Abstract
The degradation process of virgin olive oil (VOO) is related to storage time and the type of storage container used. The aim of this work is to explore the evolution of the VOO quality stored in different container types over a defined storage period in order to predict the organoleptic characteristics using a non-destructive technique such as the electronic-nose (E-nose). The “Picual” variety VOO was stored in different containers over a period of 21 months and monitored using sensory analysis, volatile compounds, and an E-nose. The panelists showed that oil stored in dark glass bottles and in green polyethylene bottles began to show defects after 12 and 15 weeks, respectively. However, oil stored in tin containers retained its quality throughout the 21 months studied. A total of 31 volatile compounds were identified, and the evolution of the volatile profile in the different containers during the storage period was studied. The E-nose data were able to classify oil quality by container using principal component analysis (PCA). Furthermore, the E-nose data combined with partial least squares (PLS) regression enabled the building of a predictive model to quantify sensory defect values ( = 0.92; = 0.86), evidencing that this technique would be an appropriate screening tool to support a sensory panel.
1. Introduction
Virgin olive oil is considered to be of great importance among the agricultural products that make up the Mediterranean diet. It is characterized by a high nutritional value and sensory qualities [1]. Currently, the quality of olive oil is regulated by European legislation (EC), the International Olive Oil Council (IOC), and the Codex Alimentarius. The Commission Regulation [2] has established different categories of olive oil, with extra virgin olive oil (EVOO) considered to be of the highest quality based on analytical parameters and sensory criteria and is, therefore, placed in the highest commercial category.
The quality of olive oil and its shelf life is greatly influenced by different aspects such as agronomical conditions, raw materials, harvesting, and fruit storage [3]. In addition, storage time and packaging type are two further critical factors that determine quality [4]. Quality decreases during storage, and one of the principal factors to affect it is oxidation, the intensity of which depends on the antioxidant activity of the product [5]. Auto-oxidation, photo-oxygenation, or photo-oxidation are processes that are difficult to control. Auto-oxidation takes place when oxidation occurs in the absence of light, which follows a free radical mechanism, where hydroperoxides are formed due to the absorption of oxygen. Once EVOO is exposed to light, photo-oxidation occurs through natural photosensitizers that react with triplet oxygen to form singlet oxygen in the excited state [6]. Flavor, aroma, and quality can be affected as a result of the oxidation of lipids to hydroperoxides. This results in ketones, aldehydes, acids, and alcohols. The influence of light transmission and the effect of temperature on physico-chemical quality parameters, sensory attributes, and the shelf life of EVOO has been studied [5,7,8,9]. The deterioration of EVOO is caused by different variables such as oxygen, light, and temperature that cause oxidative and hydrolytic reactions. This research has shown that EVOO can have a shelf life of between 12 and 18 months if the different variables are controlled [10]. Thus, to minimize oxidation processes, different containers have been proposed to protect olive oil from both oxygen and light. Industrialists use different types of containers made from dark packaging that can prevent the penetration of light and, in turn, prevent the oxidation process [5,6,7,9]. As a result, the Royal Decree 760/2021 of 31 August [11], which approves the quality standard for olive and olive-pomace oils, advises to protect oils from oxidative processes with regard to the product within which it is packaged.
The most commonly used techniques to identify and quantify volatile compounds are a panel test and gas chromatography. These procedures of analysis require specialized technical personnel and long laboratory analysis times to determine the quality of the stored oil. Therefore, an alternative to these traditional methods is an artificial olfactory system such as the electronic nose (E-nose). This non-destructive device represents a powerful sensory tool that enables the successful qualitative and quantitative evaluation of the volatile compounds of the product. An E-nose has been used during the elaboration process or in the final product in several industrial sectors. There are some references of its use related to olives and table olive products [12,13] in the olive sector. In the case of olive oil, this tool has previously been used to discriminate VOO with varying qualities with high accuracy (67–77%) [14] and to differentiate between VOO with different sensory qualities obtained from healthy and infested olives [15]. With the use of an E-nose, Martínez et al. [16] predicted the olive oil quality based on the fruit characteristic after harvest. Moreover, studies have demonstrated the capability of the E-nose in the monitoring of the evolution of volatile compounds during storage [17]. An E-nose consisting of 12 metal oxide semiconductor (MOS) sensors and 10 metal oxide semiconductor field-effect transistors (MOS-FET) was able to discriminate olive oil samples based on aging time, and the E-nose data correlated strongly with peroxide values [18]. Other authors have studied the use of the E-nose for the evaluation of rancidity in virgin olive oils, to monitor the shelf life of bottled virgin olive oils [19] or to predict the intensity of the fruity aroma and off-flavors in VOOs [20]. However, to the best of our knowledge, there are no studies of the use of such electronic devices to monitor the storage of olive oil in different containers and to quantify any defect perceived.
The aim of this work was to discriminate the quality of virgin olive oils kept in different containers during the storage period by using an E-nose and validating the results using sensory and gas chromatography analysis.
