Discovering a Dihydrofluorescein Analogue as a Promising Fluorescence Substrate to HRP
Abstract
1. Introduction
2. Experimental Method
2.1. Synthesis and Characterization
2.2. Kinetic and Spectroscopy Measurement
2.3. Examination of the Reactivity of DCFH-1 and Amplex Red toward Carboxylesterase
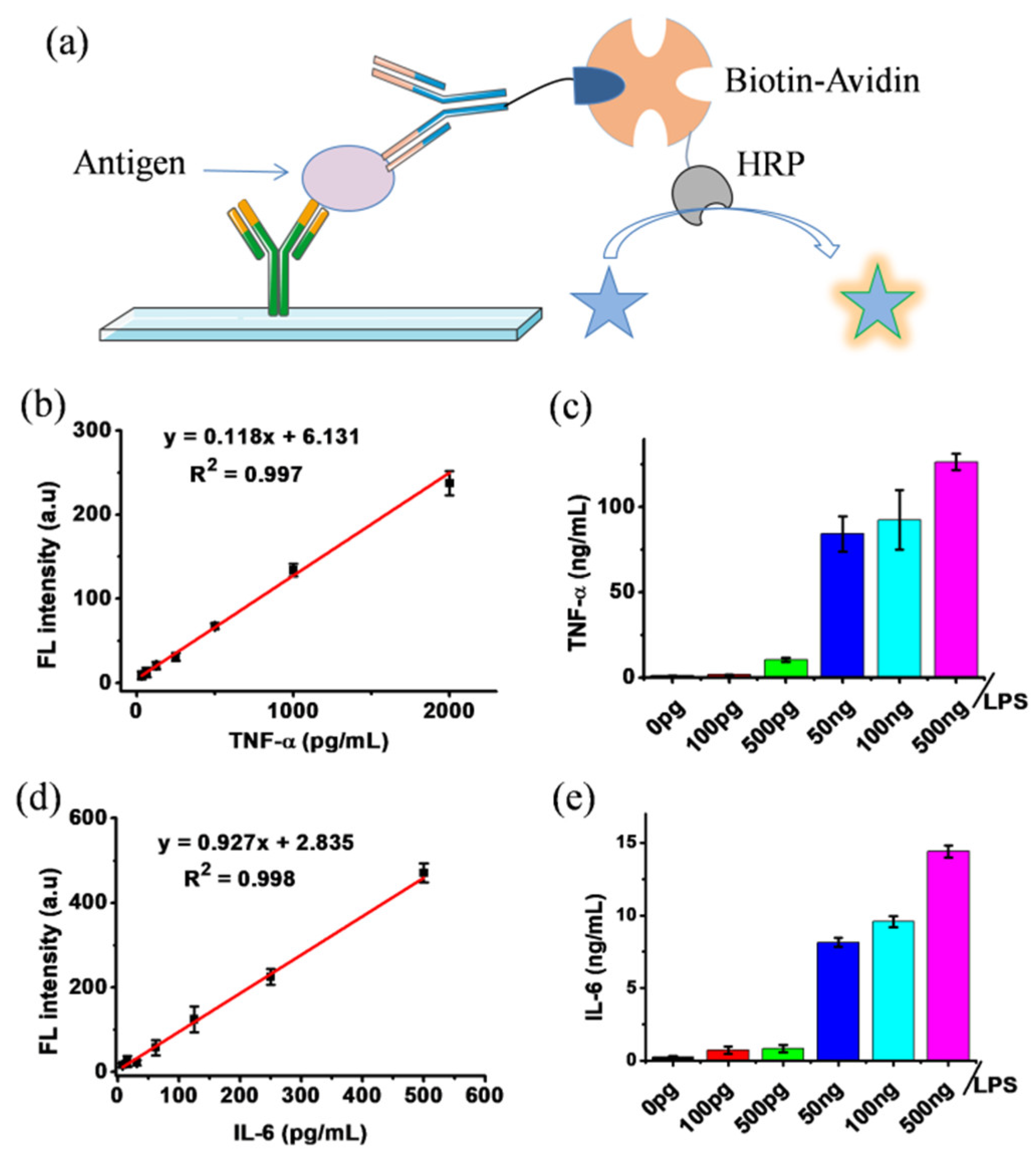
2.4. Fluorescence ELISA Using DCFH-1 as HRP Substrate
3. Result and Discussion
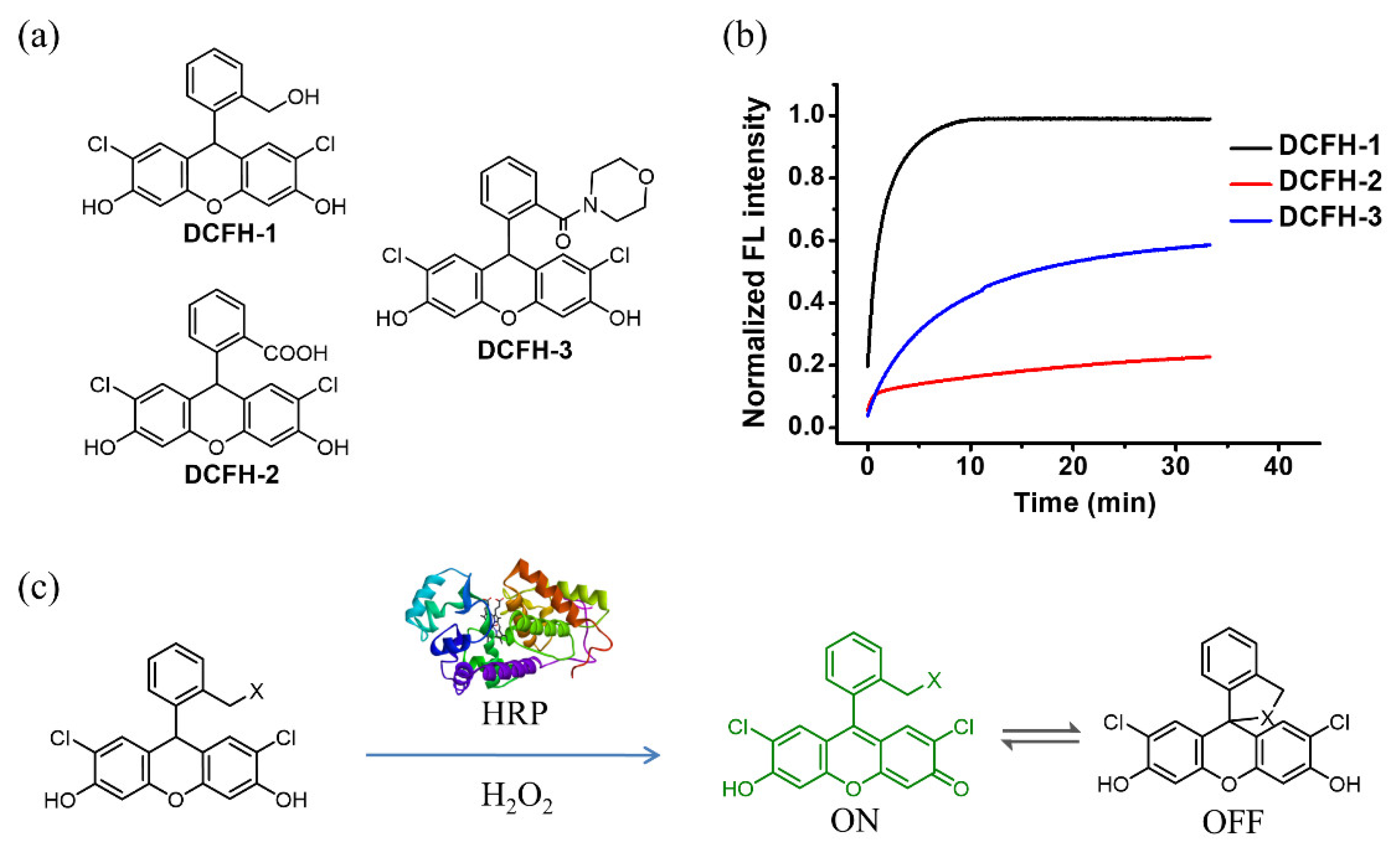
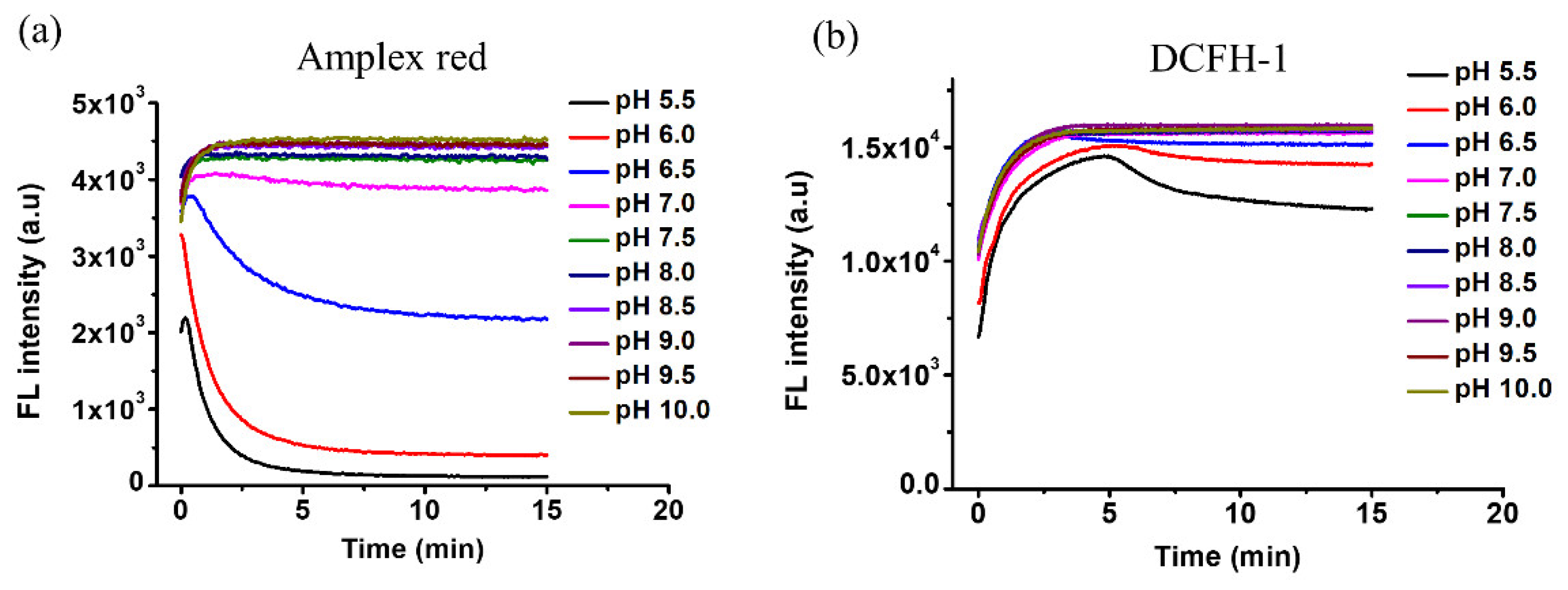
3.1. DCFH-1 Serves as an Excellent Fluorescence Substrate to HRP
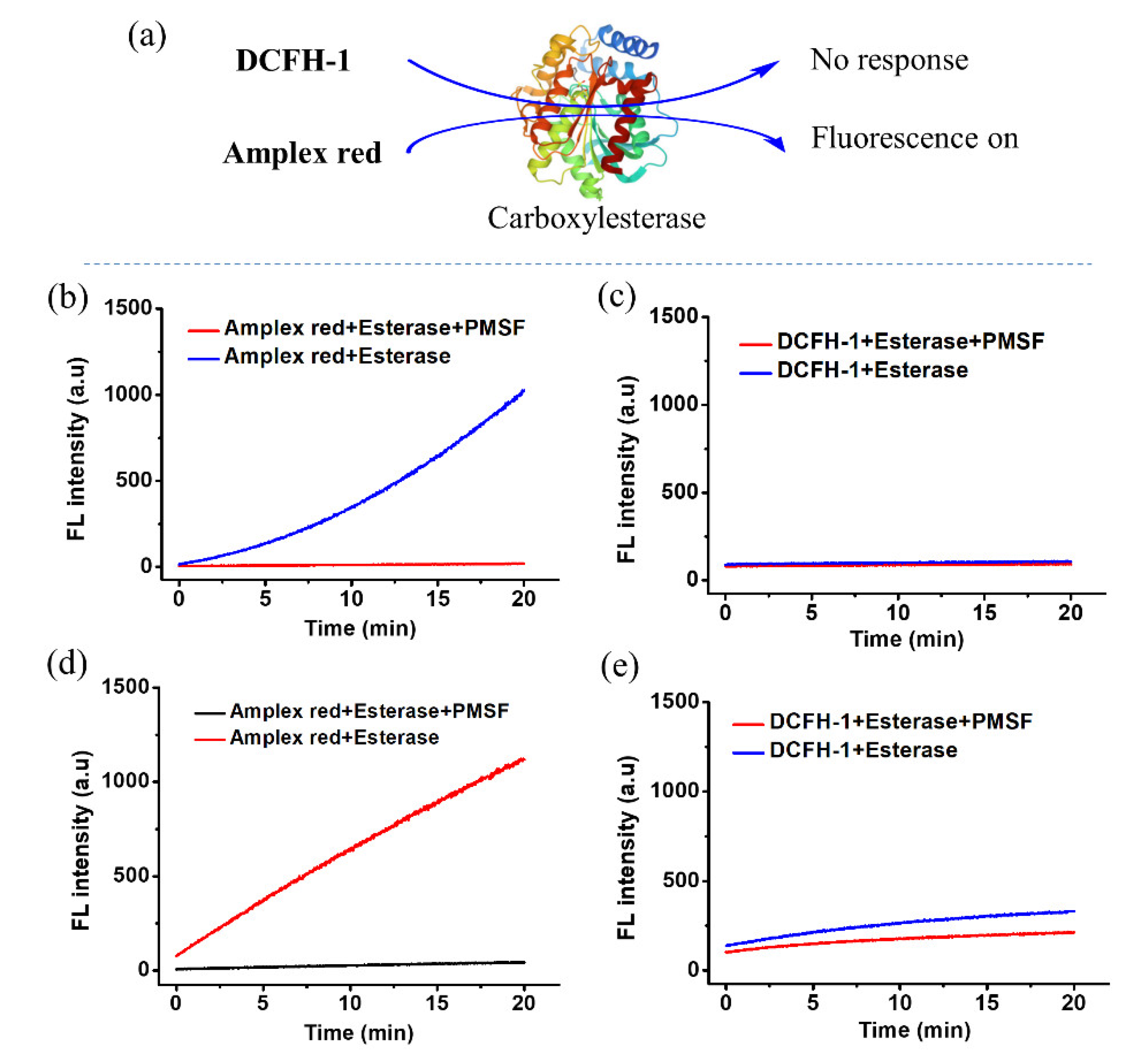
3.2. Nonspecific Esterase Reactivity of DCFH-1 and Amplex Red
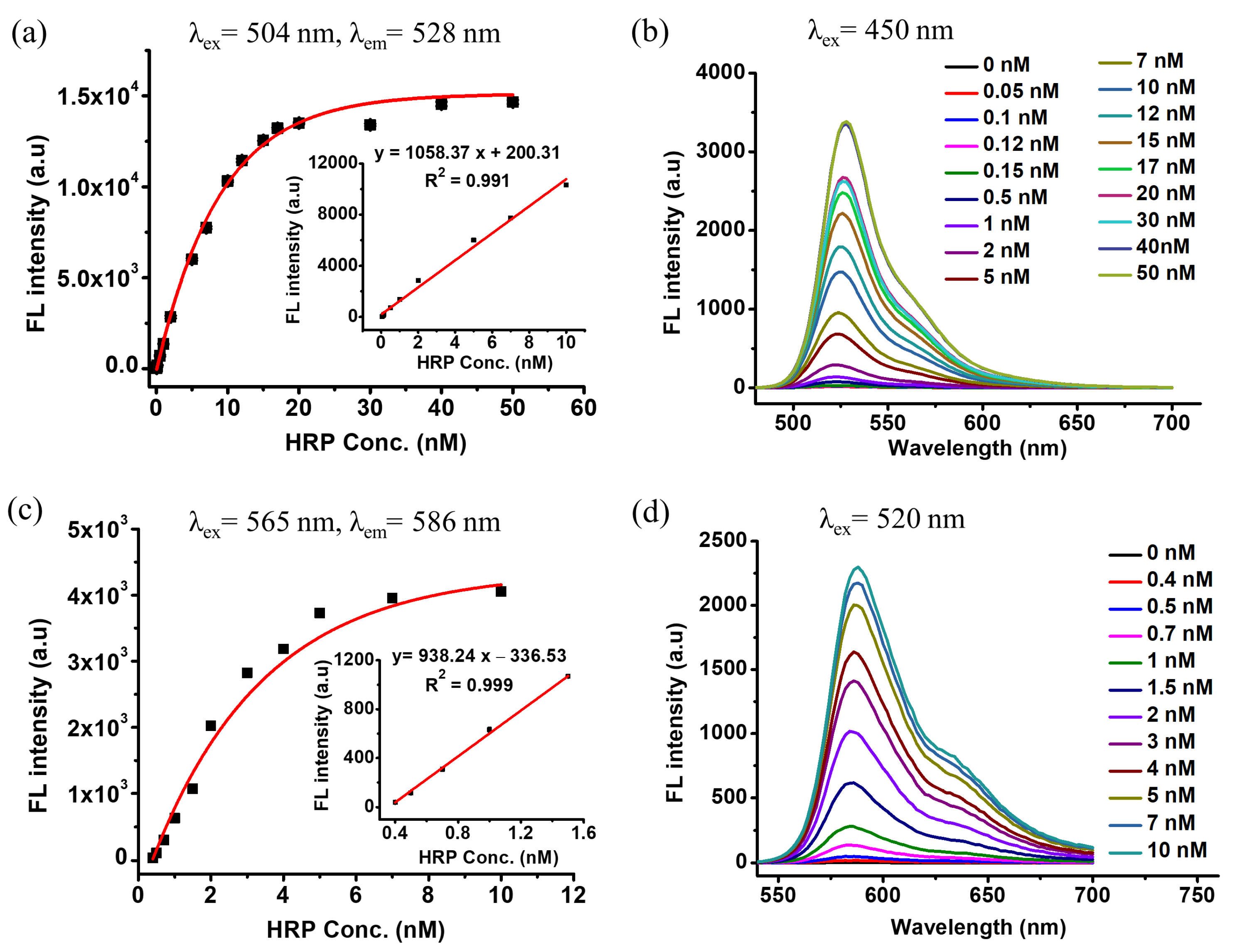
3.3. Fluorescence ELISA Using DCFH-1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Gorris, H.H.; Walt, D.R. Mechanistic aspects of horseradish peroxidase elucidated through single-molecule studies. J. Am. Chem. Soc. 2009, 131, 6277–6282. [Google Scholar] [CrossRef]
- Azevedo, A.M.; Martins, V.C.; Prazeres, D.M.; Vojinović, V.; Cabral, J.M.; Fonseca, L.P. Horseradish peroxidase: A valuable tool in biotechnology. Biotechnol. Annu. Rev. 2003, 9, 199–247. [Google Scholar] [CrossRef]
- Sellami, K.; Couvert, A.; Nasrallah, N.; Maachi, R.; Abouseoud, M.; Amrane, A. Peroxidase enzymes as green catalysts for bioremediation and biotechnological applications: A review. Sci. Total Environ. 2022, 806, 150500. [Google Scholar] [CrossRef]
