Solid-Phase Extraction Followed by Gas Chromatography–Mass Spectrometry for Revealing the Effects of the Application of Bentonite, Tannins, and Their Combination during Fermentation in the Production of White Wine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Winemaking and Treatments
2.2. Post-Fermentation Procedures
2.3. Protein Stability Tests
2.4. Standard Physicochemical Analyses
2.5. Analysis of Phenolic Compounds by High-Performance Liquid Chromatography with Diode-Array Detection (HPLC-DAD)
2.6. Isolation of Free and Bound Volatile Aroma Compounds by Solid-Phase Extraction (SPE) and Analysis by Gas Chromatography–Mass Spectrometry (GC-MS)
2.7. Data Elaboration
3. Results and Discussion
3.1. Standard Physicochemical Parameters
3.2. Phenols
3.3. Free Volatile Aroma Compounds
3.4. Bound Aroma Compounds
3.5. Partial Least Squares-Discriminant Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, J.A.; Saucier, C.; Glories, Y. Grape and wine phenolics: History and perspective. Am. J. Enol. Vitic. 2006, 57, 239–248. [Google Scholar] [CrossRef]
- Sáenz-Navajas, M.-P.; Avizcuri, J.-M.; Ferreira, V.; Fernández-Zurbano, P. Insights on the chemical basis of the astringency of Spanish red wines. Food Chem. 2012, 134, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Gris, E.F.; Mattivi, F.; Ferreira, E.A.; Vrhovsek, U.; Filho, D.W.; Pedrosa, R.C.; Bordignon-Luiz, M.T. Phenolic profile and effect of regular consumption of Brazilian red wines on in vivo antioxidant activity. J. Food Compos. Anal. 2013, 31, 31–40. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, Volume 2: The Chemistry of Wine, Stabilization and Treatments, 2nd ed.; John Wiley & Sons: Chichester, UK, 2006; ISBN 0-470-01037-1. [Google Scholar]
- Marangon, M.; Stockdale, V.J.; Munro, P.; Trethewey, T.; Schulkin, A.; Holt, H.E.; Smith, P.A. Addition of Carrageenan at Different Stages of Winemaking for White Wine Protein Stabilization. J. Agric. Food Chem. 2013, 61, 6516–6524. [Google Scholar] [CrossRef]
- Chagas, R.; Monteiro, S.; Ferreira, R.B. Assessment of potential effects of common fining agents used for white wine protein stabilization. Am. J. Enol. Vitic. 2012, 63, 574–578. [Google Scholar] [CrossRef]
- Lira, E.; Rodríguez-Bencomo, J.J.; Salazar, F.N.; Orriols, I.; Fornos, D.; López, F. Impact of Bentonite Additions during Vinification on Protein Stability and Volatile Compounds of Albariño Wines. J. Agric. Food Chem. 2015, 63, 3004–3011. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.J.; Alexander, G.; Muhlack, R.; Pocock, K.F.; Colby, C.; O’Neill, B.K.; Høj, P.B.; Jones, P. Preventing protein haze in bottled white wine. Aust. J. Grape Wine Res. 2005, 11, 215–225. [Google Scholar] [CrossRef]
- Puig-Deu, M.; López-Tamames, E.; Buxaderas, S.; Torre-Boronat, M.C. Influence of must racking and fining procedures on the composition of white wine. Vitis 1996, 35, 141–145. [Google Scholar] [CrossRef]
- Majewski, P.; Barbalet, A.; Waters, E. $1 billion hidden costs of bentonite fining. Aust. N. Z. Grapegrow. Winemak. 2011, 569, 58–62. [Google Scholar]
- Vincenzi, S.; Panighel, A.; Gazzola, D.; Flamini, R.; Curioni, A. Study of Combined Effect of Proteins and Bentonite Fining on the Wine Aroma Loss. J. Agric. Food Chem. 2015, 63, 2314–2320. [Google Scholar] [CrossRef]
- Pocock, K.F.; Salazar, F.N.; Waters, E.J. The effect of bentonite fining at different stages of white winemaking on protein stability. Aust. J. Grape Wine Res. 2011, 17, 280–284. [Google Scholar] [CrossRef]
- Lira, E.; Salazar, F.N.; Rodríguez-Bencomo, J.J.; Vincenzi, S.; Curioni, A.; López, F. Effect of using bentonite during fermentation on protein stabilisation and sensory properties of white wine. Int. J. Food Sci. Technol. 2014, 49, 1070–1078. [Google Scholar] [CrossRef]
- Horvat, I.; Radeka, S.; Plavša, T.; Lukić, I. Bentonite fining during fermentation reduces the dosage required and exhibits significant side-effects on phenols, free and bound aromas, and sensory quality of white wine. Food Chem. 2019, 285, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Vignault, A.; Pascual, O.; Jourdes, M.; Moine, V.; Fermaud, M.; Roudet, J.; Canals, J.M.; Teissedre, P.-L.; Zamora, F. Impact of enological tannins on laccase activity. OENO One 2019, 53, 27–38. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Parpinello, G.P.; Heymann, H.; Downey, M.O. Impact of exogenous tannin additions on wine chemistry and wine sensory character. Food Chem. 2012, 131, 999–1008. [Google Scholar] [CrossRef]
- OIV (International Organisation of Vine and Wine). COEI-1-TANINS, Oenological Tannins (OIV-OENO 554-2015); International Oenological CODEX; Organisation Internationale de la Vigne et du Vin: Paris, France, 2015; Available online: https://www.oiv.int/public/medias/4093/e-coei-1-tanins.pdf (accessed on 28 June 2020).
- Sonni, F.; Cejudo Bastante, M.J.; Chinnici, F.; Natali, N.; Riponi, C. Replacement of sulfur dioxide by lysozyme and oenological tannins during fermentation: Influence on volatile composition of white wines. J. Sci. Food Agric. 2009, 89, 688–696. [Google Scholar] [CrossRef]