2. Materials and Methods
2.1. EVOO Samples
Olives (Olea europaea L.) from an experimental field of the “Picual” variety were harvested in the southwest of Extremadura (Spain) at the end of October at the veraison stages of maturation. Olives were transported to a mill to be submitted to the industrial elaboration process by a local oil producer (Pieralisi, Jesi AN, Italy), following the methodology proposed by Martín-Tornero et al. [21]. The VOO was then bottled into three containers of 250 mL capacity: (i) opaque crystal bottles (Dark crystal); (ii) opaque green polyethylene bottles (Green PET); and (iii) tin containers (Tinplate). The samples were stored in the laboratory at 20 °C. EVOO samples were taken every three months (t0–t6) over a period of 21 months.
2.2. Analyses
2.2.1. Sensory Analysis
The sensory analysis was evaluated by a group of 12 panelists specially trained to describe the taste and odor properties of VOO. The panel belongs to the Scientific and Technological Research Center of Extremadura (CICYTEX). The evaluation was carried out in a tasting room, where 15 mL of each sample was placed into standard tasting glasses covered with a watch glass and placed on a heating block at 28 ± 2 °C [2]. The descriptive profile was assessed using a test sheet, with each attribute scored using an unstructured scale of 10 cm. Olfactory aspects were assessed in this study. The intensity of the fruitiness and the main perceived defect (rancidity) of VOO were evaluated. The results of the sensory evaluation were expressed as mean values for each attribute studied. The coefficient of variation such as the standard deviation of the sample divided by the mean values and multiplied by 100, was also calculated in each sensory evaluation. When this value was less than 20%, the results were considered valid.
2.2.2. Volatile Compound Analysis
Volatile compounds were analyzed by gas chromatography previous static headspace sampling with solid phase microextraction [12,22]. Aliquots of 2.0 g of VOO were placed in glass vials capped with polytetrafluoroethylene septum. A polydimethylsiloxane/divinylbenzene (PDMS/DVB) StableFlex fiber (65 μm, Supelco, Madrid, Spain) was manually inserted into the vials at 40 °C for 30 min for headspace removal. After extraction, desorption was carried out, whereby the fiber was introduced into the injection port of the GC at 250 °C for 40 min. Analyses were performed using a Bruker Scion 456-GC triple quadrupole gas chromatograph with a capillary column (DB WAXETR, 60 m × 0.25 mm; ID: 0.25 mm). The compound identification was carried out in accordance with the NIST standard reference database through mass spectra.
2.2.3. Electronic Nose
The miniaturized electronic device (with an electronic board measuring 39 mm × 33 mm) was used in this study, and it was designed and developed in-house by the Department of Electrical, Electronic and Automatic Engineering of the University of Extremadura (Spain) [23]. The electronic device is shown in Figure 1 and has the desired features of portable equipment: it has very low power consumption (185 mA), it is powered by a 3.7 VDC and 2000 mAh rechargeable LiPo battery, it is lightweight and small in size, and can transfer data wirelessly to a smartphone. All these features make it manageable when taking measurements from the tasting glasses. The E-nose board has two different power supplies (+3.3 VDC and +1.8 VDC) for the different sensors and is governed by a powerful microcontroller (PIC32MM0256GPM048 from Microchip Technology Inc., Chandler, AZ, USA). Among other features, this microcontroller has been chosen because of its memory (256 KB of program memory and 32 KB of data memory) and its communications modules (several I2C and UART). The E-nose also has a Bluetooth module (Microchip’s RN4871), a UART port serial communication, and a battery charger via micro-USB-B connector. The sensors used (Sánchez et al., 2021a) are grouped into an array of 11 Metal Oxide Semiconductor (MOS) sensors inserted into four chips with integrated analog-to-digital converter and I2C bus communications: BME680 from Bosch Sensortec GmbH (Reutlingen, Germany), SGP30 from Sensirion AG (Stäfa, Switzerland) and CCS811 and iAQ-Core from ScioSense B.V. (AE Eindhoven, The Netherlands). These sensors send a total of 14 signals, distributed as follows:
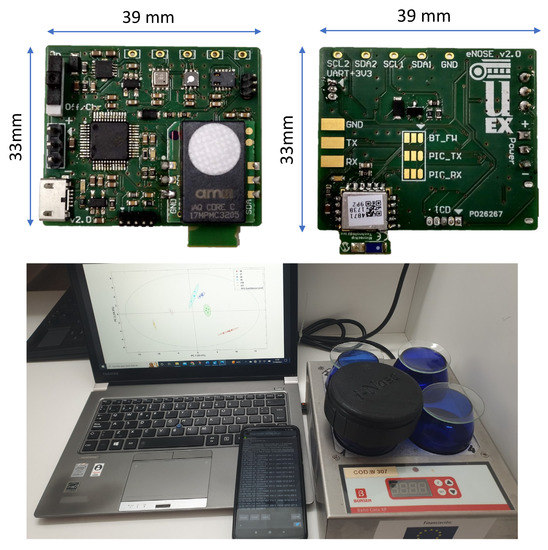
Figure 1.
E-nose electronic board (view from front and above) and the different components involved in the measurements.
- BME680: ambient temperature (oC), pressure (hPa), relative humidity (% RH), and gas measurement (Ω).
- SGP30: equivalent CO2 concentration (eCO2) (ppm), total volatile organic compounds concentration (TVOC) (ppb), and the raw measurements of H2 and ethanol.
- CCS811: eCO2 (ppm), TVOC (ppb), and sensor resistance (Ω).
- iAQ-Core: eCO2 (ppm), TVOC (ppb), and sensor resistance (Ω).