- Lin, L.; Yi, Z.; Liang, S.; Yongxin, L.; Wei, H.; Mengda, C.; Naidi, Y.; Qiong, W.; Zhongxi, H.; Changmin, Y.; et al. Ultrasensitive detection of IgE levels based on magnetic nanocapturer linked immunosensor assay for early diagnosis of cancer. Chin. Chem. Lett. 2022, 33, 1855–1860. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Zhu, F.; Liu, Q.; Feng, Y.; Zhao, X.; Chen, M.; Chen, X. Facile construction of a reusable multi-enzyme cascade bioreactor for effective fluorescence discrimination and quantitation of amino acid enantiomers. Chem. Eng. J. 2022, 428, 131975. [Google Scholar] [CrossRef]
- Faqin, D.; Jin, L.; Yevgeny, A.G.; Shiyong, S.; Yan, L.; Rui, L.; Ke, W.; Daoyong, T.; Olga, B.K.; Xiaoqin, N. High-performance cascade nanoreactor based on halloysite nanotubes-integrated enzyme-nanozyme microsystem. Chin. Chem. Lett. 2022, 33, 807–811. [Google Scholar] [CrossRef]
- Li, M.; Su, H.; Tu, Y.; Shang, Y.; Liu, Y.; Peng, C.; Liu, H. Development and Application of an Efficient Medium for Chromogenic Catalysis of Tetramethylbenzidine with Horseradish Peroxidase. ACS Omega 2019, 4, 5459–5470. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Y.; Liu, J.; He, J. Luminol, horseradish peroxidase and antibody ternary codified gold nanoparticles for a label-free homogenous chemiluminescent immunoassay. Anal. Methods 2018, 10, 722–729. [Google Scholar] [CrossRef]
- Wang, N.; Miller, C.J.; Wang, P.; Waite, T.D. Quantitative determination of trace hydrogen peroxide in the presence of sulfide using the Amplex Red/horseradish peroxidase assay. Anal. Chim. Acta 2017, 963, 61–67. [Google Scholar] [CrossRef]
- Taylor, S.C.; Rosselli-Murai, L.K.; Crobeddu, B.; Plante, I. A critical path to producing high quality, reproducible data from quantitative western blot experiments. Sci. Rep. 2022, 12, 17599. [Google Scholar] [CrossRef]
