Detection of Lysosomal Hg2+ Using a pH-Independent Naphthalene Monoimide-Based Fluoroprobe
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Steady-State Measurement
2.3. Time-Resolved Single Photon Counting Studies (TCSPC)
2.4. Solid-State Photophysical Studies
2.5. pH Titration and Fluorescence Titration with Metal Ions
2.6. Job’s Plot
2.7. NMR Spectroscopy and Mass Spectrometry
2.8. Density Functional Theory (DFT) Calculation
2.9. Cell Culture and Imaging
2.10. Culture Method
2.11. Confocal Microscopy
3. Results
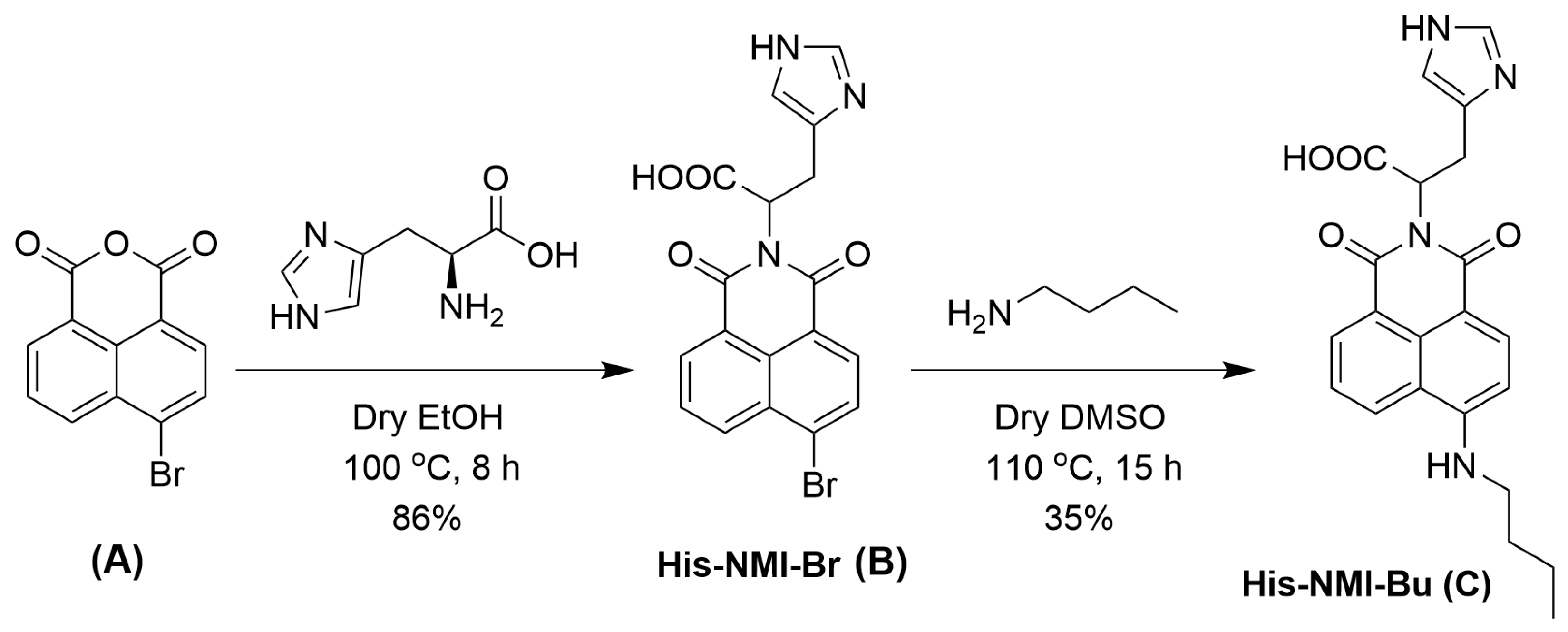
3.1. Synthesis
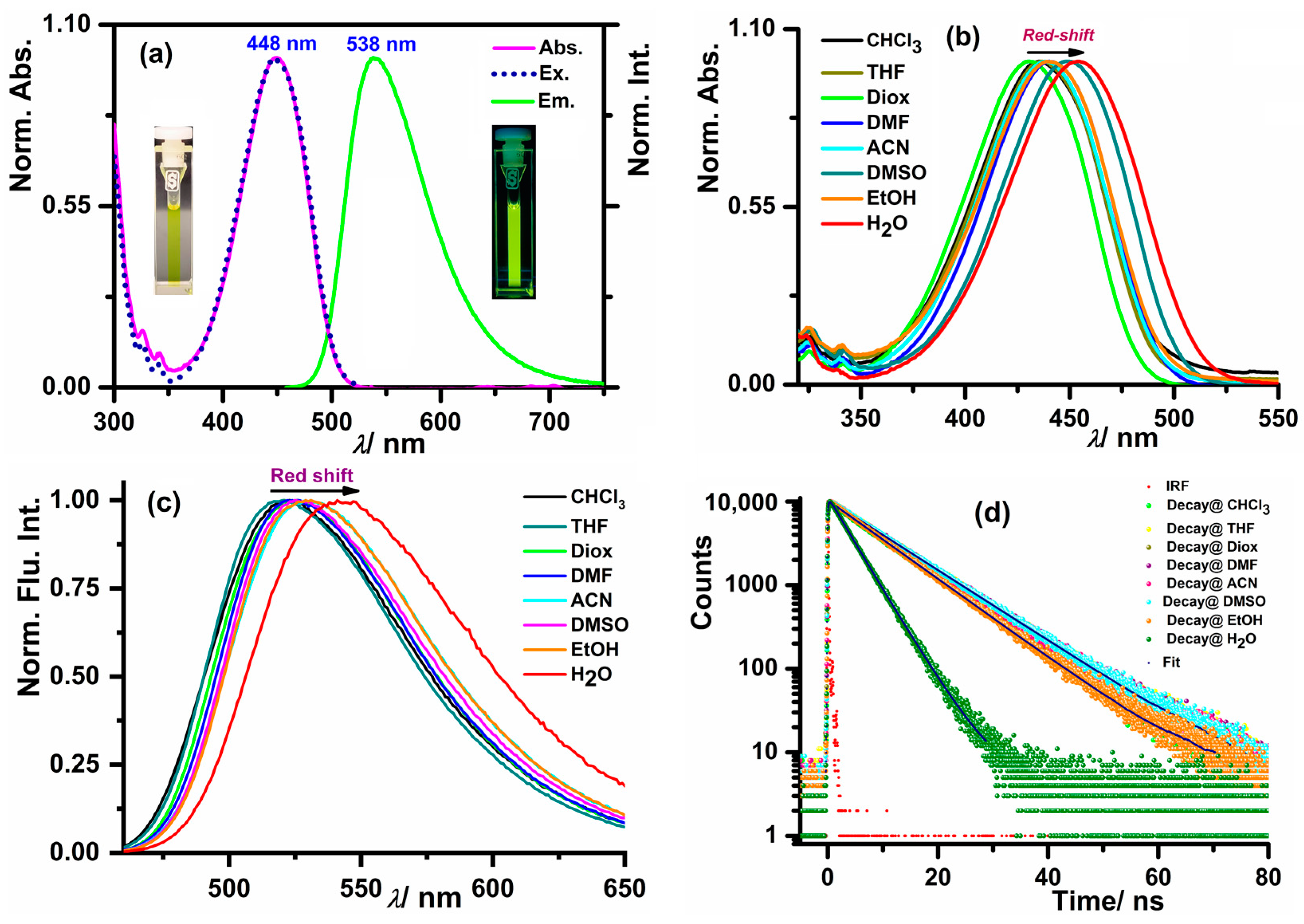
3.2. Photophysical Properties of the Probe
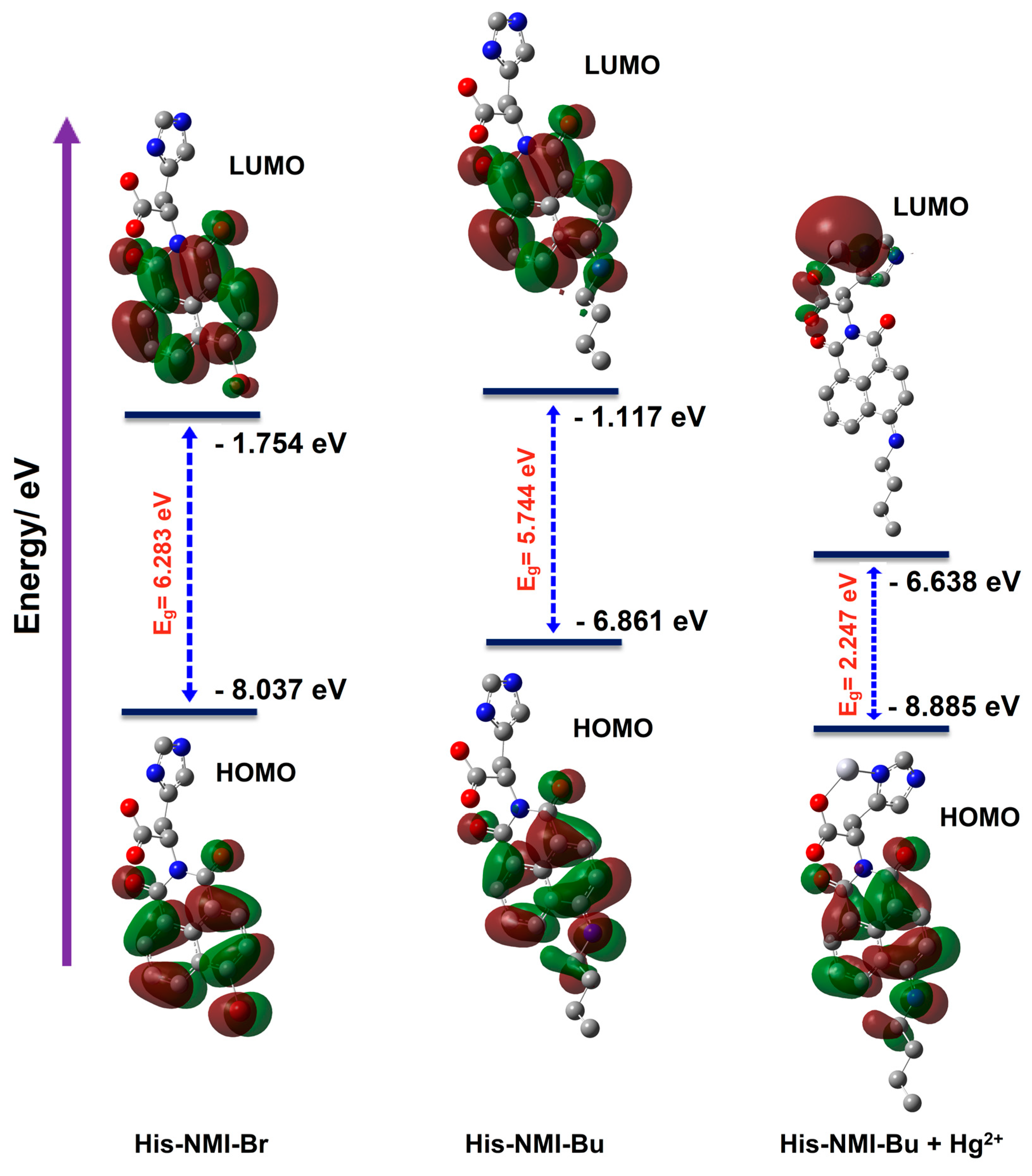
3.3. Computational Investigations
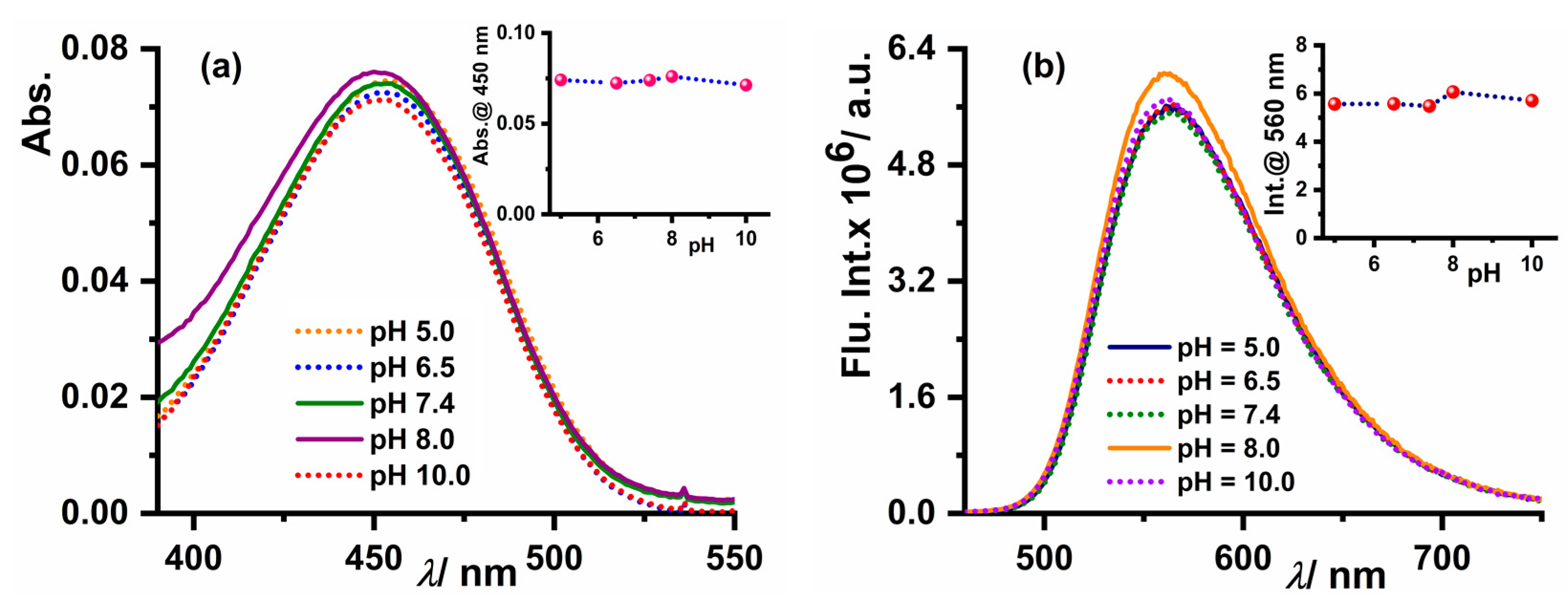
3.4. pH-Dependent Absorption and Emission Studies
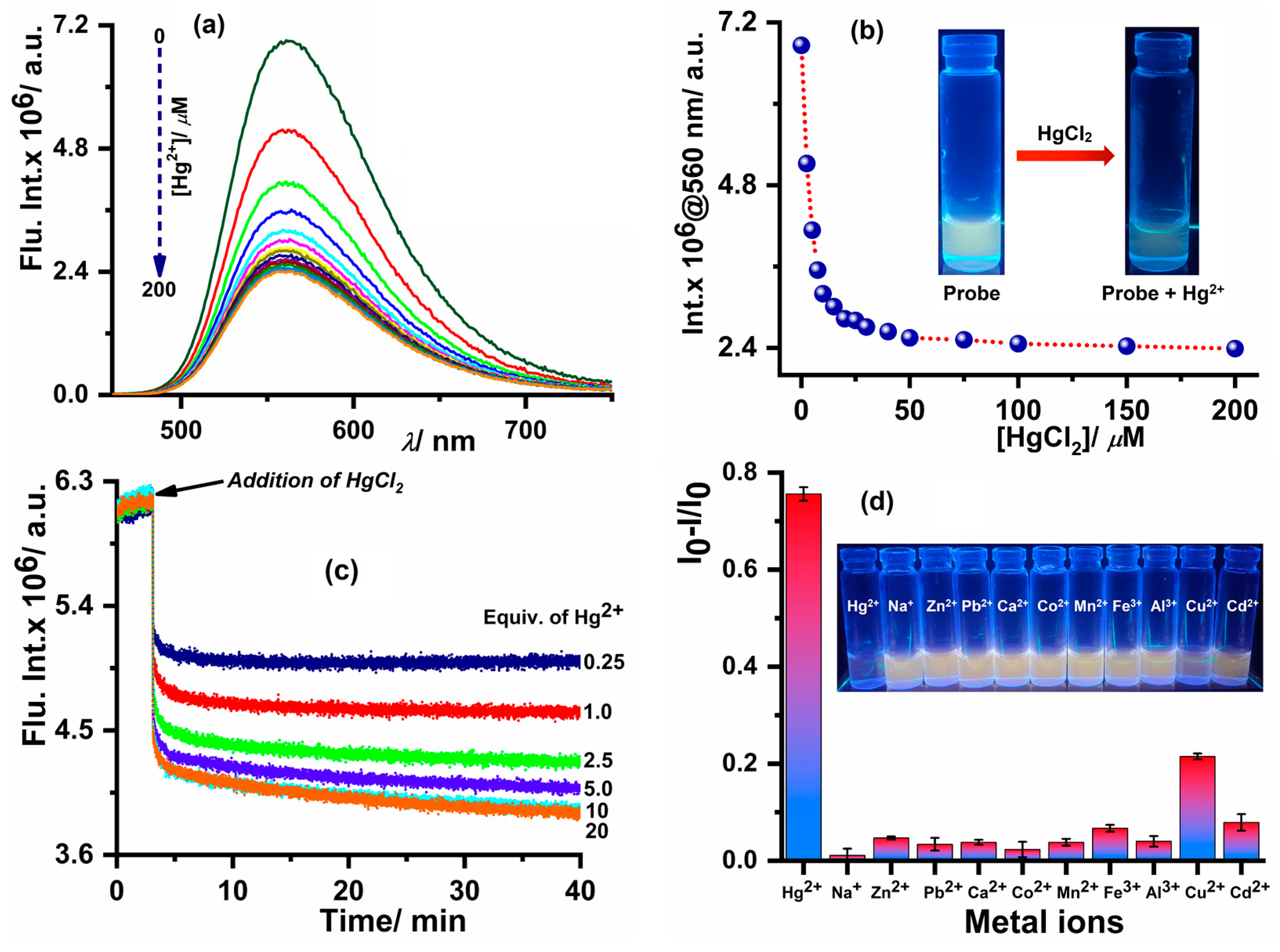
3.5. Spectroscopic Studies for Hg2+ Sensing
3.6. Selectivity Assay
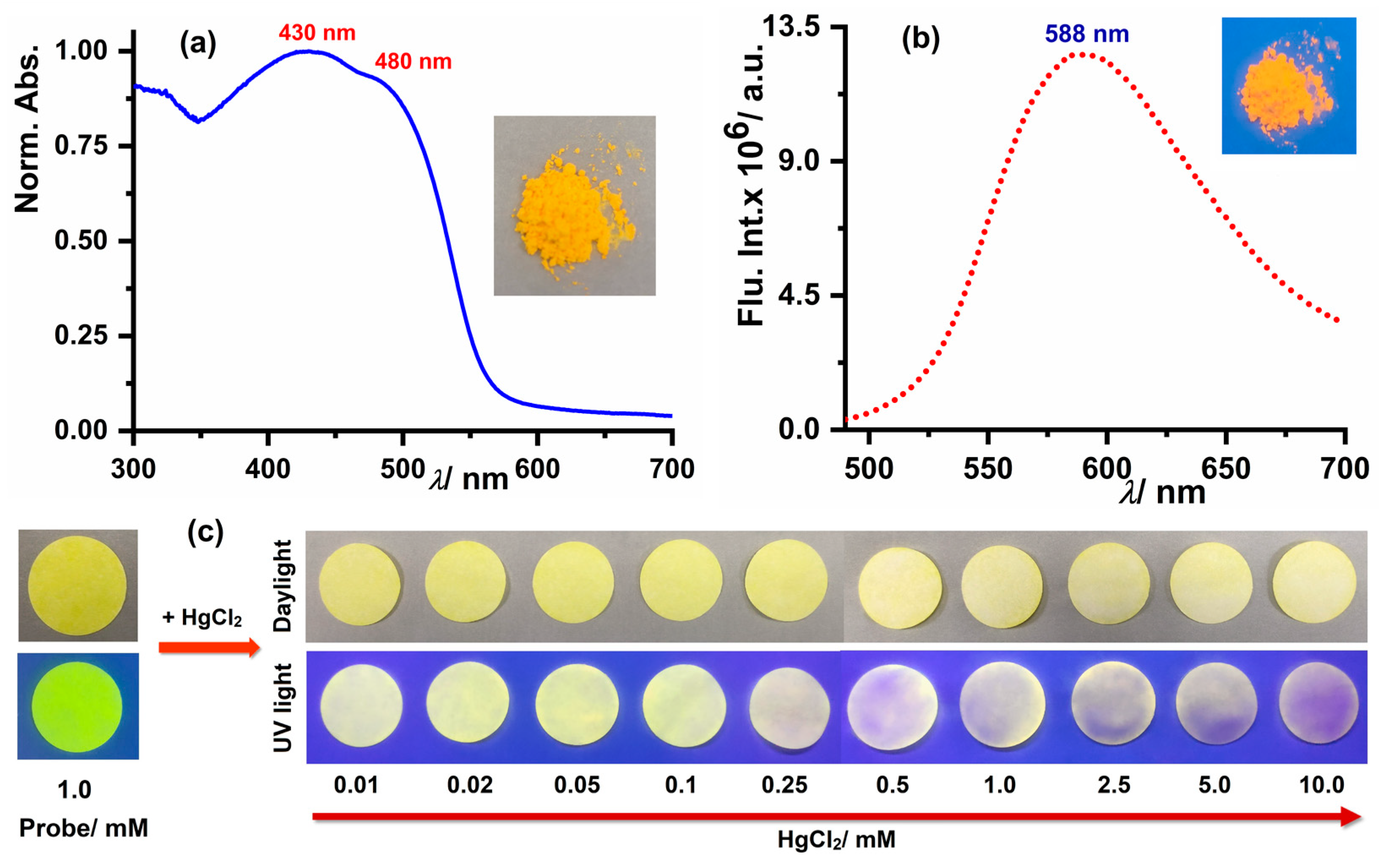
3.7. Solid-State Properties of Probe and Paper-Based Sensing
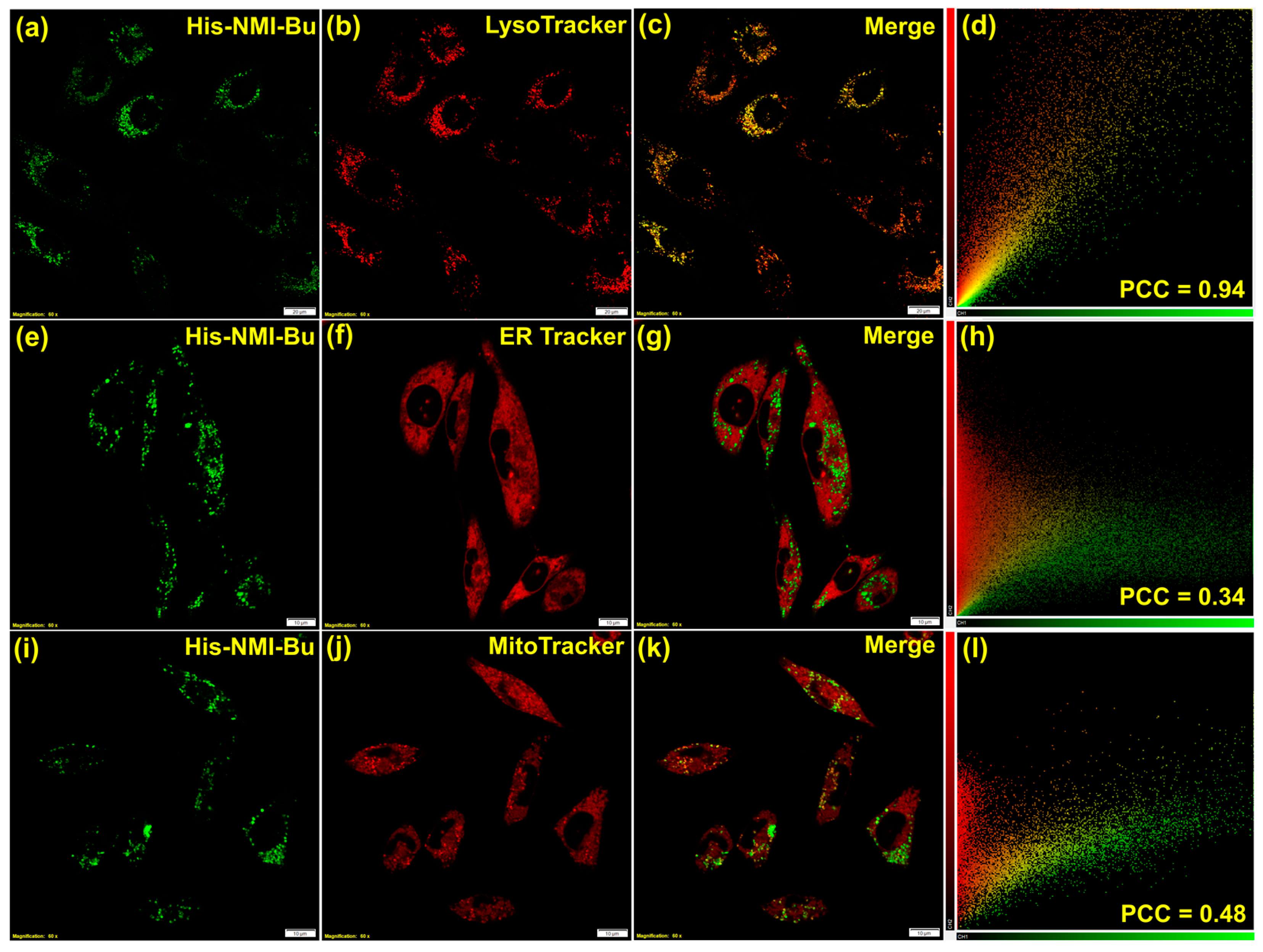
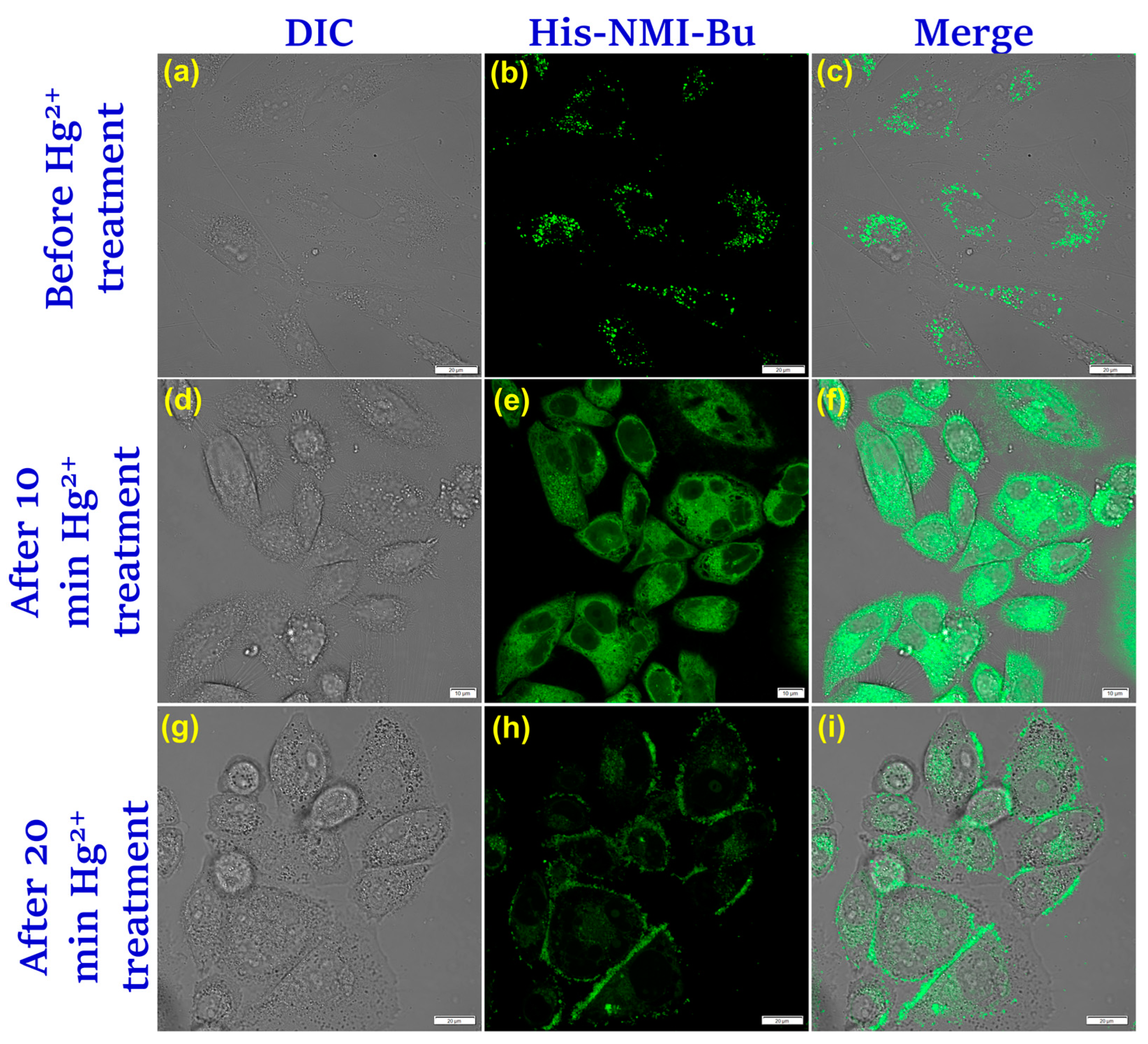
3.8. Bio-Imaging and Detection of Hg2+ Inside Lysosomes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernhoft, R.A. Mercury toxicity and treatment: A review of the literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.M.; Walker, E.M., Jr.; Wu, M.; Gillette, C.; Blough, E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Azevedo, B.; Barros Furieri, L.; Peçanha, F.M.; Wiggers, G.A.; Frizera Vassallo, P.; Ronacher Simões, M.; Fiorim, J.; Rossi de Batista, P.; Fioresi, M.; Rossoni, L.; et al. Toxic effects of mercury on the cardiovascular and central nervous systems. J. Biomed. Biotechnol. 2012, 2012, 949048. [Google Scholar] [CrossRef]
