | 0–600 μM; 15.1 μA/mMcm−2 | 0.9 μM | Iontophoresis | Bluetooth | 60 s | 10-h; NA | [72] | |||
| Lactate | 5–20 mM [2] | LOx | 1,2-naphthoquinone | Carbon/GMgOC (WE), carbon (CE), and Ag/AgCl (RE) | PDMS | 0–50 mM (36.2 μA mM−1 cm−2) | 0.3 mM | Artificial sweat | Wires and sticker-based connector | 2–6 min | NA | Cystic fibrosis, stress ischemia, lactic acidosis | [69] |
| LOx, HRP | Os-complex | Ag/AgCl (RE), graphite paste (CE, WE), and WE modified with MWCNTs | PP | 0–25 mM (0.74 μA mM−1) | 0.04 mM | Sweat from forehead during cycling | Wires | 60 s | Intervals during 6-h; NA | [24] | |||
| LOx | Prussian Blue | Ag/AgCl (RE), SPCE (CE) | Flexible substrate | 1–222 μM (40.6 μA mM−1 cm−2) 0.222–25 mM (1.9 μA mM−1 cm−2) | 0.8 μM | Sweat from forehead while walking | Bluetooth | 100 s | Sensitivity remained 88.3% after multiple use in 20 days; NA | [68] | |||
| Ethanol | 2–30 mM | AOx | Prussian Blue | SPPB conducting carbon, AOx, chitosan (WE), SPPB conducting carbon (CE), Ag/AgCl (RE) | Tattoo | 0–40 × 10−3 M; NA | NA | Sweat by 5-min iontophoresis | Bluetooth | 60 s | At least 10 repetitions; NA | Alcoholism, hepatitis B, diabetes, drunk driving | [29] |
| Ascorbic acid | 10–50 mM [2] | Ascorbate oxidase | Mediator-free | Ag/AgCl (RE), SPCE (CE), and Rh-SPCE (WE) | Tattoo polyurethane | 0−1000 μM; NA | NA | Sweat stimulation of forearm | Wires | 60 s | 2 h after ingesting vitamin C; NA | Tumours, cancer, kidney disease, thrombosis, stones | [73] |
| Levodopa | Dose dependent | Tyrosinase | Thionine acetate | Au nano dendrites on Au/Cr tyrosinase (WE), Ag/AgCl (RE), and Au (CE) | PET | 1–1000 mM; 1.7 nAmM−1 | 1 μM | Sweat by 5-min iontophoresis | Wires | 200 s | NA | Parkinson’s disease monitoring and optimization | [70] |
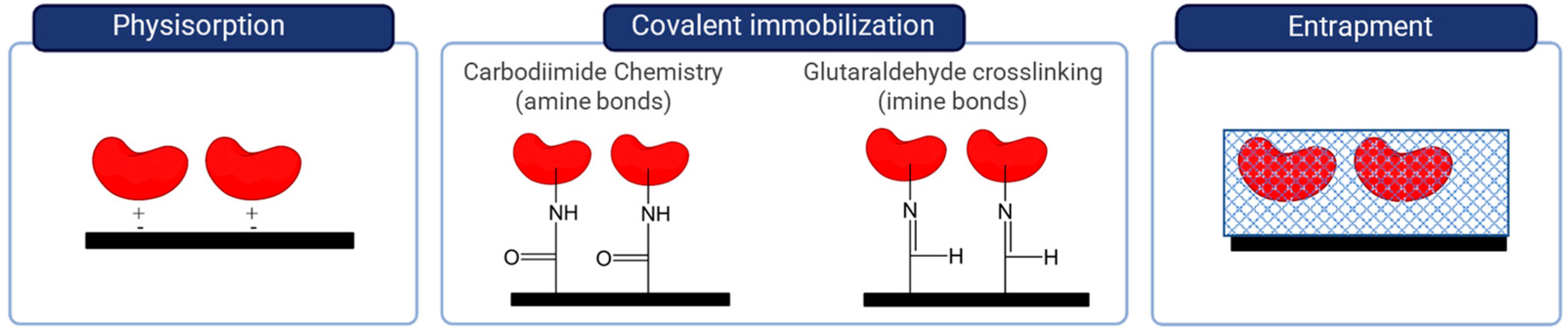
4.1. Immobilization Strategies in the Development of Enzymatic Biosensors
4.2. Stability of Enzymatic Biosensors
4.3. Selectivity: Chemical Interferences
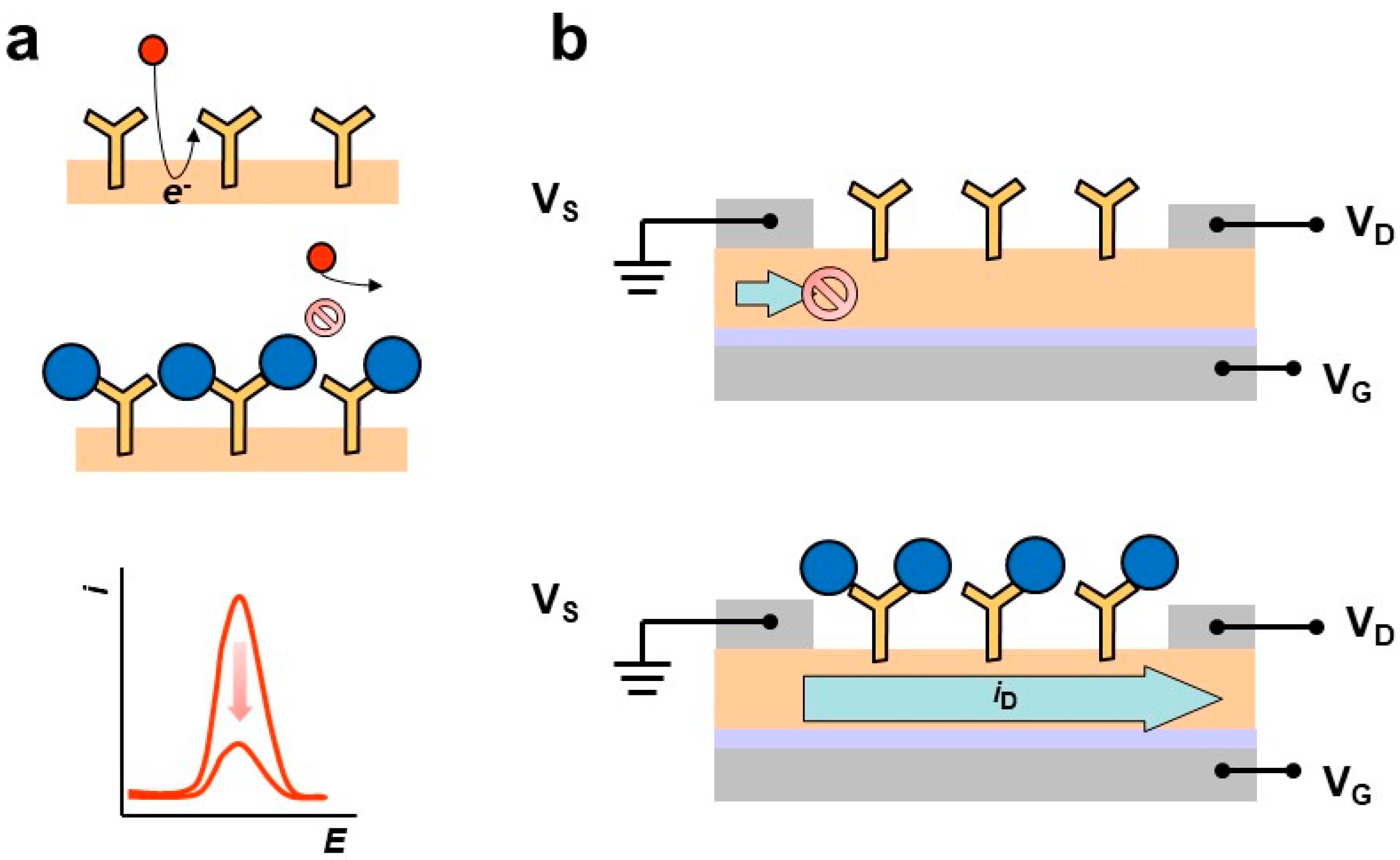
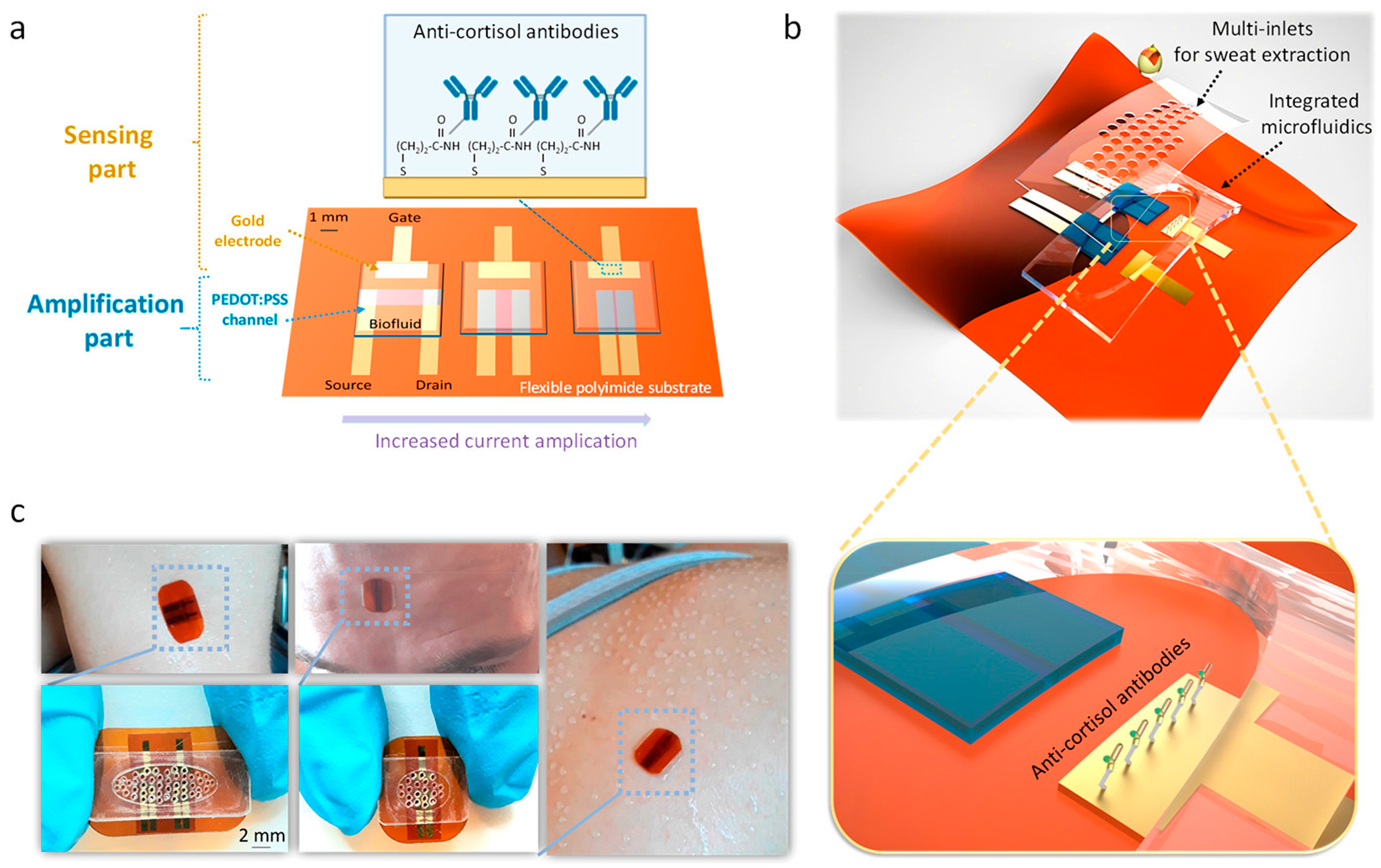
5. Sensing Biomolecules in Sweat with Affinity Electrochemical Biosensors
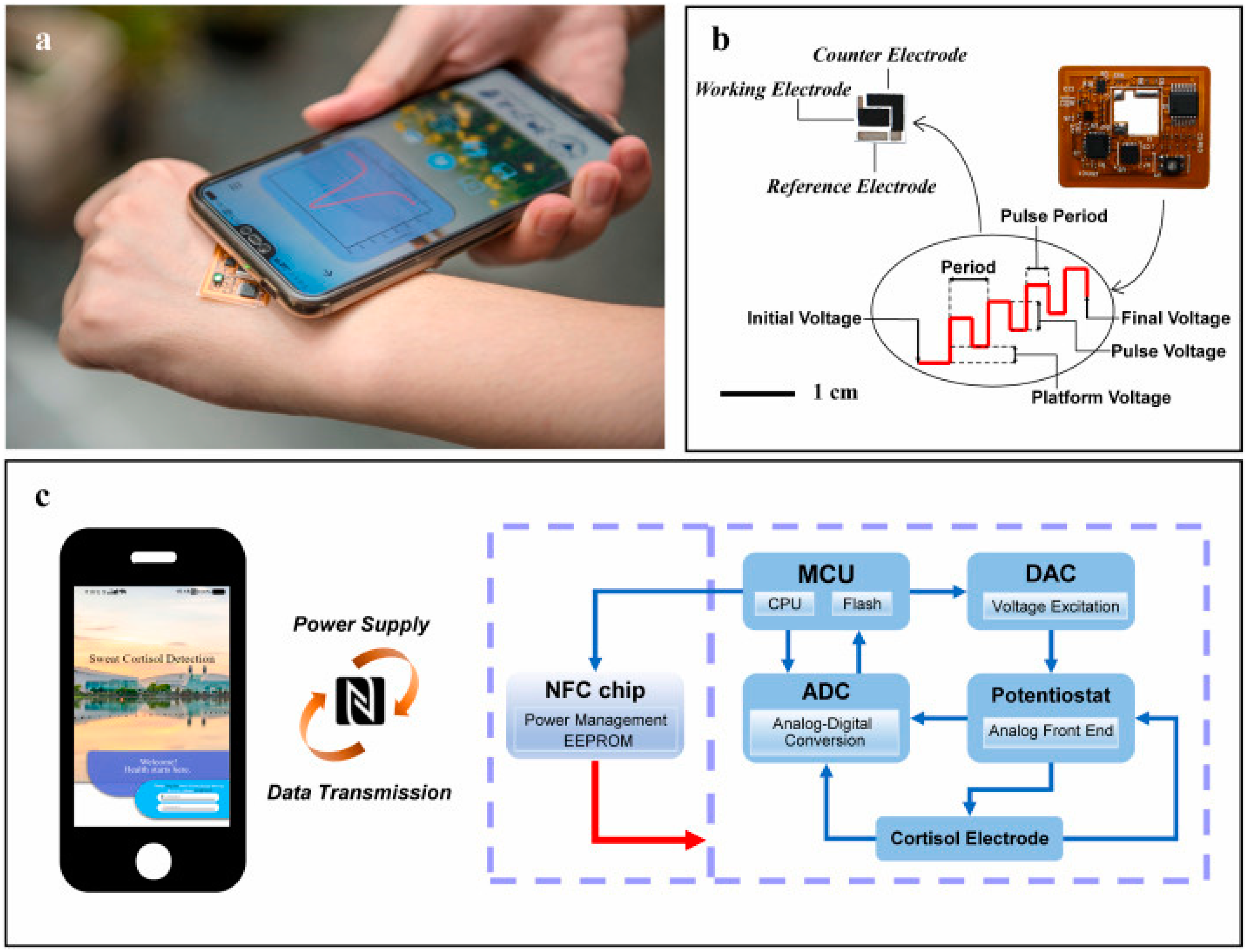
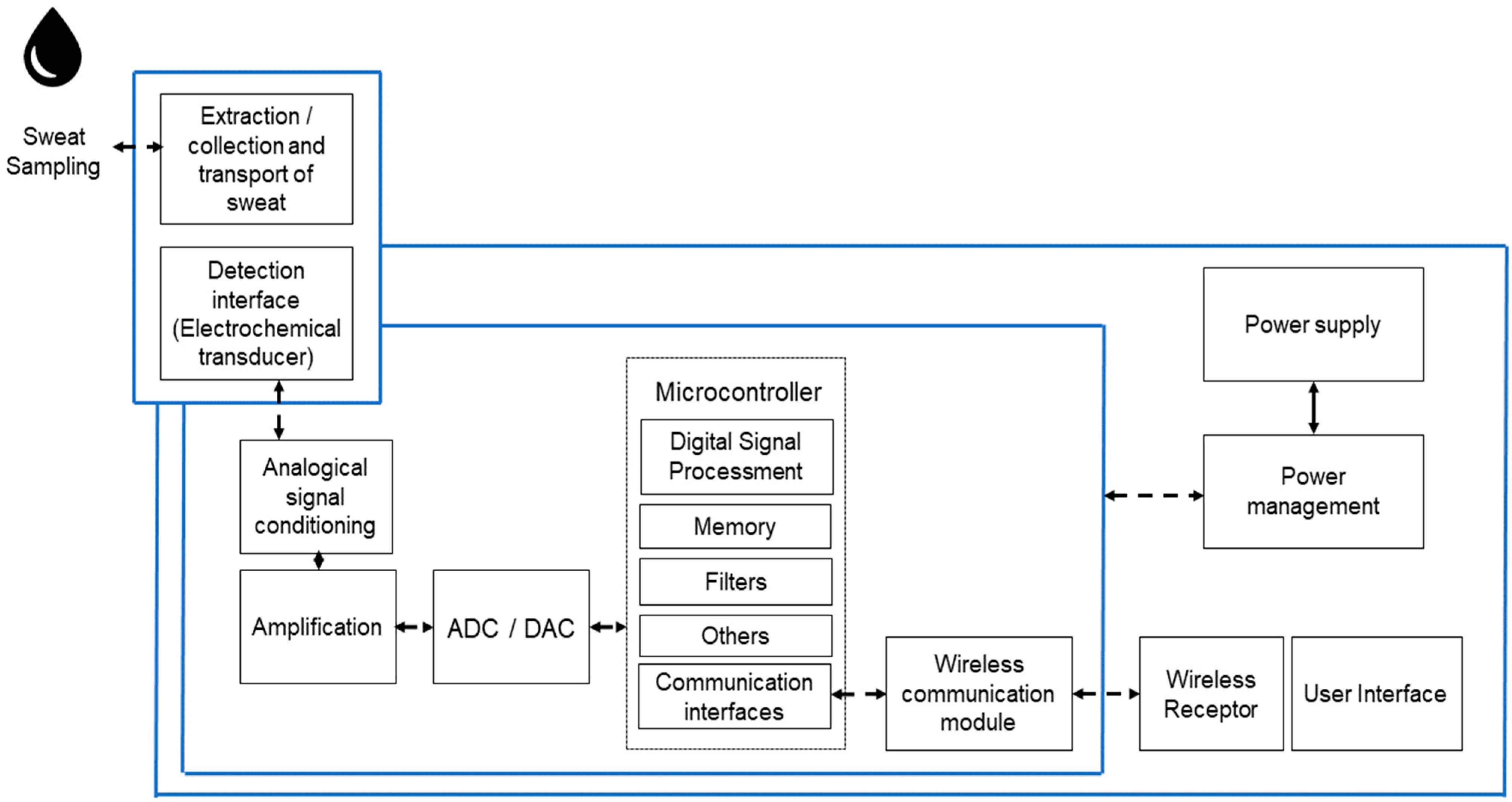
6. Electronic Instrumentation
6.1. Integrated Electronics
6.2. Power Supply
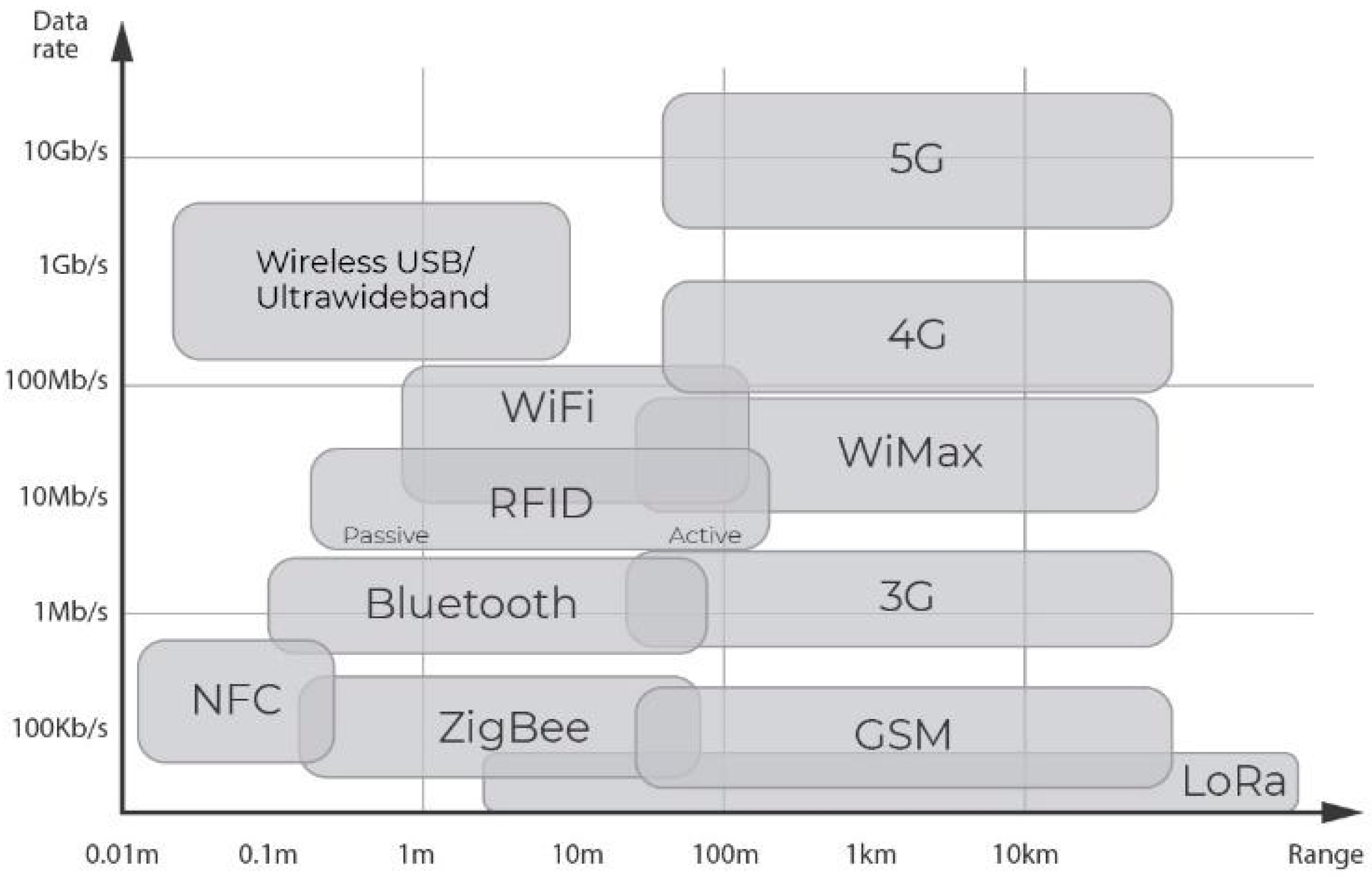
6.3. Wireless Communication
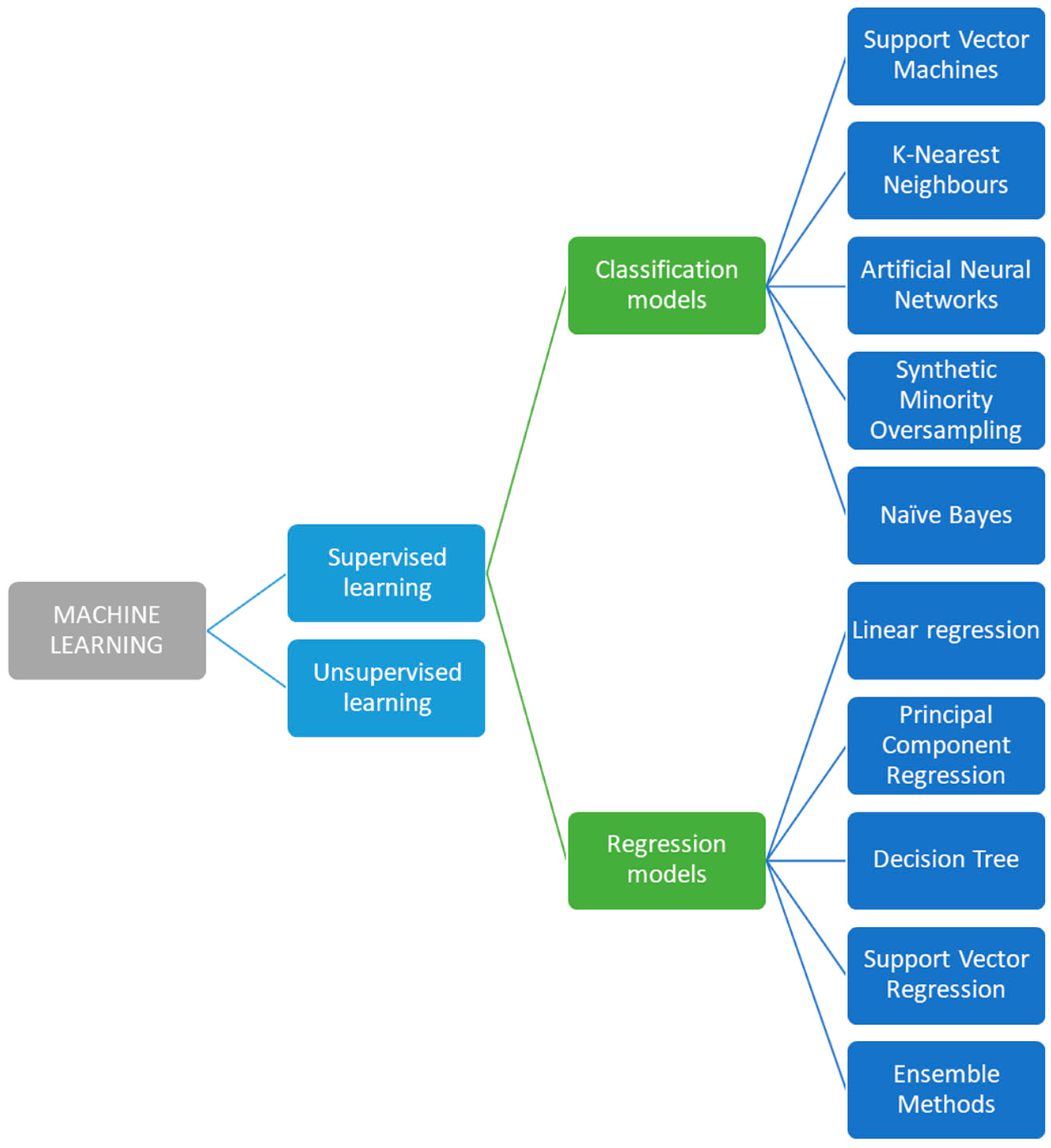
7. Machine Learning Signal Processing of Electrochemical Sweat Sensors
8. Regulatory Aspects
9. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Baker, L.B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Qiao, L.; Chen, Z.; Liu, B.; Gao, L.; Zhang, L. Wearable Sensor for Continuous Sweat Biomarker Monitoring. Chemosensors 2022, 10, 273. [Google Scholar] [CrossRef]
- Van Hoovels, K.; Xuan, X.; Cuartero, M.; Gijssel, M.; Swarén, M.; Crespo, G.A. Can Wearable Sweat Lactate Sensors Contribute to Sports Physiology? ACS Sens. 2021, 6, 3496–3508. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Nyein, H.Y.Y.; Shahpar, Z.; Fahad, H.M.; Chen, K.; Emaminejad, S.; Gao, Y.; Tai, L.C.; Ota, H.; Wu, E.; et al. Wearable Microsensor Array for Multiplexed Heavy Metal Monitoring of Body Fluids. ACS Sens. 2016, 1, 866–874. [Google Scholar] [CrossRef]
- Xu, J.; Fang, Y.; Chen, J. Wearable biosensors for non-invasive sweat diagnostics. Biosensors 2021, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, C.; Zhang, L.; Liu, T.; Wang, Z.; Song, Z.; Cai, H.; Fang, Z.; Chen, J.; Wang, J.; et al. Wearable and flexible electrochemical sensors for sweat analysis: A review. Microsyst. Nanoeng. 2023, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Nyein, H.Y.Y.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef]
- Patterson, M.J.; Galloway, S.D.R.; Nimmo, M.A. Variations in regional sweat composition in normal human males. Exp. Physiol. 2000, 85, 869–875. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Gao, W.; Shahpar, Z.; Emaminejad, S.; Challa, S.; Chen, K.; Fahad, H.M.; Tai, L.C.; Ota, H.; Davis, R.W.; et al. A Wearable Electrochemical Platform for Noninvasive Simultaneous Monitoring of Ca2+ and pH. ACS Nano 2016, 10, 7216–7224. [Google Scholar] [CrossRef]
- Anastasova, S.; Crewther, B.; Bembnowicz, P.; Curto, V.; Ip, H.M.D.; Rosa, B.; Yang, G.-Z. A wearable multisensing patch for continuous sweat monitoring. Biosens. Bioelectron. 2017, 93, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeerapan, I.; Imani, S.; Cho, T.N.; Bandodkar, A.; Cinti, S.; Mercier, P.P.; Wang, J. Noninvasive Alcohol Monitoring Using a Wearable Tattoo-Based Iontophoretic-Biosensing System. ACS Sens. 2016, 1, 1011–1019. [Google Scholar] [CrossRef]