- Karatani, H. Luminol–hydrogen peroxide–horseradish peroxidase chemiluminescence intensification by kosmotrope ammonium sulfate. Anal. Sci. 2022, 38, 613–621. [Google Scholar] [CrossRef]
- Ye, Q.; Ren, S.; Huang, H.; Duan, G.; Liu, K.; Liu, J.-B. Fluorescent and Colorimetric Sensors Based on the Oxidation of o-Phenylenediamine. ACS Omega 2020, 5, 20698–20706. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, H.; Dong, T.; Mansour, H.; Zhang, X.; Li, F.; Wu, P. Double-Stranded DNA Matrix for Photosensitization Switching. CCS Chem. 2021, 3, 2394–2404. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Lu, C.; Yang, Z.; Wu, W.; Wang, X. Novel colorimetric, photothermal and up-conversion fluorescence triple-signal sensor for rosmarinic acid detection. Chin. Chem. Lett. 2022, 108099. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Zhang, Y.; Guan, X.; Mei, L.; Feng, H.; Li, J.; Tu, L.; Feng, G.; Deng, G.; et al. Construction of Long-Wavelength Emissive Organic Nanosonosensitizer Targeting Mitochondria for Precise and Efficient In Vivo Sonotherapy. Adv. Funct. Mater. 2022, 32, 2207259. [Google Scholar] [CrossRef]
- Li, K.; Ren, T.-B.; Huan, S.; Yuan, L.; Zhang, X.-B. Progress and Perspective of Solid-State Organic Fluorophores for Biomedical Applications. J. Am. Chem. Soc. 2021, 143, 21143–21160. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, L.; Li, C.; Jiang, Y.; Wang, F. Direct Z-scheme In2S3/Bi2S3 heterojunction-based photoelectrochemical aptasensor for detecting oxytetracycline in water. J. Environ. Chem. Eng. 2022, 10, 107404. [Google Scholar] [CrossRef]