- Udhayakumari, D. Review on fluorescent sensors-based environmentally related toxic mercury ion detection. J. Incl. Phenom. Macrocycl. Chem. 2022, 102, 451–476. [Google Scholar] [CrossRef]
- Ratner, N.; Mandler, D. Electrochemical Detection of Low Concentrations of Mercury in Water Using Gold Nanoparticles. Anal. Chem. 2015, 87, 5148–5155. [Google Scholar] [CrossRef] [PubMed]
- Kalate Bojdi, M.; Behbahani, M.; Omidi, F.; Hesam, G. Application of a novel electrochemical sensor based on modified siliceous mesocellular foam for electrochemical detection of ultra-trace amounts of mercury ions. New J. Chem. 2016, 40, 4519–4527. [Google Scholar] [CrossRef]
- Hsu, I.H.; Hsu, T.-C.; Sun, Y.-C. Gold-nanoparticle-based graphite furnace atomic absorption spectrometry amplification and magnetic separation method for sensitive detection of mercuric ions. Biosens. Bioelectron. 2011, 26, 4605–4609. [Google Scholar] [CrossRef]
- Chen, H.; Chen, J.; Jin, X.; Wei, D. Determination of trace mercury species by high performance liquid chromatography–inductively coupled plasma mass spectrometry after cloud point extraction. J. Hazard. Mater. 2009, 172, 1282–1287. [Google Scholar] [CrossRef]
- Suvarapu, L.N.; Seo, Y.-K.; Baek, S.-O. Speciation and determination of mercury by various analytical techniques. J. Anal. Methods Chem. 2013, 32, 225–245. [Google Scholar] [CrossRef]
- Thirumalai, M.; Kumar, S.N.; Prabhakaran, D.; Sivaraman, N.; Maheswari, M.A. Dynamically modified C18 silica monolithic column for the rapid determinations of lead, cadmium and mercury ions by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2018, 1569, 62–69. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, Y.; Wang, H.; Tong, Y.; Sheng, X.; Sun, Y.; Zhou, X.; Zhou, Q. Simultaneous enrichment and determination of cadmium and mercury ions using magnetic PAMAM dendrimers as the adsorbents for magnetic solid phase extraction coupled with high performance liquid chromatography. J. Hazard. Mater. 