- Windmiller, J.R.; Bandodkar, A.J.; Valdés-Ramirez, G.; Parkhomovsky, S.; Martinez, A.G.; Wang, J. Electrochemical sensing based on printable temporary transfer tattoos. Chem. Commun. 2012, 48, 6794–6796. [Google Scholar] [CrossRef] [PubMed]
- Kinnamon, D.; Ghanta, R.; Lin, K.C.; Muthukumar, S.; Prasad, S. Portable biosensor for monitoring cortisol in low-volume perspired human sweat. Sci. Rep. 2017, 7, 13312. [Google Scholar] [CrossRef]
- Montón, E.; Hernandez, J.F.; Blasco, J.M.; Hervé, T.; Micallef, J.; Grech, I.; Brincat, A.; Traver, V. Body area network for wireless patient monitoring. IET Commun. 2008, 2, 215–222. [Google Scholar] [CrossRef]
- LeGrys, V.A.; Yankaskas, J.R.; Quittell, L.M.; Marshall, B.C.; Mogayzel, P.J. Diagnostic Sweat Testing: The Cystic Fibrosis Foundation Guidelines. J. Pediatr. 2007, 151, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol. Ther. 2012, 14, 398–402. [Google Scholar] [CrossRef]
- Xue, W.; Tan, X.; Khaing Oo, M.K.; Kulkarni, G.; Ilgen, M.A.; Fan, X. Rapid and sensitive detection of drugs of abuse in sweat by multiplexed capillary based immuno-biosensors. Analyst 2020, 145, 1346–1354. [Google Scholar] [CrossRef]
- Tai, L.C.; Gao, W.; Chao, M.; Bariya, M.; Ngo, Q.P.; Shahpar, Z.; Nyein, H.Y.Y.; Park, H.; Sun, J.; Jung, Y.; et al. Methylxanthine Drug Monitoring with Wearable Sweat Sensors. Adv. Mater. 2018, 30, 1707442. [Google Scholar] [CrossRef]
- Teymourian, H.; Parrilla, M.; Sempionatto, J.R.; Montiel, N.F.; Barfidokht, A.; Van Echelpoel, R.; De Wael, K.; Wang, J. Wearable Electrochemical Sensors for the Monitoring and Screening of Drugs. ACS Sens. 2020, 5, 2679–2700. [Google Scholar] [CrossRef]
- Lam Po Tang, S. Wearable sensors for sports performance. In Textiles for Sportswear; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 169–196. ISBN 9781782422297. [Google Scholar]
- Parrilla, M.; Ortiz-Gómez, I.; Cánovas, R.; Salinas-Castillo, A.; Cuartero, M.; Crespo, G.A. Wearable Potentiometric Ion Patch for On-Body Electrolyte Monitoring in Sweat: Toward a Validation Strategy to Ensure Physiological Relevance. Anal. Chem. 2019, 91, 8644–8651. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Pérez-Ràfols, C.; Chen, C.; Cuartero, M.; Crespo, G.A. Lactate Biosensing for Reliable On-Body Sweat Analysis. ACS Sens. 2021, 6, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Fu, Z.; Qi, L. A Wearable Biosensor Based on Bienzyme Gel-Membrane for Sweat Lactate Monitoring by Mounting on Eyeglasses. J. Nanosci. Nanotechnol. 2020, 20, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, T.; Wang, D.; Zhang, X. The role of sampling in wearable sweat sensors. Talanta 2020, 212, 120801. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.W.; Chinnamani, M.V.; Lee, E.H.; Lee, N.E. Stretchable Non-Enzymatic Fuel Cell-Based Sensor Patch Integrated with Thread-Embedded Microfluidics for Self-Powered Wearable Glucose Monitoring. Adv. Mater. Interfaces 2022, 9, 2200492. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.; Gupta, V.; Arya, S. Advancements and future prospects of wearable sensing technology for healthcare applications. Sens. Diagn. 2022, 1, 387–404. [Google Scholar] [CrossRef]
- Sharma, A.; Badea, M.; Tiwari, S.; Marty, J.L. Wearable biosensors: An alternative and practical approach in healthcare and disease monitoring. Molecules 2021, 26, 748. [Google Scholar] [CrossRef]
- Kim, J.; Sempionatto, J.R.; Imani, S.; Hartel, M.C.; Barfidokht, A.; Tang, G.; Campbell, A.S.; Mercier, P.P.; Wang, J. Simultaneous Monitoring of Sweat and Interstitial Fluid Using a Single Wearable Biosensor Platform. Adv. Sci. 2018, 5, 14–23. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Liu, R.; Li, J.; Zhang, Q.; Shi, G.; Li, Y.; Hou, C.; Wang, H. A highly integrated sensing paper for wearable electrochemical sweat analysis. Biosens. Bioelectron. 2021, 174, 112828. [Google Scholar] [CrossRef]
- He, X.; Xu, T.; Gu, Z.; Gao, W.; Xu, L.P.; Pan, T.; Zhang, X. Flexible and Superwettable Bands as a Platform toward Sweat Sampling and Sensing. Anal. Chem. 2019, 91, 4296–4300. [Google Scholar] [CrossRef]
- Dai, B.; Li, K.; Shi, L.; Wan, X.; Liu, X.; Zhang, F.; Jiang, L.; Wang, S. Bioinspired Janus Textile with Conical Micropores for Human Body Moisture and Thermal Management. Adv. Mater. 2019, 31, 1904113. [Google Scholar] [CrossRef]
- Cao, Q.; Liang, B.; Tu, T.; Wei, J.; Fang, L.; Ye, X. Three-dimensional paper-based microfluidic electrochemical integrated devices (3D-PMED) for wearable electrochemical glucose detection. RSC Adv. 2019, 9, 5674–5681. [Google Scholar] [CrossRef]
- Sophocleous, M.; Atkinson, J.K. A review of screen-printed silver/silver chloride (Ag/AgCl) reference electrodes potentially suitable for environmental potentiometric sensors. Sens. Actuators A Phys. 2017, 267, 106–120. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jeang, W.J.; Ghaffari, R.; Rogers, J.A. Wearable Sensors for Biochemical Sweat Analysis. Annu. Rev. Anal. Chem. 2019, 12, 201–224. [Google Scholar] [CrossRef]