The same procedure described for the sensory analysis was followed to carry out the E-nose analysis of the VOO samples. The samples selected for each sampling date during the shelf life were frozen at −80 °C until the time of analysis. Samples were then subjected to a cold defrost for 48 h and placed into the tasting glass as previously described in the sensory analysis epigraph. The E-nose data consisted of a static headspace adsorption phase, where the aroma of the samples was put into contact with the E-nose sensors for 60 s. The E-nose was then placed into a tasting glass containing a silica gel cup for 30 s until the desorption phase took place and to bring the gas sensor signal back to the baseline. The operational temperature of the sensors (around 400 °C) was optimized for this application and selected through the user interface. The E-nose recorded data at one second intervals and the system took a reading of the resistive value supplied by each sensor. The E-nose measurements were made randomly on the tasting glasses, making ten measurements for each VOO sample. The values detected by the sensors were read by a microprocessor, which sent them to a smartphone via Bluetooth using an ASCII-based protocol. All signals were sent at the same time separated by tabs in a single row, and each measurement was sent every second, resulting in a table in which each column corresponded to a signal and each row to a measurement. An Android application with an easy user interface was developed to fetch and save this data for further processing. The resulting data were then sent to a computer and organized into columns in a spreadsheet for chemometric analysis.
2.3. Statistical Analysis
A one-way ANOVA was used followed by Tukey’s multiple range test of sensorial analysis data obtained by panelists to establish statistically significant differences between the VOO samples stored for 21 months in the different containers studied. For this statistical analysis, SPSS software Version 18.0 was used (SPSS Inc., Chicago, IL, USA). The level of significance was set at p < 0.05. The results were considered as mean values with their standard deviations.
With reference to the E-nose data, a vector of data with 11 columns (one for each gas sensor) was generated for each sample using the relative resistance feature extraction method. The data were processed using an unsupervised exploratory principal component analysis (PCA) [24]. This analysis was used to detect outliers and to evaluate if the samples were grouped according to their respective volatile compounds. By performing a PCA, a reduction in the dimension of the input variables could be carried out, thereby obtaining principal components that were linear combinations of original response vectors. Since the studied variables were measured according to different units, the original variables were auto scaled.
The defects perceived by the tasting panel could be determined and quantified by using the partial least squares (PLS) method [25]. A total of 18 samples were used to establish the PLS model. The model performance was set using a calibration set which included 70% of the samples. The accuracy and robustness of the model were validated with 30% of the samples.
Data analysis was performed using Matlab R2016 Version 9.1 (The Mathworks Inc., Natick, MA, USA) with PLS_Toolbox 8.2.1 (Eigenvector Research Inc., Wenatchee, WA, USA).
3. Results and Discussion
Figure 2 shows a diagram of the general study workflow. The process commenced with the organoleptic evaluation by a tasting panel, followed by the gas chromatographic analysis. An electronic device was then used to obtain data which were chemometrically treated to discriminate between storage times and to quantify the organoleptic defects in the olive samples.
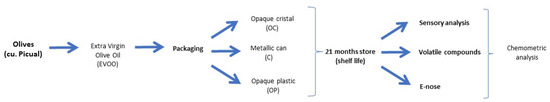
Figure 2.
Study workflow.
3.1. Sensory Aroma of Olive Oils
A tasting panel evaluated the sensory attributes of VOO in accordance with the official method, and positive and/or negative sensory attributes were rated. Positive sensory attributes related to fruity odor, while negative sensory attributes related to the principal defect, namely rancidity (Table 1).

Table 1.
Most common sensory attributes identified through panel testing (mean ± standard deviation), split by commercial category of EVOO and packaging in different container types over 21 months of storage. The small letters refer to significant statistical differences (Tukey’s Test, p < 0.05) according to the storage time (t0–t6) for each container type. The capital letters represent significant statistical differences (Tukey’s Test, p < 0.05) according to the different container types (dark crystal, green PET and Tinplate) relative to storage time (t0–t6).
At t0, all samples were classified into the highest commercial category, EVOO, as a result of the positive attributes and the lack of defects identified. Olive oil stored in Tinplate containers presented the highest proportion of positive attributes during the conservation period. The intensity of fruitiness decreased for all packaging types during storage time. This decrease was slightly higher in oil packaged in dark crystal bottles and green PET containers. Polyethylene can adsorb considerable amounts of aroma compounds, resulting in the loss of aroma intensity with an unbalanced flavor [26].
The negative attributes shown in Table 1 also demonstrate that defects were detected during storage time in two of the three assessed packaging types. EVOO stored in Tinplate containers showed no defects during the 21 months of storage. In contrast, the dark crystal and green PET containers showed defects related to rancidity at t5 and t6, respectively. These defects resulted in the olive oil changing its commercial category from EVOO to VOO. Furthermore, at the end of the storage time (t6), the quality of the olive oil stored in dark crystal bottles decreased to the lowest commercial category: lampante olive oil (LOO). The Tinplate containers avoided these defects as they provided total protection against light, oxygen, and water vapor, thereby allowing the olive oil to maintain the highest commercial category: EVOO. Transparent or translucent containers could not protect the olive oil from photo-oxidation, hence the presence of the rancid aroma. According to Flori et al. [27] a longer shelf life is obtained during storage at a lower temperature. They also suggested that the best packaging types to maintain the storage stability of olive oil are containers of tin, stainless-steel, and dark glass.
