Abstract
This work demonstrates the successful application of the picein wax carbon composite electrode (PWCCE) for profiling both commercial and homemade plant milks. Picein wax was utilized as an unconventional binder. The resulting electrode paste exhibited a solidified and hard texture, enabling its use in a manner analogous to that of the glassy carbon electrode. Differential pulse voltammetry (DPV) with an automated measurement and recording procedure was employed to obtain plant-based milk profiles. The utilization of operator-independent measurement procedures yielded high-quality electrochemical fingerprints suitable for subsequent calculations. To interpret the data, unsupervised machine learning methods were implemented, such as principal component analysis (PCA) and cluster analysis. These chemometric techniques confirmed the electrode effectiveness of the construction for this type of research. Moreover, they proved valuable in distinguishing between plant-based milk and cow’s milk, including two different variants: whole milk and lactose-free milk.
1. Introduction
Plant-based or vegan milks are a food product that is an alternative to cow’s milk. According to EU law, plant-based milks cannot be called milk, but plant-based drinks [1]. However, it is common practice to refer to these products as milk. Production of this type of food is necessary due to the large and constantly growing demand in the consumer market. These milks are the basis of the diet for people who follow a vegan diet [2] and promote an ecological lifestyle [3], but they are also an excellent substitute for animal milk for people who suffer from lactose intolerance [4]. Vegan milks are also a great choice for people with heart disease due to their low cholesterol content [5]. The use of plant-based drinks as a substitute for cow’s milk may affect the supplementation of micronutrients provided to the body. Vegan milks have lower levels of natural proteins, calcium, and vitamins [6]. Plant-based drinks can be produced in two different ways. The first is to grind the plant material and create an aqueous slurry. The second consists of creating a homogeneous emulsion of water, oil, and emulsifiers [7]. The technological development of ways of producing vegan milk is caused by social pressure and awareness. Consumers want to be able to use plant-based substitutes exactly the same way cow’s milk is used (heating or foaming) without changing its texture, taste, or appearance [8].
In recent years, a significant increase in the consumption of plant-based milk has been observed [9]. This situation creates the need to propose new methods to evaluate the quality of the product, verify its authenticity, and detect adulteration in the drinks. Such strategies must be characterized by high efficiency and a low price of analysis to be widely used in the food industry. One of these approaches is the combination of voltammetry and chemometrics. Numerous examples of the use of electrochemical fingerprints for profiling food samples can be found in literature reports. Profiles can be recorded using a set of electrodes or a single sensor, and the response in the form of a signal is the subject of further multivariate analysis. In [10], voltammetric measurements were applied using the cyclic voltammetry (CV) method with the use of an electronic tongue (ET) to create a database of vinegar fingerprints for subsequent chemometric analysis. Utilizing the differential pulse voltammetry technique (DPV) and a glassy carbon electrode (GC), the data was obtained during the study of medical plant profiles [11]. The same technique, but in combination with ET, was used during the analysis of seasonal changes in honeys [12] and while observing the maturation process of young wine [13]. An e-tongue consisting of eight metal wires (Au, Pt, Rh, Ir, Cu, Co, Ag, and Ni) was utilized in conjunction with LAPV to profile five vegan milks [14]. In addition to profiling and classifying food samples, voltammetry was also used to detect adulteration of food products. Voltammograms obtained using the CV measurement technique and a GC electrode were used to study the adulteration of olive oil [15], and a novel graphite/SiO2 hybrid electrode was used in cow milk analysis of the adulteration [16]. The DPV technique, or DPV combined with a voltammetric ET, has also been applied to detect the presence of glucose-fructose syrup added to apple juices [17].
In the most recently published articles, voltammetric food profiling uses a combination of several types of ET with supervised and unsupervised machine learning methods. However, there are examples of the use of a different type of working electrode. The idea of a CPE has been known for many years [18]. Over the last 60 years, many works have been published on its varieties, modifications, and proposals for new fabrication methods [19,20]. Therefore, newly emerging CPEs are dedicated electrochemical sensors for specific applications. Electrodes that use carbon paste in their construction can be modified not only by adding various organic or inorganic compounds but also by changing the form of carbon used to prepare the paste [21] or modifying the binder [22]. Such treatments aim to increase the sensitivity of the electrode to specific substances. Conventional carbon pastes consist of organic fluids that mechanically connect the individual graphite particles. Apart from its primary role, the binder also contributes to the development of electrode properties. Common criteria for selecting binder materials include chemical stability, electroinactivity, high viscosity, low volatility, minimal solubility in water-based solutions, and immiscibility with organic solvents. Mineral oils, such as paraffin, stand out as the most widely employed binding agents for the preparation of carbon pastes [23]. Additionally, unconventional substances as binders have been explored and described in the literature.