- Kumar, A.K.S.; Zhang, Y.; Li, D.; Compton, R.G. A mini-review: How reliable is the drop casting technique? Electrochem. Commun. 2020, 121, 106867. [Google Scholar] [CrossRef]
- Suresh, R.R.; Lakshmanakumar, M.; Arockia Jayalatha, J.B.B.; Rajan, K.S.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. Fabrication of screen-printed electrodes: Opportunities and challenges. J. Mater. Sci. 2021, 56, 8951–9006. [Google Scholar] [CrossRef]
- Gan, S.; Liao, C.; Liang, R.; Du, S.; Zhong, L.; Tang, Y.; Han, T.; Bao, Y.; Sun, Z.; Ma, Y.; et al. A Solid-Contact Reference Electrode Based on Silver/Silver Organic Insoluble Salt for Potentiometric Ion Sensing. ACS Meas. Sci. Au 2022, 2, 568–575. [Google Scholar] [CrossRef]
- Dawkins, R.C.; Wen, D.; Hart, J.N.; Vepsäläinen, M. A screen-printed Ag/AgCl reference electrode with long-term stability for electroanalytical applications. Electrochim. Acta 2021, 393, 139043. [Google Scholar] [CrossRef]
- Macedo, D.S.; Vepsäläinen, M.; Acharya, D.; Wood, C.D.; Wen, D.; Thomson, L.; Peacock, S.; Rodopoulos, T.; Hogan, C.F. An unusually stable solid state Ag|AgCl reference electrode for long term continuous measurements based on a crosslinked poly(vinyl acetate)/KCl composite. Electrochim. Acta 2021, 368, 137636. [Google Scholar] [CrossRef]
- Moya, A.; Pol, R.; Martínez-Cuadrado, A.; Villa, R.; Gabriel, G.; Baeza, M. Stable Full-Inkjet-Printed Solid-State Ag/AgCl Reference Electrode. Anal. Chem. 2019, 91, 15539–15546. [Google Scholar] [CrossRef]
- Komoda, M.; Shitanda, I.; Hoshi, Y.; Itagaki, M. Fabrication and characterization of a fully screen-printed Ag/AgCl reference electrode using silica gel inks exhibiting instantaneous usability and long-term stability. Electrochemistry 2019, 87, 65–69. [Google Scholar] [CrossRef]
- Manjakkal, L.; Shakthivel, D.; Dahiya, R. Flexible Printed Reference Electrodes for Electrochemical Applications. Adv. Mater. Technol. 2018, 3, 1800252. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Y.; Gu, Y.; Li, T.; Luo, H.; Li, L.-H.; Bai, Y.; Li, L.; Liu, L.; Cao, Y.; et al. Wearable Sweatband Sensor Platform Based on Gold Nanodendrite Array as Efficient Solid Contact of Ion-Selective Electrode. Anal. Chem. 2017, 89, 10224–10231. [Google Scholar] [CrossRef]
- Alva, S.; Heng, L.Y.; Ahmad, M. Optimization of Screen Printed Reference Electrode Based on Charge Balance and Poly (Butyl Acrylate) Photocurable Membrane. Int. J. Innov. Mech. Eng. Adv. Mater. 2016, 2, 10. [Google Scholar] [CrossRef]
- Tangkuaram, T.; Ponchio, C.; Kangkasomboon, T.; Katikawong, P.; Veerasai, W. Design and development of a highly stable hydrogen peroxide biosensor on screen printed carbon electrode based on horseradish peroxidase bound with gold nanoparticles in the matrix of chitosan. Biosens. Bioelectron. 2007, 22, 2071–2078. [Google Scholar] [CrossRef]
- Guan, W.-J.; Li, Y.; Chen, Y.-Q.; Zhang, X.-B.; Hu, G.-Q. Glucose biosensor based on multi-wall carbon nanotubes and screen printed carbon electrodes. Biosens. Bioelectron. 2005, 21, 508–512. [Google Scholar] [CrossRef]
- Guinovart, T.; Crespo, G.A.; Rius, F.X.; Andrade, F.J. A reference electrode based on polyvinyl butyral (PVB) polymer for decentralized chemical measurements. Anal. Chim. Acta 2014, 821, 72–80. [Google Scholar] [CrossRef]
- Parrilla, M.; Ferré, J.; Guinovart, T.; Andrade, F.J. Wearable Potentiometric Sensors Based on Commercial Carbon Fibres for Monitoring Sodium in Sweat. Electroanalysis 2016, 28, 1267–1275. [Google Scholar] [CrossRef]
- Bananezhad, A.; Jović, M.; Villalobos, L.F.; Agrawal, K.V.; Ganjali, M.R.; Girault, H.H. Large-scale fabrication of flexible solid-state reference electrodes. J. Electroanal. Chem. 2019, 847, 113241. [Google Scholar] [CrossRef]
- Veiga, L.S.; Garate, O.; Giménez, G.; Ybarra, G.; Monsalve, L.N. Nanomaterial-Based Multifunctional Inks for the Fabrication of Printed Biosensors, 1st ed.; Gupta, R.K., Nguyen, T., Eds.; Elservier: Amsterdam, The Netherlands, 2022; ISBN 9780323911450. [Google Scholar]
- Lou, S.; Chen, Q.; Wang, W.; Wang, Y.; Zhou, S. Template-assisted synthesis of Ag/AgCl hollow microcubes and their composition-dependent photocatalytic activity for the degradation of phenol. RSC Adv. 2021, 11, 26311–26318. [Google Scholar] [CrossRef]
- Bieg, C.; Fuchsberger, K.; Stelzle, M. Introduction to polymer-based solid-contact ion-selective electrodes—Basic concepts, practical considerations, and current research topics. Anal. Bioanal. Chem. 2017, 409, 45–61. [Google Scholar] [CrossRef]