2020, 386, 121658. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Meher, N.; Barman, D.; Parui, R.; Iyer, P.K. Recent development of the fluorescence-based detection of volatile organic compounds: A mechanistic overview. J. Mater. Chem. C 2022, 10, 10224–10254. [Google Scholar] [CrossRef]
- Biswas, S.; Sharma, V.; Kumar, P.; Koner, A.L. Selective sensing of lysosomal iron(III) via three-component fluorescence-based strategy in living cells. Sens. Actuators B Chem. 2018, 260, 460–464. [Google Scholar] [CrossRef]
- Parambil, A.R.U.; Kavyashree, P.; Silswal, A.; Koner, A.L. Water-soluble optical sensors: Keys to detect aluminium in biological environment. RSC Adv. 2022, 12, 13950–13970. [Google Scholar] [CrossRef]
- Dey, N. An anthraimidazoledione-based charge transfer probe for dual mode sensing of calcium ions: Role of the counter ion in signal improvement. J. Mater. Chem. B 2023, 11, 1222–1231. [Google Scholar] [CrossRef]
- Sudheesh, K.V.; Joseph, M.M.; Philips, D.S.; Samanta, A.; Kumar Maiti, K.; Ajayaghosh, A. pH-Controlled Nanoparticles Formation and Tracking of Lysosomal Zinc Ions in Cancer Cells by Fluorescent Carbazole–Bipyridine Conjugates. ChemistrySelect 2018, 3, 2416–2422. [Google Scholar] [CrossRef]
- Shuai, H.; Xiang, C.; Qian, L.; Bin, F.; Xiaohui, L.; Jipeng, D.; Chang, Z.; Jiahui, L.; Wenbin, Z. Fluorescent sensors for detection of mercury: From small molecules to nanoprobes. Dyes Pigm. 2021, 187, 109125. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Li, Z.; Li, K.; Yu, X.-Q. Small molecular fluorescent probes for the detection of lead, cadmium and mercury ions. Coord. Chem. Rev. 2021, 429, 213691. [Google Scholar] [CrossRef]