3.2. Gas Chromatographic Analysis of Volatile Compounds
The volatile organic compounds (VOCs) that contribute to taste and flavor belong to several chemical classes, namely aldehydes, alcohols, hydrocarbons, ethers, esters, carboxylic acids, and ketones [28]. Those most responsible for the off-flavors in olive oils are aldehydes, ketones, and alcohols. In this research, the olive oil samples analyzed by gas chromatographic and the evolution of the contents of the different VOCs during storage time in the three containers assayed are summarized in Table S1.
In total, 31 compounds were identified, which were grouped according to different chemical classes (Table 2). At t0, the families most prominent in appearance were aldehydes, alcohols, and esters. During storage, and independently of the container, their prominence decreased, while the appearance of hydrocarbons and carboxylic acids increased.

Table 2.
Chemical distribution of VOCs of oils stored in different containers.
Figure 3 summarizes the evolution of the most prominent VOCs. In relation to aldehydes, (E)-2-hexenal associated with fruity attributes [29,30], is the most common, and its concentration decreased throughout storage in all containers. At t6, the lowest concentrations were found in the dark crystal and green PET containers and the highest in the Tinplate containers (Figure 3A). Hexanal and nonanal were identified as compounds associated with the increase of defects during storage. At t0, the presence of these compounds was not detected in any of the containers, but the hexanal concentration increased in Dark glass bottles from t4 and in Green PET from t5. However, they were not detected in the Tinplate containers over the storage time. These aldehydes are produced by the oxidation of unsaturated fatty acids; this leads to a loss of quality and develops a rancid taste and smell [31]. The second family most prominently represented in fresh olive oil was alcohols (Table 2), with a similar percentage to the aldehydes group. (Z)-2-penten-1-ol, (E)-2-hexen-1-ol, and (E)-3-hexen-1-ol are related to fruit and the green odor in fresh oils. The concentration of the first two practically did not vary, but the concentration of (E)-3-hexen-1-ol decreased in all containers (Figure 3B).
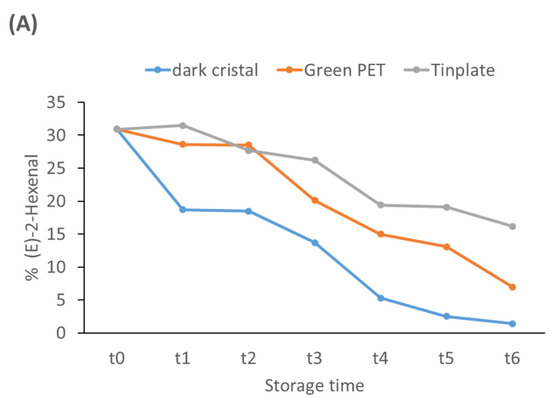
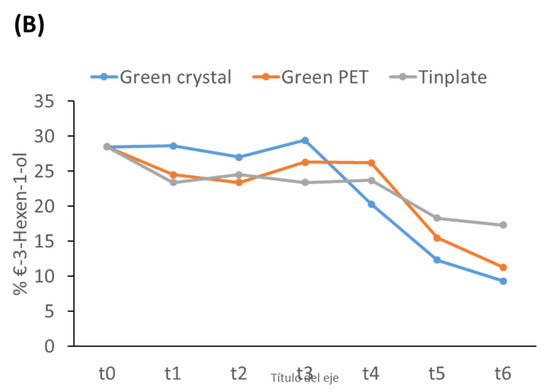
Figure 3.
Evolution of the most prominent VOCs during the storage time. (A) (E)-2-Hexenal; (B) (E)-3-Hexen-1-ol.
Although hydrocarbons have a negligible impact on the odor of olive oils, compounds such as octane and α-farnesene, which contribute to a positive odor with a strawberry aroma [32], appeared in very low proportion in the fresh oil and decreased at the end of the storage time (Table S1). Other hydrocarbons such as 4-methylheptane and 2,4-dimethylhexane, which are related to negative attributes with an unpleasant odor [33], increased slightly during storage, except in the Tinplate containers. In the case of ethers, which contribute to a positive aroma [33], their values did not vary significantly in any of the containers. Similar behavior was observed with esters. As with the previous compounds, they contributed to positive aromas, a green grassy and mild sweet smell [34], and their values did not vary significantly in any of the containers either. Acetic acid, belonging to the group of carboxylic acids and contributing with vinegary and sour sensory attributes [35], significantly increased its concentration in the Dark crystal bottles and the PET containers and slightly in the Tinplate containers. In the case of the ketones group, both 6-methyl-5-hepten-2-one and geranylacetone, which are positive compounds with a green odor [35,36], showed a similar concentration throughout the study (Table S1).
In line with what has been commented on in the study on the evolution of volatiles, the container that best preserved the aroma of the olive oil was Tinplate. (E)-2-hexenal, (E)-3-hexen-1-ol, and 1-hexanol in t6 had a higher percentage representation in this container type and all had a positive aroma. However, hexanal, 2-methyl-2-propanol, 4-methyl-1-(1-methylethyl) cyclohexanol, 4-methylheptane, 3-decyne, 3,5-dimethyloctane, 3-ethyloctane and acetic acid had a negative odor. These VOCs represented the highest percentages in Dark crystal and Green PET containers.