In [24], a novel heterogeneous carbon electrode was introduced, utilizing a solid binder in the form of phenanthrene. This choice of binder offered significant advantages in terms of its physical characteristics, including a melting point within the range of 98–100 °C and minimal volatility in both solid and liquid states. Since the binder remained in a solid state at room temperature, it allowed for the use of higher binder-to-carbon powder ratios, resulting in a strong affinity for lipophilic substances. The paper [25] described the construction and application of a mixed binder carbon paste electrode (MBCPE) containing a dimethylglyoxime system, where the binder consisted of liquid paraffin and glycerol. This sensor was used for the cathodic stripping voltammetric determination of mercury(II), cobalt(II), nickel(II), and palladium(II) in rice, tea, and human hair samples. The dispersion of zeolite particles in the bulk of a carbon paste matrix containing solid paraffin as a binder was proposed in [26]. This project displayed superior electrochemical performance in comparison to corresponding classical zeolite-modified carbon paste electrodes using mineral oil as a binder. The proposed electrode was applied in the voltammetric detection of Cu(II) ions after accumulation by ion exchange at open circuit and the indirect amperometric detection of non-electroactive species (i.e., Na(I)) in flow injection analysis. The replacement of a non-conductive organic binder with a conductive room-temperature ionic liquid in fabricating carbon paste electrodes was described in [27]. This new electrode, due to its enhanced conductivity, presented a very large current response from electroactive substrates and may be applied in physical chemistry and electroanalytical chemistry fields. In the paper [28], the authors studied ion transfer across a liquid–liquid interface by employing a carbon nanotube paste electrode in which an electroactive oil serves as the binding agent. This approach was illustrated through the investigation of electrochemically facilitated ion transfer processes at the interface between an aqueous solution and a redox liquid, specifically N,N-didodecyl-N′,N′-diethylphenylenediamine (DDPD). A new approach to constructing a carbon paste electrode involved mixing an ionic liquid, specifically 1-butyl-3-methylimidazolium hexafluorophosphate, with paraffin oil as a binding agent [23]. This electrode successfully addressed the challenge of high background current observed in carbon paste electrodes that exclusively employed ionic liquid as the binder. Notably, it exhibited superior signal-to-noise ratios and demonstrated enhanced electrochemical activity when compared to the conventional carbon paste electrode, which relied solely on paraffin oil as the binder. The research outlined in [29] detailed the utilization of uncured polydimethylsiloxane (PDMS) as a binding agent in the construction of carbon paste electrodes. The investigation demonstrated that PDMS stands out as a promising alternative binder, characterized by enhanced chemical and electrochemical stability, leading to improved analytical performance. Experimental techniques such as cyclic voltammetry, electrochemical impedance spectroscopy, and chronoamperometry consistently revealed that PDMS-based CPEs exhibited superior attributes, including higher electrical conductivity, an increased active electrochemical surface area, and a broader useful potential range when compared to Nujol®-based CPEs. They displayed enhanced stability when exposed to aqueous mixtures containing 50% (v/v) of ethanol or methanol. Furthermore, they exhibited higher sensitivity and a lower limit of detection in the analysis of propranolol. In the paper [22], a novel approach was introduced for the enhancement of carbon paste electrodes using natural deep eutectic solvents (NADES) as a modified binder. The cyclic voltammograms revealed notable improvements in both conductivity and charge transfer rates compared to the unmodified carbon paste electrodes, as evidenced by the reduced peak potential separation. The incorporation of a KCl solution into the NADES modifier effectively decreased the viscosity of the binder layer, leading to improved diffusion of the probe material to the graphite surface. The resultant carbon paste electrode, thus modified, exhibited superior performance in the oxidation of both dopamine and ascorbic acid. This enhancement was characterized by increased peak current values and a reduction in both the half-peak width and potential peak separation, particularly for dopamine.
In the study [30], a total of 12 distinct ionic liquids (ILs) were incorporated as co-binders in the preparation of modified carbon paste electrodes used for voltammetric analysis of dopamine in Britton-Robinson buffer. This approach yielded a consistent improvement in both the sensitivity and reversibility of dopamine oxidation. Notably, in square wave voltammetry experiments, the peak current exhibited a remarkable up to 400% increase when 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide was employed as the co-binder, as compared to the response obtained with the unmodified CPE. The measured data support the notion that electrostatic and steric effects play a predominant role as electrocatalytic factors in the anodic oxidation of dopamine on IL-CPEs. The paper [31] introduced the development of a cost-effective electrode featuring a renewable surface for the determination of sulfite via electroreduction. This innovative carbon paste electrode was composed of multi-walled carbon nanotubes (MWCNTs) and employed a binder comprising a blend of mineral oil and an ionic liquid, N-octylpyridinium hexafluorophosphate. This novel electrode design facilitated the creation of high-conductivity sensors that are not only economical and straightforward to prepare but also possess electrocatalytic properties. In [32], an electrochemical sensor was developed using carbon paste modified with CdO/SWCNT as a catalyst and 1-ethyl-3-methylimidazolium trifluoromethanesulfonate (1E3MITFS) as a binder for the quantification of bisphenol-A. The heightened effectiveness of this new electrode can be attributed to the synergistic interaction between the nanocatalyst and 1E3MITFS, resulting in an approximately 3.5-fold increase in the detected signal for bisphenol-A. The augmentation of electrical conductivity facilitated the electron transfer process of bisphenol-A, consequently amplifying the electrochemical sensitivity of the sensor.
