Continuous Glucose Monitoring in Hypoxic Environments Based on Water Splitting-Assisted Electrocatalysis
Abstract
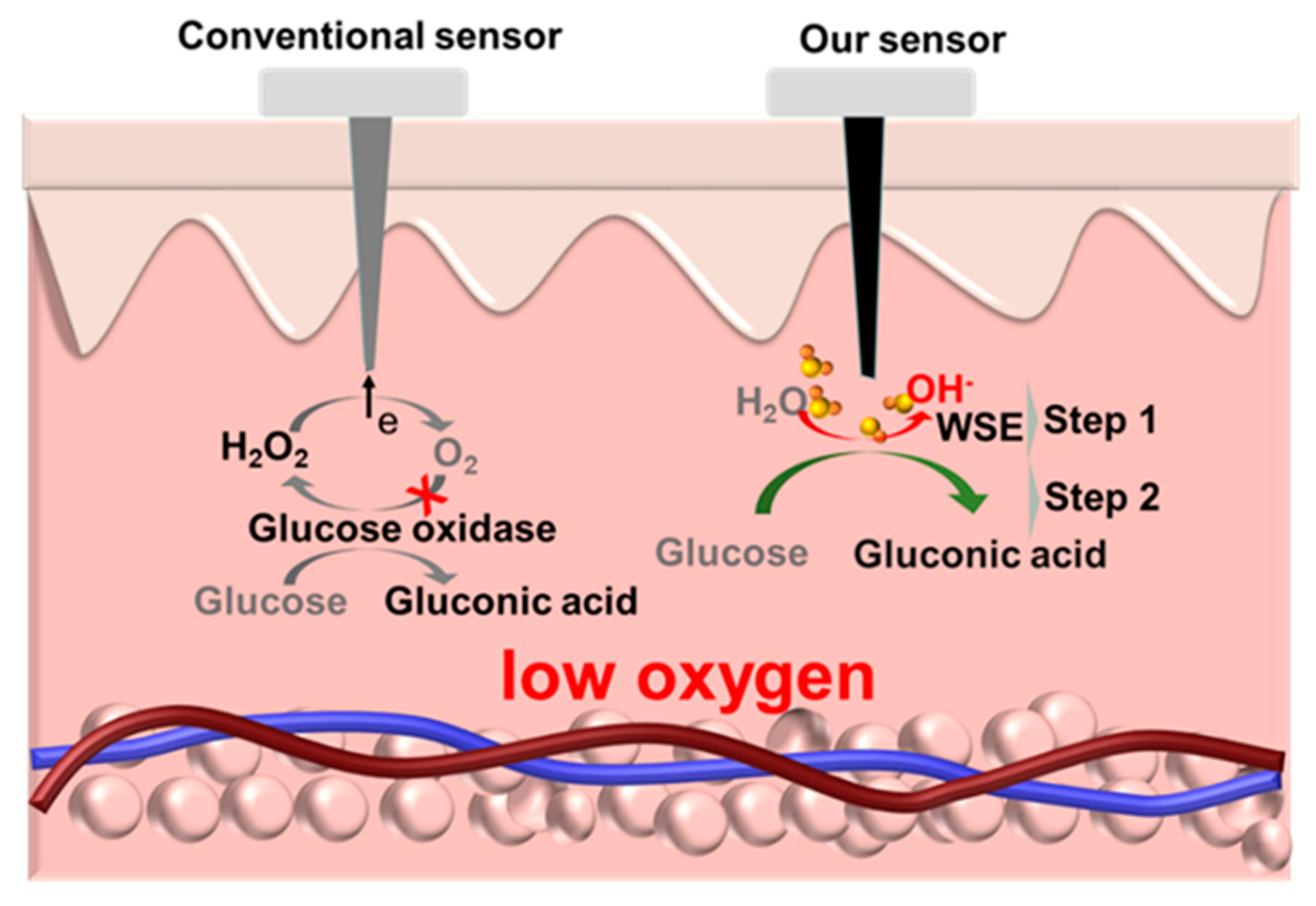
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sensor Fabrication
2.3. In Vitro Glucose Detection
2.4. In Vitro Cytotoxicity Tests
2.5. In Vivo Biocompatibility Test
2.6. Glucose Detection in Normal Rat
2.7. Animal Models
3. Results and Discussion
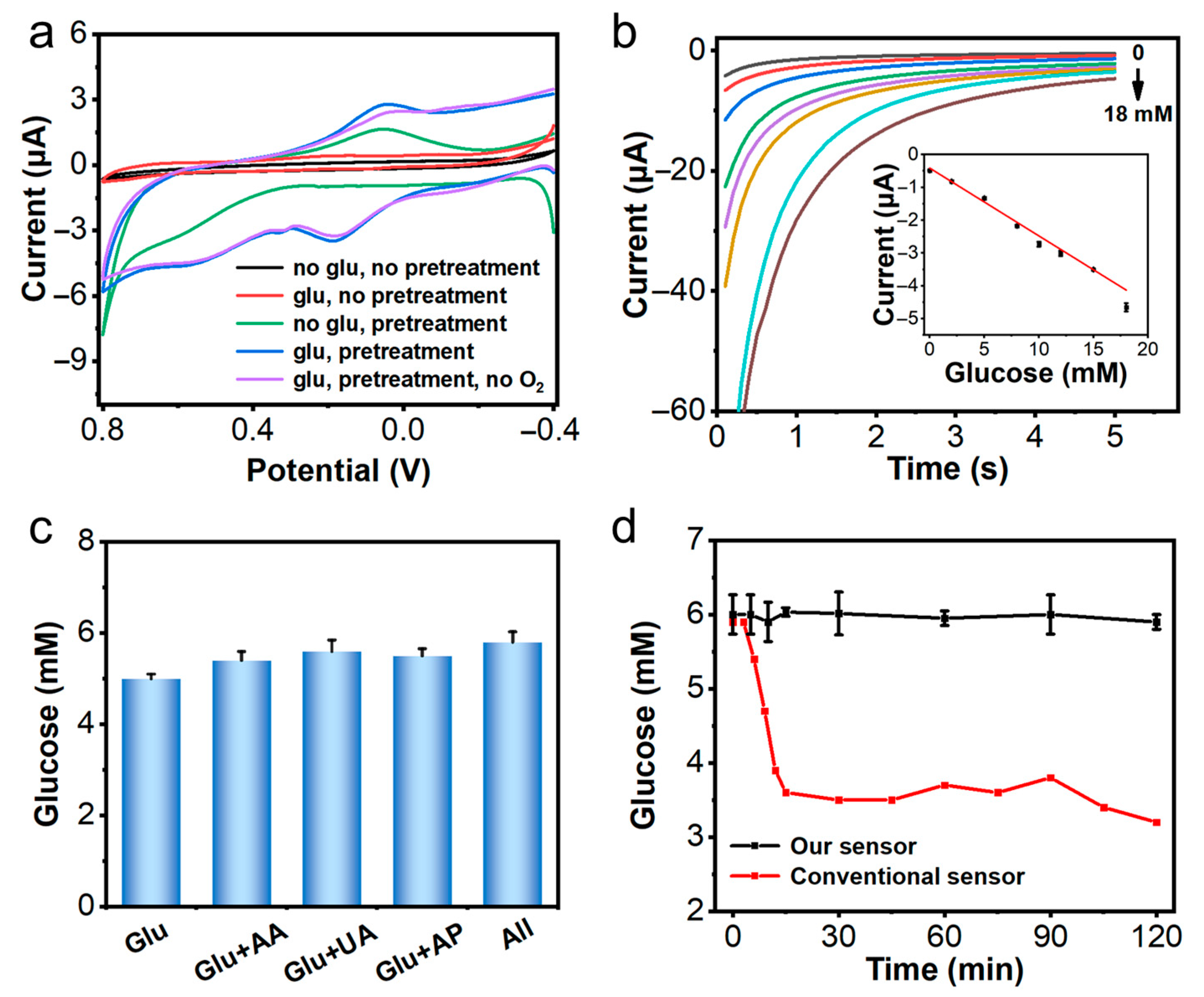
3.1. Electrochemical Performance of the Sensor In Vitro
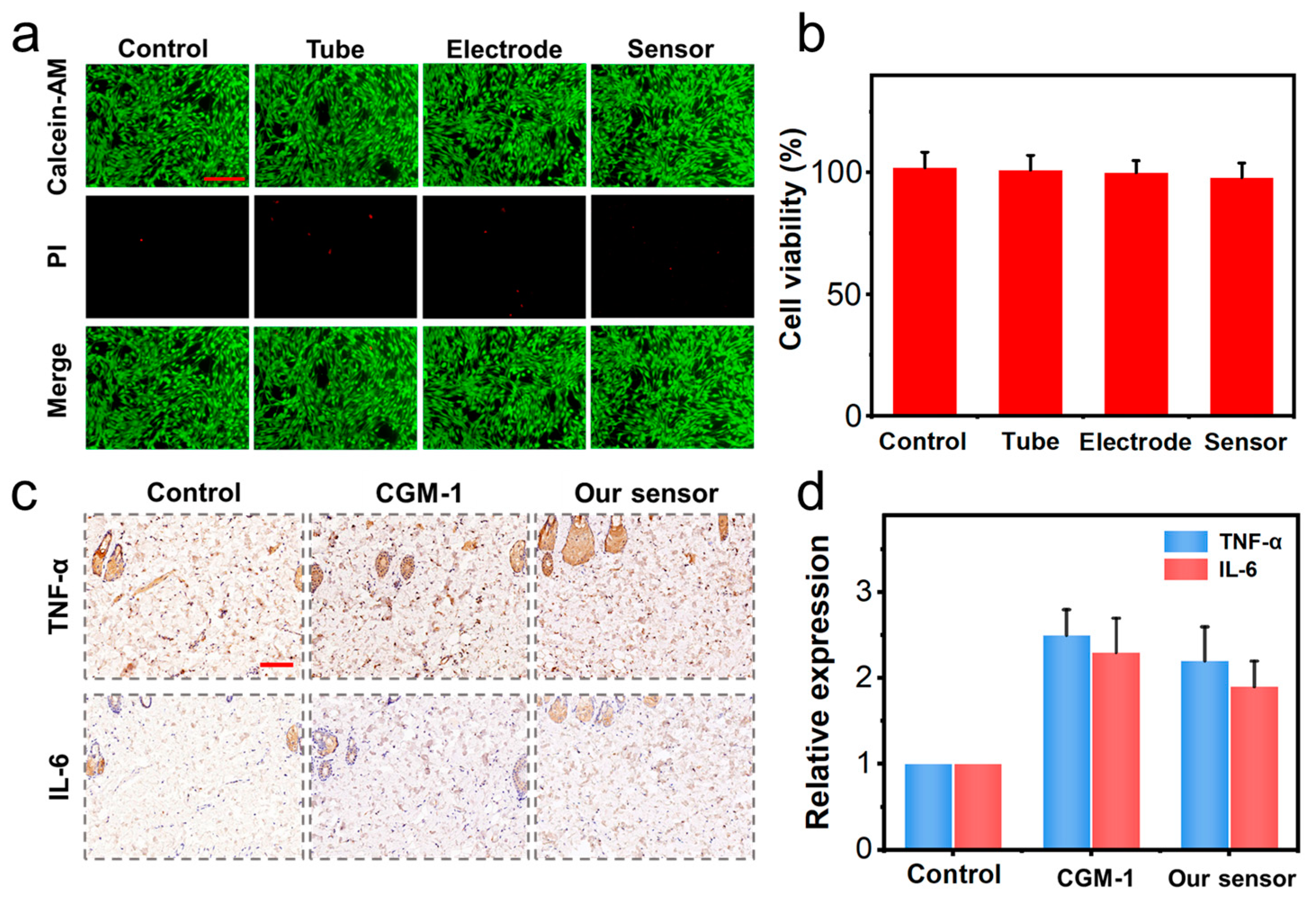
3.2. Cytotoxicity and Biocompatibility Test
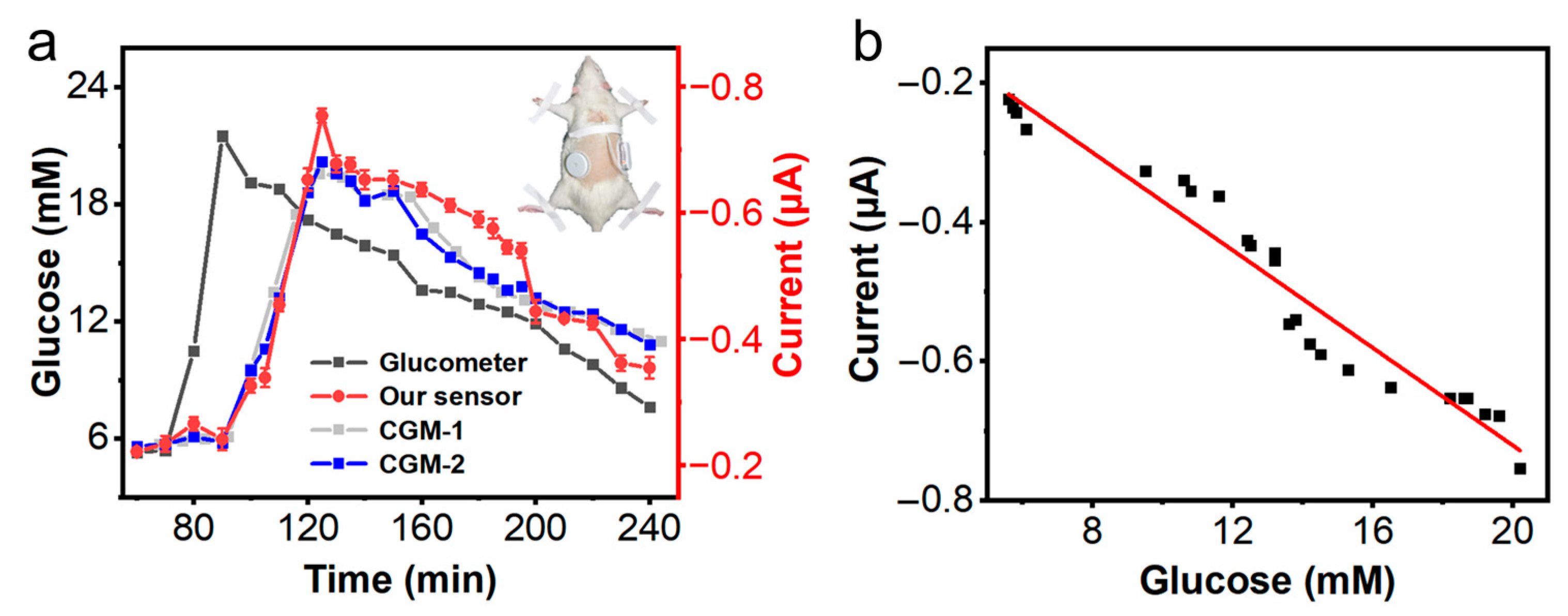
3.3. Glucose Detection in Normal Rat
3.4. Glucose Detection in Systemic Hypoxic Rat
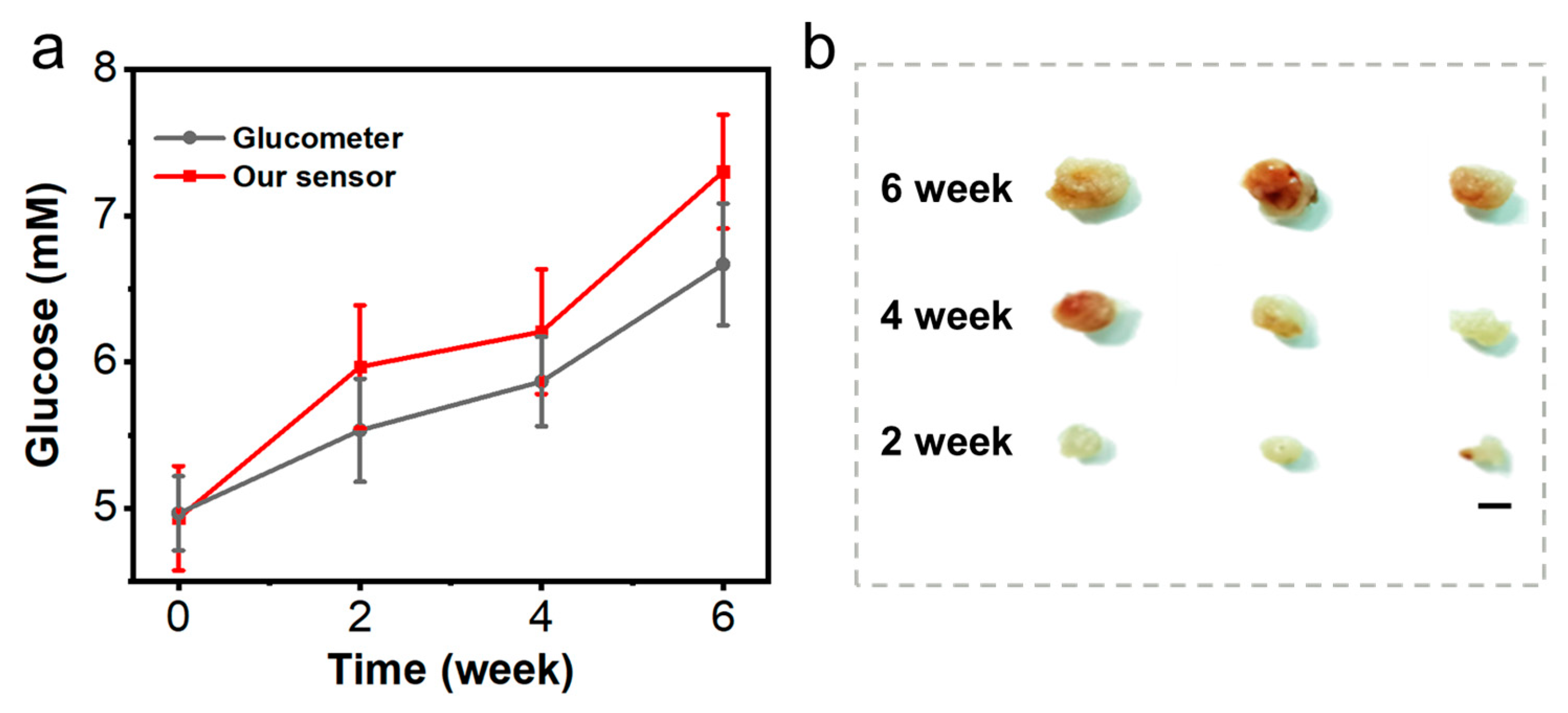
3.5. Glucose Detection in Local Tissue Hypoxic Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Probst, D.; Klonoff, D.; Sode, K. Continuous glucose monitoring systems-Current status and future perspectives of the flagship technologies in biosensor research. Biosens. Bioelectron. 2021, 181, 113054. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, Z.; Del Favero, S.; Jacob, P.; Choudhary, P.; Hypo, R.C. Toward an optimal definition of hypoglycemia with continuous glucose monitoring. Comput. Methods Programs Biomed. 2021, 209, 106303. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Brown, F.; Ekinci, E.I. The ambulatory glucose profile and its interpretation. Med. J. Aust. 2022, 217, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Ghadiya, S.; Jain, A.; Pandya, H.; Lakhani, J.D. Continuous blood glucose monitoring to determine the glycemic variability in patients having SARS CoV-2 infection with ARDS and its bearing on the severity of the disease. J. Adv. Med. Med. Res. 2020, 116, 51–56. [Google Scholar] [CrossRef]
- Bando, H.; Ebe, K.; Muneta, T.; Bando, M.; Yonei, Y. Detail glucose fluctuation and variability by continuous glucose monitoring (CGM). J. Diabetes Metab. Disord. Control 2020, 7, 31–35. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.H. Enzyme-based glucose sensor: From invasive to wearable device. Adv. Healthc. Mater. 2018, 7, e1701150. [Google Scholar] [CrossRef]
- DeHennis, A.; Mortellaro, M. CGM sensor technology. In Glucose Monitoring Devices; Academic Press: Cambridge, MA, USA, 2020; pp. 111–134. [Google Scholar] [CrossRef]
- Lim, S.; Bae, J.H.; Kwon, H.S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30. [Google Scholar] [CrossRef]
- Velavan, T.P.; Meyer, C.G. The COVID-19 epidemic. Trop. Med. Int. Health 2020, 25, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, S.; Herbert, J.A.; McNamara, P.S.; Hedrich, C.M. COVID-19: Immunology and treatment options. Immunol. Treat. Options 2020, 215, 108448. [Google Scholar] [CrossRef]
- Bein, T.; Grasso, S.; Moerer, O.; Quintel, M.; Guerin, C.; Deja, M.; Brondani, A.; Mehta, S. The standard of care of patients with ARDS: Ventilatory settings and rescue therapies for refractory hypoxemia. Intensive Care Med. 2016, 42, 699–711. [Google Scholar] [CrossRef]
