Abstract
NAD(P)H: quinone oxidoreductase isozyme 1 (NQO1) is a flavoenzyme and involved in protection against oxidative stress and the regulation of metabolic functions, which is strongly implicated in neurodegenerative diseases and carcinogenic processes. Furthermore, NQO1 is also involved in the modes of action of redox-active drugs (e.g., antimalarials). Determining the activity and localization of NQO1 in living organisms is of great importance for early disease diagnosis and therapy. As a promising and convenient biosensing technique, trimethyl lock quinone-based organic molecular probes have been well established for the specific detection and imaging of NQO1 in living cells and in vivo. In this review, the recent progress of NQO1 probes based on organic small molecules is summarized from the perspectives of molecular design strategies, sensing mechanisms and bioimaging applications. We also elucidate the potential limitations and prospects of current NQO1 probes to further promote the development of versatile imaging tools for NQO1-related biomedical investigation.
1. Introduction
NAD(P)H: quinone oxidoreductase isozyme 1 (NQO1), also known as DT-diaphorase, catalyzes the two-electron reduction of quinones to the corresponding hydroquinones [1,2]. Owing to the specific chemical, electronic and biological features of quinones and their derivatives, these biomarkers have attracted increasing attention for over a century. Quinones are ubiquitously distributed in mammalian cells and overexpressed quinones can induce carcinogenicity, mutagenicity and cell necrosis [3]. Catalyzing the one-electron reduction of a quinone to semiquinone can result in the formation of various reactive oxygen species (e.g., superoxide anion radical, hydrogen peroxide), which is the main mechanism responsible for the toxicity of intracellular quinones [2]. However, NQO1 has been reported to reduce the level of free quinone for single-electron reduction to decrease quinone-induced carcinogenicity and cell necrosis, thereby protecting against oxidative stress and regulating metabolic functions [1].
As an antioxidant enzyme, NQO1 is widely present in cellular environments including the mitochondria, Golgi, endoplasmic reticulum and cytosol [4]. Usually, NQO1 is over-expressed after exposure to quinone-containing environments (e.g., cigarettes, pollutants), but it is found in low levels in most normal tissues [3]. The increased expression of NQO1 in normal cells is closely related to the NF-E2-related factor 2 (NRF2), c-fos/AP1 and c-jun transcription factors, which regulate the NQO1 gene under human antioxidant response element [5]. Moreover, abnormal NQO1 activities are responsible for various diseases, and can act as a key biomarker in disease diagnosis and therapy [6,7]. For instance, NQO1 plays significant roles in cancer formation and metastasis, and is overexpressed in diverse human primary tumors, such as prostate, pancreatic, colon and breast cancers [7,8,9]. The increased metastatic capacity of cancer cells was reported to be mainly due to the abnormal expression of hypoxia-inducible factor 1α (HIF1-α), which is regulated by the proteins p53 and NQO1 [9]. Further studies of the roles in melanoma cells also indicate that NQO1 could promote cancer cell proliferation by protecting against oxidative stress under accelerating expression of cyclins [8]. Owing to the integral roles of NQO1 in cancer formation, quinone moieties also serve as a common feature in the development of anti-cancer drugs, which can be converted to hydroquinone by NQO1, delivering ROS to cancer cells [10]. In addition, accumulating evidence indicates that the overexpression of NQO1 promotes cancer formation, metastasis and chemoresistance [5,11]. Accordingly, NQO1 may be a promising target for the construction of novel anticancer therapies based on quinone-containing activatable prodrugs. Therefore, there is no doubt that obtaining the concentration and distribution information of NQO1 in living systems will strengthen our understanding of tumor diagnosis and therapy.
Currently, a variety of canonical methods have been used for the quantification or detection of NQO1 in diverse biological samples through electrochemistry, HPLC, absorption and proteomics [12,13,14,15]. However, because of the poor specificity and limited spatial resolution of these detection modalities, obtaining real-time imaging information of NQO1 in living systems is challenging. As a typical biological labeling technique, antibody conjugates have been widely used to determine the local distribution of specific biomarkers in biological samples of interest [16]. Nevertheless, antibody conjugates are hardly applied for the real-time imaging of NQO1 activities in living cells or in vivo because this method requires the introduction of the proteins into cells by fixing cells and promoting cell permeability [16].
Among the various imaging methods, organic molecular probes based on optical imaging techniques can be easily diffused into living cells to image biological processes with noninvasive and convenient properties, which have aroused unprecedented attention in achieving real-time imaging and profiling of biomolecules in living systems and organisms [17,18]. Over the past decades, scientists have made great progress in the construction of various small molecular probes to measure the activity and concentration of NQO1 within living cells [19,20], Compared to other substituted quinone propionic acids derivatives, trimethyl lock quinones were reported to be a more ideal trigger group for NQO1 detection [21]. Therefore, these activatable NQO1 probes, devoted to fluorescence, chemiluminescence or photoacoustic applications, are constructed by using trimethyl lock quinones as the recognition moiety, thereby enabling highly specific and selective detection of NQO1 in complex living systems. Moreover, these smart imaging probes can be modified with different targeting groups for accurate imaging of NQO1 in specific subcellular organelles, or equipped with a long-wavelength fluorophore scaffold for in vivo imaging with high temporal and spatial resolution [22]. Therefore, organic fluorescent probes open a promising avenue for real-time imaging of NQO1 in complex living systems, with the potential to systematically clarify the undiscovered functional mechanism of NQO1 in relevant biological processes and diagnose the early stages of NQO1-related diseases.
In this review, we present and summarize the significant advances in the accurate detection and imaging of NQO1 activity in living cells and in vivo by using organic probes. Subsequently, we discuss the design strategies and mechanisms of relevant NQO1 probes that can be utilized for imaging enzyme activity in different environments. Finally, the potential challenges and opportunities of NQO1-activated organic probes are outlined.
2. The General Design Strategies of Organic Molecular Probes for NQO1
NQO1, as an antioxidant enzyme, can specifically reduce quinones to the corresponding hydroquinones [1]. In 2012, McCarley and co-workers investigated the impact of all the factors associated with the enzymatic reduction of a family of substituted quinone propionic acids derivatives and NQO1 through enzyme kinetic assays and docking studies, indicating that trimethyl lock quinone propionic acid may be an ideal trigger group for NQO1 detection [21]. Consequently, “trimethyl lock quinone” of quinone derivatives are appointed as effective recognition substrates of NQO1, which is also beneficial to the formation of intramolecular lactonization, thereby leading to the turning on of reporter unit signals [3]. In general, the electron-poor nature of the “trimethyl lock quinone” group can efficiently quench the fluorescence signal of the reporter moiety, which is one of the main strategies for constructing of activatable NQO1 probes (Figure 1A).Furthermore, the NQO1-activated probes also can be developed by introducing self-immolative linkers between “trimethyl lock quinone” and reporter moiety. The enzymatic reduction by NQO1 is first to remove the quinone substrate, triggering the elimination of the self-immolative linker, followed by releasing the free signal reporter moiety (Figure 1B). Compared to canonical activatable NQO1 probes, the probes containing self-immolative linkers not only improve the stability of probes, but also enhance the reactivity between substrates and NQO1. In addition, a variety of NQO1 probes behave as prodrugs by introducing anti-cancer drugs to enable cancer diagnosis and treatment through the activation of overexpressed NQO1 in the lesion site.
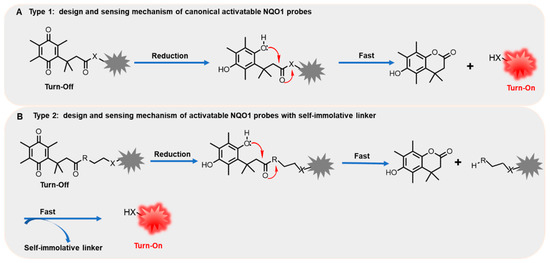
Figure 1.
The general design and sensing mechanism of canonical activatable NQO1 probes (A) and self-immolative linker-based activatable NQO1 probes (B).
3. Fluorescent Probes for NQO1
Generally, these NQO1 probes can be classified as visible-range and near-infrared (NIR) probes according to the different emission wavelengths of the fluorescent reporter units. In this section, we discuss the different bio-applications of diverse NQO1 fluorescent probes in living cells and in vivo.
3.1. Visible-Range Fluorescent Probes
3.1.1. Fluorescent Probes for Detection of NQO1 in Living Cells
A great body of chemo-functional investigations on NQO1-activated probes in the naphthimide series was achieved by the Robin L. McCarley group. In 2012, McCarley and co-workers reported a fluorescence probe (probe 1) based on naphthalimide dye operating by the photoinduced electron transfer (PET) mechanism, which was covalently attached with quinone as the enzyme substrate (Figure 2A). The fluorescence of Q3NI was firstly quenched via the PET mechanism and recovered by the reduction-initiated removal of quinone [19]. When the probe reacted with NQO1, it quickly released fluorescence, with a maximum emission wavelength at 470 nm (λex = 370 nm). Moreover, the Q3NI probe was activated by NQO1 at a high rate, and fluorescent intensity could reach 22% of the saturation state within 5 min (Figure 2B). The authors also used such a probe with excellent properties to distinguish cancer cells with different expression levels of NQO1 (Figure 2C). As shown in Figure 2D, the probes were incubated with A549 and HT-29 cells (high expression) and showed a significant increasing fluorescence signal, whereas no obvious fluorescence signal was observed in the negative cells (H446 and H596 cells). Two-photon confocal experiments revealed that in NQO1-positive cells (A549 and HT-29 cells), a clear and strong two-photon fluorescence signal could be observed, while almost no fluorescence signal could be seen in the NQO1-negtive cells (H446 and H596 cells). However, the excitation wavelength of the Q3NI was in the ultraviolet region, which is not amenable for deep tissue imaging.