The aim of the presented work is to introduce a picein wax carbon composite electrode (PWCCE) made of graphite powder and picein wax for food profiling. In this study, we are testing a single electrode for this purpose; however, chemometric calculations take into consideration the entire recorded signal (voltammogram). We have previously employed a similar construction in quantitative analysis for the determination of europium in aqueous solutions [33]. For the purposes of this study, the design and manufacture of the sensor have been optimized. Now, this electrode has been used as a sensor to distinguish between various commercial and homemade plant-based milks. The study presents the profiling of nine commercial vegan milks representing various groups of plants from which such drinks are obtained, as well as five other drinks that were made in the laboratory just before the experiment. For comparison purposes, two non-vegan milks, regular cow’s milk and lactose-free milk, were considered.
2. Experimental
2.1. Apparatus
In this work, a multifunctional electrochemical analyzer M161 (MTM-ANKO, Cracow, Poland) with a compatible electrode stand (MTM-ANKO, Cracow, Poland) was used to record all voltammetric profiles of plant milk. The signals were measured using a standard electrode system. A PWCCE was applied as the working electrode, a double junction Ag|AgCl|Cl (3 M) as the reference electrode, and a platinum wire as the auxiliary electrode.
2.2. Preparation of the Working Electrode
While using carbon paste electrodes, the key problem is preparing the appropriate paste. In the construction of the proposed sensor, an electrode paste with picein (ROTH) was applied as a binder, which is a solid, hard black wax and graphite powder (Acros Organics, Germany; CAS: 7782-42-5). Previous experience has shown that the best quality paste is made by mixing graphite powder with a binder in a 1:1 ratio. For this purpose, exactly 1 g of both substrates were weighed. The portions were quantitatively transferred to a quartz crucible, and the whole was flooded with about 5 mL of ether (Sigma-Aldrich, St. Louis, MO, USA; CAS: 60-29-7). The addition of ether at this stage was intended to dissolve the wax, ensure the best possible homogenization of the mixture, and not introduce modifications to the final product. After 3 h and partial evaporation of the solvent, the mixture was warmed in a heated sand bath at 100 °C until the wax dissolved while stirring. The hot paste obtained in this way was placed in a previously prepared Teflon body with a hole cut with a diameter of 3 mm and a depth of 8 mm for the electrode paste, and a steel wire with a diameter of 1.4 mm passed through the center to ensure electrical contact between the stand and the paste. After first smoothing the hot mixture, the filled body was left at room temperature for 24 h to cool and harden the electrode paste.
The next day, the electrode was ground using abrasive papers with decreasing granularity. After grinding, the electrode was polished on a polishing cloth using aluminum oxide polishing pastes in a manner analogous to that used for traditional glassy carbon electrodes.
2.3. Automatic Measuring Procedure
To ensure the highest quality of the results, the profiles were recorded automatically. To obtain a greater conductivity of the solution and, as a result, a higher profile resolution, it was necessary to use a supporting electrolyte. The best results were obtained using 4.5 mL of 0.1 M ammonium buffer, pH = 9.0, and 0.5 mL of sample. The voltammograms were recorded in a wide window of potentials, from −1200 to 1000 mV, with a potential step of 2 mV. The waiting and sampling times were the same and were 20 ms each. Additionally, a 15-second preconcentration time at the initial potential was used. Under such conditions, 12 cycles were recorded for each milk sample.
2.4. Plant Milk Sample and Preparation
In this work, nine commercially available plant milks from various producers were used (Table 1). Milks have been selected so that none of them have added sugar and that they represent all six types of plant raw materials used to produce this type of beverage [34]. The oat and rice milks belong to the plant milks obtained from grains (cereals). Quinoa milk is a drink obtained from pseudocereals. Soy milk is obtained from legumes. Hemp milk represents a group of milks obtained from seeds. Coconut, almond, and cashew milk are groups obtained from nuts, while tiger nut milk belongs to the group obtained from tubers.

Table 1.
Summary of the tested plant milks and the composition declared by the producer.
In addition, plant milk produced in the laboratory from various plant materials was tested. For this purpose, according to the procedure described in Table 2, five different plant milks were prepared, which also represented various sources of plant raw materials. For comparison, during the research, two commercial cow’s milks (regular and lactose-free) were used, which, according to the manufacturer’s declaration, contained 3.2% fat.

Table 2.
The procedure for the preparation of homemade plant milk used in this work.
2.5. Software
In all measurements, the EAQt program with the EACfg extension (MTM-ANKO, Cracow, Poland) was used for the automatic procedure to control the electrochemical analyzer and record the voltammetric profiles. All calculations were carried out using Matlab 2014b with PLS Toolbox and Matlab 2021a.
3. Results and Discussion
The concept of the entire work was to use a new carbon composite electrode to record the voltammetric profiles of vegan milks of various origins. For this purpose, an electrode paste with picein wax as a binder was prepared. It was used to obtain the electrochemical fingerprints of each tested sample. The DPV technique was utilized with optimized parameters. Measurements were performed automatically, recording the entire current in the anodic and cathodic directions. The results obtained were analyzed using unsupervised machine learning methods. A graphical presentation of the workflow is shown in Figure 1.
