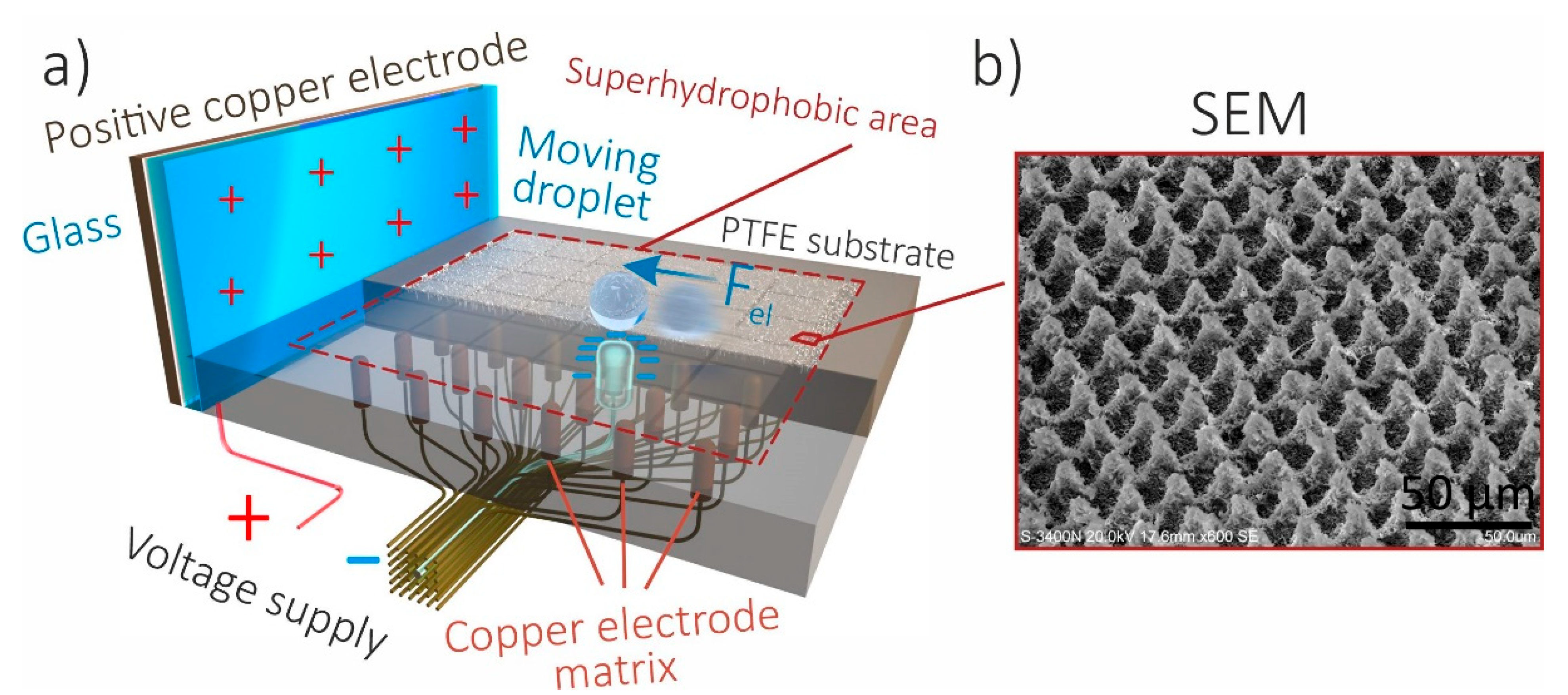
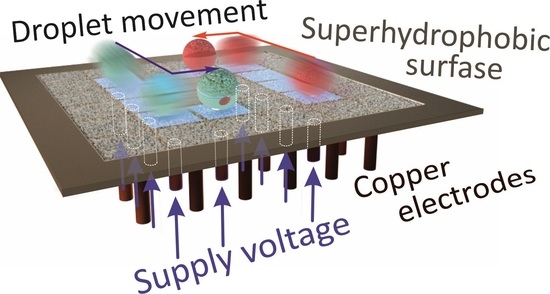
3.1. Controllable Movements of Droplets
Below, the threshold voltage
Uth will be understood as the minimum voltage at the electrode at which the droplet starts moving towards it. As experiments have shown, for a droplet located at a distance of one step from the control electrode, the value of
Uth depends only on the volume of the droplet wherein
Uth does not depend on the polar angle at which the droplet is located with respect to the CE. In other words, the CE-created force field is practically axisymmetric in the local vicinity of the electrode. The experimental dependence of
Uth on the volume of a deionized water droplet located at one step distance from the control electrode is illustrated by curve 1 in
Figure 2.