3.3. E-Nose Discrimination of VOO Stored in Different Containers
VOO stored in different containers was analyzed using an E-nose. Through sensor measurements versus time, a response curve measuring responses over one second intervals was generated to characterize the sensor response curves. An algorithm function was then used to extract the data of each sensor in the samples studied: the maximum value of the signal minus the minimum, plus 100, minus one in our case was used to obtain a data vector with 11 rows for each sample as a result of a feature extraction algorithm. Once all measurements were obtained, a baseline manipulation pre-processing was performed following Equation (1).
where Characteristic value is the pre-processed value, Reference is the steady value (average of the last five values) reached during the clean air measurement phase, and Sample is the steady value during the sample measurement phase.
Characteristic value = (Reference/Sample) · 100,
E-nose data of each container type were then individually processed using principal component analysis (PCA) with the objective of exploring whether there were any natural groupings throughout the storage time (Figure 4).
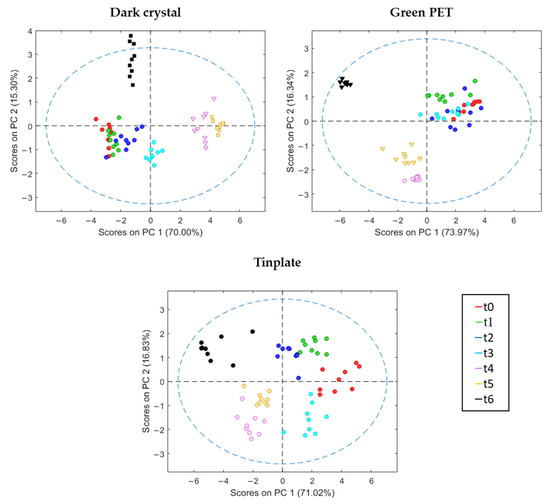
Figure 4.
Score plot of the PCA analysis for oils stored in different containers. Dot: extra virgin olive oil; triangle: virgin olive oil; square: lampante olive oil.
The PCA results showed that more than 70.0% of the total variance of data was explained by only PC1 in all the containers studied. When comparing the two first principal components, a clear progression of the scores over storage time could be observed, regardless of the containers used. This was most notable with the Dark crystal bottles, where four groups of samples correlated with the storage time (Figure 3). The first group, with negative scores for both components, included samples of t0, t1, and t2; the second (positive values for PC1 and negative values for PC2) with t3 samples; the third (positive and negative scores for PC1 and PC2) with samples of t4 and t5; and the fourth (negative scores for PC1 and positive scores for PC2) with samples of t6. Groups three and four clearly differed from the other two. These results support the sensory analysis carried out by the tasting panel, which showed that in the last three samplings (t4–t6), the oils lost fruity aromatic intensity, defects increased, and the Extra category was lost.
In the case of the Green PET bottles, only three different groups can be observed in the PCA scores biplot (Figure 3): the first group (positive scores values for PC1 and PC2) with samples from t0 to t3, the second group (negative scores for both PC1 and PC2) with t4 and t5 samples, and the third group (negative scores for PC1 and positive scores for PC2) with samples of t6. In this container, and in line with the results of the sensorial analysis, samples from t0 to t3 continued to maintain the extra category (EVOO). However, the E-nose classified samples from t4 as VOO, while the tasting panel still classified t4 as EVOO. This contradiction could be considered as supportive of the fact that many authors have questioned the accuracy of human panels and indicate that they should be aided by instrumental tools [37].
Lastly, in the case of olive oil samples conserved in Tinplate, although the same three groups could be observed as with the Green PET bottles, the differences between them were not significant, except for the samples t6. In this case, samples did not show any variation over storage time, in line with the sensorial analysis and, as a result, all samples were categorized as EVOO.
It is notable that the behavior of the t6 samples was very similar in all containers, with identical score values (negative scores for PC1 and positive scores for PC2), corresponding to a loss of quality in all cases. Samples from the Tinplate containers, as concluded by the tasting panel, were still considered to be of EVOO category, although the intensity of the fruitiness had decreased (Table 1). However, the results obtained from the E-nose indicated that the quality of EVOO had significantly decreased. Thus, these results suggest once again that the electronic device provided better results than those provided by sensory analysis.
Partial least squares (PLS) regression was selected to establish a quantitative model consisting of the E-nose data obtained from VOO stored in different containers and the known defects identified by the tasting panel (Figure 5). A calibration set (18 samples) was used to build the model and to perform the cross validation. Through Haaland and Thomas’s criterion [38], the optimal number of components was taken. This occurred when the value of PRESS did not statistically differ from the minimum value of PRESS. The optimum number of latent variables was 3. The coefficient of determination in the cross validation was 0.92, while low RMSECV values of 0.65 were also obtained. The model was validated across the validation set (12 samples). The validation parameters were acceptable, obtaining a value of 0.86 for perceived negative aroma, while a value of 0.54 was obtained for RMSEP.