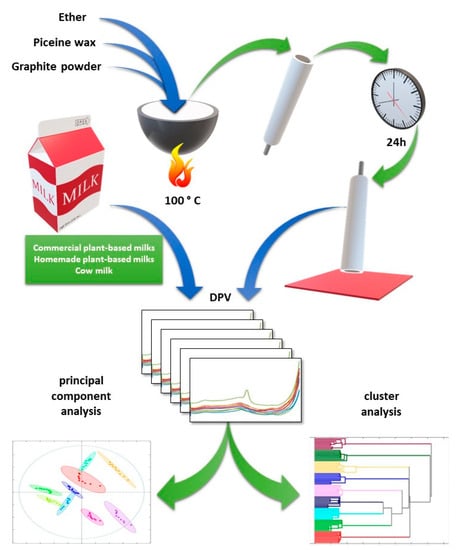
Figure 1.
Workflow of the proposed approach to plant milk voltammetric profiling with the construction of a picein wax carbon paste electrode.
Figure 2 shows examples of voltammetric signals for all milks that were used in the work. The presented voltammograms did not undergo any preprocessing, data filtering, averaging, or baseline correction. All voltammetric profiles were recorded using an automatic procedure using a supporting electrolyte. The application of an automatic procedure made it possible to ensure an ideal time regime during profile registration. The importance of this approach to the registration of voltammetric fingerprints has been confirmed by numerous previous experiences working with this type of sample. Figure 2a,b shows the recorded anodic signals (Figure 2a) and cathodic signals (Figure 2b) for the nine tested plant-based milks commercially available in the local supermarket. The recorded signals indicated the great potential of the electrochemical sensor used to obtain high-quality data for profiling this type of food product. Differences between recorded profiles can be observed in both the anodic and cathodic directions, which proves the effectiveness of the applied approach. Figure 2c,d shows the recorded anodic and cathodic signals for home-made plant milks prepared just before the measurements. When comparing the milk profiles obtained in the laboratory with their analogous commercial counterparts, it can be noticed that the signal for the milk produced immediately before the measurements is more extensive and the recorded current has higher levels. For comparison and control of the results, cow’s milk profiles were also recorded in two variants: regular cow’s milk and lactose-free milk (Figure 2e,f). It can be observed that the milk profiles clearly differ from the profiles of its vegetable substitutes.
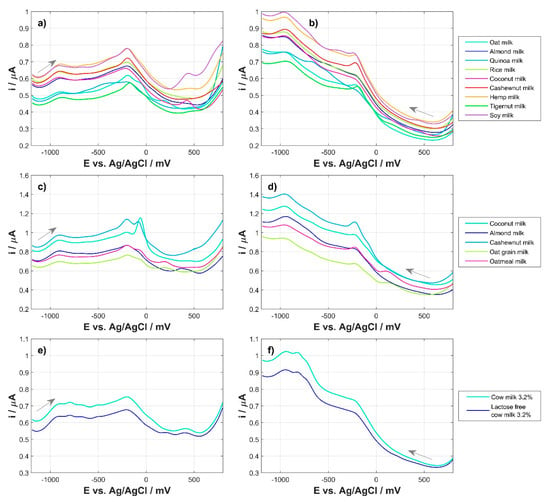
Figure 2.
Averaged DPV runs for: commercial plant milk: (a) anodic and (b) cathodic; homemade plant milk: (c) anodic and (d) cathodic; cow’s milk: (e) anodic and (f) cathodic.
Before comparing the DPV profiles of plant milk, a baseline correction with a third-degree polynomial was applied to each recorded signal. The background was adjusted for each of the plant milks individually, both for the anodic and cathodic directions. The anodic signals that were used in further analysis are shown in Figure 3a–i. The profiles of all milks have one thing in common: a clear peak at a potential of about −200 mV. The elements that distinguish individual milks are other smaller peaks occurring at a potential of about 200 mV (oat milk) or 450 mV (coconut and soy milk). It is possible to discern the characteristic, asymmetric shape of the quinoa milk profile. Figure 3j shows the projection of points on the PC1/PC2 plane that describes 61.22% of the data variability. The effect of using the PCA can be considered beneficial because individual samples form homogeneous clusters at clear distances from each other.
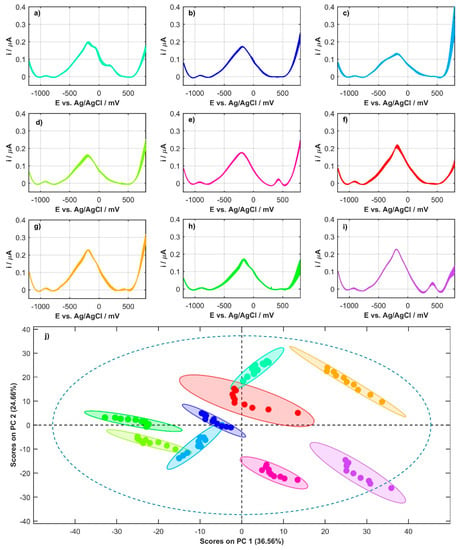
Figure 3.