- Sonni, F.; Chinnici, F.; Natali, N.; Riponi, C. Pre-fermentative replacement of sulphur dioxide by lysozyme and oenological tannins: Effect on the formation and evolution of volatile compounds during the bottle storage of white wines. Food Chem. 2011, 129, 1193–1200. [Google Scholar] [CrossRef]
- Canuti, V.; Puccioni, S.; Giovani, G.; Salmi, M.; Rosi, I.; Bertuccioli, M. Effect of Oenotannin Addition on the Composition of Sangiovese Wines from Grapes with Different Characteristics. Am. J. Enol. Vitic. 2012, 63, 220–231. [Google Scholar] [CrossRef]
- Larcher, R.; Tonidandel, L.; Villegas, T.R.; Nardin, T.; Fedrizzi, B.; Nicolini, G. Pre-Fermentation Addition Of Grape Tannin Increases The Varietal Thiols Content In Wine. Food Chem. 2015, 166, 56–61. [Google Scholar] [CrossRef]
- Jeremic, J.; Vongluanngam, I.; Ricci, A.; Parpinello, G.P.; Versari, A. The Oxygen Consumption Kinetics of Commercial Oenological Tannins in Model Wine Solution and Chianti Red Wine. Molecules 2020, 25, 1215. [Google Scholar] [CrossRef]
- Hagerman, A.E. Chemistry of tannin-protein complexation. In Chemistry and Significance of Condensed Tannins, 1st ed.; Hemingway, R.W., Karchesy, J.J., Eds.; Plenum Press: New York, NY, USA, 1989; pp. 323–331. ISBN 978-1-4684-7513-5. [Google Scholar]
- Haslam, E.; Lilley, T.H.; Warminski, E.; Liao, H.; Cai, Y.; Martin, R.; Gaffney, S.H.; Goulding, P.N.; Luck, G. Polyphenol complexation. A study in molecular recognition. In Phenolic Compounds in Food and Their Effects on Health I: Analysis, Ocurrence, & Chemistry, 1st ed.; Ho, C.-T., Lee, C.Y., Huang, M.-T., Eds.; American Chemical Society: Washington, DC, USA, 1992; pp. 8–50. ISBN 978-0-8412-2475-9. [Google Scholar]
- Terrier, N.; Poncet-Legrand, C.; Cheynier, V. Flavanols, Flavonols and Dihydroflavonols. In Wine Chemistry and Biochemistry, 1st ed.; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 463–507. ISBN 978-0-387-74116-1. [Google Scholar]
- Hagerman, A.E.; Rice, M.E.; Richard, N.T. Mechanisms of protein precipitation for two tannins, pentagalloylglucose and epicatechin16(4→8) catechin (procyanidin). J. Agric. Food Chem. 1998, 46, 2590–2595. [Google Scholar] [CrossRef]
- Radeka, S.; Peršurić, Đ.; Lukić, I.; Bocca, E.; Plavša, T. Influence of the addition of tannins of different origin on protein stability and aromatic profile of Malvazija istarska wine. In Proceedings of the 32nd World Congress of Vine and Wine and 7th General Assembly of the OIV, Zagreb, Croatia, 28 June–3 July 2009; Kurbanovic, V., Ed.; Ministry of Agriculture, Fisheries and Rural Development: Zagreb, Croatia, 2009. [Google Scholar]
- Lukić, I.; Horvat, I. Moment of Bentonite Addition, Co-Addition of Tannins, and Bentonite Type Affect the Differential Affinity of Pathogenesis-Related Grape Proteins towards Bentonite during Fermentation. Foods 2020, 9, 1534. [Google Scholar] [CrossRef]
- Versari, A.; du Toit, W.; Paripinello, G.P. Oenological tannins: A review. Aust. J. Grape Wine Res. 2013, 19, 1–10. [Google Scholar] [CrossRef]
- Pati, S.; Crupi, P.; Benucci, I.; Antonacci, D.; Di Luccia, A.; Esti, M. HPLC-DAD–MS/MS characterization of phenolic compounds in white wine stored without added sulfite. Food Res. Int. 2014, 66, 207–215. [Google Scholar] [CrossRef]
- Lukić, I.; Budić-Leto, I.; Bubola, M.; Damijanić, K.; Staver, M. Pre-fermentative cold maceration, saignée, and various thermal treatments for more options in modulating volatile aroma and phenol profiles of red wine. Food Chem. 2017, 224, 251–261. [Google Scholar] [CrossRef]
- Di Stefano, R.; Cravero, M.C.; Gentilini, N. Metodi per lo studio dei polifenoli dei vini. L’Enotecnico 1989, 5, 83–89. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phoshotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Lotti, C.; Masuero, D.; Carlin, S.; Weingart, G.; Mattivi, F. Quantitative metabolic profiling of grape, apple and raspberry volatile compounds (VOCs) using a GC/MS/MS method. J. Chromatogr. B 2014, 966, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Carlin, S.; Lotti, C.; Correggi, L.; Mattivi, F.; Arapitsas, P.; Vrhovšek, U. Measurement of the Effect of Accelerated Aging on the Aromatic Compounds of Gewürztraminer and Teroldego Wines, Using a SPE-GC-MS/MS Protocol. Metabolites 2022, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Zoecklein, B.W.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. Production Wine Analysis, 1st ed.; Van Nostrand Reinhold: New York, NY, USA, 1990; ISBN 0-442-23463-5. [Google Scholar]
- Main, G.L.; Morris, J.R. Color of Riesling and Vidal Wines as Affected by Bentonite, Cufex®, and Sulfur Dioxide Juice Treatments. Am. J. Enol. Vitic. 1991, 42, 354–357. [Google Scholar] [CrossRef]
- Salazar, F.N.; Achaerandio, I.; Labbé, M.A.; Güell, C.; López, F. Comparative study of protein stabilization in white wine using zirconia and bentonite: Physicochemical and wine sensory analysis. J. Agric. Food Chem. 2006, 54, 9955–9958. [Google Scholar] [CrossRef]