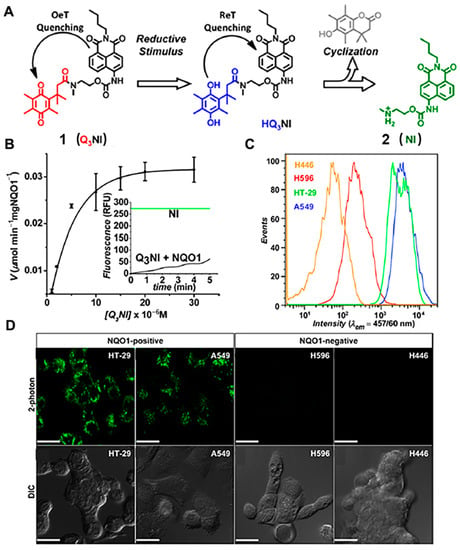
Figure 2.
Schematic representation of the reaction mechanism of NQO1 probe Q3NI (A) and kinetics plot of NQO1 toward Q3NI probe (B). Flow cytometry assays (C) and confocal microscopy imaging (D) of Q3NI activation by NQO1-positive (A549 and HT-29) and NQO1-negative (H596 and H446) cancer cells. Adapted with permission from ref. [19]. Copyright (2013) American Chemical Society.
In 2014, they developed a “Turn-On” probe, (probe 3) to monitor the activity of NQO1 in cancer cells, which consisted of a trimethyl-locked quinone propionic acid trigger group connected to a locked naphthalimide fluorophore (reporter 4) and a self-immolative cleavable linker N-methyl-p-aminobenzyl alcohol (NMPABA) [20]. The specific reaction mechanism is shown in Figure 3A. The absorption of reporter 4 was red-shifted 50 nm compared with the absorption of probe 3 due to the enhanced internal charge transfer (ICT) effect (Figure 3B). Moreover, the self-immolation linker NMPABA accelerated the release of reporter 4. The initial rate of reporter 4 formation (Figure 3C) was calculated, and the maximum velocity (Vmax) could reach 0.00225 ± 0.00008 μmol min−1mg NQO1−1 Such highly selective and rapidly activated probes were also successfully used for differentiating different cancer cells based on NQO1 activation. As shown in Figure 3D, the NQO1-positive cells (HT-29) were incubated with probe 3, resulting in the bright fluorescence signal. However, when the NQO1-negtive cells (H596) were treated with probe 3, there was no obvious fluorescence signal to be seen in the confocal fluorescence microscopy images, presenting an effective positive-to-negative (PNR) ratio.
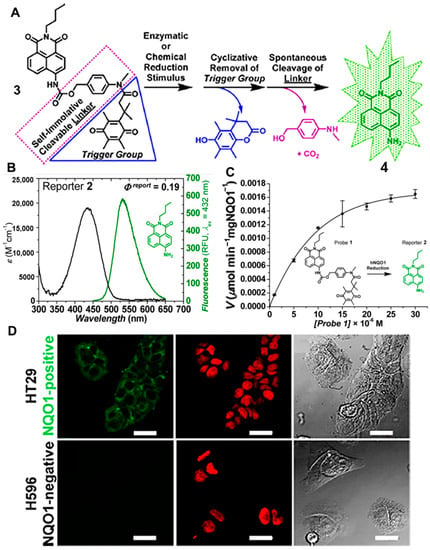
Figure 3.
Schematic representation of the reaction mechanism of probe 3 (A) and the absorbance/emission spectra of compound 4 (B). Kinetics of human NQO1 with probe 3 (C) and microscopy images of cancer cells (D). Adapted with permission from ref. [20]. Copyright (2014) American Chemical Society.
In 2015, the group reported an NQO1 probe (probe 5), which could be rapidly turned on in the presence of NQO1 (Figure 4A). The highly sensitive probe was successfully used in the differentiation of NQO1-positive cells from negative cells [23]. The high positive-to-negative fluorescence intensity ratio (PNR) was up to ∼135-fold, which was due to the proximity of the NQO1 substrate (quinone propionic acid) quenching group to mask the fluorophore and the thermodynamic driving force of the quenching process. Analogously, this group also described a new naphthalimide-based probe (Q3HMRG) for sensing the activity of NQO1 in 2017 [24]. The Q3HMRG probe was successfully used to distinguish NQO1-positive cells from low enzyme activity cells with a high turn-on ratio (~200-fold). In 2019, Lee’ group developed a coumarin-naphthalimide hybrid as a dual emissive fluorescent probe (probe 7) to obtain a ratiometric fluorescence signal for NQO1 activity (Figure 4B). Probe 7 displayed two absorption bands at 345 and 405 nm for the coumarin and naphthalimide moieties, respectively [25]. In the presence of NQO1, the blue fluorescence signal produced by coumarin increased slightly, while the yellow fluorescence signal produced by naphthalimide moiety increased significantly under the excitation of 345 nm. Based on these spectral properties, probe 7 was used for real-time monitoring of NQO1 activity in living cells efficiently and sensitively.
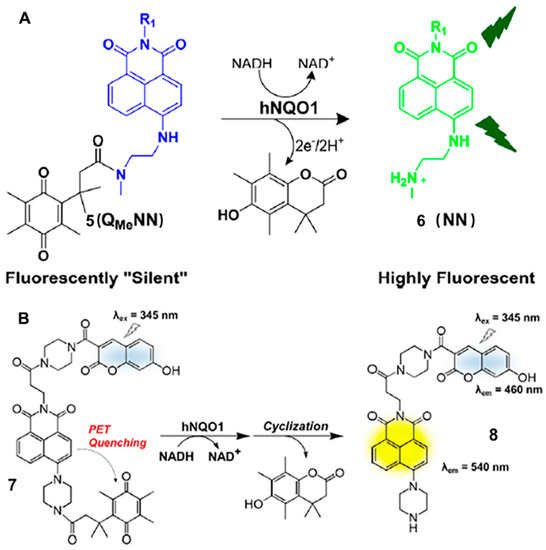
Figure 4.
Schematic representation of the reaction mechanism of probe 5 (A) and probe 7 (B). Adapted with permission from ref. [23]. Copyright (2015) American Chemical Society. Adapted with permission from ref. [25]. Copyright (2019) Elsevier Ltd.
In addition, several highly sensitive organic probes also have been developed based on rhodamine for the detection and imaging of NQO1 [26]. For instance, in 2011, Robin L. McCarley’s group synthesized a probe based on quinone propionic acid-cloaked rhodamine fluorophore [27]. The reaction mechanism was divided into two steps (Figure 5A). Firstly, in the presence of NQO1, the Q3PA trigger group of probe 9 was reduced and formed the trimethyl-locked hydroquinone. Subsequently, the intermediate product underwent self-removal to form the lactone and released emissive fluorophore. On this basis, in 2015, Robin L. McCarley and co-workers modified the molecular structure of the probe and developed a new rhodamine-based probe to monitor the activity of NQO1 in different cancer cells (Figure 5B). This work explored the optical performance of rhodamine analogs (MJSNR) [28]. MJSNR served as the fluorescent reporter of probe 11 (Q3MJSNR) because of its good chemical stability (t1/2 approximately 39 h) and attractive photophysical properties, e.g., large Stokes shift, long emission wavelength and high brightness. Most importantly, there was a clear fluorescence difference between the NQO1-positive and negative cells when they were incubated with probe 11 (Q3MJSNR) for only 10 min (Figure 5C). NQO1-positive HT29 cells displayed bright red fluorescence; in contrast, there was no obvious signal to be seen in the NQO1-negtive H596 cells. This probe displayed superior performance for the detection and imaging of NQO1 in living cells.
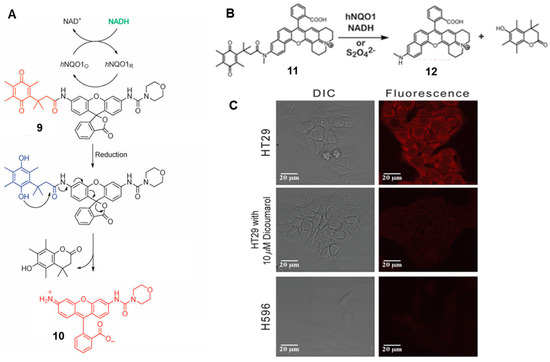
Figure 5.
Schematic representation of the reaction mechanism of probe 9 (A) and probe 11 (B). Confocal images (C) of NQO1-positive (HT-29) and NQO1-negative (H596) cancer cells. Scale bar = 20 μm. Adapted with permission from ref. [27]. Copyright (1996) the Royal Society of Chemistry. Adapted with permission from ref. [28]. Copyright (2016) American Chemical Society.
Li’s group reported a nanoprobe that was designed to improve the water solubility, chemical stability and photostability of probe 13 by assembling small molecular probes based on 6-hydroxylphenol-BODIPY dye into micelles (Figure 6) [29]. The nanoprobe enabled differentiation of NQO1 activity in different cancer cells. Based on the optical properties of different dyes, Simone Cuffa and co-workers designed an NQO1-actived probe (probe 14) based on 4-methylumbelliferone (4-MU). It could be used for discriminating NQO1 activity between various cancer cells within less than 10 min (Figure 6) [30]. Meanwhile, Shao’s group synthesized a probe (probe 15) based on benzothiazole dye HBTM (Figure 6), which showed long wavelength emission (~600 nm) and a large Stokes shift (~203 nm), and achieved a highly sensitive and selective response to NQO1 with a linear relationship in the range of 60–180 ng/mL [31]. Furthermore, a novel NQO1-activatable probe (probe 16) (SYZ-30) was rationally designed based on 7-nitro-2,1,3-benzoxadiazole (NBD) fluorophore by Chen’s group (Figure 6). SYZ-30 exhibited a selective response to NQO1 in vitro within 5 min and could be applied for imaging of NQO1-positive cancer cells [32].
