Impedance In Vitro Assessment for the Detection of Salmonella typhimurium Infection in Intestinal Human Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions of Bacterial Strain and Preparation of MOI
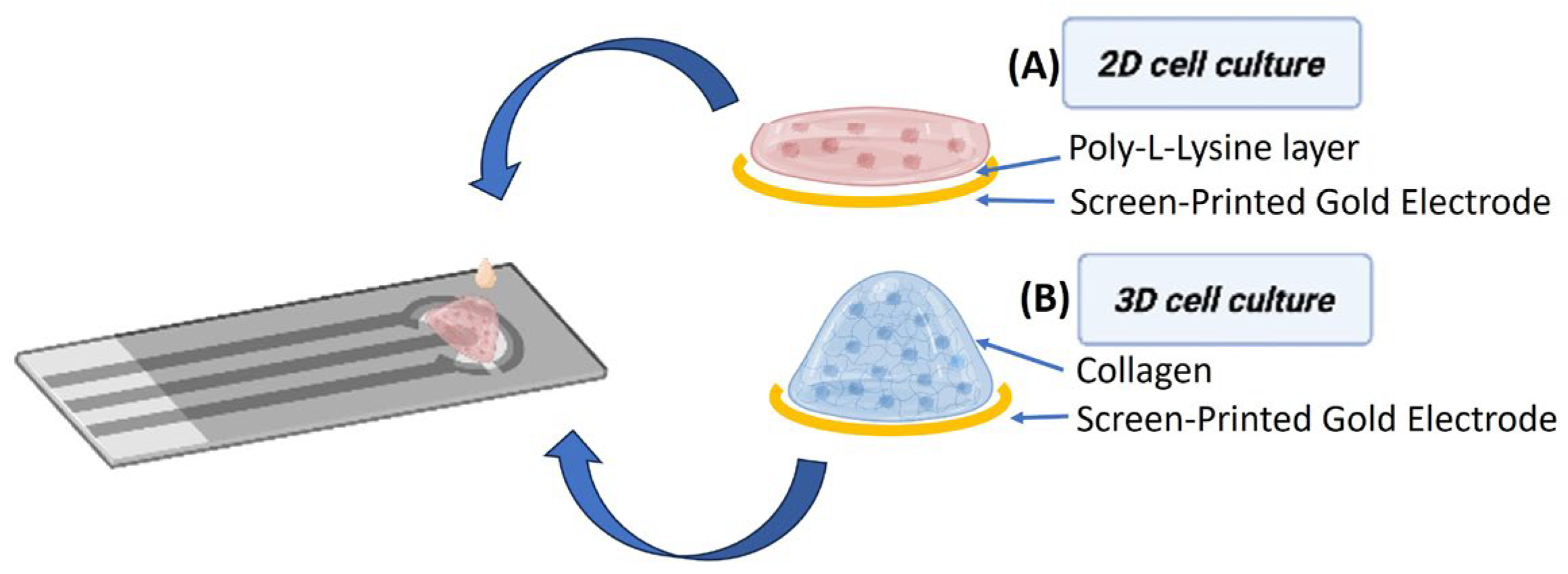
2.2. Intestinal Human Cell Culture
2.3. Adhesion and Invasion Assay
2.4. Cell Viability Assessment
2.5. Monitoring Mitochondrial Membrane Potential (ΔΨm) in Live Cells
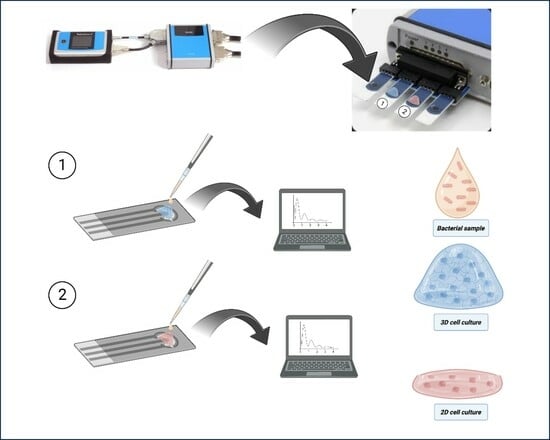
2.6. Electrode Preparation
2.7. Impedance Measurements
3. Results
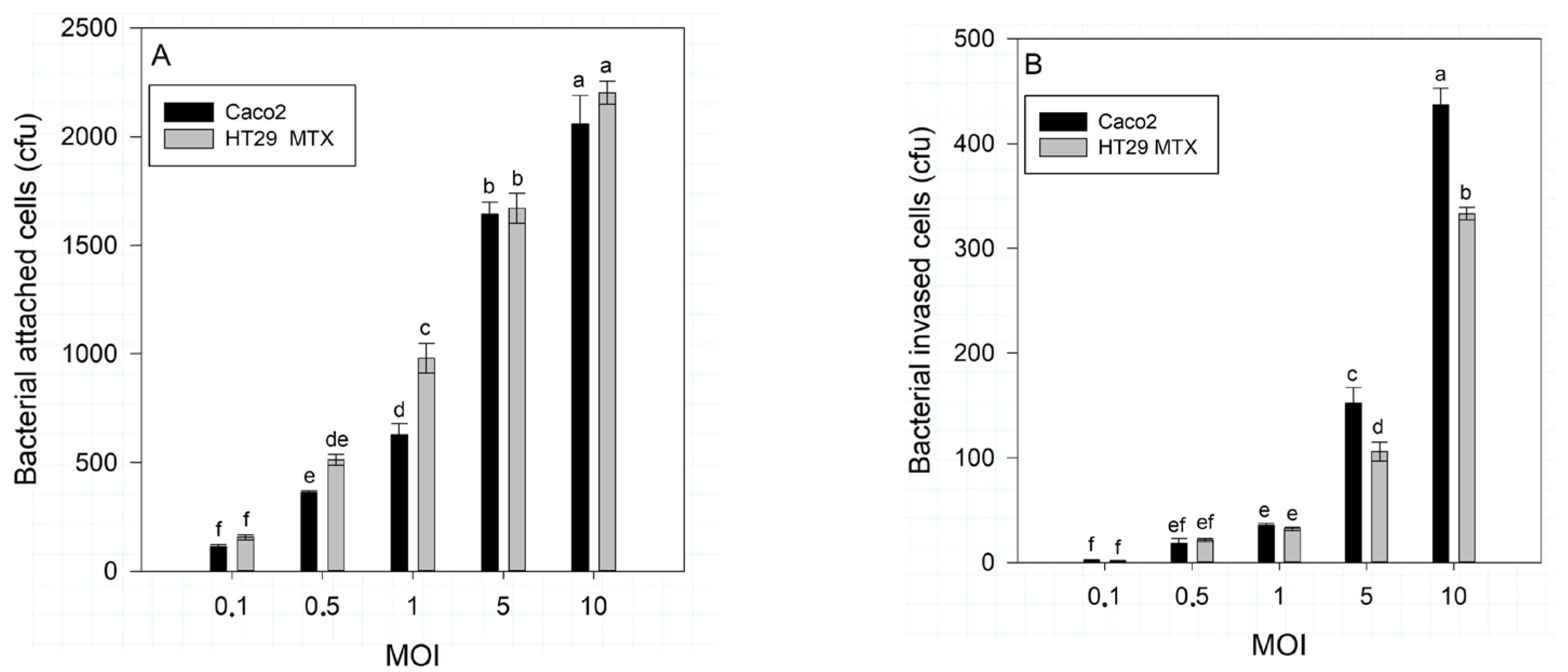
3.1. S. typhimurium Attachment and Invasion Effect on Caco-2 and HT29-MTX-E12 Cells
3.2. Non-Cytotoxic Effect of S. typhimurium Infection on Caco-2 and HT29-MTX Cells
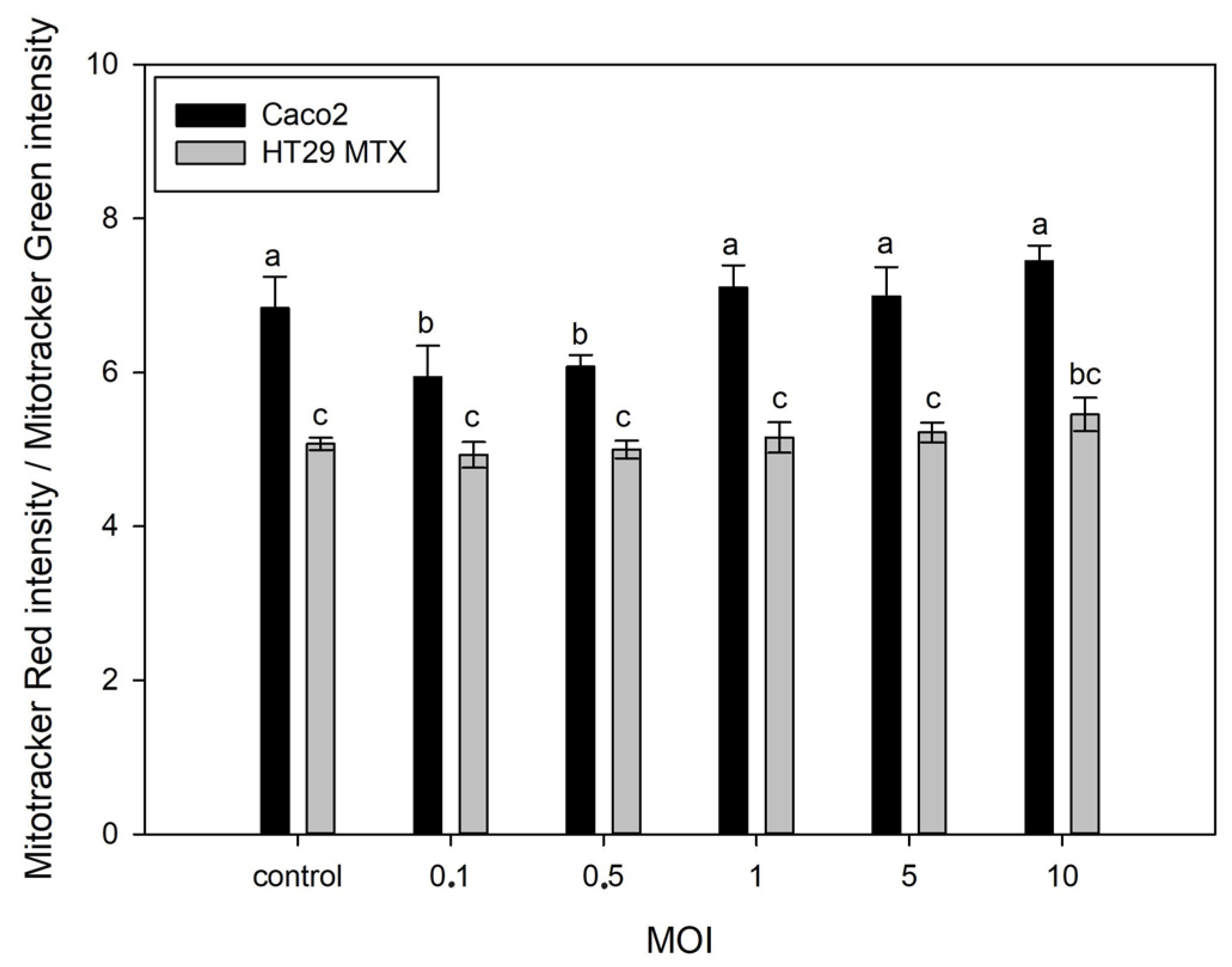
3.3. Effect of S. typhimurium on Mitochondrial Potential on Caco-2 and HT29-MTX Cells
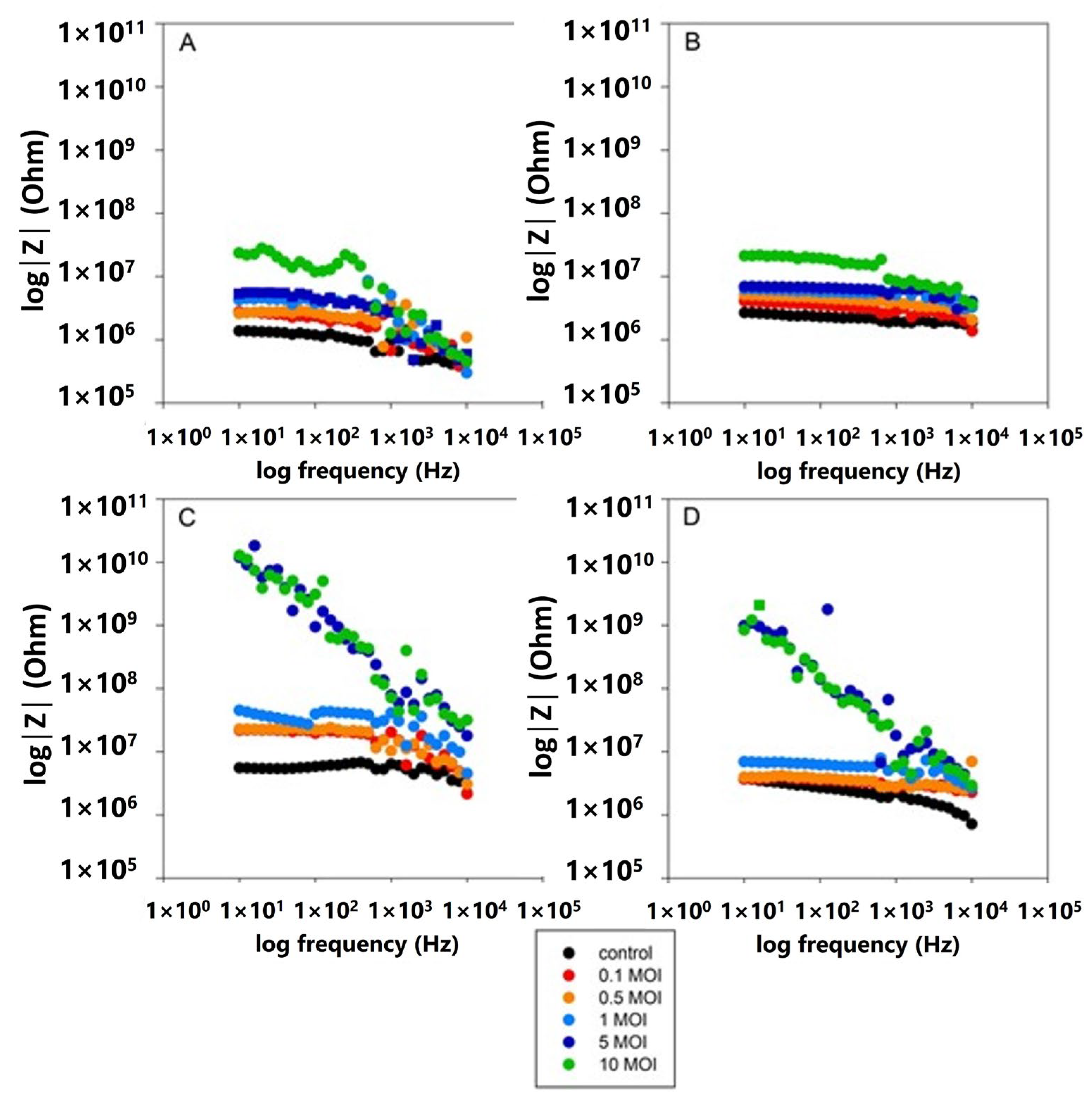
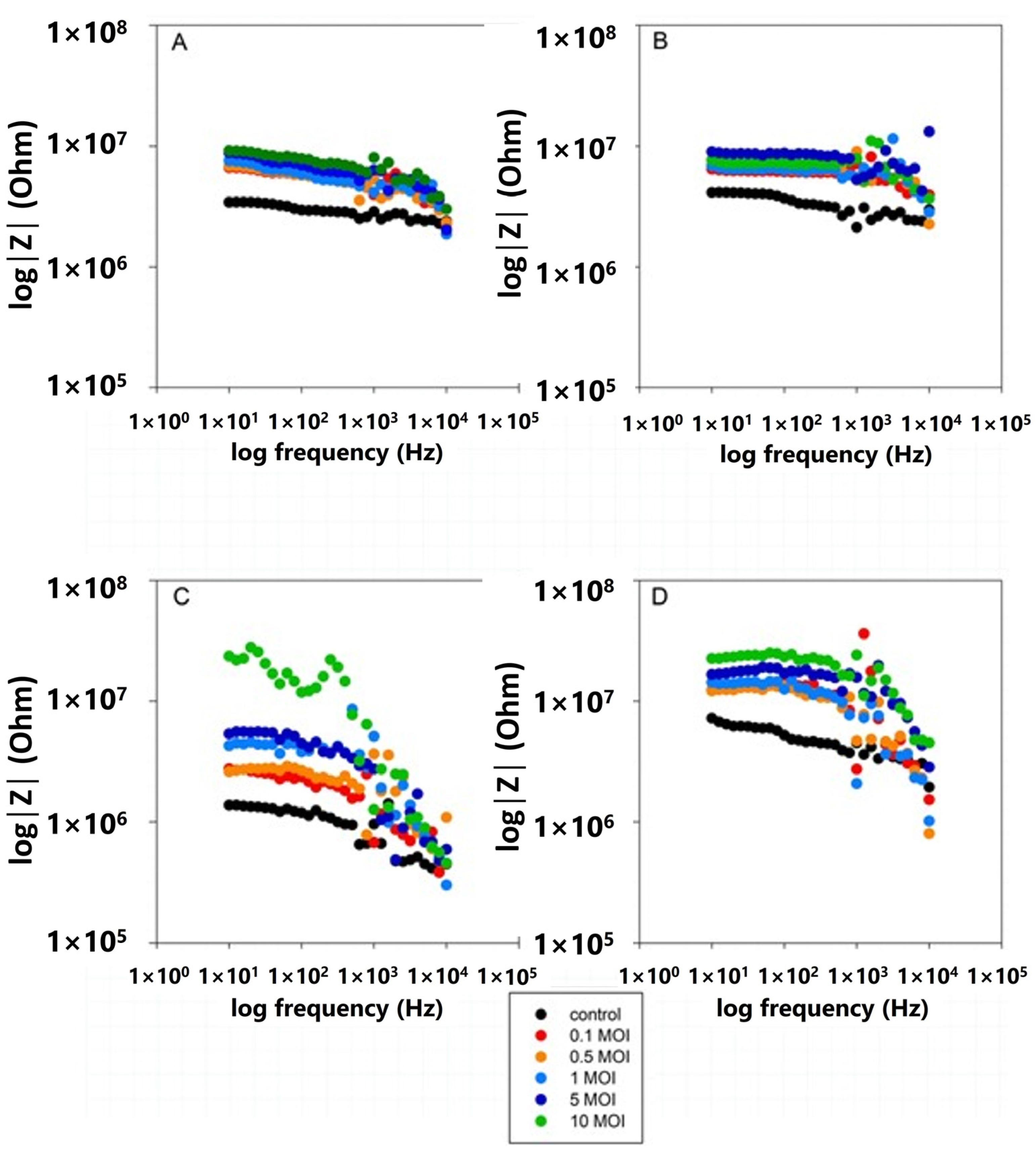
3.4. Impedance Comparative Studies on the Effects of S. typhimurium on 2D and 3D Cell Cultures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banerjee, P.; Bhunia, A.K. Mammalian Cell-Based Biosensors for Pathogens and Toxins. Trends Biotechnol. 