- Serrano, J.; Jové, M.; Boada, J.; Bellmunt, M.J.; Pamplona, R.; Portero-Otín, M. Dietary antioxidants interfere with Amplex Red-coupled-fluorescence assays. Biochem. Biophys. Res. Commun. 2009, 388, 443–449. [Google Scholar] [CrossRef]
- Miwa, S.; Treumann, A.; Bell, A.; Vistoli, G.; Nelson, G.; Hay, S.; von Zglinicki, T. Carboxylesterase converts Amplex red to resorufin: Implications for mitochondrial H2O2 release assays. Free Radical Biol. Med. 2016, 90, 173–183. [Google Scholar] [CrossRef]
- Wang, T.; Xiang, Y.; Liu, X.; Chen, W.; Hu, Y. A novel fluorimetric method for laccase activities measurement using Amplex Red as substrate. Talanta 2017, 162, 143–150. [Google Scholar] [CrossRef]
- Zhao, B.; Ranguelova, K.; Jiang, J.; Mason, R.P. Studies on the photosensitized reduction of resorufin and implications for the detection of oxidative stress with Amplex Red. Free Radical Biol. Med. 2011, 51, 153–159. [Google Scholar] [CrossRef]
- Shatsauskas, A.; Shatalin, Y.; Shubina, V.; Zablodtskii, Y.; Chernenko, S.; Samsonenko, A.; Kostyuchenko, A.; Fisyuk, A. Synthesis and application of new 3-amino-2-pyridone based luminescent dyes for ELISA. Dyes Pigm. 2021, 187, 109072. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Ren, X.; Li, W.; Sun, J.; Wang, X.; Huang, Y.; Guo, Y.; Zeng, H. Novel fluorescence immunoassay for the detection of zearalenone using HRP-mediated fluorescence quenching of gold-silver bimetallic nanoclusters. Food Chem. 2021, 355, 129633. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, J.; Wang, L.; Li, H.; Yang, F.; Yang, X. Inner Filter Effect-Based Sensor for Horseradish Peroxidase and Its Application to Fluorescence Immunoassay. ACS Sens. 2018, 3, 183–190. [Google Scholar] [CrossRef]