- Lambri, M.; Dordoni, R.; Silva, A.; De Faveri, D.M. Comparing the impact of bentonite addition for both must clarification and wine fining on the chemical profile of wine from Chambave Muscat grapes. Int. J. Food Sci. Technol. 2012, 47, 1–12. [Google Scholar] [CrossRef]
- Ma, T.-Z.; Gong, P.-F.; Lu, R.-R.; Zhang, B.; Morata, A.; Han, S.-Y. Effect of Different Clarification Treatments on the Volatile Composition and Aromatic Attributes of ‘Italian Riesling’ Icewine. Molecules 2020, 25, 2657. [Google Scholar] [CrossRef] [PubMed]
- Salazar, F.N.; Marangon, M.; Labbé, M.; Lira, E.; Rodríguez-Bencomo, J.J.; López, F. Comparative study of sodium bentonite and sodium-activated bentonite fining during white wine fermentation: Its effect on protein content, protein stability, lees volume, and volatile compounds. Eur. Food Res. Technol. 2017, 243, 2043–2054. [Google Scholar] [CrossRef]
- Vela, E.; Hernández-Orte, P.; Castro, E.; Ferreira, V.; Lopez, R. Effect of Bentonite Fining on Polyfunctional Mercaptans and Other Volatile Compounds in Sauvignon blanc Wines. Am. J. Enol. Vitic. 2017, 68, 30–38. [Google Scholar] [CrossRef]
- Wu, Z.S.; Sun, X.F.; Li, C. Effects of Bentonite Clarificants on Organic Acids Contents in Red Wine during Clarification. Adv. Mat. Res. 2011, 194–196, 802–805. [Google Scholar] [CrossRef]
- Berg, H.W.; Akiyoshi, M. The utility of potassium bitartrate concentration product values in wine processing. Am. J. Enol. Vitic. 1971, 22, 127–134. [Google Scholar] [CrossRef]
- Lambri, M.; Colangelo, D.; Dordoni, R.; Torchio, F.; De Faveri, D.M. Innovations in the Use of Bentonite in Oenology: Interactions with Grape and Wine Proteins, Colloids, Polyphenols and Aroma Compounds. In Grape and Wine Biotechnology; Morata, A., Loira, I., Eds.; IntechOpen: London, UK, 2016; pp. 381–400. [Google Scholar]
- Jourdes, M.; Pouységu, L.; Deffieux, D.; Teisseder, P.-L.; Quideau, S. Hydrolyzable Tannins: Gallotannins and Ellagitannins. In Natural Products; Ramawat, K.G., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1975–2010. [Google Scholar]
- Neves, A.C.; Spranger, M.I.; Zhao, Y.; Leandro, M.C.; Sun, B. Effect of Addition of Commercial Grape Seed Tannins on Phenolic Composition, Chromatic Characteristics, and Antioxidant Activity of Red Wine. J. Agric. Food Chem. 2010, 58, 11775–11782. [Google Scholar] [CrossRef]
- Jaeckels, N.; Tenzer, S.; Rosch, A.; Scholten, G.; Decker, H.; Fronk, P. β-Glucosidase removal due to bentonite fining during winemaking. Eur. Food Res. Technol. 2015, 241, 253–262. [Google Scholar] [CrossRef]
- Cheynier, V.; Souquet, J.-M.; Samson, A.; Moutounet, M. Hyperoxidation: Influence of various oxygen supply levels on oxidation kinetics of phenolic compounds and wine quality. Vitis 1991, 30, 107–115. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Ritchard, N.T.; Hatzfeld, P.W.; Riechel, T.L. High Molecular Weight Plant Polyphenolics (Tannins) as Biological Antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, C.; Taillandier, P.; Rizk, M.; Rizk, Z.; Nehme, N.; Souchard, J.P.; El Rayess, Y. Analysis of the impact of fining agents types, oenological tannins and mannoproteins and their concentrations on the phenolic composition of red wine. LWT—Food Sci. Technol. 2017, 83, 101–109. [Google Scholar] [CrossRef]
- Haslam, E. In vino veritas: Oligomeric procyanidins and the ageing of red wines. Phytochemistry 1980, 19, 2577–2582. [Google Scholar] [CrossRef]
- Obreque-Slíer, E.; Peña-Neira, A.; López-Solís, R.; Ramírez-Escudero, C.; Zamora-Marín, F. Phenolic characterization of commercial enological tannins. Eur. Food Res. Technol. 2009, 229, 859–866. [Google Scholar] [CrossRef]
- Yɪldɪrɪm, H.K. Effects of Fining Agents on Antioxidant Capacity of Red Wines. J. Inst. Brew. 2011, 117, 55–60. [Google Scholar] [CrossRef]
- Dordoni, R.; Galasi, R.; Colangelo, D.; De Faveri, D.M.; Lambri, M. Effects of fining with different bentonite labels and doses on colloidal stability and colour of a Valpolicella red wine. Int. J. Food Sci. Technol. 2015, 50, 2246–2254. [Google Scholar] [CrossRef]
- Main, G.L.; Morris, J.R. Color of Seyval blanc juice and wine as affected by juice fining and bentonite fining during fermentation. Am. J. Enol. Vitic. 1994, 45, 417–422. [Google Scholar] [CrossRef]
- Monagas, M.; Bartolomé, B.; Gómez-Cordovés, C. Evolution of polyphenols in red wines from Vitis vinifera L. during aging in the bottle. Eur. Food Res. Technol. 2005, 220, 331–340. [Google Scholar] [CrossRef]
- Armada, L.; Falqué, E. Repercussion of the clarification treatment agents before the alcoholic fermentation on volatile composition of white wines. Eur. Food Res. Technol. 2007, 225, 553–558. [Google Scholar] [CrossRef]
- Burin, V.M.; Caliari, V.; Bordignon-Luiz, M.T. Nitrogen compounds in must and volatile profile of white wine: Influence of clarification process before alcoholic fermentation. Food Chem. 2016, 202, 417–425. [Google Scholar] [CrossRef]
- Lambri, M.; Dordoni, R.; Silva, A.; De Faveri, D.M. Effect of bentonite fining on odor-active compounds in two different white wine styles. Am. J. Enol. Vitic. 2010, 61, 225–233. [Google Scholar] [CrossRef]