2009, 27, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Reverté, L.; Soliño, L.; Carnicer, O.; Diogène, J.; Campàs, M. Alternative Methods for the Detection of Emerging Marine Toxins: Biosensors, Biochemical Assays and Cell-Based Assays. Mar. Drugs 2014, 12, 5719–5763. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhu, P.; Pi, F.; Zhang, Y.; Li, Y.; Wang, J.; Sun, X. Development of a Simple and Convenient Cell-Based Electrochemical Biosensor for Evaluating the Individual and Combined Toxicity of Don, Zen, and Afb1. Biosens. Bioelectron. 2017, 97, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Ge, P.; Wang, L.; Jiang, H.; Yang, M.; Yuan, L.; Ge, Q.; Fang, W.; Ju, X. A Novel Electrochemical Mast Cell-Based Paper Biosensor for the Rapid Detection of Milk Allergen Casein. Biosens. Bioelectron. 2019, 130, 299–306. [Google Scholar] [CrossRef]
- Yazgan Karacaglar, N.N.; Topcu, A.; Dudak, F.C.; Boyaci, I.H. Development of a Green Fluorescence Protein (Gfp)-Based Bioassay for Detection of Antibiotics and Its Application in Milk. J. Food Sci. 2020, 85, 500–509. [Google Scholar] [CrossRef]
- Gagnon, M.; Zihler Berner, A.; Chervet, N.; Chassard, C.; Lacroix, C. Comparison of the Caco-2, HT-29 and the Mucus-Secreting HT29-MTX Intestinal Cell Models to Investigate Salmonella Adhesion and Invasion. J. Microbiol. Methods 2013, 94, 274–279. [Google Scholar] [CrossRef]