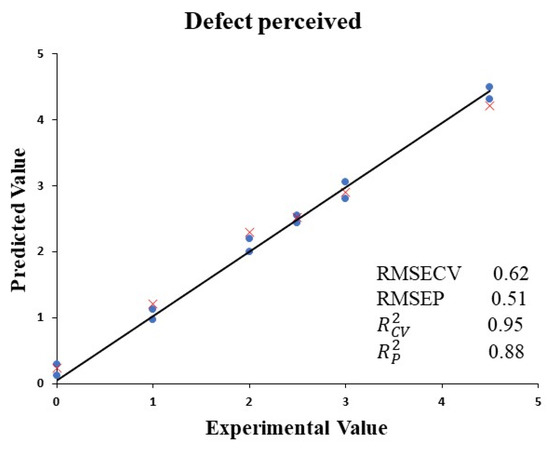
Figure 5.
Experimental values against PLS cross-validation predictions (●) and validation set predictions (×) for VOO defects identified by panelists.
These initial results indicate that this model enables the intensity of VOO defects to be quantified, which could be used by the oleic sector to evaluate the quality of its product during storage. Similar models have been established in other matrices to detect fermentation defects [12,13] in the olive growing sector, to classify oils according to their quality [14,21] or to detect the presence of unpleasant cooking odors in Californian-style black olives [39,40]. This information will allow producers to recognize changes in the quality of the product and to classify it accordingly. This model could predict incipient alterations to the oil that may occur during its conservation through continuous analysis using electronic equipment. These results also demonstrate the accuracy and high capacity of the E-nose to determine the quality of the VOO. The outcomes show that the E-nose is able to differentiate oils according to their aroma. These findings are consistent with those obtained from the sensorial analysis (Table 1) and volatile organic compounds (Figure 2 and Table 2), differentiating between VOO quality and confirming the suitability of the E-nose to be used in this sector. This is an interesting result given that this tool could be useful at industrial level in detecting positive and negative attributes after the elaboration process. Therefore, oils could be marketed with a higher quality.
4. Conclusions
When preserving EVOO at room temperature, the extent of its quality during storage is dependent on the type of container used. EVOO loses its quality after 21 months of storage but remains in a better condition in a tinplate container (EVOO) than in dark glass bottles (VOO or LOO). The oil stored in green PET bottle containers was classified as VOO and in dark glass bottle containers as LOO. However, positive aromas started to be lost in all of them and, therefore, defects related to rancidity appeared. The volatile compounds related to the positive aroma of the oil were: (E)-2-hexenal, (E)-3-hexen-1-ol and (Z)-3-hexen-1-ol, acetate. Those related to negative aromas were hexanal, 2-methyl-2-propanol, and acetic acid. The E-nose differentiated the aroma perceived of the VOO in different groups, demonstrating it to be a powerful tool with analytical capabilities. The results provided by the tasting panel and the volatile compounds profile matched the classification provided by this device. This proves that, combined with chemometric tools, and as an auxiliary tool for a tasting panel, the E-nose represents a fast, simple, reliable, and low-cost method suitable for use at industrial level to control the quality of this product.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors11020085/s1, Table S1:Content of volatile compounds (mean %, n = 3) obtained of VOO packed in different containers during 21 months of storage. R.T.: retention time.
Author Contributions
Conceptualization, E.M.-T., I.D.-M. and D.M.-V.; data curation, J.D.B.-R., J.L. and D.M.-V.; formal analysis, J.D.B.-R., E.M.-T., I.D.-M. and D.M.-V.; funding acquisition, I.D.-M., J.L. and D.M.-V.; investigation, all the authors; methodology, E.M.-T., I.D.-M., J.L. and D.M.-V.; project administration, I.D.-M. and D.M.-V.; resources, I.D.-M. and D.M.-V.; supervision, I.D.-M., J.L. and D.M.-V.; validation, all the authors.; visualization, J.D.B.-R. and D.M.-V.; writing—original draft, J.D.B.-R. and D.M.-V.; writing—review and editing, all the authors. All authors have read and agreed to the published version of the manuscript.
Funding
E. Martín-Tornero and I. Durán-Merán wish to thank the Ministerio de Ciencia e Innovación de España (Project PID2020-112996GB-I00 funded by MCIN/AEI/10.13039/501100011033) and Junta de Extremadura (Ayuda a Grupos ref. GR21048 and Project IB20016) co-financed by European Funds for Regional Development. D. Martín-Vertedor also thanks the Junta de Extremadura (Ayuda a Grupos ref. GR21121) co-financed by European Funds for Regional Development.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and the raw data that support the findings are available from the corresponding author, upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Inarejos-García, A.M.; Santacatterina, M.; Salvador, M.D.; Fregapane, G.; Gómez-Alonso, S. PDO virgin olive oil quality—Minor components and organoleptic evaluation. Food Res. Int. 2010, 43, 2138–2146. [Google Scholar] [CrossRef]
- Commission Regulation (EEC) No 2568/91 of 11 July 1991. Available online: https://eur-lex.europa.eu/legal-content/ES/TXT/PDF/?uri=CELEX:01991R2568-20151016&from=EN (accessed on 1 April 2022).