- Ough, C.S.; Berg, H.; Amerine, M.A. Substances extracted during skin contact with white musts. II. Effect of bentonite additions during and after fermentation on wine composition and sensory quality. Am. J. Enol. Vitic. 1969, 20, 101–107. [Google Scholar] [CrossRef]
- Chen, K.; Escott, C.; Loira, I.; Del Fresno, J.M.; Morata, A.; Tesfaye, W.; Calderon, F.; Benito, S.; Suárez-Lepe, J.A. The Effects of Pre-Fermentative Addition of Oenological Tannins on Wine Components and Sensorial Qualities of Red Wine. Molecules 2016, 21, 1445. [Google Scholar] [CrossRef]
- Martin, V.; Boido, E.; Giorello, F.; Mas, A.; Dellacassa, E.; Carrau, F. Effect of yeast assimilable nitrogen on the synthesis of phenolic aroma compounds by Hanseniaspora vineae strains. Yeast 2016, 33, 323–328. [Google Scholar] [CrossRef]
- Bellachioma, A.; Riponi, C.; Sonni, F.; Chinnici, F. Applicazione di un protocollo sperimentale per la produzione di vini bianchi in assenza di SO2. L’Enologo 2008, 5, 77–81. [Google Scholar]
- Patrianakou, M.; Roussis, I.G. Decrease of wine volatile aroma esters by oxidation. S. Afr. J. Enol. Vitic. 2013, 34, 241–245. [Google Scholar] [CrossRef]
- Zoecklein, B.W.; Marcy, J.E.; Jasinski, Y. Effect of fermentation, storage sur lie or post-fermentation thermal processing on White Riesling (Vitis vinifera L.) glycoconjugates. Am. J. Enol. Vitic. 1997, 48, 397–402. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Oliveira, P.; Baumes, R.L.; Maia, O. Changes in aromatic characteristics of Loureiro and Alvarinho wines during maturation. J. Food Compos. Anal. 2008, 21, 695–797. [Google Scholar] [CrossRef]
- Lambri, M.; Dordoni, R.; Silva, A.; De Faveri, D.M. Odor-active compound adsorption onto bentonite in a model white wine solution. Chem. Eng. Trans. 2013, 32, 1741–1746. [Google Scholar] [CrossRef]
- Di Gaspero, M.; Ruzza, P.; Hussain, R.; Vincenzi, S.; Biondi, B.; Gazzola, D.; Siligardi, G.; Curioni, A. Spectroscopy reveals that ethyl esters interact with proteins in wine. Food. Chem. 2017, 217, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Maroto, M.C.; Schneider, R.; Baumes, R. Formation Pathways of Ethyl Esters of Branched Short-Chain Fatty Acids during Wine Aging. J. Agric. Food Chem. 2005, 53, 3503–3509. [Google Scholar] [CrossRef] [PubMed]
- McMahon, H.M.; Zoecklein, B.W.; Jasinski, Y.W. The effects of prefermentation maceration temperature and percent alcohol (v/v) at press on the concentration of Cabernet Sauvignon grape glycosides and glycoside fractions. Am. J. Enol. Vitic. 1999, 50, 385–390. [Google Scholar] [CrossRef]
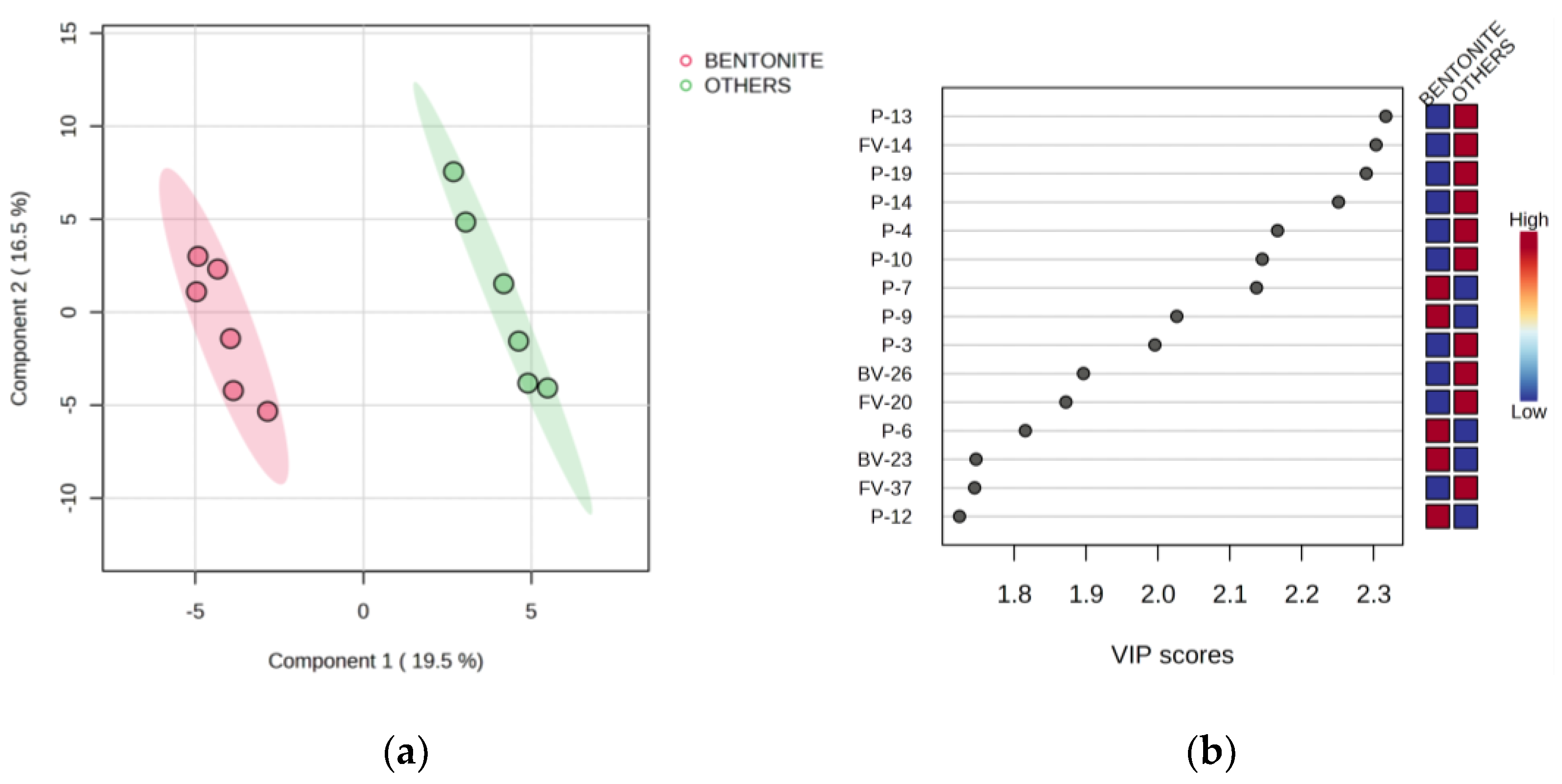
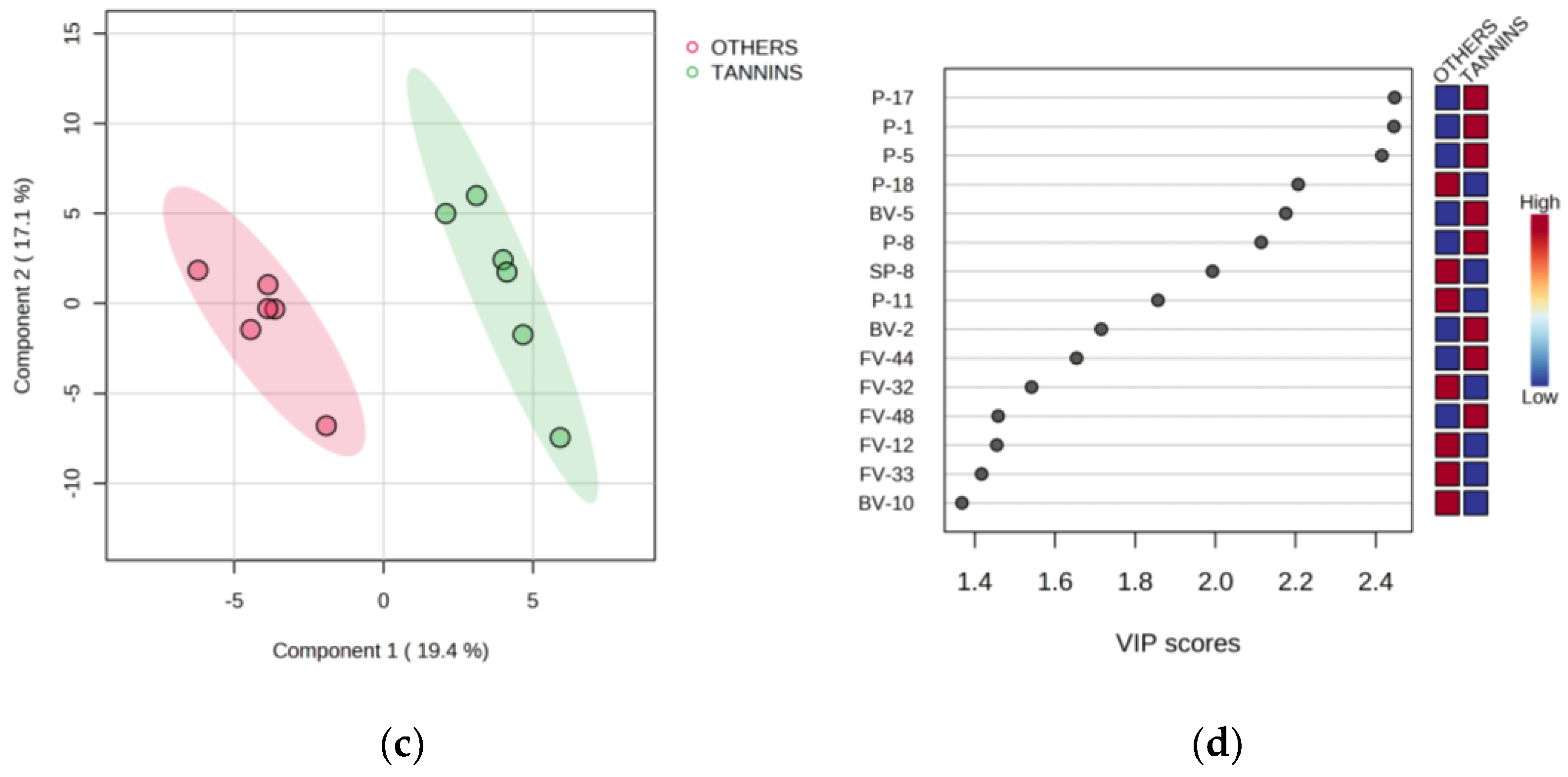
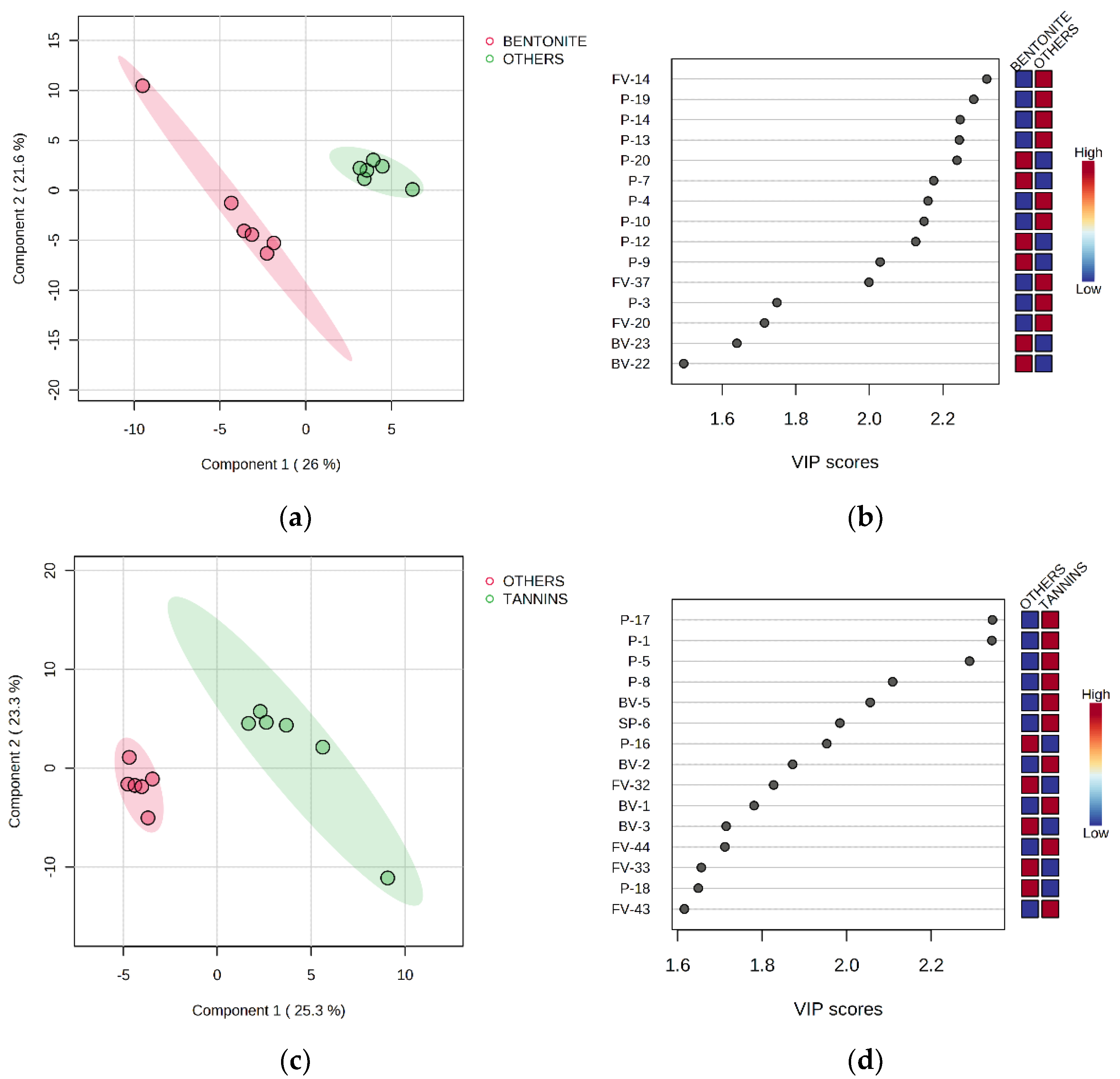
| Code | Parameter | Stage | Treatment | |||
|---|---|---|---|---|---|---|
| CO | B | T | BT | |||
| SP-1 | Relative density | AFerm | 0.9895 ± 0.0002 ab | 0.9893 ± 0.0002 b | 0.9898 ± 0.0003 a | 0.9895 ± 0.0001 ab |
| ProStab | 0.9894 ± 0.0002 b | 0.9895 ± 0.0002 ab | 0.9898 ± 0.0002 a | 0.9896 ± 0.0002 ab | ||
| SP-2 | Alcoholic strength (% vol.) | AFerm | 15.04 ± 0.08 A | 15.09 ± 0.07 A | 14.98 ± 0.13 A | 14.94 ± 0.04 A |
| ProStab | 14.69 ± 0.09 B | 14.66 ± 0.06 B | 14.61 ± 0.03 B | 14.67 ± 0.04 B | ||
| SP-3 | Total extract (g/L) | AFerm | 22.6 ± 0.4 bA | 22.4 ± 0.5 b | 23.4 ± 0.4 a | 22.5 ± 0.3 b |
| ProStab | 21.5 ± 0.4 B | 21.7 ± 0.4 | 22.3 ± 0.8 | 22.0 ± 0.4 | ||
| SP-4 | Reducing sugars (g/L) | AFerm | 3.4 ± 0.2 b | 3.7 ± 0.3 b | 4.2 ± 0.4 a | 3.5 ± 0.2 b |
| ProStab | 3.4 ± 0.3 b | 3.5 ± 0.2 ab | 4.0 ± 0.5 a | 3.6 ± 0.2 ab | ||
| SP-5 | Total extract without reducing sugars (g/L) | AFerm | 19.2 ± 0.2 aA | 18.7 ± 0.3 bA | 19.1 ± 0.2 aA | 19.0 ± 0.1 abA |
| ProStab | 18.2 ± 0.1 B | 18.2 ± 0.2 B | 18.3 ± 0.3 B | 18.4 ± 0.2 B | ||
| SP-6 | Total acidity (as g/L tartaric acid) | AFerm | 5.5 ± 0.1 aA | 5.2 ± 0.1 bA | 5.3 ± 0.2 b | 5.4 ± 0.1 abA |
| ProStab | 5.0 ± 0.1 bB | 5.0 ± 0.0 bB | 5.1 ± 0.0 a | 5.1 ± 0.0 aB | ||
| SP-7 | Volatile acidity (as g/L acetic acid) | AFerm | 0.67 ± 0.03 | 0.69 ± 0.06 | 0.65 ± 0.04 | 0.62 ± 0.06 |
| ProStab | 0.65 ± 0.04 | 0.66 ± 0.04 | 0.63 ± 0.04 | 0.65 ± 0.02 | ||
| SP-8 | pH | AFerm | 3.33 ± 0.01 bB | 3.34 ± 0.01 a | 3.31 ± 0.01 c | 3.31 ± 0.01 c |
| ProStab | 3.36 ± 0.01 aA | 3.31 ± 0.03 b | 3.32 ± 0.01 ab | 3.32 ± 0.01 ab | ||
| Code | Phenols | Stage | Treatment | |||
|---|---|---|---|---|---|---|
| CO | B | T | BT | |||
| Hydroxybenzoic acids | ||||||
| P-1 | Gallic acid | AFerm | 2.38 ± 0.57 c | 2.90 ± 0.17 c | 46.62 ± 0.39 aB | 43.96 ± 1.35 b |
| ProStab | 2.97 ± 0.07 c | 2.98 ± 0.15 c | 50.51 ± 0.75 aA | 47.32 ± 1.54 bA | ||
| P-2 | Protocatechuic acid | AFerm | 1.72 ± 0.44 a | 1.05 ± 0.03 bB | 1.14 ± 0.02 bB | 0.96 ± 0.01 bB |
| ProStab | 1.20 ± 0.33 | 1.19 ± 0.02 A | 1.38 ± 0.06 A | 1.15 ± 0.03 A | ||
| P-3 | p-Hydroxybenzoic acid | AFerm | 0.54 ± 0.01 a | 0.34 ± 0.02 cB | 0.53 ± 0.04 a | 0.45 ± 0.01 bB |
| ProStab | 0.52 ± 0.03 b | 0.38 ± 0.01 cA | 0.59 ± 0.02 a | 0.50 ± 0.01 bA | ||
| P-4 | 2,5-Dihydroxybenzoic acid | AFerm | 0.12 ± 0.02 a | 0.05 ± 0.01 cB | 0.09 ± 0.01 bB | 0.04 ± 0.00 c |