- Singha, S.; Jun, Y.W.; Sarkar, S.; Ahn, K.H. An Endeavor in the Reaction-Based Approach to Fluorescent Probes for Biorelevant Analytes: Challenges and Achievements. Acc. Chem. Res. 2019, 52, 2571–2581. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Gong, D.; Han, S.-C.; Zhu, X.; Iqbal, A.; Liu, W.; Qin, W.; Guo, H. BODIPY based phenylthiourea derivatives as highly selective MeHg+ and Hg2+ ions fluorescent chemodosimeter and its application to bioimaging. Sens. Actuators B Chem. 2017, 243, 195–202. [Google Scholar] [CrossRef]
- Yang, H.; Han, C.; Zhu, X.; Liu, Y.; Zhang, K.Y.; Liu, S.; Zhao, Q.; Li, F.; Huang, W. Upconversion Luminescent Chemodosimeter Based on NIR Organic Dye for Monitoring Methylmercury In Vivo. Adv. Funct. Mater. 2016, 26, 1945–1953. [Google Scholar] [CrossRef]
- Nolan, E.M.; Lippard, S.J. Tools and Tactics for the Optical Detection of Mercuric Ion. Chem. Rev. 2008, 108, 3443–3480. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Dutta, T.; Koner, A.L. An Enumerated Outlook of Intracellular Micropolarity Using Solvatochromic Organic Fluorescent Probes. ACS Omega 2021, 6, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.C.; Bartlett, J.J.; Pulinilkunnil, T. Lysosomal Biology and Function: Modern View of Cellular Debris Bin. Cells 2020, 9, 1131. [Google Scholar] [CrossRef] [PubMed]
- Saftig, P.; Klumperman, J. Lysosome biogenesis and lysosomal membrane proteins: Trafficking meets function. Nat. Rev. Mol. Cell Biol. 2009, 10, 623–635. [Google Scholar] [CrossRef]
- Bonam, S.R.; Wang, F.; Muller, S. Lysosomes as a therapeutic target. Nat. Rev. Drug Discov. 2019, 18, 923–948. [Google Scholar] [CrossRef]
- Silswal, A.; Kanojiya, A.; Koner, A.L. A Fluorogenic Far Red-Emitting Molecular Viscometer for Ascertaining Lysosomal Stress in Live Cells and Caenorhabditis elegans. Front. Chem. 2022, 10, 72. [Google Scholar] [CrossRef]
- Sun, A. Lysosomal storage disease overview. Ann. Transl. Med. 2018, 6, 476. [Google Scholar] [CrossRef]