All anodic signals for commercial plant milks with a subtracted background: (a) oat milk, (b) almond milk, (c) quinoa milk, (d) rice milk, (e) coconut milk, (f) cashew milk, (g) hemp milk, (h) tiger nut milk, and (i) soy milk; (j) projection of points in the PC1/PC2 plane for all milks (color of points corresponds with color of voltammograms).
In the next stage of work, all home-made milks were analyzed. Various types of nuts (almonds, cashews, and coconut) and seeds (oats) were used to prepare the plant drinks according to the recipe in Table 2. The raw materials that have been used to produce homemade vegan milks have been selected so that they have commercial equivalents. Profile registration and baseline correction were analogous to commercial milk. Figure 4a–d shows the averaged cathodic profiles of commercial milk (darker colors) and homemade milk (lighter colors). A comparison of homemade milk and its commercial counterparts shows clear differences between them. The greatest differences are in the case of coconut milk and cashew milk, while the smallest differences are in the case of almond drinks. The differences between the profiles may be due to the composition of commercial milks, which are very complex and extensive compared to their domestic counterparts. It should be noted that homemade milk was prepared immediately before measurements and can be characterized by an increased content of substances that can be subject to oxidation or degradation. The observations were confirmed by PCA analysis and projection of points onto the PC1/PC2 plane. The projection presented in Figure 4e describes 76.66% of the data variability that confirms previous observations of differences between commercial and homemade milks. Furthermore, the projection of points onto the plane of the first two principal components, PC1 and PC2, shows that points representing commercial milks form clusters at small distances from each other, whereas domestic milks form clusters clearly separated from commercial milks and each other. An exceptional case is the group of oat milks, in which two different sources of plant material, oat grains and oat flakes, were used to produce home-made milk. Despite the significant differences between the substrates used to prepare vegan drinks, the projections of these points on the plane of the principal components are very close and form a single cluster of objects.
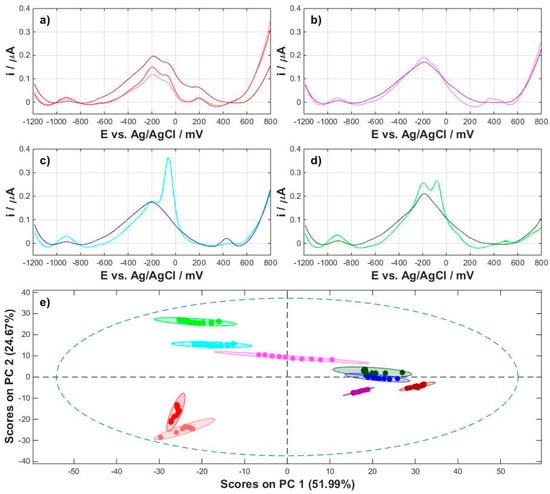
Figure 4.
Comparison of the averaged cathodic signals for commercial and homemade plant milks with a subtracted background: (a) dark red—commercial oat milk, red—homemade oat mil, light red—homemade oatmeal milk; (b) dark magenta—commercial almond milk, magenta—homemade almond milk; (c) dark blue—commercial coconut milk, blue—homemade coconut milk; (d) dark green—commercial cashew milk, green—homemade cashew milk; (e) projection of points in the PC1/PC2 plane for all milks (color of points corresponds to color of voltammograms).
Since plant milks were created as a market response to consumer demand and are a substitute for cow’s milk in the daily diet, the next step was to compare cow’s milk with its vegetable substitutes. Cow’s milk was tested in two variants: regular milk with 3.2% fat content and lactose-free milk, also with 3.2% fat. The cow’s milk profiles were registered in a similar way as the others. The same was carried out with the baseline correction. The anodic and cathodic profiles are shown for regular milk in Figure 5a and for milk without lactose in Figure 5b. Most important, however, is the comparison of cow’s and plant milk profiles, which was performed using a principal component analysis. The projection of the points on the plane of the principal components (Figure 5c), describing 81.32% of the data variability for the cathodic profiles, shows the effect of plant milk grouping into one cluster with clearly spaced points representing cow’s milk in both variants.
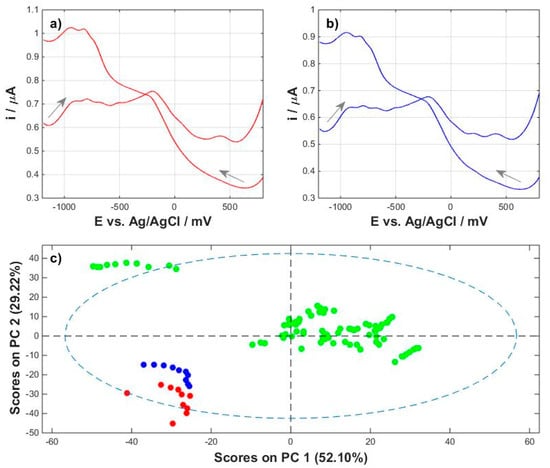
Figure 5.