| ProStab | 0.13 ± 0.01 a | 0.08 ± 0.02 bA | 0.12 ± 0.01 aA | 0.06 ± 0.01 b | ||
| P-5 | Syringic acid | AFerm | 0.11 ± 0.01 cB | 0.11 ± 0.00 cB | 1.52 ± 0.02 bA | 1.89 ± 0.02 aA |
| ProStab | 0.14 ± 0.00 cA | 0.16 ± 0.01 cA | 0.75 ± 0.03 bB | 0.97 ± 0.03 aB | ||
| Hydroxycinnamic acids | ||||||
| P-6 | cis-Caftaric acid * | AFerm | 1.72 ± 0.06 abA | 1.81 ± 0.05 aA | 1.67 ± 0.04 bA | 1.79 ± 0.04 aA |
| ProStab | 1.19 ± 0.08 B | 1.10 ± 0.04 B | 1.12 ± 0.08 B | 1.11 ± 0.06 B | ||
| P-7 | trans-Caftaric acid | AFerm | 12.76 ± 1.53 d | 19.13 ± 0.29 b | 15.54 ± 0.29 c | 21.11 ± 0.35 a |
| ProStab | 12.79 ± 1.37 d | 19.23 ± 0.47 b | 15.43 ± 0.05 c | 21.35 ± 0.21 a | ||
| P-8 | cis-Coutaric acid * | AFerm | 0.82 ± 0.00 cA | 0.87 ± 0.01 bA | 0.93 ± 0.02 aA | 0.93 ± 0.03 aA |
| ProStab | 0.75 ± 0.01 bB | 0.77 ± 0.00 bB | 0.82 ± 0.02 aB | 0.82 ± 0.02 aB | ||
| P-9 | trans-Coutaric acid * | AFerm | 0.08 ± 0.01 d | 0.23 ± 0.01 b | 0.13 ± 0.02 c | 0.39 ± 0.03 a |
| ProStab | 0.09 ± 0.00 d | 0.23 ± 0.01 b | 0.13 ± 0.01 c | 0.39 ± 0.02 a | ||
| P-10 | Caffeic acid | AFerm | 4.88 ± 0.26 aB | 3.64 ± 0.06 cB | 4.45 ± 0.05 bB | 2.95 ± 0.03 dB |
| ProStab | 5.54 ± 0.29 aA | 4.04 ± 0.08 cA | 5.00 ± 0.03 bA | 3.13 ± 0.06 dA | ||
| P-11 | cis-Fertaric acid * | AFerm | 0.37 ± 0.01 aA | 0.37 ± 0.01 aA | 0.34 ± 0.02 bA | 0.35 ± 0.01 abA |
| ProStab | 0.25 ± 0.02 aB | 0.25 ± 0.01 aB | 0.23 ± 0.01 abB | 0.22 ± 0.01 bB | ||
| P-12 | trans-Fertaric acid * | AFerm | 1.64 ± 0.02 cB | 1.77 ± 0.01 abB | 1.72 ± 0.09 bcB | 1.85 ± 0.01 aB |
| ProStab | 1.90 ± 0.03 bA | 1.95 ± 0.02 aA | 1.89 ± 0.01 bA | 1.96 ± 0.08 aA | ||
| P-13 | p-Coumaric acid | AFerm | 0.85 ± 0.01 aB | 0.56 ± 0.01 bB | 0.88 ± 0.05 aB | 0.55 ± 0.04 b |
| ProStab | 1.12 ± 0.17 aA | 0.59 ± 0.01 bA | 1.01 ± 0.04 aA | 0.60 ± 0.01 b | ||
| P-14 | Ferulic acid | AFerm | 1.05 ± 0.03 a | 0.88 ± 0.02 b | 1.05 ± 0.03 a | 0.85 ± 0.04 b |
| ProStab | 1.02 ± 0.03 a | 0.86 ± 0.03 b | 1.00 ± 0.03 a | 0.83 ± 0.03 b | ||
| Flavan-3-ols | ||||||
| P-15 | Catechin + tyrosol (as catechin) | AFerm | 21.48 ± 0.20 | 20.80 ± 0.24 | 20.73 ± 0.20 | 20.67 ± 0.82 |
| ProStab | 21.28 ± 0.30 a | 20.35 ± 0.27 b | 20.42 ± 0.31 b | 20.45 ± 0.67 b | ||
| P-16 | Epicatechin | AFerm | 0.78 ± 0.22 | 0.79 ± 0.01 B | 0.65 ± 0.05 B | 0.85 ± 0.14 |
| ProStab | 0.94 ± 0.13 b | 1.17 ± 0.03 aA | 0.78 ± 0.05 cA | 0.74 ± 0.03 c | ||
| P-17 | Procyanidin B1 | AFerm | 1.61 ± 0.04 b | 1.70 ± 0.03 b | 7.39 ± 0.09 a | 7.59 ± 0.18 a |
| ProStab | 1.60 ± 0.13 b | 1.75 ± 0.04 b | 7.18 ± 0.20 a | 7.28 ± 0.15 a | ||
| P-18 | Procyanidin B2 | AFerm | 0.50 ± 0.02 bB | 0.60 ± 0.03 aB | 0.25 ± 0.03 dB | 0.37 ± 0.01 cB |
| ProStab | 0.74 ± 0.02 bA | 1.52 ± 0.07 aA | 0.55 ± 0.08 cA | 0.61 ± 0.01 cA | ||
| Other | ||||||
| P-19 | Taxifolin | AFerm | 0.16 ± 0.00 aB | 0.11 ± 0.00 cB | 0.15 ± 0.00 bB | 0.11 ± 0.01 c |
| ProStab | 0.17 ± 0.00 aA | 0.11 ± 0.00 cA | 0.15 ± 0.00 bA | 0.11 ± 0.00 c | ||
| P-20 | trans-Piceid | AFerm | 0.13 ± 0.01 cA | 0.17 ± 0.01 a | 0.16 ± 0.01 bA | 0.16 ± 0.00 bB |
| ProStab | 0.11 ± 0.01 dB | 0.17 ± 0.01 b | 0.13 ± 0.01 cB | 0.20 ± 0.00 aA | ||
| Unidentified * | ||||||
| P-21 | T1 | AFerm | n.d. | n.d. | 22.93 ± 0.24 aB | 22.24 ± 0.26 bB |
| ProStab | n.d. | n.d. | 24.17 ± 0.23 aA | 23.33 ± 0.19 bA | ||
| P-22 | T2 | AFerm | n.d. | n.d. | 15.08 ± 0.22 aB | 13.49 ± 0.50 bB |
| ProStab | n.d. | n.d. | 18.62 ± 0.13 aA | 16.49 ± 0.63 bA | ||
| P-23 | T3 | AFerm | n.d. | n.d. | 15.64 ± 0.36 aB | 13.55 ± 0.60 bB |
| ProStab | n.d. | n.d. | 19.84 ± 0.33 aA | 17.23 ± 0.58 bA | ||
| P-24 | T4 | AFerm | n.d. | n.d. | 79.28 ± 0.56 A | 79.52 ± 2.74 |
| ProStab | n.d. | n.d. | 76.19 ± 1.39 B | 77.64 ± 2.99 | ||
| P-25 | T5 | AFerm | n.d. | n.d. | 37.36 ± 0.20 A | 38.92 ± 2.22 |
| ProStab | n.d. | n.d. | 35.06 ± 0.78 B | 36.29 ± 2.17 | ||
| P-26 | T6 | AFerm | n.d. | n.d. | 5.56 ± 0.02 B | 5.93 ± 0.26 B |
| ProStab | n.d. | n.d. | 6.97 ± 0.12 A | 6.84 ± 0.41 A | ||
| P-27 | T7 | AFerm | n.d. | n.d. | 3.63 ± 0.05 bA | 4.64 ± 0.11 aA |
| ProStab | n.d. | n.d. | 1.40 ± 0.08 bB | 2.02 ± 0.11 aB | ||
| P-28 | T8 | AFerm | n.d. | n.d. | 3.39 ± 0.07 bA | 4.01 ± 0.29 aA |
| ProStab | n.d. | n.d. | 2.19 ± 0.10 B | 2.42 ± 0.14 B | ||
| P-29 | T9 | AFerm | n.d. | n.d. | 33.38 ± 0.41 B | 29.01 ± 2.78 |
| ProStab | n.d. | n.d. | 38.56 ± 0.67 aA | 33.59 ± 2.89 b | ||
| P-30 | Total flavonoids | AFerm | 166.3 ± 6.8 bA | 115.9 ± 0.0 cA | 585.0 ± 37.1 a | 558.2 ± 13.6 aA |
| ProStab | 86.1 ± 5.1 bB | 59.4 ± 6.8 cB | 540.4 ± 10.3 a | 522.6 ± 13.6 aB | ||
| P-31 | Total phenols ** | AFerm | 195.6 ± 1.7 bB | 181.7 ± 4.5 bB | 309.2 ± 21.8 a | 314.9 ± 4.1 a |
| ProStab | 215.0 ± 2.0 bA | 200.3 ± 6.0 cA | 331.5 ± 8.4 a | 330.8 ± 10.7 a | ||
| Code | Free Aroma Compounds | Stage | Treatment | |||
|---|---|---|---|---|---|---|
| CO | B | T | BT | |||
| Monoterpenes | ||||||
| FV-1 | Linalool | AFerm | 24.41 ± 0.74 ab | 22.92 ± 0.45 bB | 24.78 ± 1.16 aB | 24.79 ± 1.27 a |
| ProStab | 29.68 ± 4.92 | 29.24 ± 2.27 A | 29.56 ± 2.52 A | 28.70 ± 3.44 | ||
| FV-2 | α-Terpineol | AFerm | 9.51 ± 0.24 | 10.18 ± 0.09 B | 10.45 ± 0.94 | 9.97 ± 0.50 B |
