Facile Electrodeposition-Based Chemosensors Using PANI and C-Hybrid Nanomaterials for the Selective Detection of Ammonia and Nitrogen Dioxide at Room Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
2.3. Sensor Fabrication
2.4. Electrodeposition
2.4.1. PANI-rGO-ZnO Electrodeposition
- Solution 1 (S1): add 20 mL of GO (2 mg/mL) and 0.038 g of MgSO4 to dispersed water;
- Solution 2 (S2): mix 182 µL aniline, 197 µL HCl, and 17.818 mL H2O;
- Solution 3 (S3): dissolve 0.08 g NaOH in 20 mL H2O;
- Solution 4 (S4): dissolve 0.08 M Zn(NO3)2 and 0.27 g KNO3 in 20 mL H2O.
2.4.2. PANI-MWCNT-NH2 Electrodeposition
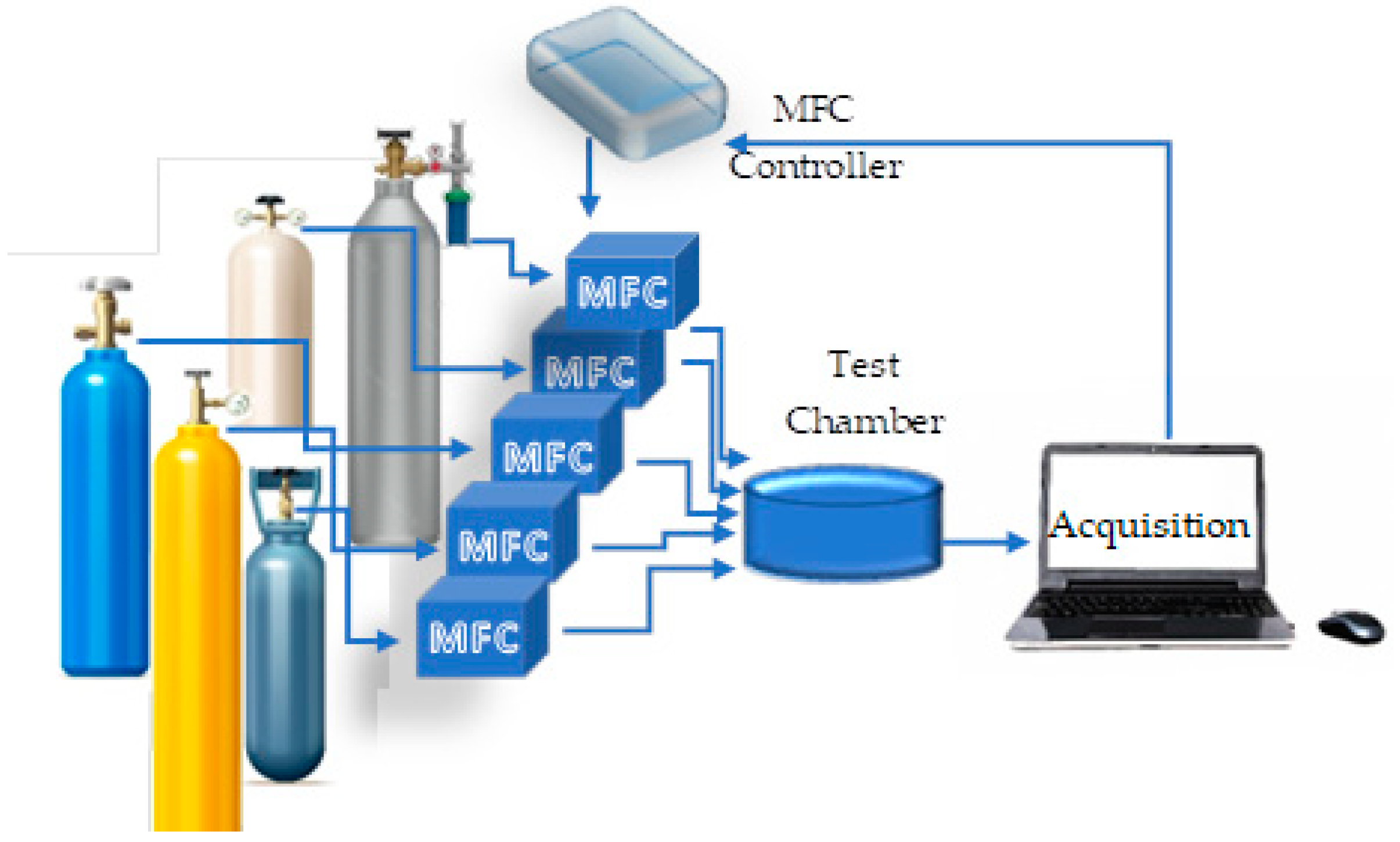
2.5. Testing Method
2.6. Sensor Analytical Performance
3. Results and Discussions