It can be seen that, with an increase in volume from 0.5 to 7 μL, an approximately twofold increase in threshold voltages is observed. With further increase in volume, however, this trend is reversed so that at Vd = 35 µL, the threshold voltages again decrease to the level Uthr for Vd = 0.5 µL.
It also follows from
Figure 2 that the necessary and sufficient condition for the movement of droplets of all considered volumes (0.5 ÷ 35 μL) by one step is the application of voltage
Uw ≈ 3.5 kV to the CE. We shall, therefore, hereinafter call this voltage the working voltage (WV).
It is important to emphasize that relatively high values of the control voltages are not a significant problem. Indeed, technically it can be easy realized. In addition, a very large internal resistance can be choosen for the voltage sources (in our case, 109 Ω). This implies that currents are so small that in the event of short circuits they do not pose a serious threat to equipment or personnel.
The nature of the dependences presented in
Figure 2 is discussed within the framework of the following model. We will assume that the absolute value of the electric field strength E near the control electrode is proportional to the charge acquired by this electrode q = CU, where C is the capacitance of the CE. Let us assume that a function f(r) describes a certain law of decrease in the field strength with change in distance r from the end of the electrode, taking into account the distribution of the charge density on the electrode and the contribution of the PMD polarization. In other words,
E = CUf(r).
The force acting on a spherical droplet in the non-uniform field of CE can be written as , where —is the polarizability of a spherical drop; a—is its diameter; —is the permittivity of water; —is the permittivity of the environment; —is the gradient operator. Taking into account that , this force can be written as , where .
Due to their mobility, excess like charges are not distributed uniformly in an extended conductor but are concentrated at the greatest possible distance from each other. In accordance with this statement, the highest excess charge density will be observed near the upper end of the CE. Accordingly, in this region, the highest field strength gradients will be achieved and, as a result, the force
acting on the droplet will be directed towards it. Therefore, the horizontal component of this force will be equal to
where
,
is the angle at which the center of the droplet is visible from the top of the CE. When the threshold voltage
Uth on the CE is reached, this component exceeds the pinning force, which, based on [
28], can be represented as:
where the coefficient
depends on the value of the contact angle, its hysteresis and other vapor-liquid surface tension parameters, while
specifies the contact zone perimeter up to a constant coefficient. Based on this, the value of the threshold voltage can be found as:
Strictly speaking, and, consequently, the value of depend on the volume of the drop. However, for a droplet located a step away from the CE, and . Therefore, the value of can be considered a constant. If, in addition, the coefficient is also assumed to be constant, then, as can be seen from Expression (1), the threshold voltage should decrease monotonically with an increase in the droplet volume, which is experimentally observed only at Vd > 7 μL.
Due to the complexity of calculating the exact value of the coefficient
, the ratio
in Expression (1) can be viewed as a fitting parameter. It is chosen in such a way that the calculated dependence
, illustrated by curve 2 in
Figure 2, agrees with the experimental curve 1 for droplets of the largest volume. As can be seen, a significant discrepancy between the experimental and calculated curves is already observed at
Vd < 15 µL. This discrepancy can be explained by the fact that, in the presence of voltage on the CE, the coefficient
in Expression (2) becomes a function of the volume
Vd.
It was noted above that we exclude the possibility of direct leakage of excess charge onto the droplet. Therefore, the phenomena caused by the NEF on the PMD should be associated with the polarization of the droplet in an electric field. This effect leads to the appearance of polarization charges in the zone of its contact with the dielectric surface. As a result, conditions for a phenomenon known as electrowetting on a dielectric (EWOD) are realized at the solid/liquid interface [
29,
30].
In contrast to the classical case of EWOD [
30,
31], in our case there is no direct electrical contact between the voltage source and the droplet. In addition, the electrode located below the droplet is not flat but rod-shaped, with the diameter of no more than 500 μm. Therefore, the electric field created by it is highly non-uniform even on the scale of the smaller droplets with a diameter of ~1 mm. This leads to the appearance of higher-order multipole charges on the droplet. This process leads to a strong non-uniformity of the surface charge density in the region of its contact with the dielectric. These features significantly complicate the mathematical description of the features of electrowetting in our case, in comparison with the classical EWOD. Such a description is possible only on the basis of additional studies, which, in our opinion, are beyond the scope of this work.