- Acharya, A.P.; Sen, P.; Aran, K.; Gardner, A.B.; Rafi, M.; Dean, D.; Murthy, N. A turn-off fluorescent substrate for horseradish peroxidase improves the sensitivity of ELISAs. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 206–210. [Google Scholar] [CrossRef]
- Wu, M.; Wong, G.T.F.; Wu, Y.C. The Scopoletin-HRP Fluorimetric Determination of H(2)O(2) in Seawaters-A Plea for the Two-Stage Protocol. Methods Protoc. 2017, 1, 4. [Google Scholar] [CrossRef]
- Liu, J.; Ruan, G.; Ma, W.; Sun, Y.; Yu, H.; Xu, Z.; Yu, C.; Li, H.; Zhang, C.-w.; Li, L. Horseradish peroxidase-triggered direct in situ fluorescent immunoassay platform for sensing cardiac troponin I and SARS-CoV-2 nucleocapsid protein in serum. Biosens. Bioelectron. 2022, 198, 113823. [Google Scholar] [CrossRef]
- Chen, X.-L.; Li, D.-H.; Yang, H.-H.; Zhu, Q.-Z.; Zheng, H.; Xu, J.-G. Study of tetra-substituted amino aluminum phthalocyanine as a new red-region substrate for the fluorometric determination of peroxidase and hydrogen peroxide. Anal. Chim. Acta 2001, 434, 51–58. [Google Scholar] [CrossRef]
- Khosravi, M.; Nouri, M.; Mohammadi, A.; Mosavari, N.; Constable, P.D. Preparation of immunomagnetic beads coupled with a rhodamine hydrazine immunosensor for the detection of Mycobacterium avium subspecies paratuberculosis in bovine feces, milk, and colostrum. J. Dairy Sci. 2021, 104, 6944–6960. [Google Scholar] [CrossRef]