- Mistry, N.; Mazer, C.D.; Sled, J.G.; Lazarus, A.H.; Cahill, L.S.; Solish, M.; Zhou, Y.Q.; Romanova, N.; Hare, A.G.M.; Doctor, A.; et al. Red blood cell antibody-induced anemia causes differential degrees of tissue hypoxia in kidney and brain. J. Physiol.-Regul. Integr. Comp. Physiol. 2018, 314, R611–R622. [Google Scholar] [CrossRef]
- Siwaponanan, P.; Fucharoen, S.; Sirankapracha, P.; Winichagoon, P.; Umemura, T.; Svasti, S. Elevated levels of miR-210 correlate with anemia in beta-thalassemia/HbE patients. Int. J. Hematol. 2016, 104, 338–343. [Google Scholar] [CrossRef] [PubMed]
- He, L.N.; Chen, W.; Yang, Y.; Xie, Y.J.; Xiong, Z.Y.; Chen, D.Y.; Lu, D.; Liu, N.Q.; Yang, Y.H.; Sun, X.F. Elevated prevalence of abnormal glucose metabolism and other endocrine disorders in patients with beta-thalassemia major: A meta-analysis. BioMed Res. Int. 2019, 2019, 6573497. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.T.; Yasin, M.; El-Awwa, A.; De Sanctis, V. Detection of glycemic abnormalities in adolescents with beta thalassemia using continuous glucose monitoring and oral glucose tolerance in adolescents and young adults with beta-thalassemia major: Pilot study. Indian J. Endocrinol. Metab. 2013, 17, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Liu, P.; Pan, W.; Singh, S.R.; Wei, Y. Hypoxia and hypoxia inducible factors in tumor metabolism. Cancer Lett. 2015, 356, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Mao, L. Electrochemical Sensors Dancing with the Brain. ACS Sens. 2020, 5, 2659–2660. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent advances in electrochemical glucose biosensors: A review. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Feldman, B.; Brazg, R.; Schwartz, S.; Weinstein, R. A continuous glucose sensor based on wired enzyme technology—Results from a 3-day trial in patients with type 1 diabetes. Diabetes Technol. Ther. 2003, 5, 769–779. [Google Scholar] [CrossRef]
- Harris, J.M.; Reyes, C.; Lopez, G.P. Common causes of glucose oxidase instability in in vivo biosensing: A brief review. J. Diabetes Sci. Technol. 2013, 7, 1030–1038. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, F.; Zhang, Y.; Djire, A. Recent development in electrocatalysts for hydrogen production through water electrolysis. Int. J. Hydrog. Energy 2021, 46, 32284–32317. [Google Scholar] [CrossRef]
- Tian, L.; Li, Z.; Xu, X.; Zhang, C. Advances in noble metal (Ru, Rh, and Ir) doping for boosting water splitting electrocatalysis. J. Mater. Chem. A 2021, 9, 13459–13470. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Sakthikumar, K.; Karthick, K.; Mishra, S.; Kundu, S. Recent trends and perspectives in electrochemical water splitting with an emphasis on sulfide, selenide, and phosphide catalysts of Fe, Co, and Ni: A review. Acs Catal. 