- Martín-Tornero, E.; Fernández, A.; Pérez-Rodríguez, J.M.; Durán-Merás, I.; Prieto, M.H.; Martín-Vertedor, D. Non-destructive fluorescence spectroscopy as a tool for discriminating between olive oils according to agronomic practices and for assessing quality parameters. Food Anal. Methods 2022, 15, 253–265. [Google Scholar] [CrossRef]
- Stefanoudaki, E.; Williams, M.; Harwood, J. Changes in virgin olive oil characteristics during different storage conditions. Eur. J. Lipid Sci. Technol. 2010, 112, 906–914. [Google Scholar] [CrossRef]
- Pristouri, G.; Badeka, A.; Kontominas, M.G. Effect of packaging material headspace, oxygen and light transmission, temperature and storage time on quality characteristics of extra virgin olive oil. Food Control 2010, 21, 412–418. [Google Scholar] [CrossRef]
- Gargouri, B.; Zribi, A.; Bouaziz, M. Effect of containers on the quality of Chemlali olive oil during storage. J. Food Sci. Technol. 2015, 52, 1948–1959. [Google Scholar] [CrossRef]
- Caponio, F.; Bilancia, M.T.; Pasqualone, A.; Sikorska, E.; Gomes, T. Influence of the exposure to light on extra virgin olive oil quality during storage. Eur. Food Res. Technol. 2005, 221, 92–98. [Google Scholar] [CrossRef]
- Méndez, A.I.; Falqué, E. Effect of storage time and container type on the quality of extra-virgin olive oil. Food Control 2007, 18, 521–529. [Google Scholar] [CrossRef]
- Cecchi, T.; Passamonti, P.; Cecchi, P. Study of the quality of extra virgin olive oil stored in PET bottles with or without an oxygen scavenger. Food Chem. 2010, 120, 730–735. [Google Scholar] [CrossRef]
- Cicerale, S.; Conlan, X.A.; Barnett, N.W.; Keast, R.S. Storage of extra virgin olive oil and its effect on the biological activity and concentration of oleocanthal. Food Res. Int. 2013, 50, 597–602. [Google Scholar] [CrossRef]
- Royal Decree 760/2021, de 31 de August. Available online: https://www.boe.es/buscar/pdf/2021/BOE-A-2021-14318-consolidado.pdf (accessed on 15 October 2022).
- Sánchez, R.; Martín-Tornero, E.; Lozano, J.; Boselli, E.; Arroyo, P.; Meléndez, F.; Martín-Vertedor, D. E-Nose discrimination of abnormal fermentations in Spanish-Style Green Olives. Molecules 2021, 26, 5353. [Google Scholar] [CrossRef]
- Sánchez, R.; Boselli, E.; Fernández, A.; Arroyo, P.; Lozano, J.; Martín-Vertedor, D. Determination of the Masking Effect of the ‘Zapateria’ Defect in Flavoured Stuffed Olives Using E-Nose. Molecules 2022, 27, 4300. [Google Scholar] [CrossRef] [PubMed]
- Oates, M.J.; Fox, P.; Sanchez-Rodriguez, L.; Carbonell-Barrachina, Á.A.; Ruiz-Canales, A. DFT based classification of olive oil type using a sinusoidally heated, low cost electronic nose. Comput. Electron. Agric. 2018, 155, 348–358. [Google Scholar] [CrossRef]
- Kishimoto, N.; Kashiwagi, A. Prediction of Specific Odor Markers in Oil from Olive Fruit Infested with Olive Scale Using an Electronic Nose. In Proceedings of the IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Fukuoka, Japan, 26–29 May 2019; pp. 1–3. [Google Scholar]
- Martínez, D.M.; Gámez, J.; Bellincontro, A.; Mencarelli, F.; Gómez, J. Fast tool based on electronic nose to predict olive fruit quality after harvest. Postharvest Biol. Technol. 2020, 160, 111058. [Google Scholar] [CrossRef]
- Modesti, M.; Taglieri, I.; Bianchi, A.; Tonacci, A.; Sansone, F.; Bellincontro, A.; Venturi, F.; Sanmartin, C. E-nose and Olfactory assessment: Teamwork or a challenge to the last data? The case of virgin olive oil stability and shelf life. Appl. Sci. 2021, 11, 8453. [Google Scholar] [CrossRef]
- Buratti, S.; Benedetti, S.; Cosio, S. An Electronic Nose to Evaluate Olive Oil Oxidation during Storage. Ital. J. Food Sci. 2005, 17, 203–210. [Google Scholar]
- Battimo, I.; Savarese, M.; Parisini, C.; Malagrinò, G.; Paduano, A.; Marco, E.D.; Sacchi, R. Rancidity Evaluation and Shelf-Life Monitoring of Virgin Olive Oil by Electronic-Nose. Ital. J. Food Sci. 2006, 18, 278–287. [Google Scholar]
- Marchal, P.C.; Sanmartin, C.; Martínez, S.S.; Ortega, J.G.; Mencarelli, F.; García, J.G. Prediction of Fruity Aroma Intensity and Defect Presence in Virgin Olive Oil Using an Electronic Nose. Sensors 2021, 21, 2298. [Google Scholar] [CrossRef]
- Martín-Tornero, E.; Fernández, A.; Durán-Merás, I.; Martín-Vertedor, D. Fluorescence Monitoring Oxidation of Extra Virgin Olive Oil Packed in Different Containers. Molecules 2022, 27, 7254. [Google Scholar] [CrossRef]