- Zihler, A.; Gagnon, M.; Chassard, C.; Lacroix, C. Protective Effect of Probiotics on Salmonella Infectivity Assessed with Combined in Vitro Gut Fermentation-Cellular Models. BMC Microbiol. 2011, 11, 264. [Google Scholar] [CrossRef]
- Ostovan, R.; Pourmontaseri, M.; Hosseinzadeh, S.; Shekarforoush, S.S. Interaction between the Probiotic Bacillus subtilis and Salmonella typhimurium in Caco-2 Cell Culture. Iran. J. Microbiol. 2021, 13, 91–97. [Google Scholar] [CrossRef]
- Rousset, M. The Humancolon Carcinoma Cell Lines HT-29 and Caco-2: Two in Vitro Models for the Study of Intestinal Differentiation. Biochimie 1986, 68, 1035–1040. [Google Scholar] [CrossRef]
- Reddy, S.; Austin, F. Adhesion and Invasion Assay Procedure Using Caco-2 Cells for Listeria monocytogenes. Bio-Protocol 2017, 7, e2267. [Google Scholar] [CrossRef]
- Giannasca, K.T.; Giannasca, P.J.; Neutra, M.R. Adherence of Salmonella typhimurium to Caco-2 Cells: Identification of a Glycoconjugate Receptor. Infect. Immun. 1996, 64, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, A.; Laubitz, D.; Antushevich, H.; Zabielski, R.; Grzesiuk, E. Competition of Lactobacillus paracasei with Salmonella enterica for Adhesion to Caco-2 cells. J. Biomed. Biotechnol. 2008, 2008, 357964. [Google Scholar] [CrossRef] [PubMed]