2016, 6, 8069–8097. [Google Scholar] [CrossRef]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Antiochia, R. Minimally-invasive microneedle-based biosensor array for simultaneous lactate and glucose monitoring in artificial interstitial fluid. Electroanalysis 2019, 31, 374–382. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, S.; Liu, Y.; Xu, Z.; Zhang, K.; Guo, Y. Development of Cu nanoflowers modified the flexible needle-type microelectrode and its application in continuous monitoring glucose in vivo. Biosens. Bioelectron. 2018, 110, 44–51. [Google Scholar] [CrossRef]
- Lu, X.; Qin, L.; Guo, M.; Geng, J.; Dong, S.; Wang, K.; Xu, H.; Qu, C.; Miao, J.; Liu, M. A novel alginate from Sargassum seaweed promotes diabetic wound healing by regulating oxidative stress and angiogenesis. Carbohydr. Polym. 2022, 289, 119437. [Google Scholar] [CrossRef]
- Song, X.; Feng, L.; Liang, C.; Yang, K.; Liu, Z. Ultrasound triggered tumor oxygenation with oxygen-shuttle nanoperfluorocarbon to overcome hypoxia-associated resistance in cancer therapies. Nano Lett. 2016, 16, 6145–6153. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, X.; Cai, P.; Yu, X. Application of a platinum sheet for pH measurement based on chronopotentiometry. Sens. Actuators B Chem. 2015, 216, 409–411. [Google Scholar] [CrossRef]
- Lei, L.; Zhao, C.; Zhu, X.; Yuan, S.; Dong, X.; Zuo, Y.; Liu, H. Nonenzymatic electrochemical sensor for wearable interstitial fluid glucose monitoring. Electroanalysis 2022, 34, 415–422. [Google Scholar] [CrossRef]
- Park, S.; Boo, H.; Chung, T.D. Electrochemical non-enzymatic glucose sensors. Anal. Chim. Acta 2006, 556, 46–57. [Google Scholar] [CrossRef]