- Bilbao, E.; Kapadia, S.; Riechert, V.; Amalvy, J.; Molinari, F.N.; Escobar, M.M.; Baumann, R.R.; Monsalve, L.N. Functional aqueous-based polyaniline inkjet inks for fully printed high-performance pH-sensitive electrodes. Sens. Actuators B Chem. 2021, 346, 130558. [Google Scholar] [CrossRef]
- Kumar, P.A.; Pradeep, A.; Nair, B.K.G.; Babu, T.G.S.; Suneesh, P.V. Silver-manganese nanocomposite modified screen-printed carbon electrode in the fabrication of an electrochemical, disposable biosensor strip for cystic fibrosis. Microchim. Acta 2022, 189, 327. [Google Scholar] [CrossRef]
- Cui, X.; Bao, Y.; Han, T.; Liu, Z.; Ma, Y.; Sun, Z. A wearable electrochemical sensor based on β-CD functionalized graphene for pH and potassium ion analysis in sweat. Talanta 2022, 245, 123481. [Google Scholar] [CrossRef]
- Ozer, T.; Agir, I.; Henry, C.S. Low-cost Internet of Things (IoT)-enabled a wireless wearable device for detecting potassium ions at the point of care. Sens. Actuators B Chem. 2022, 365, 131961. [Google Scholar] [CrossRef]
- Wang, S.; Liu, M.; Shi, Y.; Yang, X.; Li, L.; Lu, Q.; Zheng, H.; Feng, S.; Bai, Y.; Zhang, T. Vertically aligned conductive metal-organic framework nanowires array composite fiber as efficient solid-contact for wearable potentiometric sweat sensing. Sens. Actuators B Chem. 2022, 369, 132290. [Google Scholar] [CrossRef]
- Yeung, K.K.; Li, J.; Huang, T.; Hosseini, I.I.; Al Mahdi, R.; Alam, M.M.; Sun, H.; Mahshid, S.; Yang, J.; Ye, T.T.; et al. Utilizing Gradient Porous Graphene Substrate as the Solid-Contact Layer To Enhance Wearable Electrochemical Sweat Sensor Sensitivity. Nano Lett. 2022, 22, 6647–6654. [Google Scholar] [CrossRef]
- Iannazzo, D.; Espro, C.; Ferlazzo, A.; Celesti, C.; Branca, C.; Neri, G. Electrochemical and fluorescent properties of crown ether functionalized graphene quantum dots for potassium and sodium ions detection. Nanomaterials 2021, 11, 2897. [Google Scholar] [CrossRef] [PubMed]
- Napier, B.S.; Matzeu, G.; Lo Presti, M.; Omenetto, F.G. Dry Spun, Bulk-Functionalized rGO Fibers for Textile Integrated Potentiometric Sensors. Adv. Mater. Technol. 2022, 7, 2101508. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, X.; Sun, Z.; Chen, H.; Fu, J.; Si, H.; Ge, C.; Lin, S. Laser-Induced Graphene-Based Wearable Epidermal Ion-Selective Sensors for Noninvasive Multiplexed Sweat Analysis. Biosensors 2022, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Ghoorchian, A.; Kamalabadi, M.; Moradi, M.; Madrakian, T.; Afkhami, A.; Bagheri, H.; Ahmadi, M.; Khoshsafar, H. Wearable Potentiometric Sensor Based on Na 0.44 MnO 2 for Non-invasive Monitoring of Sodium Ions in Sweat. Anal. Chem. 2022, 94, 2263–2270. [Google Scholar] [CrossRef]
- Jiang, T.; Yin, B.; Liu, X.; Yang, L.; Pang, H.; Song, J.; Wu, S. Porous carbon-based robust, durable, and flexible electrochemical device for K+ detection in sweat†. Analyst 2022, 147, 1144–1151. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Nakagawa, T.; Pavinatto, A.; Mensah, S.T.; Imani, S.; Mercier, P.; Wang, J. Eyeglasses based wireless electrolyte and metabolite sensor platform. Lab Chip 2017, 17, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Moina, C.; Ybarra, G. Fundamentals and applications of immunosensors. In Advances in Immunoassay Technology; Theodore, K., Chiu, N., Eds.; InTech: Zagreb, Croatia, 2012; pp. 65–80. [Google Scholar]
- Wang, L.; Wang, L.; Zhang, Y.; Pan, J.; Li, S.; Sun, X.; Zhang, B.; Peng, H. Weaving Sensing Fibers into Electrochemical Fabric for Real-Time Health Monitoring. Adv. Funct. Mater. 2018, 28, 1804456. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, C.; Zhang, Q.; Ye, Y.; Cai, Y.; Li, K.; Li, Y.; Huang, X.; Wang, Y. In-situ preparation of lactate-sensing membrane for the noninvasive and wearable analysis of sweat. Biosens. Bioelectron. 2022, 210, 114303. [Google Scholar] [CrossRef] [PubMed]
- Shitanda, I.; Mitsumoto, M.; Loew, N.; Yoshihara, Y.; Watanabe, H.; Mikawa, T.; Tsujimura, S.; Itagaki, M.; Motosuke, M. Continuous sweat lactate monitoring system with integrated screen-printed Mgo-templated carbon-lactate oxidase biosensor and microfluidic sweat collector. Electrochim. Acta 2021, 368, 137620. [Google Scholar] [CrossRef]
- Tai, L.C.; Liaw, T.S.; Lin, Y.; Nyein, H.Y.Y.; Bariya, M.; Ji, W.; Hettick, M.; Zhao, C.; Zhao, J.; Hou, L.; et al. Wearable Sweat Band for Noninvasive Levodopa Monitoring. Nano Lett. 2019, 19, 6346–6351. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Kitazumi, Y.; Shirai, O.; Kano, K. Direct Electron Transfer-Type Bioelectrocatalysis of Redox Enzymes at Nanostructured Electrodes. Catalysts 2020, 10, 236. [Google Scholar] [CrossRef]
- Hojaiji, H.; Zhao, Y.; Gong, M.C.; Mallajosyula, M.; Tan, J.; Lin, H.; Hojaiji, A.M.; Lin, S.; Milla, C.; Madni, A.M.; et al. An autonomous wearable system for diurnal sweat biomarker data acquisition. Lab Chip 2020, 20, 4582–4591. [Google Scholar] [CrossRef] [PubMed]