- Acharya, A.P.; Nafisi, P.M.; Gardner, A.; Mackay, J.L.; Kundu, K.; Kumar, S.; Murthy, N. A fluorescent peroxidase probe increases the sensitivity of commercial ELISAs by two orders of magnitude. Chem. Commun. 2013, 49, 10379–10381. [Google Scholar] [CrossRef]
- Yoo, S.; Kim, S.; Jeon, S.; Han, M.S. Aldehyde N,N-dimethylhydrazone-based fluorescent substrate for peroxidase-mediated assays. RSC Adv. 2022, 12, 8668–8673. [Google Scholar] [CrossRef]
- Reiniers, M.J.; van Golen, R.F.; Bonnet, S.; Broekgaarden, M.; van Gulik, T.M.; Egmond, M.R.; Heger, M. Preparation and Practical Applications of 2′,7′-Dichlorodihydrofluorescein in Redox Assays. Anal. Chem. 2017, 89, 3853–3857. [Google Scholar] [CrossRef]
- Deng, T.; Bao, H.; Huang, W.; Wang, X.; Hu, S.; Wu, S.; Zhao, L.; Cai, C.; Hu, Y.; Liu, F. Easy access of dihydrofluoresceins as advanced fluorescence turn-on probes for oxidative stress. Dyes Pigm. 2020, 173, 107915. [Google Scholar] [CrossRef]
- Haldar, P.; Barman, G.; Ray, J.K. Sodium borohydride–iodine mediated reduction of γ-lactam carboxylic acids followed by DDQ mediated oxidative aromatisation: A simple approach towards N-aryl-formylpyrroles and 1,3-diaryl-formylpyrroles. Tetrahedron 2007, 63, 3049–3056. [Google Scholar] [CrossRef]
- Kessler, A.; Hedberg, J.; McCarrick, S.; Karlsson, H.L.; Blomberg, E.; Odnevall, I. Adsorption of Horseradish Peroxidase on Metallic Nanoparticles: Effects on Reactive Oxygen Species Detection Using 2′,7′-Dichlorofluorescin Diacetate. Chem. Res. Toxicol. 2021, 34, 1481–1495. [Google Scholar] [CrossRef]