- Ballabio, A.; Gieselmann, V. Lysosomal disorders: From storage to cellular damage. Biochim. Biophys. Acta—Mol. Cell Res. 2009, 1793, 684–696. [Google Scholar] [CrossRef]
- Zhang, Z.; Yue, P.; Lu, T.; Wang, Y.; Wei, Y.; Wei, X. Role of lysosomes in physiological activities, diseases, and therapy. J. Hematol. Oncol. 2021, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.-Y.; Zoncu, R. The lysosome as a command-and-control center for cellular metabolism. J. Cell Biol. 2016, 214, 653–664. [Google Scholar] [CrossRef]
- Platt, F.M.; Boland, B.; van der Spoel, A.C. Lysosomal storage disorders: The cellular impact of lysosomal dysfunction. J. Cell Biol. 2012, 199, 723–734. [Google Scholar] [CrossRef]
- Saffi, G.T.; Tang, E.; Mamand, S.; Inpanathan, S.; Fountain, A.; Salmena, L.; Botelho, R.J. Reactive oxygen species prevent lysosome coalescence during PIKfyve inhibition. PLoS ONE 2021, 16, e0259313. [Google Scholar] [CrossRef]
- Sulak, M.; Kursunlu, A.N.; Girgin, B.; Karakuş, Ö.Ö.; Güler, E. A highly selective fluorescent sensor for mercury (II) ion based on Bodipy and Calix[4]arene bearing triazolenaphthylene groups; synthesis and photophysical investigations. J. Photochem. Photobiol. A Chem. 2017, 349, 129–137. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Chen, D.; Wu, D.; Chen, Z.; Zhang, J.; Chen, X.; Liu, S.; Yin, J. A colorimetric and ratiometric fluorescent probe for mercury (II) in lysosome. Sens. Actuators B Chem. 2016, 224, 907–914. [Google Scholar] [CrossRef]
- Ding, J.; Li, H.; Wang, C.; Yang, J.; Xie, Y.; Peng, Q.; Li, Q.; Li, Z. “Turn-On” Fluorescent Probe for Mercury(II): High Selectivity and Sensitivity and New Design Approach by the Adjustment of the π-Bridge. ACS Appl. Mater. Interfaces 2015, 7, 11369–11376. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Meng, X.; Wang, L.; Zhou, J.; Qin, D.; Duan, H. A Naphthalimide-Based Fluorescent Probe for the Detection and Imaging of Mercury Ions in Living Cells. ChemistryOpen 2021, 10, 1116–1122. [Google Scholar] [CrossRef]