- Sempionatto, J.R.; Khorshed, A.A.; Ahmed, A.; De Loyola E Silva, A.N.; Barfidokht, A.; Yin, L.; Goud, K.Y.; Mohamed, M.A.; Bailey, E.; May, J.; et al. Epidermal Enzymatic Biosensors for Sweat Vitamin C: Toward Personalized Nutrition. ACS Sens. 2020, 5, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Rinawati, M.; De Zhan, J.; Lin, K.Y.; Huang, C.J.; Chen, K.J.; Mizuguchi, H.; Jiang, J.C.; Hwang, B.J.; Yeh, M.H. Boron-Doped Graphene Quantum Dots Anchored to Carbon Nanotubes as Noble Metal-Free Electrocatalysts of Uric Acid for a Wearable Sweat Sensor. ACS Appl. Nano Mater. 2022, 5, 11100–11110. [Google Scholar] [CrossRef]
- Jang, J.H.; Lim, H.B. Characterization and analytical application of surface modified magnetic nanoparticles. Microchem. J. 2010, 94, 148–158. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Economou, A.; Karapetis, S.K.; Nikoleli, G.-P.; Nikolelis, D.P.; Bratakou, S.; Varzakas, T.H. Enzyme-based Sensors. In Advances in Food Diagnostics; Toldrá, F., Nollet, L.M.L., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 231–250. ISBN 9781119105916. [Google Scholar]
- Sonawane, A.; Manickam, P.; Bhansali, S. Stability of Enzymatic Biosensors for Wearable Applications. IEEE Rev. Biomed. Eng. 2017, 10, 174–186. [Google Scholar] [CrossRef]
- Balcão, V.M.; Vila, M.M.D.C. Structural and functional stabilization of protein entities: State-of-the-art. Adv. Drug Deliv. Rev. 2015, 93, 25–41. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Rathee, K.; Dhull, V.; Dhull, R.; Singh, S. Biosensors based on electrochemical lactate detection: A comprehensive review. Biochem. Biophys. Rep. 2016, 5, 35–54. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Silva, H.; Pires, F.; Dias-Cabral, A.C.; Arcos-Martinez, M.J. Inhibited enzymatic reaction of crosslinked lactate oxidase through a pH-dependent mechanism. Colloids Surf. B Biointerfaces 2019, 184, 110490. [Google Scholar] [CrossRef]
- Klimuntowski, M.; Alam, M.M.; Singh, G.; Howlader, M.M.R. Electrochemical Sensing of Cannabinoids in Biofluids: A Noninvasive Tool for Drug Detection. ACS Sens. 2020, 5, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, D.J.; Cai, A.; Dorval Courchesne, N.M. The Evolving Role of Proteins in Wearable Sweat Biosensors. ACS Biomater. Sci. Eng. 2021; Article in press. [Google Scholar] [CrossRef]
- Santiago, E.; Poudyal, S.S.; Shin, S.Y.; Yoon, H.J. Graphene Oxide Functionalized Biosensor for Detection of Stress-Related Biomarkers. Sensors 2022, 22, 558. [Google Scholar] [CrossRef]
- Laochai, T.; Yukird, J.; Promphet, N.; Qin, J.; Chailapakul, O.; Rodthongkum, N. Non-invasive electrochemical immunosensor for sweat cortisol based on L-cys/AuNPs/MXene modified thread electrode. Biosens. Bioelectron. 2022, 203, 114039. [Google Scholar] [CrossRef]
- Madhu, S.; Anthuuvan, A.J.; Ramasamy, S.; Manickam, P.; Bhansali, S.; Nagamony, P.; Chinnuswamy, V. ZnO Nanorod Integrated Flexible Carbon Fibers for Sweat Cortisol Detection. ACS Appl. Electron. Mater. 2020, 2, 499–509. [Google Scholar] [CrossRef]
- Madhu, S.; Ramasamy, S.; Manickam, P.; Nagamony, P.; Chinnuswamy, V. TiO2 anchored carbon fibers as non-invasive electrochemical sensor platform for the cortisol detection. Mater. Lett. 2022, 308, 131238. [Google Scholar] [CrossRef]
- Naik, A.R.; Zhou, Y.; Dey, A.A.; Arellano, D.L.G.; Okoroanyanwu, U.; Secor, E.B.; Hersam, M.C.; Morse, J.; Rothstein, J.P.; Carter, K.R.; et al. Printed microfluidic sweat sensing platform for cortisol and glucose detection. Lab Chip 2022, 22, 156–169. [Google Scholar] [CrossRef]
- Nah, J.S.; Barman, S.C.; Zahed, M.A.; Sharifuzzaman, M.; Yoon, H.; Park, C.; Yoon, S.; Zhang, S.; Park, J.Y. A wearable microfluidics-integrated impedimetric immunosensor based on Ti3C2Tx MXene incorporated laser-burned graphene for noninvasive sweat cortisol detection. Sens. Actuators B Chem. 2021, 329, 129206. [Google Scholar] [CrossRef]
- Cheng, C.; Li, X.; Xu, G.; Lu, Y.; Low, S.S.; Liu, G.; Zhu, L.; Li, C.; Liu, Q. Battery-free, wireless, and flexible electrochemical patch for in situ analysis of sweat cortisol via near field communication. Biosens. Bioelectron. 2021, 172, 112782. [Google Scholar] [CrossRef]
- Ganguly, A.; Lin, K.C.; Muthukumar, S.; Prasad, S. Autonomous, Real-Time Monitoring Electrochemical Aptasensor for Circadian Tracking of Cortisol Hormone in Sub-microliter Volumes of Passively Eluted Human Sweat. ACS Sens. 2021, 6, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Demuru, S.; Kim, J.; El Chazli, M.; Bruce, S.; Dupertuis, M.; Binz, P.A.; Saubade, M.; Lafaye, C.; Briand, D. Antibody-Coated Wearable Organic Electrochemical Transistors for Cortisol Detection in Human Sweat. ACS Sens. 2022, 6, 16472–16475. [Google Scholar] [CrossRef]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Guk, K.; Han, G.; Lim, J.; Jeong, K.; Kang, T.; Lim, E.K.; Jung, J. Evolution of wearable devices with real-time disease monitoring for personalized healthcare. Nanomaterials 2019, 9, 813. [Google Scholar] [CrossRef]