- López-López, A.; Cortés-Delgado, A.; de Castro, A.; Sánchez, A.H.; Montaño, A. Changes in volatile composition during the processing and storage of black ripe olives. Food Res. Int. 2019, 125, 108568. [Google Scholar] [CrossRef]
- Sánchez, R.; Fernández, A.; Martín-Tornero, E.; Meléndez, F.; Lozano, J.; Martín-Vertedor, D. Application of Digital Olfaction for Table Olive Industry. Sensors 2022, 22, 5702. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Geladi, P.; Kowalski, B. Partial least-squares regression: A tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Kontominas, M.G. Olive oil packaging: Recent developments. In Olives and Olive Oil as Functional Foods: Bioactivity, Chemistry and Processing, 1st ed.; Kiritsakis, P., Shahidi, F., Eds.; John Wiley & Sons: New York, NY, USA, 2017; pp. 279–294. [Google Scholar]
- Flori, L.; Donnini, S.; Calderone, V.; Zinnai, A.; Taglieri, I.; Venturi, F.; Testai, L. The nutraceutical value of olive oil and its bioactive constituents on the cardiovascular system. Focusing on main strategies to slow down its quality decay during production and storage. Nutrients 2019, 11, 1962. [Google Scholar] [PubMed]
- Kiritsakis, A.K. Flavor components of olive oil—A review. J. Am. Oil Chem. Soc. 1998, 75, 673–681. [Google Scholar] [CrossRef]
- Meneses, D.A.; Bejarano, A.; Juan, C. Vapor pressure data for ethyl-2-methylbutyrate, hexanal and (E)-2-hexenal at a pressure range of (25 to 190) kPa. J. Chem. Thermodyn. 2014, 74, 16–21. [Google Scholar] [CrossRef]
- Lobo-Prieto, A.; Tena, N.; Aparicio-Ruiz, R.; Morales, M.T.; García-González, D.L. Tracking sensory characteristics of virgin olive oils during storage: Interpretation of their changes from a multiparametric perspective. Molecules 2020, 25, 1686. [Google Scholar] [CrossRef]
- Benincasa, C.; Russo, A.; Romano, E.; Elsorady, M.E.; Perri, E.; Muzzalupo, I. Chemical and sensory analysis of some Egyptian virgin olive oils. J. Nutr. Food Sci. 2011, 5, 118. [Google Scholar] [CrossRef]
- Sánchez, R.; Pérez-Nevado, F.; Martillanes, S.; Montero-Fernández, I.; Lozano, J.; Martín-Vertedor, D. Machine olfaction discrimination of Spanish-style green olives inoculated with spoilage mold species. Food Control 2023, 147, 109600. [Google Scholar] [CrossRef]
- Yu, H.; Seow, Y.X.; Ong, P.K.; Zhou, W. Effects of high-intensity ultrasound and oil type on the Maillard reaction of d-glucose and glycine in oil-in-water systems. NPJ Sci. Food 2018, 2, 2. [Google Scholar] [CrossRef]
- McRae, J.F.; Mainland, J.D.; Jaeger, S.R.; Adipietro, K.A.; Matsunami, H.; Newcomb, R.D. Genetic variation in the odorant receptor OR2J3 is associated with the ability to detect the “grassy” smelling odor, cis-3-hexen-1-ol. Chem. Senses 2012, 37, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Lejková, N.Š.J.S.J.; Kolek, A.P.J.K.E.; Pangallo, Ľ.V.T.K.D. Characterization of May bryndza cheese from various regions in Slovakia based on microbiological, molecular and principal volatile odorants examination. J. Food Nutr. Res. 2015, 54, 239–251. [Google Scholar]
- Garcia-Oliveira, P.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Chamorro, F.; Pereira, A.G.; Carrera-Casais, A.; Fraga-Corral, M.; Carpena, M.; Simal-Gandara, J.; Prieto, M.A. Evolution of flavors in extra virgin olive oil shelf-life. Antioxidants 2021, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Conte, L.; Bendini, A.; Valli, E.; Lucci, P.; Moret, S.; Maquet, A.; Lacoste, F.; Brereton, P.; García-González, D.L.; Moreda, W.; et al. Olive oil quality and authenticity: A review of current EU legislation, standards, relevant methods of analyses, their drawbacks and recommendations for the future. Trends Food Sci. Technol. 2020, 105, 483–493. [Google Scholar] [CrossRef]
- Haaland, D.M.; Thomas, E.V. Partial least-squares methods for spectral analyses. Relation to other quantitative calibration methods and the extraction of qualitative information. Anal. Chem. 1988, 60, 1193–1202. [Google Scholar] [CrossRef]
- Martín-Tornero, E.; Sánchez, R.; Lozano, J.; Martínez, M.; Arroyo, P.; Martín-Vertedor, D. Characterization of polyphenol and volatile fractions of Californian-style black olives and innovative application of E-nose for acrylamide determination. Foods 2021, 10, 2973. [Google Scholar] [CrossRef]
- Sánchez, R.; Martín-Tornero, E.; Lozano, J.; Fernández, A.; Arroyo, P.; Meléndez, F.; Martín-Vertedor, D. Electronic nose application for the discrimination of sterilization treatments applied to Californian-style black olive varieties. J. Sci. Food Agric. 2022, 102, 2232–2241. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).