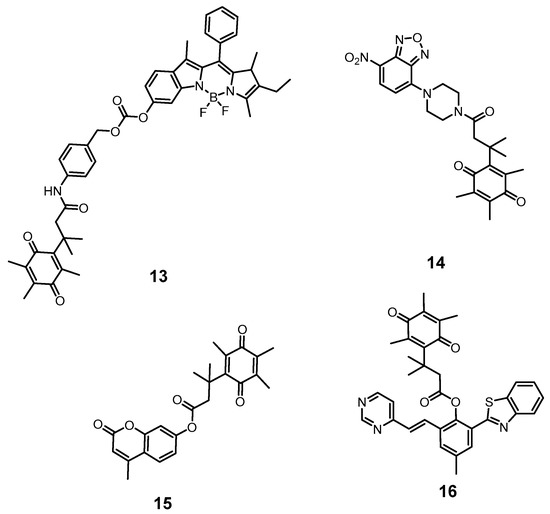
Figure 6.
Different activatable molecular probes based on classical dyes for the detection of NQO1.
The sensitivity and selectivity of the probes can be improved by changing the fluorescence reporter or adjusting the intermediate linker. Such fluorescence imaging probes have become an attractive tool for detection of NQO1 due to simple operation and noninvasiveness, etc. It should be noted that the above probes detected NQO1 through single-photon imaging, which was restricted by limited tissue penetration and high photodamage. Thus, two-photon imaging has attracted increasing attention due to the superiorities of higher spatiotemporal resolution, lower photodamage and deeper tissue penetration [33,34,35,36]. In 2017, Yoon and co-workers synthesized a novel two-photon probe (probe 18) for the detection of NQO1 (Figure 7A) [37]. Probe 18 was designed by conjugating an amino-acetyl-naphthalene motif with trimethyl lock quinone to obtain two-photon excitation, and it exhibited rapid response capability and high selectivity for NQO1 (Figure 7B), with an approximately 8-fold increase in fluorescence intensity after only 4 min (Figure 7C). Moreover, the probe was used to monitor NQO1 in different cancer cells with two-photon excitation, exhibiting high photo stability. In addition, Jiang and Kima also developed some TP probes that have been used to distinguish cancer cells and normal cells based on the expression level of NQO1 [38].
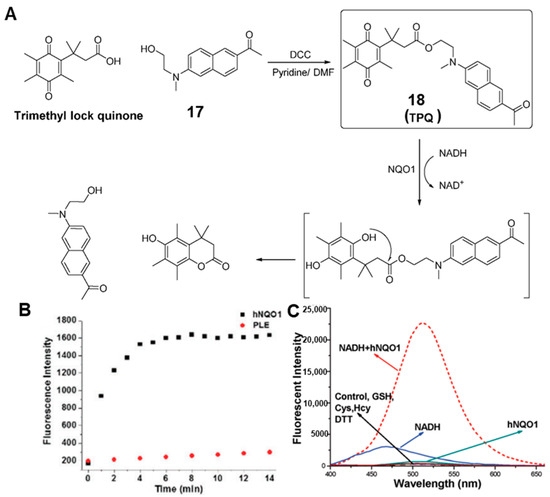
Figure 7.
Proposed mechanisms (A) for probe 18 prior to and after chemical/NQO1-catalyzed reduction and subsequent production of the fluorescent reporter. Reactivity of probe 18 with porcine liver esterase (PLE) and NQO1 (B). Fluorescence spectra with the biological reductants (C). Adapted with permission from ref. [37]. Copyright (2017) the Royal Society of Chemistry.
3.1.2. Fluorescent Probes for Detection of NQO1 in Specific Organelles
It is worth noting that NQO1 is widely distributed in diverse organelles. As the powerhouses of living cells, the maintenance of mitochondrial physiological function is closely related to the redox states [39]. Therefore, it is extremely necessary to develop molecular tools for imaging mitochondrial NQO1 at the cell level. In 2020, Zhou’s group reported a ratiometric two-photon probe (probe 19) for imaging of NQO1 in mitochondria and in vivo [39]. Probe 19 (QBMP) was designed by coupling hydroxylphenylpolyenyl pyridinium fluorophores with trimethyl lock quinone (Figure 8A). As shown in Figure 8B, the green fluorescence signal of QBMP red-shifted to 566 nm with an excitation wavelength of 407 nm. Moreover, QBMP showed many superior performances, including rapid response (4 min) and a large Stokes shift (162 nm). It was applied for ratiometric two-photon imaging of NQO1 in different cancer cells (Figure 8D). Most importantly, it was successfully used for revealing the dynamics of NQO1 activities in a Parkinson’s disease model and imaging of NQO1 in fixed brain tissue. The depth of tissue penetration could be up to 225 μm in fixed brain tissues (Figure 8C). These superior properties make the probe promising for early disease diagnosis associated with NQO1.
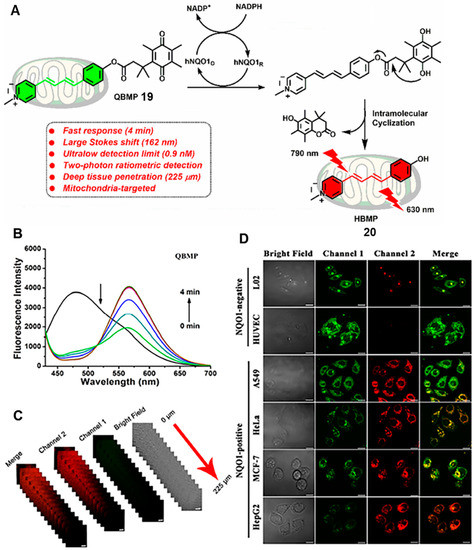
Figure 8.
(A) Proposed reaction mechanisms of probe 20 with NQO1. (B) Time-dependent fluorescence changes in QBMP in the presence of NQO1. Two-photon fluorescence images of brain tissues incubated with NQO1 (C) and two-photon confocal fluorescence images of cancer cells and normal cells (D). Adapted with permission from ref. [39]. Copyright (2021) American Chemical Society.
In 2016, Kim and co-workers described a mitochondria-targeting probe for theranostics [40]. As shown in Figure 9, probe 21 was synthesized by introducing a triphenylphosphonium group, the mitochondria-targeted unit, and an aggregation induced emission (AIE) generator, tetraphenylethene, into an NQO1-responsive quinone moiety. When the probe was targeted to mitochondria in cancer cells, the AIE dyes could be released, and formed AIE aggregates at mitochondrial sites with the action of NQO1. Then, the AIE aggregates disrupted mitochondrial metabolism, resulting in cell death. Moreover, the AIE property of the reporter made the background fluorescence extremely low, and the signal increased by 500-fold after NQO1 activation. Unfortunately, the excitation wavelength is in the ultraviolet region, which hinders its application in in vivo.
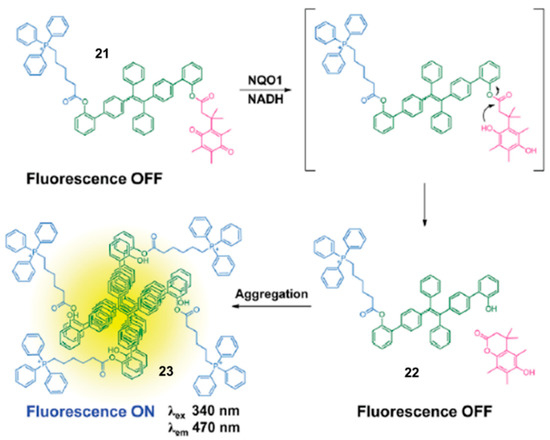
Figure 9.
Illustration for the NQO1-sensing mechanism of probe 21. Adapted with permission from ref. [40]. Copyright (2016) the Royal Society of Chemistry.
3.1.3. NQO1 Fluorescent Probes for Theranostics
In another perspective, NQO1 was used as a trigger to develop activatable prodrugs that can be applied for monitoring drug delivery in living cells. In 2016, Kim and co-workers developed a novel probe (probe 24) by introducing 7-ethyl-10-hydroxycamptothecin (SN-38) as an anticancer drug, [41] hydroquinone as an NQO1-triggered unit and biotin as a cancer-targeting moiety (Figure 10A). In the presence of NQO1, the fluorescence signal was clearly increased within 30 min at 550 nm, which suggested the release of SN-38 (Figure 10B,C). Moreover, the toxicity to tumor cells was cell-selective, including not only the difference in the expression of biotin receptors, but also the difference in NQO1 expression. As shown in Figure 10D, the cell viability of two cancer cell lines (A549 and HeLa) was significantly decreased due to the high expression of biotin receptor and NQO1. However, there was no effect on human normal fibroblast cell lines (WI-38 and BJ) lacking biotin receptor and NQO1. The same inhibition can be observed in tumor growth with the injection of a probe or SN-38 (Figure 10E). This brings a new avenue for the design of prodrug-based molecule probes. Beharry and colleagues utilized phenalenone (PN) as a photosensitizer that can be quenched by quinone via the PET effect (Figure 10F) [42]. In the presence of NQO1, probe 26 initiated intramolecular cyclization and produced free PN, and the fluorescence intensity increased ~5.5 fold at 500 nm after 5 min (Figure 10G). The released PN could selectively cause cellular toxicity by releasing 1O2 upon irradiation, followed by use for photodynamic therapy (PDT). As shown in Figure 10H, a bright green fluorescence signal was observed in A549 cells, which was produced by dichlorofluorescein (DCF), an indicator of ROS. In contrast, no obvious fluorescence signal could be observed in H596 cells and MRC9 cells due to the low levels of NQO1. In this way, this probe can be used not only to detect NQO1 activities, but also to improve the selectivity of photodynamic therapy. Similarly, Wu’s group also developed an NQO1-activated prodrug for tumor theranostics. The prodrug could release 2,6-dihydroxymethyl-4-methylphenol and fluorophore camptothecin (CPT) moieties in the presence of NQO1, which was used for bioimaging and chemotherapy in A549 and L929 cells [43].