- Ling, Y.; An, T.; Yap, L.W.; Zhu, B.; Gong, S.; Cheng, W. Disruptive, Soft, Wearable Sensors. Adv. Mater. 2020, 32, 1904664. [Google Scholar] [CrossRef]
- Brothers, M.C.; Debrosse, M.; Grigsby, C.C.; Naik, R.R.; Hussain, S.M.; Heikenfeld, J.; Kim, S.S. Achievements and Challenges for Real-Time Sensing of Analytes in Sweat within Wearable Platforms. Acc. Chem. Res. 2019, 52, 297–306. [Google Scholar] [CrossRef]
- Swetha, P.; Balijapalli, U.; Feng, S.P. Wireless accessing of salivary biomarkers based wearable electrochemical sensors: A mini-review. Electrochem. Commun. 2022, 140, 107314. [Google Scholar] [CrossRef]
- Hong, Y.J.; Lee, H.; Kim, J.; Lee, M.; Choi, H.J.; Hyeon, T.; Kim, D.H. Multifunctional Wearable System that Integrates Sweat-Based Sensing and Vital-Sign Monitoring to Estimate Pre-/Post-Exercise Glucose Levels. Adv. Funct. Mater. 2018, 28, 1805754. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Colburn, A.W.; Levey, K.J.; O’Hare, D.; Macpherson, J.V. Lifting the lid on the potentiostat: A beginner’s guide to understanding electrochemical circuitry and practical operation. Phys. Chem. Chem. Phys. 2021, 23, 8100–8117. [Google Scholar] [CrossRef]
- Metrohm. Portable Potentiostats/Galvanostats. Available online: https://www.metrohm.com/en/products/electrochemistry/portable-line.html (accessed on 23 December 2022).
- PalmSens. Available online: https://www.palmsens.com/product-select/ (accessed on 1 April 2023).
- Ilvium. Available online: https://www.ivium.com/ (accessed on 1 April 2023).
- Irving, P.; Cecil, R.; Yates, M.Z. MYSTAT: A compact potentiostat/galvanostat for general electrochemistry measurements. HardwareX 2021, 9, e00163. [Google Scholar] [CrossRef]
- Ainla, A.; Mousavi, M.P.S.; Tsaloglou, M.N.; Redston, J.; Bell, J.G.; Fernández-Abedul, M.T.; Whitesides, G.M. Open-Source Potentiostat for Wireless Electrochemical Detection with Smartphones. Anal. Chem. 2018, 90, 6240–6246. [Google Scholar] [CrossRef] [PubMed]
- Dobbelaere, T.; Vereecken, P.M.; Detavernier, C. A USB-controlled potentiostat/galvanostat for thin-film battery characterization. HardwareX 2017, 2, 34–49. [Google Scholar] [CrossRef]
- Dryden, M.D.M.; Wheeler, A.R. DStat: A versatile, open-source potentiostat for electroanalysis and integration. PLoS ONE 2015, 10, e0140349. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.A.; Bonham, A.J.; White, R.J.; Zimmer, M.P.; Yadgar, R.J.; Hobza, T.M.; Honea, J.W.; Ben-Yaacov, I.; Plaxco, K.W. Cheapstat: An open-source, “do-it-yourself” potentiostat for analytical and educational applications. PLoS ONE 2011, 6, e23783. [Google Scholar] [CrossRef]
- Tahernia, M.; Mohammadifar, M.; Liu, L.; Choi, S. A Disposable, Papertronic Three-Electrode Potentiostat for Monitoring Bacterial Electrochemical Activity. ACS Omega 2020, 5, 24717–24723. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Santos, Q. A Wearable Cortisol Monitoring Microsystem. Ph.D. Thesis, Universidade do Porto (Portugal), Porto, Portugal, 2021. [Google Scholar]
- Hoilett, O.S.; Walker, J.F.; Balash, B.M.; Jaras, N.J.; Boppana, S.; Linnes, J.C. Kickstat: A coin-sized potentiostat for high-resolution electrochemical analysis. Sensors 2020, 20, 2407. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Tan, J.; Zhu, J.; Lin, S.; Zhao, Y.; Yu, W.; Hojaiji, H.; Wang, B.; Yang, S.; Cheng, X.; et al. A programmable epidermal microfluidic valving system for wearable biofluid management and contextual biomarker analysis. Nat. Commun. 2020, 11, 4405. [Google Scholar] [CrossRef]
- Texas Instruments. LMP91000. Available online: https://www.ti.com/product/LMP91000 (accessed on 1 April 2023).
- Brown, E.W.; Glasscott, M.W.; Conley, K.; Barr, J.; Ray, J.D.; Moores, L.C.; Netchaev, A. ACEstat: A DIY Guide to Unlocking the Potential of Integrated Circuit Potentiostats for Open-Source Electrochemical Analysis. Anal. Chem. 2022, 94, 4906–4912. [Google Scholar] [CrossRef]
- Mintah Churcher, N.K.; Upasham, S.; Rice, P.; Bhadsavle, S.; Prasad, S. Development of a flexible, sweat-based neuropeptide y detection platform. RSC Adv. 2020, 10, 23173–23186. [Google Scholar] [CrossRef]
- Analog Devices. ADuCM355. Available online: https://www.analog.com/en/products/ADUCM355.html#product-overview (accessed on 1 April 2023).
- Analog Devices. AD5933. Available online: https://www.analog.com/en/products/ad5933.html (accessed on 1 April 2023).