| ProStab | 12.44 ± 0.43 | 11.92 ± 0.39 A | 11.98 ± 0.34 | 12.23 ± 0.27 A | ||
| FV-3 | α-Terpinolene * | AFerm | 0.84 ± 0.73 | 0.97 ± 0.92 | 0.86 ± 0.76 | 1.20 ± 1.16 |
| ProStab | 0.89 ± 0.77 | 1.35 ± 0.53 | 0.86 ± 0.75 | 0.85 ± 0.75 | ||
| FV-4 | Citronellol | AFerm | 7.81 ± 0.78 | 8.04 ± 0.25 | 7.85 ± 0.22 | 7.73 ± 0.74 |
| ProStab | 7.68 ± 0.31 | 7.59 ± 0.33 | 7.39 ± 0.56 | 7.44 ± 0.64 | ||
| FV-5 | Terpendiol I * | AFerm | 86.78 ± 1.00 A | 85.80 ± 5.46 A | 83.70 ± 2.93 A | 86.62 ± 5.45 A |
| ProStab | 77.59 ± 1.28 B | 76.09 ± 2.41 B | 75.70 ± 3.52 B | 73.62 ± 1.03 B | ||
| C13-norisoprenoid | ||||||
| FV-6 | β-Damascenone | AFerm | 3.10 ± 0.49 | 3.51 ± 0.28 A | 3.20 ± 0.16 A | 3.35 ± 0.27 A |
| ProStab | 2.29 ± 0.22 | 2.32 ± 0.22 B | 2.30 ± 0.28 B | 2.45 ± 0.19 B | ||
| Alcohols | ||||||
| FV-7 | 1-Propanol (mg/L) | AFerm | 28.31 ± 3.18 | 28.25 ± 3.55 | 27.14 ± 1.94 | 26.67 ± 1.53 |
| ProStab | 29.15 ± 0.48 a | 29.37 ± 2.35 a | 29.02 ± 0.99 a | 25.58 ± 1.07 b | ||
| FV-8 | Isobutanol (mg/L) | AFerm | 18.50 ± 0.96 | 18.85 ± 1.58 | 18.80 ± 1.37 | 20.59 ± 4.74 |
| ProStab | 19.63 ± 0.02 | 19.78 ± 0.39 | 19.32 ± 0.29 | 19.70 ± 3.74 | ||
| FV-9 | Isoamyl alcohol (mg/L) | AFerm | 197.0 ± 9.7 | 199.8 ± 16.5 | 204.4 ± 13.6 | 211.4 ± 40.8 |
| ProStab | 206.0 ± 0.5 | 205.5 ± 4.6 | 206.4 ± 3.6 | 205.9 ± 31.6 | ||
| FV-10 | 1-Hexanol (mg/L) | AFerm | 2.02 ± 0.07 | 1.97 ± 0.02 A | 1.94 ± 0.06 | 1.99 ± 0.14 |
| ProStab | 1.98 ± 0.06 | 1.91 ± 0.02 B | 1.87 ± 0.04 | 1.96 ± 0.16 | ||
| FV-11 | trans-3-Hexenol * | AFerm | 134.3 ± 1.9 | 133.4 ± 2.6 A | 133.1 ± 5.2 | 132.4 ± 3.5 |
| ProStab | 133.0 ± 4.2 | 127.3 ± 0.8 B | 128.1 ± 4.2 | 129.2 ± 3.4 | ||
| FV-12 | cis-3-Hexenol * | AFerm | 84.70 ± 1.56 a | 83.26 ± 0.89 aA | 80.92 ± 2.24 ab | 74.11 ± 8.39 b |
| ProStab | 83.09 ± 3.01 a | 78.59 ± 1.65 abB | 77.74 ± 1.65 ab | 72.53 ± 8.87 b | ||
| FV-13 | 1-Octanol * | AFerm | 14.83 ± 3.13 | 14.46 ± 1.35 | 12.69 ± 1.26 | 15.92 ± 3.65 |
| ProStab | 18.62 ± 4.93 | 15.01 ± 3.57 | 11.96 ± 1.34 | 14.02 ± 5.76 | ||
| FV-14 | Benzyl alcohol * | AFerm | 51.59 ± 0.49 aB | 28.12 ± 0.47 c | 45.84 ± 0.78 bB | 28.24 ± 1.20 c |
| ProStab | 60.79 ± 1.02 aA | 28.23 ± 0.84 c | 52.83 ± 1.82 bA | 27.78 ± 1.52 c | ||
| FV-15 | 2-Phenyletanol (mg/L) | AFerm | 29.90 ± 1.03 A | 28.77 ± 0.79 | 29.64 ± 0.81 A | 29.98 ± 0.84 |
| ProStab | 27.99 ± 0.52 B | 28.14 ± 0.68 | 27.52 ± 0.10 B | 28.62 ± 1.29 | ||
| Fatty acids | ||||||
| FV-16 | Butyric acid (mg/L) | AFerm | 1.78 ± 0.06 a | 1.84 ± 0.10 a | 1.79 ± 0.02 a | 1.57 ± 0.13 b |
| ProStab | 1.75 ± 0.15 | 1.74 ± 0.06 | 1.71 ± 0.06 | 1.54 ± 0.15 | ||
| FV-17 | Hexanoic acid (mg/L) | AFerm | 3.92 ± 0.16 | 4.11 ± 0.18 | 4.29 ± 0.15 | 3.88 ± 0.48 |
| ProStab | 3.90 ± 0.26 | 3.94 ± 0.02 | 4.05 ± 0.24 | 3.67 ± 0.39 | ||
| FV-18 | Octanoic acid (mg/L) | AFerm | 5.36 ± 0.25 | 5.96 ± 0.16 | 6.04 ± 0.33 | 5.51 ± 0.68 |
| ProStab | 5.62 ± 0.56 | 5.68 ± 0.11 | 5.81 ± 0.47 | 5.20 ± 0.78 | ||
| FV-19 | Decanoic acid (mg/L) | AFerm | 1.60 ± 0.08 b | 1.76 ± 0.05 ab | 1.91 ± 0.06 a | 1.67 ± 0.16 b |
| ProStab | 1.84 ± 0.33 | 1.77 ± 0.14 | 1.89 ± 0.11 | 1.68 ± 0.23 | ||
| FV-20 | Dodecanoic acid * | AFerm | 52.43 ± 8.08 aA | 27.41 ± 13.10 b | 42.71 ± 4.90 ab | 27.26 ± 4.89 b |
| ProStab | 36.63 ± 1.93 aB | 22.64 ± 5.35 b | 32.21 ± 5.98 ab | 24.05 ± 9.11 b | ||
| Ethyl esters | ||||||
| FV-21 | Ethyl butyrate (mg/L) | AFerm | 0.29 ± 0.02 | 0.33 ± 0.04 | 0.31 ± 0.01 | 0.27 ± 0.07 |
| ProStab | 0.31 ± 0.04 | 0.32 ± 0.03 | 0.32 ± 0.02 | 0.26 ± 0.06 | ||
| FV-22 | Ethyl hexanoate (mg/L) | AFerm | 0.49 ± 0.04 b | 0.57 ± 0.03 ab | 0.58 ± 0.06 a | 0.51 ± 0.06 ab |
| ProStab | 0.60 ± 0.10 | 0.59 ± 0.04 | 0.62 ± 0.05 | 0.55 ± 0.07 | ||
| FV-23 | Ethyl octanoate (mg/L) | AFerm | 0.21 ± 0.02 B | 0.24 ± 0.03 | 0.25 ± 0.05 | 0.23 ± 0.05 |
| ProStab | 0.27 ± 0.02 A | 0.25 ± 0.03 | 0.27 ± 0.05 | 0.26 ± 0.07 | ||
| FV-24 | Ethyl decanoate (mg/L) | AFerm | 0.04 ± 0.00 ab | 0.03 ± 0.00 b | 0.04 ± 0.01 a | 0.03 ± 0.01 b |
| ProStab | 0.04 ± 0.01 | 0.04 ± 0.00 | 0.04 ± 0.01 | 0.04 ± 0.01 | ||
| Acetate esters | ||||||
| FV-25 | Ethyl acetate (mg/L) | AFerm | 18.00 ± 2.79 B | 20.27 ± 0.34 | 20.16 ± 3.62 | 23.53 ± 3.80 |
| ProStab | 29.18 ± 3.28 A | 21.86 ± 4.55 | 22.76 ± 2.81 | 22.17 ± 2.58 | ||
| FV-26 | Isoamyl acetate (mg/L) | AFerm | 0.91 ± 0.03 | 1.14 ± 0.01 A | 1.12 ± 0.14 | 0.89 ± 0.28 |
| ProStab | 0.99 ± 0.20 | 0.99 ± 0.04 B | 1.01 ± 0.09 | 0.79 ± 0.24 | ||
| FV-27 | Hexyl acetate | AFerm | 67.14 ± 4.63 b | 90.82 ± 3.86 ab | 95.09 ± 14.65 a | 70.29 ± 21.20 ab |
| ProStab | 82.90 ± 24.11 | 80.06 ± 9.95 | 89.11 ± 11.68 | 69.20 ± 24.48 | ||
| FV-28 | 2-Phenethyl acetate (mg/L) | AFerm | 0.12 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.13 ± 0.03 |
| ProStab | 0.14 ± 0.03 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.12 ± 0.03 | ||
| Other esters | ||||||
| FV-29 | Ethyl lactate (mg/L) | AFerm | 5.76 ± 0.32 B | 5.51 ± 0.56 B | 5.77 ± 0.47 B | 5.65 ± 0.97 B |
| ProStab | 7.60 ± 0.45 A | 7.60 ± 0.62 A | 7.63 ± 0.20 A | 7.91 ± 0.95 A | ||
| FV-30 | Ethyl 3-hydroxybutanoate * (mg/L) | AFerm | 0.25 ± 0.00 | 0.26 ± 0.00 A | 0.25 ± 0.01 | 0.28 ± 0.04 |