- Ghéczy, N.; Sasaki, K.; Yoshimoto, M.; Pour-Esmaeil, S.; Kröger, M.; Stano, P.; Walde, P. A two-enzyme cascade reaction consisting of two reaction pathways. Studies in bulk solution for understanding the performance of a flow-through device with immobilised enzymes. RSC Adv. 2020, 10, 18655–18676. [Google Scholar] [CrossRef]
- Le Guern, F.; Mussard, V.; Gaucher, A.; Rottman, M.; Prim, D. Fluorescein Derivatives as Fluorescent Probes for pH Monitoring along Recent Biological Applications. Int. J. Mol. Sci. 2020, 21, 9217. [Google Scholar] [CrossRef]
- Klonis, N.; Sawyer, W.H. Spectral properties of the prototropic forms of fluorescein in aqueous solution. J. Fluoresc. 1996, 6, 147–157. [Google Scholar] [CrossRef]
- Keller, S.; Kamiya, M.; Urano, Y. Recent Progress in Small Spirocyclic, Xanthene-Based Fluorescent Probes. Molecules 2020, 25, 5964. [Google Scholar] [CrossRef]
- Turnbull, J.L.; Benlian, B.R.; Golden, R.P.; Miller, E.W. Phosphonofluoresceins: Synthesis, Spectroscopy, and Applications. J. Am. Chem. Soc. 2021, 143, 6194–6201. [Google Scholar] [CrossRef]
- Facchin, B.M.; Dos Reis, G.O.; Vieira, G.N.; Mohr, E.T.B.; da Rosa, J.S.; Kretzer, I.F.; Demarchi, I.G.; Dalmarco, E.M. Inflammatory biomarkers on an LPS-induced RAW 264.7 cell model: A systematic review and meta-analysis. Inflamm. Res. 2022, 71, 741–758. [Google Scholar] [CrossRef]
- He, L.; He, L.-H.; Xu, S.; Ren, T.-B.; Zhang, X.-X.; Qin, Z.-J.; Zhang, X.-B.; Yuan, L. Engineering of Reversible NIR-II Redox-Responsive Fluorescent Probes for Imaging of Inflammation In Vivo. Angew. Chem. Int. Ed. 2022, 61, e202211409. [Google Scholar] [CrossRef]