- Finlay, B.B.; Brumell, J.H. Salmonella Interactions with Host Cells: In Vitro to In Vivo. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Mellor, G.E.; Goulter, R.M.; Raymond Chia, T.W.; Dykes, G.A. Comparative Attachment of Shiga Toxigenic Escherichia coli and Salmonella to Cultured HT-29 and Caco2 Cell Lines. Appl. Environ. Microbiol. 2009, 75, 1796–1799. [Google Scholar] [CrossRef]
- Steele-Mortimer, O. Infection of Epithelial Cells with Salmonella enterica. Methods Mol. Biol. 2008, 431, 201–211. [Google Scholar]
- Le Blay, G.; Fliss, I.; Lacroix, C. Comparative Detection of Bacterial Adhesion to Caco-2 Cells with ELISA, Radioactivity and Plate Count Methods. J. Microbiol. Methods 2004, 59, 211–221. [Google Scholar] [CrossRef]
- Cacopardo, L.; Costa, J.; Giusti, S.; Buoncompagni, L.; Meucci, S.; Corti, A.; Mattei, G.; Ahluwalia, A. Real-Time Cellular Impedance Monitoring and Imaging of Biological Barriers in a Dual-Flow Membrane Bioreactor. Biosens. Bioelectron. 2019, 140, 111340. [Google Scholar] [CrossRef]
- Fajdiga, S.; Koninkx, J.F.; Tooten, P.C.; Marinsek-Logar, R. Interference of Salmonella enteritidis and Lactobacillus spp. with IL-8 Levels and Transepithelial Electrical Resistance of Enterocyte-like Caco-2 Cells. Folia Microbiol. 2006, 51, 268–272. [Google Scholar] [CrossRef]