However, some correction of the analytical curve 2 in
Figure 2 can be performed using the results of the following experiment.
Droplets of various volumes are deposited from a capillary onto an inclined PMD at a point located above the selected CE. A short-term voltage pulse (~1 s) with an amplitude of 4 kV is applied to it, which initiates the deposition process and the initial retention of the droplet on the PMD. In the case when the angle of inclination of the PMD exceeds the roll-off angle modified by the electric field, the droplet begins to move immediately after the end of the pulse. Otherwise, the droplet remains “fixed” for another ~1000 ms. We interpret this delay as the time required for the transition from electrowetting to normal conditions.
The obtained results of measuring the angle
modified as a result of the EWOD phenomenon (
Vd) are shown by points 1 in
Figure 3a. For comparison, the same
Figure 2 shows curve 2, which illustrates the dependence of the roll-off angle
in the absence of voltage. Let us assume that
depends on the volume of the droplet according to the power law
, as is the case for the “usual” roll-off angle
[
32,
33,
34]. It can be seen from
Figure 3 that, with an increase in the droplet volume, the angle
decreases more slowly than does
. Therefore, the exponent p in
will be somewhat smaller for
than for
. The best fit of the experimental data for
is achieved with
p = 1/2 (curve 2 in
Figure 3).
The droplet starts rolling at the moment when the force of gravity
acting on it (where
is the density of water) becomes equal to the modified pinning force
. Based on this statement, it is easy to recalculate the data in
Figure 3a to obtain the dependence of the modified pinning force on the droplet volume. The corresponding results of processing the experimental data (points) are shown in
Figure 3b along with the approximating dependence, which, in this case, is given by the expression
This character of the increase in the pinning force, in the presence of NEF, in turn requires the modification of Expressions (2) and (3), so that the coefficient
in them becomes dependent on the volume of the droplet according to the law
. This leads to the correction of the dependence
), which is illustrated by curve 3 in
Figure 2. As can be seen, the corrected analytical dependence correlates somewhat better with the experimental curve 1, although, as before, it does not explain its behavior at
Vd ≲ 7 µL. Apparently, this is because the model being used becomes inaccurate for such volumes of the droplet.
In the experiments on the movement of droplets on the PMD, the movement by one step distance takes time
, after which the droplet performs damped oscillations for the time
near the CE that attracts it. Microscopic observations show that, in the course of oscillations, the droplet slides back and forth about the equilibrium position as a whole without visible shape deformations. This process is illustrated in the high-speed footage in the
Supplementary Materials (
Video S1). It increases the total time to complete one step, which becomes equal to
. This, accordingly, reduces the average speed of movement by one step to the value
, approximately three times less than the translational speed
.
The dependences of the times
,
and
on the droplet volume in the case when
Uw = 3.5 kV are shown in
Figure 4, curves 1, 2, and 3, respectively. It is also seen that the total time of one step
tpass for droplets in the volume range of 3–15 μL, which is the most interesting from a practical point of view, does not exceed ~300 ms. This sets the lower limit of the average droplet velocity
for this range of volumes to ~17 mm/s. The upper limit of the average velocity is reached for droplets of the smallest volume of 3 µL, and is 50 mm/s. In addition, the maximum droplet movement velocity
reaches 150 mm/s.
We also studied the possibility of controlling droplets whose volumes exceed those considered above by more than an order of magnitude. As it turned out, the possibilities of fail-safe control are exhausted when the volume
Vd exceeds 200 μm. In this case, a too massive droplet does not always stop above the selected CE, and, while continuing to move, it can roll out of the working area of the PMD or coalesce uncontrollably with another droplet. The
Video S2 demonstrates the process of controlled movement of a 200-µL droplet. It can be seen that, for this droplet, it is still possible to implement a complex broken trajectory of travel, with the average velocity reduced to 10 mm/s.