- Li, X.-H.; Han, X.-F.; Wu, W.-N.; Wang, Y.; Fan, Y.-C.; Zhao, X.-L.; Xu, Z.-H. Simple thiosemicarbazone “switch” sensing of Hg2+ and biothiols in pure aqueous solutions and application to imaging in lysosomes. J. Mol. Struct. 2022, 1250, 131811. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, D.-X.; Zhu, L.-N.; Lan, Y.-L.; Cheng, M.; Zhang, L.-M.; Chu, J.-Q.; Li, X.-Z.; Kong, D.-M. Dinuclear HgII tetracarbene complex-triggered aggregation-induced emission for rapid and selective sensing of Hg2+ and organomercury species. Chem. Sci. 2019, 10, 4220–4226. [Google Scholar] [CrossRef]
- Weil, T.; Vosch, T.; Hofkens, J.; Peneva, K.; Müllen, K. The Rylene Colorant Family—Tailored Nanoemitters for Photonics Research and Applications. Angew. Chem. Int. Ed. 2010, 49, 9068–9093. [Google Scholar] [CrossRef] [PubMed]
- Gopikrishna, P.; Meher, N.; Iyer, P.K. Functional 1,8-Naphthalimide AIE/AIEEgens: Recent Advances and Prospects. ACS Appl. Mater. Interfaces 2018, 10, 12081–12111. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Thilagar, P. Insights into the AIEE of 1,8-Naphthalimides (NPIs): Inverse Effects of Intermolecular Interactions in Solution and Aggregates. Chem. Eur. J. 2014, 20, 8012–8023. [Google Scholar] [CrossRef]
- Biswas, S.; Dutta, T.; Silswal, A.; Bhowal, R.; Chopra, D.; Koner, A.L. Strategic engineering of alkyl spacer length for a pH-tolerant lysosome marker and dual organelle localization. Chem. Sci. 2021, 12, 9630–9644. [Google Scholar] [CrossRef]