- Smith, I.M.; Baker, A.; Arneborg, N.; Jespersen, L. Non-Saccharomyces Yeasts Protect against Epithelial Cell Barrier Disruption Induced by Salmonella enterica subsp. enterica serovar typhimurium. Lett. Appl. Microbiol. 2015, 61, 491–497. [Google Scholar] [CrossRef]
- Hassan, Q.; Ahmadi, S.; Kerman, K. Recent Advances in Monitoring Cell Behavior Using Cell-Based Impedance Spectroscopy. Micromachines 2020, 11, 590. [Google Scholar] [CrossRef]
- Benson, K.; Cramer, S.; Galla, H.-J. Impedance-Based Cell Monitoring: Barrier Properties and Beyond. Fluids Barriers CNS 2013, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, N.; Abdolrazzaghi, M.; Light, P.; Musilek, P. In–Human Testing of a Non-Invasive Continuous Low–Energy Microwave Glucose Sensor with Advanced Machine Learning Capabilities. Biosens. Bioelectron. 2023, 241, 115668. [Google Scholar] [CrossRef] [PubMed]
- Abdolrazzaghi, M.; Kazemi, N.; Nayyeri, V.; Martin, F. AI-Assisted Ultra-High-Sensitivity/Resolution Active-Coupled CSRR-Based Sensor with Embedded Selectivity. Sensors 2023, 23, 6236. [Google Scholar] [CrossRef] [PubMed]