3.1. PANI-MWCNT-NH2 Electrodeposition
- ANI monomer and MWCNT-NH2 concentrations
- Deposition cycle number
3.1.1. PANI-MWCNT-NH2 Morphological and Structural Characterization
- SEM, Raman, and XPS
3.1.2. Mechanisms of the PANI-MWCNT-NH2 Sensor Interaction with NH3
3.2. PANI-rGO-ZnO
3.2.1. PANI-rGO-ZnO Electrodeposition
3.2.2. PANI-rGO-ZnO Structural and Morphological Characterization
- SEM, Raman, and Energy-dispersive X-ray
3.2.3. Mechanisms of the PANI-rGO-ZnO Sensor Interaction with NO2
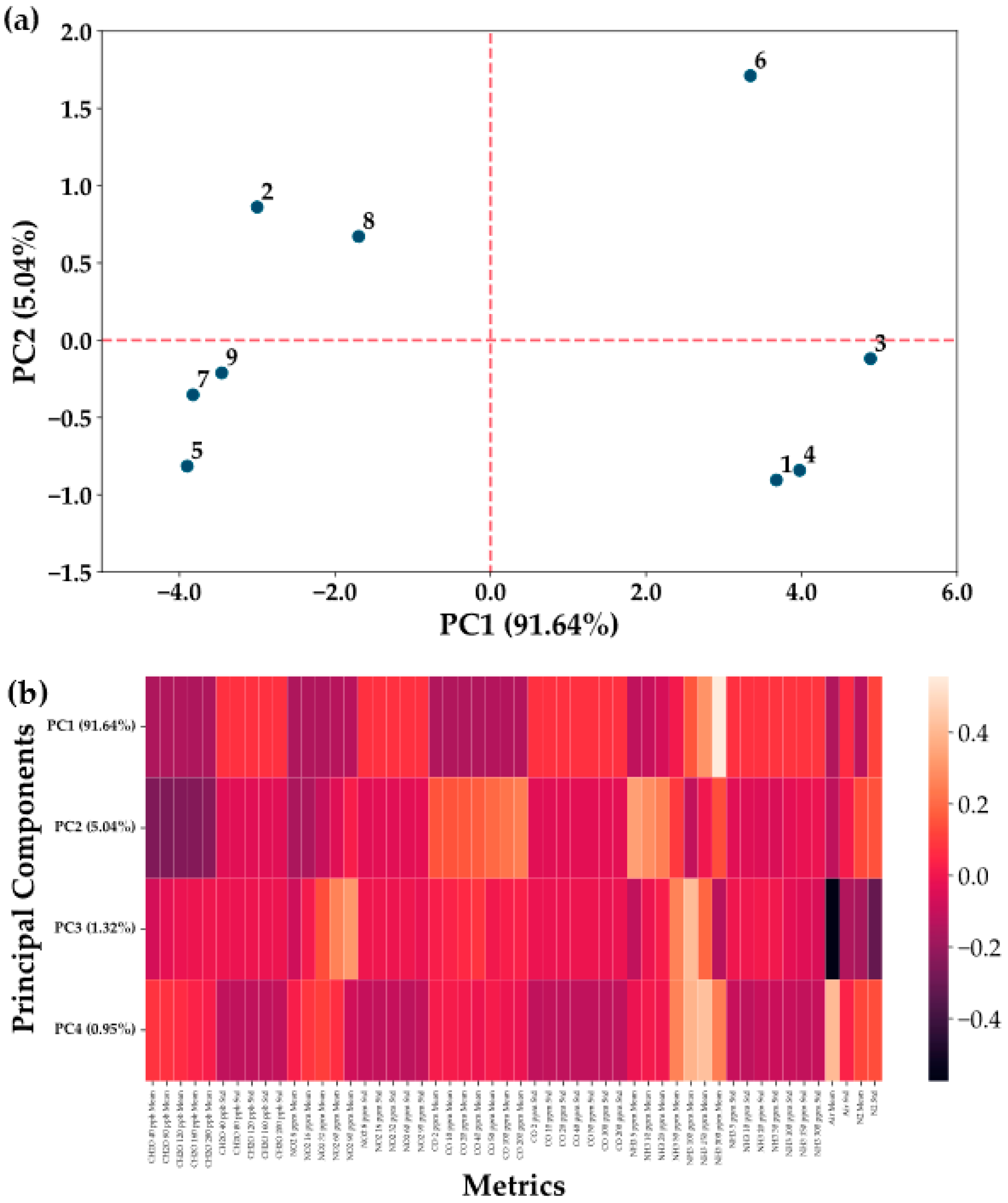
3.3. Data Analysis
- Materials selection
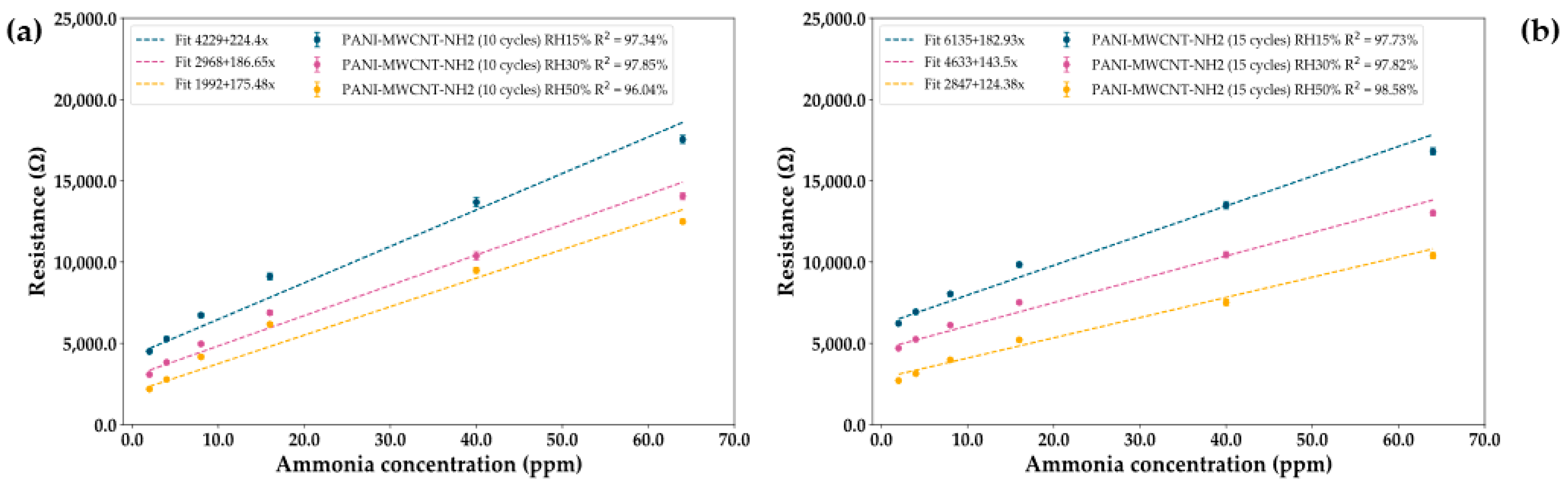
- PANI-MWCNT-NH2 sensor
- PANI-rGO-ZnO sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- U.S. Environmental Protection Agency. Report to Congress on Indoor Air Quality: Volume 2; EPA/400/1-89/001C; U.S. Environmental Protection Agency: Washington, DC, USA, 1989. [Google Scholar]
- Valera-Medina, A.; Amer-Hatem, F.; Azad, A.K.; Dedoussi, I.C.; De Joannon, M.; Fernandes, R.X.; Glarborg, P.; Hashemi, H.; He, X.; Mashruk, S.; et al. Review on ammonia as a potential fuel: From synthesis to economics. Energy Fuels 2021, 35, 6964–7029. [Google Scholar] [CrossRef]
- Szmant, H.H. Organic Building Blocks of the Chemical Industry; John Wiley & Sons: Hoboken, NJ, USA, 1989. [Google Scholar]
- Ammonia, National Institute for Occupational Safety and Health. Available online: https://www.cdc.gov/niosh/idlh/7664417.html (accessed on 15 November 2022).