- Deng, T.; Hu, S.; Huang, X.-a.; Song, J.; Xu, Q.; Wang, Y.; Liu, F. A novel strategy for colorimetric detection of hydroxyl radicals based on a modified Griess test. Talanta 2019, 195, 152–157. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Deng, T.; Wang, X.; Wu, S.; Hu, S.; Liu, W.; Chen, T.; Yu, Z.; Xu, Q.; Liu, F. A new FRET probe for ratiometric fluorescence detecting mitochondria-localized drug activation and imaging endogenous hydroxyl radicals in zebrafish. Chem. Commun. 2020, 56, 4432–4435. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Li, T.; Zhang, S.; Zou, X.; Zhou, Y.; Lu, W.; Liu, Z.; Deng, T.; Liu, F. Discovering a Dihydrofluorescein Analogue as a Promising Fluorescence Substrate to HRP. Chemosensors 2023, 11, 152. https://doi.org/10.3390/chemosensors11020152
Zhu J, Li T, Zhang S, Zou X, Zhou Y, Lu W, Liu Z, Deng T, Liu F. Discovering a Dihydrofluorescein Analogue as a Promising Fluorescence Substrate to HRP. Chemosensors. 2023; 11(2):152. https://doi.org/10.3390/chemosensors11020152
Chicago/Turabian StyleZhu, Jiayan, Ting Li, Shihui Zhang, Xiaomei Zou, Yingchun Zhou, Weiguo Lu, Zhihui Liu, Tao Deng, and Fang Liu. 2023. "Discovering a Dihydrofluorescein Analogue as a Promising Fluorescence Substrate to HRP" Chemosensors 11, no. 2: 152. https://doi.org/10.3390/chemosensors11020152
APA StyleZhu, J., Li, T., Zhang, S., Zou, X., Zhou, Y., Lu, W., Liu, Z., Deng, T., & Liu, F. (2023). Discovering a Dihydrofluorescein Analogue as a Promising Fluorescence Substrate to HRP. Chemosensors, 11(2), 152. https://doi.org/10.3390/chemosensors11020152