Comparison of cow’s milk profiles with plant milk profiles: (a) averaged anodic and cathodic waveforms for regular cow’s milk 3.2%; (b) averaged anodic and cathodic waveforms for lactose-free cow’s milk 3.2%; (c) projection of points in the PC1/PC2 plane, cathodic waveform: green—plant milk, red—regular cow’s milk 3.2%, blue—lactose-free cow’s milk 3.2%.
To assess the repeatability of the electrode signal, the coefficient of variation (CV) for milk and vegan profiles was examined. This coefficient was calculated as the ratio of the standard deviation to the average value calculated from the values at maximum current. The results obtained for all groups of signals are presented in Table 3. CV values oscillate within the range of 0.26–3.97%, which, according to established standards, is within the acceptable level of 5% variability.

Table 3.
Evaluation of electrode signal repeatability. The values of the coefficient of variation for the tested commercial plant and cow’s milk were calculated for the maximum value of the signal.
In the last stage, the collected profiles were analyzed using cluster analysis. The dendrogram in Figure 6 shows that the tested commercial plant milks are clearly grouped within their respective categories, and differences between individual classes of objects are visible. A similar approach was applied to homemade plant milk, and the corresponding dendrogram is illustrated in Figure 7. This visualization confirms the conclusions that can be drawn from the analysis of Figure 4e. Homemade oat milks form a cluster on the PCA plane and one branch of the dendrogram, while cashew, coconut, and almond milk form the second group of objects.
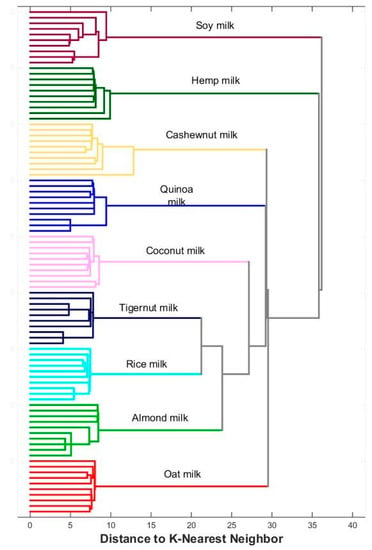
Figure 6.
Dendrogram for commercial plant milks.
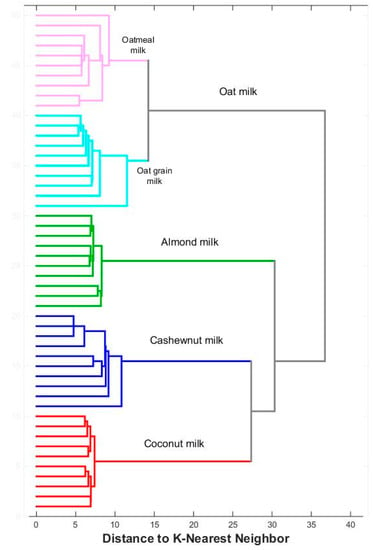
Figure 7.
Dendrogram for homemade plant milks.
4. Conclusions
This work shows that the proposed picein–carbon composite electrode can be used as a dedicated sensor to profile milks of various origins. The optimized approach covers the entire research process, from the production of the electrode paste to the optimization of the measurement parameters and the performance of the measurements themselves, and finally to the chemometric analysis. Considering the limited number of reports in the literature on the electrochemical profiling of vegan milks, this study can be considered an introductory step in developing the proposed topic.
The use of the DPV technique combined with a dedicated automatic measurement procedure ensured high-quality data. Plant-based milk profile analysis using electrochemical fingerprints was conducted in two stages. The first stage involved the study of nine vegan milks from a local supermarket. It was observed that all milks under consideration exhibited different profiles, which were visualized using the PCA technique. The second stage focused on preparing homemade plant milks and subjecting them to similar analysis. Each of the prepared homemade milks had a corresponding commercial milk counterpart. All homemade plant-based drinks were prepared according to a standardized recipe just before the start of measurements. The results showed that homemade plant-based milks differed from their commercial counterparts. Particular attention should be paid to the amount of ingredients used to prepare a homemade plant-based drink and the composition of its commercial equivalent, as declared by the manufacturer.
Because plant milks were developed to replace cow’s milk in the daily diet, the profiles of both groups of milks were compared. For this purpose, the profiles of regular milk and lactose-free milk were recorded. The voltammograms collected in this way were compared with the fingerprints of the plant milk. The profiles of plant drinks and cow’s milk were shown to clearly differ from each other, while the difference in profiles between regular milk and lactose-free milk was found to be small.
Author Contributions
S.W.: data curation; formal analysis; investigation; resources; visualization; and writing—original draft. J.W.: conceptualization; methodology; and investigation; F.C.: conceptualization; control software; funding acquisition; and methodology. M.J.: conceptualization; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; supervision; validation; and writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
A research project supported by the program “Excellence Initiative—Research University” from the AGH University of Science and Technology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available upon request to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- European Commission Regulation (EU) No 605/2010: Laying down Animal and Public Health and Veterinary Certification for the Introduction into the European Union of Raw Milk and Dairy Products Intended for Human Consumption. Off. J. Eur. Union 2010, 175, 1–24.