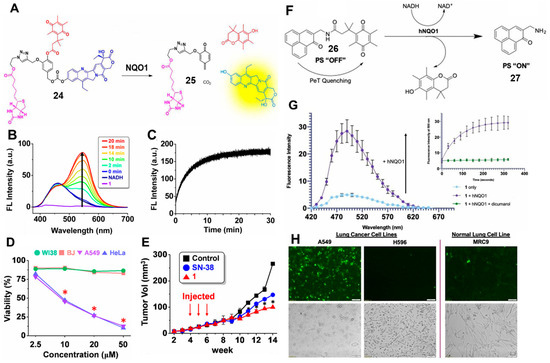
Figure 10.
(A) Proposed mechanisms of probe 24 with NQO1. Fluorescence spectra of probe 24 with NQO1 and NADH (B) and time-dependent changes in fluorescence intensity (C). Cell viability (D) of cancer cell lines (A549 and HeLa) and normal cell lines (WI-38 and BJ). Tumor size changes with probe 24 treatment in vivo in a xenograft mouse model (E). Design of an NQO1-activatable photosensitizer (F) and fluorescence spectra with NQO1 and NADH (G). ROS detection (H) in lung cancer cells (A549 and H596) and a normal lung cell line (MRC9) with incubation with probe 26. Adapted with permission from ref. [41]. Copyright (2016) American Chemical Society. Adapted with permission from ref. [42]. Copyright (2020) Wiley.
3.2. NIR Fluorescent Probes for NQO1
Although the visible-range probe is widely used, it is still facing the problems of low signal-to-background ratio and limited tissue penetration depth [43]. To overcome these challenges, NIR NQO1 probes were developed by introducing long-wavelength fluorophores to enable imaging of NQO1-related physiological and pathological processes in vivo [44,45]. In 2016, McCarley and co-workers reported a probe (probe 28), Q3STCy, whose NIR fluorescence was selectively activated by NQO1 reduction to yield the reporter TCy (Figure 11A) [46]. The large Stokes shift of the TCy reporter (149 nm) was attributed to the formation of an ICT state (Figure 11B–E). The capability of Q3STCy to differentiate tumor cells was successfully confirmed in confocal experiments. As shown in Figure 11F, the obvious bright fluorescence can be observed in the colorectal carcinoma cell line HT-29 and the ovarian cancer cell line OVCAR-3 based on the overexpression of NQO1. In contrast, the non-small cell lung carcinoma (NSCLC) cell line H596 showed no fluorescence because of the low expression of NQO1. As a middle-expression cell line, the SHIN3 cell line showed brighter fluorescence than H596 cells and darker fluorescence than H-29 cells. In addition, the fluorescence signal generated by Q3STCy was mostly coincident with the RFP-positive area, suggesting the capabilities of the NIR probe to verify the presence of the NQO1-overexpressing SHIN3 cells (Figure 11G). Meanwhile, the advantage of near-infrared fluorescence imaging enabled it to be successfully used for imaging three-dimensional colorectal tumor models. All of these NIR probes showed good performance in the sensitive detection of NQO1 activity in living cells and in vivo.
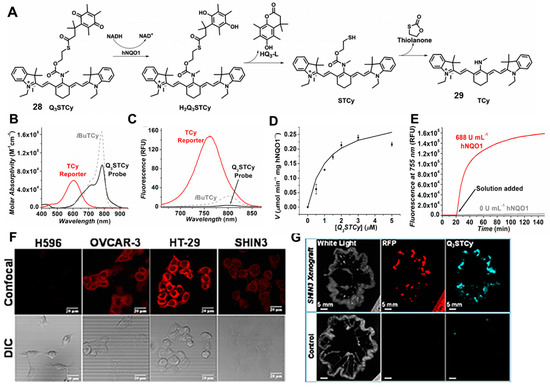
Figure 11.
Proposed mechanisms for Q3STCy probe toward NQO1 (A). Absorption (B) and emission (C) spectra of probe. Kinetic plot (D) and fluorescence intensities (E) of NQO1 with Q3STCy probe. Fluorescence imaging of endogenous NQO1 in cancer cells (F) and SHIN3 mouse model of human ovarian cancer (G). Adapted with permission from ref. [46]. Copyright (2017) American Chemical Society.
In 2019, Srivenugopal’s group developed a turn-on NIR fluorescent probe (probe 30) (NIR-ASM), which consisted of the dicyanoisophorone (ASM) fluorophore and quinone propionic acid (QPA), to detect the activity of NQO1 and diagnose non-invasive lung cancer (Figure 12A) [47]. NIR-ASM was able to monitor NQO1 activity in vitro and in vivo with high sensitivity and selectivity. As shown in Figure 12B. in the presence of NQO1, a clear NIR fluorescence (646 nm) was observed with a large Stokes shift (186 nm). The proposed reaction mechanism was explained by the cleavage of the amide bond, which triggered the release of strong fluorescence. NIR-ASM displayed excellent selectivity for NQO1 in PBS buffer, as well as good performance in cancer cells and mice. Moreover, a clear fluorescence decrease was observed when NQO1-positive cells (A549 and H460) were treated with an NQO1 inhibitor (ES936), which further identified the excellent specificity of the NIR-ASM (Figure 12C). Satisfyingly, as shown in Figure 12D, the NIR-ASM was capable of detecting NQO1 activity in tumor-bearing nude mice, as well as in an orthotopic lung cancer model. This work provides ample evidence of the potential of small molecular-based probes to sense NQO1 activity in vitro and in vivo.
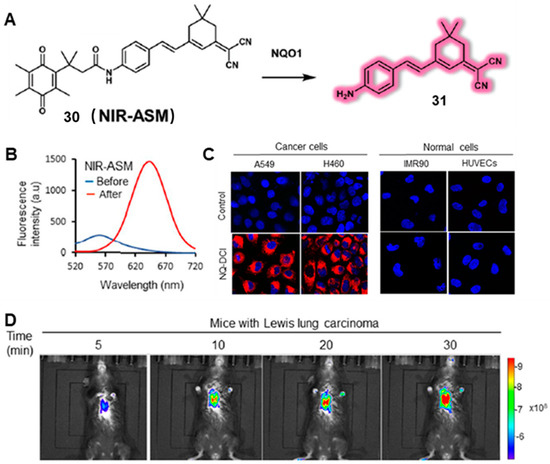
Figure 12.
Representation of reaction mechanisms of probe NIR-ASM with NQO1 (A). (B) The fluorescence response intensity of the probe prior to and after NQO1 treatment. (C) Fluorescence images of NQO1-positive cancer cells and NQO1-negative normal cells after incubation with NIR-ASM probe. (D) Real-time fluorescence imaging in Lewis lung carcinoma (LLC)-bearing BALB/c57 mice after injection with probe. Adapted with permission from ref. [47]. Copyright (2019) Springer Nature.
Shen and co-workers developed an ultrasensitive hemicyanine-based NIR fluorescence probe (probe HCYSN) for the detection of NQO1 activity in different human cancer cells. The maximum fluorescence emission wavelength was 704 nm and the detection limit (LOD) was 4.9 ng/mL (0.49 mU/mL) [48]. In 2021, Zhang’s group designed an NIR probe based on the naphthoquinone trigger group and methylene blue (MB) fluorophore for effective bioimaging of NQO1 in vitro and in vivo. The maximum fluorescence emission wavelength was 695 nm. Most interestingly, the probe was successfully used to monitor the process of cancer treatment in vivo [49].
In order to further enhance the sensitivity and selectivity or tissue penetration depth of NIR probes, researchers have also developed two-photon NIR probes, ratiometric NIR small molecular probes and even NIR fluorescence–photoacoustic multimode imaging probes for sensing NQO1, which showed excellent advantages for bioimaging applications in vivo [50,51,52,53]. For example, in 2022, Zhao and co-workers designed and synthesized an NIR two-photon fluorescence probe for the selective detection of NQO1 [54]. Probe 32 showed superior imaging performance with deep tissue penetration. The long excitation and emission wavelength of the TP fluorescent probe were able to reduce the light damage and avoid the interference of biological spontaneous fluorescence. In this work, the NIR TP probe showed an emission wavelength in the NIR region (680 nm) and a large Stokes shift (120 nm), which effectively improved the selectivity and sensitivity for NQO1. As shown in Figure 13A, probe 32 (DCM-NQO1) was developed by connecting dicyanomethylene-4H-pyran (DCM-OH) with Q3PA (the responsive group for NQO1). In addition to imaging NQO1 levels in human cervical and colorectal cancer cell lines (HeLa and LoVo, respectively) with good properties, probe 32 was also used for two-photon fluorescence imaging of tumor tissues up to 290 µm. In addition to the strategy of two-photon NIR probes, researchers have developed multimode imaging to achieve more sensitive detection and deeper tissue penetration. In 2022, Lin’s group synthesized an activatable hemi-cyanine dye-based probe (probe 34) (the LET-10 probe) for NIR fluorescence and photoacoustic imaging of NQO1 (Figure 13B) [55]. Taking the naphthalocyanine in the LET-10 probe as a built-in reference signal, the LET-10 probe further showed a double-signal self-calibration process for ratiometric photoacoustic imaging. The LET-10 probe was caged by Q3PA (the responsive group for NQO1) because the hydroxyl acylation on Et-HCyOH hindered the electron-donating ability of the phenolic hydroxyl. In the presence of NQO1, HCyOH was released from probe LET-10 to produce NIR fluorescence and absorption signals, enabling NIRF/PA dual-modal imaging. The NIR fluorescence signal at 730 nm (NIRF730) was turned on, while the PA signal was red-shifted from 700 to 740 nm. Moreover, the probe was encapsulated with ONc by using DSPE-mPEG2000 as coating to improve the practicability for NIRF/PA dual-modal imaging in vivo. The probe showed two characteristic peaks, with 735/863 nm for absorption and 722/865 nm for PA, which was beneficial for ratiometric imaging. In 2016, Liang and co-workers reported a novel molecular probe (Rh-QL) based on quinoline, which displayed an NIR fluorescence at 750 nm and a visible fluorescence at 580 nm [56]. In the presence of NQO1, the fluorescence signal in the visible region was enhanced and the signal in the NIR region was turned on. Benefiting from dual-channel fluorescence signal, Rh-QL was successfully used to detect the fluctuations of NQO1 in tumor cells, and further applied for NQO1 bioimaging in vivo.