Usually, the damping of the pendulum oscillations, which, in our case, is a droplet of water near the CE, is considered a result of viscous friction. In this case, there is a gradual damping of the oscillation amplitude according to an exponential law. Moreover, weak oscillations of the system can be detected for a prolonged period of time, much longer than the characteristic decay time. In our case, however, the droplet oscillation amplitude decays almost linearly down to a complete stop. This damping process is typical for oscillations occurring with a constant friction force, for which the pinning force
given by Expression (4) can be taken as an estimate. This expression can also be recast as
, where
md is the mass of the droplet,
g is the free fall acceleration, and the coefficient of sliding friction
μ depends on the volume, according to the law
As follows from the solution of the problem of free oscillations of a spring pendulum weighing m
d, the time until it stops completely under the action of a constant friction force turns out to be inversely proportional to the product
. For the law of change of the coefficient μ obtained above, this product turns out to be equal to
. As a result, the deceleration time ta should be almost independent of the droplet volume. Such (or similar) character of the
tvibr(
Vd) dependence is observed only for droplets larger than 7 μL (curve 2 in
Figure 4). A different character of this dependence for droplets of smaller volumes is obviously due to the poor accuracy of the model being used at
Vd ≲ 7 µL as discussed above.
Sequential application of the control voltage
Uw to a series of electrodes makes it possible to achieve a step-by-step movement of a selected droplet along predetermined, including non-rectilinear trajectories (
Video S3). To exclude accidental movement of all other droplets, a holding voltage equal to
Ufix = 2 kV is applied to the electrodes located under them (
Video S4). In this case, this technique allows one to selectively move selected droplets along straight, broken, zigzag, including closed trajectories, similar to how pieces move in a chess game. In addition, due to the combination of holding and control voltages on the CE, it is possible to implement parallel control of the movements of several droplets at once (
Video S5).
Generally speaking, the time of complete movement of the droplet by several steps N
pass grows as t
passN, where N is the number of steps. However, in the case where it is required to move the droplet along a straight line, this time can be significantly reduced by simultaneously switching on a series of CEs along this straight line at once. With such a movement, the droplet “flies” over the next control electrode due to its inertia, enters the field of the next one and then moves under its influence (
Video S6). In this case, the damped vibration of the droplet occurs only at the last of the switched-on CEs. As a result, the average velocity of the droplet in this mode v
Npass increases significantly compared to the velocity of movement by one step. In
Figure 4b the dependence of v
Npass on the distance traveled by a 4 μL droplet is shown. As might be expected, the average speed v
Npass increases with the number of steps. For 4 steps, it reaches 75 mm/s, at
Vd = 4 µL.
3.2. Controllable Coalescence and Microliter Reactions
In the proposed PMD, the coalescence of two or more liquid droplets can be realized. It is carried out in the following ways. The first of them (
Figure 5a,b,
Video S7) consists of applying a control voltage (3.5 kV) to the CE located in the gap between two or more droplets at a distance of about a step from each of them. Under the action of the NEF, all droplets move towards this CE, above which the droplets merge. The second method is suitable only for manipulations with two droplets (
Figure 5c,d). It consists in applying a voltage to the CE located under one of them, and the second one is located at a distance of no more than one step h. In this case, successful coalescence requires higher values of the CE voltage (6 kV), since the fixed droplet is reducing the field gradient in the region of the moving droplet. For the coalescence of three droplets in this way, higher voltages on the CE are needed, which, as mentioned above, leads to negative consequences.
Actually, in both approaches, the droplets can be located at a greater distance than h, but this also requires increasing voltages beyond acceptable levels. This implies the need to set certain starting positions of the droplets before coalescence, which can have been achieved by the preliminary selective movement of the droplets.
Using the proposed PMD, various chemical reactions can be implemented in microliter volumes, which obviously requires much smaller quantities of reagents than in the usual case of reactions “in test tubes”. In the
Video S8, an example of the implementation of a chemical reaction is demonstrated when droplets of solutions of copper sulfate and sodium hydroxide merge. The formation of a blue precipitate of copper hydroxide is visible.
Figure 5c,d and
Video S9 illustrate an example of the implementation of an analytical reaction for the detection of a hydroxyl radical. In this case, droplets of sodium hydroxide and methyl orange solutions merge. As can be seen, immediately after coalescence, the less dense methyl orange is concentrated in the upper part of the merged droplet, while sodium hydroxide is in the lower part. Further, during the diffusion of hydroxide, a gradual change in the color of the upper half of the droplet to yellow is observed, which demonstrates the detection of the desired radicals.
It is known that an important problem of superhydrophobic surfaces is the initial deposition of droplets at a given point. Due to the low adhesion of such surfaces, even slight vibrations of the capillary can lead to uncontrolled movement of the drop or even its rolling off. In our platform, this problem is completely solved by applying a holding voltage (2.5 kV is enough) to the CE located at the surface point chosen for deposition. Even with an inaccurate location of the capillary or an accidental jump of a droplet during the deposition process, the latter will still be deposited in the selected position.