| ProStab | 0.25 ± 0.02 | 0.24 ± 0.01 B | 0.24 ± 0.02 | 0.27 ± 0.04 | ||
| FV-31 | Diethyl succinate * (mg/L) | AFerm | 0.44 ± 0.01 B | 0.41 ± 0.05 B | 0.42 ± 0.01 B | 0.46 ± 0.06 B |
| ProStab | 0.69 ± 0.03 abA | 0.67 ± 0.05 bA | 0.71 ± 0.02 abA | 0.78 ± 0.09 aA | ||
| FV-32 | Methyl 4-hydroxybutanoate * | AFerm | 22.82 ± 1.44 bA | 25.06 ± 0.93 aA | 22.90 ± 0.59 bA | 19.62 ± 1.15 cA |
| ProStab | 18.08 ± 2.21 abB | 19.61 ± 0.75 a | 15.73 ± 0.73 bcB | 14.23 ± 2.27 cB | ||
| FV-33 | Ethyl 4-hydroxybutanoate * (mg/L) | AFerm | 6.71 ± 0.66 ab | 7.46 ± 0.40 aA | 6.63 ± 0.12 abA | 5.80 ± 0.83 b |
| ProStab | 5.44 ± 0.67 a | 5.59 ± 0.30 aB | 4.86 ± 0.22 abB | 4.24 ± 0.72 b | ||
| FV-34 | Diethyl malate * (mg/L) | AFerm | 0.31 ± 0.01 B | 0.29 ± 0.02 B | 0.30 ± 0.02 B | 0.32 ± 0.02 B |
| ProStab | 0.59 ± 0.01 A | 0.56 ± 0.03 A | 0.60 ± 0.03 A | 0.60 ± 0.02 A | ||
| FV-35 | Monomethyl succinate * | AFerm | 80.31 ± 9.81 | 65.99 ± 2.87 | 77.14 ± 9.44 | 84.81 ± 15.23 |
| ProStab | 79.26 ± 5.57 ab | 72.43 ± 6.61 b | 78.80 ± 4.89 ab | 89.67 ± 11.04 a | ||
| FV-36 | Monoethyl succinate *(mg/L) | AFerm | 21.27 ± 0.95 B | 19.55 ± 0.56 B | 19.01 ± 0.97 B | 21.06 ± 1.94 B |
| ProStab | 26.48 ± 1.04 A | 26.30 ± 0.87 A | 25.85 ± 1.57 A | 26.89 ± 1.45 A | ||
| FV-37 | Ethyl p-coumarate *(mg/L) | AFerm | 0.45 ± 0.02 aB | 0.40 ± 0.02 a | 0.45 ± 0.06 a | 0.32 ± 0.02 b |
| ProStab | 0.49 ± 0.02 aA | 0.42 ± 0.04 b | 0.51 ± 0.04 a | 0.33 ± 0.03 c | ||
| Other | ||||||
| FV-38 | Acetaldehyde (mg/L) | AFerm | 43.04 ± 5.08 | 44.22 ± 6.12 | 42.95 ± 3.77 | 47.06 ± 7.42 |
| ProStab | 44.19 ± 1.93 | 48.92 ± 2.81 | 46.78 ± 4.19 | 49.49 ± 7.06 | ||
| FV-39 | Benzaldehyde * | AFerm | 9.26 ± 5.28 | 9.22 ± 5.24 | 9.03 ± 5.25 | 9.11 ± 4.46 |
| ProStab | 8.52 ± 4.91 | 8.80 ± 5.42 | 8.33 ± 5.19 | 8.68 ± 5.16 | ||
| FV-40 | Methanol (mg/L) | AFerm | 48.31 ± 0.80 | 44.42 ± 6.22 | 44.63 ± 4.10 | 51.65 ± 1.57 |
| ProStab | 48.91 ± 2.62 | 52.16 ± 6.24 | 49.27 ± 6.32 | 47.12 ± 10.00 | ||
| FV-41 | Guaiacol * | AFerm | 0.95 ± 0.04 | 0.97 ± 0.20 | 0.95 ± 0.13 | 0.70 ± 0.67 |
| ProStab | 0.94 ± 0.24 | 1.24 ± 0.66 | 0.97 ± 0.12 | 0.96 ± 0.17 | ||
| FV-42 | 4-Vinylguaiacol * | AFerm | 366.1 ± 19.7 | 439.5 ± 74.6 | 433.1 ± 21.8 A | 435.8 ± 30.5 |
| ProStab | 382.4 ± 26.6 | 417.9 ± 112.9 | 324.5 ± 38.9 B | 392.9 ± 8.2 | ||
| FV-43 | Acetoin * | AFerm | 29.51 ± 3.44 ab | 24.28 ± 1.03 b | 33.48 ± 2.32 a | 29.86 ± 6.12 ab |
| ProStab | 28.76 ± 2.30 ab | 24.62 ± 1.60 b | 32.06 ± 2.02 a | 31.46 ± 4.22 a | ||
| FV-44 | 3-Hydroxy-2-pentanone * | AFerm | 38.30 ± 2.73 ab | 34.83 ± 4.18 b | 43.73 ± 2.52 ab | 48.91 ± 10.48 a |
| ProStab | 36.90 ± 2.17 b | 35.03 ± 3.44 b | 42.48 ± 4.24 ab | 49.62 ± 7.68 a | ||
| FV-45 | γ-Butyrolactone * | AFerm | 191.0 ± 13.0 | 215.5 ± 25.7 | 201.8 ± 18.2 | 186.0 ± 14.0 |
| ProStab | 232.5 ± 23.8 ab | 234.6 ± 8.5 a | 229.5 ± 18.4 ab | 200.7 ± 15.7 b | ||
| FV-46 | Pantolactone * | AFerm | 45.07 ± 2.76 | 46.79 ± 4.45 | 42.51 ± 2.26 | 46.82 ± 4.19 |
| ProStab | 46.88 ± 2.67 | 45.90 ± 1.58 | 44.80 ± 0.70 | 44.78 ± 1.61 | ||
| FV-47 | 3-Methylthiopropanol * (mg/L) | AFerm | 1.06 ± 0.05 | 1.05 ± 0.04 | 1.11 ± 0.04 | 1.12 ± 0.17 |
| ProStab | 1.06 ± 0.06 | 1.02 ± 0.04 | 1.06 ± 0.03 | 1.08 ± 0.14 | ||
| FV-48 | Tryptophol * (mg/L) | AFerm | 0.75 ± 0.05 b | 1.07 ± 0.02 aA | 1.08 ± 0.07 a | 1.15 ± 0.16 a |
| ProStab | 0.92 ± 0.18 | 0.84 ± 0.06 B | 0.92 ± 0.14 | 0.98 ± 0.03 | ||
| Code | Bound Aroma Compounds | Stage | Treatment | |||
|---|---|---|---|---|---|---|
| CO | B | T | BT | |||
| Monoterpenes | ||||||
| BV-1 | trans-furan Linalool oxide * | AFerm | 2.27 ± 0.21 | 2.31 ± 0.19 | 2.39 ± 0.51 | 2.42 ± 0.10 |
| ProStab | 2.34 ± 0.12 b | 2.30 ± 0.13 b | 2.55 ± 0.17 ab | 2.68 ± 0.15 a | ||
| BV-2 | cis-furan Linalool oxide * | AFerm | 0.77 ± 0.05 b | 0.80 ± 0.07 ab | 0.89 ± 0.07 a | 0.87 ± 0.06 abB |
| ProStab | 0.84 ± 0.01 b | 0.76 ± 0.04 c | 0.91 ± 0.02 b | 1.04 ± 0.07 aA | ||
| BV-3 | Linalool | AFerm | 8.61 ± 0.69 A | 7.96 ± 1.40 | 7.93 ± 1.16 A | 5.80 ± 2.36 |
| ProStab | 6.48 ± 0.29 aB | 6.03 ± 0.49 a | 4.78 ± 1.07 abB | 3.80 ± 1.64 b | ||
| BV-4 | Hotrienol * | AFerm | 1.85 ± 0.63 | 2.25 ± 0.35 | 2.48 ± 0.42 | 2.43 ± 0.53 |
| ProStab | 1.81 ± 0.62 | 2.35 ± 0.38 | 2.38 ± 0.08 | 2.27 ± 1.02 | ||
| BV-5 | α-Terpineol | AFerm | 1.35 ± 0.02 b | 1.40 ± 0.07 b | 1.86 ± 0.29 a | 2.08 ± 0.13 a |
| ProStab | 1.33 ± 0.02 c | 1.33 ± 0.02 c | 1.71 ± 0.17 b | 2.02 ± 0.17 a | ||
| BV-6 | trans-pyran Linalool oxide * | AFerm | 5.72 ± 0.43 | 5.85 ± 0.39 | 6.09 ± 1.13 | 5.89 ± 0.16 |
| ProStab | 5.72 ± 0.49 | 5.77 ± 0.12 | 5.91 ± 0.62 | 6.27 ± 0.66 | ||
| BV-7 | Citronellol | AFerm | 1.03 ± 0.04 ab | 0.66 ± 0.58 ab | 1.18 ± 0.26 a | 0.31 ± 0.54 b |
| ProStab | 0.69 ± 0.60 | 1.04 ± 0.18 | 0.30 ± 0.52 | 0.62 ± 0.54 | ||
| BV-8 | Nerol | AFerm | 7.45 ± 0.04 | 7.69 ± 0.72 | 7.46 ± 0.45 | 7.74 ± 0.87 |
| ProStab | 7.61 ± 0.48 | 7.52 ± 0.35 | 7.25 ± 0.31 | 7.87 ± 1.69 | ||
| BV-9 | Geraniol | AFerm | 43.49 ± 0.89 | 45.48 ± 7.90 | 40.04 ± 2.34 | 43.35 ± 7.12 |
| ProStab | 41.76 ± 1.99 | 43.13 ± 2.85 | 39.07 ± 1.77 | 39.94 ± 5.41 | ||