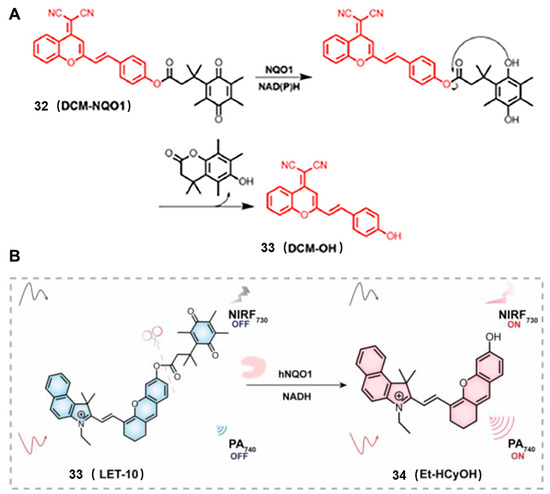
Figure 13.
(A) Representation of two-photon NIR probes for sensing NQO1. Fluorescence/ photoacoustic dual imaging probe LET-10 for sensing NQO1 (B). Adapted with permission from ref. [54]. Copyright (2022) the Royal Society of Chemistry. Adapted with permission from ref. [55]. Copyright (2022) American Chemical Society.
Compared with the visible and NIR-I (650–900 nm) probe, the probe based on second NIR (NIR-II, 1000–1700 nm) fluorophores has attracted increasing attention due to lower background autofluorescence, deeper tissue penetration (5–20 mm) and improved signal-to-noise ratio [57,58,59,60]. For example, in 2019, Zhao and co-workers reported an NIR-II fluorescent probe platform to detect enzyme activity. The strategy showed the advantages of two-channel ratiometric fluorescence, controllable NIR (I and II) fluorescence emission and large Stokes shifts. These activatable probes were fabricated by coupling BODIPY dyes to enzymic substrates via a self-immolative benzyl thioether linker [61]. In this work, the NQO1-activitable probe 36 (NQO-ImI) was constructed using the p-aminobenzyl thioether linker, which was able to form a 1,6-elimination reaction to release the desired fluorophore in the presence of NQO1 (Figure 14A). In the presence of NQO1, a clear absorption band around 675 nm emerged gradually, while the original absorption band at 557 nm was decreased, resulting in a remarkable red shift of 118 nm. Interestingly, the probe NQO-ImI showed two-channel ratiometric fluorescence signal and controllable NIR fluorescence signal. A similar phenomenon was observed in NQO1-positive cell lines (HT-29) and HT-29 tumor-bearing mice, which is shown in Figure 14D. To improve the tissue penetration depth of the small molecule probe NQO-ImI, this work synthesized an NIR-II fluorescence probe (probe 37) (NQO-InD) to monitor the NQO1 in vivo. The typical fluorescence signal can be observed at the wavelength of 910 nm (λex = 760 nm) upon the addition of NQO1 (Figure 14C). Notably, the tumor could be specifically illuminated within 40 min with the release of NIR-II fluorescent signal (Figure 14E). When mice were treated with dicoumarol, an inhibitor of NQO1, the fluorescence was significantly decreased, suggesting the high specificity of NQO-InD toward NQO1.
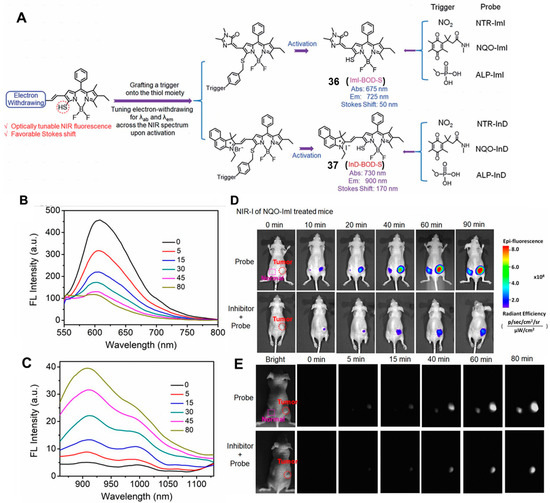
Figure 14.
(A) Schematic representation of the strategy for enzyme-activated NIR fluorescent probes. Fluorescence spectra of NQO-Iml activated by NQO1 (B) and NQO1-InD in NIR-II region (C). NIR-I imaging with NQO-Iml probe (D) and NIR-II imaging with NQO1-InD probe (E). Adapted with permission from ref. [61]. Copyright (2019) the Royal Society of Chemistry.
4. Chemiluminescent Probes for NQO1
Chemiluminescence (CL) without an external light source offers several advantages over the traditional fluorescence-based methods, including low background, deep tissue penetration and high sensitivity [62,63,64,65,66]. In 2008, Kim and co-workers reported an NQO1-specific chemiluminescent probe (probe 38). This probe allowed NQO1 activity to be detected in human lung cancer models both in vitro and in vivo [67]. The NQO1-specific chemiluminescent probe was constructed based on phenoxy-dioxetanes further equipped with an enzymatic substrate to design a turn-on probe. Upon the NQO1-mediated reduction of the quinone, followed by intramolecular lactonization and elimination of the benzyl aniline tether, the chemiluminescence was harvested (Figure 15A). Probe 38 showed good reactivity toward NQO1 in the presence of NAD(P), and a 130-fold increased chemiluminescence was observed at 515 nm. The minimum limit of detection (LOD) for probe 38 was found to be 51 ng/mL. The reactivity rate of the probe was very fast, with a rapidly increased signal intensity during the first 5 min. As shown in Figure 15B, the chemiluminescence signal of A549 cells was significantly higher than that of H596 cells. Compared with fluorescence imaging models, probe 38 showed a higher signal-to-noise ratio in the chemiluminescent model (Figure 15C,D). Most importantly, the chemiluminescent probe 38 showed good imaging ability in vivo, and a strong chemiluminescence signal was seen over the tumor area in the case of the A549 tumor-bearing mice, but no discernible signal was observed in the case of the NQO1-negative H596 cell line-derived xenografts (Figure 15E). However, probe 38 emits exclusively visible light with limited penetration depth, hindering its broad application for deep tissue imaging in vivo.
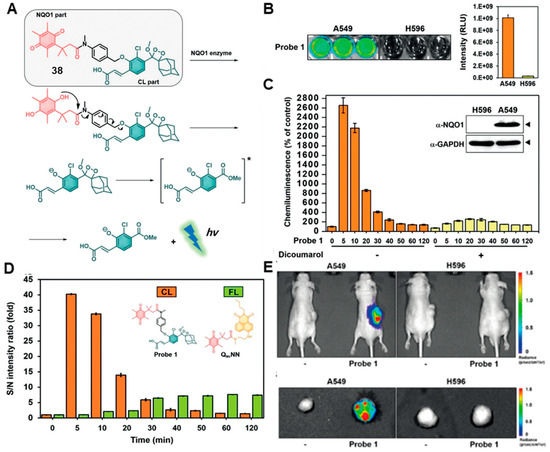
Figure 15.
(A) Proposed mechanisms of chemiluminescence release activation by NQO1. (B) Chemiluminescence images and signal intensity quantification of probe incubated with cancer cells. (C) Time-dependent chemiluminescence signal of probe incubated with A549 cells. (D) Signal-to-noise intensity ratios for probe compared to other probes. (E) Chemiluminescence images in vivo. Adapted with permission from ref. [67]. Copyright (2019) Wiley..
To this end, in 2019, Song and co-workers developed an activatable unimolecular CL probe to detect NQO1 in vivo, which emitted light in the NIR region. In the presence of NQO1, the NIR CL probe (CL-P) released chemiluminescence with a maximum emission wavelength at 725 nm (Figure 16A) [68]. Probe 39 showed high sensitivity to NQO1 under physiological conditions, with a moderate limit of detection (LOD) of 0.134 μg/mL. Moreover, CL-P produced an NIR CL signal within 10 min, indicating its rapid reaction kinetics, which is beneficial for practical bioimaging applications. The NIR CL probe was successfully used to detect NQO1 in living cells. The NIR CL intensity remarkably increased in A549 cells (NQO1 high expression) compared to that in LO2 cells (NQO1 low expression).
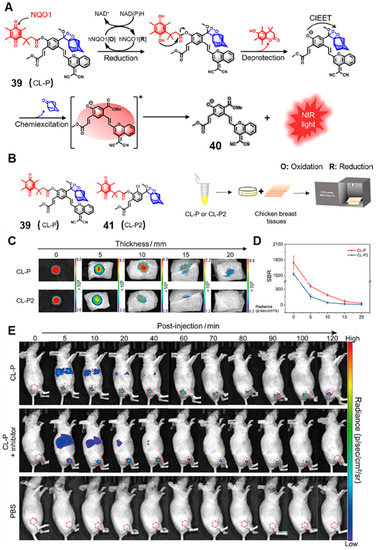
Figure 16.
(A) The reaction mechanism of the NQO1-activated CL probe (CL-P). (B) The molecular structure of CL-P and CL-P2. NIR CL imaging of probes under different thicknesses of chicken breast tissues (C) and the SBR of tissue depth (D). NIR CL images of tumor-bearing mice at different times after injection of CL-P (E). Adapted with permission from ref. [68]. Copyright (2022) Wiley.