- Dotson, G.S.; Maier, A.; Parker, A.; Haber, L. TImmediately Dangerous to Life or Health (IDLH) Value Profile: Nitrogen Dioxide; CAS No. 10102-44-0; Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health: Washington, DC, USA, 2017. [Google Scholar]
- Fernández-Ramos, M.D.; Capitán-Vallvey, L.F.; Pastrana-Martínez, L.M.; Morales-Torres, S.; Maldonado-Hódar, F.J. Chemoresistive NH3 gas sensor at room temperature based on the Carbon gel-TiO2 nanocomposites. Sens. Actuators B Chem. 2022, 368, 132103. [Google Scholar] [CrossRef]
- Serafini, M.; Mariani, F.; Gualandi, I.; Decataldo, F.; Possanzini, L.; Tessarolo, M.; Fraboni, B.; Tonelli, D.; Scavetta, E. A Wearable Electrochemical Gas Sensor for Ammonia Detection. Sensors 2021, 21, 7905. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhang, B.Y.; Mohiuddin, M.; Ha, N.; Wen, X.; Zhou, C.; Li, Y.; Ren, G.; Zhang, H.; Zavabeti, A.; et al. Free-standing ultra-thin Janus indium oxysulfide for ultrasensitive visible-light-driven optoelectronic chemical sensing. Nano Today 2021, 37, 101096. [Google Scholar] [CrossRef]
- Yao, Q.; Ren, G.; Xu, K.; Zhu, L.; Khan, H.; Mohiuddin, M.; Khan, M.W.; Zhang, B.Y.; Jannat, A.; Haque, F.; et al. 2D plasmonic tungsten oxide enabled ultrasensitive fiber optics gas sensor. Adv. Opt. Mater. Mater. 2019, 7, 1901383. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B-Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.; Kim, T.; Eom, T.H.; Kim, S.Y.; Jang, H.W. Chemoresistive materials for electronic nose: Progress, perspectives, and challenges. InfoMat 2019, 1, 289–316. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef]
- Cheng, Y.; Ren, B.; Xu, K.; Jeerapan, I.; Chen, H.; Li, Z.; Ou, J.Z. Recent progress in intrinsic and stimulated room-temperature gas sensors enabled by low-dimensional materials. J. Mater. Chem. C 2021, 9, 3026–3051. [Google Scholar] [CrossRef]
- Savin, M.; Mihailescu, C.M.; Avramescu, V.; Dinulescu, S.; Firtat, B.; Craciun, G.; Brasoveanu, C.; Pachiu, C.; Romanitan, C.; Serban, A.B.; et al. A New Hybrid Sensitive PANI/SWCNT/Ferrocene-Based Layer for a Wearable CO Sensor. Sensors 2021, 21, 1801. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Duan, Z.; Zhang, B.; Zhao, Y.; Yuan, Z.; Zhang, Y.; Wu, Y.; Jiang, Y.; Tai, H. Local Gaussian process regression with small sample data for temperature and humidity compensation of polyaniline-cerium dioxide NH3 sensor. Sens. Actuators B-Chem. 2023, 378, 133113. [Google Scholar] [CrossRef]
- Javadian-Saraf, A.; Hosseini, E.; Wiltshire, B.D.; Zarifi, M.H.; Arjmand, M. Graphene oxide/polyaniline-based microwave split-ring resonator: A versatile platform towards ammonia sensing. J. Hazard. Mater. 2021, 418, 126283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cao, S.; Wu, Z.; Zhang, M.; Cao, Y.; Guo, J.; Zhong, F.; Duan, H.; Jia, D. High-performance gas sensor of polyaniline/carbon nanotube composites promoted by interface engineering. Sensors 2019, 20, 149. [Google Scholar] [CrossRef]
- Elizalde-Torres, J.; Hu, H.; Saniger, J.M. Comparison of NO2 and NH3 gas adsorption on semiconductor polyaniline thin films. Rev. Mex. Fís 2005, 51, 482–487. [Google Scholar]
- Chen, X.; Chen, X.; Ding, X.; Yu, X. Gas Sensitive Characteristics of Polyaniline Decorated with Molybdenum Ditelluride Nanosheets. Chemosensors 2022, 10, 264. [Google Scholar] [CrossRef]
- Duan, X.; Duan, Z.; Zhang, Y.; Liu, B.; Li, X.; Zhao, Q.; Yuan, Z.; Jiang, Y.; Tai, H. Enhanced NH3 sensing performance of polyaniline via a facile morphology modification strategy. Sens. Actuators B-Chem. 2022, 369, 132302. [Google Scholar] [CrossRef]
- Maity, D.; Kumar, R.T.R. Polyaniline anchored MWCNTs on fabric for high performance wearable ammonia sensor. ACS Sens. 2018, 3, 1822–1830. [Google Scholar] [CrossRef]