- Paivana, G.; Barmpakos, D.; Mavrikou, S.; Kallergis, A.; Tsakiridis, O.; Kaltsas, G.; Kintzios, S. Evaluation of Cancer Cell Lines by Four-Point Probe Technique, by Impedance Measurements in Various Frequencies. Biosensors 2021, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Paivana, G.; Mavrikou, S.; Kaltsas, G.; Kintzios, S. Bioelectrical Analysis of Various Cancer Cell Types Immobilized in 3D Matrix and Cultured in 3D-Printed Well. Biosensors 2019, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Paivana, G.; Apostolou, T.; Mavrikou, S.; Barmpakos, D.; Kaltsas, G.; Kintzios, S. Impedance Study of Dopamine Effects after Application on 2D and 3D Neuroblastoma Cell Cultures Developed on a 3D-Printed Well. Chemosensors 2019, 7, 6. [Google Scholar] [CrossRef]
- Pinto, M.; Robine-Leon, S.; Appay, M.D.; Kedinger, M.; Triadou, N.; Dussaulx, E.; Lacroix, B.; Simon-Assman, P.; Haffen, K.; Fogh, J.; et al. Enterocyte-like Differentiation and Polarization of the Human Colon Carcinoma Cell Line Caco-2 in Culture. Biol. Cell 1983, 47, 323–330. [Google Scholar]
- Zweibaum, A.; Pinto, M.; Chevalier, G.; Dussaulx, E.; Triadou, N.; Lacroix, B.; Haffen, K.; Brun, J.L.; Rousset, M. Enterocytic Differentiation of a Subpopulation of the Human Colon Tumor Cell Line HT-29 Selected for Growth in Sugar-Free Medium and Its Inhibition by Glucose. J. Cell. Physiol. 1985, 122, 21–29. [Google Scholar] [CrossRef]