In addition, dicoumarol was used to inhibit the activities of NQO1, and the CL signal was obviously decreased in dicoumarol pre-treatment cells compared to that of only CL-P in A549 cells, demonstrating the specific activation of the probe for NQO1. In particular, probe 39 showed a superior advantage of NIR CL imaging in vivo compared with probe 41 (CL-P2), which emitted visible light (Figure 16B). As shown in Figure 16C, the NIR CL produced by activated CL-P could be detected at a tissue depth of 20 mm, while the visible light of the activated CL-P2 could not be detected at 15 mm. The imaging signal-to-background ratio (SBR) of CL-P was 4.4-fold higher than CL-P2 (Figure 16D). These results indicated that NIR CL-P had better tissue penetration depth and resolution than the corresponding visible CL probe. Moreover, it can be seen in Figure 16E that the CL-P showed excellent imaging ability to detect NQO1 in A549 tumor-bearing mice.
5. Bioluminescent Probes for NQO1
Some living organisms are able to convert chemical energy into light. This natural phenomenon has led to a field of research called bioluminescence imaging (BLI) [69,70]. Thus, BLI shows great potential in tumor imaging, detection and therapeutic drug screening because it does not require any other external excitation light source [71]. It has many intrinsic advantages, such as better penetration depths, reduced autofluorescence and light scattering and high signal-to-ratio [72,73,74]. Bioluminescence is a biological process that requires luciferase, a substrate (luciferin) and oxygen. Some luciferases require other cofactors such as ATP and Mg2+ for full activities. In the enzymatic reaction, the key position of luciferin is the 6′-hydroxy/amino group. The bioluminescence can be regulated by modifying the substrate. It can be quenched when the 6′-position is caged, and instantly recovered when it reacts with specific targets [75].
In 2013, Zhou and co-workers developed a series of quinone-luciferins to detect NQO1 based on trimethyl lock quinone luciferin molecules [76]. As shown in Figure 17, compounds 42 and 45 were obtained by modifying the quinone-luciferin with an N, N’-dimethylethylenediamine spacer to overcome the high background bioluminescence. To develop a homogeneous assay for the specific quantification of NAD/NADH, NADP/NADPH or total NADH/NADPH in biological samples, the researchers ascertained the cell count before treating with compounds 42 and 45, respectively. Compounds 42 and 45 incubated with HepG2 cells generated significant bioluminescent signals. However, compound 45 showed a poor linear correlation of bioluminescent signals with cell numbers, and the bioluminescent signals of compound 42 increased with cell numbers at low cell density (<10,000 cells), suggesting that compound 42 can be effectively used for measuring living cells. It is not intuitive to detect NQO1 activity by measuring cell viability, and more intelligent bioluminescence probes should be designed to detect NQO1.
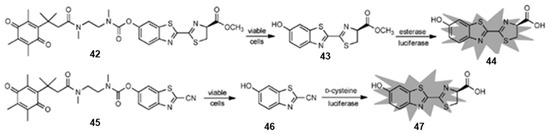
Figure 17.
The release mechanisms of luciferin from N, N’-dimethyldiamine linker-modified quinone luciferins. Adapted with permission from ref. [76]. Copyright (2014) Wiley.
In 2022, Yang and co-workers developed a new NQO1-activatable BL probe (probe 48), NQO1-luc, which was designed by equipping trimethyl-locked quinone propionic acid (QPA), a special substrate of NQO1, at the phenolic position of luciferins (Figure 18A) [77]. As shown in Figure 18B, the bioluminescence signal intensity was proportional to the NQO1 concentration in the concentration range of 0~40 μg mL−1, and the relationship between the luminescence intensity and NQO1 concentration was well fitted with the linear regression equation. In addition, NQO1-luc showed excellent selectivity to NQO1. The results in Figure 18C showed that NQO1-luc mixing with other bio-interferents showed almost no obvious bioluminescence signal, indicating that NQO1-luc had a good specificity for NQO1, which may be attributed to the specific reduction of the QPA group by NQO1. Importantly, NQO1-luc was more than 11-fold higher than in the absence of NQO1 in the simulated intracellular environment, demonstrating the excellent selectivity of NQO1-luc towards NQO1. More importantly, the NQO1-Luc bioluminescence probe can be used in in vivo, and the results indicated that NQO1-Luc could be rapidly taken up by the tumor tissue with good permeability and biocompatibility. In contrast, mice pre-injected with dicoumarol showed a clear bioluminescence inhibition, demonstrating that the signal of NQO1-Luc was NQO1-dependent (Figure 18D). These results suggested that the NQO1-Luc probe was a promising indicator to dynamically reflect the endogenous NQO1 activities and a prospective probe for the imaging of NQO1-overexpressed tumors.
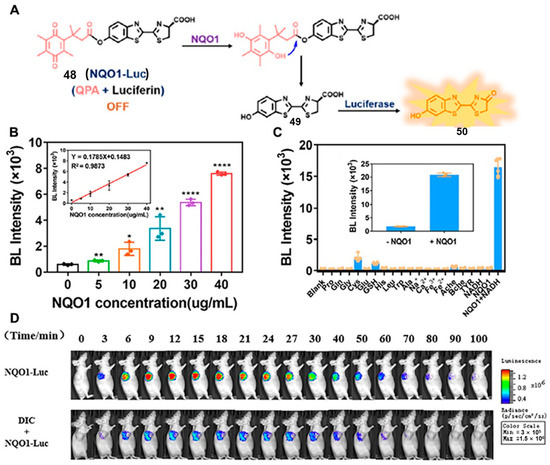
Figure 18.
Reaction mechanisms of NQO1-Luc for NQO1 detection (A). (B) Bioluminescence intensity changes in NQO1-Luc with different concentrations of NQO1. Selectivity of NQO1-Luc to biological molecules (C) and BL images (D) of nude mice bearing Luc-transfected A549 cell tumors. Adapted with permission from ref. [77]. Copyright (2022) the Royal Society of Chemistry.
6. Conclusions and Perspective
Quinones are widely distributed in mammalian cells and display versatile biological functions due to their unique chemical structures. Furthermore, quinones are involved in various physiological and pathological processes by acting as electrophilic species and inducing oxidative stress. As an antioxidant enzyme, NQO1 has been reported to reduce the level of free quinones for single-electron reduction to decrease quinone-induced carcinogenicity and cell necrosis, thereby protecting against oxidative stress and regulating metabolic functions. By focusing on the challenge of real-time imaging and monitoring of NQO1 in living organisms, there has been increasing interest in the development of various chemical/biological strategies to construct novel molecular tools and to profile their properties in diverse biological processes.
Based on the advanced optical imaging methods, diverse versatile molecular probes have been constructed, which can be used to image endogenous NQO1 activities in living cells and in vivo in real time. In particular, many probes have been applied to decipher the intrinsic relationships between abnormally expressed NQO1 and carcinogenic processes. In this review, we have summarized the rise of NQO1-based organic probes from the perspectives of molecular design strategies, sensing mechanisms and biomedical application. Recent works presenting the obtaining of new molecular probes to target subcellular organelles and monitor specific intracellular environments illustrate the promising value of these NQO1-activated probes.
In the future, more efforts should focus on constructing in situ imaging probes with self-localizable ability, thereby obtaining the distribution of NQO1 activates within their native habitat. Furthermore, considering the significant expression difference of NQO1 between tumor and normal tissues, NQO1 probes with high signal-to-noise ratio and long wavelengths should be developed to distinguish cancer cells with high resolution, offering a precise tumor imaging tool for imaging-guided surgery. We believe that the aforementioned molecular probe design strategies can also be modified and improved to construct versatile tools that may better meet the application requirements of NQO1-related physiological and pathological studies.