- Chaudhary, V.; Gautam, A.; Mishra, Y.K.; Kaushik, A. Emerging MXene–polymer hybrid nanocomposites for high-performance ammonia sensing and monitoring. Nanomaterials 2021, 11, 2496. [Google Scholar] [CrossRef]
- Kałużyński, P.; Mucha, W.; Capizzi, G.; Lo Sciuto, G. Chemiresistor gas sensors based on conductive copolymer and ZnO blend–prototype fabrication, experimental testing, and response prediction by artificial neural networks. J. Mater. Sci.—Mater. El. 2022, 33, 26368–26382. [Google Scholar] [CrossRef]
- Tohidi, S.; Parhizkar, M.; Bidadi, H.; Mohamad-Rezaei, R. Electrodeposition of polyaniline/three-dimensional reduced graphene oxide hybrid films for detection of ammonia gas at room temperature. IEEE Sens. J. 2020, 20, 9660–9667. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Lei, S.; Song, Y. Graphene-based polyaniline nanocomposites: Preparation, properties and applications. J. Mater. Chem. A 2014, 2, 4491–4509. [Google Scholar] [CrossRef]
- Chakrabarti, M.H.; Low, C.T.J.; Brandon, N.P.; Yufit, V.; Hashim, M.A.; Irfan, M.F.; Akhtar, J.; Ruiz-Trejo, E.; Hussain, M.A. Progress in the electrochemical modification of graphene-based materials and their applications. Electrochim. Acta 2013, 107, 425–440. [Google Scholar] [CrossRef]
- Norizan, M.N.; Moklis, M.H.; Demon, S.Z.N.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef]
- Lee, C.T.; Wang, Y.S. High-performance room temperature NH3 gas sensors based on polyaniline-reduced graphene oxide nanocomposite sensitive membrane. J. Alloys Compd. 2019, 789, 693–696. [Google Scholar] [CrossRef]
- Suhail, M.H.; Abdullah, O.G.; Kadhim, G.A. Hydrogen sulfide sensors based on PANI/f-SWCNT polymer nanocomposite thin films prepared by electrochemical polymerization. J. Sci. Adv. Mater. Devices 2019, 4, 143–149. [Google Scholar] [CrossRef]
- Wulandari, S.A.; Widiyandari, H. Subagio, Synthesis and characterization carboxyl functionalized multi-walled carbon nanotubes (MWCNT-COOH) and NH2 functionalized multi-walled carbon nanotubes (MWCNT-NH2). J. Phys. Conf. Ser. 2018, 1025, 012005. [Google Scholar] [CrossRef]
- Alharbi, N.D.; Shahnawaze Ansari, M.; Salah, N.; Khayyat, S.A.; Khan, Z.H. Zinc oxide-multi walled carbon nanotubes nanocomposites for carbon monoxide gas sensor application. J. Nanosci. Nanotechnol. 2016, 16, 439–447. [Google Scholar] [CrossRef]
- Franco, M.A.; Conti, P.P.; Andre, R.S.; Correa, D.S. A review on chemiresistive ZnO gas sensors. Sensor. Actuator. Rep. 2022, 4, 100100. [Google Scholar] [CrossRef]
- Xu, K.; Ha, N.; Hu, Y.; Ma, Q.; Chen, W.; Wen, X.; Ou, R.; Trinh, V.; McConville, C.F.; Ou, J.Z. A room temperature all-optical sensor based on two-dimensional SnS2 for highly sensitive and reversible NO2 sensing. J. Hazard. Mater. 2022, 426, 127813. [Google Scholar]
- Bonyani, M.; Zebarjad, S.M.; Janghorban, K.; Kim, J.Y.; Kim, H.W.; Kim, S.S. Au-Decorated Polyaniline-ZnO Electrospun Composite Nanofiber Gas Sensors with Enhanced Response to NO2 Gas. Chemosensors 2022, 10, 388. [Google Scholar] [CrossRef]
- Talwar, V.; Singh, O.; Singh, R.C. ZnO assisted polyaniline nanofibers and its application as ammonia gas sensor. Sens. Actuators B-Chem. 2014, 191, 276–282. [Google Scholar] [CrossRef]
- Al-Mashat, L.; Shin, K.; Kalantar-Zadeh, K.; Plessis, J.D.; Han, S.H.; Kojima, R.W.; Kaner, R.B.; Li, D.; Gou, X.; Ippolito, S.J.; et al. Graphene/polyaniline nanocomposite for hydrogen sensing. J. Phys. Chem. C 2010, 114, 16168–16173. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Zong, X. Flexible and highly sensitive H2S gas sensor based on in-situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sens. Actuators B-Chem. 2019, 289, 32–41. [Google Scholar] [CrossRef]