- Lorenz, C.; Sandoval, W.; Mortellaro, M. Interference assessment of various endogenous and exogenous substances on the performance of the eversense long-term implantable continuous glucose monitoring system. Diabetes Technol. Ther. 2018, 20, 344–352. [Google Scholar] [CrossRef]
- Tang, L.; Cui, T.; Wu, J.J.; Liu-Mares, W.; Huang, N.; Li, J. A rice-derived recombinant human lactoferrin stimulates fibroblast proliferation, migration, and sustains cell survival. Wound Repair Regen. 2010, 18, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Qin, X.; Xu, Z.; Song, Y.; Jiang, H.; Wu, Y.; Ruan, H.; Chen, J. Comparison of cytotoxicity evaluation of anticancer drugs between real-time cell analysis and CCK-8 method. Acs Omega 2019, 4, 12036–12042. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Louie, R.F.; Lee, J.H.; Lee, D.M.; Miller, E.E.; Kost, G.J. Oxygen effects on glucose meter measurements with glucose dehydrogenase- and oxidase-based test strips for point-of-care testing. Crit. Care Med. 2001, 29, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Liang, Y.; Tu, Y.; Chen, J.; Xie, Y. Hypoxic preconditioning reduces propofol-induced neuroapoptosis via regulation of Bcl-2 and Bax and downregulation of activated caspase-3 in the hippocampus of neonatal rats. Neurol. Res. 2018, 40, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Stetler, R.A.; Leak, R.K.; Gan, Y.; Li, P.; Zhang, F.; Hu, X.; Jing, Z.; Chen, J.; Zigmond, M.J.; Gao, Y. Preconditioning provides neuroprotection in models of CNS disease: Paradigms and clinical significance. Prog. Neurobiol. 2014, 114, 58–83. [Google Scholar] [CrossRef] [PubMed]