Author Contributions
Conceptualization, K.L.; investigation and writing—original draft preparation, K.C. and S.X.; validation and formal analysis, K.C. and Z.S.; data curation, writing and editing review, S.X. and K.L.; project administration, supervision and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (22204177, 22204061) and Shandong Key Laboratory of Biochemical Analysis (SKLBA2107).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We gratefully acknowledge the support of this work by the National Natural Science Foundation of China (22204177, 22204061) and Shandong Key Laboratory of Biochemical Analysis (SKLBA2107).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schlager, J.J.; Powis, G. Cytosolic NAD(P)H:(quinone-acceptor)oxidoreductase in human normal and tumor tissue: Effects of cigarette smoking and alcohol. Int. J. Cancer 2010, 45, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.; Siegel, D. NAD(P)H:quinone oxidoreductase 1 (NQO1, DT-diaphorase), functions and pharmacogenetics. Meth. Enzymol. 2004, 382, 115. [Google Scholar]
- Dias, G.G.; King, A.; Moliner, F.D.; Vendrell, M.; Júnior, E. Quinone-based fluorophores for imaging biological processes. Chem. Soc. Rev. 2018, 47, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Winski, S.L.; Koutalos, Y.; Bentley, D.L.; Ross, D. Subcellular localization of NAD(P)H:quinone oxidoreductase 1 in human cancer cells. Cancer Res. 2002, 62, 1420–1424. [Google Scholar]
- Madajewski, B.; Boatman, M.A.; Chakrabarti, G.; Boothman, D.A.; Bey, E.A. Depleting tumor-NQO1 potentiates anoikis and inhibits growth of NSCLC. Mol. Cancer Res. 2016, 14, 14–25. [Google Scholar] [CrossRef]
- Su, D.; Chen, X.; Zhang, Y.; Gao, X. Activatable imaging probes for cancer-linked NAD(P)H:quinone oxidoreductase-1 (NQO1): Advances and future prospects. Trends Analyt. Chem. 2020, 133, 116112. [Google Scholar] [CrossRef]
- Asher, G.; Lotem, J.; Cohen, B. Regulation of p53 stability and p53-dependent apoptosis by NADH quinone oxidoreductase. Proc. Natl. Acad. Sci. USA 2001, 98, 1188–1193. [Google Scholar] [CrossRef]
- Johansson, H.; Malmnas, P.E. NQO1 suppresses NF-κB-p300 interaction to regulate inflammatory mediators associated with prostate tumorigenesis. Cancer Res. 2014, 74, 5644. [Google Scholar]
- Sharp, S.Y.; Kelland, L.R.; Valenti, M.R.; Brunton, L.A.; Hobbs, S.; Workman, P. Establishment of an isogenic human colon tumor model for NQO1 gene expression: Application to investigate the role of DT-diaphorase in bioreductive drug activation in vitro and in vivo. Mol. Pharmacol. 2000, 58, 1146–1155. [Google Scholar] [CrossRef]
- Blanche, E.A.; Maskell, L.; Colucci, M.A.; Whatmore, J.L.; Moody, C.J. Synthesis of potential prodrug systems for reductive activation. Prodrugs for anti-angiogenic isoflavones and VEGF receptor tyrosine kinase inhibitory oxindoles. Tetrahedron 2009, 65, 4894–4903. [Google Scholar] [CrossRef]
- Glorieux, C.; Sandoval, J.M.; Dejeans, N.; Ameye, G.; Poirel, H.A.; Verrax, J.; Calderon, P.B. Overexpression of NAD(P)H: Quinone oxidoreductase 1 (NQO1) and genomic gain of the NQO1 locus modulates breast cancer cell sensitivity to quinones. Life Sci. 2016, 145, 57–65. [Google Scholar] [CrossRef]
- Zerez, C.R.; Lee, S.J.; Tanaka, K.R. Spectrophotometric determination of oxidized and reduced pyridine nucleotides in erythrocytes using a single extraction procedure. Anal. Biochem. 1987, 164, 367–373. [Google Scholar] [CrossRef]
- Li, J.; Rassi, Z.E. High performance liquid chromatography of phenolic choline ester fragments derived by chemical and enzymatic fragmentation processes: Analysis of sinapine in rape seed. J. Agric. Food Chem. 2002, 50, 1368–1373. [Google Scholar] [CrossRef]
- Torabi, F.; Ramanathan, K.; Larsson, P.O.; Gorton, L.; Khayyami, M. Coulometric determination of NAD(+) and NADH in normal and cancer cells using LDH, RVC and a polymer mediator. Talanta 1999, 50, 787–797. [Google Scholar] [CrossRef]
- Kerr, R.G.; Kelly, K. An enzyme-based pormaldehyde assay and its utility in a sponge sterolbiosynthetic pathway. J. Nat. Prod. 1999, 62, 201–202. [Google Scholar] [CrossRef]
- Prescher, J.A.; Bertozzi, C.R. Chemistry in living systems. Nat. Chem. Biol. 2005, 1, 13–21. [Google Scholar] [CrossRef]
- Li, K.; Xu, S.; Xiong, M.; Huan, S.-Y.; Yuan, L.; Zhang, X.-B. Molecular engineering of organic-based agents for in situ bioimaging and phototherapeutics. Chem. Soc. Rev. 2021, 50, 11766–11784. [Google Scholar] [CrossRef]
- Li, K.; Ren, T.B.; Huan, S.; Yuan, L.; Zhang, X.B. Progress and perspective of solid-state organic fluorophores for biomedical applications. J. Am. Chem. Soc. 2021, 143, 21143–21160. [Google Scholar] [CrossRef]
- Hettiarachchi, S.U.; Prasai, B.; Mccarley, R.L. Detection and cellular imaging of human cancer enzyme using a turn-on, wavelength-shiftable, self-immolative profluorophore. J. Am. Chem. Soc. 2014, 136, 7575–7578. [Google Scholar] [CrossRef]
- Silvers, W.C.; Prasai, B.; Burk, D.H.; Brown, M.L.; Mccarley, R.L. Profluorogenic reductase substrate for rapid, selective, and sensitive visualization and detection of human cancer cells that overexpress NQO1. J. Am. Chem. Soc. 2013, 135, 309–314. [Google Scholar] [CrossRef]
- Mendoza, M.F.; Hollabaugh, N.M.; Hettiarachchi, S.U.; McCarley, R.L. Human NAD(P)H:quinone oxidoreductase type I (hNQO1) activation of quinone propionic acid trigger groups. Biochemistry 2012, 51, 8014–8026. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; pp. 331–351. [Google Scholar]
- Prasai, B.; Silvers, W.C.; McCarley, R.L. Oxidoreductase-facilitated visualization and detection of human cancer cells. Anal. Chem. 2015, 87, 6411–6418. [Google Scholar] [CrossRef] [PubMed]
- Best, Q.A.; Prasai, B.; Rouillere, A.; Johnson, A.E.; McCarley, R.L. Efficacious fluorescence turn-on probe for high-contrast imaging of human cells overexpressing quinone reductase activity. Chem. Comm. 2017, 53, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Won, M.; Kang, C.; Kim, J.S.; Lee, M.H. A coumarin-naphthalimide hybrid as a dual emissive fluorescent probe for NQO1. Dyes Pigm. 2019, 164, 341–345. [Google Scholar] [CrossRef]
- Beija, M.; Afonso, C.A.M.; Martinho, J.M.G. Synthesis and applications of Rhodamine derivatives as fluorescent probes. Chem. Soc. Rev. 2009, 38, 2410–2433. [Google Scholar] [CrossRef]
- Silvers, W.C.; Payne, A.S.; McCarley, R.L. Shedding light by cancer redox-human NAD (P) H: Quinone oxidoreductase 1 activation of a cloaked fluorescent dye. Chem. Comm. 2011, 47, 11264–11266. [Google Scholar] [CrossRef]
- Best, Q.A.; Johnson, A.E.; Prasai, B.; Rouillere, A.; McCarley, R.L. Environmentally robust rhodamine reporters for probe-based cellular detection of the cancer-linked oxidoreductase NQO1. ACS Chem. Biol. 2016, 11, 231–240. [Google Scholar] [CrossRef]
- Fei, Q.; Zhou, L.; Wang, F.; Shi, B.; Li, C.; Wang, R.; Zhao, C. Rational construction of probes rendering ratiometric response to the cancer-specific enzyme NQO1. Dyes Pigm. 2017, 136, 846–851. [Google Scholar] [CrossRef]
- Cuff, S.; Lewis, R.D.; Chinje, E.; Jaffar, M.; Knox, R.; Weeks, I. An improved cell-permeable fluorogenic substrate as the basis for a highly sensitive test for NAD (P) H quinone oxidoreductase 1 (NQO1) in living cells. Free Radic. Biol. Med. 2018, 116, 141–148. [Google Scholar] [CrossRef]
- Yang, Q.; Wen, Y.; Xu, J.; Shao, S. An HBT-based fluorescent dye with enhanced quantum yield in water system and its application for constructing NQO1 fluorescent probe. Talanta 2020, 216, 120982. [Google Scholar] [CrossRef]
- Yuan, Z.; Xu, M.; Wu, T.; Zhang, X.; Shen, Y.; Ernest, U.; Chen, H. Design and synthesis of NQO1 responsive fluorescence probe and its application in bio-imaging for cancer diagnosis. Talanta 2019, 198, 323–329. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.W.; Hu, X.X.; Li, J.; Liang, L.H.; Zhang, X.B.; Tan, W. Efficient two-photon fluorescent probe for nitroreductase detection and hypoxia imaging in tumor cells and tissues. Anal. Chem. 2015, 87, 11832–11839. [Google Scholar] [CrossRef]
- Kim, H.M.; Cho, B.R. Small-molecule two-photon probes for bioimaging applications. Chem. Rev. 2015, 115, 5014–5055. [Google Scholar] [CrossRef]
- Cho, M.K.; Juvekar, V.; Lim, C.S.; Noh, C.K.; Shin, S.J.; Kim, H.M. A highly sensitive two-photon ratiometric probe for rapid detection of the NQO1 enzyme in colon cancer tissue. Asian J. Org. Chem. 2019, 8, 1707–1712. [Google Scholar] [CrossRef]
- Cho, M.K.; Lim, C.S.; Sarkar, A.R.; Lee, H.W.; Choi, H.J.; Noh, C.K.; Kim, H.M. A two-photon ratiometric probe for detection of NQO1 enzyme activity in human colon tissue. Sens. Actuators B Chem. 2018, 272, 203–210. [Google Scholar] [CrossRef]
- Kwon, N.; Cho, M.K.; Park, S.J.; Kim, D.; Nam, S.J.; Cui, L.; Yoon, J. An efficient two-photon fluorescent probe for human NAD (P) H: Quinone oxidoreductase (NQO1) detection and imaging in tumor cells. Chem. Comm. 2017, 53, 525–528. [Google Scholar] [CrossRef]
- Pan, D.; Luo, F.; Liu, X.; Liu, W.; Chen, W.; Liu, F.; Jiang, J.H. A novel two-photon fluorescent probe with a long stokes shift and a high signal-to-background ratio for human NAD (P) H: Quinone oxidoreductase 1 (NQO1) detection and imaging in living cells and tissues. Analyst 2017, 142, 2624–2630. [Google Scholar] [CrossRef]