- Ou, J.Z.; Ge, W.; Carey, B.; Daeneke, T.; Rotbart, A.; Shan, W.; Wang, Y.; Fu, Z.; Chrimes, A.F.; Wlodarski, W.; et al. Physisorption-based charge transfer in two-dimensional SnS2 for selective and reversible NO2 gas sensing. ACS Nano 2015, 9, 10313–10323. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Park, J.H.; Ahn, B.W.; Ro, J.C.; Suh, S.J. Facile Synthesis of Template-Free SnS2 with Different Morphologies and Excellent Gas-Sensing Performance for NO2 Gas-Sensor Applications. Phys. Status Solidi (A) 2022, 219, 2100827. [Google Scholar] [CrossRef]
- Kuo, C.G.; Chen, J.H.; Chao, Y.C.; Chen, P.L. Fabrication of a P3HT-ZnO nanowires gas sensor detecting ammonia gas. Sensors 2017, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xu, L.; Wang, X.; Li, Q.; Cui, Q.; Suo, H.; Zhao, C. A simple dip-coating method of SnO2-NiO composite thin film on a ceramic tube substrate for methanol sensing. Crystals 2019, 9, 621. [Google Scholar] [CrossRef]
- Caricato, A.P.; Luches, A.; Rella, R. Nanoparticle thin films for gas sensors prepared by matrix assisted pulsed laser evaporation. Sensors 2009, 9, 2682–2696. [Google Scholar] [CrossRef]
- Chou, T.I.; Chiu, S.W.; Tang, K.T. A CMOS compatible miniature gas sensing system. In Chemical, Gas, and Biosensors for Internet of Things and Related Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 237–252. [Google Scholar]
- Guang, Q.; Huang, B.; Li, X. Au-decorated WS2 microflakes based sensors for selective ammonia detection at room temperature. Chemosensors 2021, 10, 9. [Google Scholar] [CrossRef]
- Baharuddin, A.A.; Ang, B.C.; Haseeb, A.S.M.A.; Wong, Y.C.; Wong, Y.H. Advances in chemiresistive sensors for acetone gas detection. Mat. Sci. Semicon. Proc. 2019, 103, 104616. [Google Scholar] [CrossRef]
- Anisimov, Y.A.; Evitts, R.W.; Cree, D.E.; Wilson, L.D. Polyaniline/biopolymer composite systems for humidity sensor applications: A review. Polymers 2021, 13, 2722. [Google Scholar] [CrossRef]
- El Aggadi, S.; Loudiyi, N.; Chadil, A.; El Abbassi, Z.; El Hourch, A. Electropolymerization of aniline monomer and effects of synthesis conditions on the characteristics of synthesized polyaniline thin films. Mediterr. J. Chem. 2020, 10, 138–145. [Google Scholar] [CrossRef]
- Tang, H.; Ding, Y.; Zang, C.; Gu, J.; Shen, Q.; Kan, J. Effect of temperature on electrochemical degradation of polyaniline. Int. J. Electrochem. Sci 2014, 9, 7239–7252. [Google Scholar]
- Sohn, J.H.; Atzeni, M.; Zeller, L.; Pioggia, G. Characterisation of humidity dependence of a metal oxide semiconductor sensor array using partial least squares. Sens. Actuators B-Chem. 2008, 131, 230–235. [Google Scholar] [CrossRef]
- Liu, C.; Noda, Z.; Sasaki, K.; Hayashi, K. Development of a polyaniline nanofiber-based carbon monoxide sensor for hydrogen fuel cell application. Int. J. Hydrogen Energ. 2012, 37, 13529–13535. [Google Scholar] [CrossRef]
- Liu, C.; Hayashi, K.; Toko, K. Electrochemical deposition of nanostructured polyaniline on an insulating substrate. Electrochem. Commun. 2010, 12, 36–39. [Google Scholar] [CrossRef]
- Van Tuan, C.; Tuan, M.A.; Van Hieu, N.; Trung, T. Electrochemical synthesis of polyaniline nanowires on Pt interdigitated microelectrode for room temperature NH3 gas sensor application. Curr. Appl. Phys. 2012, 12, 1011–1016. [Google Scholar] [CrossRef]
- Koluaçık, E.; Karabiberoğlu, Ş.U.; Dursun, Z. Electrochemical Determination of Serotonin Using Pre-treated Multi-walled Carbon Nanotube-polyaniline Composite Electrode. Electroanalysis 2018, 30, 2977–2987. [Google Scholar] [CrossRef]
- Hassan, A.A.; Abdulazeez, I.; Salawu, O.A.; Al-Betar, A.R. Electrochemical deposition and characterization of polyaniline-grafted graphene oxide on a glassy carbon electrode. SN Appl. Sci. 2020, 2, 1257. [Google Scholar] [CrossRef]