- Wee, J.; Climstein, M. Hypoxic training: Clinical benefits on cardiometabolic risk factors. J. Sci. Med. Sport 2015, 18, 56–61. [Google Scholar] [CrossRef]
- Gerber, P.A.; Rutter, G.A. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, L.; Xu, C.; Dong, X.; Ma, B.; Chen, Y.; Hao, Q.; Zhao, C.; Liu, H. Continuous Glucose Monitoring in Hypoxic Environments Based on Water Splitting-Assisted Electrocatalysis. Chemosensors 2023, 11, 149. https://doi.org/10.3390/chemosensors11020149
Lei L, Xu C, Dong X, Ma B, Chen Y, Hao Q, Zhao C, Liu H. Continuous Glucose Monitoring in Hypoxic Environments Based on Water Splitting-Assisted Electrocatalysis. Chemosensors. 2023; 11(2):149. https://doi.org/10.3390/chemosensors11020149
Chicago/Turabian StyleLei, Lanjie, Chengtao Xu, Xing Dong, Biao Ma, Yichen Chen, Qing Hao, Chao Zhao, and Hong Liu. 2023. "Continuous Glucose Monitoring in Hypoxic Environments Based on Water Splitting-Assisted Electrocatalysis" Chemosensors 11, no. 2: 149. https://doi.org/10.3390/chemosensors11020149
APA StyleLei, L., Xu, C., Dong, X., Ma, B., Chen, Y., Hao, Q., Zhao, C., & Liu, H. (2023). Continuous Glucose Monitoring in Hypoxic Environments Based on Water Splitting-Assisted Electrocatalysis. Chemosensors, 11(2), 149. https://doi.org/10.3390/chemosensors11020149