| BV-10 | Terpendiol I * | AFerm | 1.68 ± 0.33 | 1.33 ± 0.63 | 1.10 ± 0.27 | 0.94 ± 0.33 |
| ProStab | 1.44 ± 0.41 | 1.32 ± 0.36 | 0.92 ± 0.06 | 1.82 ± 2.03 | ||
| BV-11 | trans-8-Hydroxy-linalool * | AFerm | 29.60 ± 6.73 | 29.55 ± 3.55 | 27.04 ± 8.40 | 24.57 ± 1.56 |
| ProStab | 21.40 ± 3.04 | 26.67 ± 4.45 | 19.25 ± 4.66 | 31.37 ± 19.12 | ||
| BV-12 | 7-Hydroxy-geraniol * | AFerm | 21.92 ± 1.83 | 23.23 ± 1.82 | 21.85 ± 3.74 | 22.97 ± 3.12 |
| ProStab | 19.40 ± 4.85 | 23.48 ± 0.70 | 21.59 ± 3.13 | 41.95 ± 34.49 | ||
| BV-13 | cis-8-Hydroxy-linalool * | AFerm | 92.78 ± 10.23 | 99.87 ± 9.73 | 91.86 ± 6.91 | 91.94 ± 19.18 |
| ProStab | 83.5 ± 17.1 | 100.3 ± 1.3 | 82.2 ± 9.3 | 158.3 ± 134.6 | ||
| BV-14 | trans-Geranic acid * | AFerm | 8.37 ± 1.53 | 9.37 ± 1.87 | 8.69 ± 1.88 | 9.36 ± 1.79 |
| ProStab | 8.12 ± 0.65 | 9.54 ± 0.54 | 7.98 ± 1.33 | 9.88 ± 3.25 | ||
| C13-norisoprenoids | ||||||
| BV-15 | β-Damascenone | AFerm | 0.14 ± 0.02 | 0.10 ± 0.10 | 0.17 ± 0.02 A | 0.17 ± 0.02 |
| ProStab | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.12 ± 0.01 B | 0.07 ± 0.07 | ||
| BV-16 | 3-Hydroxy-β-damascone * | AFerm | 177.6 ± 8.1 A | 179.8 ± 39.3 | 183.2 ± 4.6 A | 183.4 ± 45.7 |
| ProStab | 130.1 ± 10.3 B | 135.2 ± 14.9 | 130.4 ± 17.0 B | 129.3 ± 29.7 | ||
| BV-17 | 3-Oxo-α-ionol * | AFerm | 59.23 ± 4.77 | 62.71 ± 7.84 | 61.11 ± 12.39 | 58.10 ± 6.42 |
| ProStab | 59.10 ± 1.31 | 60.96 ± 4.86 | 54.79 ± 8.24 | 58.88 ± 7.24 | ||
| BV-18 | 3-Hydroxy-7,8-dihydro-β-ionol * | AFerm | 50.45 ± 3.57 | 53.45 ± 4.93 | 51.14 ± 5.23 | 53.08 ± 4.95 |
| ProStab | 47.42 ± 5.02 | 52.07 ± 2.55 | 48.45 ± 6.70 | 66.34 ± 25.02 | ||
| BV-19 | Vomifoliol * | AFerm | 20.28 ± 4.01 ab | 25.32 ± 4.41 a | 22.51 ± 2.62 ab | 18.84 ± 1.81 b |
| ProStab | 17.07 ± 5.21 | 20.81 ± 2.94 | 18.19 ± 1.65 | 47.68 ± 49.76 | ||
| Alcohols | ||||||
| BV-20 | 1-Hexanol | AFerm | 88.11 ± 2.15 | 86.98 ± 9.32 | 89.53 ± 5.73 | 90.31 ± 2.08 A |
| ProStab | 86.35 ± 3.27 | 87.49 ± 1.62 | 84.53 ± 2.08 | 86.45 ± 0.82 B | ||
| BV-21 | trans-3-Hexenol * | AFerm | 1.16 ± 0.04 | 1.10 ± 0.10 | 1.20 ± 0.09 | 1.13 ± 0.05 |
| ProStab | 1.18 ± 0.05 | 1.16 ± 0.10 | 1.18 ± 0.05 | 1.10 ± 0.05 | ||
| BV-22 | cis-3-Hexenol * | AFerm | 13.29 ± 1.09 | 13.46 ± 0.54 | 13.94 ± 0.83 | 14.82 ± 0.96 |
| ProStab | 12.81 ± 0.88 b | 13.17 ± 0.29 b | 12.74 ± 0.24 b | 14.31 ± 0.47 a | ||
| BV-23 | trans-2-Hexenol * | AFerm | 15.18 ± 0.47 b | 16.37 ± 0.95 ab | 15.63 ± 0.63 b | 17.46 ± 0.49 a |
| ProStab | 15.27 ± 1.08 | 16.88 ± 0.22 | 15.51 ± 1.47 | 16.81 ± 0.20 | ||
| BV-24 | 1-Octen-3-ol * | AFerm | 2.49 ± 0.30 b | 2.60 ± 0.05 ab | 2.89 ± 0.28 a | 2.62 ± 0.05 ab |
| ProStab | 2.53 ± 0.18 | 2.51 ± 0.04 | 2.52 ± 0.22 | 2.75 ± 0.54 | ||
| BV-25 | 1-Octanol * | AFerm | 200.4 ± 309.8 | 192.8 ± 302.5 | 191.8 ± 295.0 | 164.0 ± 252.8 |
| ProStab | 210.6 ± 323.9 | 180.6 ± 280.9 | 199.7 ± 309.7 | 166.1 ± 255.7 | ||
| BV-26 | 2-Phenylethanol * | AFerm | 322.7 ± 4.6 a | 266.7 ± 12.6 b | 318.1 ± 36.2 a | 279.6 ± 13.1 b |
| ProStab | 305.4 ± 32.6 | 273.1 ± 8.4 | 309.1 ± 17.7 | 287.0 ± 22.1 | ||
| Other | ||||||
| BV-27 | Benzaldehyde * | AFerm | 5.09 ± 2.80 a | 2.58 ± 0.75 ab | 1.85 ± 0.90 b | 2.82 ± 0.54 ab |
| ProStab | 3.12 ± 0.97 ab | 2.31 ± 0.06 b | 3.15 ± 0.26 ab | 4.40 ± 1.69 a | ||
| BV-28 | 4-Vinylguaiacol * | AFerm | 12.29 ± 2.94 | 16.20 ± 6.16 | 14.83 ± 1.52 A | 12.43 ± 2.14 |
| ProStab | 10.36 ± 2.64 | 13.41 ± 2.20 | 10.13 ± 1.31 B | 47.28 ± 59.71 | ||
| BV-29 | Tryptophol * | AFerm | 10.09 ± 1.90 b | 13.79 ± 1.75 ab | 13.10 ± 2.45 ab | 14.40 ± 2.28 a |
| ProStab | 10.62 ± 1.27 | 12.64 ± 0.22 | 11.98 ± 2.61 | 15.01 ± 6.23 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukić, I.; Horvat, I.; Radeka, S.; Vrhovsek, U. Solid-Phase Extraction Followed by Gas Chromatography–Mass Spectrometry for Revealing the Effects of the Application of Bentonite, Tannins, and Their Combination during Fermentation in the Production of White Wine. Chemosensors 2023, 11, 545. https://doi.org/10.3390/chemosensors11100545
Lukić I, Horvat I, Radeka S, Vrhovsek U. Solid-Phase Extraction Followed by Gas Chromatography–Mass Spectrometry for Revealing the Effects of the Application of Bentonite, Tannins, and Their Combination during Fermentation in the Production of White Wine. Chemosensors. 2023; 11(10):545. https://doi.org/10.3390/chemosensors11100545
Chicago/Turabian StyleLukić, Igor, Ivana Horvat, Sanja Radeka, and Urska Vrhovsek. 2023. "Solid-Phase Extraction Followed by Gas Chromatography–Mass Spectrometry for Revealing the Effects of the Application of Bentonite, Tannins, and Their Combination during Fermentation in the Production of White Wine" Chemosensors 11, no. 10: 545. https://doi.org/10.3390/chemosensors11100545
APA StyleLukić, I., Horvat, I., Radeka, S., & Vrhovsek, U. (2023). Solid-Phase Extraction Followed by Gas Chromatography–Mass Spectrometry for Revealing the Effects of the Application of Bentonite, Tannins, and Their Combination during Fermentation in the Production of White Wine. Chemosensors, 11(10), 545. https://doi.org/10.3390/chemosensors11100545