- Burgos-Flórez, F.; Rodríguez, A.; Cervera, E.; Zucolotto, V.; Sanjuán, M.; Villalba, P.J. TBISTAT: An open-source, wireless portable, electrochemical impedance spectroscopy capable potentiostat for the point-of-care detection of S100B in plasma samples. PLoS ONE 2022, 17, e0263738. [Google Scholar] [CrossRef]
- Jenkins, D.M.; Lee, B.E.; Jun, S.; Reyes-De-Corcuera, J.; McLamore, E.S. ABE-Stat, a Fully Open-Source and Versatile Wireless Potentiostat Project Including Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2019, 166, B3056–B3065. [Google Scholar] [CrossRef]
- Vinoth, R.; Nakagawa, T.; Mathiyarasu, J.; Mohan, A.M.V. Fully Printed Wearable Microfluidic Devices for High-Throughput Sweat Sampling and Multiplexed Electrochemical Analysis. ACS Sens. 2021, 6, 1174–1186. [Google Scholar] [CrossRef]
- Nag, A.; Mukhopadhyay, S.C.; Kosel, J. Wearable Flexible Sensors: A Review. IEEE Sens. J. 2017, 17, 3949–3960. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jeerapan, I.; Wang, J. Wearable Chemical Sensors: Present Challenges and Future Prospects. ACS Sens. 2016, 1, 464–482. [Google Scholar] [CrossRef]
- Krorakai, K.; Klangphukhiew, S.; Kulchat, S.; Patramanon, R. Smartphone-based nfc potentiostat for wireless electrochemical sensing. Appl. Sci. 2021, 11, 392. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Gutruf, P.; Choi, J.; Lee, K.; Sekine, Y.; Reeder, J.T.; Jeang, W.J.; Aranyosi, A.J.; Lee, S.P.; Model, J.B.; et al. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 2019, 5, eaav3294. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, D.H.; Lee, Y.Y.; Shin, H.W.; Han, G.S.; Hong, J.S.; Mahmood, K.; Ahn, T.K.; Joo, Y.C.; Hong, K.S.; et al. Highly efficient and bending durable perovskite solar cells: Toward a wearable power source. Energy Environ. Sci. 2015, 8, 916–921. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Molinnus, D.; Mirza, O.; Guinovart, T.; Windmiller, J.R.; Valdés-Ramírez, G.; Andrade, F.J.; Schöning, M.J.; Wang, J. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 2014, 54, 603–609. [Google Scholar] [CrossRef]
- Gai, Y.; Wang, E.; Liu, M.; Xie, L.; Bai, Y.; Yang, Y.; Xue, J.; Qu, X.; Xi, Y.; Li, L.; et al. A Self-Powered Wearable Sensor for Continuous Wireless Sweat Monitoring. Small Methods 2022, 6, 2200653. [Google Scholar] [CrossRef]
- Song, Y.; Min, J.; Yu, Y.; Wang, H.; Yang, Y.; Zhang, H.; Gao, W. Wireless battery-free wearable sweat sensor powered by human motion. Sci. Adv. 2020, 6, eaay9842. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, Y.; Chen, S.; Yuan, Q. Smart, stretchable and wearable supercapacitors: Prospects and challenges. CrystEngComm 2016, 18, 4218–4235. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Cho, J.; Lee, J.; Huang, X.; Jia, L.; Fan, J.A.; Su, Y.; Su, J.; Zhang, H.; et al. Stretchable batteries with self-similar serpentine interconnects and integrated wireless recharging systems. Nat. Commun. 2013, 4, 1543. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Tang, C.; Peng, H.; Huang, J.; Zhang, Q. Advanced energy materials for flexible batteries in energy storage: A review. SmartMat 2020, 1, e1007. [Google Scholar] [CrossRef]
- Kong, T.; Flanigan, S.; Weinstein, M.; Kalwa, U.; Legner, C.; Pandey, S. A fast, reconfigurable flow switch for paper microfluidics based on selective wetting of folded paper actuator strips. Lab Chip 2017, 17, 3621–3633. [Google Scholar] [CrossRef] [PubMed]
- Nemiroski, A.; Christodouleas, D.C.; Hennek, J.W.; Kumar, A.A.; Maxwell, E.J.; Fernández-Abedul, M.T.; Whitesides, G.M. Universal mobile electrochemical detector designed for use in resource-limited applications. Proc. Natl. Acad. Sci. USA 2014, 111, 11984–11989. [Google Scholar] [CrossRef]
- Rodríguez, Y.A. (Ed.) 2017 IEEE Colombian Conference on Communications and Computing (COLCOM); Conference Proceedings, 16–18 August 2017, Cartagena, Colombia; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2017; ISBN 9781538610602. [Google Scholar]
- Zhang, T.; Lu, J.; Hu, F.; Hao, Q. Bluetooth low energy for wearable sensor-based healthcare systems. In Proceedings of the 2014 IEEE Healthcare Innovation Conference (HIC), Seattle, WA, USA, 8–10 October 2014; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2014; pp. 251–254. [Google Scholar]
- Kovalska, E.; Baldycheva, A.; Somov, A. Wireless graphene-enabled wearable temperature sensor. In Proceedings of the Journal of Physics: Conference Series; IOP Publishing Ltd.: Bristol, UK, 2020; Volume 1571. [Google Scholar]
- Bianchi, V.; Ciampolini, P.; De Munari, I. RSSI-Based Indoor Localization and Identification for ZigBee Wireless Sensor Networks in Smart Homes. IEEE Trans. Instrum. Meas. 2019, 68, 566–575. [Google Scholar] [CrossRef]
- Kim, S.B.; Lee, K.H.; Raj, M.S.; Lee, B.; Reeder, J.T.; Koo, J.; Hourlier-Fargette, A.; Bandodkar, A.J.; Won, S.M.; Sekine, Y.; et al. Soft, Skin-Interfaced Microfluidic Systems with Wireless, Battery-Free Electronics for Digital, Real-Time Tracking of Sweat Loss and Electrolyte Composition. Small 2018, 14, 1802876. [Google Scholar] [CrossRef]
- Rose, D.P.; Ratterman, M.E.; Griffin, D.K.; Hou, L.; Kelley-Loughnane, N.; Naik, R.R.; Hagen, J.A.; Papautsky, I.; Heikenfeld, J.C. Adhesive RFID Sensor Patch for Monitoring of Sweat Electrolytes. IEEE Trans. Biomed. Eng. 2015, 62, 1457–1465. [Google Scholar] [CrossRef]
- Baumbauer, C.L.; Anderson, M.G.; Ting, J.; Sreekumar, A.; Rabaey, J.M.; Arias, A.C.; Thielens, A. Printed, flexible, compact UHF-RFID sensor tags enabled by hybrid electronics. Sci. Rep. 2020, 10, 16543. [Google Scholar] [CrossRef]
- Bassoli, M.; Bianchi, V.; De Munari, I. A plug and play IoT Wi-Fi smart home system for human monitoring. Electronics 2018, 7, 200. [Google Scholar] [CrossRef]
- Zhang, X.; Grajal, J.; Vazquez-Roy, J.L.; Radhakrishna, U.; Wang, X.; Chern, W.; Zhou, L.; Lin, Y.; Shen, P.C.; Ji, X.; et al. Two-dimensional MoS2-enabled flexible rectenna for Wi-Fi-band wireless energy harvesting. Nature 2019, 566, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Malik, H.; Alam, M.M.; Le Moullec, Y.; Kuusik, A. NarrowBand-IoT Performance Analysis for Healthcare Applications. Procedia Comput. Sci. 2018, 130, 1077–1083. [Google Scholar] [CrossRef]
- Reeder, J.T.; Choi, J.; Xue, Y.; Gutruf, P.; Hanson, J.; Liu, M.; Ray, T.; Bandodkar, A.J.; Avila, R.; Xia, W.; et al. Waterproof, electronics-enabled, epidermal microfluidic devices for sweat collection, biomarker analysis, and thermography in aquatic settings. Sci. Adv. 2019, 5, eaau6356. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable sensors: Modalities, challenges, and prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef]
- Brueck, A.; Iftekhar, T.; Stannard, A.B.; Yelamarthi, K.; Kaya, T. A Real-Time Wireless Sweat Rate Measurement System for Physical Activity Monitoring. Sensors 2018, 18, 533. [Google Scholar] [CrossRef]
- Barua, A.; Al Alamin, M.A.; Hossain, M.S.; Hossain, E. Security and Privacy Threats for Bluetooth Low Energy in IoT and Wearable Devices: A Comprehensive Survey. IEEE Open J. Commun. Soc. 2022, 3, 251–281. [Google Scholar] [CrossRef]
- Wen, C.; Dematties, D.; Zhang, S.L. A Guide to Signal Processing Algorithms for Nanopore Sensors. ACS Sens. 2021, 6, 3536–3555. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Bailey, T.; Bode, B.W.; Christiansen, M.P.; Klaff, L.J.; Alva, S. The Performance and Usability of a Factory-Calibrated Flash Glucose Monitoring System. Diabetes Technol. Ther. 2015, 17, 787–794. [Google Scholar] [CrossRef]
- Bertachi, A.; Viñals, C.; Biagi, L.; Contreras, I.; Vehí, J.; Conget, I.; Giménez, M. Prediction of nocturnal hypoglycemia in adults with type 1 diabetes under multiple daily injections using continuous glucose monitoring and physical activity monitor. Sensors 2020, 20, 1705. [Google Scholar] [CrossRef]
- Yadav, J.; Rani, A.; Singh, V.; Murari, B.M. Investigations on multisensor-based noninvasive blood glucose measurement system. J. Med. Devices Trans. ASME 2017, 11, 031006. [Google Scholar] [CrossRef]
- Staples, E.; Ingram, R.J.M.; Atherton, J.C.; Robinson, K. Optimising the quantification of cytokines present at low concentrations in small human mucosal tissue samples using Luminex assays. J. Immunol. Methods 2013, 394, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Kitchen, G.; Kim, J.S.; Li, Y.; Kim, K.; Jeong, I.C.; Nguyen, J.; Stewart, K.J.; Zeger, S.L.; Searson, P.C. Two Distinct Types of Sweat Profile in Healthy Subjects While Exercising at Constant Power Output Measured by a Wearable Sweat Sensor. Sci. Rep. 2019, 9, 17877. [Google Scholar] [CrossRef]
- Sabilla, I.A.; Cahyaningtyas, Z.A.; Sarno, R.; Al Fauzi, A.; Wijaya, D.R.; Gunawan, R. Classification of Human Gender from Sweat Odor using Electronic Nose with Machine Learning Methods. In Proceedings of the 2021 IEEE Asia Pacific Conference on Wireless and Mobile (APWiMob), Bandung, Indonesia, 8–9 April 2021; pp. 109–115. [Google Scholar] [CrossRef]
- Sabilla, I.A.; Purbawa, D.P.; Sarno, R.; Al Fauzi, A.; Wijaya, D.R.; Gunawan, R. Men and Women Classification at Night through the Armpit Sweat Odor using Electronic Nose. In Proceedings of the 2021 IEEE Asia Pacific Conference on Wireless and Mobile (APWiMob), Bandung, Indonesia, 8–9 April 2021; pp. 121–127. [Google Scholar] [CrossRef]
- Sabilla, I.A.; Irawan, R.A.; Sarno, R.; Al Fauzi, A.; Wijaya, D.R.; Gunawan, R. Classification of Male and Female Sweat Odor in the Morning Using Electronic Nose. In Proceedings of the 2021 3rd East Indonesia Conference on Computer and Information Technology (EIConCIT), Surabaya, Indonesia, 9–11 April 2021; pp. 320–324. [Google Scholar]
- Sankhala, D.; Sardesai, A.U.; Pali, M.; Lin, K.C.; Jagannath, B.; Muthukumar, S.; Prasad, S. A machine learning-based on-demand sweat glucose reporting platform. Sci. Rep. 2022, 12, 2442. [Google Scholar] [CrossRef] [PubMed]