Another problem with superhydrophobic surfaces is the capillary deposition of droplets with a volume of less than 1 μL. In this case, the total action of gravity and pinning forces on the droplet from the superhydrophobic surface is insufficient to overcome the pinning forces from the capillary, which prevents its deposition on the substrate. This problem can also be solved by applying voltage to the CE at the selected point of the PMD. In our case, it was possible to deposit droplets with a volume down to 0.1 µL in this way.
The use of holding voltages makes it possible not only to solve the problems of uncontrolled movement and rolling of droplets under random vibrations and inclinations of superhydrophobic substrates but also to realize controlled movement and even coalescence of droplets on an inclined PMD, at an inclination angle
of up to 20° (
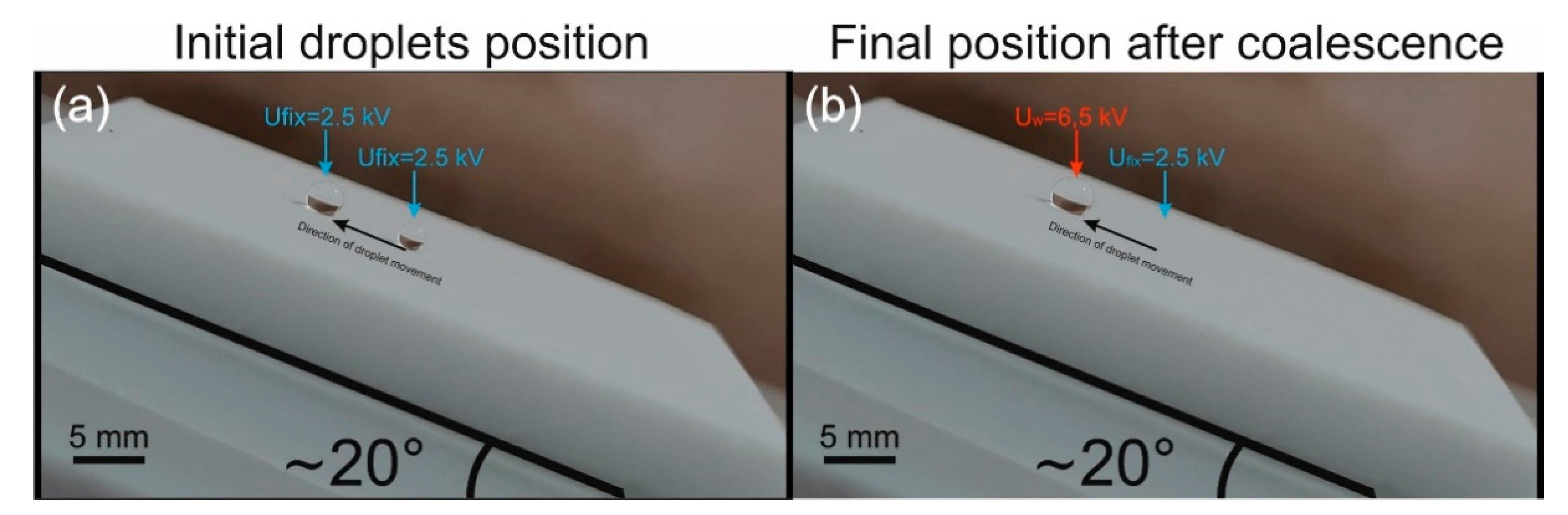
Figure 6a,b. In the case where
> 5°, moving the droplets by several steps at once by simultaneous activation of several CEs is no longer possible. Apparently, this is due to the fact that, when moving up the slope after moving to a nearby CE, the inertia of the droplet is not enough to get into the area of action of the next electrode. When moving down, a droplet gets accelerated too much and rolls off the working area. Therefore, in this case, the movement of the droplet can only be step-by-step.
On an inclined PMD, the mode of droplet coalescence can also be achieved, which is illustrated in
Figure 6 and
Video S10. Merging of droplets in this case is realized only in the second way, when both droplets are initially held on the PMD by the application of holding voltages
Ufix = 2.5 kV. Then there is a pulsed increase in voltage to 6 kV under the selected motionless droplet, as a result of which the second droplet is attracted to it.
Based on experiments with inclined PMDs, it is possible to estimate the maximum allowed accelerations aimp caused by lateral displacements of the horizontal platform, its vibrations and mechanical impacts. Based on the value of the maximum allowable slope angle , we obtain that .