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-Based Milk Alternatives an Emerging Segment of Functional Beverages: A Review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef] [PubMed]
- Alae-Carew, C.; Green, R.; Stewart, C.; Cook, B.; Dangour, A.D.; Scheelbeek, P.F.D. The Role of Plant-Based Alternative Foods in Sustainable and Healthy Food Systems: Consumption Trends in the UK. Sci. Total Environ. 2022, 807, 151041. [Google Scholar] [CrossRef] [PubMed]
- Suri, S.; Kumar, V.; Prasad, R.; Tanwar, B.; Goyal, A.; Kaur, S.; Gat, Y.; Kumar, A.; Kaur, J.; Singh, D. Considerations for Development of Lactose-Free Food. J. Nutr. Intermed. Metab. 2019, 15, 27–34. [Google Scholar] [CrossRef]
- Chalupa-Krebzdak, S.; Long, C.J.; Bohrer, B.M. Nutrient Density and Nutritional Value of Milk and Plant-Based Milk Alternatives. Int. Dairy J. 2018, 87, 84–92. [Google Scholar] [CrossRef]
- Haas, R.; Schnepps, A.; Pichler, A.; Meixner, O. Cow Milk versus Plant-Based Milk Substitutes: A Comparison of Product Image and Motivational Structure of Consumption. Sustainability 2019, 11, 5046. [Google Scholar] [CrossRef]
- McClements, D.J.; Newman, E.; McClements, I.F. Plant-Based Milks: A Review of the Science Underpinning Their Design, Fabrication, and Performance. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Development of Next-Generation Nutritionally Fortified Plant-Based Milk Substitutes: Structural Design Principles. Foods 2020, 9, 421. [Google Scholar] [CrossRef]
- Islam, N.; Shafiee, M.; Vatanparast, H. Trends in the Consumption of Conventional Dairy Milk and Plant-Based Beverages and Their Contribution to Nutrient Intake among Canadians. J. Hum. Nutr. Diet. 2021, 34, 1022–1034. [Google Scholar] [CrossRef]
- Cetó, X.; Pérez, S. Voltammetric Electronic Tongue for Vinegar Fingerprinting. Talanta 2020, 219, 121253. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhou, Z.; Shen, Z.; Chen, J.; Gu, C.; Li, L.; Chen, F.; Liu, H. Electrochemical Fingerprinting Combined with Machine Learning Algorithm for Closely Related Medicinal Plant Identification. Sens. Actuators B Chem. 2023, 375, 132922. [Google Scholar] [CrossRef]
- Wójcik, S.; Ciepiela, F.; Baś, B.; Jakubowska, M. Deep Learning Assisted Distinguishing of Honey Seasonal Changes Using Quadruple Voltammetric Electrodes. Talanta 2022, 241, 123213. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, S.; Górski, Ł.; Jakubowska, M. Observation of Spontaneous Maturation Process of Young Wine by Application of the Voltammetric Quadruple Disk Iridium Sensor Combined with Chemometrics. J. Electrochem. Soc. 2021, 168, 026514. [Google Scholar] [CrossRef]
- Pascual, L.; Gras, M.; Vidal-Brotóns, D.; Alcañiz, M.; Martínez-Máñez, R.; Ros-Lis, J.V. A Voltammetric E-Tongue Tool for the Emulation of the Sensorial Analysis and the Discrimination of Vegetal Milks. Sens. Actuators B Chem. 2018, 270, 231–238. [Google Scholar] [CrossRef]
- Tsopelas, F.; Konstantopoulos, D.; Kakoulidou, A.T. Voltammetric Fingerprinting of Oils and Its Combination with Chemometrics for the Detection of Extra Virgin Olive Oil Adulteration. Anal. Chim. Acta 2018, 1015, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, P.; Deskoulidis, E.; Topoglidis, E.; Kakoulidou, A.T.; Tsopelas, F. Application of Chemometrics for Detection and Modeling of Adulteration of Fresh Cow Milk with Reconstituted Skim Milk Powder Using Voltammetric Fingerpriting on a Graphite/ SiO2 Hybrid Electrode. Talanta 2020, 206, 120223. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, S.; Jakubowska, M. Deep Neural Networks in Profiling of Apple Juice Adulteration Based on Voltammetric Signal of the Iridium Quadruple-Disk Electrode. Chemom. Intell. Lab. Syst. 2021, 209, 104246. [Google Scholar] [CrossRef]
- Adams, R.N. Carbon Paste Electrodes. Anal. Chem. 1958, 30, 1576. [Google Scholar] [CrossRef]
- Švancara, I.; Vytřas, K.; Kalcher, K.; Walcarius, A.; Wang, J. Carbon Paste Electrodes in Facts, Numbers, and Notes: A Review on the Occasion of the 50-Years Jubilee of Carbon Paste in Electrochemistry and Electroanalysis. Electroanalysis 2009, 21, 7–28. [Google Scholar] [CrossRef]