- Banerjee, S.; Veale, E.B.; Phelan, C.M.; Murphy, S.A.; Tocci, G.M.; Gillespie, L.J.; Frimannsson, D.O.; Kelly, J.M.; Gunnlaugsson, T. Recent advances in the development of 1,8-naphthalimide based DNA targeting binders, anticancer and fluorescent cellular imaging agents. Chem. Soc. Rev. 2013, 42, 1601–1618. [Google Scholar] [CrossRef]
- Sheng, Y.; Ma, J.; Liu, S.; Wang, Y.; Zhu, C.; Cheng, Y. Strong and Reversible Circularly Polarized Luminescence Emission of a Chiral 1,8-Naphthalimide Fluorophore Induced by Excimer Emission and Orderly Aggregation. Chem. Eur. J. 2016, 22, 9519–9522. [Google Scholar] [CrossRef]
- Do, T.T.; Pham, H.D.; Manzhos, S.; Bell, J.M.; Sonar, P. Molecular Engineering Strategy for High Efficiency Fullerene-Free Organic Solar Cells Using Conjugated 1,8-Naphthalimide and Fluorenone Building Blocks. ACS Appl. Mater. Interfaces 2017, 9, 16967–16976. [Google Scholar] [CrossRef]
- Masimukku, N.; Gudeika, D.; Volyniuk, D.; Bezvikonnyi, O.; Simokaitiene, J.; Matulis, V.; Lyakhov, D.; Azovskyi, V.; Gražulevičius, J.V. Bipolar 1,8-naphthalimides showing high electron mobility and red AIE-active TADF for OLED applications. Phys. Chem. Chem. Phys. 2022, 24, 5070–5082. [Google Scholar] [CrossRef] [PubMed]
- Mahato, S.K.; Bhattacherjee, D.; Bhabak, K.P. The biothiol-triggered organotrisulfide-based self-immolative fluorogenic donors of hydrogen sulfide enable lysosomal trafficking. Chem. Commun. 2020, 56, 7769–7772. [Google Scholar] [CrossRef]
- Verma, M.; Kaur, N.; Singh, N. Naphthalimide-Based DNA-Coupled Hybrid Assembly for Sensing Dipicolinic Acid: A Biomarker for Bacillus anthracis Spores. Langmuir 2018, 34, 6591–6600. [Google Scholar] [CrossRef]
- Dong, H.-Q.; Wei, T.-B.; Ma, X.-Q.; Yang, Q.-Y.; Zhang, Y.-F.; Sun, Y.-J.; Shi, B.-B.; Yao, H.; Zhang, Y.-M.; Lin, Q. 1,8-Naphthalimide-based fluorescent chemosensors: Recent advances and perspectives. J. Mater. Chem. C 2020, 8, 13501–13529. [Google Scholar] [CrossRef]