- Korent, A.; Žagar Soderžnik, K.; Šturm, S.; Žužek Rožman, K.; Redon, N.; Wojkiewicz, J.L.; Duc, C. Facile Fabrication of an ammonia-gas sensor using electrochemically synthesised polyaniline on commercial screen-printed three-electrode systems. Sensors 2020, 21, 169. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Phiboolsirichit, N.; Mubeen, S.; Deshusses, M.A.; Mulchandani, A.; Myung, N.V. Electrical and gas sensing properties of polyaniline functionalized single-walled carbon nanotubes. Nanotechnology 2010, 21, 075502. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.L.; Couzi, M. Raman spectra of carbon-based materials (from graphite to carbon black) and of some silicone composites. C 2015, 1, 77–94. [Google Scholar] [CrossRef]
- Korusenko, P.M.; Nesov, S.N.; Iurchenkova, A.A.; Fedorovskaya, E.O.; Bolotov, V.V.; Povoroznyuk, S.N.; Smirnov, D.A.; Vinogradov, A.S. Comparative study of the structural features and electrochemical properties of nitrogen-containing multi-walled carbon nanotubes after ion-beam irradiation and hydrochloric acid treatment. Nanomaterials 2021, 11, 2163. [Google Scholar] [CrossRef]
- Patil, D.S.; Shaikh, J.S.; Pawar, S.A.; Devan, R.S.; Ma, Y.R.; Moholkar, A.V.; Kim, J.H.; Kalubarme, R.S.; Park, C.J.; Patil, P.S. Investigations on silver/polyaniline electrodes for electrochemical supercapacitors. Phys. Chem. Chem. Phys. 2012, 14, 11886–11895. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, S.; Ponnuvelu, D.V.; Pullithadathil, B. Rapid, trace-level ammonia gas sensor based on surface-engineered Ag nanoclusters@ polyaniline/multiwalled carbon nanotubes and insights into their mechanistic pathways. Chem. Sel. 2017, 2, 4277–4289. [Google Scholar] [CrossRef]
- Norizan, M.N.; Zulaikha, N.S.; Norhana, A.B.; Syakir, M.I.; Norli, A. Carbon nanotubes-based sensor for ammonia gas detection–an overview. Polimery 2021, 66, 175–186. [Google Scholar] [CrossRef]
- Dakshayini, B.S.; Reddy, K.R.; Mishra, A.; Shetti, N.P.; Malode, S.J.; Basu, S.; Naveen, S.; Raghu, A.V. Role of conducting polymer and metal oxide-based hybrids for applications in ampereometric sensors and biosensors. Microchem. J. 2019, 147, 7–24. [Google Scholar] [CrossRef]
- Prasad, B.E.; Kamath, P.V.; Ranganath, S. Electrodeposition of ZnO coatings from aqueous Zn (NO3)2 baths: Effect of Zn concentration, deposition temperature, and time on orientation. J. Solid State Electr. 2012, 16, 3715–3722. [Google Scholar] [CrossRef]
- Chang, C.M.; Hon, M.H.; Leu, I.C. Preparation of ZnO nanorod arrays with tailored defect-related characterisitcs and their effect on the ethanol gas sensing performance. Sens. Actuators B-Chem. 2010, 151, 15–20. [Google Scholar] [CrossRef]
- Izaki, M.; Omi, T. Electrolyte optimization for cathodic growth of zinc oxide films. J. Electrochem. Soc. 1996, 143, L53. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Zhang, X. Novel 3D graphene aerogel–ZnO composites as efficient detection for NO2 at room temperature. Sens. Actuators B-Chem. 2015, 211, 220–226. [Google Scholar] [CrossRef]
- Gu, C.; Shanshan, L.; Huang, J.; Shi, C.; Liu, J. Preferential growth of long ZnO nanowires and its application in gas sensor. Sens. Actuators B-Chem. 2013, 177, 453–459. [Google Scholar] [CrossRef]
- Hsu, C.L.; Chen, K.C.; Tsai, T.Y.; Hsueh, T.J. Fabrication of gas sensor based on p-type ZnO nanoparticles and n-type ZnO nanowires. Sens. Actuators B-Chem. 2013, 182, 190–196. [Google Scholar] [CrossRef]
- Scardaci, V.; Compagnini, G. Raman spectroscopy investigation of graphene oxide reduction by laser scribing. C 2021, 7, 48. [Google Scholar] [CrossRef]
- Quillard, S.; Louarn, G.; Lefrant, S.; MacDiarmid, A.G. Vibrational analysis of polyaniline: A comparative study of leucoemeraldine, emeraldine, and pernigraniline bases. Phys. Rev. B 1994, 50, 12496. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Umar, A.; Ibrahim, A.A.; Algadi, H.; Albargi, H.; Alsairi, M.A.; Wang, Y.; Akbar, S. Enhanced NO2 gas sensor device based on supramolecularly assembled polyaniline/silver oxide/graphene oxide composites. Ceram. Int. 2021, 47, 25696–25707. [Google Scholar] [CrossRef]