- Connell, H.; Hedlund, M.; Agace, W.; Svanborg, C. Bacterial Attachment to Uro-Epithelial Cells: Mechanisms and Consequences. Adv. Dent. Res. 1997, 11, 50–58. [Google Scholar] [CrossRef]
- Ahmed, A.; Rushworth, J.V.; Hirst, N.A.; Millner, P.A. Biosensors for Whole-Cell Bacterial Detection. Clin. Microbiol. Rev. 2014, 27, 631–646. [Google Scholar] [CrossRef]
- Cencic, A.; Langerholc, T. Functional Cell Models of the Gut and Their Applications in Food Microbiology—A Review. Int. J. Food Microbiol. 2010, 141, S4–S14. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.R. Impedance Spectroscopy. Ann. Biomed. Eng. 1992, 20, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Ates, M. Review Study of Electrochemical Impedance Spectroscopy and Equivalent Electrical Circuits of Conducting Polymers on Carbon Surfaces. Prog. Org. Coat. 2011, 71, 1–10. [Google Scholar] [CrossRef]
- Singh, J.; Batish, V.K.; Grover, S. Simultaneous Detection of Listeria monocytogenes and Salmonella spp. in Dairy Products Using Real Time PCR-Melt Curve Analysis. J. Food Sci. Technol. 2012, 49, 234–239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rivnay, J.; Ramuz, M.; Leleux, P.; Hama, A.; Huerta, M.; Owens, R.M. Organic Electrochemical Transistors for Cell-Based Impedance Sensing. Appl. Phys. Lett. 2015, 106, 043301. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marka, S.; Zografaki, M.-E.; Papaioannou, G.-M.; Mavrikou, S.; Flemetakis, E.; Kintzios, S. Impedance In Vitro Assessment for the Detection of Salmonella typhimurium Infection in Intestinal Human Cancer Cells. Chemosensors 2023, 11, 534. https://doi.org/10.3390/chemosensors11100534
Marka S, Zografaki M-E, Papaioannou G-M, Mavrikou S, Flemetakis E, Kintzios S. Impedance In Vitro Assessment for the Detection of Salmonella typhimurium Infection in Intestinal Human Cancer Cells. Chemosensors. 2023; 11(10):534. https://doi.org/10.3390/chemosensors11100534
Chicago/Turabian StyleMarka, Sofia, Maria-Eleftheria Zografaki, George-Marios Papaioannou, Sofia Mavrikou, Emmanouil Flemetakis, and Spyridon Kintzios. 2023. "Impedance In Vitro Assessment for the Detection of Salmonella typhimurium Infection in Intestinal Human Cancer Cells" Chemosensors 11, no. 10: 534. https://doi.org/10.3390/chemosensors11100534
APA StyleMarka, S., Zografaki, M.-E., Papaioannou, G.-M., Mavrikou, S., Flemetakis, E., & Kintzios, S. (2023). Impedance In Vitro Assessment for the Detection of Salmonella typhimurium Infection in Intestinal Human Cancer Cells. Chemosensors, 11(10), 534. https://doi.org/10.3390/chemosensors11100534