- Shahub, S.; Upasham, S.; Ganguly, A.; Prasad, S. Machine learning guided electrochemical sensor for passive sweat cortisol detection. Sens. Bio-Sens. Res. 2022, 38, 100527. [Google Scholar] [CrossRef]
- Veeralingam, S.; Khandelwal, S.; Badhulika, S. AI/ML-Enabled 2-D—RuS 2 Nanomaterial-Based Multifunctional, Low Cost, Wearable Sensor Platform for Non-Invasive Point of Care Diagnostics. IEEE Sens. J. 2020, 20, 8437–8444. [Google Scholar] [CrossRef]
- Kalasin, S.; Sangnuang, P.; Surareungchai, W. Satellite-Based Sensor for Environmental Heat-Stress Sweat Creatinine Monitoring: The Remote Artificial Intelligence-Assisted Epidermal Wearable Sensing for Health Evaluation. ACS Biomater. Sci. Eng. 2021, 7, 322–334. [Google Scholar] [CrossRef]
- Kalasin, S.; Sangnuang, P.; Surareungchai, W. Intelligent Wearable Sensors Interconnected with Advanced Wound Dressing Bandages for Contactless Chronic Skin Monitoring: Artificial Intelligence for Predicting Tissue Regeneration. Anal. Chem. 2022, 94, 6842–6852. [Google Scholar] [CrossRef]
- Kammarchedu, V.; Butler, D.; Ebrahimi, A. A machine learning-based multimodal electrochemical analytical device based on eMoSx-LIG for multiplexed detection of tyrosine and uric acid in sweat and saliva. Anal. Chim. Acta 2022, 1232, 340447. [Google Scholar] [CrossRef]
- Kalasin, S.; Sangnuang, P.; Surareungchai, W. Lab-on-Eyeglasses to Monitor Kidneys and Strengthen Vulnerable Populations in Pandemics: Machine Learning in Predicting Serum Creatinine Using Tear Creatinine. Anal. Chem. 2021, 93, 10661–10671. [Google Scholar] [CrossRef]
- Hanitra, I.N.; Criscuolo, F.; Carrara, S.; De Micheli, G. Multi-Ion-Sensing Emulator and Multivariate Calibration Optimization by Machine Learning Models. IEEE Access 2021, 9, 46821–46836. [Google Scholar] [CrossRef]
- Ravizza, A.; De Maria, C.; Di Pietro, L.; Sternini, F.; Audenino, A.L.; Bignardi, C. Comprehensive Review on Current and Future Regulatory Requirements on Wearable Sensors in Preclinical and Clinical Testing. Front. Bioeng. Biotechnol. 2019, 7, 313. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Hui, J.; Zhou, L.; Lin, B.; Sun, H.; Bai, Y.; Zhao, J.; Mao, H. A low-cost and simple-fabricated epidermal sweat patch based on “cut-and-paste” manufacture. Sens. Actuators B Chem. 2022, 368, 132184. [Google Scholar] [CrossRef]

| Structure | Method | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Ag|AgTPB|PVC + TBATPB | SP, DC | Suitable structure for solid state and flexible REs. Remarkable lifetime and negligible potential drift towards various electrolytes even under mechanical stress. | Time/cost differences between the development of reservoir layers with hydrophilic or organic insoluble salts require further addressing. | [38] |
| Ag|AgCl|KCl + PVA|PDMS | SP | PDMS junction suppresses electrolyte leaking, conferring potential stability over a month and ion insensitivity. | Electrolyte stability comes at the expense of long hydration times (30 min). | [39] |
| Ag|AgCl|KCl + PVA | DC | Stable Nernstian response even after five months over a wide pH range (1–10). | Crosslinked PVA increases hydration time. There are no assays regarding sensitivity to ions. | [40] |
| Ag|AgCl|KCl + PVB | IJP | Negligible potential drift under a wide range of pH and Cl− concentration ranges. Three-month stability. | PVB cocktail requires a mix of four solvents. A limited library of interferents has been evaluated. | [41] |
| Ag|AgCl|SG + PVdF | SP | Paper-based SPRE as an alternative to counterparts developed in plastic substrates. | Complicated structural assembly. Very limited lifetime and stability during measurements. | [42] |
| Ag|AgCl|PVC+ NaTFPB | IJP, DC | Insensitive near-Nernstian potentiometric response to a wide range of pH (±0.02 mV) and Cl− concentrations. | Requires extended conditioning and may suffer from unwanted structural changes in the process. | [50] |
| Ag|AgCl|KC + MM:BM | SP | Negligible potential drift (±4 mV) under mechanical stress suitable for in-vivo pH sensing. | Not enough experimental data have been presented to define whether the proposed electrode is a pseudo-RE or an RE. | [43] |
| Ag|AgCl|KCl + PVA | DC | Negligible potential drift under light exposure and a wide range of pH. Successful integration into a miniaturized chip wearable device. | Considerable technical and cost requirements given the characteristics of the electrochemical device. | [44] |
| Ag|AgCl|PBA + NaTFPB:TDMA | SP | Polymeric and lipophilic salt reservoir confers low interference (9 mV) against a wide spectrum of anions. | Short lifetime and high potential drift under the presence of perchlorate ions. | [45] |
| Analyte | Substrate | Ion-To-Electron Transducer | Ion-Sensitive Membrane | Figures of Merit | Ref. |
|---|---|---|---|---|---|
| K+ | Commercial conductive ink printed on PET | Mix: β-cyclodextrin and rGO (pH-sensitive) | K+: valynomicin, KTPFB, PVC, DOS | pH: sensitivity = 54 mV/dec LOD = pH 10 K+: sensitivity = 56 mV/dec LOD = 10−6.2 | [56] |
| K+ | Stencil-patterned carbon electrode on PET, carbon black-modified | K+: valynomicin, KTClPB, PVC, DOS | K+: sensitivity = 56.1 mV/dec LOD = 10−5 M LR = 10−4 to 10−1 M | [57] | |
| Na+ | CNT fibres on an elastic band | NiCAT coated with Nafion | Na+: sodium ionophore X, NaTFPB, PVC, DOS | Na+: sensitivity = 58.7 mV/dec LOD = 10−6 M LR = 10−5 to 10−1 M | [58] |
| Na+ | Gold electrodes on PET | Nafion-covered porous 3D graphene | Na+: Na ionophore X, NaBARF, PVC, NPOE | Na+: sensitivity = 65.1 mV/dec | [59] |
| Na+ and K+ | Screen-printed commercial electrode | Crown-ether-functionalized graphene quantum dots | Na+ and K+: 42 mV/dec, but no selectivity | [60] | |
| H+ | RGO dry-spun fibres | Ferrocene | pH: 4-nonadecylpyridine, PVC | pH: 55 mV/dec | [61] |
| Cl− | Screen-printed carbon electrodes | PANI, acrylic binder | pH: sensitivity = 66 mV/dec | [54] | |
| H+, Na+ and K+ | Laser-induced-graphene on Kapton | pH: PANI Na+ and K+: PEDOT:PSS | Na+: Na ionophore X, NaTFPB, PVC, DOS K+: valynomicin, KTClPB, PVC, DOS | pH: sensitivity = 51.5 mV/dec Na+: sensitivity = 45.4 mV/dec K+: sensitivity = 43.3 mV/dec | [62] |
| Na+ | Leather | Graphite-Na0.44MnO2 mix | Na+: sensitivity = 58 mV/dec | [63] |
| Name | Power | Communication | User Interface | Range | Resolution | Ref. |
|---|---|---|---|---|---|---|
| UWED | Lithium polymer rechargeable battery (LiPo) | Wireless with protocol “Bluetooth Low Energy” (BLE) | Smart phone/tablet | ±1.5 V, ±180 μA | 67 μV (40 μV noise); 6.4 nA (30 nA noise + nonlinearity) | [109] |
| DStat | USB | Wired connection via USB/serial port | PC—multiplatform software | ±1.5 V; lowest limit of detection 600 fA (7 ranges) | 46 μV; ~pA | [111] |
| CheapStat | USB | Wired connection via USB/serial port | PC and three-line display + joystick | 2990 mV a 990 mV (~±1 V); ±100 nA and ±10 μA (2 ranges); 1 to 1000 Hz | ~mV; ~nA | [112] |
| USB-controlled potentiostat/galvanostat | USB | Wired connection via USB/Serial port | PC—multiplatform software | ±8 V, ±20 mA, ±200 μA and ±2 μA; sample rate: 90 ms | (DAC: 20 bits/ADC: 22 bits) 15.3 μV; 12 nA, 120 pA and 1.2 pA and noise: 88 nA, 1.1 nA and 9.9 pA | [110] |
| MYSTAT | External 15 volt DC | Wired connection via USB/serial port | PC—multiplatform software | ±12 V; ±200 mA | - | [108] |
| Type of Sensor | Monitored Parameters | Monitored Fluids | Calibration/Prediction Strategy | Reference |
|---|---|---|---|---|
| Potentiometric | Chloride, skin temperature, core temperature, heart rate | Sweat | External calibration, principal component analysis, random forest | [159] |
| Chemoresistive (MOS type gas sensor) | Ethanol, methanethiol, ammonia, trimethylamine | Sweat (gas phase) | Principal component analysis, synthetic minority oversampling technique, support vector machine, decision tree, K-nearest neighbours, naïve Bayes classifier | [160,161,162] |
| EIS | Glucose, skin temperature, relative humidity | Sweat | Linear regression, ensemble regression, decision tree | [163] |
| EIS | Cortisol, skin temperature, relative humidity | Synthetic sweat | External calibration, K-nearest neighbours | [164] |
| EIS, Amperometric, Chemoresistive | Glucose, pH, relative humidity | Sweat | External calibration, K-nearest neighbours | [165] |
| Amperometric | Creatinine, heart rate | Sweat | External calibration, algorithm not specified | [166] |
| Potentiometric | pH | Wound | Linear regression, K-nearest neighbours, decision tree, random forest, gradient boosting, artificial neural network | [167] |
| Amperometric | Tyrosine, uric acid | Sweat, saliva | Linear regression, support vector regression, Bayesian regression, K-nearest neighbours, decision tree, random forest | [168] |
| Amperometric | Creatinine | Tears | Linear regression, K-nearest neighbours, decision tree, random forest, gradient boosting, artificial neural network | [169] |
| Potentiometric | Na+, K+, Li+, Pb2+ | Emulated dataset | Support vector regression, artificial neural network | [170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilbao, E.; Garate, O.; Rodríguez Campos, T.; Roberti, M.; Mass, M.; Lozano, A.; Longinotti, G.; Monsalve, L.; Ybarra, G. Electrochemical Sweat Sensors. Chemosensors 2023, 11, 244. https://doi.org/10.3390/chemosensors11040244
Bilbao E, Garate O, Rodríguez Campos T, Roberti M, Mass M, Lozano A, Longinotti G, Monsalve L, Ybarra G. Electrochemical Sweat Sensors. Chemosensors. 2023; 11(4):244. https://doi.org/10.3390/chemosensors11040244
Chicago/Turabian StyleBilbao, Emanuel, Octavio Garate, Theo Rodríguez Campos, Mariano Roberti, Mijal Mass, Alex Lozano, Gloria Longinotti, Leandro Monsalve, and Gabriel Ybarra. 2023. "Electrochemical Sweat Sensors" Chemosensors 11, no. 4: 244. https://doi.org/10.3390/chemosensors11040244
APA StyleBilbao, E., Garate, O., Rodríguez Campos, T., Roberti, M., Mass, M., Lozano, A., Longinotti, G., Monsalve, L., & Ybarra, G. (2023). Electrochemical Sweat Sensors. Chemosensors, 11(4), 244. https://doi.org/10.3390/chemosensors11040244