- Yu, H.; Guo, Y.; Zhu, W.; Havener, K.; Zheng, X. Recent advances in 1,8-naphthalimide-based small-molecule fluorescent probes for organelles imaging and tracking in living cells. Coord. Chem. Rev. 2021, 444, 214019. [Google Scholar] [CrossRef]
- Dai, Z.-R.; Ge, G.-B.; Feng, L.; Ning, J.; Hu, L.-H.; Jin, Q.; Wang, D.-D.; Lv, X.; Dou, T.-Y.; Cui, J.-N.; et al. A Highly Selective Ratiometric Two-Photon Fluorescent Probe for Human Cytochrome P450 1A. J. Am. Chem. Soc. 2015, 137, 14488–14495. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Biswas, S.; Koner, A.L. Fast tyrosinase detection in early stage melanoma with nanomolar sensitivity using a naphthalimide-based fluorescent read-out probe. New J. Chem. 2020, 44, 10771–10775. [Google Scholar] [CrossRef]
- Silswal, A.; Weslie, P.; Koner, A.L. Bioimaging of labile lysosomal iron through naphthalimide-based fluorescent probe. Talanta 2023, 254, 124147. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, R.; Dutta, T.; Nema, S.; Koner, A.L. Detection of Lysosomal Hg2+ Using a pH-Independent Naphthalene Monoimide-Based Fluoroprobe. Chemosensors 2023, 11, 184. https://doi.org/10.3390/chemosensors11030184
Roy R, Dutta T, Nema S, Koner AL. Detection of Lysosomal Hg2+ Using a pH-Independent Naphthalene Monoimide-Based Fluoroprobe. Chemosensors. 2023; 11(3):184. https://doi.org/10.3390/chemosensors11030184
Chicago/Turabian StyleRoy, Rupam, Tanoy Dutta, Shruti Nema, and Apurba Lal Koner. 2023. "Detection of Lysosomal Hg2+ Using a pH-Independent Naphthalene Monoimide-Based Fluoroprobe" Chemosensors 11, no. 3: 184. https://doi.org/10.3390/chemosensors11030184
APA StyleRoy, R., Dutta, T., Nema, S., & Koner, A. L. (2023). Detection of Lysosomal Hg2+ Using a pH-Independent Naphthalene Monoimide-Based Fluoroprobe. Chemosensors, 11(3), 184. https://doi.org/10.3390/chemosensors11030184