- Svancara, I.; Kalcher, K.; Walcarius, A.; Vytras, K. Electroanalysis with Carbon Paste Electrodes; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9780429152078. [Google Scholar]
- Kalinke, C.; de Oliveira, P.R.; Bonet San Emeterio, M.; González-Calabuig, A.; del Valle, M.; Salvio Mangrich, A.; Humberto Marcolino Junior, L.; Bergamini, M.F. Voltammetric Electronic Tongue Based on Carbon Paste Electrodes Modified with Biochar for Phenolic Compounds Stripping Detection. Electroanalysis 2019, 31, 2238–2245. [Google Scholar] [CrossRef]
- Cariati, L.S.S.; Buoro, R.M. Evaluation of Ionic Natural Deep Eutectic Solvents (NADES) Modified Binders towards the Chemical Properties of Carbon Paste Electrodes. Electrochem. Commun. 2019, 109, 106605. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Zhang, L.; Wang, H. A Bismuth Modified Hybrid Binder Carbon Paste Electrode for Electrochemical Stripping Detection of Trace Heavy Metals in Soil. Int. J. Electrochem. Sci. 2012, 7, 12326–12339. [Google Scholar] [CrossRef]
- Diewald, W.; Kalcher, K.; Neuhold, C.; Švancara, I.; Cai, X. Voltammetric Behaviour of Thallium at a Solid Heterogeneous Carbon Electrode Using Ion-Pair Formation. Analyst 1994, 119, 299–304. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Liu, H.; Zhang, H.; Li, Y.-F. Simultaneous Cathodic Stripping Voltammetric Determination of Mercury, Cobalt, Nickel and Palladium by Mixed Binder Carbon Paste Electrode Containing Dimethylglyoxime. Anal. Chim. Acta 1996, 333, 119–124. [Google Scholar] [CrossRef]
- Walcarius, A.; Mariaulle, P.; Lamberts, L. Zeolite-Modified Solid Carbon Paste Electrodes. J. Solid State Electrochem. 2003, 7, 671–677. [Google Scholar] [CrossRef]
- Liu, H.; He, P.; Li, Z.; Sun, C.; Shi, L.; Liu, Y.; Zhu, G.; Li, J. An Ionic Liquid-Type Carbon Paste Electrode and Its Polyoxometalate-Modified Properties. Electrochem. Commun. 2005, 7, 1357–1363. [Google Scholar] [CrossRef]
- Kachoosangi, R.T.; Xiao, L.; Wildgoose, G.G.; Marken, F.; Bulman Page, P.C.; Compton, R.G. A New Method of Studying Ion Transfer at Liquid|Liquid Phase Boundaries Using a Carbon Nanotube Paste Electrode with a Redox Active Binder. J. Phys. Chem. C 2007, 111, 18353–18360. [Google Scholar] [CrossRef]
- Araújo, D.A.G.; Pradela-Filho, L.A.; Santos, A.L.R.; Faria, A.M.; Takeuchi, R.M.; Karimi-Maleh, H.; Santos, A.L. Uncured Polydimethylsiloxane as Binder Agent for Carbon Paste Electrodes: Application to the Quantification of Propranolol. J. Braz. Chem. Soc. 2019, 30, 1988–1998. [Google Scholar] [CrossRef]
- Hernández-Vargas, S.G.; Alberto Cevallos-Morillo, C.; Aguilar-Cordero, J.C. Effect of Ionic Liquid Structure on the Electrochemical Response of Dopamine at Room Temperature Ionic Liquid-modified Carbon Paste Electrodes (IL–CPE). Electroanalysis 2020, 32, 1938–1948. [Google Scholar] [CrossRef]
- Villalobos, M.B.; Ibarra, J.; Gidi, L.; Cavieres, V.; Aguirre, M.J.; Ramírez, G.; Arce, R. Electrochemical Detection of Sulfite by Electroreduction Using a Carbon Paste Electrode Binder with N-Octylpyridinium Hexafluorophosphate Ionic Liquid. Catalysts 2022, 12, 1675. [Google Scholar] [CrossRef]
- Mosavi, S.M.; Ebrahimi, M.; Beyramabadi, S.A.; Mohseni, S. A Sensitive Method for Determination of Bisphenol-a Based on Carbon Paste Electrode Modified by CdO/SWCNT and 1-Ethyl-3-Methylimidazolium Trifluoromethanesulfonat as a Binder. J. Food Meas. Charact. 2023, 17, 5352–5359. [Google Scholar] [CrossRef]
- Wójcik, S.; Wyrwa, J.; Dziubaniuk, M.; Jakubowska, M. Unconventional Solid-like Picein Wax Carbon Paste Electrode in Europium Determination. Ionics 2020, 26, 2643–2653. [Google Scholar] [CrossRef]
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of Plant-Based Milk Alternatives for Improved Flavour and Nutritional Value. Appl. Microbiol. Biotechnol. 2019, 103, 9263–9275. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).