- Chen, H.; Qu, Y.; Ding, J.; Fu, H. Adsorption behavior of graphene-like ZnO monolayer with oxygen vacancy defects for NO2: A DFT study. Superlattices Microst. 2019, 134, 106223. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, K.; Ha, N.; Cheng, Y.; Ou, R.; Ma, Q.; Hu, Y.; Trinh, V.; Ren, G.; Li, Z.; et al. Reversible room temperature H2 gas sensing based on self-assembled cobalt oxysulfide. Sensors 2022, 22, 303. [Google Scholar] [CrossRef]
- Fioravanti, A.; Marani, P.; Morandi, S.; Lettieri, S.; Mazzocchi, M.; Sacerdoti, M.; Carotta, M.C. Growth mechanisms of ZnO micro-nanomorphologies and their role in enhancing gas sensing properties. Sensors 2021, 21, 1331. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Pan, Q.; Tian, Z. Grain size control and gas sensing properties of ZnO gas sensor. Sens. Actuators B-Chem. 2000, 66, 277–279. [Google Scholar] [CrossRef]
- Wang, J.X.; Sun, X.W.; Yang, Y.; Wu, C.M.L. N–P transition sensing behaviors of ZnO nanotubes exposed to NO2 gas. Nanotechnology 2009, 20, 465501. [Google Scholar] [CrossRef]
- Sanchez-Martın, S.; Olaizola, S.M.; Castaño, E.; Mandayo, G.G.; Ayerdi, I. Low temperature NO2 gas sensing with ZnO nanostructured by laser interference lithography. RSC Adv. 2021, 11, 34144–34151. [Google Scholar] [CrossRef]








| Sensing Layer | Concentration (ppm) | S (%) | LoD (ppm) | RSD (%) |
|---|---|---|---|---|
| PANI-MWCNT-NH2 (10 cycles) | NH3: 8 | 96.99 | 0.85 | 10.06 |
| PANI-MWCNT-NH2 (15 cycles) | NH3: 8 | 55.82 | 0.76 | 11.79 |
| PANI-rGO-ZnO | NO2: 8 | −10.71 | 1.17 | 8.85 |
| Sensing Layer | Slope (Ω/ppm) | R2 (%) | Linear Domain (ppm) |
|---|---|---|---|
| PANI-MWCNT-NH2 (10 cycles) | 186.65 ± 13.01 | 97.85 ± 0.86 | 2–64 |
| PANI-MWCNT-NH2 (15 cycles) | 143.5 ± 11.87 | 97.82 ± 0.92 | 2–64 |
| PANI-rGO-ZnO | −294.27 ± 21.32 | 98.77 ± 0.89 | 0.4–90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoroiu, A.; Mihailescu, C.-M.; Savin, M.; Moldovan, C.A.; Brasoveanu, C.; Dinulescu, S.; Djourelov, N.; Cristian, G.V.; Brincoveanu, O.; Craciun, G.; et al. Facile Electrodeposition-Based Chemosensors Using PANI and C-Hybrid Nanomaterials for the Selective Detection of Ammonia and Nitrogen Dioxide at Room Temperature. Chemosensors 2023, 11, 132. https://doi.org/10.3390/chemosensors11020132
Grigoroiu A, Mihailescu C-M, Savin M, Moldovan CA, Brasoveanu C, Dinulescu S, Djourelov N, Cristian GV, Brincoveanu O, Craciun G, et al. Facile Electrodeposition-Based Chemosensors Using PANI and C-Hybrid Nanomaterials for the Selective Detection of Ammonia and Nitrogen Dioxide at Room Temperature. Chemosensors. 2023; 11(2):132. https://doi.org/10.3390/chemosensors11020132
Chicago/Turabian StyleGrigoroiu, Alexandru, Carmen-Marinela Mihailescu, Mihaela Savin, Carmen Aura Moldovan, Costin Brasoveanu, Silviu Dinulescu, Nikolay Djourelov, Georgescu Vlad Cristian, Oana Brincoveanu, Gabriel Craciun, and et al. 2023. "Facile Electrodeposition-Based Chemosensors Using PANI and C-Hybrid Nanomaterials for the Selective Detection of Ammonia and Nitrogen Dioxide at Room Temperature" Chemosensors 11, no. 2: 132. https://doi.org/10.3390/chemosensors11020132
APA StyleGrigoroiu, A., Mihailescu, C.-M., Savin, M., Moldovan, C. A., Brasoveanu, C., Dinulescu, S., Djourelov, N., Cristian, G. V., Brincoveanu, O., Craciun, G., Pachiu, C., Stan, I., Firtat, B., Muscalu, G. S., Ion, M., & Anghelescu, A. (2023). Facile Electrodeposition-Based Chemosensors Using PANI and C-Hybrid Nanomaterials for the Selective Detection of Ammonia and Nitrogen Dioxide at Room Temperature. Chemosensors, 11(2), 132. https://doi.org/10.3390/chemosensors11020132