- Yang, Y.P.; Qi, F.J.; Qian, Y.P.; Bao, X.Z.; Zhang, H.C.; Ma, B.; Zhou, B. Developing push–pull hydroxylphenylpolyenylpyridinium chromophores as ratiometric two-photon fluorescent probes for cellular and intravital imaging of mitochondrial NQO1. Anal. Chem. 2021, 93, 2385–2393. [Google Scholar] [CrossRef]
- Shin, W.S.; Lee, M.G.; Verwilst, P.; Lee, J.H.; Chi, S.G.; Kim, J.S. Mitochondria-targeted aggregation induced emission theranostics: Crucial importance of in situ activation. Chem. Sci. 2016, 7, 6050–6059. [Google Scholar] [CrossRef]
- Shin, W.S.; Han, J.; Verwilst, P.; Kumar, R.; Kim, J.H.; Kim, J.S. Cancer targeted enzymatic theranostic prodrug: Precise diagnosis and chemotherapy. Bioconjug. Chem. 2016, 27, 1419–1426. [Google Scholar] [CrossRef]
- Digby, E.M.; Sadovski, O.; Beharry, A.A. An activatable photosensitizer targeting human NAD (P) H: Quinone oxidoreductase. Chem. Eur. J. 2020, 26, 2713–2718. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, P.; Yan, D.; Zeng, F.; Wu, S. A self-immolative and DT-diaphorase-activatable prodrug for drug-release tracking and therapy. J. Mater. Chem. B 2017, 5, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Song, C.W.; Yang, Y.J.; Kim, H.R.; Reo, Y.J.; Ahn, K.H. Toward ratiometric detection of NAD (P) H quinone oxidoreductase-1: Benzocoumarin-based fluorescent probes. Sens. Actuators B Chem. 2021, 330, 129277. [Google Scholar] [CrossRef]
- Zhang, C.; Zhai, B.B.; Peng, T.; Zhong, Z.; Xu, L.; Zhang, Q.Z.; Xi, Z. Design and synthesis of near-infrared fluorescence-enhancement probes for the cancer-specific enzyme NQO1. Dyes Pigm. 2017, 143, 245–251. [Google Scholar] [CrossRef]
- Shen, Z.; Prasai, B.; Nakamura, Y.; Kobayashi, H.; Jackson, M.S.; McCarley, R.L. A near-infrared, wavelength-shiftable, turn-on fluorescent probe for the detection and imaging of cancer tumor cells. ACS Chem. Biol. 2017, 12, 1121–1132. [Google Scholar] [CrossRef]
- Punganuru, S.R.; Madala, H.R.; Arutla, V.; Zhang, R.; Srivenugopal, K.S. Characterization of a highly specific NQO1-activated near-infrared fluorescent probe and its application for in vivo tumor imaging. Sci. Rep. 2019, 9, 8577. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, D.; Zhang, Y.; Zhang, Y.; Shen, Y. Hemicyanine-based near-infrared fluorescent probe for the ultrasensitive detection of NQO1 activity and discrimination of human cancer cells. Anal. Chim. Acta 2019, 1090, 125–132. [Google Scholar] [CrossRef]
- Gong, Q.; Yang, F.; Hu, J.; Li, T.; Wang, P.; Li, X.; Zhang, X. Rational designed highly sensitive NQO1-activated near-infrared fluorescent probe combined with NQO1 substrates in vivo: An innovative strategy for NQO1-overexpressing cancer theranostics. Eur. J. Med. Chem. 2021, 224, 113707. [Google Scholar] [CrossRef]
- Singh, A.K.; Nair, A.V.; Singh, N.P. Small two-photon organic fluorogenic probes: Sensing and bioimaging of cancer relevant biomarkers. Anal. Chem. 2021, 94, 177–192. [Google Scholar] [CrossRef]
- Juvekar, V.; Lee, H.W.; Kim, H.M. Two-photon fluorescent probes for detecting enzyme activities in live tissues. ACS Appl. Bio Mater. 2021, 4, 2957–2973. [Google Scholar] [CrossRef]
- Juvekar, V.; Lee, H.W.; Lee, D.J.; Kim, H.M. Two-photon fluorescent probes for quantitative bio-imaging analysis in live tissues. TrAC Trends Anal. Chem. 2022, 157, 116787. [Google Scholar] [CrossRef]
- Li, G.; Wu, S.; Chen, W.; Duan, X.; Sun, X.; Li, S.; Chen, T. Designing intelligent nanomaterials to achieve highly sensitive diagnoses and multimodality therapy of bladder cancer. Small Methods 2023, 7, 2201313. [Google Scholar] [CrossRef]
- Wu, W.; Li, X.; Zhao, L.; Li, S.; Han, J.; Zhang, Y.; Zhao, Z. Design and synthesis of a deep tissue penetrating near-infrared two-photon fluorescence probe for the specific detection of NQO1. Chem. Comm. 2022, 58, 5634–5637. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, K.; Jiang, S.; Zhao, F.; Chen, P.; Huang, P.; Lin, J. In vivo near-infrared fluorescence/ratiometric photoacoustic duplex imaging of lung cancer-specific NQO1. Anal. Chem. 2022, 94, 13770–13776. [Google Scholar] [CrossRef]
- Guan, L.; Sun, H.; Xiong, J.; Hu, W.; Ding, M.; Liang, Q. A quinoline-based indicator for NAD (P) H multimodal detection in vitro and in vivo: Spectrophotometry and visible near-infrared dual-channel lighting-up fluorescence imaging. Sens. Actuators B Chem. 2022, 373, 132694. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Z.; Wang, H.; Zhou, B.; Zhu, S.; Zhong, Y.; Dai, H. Rational design of molecular fluorophores for biological imaging in the NIR-II window. Adv. Mater. 2017, 29, 1605497. [Google Scholar] [CrossRef]
- Mu, J.; Xiao, M.; Shi, Y.; Geng, X.; Li, H.; Yin, Y.; Chen, X. The chemistry of organic contrast agents in the NIR-II window. Angew. Chem. Int. Ed. 2022, 61, e202114722. [Google Scholar] [CrossRef]
- Li, C.; Chen, G.; Zhang, Y.; Wu, F.; Wang, Q. Advanced fluorescence imaging technology in the near-infrared-II window for biomedical applications. J. Am. Chem. Soc. 2020, 142, 14789–14804. [Google Scholar] [CrossRef]
- Hong, G.; Lee, J.C.; Robinson, J.T.; Raaz, U.; Xie, L.; Huang, N.F.; Dai, H. Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nat. Med. 2012, 18, 1841–1846. [Google Scholar] [CrossRef]
- Wang, R.; Chen, J.; Gao, J.; Chen, J.A.; Xu, G.; Zhu, T.; Zhao, C. A molecular design strategy toward enzyme-activated probes with near-infrared I and II fluorescence for targeted cancer imaging. Chem. Sci. 2019, 10, 7222–7227. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, Y.; Li, J.; Huang, J.; Pu, K. Molecular chemiluminescent probes with a very long near-infrared emission wavelength for in vivo imaging. Angew. Chem. Int. Ed. 2021, 60, 3999–4003. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Hananya, N.; Green, O.; Chen, H.; Zhao, A.Q.; Shen, J.; Yang, D. A highly selective and sensitive chemiluminescent probe for real-time monitoring of hydrogen peroxide in cells and animals. Angew. Chem. Int. Ed. 2020, 59, 14326–14330. [Google Scholar] [CrossRef] [PubMed]
- Bruemmer, K.J.; Green, O.; Su, T.A.; Shabat, D.; Chang, C.J. Chemiluminescent probes for activity-based sensing of formaldehyde released from folate degradation in living mice. Angew. Chem. Int. Ed. 2018, 130, 7630–7634. [Google Scholar] [CrossRef]
- Yang, M.; Huang, J.; Fan, J.; Du, J.; Pu, K.; Peng, X. Chemiluminescence for bioimaging and therapeutics: Recent advances and challenges. Chem. Soc. Rev. 2020, 49, 6800–6815. [Google Scholar] [CrossRef] [PubMed]
- Hananya, N.; Reid, J.P.; Green, O.; Sigman, M.S.; Shabat, D. Rapid chemiexcitation of phenoxy-dioxetane luminophores yields ultrasensitive chemiluminescence assays. Chem. Sci. 2019, 10, 1380–1385. [Google Scholar] [CrossRef]
- Son, S.; Won, M.; Green, O.; Hananya, N.; Sharma, A.; Jeon, Y.; Kim, J.S. Chemiluminescent probe for the in vitro and in vivo imaging of cancers over-expressing NQO1. Angew. Chem. Int. Ed. 2019, 58, 1739–1743. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Huo, H.; Chen, L.; Wu, Y.; Zhang, X.; Song, J. An activatable near-infrared molecular chemiluminescence probe for visualization of NQO1 activity in vivo. Chin. J. Chem. 2022, 40, 2400–2406. [Google Scholar] [CrossRef]
- Sadikot, R.T.; Blackwell, T.S. Bioluminescence imaging. Proc. Am. Thorac. Soc. 2005, 2, 537–540. [Google Scholar] [CrossRef]
- Ozawa, T.; Yoshimura, H.; Kim, S.B. Advances in fluorescence and bioluminescence imaging. Anal. Chem. 2013, 85, 590–609. [Google Scholar] [CrossRef]
- Welsh, D.K.; Kay, S.A. Bioluminescence imaging in living organisms. Curr. Opin. Biotechnol. 2005, 16, 73–78. [Google Scholar] [CrossRef]
- Cohen, A.S.; Dubikovskaya, E.A.; Rush, J.S.; Bertozzi, C.R. Real-time bioluminescence imaging of glycans on live cells. J. Am. Chem. Soc. 2010, 132, 8563–8565. [Google Scholar] [CrossRef]
- Liu, R.; Tang, J.; Xu, Y.; Dai, Z. Bioluminescence imaging of inflammation in vivo based on bioluminescence and fluorescence resonance energy transfer using nanobubble ultrasound contrast agent. ACS Nano 2019, 13, 5124–5132. [Google Scholar] [CrossRef]
- Lu, L.; Li, B.; Ding, S.; Fan, Y.; Wang, S.; Sun, C.; Zhang, F. NIR-II bioluminescence for in vivo high contrast imaging and in situ ATP-mediated metastases tracing. Nat. Commun. 2020, 11, 4192. [Google Scholar] [CrossRef]
- Gross, S.; Gammon, S.T.; Moss, B.L.; Rauch, D.; Harding, J.; Heinecke, J.W.; Piwnica-Worms, D. Bioluminescence imaging of myeloperoxidase activity in vivo. Nat. Med. 2009, 15, 455–461. [Google Scholar] [CrossRef]
- Zhou, W.; Leippe, D.; Duellman, S.; Sobol, M.; Vidugiriene, J.; O’Brien, M.; Meisenheimer, P. Self-immolative bioluminogenic quinone luciferins for NAD (P) H assays and reducing capacity-based cell viability assays. ChemBioChem 2014, 15, 670–675. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, W.; Zeng, Y.; Wang, S.; Guo, X.; Hu, R.; Yang, G. A bioluminescent probe for NQO1 overexpressing cancer cell imaging in vitro and in vivo. Analyst 2022, 147, 5264–5268. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).